Since the SARS‐CoV‐2 pandemic began, countries across the globe have seen repeated periods of government‐enforced social distancing to restrict transmission in response to rises in COVID‐19 incidence. 1 Social distancing in the UK was first introduced in March 2020, and for six of the following 12 months the population was under strict national restrictions. This led to substantially reduced contact between different households, 2 with mass gatherings prohibited.
Prior to this pandemic, respiratory viral infections were a common cause of morbidity amongst haematology patients, 3 including those immunosuppressed or post haematopoietic stem cell transplantation (HSCT), with reported incidences of 3–30% per year. 4 , 5 Respiratory syncytial virus (RSV) is the virus most likely to progress from upper respiratory tract infection to pneumonia in patients who have undergone HSCT, 6 , 7 , 8 and the British Society of Haematology recommends ribavirin and intravenous immunoglobulin in this group. 9 Influenza, parainfluenza, and adenovirus can all cause severe disease in the immunocompromised, and SARS‐CoV‐2 has been shown to cause severe disease and excess mortality in those who have undergone HSCT and some haematological malignancies. 10 , 11
Regional surveillance networks have noted a significant reduction in influenza transmission in both southern 12 and northern hemispheres 13 (https://www.cdc.gov/flu/weekly/index.html) during their respective winter seasons in 2020–21. This has been attributed to non‐pharmacological interventions and social distancing introduced to reduce SARS‐CoV‐2 transmission.
Here, we describe the impact of UK social distancing measures on the incidence of non‐SARS‐CoV‐2 respiratory viral infection in a cohort of haematology patients.
Methods
Data were obtained from Oxford University Hospitals (OUH), a large UK teaching hospital. BioFire FilmArray (bioMérieux, Basingstoke) multiplex polymerase chain reaction (PCR) was performed on respiratory samples to detect the presence of respiratory viruses (influenza A & B, parainfluenza 1–4, RSV, human enterovirus/rhinovirus, coronaviruses 229E, HKU1, NL63, OC43, adenovirus, as well as Mycoplasma pneumoniae) as part of routine care of haematology patients presenting with respiratory symptoms or fever. It is comparable in performance to several other similar platforms, 14 and has been updated to include SARS‐CoV‐2 detection.
All PCR results performed during haematology inpatient admissions (including encounters to the triage unit) were obtained from the Infections in Oxfordshire Research Database which has generic Research Ethics Committee, Health Research Authority and Confidentiality Advisory Group approvals (19/SC/0403, 19/CAG/0144). Data were also obtained on baseline characteristics including gender, age, ethnicity, haemato‐oncological or benign haematology specialist care, and comorbidity indices (Charlson comorbidity index, CCI).
We used piecewise linear regression to model changes in admissions over time, and Poisson regression to model changes over time in incidence of testing per 100 admissions for respiratory viruses, allowing for a step change in incidence from March to April 2020, that is, around the introduction of the first UK national lockdown on 16 March 2020, and a linear trend before and after this. We do not adjust for seasonality and so trends presented represent averages across seasonal peaks and troughs.
Results
Between 1 January 2016 and 28 February 2021, across all haematological specialities at OUH, there were 22 536 inpatient encounters. Median age of patients was 59 (interquartile range, IQR, 18–71), 13 534 (60%) were male, 14 940/16 502 (91%) with recorded ethnicity were white and 21 817 (97%) were managed by a haemato‐oncologist. Across this period, 3 380 BioFire PCR tests were performed in 2 719 haematology inpatients. Tested patients were older than untested patients, median (IQR) age 61 (49–70) vs 58 (16–71) years (P < 0·001) and had more comorbidities, CCI median (IQR) 8 (0–8) vs 0 (0–0) (P < 0·001; Table SI). There was no evidence of a difference by sex or ethnicity.
The number of encounters remained stable across 2016–2019 [estimated change per month in daily rate 0·00 (95% CI −0·02, 0·02; P = 0·88)] but fell markedly at the start of the pandemic [change in daily rate −4·99 (−6·25, −3·74)], and there was a trend towards a rise following [0·17 (−0·02, 0·36; P = 0·08); Fig 1A]. Between January 2016 and March 2020 there were 12·5 admissions per day, and 8·5 admission per day from April 2020 to Febuary 2021.
Fig 1.
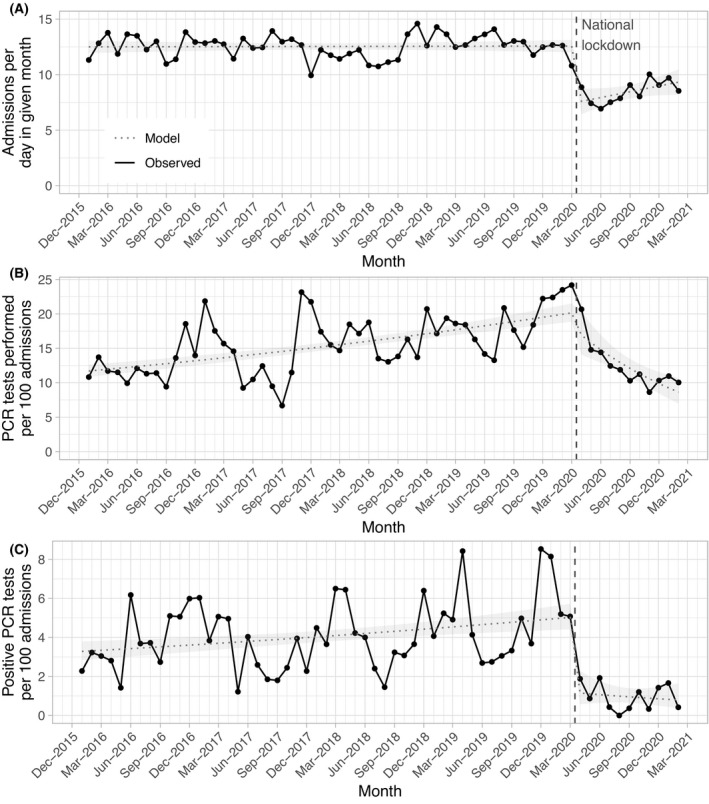
(A) Monthly haematology inpatient admissions. (B)Monthly respiratory virus polymerase chain reaction (PCR) testing rates. (C)Monthly respiratory virus detection rates. The black line indicates observed values and the blue dotted line the modelled incidence, with the ribbon indicating the 95% confidence interval. The start of UK national social distancing restrictions is shown as a dashed red vertical line.
Use of PCR testing was rising prior to the pandemic [monthly increase in tests per 100 admissions, 0·01 (95% CI 0·01, 0·01; P < 0·001] but fell during the pandemic [−0·08 (−0·11, −0·04; P < 0·001)], with marginal evidence for a step change at the start [−0·19 (−0·38, 0·01; P = 0·06); Fig 1B]. The number of positive PCR tests was rising similarly prior to the pandemic [monthly increase in positive tests per 100 admissions 0·01 (95% CI 0·00, 0·01; P < 0·001)] but fell by substantially more than could be explained by reduced testing alone at the start of the pandemic [−1·48 (−2·18, −0·78; P < 0·001)] and remained stably low following this [−0·05 (−0·16, 0·07; P = 0·46; Fig 1C].
Overall, there were 974 positive PCR tests, 943 during 19 465 admissions (4·8/100 admissions) between January 2016 and March 2020 and 31 during 2 951 admissions (1·1/100 admissions) between April 2020 and Febuary 2021. The most frequently detected pathogens were rhinovirus/enterovirus (detected using the same PCR target; 385, 40%), parainfluenza virus (139, 14%), non‐SARS‐CoV‐2 coronaviruses (128, 13%) and RSV (102, 10%; Fig 2). Our data also show expected seasonal variation in common and clinically relevant viruses. Typical seasonal patterns are notable during 2016–2019 with winter peaks of RSV, rhinovirus/enterovirus and coronaviruses. A rapid and sustained fall in all viruses is seen from the second quarter of 2020 until last follow‐up when UK social distancing measures remain in place.
Fig 2.
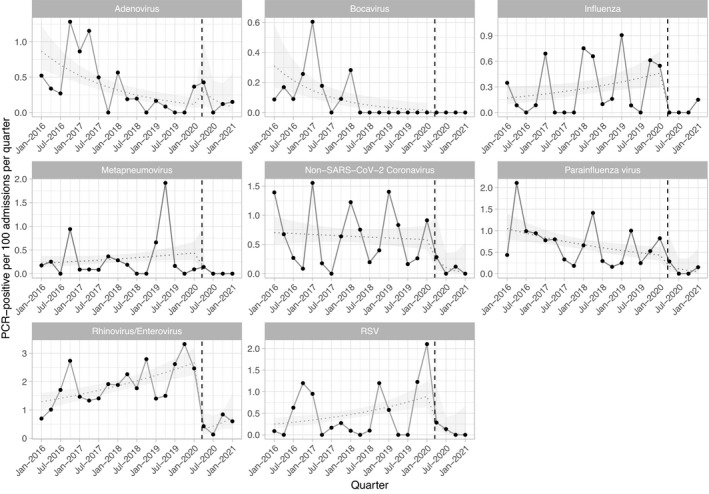
Quarterly incidence of respiratory virus infection in haematology inpatients. For each virus the dashed line indicates the modelled incidence, and the ribbon the 95% confidence interval, allowing for a linear trend before and after the onset of UK national social distancing restrictions, and a step change at this point (shown with a vertical dashed red line). PCR, polymerase chain reaction.
Discussion
Our results show a clear, rapid decline in the incidence of pathogenic non‐COVID‐19 respiratory viruses in our cohort of haematology patients since the introduction of UK social distancing and other infection control measures. This mirrors a decline in detection rates of other respiratory viruses, including influenza, in the general population. 12 , 13 RSV, influenza and adenovirus can be particularly pathogenic in the immunosuppressed, including those who have undergone HSCT or other T‐cell‐depleting therapies, who form a significant proportion of our cohort. Although we have not directly studied mortality overall or in particular patient groups, based on historical data 6 , 7 , 8 it can reasonably be inferred that the reduction in incidence seen may have led to a concurrent reduction in viral‐related mortality.
It is predicted that there may be a rebound in RSV incidence once social distancing measures are relaxed, 15 due to increased RSV susceptibility following reduced transmission during COVID‐19‐related restrictions. If this occurs, ongoing risk mitigation policies regarding personal social distancing and face mask use by subsets of haematology patients could be considered. However, immunosuppressed patients and their clinicians will need to weigh infection‐related risks against the impact of social restrictions on patients’ overall well‐being, particularly given the restrictions already faced during the pandemic. We advocate for the ongoing surveillance of these pathogenic respiratory viral infections in the general population, to allow informed discussions with our patients, and counselling of the risks of complications of these infections considering current prevalence.
Funding information
TAE recognizes support from the Oxford NIHR Biomedical Research Centre. DWE is a Robertson Foundation Fellow and an NIHR Oxford BRC Senior Fellow.
Author contributions
TAE and DWE: study design, data collection, coordination, analysis, and interpretation, manuscript writing, editing. LP: analysis, and interpretation, manuscript writing. MIA and AP: interpretation, manuscript editing. DWE: statistical analysis.
Conflicts of interest
TAE declares personal advisory and/or lecture fees following Roche, KITE Gilead, Janssen, Abbvie, AstraZeneca, Loxo Oncology, Beigene and Incyte and research funding from Gilead, AstraZeneca and Beigene. DWE declares lecture fees from Gilead outside the submitted work. MIA declares research and consultant fees from Prenetics. No other authors have any relevant conflict of interest to declare.
Supporting information
Table SI. Patient characteristics for haematology inpatients tested and not tested for respiratory viruses between January 2016 and February 2021.
Acknowledgements
This work uses data provided by patients and collected by the UK’s National Health Service as part of their care and support. We thank all the people of Oxfordshire who contribute to the Infections in Oxfordshire Research Database. Research Database Team: L. Butcher, H. Boseley, C. Crichton, D. W. Crook, D. W. Eyre, O. Freeman, J. Gearing (community), R. Harrington, K. Jeffery, M.Landray, A. Pal, T. E. A. Peto, T. P. Quan, J. Robinson (community), J. Sellors, B. Shine, A. S. Walker, D. Waller. Patient and Public Panel: G. Blower, C, Mancey, P. McLoughlin, B. Nichols.
References
- 1. Thu TP, Ngoc PN, Hai NM. Effect of the social distancing measures on the spread of COVID‐19 in 10 highly infected countries. Sci Total Environ. 2020;10(742):140430. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Jarvis CI, Van Zandvoort K, Gimma A, Prem K, Klepac P, Rubin GJ, et al. Quantifying the impact of physical distance measures on the transmission of COVID‐19 in the UK. BMC Med. 2020;18:1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Mikulska M, Del Bono V, Gandolfo N, Dini S, Dominietto A, Di Grazia C, et al. Epidemiology of viral respiratory tract infections in an outpatient haematology facility. Ann Hematol. 2014;93(4):669–76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Ljungman P, Ward KN, Crooks B, Parker A, Martino R, Shaw PJ, et al. Respiratory virus infections after stem cell transplantation: a prospective study from the infectious diseases working party of the European group for blood and marrow transplantation. Bone Marrow Transplant. 2001;28(5):479–84. [DOI] [PubMed] [Google Scholar]
- 5. Martino R, Porras RP, Rabella N, Williams JV, Rámila E, Margall N, et al. Prospective study of the incidence, clinical features, and outcome of symptomatic upper and lower respiratory tract infections by respiratory viruses in adult recipients of hematopoietic stem cell transplants for hematologic malignancies. Biol Blood Marrow Transplant. 2005;11(10):781–96. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Khanna N, Widmer AF, Decker M, Steffen I, Halter J, Heim D, et al. Respiratory syncytial virus infection in patients with hematological diseases: single‐center study and review of the literature. Clin Infect Dis. 2008;46(3):402–12. [DOI] [PubMed] [Google Scholar]
- 7. Small TN, Casson A, Malak SF, Boulad F, Kiehn TE, Stiles J, et al. Respiratory syncytial virus infection following hematopoietic stem cell transplantation. Bone Marrow Transplant. 2002;29(4):321–7. [DOI] [PubMed] [Google Scholar]
- 8. Avetisyan G, Mattsson J, Sparrelid E, Ljungman P. Respiratory syncytial virus infection in recipients of allogeneic stem‐cell transplantation: a retrospective study of the incidence, clinical features, and outcome. Transplantation. 2009;88(10):1222–6. [DOI] [PubMed] [Google Scholar]
- 9. Dignan FL, Clark A, Aitken C, Gilleece M, Jayakar V, Krishnamurthy P, et al. BCSH/BSBMT/UK clinical virology network guideline: diagnosis and management of common respiratory viral infections in patients undergoing treatment for haematological malignancies or stem cell transplantation. Br J Haematol. 2016;173(3):380–93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Mato AR, Roeker LE, Lamanna N, Allan JN, Leslie L, Pagel JM, et al. Outcomes of COVID‐19 in patients with CLL: a multicenter international experience. Blood J Am Soc Hematol. 2020;136(10):1134–43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Bailén R, Kwon MI, Aguado B, Duarte RF, Calbacho M, Chinea A, et al. Impact of Sars‐Cov‐2 infection in hematopoietic transplant patients: experience from the Madrid group. Blood. 2020;136(Supplement 1):12–3. [Google Scholar]
- 12. Olsen SJ, Azziz‐Baumgartner E, Budd AP, Brammer L, Sullivan S, Pineda RF, et al. Decreased influenza activity during the COVID‐19 pandemic—United States, Australia, Chile, and South Africa, 2020. Am J Transplant. 2020;20(12):3681–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Adlhoch C, Mook P, Lamb F, Ferland L, Melidou A, Amato‐Gauci AJ, et al. Very little influenza in the WHO European Region during the 2020/21 season, weeks 40 2020 to 8 2021. Eurosurveillance. 2021;26(11):2100221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Leber AL, Everhart K, Daly JA, Hopper A, Harrington A, Schreckenberger P, et al. Multicenter evaluation of BioFire FilmArray Respiratory Panel 2 for detection of viruses and bacteria in nasopharyngeal swab samples. J Clin Microbiol. 2018;56(6):2251–2261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Baker RE, Park SW, Yang W, Vecchi GA, Metcalf CJ, Grenfell BT. The impact of COVID‐19 nonpharmaceutical interventions on the future dynamics of endemic infections. Proc Natl Acad Sci. 2020;117(48):30547–53. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Table SI. Patient characteristics for haematology inpatients tested and not tested for respiratory viruses between January 2016 and February 2021.


