Abstract
Simple tests of routine data are needed for those with severe acute respiratory syndrome coronavirus 2, which causes corona virus disease 2019 (COVID‐19), to help identify those who may need mechanical ventilation (MV). In this study, we aimed to determine if fibrosis‐4 (FIB‐4) is associated with the need for MV in patients with COVID‐19 and if there is an association to determine the optimal FIB‐4 cutoff. This was a retrospective, national, multiethnic cohort study of adults seen in an ambulatory or emergency department setting who were diagnosed with COVID‐19. We used the TriNetX platform for analysis. Measures included demographics, comorbid diseases, and routine laboratory tests. A total of 4,901 patients with COVID‐19 were included. Patients had a mean age of 56, 48% were women, 42% were obese, 38% were white, 40% were black, 15% had cardiac disease, 39% had diabetes mellitus, 20% had liver disease, and 50% had respiratory disease. The need for MV was 6%. The optimal FIB‐4 cutoff for the need for MV was 3.04 (area under the curve, 0.735), which had sensitivity, specificity, and positive and negative predictive values of 42%, 77%, 11%, and 95%, respectively, with 93% accuracy. When stratified by race, increased FIB‐4 remained associated with the need for MV in both white and black patients. Conclusion: FIB‐4 can be used by frontline providers to identify patients that may require MV.
The FIB‐4 index, a simple tool of routine clinical data, was found to independently be associated with need for mechanical ventilation in patients with COVID‐19.

Abbreviations:
- ALT
alanine aminotransferase
- AST
aspartate aminotransferase
- AUC
under the curve
- BMI
body mass index
- CI
confidence interval
- COVID‐19
corona virus disease 2019
- CRP
C‐reactive protein
- FIB‐4
fibrosis‐4
- ICD‐10
International Classification of Diseases, Tenth Revision
- ICU
intensive care unit
- IQR
interquartile range
- MV
mechanical ventilation
- NAFLD
nonalcoholic fatty liver disease
- NPV
negative predictive value
- OR
odds ratio
- PLT
platelet
- PPV
positive predictive value
- RDW
red cell distribution width
- ROC
receiver operating characteristic
- SARS‐CoV‐2
severe acute respiratory syndrome coronavirus 2
Corona virus disease 2019 (COVID‐19), caused by severe acute respiratory syndrome coronavirus 2 (SARS‐CoV‐2), is a global pandemic leading to respiratory failure and the need for mechanical ventilation (MV).( 1, 2 ) While many are asymptomatic or have only mild symptoms, others present to emergency rooms and frontline providers with more severe symptoms.( 3, 4 ) Although the clinical course varies, observational studies have identified several comorbid conditions, such as diabetes mellitus, respiratory diseases, and obesity, in hospitalized patients that are linked to disease severity, including respiratory failure, a need for ventilator support, and mortality.( 5, 6, 7, 8 ) Therefore, there is interest in developing prediction tools to help in the early identification of patients infected with COVID‐19 who might require more intensive monitoring and treatments.
Several complex models have been developed to predict mortality of hospitalized patients with COVID‐19 by using clinical data and markers of increased inflammation associated with infection; however, few have focused on the need for MV, which is often a precursor to mortality.( 2, 9, 10, 11, 12, 13, 14 ) One limitation of most of these models is the inclusion of nonroutine data; this makes their applicability to routine care challenging. To overcome these limitations, simple tests are needed. One simple test, increased red cell distribution width (RDW), was recently shown to be associated with increased mortality from COVID‐19, but no data were provided on its association with MV.( 15 )
Infection with COVID‐19 is associated with systemic inflammation and increased liver enzymes, which can be associated with poor prognosis regardless of preexisting liver disease.( 14, 16, 17, 18, 19 ) The fibrosis‐4 index (FIB‐4), a simple index consisting of age, platelet (PLT) count, and two liver enzymes (aspartate aminotransferase [AST] and alanine aminotransferase [ALT]), was developed to predict advanced fibrosis in chronic liver disease. It has been hypothesized to be a surrogate for severity of the inflammatory response and to be associated with the need for MV and mortality in patients hospitalized with COVID‐19.( 7, 20, 21, 22 ) However, because these studies were limited to single centers or unique populations and used established FIB‐4 thresholds developed in viral hepatitis and nonalcoholic fatty liver disease (NAFLD), the utility of FIB‐4 to help frontline providers identify who might be at higher risk for intensive care requires broad validation.( 21, 23 ) To address this gap in knowledge, our aims were 1) to determine if FIB‐4 is associated with the need for MV in a national multiethnic cohort of patients seen for COVID‐19 symptoms and 2) if an association exists, to determine the optimal FIB‐4 cutoff associated with the need for MV.
Patients and Methods
Data Source
This was a retrospective analysis of a national multiethnic cohort of patients with COVID‐19 in the United States, using the TriNetX platform (www.trinetx.com). We used the federated research network platform TriNetX (Supporting Appendix S1) to identify our initial cohort of 28,610 distinct patients with COVID‐19 based on International Classification of Diseases, Tenth Revision (ICD‐10) codes from the index visit or related hospitalizations. For the TriNetX platform, we queried all patients who had been diagnosed with either COVID‐19 (U07.1) or pneumonia due to SARS‐associated coronavirus (J12.81) between January and June 2020 in an ambulatory or emergency department setting. TriNetX does not allow data downloads or the review of individual patient data to assess when supplemental oxygen or MV occurred during the hospitalization. However, this platform allows analysis in the form of queries. TriNetX has previously been described in detail in several similar studies of COVID‐19 that used this platform.( 24, 25, 26, 27 ) For all but 2 of these patients, we were able to identify the clinical encounter during which they were either diagnosed with COVID‐19 or received a test for COVID‐19 that yielded a positive result. Out of these encounters, we were able to identify 4,901 distinct patients who had recorded AST, ALT, and PLT values within the first 24 hours of the encounter that we could use to calculate a FIB‐4 score. Because the data were de‐identified, we were unable to obtain a reliable death date that could be used to assess 30‐day mortality. Institutional review board approval was not required for the de‐identified data.
Demographic data (age, sex, race/ethnicity), body mass index (BMI) if available, use of supplemental O2, comorbid conditions (diabetes mellitus, cardiac disease, respiratory disease, and liver disease), and the need for intensive care unit (ICU) were identified by ICD‐10 codes from the index visit or related hospitalizations (Supporting Table S1). The primary outcome, which was the need for MV, was identified by ICD‐10 codes. AST, ALT, and PLT counts obtained from the day of the visit were used to determine the FIB‐4 score, calculated as (age × AST) / (PLT × ALT1/2).( 20 )
Statistical Analysis
Patient demographics (age, race, sex), comorbidities (cardiac, liver, respiratory, and diabetes), increased RDW (RDW >14.5%), obesity (BMI ≥30 kg/m2), supplemental O2 use, and FIB‐4 (greater than or equal to the optimized cutoff) were analyzed as covariates. Data are presented as mean and SD for normally distributed data, median and interquartile range (IQR) for skewed continuous data, or frequency and percentage for categorical data, as appropriate. A multiple logistic regression model was used to estimate the impact of FIB‐4 on the need for MV adjusted for covariates. We categorized age (ranging between 19 and 81 years) into four quartiles and analyzed their effects on the need for MV because the categorical age produced a better fit than the continuous one. We first used the established FIB‐4 cutoffs developed for advanced fibrosis in NAFLD and chronic hepatitis C to build on previous analyses that estimated the effect of binary FIB‐4 dichotomized at ≥2.67 or ≥3.25 on the need for MV.( 20, 23 ) To determine our sample’s optimal binary FIB‐4 similar to how FIB‐4 is used in chronic liver disease, we investigated the FIB‐4 dichotomizing cutoffs between 2.50 and 4.00 and found the FIB‐4 cutoff that maximized power to detect its effect on the need for MV. To assess the optimal cutoff against other cutoffs (2.67 and 3.25), we plotted the receiver operating characteristic (ROC) curve; obtained the area under the curve (AUC, also known as the C statistic), given the estimated multiple logistic regression model of interest with the binary FIB‐4 based on each cutoff; and compared the resulting AUCs.
Each covariate was tested independently in a simple logistic regression model. Those that achieved a significance level with P < 0.10 were included as the predictors in a subsequent multiple logistic regression model of interest. The effects, odds ratios (ORs), and 95% confidence intervals (CIs) for the ORs were estimated by maximum likelihood for the need for MV (the primary outcome) and increased FIB‐4 (a secondary outcome). The Hosmer‐Lemeshow goodness‐of‐fit test was performed to assess the adequacy of the model.( 28 ) Lastly, a 10‐fold cross‐validation was performed to evaluate the accuracy of the model. All statistical analyses were performed using R version 4.0 and SAS version 9.4.
Results
Characteristics of Patients
Patient characteristics for the entire sample of 28,608 patients with COVID‐19 and a subsample of 4,901 patients who had all the components to calculate FIB‐4 at the time of evaluation are summarized in Table 1. The patients with FIB‐4 data were more likely to be older, men, black, and obese; had a higher proportion with increased RDW, AST, and comorbid diseases; were more likely to use supplemental O2; and were more likely to require ICU or MV than subjects who did not have the components to calculate FIB‐4.
Table 1.
Characteristics of the cohort
| Variable | FIB‐4 Patients (n = 4,901) | Entire Sample (n = 28,608) | Mean Difference (P Value) | |
|---|---|---|---|---|
| Categorical variables: Proportion (%) | ||||
| Female (%) | 48.45 | 54.69 | −6.24 (<0.01) | |
| Comorbid cardiac disease (%) | 15.22 | 8.76 | 6.46 (<0.01) | |
| Comorbid diabetes mellitus (%) | 38.80 | 22.51 | 16.29 (<0.01) | |
| Comorbid liver disease (%) | 20.40 | 15.61 | 4.79 (<0.01) | |
| Comorbid respiratory disease (%) | 50.35 | 39.69 | 10.66 (<0.01) | |
| Supplemental O2 use (%) | 3.71 | 1.12 | 2.58 (<0.01) | |
| MV (%) | 6.08 | 1.47 | 4.61 (<0.01) | |
| ICU (%) | 14.22 | 3.90 | 10.32 (<0.01) | |
| Race (%) | American Indian or Alaska Native | 0.45 | 0.33 | 391.32 (<0.01) chi‐square test |
| Asian | 1.96 | 2.44 | ||
| Black | 39.58 | 26.13 | ||
| Native Hawaiian or Pacific Islander | 0.39 | 0.24 | ||
| White | 38.28 | 48.82 | ||
| Unknown | 19.34 | 22.04 | ||
| RDW (>14.5%) (%) | 32.04 (17%) | 29.68 (74%) | 2.36 (<0.01) | |
| Obesity (BMI, ≥30 kg/m2) (%) | 42.43 (78%) | 38.48 (81%) | 3.95 (0.02) | |
| Continuous variables | ||||
| Age (years)* | 55.74 (15.6, 0%) | 48.32 (16.51, 0%) | 7.42 (<0.01) | |
| AST (U/L)† | 37 (25‐58, 0%) | 34 (23‐54, 89%) | 3.76 (<0.01) | |
| ALT (U/L)† | 28 (18‐47, 0%) | 28 (18‐47, 84%) | −1.79 (0.92) | |
| (×1,000)† | 220.0 (169‐282, 0%) | 217 (168‐280, 73%) | 3.08 (0.13) |
Mean (SD, %).
Median (IQR, %).
Need for MV
Of the 4,901 patients with FIB‐4 data, 298 patients (6.08%) required MV. The difference in patient characteristics between patients who used the ventilator support and patients who did not during admission is compared in Table 2. Patients with ventilator support had a higher OR of being men and older; had more comorbid diseases; were obese; had higher FIB‐4, AST, and use of supplemental O2; and had lower PLT than those that did not require MV. However, the proportion with increased RDW and median ALT did not differ in means across the two groups of patients.
Table 2.
FIB‐4 patients who used and did not use ventilator support
| Variable | Used MV (n = 298) | No MV (n = 4,603) | Mean Difference (P Value) | |
|---|---|---|---|---|
| Categorical variables: Proportion (%) | ||||
| Female (%) | 40.6 | 48.96 | −8.36 (<0.01) | |
| Comorbid cardiac disease (%) | 25.5 | 14.55 | 10.95 (<0.01) | |
| Comorbid diabetes mellitus (%) | 59.73 | 37.45 | 22.28 (<0.01) | |
| Comorbid liver disease (%) | 27.51 | 19.94 | 7.57 (<0.01) | |
| Comorbid respiratory disease (%) | 67.11 | 49.27 | 17.84 (<0.01) | |
| Supplemental O2 use (%) | 8.05 | 3.43 | 4.62 (<0.01) | |
| ICU (%) | 74.16 | 10.34 | 6.38 (<0.01) | |
| Race (%) | American Indian or Alaska Native | 0.67 | 0.43 | 6.92 (0.23) chi‐square test |
| Asian | 2.35 | 1.93 | ||
| Black | 42.95 | 39.37 | ||
| Native Hawaiian or Pacific Islander | 0.67 | 0.37 | ||
| White | 31.54 | 38.71 | ||
| Unknown | 21.81 | 19.18 | ||
| FIB‐4 ≥3.04) (%) | 42.28 | 22.33 | 19.95 (<0.01) | |
| RDW >14.5% (%) | 36.92 (13%) | 31.17 (17%) | 5.21 (0.09) | |
| Obesity (BMI ≥30 kg/m2) (%) | 54.61 (56%) | 40.77 (79%) | 13.84 (<0.01) | |
| Continuous variables | ||||
| Age (years)* | 58.96 (13.3, 0%) | 55.53 (15.4, 0%) | 3.43 (<0.01) | |
| AST (U/L)† | 45 (31‐72, 0%) | 36 (25‐57, 0%) | 65.54 (<0.01) | |
| ALT (U/L)† | 28.5 (19‐46, 0%) | 28 (18‐47, 0%) | 11.97 (0.73) | |
| (×1,000)† | 199 (148‐272, 0%) | 221 (170‐282, 0%) | −16.74 (<0.01) |
Mean (SD, %).
Median (IQR, %).
Determining the Optimal FIB‐4 Cutoff
We found the optimal FIB‐4 threshold associated with the need for MV at 3.04 among cutoffs ranging from 2.50 to 4.00 that maximized the power to detect the effect of FIB‐4 on MV in a simple logistic regression model. To evaluate the optimal FIB‐4 threshold 3.04 against other competing ones (2.67 and 3.25), we used the ROC curve of the estimated model in Table 3 to produce the ROC curve of each cutoff. Our threshold was one of the four FIB‐4 cutoffs (2.91, 2.92, 3.03, and 3.04) that produced the maximum AUC of 0.7035. The analyzed cutoffs 2.67 and 3.25 noted in the literature produced comparative AUCs of 0.7014 and 0.7007, respectively (Fig. 1).20, 21 Therefore, the FIB‐4 cutoff 3.04 not only achieved the maximum power to detect the FIB‐4 effect but also produced the highest AUC among the competing dichotomizations of FIB‐4. Using this cutoff, the sensitivity, specificity, positive predictive value (PPV), and negative predictive value (NPV) were 42%, 78%, 11%, and 95%, respectively. When setting the sensitivity or specificity at 90%, the cutoff FIB‐4, PPV, and NPV were unchanged across the cutoffs (data not shown).
Table 3.
Logistic Regression Analysis Outcome of patients with FIB‐4 on the need for MV
| Variables | Simple Logistic Regression (n = 4,901) | Multiple Logistic Regression (n = 3,953)* | Multiple Logistic Regression (n = 774)† | |||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Estimates (P Value) | OR | 95% CI | Estimates (P Value) | OR | 95% CI | Estimates (P Value) | OR | 95% CI | ||
| Intercept | ‐ | ‐ | ‐ | −3.84 (<0.01) | −3.29 (<0.01) | |||||
| Female | −0.34 (<0.01) | 0.71 | 0.56‐0.90 | −0.26 (0.07) | 0.77 | 0.58‐1.02 | −0.22 (0.35) | 0.80 | 0.50‐1.27 | |
| Age group (Ref. ≤33) | (34, 48) | 0.75 (0.02) | 2.12 | 1.14‐3.94 | ||||||
| (49, 60) | 1.02 (<0.01) | 2.76 | 1.52‐5.01 | |||||||
| (≥61) | 1.11 (<0.01) | 3.00 | 1.70‐5.38 | |||||||
| Race (ref. white) | Native American | 0.64 (0.39) | 1.89 | 0.43‐8.23 | 0.56 (0.46) | 1.76 | 0.39‐7.88 | 1.16 (0.33) | 3.19 | 0.30‐33.1 |
| Asian | 0.40 (0.32) | 1.49 | 0.67‐3.30 | 0.52 (0.21) | 1.68 | 0.74‐3.84 | ‐0.24 (0.76) | 0.78 | 0.17‐3.61 | |
| Black | 0.29 (0.04) | 1.34 | 1.01‐1.76 | 0.31 (0.03) | 1.36 | 1.02‐1.81 | 0.47 (0.04) | 1.60 | 1.01‐2.53 | |
| Native Hawaiian | 0.80 (0.29) | 2.23 | 0.51‐9.79 | 0.96 (0.21) | 2.63 | 0.58‐11.9 | 1.68 (0.05) | 5.4 | 0.95‐30.5 | |
| Unknown | 0.33 (0.05) | 1.40 | 1.00‐1.93 | |||||||
| Supplemental O2 | 0.90 (<0.01) | 2.46 | 1.57‐3.85 | 0.52 (0.06) | 1.68 | 0.96‐2.94 | 0.33 (0.33) | 1.40 | 0.71‐2.77 | |
| Comorbidity Cardiac disease | 0.70 (<0.01) | 2.01 | 1.53‐2.64 | 0.34 (0.04) | 1.40 | 1.01‐1.94 | 0.28 (0.27) | 1.33 | 0.80‐2.21 | |
| Comorbidity diabetes mellitus | 0.91 (<0.01) | 2.47 | 1.95‐3.14 | 0.53 (<0.01) | 1.69 | 1.28‐2.24 | 0.56 (0.02) | 1.75 | 1.09‐2.81 | |
| Comorbidity liver disease | 0.42 (<0.01) | 1.52 | 1.17‐1.98 | 0.33 (0.03) | 1.39 | 1.02‐1.91 | 0.71 (<0.01) | 2.03 | 1.25‐3.29 | |
| Comorbidity respiratory disease | 0.74 (<0.01) | 2.10 | 1.63‐2.69 | 0.38 (0.01) | 1.47 | 1.88‐1.98 | 0.11 (0.69) | 1.11 | 0.65‐1.89 | |
| AST | 2.3e‐4 (0.02) | 1.00 | 1.00‐1.00 | |||||||
| ALT | 5.5e‐4 (0.10) | 1.00 | 1.00‐1.00 | |||||||
| −1.8e‐3 (<0.01) | 1.00 | 1.00‐1.00 | ||||||||
| FIB‐4 ≥3.04 | 0.94 (<0.01) | 2.55 | 2.00‐3.24 | 0.92 (<0.01) | 2.52 | 1.92‐3.32 | 0.79 (<0.01) | 2.20 | 1.40‐3.46 | |
| BMI ≥30 kg/m2 (missing in 80%) | 0.56 (<0.01) | 1.75 | 1.21‐2.52 | ‐ | ‐ | ‐ | 0.41 (0.08) | 1.51 | 0.96‐2.37 | |
| RDW >14.5% | 0.23 (0.08) | 1.26 | 0.97‐1.64 | |||||||
P < 0.05 indicates significant association in multivariate analysis.
Includes patients with all available data not including BMI.
Includes only those with BMI data.
FIG. 1.
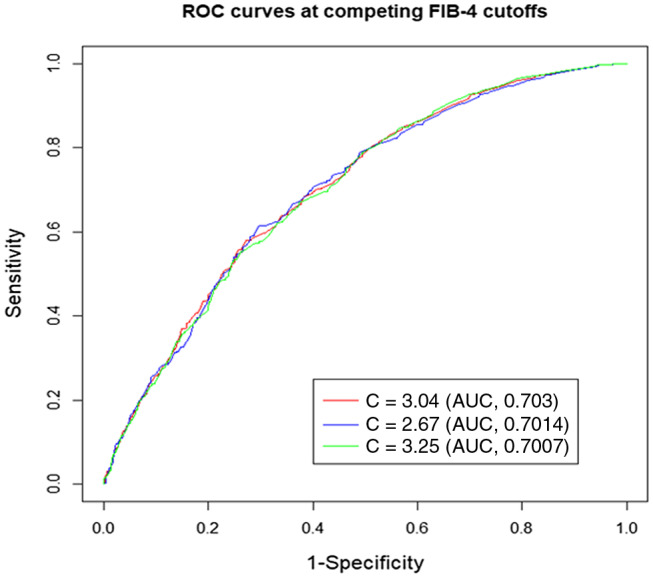
Area under the receiver operating characteristic curve at competing FIB‐4 cutoffs.
Analysis of Independent Associations With Increased FIB‐4
Compared to those with FIB‐4 <3.04 (n = 3,747), those with increased FIB‐4 (≥3.04, n = 1,154) were more likely to be men (OR, 1.64; 95% CI, 1.43‐1.92), black race (OR, 1.25; 95% CI, 1.07‐1.45), had cardiac disease (OR, 40; 95% CI, 1.15‐1.70), diabetes mellitus (OR, 1.18; 95% CI, 1.0‐1.37), respiratory disease (OR, 1.30; 95% CI, 1.11‐1.51), and used supplemental O2 (OR, 2.10; 95% CI, 1.44‐3.05). When only those with obesity recorded were assessed (n = 600), only men (OR, 1.75; 95% CI, 1.20‐2.56) and black race (OR, 1.56; 95% CI, 1.07‐2.56) were associated with increased FIB‐4 while obesity itself was inversely related (OR, 0.62; 95% CI, 0.42‐0.90). We found increased FIB‐4 was more common in those with liver disease (23.65% vs. 19.40%; P < 0.01), but liver disease was not associated with increased FIB‐4 when adjusting for demographics, obesity, and other chronic conditions.
Analysis of Independent Associations of Need for MV
We estimated the effects of sex, race, four comorbidities (cardiac disease, diabetes mellitus, liver disease, and respiratory disease), FIB‐4 (≥3.04), supplemental O2 use, and obesity on the MV outcome. We excluded RDW as insignificant and obesity severely missing for 79% of patients. Age, AST, ALT, and PLT were not included in the model due to the predictor FIB‐4 defined as a function of them. In addition, patients with unknown or missing race were excluded.
The associations with the need for MV in the cohort with these adjustments are shown in Table 3. In those patients with all available data (n = 3,953) after controlling for other covariates, positive associations with the need for MV were black race (OR, 1.36; 95% CI, 1.02‐1.81), cardiac disease (OR, 1.40; 95% CI, 1.01‐1.94), diabetes mellitus (OR, 1.69; 95% CI, 1.28‐2.24), respiratory disease (OR, 1.47; 95% CI, 1.88‐1.98), liver disease (OR, 1.39; 95% CI, 1.02‐1.91), and patients with FIB‐4 ≥3.04. These patients were more likely to use ventilator support than other patients (OR, 2.52; 95% CI, 1.92‐3.32). The goodness‐of‐fit test implied that the final model was adequate with P = 0.31. Lastly, our predictive model achieved 93% accuracy in predicting the response by 10‐fold cross validation. When data were restricted to those with obesity data (n = 774), along with underlying liver disease, black race, diabetes, and FIB‐4 remained associated with the need for MV (Table 3) while obesity, respiratory disease, and cardiac disease did not have this association.
Impact of Race on Need for MV
When we compared the entire cohort stratified by race (white, n = 1,876; black, n = 1,940; and others, n = 1,085, which included Asian, Native American, Pacific Islander, and a large proportion of unknowns), diabetes mellitus and respiratory disease remained independently associated with the need for MV across all races (Table 4) while supplemental O2 and liver diseases were significant only among whites and cardiac disease only among blacks. Importantly, increased FIB‐4 remained associated with the need for MV in both whites (OR, 2.1; 95% CI, 1.3‐3.2) and blacks (OR, 3.1; 95% CI, 2.2‐4.5) but not in others or those of unknown race.
Table 4.
Logistic Regression Analysis Outcome of patients with FIB‐4 on the need for MV by Race
| Variables | Entire Sample (n = 4,901) | White (n = 1,876) | Black (n = 1,940) | Others (n = 1,085) | ||||
|---|---|---|---|---|---|---|---|---|
| Estimate (P Value) | Odds (CI) | Estimate (P Value) | Odds (CI) | Estimate (P Value) | Odds (CI) | Estimate (P Value) | Odds (CI) | |
| Intercept | −3.58 (<0.01) | −3.82 (<0.01) | −3.79 (<0.01) | −3.22 (<0.01) | ||||
| Female | −0.22 (0.08) | 0.80 (0.6‐ 1.0) | −0.54 (0.02) | 0.58 (0.3‐ 0.9) | 0.04 (0.81) | 1.04 (0.7‐ 1.5) | −0.3 (0.15) | 0.70 (0.4‐ 1.1) |
| Supplemental O2 | 0.52 (0.03) | 1.68 (1.1‐2.7) | 0.74 (0.03) | 2.1 (1.1‐ 4.2) | 0.39 (0.43) | 1.48 (0.5‐ 3.9) | 0.17 (0.68) | 1.2 (0.5‐ 2.8) |
| Comorbidity cardiac disease | 0.19 (0.21) | 1.2 (0.9‐1.6) | 0.17 (0.49) | 1.2 (0.7‐ 1.9) | 0.49 (0.03) | 1.63 (1.1‐ 2.5) | −0.35 (0.41) | 0.7 (0.3‐ 1.6) |
| Comorbidity diabetes mellitus | 0.68 (<0.01) | 1.9 (1.5‐2.5) | 0.53 (0.02) | 1.7 (1.1‐ 2.7) | 0.55 (<0.01) | 1.73 (1.2‐ 2.5) | 1.02 (<0.01) | 2.8 (1.7‐ 4.7) |
| Comorbidity liver disease | 0.15 (0.30) | 1.2 (0.8‐ 1.5) | 0.57 (0.02) | 1.7 (1.1‐ 2.8) | 0.16 (0.48) | 1.2 (0.8‐ 1.8) | −0.57 (0.17) | 0.56 (0.2‐ 1.3) |
| Comorbidity respiratory disease | 0.46 (<0.01) | 1.6 (1.2‐2.1) | 0.52 (0.04) | 1.7 (1.0‐ 2.8) | 0.40 (0.05) | 1.5 (1.0‐ 2.2) | 0.66 (<0.01) | 1.94 (1.2‐ 3.2) |
| (FIB‐4 ≥3.04) | 0.76 (<0.01) | 2.14 (1.7‐ 2.7) | 0.74 (<0.01) | 2.1 (1.3‐ 3.2) | 1.14 (<0.01) | 3.1 (2.2‐ 4.5) | 0.006 (0.98) | 1.00 (0.5‐ 1.8) |
P < 0.05 indicates a significant association.
Entire cohort comprised white, black, others, and unknown. Others comprised Asian, Native American, Pacific Islander, and unknown.
Discussion
A major challenge for frontline providers is predicting which patients with COVID‐19 will progress to respiratory failure and the need for MV. In this national multiethnic cohort of patients with COVID‐19, we observed a need for MV of 6.08%, which is similar to other studies, and confirm the independent association of increased FIB‐4, a simple index that includes routine data, with the need for MV during hospitalization.( 19 ) When controlling for comorbid diseases, including liver disease, FIB‐4 remained associated with the need for MV across racial groups.
Recent studies have modeled associated factors with increased morbidity associated with COVID‐19. Knight and colleagues( 12 ) looked at over 35,000 patients admitted to hospitals across England, Scotland, and Wales to develop the 4C mortality score, which includes eight variables (age, sex, number of comorbidities, respiratory rate peripheral oxygen saturation, level of consciousness, urea, and C‐reactive protein [CRP]). The 4C mortality score had an AUC of 0.79. More recently, the BAS2IC score was developed, which includes age, sex, BMI, dyspnea, CRP, and lymphocyte count; this score had an AUC of 0.76, which is in line with other scores to predict mortality in COVID‐19.( 13 ) However, these models are complex and include subjective symptoms (dyspnea) and nonroutine laboratory tests (CRP), limiting their utility in clinical practice.( 10, 45 )
Simple models of routine data have the advantage over more complex models because of their ease of use. FIB‐4 was developed and cutoffs determined to predict advanced liver fibrosis; this score has been validated in those with viral hepatitis and NAFLD.( 20, 23, 46 ) Studies in patients with diabetes mellitus and in patients with NAFLD found increased FIB‐4 associated with increased mortality from COVID‐19.( 6, 7 ) However, it is unclear if these studies represent the severity of COVID‐19 or underlying liver disease. In a recent study of 287 hospitalized Veterans with COVID‐19 after adjusting for comorbid diseases, FIB‐4 >3.25 had an OR of 8.40 for ICU admission compared to FIB‐4 <1.45.( 22 ) However, because over 50% of the cohort had an indeterminate FIB‐4 value (1.45‐3.25), the applicability using this cutoff was limited. Similar to our findings in an unselected national multiethnic population, other studies found increased FIB‐4 (>2.67) to be associated with SARS‐CoV‐2 infection severity.( 21, 47 )
While other studies used published FIB‐4 thresholds in viral hepatitis (>3.25) and NAFLD (>2.67), we were able to determine that the optimal FIB‐4 threshold associated with the need for MV was 3.04, which is between the threshold used to identify advanced fibrosis in NAFLD and chronic hepatitis C.( 20, 23, 46 ) Increased FIB‐4 in our cohort was associated with male sex, black race, supplemental O2, diabetes, and cardiac and respiratory disease; it was not associated with a history of liver disease, and similar to the single‐center study by Sterling and colleagues,( 21 ) it was inversely associated with obesity in our national multiethnic cohort.
Of the components of FIB‐4, we observed increased age, increased AST, and lower PLT count were all associated with MV by analysis of simple logistic regression models; this reflects the systemic inflammation associated with COVID‐19.( 14, 16, 17, 18, 19 ) The association of increased AST to ICU admission and the need for MV has been reported.( 5, 19, 47, 48 ) However, when individually substituted with FIB‐4 in our multiple regression model, AST, ALT, and PLT count were not independently associated with the need for MV, confirming it is the FIB‐4 index and not its components that are associated with disease severity.( 21 ) Although a recent study found that increased RDW, a standard component of the complete blood count, was associated with increased mortality among hospitalized patients with COVID‐19, we did not observe an impact of increased RDW on MV, which is often a precursor to mortality.( 15 )
The impact of race on COVID‐19 severity is controversial. Despite a prevalence of 13% in the black race in the United States compared to the white race (76%), we found a similar prevalence (39%) in both races in our cohort of patients with COVID‐19.( 49 ) This suggests that, in this national cohort, Blacks may be disproportionately affected by COVID‐19. Although other studies have had mixed results on the impact of race on disease severity, we observed that black patients had a higher rate in the need for MV than white patients.( 19, 21, 22, 50 ) We did not observe an impact of obesity on COVID‐19 severity, but other studies have,( 7, 21, 47 ) and similar to others, we observed an impact of diabetes mellitus on COVID‐19 severity.( 5, 21 ) Interestingly, when obesity was included in the model, both cardiac and respiratory diseases were no longer significantly associated with the need for MV but liver disease and diabetes were.
Increased FIB‐4 may not be assessing liver fibrosis, but it most likely reflects a more global score of systemic inflammation associated with COVID‐19.( 14, 16, 18, 47 ) We observed increased FIB‐4 in those with a history of diabetes, cardiac disease, and respiratory disease but surprisingly not with liver disease. In support of the independent association of FIB‐4 to non‐liver related outcomes, increased FIB‐4 was associated with outcomes in patients with intracranial hemorrhage while the NAFLD fibrosis score was not, suggesting that it has unique properties separate from liver disease.( 48 )
The strengths of our study are the large sample size and racial diversity reflective of the national cohort. However, our study also has several limitations. Our data and outcomes were determined by a de‐identified data set obtained by TriNetX from January to June 2020 and may be subject to temporal bias. Therefore, comorbid conditions that were not properly recorded may have affected our data. While race was recorded in a large proportion of our cohort, the substantial number listed as “unknown” may have limited our ability to assess the utility of FIB‐4 stratified by race and specifically in Hispanics. Similarly, we did not have data to determine obesity (height and weight) in a majority of subjects. In addition, we did not have historical data to assess if increased liver enzymes or low PLTs were new and related to the COVID‐19 infection or chronic and due to a prior diagnosed or unrecognized chronic liver disease. We also were unable to capture reliable data on prior viral hepatitis infection or alcohol use, which may have affected our ability to diagnose known history and impact of liver disease. Although we did capture supplemental oxygen use, we were not able to differentiate between the need for high and low flow oxygen or when supplemental oxygen was used during hospitalization. Furthermore, we were not able to capture data on mortality. Because we used a de‐identified national data set, we were also not able to determine the exact time (hour or days) from admission to the need for MV, and therefore we were not able to determine if FIB‐4 was a better predictor of early or late respiratory disease associated with COVID‐19. The differences in comorbidities between patients without the components to calculate FIB‐4 (Table 1) most likely reflect a less severe COVID‐19 clinical presentation. Therefore, we did not compare the need for MV between these two cohorts. Furthermore, we did not have the data to calculate several more complex models (4C mortality score and BAS2IC score) for comparison to FIB‐4. Lastly, we did not include any experimental treatments that patients could have received for COVID‐19, which may have impacted the utility of FIB‐4 on admission to eventual need for MV during hospitalization.
In conclusion, FIB‐4, a simple clinical index of readily available clinical data measured at presentation, can be used by frontline providers to help identify which patients, regardless of race, may require more intensive care and MV. With the need for MV in our cohort of 6%, similar to published reports, we found FIB‐4 to have a very high NPV with moderate specificity, suggesting the real utility of FIB‐4 may be in identifying those who may not (ruling out) rather than those who will (ruling in) need MV.( 19 ) While FIB‐4 may not be measuring hepatic fibrosis, those with increased FIB‐4 are more than twice as likely to require MV.
Supporting information
Table S1
Appendix S1
Supported by the Virginia Commonwealth University C. Kenneth and Dianne Wright Center for Clinical and Translational Research (UL1TR002649 to R.K.S., Y.S., E.F. and A.J.S.).
Potential conflict of interest: Dr. Sterling receives grant support from Abbott, AbbVie, Roche, and Gilead and serves on a data and safety monitoring board for Pfizer and AskBio. Dr. Sanyal is President of Sanyal Biotechnology and has stock options in GENFIT, Akarna, Tiziana, Indalo, Durect Inversago, and Galmed; he has served as a consultant to Astra Zeneca, Nitto Denko, Conatus, Nimbus, Salix, Tobira, Takeda, Jannsen, Gilead, Terns, Birdrock, Merck, Valeant, Boehringer‐Ingelheim, Bristol Myers Squibb, Lilly, Hemoshear, Zafgen, Novartis, Novo Nordisk, Pfizer, Exhalenz, and GENFIT; he has been an unpaid consultant to Intercept, Echosens, Immuron, Galectin, Fractyl, Syntlogic, Affimune, Chemomab, Zydus, Nordic Bioscience, Albireo, Prosciento, and Surrozen; his institution has received grant support from Gilead, Salix, Tobira, Bristol Myers, Shire, Intercept, Merck, Astra Zeneca, Malinckrodt, Cumberland, and Novartis; he receives royalties from Elsevier and UptoDate. The other authors have nothing to report.
References
- 1.World Health Organization . WHO Director‐General’s opening remarks at the media briefing on COVID‐19 ‐ 13 March 2020. https://www.who.int/director‐general/speeches/detail/who‐director‐general‐s‐opening‐remarks‐at‐the‐mission‐briefing‐on‐covid‐19‐‐‐13‐march‐2020. Published March 13, 2020. Accessed March 2021.
- 2.Guan WJ, Ni ZY, Hu Y, Liang WH, Ou CQ, He JX, et al.; China Medical Treatment Expert Group for Covid‐19 . Clinical characteristics of coronavirus disease 2019 in China. N Engl J Med 2020;382:1708‐1720. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Wang D, Hu B, Hu C, Zhu F, Liu X, Zhang J, et al. Clinical characteristics of 138 hospitalized patients with 2019 novel coronavirus‐infected pneumonia in Wuhan, China. JAMA 2020;323:1061‐1069. Erratum in: JAMA 2021;325:1113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Grasselli G, Zangrillo A, Zanella A, Antonelli M, Cabrini L, Castelli A, et al.; COVID‐19 Lombardy ICU Network . Baseline characteristics and outcomes of 1591 patients infected with SARS‐CoV‐2 admitted to ICUs of the Lombardy region, Italy. JAMA 2020;323:1574‐1581. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Wu C, Chen X, Cai Y, Xia J, Zhou X, Xu S, et al. Risk factors associated with acute respiratory distress syndrome and death in patients with coronavirus disease 2019 pneumonia in Wuhan, China. JAMA Intern Med 2020;180:934‐943. Erratum in: JAMA Intern Med 2020;180:1031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Targher G, Mantovani A, Wang X‐B, Yan H‐D, Sun Q‐F, Pan K‐H, et al. Patients with diabetes are at higher risk for severe illness from COVID‐19. Diabetes Metab 2020;46:335‐337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Targher G, Mantovani A, Byrne CD, Wang X‐B, Yan H‐D, Sun Q‐F, et al. Risk of severe illness from COVID‐19 in patients with metabolic dysfunction‐associated fatty liver disease and increased fibrosis scores. Gut 2020;69:1545‐1547. [DOI] [PubMed] [Google Scholar]
- 8.Gao F, Zheng KI, Wang X‐B, Sun Q‐F, Pan K‐H, Wang T‐Y, et al. Obesity is a risk factor for greater COVID‐19 severity. Diabetes Care 2020;43:e72‐e74. [DOI] [PubMed] [Google Scholar]
- 9.Wynants L, Calster BV, Collins GS, Riley RD, Heinze G, Schuit E, et al. Prediction models for diagnosis and prognosis of covid‐19: systematic review and critical appraisal. BMJ 2020;369:m1328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Chen J‐H, Chang S‐S, Liu JJ, Chan R‐C, Wu J‐Y, Wang W‐C, et al. Comparison of clinical characteristics and performance of pneumonia severity score and CURB‐65 among younger adults, elderly and very old subjects. Thorax 2010;65:971‐977. [DOI] [PubMed] [Google Scholar]
- 11.Gupta RK, Marks M, Samuels THA, Luintel A, Rampling T, Chowdhury H, et al.; UCLH COVID‐19 Reporting Group . Systematic evaluation and external validation of 22 prognostic models among hospitalised adults with COVID‐19: an observational cohort study. Eur Respir J 2020;56:2003498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Knight SR, Ho A, Pius R, Buchan I, Carson G, Drake TM, et al.; ISARIC4C investigators . Risk stratification of patients admitted to hospital with COVID‐19 using the ISARIC WHO Clinical Characterisation Protocol: development and validation of the 4C Mortality Score. BMJ 2020;370:m3339. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Kaeuffer C, Ruch Y, Fabacher T, Hinschberger O, Mootien J, Eyriey M, et al. The BAS2IC score: a useful tool to identify patients at high risk of early progression to severe coronavirus disease 2019. Open Forum Infect Dis 2020;7:ofaa405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Fu Y, Cheng Y, Wu Y. Understanding SARS‐CoV‐2‐mediated inflammatory responses: from mechanisms to potential therapeutic tools. Virol Sin 2020;35:266‐271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Foy BH, Carlson JCT, Reinertsen E, Valls RP, Lopez RP, Palanques‐Tost E. Association of red cell distribution width with mortality risk in hospitalized adults with SARS‐CoV‐2 infection. JAMA Netw Open 2020;3:e2022058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Li J, Fan JG. Characteristics and mechanism of liver injury in 2019 coronavirus disease. J Clin Transl Hepatol 2020;8:13‐17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Kulkarni AV, Kumar P, Tevethia HV, Premkumar M, Arab JP, Candia R, et al. Systematic review with meta‐ analysis: liver manifestations and outcomes in COVID‐19. Aliment Pharmacol Ther 2020;52:584‐599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Kerner A, Avizohar O, Sella R, Bartha P, Zinder O, Markiewicz W, et al. Association between elevated liver enzymes and C‐reactive protein: possible hepatic contribution to systemic inflammation in the metabolic syndrome. Arterioscler Thromb Vasc Biol 2005;25:193‐197. [DOI] [PubMed] [Google Scholar]
- 19.Ioannou GN, Locke E, Green P, Berry K, O'Hare AM, Shah JA, et al. Risk factors for hospitalization, mechanical ventilation, or death among 10 131 US Veterans with SARS‐CoV‐2 infection. JAMA Netw Open 2020;3:e2022310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Sterling RK, Lissen E, Clumeck N, Sola R, Correa MC, Montaner J, et al.; APRICOT Clinical Investigators . Development of a simple noninvasive index to predict significant fibrosis in patients with HIV/HCV coinfection. Hepatology 2006;43:1317‐1325. [DOI] [PubMed] [Google Scholar]
- 21.Sterling RK, Oakes T, Gal TS, Stevens MO, deWit M, Sanyal AJ. The fibrosis‐4 index is associated with need for mechanical ventilation and 30‐day mortality in patients admitted with coronavirus disease 2019. J Infect Dis 2020;222:1794‐1797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Rentsch CT, Kidwai‐Khan F, Tate JP, Park LS, King JT, Skanderson M, et al. Covid‐19 testing, hospital admission, and intensive care among 2,026,227 United States veterans aged 54‐75 years. medRxiv 2020; 10.1101/2020.04.09.20059964. [DOI] [Google Scholar]
- 23.Shah AG, Lydecker A, Murray K, Tetri BN, Contos MJ, Sanyal AJ; Nash Clinical Research Network . Comparison of noninvasive markers of fibrosis in patients with nonalcoholic fatty liver disease. Clin Gastroenterol Hepatol 2009;7:1104‐1112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Onteddu SR, Nalleballe K, Sharma R, Brown AT. Underutilization of health care for strokes during the COVID‐19 outbreak. Int J Stroke 2020;15:NP9‐NP10. [DOI] [PubMed] [Google Scholar]
- 25.Nalleballe K, Reddy Onteddu S, Sharma R, Dandu V, Brown A, Jasti M, et al. Spectrum of neuropsychiatric manifestations in COVID‐19. Brain Behav Immun 2020;88:71‐74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Stapff M, Hilderbrand S. First‐line treatment of essential hypertension: a real‐world analysis across four antihypertensive treatment classes. J Clin Hypertens (Greenwich) 2019;21:627‐634. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Ranabothu S, Onteddu S, Nalleballe K, Dandu V, Veerapaneni K, Veerapandiyan A. Spectrum of COVID‐19 in children. Acta Paediatr 2020;109:1899‐1900. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Kutner MH, Nachtsheim CJ, Neter J, Li W. Applied Linear Statistical Models, 5th ed. New York, NY: McGraw‐Hill/Irwin; 2005. [Google Scholar]
- 29.Fine MJ, Auble TE, Yealy DM, Hanusa BH, Weissfeld LA, Singer DE, et al. A prediction rule to identify low‐risk patients with community‐acquired pneumonia. N Engl J Med 1997;336:243‐250. [DOI] [PubMed] [Google Scholar]
- 30.Miyashita N, Matsushima T, Oka M; Japanese Respiratory Society . The JRS guidelines for the management of community‐acquired pneumonia in adults: an update and new recommendations. Intern Med 2006;45:419‐428. [DOI] [PubMed] [Google Scholar]
- 31.Bauer TT, Ewig S, Marre R, Suttorp N, Welte T; CAPNETZ Study Group . CRB‐65 predicts death from community‐acquired pneumonia. J Intern Med 2006;260:93‐101. [DOI] [PubMed] [Google Scholar]
- 32.Dwyer R, Hedlund J, Henriques‐Normark B, Kalin M. Improvement of CRB‐65 as a prognostic tool in adult patients with community‐acquired pneumonia. BMJ Open Respir Res 2014;1:e000038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Liu JL, Xu F, Zhou H, Wu XJ, Shi LX, Lu RQ, et al. Expanded CURB‐65: a new score system predicts severity of community‐acquired pneumonia with superior efficiency. Sci Rep 2016;6:22911. Erratum in: Sci Rep 2018;8:47005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Royal College of Physicians . National early warning score (NEWS) 2: standardising the assessment of acute‐illness severity in the NHS. https://www.rcplondon.ac.uk/projects/outputs/national‐early‐warning‐score‐news‐2. Published December 19, 2017. Accessed March 2021.
- 35.Singer M, Deutschman CS, Seymour CW, Hari MS, Annane D, Bauer M, et al. The Third International Consensus Definitions for Sepsis and Septic Shock (Sepsis‐3). JAMA 2016;315:801‐810. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Charles P, Wolfe R, Whitby M, Fine M, Fuller A, Stirling R, et al.; Australian Community‐Acquired Pneumonia Study Collaboration . SMART‐COP: a tool for predicting the need for intensive respiratory or vasopressor support in community‐acquired pneumonia. Clin Infect Dis 2008;47:375‐384. [DOI] [PubMed] [Google Scholar]
- 37.Vincent J‐L, Moreno R, Takala J, Willatts S, De Mendonça A, Bruining H, et al. The SOFA (Sepsis‐related Organ Failure Assessment) score to describe organ dysfunction/failure. On behalf of the Working Group on Sepsis‐Related Problems of the European Society of Intensive Care Medicine. Intensive Care Med 1996;22:707‐710. [DOI] [PubMed] [Google Scholar]
- 38.Yandiola PPE, Capelastegui A, Quintana J, Diez R, Gorordo I, Bilbao A, et al. Prospective comparison of severity scores for predicting clinically relevant outcomes for patients hospitalized with community‐acquired pneumonia. Chest 2009;135:1572‐1579. [DOI] [PubMed] [Google Scholar]
- 39.Liang W, Liang H, Ou L, Chen B, Chen A, Li C, et al. Development and validation of a clinical risk score to predict the occurrence of critical illness in hospitalized patients with COVID‐19. JAMA Intern Med 2020;180:1081‐1089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Xie J, Hungerford D, Chen H, Abrams ST, Li S, Wang G, et al. Development and external validation of a prognostic multivariable model on admission for hospitalized patients with COVID‐19. medRxiv. 2020; 10.1101/2020.03.28.20045997. [DOI] [Google Scholar]
- 41.Businesswire . Surgisphere’s COVID‐19 machine learning platform receives international endorsement – now clinically in use at >1,000 hospitals worldwide. https://www.businesswire.com/news/home/20200326005199/en/Surgisphere%E2%80%99s‐COVID‐19‐Machine‐Learning‐Platform‐Receives‐International. Published March 26, 2020. Accessed March 2021.
- 42.Zhang H, Shi T, Wu X, Zhang Z, Wang K, Bean D, et al. Risk prediction for poor outcome and death in hospital in‐patients with COVID‐19: derivation in Wuhan, China and external validation in London, UK. medRxiv 2020; 10.1101/2020.04.28.2008222. [DOI] [Google Scholar]
- 43.Beigel JH, Tomashek KM, Dodd LE, Mehta AK, Zingman BS, Kalil AC; ACTT‐1 Study Group Members . Remdesivir for the treatment of Covid‐19 – final report. N Engl J Med 2020;383:1813‐1826. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Ji D, Zhang D, Xu J, Chen Z, Yang T, Zhao P, et al. Prediction for progression risk in patients with COVID‐19 pneumonia: the CALL score. Clin Infect Dis 2020;71:1393‐1399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Shi Y, Yu X, Zhao H, Wang H, Zhao R, Sheng J. Host susceptibility to severe COVID‐19 and establishment of a host risk score: findings of 487 cases outside Wuhan. Crit Care 2020;24:108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Vallet‐Pichard A, Mallet V, Nalpas B, Verkarre V, Nalpas A, Dhalluin‐Venier V, et al. FIB‐4: an inexpensive and accurate marker of fibrosis in HCV infection. comparison with liver biopsy and fibrotest. Hepatology 2007;46:32‐36. [DOI] [PubMed] [Google Scholar]
- 47.Ibanez‐Samaniego L, Bighelli F, Uson C, Caravaca C, Carrillo CF, Romero M, et al. Elevation of liver fibrosis index FIB‐4 is associated with poor clinical outcomes in patients with COVID‐19. J Infect Dis 2020;222:726‐733. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Parikh NS, Kamel H, Navi BB, Iadecola C, Merkler AE, Jesudian A, et al.; VISTA‐ICH Collaborators . Liver fibrosis indices and outcomes after primary intracerebral hemorrhage. Stroke 2020;51:830‐837. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.United States Census Bureau . Quick facts: United States. https://www.census.gov/quickfacts/fact/table/US/PST045219. Vintage year 2019. Accessed March 2021.
- 50.Price‐Haywood EG, Burton J, Fort D, Seoane L. Hospitalization and mortality among black patients and white patients with Covid‐19. N Engl J Med 2020;382:2534‐2543. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Table S1
Appendix S1


