Dear Editor,
Recurrent varicella zoster virus (VZV) infection is an uncommon phenomenon, especially in immunocompetent patients, and has only sparsely been reported in the past. Alterations in the humoral or cellular immunity function might be a predisposition to such infection. Here we present a case of an immunocompetent patient who developed recurrent varicella following vaccination with the Pfizer/BioNTech mRNA vaccine.
A 33‐year‐old generally healthy female patient presented to the emergency department due to high‐grade fever and widespread rash. The patient received the first dose of the Pfizer/BioNTech BNT162b2 mRNA vaccine 1 week prior to the appearance of the symptoms. On examination, multiple vesicular lesions with an erythematous rim, in different stages of healing, were observed on the trunk, scalp, and limbs (Fig. 1). There was no specific dermatome involved. On further questioning, the patient indicated that she had chickenpox in childhood that was treated with calamine lotion. VZV‐immunoglobulin G antibodies were positive in the serum.
Figure 1.
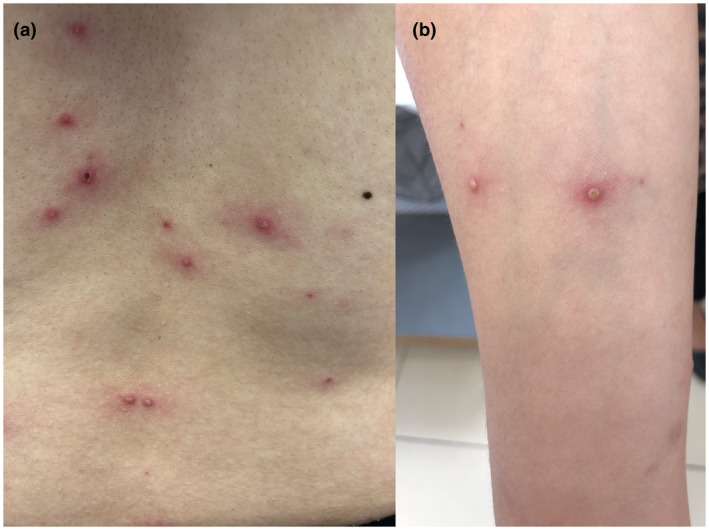
Multiple vesicular lesions with an erythematous rim, in different stages of healing on the back (a) and forearm (b) of a 33‐year‐old female patient
Although VZV infection supposedly provides lifelong immunity, recurrent infections have been reported, and it is hypothesized that second varicella infections are more prevalent than previously thought. 1 , 2 While recurrent varicella is thought to be more prevalent in immunocompromised patients, more than 40 cases of recurrent varicella have been described in immunocompetent patients, although it is still considered an uncommon phenomenon. 3 Differential diagnosis for multiple vesicular lesions (which did not fit the clinical appearance of the current case) includes other viral infections (e.g., herpes simplex, enteroviruses), bacterial infections (e.g., bullous impetigo), and other reactive eruptions, such as erythema multiforme and eczema vaccinatum. 4
Recurrent varicella may be attributed to intrinsic virulence factors and lack of protection with exposure to different strains. 5 However, intact cellular and humoral immunity plays a major role in the protection against reinfection. Pfizer/BioNTech BNT162b2 mRNA vaccine, and more generally, the mRNA group of vaccines, has only recently been used in large populations. 6 While the exact effect on the immune system of these vaccines is still being investigated, it is plausible that changes in the immune system following the vaccination can be linked to greater susceptibility to VZV infection.
Conflict of interest: None.
Funding source: None.
References
- 1. Hall S, Maupin T, Seward J, et al. Second varicella infections: are they more common than previously thought? Pediatrics 2002; 109: 1068–1073. [DOI] [PubMed] [Google Scholar]
- 2. Johnson JA, Bloch KC, Dang BN. Varicella reinfection in a seropositive physician following occupational exposure to localized zoster. Clin Infect Dis 2011; 52: 907–909. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Dyer J, Greenfield M. Recurrent varicella in an immunocompetent woman. Cutis 2016; 97: 65–69. [PubMed] [Google Scholar]
- 4. Dubey V, MacFadden D. Disseminated varicella zoster virus infection after vaccination with a live attenuated vaccine. CMAJ 2019; 191: E1025–E1027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Loparev VN, Rubtcova EN, Bostik V, et al. Identification of five major and two minor genotypes of varicella‐zoster virus strains: a practical two‐amplicon approach used to genotype clinical isolates in Australia and New Zealand. J Virol 2007; 81: 12758–12765. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Follmann D, Fintzi J, Fay MP, et al. Assessing durability of vaccine effect following blinded crossover in COVID‐19 vaccine efficacy trials. medRxiv 2020. 10.1101/2020.12.14.20248137 [DOI] [Google Scholar]


