Abstract
Background and purpose
An incremental number of cases of acute transverse myelitis (ATM) in individuals with ongoing or recent coronavirus disease 2019 (COVID‐19) have been reported.
Methods
A systematic review was performed of cases of ATM described in the context of severe acute respiratory syndrome coronavirus 2 (SARS‐CoV‐2) infection by screening both articles published and in preprint.
Results
Twenty cases were identified. There was a slight male predominance (60.0%) and the median age was 56 years. Neurological symptoms first manifested after a mean of 10.3 days from the first onset of classical, mostly respiratory symptoms of COVID‐19. Overall, COVID‐19 severity was relatively mild. Polymerase chain reaction of cerebrospinal fluid for SARS‐CoV‐2 was negative in all 14 cases examined. Cerebrospinal fluid findings reflected an inflammatory process in most instances (77.8%). Aquaporin‐4 and myelin oligodendrocyte protein antibodies in serum (tested in 10 and nine cases, respectively) were negative. On magnetic resonance imaging, the spinal cord lesions spanned a mean of 9.8 vertebral segments, necrotic‐hemorrhagic transformation was present in three cases and two individuals had additional acute motor axonal neuropathy. More than half of the patients received a second immunotherapy regimen. Over a limited follow‐up period of several weeks, 90% of individuals recovered either partially or near fully.
Conclusion
Although causality cannot readily be inferred, it is possible that cases of ATM occur para‐ or post‐infectiously in COVID‐19. All identified reports are anecdotal and case descriptions are heterogeneous. Whether the condition and the observed radiological characteristics are specific to SARS‐CoV‐2 infection needs to be clarified.
Keywords: acute transverse myelitis, autoimmune, COVID‐19, immune‐mediated, neuroinfection, neurological complication, SARS‐CoV‐2
INTRODUCTION
The ongoing severe acute respiratory syndrome coronavirus 2 (SARS‐CoV‐2) pandemic is beginning to provide further insights to infection‐related neurological manifestations [1]. The conditions reported in the context of coronavirus disease 2019 (COVID‐19) include but are not limited to encephalitis, myelitis, meningitis, acute disseminating encephalomyelitis (ADEM), metabolic and acute hemorrhagic necrotizing encephalopathy, cerebrovascular diseases, Guillain–Barré syndrome (GBS), cranial polyneuritis, dysautonomia and myopathies [2]. From a pathogenetic viewpoint, neurological manifestations can fall into any of four categories—direct virus effects on the nervous system, para‐ or post‐infectious immune‐mediated diseases and neurological conditions stemming from complications of systemic COVID‐19 [3].
Reports of neurological manifestations in observational cohorts vary widely from 3.5% to 84% [4]. One large prospective study of 4491 individuals with COVID‐19 in New York City (United States) reported neurological complications in 606 of them (13.5%) [4]. In that study, encephalopathy, seizures and stroke were the most common manifestations. At the same time, one of the earliest studies from Wuhan also considered mild and unspecific neurological symptoms like anosmia, headache and dizziness and found that 78 out of 241 individuals (36.4%) with COVID‐19 were affected [5].
Neither of the above cohort studies depicted cases of myelitis in the context of COVID‐19. Yet, there is mounting—although, at this point, largely anecdotal—evidence of individuals with acute transverse myelitis (ATM) and a history of infection with SARS‐CoV‐2. Considering that more than 50 million cases of COVID‐19 have been recorded worldwide to date and that this number will only grow, even rare complications may be important to recognize, especially if they require specific management strategies. Eight cases of acute and subacute neurological complications in the form of encephalitis, seizures, leukoencephalopathy, neuropathy or myopathy due to direct viral invasion have been reported for SARS‐CoV‐1 and Middle Eastern respiratory syndrome CoV (MERS‐CoV) [6, 7, 8]. Yet, no reports of ATM associated with these two beta‐coronaviruses, which caused epidemics in recent history, are found in the literature [9]. However, the total number of infected individuals for both viruses combined only totaled approximately 11,000 individuals; the frequency may not have been sufficient to notice potentially rarer complications [3]. For CoV‐OC43 or CoV‐229E, which belong to another subspecies of coronaviruses, cases of severe central nervous system (CNS) manifestations including encephalitis, ADEM or GBS in combination with detection of the virus by histological analysis of brain sections have been reported [10, 11]. There has also been a case of acute flaccid myelitis in association with respiratory CoV‐OC43 and CoV‐229E co‐infection [12].
In order to elucidate a potential occurrence of ATM in association with SARS‐CoV‐2 infections, all cases reported to date were systematically reviewed.
METHODS
This systematic review was carried out in accordance with PRISMA guidelines [13]. MEDLINE and two preprint servers (MedRxiv and BioRxiv) was searched from database inception to October 20, 2020, using the following search terms: “myelitis”, “myelopathy”, “spinal cord”, “neurologic manifestations”, “neurological manifestations” as well as “neurology“ in combination with “SARS‐CoV‐2” and “COVID‐19”. No language restrictions were applied. All types of studies were considered but only studies presenting original data were included in downstream analyses. Additionally, reference lists of included articles were also followed up to check for additional relevant studies that might have been missed.
Study bias was assessed using the Newcastle–Ottawa scale to identify possible selection bias, assessment bias, comparability issues, causality bias and reporting bias [14].
Cases were defined as “confirmed”, “probable” or “suspected” COVID‐19 cases using the case definitions put forward by the World Health Organization (WHO) and as “confirmed”, “probable” or “possible” SARS‐CoV‐2 myelitis as described previously [3]. Confidence in SARS‐CoV‐2‐associated myelopathy/myelitis was established using the four categories (“suspected myelopathy”, “myelopathy”, “possible myelitis” and “myelitis”) suggested by Ellul et al. [3]. Overall COVID‐19 severity was judged using the 0 to 10 scale of the WHO outcome criteria [15]. Wherever timeframes of disease course were not stated explicitly, a “best guess” was employed, where possible, using data derived from the case descriptions. If this was not deemed possible, the cases were left out of the analyses. Accordingly, for a reference, the number of cases which were used is stated in all analyses. Averages are reported as means ± standard deviation.
RESULTS
Systematic review and bias assessment
In total, 497 records were identified on MEDLINE, 156 records from preprint servers and four records from other sources, totaling to 657 records.
After the removal of 36 duplicates, titles and abstracts of 621 records were screened and 52 full‐length articles were assessed for eligibility. Twenty full‐length articles were included in the individual level data synthesis (Figure 1). Two case reports were identified [16, 17] which seemed to report the same patient. This notion was confirmed by at least one of the corresponding authors (personal correspondence). Accordingly, in the analyses, information was synthesized from both reports into one case presentation.
FIGURE 1.
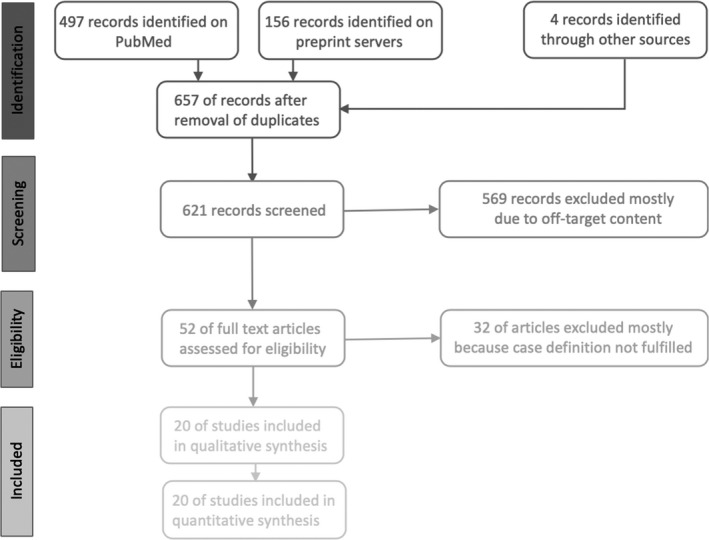
Record selection in accordance with PRISMA guidelines
Bias assessment revealed low quality for almost all studies, with only one case series with moderate quality (Appendix S1). Since most cases are reported as single case reports, the compromised quality was mainly due to selection or reporting bias.
Demographics
Over the period from January 1, 2020, to October 20, 2020, a total of 20 cases of ATM in the context of COVID‐19 were reported in the form of case reports or case series with individual level data. The first case of acute myelitis reported in Wuhan appeared on March 16, 2020, although currently still only available on a preprint server [18].
Cases were reported from 14 different countries; ancestries included European, Arabic, Native American, African and East Asian. 60.0% were men (12/20). The average age was 48.1 ± 19.2 years, with a median of 56. The comorbidities included hypertension (7/18), diabetes (4/18), obesity (2/18), hyperlipidemia (2/18) and hypothyroidism (2/18). Amongst the rarer comorbidities were human immunodeficiency virus (1/18) and glucose‐6‐phosphate‐dehydrogenase deficiency (1/18). Six out of 18 individuals did not have comorbidities (Table 1).
TABLE 1.
Demographics and clinical presentation
| Reference | Demographics | Diagnosis | Neurological presentation | Neurological findings |
Time to NLO max |
COVID−19 presenting symptoms | COVID−19 non‐NLO symptoms | Comorbidities | Latency to NLO (days) | WHO confidence | Myelitis diagnosis category | WHO severity | ||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Sex | Age | Origin | ||||||||||||
| Abdelhady [40] | M | 52 | Qatar | TM | Bilateral lower limb weakness, lower abdominal pain, urinary retention, fever | Flaccid paraparesis lower limbs, urinary retention | 4 days | Fever | P, cardiac arrest | DM II | 0 | Possible | Myelitis (1) | 2 or 10 |
| AlKetbi [49] | M | 32 | UAE (Asian) | LETM | Bliateral lower limb weakness, difficulty sitting up, difficulty on urination | Paralysis upper (3–4/5) and lower (0/5) limbs, truncal weakness, urinary retention | 8 h | Fever, flu‐like symptoms | PE | None | 2 | Probable | Myelitis (1) | 3 |
| Baghbanian [50] | F | 53 | Iran | LETM | Bilateral lower limb weakness, lower back pain, transient urinary incontinence | Asymmetrical hypotonic paraparesis lower limbs, areflexia, up‐going plantars, sensory level Th11/Th12 | 48–96 h | NA | None | DM, HTN, IHD | 14 | Probable | Myelitis (1) | 4 to 5 |
| Chakraborty [51] | F | 59 | India | TM | Progressive bilateral lower limb weakness, urinary retention, constipation | Flaccid paraplegia (0/5) and areflexia lower limbs, no plantar responses, sensory level Th10, urinary retention, constipation | 4 days | Fever | RF, cardiac arrest | None | 4 | Possible | Myelitis (1) | 5 or 10 |
| Chow [52] | M | 60 | Australia | LETM | Bilateral lower limb weakness, urinary retention, constipation | Global weakness, increased muscle tone, hyperreflexia, reduced proprioception lower limbs, paraesthesia to umbilicus | 48 h | Fever, cough, dysgeusia, anosmia | P | HTN, HLP | 16 | Probable | Myelitis (1) | 2 |
| Durrani [53] | M | 24 | USA | TM | Bilateral lower limb weakness, urinary incontinence | Flaccid paraplegia and areflexia lower limbs, urinary incontinence | Few days | Fever, chills, vomiting, tachypnea | P | None | ~14 | Probable | Myelitis (1) | 4 to 5 |
| Giorgianni [54] | F | 22 | Italy | Flaccid tetraparesis | Flaccid tetraparesis, fecal and urinary incontinence, fluctuating dysaesthesias | Acute flaccid tetraparesis, hyperreflexia, hypo‐/dysaesthesias lower limbs, fecal/urinary incontinence | <15 days | Fever, dyspnea | P, RF | DM I, keto‐ acidotic coma | 5 to 20 | Possible | Possible myelitis (2) | 8 |
| Kaur [19] | F | 3 | USA (Navajo) | LETM | Progressive weakness and decreased sensation of all limbs | Flaccid tetraparesis, neurogenic respiratory failure, generalized areflexia, no response to pain below neck | 12 h | Asymptomatic | None | None | 14 to 21 | Probable | Myelitis (1) | 1 |
| Lisnic [55] | M | 27 | Moldova | LETM | Bilateral lower limbs paralysis, numbness lower limbs and right arm, bladder/bowel dysfunction | Spastic tetraparesis lower > upper extremities, sensory level Th7 | 15 h | Subfebrile | P | HIV on antivirals | Asymptomatic | Possible | Myelitis (1) | 1 to 2 |
| Maideniuc [16]* Valiuddin [17]* | F | 61 | USA | TM + AMAN | Weakness all limbs, loss of ability to walk, numbness from chest down, urinary retention | Spastic weakness limbs, hyperreflexia/up‐going plantars lower limbs, sensory level C3 ⇢ areflexive tetraparalysis | 36 h | Rhinorrhea, chills | P, AMAN | HTN, HLP, HT, post‐solid tumor | 5 | Possible | Myelitis (1) | 2 |
| Masuccio [20] | F | 70 | Italy | TM + AMAN | Progressive weakness of all limbs, inability to walk, ascending paraesthesias | Hyperreflexia, up‐going plantars, tetraparesis upper (3/5) and lower (0/5) limbs, urinary retention, perineal areflexia | 5–10 days | Fever, myalgia, anosmia | P, AMAN | HTN, obesity | 15 | Possible | Myelitis (1) | 2 |
| Munz [22] | M | 60 | Germany | TM | Bilateral lower limb weakness, bladder dysfunction | Moderate spastic paraparesis lower limbs, hypaesthesia below Th9, hyperreflexia, up‐going plantars | 48 h | Respiratory symptoms | P | HTN, urolithiasis | ~13 | Probable | Myelitis (1) | 4 |
| Paterson [42] | M | 48 | UK | LETM | Unsteady gait, numbness hands and feet, band of itching sensation at level of umbilicus | Weakness of hip flexion, hyperreflexia, extensor plantars, vibration/pinprick to Th10, sensory ataxia | NA | Fever, cough, dyspnea | P | HTN, DM | 19 | Possible | Myelitis (1) | 4 |
| Rifino [21] | M | 66 | Italy | TM | Unsteady gait, numbness of lower limbs | Spastic paraparesis (4/5) lower limbs, reduced sensation to touch, acroparaesthesia, hyperreflexia with bilateral distal clonus | NA | Fever, anosmia, ageusia | P | NA | 24 | Confirmed | Possible myelitis (2) | 2 |
| Rifino [21] | M | 62 | Italy | TM | Lower limb weakness, back pain radiating to lower limbs, sensory changes, constipation | Paraparesis (4/5) lower limbs, sensory level Th11 | NA | NA | NA | None | NA | Confirmed | Possible myelitis (2) | NA |
| Sarma [56] | F | 28 | Denmark | LETM | Lower back pain, bilateral symmetric numbness of all limbs and chest and tip of tongue, urinary retention, nausea/vomitting | Sensory level Th5, paraparesis upper limbs (4/5), wide‐based gait, Lhermitte’s sign positive, urinary retention | 8 days | Fever, productive cough, lower back pain, myalgias, rhinorrhea | None | HT | 8 | Possible | Myelitis (1) | 2 |
| Sotoca [23] | F | 69 | Spain | Acute necrotizing TM | Cervical pain, tetraparesis, numbness both hands, incontinence | Right facial and left hand hypoaesthesia, interosseus weakness left hand, general hyperreflexia | 7 days | Fever, dry cough | None | None | 8 | Probable | Myelitis (1) | 2 to 3 |
| Wong [24] | M | 40 | UK (African) | Rhombencephalomyelitis | Unsteady gait, limb ataxia, altered sensation right arm, hiccups, diplopia, oscillopsia | Bilatl facial weakness, tongue weakness, upbeat nystagmus, limb ataxia greater on right and lower limbs | 24 h | Fever, dyspnea, cough, diarrhea | P, hepatitis, rhombencephalitis | HTN, glaucoma | 13 | Possible | Myelitis (1) | 4 to 5 |
| Zachariadis [57] | M | 60 | Switzerland | TM | Lower limb weakness, par‐ and hypoaesthesias of both feet progressing to abdominal area | Moderate proximal paraparesis lower limbs, pyramidal signs, sensory level Th10 ⇢ paraplegia, sphincter dysfunction | 7 days | Headache, rhinorrhea, myalgia, subfebrile | P | Obesity, smoking, alcohol abuse | 12 | Probable | Myelitis (1) | 2 |
| Zhao [18] | M | 66 | China | TM | Bilateral lower > upper limb weakness, reduced sensation lower limbs, urinary/bowel incontinence | Tetraparesis (3/5 arms, 0/5 legs), hyporeflexia lower limbs, sensory level at Th10 | 4–8 h | Fever, fatigue, cough, dyspnea | P | NA | 8 | Possible | Myelopathy (3) | 5 |
Abbreviations: AMAN, acute motor axonal neuropathy; DM, diabetes mellitus; F, female; HIV, human immunodeficiency virus; HLP, hyperlipidemia; HT, hypothyroidism; HTN, hypertension; IHD, ischaemic heart disease; LETM, longitudinally extensive transverse myelitis; M, male; NA, not available; NLO, neurological; P, pneumonia; PE, pulmonary embolism; RF, respiratory failure; TM, transverse myelitis; WHO, World Health Organization.
Same case reported in two publications.
Clinical presentation
In most instances, neurological symptoms consisted of the classical triad of weakness of the lower extremities, sensory deficits in the form of a sensory level, and bladder or bowel dysfunction. Details on the neurological presentation and individual findings are presented in Tables 1 and 2.
TABLE 2.
Neuroimaging, CSF findings and ancillary investigations
| Reference | MRI spinal cord | MRI brain | CSF | NCS/EMG | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| T2 hyperintensity | Enhancement | Spinal cord subsegment | Lesion length (vertebral segments) | Pattern | Presence of pleocytosis (cell count) | Protein | Glucose | Pathogens | Other | |||
| Abdelhady [40] | + | – | Thoracic | 6 | Continuous | WNL | + | + | NA | Cultures neg, SARS‐CoV‐2 PCR neg | None | NA |
| AlKetbi [49] | + | – | Cervical, thoracic, lumbar | 23 | Patchy | NA | NA | NA | NA | NA | NA | NA |
| Baghbanian [50] | + | NA | Thoracic | 3 | Continuous | WNL | + (13/μl) | WNL | WNL | PCR for HSV, CMV and SARS‐CoV‐2 neg | No CSF‐specific OCBs, IgG index upper limit of normal, MOG/AQP4 Abs neg | NA |
| Chakraborty [51] | + | NA | Thoracic | 2 | Continuous | NA | WNL | + (71.4 mg/dl) | WNL | Ziehl–Neelsen and Gram‐stain neg, SARS‐CoV‐2 PCR neg | None | NA |
| Chow [52] | + | NA | Thoracic | 4 | Continuous | WNL | WNL | + (79 mg/dl) | WNL | Cultures neg, SARS‐CoV‐2 PCR neg | MOG/AQP4 Abs neg | NA |
| Durrani [53] | + | NA | Thoracic | 6 | Continuous | NA | + | WNL | WNL | NA | No CSF‐specific OCBs, IgG index normal, AQP4 Abs neg | NA |
| Giorgianni [54] | WNL | NA | NA | NA | NA | Tiny subacute frontal hemorrhage | WNL | WNL | + | SARS‐CoV‐2/VZV/HSV PCR, Borrelia Abs, microbial culture, and Tbc neg | None | NA |
| Kaur [19] | +/+, necrosis, hemorrhages | –/+ | Medulla, cervical, thoracic | 13 | Continuous | WNL | + (42/μl), 96% neutrophilic | + (58 mg/dl (15–45 mg/dl)) | NA | SARS‐CoV‐2, viral, and microbacterial panels neg | Including MOG/AQP4 Abs neg; hemorrhagic (282/mm3) | NA |
| Lisnic [55] | + | – | Cervical, thoracic | 9 | Continuous | WNL | WNL | WNL | WNL | SARS‐CoV‐2, viral, and bacterial tests neg | No CSF‐specific OCBs, MOG/AQP4 Abs neg | NA |
| Maideniuc [16] * Valiuddin [17]* | + | + | Medulla, cervical, thoracic, lumbar | 24 | Continuous | WNL | d10: WNL d21: WNL |
d10: + (87 mg/dl) d21: + (153 mg/dl) |
WNL | d10: SARS‐CoV‐2 negative, other viral pathogens not done, VDRL/culture neg | No CSF‐specific OCBs, IgG index normal, ganglioside Abs not tested, MOG/AQP4/anti‐neuronal Abs neg; d10: hemorrhagic (312/ul) | NCS/EMG: acute motor axonal neuropathy |
| Masuccio [20] | + | – | Cervical, thoracic | 3 | Continuous | WNL | WNL | WNL | NA | Viral and bacterial work‐up neg | Anti‐GD1b‐IgM pos, no CSF‐specific OCBs, viral/bacterial work‐up neg in serum | NCS/EMG: acute motor axonal neuropathy |
| Munz [22] | + | – | Thoracic | 3 plus 2 | Patchy | WNL | d1: + (16/μl) d6: + (27/μl) |
d1: + (79 mg/dl) d6: + (118 mg/dl) |
NA | HSV, VZV, HHV‐6, EBV, HEV, SARS‐CoV‐2 neg, anti‐SARS‐CoV‐2 IgG neg | No CSF‐specific OCBs, MOG/AQP4/anti‐neuronal Abs neg | NA |
| Paterson [42] | + | – | Thoracic, lumbar | > 4 | Patchy | WNL | + (10/μl) | + (70 mg/dl) | + | Culture and viral PCRs neg | No CSF‐specific OCBs | NCS/EMG: WNL |
| Rifino [21] | +, diffuse degeneration | – | NA | NA | NA | WNL | WNL | + | NA | PCR for bacteria/neurotropic viruses/SARS‐CoV‐2 neg, anti‐SARS‐CoV‐2 IgG pos | None | NCS/EMG: reduction of maximal voluntary activity; SEP/MEP lower limbs: bilateral medullar conduction block |
| Rifino [21] | Diffuse degeneration | – | NA | NA | NA | WNL | WNL | + | NA | PCR for bacteria/neurotropic viruses/SARS‐CoV‐2 neg, anti‐SARS‐CoV‐2 IgG pos | None | NCS/EMG: reduction of maximal voluntary activity; SEP/MEP lower limbs: bilateral medullar conduction block |
| Sarma [56] | + | + | Medulla, cervical, thoracic, lumbar | 24 | Continuous | NA | + (125/μl) | (+) | WNL | Gram‐stain and cultures unremarkable | Abs neg | NA |
| Sotoca [23] | +, necrosis, hemorrhages | + | Medulla, cervical, thoracic | 13 | Continuous | WNL | + (75/μl) | + (283 mg/dl) | WNL | Bacterial culture, viral multi‐PCR neg | No CSF‐specific OCBs, IgG index normal, MOG/AQP4/anti‐neuronal Abs neg | NA |
| Wong [24] | +, hemorrhages | NA | Rhomencephalic, medulla, cervical | NA | Continuous | T2 hyperintensity right inferior cerebellar peduncle, microhemorrhages | WNL | WNL | NA | Bacterial culture neg | MOG/AQP4 Abs neg | NA |
| Zachariadis [57] | WNL | NA | NA | NA | NA | WNL | d1: + (16/μl) d6: + (36/μl) |
d1: + (57.3 mg/dl) d6: + (60.0 mg/dl) |
WNL | Neg for bacteria and viruses including SARS‐CoV‐2 | MOG/AQP4/anti‐neuronal/anti‐ganglioside neg | NA |
| Zhao [18] | NA | NA | No MRI | No MRI | No MRI | Lacunar infarctions, atrophy | NA | NA | NA | NA | NA | NA |
Abbreviations: Ab, antibody; AQP4, aquaporin 4; CMV, Cytomegalovirus; CSF, cerebrospinal fluid; EBV Epstein‐Barr virus; GD1b, ganglioside 1b; HHV‐6, Human Herpesvirus‐6; Hepatitis E virus, HEV, SEP/MEP, somatosensory/motor evoked potentials; HSV, Herpes‐simplex virus (HSV); IgG, Immunoglobulin G; IgM, Immunoglobulin M; MOG, myelin oligodendrocyte glycoprotein; MRI, magnetic resonance imaging; NA, not available; neg, negative; NNCS/EMG, nerve conduction study/Electromyography; OCB, oligoclonal bands; PCR, polymerase chain reaction; pos, positive; Tb, tuberculosis; VDRL, Venereal Disease Research Laboratory; VZV, Varicella‐zoster virus; WNL, within normal limits.
Same case reported in two publications.
Progression from onset of neurological symptoms to maximum symptom severity was approximately 80.8 ± 66.9 h, range 6 h to approximately 7 days, median 48 h (data available for 17/20 cases; Table 1). Neurological symptoms first manifested on average 10.3 ± 5.8 days after the first onset of classical, mostly respiratory, symptoms of COVID‐19 (range 0–19 days, data available for 15/20 polymerase chain reaction [PCR] positive cases; Table 1). The most frequently reported symptoms of the initial manifestation of SARS‐CoV‐2 infection included fever/subfebrile temperatures (15/18 cases), cough (7/18 cases), dyspnea (5/18), rhinorrhea (3/18) and myalgia (4/18) (also see Table 1). Only in the instance of a 3‐year‐old child were no symptoms of respiratory tract infection or fever reported prior to the onset of neurological symptoms [19]. COVID‐19 manifestation overall was relatively mild with an average WHO score [15] of 3.2 ± 1.7 (median 2, range 1–8), equivalent to respiratory symptoms that can be treated at home without need for hospitalization. Two cases with mild respiratory symptoms and sudden death from cardiac arrest were not included in the analysis due to the fact that the association between COVID‐19 and the deaths could not be established. Pneumonia was diagnosed in 68.4% of cases (13/19). Additional possibly COVID‐19‐related complications included cardiac arrest (2/19), hepatic inflammation and failure (1/19) and pulmonary embolism (1/19). Of note, distal axonal motor neuropathies were reported in two individuals with myelitis (2/19; 10.5%). No individual was described as having had a prior episode of transverse myelitis (TM) or other autoimmune conditions of the CNS.
Laboratory and cerebrospinal fluid findings
Polymerase chain reaction (PCR) from nasopharyngeal swabs was performed in all cases. It was positive at some point in the disease course in 75.0% of cases (16/20 cases). In the four cases without a positive SARS‐CoV‐2 PCR, anti‐SARS‐CoV‐2 antibodies were present in serum (immunoglobulin G [IgG] only in 2/4; IgG/IgM in 1/4; IgG/IgM/IgA in 1/4). At the time of onset of neurological symptoms, 8/16 cases were PCR positive. Of the 8/16 negative cases, two turned positive 2 or 3 days later; the others remained negative or were not tested again but had had a positive SARS‐CoV‐2 PCR prior to the onset of neurological symptoms. Chest X‐rays (8/18 cases) or computed tomography (10/18 cases) were performed in 90.0% of individuals (18/20 cases). In five cases, these were within normal limits (5/18 cases; 27.8%). Thirteen of 18 showed patchy infiltrations, which were unilateral in 3/13 cases and bilateral in 7/13 cases. Data regarding the extent of infiltrations was lacking in 3/13 cases.
Blood laboratory findings were reported in 15/20 cases although the extent of what was reported was very heterogeneous (Table S2). In the majority of cases (13/15 cases), laboratory changes showed a mild, often incomplete, systemic inflammatory syndrome with slightly elevated white blood cell counts (5/14 cases), erythrocyte sedimentation rates (1/5 cases) and C‐reactive protein (8/13 cases) as well as lymphocytopenia (3/11 cases). In five out of 15 cases, additional changes often seen in the context of SARS‐CoV‐2 infection, such as elevated D‐dimer levels or liver enzymes, were also observed. In half of the cases, serological work‐up for autoimmune diseases was performed. Where tested, anti‐ aquaporin 4 (anti‐AQP4) antibodies (10/20 cases) and anti‐myelin oligodendrocyte glycoprotein (anti‐MOG) antibodies (9/20 cases) were not present and panels for systemic autoimmune diseases (9/20 cases) or anti‐neuronal antibodies (3/20) returned negative results. In one individual who also had axonal motor neuropathy, anti‐GD1b IgM ganglioside antibodies were detected [20].
Cerebrospinal fluid (CSF) findings were heterogeneous but reflected some form of inflammatory process in most cases (14/18 cases): lymphocytic pleocytosis with elevated protein was seen in 6/18 cases, isolated lymphocytic pleocytosis was present in 3/18 cases and isolated protein elevation was found in 5/18 cases. CSF glucose levels were available in 11 of 18 cases and were within normal limits in nine and increased in two individuals with diabetes. If evaluated, CSF‐specific oligoclonal bands were not detected (8/18) and IgG index was unremarkable or at the upper limit of normal (5/18) (Table 2). In all 14 cases examined, no SARS‐CoV‐2 RNA was detected by real‐time PCR in the CSF. In two of three cases, CSF was positive for anti‐SARS‐CoV‐2 IgG [21] in one case, it was negative [22]. No other viral or bacterial infections were detected in the CSF or serum of those 19 individuals for whom it was reported (Tables 2 and 3 for details).
TABLE 3.
SARS‐CoV‐2 diagnostics, treatment and outcome
| Reference | SARS‐CoV‐2 diagnostics | SARS‐CoV‐2 at NLO onset | Chest imaging | Blood laboratory findings | Additional pathogens tested (all negative) | Additional antibodies | Treatment of myelitis | Recovery |
|---|---|---|---|---|---|---|---|---|
| Abdelhady [40] | NPS PCR pos | Positive | Chest X‐ray: bilateral scattered infiltrations | NA | HSV, HBV, HCV, Tbc | ANA/ANCA neg | ACV, iv MP | Death |
| AlKetbi [49] | NPS PCR pos | Positive | Chest CT: no consolidations/pleural effusions, PE | CRP +, Hb –, D‐dimer +, CK + | AdV, HSV, EBV, CMV, HIV, IAV/IBV, PIV 1–4, RSV, EV, RV, Chlamydia pneumoniae, Bordetella pertussis, Mycoplasma pneumoniae, Borrelia burgdorferi | AID panel neg | iv MP, ACV, LMWH | Partial |
| Baghbanian [50] | NPS PCR pos | Not done | Chest CT: patchy ground‐glass consolidation right lung | WNL | HSV, CMV | NA | PEX | Partial |
| Chakraborty [51] | d1: NPS PCR neg d2: NPS PCR pos |
d1: neg d2: pos |
Chest X‐ray: WNL | WNL | HIV, HBV, HCV | NA | iv MP | Death |
| Chow [52] | NPS PCR pos; SARS‐CoV‐2 IgG/IgM/IgA sero‐pos | Negative | Chest CT: bilateral peripheral ground‐glass opacities and consolidation | ESR +, CRP (+), D‐dimers +, lympho – | EBV, CMV, HIV, HBV, HCV, M. pneumoniae | AID panel neg | iv MP | Full |
| Durrani [53] | NPS PCR pos |
d1: neg d4: pos |
Chest CT: multifocal pneumonia | NA | HIV, Legionella pneumophila, blood and respiratory cultures | AID panel neg | iv MP | Partial to full |
| Giorgianni [54] | NPS and BAL PCR pos | NA | Chest CT: extensive bilateral ground‐glass opacities | WBC +, CRP +, D‐dimers +, LDH +, ASAT +, glucose ++, pH + | HIV, VZV, HSV, M. pneumoniae, L. pneumophila, C. pneumoniae, B. burgdorferi, Mycobacterium tuberculosis, CSF bacterial culture | NA | Antiviral, immune modulatory for respiratory/metabolic syndromes | Partial |
| Kaur [19] | NPS PCR pos | Positive | Chest X‐ray: WNL | NA | VZV, HSV, EV, HIV, EBV, CMV, IAV/IBV, Treponema pallidum, M. tuberculosis, M. pneumoniae | AID panel neg | iv MP and IVIG ⇢ PEX ⇢ rituximab | Partial |
| Lisnic [55] | d1: NPS PCR neg d19: NPS PCR pos | Positive | Chest CT: slight patchy ground‐glass opacities basal on the left (d19) | WBC +, ESR +, HIV <40 copies, CD4 340/ul | HSV 1/2/6, CMV, EBV, HIV, HBV, HCV, B. burgdorferi, T. pallidum, Toxoplasma gondii, Chlamydia trachomatis, M. pneumoniae, Ureaplasma urolyticum | AID panel neg | iv MP ⇢ PEX | Partial |
| Maideniuc [16] * Valiuddin [17]* | NPS PCR pos | Positive | NA | ANA 1:80; WBC (+); CRP (+) | Infectious pathogens in serum | AID panel neg | iv MP ⇢ PEX | Partial |
| Masuccio [20] | NPS PCR neg; SARS‐CoV‐2 IgG sero‐pos | Negative | Chest CT: interstitial pneumonia with ground‐class opacities | CRP +, lympho – | EBV, CMV, HSV, VZV, HIV, B. burgdorferi, C. pneumoniae, M. pneumoniae | NA | PEX, IVIG | Partial |
| Munz [22] | NPS PCR pos | Negative | Chest X‐ray: bilateral mild ground‐glass opacities | CRP (+) | HSV, VZV, HHV‐6, EBV, HEV | NA | MP, ceftriaxone, ACV | Partial |
| Paterson [42] | NPS PCR pos | NA | Chest X‐ray: patchy infiltrates | Ferritin (+) | Viral PCRs, blood/urine/CSF cultures, HTLV‐1/2, syphilis neg | NA | iv MP, antibiotics for secondary bacterial pneumonia | Partial |
| Rifino [21] | NPS PCR neg; SARS‐CoV‐2 IgG sero‐pos | Negative | Chest CT: small ground‐glass opacities | NA | Bacteria, common neurotropic viruses | NA | iv MP ⇢ PEX | Partial |
| Rifino [21] | NPS PCR neg; SARS‐CoV‐2 IgG sero‐pos | Negative | Chest X‐ray: WNL | NA | Bacteria, common neurotropic viruses | NA | iv MP ⇢ IVIG ⇢ PEX | Partial |
| Sarma [56] | NPS PCR pos | NA | NA | NA | NA | NA | iv MP ⇢ PEX | Near full |
| Sotoca [23] | NPS PCR pos | Positive | Chest X‐ray: WNL | WNL | Panviral PCR, CSF culture | AID panel neg | iv MP ⇢ 2nd iv MP ⇢ PEX | Partial |
| Wong [24] | NPS PCR pos | Positive | Chest X‐ray: right lower zone consolidation | CRP (+), GGT (+), ALAT (+) | HAV, HBV, HCV, HIV 1/2, syphilis | NA | Amoxicillin, paracetamol, gabapentin | Partial |
| Zachariadis [57] | NPS PCR neg; SARS‐CoV‐2 IgG/IgM sero‐pos | Negative | Chest CT: bilateral ground‐glass opacities; PET‐CT: non‐revealing | WBC (+), CRP (+) | Broad serology panel | AID panel neg | IVIG ⇢ iv MP | Partial |
| Zhao [18] | NPS PCR pos | Positive | Chest CT: bilateral patchy infiltrations | WBC +, lympho/eosino –, CRP ++, Hb –, ALAT/ASAT (+), CK +, iron – | EBV, IAV/IBV, AdV, EV, PIV, CMV, RSV, M. pneumoniae, C. pneumoniae, Tbc | NA | Dexamethason, IVIG, ganciclovir, lopinavir/ritonavir, moxifloxacin | Partial |
Abbreviations: ACV, acyclovir; AdV, adenovirus; AID, autoimmune disease; ALAT, alanine transaminase; ANA, antinuclear antibodies; ANCA, antineutrophil cytoplasmic antibodies; ASAT, aspartate transaminase; BAL, bronchoalveolar lavage; CK, creatinine kinase; CMV, cytomegalovirus; CRP, C‐reactive protein; CSF, cerebrospinal fluid; CT, computed tomography; EBV, Epstein–Barr virus; eosino, eosinophils; ESR, erythrocyte sedimentation rate; EV, enterovirus; GGT, gamma‐glutamyltransferase; HAV, Hepatitis A virus; Hb, hemoglobin; HBV, hepatitis B virus; HCV, hepatitis C virus; HEV, hepatitis E virus; HHV, human hepatitis virus; HIV, human immunodeficiency virus; HSV, herpes simplex virus; HTLV, human T‐lymphotropic virus type 1; IAV/IBV, influenza virus A/B; IgA immunoglobulin A; IgG, immunoglobulin G; IgM, immunoglobulin M; iv, intravenous; IVIG, intravenous immunoglobins; LDH, lactate dehydrogenase; LMWH, low molecular weight hemoglobin; lympho, lymphocytes; MP, methylprednisolone; NA, not available; neg, negative; NLO, neurological; NPS, nasopharyngeal swab; PCR, polymerase chain reaction; PE, pulmonary embolism; PET, positron emission tomography; PEX, plasma exchange; PIV, parainfluenza virus 1–4; pos, positive; RSV, respiratory syncytial virus; RV, rhinovirus; sero‐pos, sero‐positive; Tbc, tuberculosis; VZV, varicella zoster virus; WBC, white blood cell count; WNL, within normal limits; +, above normal limits; ++, greatly above normal limits; – , below normal limits; (+), upper limit of normal or slightly above normal.
Same case reported in two publications.
Based on the WHO definitions for describing associations between SARS‐CoV‐2 and myelitis/myelopathy, the association was “confirmed” in two cases based on the presence of anti‐SARS‐CoV‐2 IgG in CSF. In 40% of cases, it was “probable” (8/20 cases) with neither viral RNA or SARS‐CoV‐2 antibodies found in CSF but clear evidence of SARS‐CoV‐2 infection and no evidence of alternative causes for TM. In the remaining 50%, association was “possible” (10/20 cases) due to incomplete exclusion of potential alternative causes of TM (Tables 1, 2, 3).
Neuroradiological imaging
Nineteen out of 20 cases had a magnetic resonance imaging (MRI) of the spinal cord; two of these (10%) were unrevealing and two showed degenerative changes, in one instance with concomitant T2 hyperintensities of the cauda. In the remaining 78.9% (15/19), classical T2 hyperintensities of the spinal cord were observed. At the time of first spinal cord imaging, lesions in nine individuals were described as non‐enhancing whilst in three individuals they were enhancing. In the remaining cases, it is unclear whether MRIs were performed with contrast (Table 2). In three instances, there was also evidence of hemorrhagic transformation and necrosis of the spinal cord lesion [19, 23, 24].
Transverse localization was central, frequently with extension throughout most of the transverse diameter, in 7/11 cases. The thoracic subsegment of the spinal cord was affected most frequently (93.3%; 14/15 cases), followed by the cervical subsegment (53.3%; 8/15) (Table 2). Interestingly, in most cases lesions were longitudinally extensive extending over an average of 9.8 ± 8.3 vertebral body segments (median 6; range 2–24) (Table 2). In 93.3% of individuals with a clear lesion on spinal cord imaging, the lesion extended over three or more spinal cord segments, fulfilling the criteria for longitudinally extensive transverse myelitis (LETM). In five out of 15 individuals with a spinal cord lesion, the lesion spanned more than half the spinal cord. Whenever performed (16/20 cases), brain MRI showed no additional supratentorial inflammatory lesions (Table 2). In several instances, T2 hyperintensities were described as “patchy” (3/15) or with “patchy enhancement” (2/15), whilst they were continuous in the other cases (10/15).
Applying the diagnostic levels set forth by Ellul et al. specifically for myelopathy/myelitis in the context of infections with SARS‐CoV‐2 [3], 16/20 cases were classified as having “myelitis”, three as having “possible myelitis” and one as having “myelopathy” only, due to the lack of both CSF analysis and imaging studies (Table 1).
Treatment and outcome
The majority of cases (90.0%, 18/20 cases) received some form of immune therapy. Eight of 18 cases, received either intravenous methylprednisolone (MP) (seven cases) or plasma exchange (one case) alone. However, in 10 of 18 cases, more than one immune therapy was administered. Eight individuals were treated with a combination of two different immune therapies—in most instances, intravenous MP followed by plasma exchange—, in one case a combination of three and in another of four different immune therapies (Table 3). Additional antivirals or antibiotics were administered in five cases each (Table 3). Whilst antibiotic regimes varied, acyclovir was the most commonly used antiviral (4/5 cases). Only one of the reported individuals received an antiviral combination specifically geared at SARS‐CoV‐2 (ritonavir/lopinavir). Other SARS‐CoV‐2‐specific therapies such as remdesivir, convalescent plasma or monoclonal antibodies were not administered to any of the individuals.
Follow‐up was mostly limited to several weeks of in‐hospital treatment. Over this limited time period, 90.0% of individuals recovered either partially (15/20 cases) or near fully (3/20 cases). Two patients died from sudden cardiac arrest on day 5 after onset of myelitis symptoms whilst undergoing treatment in the hospital. In one case, cardiac arrest occurred immediately following sudden‐onset respiratory failure; in the other, no additional details are provided (Table 3).
DISCUSSION
In most of the reported cases reviewed herein, the diagnosis of myelitis in the context of SARS‐CoV‐2 infection was undisputed. Yet, since the majority of cases were reported as single case reports, there is likely to be reporting bias and one needs to be wary of inferring causality directly from the anecdotal data provided. Although presented as a set of cases for reasons of practicality, one needs to be wary of seeing them as a uniform cohort as they really represent single cases documented in unique individual settings. Further, interpretability is hampered by the limited number of available cases, the highly diverse patient population spanning many ages and ancestries, and the heterogeneous and, in many cases, incomplete work‐up.
None of the 20 cases could be classified as confirmed SARS‐CoV‐2 myelitis. Most frequently, this was due to the fact that SARS‐CoV‐2 could not be detected by PCR in the CSF, and the work‐up did not include analysis of spinal cord specimens. Of note, at least one large prospective cohort study of neurological manifestations in COVID‐19 did not identify any cases of myelitis amongst 4491 individuals with COVID‐19 [4]. At the same time, even though the follow‐up period was not long enough to fully exclude this possibility, none of the reported cases showed signs of laboratory or imaging findings suggestive of other underlying autoimmune diseases that could manifest with LETM such as neuromyelitis optica spectrum disorders or spinal cord manifestation of systemic autoimmune disease [25, 26]. The work‐up included the exclusion of viruses with neuroinvasive potential as well as other viruses known for para‐ or post‐infectious spinal cord complications.
Another possible way to assess whether SARS‐CoV‐2 is actually responsible for cases of TM would be to compare the incidence of myelitis cases pre‐SARS‐CoV‐2 pandemic to the current incidence to see if there is an overall increase in cases of TM. The incidence of TM has been reported to range somewhere between 1 and 8 cases per 1 million population per year, with rates relatively stable across ancestries [27, 28]. If cases that are later diagnosed as having an underlying autoimmune disorder are included, this number increases to around 32 cases per 1 million population per year [29]. Extrapolated to the 107 million reported infections to date, this would mean that between 107 and 3317 cases of TM would be expected to occur amongst these individuals from causes unrelated to COVID‐19. Not least due to this large number and the wide range of TM cases that can be ascribed to causes other than potentially COVID‐19, it will be very important to continue to survey the situation of myelitis in COVID‐19 both by systematically including neurological manifestations as outcomes in large COVID‐19 cohort studies and by collecting myelitis cases in the context of COVID‐19 in Neuro‐COVID‐19 registries [30].
A number of demographic characteristics reinforce the notion that the cases depicted herein, for the most part, truly represent myelitis caused by SARS‐CoV‐2 infection. First, affected individuals were of all different ages and ancestries, and had a slight predominance for males. Male predominance and a median age of 56 are not in line with TM as the first manifestation of autoimmune disorders of the CNS, where patients are predominantly female and much younger. Observational cohorts of ATM of any cause show a bimodal age distribution with peaks between 10 and 20 years of age and 30 and 40 years of age with the mean age of onset between 35 and 40 [29]. Sex and age distributions of the cases herein, on the other hand, are in line with more complicated presentations of COVID‐19 and the finding that neurological symptoms in COVID‐19 have been reported to occur more frequently in men and older individuals [5]. As opposed to other neurological manifestations in the context of SARS‐CoV‐2 infection, overall individuals with myelitis did not seem to have very severe respiratory COVID‐19.
Several possible mechanisms exist by which SARS‐CoV‐2 could lead to spinal cord manifestations. Coronaviruses have been shown to be both neuroinvasive and neurovirulent and can lead to demyelination and as well as an inflammatory response [31]. One possible mechanism for myelitis in the context of SARS‐CoV‐2 infection is the direct invasion of and replication in spinal cord neurons by the virus itself [32]. The presence of angiotensin‐converting enzyme 2, SARS‐CoV‐2’s primary entry receptor, on membranes of spinal cord neurons further renders this possible [33]. The fact that no viral RNA was detected in the CSF of the cases reviewed herein or in the vast majority of other individuals with neurological manifestations of COVID‐19 depicted in the literature [4, 21, 34] and that SARS‐CoV‐2 RNA has only very rarely been detected in the CSF or in CNS tissue argues against this as the primary mechanism [35, 36, 37]. Yet, the presence of very low viral copies in general or following degradation, as well as the examination of CSF specimens outside the peak of viral copy numbers in CSF, as potential explanations for the rare detection of SARS‐CoV‐2 in CSF cannot be excluded [2]. A second possibility is indirect injury due to severe systemic disease or cytokine storm syndrome [23, 38]. In the described cases, however, COVID‐19 severity was rather mild making this possibility less likely. A third possible way by which TM in the context of COVID‐19 could arise is in the form of para‐ or post‐infectious disease. The latency of on average 11 days from the onset of the first COVID‐19 symptoms to the first signs of myelitis would speak to a para‐ or post‐infectious mechanism. In the literature, there is no clear definition as to when para‐infectious disease ends and when post‐infectious disease starts. The latency in the cases described here is a little shorter than what is commonly seen in, for example, GBS (median 23 days) [39]. Also, at least 52.9% of cases were PCR positive for SARS‐CoV‐2 on nasopharyngeal swab at the time of presentation of myelitis symptoms. Accordingly, should SARS‐CoV‐2 infection be the driver of myelitis in these cases, the most likely mechanism would be immune‐mediated or autoimmune with no clear distinction between para‐ and post‐infectious processes possible at present.
Under the conjecture of a para‐infectious mechanism of myelitis in the context of SARS‐CoV‐2 infection, the question of the most appropriate therapeutic strategy imposes itself. High‐dose intravenous MP alone was administered in 36.8% of individuals. 57.8% received more than one immune therapy—in most instances, high‐dose MP followed by plasma exchange. This argues for treatment failure with steroids in the majority of the patients. To our knowledge, there are no evidence‐based treatment guidelines available for para‐infectious ATM. If a para‐ or post‐infectious pathogenesis is corroborated, intravenous immunoglobulins (IVIG) may be another treatment option.
The large majority of cases assessed in this review had a myelopathy fulfilling LETM criteria. Whilst LETM is often perceived as characteristic of neuromyelitis optica spectrum disorder, there are many potential causes which include viral infections. Some viruses have a greater tendency to cause LETM than others. LETM is more frequently observed with flaviviruses and enteroviruses. Herpes simplex virus type 2, varicella zoster virus, Epstein–Barr virus, or cytomegalovirus tend to cause ATM of shorter longitudinal extension [40, 41]. SARS‐CoV‐2 should probably be added to that list. This is relevant not least because LETM has been associated with poorer outcome compared to short segment ATM [29].
Hemorrhagic transformation and necrosis are only rarely seen in LETM [23]. Interestingly and in line with what has been described for ADEM cases in the context of SARS‐CoV‐2 infection, which also seem to show hemorrhagic transformations relatively frequently [34, 42], this was also observed in three out of 16 of this case series.
Another interesting finding emerging from this systematic review is the co‐occurrence of TM with the GBS variant acute motor axonal neuropathy (AMAN). In two individuals, AMAN was reported in addition to TM. GBS—of the AMAN and more frequently the acute inflammatory demyelinating variant—has been described in individuals with COVID‐19 [43, 44]. Critical illness neuropathy seems unlikely as an alternative diagnosis because both patients were not severely ill from COVID‐19 (WHO score of 2—very mild symptoms) and the CSF findings were in line with immune‐mediated neuropathy (Table 2). Although the number of undiscovered cases may be much higher, less than 30 cases of GBS/ATM overlap syndromes are found in the literature, the majority having occurred after viral infections [45]. Accordingly, the fact that two of the depicted cases showed this overlap syndrome merits attention, arguing for the need to screen individuals with ATM or GBS in the context of COVID‐19 for the other disease as well. This could have important treatment implications as first‐line treatments for GBS (IVIG or plasma exchange) differ from those usually used in ATM (intravenous MP) and IVIG may be more effective in individuals with combined demyelinating disorders of the central and peripheral nervous systems [46].
Transverse myelitis has also occurred in at least one participant in a trial for a SARS‐CoV‐2 vaccine developed by AstraZeneca and the University of Oxford [47]. Although causality cannot be readily inferred, TM is known as a potential complication of several different vaccines and causality has been shown for the oral polio vaccine and ATM [47, 48]. Accordingly, with a significant portion of the world’s population going to receive a SARS‐CoV‐2 vaccine, post‐vaccination ATM will be something to watch as vaccine trials are intensifying and vaccination efforts are starting to get under way [47].
Current estimates of neurological manifestations in COVID‐19 of 7.8%–13.5% seem high and are most likely subject to significant reporting and selection bias [4, 21]. True numbers for neurological sequelae may be closer to the 0.09% and 0.36% estimated for SARS‐CoV‐1 and MERS‐CoV [3]. Nonetheless, even if the true rate of neurological manifestations in COVID‐19 remains to be established and reports of myelitis are anecdotal, at the current scale of this global pandemic they merit further scrutiny in a timely fashion.
CONFLICT OF INTERESTS
The authors have no conflicts to declare.
Supporting information
Schulte EC, Hauer L, Kunz AB, Sellner J. Systematic review of cases of acute myelitis in individuals with COVID‐19. Eur J Neurol. 2021;28:3230–3244. 10.1111/ene.14952
Kunz and Sellner shared senior authorship.
DATA AVAILABILITY STATEMENT
The entire dataset is included in the paper/supplemental material.
REFERENCES
- 1. Moro E, Priori A, Beghi E, et al. The international European Academy of Neurology survey on neurological symptoms in patients with COVID‐19 infection. Eur J Neurol. 2020;27(9):1727‐1737. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Romoli M, Jelcic I, Bernard‐Valnet R, et al. A systematic review of neurological manifestations of SARS‐CoV‐2 infection: the devil is hidden in the details. Eur J Neurol. 2020;27(9):1712‐1726. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Ellul MA, Benjamin L, Singh B, et al. Neurological associations of COVID‐19. Lancet Neurol. 2020;19(9):767‐783. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Frontera JA, Sabadia S, Lalchan R, et al. A prospective study of neurologic disorders in hospitalized COVID‐19 patients in New York City. Neurology. 2021;96(4):e575–e586. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Mao L, Jin H, Wang M, et al. Neurologic manifestations of hospitalized patients with coronavirus disease 2019 in Wuhan, China. JAMA Neurol. 2020;77(6):683‐690. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Arabi YM, Harthi A, Hussein J, et al. Severe neurologic syndrome associated with Middle East respiratory syndrome corona virus (MERS‐CoV). Infection. 2015;43(4):495‐501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Lau KK, Yu WC, Chu CM, Lau ST, Sheng B, Yuen KY. Possible central nervous system infection by SARS coronavirus. Emerg Infect Dis. 2004;10(2):342‐344. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Tsai LK, Hsieh ST, Chao CC, et al. Neuromuscular disorders in severe acute respiratory syndrome. Arch Neurol. 2004;61(11):1669‐1673. [DOI] [PubMed] [Google Scholar]
- 9. Akhvlediani T, Jelcic I, Taba P, Pfausler B, Steiner I, Sellner J. What did we learn from the previous coronavirus epidemics and what can we do better: a neuroinfectiological point of view. Eur J Neurol. 2020;27(11):e69‐e72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Morfopoulou S, Brown JR, Davies EG, et al. Human coronavirus OC43 associated with fatal encephalitis. N Engl J Med. 2016;375(5):497‐498. [DOI] [PubMed] [Google Scholar]
- 11. Yeh EA, Collins A, Cohen ME, Duffner PK, Faden H. Detection of coronavirus in the central nervous system of a child with acute disseminated encephalomyelitis. Pediatrics. 2004;113(1):e73‐e76. [DOI] [PubMed] [Google Scholar]
- 12. Turgay C, Emine T, Ozlem K, Muhammet SP, Haydar AT. A rare cause of acute flaccid paralysis: human coronaviruses. J Pediatr Neurosci. 2015;10(3):280‐281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Liberati A, Altman DG, Tetzlaff J, et al. The PRISMA statement for reporting systematic reviews and meta‐analyses of studies that evaluate healthcare interventions: explanation and elaboration. BMJ. 2009;339: b2700. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Wells G, Shea BJ, O'Connell D, et al. The Newcastle–Ottawa Scale (NOS) for assessing the quality of nonrandomised studies in meta‐analyses; 2013. Available from: http://www.ohri.ca/progams/clinical_epidemiology/oxford.asp
- 15. Characterisation WHOWGotC, Management of C‐i . A minimal common outcome measure set for COVID‐19 clinical research. Lancet Infect Dis. 2020;20(8):e192‐e197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Maideniuc C, Memon AB. Acute necrotizing myelitis and acute motor axonal neuropathy in a COVID‐19 patient. J Neurol. 2021;268(2):739. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Valiuddin H, Skwirsk B, Paz‐Arabo P. Acute transverse myelitis associated with SARS‐CoV‐2: a case‐report. Brain Behav Immun Health. 2020;5:100091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Zhao K, Jucun H, Dai D, Feng Y, Liu L, Nie S. Acute meylitis after SARS‐CoV‐2 infection: a case report. medRxiv. 2020. [Google Scholar]
- 19. Kaur H, Mason JA, Bajracharya M, et al. Transverse myelitis in a child with COVID‐19. Pediatr Neurol. 2020;112:5‐6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Masuccio FG, Barra M, Claudio G, Claudio S. A rare case of acute motor axonal neuropathy and myelitis related to SARS‐CoV‐2 infection. J Neurol. 2021;268(7):2327‐2330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Rifino N, Censori B, Agazzi E, et al. Neurologic manifestations in 1760 COVID‐19 patients admitted to Papa Giovanni XXIII Hospital, Bergamo, Italy. J Neurol. 2020;1‐8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Munz M, Wessendorf S, Koretsis G, et al. Acute transverse myelitis after COVID‐19 pneumonia. J Neurol. 2020;267(8):2196‐2197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Sotoca J, Rodriguez‐Alvarez Y. COVID‐19‐associated acute necrotizing myelitis. Neurol Neuroimmunol Neuroinflamm. 2020;7(5):e803. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Wong PF, Craik S, Newman P, et al. Lessons of the month 1: a case of rhombencephalitis as a rare complication of acute COVID‐19 infection. Clin Med (Lond). 2020;20(3):293‐294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Sellner J, Hemmer B, Muhlau M. The clinical spectrum and immunobiology of parainfectious neuromyelitis optica (Devic) syndromes. J Autoimmun. 2010;34(4):371‐379. [DOI] [PubMed] [Google Scholar]
- 26. Sellner J, Boggild M, Clanet M, et al. EFNS guidelines on diagnosis and management of neuromyelitis optica. Eur J Neurol. 2010;17(8):1019‐1032. [DOI] [PubMed] [Google Scholar]
- 27. Beh SC, Greenberg BM, Frohman T, Frohman EM. Transverse myelitis. Neurol Clin. 2013;31(1):79‐138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Berman M, Feldman S, Alter M, Zilber N, Kahana E. Acute transverse myelitis: incidence and etiologic considerations. Neurology. 1981;31(8):966‐971. [DOI] [PubMed] [Google Scholar]
- 29. Borchers AT, Gershwin ME. Transverse myelitis. Autoimmun Rev. 2012;11(3):231‐248. [DOI] [PubMed] [Google Scholar]
- 30. Beghi E, Helbok R, Crean M, et al. The EAN COVID‐19 registry (ENERGY): an international instrument for surveillance of neurological complications in patients with COVID‐19. Eur J Neurol. 2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Natoli S, Oliveira V, Calabresi P, Maia LF, Pisani A. Does SARS‐Cov‐2 invade the brain? Translational lessons from animal models. Eur J Neurol. 2020;27(9):1764‐1773. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Chu H, Chan JF, Yuen TT, et al. Comparative tropism, replication kinetics, and cell damage profiling of SARS‐CoV‐2 and SARS‐CoV with implications for clinical manifestations, transmissibility, and laboratory studies of COVID‐19: an observational study. Lancet Microbe. 2020;1(1):e14‐e23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Nemoto W, Yamagata R, Nakagawasai O, et al. Effect of spinal angiotensin‐converting enzyme 2 activation on the formalin‐induced nociceptive response in mice. Eur J Pharmacol. 2020;872:172950. [DOI] [PubMed] [Google Scholar]
- 34. Kremer S, Lersy F, Anheim M, et al. Neurologic and neuroimaging findings in patients with COVID‐19: a retrospective multicenter study. Neurology. 2020;95(13):e1868‐e1882. [DOI] [PubMed] [Google Scholar]
- 35. Moriguchi T, Harii N, Goto J, et al. A first case of meningitis/encephalitis associated with SARS‐Coronavirus‐2. Int J Infect Dis. 2020;94:55‐58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Virhammar J, Kumlien E, Fallmar D, et al. Acute necrotizing encephalopathy with SARS‐CoV‐2 RNA confirmed in cerebrospinal fluid. Neurology. 2020;95(10):445‐449. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Paniz‐Mondolfi A, Bryce C, Grimes Z, et al. Central nervous system involvement by severe acute respiratory syndrome coronavirus‐2 (SARS‐CoV‐2). J Med Virol. 2020;92(7):699‐702. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Kansagra SM, Gallentine WB. Cytokine storm of acute necrotizing encephalopathy. Pediatr Neurol. 2011;45(6):400‐402. [DOI] [PubMed] [Google Scholar]
- 39. Filosto M, Cotti Piccinelli S, Gazzina S, et al. Guillain–Barre syndrome and COVID‐19: an observational multicentre study from two Italian hotspot regions. J Neurol Neurosurg Psychiatry. 2021;92(7):751–756. [DOI] [PubMed] [Google Scholar]
- 40. Abdelhady M, Elsotouhy A, Vattoth S. Acute flaccid myelitis in COVID‐19. BJR Case Rep. 2020;6(3):20200098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Kincaid O, Lipton HL. Viral myelitis: an update. Curr Neurol Neurosci Rep. 2006;6(6):469‐474. [DOI] [PubMed] [Google Scholar]
- 42. Paterson RW, Brown RL, Benjamin L, et al. The emerging spectrum of COVID‐19 neurology: clinical, radiological and laboratory findings. Brain. 2020;143(10):3104‐3120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Toscano G, Palmerini F, Ravaglia S, et al. Guillain–Barre syndrome associated with SARS‐CoV‐2. N Engl J Med. 2020;382(26):2574‐2576. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Uncini A, Vallat JM, Jacobs BC. Guillain–Barre syndrome in SARS‐CoV‐2 infection: an instant systematic review of the first six months of pandemic. J Neurol Neurosurg Psychiatry. 2020;91(10):1105‐1110. [DOI] [PubMed] [Google Scholar]
- 45. Guo F, Zhang YB. Clinical features and prognosis of patients with Guillain–Barre and acute transverse myelitis overlap syndrome. Clin Neurol Neurosurg. 2019;181:127‐132. [DOI] [PubMed] [Google Scholar]
- 46. Marchioni E, Ravaglia S, Piccolo G, et al. Postinfectious inflammatory disorders: subgroups based on prospective follow‐up. Neurology. 2005;65(7):1057‐1065. [DOI] [PubMed] [Google Scholar]
- 47. Mallapaty S, Ledford H. COVID‐vaccine results are on the way—and scientists’ concerns are growing. Nature. 2020;586(7827):16‐17. [DOI] [PubMed] [Google Scholar]
- 48. Agmon‐Levin N, Kivity S, Szyper‐Kravitz M, Shoenfeld Y. Transverse myelitis and vaccines: a multi‐analysis. Lupus. 2009;18(13):1198‐1204. [DOI] [PubMed] [Google Scholar]
- 49. AlKetbi R, AlNuaimi D, AlMulla M, et al. Acute myelitis as a neurological complication of Covid‐19: a case report and MRI findings. Radiol Case Rep. 2020;15(9):1591‐1595. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Baghbanian SM, Namazi F. Post COVID‐19 longitudinally extensive transverse myelitis (LETM)—a case report. Acta Neurol Belg. 2020;1‐2. Online ahead of print. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Chakraborty U, Chandra A, Ray AK, Biswas P. COVID‐19‐associated acute transverse myelitis: a rare entity. BMJ Case Rep. 2020;13(8):e238668. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Chow CCN, Magnussen J, Ip J, Su Y. Acute transverse myelitis in COVID‐19 infection. BMJ Case Rep. 2020;13(8):e236720. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Durrani M, Kucharski K, Smith Z, Fien S. Acute transverse myelitis secondary to severe acute respiratory syndrome coronavirus 2 (SARS‐CoV‐2): a case report. Clin Pract Cases Emerg Med. 2020;4(3):344‐348. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Giorgianni A, Vinacci G, Agosti E, et al. Transient acute‐onset tetraparesis in a COVID‐19 patient. Spinal Cord. 2020;58(9):1042‐1044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. Lisnic CN, Nemtan V, Hacina E, et al. Acute transverse myelitis in a HIV‐positive patient with COVID‐19. Research Square. 2020. https://www.researchsquare.com/article/rs‐50901/v1. [Google Scholar]
- 56. Sarma DB, Bilello L. A case report of acute transverse myelitis following novel coronavirus infection. Clin Pract Cases Emerg Med. 2020;4(3):321‐323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57. Zachariadis A, Tulbu A, Strambo D, Dumoulin A, Di Virgilio G. Transverse myelitis related to COVID‐19 infection. J Neurol. 2020;267(12):3459‐3461. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The entire dataset is included in the paper/supplemental material.


