The novel coronavirus (SARS‐CoV‐2) was first identified in humans in late 2019. As of January 2021, over 100 million cases of coronavirus disease 2019 (COVID‐19) were confirmed worldwide, with over 2% of death. It is estimated 30% of the population were to get infected. There are several vaccines with emergency use authorization worldwide. Currently, vaccination started globally, but its long‐term effectiveness is still under clinical trial. 1
The critical question is how to treat patients infected with SARS‐CoV‐2. Initially, everyone thought COVID‐19 was a flu‐like symptom and after a period of recovery back to normal health, but emerging data showed that SARS‐CoV‐2 affects the red blood cells (RBC) more than we ever thought possible. It has been reported that hospitalized patients have elevated red blood cell distribution width (RDW) coefficient variation (CV) greater than 15%. 2 However, the elevated and increasing RDW‐CV% is associated with an enhanced mortality rate in COVID‐19 patients. 2 The high RDW‐CV% means that the patients may have small and large or normal and large or normal and small RBC at the same time. In other words, patients have varied RBC volume at the same time. Some studies indicated in the delayed clearance of RBC from the bloodstream makes RBC volume smaller than normal, leading to elevated RDW‐CV%. Another possible mechanism behind elevated RDW‐CV% is the slowing of RBC turnover in the setting of elevated turnover or production of lymphocytes and platelets in inflammation. There are several controversial studies related to the role of lymphopenia and lymphocytosis as the factors of mortality rate in COVID‐19 patients. It has been reported that there is an association of lymphopenia to the mortality rate in COVID‐19 patients and also there is an association of severe COVID‐19 symptoms with lymphocytosis. Therefore, the hypothesis related to the association of elevated RDW‐CV% in the setting of lymphocytosis is controversial in COVID‐19 patients. While the increase of platelets is in accordance with the mortality rate in COVID‐19 patients and it would be one possibility of elevated RDW‐CV%. 2 There are also controversial reports related to the association of mean corpuscular volume (MCV) with COVID‐19. Some clinical studies reject this association between the MCV and COVID‐19 symptom, while others indicated in the infection is in line with the elevated and declined MCV and hamoglobin. 3 The RDW‐CV% changes are associated with RBC volume changes, in which iron serum level is playing an essential role in microcytosis, macrocytosis, and normal RBC volume.
Ferroptosis is accounted one of the central cell death mechanisms 4 in COVID‐19 patients in which iron plays a vital role in its occurrence. The iron serum level and transferrin are low, and ferritin is high in COVID‐19 patients. 5 However, the level of iron serum changes based on the disease stage and is negatively related to the severity of the infection. 6 The transferrin overexpression is higher in males and increases with age, and this is in correlation with the severity and mortality of male and elderly COVID‐19 patients. 7 Erroptosis, a type of iron dependentprogrammed cell death, plays a vital role in multiple system diseases and damages to the lung, kidney, heart, liver, gut, and nerve system 8 (Figure 1c). There is a direct association between the intracellular iron level, glutathione peroxidase 4 (GPX4), and ferroptosis. COVID‐19 patients suffer from GPX4 depletion, confirming ferroptosis. Further confirmation is related to the latency of kidney and other tissue damages upon SARS‐CoV‐2 infection. The outcome is that iron accumulation takes time to be overload in cells and triggers ferroptosis; therefore, we observe tissue damages with latency.
FIG 1.
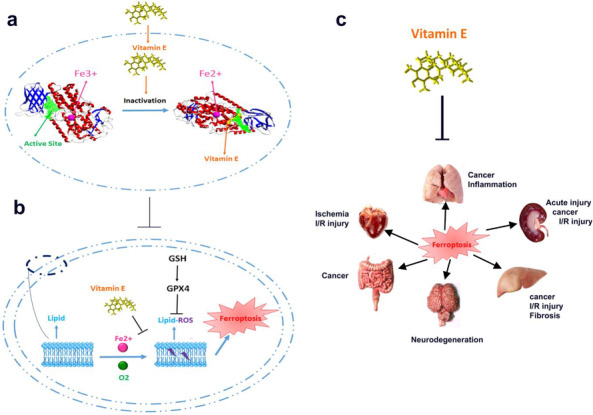
(A) The involvement of vitamin E in the inactivation of 15‐lipoxyganse through the reduction of Fe3+ to Fe2+ leading to ferroptosis prevention. (B) Involvement of oxygen and ferric iron in induction of lipid hydroperoxides leading to ferroptosis. (C) inhibition of ferroptosis using vitamin E leading to elimination of injuries to the heart, kidney, nervous system, liver, gastrointestinal, and lung
Based on the recent clinical results, there is a correlation between ferroptosis and COVID‐19 and its destructive effects on organs and its irreversible damage. Therefore, the treatment should be based on anti‐ferroptosis drugs that will reduce mortality and tissue damage. The glutathione, through the elimination of electrophiles derived from lipid peroxidation and inhibiting protein cysteine oxidation, preserves membrane integrity, and just a lipophilic antioxidant such as vitamin E can fill the void left by glutathione. The principal mechanism behind the anti‐ferroptosis of vitamin E is through the reduction of ferric iron center in 15‐lipoxygenase to inactive ferrous (Fe2+), resulting in 15‐lipoxygenase inhibition (Figure 1A). In brief, when oxygen reacts with cell membrane lipids using lipoxygenase leading to the formation of lipid hydroperoxides with the help of peroxyl radicals. While vitamin E reacts with peroxyl radicals to prevent lipid hydroperoxides formation. Afterward, glutathione, GPX4, and other antioxidant agents detoxify oxidized lipids to inhibit the triggering of ferroptosis (Figure 1B). Moreover, vitamin E can compensate for detoxification derived from GPX4 deficiency. It is worth mentioning that alpha‐tocopherol hydroquinone is a more potent antioxidant than alpha‐tocopherol. 9
Even though the neurodegeneration upon vitamin E deficiency is related to the ferroptosis. Maybe the neural damages in COVID‐19 patients are inhibited in part using vitamin E consumption through an anti‐ferroptosis mechanism. In conclusion, it might be said that RBC characteristics such as RDW‐CV% have vital importance in the prognosis of COVID‐19 patients, while the iron serum level affects RDW‐CV% and RBC volume and needs more attention to be monitored in patients. Therefore, the toxic mechanisms behind iron serum levels such as ferroptosis should be prevented by vitamin E consumption through inhibiting lipoxygenase and peroxyl radicals. Vitamin E supplement at a high dose of 500 mg/kg may also act as a treatment drug to inhibit ferroptosis in COVID‐19 patients and decline ferroptosis damages to multiple organs, including lung, kidney, liver, gut, heart, and nervous system. Based on some reports, it is leading to viral clearance and inflammation ablation through the T cells modulation.
Tavakol S, Seifalian, AM. Vitamin E at a high dose as an anti‐ferroptosis drug and not just a supplement for COVID‐19 treatment. Biotechnol Appl Biochem. 2022;69:1058–1060. 10.1002/bab.2176
REFERENCES
- 1. Tavakol S, Alavijeh MS, Seifalian A. COVID‐19 vaccines in clinical trials and their mode of action for immunity against the virus. Curr Pharm Des. 2020. doi: 10.2174/1381612826666201023143956. [DOI] [PubMed] [Google Scholar]
- 2. Foy BH, Carlson JC, Reinertsen E, Valls RPI, Lopez RP, Palanques‐Tost E, et al. Association of red blood cell distribution width with mortality risk in hospitalized adults with SARS‐CoV‐2 infection. JAMA 2020;3(9):e2022058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Khartabil T, Russcher H, van der Ven A, De Rijke Y. A summary of the diagnostic and prognostic value of hemocytometry markers in COVID‐19 patients. Crit Rev Clin Lab Sci. 2020;57(6):415–31. [DOI] [PubMed] [Google Scholar]
- 4. Ashrafizadeh M, Mohammadinejad M, Tavakol S, Ahmadi Z, Roomiani S, Katebi M. Autophagy, anoikis, ferroptosis, necroptosis, and endoplasmic reticulum stress: Potential applications in melanoma therapy. Cell Physiol. 2019;234(11):19471–79. [DOI] [PubMed] [Google Scholar]
- 5. Bellmann‐Weiler R, Lanser L, Barket R, Rangger L, Schapfl A, Schaber M, et al. Prevalence and predictive value of anemia and dysregulated iron homeostasis in patients with COVID‐19 infection. J Clin Med. 2020;9(8):2429. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Zhao K, Huang J, Dai D, Feng Y, Liu L, Nie S, et al. In Serum iron level as a potential predictor of coronavirus disease 2019 severity and mortality: a retrospective study. Open Forum Infect Dis. 2020;7(7):1–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. McLaughlin K‐M, Bechtel M, Bojkova D, Münch C, Ciesek S, Wass MN, et al. COVID‐19‐related coagulopathy—is transferrin a missing link? Diagnostics 2020;10(8):539. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Li J, Cao F, Yin H‐l, Huang Z‐j, Lin, Z‐t , Mao N, et al. Ferroptosis: past, present and future. Cell Death Dis. 2020;11(2):1–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Hinman A, Holst CR, Latham JC, Bruegger JJ, Ulas G, McCusker KP, et al. Vitamin E hydroquinone is an endogenous regulator of ferroptosis via redox control of 15‐lipoxygenase. PLoS One 2018;13(8):e0201369. [DOI] [PMC free article] [PubMed] [Google Scholar]


