COVID‐19 is associated with haemostatic dysregulation, 1 with thromboembolism occurring in 25% of hospitalised COVID‐19 patients and microvascular thrombi reported at autopsy. 2 Platelets are activated in COVID‐19 patients requiring intensive care, 3 while limited data in mild COVID‐19 shows no changes in platelet phenotype. 4
Children have low‐risk of severe COVID‐19 and thrombosis. 5 If haemostasis is fundamental to COVID‐19 pathogenesis, then age‐related haemostatic differences may protect children from COVID‐19. While there is evidence of changes in leucocyte populations of non‐hospitalised children and adults infected and exposed to SARS‐CoV‐2, 6 the effect on paediatric platelets is unknown.
We investigated platelet surface‐markers in adults and children who were SARS‐CoV‐2‐positive and their household contacts. We aimed to establish whether SARS‐CoV‐2 induced changes in platelet phenotype in individuals with mild COVID‐19.
Participants were recruited from the Respiratory Infection Clinic at The Royal Children’s Hospital (RCH), Melbourne, using the enrollment protocol and participant pool previously described. 6 Upon a positive SARS‐CoV‐2 polymerase chain reaction (PCR) test, blood collection was arranged for family members at two time points: ‘acute’, within two weeks of post‐test and ‘convalescent’, 4–7 weeks post‐test. Individuals were classified ‘SARS‐CoV‐2‐positive’ if they tested positive, and ‘SARS‐CoV‐2‐exposed’ if they tested negative on repeated tests but remained in close household contact with individuals who tested positive. All participants recovered at home. This study was approved by the RCH Human Research Ethics Committee (QA/63666/RCHM‐2020).
Healthy samples were collected prior to the COVID‐19 pandemic, under the Royal Children’s Hospital Human Research and Ethics Committee 34183/32287. Controls were treated identically to COVID‐19‐related samples.
Blood was collected into tubes containing one part sodium citrate to nine parts blood (Sarstedt, US). Samples from COVID‐19 families and healthy adults were collected by venepuncture, while samples from healthy children were collected by cannula during elective day surgery. The platelet count was determined using ADVIA 2120i (Siemens, Germany).
Platelet surface‐markers were determined by the International Society of Thrombosis and Haemostasis guidelines for platelet function disorders 7 (Table SI).
Platelet count was standardised to 4 × 105 platelets/µl in 1% BSA‐HEPES. Samples were stained and fixed with 1% formaldehyde. 8 An isotype control included 20 μM eptifibatide‐acetate (Sigma‐Aldrich, USA) to inhibit platelet activation.
Samples were analysed using BD‐LSR Fortessa X‐20 (BD Biosciences, United States), calibrated using Cytometer Set‐Up and Tracking beads (BD Biosciences, United States) and compared to a baseline. Platelets were identified by side scatter (SSC) and platelet identifier (CD42b‐FITC in Panel 1/3, CD42a‐BV421 in Panel 2), with 10,000 events collected. Single platelets were identified by gating SSC and forward scatter (FSC) (Figure S1A–D).
Analysis was performed using FlowJo (United States). Baseline activation was determined by the percentage of platelets expressing activation markers CD62P and PAC1. The positive percentage was determined by setting a gate to the top 1% of events in the isotype control (Figure S1E–H). Expression of other markers are presented as median fluorescence intensity (MFI).
Data was managed using REDCap data‐capture tools. 9 , 10 Normality was established by plotting histograms for each parameter. Inter‐group differences were tested using ANOVA with Tukey’s multiple‐comparison test. Differences between adults and children within each group were compared using an unpaired t‐test (Bonferroni‐adjusted P = 0·01). Analysis was performed using GraphPad‐Prism 8 (GraphPad, United States).
Sixty‐seven individuals were enrolled (Table I) and all had only mild symptoms. Twenty‐five controls were included in the study. Platelet counts are presented in Figure S2. In acute SARS‐CoV‐2‐exposed individuals, children had higher platelet counts compared to adults (P = 0·0026), but all were normal.
Table I.
Participant demographic information, including age and sex distribution between groups. Platelet counts were collected on prospectively enrolled patients. Healthy individuals were age‐ and gender‐matched based on the entire prospectively enrolled cohort. Participant ages presented are mean and standard deviation.
| Enrollment | ||||
|---|---|---|---|---|
| Category | Prospectively enrolled | Healthy controls | ||
| 67 | 25 | |||
| Participants | ||||
| Group | Children | Mean age ± SD (years) | Adults | Mean age ± SD (years) |
| Healthy | 16 (9 M/7F) | 9·2 ± 7·4 | 9 (4 M/5F) | 28·1 ± 9 |
| Acute SARS‐CoV‐2‐exposed | 6 (3 M/3F) | 8·3 ± 2·9 | 15 (8 M/7F) | 39·7 ± 4·1 |
| Acute SARS‐CoV‐2‐infected | 7 (5 M/2F) | 2·4 ± 1·3 | 6 (2 M/4F) | 44·5 ± 14·6 |
| Convalescent SARS‐CoV‐2‐exposed | 10 (5 M/5F) | 7·3 ± 3·2 | 6 (3 M/3F) | 42 ± 4·7 |
| Convalescent SARS‐CoV‐2‐infected | 8 (4 M/4F) | 9·4 ± 6·7 | 9 (3 M/6F) | 37·8 ± 7·6 |
| Total | 47 (26 M/21F) | 7·7 ± 5·6 | 45 (20 M/25F) | 37·9 ± 9·5 |
Platelet Flow Cytometry results are summarised in Fig 1. PAC1‐positive platelets increased in acute SARS‐CoV‐2‐exposed (P = 0·0006) and ‐positive (P = 0·0035) children. CD62P‐positive platelets increased in acute SARS‐CoV‐2‐exposed (P = 0·0094) and ‐positive (P < 0·0001) children.
Fig 1.
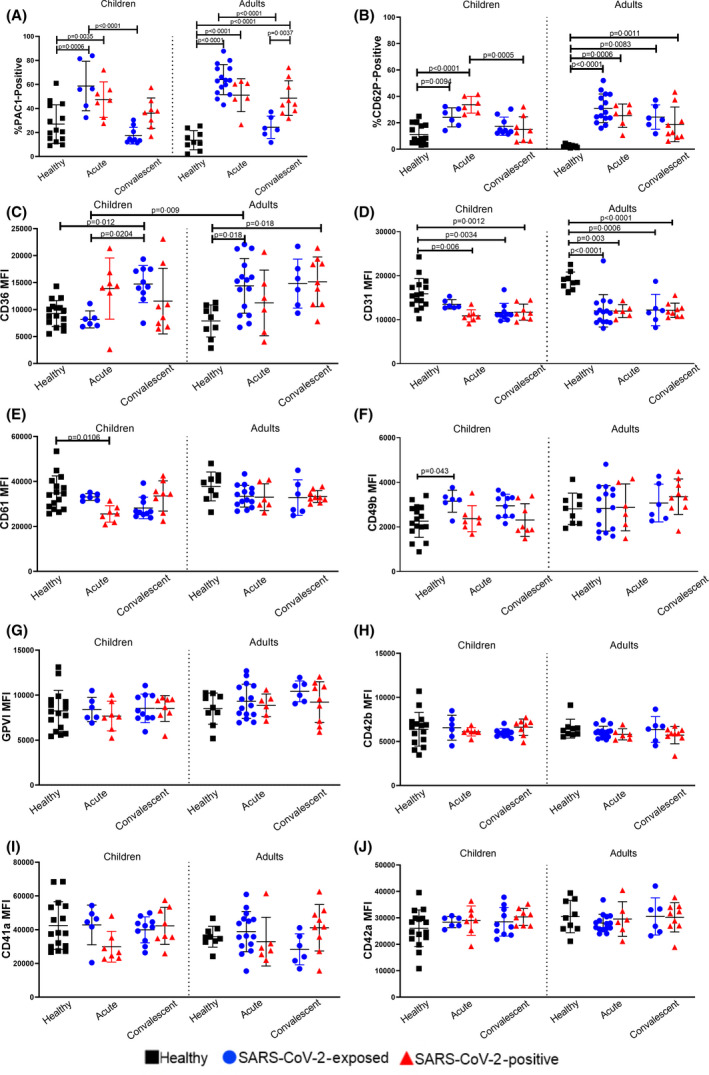
Flow Cytometry Results summary of platelet markers. Values are expressed as mean ± standard deviation. A) Percentage of platelets positive for PAC1. B) Percentage of platelets positive for CD62P. C) CD36 MFI. D) CD31 MFI. E) CD61 MFI. F) CD49b MFI. G) GPVI MFI. H) CD42b MFI. I) CD41a MFI. J) CD42a MFI. Statistically significant results are indicated by a connecting line and P‐value. Within each graph, results from child platelets are reported on the left and results from adults are reported on the right. Black squares denote healthy controls, blue circles denote SARS‐CoV‐2‐exposed individuals and red triangles denote SARSCoV‐2‐positive individuals. Activation markers (A–B) are presented as percentage positive, all other platelet surface markers (C–J) are presented as Median Fluorescence Intensity (MFI). Differences within age groups were determined using ANOVA with Tukey’s multiple comparison test, while differences between age groups were determined using a t‐test with Bonferroni correction. [Colour figure can be viewed at wileyonlinelibrary.com]
The PAC1‐positive platelets increased in acute SARS‐CoV‐2‐exposed adults (P < 0·0001) and SARS‐CoV‐2‐positive adults at (P < 0·0001) and convalescent (P < 0·0001) stages. CD62P‐positive platelets increased in acute SARS‐CoV‐2‐exposed (P < 0·0001) and ‐positive (P = 0·0006) adults, and in convalescent SARS‐CoV‐2‐exposed (P = 0·0083) and ‐positive adults (P = 0·011).
CD31‐expression decreased in acute SARS‐CoV‐2‐positive (P = 0·006), and in convalescent SARS‐CoV‐2‐exposed (P = 0·0034) and ‐positive (P = 0·0012) children. CD31‐expression decreased in acute SARS‐CoV‐2‐exposed (P < 0·0001) and ‐positive (P = 0·003) adults, as well as convalescent SARS‐CoV‐2‐exposed (P = 0006) and ‐positive (P < 0·0001) adults.
CD36‐expression increased in acute SARS‐CoV‐2‐exposed adults compared to children (P = 0·009). Convalescent SARS‐CoV‐2‐exposed children had decreased CD36‐expression (P = 0·012). CD36‐expression increased in acute SARS‐CoV‐2‐exposed adults (P = 0·018) and convalescent SARS‐CoV‐2‐positive adults (P = 0·018).
CD49b‐expression increased in acute SARS‐CoV‐2‐exposed children (P = 0·0042), while‐expression increased in acute SARS‐CoV‐2‐positive children (P = 0·0106).
Thrombosis is considered a late‐stage comorbidity of severe COVID‐19. 11 We demonstrate platelet activation in mild COVID‐19 and SARS‐CoV‐2‐negative household contacts. Platelet activation returned to normal at convalescence in SARS‐CoV‐2‐exposed individuals, suggesting that the effects of SARS‐CoV‐2‐exposure alone on platelet activation are short‐lived. Our results suggest that SARS‐CoV‐2‐exposed individuals may have a SARS‐CoV‐2 infection below the detection threshold, sufficient however for platelet response, and show that platelets are sensitive to SARS‐CoV‐2.
Children experience less severe COVID‐19 symptoms compared to adults. 12 We show few age‐specific differences in platelet response to SARS‐CoV‐2, suggesting that age‐specific differences in platelet phenotype do not play a role in mild COVID‐19. The fact that platelet counts remained normal suggests that thrombocytopenia is a unique symptom of severe COVID‐19. 13
Our finding of increased CD36 (a marker involved in immunity and neutrophil‐signalling 14 ) in SARS‐CoV‐2‐exposed and ‐positive children, is consistent with an immunological study performed on the same population which showed increased low‐density neutrophils in SARS‐CoV‐2‐exposed individuals at convalescence. 6
Changes in platelet phenotype that persist into convalescence is another novel finding. As platelets circulate for approximately 10 days, 15 it is an unexpected finding that SARS‐CoV‐2 is directly interacting with platelets, and we hypothesise that the SARS‐CoV‐2 effect on neutrophil and monocyte populations may be the source of these prolonged platelet changes.
Three limitations are low participant numbers, which reflect the low COVID‐19 rates in Australia, the fact that participants may have been at variable time points in their illness and that the ‘dose’ of virus‐exposure was not comparable.
In conclusion, platelet activation occurs in mild COVID‐19 and in SARS‐CoV‐2 negative close contacts, and that it persists into convalescence for SARS‐CoV‐2‐positive individuals, suggesting that mild COVID‐19 may have a long‐lasting thrombotic risk.
Acknowledgements
We thank the Royal Children’s Hospital Foundation for funding this study, as well as our previous study (the HAPPI Kids study) enabling the collection of samples from healthy controls. The FFX study has Australian Commonwealth government support for identification of positive samples and database management.
We thank study participants and their families for their time in participating in this study.
Conflict of interest
There are no conflicts of interest to declare.
Author contributions
CM wrote the manuscript. CM, SV, NL performed experiments and analysed data. KD coordinated sample collection. ST, NC, VI and PM coordinated the study. VK, LC, CA, SP, TC, ES, MN, DB, VI and PM reviewed the manuscript.
Supporting information
Fig S1. Gating strategy for flow‐cytometry acquisition. Platelets were identified by side scatter (SSC) and a platelet‐specific marker, and single non‐aggregated platelets were then identified using SSC and forward scatter (FSC). The percentage of platelets positive for PAC1 and CD62P was determined by gating 1% of events of a negative isotype control. A) Platelet gating for Panel 1 and 3, using SSC and CD42b‐FITC fluorescence. B) Single platelet gating for panel 1 and 3, using SSC and FSC. C) Platelet gating for Panel 2 using SSC and CD42a‐BV421. D) Single platelet gating for Panel 2 using SSC and FSC. E) PAC1‐FITC+ gate based on 1% of events in negative isotype. F) PAC1‐FITC+ G gate on a sample tube. G) CD62P‐PE+ gate based on 1% of events in negative isotype. H) CD62P‐PE+ gate on a sample tube.
Fig S2. Platelet counts for prospectively collected participants, analysed using Advia 2120i Cell Counter. Values are presented as mean ± standard deviation, and units are x109 Platelets/L. Differences within age groups were determined using ANOVA with Tukey’s multiple comparison test, and differences between age groups were determined using a t‐test with Bonferroni adjustment. Significant differences between groups are denoted with a black line and P‐value. Blue circles represent SARS‐CoV‐2‐exposed individuals and black triangles represent SARS‐CoV‐2‐positive individuals.
Table S1. Summary of targets for flow cytometry.
References
- 1. Becker RC. COVID‐19 update: Covid‐19‐associated coagulopathy. J Thromb Thrombolysis. 2020:1–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Rapkiewicz AV, Mai X, Carsons SE, Pittaluga S, Kleiner DE, Berger JS, et al. Megakaryocytes and platelet‐fibrin thrombi characterize multi‐organ thrombosis at autopsy in COVID‐19: A case series. EClinicalMedicine [Internet]. 2020. Jun 25 [cited 2020 Jun 28];0(0). Available from: https://www.thelancet.com/journals/eclinm/article/PIIS2589‐5370(20)30178‐4/abstract [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Manne BK, Denorme F, Middleton EA, Portier I, Rowley JW, Stubben CJ, et al. Platelet gene expression and function in COVID‐19 patients. Blood. 2020;136(11):1317–1329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Hottz ED, Azevedo‐Quintanilha IG, Palhinha L, Teixeira L, Barreto EA, Pão CRR, et al. Platelet activation and platelet‐monocyte aggregates formation trigger tissue factor expression in severe COVID‐19 patients. Blood. 2020;136(11):1330–1341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Attard C, van der Straaten T, Karlaftis V, Monagle P, Ignjatovic V. Developmental hemostasis: age‐specific differences in the levels of hemostatic proteins. J Thromb Haemost JTH. 2013;11(10):1850–4. [DOI] [PubMed] [Google Scholar]
- 6. Neeland MR, Bannister S, Clifford V, Dohle K, Mulholland K, Sutton P, et al. Innate cell profiles during the acute and convalescent phase of SARS‐CoV‐2 infection in children. Nat. Commun. 2021;12(1):1084. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Gresele P. Diagnosis of inherited platelet function disorders: guidance from the SSC of the ISTH. J Thromb Haemost. 2015;13(2):314–22. [DOI] [PubMed] [Google Scholar]
- 8. Busuttil‐Crellin X, McCafferty C, Van Den Helm S, Yaw HP, Monagle P, Linden M, et al. Guidelines for panel design, optimization, and performance of whole blood multi‐color flow cytometry of platelet surface markers. Platelets. 2020;31(7):845–52. [DOI] [PubMed] [Google Scholar]
- 9. Harris PA, Taylor R, Thielke R, Payne J, Gonzalez N, Conde JG. Research electronic data capture (REDCap)—a metadata‐driven methodology and workflow process for providing translational research informatics support. J Biomed Inform. 2009;42(2):377–81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Harris PA, Taylor R, Minor BL, Elliott V, Fernandez M, O'Neal L, et al. The REDCap consortium: building an international community of software platform partners. J Biomed Inform. 2019;95:103208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Thachil J, Tang N, Gando S, Falanga A, Cattaneo M, Levi M, et al. ISTH interim guidance on recognition and management of coagulopathy in COVID‐19. J Thromb Haemost. 2020;18(5):1023–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Mehta N, Mytton O, Mullins E, Fowler T, Falconer C, Murphy O, et al. SARS‐CoV‐2 (COVID‐19): what do we know about children? a systematic review. Clin Infect Dis. 2020;71(9):2469–2479. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Xu P, Zhou Q, Xu J. Mechanism of thrombocytopenia in COVID‐19 patients. Ann Hematol. 2020:1–4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Castleman MJ, Febbraio M, Hall PR. CD36 is essential for regulation of the host innate response to Staphylococcus aureus alpha‐toxin‐mediated dermonecrosis. J Immunol. 2015;195(5):2294–302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. LeBrasseur N. Platelets’ preset lifespan. J Cell Biol. 2007;177(2):186a. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Fig S1. Gating strategy for flow‐cytometry acquisition. Platelets were identified by side scatter (SSC) and a platelet‐specific marker, and single non‐aggregated platelets were then identified using SSC and forward scatter (FSC). The percentage of platelets positive for PAC1 and CD62P was determined by gating 1% of events of a negative isotype control. A) Platelet gating for Panel 1 and 3, using SSC and CD42b‐FITC fluorescence. B) Single platelet gating for panel 1 and 3, using SSC and FSC. C) Platelet gating for Panel 2 using SSC and CD42a‐BV421. D) Single platelet gating for Panel 2 using SSC and FSC. E) PAC1‐FITC+ gate based on 1% of events in negative isotype. F) PAC1‐FITC+ G gate on a sample tube. G) CD62P‐PE+ gate based on 1% of events in negative isotype. H) CD62P‐PE+ gate on a sample tube.
Fig S2. Platelet counts for prospectively collected participants, analysed using Advia 2120i Cell Counter. Values are presented as mean ± standard deviation, and units are x109 Platelets/L. Differences within age groups were determined using ANOVA with Tukey’s multiple comparison test, and differences between age groups were determined using a t‐test with Bonferroni adjustment. Significant differences between groups are denoted with a black line and P‐value. Blue circles represent SARS‐CoV‐2‐exposed individuals and black triangles represent SARS‐CoV‐2‐positive individuals.
Table S1. Summary of targets for flow cytometry.


