Abstract
COVID‐19 (coronavirus disease 2019) represents a prothrombotic disorder, and there have been several reports of platelet factor 4/heparin antibodies being present in COVID‐19‐infected patients. This has thus been identified in some publications as representing a high incidence of heparin‐induced thrombocytopenia (HIT), whereas in others, findings have been tempered by general lack of functional reactivity using confirmation assays of serotonin release assay (SRA) or heparin‐induced platelet aggregation (HIPA). Moreover, in at least two publications, data are provided suggesting that antibodies can arise in heparin naïve patients or that platelet activation may not be heparin‐dependent. From this literature, we would conclude that platelet factor 4/heparin antibodies can be observed in COVID‐19‐infected patients, and they may occur at higher incidence than in historical non‐COVID‐19‐infected cohorts. However, the situation is complex, since not all platelet factor 4/heparin antibodies may lead to platelet activation, and not all identified antibodies are heparin‐dependent, such that they do not necessarily reflect “true” HIT. Most recently, a “HIT‐like” syndrome has reported in patients who have been vaccinated against COVID‐19. Accordingly, much more is yet to be learnt about the insidious disease that COVID‐19 represents, including autoimmune outcomes in affected patients.
Keywords: COVID‐19, heparin‐induced thrombocytopenia, platelet factor 4/heparin antibodies
1. INTRODUCTION
COVID‐19 (coronavirus disease 2019) is a recognized global pandemic caused by infection with SARS‐CoV‐2 (severe acute respiratory syndrome coronavirus 2). This infectious disease is thought to have originated in Wuhan, China, in late 2019, and at time of writing has infected over 141 million people and caused over 3 million deaths. 1 Severe COVID‐19 reflects primarily a prothrombotic disorder, with thrombosis appearing in various forms. 2 , 3 , 4 For example, a recent meta‐analysis has indicated a venous thromboembolism (VTE) rate, including deep vein thrombosis (DVT) and pulmonary thrombosis (PE), of close to 30% in patients with severe COVID‐19. 5 Acute myocardial ischaemia (infarction) and cerebrovascular accidents may also develop in as many as 8% and 3% of COVID‐19‐infected patients needing intensive care, 6 whilst systemic coagulopathy and disseminated intravascular coagulation (DIC) may occur in as many as 7% of such patients. 7 Evidence of microthrombosis in multiple organs including lungs, kidneys and liver, also occurs, although only identifiable on autopsy in patients who have died due to COVID‐19. 8 , 9 , 10 , 11 Indeed, COVID‐19 appears to affect all facets of haemostasis, including primary haemostasis (ie platelets, von Willebrand factor, endothelium), secondary haemostasis and fibrinolysis. 12 , 13 , 14 , 15 In addition, thromboses may arise from disturbances in immune response, creating cytokine disturbance (so‐called “cytokine storm”), according to immunothrombosis/endotheliitis type mechanisms. 2
Perhaps unsurprisingly, then, a series of autoimmune events have also been reported in patients with COVID‐19, including for example the presence of antiphospholipid antibodies potentially associated with antiphospholipid syndrome. 16 Of relevance to the current narrative review is that there have been several reports of platelet factor 4/heparin (PF4/H) antibodies being present in COVID‐19‐infected patients. Accordingly, we critically appraise this literature to answer the question: is heparin‐induced thrombocytopenia (HIT) a feature of COVID‐19?
2. MATERIALS AND METHODS
This is a narrative review. The PubMed database (https://pubmed.ncbi.nlm.nih.gov) was searched as required for both background information on PF4/H antibodies and HIT as well as specific papers related to COVID‐19. For the latter, we primarily used the search term (heparin AND (antibodies OR thrombocytopenia OR thrombocytopaenia OR HIT)) AND (COVID OR SARS)). An initial search performed on 24 March 2021 was later updated to be current as of 1 April 2021. Of 102 separate articles identified by this specific search, we then excluded general reviews and commentaries (ie not presenting original data), and papers otherwise found to be irrelevant to the topic.
3. RESULTS
3.1. Background information on platelet factor 4/heparin antibodies vs HIT
PF4/H antibodies develop in some individuals after exposure to heparin, primarily unfractionated heparin (UFH), but in some cases also to low molecular weight heparin (LMWH). 17 These antibodies can be detected immunologically via a wide range of assays, including ELISA (enzyme‐linked immunosorbent assays), LIA (latex immunoassay), CLIA (chemiluminescence immunoassays) and particle/lateral flow‐based methods. 18 , 19 There is some considerable inter‐assay variability regarding the detection of these PF4/H antibodies. For example, ELISA assays tend to detect a proportionally greater number of PF4/H antibodies than does CLIA. 20 Irrespective, the presence of these PF4/H antibodies in themselves does not identify pathological HIT, which also requires resultant activation of platelets. 21 This represents a functional event detected by functional assays, such as SRA (serotonin release assay) and HIPA (heparin‐induced platelet aggregation). 22 , 23 Only a small proportion of detected PF4/H antibodies will cause platelet activation, and thus “positivity” in the functional assays, and therefore, the anticipated pathological consequence of thrombosis. 21 , 24 There is also an association between the level of detected PF4/H antibodies and the likelihood of “positivity” in a functional assay. For example, an ELISA OD >1.0 or a CLIA value >10 U/mL may be characterized as more likely associated with functional assay positivity. 18 , 20 , 21 , 24 However, it is generally accepted that a functional assay is required to confirm pathological HIT in a patient with immunologically detected PF4/H antibodies.
It may also be important to note some nomenclature issues and arising confusion in the literature. The development of PF4/H antibodies is variously identified by authors as “immunological HIT,” or just HIT. A transient heparin‐induced nonimmune reduction in platelet count is sometimes called “HIT type I.” The secondary confirmation by antibody‐mediated platelet activation causing pathological HIT is variously called “HIT Type II,” “HITT” (ie HIT with thrombosis) or else may also just be referred to as HIT. In this review, we will refer to the first and last of these entities as (immunologically detected) PF4/H antibodies and pathological HIT, respectively. Where it is not clear from the literature whether pathological HIT has been appropriately defined, we will use the term “HIT” to denote this uncertainty.
The background risk of developing PF4/H antibodies and subsequent pathological HIT depends on the clinical setting. The overall reported incidence of “HIT” in patients exposed to heparin varies from 0.2% to up to 5%. 25 , 26 Interestingly, “HIT” is relatively rare in intensive care unit (ICU) populations, despite high use of heparin, with estimates approximating 0.5%. 27 The risk of HIT is higher amongst surgical compared with medical patients. 28 Amongst surgical patients, post‐cardiac surgery patients may have a higher risk of developing PF4/H antibodies than do post‐orthopaedic surgical patients, but the development of pathological HIT was historically potentially more likely after orthopaedic surgery when UFH was used. 29 Patients with major trauma are at higher risk of being PF4/H antibody positive and also developing pathological HIT as compared to those with minor trauma. 30 Pathological HIT is very rare in obstetric or paediatric patients. The risk of developing “HIT” is also higher amongst women than men, 31 and in patients on UFH vs patients receiving LMWH. 17 , 32
The incidence of PF4/H antibodies and pathological HIT with ECMO (extracorporeal membrane oxygenation) is also not insignificant, although accurate numbers are difficult to determine. However, Sokolovic et al 33 reported that in a cohort of adult ECMO patients, 8/96 (8.3%) were positive for “HIT” (positive SRA or PF4/H antibodies by ELISA with optical density [OD] >1) and 7/9 had documented thrombotic events (HITT or pathological HIT) based on predefined criteria (thus, “HIT” and “HITT” incidence in the study group were 8.3% and 7.3%, respectively). Vayne et al, 34 in a single‐centre prospective trial, reported on 57 adult patients who were supported by ECMO for at least 5 days. HIT was suspected in two patients (ie 3.5%) with ECMO circuit dysfunction and unexpected platelet count decrease after day 5. High levels of PF4‐specific IgG were detected in both patients, and HIT was confirmed by SRA. Additional data on such patients have been reported in published reviews. 35 , 36
3.2. COVID‐19 and “HIT”—results of our specific literature search—variation in reporting
The arising literature from our specific literature search is listed and summarized in Table 1. 37 , 38 , 39 , 40 , 41 , 42 , 43 , 44 , 45 , 46 , 47 , 48 , 49 , 50 , 51 , 52 , 53 , 54 , 55 Most published reports were individual case reports, or small case series. The information specifically provided by each publication varied considerably. Of interest, not all studies performed laboratory testing for PF4/H antibodies—some “diagnosed” “HIT” based on clinical grounds, such as the 4T score (4Ts; being evidence of Thrombocytopenia, and additional information about the Timing of platelet count fall, the presence of Thrombosis or other sequelae and Other causes for thrombocytopenia). Alternatively, some publications did not report 4Ts data, and instead “diagnosed” “HIT” on the basis of positive PF4/H antibody tests, only sometimes confirmed by additional testing using functional assays such as SRA or HIPA. Not all publications reported on the anticoagulant used ahead of the “HIT” “diagnosis.” Where anticoagulant use was identified, this typically being heparin, it was not always specified if this was UFH or LMWH. Sometimes, anticoagulant use was changed for patients according to clinical need; for example, LMWH use initiated as standard prophylaxis for prevention of thrombosis in COVID‐19 might be changed to UFH for use in ECMO, with this being a common treatment in severe COVID‐19.
TABLE 1.
Summary data for case reports and case series related to heparin (and non‐heparin)‐induced thrombocytopenia in COVID‐19 a
| Reference | Study type | Main findings | Case details | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Age | Sex | AC b | Indication, treatment, or HIT event c | 4Ts | ELISA OD | LIA or CLIA (U/mL) | Func Assay? d | HD e | AC switch f | Notes g | |||
| Lingamaneni et al 37 | Case series (n = 5) | 5 COVID‐19‐infected patients suspected of HIT; 1 confirmed | 63 | M |
LMWH/UFH |
right femoral DVT | 6 | 1.243 | NT | SRA + | 11 | arg | anti‐PF4/heparin antibody ELISA, method otherwise unspecified; 4 SRA – cases considered HIT false positives; DIC possible in 2/4 SRA‐ with lowest ELISA OD based on ISTH DIC score |
| 53 | M | H (NS) | ACS, AF | 5 | 0.707 | SRA ‐ | 7 | ||||||
| 63 | M | H (NS) | DVT | 7 | 0.767 | SRA ‐ | 6 | ||||||
| 70 | F | H (NS) | DVT | 7 | 0.042 | SRA ‐ | 8 | ||||||
| 46 | F | H (NS) | Suspected PE | 4 | 0.307 | SRA ‐ | 2 | ||||||
| Riker et al 38 | Case series (n = 3) from 16 patients with COVID‐19 ARDS | 3 HIT/16 (higher than expected incidence of 19%) | 70 | M | UFH | PE | 6 | 2.0 | NT | SRA + | 20 | biv | ELISA method polyspecific Stago assay with cut‐off 0.40; 1 case SRA‐ considered potential SRA‐ HIT; other case possible false HIT |
| 74 | M | UFH | UEVT | 4 | 1.3 | SRA ‐ | 9 | biv | |||||
| 53 | M | UFH | skin necrosis | 6 | 0.48 | SRA ‐ | 11 | arg | |||||
| Dragonetti et al 39 | Case series (n = 3) from 16 patients with COVID‐19 | Heparin antibodies present in 3/6 UFH treated, vs 0/10 LMWH treated | 77 | F | UFH | NR | NR | NT | positive | NR | 10 | fon |
6 patients in ICU treated with UFH, 10 patients other wards treated with LMWH. LIA test for PF4‐heparin (1). |
| 70 | M | UFH | NR | NR | positive | NR | 10 | fon | |||||
| 73 | M | UFH | NR | NR | positive | NR | 10 | fon | |||||
| Patell et al 40 | Case series (n = 5). 439 hospitalized COVID‐19‐infected patients, 88 receiving at least 5 days UFH, with 8 suspected of HIT. Cumulative incidence of detectable HIT antibodies of 12% at 25 days. Historical non‐COVID‐19 cohort ~3%. | 68 | F | UFH | No thrombosis | 4 | NT | 1.8 | SRA + | 7 | arg x4, biv x1 | LIA test for PF4‐heparin (1). 3 patients developed bleeding complications after AC switch | |
| 71 | F | UFH | No thrombosis | 6 | 1.1 | SRA ‐ | 6 | ||||||
| 63 | M | UFH | Spleen infarct and cerebral infarct post HIT | 8 | 1.6 | SRA bord + | 6 | ||||||
| 49 | M | UFH | No thrombosis | 6 | 1.9 | SRA bord + | 12 | ||||||
| 82 | M | UFH | No thrombosis | 5 | >16 | NA | 14 | ||||||
| Lozano et al 41 | Case series. 3/43 (6.9%) COVID‐19‐infected patients with possible HIT. | 45 | M | LMWH | No thrombosis | 6 | NT | NT | NT | NR | NR | No testing performed; HIT based on clinical suspicion (4Ts) | |
| 71 | M | LMWH | No thrombosis | 6 | NT | NT | NT | NR | NR | ||||
| 90 | M | LMWH | No thrombosis | 6 | NT | NT | NT | NR | NR | ||||
|
May et al 42 |
Case series. 7 COVID‐19‐infected patients suspected of HIT and positive by screen; only 1 was SRA positive | 50 | M | UFH |
Prophylaxis ECMO |
5 | 0.626 | NT | SRA ‐ | NR | NR | HIT testing by Asserachrom HPIA ELISA Kit, Diagnostica Stago, Parsippany, NJ, USA). All SRA‐ considered false positive HIT. | |
| 79 | F | LMWH | Prophylaxis | 3 | 1.881 | SRA ‐ | NR | NR | |||||
| 58 | F | LMWH |
Prophylaxis (PE) |
3 | 0.505 | SRA ‐ | NR | NR | |||||
| 61 | F | UFH IV | CRRT | 4 | 0.95 | SRA + | NR | NR | |||||
| 38 | M |
LMWH UFH |
Prophylaxis ECMO |
3 | 0.828 | SRA ‐ | NR | NR | |||||
| 71 | F | UFH |
Prophylaxis CRRT (Stroke) |
6 | 0.465 | SRA ‐ | NR | NR | |||||
| 46 | M | LMWH |
Prophylaxis (DVT) |
5 | 0.828 | SRA ‐ | NR | NR | |||||
| Huang et al 43 | Case report; patient died of cardiac failure/ acute myocardial infarction 23 h after applying ECMO with heparinization | 44 | M | UFH | ECMO | 2 > 6 | >2.0 | NT | NA |
9 12 |
NR |
anti‐PF4‐heparin antibody ELISA =0.38 ng/mL Day 9, 17.14 ng/mL Day 12 with OD >2.0 |
|
| Ogawa et al 44 | Case report. HIT based on 4Ts and positive PF4/H antibody | 37 | M | UFH | VA‐ECMO; acute pulmonary thrombosis | 6 | NT | 3.1 | NR | 15 | arg | Latex agglutination method for PF4/heparin antibody | |
| Bidar et al 45 | Case series. N = 2 COVID‐19 ICU severe ARDS | HIPA confirmed HIT | 62 | F | UFH |
VV‐ECMO support |
NR | 0.5 | NT | HIPA + | 11 | arg | Anti‐PF4 antibody ELISA (ZYMUTEST HIA IgGAM, HYPHEN BioMed) |
| 38 | M | UFH |
VV‐ECMO support |
NR | 0.12 | HIPA + | 12 | arg | |||||
| Tran et al 46 | Case report | HIPA confirmed HIT | 41 | M |
LMWH UFH |
VTE prophylaxis Suspected PE |
4 | 1.08 | NT | HIPA + | 4 (+UFH) | biv | IgG specific anti‐PF4‐heparin ELISA |
| Gubitosa et al 47 | Case report | “Prob not HIT” | 65 | M | LMWH | prophylaxis | 2 | "Qualitative anti‐heparin antibodies were sent and found to be positive" Method unspecified | SRA ‐ | <4 | “DIC was suspected given the patient's clinical findings” | ||
| Daviet et al 48 | Case series. All HIT cases (n = 7) amongst all COVID‐19‐infected patients (n = 86) with ARDS in 2 ICU. HIT incidence of 8%. | 46 | M | LMWHUFH | Multiple DVT | 6 | NT | 46 | All 7 HIPA+ | 16 | arg |
HemosIL AcuStar HIT immunoglobulin G, PF4‐ H, normal value <1 U/ml |
|
| 50 | M | LMWHUFH |
Intracardiac thrombosis ECMO membrane thrombosis |
6 | 11 | 13 | arg | ||||||
| 43 | F | LMWH UFH |
Multiple DVT, ECMO pump thrombosis |
6 | 39 | 15 | arg | ||||||
| 63 | M | LMWHUFH | Stroke | 4 | 60 | 14 | dan | ||||||
| 59 | M | LMWH UFH | DVT | 5 | 4 | 9 | dan | ||||||
| 57 | M | UFH | None | 5 | 21 | 11 | dan | ||||||
| 69 | M | UFH | None | 4 | 2 | 16 | dan | ||||||
| Parzy et al 49 | 13 severe ARDS COVID‐19 requiring VV‐ECMO. All developed VTE. 3 (23.1%) had laboratory confirmed HIT |
NR |
NR |
UFH |
VTE VV‐ECMO |
NR |
NR | NR | NR | NR | arg | Lab HIT test method not indicated; no confirmatory test indicated. HIT cases not further specifically identified or elaborated in report. | |
| Phan et al 50 | Case report (HIT post‐ECMO) | 43 | M |
LMWH UFH (ECMO) |
ECMO thrombi | 5 | NT |
2.9 4.0 |
NR | 4 (post ECMO) | riv, arg |
anti‐PF4/Heparin antibody (HemosIL HIT‐Ab, Instrumentation Laboratory, Bedford, MA) |
|
| Friedrich et al 51 | 31 adult COVID‐19‐infected patients. 2 (6%) developed HIT | NR | NR | LMWH or UFH | NR | NR | NR | NR | NR | NR | arg | no other specific details on the HIT cases, or HIT methods | |
| Brodard et al 52 |
12 COVID‐19‐infected patients with suspected HIT. 3 tested negative in all assays; 9 tested positive by antigen tests. Only 3 tested positive by HIPA. |
54 | M | NR | NR | 5 | 0.26 | 0.13 | HIPA ‐ | NR | NR | PF4/H antibodies by AcuStar CLIA and GTI‐PF4 ELISA; purified IgG fractions of COVID‐19 sera testing strongly positive by PF4/heparin antigen tests but negative by HIPA did not show increased reactivity by HIPA compared with original serum. Both results make a functionally inhibitory factor in the serum/plasma of COVID‐19‐infected patients highly unlikely. | |
| 73 | M | NR | NR | 6 | 0.5 | 0.3 | HIPA ‐ | NR | NR | ||||
| 59 | M | NR | NR | 4 | 0.56 | 0.4 | HIPA ‐ | NR | NR | ||||
| 56 | M | NR | NR | 4 | 0.13 | 0 | HIPA ‐ | NR | NR | ||||
| 58 | M | NR | NR | 4 | 1.71 | 2.6 | HIPA ‐ | NR | NR | ||||
| 54 | F | NR | NR | 4 | 2.43 | 41.3 | HIPA ‐ | NR | NR | ||||
| Nazy et al 53 |
10 critically ill COVID‐19 suspected of HIT, assessed by anti‐PF4/heparin antibodies and functional platelet activation by SRA. HIT excluded in all samples based on SRA. |
58 | M | UFH | thrombosis + | NR | 0.506 | NT | SRA NHD | NR | NR | HIT screen using multipanel (IgG, IgA, IgM) assay; positive (OD >0.4) confirmed by IgG‐specific assay, with 3/5 also positive (OD>0.4) (respectively, 0.235, 0.495, 0.583, 0.103, 0.931). SRA showed reactivity in 6 cases but this was not heparin dependent. Indeed, heparin caused inhibition of SRA | |
| 64 | M | LMWH | NR | NR | 0.102 | NT | NR | NR | |||||
| 49 | M | UFH | NR | NR | 0.867 | NT | NR | NR | |||||
| 53 | M | UFH | thrombosis + | NR | 0.168 | NT | NR | NR | |||||
| 65 | M | UFH | NR | NR | 3.155 | NT | NR | NR | |||||
| 80 | M | UFH | NR | NR | 0.456 | NT | NR | NR | |||||
| 51 | M | UFH | thrombosis + | NR | 1.64 | NT | NR | NR | |||||
| 70 | M | UFH | thrombosis + | NR | 0.086 | NT | NR | NR | |||||
| 77 | F | UFH | NR | NR | 0.261 | NT | NR | NR | |||||
| 71 | F | UFH | thrombosis ‐ | NR | 0.049 | NR | NR | ||||||
| Sartori et al 54 | Case report. HIT positive by CLIA | 78 | M |
UFH |
DVT | 4 | NT | 9.44 | NA | 17 | arg | AcuStar CLIA HIT‐IgGPF4‐H | |
| Liu et al 55 |
Case series. 61 critical ICU COVID‐19 and 93 severe non‐ICU patients. A high level of anti‐heparin‐PF4 antibodies, a marker of HIT, was observed in most ICU patients. Surprisingly, HIT occurred not only in patients with heparin exposure, but also in heparin‐naïve patients |
NR | NR | UFH & LMWH | NR | NR | Shown graphically | NT | NT | NR | NR | Commercial (Chinese) ELISA to Human anti‐heparin‐PF4 complex antibodies (IgG). No further details on individual cases. No confirmation assay. | |
Abbreviations: ACS, Acute coronary syndrome; AF, atrial fibrillation; ARDS, adult respiratory distress syndrome; CRRT, continuous renal replacement therapy; DIC, disseminated intravascular coagulation; DVT, deep vein thrombosis; H, heparin; HIT, heparin‐induced thrombocytopenia; ICU, Intensive Care Unit; ISTH, International Society on Thrombosis and Haemostasis; NA, not available; NR, not reported; NS, not specified; NT, not tested; PE, pulmonary embolism; PF4, platelet factor 4; UEVT, upper extremity venous thromboses; UFH, unfractionated heparin; VA‐ECMO, veno‐arterial extracorporeal membrane oxygenation; VTE, Venous thromboembolism; VV‐ECMO, venovenous extracorporeal membrane oxygenation.
Studies listed in order of PubMed listing, except reference [55], a non‐peer‐reviewed paper, which was an additional reference identified from the reference list of other publications.
AC, anticoagulation, as used in the patient and thus the potential trigger for HIT. Sometimes patients were switched to a different anticoagulant according to change in clinical situation (eg, LMWH for prophylaxis switched to UFH for ECMO). Sometimes the AC used was not identified.
Indication for heparin use (eg, prophylaxis or treatment) or the HIT event (eg, thrombosis) as reported in the cited publication.
Functional assay for HIT confirmation, if reported; HIPA, heparin induced platelet aggregation; SRA, serotonin release assay; NA, not available; NR, not reported; NHD, not heparin dependent
hospital day triggering HIT test or HIT diagnosis, either due to platelet fall, or 4Ts, or clinical suspicion; sometimes reported as day post admission, and sometimes as post heparin exposure
anticoagulant switched to (from heparin)—arg, argatroban; biv, bivalirudin; dan, danaparoid; fon, fondaparinux; riv, rivaroxaban
(1) latex immunological test for the determination of total immunoglobulins (IgA; IgM; IgG) against the PF4‐heparin complex, using an automated instrumental system (HemosIL HIT‐Ab(PF4‐H), Instrumentation Laboratory Bedford, MA, USA)
Not all publications identified the day of “HIT” diagnosis in regard to hospital day (HD) or day post‐heparin initiation, or subsequent to progression of thrombocytopenia. When laboratory testing was performed for PF4/H antibodies, methodologies were not always identified, or test results were not always reported.
3.3. Selection bias in the literature
One could hypothesis that the reported incidence of COVID‐19‐associated anti‐PF4/H antibodies or HIT would be highly biased due to the type of reported study. In particular, case reports and small cases series, comprising the current literature (Table 1), would be biased simply due to patient selection. Thus, authors are more likely to publish positive rather than negative findings. Second, researchers may actively look for anti‐PF4/H antibodies or HIT in select COVID‐19‐infected patient cohorts, such as those with clinical suspicion, either due to thrombocytopenia or 4Ts. It can be noted that (mild) thrombocytopenia is common in COVID‐19. 15 Third, there may be anticoagulation bias, where, given that UFH is more often reported to be associated with “HIT” than LMWH, studies may be limited to investigation only of UFH‐treated COVID‐19‐infected patients. In total, in such studies, a relatively high incidence of anti‐PF4/H antibodies or “HIT” may be identified, as might be anticipated, irrespective of the presence of COVID‐19. This is important to note in any evaluation of anti‐PF4/H antibodies or “HIT” in COVID‐19.
3.4. Gender and age of “HIT” “diagnosed” patients in COVID‐19
Details provided in Table 1 are further summarized in Table 2. The median age of patients reported in the literature search was 61 years, with interquartile range (IQR) of 50‐70 years. The median age of patients positive by functional HIT assays (SRA or HIPA) vs negative by functional HIT assay was similar to the entire cohort, being 61 and 58 years, respectively. Most patients reported in the literature were male (75.4%), which was also similar according to being positive (70.6%) or negative (68.4%) by functional assay. It can be noted that this distribution follows the anticipated pattern of hospitalized patients with COVID‐19, being predominantly male, and above the age of 55 years. 2
TABLE 2.
Summary of data from Table 1
| Cohort | Parameter | ||||
|---|---|---|---|---|---|
| Age | 4Ts | ELISA OD | CLIA (U/mL) | ||
| All cases | Median | 61 | 5 | 0.67 | 1.9 |
| IQR | 50‐70 | 4‐6 | 0.47‐1.20 | 1.9‐18.5 | |
|
Functional +ve cases |
Median | 61 | 6 | 1.02 | 21.0 |
|
Functional ‐ve cases |
Median | 58 | 4 | 0.59 | 0.35 |
| M | F | UFH | LMWH | ||
|---|---|---|---|---|---|
| All cases | n (%) | 43 (75.4) | 14 (24.6) | 34 (55.7) | 27 (44.3) |
|
Functional +ve cases |
n (%) | 12 (70.6) | 5 (29.4) | 11 (45.8) | 13 (54.2) |
| Functional ‐ve cases | n (%) | 13 (68.4) | 6 (31.6) | 4 (40.0) | 6 (60.0) |
Abbreviations: 4T, 4T score; ELISA, enzyme‐linked immunosorbent assay; CLIA, chemiluminescence immunoassay; LIA, latex immunoassay; M, male; F, female; UFH, unfractionated heparin; LMWH, low molecular weight heparin; IQR, interquartile range.
3.5. UFH or LMWH as the “trigger” for anti‐PF4/H antibodies or “HIT” in COVID‐19
As noted earlier, the COVID‐19 literature is sometimes unclear regarding whether UFH or LMWH was the anticoagulant in use prior to development of anti‐PF4/H antibodies or “HIT,” or the time course of their development. However, based on the information gleaned from the literature, UFH (55.7%) and LMWH (44.3%) seemed “similarly” involved in development of anti‐PF4/H antibodies or “HIT.” Note here, however, that selection bias is particularly problematic, as some studies only evaluated cases under UFH therapy. The number of cases according to functional assay positivity vs negativity is numerically low, but similar “equality” was seen for LMWH vs UFH in the separated cohorts (Table 2).
3.6. 4Ts in the anti‐PF4/H antibody or HIT COVID‐19 cohorts
Again, accepting that selection bias would play a part in findings, the median 4Ts for cases identified in Table 1 was 5, with an IQR of 4‐6 (Table 2). Interestingly, the median value for those positive in functional assays was 6, and higher than the median (ie 4) for those with negative functional assay findings. Although this would be as expected, study numbers are low overall, and the difference was not statistically significant (Figure 1). Also, the 4Ts is noted to be less useful in ICU cohorts, and many of the “HIT” assessed population derived from ICU.
FIGURE 1.
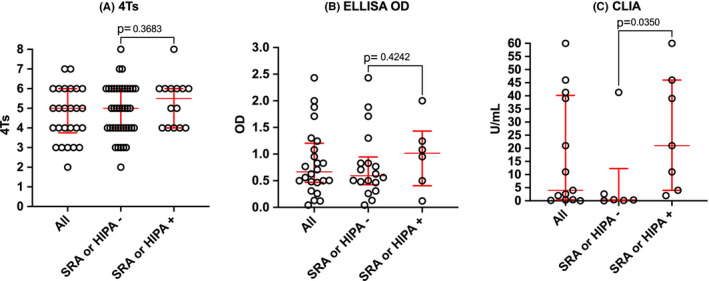
Comparative findings between functional assay (SRA or HIPA) confirmed HIT vs not confirmed in the COVID‐19 literature. A, 4T score. B, ELISA OD readings. C, Antibody levels according to CLIA assays
3.7. Anti‐PF4/H antibody levels in COVID‐19 cohorts
Again, cognizant of study bias, the median (IQR) for the whole cohort for ELIZA OD was 0.67 (0.47‐1.20), with higher median in functionally positive patients (1.02) than negative patients (0.59). However, the difference was not statistically significant (Figure 1). Similarly, using CLIA assays, the median (IQR) for the whole cohort was 1.9 (1.9‐18.5) U/mL, with higher median in functionally positive than negative patients (21 vs 0.35). Again, this fits expectations, although study numbers are low. Here, however, the difference was statistically significant (Figure 1).
3.8. Incidence of “HIT” in COVID‐19
It is not possible to be entirely sure of the true incidence of pathological HIT in COVID‐19, given selection bias noted above and limited information provided in most publications. However, a few studies reported incidence as a proportion of investigated COVID‐19 cases. For example, Dragonetti et al investigated 6 patients in ICU treated with UFH, and 10 patients in other wards treated with LMWH, and used a LIA test for PF4/H antibodies to “diagnose” “HIT.” 39 Heparin antibodies were present in 3/6 (50%) UFH treated, vs 0/10 managed with LMWH. Functional HIT testing was not reported. Patell et al reported a case series of HIT patients (n = 5) diagnosed with “HIT” also based on the LIA assay, and derived from a COVID‐19 cohort of 439 hospitalized patients, 88 receiving at least 5 days UFH, with 8 suspected of HIT. 40 The cumulative incidence of detectable PF4/H antibodies in the cohort was 12% at 25 days, compared with a historical non‐COVID‐19 cohort of ~3%. However, the incidence was calculated based on selection of cases receiving at least 5 days UFH, and the functional assay (SRA) was only strongly positive in 1/5 patients. Lozano and Franco reported a case series of 43 COVID‐19‐infected patients, of which 3 (6.9%) had possible “HIT.” 41 However, this study defined HIT solely based on 4Ts, and no laboratory assays were performed. Daviet et al reported a case series of “HIT” cases (n = 7) amongst all COVID‐19‐infected patients (n = 86) with acute respiratory distress syndrome (ARDS) in 2 ICUs, and thus, a pathological HIT incidence of 8%. 48 HIT was defined by positive CLIA and confirmed by HPIA. Parzy et al reported on 13 severe ARDS COVID‐19‐infected patients requiring venovenous (VV)‐ECMO. 49 All developed VTE, and 3 (23.1%) had laboratory confirmed “HIT.” However, the laboratory HIT test method was not indicated, and no confirmatory test was performed. Further, the HIT cases were not further specifically identified or elaborated in the report. Friedrich et al reported 2/31 (6%) adult COVID‐19‐infected patients developed “HIT.” 51 However, no other specific details were provided on the “HIT” cases or “HIT” diagnosing methods.
Conversely, May et al reported a case series of 7 COVID‐19‐infected patients suspected of HIT and positive using an immunological screen by ELISA, only one of which was SRA positive. 42 Thus, they indicated a low incidence of pathological HIT in COVID‐19‐infected patients. Similarly, Brodard et al reported on 12 COVID‐19‐infected patients with suspected HIT. 52 Three tested negative in all laboratory assays; 9 tested positive by antigen tests, but only 3 tested positive by HIPA. Sartori et al reported on 10 critically ill COVID‐19‐infected patients suspected of HIT, assessed by anti‐PF4/H antibodies and functional platelet activation by SRA. 53 HIT was excluded in all samples based on SRA. Interestingly, SRA showed reactivity in 6 cases, but this was not heparin‐dependent, and heparin actually caused inhibition of SRA. Finally, Liu et al, in a non‐peer‐reviewed preprint, presented another case series comprising 61 critical ICU COVID‐19 and 93 severe non‐ICU patients. 55 A high level of anti‐PF4/H antibodies was observed in most ICU patients. Surprisingly, “HIT” occurred not only in patients with heparin exposure, but also in several heparin‐naïve patients.
4. DISCUSSION
The literature on “HIT” in COVID‐19 is conflicting. Some researchers find a high level of “HIT” in COVID‐19‐infected patients, but these findings need to be tempered by methodological flaws, where patients were either not fully worked up, or else key details were missing. It needs to be noted that in non‐COVID‐19 literature, many patients may be found to have PF4/H antibodies, but most of these will not develop pathological HIT. 56 Moreover, a high level of PF4/H antibodies (especially as detected by ELISA) will not be positive by a functional assay such as SRA. 20 , 24
In contrast, some researchers reported a low level of “HIT” in COVID‐19‐infected patients, in particular when functional assays were performed. Moreover, some researchers 53 , 55 actually reflected that in a significant proportion of their SRA‐positive patients, therapeutic levels of heparin either inhibited the assay, or else heparin did not appear to be a trigger for the event as patients were heparin naïve.
Thus, the overall situation in regards to identification of anti‐PF4/H antibodies or “HIT” is complex. Indeed, the situation has recently become even more complex, given a recent publication reporting a rare phenomenon in 9 patients recently vaccinated against COVID‐19, primarily leading to venous thrombotic episodes (especially cerebral venous thrombosis, CVT) accompanied by relevant thrombocytopenia (ie 39 ± 30×109/L) 4‐16 days after receiving the vaccine. 57 Interestingly, the German researchers indicated a mechanism similar to HIT, with the serum of 4 such patients referred for investigation of platelet‐activating antibodies directed against PF4/H. All test results revealed strong positivity for “PF4/‐H antibodies” using an immunoassay (ELISA) and also activated platelets using a platelet activation assay, but these events appeared to occur independently of heparin, and reactivity against PF4 (without heparin) was later also shown. Although a causal link between COVID‐19 vaccination (especially with AstraZeneca AZD1222) and CVT has yet to be clarified, these patients (mostly young women in the German study) appeared to develop “PF4 antibodies,” presumably without heparin exposure. The authors termed this clinical syndrome “vaccine‐induced prothrombotic immune thrombocytopenia” (VIPIT). The authors have since republished these findings, with some amendments, in the peer reviewed literature, as well as changing the name to “VITT” (ie vaccine induced immune thrombotic thrombocytopenia). 58 A few other groups from other countries have since also published on this phenomenon, mostly observed post AstraZeneca AZD1222, 59 , 60 except for one published case with the Ad26.COV2.S vaccine (Johnson & Johnson/Janssen). 61 In the UK series, the phenomenon was more generally observed in both men and women, and in both “young” and “old.” 60 The events are unquestionably rare, but physicians should be advised about its possible development after COVID‐19 vaccination. 62
5. CONCLUSION
Taking all this information into consideration, we need to accept that PF4/H antibody development does represent a feature of COVID‐19, at least in some patients. However, this does not always indicate pathological HIT for most. Also, there appears to be a question over the role of heparin in some of the cases reported in the literature, and instead, a “HIT‐like” event may be occurring in some patients without heparin exposure. This may be linked to theoretical concepts around PF4/H mimicry in infectious disease, although additional confounders may exist in relation to COVID‐19. The binding of SARS‐CoV‐2 spike protein to platelet surface receptor(s), leading to platelet activation, is another potential mechanism that deserves further scrutiny in the pathogenesis of thrombosis in COVID‐19. 63 However, whether pathological HIT truly develops in a high proportion of COVID‐19‐infected patients remains unresolved, and more needs to be done to fully investigate the phenomena of PF4/H antibody development, and arising pathophysiology in COVID‐19. We encourage further studies on the development of HIT or PF4(/H) antibodies, either following heparin exposure or not. These studies need to document all aspects of patient exposure to heparin (or not), the type of heparin, the time course for antibody development or arising pathology, and the methods employed for laboratory testing, including any employed modifications (eg heparin inhibition).
CONFLICT OF INTEREST
The authors have no competing interests.
ACKNOWLEDGEMENTS
The opinions expressed in this review are those of the authors and do not necessarily reflect the opinions of their respective employers, NSW Health Pathology, The Heart Institute, Cincinnati Children's Hospital Medical Center and the University of Verona.
Favaloro EJ, Henry BM, Lippi G. The complicated relationships of heparin‐induced thrombocytopenia and platelet factor 4 antibodies with COVID‐19. Int J Lab Hematol. 2021;43:547–558. 10.1111/ijlh.13582
DATA AVAILABILITY STATEMENT
Data is summarized in Table 1, which also identifies reference cited.
REFERENCES
- 1. COVID‐19 Dashboard by the Center for Systems Science and Engineering (CSSE) at Johns Hopkins University. 2021. <https://www.arcgis.com/apps/opsdashboard/index.html#/bda7594740fd40299423467b48e9ecf6> accessed 30th March
- 2. Lippi G, Sanchis‐Gomar F, Favaloro EJ, Lavie CJ, Henry BM. Coronavirus disease 2019‐associated coagulopathy. Mayo Clin Proc. 2021;96(1):203‐217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Thachil J, Srivastava A. SARS‐2 coronavirus‐associated hemostatic lung abnormality in COVID‐19: is it pulmonary thrombosis or pulmonary embolism? Semin Thromb Hemost. 2020;46(7):777‐780. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Schulman S. Coronavirus disease 2019, prothrombotic factors, and venous thromboembolism. Semin Thromb Hemost. 2020;46(7):772‐776. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Di Minno A, Ambrosino P, Calcaterra I, Di Minno MND. COVID‐19 and venous thromboembolism: a meta‐analysis of literature studies. Semin Thromb Hemost. 2020;46(7):763‐771. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Jenner WJ, Kanji R, Mirsadraee S, et al. Thrombotic complications in 2928 patients with COVID‐19 treated in intensive care: a systematic review. J Thromb Thrombolysis. 2021;14:1‐13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Uaprasert N, Moonla C, Sosothikul D, Rojnuckarin P, Chiasakul T. Systemic coagulopathy in hospitalized patients with coronavirus disease 2019: a systematic review and meta‐analysis. Clin Appl Thromb Hemost. 2021;27:107602962098762. 10.1177/1076029620987629 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Carsana L, Sonzogni A, Nasr A, et al. Pulmonary post‐mortem findings in a series of COVID‐19 cases from northern Italy: a two‐centre descriptive study. Lancet Infect Dis. 2020;20(10):1135‐1140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Wichmann D, Sperhake J, Lutgehetmann M, Steurer S, Edler C. Autopsy findings and venous thromboembolism in patients with COVID‐19. Ann Clin Med. 2020;173(4):268‐277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Bradley BT, Maioli H, Johnston R, et al. Histopathology and ultrastructural findings of fatal COVID‐19 infections in Washington State: a case series. The Lancet. 2020;396(10247):320‐332. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Varga Z, Flammer AJ, Steiger P, et al. Endothelial cell infection and endotheliitis in COVID‐19. The Lancet. 2020;395(10234):1417‐1418. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Favaloro EJ, Henry BM, Lippi G. Increased VWF and decreased ADAMTS13 in COVID‐19: creating a milieu for (micro)thrombosis? Semin Thromb Hemost. 2021;47:400‐418. 10.1055/s-0041-1727282 [DOI] [PubMed] [Google Scholar]
- 13. Levi M, Thachil J. Coronavirus disease 2019 coagulopathy: disseminated intravascular coagulation and thrombotic microangiopathy‐either, neither, or both. Semin Thromb Hemost. 2020;46(7):781‐784. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Kwaan HC. Coronavirus disease 2019: the role of the fibrinolytic system from transmission to organ injury and sequelae. Semin Thromb Hemost. 2020;46(7):841‐844. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Larsen JB, Pasalic L, Hvas AM. Platelets in coronavirus disease 2019. Semin Thromb Hemost. 2020;46(7):823‐825. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Favaloro EJ, Henry BM, Lippi G. COVID‐19 and antiphospholipid antibodies: time for a reality check? Semin Thromb Hemost. 2021. 10.1055/s-0041-1728832 [DOI] [PubMed] [Google Scholar]
- 17. Junqueira DR, Zorzela LM, Perini E. Unfractionated heparin versus low molecular weight heparins for avoiding heparin‐induced thrombocytopenia in postoperative patients. Cochrane Database Syst Rev. 2017;4(4):CD007557. 10.1002/14651858 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Favaloro EJ, McCaughan G, Pasalic L. Clinical and laboratory diagnosis of heparin induced thrombocytopenia: an update. Pathology. 2017;49(4):346‐355. [DOI] [PubMed] [Google Scholar]
- 19. Warkentin TE. Heparin‐induced thrombocytopenia. Curr Opin Crit Care. 2015;21(6):576‐585. [DOI] [PubMed] [Google Scholar]
- 20. Favaloro EJ, McCaughan G, Mohammed S, et al. HIT or miss? A comprehensive contemporary investigation of laboratory tests for heparin induced thrombocytopenia. Pathology. 2018;50(4):426‐436. [DOI] [PubMed] [Google Scholar]
- 21. Nazi I, Arnold DM, Warkentin TE, Smith JW, Staibano P, Kelton JG. Distinguishing between anti–platelet factor 4/heparin antibodies that can and cannot cause heparin‐induced thrombocytopenia. J Thromb Haemost. 2015;13:1900‐1907. [DOI] [PubMed] [Google Scholar]
- 22. Warkentin TE, Arnold DM, Nazi I, Kelton JG. The platelet serotonin‐release assay. Am J Hematol. 2015;90(6):564‐572. [DOI] [PubMed] [Google Scholar]
- 23. Joseph J, Rabbolini D, Enjeti AK, et al. Diagnosis and management of heparin‐induced thrombocytopenia: a consensus statement from the Thrombosis and Haemostasis Society of Australia and New Zealand HIT Writing Group. Med J Aust. 2019;210(11):509‐516. [DOI] [PubMed] [Google Scholar]
- 24. Favaloro EJ, Mohammed S, Donikian D, et al. A multicentre assessment of contemporary laboratory assays for heparin induced thrombocytopenia. Pathology. 2021;53(2):247‐256. [DOI] [PubMed] [Google Scholar]
- 25. Gupta S, Tiruvoipati R, Green C, Botha J, Tran H. Heparin induced thrombocytopenia in critically ill: diagnostic dilemmas and management conundrums. World J Crit Care Med. 2015;4(3):202‐212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Cuker A. Recent advances in heparin‐induced thrombocytopenia. Curr Opin Hematol. 2011;18:315‐322. [DOI] [PubMed] [Google Scholar]
- 27. Selleng K, Warkentin TE, Greinacher A. Heparin‐induced thrombocytopenia in intensive care patients. Crit Care Med. 2007;35:1165‐1176. [DOI] [PubMed] [Google Scholar]
- 28. Warkentin TE. Heparin‐induced thrombocytopenia in critically ill patients. Semin Thromb Hemost. 2015;41(1):49‐60. [DOI] [PubMed] [Google Scholar]
- 29. Warkentin TE, Sheppard JA, Horsewood P, Simpson PJ, Moore JC, Kelton JG. Impact of the patient population on the risk for heparin‐induced thrombocytopenia. Blood. 2000;96:1703‐1708. [PubMed] [Google Scholar]
- 30. Lubenow N, Hinz P, Thomaschewski S, et al. The severity of trauma determines the immune response to PF4/heparin and the frequency of heparin induced thrombocytopenia. Blood. 2010;115:1797‐1803. [DOI] [PubMed] [Google Scholar]
- 31. Warkentin TE, Sheppard JA, Sigouin CS, Kohlmann T, Eichler P, Greinacher A. Gender imbalance and risk factor interactions in heparin‐induced thrombocytopenia. Blood. 2006;108:2937‐2941. [DOI] [PubMed] [Google Scholar]
- 32. Cuker A. Clinical and laboratory diagnosis of heparin‐induced thrombocytopenia: an integrated approach. Semin Thromb Hemost. 2014;40(1):106‐114. [DOI] [PubMed] [Google Scholar]
- 33. Sokolovic M, Pratt AK, Vukicevic V, Sarumi M, Johnson LS, Shah NS. Platelet count trends and prevalence of heparin‐induced thrombocytopenia in a cohort of extracorporeal membrane oxygenator patients. Crit Care Med. 2016;44:e1031‐e1037. [DOI] [PubMed] [Google Scholar]
- 34. Vayne C, May MA, Bourguignon T, et al. Frequency and clinical impact of platelet factor 4‐specific antibodies in patients undergoing extracorporeal membrane oxygenation. Thromb Haemost. 2019;119:1138‐1146. [DOI] [PubMed] [Google Scholar]
- 35. Pollak U. Heparin‐induced thrombocytopenia complicating extracorporeal membrane oxygenation support: Review of the literature and alternative anticoagulants. J Thromb Haemost. 2019;17(10):1608‐1622. [DOI] [PubMed] [Google Scholar]
- 36. Choi JH, Luc JG, Weber MP, et al. Heparin‐induced thrombocytopenia during extracorporeal life support: incidence, management and outcomes. Ann Cardiothorac Surg. 2019;8(1):19‐31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Lingamaneni P, Gonakoti S, Moturi K, Vohra I, Zia M. Heparin‐induced thrombocytopenia in COVID‐19. J. Investig. Med. High Impact Case Rep. 2020;8:232470962094409. 10.1177/2324709620944091 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Riker RR, May TL, Fraser GL, et al. Heparin‐induced thrombocytopenia with thrombosis in COVID‐19 adult respiratory distress syndrome. Res Pract Thromb Haemost. 2020;4(5):936‐941. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Dragonetti D, Guarini G, Pizzuti M. Detection of anti‐heparin‐PF4 complex antibodies in COVID‐19 patients on heparin therapy. Blood Transfus. 2020;18(4):328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Patell R, Khan AM, Bogue T, et al. Heparin induced thrombocytopenia antibodies in Covid‐19. Am J Hematol. 2020;95(10): 10.1002/ajh.25935 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Lozano R, Franco ME. Incidence of heparin‐induced thrombocytopenia in patients with 2019 coronavirus disease. Med Clin (Barc). 2020;155(9):409‐410. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. May JE, Siniard RC, Marques M. The challenges of diagnosing heparin‐induced thrombocytopenia in patients with COVID‐19. Res Pract Thromb Haemost. 2020;4(6):1066‐1067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Huang CT, Hsu SY, Chang KW, Huang CG, Yang CT, Cheng MH. Heparin‐induced thrombocytopenia and thrombosis in a patient with COVID‐19. Thromb Res. 2020;196:11‐14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Ogawa Y, Nagata T, Akiyama T, et al. Argatroban therapy for heparin‐induced thrombocytopenia in a patient with coronavirus disease 2019. J Thromb Thrombolysis. 2020;50(4):1012‐1014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Bidar F, Hékimian G, Martin‐Toutain I, Lebreton G, Combes A, Frère C. Heparin‐induced thrombocytopenia in COVID‐19 patients with severe acute respiratory distress syndrome requiring extracorporeal membrane oxygenation: two case reports. J Artif Organs. 2020. 10.1007/s10047-020-01203-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Tran M, Sheth C, Bhandari R, Cameron SJ, Hornacek D. SARS‐CoV‐2 and pulmonary embolism: who stole the platelets? Thromb J. 2020;3(18):16. 10.1186/s12959-020-00229-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Gubitosa JC, Xu P, Ahmed A, Pergament K. COVID‐19‐associated acute limb ischemia in a patient on therapeutic anticoagulation. Cureus. 2020;12(9):e10655. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Daviet F, Guervilly C, Baldesi O, et al. Heparin‐induced thrombocytopenia in severe COVID‐19. Circulation. 2020;142(19):1875‐1877. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Parzy G, Daviet F, Puech B, et al. Venous thromboembolism events following venovenous extracorporeal membrane oxygenation for severe acute respiratory syndrome coronavirus 2 based on CT scans. Crit Care Med. 2020;48(10):e971‐e975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Phan XT, Nguyen TH, Tran TT, et al. Suspected heparin‐induced thrombocytopenia in a COVID‐19 patient on extracorporeal membrane oxygenation support: a case report. Thromb J. 2020;18(1):37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Friedrich MS, Studt JD, Braun J, Spahn DR, Kaserer A. Coronavirus‐induced coagulopathy during the course of disease. PLoS One. 2020;15(12):e0243409. 10.1371/journal.pone.0243409 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Brodard J, Kremer Hovinga JA, Fontana P, Studt JD, Gruel Y, Greinacher A. COVID‐19 patients often show high‐titer non‐platelet‐activating anti‐PF4/heparin IgG antibodies. J Thromb Haemost. 2021;19(5):1294‐1298. 10.1111/jth.15262 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Nazy I, Jevtic SD, Moore JC, et al. Platelet‐activating immune complexes identified in critically ill COVID‐19 patients suspected of heparin‐induced thrombocytopenia. J Thromb Haemost. 2021;19(5):1342‐1347. 10.1111/jth.15283 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Sartori M, Cosmi B. Heparin‐induced thrombocytopenia and COVID‐19. Hematol Rep. 2021;13(1):8857. 10.4081/hr.2021.8857 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. Liu X, Zhan X, Xiao Y, et al. Heparin‐induced thrombocytopenia is associated with a high risk of mortality in critical COVID‐19 patients receiving heparin‐involved treatment. medRxiv preprint. 10.1101/2020.04.23.20076851 [DOI]
- 56. Hvas AM, Favaloro EJ, Hellfritzsch M. Heparin‐induced thrombocytopenia: pathophysiology, diagnosis and treatment. Expert Rev Hematol. 2021;30:1‐12. 10.1080/17474086.2021.1905512 [DOI] [PubMed] [Google Scholar]
- 57. Greinacher A, Thiele T, Warkentin TE, Weisser K, Kyrle P, Eichinger S. Heparin‐Induced Thrombocytopenia Following Coronavirus‐19 Vaccination. Research Square. Preprint. 10.21203/rs.3.rs-362354/v1 [DOI]
- 58. Greinacher A, Thiele T, Warkentin TE, Weisser K, Kyrle PA, Eichinger S. Thrombotic Thrombocytopenia after ChAdOx1 nCov‐19 Vaccination. N Engl J Med. 2021. 10.1056/NEJMoa2104840 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59. Schultz NH, Sørvoll IH, Michelsen AE, et al. Thrombosis and Thrombocytopenia after ChAdOx1 nCoV‐19 Vaccination. N Engl J Med. 2021. 10.1056/NEJMoa2104882 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60. Scully M, Singh D, Lown R, et al. Pathologic antibodies to platelet factor 4 after ChAdOx1 nCoV‐19 vaccination. N Engl J Med. 2021. 10.1056/NEJMoa2105385 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61. Muir KL, Kallam A, Koepsell SA, Gundabolu K. Thrombotic thrombocytopenia after Ad26.COV2.S vaccination. N Engl J Med. 2021. 10.1056/NEJMc2105869 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62. Kupferschmidt K, Vogel G. A rare clotting disorder may cloud the world’s hopes for AstraZeneca’s COVID‐19 vaccine. Science. 2021. 10.1126/science.abi7283 [DOI] [Google Scholar]
- 63. Zhang S, Liu Y, Wang X, et al. SARS‐CoV‐2 binds platelet ACE2 to enhance thrombosis in COVID‐19. J Hematol Oncol. 2020;13(1):120. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Data is summarized in Table 1, which also identifies reference cited.


