Summary
There is concern that COVID‐19 vaccination may adversely affect immune thrombocytopenia (ITP) patients. Fifty‐two consecutive chronic ITP patients were prospectively followed after COVID‐19 vaccination. Fifteen percent had no worsening of clinical symptoms but no post‐vaccination platelet count; 73% had no new symptoms and no significant platelet count decline. However, 12% had a median platelet count drop of 96% within 2–5 days post vaccination with new bleeding symptoms; after rescue therapy with corticosteroids +/− intravenous immunoglobulin (IVIG), platelets recovered to >30 × 109/l a median three days later. ITP exacerbation occurred independently of remission status, concurrent ITP treatment, or vaccine type. Safety of a second vaccine dose needs careful assessment.
Keywords: immune thrombocytopenia, vaccination, COVID‐19, bleeding, rescue
Introduction
The effect of vaccination in immune thrombocytopenia (ITP) is of concern to both patients and their physicians. 1 Although vaccine‐induced thrombocytopenia has been associated with many types of vaccine, studies in adults have shown no difference in prior vaccine exposure in newly diagnosed ITP patients when compared to matched control patients without ITP. 2 The only notable exception is in children 13–24 months old who experience a 6·3‐fold increased risk of ITP in the six weeks following measles–mumps–rubella (MMR) vaccination. 3 , 4
The recent report of thrombocytopenia and the eventual death of a healthy person who received an mRNA‐based COVID‐19 vaccine 5 has again raised concerns about whether vaccines trigger ITP. But in a very preliminary report of 17 episodes reported out of over 20 million mRNA‐based COVID‐19 vaccinations in North America, the authors suggest that the rate of newly diagnosed ITP, including the one fatal case referenced above, was close to the background occurrence rate of ITP. 6
Less understood but of equal concern is whether vaccination, like infection, can exacerbate thrombocytopenia in patients who already have ITP. There are very few data with other vaccines to inform this concern. The only relevant data come from 65 children who developed ITP after an initial MMR vaccination; upon receipt of a second MMR vaccine, none developed a recurrence. 3 , 4 , 7
After the fatal vaccine reaction, many of the patients in the Massachusetts General Hospital ITP Center had great trepidation about receiving any of the three available COVID‐19 vaccines. Since we recommend all of our patients undergo COVID‐19 vaccination, we initiated a prospective analysis of all of our ITP patients after January 12, 2021, to assess the frequency of worsening ITP symptoms and exacerbation of thrombocytopenia in those who chose to receive COVID‐19 vaccination.
Results
Between January 12, 2021, and May 1, 2021, we prospectively evaluated all of the routinely scheduled ITP patients who were seen in person or via virtual visitation and assessed their COVID‐19 vaccination status. Fifty‐two consecutive patients who received COVID‐19 vaccination were identified and followed. Patients were advised to obtain platelet counts 1–7 days before and 3–14 days after vaccination and assessed for the exacerbation of their thrombocytopenia and clinical signs of bleeding after vaccination. To minimize ascertainment bias, we excluded from this analysis patients who were specifically referred to us because of de novo thrombocytopenia in the context of COVID‐19 infection or vaccination and those with another concurrent infection. Acknowledging that many ITP patients have significant week‐to‐week fluctuations in platelet counts, exacerbation of thrombocytopenia was defined as a platelet count reduced by 66% from the pre‐vaccination baseline along with new bleeding symptoms. Additional demographic data included age, gender, the most recent platelet count, current ITP medications, lowest platelet count observed after vaccination, any treatment increase (‘rescue’) received in response to the thrombocytopenia, thrombocytopenia with prior vaccinations and the time to recovery to a platelet count of over 30 × 109/l for those who needed rescue therapy.
All 52 patients had chronic ITP per current guidelines 8 , 9 ; ITP had been present for a median (range) of 12 (1–49) years; 18 (35%) were treatment‐free with platelets >100 × 109/l; only 16 (31%) were on active treatment; 37 (71%) were female and the median (range) age was 65·5 (19–85) years. While only four patients (8%) had received the Johnson & Johnson vaccine, 24 each (46%) received Pfizer or Moderna vaccines.
As shown in Fig 1, 8/52 (15%) had no worsening of ITP symptoms but failed to have platelet counts drawn within 14 days after vaccination. 38/52 (73%) had no worsening of ITP symptoms and no significant change in platelet counts in the 14 days following vaccination; some (˜50% of the total group) had decreases in platelet count after vaccination that could not be distinguished from their usual fluctuations in number but none decreased by more than 50% from pre‐vaccination counts. 6/52 (12%) of patients developed a severe exacerbation of their thrombocytopenia along with worsening bleeding symptoms.
Fig 1.
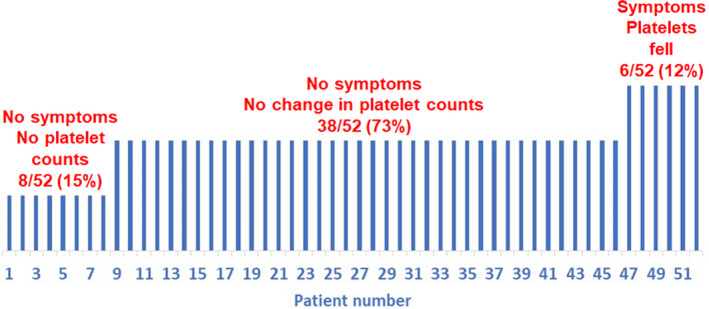
Incidence of the exacerbation of immune thrombocytopenia after COVID‐19 vaccination.
In the six patients who had exacerbation of their thrombocytopenia, median (range) pre‐vaccination platelet counts of 164 × 109/l (12–270 × 109/l) dropped by 96% to a median (range) count of 7 × 109/l (1–17 × 109/l) a median (range) of 2 (2–5) days after the most recent vaccination (Table I). All six patients required treatment and 5/6 responded to prednisone +/− intravenous immunoglobulin (IVIG). The response was rapid except for two patients, with platelets rising to >30 × 109/l after a median (range) of 3 (1–33) days. Except for the finding that 2/6 had previously dropped their platelets after prior non‐COVID‐19 vaccination, there seemed to be no predictors of this adverse response (Table SI). Four out of six had been in remission (three on no therapy) for over one year; only two were on active treatment. Exacerbation of thrombocytopenia occurred with all three vaccines. Two patients had received a second vaccination dose before platelet counts were obtained but both had increased bleeding symptoms after the first that had been wrongly attributed to their concurrent warfarin anticoagulation. The remaining four patients did not receive a second vaccination.
Table I.
Patient demographics and outcomes.
| Patient number | Age, years | Gender, female | Duration ITP, years | On active treatment, (%) | Platelet count prior to vaccination, ×109/l | Vaccine type, M/P/J (%) | Lowest platelet count after vaccination, ×109/l | Onset of worsened thrombocytopenia, days after vaccine administered | Worsened ITP symptoms, (%) | Rescue treatment, (%) | Days until platelets >30 × 109/l | Patient details |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 1–8 | 57 | 75% | 14 | 25% | 196 | 63/25/13 | 0% | 0% | ||||
| (19–78) | (3–32) | (72–330) | ||||||||||
| 9–46 | 69 | 71% | 10 | 32% | 82 | 47/47/5 | 75 | 0% | 0% | |||
| (25–85) | (1–49) | (7–582) | (10–407) | |||||||||
| 47–52 | 47 | 67% | 14 | 33% | 164 | 17/67/17 | 7 | 3 | 100% | 100% | 3 | |
| (28–85) | (4––22) | (12––270) | (1–17) | (2–5) | (1–33) | |||||||
| 47 | 28 | Female | 12 | No | 130 | P | 17 | 3 | Petechiae, ecchymoses | Prednisone, IVIG | 1 | Platelet count 130 × 109/l after first dose; dropped to 17 × 109/l three days after the second dose |
| 48 | 71 | Female | 22 | No | 197 | P | 12 | 4 | Petechiae, ecchymoses, fatigue | Prednisone | 2 | Five days after the first dose platelets dropped from 197 × 109/l to 113 × 109/l; recovered to 193 × 109/l one day before the second dose; four days after the second dose platelets 12 × 109/l |
| 49 | 34 | Female | 17 | Azathioprine | 227 | P | 7 | 2 | Petechiae, ecchymoses, oral blood blisters | Prednisone, IVIG | 4 | After first dose, increased ecchymoses and platelets 149 × 109/l 14 days later; two days after the second dose, platelets 19 × 109/l; patient on warfarin for prior DVT. Identical decline after Pneumococcal vaccine |
| 50 | 60 | Male | 16 | No | 270 | J | 1 | 5 | Petechiae, ecchymoses, epistaxis, oral blood blisters | Prednisone, IVIG, romiplostim, rituximab | 33 | Patient with multiple prior severe relapses every 3–5 years often lasting for months; one triggered by meningitis vaccine. In complete remission for three years. Prior splenectomy and multiple courses of rituximab. Five days after vaccine, platelets 7 × 109/l with epistaxis |
| 51 | 85 | Female | 4 | No | 61 | P | 7 | 2 | Petechiae | Prednisone | 2 | After first vaccine, increased ecchymoses but thought it was due to warfarin; improved over one week. Two days after the second dose, worse ecchymoses and platelets 7 × 109/l. On warfarin because of atrial fibrillation |
| 52 | 34 | Male | 12 | Mycophenolate | 12 | M | 1 | 3 | Petechiae, ecchymoses | Prednisone, IVIG | 21 | Highly refractory, asplenic patient stable at platelet count of 12 × 109/l with no bleeding for over three years at these counts. Three days after first dose, markedly increased ecchymoses and petechiae; platelets 1 × 109/l |
Unless otherwise stated, all data are median (range). M, Moderna; P, Pfizer; J, Johnson & Johnson; ITP, immune thrombocytopenia; IVIG, intravenous immunoglobulin; DVT, deep vein thrombosis.
There were no thromboses.
Discussion
Following COVID‐19 vaccination, exacerbation of thrombocytopenia occurred in 12% of our chronic ITP patients. Exacerbation of thrombocytopenia usually occurred 2–5 days after the vaccination, was severe with most platelet counts under 10 × 109/l and was associated with bleeding. Upon treatment, mostly with corticosteroids and sometimes other agents, all recovered from their thrombocytopenia within a median of two days. Only one patient had a prolonged course of thrombocytopenia necessitating the institution of rituximab and thrombopoietin receptor agonist therapy. Except for the two patients who were identified after the second exposure to the Pfizer vaccine, none have received a second vaccination dose. Of the six patients with worsening thrombocytopenia, two previously had similar declines in platelet counts with prior vaccinations for Neisseria meningitis and Streptococcus pneumoniae. Compared with the patients who had not developed clinical or laboratory exacerbation of their ITP, there was no apparent association of exacerbation of thrombocytopenia after COVID‐19 vaccination with age, gender, duration of ITP, baseline platelet count, remission status, concurrent ITP therapy, or vaccination type (Table SI).
Although worsening thrombocytopenia after infection is well known to those who take care of ITP patients, exacerbation of thrombocytopenia by infection or vaccination has not been well quantified or the risk factors for such identified.
Since ITP is a disorder of both increased platelet destruction as well as impaired platelet production, 10 the rapid exacerbation of thrombocytopenia by vaccination might occur by either mechanism. Vaccines prepared from dead bacteria or viruses are potent stimulators to the immune system in general and many adjuvants (for example aluminium salts, DNA and lipids) are commonly added to other vaccines to enhance the immune system. 11 , 12 , 13 mRNA is a potent immune activator 14 , 15 as is the nanoparticle lipid component of the mRNA COVID‐19 vaccines. 16 , 17 Recent publications show that mRNA is taken up by cellular RNA receptors leading to the upregulation of toll‐like receptors (TLR) 7 and 8 followed by the activation and maturation of immune cells and secretion of cytokines and chemokines. 14
The goal of the study was to provide ITP patients and their physicians with some guidance as to the frequency of the exacerbation of thrombocytopenia after COVID‐19 vaccination. In so doing, the strength of the study is that it prospectively evaluated all consecutive patients at one ITP centre who were undergoing COVID‐19 vaccination; patients referred to us from other centres because of worsening thrombocytopenia were excluded from this analysis to avoid an overestimate of this adverse reaction. The major weaknesses of the study are several. First, it did not have standardized timing of platelet count assessments so we might have missed some adverse events in that not all patients had platelet counts drawn within 3–7 days after vaccination; most patients did not have platelet counts drawn 14–28 days after vaccination so late events might have been missed. However, all six patients who experienced a significant exacerbation of thrombocytopenia after vaccination also had worsened clinical symptoms and such were not reported in the other 46 patients up to 28 days after vaccination. The second potential weakness is that we might be overestimating the frequency of adverse events since many patients had a heightened focus on this issue given the recent reported death and recommendations from ITP patient support organizations to obtain more than routine platelet counts. A final limitation to this study is that it did not analyze all vaccine types; few patients received the Johnson & Johnson vaccine and none received the AstraZeneca vaccine. Event rates with these two vaccines might be different than with the mRNA vaccines which comprised the majority of vaccines received by patients in this study.
These results suggest that some patients with ITP might have a transient exacerbation of their thrombocytopenia, some with symptoms, within one week of their COVID‐19 vaccination. Thrombocytopenia, in general, was responsive to IVIG and corticosteroids and it remains our recommendation that ITP patients should still undergo at least the initial vaccination for COVID‐19. ITP patients might want to consider obtaining a platelet count 3–7 days before and after vaccination especially if there is a history of thrombocytopenia with prior vaccination, if the patient is receiving anticoagulation, has other increased bleeding risks, or if there is heightened anxiety over the vaccine risk. In those who do have a major exacerbation of their ITP, we are hesitant to administer the second dose of vaccine.
Conflicts of interest
Research: Actelion (Syntimmune), Agios, Alnylam, Amgen, Argenx, Bristol Myers Squibb (BMS), Immunovant, Kezar, Principia, Protalex, Rigel, Takeda (Bioverativ), UCB. Consulting: Actelion (Syntimmune), Agios, Alnylam, Amgen, Argenx, BioCryst, Bristol Myers Squibb (BMS), Caremark, CRICO, Daiichi Sankyo, Dova, Genzyme, Immunovant, Incyte, Kyowa‐Kirin, Merck Sharp Dohme, Momenta, Novartis, Pfizer, Platelet Disorder Support Association, Principia, Protalex, Protalix, Rigel, Sanofi, Genzyme, Shionogi, Shire, Takeda (Bioverativ), UCB, Up‐To‐Date, Zafgen.
Supporting information
Table SI. Association of patient variables with exacerbation of thrombocytopenia.
Acknowledgements
This work was not funded by any agency nor was there any writing support. The study was designed, executed and written by the author. I am pleased to acknowledge the cooperation of all of the ITP patients who participated in this analysis.
References
- 1. David P, Shoenfeld Y. ITP following vaccination. Int J Infect Dis. 2020;99:243–4. [DOI] [PubMed] [Google Scholar]
- 2. Grimaldi‐Bensouda L, Michel M, Aubrun E, Leighton P, Viallard JF, Adoue D, et al. A case‐control study to assess the risk of immune thrombocytopenia associated with vaccines. Blood. 2012;120(25):4938–44. [DOI] [PubMed] [Google Scholar]
- 3. Miller E, Waight P, Farrington CP, Andrews N, Stowe J, Taylor B. Idiopathic thrombocytopenic purpura and MMR vaccine. Arch Dis Child. 2001;84(3):227–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Black C, Kaye JA, Jick H. MMR vaccine and idiopathic thrombocytopaenic purpura. Br J Clin Pharmacol. 2003;55(1):107–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Grady D, Mazzei P. Doctor's death after Covid vaccine is being investigated. The New York Times. 2021.
- 6. Lee EJ, Cines DB, Gernsheimer T, Kessler C, Michel M, Tarantino MD, et al. Thrombocytopenia following Pfizer and Moderna SARS‐CoV‐2 vaccination. Am J Hematol. 2021;96(5):534–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Stowe J, Kafatos G, Andrews N, Miller E. Idiopathic thrombocytopenic purpura and the second dose of MMR. Arch Dis Child. 2008;93(2):182–3. [DOI] [PubMed] [Google Scholar]
- 8. Neunert C, Terrell DR, Arnold DM, Buchanan G, Cines DB, Cooper N, et al. American Society of Hematology 2019 guidelines for immune thrombocytopenia. Blood Adv. 2019;3(23):3829–66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Provan D, Arnold DM, Bussel JB, Chong BH, Cooper N, Gernsheimer T, et al. Updated international consensus report on the investigation and management of primary immune thrombocytopenia. Blood Adv. 2019;3(22):3780–817. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Kuter DJ. The treatment of immune thrombocytopenia (ITP)—focus on thrombopoietin receptor agonists. Ann Blood. 2021;6:7. [Google Scholar]
- 11. Mbow ML, De Gregorio E, Valiante NM, Rappuoli R. New adjuvants for human vaccines. Curr Opin Immunol. 2010;22(3):411–6. [DOI] [PubMed] [Google Scholar]
- 12. Coffman RL, Sher A, Seder RA. Vaccine adjuvants: putting innate immunity to work. Immunity. 2010;33(4):492–503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Awate S, Babiuk LA, Mutwiri G. Mechanisms of action of adjuvants. Front Immunol. 2013;4:114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Edwards DK, Jasny E, Yoon H, Horscroft N, Schanen B, Geter T, et al. Adjuvant effects of a sequence‐engineered mRNA vaccine: translational profiling demonstrates similar human and murine innate response. J Transl Med. 2017;15(1):1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Lorenz C, Fotin‐Mleczek M, Roth G, Becker C, Dam TC, Verdurmen WP, et al. Protein expression from exogenous mRNA: uptake by receptor‐mediated endocytosis and trafficking via the lysosomal pathway. RNA Biol. 2011;8(4):627–36. [DOI] [PubMed] [Google Scholar]
- 16. Ndeupen S, Qin Z, Jacobsen S, Estanbouli H, Bouteau A, Igyártó BZ. The mRNA‐LNP platform’s lipid nanoparticle component used in preclinical vaccine studies is highly inflammatory. bioRxiv. 2021:2021.03.04.430128. [DOI] [PMC free article] [PubMed]
- 17. Thi TTH, Suys EJA, Lee JS, Nguyen DH, Park KD, Truong NP. Lipid‐based nanoparticles in the clinic and clinical trials: from cancer nanomedicine to COVID‐19 vaccines. Vaccines (Basel). 2021;9(4):359. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Table SI. Association of patient variables with exacerbation of thrombocytopenia.


