Short abstract
Coronavirus disease 2019 (COVID‐19) is a new disease whose clinical presentation and potential complications are not yet fully understood. One of these possible reported complications is the onset of subacute (De Quervain) thyroiditis (SAT). Here a series of cases of SAT during the COVID‐19 pandemic are reported, and a possible correlation between these 2 entities is extrapolated.
Keywords: COVID‐19, cytology, fine‐needle aspiration, subacute thyroidits, thyroid
In December 2019, the first case of a pneumonia of an unknown etiology was reported in Wuhan, China. Subsequently, the pathogen was recognized as a new enveloped RNA β‐coronavirus called severe acute respiratory syndrome coronavirus 2 (SARS‐CoV‐2), which causes coronavirus disease 2019 (COVID‐19). 1 The disease then spread across the world quickly, and in March 2020, it was declared a pandemic by the World Health Organization. 2 Since then, heterogeneous clinical developments, ranging from the asymptomatic to the lethal with multiple organ complications, have been observed, and there have been worldwide efforts to better characterize diagnostic and therapeutic methods, risk factors, and possible complications. One of these possible reported complications is the onset of subacute (De Quervain) thyroiditis (SAT). 3 , 4 , 5 , 6 , 7 SAT is a self‐limited inflammatory condition of the thyroid gland that was first described by De Quervain in 1904. The diagnosis is usually made on the basis of clinical data, and the relevant factor is a previous history of viral infection, with influenza, adenovirus, and coxsackievirus being the most common triggers. 8 The thyroid follicles are often infiltrated, and disruption of the basement membrane of the cells occurs. The thyroid injury is thought to be the result of cytolytic T‐cell recognition of viral and cell antigens present in an appropriate complex. 9
In our experience at 2 major services (a cancer center and a private laboratory) in São Paulo, Brazil, one of the countries most severely affected by the pandemic, we documented 3 cases with a confirmed SARS‐CoV‐2 infection and a subsequent onset of SAT. The first case was a 34‐year‐old female who underwent reverse transcriptase–polymerase chain reaction for SARS‐CoV‐2 as an institutional protocol during the preoperative preparation for breast cancer. The patient had only dyspnea on medium exertion for 2 weeks and had no need for hospitalization. Twenty‐eight days after her COVID‐19 diagnosis, she developed mild pain in the anterior cervical region and a mild fever (38 °C). An ultrasound examination of the region was performed, with a hypoechogenic area measuring 1.6 cm × 0.6 cm in the right thyroid lobe. Fine‐needle aspiration (FNA) was performed and confirmed SAT. The patient received prednisone at 15 mg/d, and her symptoms disappeared after 4 days. The second case was an asymptomatic 34‐year‐old female who tested positive for SARS‐CoV‐2 after her husband had a positive test. She also had no symptoms related to SAT, but a screening neck ultrasound 10 days after her diagnosis showed an irregular and hypoechogenic area in her right thyroid lobe, which was confirmed as SAT by FNA. The patient was previously euthyroid but, after the SAT diagnosis, presented with a high free T4 level of 1.8 ng/dL. The patient also received prednisone, and in 18 days, the laboratory tests returned to reference values. The third case was a 39‐year‐old female who presented with only mild symptoms (mild fever and anosmia), which prompted her to be tested for SARS‐CoV‐2. Twenty‐six days after her positive test, she complained of pain in the anterior cervical region, and an ultrasound revealed a solid hypoechogenic nodule in her left thyroid lobe, which was confirmed as SAT by FNA. No information was available for her therapy, but follow‐up ultrasound imaging 2 weeks later was normal.
The cytological pictures of all 3 cases were very similar, and they all showed clusters of epithelioid histiocytes forming loose granulomas and scattered giant cells amid rare inflammatory and follicular cells (Fig. 1). They were compatible with the classic cytological examination, which typically presents granulomas along with many multinucleated giant cells. Cellularity, however, is variable and depends on the stage of the disease. In the early stages, there are more neutrophils and eosinophils, which are reminiscent of acute thyroiditis. The late stages are hypocellular and show giant cells surrounding and engulfing colloid in addition to epithelioid cells, lymphocytes, and scant degenerated follicular cells. 10 , 11
Figure 1.
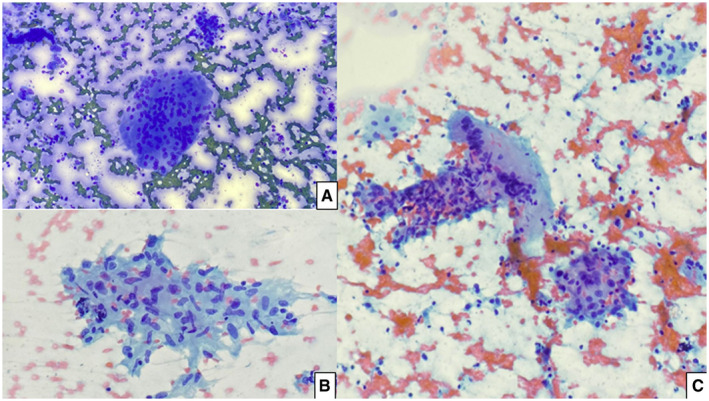
Classic morphology of subacute thyroiditis seen in our cases. (A) Multinucleated giant cell (Diff‐Quik, ×40). (B) Granuloma (Papanicolaou, ×40). (C) Scattered epithelioid histocytes, lymphocytic infiltrate, and a multinucleated giant cell (Papanicolaou, ×20).
In previously reported cases in the literature, similarly to our reported experience, the majority of patients were female with an average age of 35 years (range, 18‐43 years). Patients were mostly treated with corticotherapy for approximately 4 weeks and had good outcomes. 3 , 4 , 5 , 6 , 7 , 12 The average time between the confirmation of infection and FNA was 25 days (range, 9‐42 days). 4 , 5 , 6 , 7 , 12 The most common symptoms included cervical pain with irradiation to the jaw, asthenia, and palpitation. Importantly, these symptoms could be confused with those commonly associated with sepsis, cardiopulmonary dysfunction, or even progression of the respiratory syndrome caused by COVID‐19 7 ; therefore, SAT should be included in the clinical differential diagnosis as a possible complication in these patients.
Although the pathophysiology of possibly COVID‐associated SAT has not yet been fully explored, angiotensin‐converting enzyme 2 (ACE‐2) messenger RNA has been detected recently by reverse transcriptase–polymerase chain reaction in human follicular thyroid cells. Because ACE‐2 is the receptor for the cellular entry of SARS‐CoV‐2, this could partially explain the onset of COVID‐19–related SAT by direct virial tissue damage. 13 As a matter of fact, many changes attributed to SARS‐CoV‐2 infection have been described in several organs besides the respiratory system 14 , 15 , 16 (eg, dermatological and cardiac disorders; kidney, neural, and gastrointestinal disorders 2 , 16 , 17 , 18 ; and diabetogenic effects 19 )
To document a possible increase in the cases of SAT during the pandemic period, we also collected data on all cases of SAT diagnosed via FNA in 2019 and 2020. We observed 11 cases in 2019 (0.04% of total cases) and 15 in 2020 (0.07% of total cases). Although there was a slight increase in number potentialized by the decrease in cases in 2020 due to the lockdown established by local authorities, 20 this difference was not statistically significant. In addition, laboratory confirmation of SARS‐CoV‐2 infection was seen for only 3 of the cases, and this limits a cause‐effect association. Larger future studies with robust correlations between pathological, clinical, and imaging data of COVID‐19–associated SAT, as well as molecular and microbiological confirmation of the virus in the thyroid tissue, are needed to further elucidate its pathophysiology and clarify this possible, intriguing association.
In conclusion, although SAT is a self‐limited inflammatory condition of the thyroid gland with a usually benign course, its symptoms could be confused with those of COVID‐19's worrisome progression. Knowledge of a possible association between these 2 entities is, therefore, of the utmost importance in the current epidemic scenario and could help us to achieve accurate diagnoses and proper therapy for these patients.
Funding Support
No specific funding was disclosed.
Conflict of Interest Disclosures
The authors made no disclosures.
Abreu R, Miguel R, Saieg M. Subacute (De Quervain) thyroiditis during the COVID‐19 pandemic. Cancer Cytopathol. 2021. 10.1002/cncy.22449
References
- 1. Lu R, Zhao X, Li J, et al. Genomic characterisation and epidemiology of 2019 novel coronavirus: implications for virus origins and receptor binding. Lancet. 2020;395:565‐574. doi: 10.1016/S0140-6736(20)30251-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Guan WJ, Ni ZY, Hu Y, et al. Clinical characteristics of coronavirus disease 2019 in China. N Engl J Med. 2020;382:1708‐1720. doi: 10.1056/NEJMoa2002032 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Asfuroglu Kalkan E, Ates I. A case of subacute thyroiditis associated with Covid‐19 infection. J Endocrinol Invest. 2020;43:1173‐1174. doi: 10.1007/s40618-020-01316-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Ruggeri RM, Campennì A, Siracusa M, Frazzetto G, Gullo D. Subacute thyroiditis in a patient infected with SARS‐COV‐2: an endocrine complication linked to the COVID‐19 pandemic. Hormones (Athens). 2021;20:219‐221. doi: 10.1007/s42000-020-00230-w [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Brancatella A, Ricci D, Viola N, Sgrò D, Santini F, Latrofa F. Subacute thyroiditis after SARS‐COV‐2 infection. J Clin Endocrinol Metab. 2020;105:dgaa276. doi: 10.1210/clinem/dgaa276 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Brancatella A, Ricci D, Cappellani D, et al. Is subacute thyroiditis an underestimated manifestation of SARS‐CoV‐2 infection? Insights from a case series. J Clin Endocrinol Metab. 2020;105:dgaa537. doi: 10.1210/clinem/dgaa537 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Mattar SAM, Koh SJQ, Rama Chandran S, Cherng BPZ. Subacute thyroiditis associated with COVID‐19. BMJ Case Rep. 2020;13:e237336. doi: 10.1136/bcr-2020-237336 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Desailloud R, Hober D. Viruses and thyroiditis: an update. Virol J. 2009;6:5. doi: 10.1186/1743-422X-6-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Kojima M, Nakamura S, Oyama T, Sugihara S, Sakata N, Masawa N. Cellular composition of subacute thyroiditis. an immunohistochemical study of six cases. Pathol Res Pract. 2002;198:833‐837. doi: 10.1078/0344-0338-00344 [DOI] [PubMed] [Google Scholar]
- 10. García Solano J, Giménez Bascuñana A, Sola Pérez J, et al. Fine‐needle aspiration of subacute granulomatous thyroiditis (De Quervain's thyroiditis): a clinico‐cytologic review of 36 cases. Diagn Cytopathol. 1997;16:214‐220. doi: [DOI] [PubMed] [Google Scholar]
- 11. Cibas ES, Ali SZ. The 2017 Bethesda System for Reporting Thyroid Cytopathology. Thyroid. 2017;27:1341‐1346. doi: 10.1089/thy.2017.0500 [DOI] [PubMed] [Google Scholar]
- 12. Campos‐Barrera E, Alvarez‐Cisneros T, Davalos‐Fuentes M. Subacute thyroiditis associated with COVID‐19. Case Rep Endocrinol. 2020;2020:8891539. doi: 10.1155/2020/8891539 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Rotondi M, Coperchini F, Ricci G, et al. Detection of SARS‐COV‐2 receptor ACE‐2 mRNA in thyroid cells: a clue for COVID‐19–related subacute thyroiditis. J Endocrinol Invest. 2021;44:1085‐1090. doi: 10.1007/s40618-020-01436-w [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Singer PA. Thyroiditis. Acute, subacute, and chronic. Med Clin North Am. 1991;75:61‐77. doi: 10.1016/s0025-7125(16)30472-2 [DOI] [PubMed] [Google Scholar]
- 15. Oláh R, Hajós P, Soós Z, Winkler G. De Quervain thyroiditis. Corner points of the diagnosis. Article in Hungarian. Orv Hetil. 2014;155:676‐680. doi: 10.1556/OH.2014.29865 [DOI] [PubMed] [Google Scholar]
- 16. Gupta A, Madhavan MV, Sehgal K, et al. Extrapulmonary manifestations of COVID‐19. Nat Med. 2020;26:1017‐1032. doi: 10.1038/s41591-020-0968-3 [DOI] [PubMed] [Google Scholar]
- 17. Gulati A, Pomeranz C, Qamar Z, et al. A comprehensive review of manifestations of novel coronaviruses in the context of deadly COVID‐19 global pandemic. Am J Med Sci. 2020;360:5‐34. doi: 10.1016/j.amjms.2020.05.006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Abobaker A, Raba AA, Alzwi A. Extrapulmonary and atypical clinical presentations of COVID‐19. J Med Virol. 2020;92:2458‐2464. doi: 10.1002/jmv.26157 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Rubino F, Amiel SA, Zimmet P, et al. New‐onset diabetes in Covid‐19. N Engl J Med. 2020;383:789‐790. doi: 10.1056/NEJMc2018688 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Vigliar E, Cepurnaite R, Alcaraz‐Mateos E, et al. Global impact of the COVID‐19 pandemic on cytopathology practice: results from an international survey of laboratories in 23 countries. Cancer Cytopathol. 2020;128:885‐894. doi: 10.1002/cncy.22373 [DOI] [PubMed] [Google Scholar]


