The COVID‐19 pandemic caused by severe acute respiratory syndrome coronavirus 2 (SARS‐CoV‐2) has raised several concerns regarding patients with haematologic and immune‐mediated diseases. This also includes paroxysmal nocturnal haemoglobinuria (PNH), due to its known association with bone marrow failures and to therapy with complement inhibitors in the classic haemolytic form. Moreover, SARS‐CoV‐2 infection has been reported as a possible trigger for haemolytic crises in PNH. 1 Italy has been heavily affected by the COVID‐19 outbreak with two peaks of contagions, the first at the end of March 2020 and the second, much more severe, in mid‐November 2020. As of 12 December 2020 more than 1 825 000 infected cases have been registered in Italy, with a cumulative incidence of 3% (ranging from 1·7% in southern Italy to 4·3% in Lombardy). We conducted a survey on 156 patients with PNH (median age 50 years, range 19–86; 88 females and 68 males) among eight Italian reference centres to evaluate the occurrence and clinical characteristics of SARS‐CoV‐2 infection from the outbreak (24 February 2020) until the time of writing (15 December 2020). Most patients suffered from classic haemolytic PNH (n = 111, 71%), a minority had a concomitant aplastic anaemia (AA) or myelodysplastic syndrome (n = 31, 20%), and the remainder subclinical disease. Regarding therapy, 105 subjects (67·3%) were on stable treatment with complement inhibitors at the time of COVID‐19 outbreak (n = 80 eculizumab, n = 21 ravulizumab, n = 3 crovalimab and n = 1 iptacopan). Comorbidities were present in 35 patients (22·4%), and arterial hypertension was the most frequent (n = 15); other pathologies included solid tumours (n = 6), type‐2 diabetes mellitus (n = 5), ischaemic cardiomyopathy (n = 3), autoimmune diseases (n = 3), atrial fibrillation (n = 2) and chronic kidney disease (n = 2). During the observation period, 101 patients underwent at least one surveillance nasal‐pharyngeal swab (35/101 were regularly swabbed every 2–4 weeks). Four patients were found to be positive, two asymptomatic and two with symptoms (cumulative incidence 2·5%), mostly concomitant to the second wave of infections (Fig 1). The two symptomatic patients experienced only mild disease, and did not require hospital admission. Three out of four patients were on treatment with complement inhibitors, and one experienced breakthrough haemolysis (BTH). No thrombotic complications were observed. Particularly, patient #1 had an estimated high thrombotic risk due to a history of breast cancer and active haemolytic PNH (lactate dehydrogenase [LDH] levels fivefold the upper limit of normal) without complement inhibition therapy because of patient’s choice. Nonetheless, she recovered without complications, receiving only prophylactic heparin. As regards the remaining 152 patients without a demonstrated SARS‐CoV‐2 infection, eight experienced a febrile episode, two haemolytic crises in untreated patients (n = 50), and two a BTH event (101 patients on C5 inhibitor); four patients required transfusion support. This is the first survey on a large cohort of PNH patients (more than half of all Italian patients), suggesting that PNH subjects, either on complement inhibition or not, are not at significantly higher risk of SARS‐CoV‐2 infection compared with the general population. As a matter of fact we could not exclude that some asymptomatic or pauci‐symptomatic PNH cases (fever/mild haemolytic crises) might have SARS‐CoV‐2 infection, since not all patients had been swabbed. This may have resulted in an underestimation of SARS‐CoV‐2 infection in PNH patients. However, this does not bias the comparison with the general population, since extended testing in asymptomatic subjects has only been introduced recently in Italy (October 2020). The strict compliance with basic protective measures against SARS‐CoV‐2 may have played a role, since PNH patients are well educated about infectious risks, particularly if under complement inhibition. These measures also guaranteed a safe continuity of care of PNH patients. Notably, a young male patient on eculizumab, working as medical doctor in a COVID‐19 ward, did not contract the infection. Our findings are in line with previous reports in the literature, consisting of case reports or small case series, which described a mild course of COVID‐19 in PNH patients, especially if under complement inhibition; the only fatal case was in a AA‐associated PNH patient. 2 , 3 Immune activation and inflammation are thought to play an important role in the clinical severity of COVID‐19 pneumonia. The critical and harmful role of complement activation has been previously shown in experimental models of SARS‐CoV and Middle East Respiratory Syndrome (MERS)‐CoV pneumonia, as well as in SARS‐CoV‐2 infection in humans. 4 Consistently, complement inhibition appears to be beneficial both in in vitro models of SARS‐CoV‐2 infection and in in vivo COVID‐19, 5 , 6 since it may be advantageous in reducing thrombo‐inflammation. In conclusion, our results suggest that complement inhibition did not increase SARSCoV‐2 infectious risk in PNH, and may also contribute to smother the clinical severity of COVID‐19.
Fig 1.
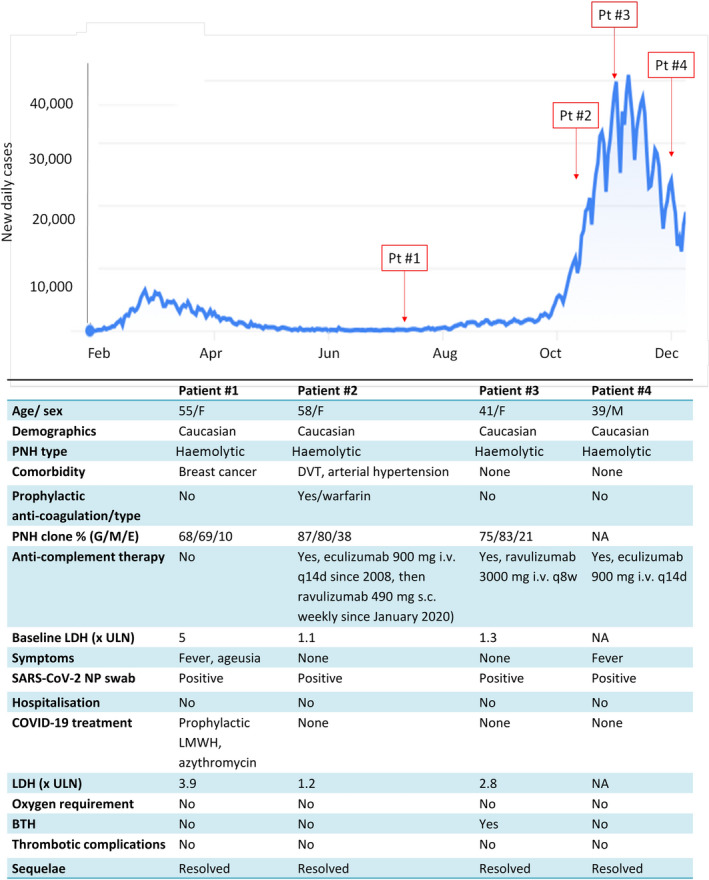
Paroxysmal nocturnal haemoglobinuria (PNH) patients with SARS‐CoV‐2 infection in Italy from the outbreak until the time of writing. The blue line shows the daily number of subjects infected with SARS‐CoV‐2 in Italy. Main clinical characteristics of PNH patients affected by SARS‐CoV‐2 infection are reported in the table. Pt, patient; DVT, deep venous thrombosis; G, granulocyte clone; M, monocyte clone; E, erythrocyte clone (both type II and type III); LDH, lactate dehydrogenase; ULN, upper limit of normal; NP, nasal‐pharyngeal; LMWH, low molecular weight heparin; BTH, breakthrough haemolysis; NA, not available.
Author contributions
WB conceived of the study, analyzed and interpreted the data, wrote the manuscript and followed patients. JAG and BF analyzed and interpreted the data, wrote the manuscript and followed patients. All other authors performed the research, followed patients and critically revised the manuscript.
Conflict of interest
The authors declare no competing financial interests.
References
- 1. Schüller H, Klein F, Lübbert M, Prager EP. Hemolytic crisis in a patient treated with eculizumab for paroxysmal nocturnal hemoglobinuria possibly triggered by SARS‐CoV‐2 (COVID‐19): a case report. Ann Hematol. 2021;100(3):841–2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Kulasekararaj AG, Lazana I, Large J, Posadas K, Eagleton H, Lord Villajin J, et al. Terminal complement inhibition dampens the inflammation during COVID‐19. Br J Haematol. 2020;190(3):e141–e143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Pike A, Muus P, Munir T, Mitchell L, Arnold L, Riley K, et al. COVID‐19 infection in patients on anti‐complement therapy: the Leeds National Paroxysmal Nocturnal Haemoglobinuria service experience. Br J Haematol. 2020;191(1):e1–4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Risitano AM, Mastellos DC, Huber‐Lang M, Yancopoulou D, Garlanda C, Ciceri F, et al. Complement as a target in COVID‐19? Nat Rev Immunol. 2020;20(6):343–4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Yu J, Yuan X, Chen H, Chaturvedi S, Braunstein EM, Brodsky RA. Direct activation of the alternative complement pathway by SARS‐CoV‐2 spike proteins is blocked by factor D inhibition. Blood. 2020;136(18):2080–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. de Latour RP, Bergeron A, Lengline E, Dupont T, Marchal A, Galicier L, et al. Complement C5 inhibition in patients with COVID‐19 ‐ a promising target? Haematologica. 2020;105(12):2847–50. [DOI] [PMC free article] [PubMed] [Google Scholar]


