Dear Editor, Reports from several European countries suggest that COVID‐19 had a profound impact on referral for cancer diagnoses. A Dutch study using their National Cancer Registry reported that skin cancer diagnosis fell by 60% 6 weeks after the first confirmed case of COVID‐19 was identified in the Netherlands, while for other cancer types, rates of diagnosis fell by 26%.1 In the UK, reductions of 56% for referrals of all skin cancers and 53% in diagnoses for skin cancers were reported,2 with similar results found in one Australian state.3
Even though Australia has been spared much of the devastating consequences of the COVID‐19 pandemic relative to other countries (i.e. approximately 29 000 confirmed cases and 909 deaths as of 1 April 2021), the impact of lockdowns changed the way primary care is provided, with a shift to telehealth.4 Data from MedicineInsight – a large general practice database involving general practices from all Australian regions and states5 – and the national Medicare Benefits Schedule (MBS) service were used to explore trends and the impact of the pandemic on the prevalence of recorded skin checks (i.e. screening), recorded skin cancer diagnosis and recorded skin lesion removals.
Deidentified electronic health records from 370 general practices and 241 468 ‘regular’ adult patients (i.e. three or more visits in two consecutive years, with at least one in each of these years; 58·8% female patients, mean age 53·5 ± 19·3 years) within MedicineInsight were used to identify consultations where skin checks were recorded, or a diagnosis of skin cancer was recorded as a diagnosis or encounter reason. We excluded all patients with a diagnosis of skin cancer [i.e. melanoma, basal cell carcinoma (BCC), squamous cell carcinoma (SCC) or nonspecified skin cancer] in the preceding 12 months and thus restricted our analysis to those considered ‘at risk’ of skin cancer. The proportion of recorded skin checks with a positive reported skin cancer diagnosis (i.e. reported skin cancer diagnosis within 6 months of skin check) was then estimated. The prevalence of recorded skin checks (per 1000 patients), reported skin cancer diagnosis (per 1000 patients) and proportion of skin checks that led to a subsequent cancer diagnosis (%) were analysed quarterly (age‐ and sex‐adjusted). MBS data related to claims for skin lesion removals (i.e. items 31356–31376) were extracted for 2017–2020 only, as data were unavailable for previous years.6 MedicineInsight data were analysed in Stata 16·0 (StataCorp, College Station, TX, USA), using the ‘variance covariance (vce) cluster’ method, with practices as clusters.
Between January 2011 and September 2020, a total of 67 933 recorded skin screening checks and 28 762 records of new skin cancer diagnosis (12·7% melanoma, 43·3% BCC, 38·2% SCC, 5·8% nonspecified skin cancer) were identified.
Figure 1a reflects the seasonal pattern of recorded skin cancer diagnosis, i.e. higher rates in quarter one of each year (i.e. summer in the southern hemisphere), decreasing in quarters two and three. The peak of any skin cancer diagnosis in quarter one of 2020 (6·9 per 1000 adults) was 20% lower than the prevalence observed in the same quarter in the three previous years (8·6 per 1000 adults), and remained lower in quarter two of 2020. A similar pattern was observed for BCC and SCC, with a greater reduction for melanoma (32%). Figure 1b shows that, compared with previous years, the expected peak of screening checks in quarter one of 2020 did not occur. A 29% decrease in the rate of skin checks was identified in the second quarter of 2020 compared with the second quarter of 2019, which coincided with 14% fewer MBS claims for skin lesion removals than the same period for the previous year. Nonetheless, the proportion of patients with a recorded skin cancer diagnosis after screening remained steady in 2020.
Figure 1.
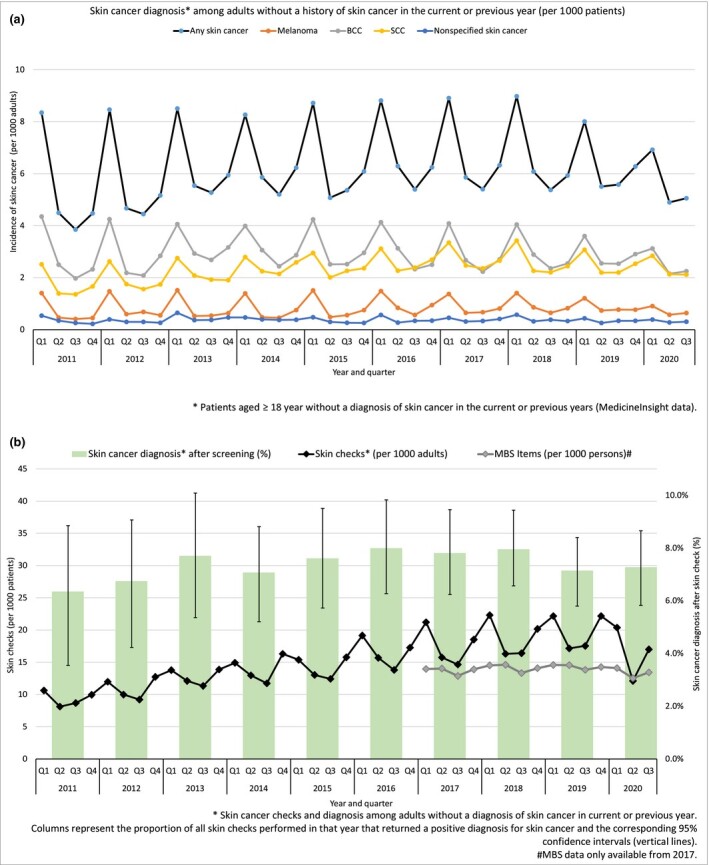
(a) Skin cancer diagnosis recorded by skin cancer type in ‘at risk’ patients. (b) Skin checks recorded per 1000 patients by quarter for 2011 to 2020, Medicare Benefits Schedule (MBS) items claimed per 1000 persons enrolled in Medicare, and skin cancer diagnosis recorded within 6 months after the screening diagnosis.
MBS data reflects the period in which the service was claimed and not the period in which the service was performed.
The reduction in skin checks performed by general practitioners in Australia could account for the reduction in melanoma notifications (and, by extrapolation, a similar reduction in skin checks could account for the reductions noted in England).2 In Australia, these checks peak in the late summer months, but COVID‐related changes affected this pattern in 2020, reducing the number of skin cancer diagnoses. Apart from the personal impact, delayed diagnosis can have a profound impact on health cost, as the average annual cost of melanoma increases from AU$1681 per patient for stage 0–II to AU$115 109 for stage III unresectable/stage IV.7 Although the total number of general practice consultations in Australia remained steady in 2020, these consultations rapidly switched from face‐to‐face to telephone consultations4 – an approach that may be permanent because telehealth is now government‐funded. Therefore, the potential negative impact of telehealth on skin cancer diagnosis requires monitoring, as poor image quality of photographs obtained by patients8 and missed opportunistic skin checks during face‐to‐face consultations for another reason can undermine the identification of malignant lesions. Dermatologists and general practitioners should work together to ensure adequate case finding and opportunistic skin cancer screening.
Acknowledgments
We are grateful to the general practices and general practitioners that participate in MedicineInsight, and the patients who allow the use of their deidentified information. Access to the data for this study was approved by the MedicineInsight Data Governance Committee (project 2020‐011).
Author Contribution
Jacqueline Roseleur: Conceptualization (equal); Formal analysis (equal); Methodology (equal); Writing‐original draft (equal); Writing‐review & editing (equal). David Gonzalez‐Chica: Conceptualization (equal); Formal analysis (equal); Methodology (equal); Writing‐original draft (equal); Writing‐review & editing (equal). Jon Emery: Writing‐review & editing (equal). Nigel Stocks: Conceptualization (equal); Data curation (lead); Writing‐review & editing (equal).
Contributor Information
J. Roseleur, Discipline of General Practice Adelaide Medical School Adelaide SAAustralia
D.A. Gonzalez‐Chica, Discipline of General Practice Adelaide Medical School Adelaide SAAustralia Adelaide Rural Clinical School The University of Adelaide Adelaide SA Australia.
J. Emery, Centre for Cancer Research and Department of General Practice University of Melbourne Melbourne VIC Australia
N.P. Stocks, Discipline of General Practice Adelaide Medical School Adelaide SAAustralia
References
- Dinmohamed AG, Visser O, Verhoeven RHA et al. Fewer cancer diagnoses during the COVID‐19 epidemic in the Netherlands. Lancet Oncol 2020; 21:750–1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Earnshaw CH, Hunter HJA, McMullen E et al. Reduction in skin cancer diagnosis, and overall cancer referrals, during the COVID‐19 pandemic. Br J Dermatol 2020; 183:792–4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Te Marvelde L, Wolfe R, McArthur G et al. Decline in cancer pathology notifications during the 2020 COVID‐19‐related restrictions in Victoria. Med J Aust 2021; 214:281–3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Snoswell CL, Caffery LJ, Haydon HM et al. Telehealth uptake in general practice as a result of the coronavirus (COVID‐19) pandemic. Aust Health Rev 2020; 44:737–40. [DOI] [PubMed] [Google Scholar]
- Busingye D, Gianacas C, Pollack A et al. Data resource profile: MedicineInsight, an Australian national primary health care database. Int J Epidemiol 2019; 48:1741–h. [DOI] [PubMed] [Google Scholar]
- Services Australia. Medicare Item Reports, vol. 2, March 2021. Canberra: Australian Government, 2021. [Google Scholar]
- Elliott TM, Whiteman DC, Olsen CM, Gordon LG. Estimated healthcare costs of melanoma in Australia over 3 years post‐diagnosis. Appl Health Econ Health Policy 2017; 15:805–16. [DOI] [PubMed] [Google Scholar]
- Freeman K, Dinnes J, Chuchu N et al. Algorithm based smartphone apps to assess risk of skin cancer in adults: systematic review of diagnostic accuracy studies. BMJ 2020; 368:m127. [DOI] [PMC free article] [PubMed] [Google Scholar]


