Abstract
Heroin intake decreases markedly during proestrus in normally cycling female rats; however, it is not known whether estradiol, progesterone, or both hormones are responsible for these decreases in heroin intake. The purpose of the present study was to examine the roles of estradiol and progesterone in heroin intake by artificially inducing a proestrus state in ovariectomized rats. To this end, ovariectomized female rats were implanted with intravenous catheters and trained to self-administer heroin (0.0075 mg/kg/infusion) on a fixed ratio (FR1) schedule of reinforcement. After one week of training, rats were tested at weekly intervals with estradiol (0.005 mg, sc) or vehicle 22 hr before a test session and progesterone (0.125 mg, sc) or vehicle 0.5 hr before a test session to artificially mimic the naturally occurring hormone concentrations characteristic of late proestrus. Administration of estradiol 22 hr prior to testing and progesterone 0.5 hr prior to testing significantly reduced heroin intake relative to the previous training day and vehicle control. Interestingly, this same effect was observed when only estradiol, but not progesterone, was administered. These data suggest that estradiol but not progesterone is responsible for the proestrus-induced decreases in heroin intake previously reported in normally cycling female rats. These findings differ from those reported previously with stimulants and suggest that estrogen-based pharmacotherapies may be of value in women with opioid use disorder.
Keywords: estradiol, female, opioid, proestrus, progesterone, rats, self-administration
We previously reported that heroin intake decreases markedly (~70%) during proestrus in normally cycling female rats (Lacy et al., 2016). This effect was replicated in individual rats across 4–7 estrous cycles and observed over a 100-fold dose range of heroin. Circulating concentrations of estradiol and progesterone reach their peak during proestrus, with estradiol reaching a peak 8–24 hr prior to progesterone (Freeman, 1994; Smith et al., 1975). In our original study, testing took place during late proestrus, when estradiol levels are falling and progesterone levels are just beginning to peak; however, it was not known whether estradiol, progesterone, or both hormones were responsible for the observed effects on heroin intake. The purpose of this study was to determine the role of estradiol and progesterone in proestrus-induced decreases in heroin intake by artificially inducing proestrus in ovariectomized rats. To mimic hormone concentrations during late proestrus, ovariectomized rats were administered estradiol (or vehicle) 22 hr prior to, and progesterone (or vehicle) 0.5 hr prior to, a heroin self-administration test.
Methods
Animals
Female, Long-Evans rats were ovariectomized by the vender (Charles River Laboratories, Raleigh, NC, USA) at 42 days of age and arrived at the laboratory at 49 days of age. Upon arrival, all rats were housed individually in a colony room on a 12-hr light/dark cycle (lights on: 0500). Food and drinking water were freely available in the home cage, except during a brief period of lever-press training (see below). All rats were maintained in accordance with the guidelines of the Animal Care and Use Committee of Davidson College.
Apparatus
Heroin self-administration training and testing took place in operant conditioning chambers from Med Associates, Inc. (St. Albans, VT, USA). Each chamber contained a houselight, two response levers, two white stimulus lights located above the two levers, a food receptacle located between the two levers, a pellet dispenser located behind the forward wall, and an infusion pump located outside the chamber.
Lever-Press Training
Approximately one week after arrival, rats were restricted to approximately 90% of their free-feeding body weight and trained to press a response lever using food reinforcement. In these sessions, each lever press was reinforced on a fixed ratio (FR1) schedule. Sessions terminated automatically once 40 reinforcers were delivered or 2 hr elapsed, whichever occurred first. Training continued in this manner until 40 reinforcers were obtained in four nonconsecutive sessions.
Catheter Surgery
Approximately two weeks after arrival, rats were anesthetized with a combination of ketamine (100 mg/kg, ip) and xylazine (8.0 mg/kg, ip) and surgically implanted with intravenous catheters as described previously (Smith et al. 2008). Rats were given Ketoprofen (3.0 mg/kg, sc) after surgery as a post-operative analgesic and wounds were treated with a topical antibiotic for two days. Catheters were flushed daily with ticarcillin (20 mg/kg, iv) and heparinized saline to prevent infection and maintain patency, respectively. After seven days, ticarcillin was discontinued and only heparinized saline was used to maintain patency.
Self-Administration Training
Approximately three weeks after arrival and one week after surgery, rats were placed in operant conditioning chambers and trained to self-administer heroin on an FR1 schedule of reinforcement. Each session began with illumination of the houselight, illumination of the stimulus light above the active response lever, and a noncontingent infusion of heroin. Each lever press produced an infusion of heroin (0.0075 mg/kg/infusion) that was followed by a 20-s timeout in which the stimulus light was turned off and responding had no programmed consequences. All sessions were 2 hr in duration and no limit was placed on the maximum number of infusions that could be obtained. Rats were returned to their home cages immediately after the training session. Training continued in this manner for five consecutive days, Monday through Friday.
Self-Administration Testing
Testing took place over the course of four weeks, beginning four weeks after arrival and one week after the initiation of self-administration training. Throughout this period, training sessions continued in the manner described above Monday through Thursday. Test sessions were always conducted on Fridays following administration of estradiol (or vehicle) 22 hr before the session and progesterone (or vehicle) 0.5 hr before the session. Specifically, estradiol (0.005 mg, sc) or vehicle (peanut oil, sc) was administered immediately after the training session on Thursday, and thus 22 hr prior to the test session on Friday. Progesterone (0.125 mg, sc) or vehicle (peanut oil, sc ) was administered 30 min before the test session on Friday to mimic the hormonal concentrations of late proestrus (i.e., falling estradiol and rising progesterone). All test sessions were otherwise identical to training sessions. The order of estradiol (or vehicle) and progesterone (or vehicle) tested was counterbalanced across rats. Rats that lost catheter patency before completing all tests were removed from the study and did not contribute to the data analysis. Fourteen rats began self-administration training and five rats lost catheter patency before the end of testing. A total of nine rats completed the study and were included in the data analysis. Inactive lever responses were recorded but had no programmed consequences.
Data Analysis
In the primary analysis, data were expressed as the change in heroin intake (measured by the difference in the number of infusions) relative to the previous training session. Differences in heroin intake relative to the previous training session were considered statistically significant if 95% confidence limits did not overlap 0. In a secondary analysis, data were expressed as number of infusions and were analyzed via two-way, repeated-measures ANOVA, with estradiol and progesterone serving as factors. Post hoc tests were then conducted via paired-samples t-tests with the Holms-Bonferroni correction for multiple comparisons. All statistical tests were two-tailed, and the alpha level was set to .05. Responses on the inactive lever were analyzed in a similar manner.
Results
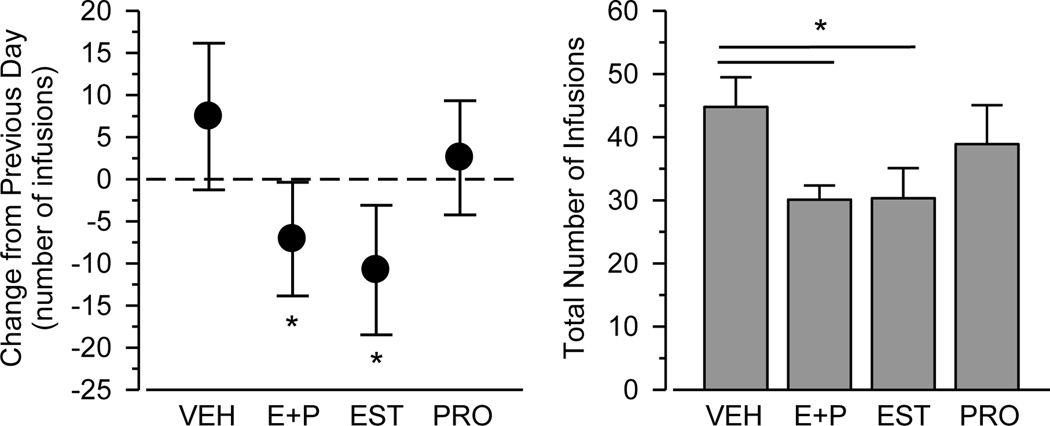
In the primary analysis (Figure 1A), estradiol (- 22 hr) followed by progesterone (- 0.5 hr) significantly decreased heroin intake relative to the previous training day, thus mimicking the proestrus-induced decreases in heroin intake reported previously in intact rats. Interestingly, estradiol (- 22 hr) followed by vehicle (- 0.5 hr) also significantly decreased heroin intake relative to the previous training day, and the magnitude of this effect was similar to that observed with estradiol + progesterone. In contrast, progesterone did not alter heroin intake when administered in the absence of estradiol.
Figure 1.
Heroin intake during a test session as measured by change in number of infusions from previous training day (Figure 1A, left) and total number of infusions during the test session (Figure 1B, right). Horizontal axes depict testing condition: vehicle + vehicle (VEH), estradiol + progesterone (E+P), estradiol + vehicle (EST), and vehicle + progesterone (PRO). Vertical lines indicated 95% confidence limits (left) and SEM (right). Asterisks indicate significant difference from previous day (left) and vehicle control (right).
Similar effects were apparent in the secondary analysis (Figure 1B). A repeated-measures ANOVA revealed a main effect of estradiol [F(1, 8) = 7.846, p = .023), but there was neither a significant effect of progesterone nor a significant estradiol x progesterone interaction. Paired t-tests corrected for multiple pairwise comparisons revealed that heroin intake was significantly lower in both the estradiol + progesterone and estradiol only conditions relative to vehicle control (p’s < .05).
Responses on the inactive lever were approximately half of that observed on the active lever (Supplemental File 1). Importantly, no differences were observed in the number of inactive levers responses across conditions.
Discussion
The main finding of this study is that administering estradiol and progesterone in a temporal pattern that mimics concentrations found during late proestrus decreases heroin intake in ovariectomized rats in a manner similar to that reported previously during proestrus in intact rats. One unexpected finding of this study is that these same effects are produced by administering estradiol alone, and that progesterone does not alter the magnitude of this effect. These data suggest that estradiol, and not progesterone, is responsible for the proestrus-induced decreases in heroin intake reported previously.
In our original study (Lacy et al., 2016), the effects of proestrus were robust (~ 70%) and replicable in individual animals across 4–7 estrous cycles. Moreover, the magnitude of the effect was similar in both high and low responding rats. The effect was observed over a 100-fold dose range of heroin, resulting in a downward shift in the dose-effect curve. Finally, the effect was independent of housing condition and observed in females housed individually, females housed with another female, and females housed with a male (note: rats were separated by a wire screen and not allowed to mate).
Estradiol, administered either alone or in combination with progesterone in the present study, decreased heroin intake by ~35% relative to control, which is half of that observed during natural proestrus. A number of factors inherent to the model may have contributed to the differences between natural and artificially induced proestrus, including compensatory changes in estrogen receptors and/or downstream effectors following ovariectomy. Alternatively, the difference could be due to a failure to reproduce completely the hormonal milieu during proestrus.
Circulating concentrations of estradiol and progesterone have been well characterized across the estrous cycle (e.g., Freeman, 1994; Smith et al., 1975). These hormones follow a diurnal pattern with estradiol peaking 8–24 hr before progesterone. Importantly, progesterone peaks during late proestrus at the beginning of the dark (active) cycle. In Lacy et al. (2016), test sessions were conducted immediately at the onset of the dark cycle during late proestrus. An abundance of data suggest that estradiol concentrations were falling and progesterone concentrations were just reaching their peak in both this and our previous study.
The selected dose of estradiol for this study produces serum concentrations similar to that naturally observed during proestrus (Hu et al., 2004), and the selected dose of progesterone reliably induces sexual receptivity in ovariectomized rats primed with estradiol (White & Uphouse, 2004). Both doses have been used previously in ovariectomized rats to examine their effects on cocaine self-administration (Jackson et al., 2006). A bolus administration of estradiol has a half-life of 8–13 hours and is cleared within 72 hours in ovariectomized rats, and a bolus administration of progesterone is rapidly absorbed and reaches maximum serum concentrations in less than 1 hr in ovariectomized rats (Petroff & Mizinga, 2003). Importantly, similar doses and temporal patterns of estradiol and progesterone administration have been used to induce sexually receptive states characteristic of late proestrus in ovariectomized rats (e.g., Herath et al., 2001; Bivens & Olster, 1997; Saito, 1987; Witcher & Freeman, 1985). Although we are confident that we tested during a time of falling estradiol and peaking progesterone concentrations in the present study, we cannot be confident that brain tissue concentrations were equivalent to that found during the natural proestrus state, nor can we be confident that the model adequately reproduced the biologically active precursors and metabolites of these hormones found in intact animals. Both possibilities likely contributed to the diminished effect observed in this model relative to the intact organism.
The primary limitation of the present study is that only a single dose of heroin was tested. We chose this dose on the basis of our previous study in which the effects of proestrus on heroin intake at this dose were replicated both within and across individual rats (Lacy et al., 2016). Moreover, in that study, we observed proestrus-induced decreases in heroin intake across a 100-fold range of heroin doses, resulting in downward shifts in both the ascending and descending limbs of the dose-effect curve. The downward shift in the dose-effect curve in the previous study indicates that the efficacy (rather than merely the potency) of heroin to serve as a positive reinforcer was reduced during proestrus. We similarly assume that estradiol reduced the efficacy of heroin to serve as a positive reinforcer in the present study; however, additional tests with a range of heroin doses that capture both the ascending and descending limbs of the dose-effect curve are necessary to confirm this possibility. Alternatively, other schedules of reinforcement that are better suited to measure changes in motivational strength (e.g., progressive ratio, second order) or that are less sensitive to nonspecific motoric effects (e.g., concurrent) could be used to confirm assumptions about reinforcing efficacy.
The finding that estradiol but not progesterone decreases heroin intake differs from that reported previously for stimulants. For instance, cocaine intake and cocaine seeking increase during the estrus phase of the estrous cycle when estradiol levels are high relative to progesterone (Feltenstein & See, 2007; Roberts et al., 1989). Moreover, estradiol increases cocaine self-administration and cocaine-primed reinstatement following ovariectomy (Jackson et al., 2006; Larson et al., 2005; Yang et al., 2007), whereas progesterone reverses both of these effects (Jackson et al., 2006; Anker et al., 2007). In freely cycling female rats, progesterone concentrations are negatively correlated with cocaine seeking (Feltenstein & See, 2007), and exogenous progesterone decreases cocaine-primed reinstatement during estrus (Feltenstein et al., 2009).
Previous studies examining the effects of estradiol and progesterone on heroin intake have been somewhat equivocal. Early studies reported that estradiol either increases the acquisition of heroin self-administration (Roth et al., 2002) or does not alter heroin self-administration (Stewart et al., 1996). More recent studies, however, have reported that estradiol decreases heroin-seeking during extinction (Vazquez et al., 2020) and heroin-seeking during food restriction (Sedki et al., 2015). Taken with the present data and those of Lacy et al., (2016), such findings suggest that estradiol and progesterone influence heroin intake in a manner differently from their influence on stimulant intake.
Estradiol administered either alone or in combination with progesterone did not decrease inactive lever responding. Estradiol also does not decrease responding maintained by other drugs (e.g., cocaine, see above) or by nondrug reinforcers (e.g., food, Rodriguez-Sierra et al., 1984). Such findings suggest that the ability of estradiol to decrease responding maintained by heroin is not due to nonspecific motoric effects or to general decreases in motivated behavior.
In conclusion, administration of estradiol and progesterone in a temporal pattern that mimics their concentrations during late proestrus significantly decreased heroin intake in ovariectomized rats. Interestingly, these effects were reproduced when estradiol but not progesterone was administered alone, suggesting that estradiol was responsible for these effects. These effects differ from those reported previously for stimulants, indicating that hormonal regulation of drug intake varies across pharmacological class. These data also suggest that estrogen-based pharmacotherapies should be explored in women with heroin use disorders.
Supplementary Material
Public Health Significance:
This study demonstrates that estradiol decreases heroin intake, suggesting that estrogen-based pharmacotherapies may be beneficial to women with opioid use disorders.
Acknowledgements:
The authors thank Kenzie Potter for expert technical assistance and the National Institute on Drug Abuse for supplying the study drug.
Role of Funding Source: This work was supported by NIH Grants DA045364, DA031725, and DA045714. The NIH had no role in the writing of the manuscript or in the decision to submit the manuscript for publication.
Footnotes
Conflict of Interest: No conflict declared.
References
- Anker JJ, Larson EB, Gliddon LA, & Carroll ME (2007). Effects of progesterone on the reinstatement of cocaine-seeking behavior in female rats. Experimental and clinical psychopharmacology, 15(5), 472. [DOI] [PubMed] [Google Scholar]
- Bivens CLM, & Olster DH (1997). Abnormal estrous cyclicity and behavioral hyporesponsiveness to ovarian hormones in genetically obese Zucker female rats. Endocrinology, 138(1), 143–148. [DOI] [PubMed] [Google Scholar]
- Feltenstein MW, & See RE (2007). Plasma progesterone levels and cocaine-seeking in freely cycling female rats across the estrous cycle. Drug and alcohol dependence, 89(2–3), 183–189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Feltenstein MW, Byrd EA, Henderson AR, & See RE (2009). Attenuation of cocaine-seeking by progesterone treatment in female rats. Psychoneuroendocrinology, 34(3), 343–352. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Freeman ME (1994). The neuroendocrine control of the ovarian cycle of the rat. The physiology of reproduction, 613–658. [Google Scholar]
- Herath CB, Watanabe G, Katsuda SI, Yoshida M, Suzuki AK, & Taya K. (2001). Exposure of neonatal female rats to p-tert-octylphenol disrupts afternoon surges of luteinizing hormone, follicle-stimulating hormone and prolactin secretion, and interferes with sexual receptive behavior in adulthood. Biology of reproduction, 64(4), 1216–1224. [DOI] [PubMed] [Google Scholar]
- Hu M, Crombag HS, Robinson TE, & Becker JB (2004). Biological basis of sex differences in the propensity to self-administer cocaine. Neuropsychopharmacology : official publication of the American College of Neuropsychopharmacology, 29(1), 81–85. 10.1038/sj.npp.1300301 [DOI] [PubMed] [Google Scholar]
- White S, & Uphouse L. (2004). Estrogen and progesterone dose-dependently reduce disruptive effects of restraint on lordosis behavior. Hormones and behavior, 45(3), 201–208. 10.1016/j.yhbeh.2003.10.001 [DOI] [PubMed] [Google Scholar]
- Petroff BK, & Mizinga KM (2003). Pharmacokinetics of ovarian steroids in Sprague-Dawley rats after acute exposure to 2,3,7,8-tetrachlorodibenzo-p-dioxin (TCDD). Reproductive biology, 3(2), 131–141. [PubMed] [Google Scholar]
- Jackson LR, Robinson TE, & Becker JB (2006). Sex differences and hormonal influences on acquisition of cocaine self-administration in rats. Neuropsychopharmacology, 31(1), 129–138. [DOI] [PubMed] [Google Scholar]
- Lacy RT, Strickland JC, Feinstein MA, Robinson AM, & Smith MA (2016). The effects of sex, estrous cycle, and social contact on cocaine and heroin self-administration in rats. Psychopharmacology, 233(17), 3201–3210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Larson EB, Roth ME, Anker JJ, & Carroll ME (2005). Effect of short-vs. long-term estrogen on reinstatement of cocaine-seeking behavior in female rats. Pharmacology Biochemistry and Behavior, 82(1), 98–108. [DOI] [PubMed] [Google Scholar]
- Petroff BK, & Mizinga KM (2003). Pharmacokinetics of ovarian steroids in Sprague-Dawley rats after acute exposure to 2,3,7,8-tetrachlorodibenzo-p-dioxin (TCDD). Reproductive biology, 3(2), 131–141. [PubMed] [Google Scholar]
- Roberts DCS, Bennett SAL, & Vickers GJ (1989). The estrous cycle affects cocaine self-administration on a progressive ratio schedule in rats. Psychopharmacology, 98(3), 408–411. [DOI] [PubMed] [Google Scholar]
- Rodriguez-Sierra JF, Howard JL, Pollard GT, & Hendricks SE (1984). Effect of ovarian hormones on conflict behavior. Psychoneuroendocrinology, 9(3), 293–300. 10.1016/0306-4530(84)90008-8 [DOI] [PubMed] [Google Scholar]
- Roth ME, Casimir AG, & Carroll ME (2002). Influence of estrogen in the acquisition of intravenously self-administered heroin in female rats. Pharmacology Biochemistry and Behavior, 72(1–2), 313–318. [DOI] [PubMed] [Google Scholar]
- Saito TR (1987). Copulatory Behavior of Male Rats Paired with Natural Proestrous and Hormone-treated Ovariectomized Females. Experimental Animals, 36(1), 91–93. [DOI] [PubMed] [Google Scholar]
- Sedki F, Gregory JG, Luminare A, D’Cunha TM, & Shalev U. (2015). Food restriction-induced augmentation of heroin seeking in female rats: manipulations of ovarian hormones. Psychopharmacology, 232(20), 3773–3782. [DOI] [PubMed] [Google Scholar]
- Smith MA, Schmidt KT, Iordanou JC, & Mustroph ML (2008). Aerobic exercise decreases the positive-reinforcing effects of cocaine. Drug and alcohol dependence, 98(1–2), 129–135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith MS, Freeman ME, & Neill JD (1975). The control of progesterone secretion during the estrous cycle and early pseudopregnancy in the rat: prolactin, gonadotropin and steroid levels associated with rescue of the corpus luteum of pseudopregnancy. Endocrinology, 96(1), 219–226. [DOI] [PubMed] [Google Scholar]
- Stewart J, Woodside B, & Shaham Y. (1996). Ovarian hormones do not affect the initiation and maintenance of intravenous self-administration of heroin in the female rat. Psychobiology, 24(2), 154–159. [Google Scholar]
- Vazquez M, Frazier JH, Reichel CM, & Peters J. (2020). Acute ovarian hormone treatment in freely cycling female rats regulates distinct aspects of heroin seeking. Learning & Memory, 27(1), 6–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- White S, & Uphouse L. (2004). Estrogen and progesterone dose-dependently reduce disruptive effects of restraint on lordosis behavior. Hormones and behavior, 45(3), 201–208. 10.1016/j.yhbeh.2003.10.001 [DOI] [PubMed] [Google Scholar]
- Witcher JA, & Freeman ME (1985). The proestrous surge of prolactin enhances sexual receptivity in the rat. Biology of reproduction, 32(4), 834–839. [DOI] [PubMed] [Google Scholar]
- Yang H, Zhao W, Hu M, & Becker JB (2007). Interactions among ovarian hormones and time of testing on behavioral sensitization and cocaine self-administration. Behavioural brain research, 184(2), 174–184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hu M, Crombag HS, Robinson TE, & Becker JB (2004). Biological basis of sex differences in the propensity to self-administer cocaine. Neuropsychopharmacology : official publication of the American College of Neuropsychopharmacology, 29(1), 81–85. 10.1038/sj.npp.1300301 [DOI] [PubMed] [Google Scholar]
- White S, & Uphouse L. (2004). Estrogen and progesterone dose-dependently reduce disruptive effects of restraint on lordosis behavior. Hormones and behavior, 45(3), 201–208. 10.1016/j.yhbeh.2003.10.001 [DOI] [PubMed] [Google Scholar]
- Petroff BK, & Mizinga KM (2003). Pharmacokinetics of ovarian steroids in Sprague-Dawley rats after acute exposure to 2,3,7,8-tetrachlorodibenzo-p-dioxin (TCDD). Reproductive biology, 3(2), 131–141. [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.