Abstract
Aims
Viral‐induced cardiac inflammation can induce heart failure with preserved ejection fraction (HFpEF)‐like syndromes. COVID‐19 can lead to myocardial damage and vascular injury. We hypothesised that COVID‐19 patients frequently develop a HFpEF‐like syndrome, and designed this study to explore this.
Methods and results
Cardiac function was assessed in 64 consecutive, hospitalized, and clinically stable COVID‐19 patients from April–November 2020 with left ventricular ejection fraction (LVEF) ≥50% (age 56 ± 19 years, females: 31%, severe COVID‐19 disease: 69%). To investigate likelihood of HFpEF presence, we used the HFA‐PEFF score. A low (0–1 points), intermediate (2–4 points), and high (5–6 points) HFA‐PEFF score was observed in 42%, 33%, and 25% of patients, respectively. In comparison, 64 subjects of similar age, sex, and comorbidity status without COVID‐19 showed these scores in 30%, 66%, and 4%, respectively (between groups: P = 0.0002). High HFA‐PEFF scores were more frequent in COVID‐19 patients than controls (25% vs. 4%, P = 0.001). In COVID‐19 patients, the HFA‐PEFF score significantly correlated with age, estimated glomerular filtration rate, high‐sensitivity troponin T (hsTnT), haemoglobin, QTc interval, LVEF, mitral E/A ratio, and H2FPEF score (all P < 0.05). In multivariate, ordinal regression analyses, higher age and hsTnT were significant predictors of increased HFA‐PEFF scores. Patients with myocardial injury (hsTnT ≥14 ng/L: 31%) vs. patients without myocardial injury, showed higher HFA‐PEFF scores [median 5 (interquartile range 3–6) vs. 1 (0–3), P < 0.001] and more often showed left ventricular diastolic dysfunction (75% vs. 27%, P < 0.001).
Conclusion
Hospitalized COVID‐19 patients frequently show high likelihood of presence of HFpEF that is associated with cardiac structural and functional alterations, and myocardial injury. Detailed cardiac assessments including echocardiographic determination of left ventricular diastolic function and biomarkers should become routine in the care of hospitalized COVID‐19 patients.
Keywords: COVID‐19, Diastolic dysfunction, HFA‐PEFF, High‐sensitivity troponin T, NT‐proBNP
Introduction
Coronavirus disease 2019 (COVID‐19) is known to lead to myocardial damage and vascular injury in many patients. We hypothesised that a substantial proportion of patients with COVID‐19 develop heart failure with preserved ejection fraction (HFpEF), and designed this study for further investigation.
The clinical manifestations of COVID‐19 disease range from none or mild symptoms to acute respiratory distress syndrome and death. 1 Despite respiratory symptoms, patients also present with chest pain, arrhythmias, palpitations, severe peripheral oedema and acute heart failure. 2 , 3 In particular, COVID‐19 patients with cardiac disease compared to patients without cardiac disease more often have thromboembolic events 4 and demonstrate a higher mortality. 5 , 6 In May 2020, Tavazzi et al. 7 reported the first case of acute cardiac injury with the finding of severe acute respiratory syndrome coronavirus 2 (SARS‐CoV‐2) particles and low‐grade inflammation within the myocardium but not accompanied by cardiomyocyte necrosis. Whether myocardial alterations are caused by direct viral damage to the heart or vasculature or by infection‐related cytokine storm is still under debate. 8 Principally, viral‐induced cardiac inflammatory alterations are known to be able to trigger myocarditis‐induced HFpEF and heart failure with reduced ejection fraction. 9 There is growing interest in identifying COVID‐19 patients at risk of developing viral‐related heart failure and cardiovascular (CV) impairments. 10
Methods
Patients
From April to November 2020, we prospectively enrolled clinically stable COVID‐19 patients at one of our COVID‐19 wards (at the Department of Internal Medicine/Infectious Diseases and Pulmonary Medicine, Charité – Universitätsmedizin, Campus Virchow‐Klinikum, Berlin, Germany) into the observational cohort study Pa‐COVID‐19. 11 All patients gave written informed consent. The study was approved by the ethics committee of the Charité – Universitätsmedizin Berlin (EA2/066/20) and conducted in accordance with the Declaration of Helsinki.
We consecutively recruited 71 clinically stable COVID‐19 patients in the first days following their admission to one of our COVID‐19 wards at the Charité [average number of days since first symptoms until echocardiography and biomarker assessment 11 days (interquartile range, IQR 5–16)]. Six patients were found to have a reduced systolic left ventricular (LV) function [LV ejection fraction (LVEF) <50%, mean LVEF 35 ± 6%] and were therefore excluded from this analysis. Three patients had a transthoracic echocardiogram in the 12 months before their SARS‐CoV‐2 infection, with one patient showing pre‐existing right ventricular dysfunction [tricuspid annular plane systolic excursion (TAPSE) 12 mm, right ventricular systolic excursion velocity (RV S') 0.06 m/s] with LVEF 50%, who was therefore excluded from the analysis. The final study cohort reported on here consists of 64 COVID‐19 patients with an LVEF ≥50% without prior known heart failure.
To compare the HFA‐PEFF scores of the COVID‐19 patients with that of patients without COVID‐19, we included a control group of 64 patients from the Massachusetts General Hospital Cardiopulmonary Exercise Testing cohort. Controls were consecutive patients with exertional dyspnoea and preserved ejection fraction (LVEF ≥50%) but without a prior diagnosis of heart failure who were referred for clinically‐indicated cardiopulmonary exercise testing and also had available echocardiographic and biomarker assessments to calculate HFA‐PEFF scores. 12 From this sample of 121 individuals, we group‐matched 64 controls for sex (primary matching criterion), age, and comorbidity distribution with the COVID‐19 patient cohort. All patients gave written informed consent. The study was approved by the Massachusetts General Hospital's institutional review board (2010P001704) and conducted in accordance with the Declaration of Helsinki.
Investigations
All patients received a standard blood sample from an antecubital vein, and a 12‐channel resting electrocardiogram (ECG) and a transthoracic echocardiography (VIVID E95, GE Healthcare) were performed. Data on patients' clinical condition, comorbidities and drug therapy were collected from all patients, directly from their history and medical records. Myocardial injury was defined as a high‐sensitivity troponin T (hsTnT) value exceeding the 99th percentile of a normal reference population (≥14 ng/L). 13 , 14
Echocardiographic assessment
A complete standard echocardiographic examination, including grey‐scale images for two‐dimensional strain analysis, was performed. Offline analyses were conducted with a standard imaging software (EchoPAC SW 203, GE Healthcare). LVEF was calculated using the biplane Simpson's method, 15 left atrial end‐systolic volumes were obtained in the apical 4‐chamber view according to Simpson's method. Post‐processing analysis with speckle tracking was conducted in the apical 4‐chamber, 2‐chamber, and long‐axis views at a frame rate of 50 to 70 frames/s using automated function imaging. Global longitudinal strain was calculated as the mean of all segmental strain values in the three apical views.
Linear LV measurements as well as LV mass calculation were performed according to the recommendations of the American Society of Echocardiography and the European Association of Cardiovascular Imaging. 16 LV mass index and left atrial index were obtained by adjustment for body surface area according to DuBois formula.
Left ventricular diastolic function was evaluated using: (i) the ratio of early transmitral flow velocity (E) to late transmitral flow velocity (A); (ii) the mean (E/e′ mean) of transmitral E to early diastolic medial LV tissue velocity (e′ septal) and the transmitral E to the early diastolic LV tissue velocity of the lateral wall (e′ lateral). Right ventricular function was defined by the measurement of TAPSE and RV S′.
Pulmonary artery systolic pressure was obtained from the peak velocity of the tricuspid regurgitation jet derived by continuous wave Doppler, using the modified Bernoulli equation, plus the estimated right atrial pressure, obtained from the inferior vena cava size and its collapsibility.
HFA‐PEFF score
The HFA‐PEFF score was calculated according to the Heart Failure Association (HFA) of the European Society of Cardiology (ESC) recommendations. 17 It is composed of four steps (pre‐test assessment, echocardiography and natriuretic peptide diagnostic score, functional testing, and final aetiology). According to the HFA‐PEFF score, the patients were divided into three risk groups for HFpEF probability: low (0–1 points, HFpEF unlikely), intermediate (2–4, HFpEF uncertain), and high (5–6, HFpEF diagnosis).
H2FPEF score
The H2FPEF score was calculated according to Reddy et al., 18 and derives from the integration of four clinical characteristics and two echocardiographic parameters. According to this score, the diagnosis of HFpEF is ruled out with 0–1 points and highly likely in patients with 6–9 points. The probability of HFpEF is intermediate when the score is between 2–5 points.
Statistical analysis
For statistical analyses, we used IBM Statistical Package for the Social Sciences (SPSS) version 26.0 (IBM Co., Armonk, NY, USA). The collected data were presented as mean ± standard deviation or number of patients and percentage. Where mean ± standard deviation was not appropriate to summarize the distributions, median and IQR were reported. We used unpaired t‐test/ANOVA as parametric and Mann‐Whitney U test/Kruskal–Wallis test as non‐parametric hypothesis tests. For the analysis of the contingency tables, we preferably used Chi‐square tests. If the contingency tables contained at least one cell assignment smaller than five, the Fisher's exact test was chosen. 19 We correlated the HFA‐PEFF score with clinical relevant parameters from ECG, echocardiography, and blood parameters using rank‐based Spearman correlation (none of these clinical relevant parameters were used to calculate the HFA‐PEFF score). All significant parameters from Spearman correlation analysis were included in a multivariate, ordinal regression analysis with logit link. Prior to multivariate, ordinal regression analysis, six missing ‘mitral E/A ratios’ were imputed using the expectation maximization algorithm. For the two significant parameters from multivariate regression analysis (hsTnT and age) we conducted receiver operating characteristics (ROC) analysis for predicting a high HFA‐PEFF score (5–6 points). The optimal cut‐off value was chosen by maximizing Youden's index using the R package maxstat. 20 , 21 A P‐value <0.05 was considered statistically significant in all analyses. To compare the frequencies of HFA‐PEFF scores (in the categories low, middle and high as well as low/middle and high) in COVID‐19 patients and controls, Chi‐square tests were used.
Results
The final study population included 64 COVID‐19 patients. The mean age was 56 ± 19 years and 20 (31%) patients were females. Overall, 44 (69%) patients had severe COVID‐19 pneumonia according to the current World Health Organization definition. 22 Detailed baseline characteristics are shown in Table 1 . 23 A total of 3 (5%) patients died during the hospitalization. All other patients were discharged by 7 December 2020 at the latest, with a median hospitalization duration of 10 days (IQR 5–15 days).
Table 1.
Baseline characteristics of COVID‐19 patients
| Baseline characteristics | COVID‐19 patients (n = 64) | HFA‐PEFF 0–1 points (n = 27, 42%) | HFA‐PEFF 2–4 points (n = 21, 33%) | HFA‐PEFF 5–6 points (n = 16, 25%) | P‐value |
|---|---|---|---|---|---|
| Age (years) | 56 ± 19 | 46 ± 15 ### | 53 ± 16 ### | 76 ± 11 | <0.001 |
| Female sex | 20 (31) | 6 (22) | 7 (33) | 7 (44) | 0.33 |
| BMI (kg/m2) | 27.1 ± 4.7 | 26.1 ± 4.4 | 28.3 ± 5.8 | 27.3 ± 3.5 | 0.29 |
| Systolic blood pressure (mmHg) | 126 ± 16 | 124 ± 14 # | 122 ± 19 # | 135 ± 14 | 0.041 |
| Diastolic blood pressure (mmHg) | 76 ± 11 | 75 ± 10 | 76 ± 12 | 76 ± 14 | 0.96 |
| Severe disease according to WHO | 44 (69) | 16 (59) # | 13 (62) # | 15 (94) | 0.044 |
| Dyspnoea | 38 (59) | 15 (56) | 11 (52) | 12 (75) | 0.33 |
| Peripheral oedema | 6 (9) | 2 (7) | 3 (14) | 1 (6) | 0.64 |
| Length of hospitalization (days) | 10 (5–15) | 8 (4–11) | 11 (7–19) | 13 (8–21) | 0.021 |
| Medical history | |||||
| Arterial hypertension | 28 (44) | 5 (19) ### | 7 (33) ### | 16 (100) | <0.001 |
| Atrial fibrillation | 8 (13) | 0 ## | 2 (10) # | 6 (38) | <0.001 |
| Coronary artery disease | 8 (13) | 0 ## | 2 (10) # | 6 (38) | <0.001 |
| Myocardial infarction | 3 (3) | 0 # | 0 # | 3 (19) | 0.009 |
| Diabetes mellitus type 2 | 9 (14) | 2 (7) | 3 (14) | 4 (25) | 0.28 |
| Chronic obstructive pulmonary disease | 7 (11) | 1 (4) | 3 (14) | 3 (19) | 0.26 |
| Chronic kidney disease ≥G4 (KDIGO) 23 | 0 | 0 | 0 | 0 | 1.00 |
| Cardiovascular medications | |||||
| ACE inhibitors | 7 (11) | 0 ## | 1 (5) # | 6 (38) | <0.001 |
| ARBs | 8 (13) | 1 (4) | 3 (14) | 4 (25) | 0.12 |
| Beta‐blockers a | 18 (28) | 3 (11) ### | 4 (19) ## | 11 (69) | <0.001 |
| Diuretics b | 8 (13) | 0 ### | 0 ### | 8 (50) | <0.001 |
| Blood parameters | |||||
| Sodium (mmol/L) | 137 ± 3 | 138 ± 4 | 137 ± 3 | 136 ± 4 | 0.19 |
| Potassium (mmol/L) | 3.8 ± 0.4 | 3.8 ± 0.3 | 3.8 ± 0.4 | 3.8 ± 0.4 | 0.96 |
| eGFR (mL/min) | 93.9 ± 24.4 | 107.1 ± 18.1 * , ### | 92.4 ± 25.3 ## | 73.8 ± 18.4 | <0.001 |
| hsTnT (ng/L) | 8 (5–16) | 5 (4–7) ### | 8 (6–12) ## | 25 (15–29) | <0.001 |
| NT‐proBNP (ng/L) | 153 (73–509) | 74 (21–126) ** , ### | 162 (92–498) ## | 977 (265–2817) | <0.001 |
| CRP (mg/L) | 27 (8–86) | 36 (9–99) | 12 (5–60) | 25 (10–90) | 0.36 |
| Haemoglobin (g/dL) | 12.5 ± 2.0 | 13.2 ± 2.1 ## | 12.3 ± 1.8 | 11.6 ± 1.8 | 0.032 |
| Leucocytes (/nL) | 7.3 ± 3.1 | 6.6 ± 3.2 | 8.0 ± 3.1 | 7.7 ± 2.9 | 0.30 |
| Thrombocytes (/nL) | 280 ± 117 | 259 ± 113 | 315 ± 139 | 268 ± 86 | 0.24 |
| Resting ECG parameters | |||||
| Sinus rhythm | 58 (91) | 27 (100) ## | 21 (100) ## | 10 (63) | <0.001 |
| Atrial fibrillation | 4 (6) | 0 ## | 0 # | 4 (25) | 0.002 |
| Heart rate (bpm) | 76 ± 14 | 74 ± 12 | 78 ± 19 | 74 ± 12 | 0.74 |
| QRS interval (ms) | 100 ± 16 | 99 ± 9 | 97 ± 6 | 107 ± 30 | 0.14 |
| QTc (ms) | 432 ± 28 | 423 ± 20 | 437 ± 25 | 441 ± 38 | 0.073 |
| QTc ≥440 ms | 23 (36) | 5 (19) * , # | 10 (48) | 8 (50) | 0.046 |
| Echocardiographic parameters | |||||
| LV ejection fraction (%) | 65 ± 4 | 66 ± 3 ## | 65 ± 3 | 63 ± 4 | 0.014 |
| Global longitudinal strain (%) | −16.3 ± 5.7 | −16.7 ± 7.8 | −17.4 ± 2.1 | −14.0 ± 2.8 | 0.28 |
| HFA‐PEFF score (points) | 2 (1–5) | 1 (0–1) ** , ### | 3 (2–4) # | 5 (5–6) | <0.001 |
| H2FPEF score (points) | 1 (0–3) | 1 (0–1) ### | 1 (1–2) ## | 4 (2–7) | <0.001 |
| LV wall thickness (mm) | 10.6 ± 1.6 | 9.9 ± 1.2 ### | 10.5 ± 1.6 ### | 11.8 ± 1.5 | <0.001 |
| LV mass index (g/m2) | 97 ± 28 | 85 ± 20 ### | 97 ± 23 # | 115 ± 37 | 0.003 |
| Relative wall thickness | 0.42 ± 0.09 | 0.39 ± 0.07 ### | 0.42 ± 0.11 # | 0.47 ± 0.06 | 0.006 |
| Left atrial volume index (mL/m2) | 22.9 ± 10.8 | 18.9 ± 6.1 ### | 22.7 ± 7.2 # | 29.9 ± 16.6 | 0.004 |
| Mitral E/A ratio | 1.1 ± 0.4 | 1.3 ± 0.4 * , ## | 1.0 ± 0.5 | 0.8 ± 0.3 | 0.008 |
| Diastolic dysfunction (n,%) | 27 (42) | 3 (11)*** , ### | 11 (52) | 13 (81) | <0.001 |
| Grade 1 (E/A < 1 + E/e′ mean <10) | 19 (30) | 3 (11)*** , # | 10 (48) | 6 (38) | 0.017 |
| Grade 2 (E/A ≥ 1 + E/e′ mean 10–14) | 4 (6) | 0 # | 1 (5) | 3 (19) | 0.046 |
| Grade 3 (E/A >= 1 + E/e′ mean >14) | 4 (6) | 0 ## | 0 # | 4 (25) | 0.002 |
| RV dysfunction (TAPSE <18 mm and/or RV S′ <0.10 m/s) | 6 (9) | 1 (4) ## | 0 ## | 5 (31) | 0.008 |
| Septal e′ (cm/s) | 8.7 ± 2.8 | 10.1 ± 2.7 ### | 8.8 ± 2.4 ### | 6.3 ± 1.7 | <0.001 |
| Lateral e′ (cm/s) | 11.1 ± 3.5 | 12.9 ± 3.2 ### | 11.2 ± 2.2 ### | 8.3 ± 2.7 | <0.001 |
| TR velocity (m/s) | 2.38 ± 0.42 | 2.24 ± 0.23 ### | 2.29 ± 0.29 ### | 2.70 ± 0.59 | 0.001 |
| Pulmonary artery systolic pressure (mmHg) | 28 ± 8 | 26 ± 4 ### | 26 ± 6 ### | 33 ± 11 | 0.010 |
Normal distributed variables are presented as mean ± standard deviation, non‐parametric variables as median (interquartile range), and nominal variables as n (%).
ACE, angiotensin‐converting enzyme; ARB, angiotensin receptor blocker; BMI, body mass index; CRP, C‐reactive protein; e′, mitral annulus early diastolic velocity; E/A, early filling velocity (E) and late filling velocity (A) ratio through the mitral annulus; ECG, electrocardiogram; eGFR, estimated glomerular filtration rate; hsTnT, high‐sensitivity troponin T; LV, left ventricular; NT‐proBNP, N‐terminal pro B‐type natriuretic peptide; RV, right ventricular; RV S′, right ventricular systolic excursion velocity; TAPSE, tricuspid annular plane systolic excursion, TR, tricuspid regurgitation; WHO, World Health Organization.
P < 0.05 vs. HFA‐PEFF 2–4 points.
P < 0.01 vs. HFA‐PEFF 2–4 points.
P < 0.001 vs. HFA‐PEFF 2–4 points.
P < 0.05 vs. HFA‐PEFF 5–6 points.
P < 0.01 vs. HFA‐PEFF 5–6 points.
P < 0.001 vs. HFA‐PEFF 5–6 points.
16 (89%) of 18 patients already used beta‐blockers before hospital admission.
7 (88%) of 8 patients already used diuretics before hospital admission.
In our study, we found that 42% of COVID‐19 patients (n = 27) had a low HFA‐PEFF score, 33% (n = 21) an intermediate score, and 25% (n = 16) a high HFA‐PEFF score (Figure 1 ). All COVID‐19 patients with a high HFA‐PEFF score [n = 16 (25%), 5–6 points] had arterial hypertension and six of these patients had atrial fibrillation (Table 1 ). Patients with high HFA‐PEFF score (5–6 points) had higher levels of hsTnT (+400% vs. 0–1 points on the HFA‐PEFF score and +213% vs. 2–4 points on the HFA‐PEFF score, ANOVA P‐value <0.001), lower levels of haemoglobin (−12% vs. 0–1 points' and −6% vs. 2–4 points, ANOVA P‐value =0.032), lower estimated glomerular filtration rate (eGFR) (−31% vs. 0–1 points and −20% vs. 2–4 points, ANOVA P‐value <0.001), lower LVEF (−5% vs. 0–1 points and −3% vs. 2–4 points, ANOVA P‐value =0.014), and more often severe COVID‐19 disease, diastolic dysfunction or right ventricular dysfunction.
Figure 1.
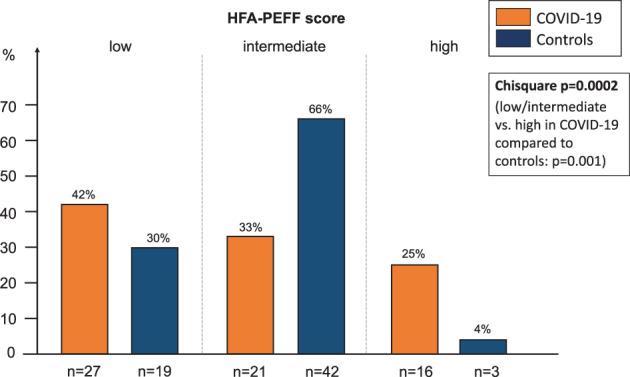
HFA‐PEFF score distribution in COVID‐19 patients and controls. Comparing the HFA‐PEFF scores in the categories low, intermediate and high (low/intermediate and high) between COVID‐19 patients and controls results in P = 0.0002 (P = 0.001).
Among the 64 controls with mean age 59 ± 16 years and 39% female, 19 (30%) showed a low HFA‐PEFF score, 42 (66%) had an intermediate score, and 3 (4%) had a high HFA‐PEFF score (Figure 1 ). The frequency distribution of low/middle/high HFA‐PEFF scores was different between COVID‐19 patients and controls (Chi‐square P‐value = 0.0002) and high HFA‐PEFF scores were more frequent in COVID‐19 patients than controls (25% vs. 4%, Chi‐square P‐value = 0.001). Additionally, COVID‐19 patients showed higher N‐terminal pro B‐type natriuretic peptide (NT‐proBNP) values (+101%, P = 0.002), and lower LVEF (−3%, P = 0.013) (Table 2 ).
Table 2.
Analysis of COVID‐19 patients vs. controls
| Baseline characteristics | COVID‐19 patients (n = 64) | Controls (n = 64) | P‐value |
|---|---|---|---|
| Age (years) | 56 ± 19 | 59 ± 16 | 0.38 |
| Female sex | 20 (31) | 25 (39) | 0.36 |
| NT‐proBNP (ng/L) | 153 (73–509) | 76 (36–133) | 0.002 |
| Sinus rhythm | 58 (91) | 49 (77) | 0.032 |
| Medical history | |||
| Arterial hypertension | 28 (44) | 36 (56) | 0.16 |
| Atrial fibrillation | 8 (13) | 15 (23) | 0.11 |
| Coronary artery disease | 8 (13) | 10 (16) | 0.61 |
| Myocardial infarction | 3 (5) | 1 (2) | 0.31 |
| Diabetes mellitus type 2 | 9 (14) | 7 (11) | 0.59 |
| Chronic obstructive pulmonary disease | 7 (11) | 5 (8) | 0.54 |
| Chronic kidney disease ≥G4 (KDIGO) | 0 | 0 | 1.00 |
| Echocardiographic parameters | |||
| LV ejection fraction (%) | 65 ± 4 | 67 ± 6 | 0.013 |
| LV wall thickness (mm) | 10.5 ± 1.6 | 10.2 ± 2.2 | 0.33 |
| LV mass index (g/m2) | 97 ± 28 | 83 ± 26 | 0.006 |
| Relative wall thickness | 0.42 ± 0.09 | 0.44 ± 0.11 | 0.36 |
| Mitral E/A ratio | 1.1 ± 0.4 | 1.2 ± 0.4 | 0.47 |
| Septal e′ (cm/s) | 8.7 ± 2.8 | 7.3 ± 2.0 | 0.002 |
| Lateral e′ (cm/s) | 1.1 ± 3.5 | 1.0 ± 3.0 | 0.048 |
| TR velocity (m/s) | 2.38 ± 0.42 | 2.51 ± 0.38 | 0.087 |
| Pulmonary artery systolic pressure (mmHg) | 29 ± 9 | 35 ± 8 | <0.001 |
Normal distributed variables are presented as mean ± standard deviation, non‐parametric variables as median (interquartile range), and nominal variables as n (%).
e′, mitral annulus early diastolic velocity; E/A, early filling velocity (E) and late filling velocity (A) ratio through the mitral annulus; LV, left ventricular; NT‐proBNP, N‐terminal pro B‐type natriuretic peptide; TR, tricuspid regurgitation.
Spearman correlation analysis showed a positive association between the HFA‐PEFF score and the following clinical parameters: age, eGFR, hsTnT, haemoglobin, leucocytes, QTc interval, LVEF, mitral E/A ratio, and additionally with the H2FPEF score. None of these parameters were used for the calculation of the HFA‐PEFF score (Table 3 ). In multivariate ordinal regression analyses, including the aforementioned significant clinical parameters, age and hsTnT were significant predictors of the HFA‐PEFF score (Table 4 , Figure 2 ). If one of these parameters increased by one unit, the HFA‐PEFF score increased by 0.06 points (age) or 0.13 points (hsTnT). Nagelkerke's R Square 24 was 0.60, indicative for a substantial goodness‐of‐fit of the multivariate model according to Cohen. 25 A chi‐square was performed to test the difference between the −2 log‐likelihood for the intercept‐only model and the final model. The statistically significant chi‐square statistic (P < 0.0001) indicates that the final model gives a significant improvement over the baseline intercept‐only model. Correlation coefficient between predicted response category and measured HFA‐PEFF score was 0.72 (Spearman's rho). According to Landis and Koch, 26 this is a substantial agreement.
Table 3.
Spearman correlation for the HFA‐PEFF score vs. clinical relevant parameters
| Measurement | rs (95% CI) | P‐value |
|---|---|---|
| Age (years) | 0.64 (0.45, 0.78) | <0.001 |
| BMI (kg/m2) | 0.16 (−0.09, 0.40) | 0.22 |
| Systolic blood pressure (mmHg) | 0.24 (−0.01, 0.45) | 0.056 |
| Diastolic blood pressure (mmHg) | 0.13 (−0.09, 0.35) | 0.32 |
| H2FPEF score (points) | 0.61 (0.41, 0.77) | <0.001 |
| Sodium (mmol/L) | −0.23 (−0.47, 0.04) | 0.070 |
| Potassium (mmol/L) | 0.06 (−0.20, 0.31) | 0.63 |
| eGFR (mL/min) | −0.59 (−0.73, −0.41) | <0.001 |
| hsTnT (ng/L) | 0.73 (0.57, 0.84) | <0.001 |
| NT‐proBNP (ng/L) | 0.77 (0.65, 0.85) | <0.001 |
| CRP (mg/L) | −0.10 (−0.36, 0.14) | 0.42 |
| Haemoglobin (g/dL) | −0.34 (−0.57, −0.08) | 0.007 |
| Leucocytes (/nL) | 0.27 (0.03, 0.47) | 0.033 |
| Thrombocytes (/nL) | 0.15 (−0.09, 0.39) | 0.23 |
| Heart rate (bpm) | −0.01 (−0.23, 0.22) | 0.95 |
| QRS interval (ms) | −0.04 (−0.30, 0.25) | 0.73 |
| QTc (ms) | 0.35 (0.08, 0.56) | 0.005 |
| LV ejection fraction (%) | −0.30 (−0.52, −0.06) | 0.017 |
| Mitral E/A ratio | −0.54 (−0.71, −0.32) | <0.001 |
BMI, body mass index; CI, confidence interval; CRP, C‐reactive protein; E/A, early filling velocity (E) and late filling velocity (A) ratio through the mitral annulus; eGFR, estimated glomerular filtration rate; hsTnT, high‐sensitivity troponin T; LV, left ventricular; NT‐proBNP, N‐terminal pro B‐type natriuretic peptide.
Table 4.
Multivariate ordinal regression analyses
| Estimate a | SE | P‐value | 95% CI | |
|---|---|---|---|---|
| Age (years) | 0.06 | 0.02 | 0.007 | 0.02, 0.11 |
| eGFR (mL/min) | 0.01 | 0.02 | 0.59 | −0.02, 0.04 |
| hsTnT (ng/L) | 0.13 | 0.04 | 0.001 | 0.05, 0.20 |
| Haemoglobin (g/dL) | 0.05 | 0.16 | 0.78 | −0.27, 0.37 |
| Leucocytes (/nL) | 0.09 | 0.08 | 0.29 | −0.07, 0.25 |
| QTc (ms) | 0.01 | 0.01 | 0.63 | −0.02, 0.03 |
| LV ejection fraction (%) | −0.06 | 0.08 | 0.44 | −0.22, 0.10 |
| Mitral E/A ratio | −0.60 | 0.74 | 0.42 | −2.05, 0.85 |
Link function: Logit. Correlation coefficient between predicted response category and measured HFA‐PEFF score: 0.72 (Spearman's rho).
CI, confidence interval; E/A, early filling velocity (E) and late filling velocity (A) ratio through the mitral annulus; eGFR, estimated glomerular filtration rate; hsTnT, high‐sensitivity troponin T; LV, left ventricular; SE, standard error.
The estimate gives the increase or decrease of the HFA‐PEFF score, if the given parameter increased by one unit.
Figure 2.
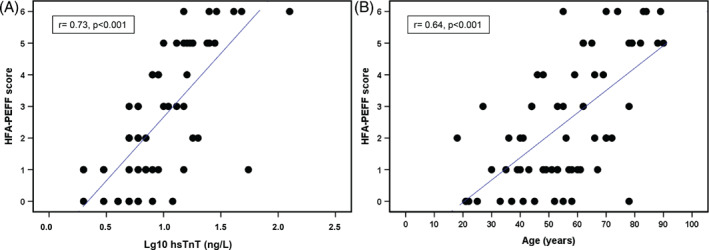
Scatter plot of the HFA‐PEFF score vs. high‐sensitivity troponin T (hsTnT) and age.
For the two significant parameters from multivariate regression analysis (age and hsTnT), we conducted ROC analysis for calculating the best cut‐offs for predicting a high HFA‐PEFF score [5–6 points; age: area under the curve 0.93 (0.87–1.00); hsTnT: area under the curve 0.94 (0.89–1.00), P < 0.001]. The best cut‐off for predicting a high HFA‐PEFF score regarding age was ≥62 years (sensitivity 94%, specificity 79%) and for hsTnT ≥10 ng/L (sensitivity 100%, specificity 79%) (Figure 3 ).
Figure 3.

Receiver operating characteristic (ROC) curves for high‐sensitivity (hs) troponin T and age for predicting a high HFA‐PEFF score (5–6 points).
Patients with vs. without myocardial injury had higher HFA‐PEFF scores [median 5 (IQR 3–6) vs. 1 (IQR 0–3), P < 0.001], elevated levels of NT‐proBNP (+455%, P < 0.001), eGFR (−30%, P < 0.001) (Table 5 ). In echocardiographic examination, patients with myocardial injury demonstrated lower LVEF, more often diastolic dysfunction, and right ventricular dysfunction.
Table 5.
Analysis of COVID‐19 patients according to myocardial injury (high‐sensitivity troponin T >99% upper limit of normal/>14 ng/L)
| Baseline characteristics | No myocardial injury (n = 44, 69%) | Myocardial injury (n = 20, 31%) | P‐value |
|---|---|---|---|
| Age (years) | 50 ± 16 | 70 ± 17 | <0.001 |
| Female sex | 11 (25) | 9 (45) | 0.11 |
| BMI (kg/m2) | 26.8 ± 4.5 | 27.8 ± 5.3 | 0.45 |
| Systolic blood pressure (mmHg) | 124 ± 17 | 131 ± 14 | 0.14 |
| Diastolic blood pressure (mmHg) | 76 ± 12 | 76 ± 11 | 0.88 |
| Severe disease according to WHO | 29 (66) | 15 (75) | 0.47 |
| Dyspnoea | 25 (57) | 13 (65) | 0.54 |
| Peripheral oedema | 4 (9) | 2 (10) | 0.91 |
| Length of hospitalization (days) | 9 (5–14) | 13 (9–21) | 0.016 |
| Medical history | |||
| Arterial hypertension | 14 (32) | 14 (70) | 0.004 |
| Atrial fibrillation | 2 (5) | 6 (30) | 0.009 |
| Coronary artery disease | 3 (7) | 5 (25) | 0.096 |
| Myocardial infarction | 1 (2) | 2 (10) | 0.23 |
| Diabetes mellitus type 2 | 2 (5) | 7 (35) | 0.003 |
| Chronic obstructive pulmonary disease | 3 (7) | 4 (20) | 0.19 |
| Chronic kidney disease ≥G4 (KDIGO) | 0 | 0 | 1.00 |
| Cardiovascular medications | |||
| ACE inhibitors | 2 (5) | 5 (25) | 0.026 |
| ARBs | 4 (9) | 4 (20) | 0.24 |
| Beta‐blockers | 7 (16) | 11 (55) | 0.001 |
| Diuretics | 0 | 8 (40) | <0.001 |
| Blood parameters | |||
| Sodium (mmol/L) | 138 ± 3 | 136 ± 3 | 0.017 |
| Potassium (mmol/L) | 3.8 ± 0.4 | 3.8 ± 0.4 | 0.86 |
| eGFR (mL/min) | 102.9 ± 20.2 | 74.4 ± 21.6 | <0.001 |
| hsTnT (ng/L) | 6 (5–8) | 22 (16–29) | <0.001 |
| NT‐proBNP (ng/L) | 97 (33–191) | 538 (234–2414) | <0.001 |
| CRP (mg/L) | 24 (7–84) | 32 (9–92) | 0.45 |
| Haemoglobin (g/dL) | 13.0 ± 1.7 | 11.4 ± 2.3 | 0.002 |
| Leucocytes (/nL) | 7.0 ± 3.1 | 8.0 ± 3.2 | 0.23 |
| Thrombocytes (/nL) | 273 ± 127 | 293 ± 94 | 0.54 |
| Resting ECG parameters | |||
| Sinus rhythm | 44 (100) | 14 (70) | 0.001 |
| Atrial fibrillation | 0 | 4 (20) | 0.008 |
| Heart rate (bpm) | 75 ± 13 | 77 ± 17 | 0.61 |
| QRS interval (ms) | 98 ± 9 | 106 ± 26 | 0.23 |
| QTc (ms) | 428 ± 23 | 440 ± 34 | 0.085 |
| QTc ≥440 ms | 13 (30) | 10 (50) | 0.11 |
| Echocardiographic parameters | |||
| LV ejection fraction (%) | 66 ± 3 | 63 ± 4 | 0.013 |
| Global longitudinal strain (%) | −16.6 ± 6.4 | −15.5 ± 3.0 | 0.53 |
| HFA‐PEFF score (points) | 1 (0–3) | 5 (3–6) | <0.001 |
| H2FPEF score (points) | 1 (0–2) | 3 (1–7) | <0.001 |
| LV wall thickness (mm) | 10.2 ± 1.5 | 11.3 ± 1.6 | 0.007 |
| LV mass index (g/m2) | 92 ± 23 | 108 ± 35 | 0.031 |
| Relative wall thickness | 0.41 ± 0.09 | 0.45 ± 0.07 | 0.061 |
| Left atrial volume index (mL/m2) | 20.6 ± 6.9 | 27.9 ± 15.5 | 0.055 |
| Mitral E/A ratio | 1.2 ± 0.5 | 0.8 ± 0.3 | 0.004 |
| Diastolic dysfunction | 12 (27) | 15 (75) | <0.001 |
| Grade 1 (E/A < 1 + E/e′ mean <10) | 10 (23) | 9 (45) | 0.071 |
| Grade 2 (E/A ≥ 1 + E/e′ mean 10–14) | 2 (5) | 2 (10) | 0.58 |
| Grade 3 (E/A >= 1 + E/e′ mean >14) | 0 | 4 (20) | 0.008 |
| RV dysfunction (TAPSE <18 mm and/or RV S′ <0.10 m/s) | 0 | 6 (30) | 0.001 |
| Septal e′ (cm/s) | 9.4 ± 2.7 | 7.2 ± 2.5 | 0.004 |
| Lateral e′ (cm/s) | 12.1 ± 3.2 | 9.4 ± 3.6 | 0.004 |
| TR velocity (m/s) | 2.28 ± 0.27 | 2.59 ± 0.57 | 0.030 |
| Pulmonary artery systolic pressure (mmHg) | 26 ± 5 | 31 ± 11 | 0.028 |
Normal distributed variables are presented as mean ± standard deviation, non‐parametric variables as median (interquartile range), and nominal variables as n (%).
ACE, angiotensin‐converting enzyme; ARB, angiotensin receptor blocker; BMI, body mass index; CRP, C‐reactive protein; e′, mitral annulus early diastolic velocity; E/A, early filling velocity (E) and late filling velocity (A) ratio through the mitral annulus; ECG, electrocardiogram; eGFR, estimated glomerular filtration rate; hsTnT, high‐sensitivity troponin T; LV, left ventricular; NT‐proBNP, N‐terminal pro B‐type natriuretic peptide; RV, right ventricular; RV S′, right ventricular systolic excursion velocity; TAPSE, tricuspid annular plane systolic excursion, TR, tricuspid regurgitation; WHO, World Health Organization.
Discussion
In this study, we describe for the first time using the HFA‐PEFF score that a substantial proportion of COVID‐19 patients showed higher risk of HFpEF. The prevalence of a high HFA‐PEFF score was increased in COVID‐19 patients in comparison to controls. In multivariate, ordinal regression analyses, age, and hsTnT were significantly associated with a raised HFA‐PEFF score. Amongst patients with biochemical evidence for myocardial injury, HFA‐PEFF score was higher and LV diastolic dysfunction and reduced global longitudinal strain were more prevalent. Based on these results using the HFA‐PEFF algorithm during the acute phase of infection may facilitate the identification of COVID‐19 patients with acute cardiac abnormalities compatible with HFpEF‐like syndrome, as is also known for other inflammatory viral diseases. 27
In the last months, great efforts have been made to accomplish better characterization of the CV profile of COVID‐19 patients, to define which factors may reveal CV complications, and how to manage CV care of these patients. 28 So far, CV risk factors like advanced age, male sex, arterial hypertension, and previous coronary artery disease have been frequently reported in COVID‐19 patients admitted to hospital. 29 , 30 , 31 The presence of arterial hypertension in 43% of our patients is slightly higher than in other large, multicentre studies conducted in China and the United States where hypertension was observed in 24% 1 and 35% 32 of patients with COVID‐19.
HFpEF is a severe medical problem with currently limited therapeutic options. The HFA‐PEFF score was recently developed to improve the diagnosis of HFpEF. 17 It has been validated in two different independent cohorts of 228 and 459 HFpEF patients, 33 describing a specificity of 93% and positive predictive value of 98% to rule in HFpEF with a high score, and a sensitivity of 99% and a negative predictive value of 73% to rule out HFpEF with a low score. The diagnostic accuracy in patients with ≥5 points was 0.90 (0.84–0.96, area under the ROC curve). Accordingly, we reasonably hypothesized the score might be helpful in the assessment of HFpEF like‐syndrome in COVID‐19 patients.
Echocardiography is an important and non‐invasive part of CV examinations in COVID‐19 patients. 28 In 1216 COVID‐19 patients assessed with echocardiography, severe cardiac disease was evident in 15%. Myocardial infarction was present in 3%, myocarditis in 3%, and Takotsubo cardiomyopathy in 2%. 34 Szekely et al. 35 observed in a prospective study of 100 COVID‐19 patients (29% with severe, 10% with critical, 61% with mild/moderate COVID‐19 disease) that LV diastolic dysfunction was present in 16% of patients, whereas we detected diastolic dysfunction in our cohort (69% of patients with severe COVID‐19 disease) to be present in 42% of patients with LVEF ≥50%. Hence, LV diastolic dysfunction might be present more often in patients with severe COVID‐19 disease, compared to patients with mild or moderate disease.
In comparison, the H2FPEF score was retrospectively derived and validated 36 to discriminate patients with HFpEF from patients with non‐cardiac dyspnoea, which is often difficult in clinical practice. Other non‐cardiac causes of dyspnoea include: chronic obstructive pulmonary disease, COVID‐19 and other pneumonia, bronchial asthma, cancer, trauma, angioedema, vocal cord dysfunction, and many others. Up to 25% of all ambulatory patients show dyspnoea 37 and therefore the HFA‐PEFF and H2FPEF score are important clinical tools in examining patients with unknown causes of dyspnoea. In this study, the HFA‐PEFF score and H2FPEF score correlated well, even though both scores are very different. While the HFA‐PEFF score includes a variety of echocardiographic parameters and laboratory values, the H2FPEF score incorporates more clinical characteristics of the patients and fewer echocardiographic parameters. Nonetheless, an intermediate risk for HFpEF according to the H2FPEF score was found in 36% of the COVID‐19 patients while 13% of patients were categorized in the high‐risk group.
Myocardial injury defined as increased values of hsTnT has been observed in about 20% of COVID‐19 patients. 6 It has been shown that higher concentrations of cardiac biomarkers (NT‐proBNP, hsTnI, hsTnT) correlate with severity of infection. 38 , 39 A non‐specific increase of cardiac enzymes in COVID‐19 patients may reveal not only a predisposition of these patients to cardiac injury, 40 but also other cardiac dysfunctions. 41 In our study, hsTnT was observed as a strong predictor of higher HFA‐PEFF score. Accordingly, increased hsTnT in COVID‐19 patients may help identifying COVID‐19‐induced cardiac diastolic aberrations.
Whether myocardial injury is only temporary is unclear and therefore clinical follow‐up studies of patients with myocardial injury are needed. Cardiac magnetic resonance and endomyocardial biopsy studies showed that in some COVID‐19 or post‐COVID‐19 patients an ongoing cardiac inflammation can be detected. 42 , 43 , 44 This is in agreement with several echocardiographic parameters, detectable in those patients. 45 , 46
The time for cardiac depolarization and repolarization (QTc interval) was prolonged in COVID‐19 patients with an intermediate or high HFA‐PEFF score. In general, a QTc prolongation is associated with a higher risk for ventricular arrhythmias and sudden cardiac death. 47 Arrhythmias and QTc prolongation are known to occur in COVID‐19 patients. 48 Our finding does not necessarily belong directly to key features of LV diastolic dysfunction in COVID‐19‐induced HFpEF, but could be an indicator of cardiac damage due to COVID‐19‐induced cardiac inflammatory stress responses as known for myocarditis and ischaemia, respectively. 49 Guo et al. 50 have shown in 187 hospitalized COVID‐19 patients that malignant arrhythmias occurred in 7% of patients and were more frequently in patients with vs. without myocardial injury (12% vs. 5%, P < 0.001). Hence, the HFA‐PEFF score could also be useful and important in detecting COVID‐19 patients more vulnerable to malignant arrhythmias.
In this COVID‐19 cohort, the in‐hospital mortality rate was at 5% (3/64 patients). There are several reasons for this low mortality: first of all, the overall mortality rate during the study period in patients in Germany with a SARS‐CoV‐2 infection was also low at about 2–5%. 51 Secondly, even though all our patients were hospitalized, we only included clinically stable patients on a normal COVID‐19 ward and examined no patients in an intermediate or intensive care unit. Lastly, as explained in the methods section, six patients with LVEF <50% and one patient with previously known right ventricular dysfunction were excluded from the study (two of these seven patients died during hospitalization). If these patients would have been included in the analysis, the in‐hospital mortality rate would have been at 7%. This in‐hospital mortality rate is comparable to that found by others in Germany, 52 when looking at hospitalized COVID‐19 patients not treated in an intensive care unit (156 deaths in 1856 patients, i.e. 8%).
Limitations
The main limitation of this study is the relatively small sample size of 64 COVID‐19 patients, but all patients were included prospectively and with detailed echocardiography assessment, which is more complicated to perform in COVID‐19 patients than usual, due to the required protection measures for the investigator. Only few patients had ever received an echocardiogram before their current COVID‐19 hospitalization (n = 3) and hence a comparison to previous echocardiograms was not possible. The reason for this is that in none of the included 64 COVID‐19 patients, HFpEF had ever been suspected or diagnosed before. We could only apply step 2 of the HFA‐PEFF algorithm in all patients. Since the HFA‐PEFF score has not been used in COVID‐19 patients before, we did not rule out patients with step 1 (i.e. pre‐test assessment), and could not apply step 3 (i.e. stress echocardiography for patients with intermediate scores to secure a final diagnosis of HFpEF) and step 4 (i.e. identify the specific aetiology for HFpEF). The HFA‐PEFF score algorithm was assessed during the acute phase of COVID‐19 infection. For this setting, the score has not been originally developed. Nevertheless, we believe that our results are representative for the CV profile of clinically stable COVID‐19 patients treated in a normal COVID‐19 ward despite the small sample size. We cannot know for sure from our analysis whether HFpEF and COVID‐19 are truly related and whether some of the COVID‐19 patients already had an elevated HFA‐PEFF score before their COVID‐19 infection, also given that patients with high HFA‐PEFF score were older and all suffered from arterial hypertension, but compared to our control group (with similar age, sex, and comorbidity status), a high HFA‐PEFF score was seen more often in COVID‐19 patients than controls. It is possible that patients had undiagnosed HFpEF before their SARS‐CoV‐2 infection. Although the control group was carefully matched for relevant potential confounders, the presence of some residual confounding cannot be excluded entirely. To establish whether the HFA‐PEFF score could be useful in predicting long‐term cardiac dysfunction and whether HFpEF is related or even caused by the SARS‐CoV‐2 infection, larger studies with long‐term follow‐up and comprehensive echocardiographic studies of COVID‐19 patients are warranted.
Conclusions
We have found evidence of cardiac structural and functional alterations compatible with HFpEF which are associated with presence of myocardial injury in hospitalized COVID‐19 patients, detectable by both recently established diagnostic HFpEF algorithms. Per definition they may belong to the group of viral‐induced HFpEF‐like syndromes, where aetiology, treatment and probably prognosis differ from classical HFpEF scenarios induced by established risk factors like hypertension or diabetes mellitus. We suggest that an assessment of cardiovascular status including NT‐proBNP, hsTnT and LV diastolic function testing by echocardiography is useful to assess the disease status of hospitalized COVID‐19 patients.
Acknowledgement
We thank the patients, investigators, nurses, supporting staff, and physicians who were involved in this study. M.W. is supported by grants from the German Research Foundation, SFB‐TR84 C6 and C9, by the German Ministry of Education and Research (BMBF) in the framework of the CAPSyS (01ZX1304B), CAPSyS‐COVID (01ZX1604B), SYMPATH (01ZX1906A) and PROVID project (01KI20160A) and by the Berlin Institute of Health (CM‐COVID).
Open Access funding enabled and organized by Projekt DEAL.
Conflict of interest: J.E.H. has received research support from NIH, Gilead Sciences, Bayer AG, and research supplies from EcoNugenics. M.W. received research support or personal fees outside the current work from German Research Council (DFG), German Ministry of Education and Research (BMBF), European Respiratory Society, Marie Curie Foundation, Else Kröner Fresenius Foundation, Capnetz Foundation, International Max Planck Research School, Actelion, Aptarion, AstraZeneca, Bayer Health Care, Berlin Chemie, Biotest, Boehringer Ingelheim, Chiesi, Glaxo Smith Kline, Novartis, Noxxon, Pantherna, Quark Pharma, Silence Therapeutics, Sinoxa, Takeda Pharma, Teva, Vaxxilon. T.F. reports personal fees from Novartis, Bayer, Janssen, SGS, Roche, Boehringer Ingelheim, Daiichi‐Sankyo, Galapagos, Penumbra, Parexel, Vifor, BiosenseWebster, CSL Behring, Fresenius Kabi, Coherex Medical, LivaNova; all outside the submitted work. J.B. is a consultant to Abbott, Adrenomed, Array, Amgen, Applied Therapeutics, AstraZeneca, Bayer, Boehringer Ingelheim, CVRx, Eli Lilly, G3 Pharma, Impulse Dynamics, Janssen, LivaNova, Luitpold, Medtronic, Merck, Novartis, Novo Nordisk, Relypsa, Sequana Medical, V‐Wave Limited, and Vifor. U.W. is supported by a Clinical Fellowship Grant from the BIH (Berlin Institute of Health) and has received speaker fees and/or contributions to congresses from Abbott, AstraZeneca, Bayer, Berlin Chemie, Bristol‐Myers Squibb, GE Healthcare, Pfizer, Philips, and Servier. S.D.A. reports grants and personal fees from Vifor Int. and Abbott Vascular, and personal fees from AstraZeneca, Bayer, Brahms, Boehringer Ingelheim, Cardiac Dimensions, Novartis, Occlutech, Servier, and Vifor Int; all outside the submitted work. C.T. has received speaker fees and/or contributions to congresses from Abbott, Abiomed, AstraZeneca, Bayer, Berlin Chemie, Novartis, Pfizer, and Servier; all outside the submitted work. M.S.A. has received personal fees from Servier, outside the submitted work. All other authors have nothing to disclose.
References
- 1. Guan WJ, Ni ZY, Hu Y, Liang WH, Ou CQ, He JX, Liu L, Shan H, Lei CL, Hui DSC, Du B, Li LJ, Zeng G, Yuen KY, Chen RC, Tang CL, Wang T, Chen PY, Xiang J, Li SY, Wang JL, Liang ZJ, Peng YX, Wei L, Liu Y, Hu YH, Peng P, Wang JM, Liu JY, Chen Z, Li G, Zheng ZJ, Qiu SQ, Luo J, Ye CJ, Zhu SY, Zhong NS; China Medical Treatment Expert Group for Covid‐19 . Clinical characteristics of coronavirus disease 2019 in China. N Engl J Med 2020;282:1708–1720. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Bromage DI, Cannata A, Rind IA, Gregorio C, Piper S, Shah AM, McDonagh TA. The impact of COVID‐19 on heart failure hospitalization and management: report from a Heart Failure Unit in London during the peak of the pandemic. Eur J Heart Fail 2020;22:978–984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Fried JA, Ramasubbu K, Bhatt R, Topkara VK, Clerkin KJ, Horn E, Rabbani L, Brodie D, Jain SS, Kirtane AJ, Masoumi A, Takeda K, Kumaraiah D, Burkhoff D, Leon M, Schwartz A, Uriel N, Sayer G. The variety of cardiovascular presentations of COVID‐19. Circulation 2020;141:1930–1936. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Inciardi RM, Adamo M, Lupi L, Cani DS, Di Pasquale M, Tomasoni D, Italia L, Zaccone G, Tedino C, Fabbricatore D, Curnis A, Faggiano P, Gorga E, Lombardi CM, Milesi G, Vizzardi E, Volpini M, Nodari S, Specchia C, Maroldi R, Bezzi M, Metra M. Characteristics and outcomes of patients hospitalized for COVID‐19 and cardiac disease in Northern Italy. Eur Heart J 2020;41:1821–1829. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Ruan Q, Yang K, Wang W, Jiang L, Song J. Clinical predictors of mortality due to COVID‐19 based on an analysis of data of 150 patients from Wuhan, China. Intensive Care Med 2020;46:846–848. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Shi S, Qin M, Shen B, Cai Y, Liu T, Yang F, Gong W, Liu X, Liang J, Zhao Q, Huang H, Yang B, Huang C. Association of cardiac injury with mortality in hospitalized patients with COVID‐19 in Wuhan, China. JAMA Cardiol 2020;5:802–810. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Tavazzi G, Pellegrini C, Maurelli M, Belliato M, Sciutti F, Bottazzi A, Sepe PA, Resasco T, Camporotondo R, Bruno R, Baldanti F, Paolucci S, Pelenghi S, Iotti GA, Mojoli F, Arbustini E. Myocardial localization of coronavirus in COVID‐19 cardiogenic shock. Eur J Heart Fail 2020;22:911–915. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Van Linthout S, Klingel K, Tschöpe C. SARS‐CoV‐2‐related myocarditis‐like syndromes Shakespeare's question: what's in a name? Eur J Heart Fail 2020;22:922–925. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Tschöpe C, Ammirati E, Bozkurt B, Caforio ALP, Cooper LT, Felix SB, Hare JM, Heidecker B, Heymans S, Hübner N, Kelle S, Klingel K, Maatz H, Parwani AS, Spillmann F, Starling RC, Tsutsui H, Seferovic P, Van Linthout S. Myocarditis and inflammatory cardiomyopathy: current evidence and future directions. Nat Rev Cardiol 2021;18:169–193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Babapoor‐Farrokhran S, Gill D, Walker J, Rasekhi RT, Bozorgnia B, Amanullah A. Myocardial injury and COVID‐19: possible mechanisms. Life Sci 2020;253:117723. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Kurth F, Roennefarth M, Thibeault C, Corman VM, Müller‐Redetzky H, Mittermaier M, Ruwwe‐Glösenkamp C, Heim KM, Krannich A, Zvorc S, Schmidt S, Kretzler L, Dang‐Heine C, Rose M, Hummel M, Hocke A, Hübner RH, Opitz B, Mall MA, Röhmel J, Landmesser U, Pieske B, Knauss S, Endres M, Spranger J, Mockenhaupt FP, Tacke F, Treskatsch S, Angermair S, Siegmund B, Spies C, Weber‐Carstens S, Eckardt KU, Schürmann D, Uhrig A, Stegemann MS, Zoller T, Drosten C, Suttorp N, Witzenrath M, Hippenstiel S, von Kalle C, Sander LE. Studying the pathophysiology of coronavirus disease 2019: a protocol for the Berlin prospective COVID‐19 patient cohort (Pa‐COVID‐19). Infection 2020;48:619–626. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Churchill TW, Li SX, Curreri L, Zern EK, Lau ES, Liu EE, Farrell R, Shoenike MW, Sbarbaro J, Malhotra R, Nayor M, Tschöpe C, de Boer RA, Lewis GD, Ho JE. Evaluation of 2 existing diagnostic scores for heart failure with preserved ejection fraction against a comprehensively phenotyped cohort. Circulation 2021;143:289–291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Huang C, Wang Y, Li X, Ren L, Zhao J, Hu Y, Zhang L, Fan G, Xu J, Gu X, Cheng Z, Yu T, Xia J, Wei Y, Wu W, Xie X, Yin W, Li H, Liu M, Xiao Y, Gao H, Guo L, Xie J, Wang G, Jiang R, Gao Z, Jin Q, Wang J, Cao B. Clinical features of patients infected with 2019 novel coronavirus in Wuhan, China. Lancet 2020;395:497–506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Gao C, Wang Y, Gu X, Shen X, Zhou D, Zhou S, Huang JA, Cao B, Guo Q; Community‐Acquired Pneumonia‐China Network . Association between cardiac injury and mortality in hospitalized patients infected with avian influenza A (H7N9) virus. Crit Care Med 2020;48:451–458. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Lang RM, Badano LP, Mor‐Avi V, Afilalo J, Armstrong A, Ernande L, Flachskampf FA, Foster E, Goldstein SA, Kuznetsova T, Lancellotti P, Muraru D, Picard MH, Rietzschel ER, Rudski L, Spencer KT, Tsang W, Voigt JW. Recommendations for cardiac chamber quantification by echocardiography in adults: an update from the American Society of Echocardiography and the European Association of Cardiovascular Imaging. J Am Soc Echocardiogr 2015;28:1–39.e14. [DOI] [PubMed] [Google Scholar]
- 16. Lang RM, Badano LP, Mor‐Avi V, Afilalo J, Armstrong A, Ernande L, Flachskampf FA, Foster E, Goldstein SA, Kuznetsova T, Lancellotti P, Muraru D, Picard MH, Rietzschel ER, Rudski L, Spencer KT, Tsang W, Voigt JU. Recommendations for cardiac chamber quantification by echocardiography in adults: an update from the American Society of Echocardiography and the European Association of Cardiovascular Imaging. Eur Heart J Cardiovasc Imaging 2015;16:233–270. [DOI] [PubMed] [Google Scholar]
- 17. Pieske B, Tschöpe C, de Boer RA, Fraser AG, Anker SD, Donal E, Edelmann F, Fu M, Guazzi M, Lam CSP, Lancellotti P, Melenovsky V, Morris DA, Nagel E, Pieske‐Kraigher E, Ponikowski P, Solomon SD, Vasan RS, Rutten FH, Voors AA, Ruschitzka F, Paulus WJ, Seferovic P, Filippatos G. How to diagnose heart failure with preserved ejection fraction: the HFA‐PEFF diagnostic algorithm: a consensus recommendation from the Heart Failure Association (HFA) of the European Society of Cardiology (ESC). Eur J Heart Fail 2020;22:391–412. [DOI] [PubMed] [Google Scholar]
- 18. Reddy YNV, Carter RE, Obokata M, Redfield MM, Borlaug BA. A simple, evidence‐based approach to help guide diagnosis of heart failure with preserved ejection fraction. Circulation 2018;138:861–870. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Fisher RA. Statistical Methods for Research Workers. In: Kotz S, Johnson NL, eds. Breakthroughs in Statistics. New York, NY: Springer; 1921. pp 66–70. [Google Scholar]
- 20. Hothorn T. R package maxstat ‐ Maximally Selected Rank Statistics, 2017. https://CRAN.R‐project.org/package=maxstat (10 May 2021).
- 21. Youden WJ. Index for rating diagnostic tests. Cancer 1950;3:32–35. [DOI] [PubMed] [Google Scholar]
- 22. World Health Organization . COVID‐19 clinical management. Living guidance, 25 January 2021. Geneva: WHO; 2021. https://apps.who.int/iris/bitstream/handle/10665/338882/WHO‐2019‐nCoV‐clinical‐2021.1‐eng.pdf?sequence=1&isAllowed=y (20 April 2021).
- 23. Kidney Disease Improving Global Outcomes (KDIGO) CKD Work Group. KDIGO 2012 clinical practice guideline for the evaluation and management of chronic kidney disease. Kidney Int Suppl 2013;3:1–150. [Google Scholar]
- 24. Nagelkerke NJD. A note on a general definition of the coefficient of determination. Biometrika 1991;78:691–692. [Google Scholar]
- 25. Cohen J. Statistical Power Analysis for the Behavioral Sciences, 2nd ed. Hillsdale, NJ: Lawrence Erlbaum Associates; 1988. [Google Scholar]
- 26. Landis JR, Koch GG. The measurement of observer agreement for categorical data. Biometrics 1977;33:159–174. [PubMed] [Google Scholar]
- 27. Tschöpe C, Bock CT, Kasner M, Noutsias M, Westermann D, Schwimmbeck PL, Pauschinger M, Poller WC, Kühl U, Kandolf R, Schultheiss HP. High prevalence of cardiac parvovirus B19 infection in patients with isolated left ventricular diastolic dysfunction. Circulation 2005;111:879–886. [DOI] [PubMed] [Google Scholar]
- 28. European Society of Cardiology . ESC Guidance for the Diagnosis and Management of CV Disease during the COVID‐19 Pandemic. Last updated on 10 June 2020. https://www.escardio.org/Education/COVID‐19‐and‐Cardiology/ESC‐COVID‐19‐Guidance (10 May 2021).
- 29. Lodigiani C, Iapichino G, Carenzo L, Cecconi M, Ferrazzi P, Sebastian T, Kucher N, Studt J‐D, Sacco C, Bertuzzi A, Sandri MT, Barco S; Humanitas COVID‐19 Task Force . Venous and arterial thromboembolic complications in COVID‐19 patients admitted to an academic hospital in Milan, Italy. Thromb Res 2020;191:9–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Zhou F, Yu T, Du R, Fan G, Liu Y, Liu Z, Xiang J, Wang Y, Song B, Gu X, Guan L, Wei Y, Li H, Wu X, Xu J, Tu S, Zhang Y, Chen H, Cao B. Clinical course and risk factors for mortality of adult inpatients with COVID‐19 in Wuhan, China: a retrospective cohort study. Lancet 2020;395:1054–1062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Chen N, Zhou M, Dong X, Qu J, Gong F, Han Y, Qiu Y, Wang J, Liu Y, Wei Y, Xia J, Yu T, Zhang X, Zhang L. Epidemiological and clinical characteristics of 99 cases of 2019 novel coronavirus pneumornia in Wuhan, China: a descriptive study. Lancet 2020;395:507–513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Reynolds HR, Adhikari S, Pulgarin C, Troxel AB, Iturrate E, Johnson SB, Hausvater A, Newman JD, Berger JS, Bangalore S, Katz SD, Fishman GI, Kunichoff D, Chen Y, Ogedegbe G, Hochman JS. Renin‐angiotensin‐aldosterone system inhibitors and risk of Covid‐19. N Engl J Med 2020;382:2441–2448. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Barandiarán Aizpurua A, Sanders‐van Wijk S, Brunner‐La Rocca HP, Henkens M, Heymans S, Beussink‐Nelson L, Shah SJ, van Empel VP. Validation of the HFA‐PEFF score for the diagnosis of heart failure with preserved ejection fraction. Eur J Heart Fail 2020;22:413–421. [DOI] [PubMed] [Google Scholar]
- 34. Dweck MR, Bularga A, Hahn RT, Bing R, Lee KK, Chapman AR, White A, Salvo GD, Sade LE, Pearce K, Newby DE, Popescu BA, Donal E, Cosyns B, Edvardsen T, Mills NL, Haugaa K. Global evaluation of echocardiography in patients with COVID‐19. Eur Heart J Cardiovasc Imaging 2020;178:949–958. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Szekely Y, Lichter Y, Taieb P, Banai A, Hochstadt A, Merdler I, Gal Oz A, Rothschild E, Baruch G, Peri Y, Arbel Y, Topilsky Y. The spectrum of cardiac manifestations in coronavirus disease 2019 (COVID‐19) – a systematic echocardiographic study. Circulation 2020;142:342–353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Segar MW, Patel KV, Berry JD, Grodin JL, Pandey A. Generalizability and implications of the H2FPEF score in a cohort of patients with heart failure with preserved ejection fraction. Circulation 2019;139:1851–1853. [DOI] [PubMed] [Google Scholar]
- 37. Berliner D, Schneider N, Welte T, Bauersachs J. The differential diagnosis of dyspnea. Dtsch Arztebl Int 2016;113:834–845. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Han H, Xie L, Liu R, Yang J, Liu F, Wu K, Chen L, Hou W, Feng Y, Zhu C. Analysis of heart injury laboratory parameters in 273 COVID‐19 patients in one hospital in Wuhan, China. J Med Virol 2020;92:819–823. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Hu L, Chen S, Fu Y, Gao Z, Long H, Wang JM, Ren HW, Zuo Y, Li H, Wang J, Xu QB, Yu WX, Liu J, Shao C, Hao JJ, Wang CZ, Ma Y, Wang Z, Yanagihara R, Deng Y. Risk factors associated with clinical outcomes in 323 COVID‐19 hospitalized patients in Wuhan, China. Clin Infect Dis 2020;71:2089–2098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Siripanthong B, Nazarian S, Muser D, Deo R, Santangeli P, Khanji MY, Cooper LT Jr, Chahal CAA. Recognizing COVID‐19‐related myocarditis: the possible pathophysiology and proposed guideline for diagnosis and management. Heart Rhythm 2020;17:1463–1471. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Shi S, Qin M, Cai Y, Liu T, Shen B, Yang F, Cao S, Liu X, Xiang Y, Zhao Q, Huang H, Yang B, Huang C. Characteristics and clinical significance of myocardial injury in patients with severe coronavirus disease 2019. Eur Heart J 2020;41:2070–2079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Puntmann VO, Carerj L, Wieters I, Fahim M, Arendt C, Hoffmann J, Shchendrygina A, Escher F, Vasa‐Nicotera M, Zeiher AM, Vehreschild M, Nagel E. Outcomes of cardiovascular magnetic resonance imaging in patients recently recovered from coronavirus disease 2019 (COVID‐19). JAMA Cardiol 2020;5:1265–1273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Rajpal S, Tong MS, Borchers J, Zareba KM, Obarski TP, Simonetti OP, Daniels CJ. Cardiovascular magnetic resonance findings in competitive athletes recovering from COVID‐19 infection. JAMA Cardiol 2021;6:116–118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Basso C, Leone O, Rizzo S, De Gaspari M, van der Wal AC, Aubry MC, Bois MC, Lin PT, Maleszewski JJ, Stone JR. Pathological features of COVID‐19‐associated myocardial injury: a multicentre cardiovascular pathology study. Eur Heart J 2020;41:3827–3835. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Liu Y, Xie J, Gao P, Tian R, Qian H, Guo F, Yan X, Song Y, Chen W, Fang L, Wu W, Zhang S. Swollen heart in COVID‐19 patients who progress to critical illness: a perspective from echo‐cardiologists. ESC Heart Fail 2020;7:3621–3632. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Stöbe S, Richter S, Seige M, Stehr S, Laufs U, Hagendorff A. Echocardiographic characteristics of patients with SARS‐CoV‐2 infection. Clin Res Cardiol 2020;109:1549–1566. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Roden DM. Keep the QT interval: it is a reliable predictor of ventricular arrhythmias. Heart Rhythm 2008;5:1213–1215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Hu TY, Lee JZ, Asirvatham SJ. Cardiovascular considerations in coronavirus disease 2019 with a special focus on arrhythmia. J Innov Card Rhythm Manag 2020;11:4191–4198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Peretto G, Sala S, Rizzo S, De Luca G, Campochiaro C, Sartorelli S, Benedetti G, Palmisano A, Esposito A, Tresoldi M, Thiene G, Basso C, Della Bella P. Arrhythmias in myocarditis: state of the art. Heart Rhythm 2019;16:793–801. [DOI] [PubMed] [Google Scholar]
- 50. Guo T, Fan Y, Chen M, Wu X, Zhang L, He T, Wang H, Wan J, Wang X, Lu Z. Cardiovascular implications of fatal outcomes of patients with coronavirus disease 2019 (COVID‐19). JAMA Cardiol 2020;5:811–818. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Worldometer. Coronavirus updates. www.worldometers.info (10 May 2021).
- 52. Ludwig M, Jacob J, Basedow F, Andersohn F, Walker J. Clinical outcomes and characteristics of patients hospitalized for influenza or COVID‐19 in Germany. Int J Infect Dis 2021;103:316–322. [DOI] [PMC free article] [PubMed] [Google Scholar]


