Abstract
The coronavirus disease 2019 (COVID‐19) pandemic necessitated down‐scaling of in‐hospital care to prohibit the spread of severe acute respiratory syndrome–coronavirus‐2. We (1) assessed patient perceptions on quality of care by telesurvey (cohort 1) and written questionnaire (cohort 2), and (2) analyzed trends in elective and nonelective admissions before (December 2019 to February 2020) and during (March to May 2020) the COVID‐19 pandemic in Austria. A total of 279 outpatients were recruited into cohort 1 and 138 patients into cohort 2. All admissions from December 2019 to May 2020 to the Division of Gastroenterology/Hepatology at the Vienna General Hospital were analyzed. A total of 32.6% (n = 91 of 279) of cohort 1 and 72.5% (n = 95 of 131) of cohort 2 had telemedical contact, whereas 59.5% (n = 166 of 279) and 68.2% (n = 90 of 132) had face‐to‐face visits. A total of 24.1% (n = 32 of 133) needed acute medical help during health care restrictions; however, 57.3% (n = 51 of 89) reported that contacting their physician during COVID‐19 was difficult or impossible. Patient‐reported satisfaction with treatment decreased significantly during restrictions in cohort 1 (visual analog scale [VAS] 0‐10: 9.0 ± 1.6 to 8.6 ± 2.2; P < 0.001) and insignificantly in cohort 2 (VAS 0‐10: 8.9 ± 1.6 to 8.7 ± 2.1; P = 0.182). Despite fewer hospital admissions during COVID‐19, the proportion of nonelective admissions (+6.3%) and intensive care unit admissions (+6.7%) increased. Patients with cirrhosis with nonelective admissions during COVID‐19 had significantly higher Model for End‐Stage Liver Disease (MELD) (25.5 [14.2] vs. 17.0 [interquartile range: 8.8]; P = 0.003) and ΔMELD (difference from last MELD: 3.9 ± 6.3 vs. 8.7 ± 6.4; P = 0.008), required immediate intensive care more frequently (26.7% vs. 5.6%; P = 0.034), and had significantly increased 30‐day liver‐related mortality (30.0% vs. 8.3%; P = 0.028). Conclusion: The COVID‐19 pandemic’s effects on quality of liver care is evident from decreased patient satisfaction, hospitalization of sicker patients with advanced chronic liver disease, and increased liver‐related mortality. Strategies for improved telemedical liver care and preemptive treatment of cirrhosis‐related complications are needed to counteract the COVID‐19‐associated restrictions of in‐hospital care.
COVID‐19‐related health care restrictions impair liver care, as evident by decreased patient satisfaction, a rising number of nonelective admissions of sicker patients, and an increased liver‐related 30‐day mortality when compared with the pre‐COVID‐19 era.

Abbreviations
- ACLD
advanced chronic liver disease
- ACLF
acute‐on‐chronic liver failure
- ALD
alcohol‐associated liver disease
- CLIF‐C AD
Chronic Liver Failure–Consortium Acute Decompensation
- COVID‐19
coronavirus disease 2019
- CTP
Child‐Turcotte‐Pugh
- HCC
hepatocellular carcinoma
- ICU
intensive care unit
- IQR
interquartile range
- LT
liver transplantation
- MELD
Model for End‐Stage Liver Disease
- SARS‐CoV‐2
severe acute respiratory syndrome‐coronavirus‐2
- VAS
visual analogue scale
The coronavirus disease 2019 (COVID‐19) pandemic caused by the rapid spread of severe acute respiratory syndrome‐coronavirus‐2 (SARS‐CoV‐2) represents a substantial threat for personal and public health.( 1 ) SARS‐CoV‐2 infection may cause severe illness and death due to disease manifestations in the respiratory, neuronal, hepatic, and intestinal systems.( 2, 3, 4, 5 ) Limiting the spread of SARS‐CoV‐2 is currently a major public health goal. On the one hand, this goal is globally pursued by physical distancing, protective equipment,( 6 ) banning of large gatherings, and travel restrictions.( 7 ) On the other hand, restrictions of health care contacts in order to minimize the risks of infection by reducing face‐to‐face visits whenever possible has become common clinical practice.( 8 ) Additionally, re‐allocation of health care resources to respiratory care and intensive care units (ICUs) was especially needed in the beginning of the pandemic, to ensure sufficient availability of health care resources to patients with COVID‐19.( 9 )
As a consequence of these modifications of health care allocations, the management of non‐COVID‐19 patients with chronic diseases, such as liver disease, was affected. To guide treatment, international societies rapidly published consensus statements on the management of patients with liver disease during COVID‐19.( 8, 10 ) These recommendations emphasize the need for continuation of guideline‐compliant treatment. Additionally, the European Association for the Study of the Liver–European Society of Clinical Microbiology and Infectious Diseases position paper and American Association for the Study of Liver Disease expert panel consensus statement recommend decreasing face‐to‐face visits by, for example, postponing visits to specialized centers( 8 ) or delaying surveillance visits for hepatocellular carcinoma (HCC) and screening for gastroesophageal varices.( 8, 10 ) Furthermore, these guidelines recommend sending follow‐up prescriptions by mail based on routine laboratory testing performed in local laboratories rather than within the centers, to further reduce personal contacts. Additionally, the number of patients evaluated for liver transplantation (LT) should be limited whenever possible.( 8, 10 ) In this context, telemedicine (i.e., contacting patients via telephone or video calls) has emerged as an essential alternative for personal visits and represents an opportunity to limit personal contact, but still ensure sufficient follow‐up of patients with liver disease.( 11 ) These telemedicine strategies were also encouraged by international consensus statements.( 8, 10 )
Despite these ongoing efforts to ensure medical care of patients with liver disease, it is most likely that the COVID‐19 pandemic will have a negative impact on the quality of care, potentially resulting in an increased rate of emergency decompensations, increased rates of acute‐on‐chronic liver failure (ACLF), delayed HCC diagnoses, increased LT wait‐list morbidity, as well as increased liver‐related mortality.( 12, 13 ) In this context, it has already been shown that COVID‐19‐related health care restrictions had a significant negative short‐term impact on LT; for example, organ availability significantly decreased in Italy,( 14 ) and the LT program was temporarily suspended by many transplant centers in the United States.( 15 ) However, apart from COVID‐19‐associated impact on LT, to date, real‐world studies evaluating the magnitude of COVID‐19‐related consequences on liver‐related “real‐life” outcomes are missing.
Therefore, the objectives of this study were to assess the quality of care of patients with chronic liver disease at a large tertiary care hospital before and during COVID‐19‐related health care restrictions. To this end, we (1) invited patients with liver disease to provide their perceptions on quality of medical care in a specifically designed survey and questionnaire, and (2) analyzed all elective and nonelective admissions to the Division of Gastroenterology and Hepatology at the Vienna General Hospital before and during the COVID‐19 pandemic.
Patients and Methods
Study Population
To evaluate patient perceptions on COVID‐19‐associated health care restrictions, we performed a telesurvey in study cohort 1 (patients with advanced chronic liver disease [ACLD] and patients after LT), distributed a specifically designed written questionnaire in study cohort 2 (patients with non‐ACLD, patients with ACLD, and patients after LT), and finally assessed trends in nonelective and elective admissions of patients with liver disease (cohort 3).
Cohort 1 included all patients with ACLD or after LT attending regular visits at the Hepatology clinic of the Vienna General Hospital before COVID‐19‐related health care restrictions, defined as at least two visits between January 2019 and February 2020. These patients were contacted by telephone in June and July 2020 with at least two attempts. Patients without ACLD or a history of LT and without regular visits at the outpatient clinic were not contacted. For analysis, patients were stratified into the three categories: (1) ACLD, (2) HCC, and (3) LT.
Cohort 2 consisted of patients who had completed a written questionnaire, which was distributed to patients attending our outpatient clinic or admitted to the inpatient ward at the Vienna General Hospital starting in June 2020. For analyses, patients were stratified into four categories of liver disease: (1) patients with non‐ACLD liver disease, (2) ACLD, (3) HCC, and (4) patients after LT.
Finally, we retrospectively evaluated cohort 3, consisting of all patients with liver disease admitted to the Division of Gastroenterology and Hepatology at the Vienna General Hospital between December 2019 and May 2020. This period was chosen because the first case of COVID‐19 in Austria was registered on February 26, 2020,( 16 ) and associated measures to adopt health care resources were initiated at the beginning of March. Moreover, along with the gradual ease of the country’s lockdown,( 17 ) health care restrictions were alleviated by the end of May. Accordingly, we compared 3 months before COVID‐19‐related health care restrictions (December 2019 to February 2020) to 3 months during COVID‐19‐related health care restrictions (March 2020 to May 2020).
Medical Care During COVID‐19‐Associated Health Care Restrictions
The strategy for liver disease–specific medical care during COVID‐19‐associated health care restrictions consisted of active contacting of patients and offering telemedicine as replacements for canceled personal visits. Moreover, information concerning COVID‐19 and liver disease was provided at the departmental website and by the Austrian Society of Gastroenterology and Hepatology online and through patient advocacy groups. In the context of the telesurvey, patients were also scheduled for future visits, if requested or necessary.
Telesurvey and Written Questionnaire
A telesurvey designed with 11 liver care–related questions was conducted in cohort 1 (Fig. 1A and Table 1 [questions translated to English]). Written questionnaires consisting of 11 questions in German language were distributed to patients at the inpatient ward and outpatient clinic of the Division of Gastroenterology and Hepatology at the Vienna General Hospital (Fig. 1B and Table 2 [questions translated to English]). Although both surveys evaluated medical treatment of patients with liver disease during COVID‐19‐related health care restrictions, the telesurvey placed more emphasis on use and acceptance of telemedicine, whereas the written questionnaire explored worries of patients concerning COVID‐19 and liver disease. Visual analog scale (VAS) ranging from 0‐10 was used to determine patient satisfaction with quality of care (10 indicating perfect quality of care). For statistical analysis, patients were stratified into VAS groups (0‐4, 5‐7, and 8‐10) before and during COVID‐19‐related restrictions.
FIG. 1.
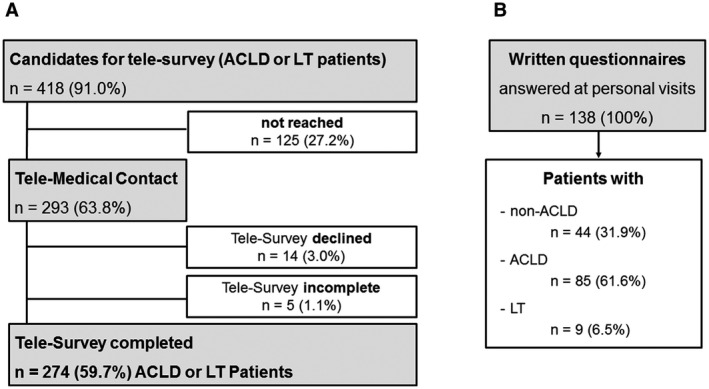
Patient flowchart. (A) Patients with ACLD or with a history of LT with regular visits at the hepatology outpatient clinic of the Vienna General Hospital were contacted for a telesurvey. Of the 418 contacted patients, n = 125 patients did not respond to the call. Most of the patients with ACLD (98.0%) and LT patients (100%) agreed to complete the survey, but n = 5 surveys were not fully completed, resulting in a final number of n = 274 patients (59.8%) completing the telesurvey. (B) Among patients who had a face‐to‐face visit at the Gastroenterology/Hepatology inpatient ward or outpatient clinic, n = 44 with nonadvanced chronic liver disease (including patients with viral hepatitis, cholestatic liver diseases, genetic and metabolic liver diseases), n = 85 with ACLD, and n = 9 patients in care after LT completed the written survey.
TABLE 1.
Telesurvey Results of All Patients, Patients With ACLD, Patients With HCC, and LT Patients
| Question | All Patients (n = 279)* | ACLD (n = 204)† | HCC (n = 41)‡ | LT (n = 34) | ||||
|---|---|---|---|---|---|---|---|---|
| Was a clinical visit canceled during COVID‐19‐related health care restrictions? yes/no (% yes) | 103/176 (36.9%) | 73/131 (35.8%) | 15/26 (36.6%) | 15/19 (44.1%) | ||||
| Did you have a telemedical contact with your treating physician during COVID‐19‐related health care restrictions? yes/no (% yes) | 91/188 (32.6%) | 54/150 (26.5%) | 23/18 (56.1%) | 14/20 (41.2%) | ||||
| Did you have a personal visit at the Vienna General Hospital during COVID‐19‐related health care restrictions? yes/no (% yes) | 166/113 (59.5%) | 113/91 (55.4%) | 31/10 (75.6%) | 22/12 (64.7%) | ||||
| Did you try to contact your treating physician at the Vienna General Hospital by telephone during COVID‐19‐related health care restrictions? yes/no (% yes) | 89/190 (31.9%) | 61/143 (29.9%) | 17/24 (41.5%) | 11/23 (32.4%) | ||||
| If yes: Was it (1) easy, (2) difficult, or (3) impossible to contact your treating physician at the Vienna General Hospital during COVID‐19‐related health care restrictions? | Easy: 38 (42.7%) | Easy: 26 (42.6%) | Easy: 8 (47.1%) | Easy: 3 (27.3%) | ||||
| Difficult: 26 (29.2%) | Difficult: 15 (24.6%) | Difficult: 7 (41.2%) | Difficult: 4 (36.4%) | |||||
| Impossible: 25 (28.1%) | Impossible: 20 (32.8%) | Impossible: 2 (11.7%) | Impossible: 4 (36.4%) | |||||
| How satisfied were you with the quality of treatment at the Vienna General Hospital before COVID‐19‐related health care restrictions on a scale of 0‐10? (mean ± SD) | 9.0 ± 1.6 | P < 0.001 | 9.0 ± 1.5 | P < 0.001 | 9.1 ± 1.3 | P = 0.159 | 8.7 ± 1.9 | P = 0.787 |
| How satisfied were you with the quality of treatment at the Vienna General Hospital during COVID‐19‐related health care restrictions on a scale of 0‐10? (mean ± SD) | 8.6 ± 2.2 | 8.5 ± 2.3 | 8.9 ± 1.5 | 8.8 ± 2.2 | ||||
| Δ satisfaction before and during COVID‐19‐related health care restrictions (mean ± SD) | 0.4 ± 1.6 | 0.6 ± 1.6 | 0.2 ± 0.9 | 0.1 ± 1.9 | ||||
| Did you experience any problems in getting your medication during COVID‐19‐related health care restrictions? yes/no (% yes) | 22/254 (8.0%) | 15/187 (7.4%) | 2/38 (5.0%) | 5/29 (14.7%) | ||||
| Do you plan to schedule another appointment at the clinic of the Vienna General Hospital now or in the future? yes/no (% yes) | 269/9 (96.8%) | 197/6 (97.0%) | 38/3 (92.7%) | 34/0 (100%) | ||||
| Would you also prefer to have telemedical contact via phone or video calls in the future? yes/no (% yes) | 151/123 (55.1%) | 106/94 (53%) | 22/18 (55.0%) | 23/11 (67.6%) | ||||
| In your opinion, how important is it to meet your treating physician in person on a scale of 0‐10? (mean ± SD) | 8.6 ± 2.2 | 8.7 ± 2.2 | 8.7 ± 1.7 | 7.7 ± 2.8 | ||||
n = 274 surveys complete; n = 5 surveys incomplete.
n = 201 surveys complete; n = 4 surveys incomplete.
n = 40 surveys complete; n = 1 survey incomplete.
TABLE 2.
Questionnaire Results of All Patients, Non‐ACLD Patients, Patients With ACLD, Patients With HCC, and LT Patients
| Question | All Patients (n = 138) | Non‐ACLD (n = 44) | ACLD (n = 63) | HCC (n = 22) | LT (n = 9) | |||||
|---|---|---|---|---|---|---|---|---|---|---|
| Would you recommend treatment in our Hepatology Clinic to a friend? scale 0‐10 (mean ± SD) | 8.9 ± 2.1 | 8.8 ± 1.9 | 8.9 ± 2.0 | 8.6 ± 3.0 | 10 ± 0.0 | |||||
| How satisfied were you with your treatment at the Vienna General Hospital before COVID‐19‐related health care restrictions on a scale of 0‐10? (mean ± SD) | 8.9 ± 1.6 | P = 0.182 | 8.8 ± 1.7 | P = 0.288 | 8.9 ± 1.8 | P = 0.359 | 9.0 ± 1.4 | P = 0.315 | 9.9 ± 0.3 | P = 0.170 |
| How satisfied were you with your treatment at the Vienna General Hospital during COVID‐19‐related health care restrictions on a scale of 0‐10? (mean ± SD) | 8.7 ± 2.1 | 8.4 ± 2.4 | 8.6 ± 2.3 | 9.3 ± 1.0 | 9.1 ± 1.7 | |||||
| Δ satisfaction before and during COVID‐19‐related health care restrictions (mean ± SD) | 0.2 ± 2.1 | 0.4 ± 2.3 | 0.3 ± 2.2 | 0.3 ± 1.3 | 0.8 ± 1.4 | |||||
| How many “in‐person” visits did you attend at the Vienna General Hospital during the COVID‐19‐pandemic? 0 = none, 1 = 1‐2, 2 = >2 | none: 42 (31.8%) | none: 15 (35.7%) | none: 18 (30.0%) | none: 6 (27.3%) | none: 3 (37.5%) | |||||
| 1‐2: 59 (44.7%) | 1‐2: 16 (38.1%) | 1‐2: 28 (46.7%) | 1‐2: 11 (50.0%) | 1‐2: 4 (50%) | ||||||
| >2: 31 (23.4%) | >2: 11 (26.2%) | >2: 14 (23.3%) | >2: 5 (22.7%) | >2: 1 (12.5%) | ||||||
| How many times did you have contact via telephone or email with doctors of the Hepatology Clinic during the COVID‐19 pandemic? 0 = none, 1 = 1‐2, 2 = >2 | none: 36 (27.5%) | none: 10 (23.8%) | none: 15 (25.9%) | none: 6 (27.3%) | none: 5 (55.6%) | |||||
| 1‐2: 74 (56.5%) | 1‐2: 23 (54.8%) | 1‐2: 33 (56.9%) | 1‐2: 14 (63.6%) | 1‐2: 4 (44.4%) | ||||||
| >2: 21 (16.0%) | >2: 9 (21.4%) | >2: 10 (17.2%) | >2: 2 (9.1%) | >2: 0 (0.0%) | ||||||
| Did you acutely need medical help during the COVID‐19 pandemic? yes/no (% yes) | 32/101 (24.1%) | 9/33 (21.4%) | 13/47 (21.7%) | 9/13 (40.9%) | 1/6 (16.7%) | |||||
| Did you encounter more difficulties in searching for medical advice during the COVID‐19 pandemic than before? yes/no (% yes) | 31/97 (24.2%) | 12/28 (30.0%) | 13/44 (22.8%) | 4/18 (18.2%) | 2/7 (22.2%) | |||||
| Did you encounter more difficulties in getting your medication during the COVID‐19 pandemic than before? yes/no (% yes) | 11/124 (8.1%) | 3/38 (7.3%) | 7/56 (11.1%) | 0/22 (0.0%) | 1/8 (12.5%) | |||||
| Do you feel sufficiently informed about the consequences of a SARS‐CoV‐2‐infection on your liver disease? yes/no (% yes) | 91/43 (67.9%) | 24/17 (58.5%) | 45/17 (72.6%) | 17/5 (77.3%) | 5/4 (55.6%) | |||||
| Do you have concerns about negative effects on your liver disease during the COVID‐19 pandemic? yes/no (% yes) | 44/89 (33.1%) | 17/24 (41.5%) | 17/44 (27.9%) | 6/16 (27.3%) | 4/5 (44.4%) | |||||
| Do you have the feeling that your medical treatment and management is worse during the COVID‐19 pandemic than before? yes/no (% yes) | 16/115 (12.2%) | 7/35 (16.7%) | 7/51 (12.1%) | 1/21 (4.5%) | 1/8 (12.5%) | |||||
Admissions to the Division of Gastroenterology and Hepatology at the Vienna General Hospital
In patients admitted to the inpatient ward, as well as the ICU, the type of admission (elective/nonelective), underlying liver disease (non‐ACLD, ACLD, HCC, and LT), as well as the incidence of (non‐)liver‐related death during hospitalization were evaluated by chart review. Liver‐related death was defined as death attributed to underlying liver disease or associated complications. Additionally, in patients with ACLD, etiology of disease, 2016 Model for End‐Stage Liver Disease (MELD),( 18 ) Child‐Turcotte‐Pugh (CTP) score, and Chronic Liver Failure–Consortium Acute Decompensation (CLIF‐C AD) score( 19 ) at the time of admission were assessed. Moreover, changes over time in MELD (ΔMELD), a well‐validated parameter for disease progression especially in advanced liver disease,( 20 ) as well as changes in CLIF‐C AD score (ΔCLIF‐C AD) compared with the last routine visit (within 1 year before admission) were assessed.
Statistical Analyses
Continuous data were presented as mean ± SD or median and interquartile range (IQR), as appropriate. D’Agostino & Pearson and Shapiro‐Wilk tests were used to check for normal distribution. Categorical variables were reported as number (n) and percent (%) of patients with the characteristic of interest. Comparisons of continuous variables between two groups were performed using unpaired t test and, where appropriate, paired t test was applied. Mann‐Whitney U test was used to compare continuous variables without normal distribution between two groups. Continuous variables in three or more groups were compared through one‐way analysis of variance. Group comparisons of categorical variables were conducted using Pearson’s chi‐squared or Fisher’s exact test. GraphPad Prism 8 (GraphPad Software, La Jolla, CA) was used for statistical analysis. A two‐sided P value < 0.050 was considered statistically significant.
Ethics
This study was approved by the local ethics committee of the Medical University of Vienna (MUV‐EKN‐1461/2020) and performed according to the current version of the Helsinki Declaration (2013). Written, informed consent was obtained from all patients completing the questionnaire.
Results
Study Population of Cohorts 1 and 2
In total, 459 patients with ACLD or a history of LT with regular visits to the Hepatology Clinic were considered for the telemedical survey (Fig. 1). Of these, 32 (7.0%) patients died during COVID‐19‐related health care restrictions and therefore could not be contacted. Additionally, no valid contact data were available for 9 (2.0%) patients. After excluding these patients, 418 (91.0%) patients were included in cohort 1 and contacted for the telesurvey. A total of 125 patients (125 of 418, 29.9%), including 99 of 317 (31.2%) patients with ACLD, 20 of 61 (32.8%) patients with HCC, and 6 of 40 (15.0%) patients after LT were not reached for the telesurvey (P = 0.093). However, this must not be overinterpreted, as contact details might have changed. Finally, two thirds of patients (n = 293, 63.8%) were reached. Of these, 14 (3.0%; ACLD: n = 14 of 218 [6.4%]) patients declined participation at the telesurvey, and 5 patients (1.8%; ACLD: n = 4 of 204 [2.0%]; and HCC: n = 1 of 41 [2.4%]) did not complete the whole survey. Thus, complete results of 274 patients (ACLD: n = 200 [73.0%]; HCC: n = 40 [14.6%]; and LT: n = 34 [12.4%]) were obtained. Cohort 2 included 138 patients (n = 123 [89.1%] outpatients and n = 15 [10.9%] inpatients) who answered the written questionnaire during personal visits. Of these, n = 44 (31.9%) were classified as non‐ACLD patients, whereas n = 63 (45.7%) had ACLD, n = 22 (15.9%) had HCC, and n = 9 (6.5%) had a history of LT. Unfortunately, some patients did not complete all questions, resulting in n = 106 (76.8%) completely answered questionnaires (non‐ACLD: n = 36 [33.9%]; ACLD: n = 43 [40.6%]; HCC: n = 20 [18.9%]; and LT: n = 7 [6.6%]).
Most of the patients in cohorts 1 and 2 were men (cohort 1: 68.5% [n = 191]; cohort 2: 65.9% [n = 91]) with a median age of 60.8 (interquartile range [IQR]: 15.7) years in cohort 1 and 59.0 (IQR: 9.0) years in cohort 2 (P = 0.004). Etiology of liver disease was alcohol‐associated liver disease (ALD; 43.0%), viral hepatitis (22.2%), nonalcoholic fatty liver disease (NAFLD; 10.9%), cholestatic liver disease (5.8%), cryptogenic etiology (6.7%), and others (10.6%) in cohort 1. The main etiologies of liver disease were viral hepatitis (31.9%), ALD (27.5%), and NAFLD (8.7%) in cohort 2.
When comparing cohort 1 patients, who were reached versus those who could not be contacted, the groups did not differ in sex, age, etiology of ACLD, MELD score, proportion of decompensated ACLD, HCC, or history of LT (Supporting Table S1). However, patients, who were not reached had significantly more advanced CTP classes (Child‐B: 31.2% [n = 39] and Child‐C: 10.4% [n = 13]) versus patients reached (Child‐B: 30.4% [n = 89] and Child‐C: 3.4% [n = 10]; P = 0.013).
Results of the Surveys: Ability to Contact Treating Physician
As indicated in Tables 1 and 2, overall, 32.6% (91 of 279) of patients in cohort 1 and 72.5% (n = 95 of 131) of cohort 2 had telemedical contact with the Hepatology Clinic (not including the telesurvey itself). Additionally, 59.5% (n = 166 of 279) of cohort 1 and 68.2% (n = 90 of 132) of cohort 2 had at least one personal visit at the outpatient clinic during COVID‐19‐related health care restrictions.
However, 103 of 279 (36.9%) patients of cohort 1 reported that at least one scheduled clinical visit was canceled during COVID‐19‐related health care restrictions. Nevertheless, 74.8% (n = 77 of 103) of these patients had telemedical contact or at least one personal visit until the telesurvey, whereas 25.2% (n = 26 of 103) had no contact with their hepatology specialist at all (data not shown). Interestingly, patients with ACLD reported significantly less telemedical contact with their hepatology specialist (ACLD: 26.5% [n = 54 of 204], HCC: 56.1% [n = 23 of 41], and LT: 41.2% [n = 14 of 34]; P < 0.001) and less personal visits in the hospital (ACLD: 55.4% [n = 113 of 204], HCC: 75.6% [n = 31 of 41], LT: 64.7% [n = 22 of 34]; P = 0.044) during COVID‐19‐related health care restrictions when compared to patients with HCC or LT patients.
Notably, 96.8% (n = 269 of 278) of cohort 1 patients planned to schedule another appointment at the Hepatology Clinic of the Vienna General Hospital.
Results of the Surveys: Acute Medical Problems and Access to Medication
Almost one quarter of cohort 2 patients (24.1%, n = 32 of 133) required acute medical help during COVID‐19‐related health care restrictions, and this percentage was even higher in patients diagnosed with HCC (non‐ACLD: 9 of 42 [21.4%], ACLD: 13 of 60 [21.7%], HCC: 9 of 22 [40.9%], and LT: 1 of 7 [16.7%]; P = 0.253). Accordingly, 89 of 279 (31.9%) patients of cohort 1 tried to contact their treating physician during COVID‐19‐related health care restrictions, and more than half of these patients (57.3%, 51 of 89) reported that achieving this was difficult or impossible. Consistently, 24.2% (n = 31 of 128) of patients in cohort 2 (non‐ACLD: 30.0% [n = 12 of 40], ACLD: 22.8% [n = 13 of 57], HCC: 18.2% [n = 4 of 22], and LT: 22.2% [n = 2 of 7]) reported increased problems in searching for medical help when compared with the situation before COVID‐19. Access to necessary medication during COVID‐19 was reported to be difficult in 8.0% (n = 22 of 276) of cohort 1 and 8.1% (n = 11 of 135) of cohort 2 patients. Consistently, one third of patients (33.1%, n = 44 of 133) reported concerns about negative effects of COVID‐19‐associated health care restrictions on their liver disease, and 12.2% (n = 16 of 131) stated that their medical treatment was worse during COVID‐19‐related health care restrictions.
Patient Satisfaction With Treatment of Liver Disease During COVID‐19‐Related Health Care Restrictions
The trends of patient perceptions on the quality of medical care before and during COVID‐19‐related health care restrictions reported by cohort 1 and cohort 2 are displayed in Fig. 2. In cohort 1, satisfaction with medical care before COVID‐19 was generally very high, as indicated by a VAS value of 9.0 ± 1.6, but lower during COVID‐19‐related health care restrictions with 8.6 ± 2.2. Notably, in cohort 1, satisfaction with medical care decreased during COVID‐19, both assessed by difference of (Δ) satisfaction (VAS: 0.4 ± 1.6; P < 0.001) and by stratifying patients for satisfaction strata before and during COVID‐19 (Fig. 2; VAS 0‐4, 5‐7, and 8‐10; P = 0.007). This decrease in satisfaction was more pronounced among patients with ACLD (n = 201, Δsatisfaction: 0.6 ± 1.6; P < 0.001), whereas Δsatisfaction was only minimal in patients with HCC (n = 40, Δsatisfaction: 0.2 ± 0.9; P = 0.159) and LT patients (n = 34, Δsatisfaction: 0.1 ± 1.9; P = 0.787). Interestingly, there was no significant difference in satisfaction with medical care before and during COVID‐19‐related health care restrictions in cohort 2 (n = 127), and satisfaction with medical care remained rather high with 8.9 ± 1.6 before COVID‐19 and 8.7 ± 2.1 during COVID‐19. Additionally, patients of cohort 2 were likely to recommend treatment at the Hepatology Clinic to a friend (VAS: 8.9 ± 2.1). However, only two thirds (67.9%, n = 91 of 143) of cohort 2 patients felt sufficiently informed about potential consequences of COVID‐19 on their liver disease.
FIG. 2.
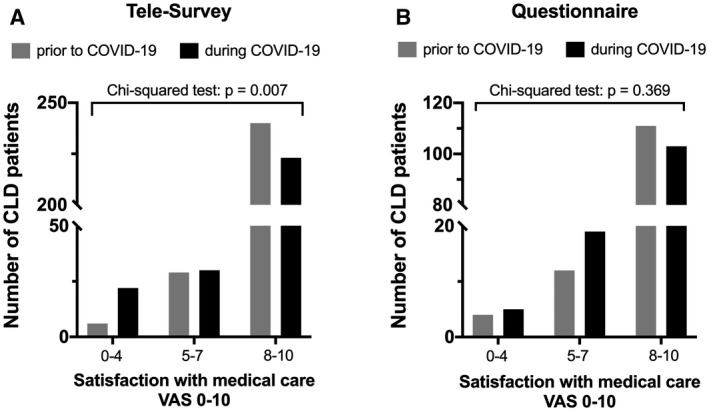
Patient perception on quality of care before and during COVID‐19‐related restrictions of health care assessed through VAS (0‐10) by telesurvey (A) and written questionnaire (B). For analyses, patients were stratified to the following VAS categories: 0‐4, 5‐7, and 8‐10.
Patient Perceptions on Telemedicine (Table 1, Supporting Table S2)
As indicated in Table 1 and Supporting Table S1, 55.1% of cohort 1 patients (n = 151 of 274) would prefer to continue telemedical contact in the future. Interestingly, especially women and younger patients (<60 years) tended to prefer telemedical contact (male: 97 of 188 [51.6%] vs. female: 54 of 86 [62.8%]; P = 0.090; <60 years: 74 of 121 [61.2%] vs. ≥60 years: 77 of 153 [50.3%]; P = 0.087). Consistently, a higher proportion of patients with ACLD ≥ 60 years reported difficulties reaching their treating physician (<60 years: 17 of 36 [47.2%] vs. ≥60 years: 34 of 53 [64.1%]; P = 0.031), and these patients were more likely to have personal visits during COVID‐19‐related health care restrictions (<60 years: 65 of 125 [52.0%] vs. ≥60 years: 101 of 154 [64.7%]; P = 0.027). Face‐to‐face contact with the treating physician was very important for all patients with liver disease (overall VAS: 8.6 ± 2.2), with numerically lower values among LT patients (ACLD: 8.7 ± 2.2; HCC: 8.7 ± 1.7; and LT: 7.7 ± 2.8; P = 0.066).
Elective and Emergency Admissions During COVID‐19‐Related Downscaling of In‐Hospital Care
As indicated in Figs. 3 and 4 and Table 3, in the three comparator months before COVID‐19 (December 2019 to February 2020), there were 513 admissions to the Gastroenterology/Hepatology wards, of which 266 (51.9%) were admissions of patients with liver disease. During the 3 months of COVID19‐related health care restrictions (March to May 2020), a total of 387 admissions, including 201 (51.9%) liver‐related admissions, were observed.
FIG. 3.
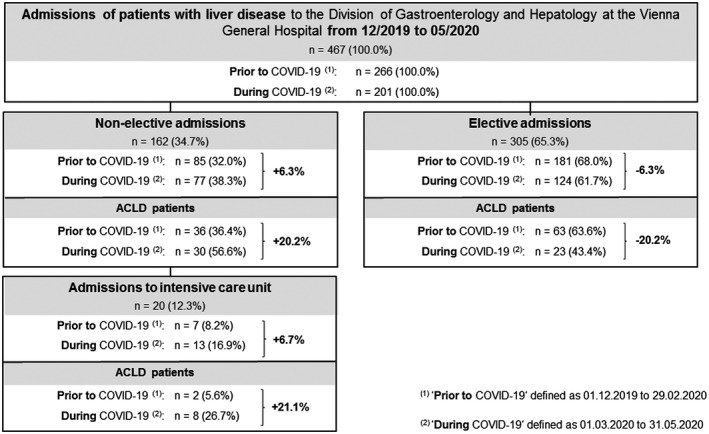
Patient admissions. Number of patient admissions with liver disease to the Division of Gastroenterology and Hepatology at the Vienna General Hospital between December 2019 and May 2020. Elective and nonelective admissions before and during COVID‐19‐related health care restrictions are shown separately.
FIG. 4.
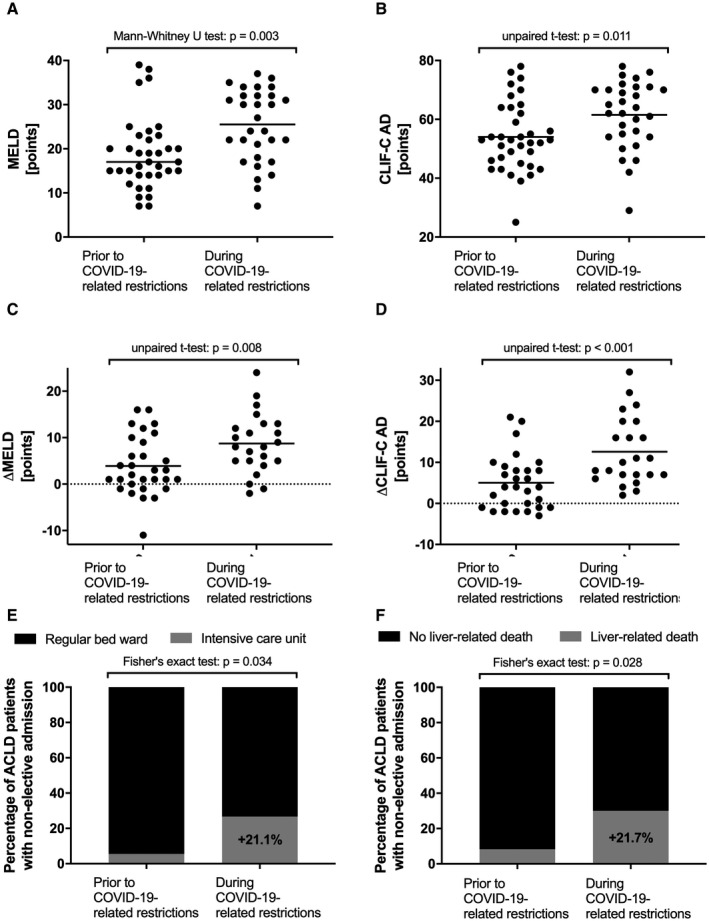
Severity of liver disease as well as outcomes of patients with ACLD nonelectively admitted to the Vienna General Hospital before and during COVID‐19‐related health care restrictions. Comparison of MELD score (A), CLIF‐C AD score (B), ΔMELD (C), and ΔCLIF‐C AD (D) of nonelectively admitted patients with ACLD before and during COVID‐19‐related health care restrictions. Comparison of rates of nonelective admissions of patients with ACLD to the regular ward versus ICU (E) and liver‐related 30‐day mortality of patients with ACLD before and during COVID‐19‐related health care restrictions (F).
TABLE 3.
Characteristics of Patients With ACLD With Nonelective and Elective Admission Before Versus During COVID‐19‐Related Health Care Restrictions
| Nonelective Admissions ( n = 66) | Elective Admissions (n = 86) | |||||
|---|---|---|---|---|---|---|
| Before COVID‐19 ( n = 36) | During COVID‐19 ( n = 30) | P Value | Before COVID‐19 ( n = 63) | During COVID‐19 ( n = 23) | P Value | |
| Sex, male/female (% male) | 26/10 (72.2%) | 18/12 (60.0%) | 0.310 | 45/18 (71.4%) | 12/11 (52.2%) | 0.123 |
| Age, years (IQR) | 60.9 (17.1) | 61.3 (13.5) | 0.840 | 57.9 (18.4) | 60.6 (9.1) | 0.230 |
| Etiology | 0.590 | 0.406 | ||||
| ALD, n (%) | 14 (39.0%) | 16 (53.3%) | 24 (38.0%) | 8 (34.8%) | ||
| Viral hepatitis, n (%) | 7 (19.4%) | 3 (10.0%) | 11 (17.5%) | 6 (26.1% | ||
| Cholestatic, n (%) | 7 (19.4%) | 4 (13.3%) | 10 (15.9%) | 1 (4.3%) | ||
| Cryptogenic, n (%) | 4 (11.1%) | 5 (16.7%) | 7 (11.1%) | 5 (21.7%) | ||
| Other, n (%) | 4 (11.1%) | 2 (6.7%) | 11(17.5%) | 3 (13.1%) | ||
| MELD, median (IQR) | 17.0 (8.8) | 25.5 (14.2) | 0.003 | 10 (8.0) | 11 (9.0) | 0.862 |
| ΔMELD, mean ± SD* | 3.9 ± 6.3 | 8.7 ± 6.4 | 0.008 | |||
| Severe/refractory ascites, n (%) | 11 (30.6%) | 19 (63.3%) | 0.012 | 11 (17.5%) | 8 (34.8%) | 0.139 |
| CTP score, median (IQR) | 9.0 (3.5) | 10.0 (3.0) | 0.230 | 7 (3) | 7 (4) | 0.876 |
| Child‐A, n (%) | 3 (8.3%) | 0 (0.0%) | 0.172 | 25 (39.7%) | 11 (47.8%) | 0.510 |
| Child‐B, n (%) | 16 (44.4%) | 11 (36.7%) | 33 (52.4%) | 9 (39.1%) | ||
| Child‐C, n (%) | 17 (47.1%) | 19 (63.3%) | 5 (7.9%) | 3 (13.1%) | ||
| CLIF‐C AD score, mean ± SD | 54.0 ± 11.7 | 61.5 ± 11.5 | 0.011 | |||
| ΔCLIF‐C AD score, mean ± SD* ) | 5.0 ± 6.6 | 12.6 ± 8.3 | <0.001 | |||
| White blood cells, per 109 cells (IQR) | 5.2 (4.2) | 7.6 (5.9) | 0.117 | |||
| Albumin, g x L‐1 (IQR) | 29.5 (8.1) | 30.5 (7.7) | 0.992 | 36.9 (6.0) | 37.7 (7.3) | 0.381 |
| Bilirubin, mg x dL‐1 (IQR) | 2.4 (5.1) | 2.2 (8.6) | 0.597 | 1.2 (1.3) | 1.1 (1.1) | 0.498 |
| International normalized ratio, median (IQR) | 1.5 (0.7) | 1.7 (0.4) | 0.157 | 1.3 (0.3) | 1.2 (0.2) | 0.186 |
| Creatinine, mg x dL‐1 (IQR) | 1.1 (0.9) | 1.3 (0.8) | 0.209 | 0.8 (0.3) | 0.8 (1.2) | 0.286 |
| Sodium, mmol x L‐1 (IQR) | 136.0 (6.0) | 130.0 (11.3) | 0.021 | 138 (5.0) | 139 (3.0) | 0.224 |
| Main reason for admission: | 0.392 | |||||
| Infection, n (%) | 7 (19.4%) | 3 (10.0%) | ||||
| Acute bleeding, n (%) | 6 (16.7%) | 10 (33.3%) | ||||
| Acute decompensation, n (%) | 20 (55.6%) | 15 (50.0%) | ||||
| ACLF, n (%) | 3 (8.3%) | 2 (6.7%) | ||||
| Median duration of hospital stay, days | 9.5 (11.8) | 13.0 (13.7) | 0.223 | |||
| Admission to ICU, n (%) | 2 (5.6%) | 8 (26.7%) | 0.034 | |||
| 30‐day mortality, n (%) | 4 (11.1%) | 9 (30.0%) | 0.068 | |||
| Liver‐related 30‐day mortality, n (%) | 3 (8.3%) | 9 (30.0%) | 0.028 | |||
Note: The boldfaced values found in Table 3 were considered statistically significant.
n = 53/66 (n = 30/36 before and n = 23/30 during COVID‐19‐related health care restrictions).
Accordingly, 467 admitted patients with liver disease were included in cohort 3. While 305 (65.3%) admissions (non‐ACLD: n = 91 [29.8%], ACLD: n = 86 [28.2%], HCC: n = 122 [40.0%], and LT: n = 6 [2.0%]) were elective, 162 (34.7%; non‐ACLD: n = 63 [38.9%], ACLD: n = 66 [40.8%], HCC: n = 26 [16.0%]; and LT: n = 7 [4.3%]) were nonelective (i.e., emergency) admissions. Unsurprisingly, elective admissions of patients with ACLD were significantly reduced (P = 0.025) during COVID‐19‐related restrictions of health care. In detail, the proportion of elective ACLD admissions decreased by 16.3%, whereas elective HCC admissions increased by +19.6% (P = 0.002; Supporting Fig. S1). These trends were not observed in patients with nonelective admissions (P = 0.838).
Interestingly, before COVID‐19, 181 (68.0%) patients were admitted electively and 85 (32.0%) nonelectively, while there were 124 (61.7%) elective and 77 (38.3%) emergency admissions (Supporting Fig. S1) during COVID‐19, resulting in a relative increase of emergency admissions of +6.3%. The main reasons for nonelective admission of patients with ACLD were infections (prior COVID‐19: 19.4% [n = 7] vs. during COVID‐19: 10.0% [n = 3]), acute gastrointestinal bleeding (prior: 16.7% [n = 6] vs. during: 33.3% [n = 10]), acute nonbleeding decompensation (prior: 55.6% [n = 20] vs. during: 50.0% [n = 15]), and ACLF (prior: 8.3% [n = 3] vs. during: 6.7% [n = 2]).
Severity of Liver Disease and Liver‐Related Mortality Before and During COVID‐19
Patients with ACLD with nonelective admissions during COVID‐19 had significantly more pronounced liver disease as compared with the time period before COVID‐19, as indicated by significantly higher median MELD (prior: 17.0 [IQR: 8.8] vs. during: 25.5 [IQR: 14.2] points; P = 0.003) and mean CLIF‐C AD score (prior: 54.0 ± 11.7 vs. during: 61.5 ± 11.5 points; P = 0.011). Importantly, differences in MELD and CLIF‐C AD scores compared with values at the last routine visit were significantly higher during COVID‐19 than before (ΔMELD [prior: 3.9 ± 6.3 vs. during: 8.7 ± 6.4 points; P = 0.008] and ΔCLIF‐C AD [prior: 5.0 ± 6.6 vs. during: 12.6 ± 8.3 points; P < 0.001]). This information was available in n = 30 of 36 (83.3%) before and n = 23 of 30 (76.7%) during COVID‐19. Moreover, the proportion of patients with severe/refractory ascites doubled (prior: n = 11 [30.6%] vs. during: n = 19 [63.3%]; P = 0.012) during COVID‐19. In contrast, in electively admitted patients with ACLD, liver disease severity was comparable in the time periods before versus during COVID‐19 (MELD: 10.0 [8.0] vs. 11.0 [9.0] points; P = 0.862) (Supporting Fig. S1).
Additionally, during COVID‐19, a significantly higher proportion of patients with ACLD had to be immediately admitted to the ICU (during: n = 8 [26.7%] vs. prior: n = 2 [5.6%]; P = 0.034). Finally, liver‐related 30‐day mortality of nonelectively admitted patients with ACLD was significantly higher during the 3 months of COVID‐19‐associated health care restrictions with 30.0% (n = 9 of 30), as compared to the 3 months prior with 8.3% (n = 3 of 36; P = 0.028).
Discussion
In this study, we present important patient perceptions on liver disease management during COVID‐19‐associated restriction of in‐hospital care. We analyzed data obtained from two large cohorts of patients with liver disease, answering a telesurvey (n = 279) and a specifically designed questionnaire (n = 138 patients). In addition, we collected critical benchmark data of in‐hospital care of patients with liver disease, including the number of elective and nonelective admissions of patients with liver disease in representative time periods before and during COVID‐19‐associated health care restrictions. Reductions of in‐hospital patient visits and other health care restrictions due to the global COVID‐19 pandemic resulted in decreased satisfaction with care, nonelective hospitalization of sicker patients with ACLD, and a higher liver‐related mortality. To our knowledge, this is the first study highlighting the negative impacts of the COVID‐19 pandemic on the quality of liver disease care on both a subjective and objective level, affirming and extending the predictions made by Tapper and Asrani( 12 ) and Pawlotsky.( 13 )
Continuous, state‐of‐the‐art care by specialists is imperative for patients with liver disease, as appropriate care improves quality of life, delays complications, and likely extends survival.( 21, 22, 23, 24, 25, 26, 27 ) This need for ongoing professional care is exceedingly problematic in the context of the COVID‐19 pandemic, as ACLD( 28, 29, 30 ) and LT patients( 31, 32 ) are possibly more susceptible for severe COVID‐19. Thus, liver disease–specific care must be maintained at the best possible level, while at the same time minimizing personal contact, to protect potentially vulnerable patients and limit the spread of the virus.( 8, 10 ) Telemedicine is an attractive means for achieving this, and telemedical contacts have been massively expanded throughout the course of COVID‐19‐related restrictions of health care.( 11, 12 ) This could be seen in cohort 2 of our study (72.5% with telemedical contact), but also in cohort 1, in which 40.8% of patients with canceled clinical visits had telemedical contact with their treating physician. Furthermore, most of the telesurvey patients expressed their openness to telemedical contact in the future, underscoring the acceptance of these measures.( 11, 33, 34, 35 ) Nevertheless, telemedicine may come with significant obstacles for specific patient groups like older or socially underprivileged patients, and indeed, patients ≥60 years of age tended to be more skeptical toward telemedical visits. Consistently, many patients with liver disease also expressed their strong desire for personal contact with their treating physician.
Overall, the level of satisfaction with liver disease management was high both before and during COVID‐19, despite many canceled visits in the outpatient and/or inpatient clinic. Nevertheless, satisfaction with medical care did decrease during COVID‐19‐related restrictions, most notably among patients with ACLD. Patients with ACLD also had significantly fewer telemedical and personal visits during health care restrictions, indicating problems that are specific to the group of patients with cirrhosis. Whether this lower number of visits is due to worse compliance, which might be explained by medical or psychosocial factors, cannot be answered by our data; however, it is an interesting topic for further research. It is also important to underline that half of the patients had difficulties in reaching their hepatology specialist after the start of the COVID‐19 pandemic. This alarming rate is likely an important reason for less patient satisfaction, as easy and reliable ways of communicating with treating physicians are essential for continuous and effective care.
Uncertainty concerning direct and indirect effects of the pandemic may be another reason for less satisfaction of patients with liver disease. One third of patients did not feel sufficiently informed about the consequences of a SARS‐CoV‐2 infection on their liver disease, despite serious efforts by informative national campaigns. Another third of patients reported having concerns about negative effects of the COVID‐19 pandemic on their liver disease. Structured patient education campaigns may help to reduce uncertainties.
Effects of the COVID‐19 pandemic on liver disease–specific care are expected to unfold in three waves: (1) a period of physical distancing, with emphasis on high‐acuity care and delay of elective procedures and routine care; (2) a return to standard course of action following the ease of physical distancing with a backlog of deferred care and increased emergency decompensations; and (3) pandemic‐related complications and suboptimal outcomes caused by missed diagnoses and incomplete follow‐up.( 12 ) Our study focuses on the alleged first wave characterized by COVID‐19‐related health care restrictions. Indeed, we found a decrease in the number of elective procedures associated with short‐term admissions to our inpatient wards. This decrease mostly affected patients with ACLD, while elective admissions of patients with HCC were relatively increased, indicating sustained care in this subgroup of patients. Importantly, these were primarily patients already diagnosed with HCC out of Milan criteria undergoing systemic therapy, whereas HCC screening, local therapies, or access to LT might still have been affected in this time period.( 13 ) Postponing elective procedures such as screening for varices is considered safe for most patients with ACLD, especially when Baveno VI criteria are applied for adequate patient preselection.( 36, 37 ) In contrast, Tapper and Asrani argue that the risk of complications on a population level could still be increased,( 12 ) as deferral of endoscopic treatments may increase the risk for severe complications such as variceal hemorrhage. Thus, deferral of endoscopic procedures should be thoroughly considered on a patient‐to‐patient level.
During COVID‐19‐related health care restrictions, we observed a relative 20.2% increase in nonelectively admitted patients with ACLD, paralleled by a 21.1% increase of immediate ICU admissions due to liver disease. Importantly, nonelectively admitted patients with ACLD exhibited not only a significantly higher MELD( 38 ) and CLIF‐C AD( 19 ) scores, but also ΔMELD and ΔCLIF‐C AD were significantly increased during COVID‐19. This indicates a more pronounced worsening of liver function before admission, as compared with before COVID‐19. Importantly, serum sodium levels were particularly low at admissions during COVID‐19, indicating increased circulatory dysfunction.( 39 ) Consistently, a significantly higher proportion of emergency ACLD admissions during health care restrictions had severe or refractory ascites, and 1 patient even had a perforated umbilical hernia, potentially due to nonevacuated large‐volume ascites, and subsequently died of secondary bacterial peritonitis. Altogether, these results show that especially decompensated patients with ascites tended to be hospitalized later (i.e., in a more advanced stage of decompensation, which likely translates into a dismal prognosis). Elective ACLD admissions also decreased significantly, which may have long‐term implications (i.e., further increased rates of emergency decompensations and liver‐related deaths in the future).( 12 )
Additionally, nonelectively admitted patients with ACLD were more frequently admitted directly to the ICU, and liver‐related mortality was considerably increased during COVID‐19‐related restrictions of health care. These increases in complications and liver‐related deaths during COVID‐19 were already predicted,( 12, 13 ) and this scenario might also extend during the continued pandemic. Unfortunately, we can only speculate about the reasons, but patients may be reluctant to contact emergency medical services due to fear of SARS‐CoV‐2 infection at the hospital, leading to hospitalization at later stages of decompensation. Similar reasons were also speculated to be responsible for the observed 50% decrease of admissions due to myocardial infarctions, with a concomitant rise in mortality and complication rates during COVID‐19‐related shutdown in Italy.( 40 )
These data indicate the need for simplifying access to in‐hospital care, especially for patients with more advanced liver decompensation (i.e., decompensated patients with ascites), and for encouraging patients to timely seek in‐hospital care despite the pandemic.
Our study has some limitations: The telesurvey and written questionnaire are assessments of subjective beliefs and feelings, and are to be recognized as such. Furthermore, recall bias cannot be ruled out completely. However, we conducted two different types of standardized surveys/questionnaires using different approaches (through phone and written questionnaires) and in different settings (exclusively outpatients and in‐patients and out‐patients combined), and both cohorts yielded similar results. Moreover, despite our efforts to contact all patients with liver disease attached to our clinic, a selection bias for cohorts 1 and 2 cannot be completely ruled out. Correspondingly, the negative impact of COVID‐19‐related health care restrictions could in fact be even more severe than shown by our data. While we assume that the changed number of admissions was caused by health care–related factors (e.g., health care policy, regulation of elective procedures), we cannot completely rule out that death or migration contributed to intrinsic changes within the patient population. The lack of monitoring of the frequency of elective procedures without short‐term inpatient stay, such as screening for HCC and varices or large‐volume paracentesis in an outpatient setting, represents another limitation of our study. Notably, this study did not assess COVID‐19‐related liver disease, and the amount of extra health care resources needed for an adequate management of this disease. Further research is required to investigate the incidence of COVID‐19‐related liver disease and potential long‐term consequences.
In conclusion, the COVID‐19 pandemic negatively affected patient’s perceptions on quality of liver‐related care. Additionally, patients often felt insufficiently informed on potential adverse effects of SARS‐CoV‐2 infections on their liver disease. In terms of clinical outcomes, we observed an increased rate of nonelective hospitalizations of sicker patients with ACLD, including emergency ICU admissions and higher liver‐related mortality. Thus, it is vital to continuously provide patient education and improve efforts for telemedical liver disease care, to allow for early treatment of complications of cirrhosis and reduce liver‐related mortality during the ongoing COVID‐19 pandemic.
Supporting information
Supplementary Material
Supported by the Medical Scientific Fund of the Mayor of the City of Vienna (MA 40‐GMWF‐485569‐2020 to T.R.) and by a Gilead International Liver Research Scholarship (FA716E0930 to T.R.).
Potential conflict of interest: Dr. Trauner consults for, is on the speakers’ bureau for, and received grants from Gilead, Intercept, and MSD. He consults and received grants from Albireo and Falk. He consults for BiomX, Boehringer Ingelheim, Genfit, Janssen, Novartis, Phenex, Shire, and Regulus. He is on speakers’ bureau for the Falk Foundation. He received grants from Cymabay, Takeda, and Abbvie. He holds intellectual property rights in a co‐inventor patent on medical use for UDCA. Dr. Mandorfer advises and received grants from AbbVie, Bristol‐Meyers Squibb, and Gilead. He advises W.L. Gores & Associates. Dr. Scheiner received grants from Ipsen, AbbVie, and Gilead. Dr. Simbrunner received travel support from AbbVie and Gilead. TR received grant support from Abbvie, Boehringer‐Ingelheim,Gilead, Gore, Intercept, MSD, Myr Pharmaceuticals, Philips Healthcare, Pliant,and Siemens; speaking honoraria from Abbvie, Gilead, Gore, Intercept, Roche,MSD; consulting/advisory board fee from Abbvie, Bayer, Boehringer‐Ingelheim,Gilead, Intercept, MSD, Siemens; and travel support from Abbvie, Boehringer‐Ingelheim,Gilead and Roche.
References
- 1.Li H, Liu SM, Yu XH, Tang SL, Tang CK. Coronavirus disease 2019 (COVID‐19): current status and future perspectives. Int J Antimicrob Agents 2020;55:105951. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Zhu NA, Zhang D, Wang W, Li X, Yang BO, Song J, et al. A novel coronavirus from patients with pneumonia in China, 2019. N Engl J Med 2020;382:727‐733. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Wang Y, Liu S, Liu H, Li W, Lin F, Jiang L, et al. SARS‐CoV‐2 infection of the liver directly contributes to hepatic impairment in patients with COVID‐19. J Hepatol 2020;73:807‐816. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Mao L, Jin H, Wang M, Hu YU, Chen S, He Q, et al. Neurologic manifestations of hospitalized patients with coronavirus disease 2019 in Wuhan, China. JAMA Neurol 2020;77:1‐9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Lax SF, Skok K, Zechner P, Kessler HH, Kaufmann N, Koelblinger C, et al. Pulmonary arterial thrombosis in COVID‐19 with fatal outcome: results from a prospective, single‐center. Clinicopathologic Case Series Ann Intern Med 2020;173:350‐361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Chu DK, Akl EA, Duda S, Solo K, Yaacoub S, Schünemann HJ, et al. Physical distancing, face masks, and eye protection to prevent person‐to‐person transmission of SARS‐CoV‐2 and COVID‐19: a systematic review and meta‐analysis. Lancet 2020;395:1973‐1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Lai CC, Wang CY, Wang YH, Hsueh SC, Ko WC, Hsueh PR. Global epidemiology of coronavirus disease 2019 (COVID‐19): disease incidence, daily cumulative index, mortality, and their association with country healthcare resources and economic status. Int J Antimicrob Agents 2020;55:105946. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Boettler T, Newsome PN, Mondelli MU, Maticic M, Cordero E, Cornberg M, et al. Care of patients with liver disease during the COVID‐19 pandemic: EASL‐ESCMID position paper. JHEP Rep 2020;2:100113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Ji Y, Ma Z, Peppelenbosch MP, Pan Q. Potential association between COVID‐19 mortality and health‐care resource availability. Lancet Glob Health 2020;8:e480. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Fix OK, Hameed B, Fontana RJ, Kwok RM, McGuire BM, Mulligan DC, et al. Clinical best practice advice for hepatology and liver transplant providers during the COVID‐19 pandemic: AASLD Expert Panel Consensus Statement. Hepatology 2020;72:287‐304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Serper M, Cubell AW, Deleener ME, Casher TK, Rosenberg DJ, Whitebloom D, et al. Telemedicine in liver disease and beyond: can the COVID‐19 crisis lead to action? Hepatology 2020;72:723‐728. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Tapper EB, Asrani SK. The COVID‐19 pandemic will have a long‐lasting impact on the quality of cirrhosis care. J Hepatol 2020;73:441‐445. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Pawlotsky JM. COVID‐19 and the liver‐related deaths to come. Nat Rev Gastroenterol Hepatol 2020;17:523‐525. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Angelico R, Trapani S, Manzia TM, Lombardini L, Tisone G, Cardillo M. The COVID‐19 outbreak in Italy: initial implications for organ transplantation programs. Am J Transplant 2020;20:1780‐1784. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Boyarsky BJ, Po‐Yu Chiang T, Werbel WA, Durand CM, Avery RK, Getsin SN, et al. Early impact of COVID‐19 on transplant center practices and policies in the United States. Am J Transplant 2020;20:1809‐1818. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.WHO . Austria: WHO Coronavirus Disease (COVID‐19) Dashboard. https://covid19.WHO.int/region/euro/country/at. Accessed September 1, 2020.
- 17.BBC . Coronavirus: how lockdown is being lifted across Europe. https://www.bbc.com/news/explainers‐52575313. Accessed September 1, 2020.
- 18.Alcorn JB. United Organ Sharing Network. Important policy note. https://optn.transplant.hrsa.gov/media/1575/policynotice_20151101.pdf. Accessed September 22, 2020.
- 19.Jalan R, Pavesi M, Saliba F, Amorós A, Fernandez J, Holland‐Fischer P, et al. The CLIF Consortium Acute Decompensation score (CLIF‐C ADs) for prognosis of hospitalised cirrhotic patients without acute‐on‐chronic liver failure. J Hepatol 2015;62:831‐840. [DOI] [PubMed] [Google Scholar]
- 20.Györi GP, Silberhumer GR, Rahmel A, de Vries E, Soliman T, Zehetmayer S, et al. Impact of dynamic changes in MELD score on survival after liver transplantation—a Eurotransplant registry analysis. Liver Int 2016;36:1011‐1017. [DOI] [PubMed] [Google Scholar]
- 21.Kanwal F, Volk M, Singal A, Angeli P, Talwalkar J. Improving quality of health care for patients with cirrhosis. Gastroenterology 2014;147:1204‐1207. [DOI] [PubMed] [Google Scholar]
- 22.Kanwal F, Kramer JR, Buchanan P, Asch SM, Assioun Y, Bacon BR, et al. The quality of care provided to patients with cirrhosis and ascites in the Department of Veterans Affairs. Gastroenterology 2012;143:70‐77. [DOI] [PubMed] [Google Scholar]
- 23.Barritt AS, Telloni SA, Potter CW, Gerber DA, Hayashi PH. Local access to subspecialty care influences the chance of receiving a liver transplant. Liver Transpl 2013;19:377‐382. [DOI] [PubMed] [Google Scholar]
- 24.Unger LW, Berlakovich GA, Trauner M, Reiberger T. Management of portal hypertension before and after liver transplantation. Liver Transpl 2018;24:112‐121. [DOI] [PubMed] [Google Scholar]
- 25.Mandorfer M, Schwabl P, Steiner S, Reiberger T, Peck‐Radosavljevic M. Advances in the management of HIV/HCV coinfection. Hepatol Int 2016;10:424‐435. [DOI] [PubMed] [Google Scholar]
- 26.Mandorfer M, Kozbial K, Schwabl P, Chromy D, Semmler G, Stättermayer AF, et al. Changes in hepatic venous pressure gradient predict hepatic decompensation in patients who achieved sustained virologic response to interferon‐free therapy. Hepatology 2020;71:1023‐1036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Reiberger T, Puspok A, Schoder M, Baumann‐Durchschein F, Bucsics T, Datz C, et al. Austrian consensus guidelines on the management and treatment of portal hypertension (Billroth III). Wien Klin Wochenschr 2017;129:135‐158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Mao R, Liang J, Shen J, Ghosh S, Zhu L‐R, Yang H, et al. Implications of COVID‐19 for patients with pre‐existing digestive diseases. Lancet Gastroenterol Hepatol 2020;5:425‐427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Gao F, Zheng KI, Wang X‐B, Yan H‐D, Sun Q‐F, Pan K‐H, et al. Metabolic associated fatty liver disease increases coronavirus disease 2019 disease severity in nondiabetic patients. J Gastroenterol Hepatol 2021;36:204‐207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Oyelade T, Alqahtani J, Canciani G. Prognosis of COVID‐19 in patients with liver and kidney diseases: an early systematic review and meta‐analysis. Trop Med Infect Dis 2020;5:80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Liu H, He XI, Wang Y, Zhou S, Zhang D, Zhu J, et al. Management of COVID‐19 in patients after liver transplantation: Beijing working party for liver transplantation. Hepatol Int 2020;14:432‐436. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Sahin TT, Akbulut S, Yilmaz S. COVID‐19 pandemic: its impact on liver disease and liver transplantation. World J Gastroenterol 2020;26:2987‐2999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.John BV, Love E, Dahman B, Kurbanova N, Konjeti VR, Sundaram LT, et al. Use of telehealth expedites evaluation and listing of patients referred for liver transplantation. Clin Gastroenterol Hepatol 2020;18:1822‐1830.e1824. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Konjeti VR, Heuman D, Bajaj JS, Gilles H, Fuchs M, Tarkington P, et al. Telehealth‐based evaluation identifies patients who are not candidates for liver transplantation. Clin Gastroenterol Hepatol 2019;17:207‐209.e201. [DOI] [PubMed] [Google Scholar]
- 35.Dobrusin A, Hawa F, Gladshteyn M, Corsello P, Harlen K, Walsh CX, et al. Gastroenterologists and patients report high satisfaction rates with telehealth services during the novel coronavirus 2019 pandemic. Clin Gastroenterol Hepatol 2020;18:2393‐2397.e2392. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.de Franchis R . Expanding consensus in portal hypertension: report of the Baveno VI Consensus Workshop: Stratifying risk and individualizing care for portal hypertension. J Hepatol 2015;63:743‐752. [DOI] [PubMed] [Google Scholar]
- 37.Paternostro R, Reiberger T, Bucsics T. Elastography‐based screening for esophageal varices in patients with advanced chronic liver disease. World J Gastroenterol 2019;25:308‐329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Kim WR, Biggins SW, Kremers WK, Wiesner RH, Kamath PS, Benson JT, et al. Hyponatremia and mortality among patients on the liver‐transplant waiting list. N Engl J Med 2008;359:1018‐1026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.John S, Thuluvath PJ. Hyponatremia in cirrhosis: pathophysiology and management. World J Gastroenterol 2015;21:3197‐3205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.De Rosa S, Spaccarotella C, Basso C, Calabrò MP, Curcio A, Filardi PP, et al. Reduction of hospitalizations for myocardial infarction in Italy in the COVID‐19 era. Eur Heart J 2020;41:2083‐2088. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary Material


