Abstract
Aims
Antihypertensive drugs have been implicated in coronavirus disease 2019 (COVID‐19) susceptibility and severity, but estimated associations may be susceptible to bias. We aimed to evaluate antihypertensive medications and COVID‐19 diagnosis and mortality, accounting for healthcare‐seeking behaviour.
Methods
A population‐based case‐control study was conducted including 16 866 COVID‐19 cases and 70 137 matched controls from the UK Clinical Practice Research Datalink. We evaluated all‐cause mortality among COVID‐19 cases. Exposures were angiotensin‐converting enzyme inhibitors (ACEIs), angiotensin receptor blockers (ARBs), beta‐blockers (B), calcium‐channel blockers (C), thiazide diuretics (D) and other antihypertensive drugs (O). Analyses were adjusted for covariates and consultation frequency.
Results
ACEIs were associated with lower odds of COVID‐19 diagnosis (adjusted odds ratio [AOR] 0.82, 95% confidence interval [CI] 0.77‐0.88) as were ARBs (AOR 0.87, 95% CI 0.80‐0.95) with little attenuation from adjustment for consultation frequency. C and D were also associated with lower odds of COVID‐19 diagnosis. Increased odds of COVID‐19 for B (AOR 1.19, 95% CI 1.12‐1.26) were attenuated after adjustment for consultation frequency (AOR 1.01, 95% CI 0.95‐1.08). Patients treated with ACEIs or ARBs had similar odds of mortality (AOR 1.00, 95% CI 0.83‐1.20) to patients treated with classes B, C, D or O or patients receiving no antihypertensive therapy (AOR 0.99, 95% CI 0.83‐1.18).
Conclusions
There was no evidence that antihypertensive therapy is associated with increased risk of COVID‐19 diagnosis or mortality; most classes of antihypertensive therapy showed negative associations with COVID‐19 diagnosis.
Keywords: angiotensin converting enzyme inhibitors, angiotensin receptor blockers, antihypertensive treatment, clinical practice research datalink, COVID‐19, renin‐angiotensin‐aldosterone system inhibitors, SARS‐CoV‐2
What is already known about this subject
Antihypertensive drugs have been implicated in coronavirus disease 2019 (COVID‐19) susceptibility and severity through upregulation of the angiotensin‐converting enzyme 2 receptor.
Studies have so far produced conflicting results, with methodological and sampling issues leading to estimated associations being susceptible to bias.
What this study adds
Associations were sensitive to adjustment for confounding and healthcare‐seeking, but there was no evidence that antihypertensive therapy is associated with increased risk of COVID‐19 diagnosis or mortality.
Most classes of antihypertensive therapy showed negative associations with COVID‐19 diagnosis.
This study adds to the evidence that antihypertensive therapy may be safely continued during the COVID‐19 pandemic.
1. INTRODUCTION
Coronavirus disease 2019 (COVID‐19) caused by the novel severe acute respiratory syndrome coronavirus 2 (SARS‐CoV‐2) has become a global pandemic. The World Health Organization's 9 February 2021 epidemiological update reported 105.4 million cumulative cases and 2.3 million cumulative deaths of COVID‐19 globally. 1 The UK is among the countries with the highest COVID‐19 incidence and death rates in the world, with figures from 14 February 2021 indicating that among those first testing positive for the virus, 117 166 died within 28 days. 2
Biomedical scientific evidence suggests a role of the renin‐angiotensin‐aldosterone system (RAAS) in COVID‐19. SARS‐CoV‐2 enters host cells via interaction with the angiotensin‐converting enzyme 2 (ACE2) receptor, which is part of the RAAS. 3 , 4 , 5 , 6 Angiotensin‐converting enzyme inhibitors (ACEIs) and angiotensin receptor blockers (ARBs) modulate the RAAS, and treatment with ACEIs and ARBs may enhance ACE2 activity, thereby increasing SARS‐CoV‐2 susceptibility and COVID‐19 severity. 7 , 8 Conversely, increased ACE2 might have a protective effect by competitive inhibition of SARS‐CoV‐2 entry into the respiratory epithelium or via negative regulation of the RAAS for anti‐inflammatory, antioxidative and vasodilatory effects. 7 , 9
Antihypertensive drugs represent the most frequently prescribed medicines in the UK, used by 15% of adults with long‐term conditions including diabetes, hypertension and cardiovascular diseases. 10 These pre‐existing conditions are frequent among those receiving healthcare for COVID‐19 6 , 11 and have also been associated with high COVID‐19 case fatality rates, 12 , 13 , 14 raising concerns about possible associations with antihypertensive treatment (AHT). There have been conflicting results from several observational studies exploring the relationship between AHT and COVID‐19 susceptibility and severity since the start of the pandemic. 15 , 16
A living systematic review, updated to 3 August 2020, included three studies with 8766 COVID‐19 patients and found no evidence of association between either ACEI or ARB treatment and positive COVID‐19 test results. 15 Two of the studies drew on patients attending hospital in the United States, 17 , 18 while the third study was population‐based, including both primary care and hospital attendances in the Lombardy region in Italy. 19 A large international study which was initially included in the review has since been retracted by the journal in which it was published. A retrospective cohort study using Danish national disease registries which found no evidence of association of prior treatment with ACEIs/ARBs with COVID‐19 diagnosis, severity or death. 20 In contrast, a systematic review and meta‐analysis of 28 872 patients found that treatment with RAAS inhibiting (RAASi) drugs was associated with lower risk of death or critical events. 16 A primary care database study in the UK reported that prior use of ACEIs and ARBs was associated with lower risk of COVID‐19 diagnosis, but there was no association with intensive care unit admission. 21
Nonrandomised studies to evaluate the association of RAASi drugs with COVID‐19 susceptibility and severity face several methodological challenges. First, there may be substantial confounding. Several previous studies have only presented ‘fully adjusted’ estimates, making it difficult to assess the possibility of residual confounding. Second, analysis of clinical data from electronic health records may be associated with misclassification of confounders, which might bias estimates in either positive or negative directions. Third, and perhaps most important, cases of COVID‐19 documented in electronic health records represent only a minority of cases occurring in the community during the first wave of the pandemic. If hypertension and COVID‐19 are both associated with increased healthcare utilisation, this might introduce spurious associations between antihypertensive therapy and COVID‐19 through collider bias. 22 , 23 Selection pressures may have biased the sample toward those with greater symptom severity 24 or those who have comorbidities that make contact with health services more likely.
This study aimed to add to the evidence concerning antihypertensive therapy and COVID‐19 diagnosis and mortality through analysis of a large population‐based sample registered in UK primary care. We aimed to evaluate the association between AHT classes, including ACEIs, ARBs, calcium‐channel blockers, beta‐blockers and thiazide diuretics, and COVID‐19 diagnosis and mortality. We aimed to explore the effects of covariate adjustment, including patient consultation frequency in primary care.
2. METHODS
2.1. Overview
We undertook a case‐control analysis of the UK Clinical Practice Research Datalink (CPRD) GOLD to estimate associations between AHT and COVID‐19 susceptibility. We examined associations between AHT classes and COVID‐19 mortality using date of death in the CPRD within 30 days as the outcome. Analyses were adjusted for covariates and consultation frequency.
2.2. Case and control selection
The CPRD GOLD is among the world's largest databases of anonymised electronic health records from primary care. It is estimated that 7% of UK general practices contribute to CPRD GOLD, enabling the database to have extensive coverage and good sociodemographic and geographic representativeness of the UK population. 25 The high quality of the CPRD GOLD data has been verified by several studies. 26 The protocol was approved by the CPRD Independent Scientific Advisory Committee (ISAC protocol 20_081RA).
Patients recorded with COVID‐19 were identified from the July 2020 release of CPRD GOLD. COVID‐19 events were identified from Read codes recorded into patients' clinical, referral and test records: ‘Suspected disease caused by 2019‐nCoV (novel coronavirus)’ 39%, ‘Suspected coronavirus infection’ 19%, ‘Telephone consultation for suspected 2019‐nCoV (novel coronavirus)’ 16%, ‘2019‐nCoV (novel coronavirus) detected’ 12%, ‘Disease caused by 2019‐nCoV (novel coronavirus)’ 7%, ‘Coronavirus infection’ 5%, ‘Coronavirus nucleic acid detection’ 3%, ‘[X] coronavirus infection, unspecified’ and ‘Coronavirus as cause of disease classified to other chapters’ less than 1%. We excluded the small number of COVID‐19 diagnosis and mortality events dated on or before 29 January 2020, the official date of the UK's first confirmed COVID‐19 case.
COVID‐19 cases were compared with a sample of matched control patients who did not have a COVID‐19 diagnosis. Up to five control patients for each case were randomly sampled from the entire registered population of CPRD GOLD, matching on age, gender, index date and general practice.
2.3. Exposures
The exposure was defined as prescription of AHT in the 6 months before the index date of the following classes: ACEIs, ARBs, beta blockers (B), calcium channel blockers (C), thiazide diuretics (D) and other antihypertensive drugs (O). Each class of AHT was coded as either prescribed or not prescribed.
2.4. Covariates
Covariates were defined using data recorded in the 5‐year period before date of diagnosis. These included smoking status (nonsmoker, current smoker, exsmoker), body mass index (BMI; underweight, normal weight, overweight, obese and missing), systolic blood pressure (SBP) and diastolic blood pressure (DBP) in categories of 10 mmHg. Ethnicity was classified as ‘white’, ‘black’, ‘Asian’, ‘mixed’, ‘other’ and ‘not known’. We used Clegg's electronic Frailty Index (eFI) to evaluate frailty into categories of nonfrail, mild, moderate and severe frailty. 27 We also evaluated the 15 comorbidities of the Charlson comorbidity index as present or absent, following the recommendations of Khan et al. 28 , 29 We evaluated healthcare‐seeking behaviour by calculating the rate of events in each patient's clinical record file, including consultations and other contacts with the practice, in the year preceding the index date. This rate per patient year was entered as a continuous variable.
2.5. Analysis
A conditional logistic regression model was employed for the case‐control analysis. Unadjusted odds ratios (ORs) were compared with covariate adjusted odds ratios (AORs) including ethnicity, BMI, blood pressure, smoking status, frailty level, comorbidities and treatment with each class of AHT. Finally, additional adjustment was made for consultation frequency. As a sensitivity analysis we repeated the analysis only including patients with confirmed COVID‐19 diagnoses, excluding patients recorded to have ‘suspected’ COVID‐19. We also explored evidence of an interaction between age group and each class of AHT, conducting a subgroup analysis to examine age as an effect modifier. We conducted subgroup analyses by BMI and frailty category.
A Cox proportional hazards model was employed to examine the association of AHT with patient survival. Survival time in days from the date of first COVID‐19 diagnosis to date of death or end of record was the outcome. Patients who were still alive at the end of the study period were censored. Covariates were month of COVID‐19 diagnosis, gender, age (0‐4, 5‐9 and 10‐14, then 10 years age groups up to 85 years and over), smoking status, BMI, blood pressure, ethnic group, eFI category comorbidities and treatment with each class of AHT. A secondary analysis also adjusted for region of practice. We did not adjust for consultation frequency because all patients in this analysis had already accessed care from their general practice. We also evaluated ACEIs and ARBs combined, as RAASi drugs, making comparisons with all other classes of AHT drugs combined, beta‐blockers, calcium channel blockers, thiazide diuretics, other hypertensive drugs (BCDO) or no AHT. The proportional hazards assumption was checked graphically and with a test for proportional hazards. All data were analysed in R, version 4.0.2.
3. RESULTS
3.1. Case‐control analysis: COVID‐19 diagnosis
There were 16 866 COVID‐19 cases identified in CPRD GOLD from 29 January to 20 June 2020 (Supporting Information Figure S1 ), which were compared to 70 137 matched controls. The age and gender distribution of COVID‐19 cases showed a higher frequency of females compared to males across all age categories, with cases peaking in the 45‐64 age group (years) (Supporting Information Figure S2 ). COVID‐19 cases had consulted their general practice more frequently over the preceding year than controls, with a mean rate of clinical record events in the preceding year of 28.0 per person year, compared to 15.4 per person year among controls.
Table 1 and Supporting Information Table S1 show descriptive data for COVID‐19 cases and controls as well as unadjusted and covariate AORs and 95% confidence intervals (CIs). COVID‐19 diagnosis was associated with current smoking, underweight and obese BMI, frailty, and black and minority ethnicity. Both low and high systolic or diastolic blood pressure values were associated with increased odds of COVID‐19 diagnosis when compared with intermediate blood pressure values. Comorbidities associated with COVID‐19 diagnosis in covariate adjusted analyses included chronic pulmonary disease, metastatic tumour, congestive heart disease, cerebrovascular disease and dementia. Diabetes and renal disease were associated with COVID‐19 diagnosis in unadjusted but not covariate adjusted analyses.
TABLE 1.
Variables associated with Covid‐19 diagnosis
| COVID‐19 cases (16866) | Controls (70137) | Risk of Covid‐19 diagnosis (adjusted OR 95% CI) a | ||
|---|---|---|---|---|
| AHT drugs | ACEI | 1789 (11) | 7314 (10) | 0.82 (0.77‐0.88) |
| ARB | 923 (5) | 3570 (5) | 0.87 (0.80‐0.95) | |
| Beta‐blocker | 2228 (13) | 7206 (10) | 1.19 (1.12‐1.26) | |
| Calcium channel | 1763 (10) | 7216 (10) | 0.97 (0.90‐1.03) | |
| Diuretics | 583 (3) | 2924 (4) | 0.83 (0.75‐0.92) | |
| Other AHT | 340 (2) | 1218 (2) | 1.06 (0.92‐1.21) | |
| Systolic blood pressure | <110 | 1487 (9) | 4652 (7) | 1.30 (1.19‐1.41) |
| 110‐119 | 2345 (14) | 8023 (11) | 1.21 (1.13‐1.29) | |
| 120‐129 | 3265 (19) | 11 966 (17) | 1.10 (1.04‐1.17) | |
| 130‐139 | 3068 (18) | 12 483 (18) | Reference | |
| 140‐149 | 1875 (11) | 7914 (11) | 0.96 (0.90‐1.03) | |
| 150‐159 | 634 (4) | 2630 (4) | 0.96 (0.87‐1.07) | |
| 160+ | 585 (3) | 2129 (3) | 1.03 (0.92‐1.16) | |
| Not known | 3607 (21) | 20 340 (29) | 1.97 (0.12‐33.32) | |
| Diastolic blood pressure | <60 | 473 (3) | 1468 (2) | 1.01 (0.89‐1.14) |
| 60‐69 | 2326 (14) | 8826 (13) | 0.87 (0.81‐0.93) | |
| 70‐79 | 4654 (28) | 18 771 (27) | 0.88 (0.84‐0.93) | |
| 80‐89 | 4368 (26) | 16 224 (23) | Reference | |
| 90‐99 | 1152 (7) | 3639 (5) | 1.18 (1.09‐1.28) | |
| 100+ | 286 (2) | 867 (1) | 1.21 (1.03‐1.41) | |
| Not known | 3607 (21) | 20 342 (29) | 0.32 (0.02‐5.51) | |
| Ethnic group | White | 8296 (49) | 33 280 (47) | Reference |
| Black | 183 (1) | 649 (1) | 1.27 (1.06‐1.53) | |
| Asian | 305 (2) | 971 (1) | 1.34 (1.17‐1.54) | |
| Mixed | 98 (1) | 350 (0) | 1.25 (0.99‐1.58) | |
| Other | 246 (1) | 931 (1) | 1.26 (1.08‐1.46) | |
| Not known | 7738 (46) | 33 956 (48) | 0.93 (0.89‐0.97) | |
| Smoking status | Nonsmoker | 10 579 (63) | 47 723 (15) | Reference |
| Smoker | 3512 (21) | 12 420 (5) | 1.12 (1.07‐1.18) | |
| Exsmoker | 2775 (16) | 10 296 (4) | 1.07 (1.01‐1.12) | |
| Frailty level | Fit | 10 029 (59) | 50 530 (14) | Reference |
| Mild | 3984 (24) | 12 759 (6) | 2.03 (1.92‐2.14) | |
| Moderate | 1865 (11) | 4961 (3) | 2.91 (2.68‐3.17) | |
| Severe | 988 (6) | 2189 (1) | 3.78 (3.35‐4.26) |
Note: Figures are frequencies (column percent) except where indicated.
Abbreviations: ACEI, angiotensin‐converting enzyme inhibitor; AHT, antihypertensive treatment; ARB, angiotensin receptor blocker; CI, confidence interval; OR, odds ratio.
All odds ratios were adjusted for each of the variables shown and body mass index category and comorbidities contributing to the Charlson index. Cases and controls were matched for age, sex, general practice and index date.
There were 4852 (29%) COVID‐19 cases prescribed an AHT in the year preceding diagnosis, compared to 17 978 (26%) of controls. Positive associations of ACEI and ARB with COVID‐19 diagnosis in unadjusted analyses (Supporting Information Table S1 ) were attenuated in the covariate adjusted analysis (Table 1), with both ACEIs and ARBs being negatively associated with COVID‐19 diagnosis (ACEI: AOR 0.82, 95% CI 0.77‐0.88; ARB: AOR 0.87, 95% CI 0.80‐0.95). Further adjustment for consultation frequency had little effect on estimated associations (ACEI: AOR 0.80, 95% CI 0.75‐0.86; ARB: AOR 0.84, 95% CI 0.77‐0.92) (Figure 1). We found no association between calcium channel blockers and COVID‐19 susceptibility in the unadjusted or adjusted models, but when including consultation frequency in the model, calcium channel blockers were associated with lower odds of COVID‐19 diagnosis (AOR 0.90, 95% CI 0.84‐0.96) (Figure 1). Analysis of thiazide diuretic treatment consistently indicated lower odds of COVID‐19 diagnosis, including in the fully adjusted model (AOR 0.87, 95% CI 0.78‐0.97) (Figure 1). Other AHT were positively associated with COVID‐19 diagnosis in the unadjusted model, but no association was indicated in adjusted models. In covariate adjusted analyses, beta‐blocker treatment was positively associated with COVID‐19 diagnosis (AOR 1.19, 95% CI 1.12‐1.26), but this was attenuated after further adjustment for consultation frequency (AOR 1.01, 95% CI 0.95‐1.08) (Figure 1). When the sample was restricted to only those cases with confirmed COVID‐19 diagnoses and their matched controls, there were 4233 cases matched to 17 700 controls, and the pattern of association was unaltered though estimates were necessarily less precise (Supporting Information Figure S3 ).
FIGURE 1.
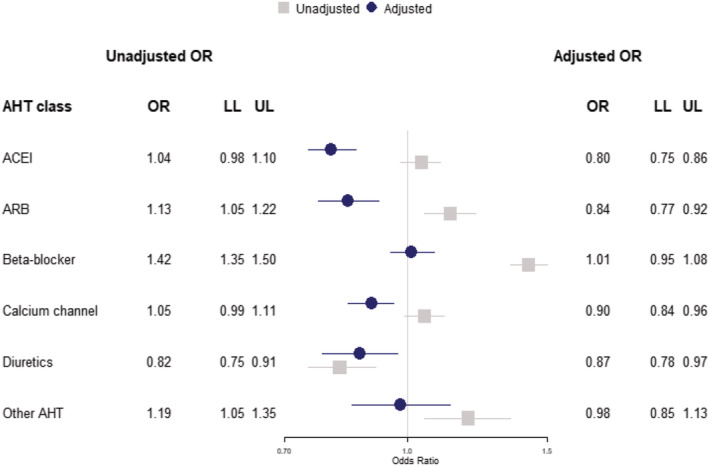
Case‐control analysis for COVID‐19 diagnosis showing unadjusted odds ratios (grey) and model adjusted for ethnicity, body mass index, blood pressure, smoking status, frailty level, comorbidities and the rate of events in each patient's clinical record in the year preceding the index date (blue). ACEI, angiotensin‐converting enzyme inhibitor; AHT, antihypertensive treatment; ARB, angiotensin receptor blocker; LL, lower limit 95% confidence interval; OR, odds ratio; UL, upper limit 95% confidence interval
Adding an interaction term for age within logistic regression models for each class of AHT improved the model goodness of fit, therefore we conducted a subgroup analysis for cases and controls by AHT and age (Supporting Information Table S3 ). AHT was more strongly associated with higher odds of COVID‐19 diagnosis at younger ages but this pattern of association was accounted for by adjusting for covariates, including consultation frequency. We conducted subgroup analyses for cases and controls by AHT and BMI category (Supporting Information Table S4 ) and by AHT and frailty category (Supporting Information Table S5 ). These analyses did not reveal any consistent difference in association between AHT and COVID‐19 diagnosis across categories of BMI or frailty in either unadjusted or adjusted analyses.
3.2. Primary cohort analysis: COVID‐19 mortality
Among the 16 866 COVID‐19 cases, 921 (5%) died within 30 days. The age and gender distribution of these fatal COVID‐19 cases showed a higher frequency of males compared to females across most age categories, with cases peaking in the highest age categories (Supporting Information Figure S2 ). Patients treated with antihypertensive drugs were more highly represented among deceased patients than the overall sample and this was true for each class of AHT drugs (Table 2 and Supporting Information Table S2 ). Table 2 and Supporting Information Table S2 provide estimates for covariates which show that male gender, older age, black and ethnic minority status, diabetes, metastatic tumour, dementia and mild liver disease were associated with greater mortality. In covariate adjusted analyses, there was no evidence that any of the classes of AHT drugs might be associated with mortality after COVID‐19 diagnosis (Table 2). Additional adjustment for practice region did not influence associations (Figure 2). There was no evidence that treatment with RAASi drugs, including ACEI and ARB drugs, might be associated with higher mortality than other classes of AHT drugs (BCDO) with adjusted hazard ratio (AHR) 1.00 (0.83 to 1.20) (Figure 3). Similarly, there was no evidence that treatment with RAASi drugs was associated with greater risk than no AHT (AHR 0.99, 95% CI 0.83‐1.18), nor was treatment with BCDO classes associated with higher mortality than no AHT treatment (AHR 0.99, 95% CI 0.84‐1.18).
TABLE 2.
Variables associated with Covid‐19 diagnosis
| COVID‐19 cases (16866) | Death in 30 days 921 (5) | Adjusted HR (95% CI) | ||
|---|---|---|---|---|
| AHT drugs | ACEI | 1789 (11) | 182 (20) | 1.08 (0.90‐1.30) |
| ARB | 923 (5) | 72 (8) | 0.84 (0.65‐1.09) | |
| Beta‐blocker | 2228 (13) | 263 (29) | 1.13 (0.97‐1.33) | |
| Calcium channel | 1763 (10) | 149 (16) | 0.87 (0.72‐1.04) | |
| Diuretics | 583 (3) | 47 (5) | 1.00 (0.74‐1.36) | |
| Other AHT | 340 (2) | 30 (3) | 0.85 (0.59‐1.24) | |
| Gender | Male | 6796 (40) | 465 (50) | 1.51 (1.32‐1.74) |
| Female | 10 070 (60) | 456 (50) | Reference | |
| Age group (years) | 0‐4 | 697 (4) | 0 (0) | 0.00 (0.00‐Inf.) |
| 5‐14 | 641 (4) | 0 (0) | 0.00 (0.00‐Inf.) | |
| 15‐24 | 889 (5) | 2 (0) | 0.10 (0.02‐0.41) | |
| 25‐34 | 1959 (12) | 0 (0) | 0.00 (0.00‐Inf.) | |
| 35‐44 | 2259 (13) | 6 (1) | 0.12 (0.05‐0.27) | |
| 45‐54 | 2663 (16) | 13 (1) | 0.23 (0.12‐0.42) | |
| 55‐64 | 2747 (16) | 56 (6) | Reference | |
| 65‐74 | 1701 (10) | 126 (14) | 3.52 (2.56‐4.85) | |
| 75‐84 | 1662 (10) | 310 (34) | 8.18 (6.02‐11.10) | |
| 85+ | 1648 (10) | 408 (44) | 10.74 (7.83‐14.72) | |
| Systolic blood pressure | <110 | 1487 (9) | 109 (12) | 1.30 (0.99‐1.70) |
| 110‐119 | 2345 (14) | 128 (14) | 1.23 (0.97‐1.56) | |
| 120‐129 | 3265 (19) | 171 (19) | 0.98 (0.79‐1.21) | |
| 130‐139 | 3068 (18) | 181 (20) | Reference | |
| 140‐149 | 1875 (11) | 130 (14) | 0.93 (0.74‐1.17) | |
| 150‐159 | 634 (4) | 45 (5) | 0.96 (0.69‐1.34) | |
| 160+ | 585 (3) | 46 (5) | 0.76 (0.54‐1.08) | |
| Not known | 3607 (21) | 111 (12) | 5.78 (0.00‐ne) | |
| Diastolic blood pressure | <60 | 473 (3) | 83 (9) | 1.42 (1.05‐1.91) |
| 60‐69 | 2326 (14) | 194 (21) | 1.05 (0.84‐1.31) | |
| 70‐79 | 4654 (28) | 276 (30) | 1.03 (0.85‐1.24) | |
| 80‐89 | 4368 (26) | 191 (21) | Reference | |
| 90‐99 | 1152 (7) | 50 (5) | 1.36 (0.99‐1.87) | |
| 100+ | 286 (2) | 16 (2) | 1.12 (0.66‐1.93) | |
| Not known | 3607 (21) | 111 (12) | 0.29 (0.00‐ne) | |
| Ethnic group | White | 8296 (49) | 396 (43) | Reference |
| Black | 183 (1) | 9 (1) | 2.60 (1.33‐5.08) | |
| Asian | 305 (2) | 9 (1) | 2.15 (1.10‐4.19) | |
| Mixed | 98 (1) | 1 (0) | 0.70 (0.10‐5.05) | |
| Other | 246 (1) | 5 (1) | 1.04 (0.43‐2.52) | |
| Not known | 7738 (46) | 501 (54) | 1.05 (0.92‐1.20) | |
| Smoking status | Nonsmoker | 10 579 (63) | 550 (60) | Reference |
| Smoker | 3512 (21) | 304 (33) | 1.12 (0.96‐1.30) | |
| Exsmoker | 2775 (16) | 67 (7) | 0.81 (0.62‐1.05) | |
| Frailty level | Fit | 10 029 (59) | 182 (20) | Reference |
| Mild | 3984 (24) | 265 (29) | 0.91 (0.74‐1.12) | |
| Moderate | 1865 (11) | 283 (31) | 1.15 (0.91‐1.45) | |
| Severe | 988 (6) | 191 (21) | 1.16 (0.88‐1.52) |
Note: Figures are frequencies (column percent) except where indicated.
Abbreviations: ACEI, angiotensin‐converting enzyme inhibitor; AHT, antihypertensive treatment; ARB, angiotensin receptor blocker; CI, confidence interval; HR, hazard ratio.
All odds ratios were adjusted for each of the variables shown and body mass index category and comorbidities contributing to the Charlson index, as well as age group, gender, general practice and month of diagnosis.
FIGURE 2.
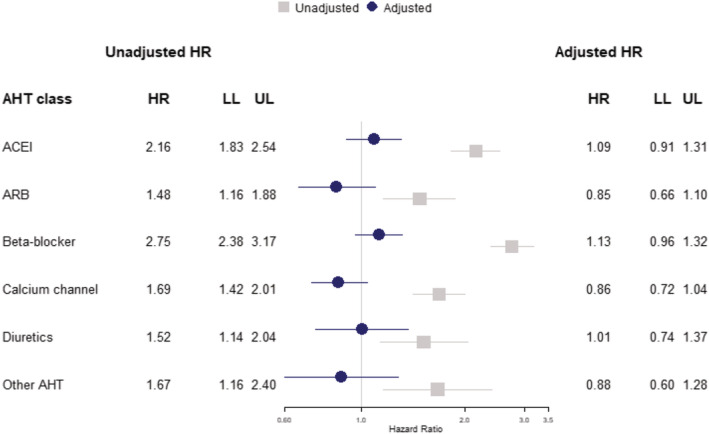
Cohort analysis for 30‐day mortality following COVID‐19 diagnosis showing unadjusted hazard ratios (grey) and model adjusted for body mass index, blood pressure, smoking status, frailty level, comorbidities and practice region (blue). ACEI, angiotensin‐converting enzyme inhibitor; AHT, antihypertensive treatment; ARB, angiotensin receptor blocker; HR, hazard ratio; LL, lower limit 95% confidence interval; UL, upper limit 95% confidence interval
FIGURE 3.
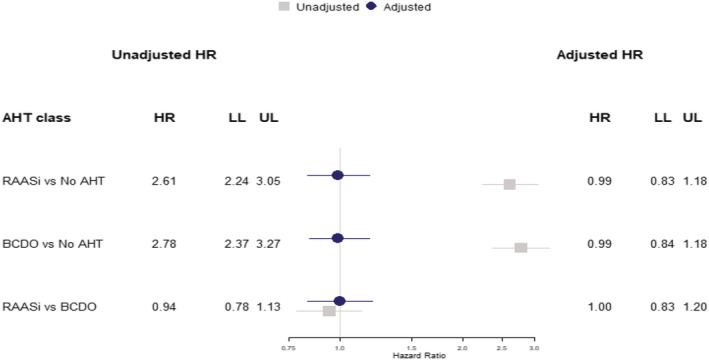
Cohort analysis for 30‐day mortality following COVID‐19 diagnosis showing unadjusted hazard ratios (grey) and model adjusted for body mass index, blood pressure, smoking status, frailty level and comorbidities (blue) comparing (i) RAASi to no AHT, (ii) BCDO AHT (non‐RAASi) to no AHT and (iii) RAASi to BCDO. AHT, antihypertensive treatment; BCDO, beta‐blockers, calcium‐channel blockers, thiazide diuretics, other antihypertensive drugs; HR, hazard ratio; LL, lower limit 95% confidence interval; RAASi, renin‐angiotensin‐aldosterone system inhibiting drugs; UL, upper limit 95% confidence interval
4. DISCUSSION
This large population‐based study included patients diagnosed with suspected or confirmed COVID‐19 in UK primary care. During the first wave of the pandemic, there was limited testing capacity and most suspected cases remained unconfirmed; however, restricting our analysis to confirmed cases yielded similar results. After adjusting for covariates that characterise case‐mix, we found no evidence that treatment with ACEIs, ARBs, calcium channel blockers, thiazide diuretics or other antihypertensive drugs might be associated with greater risk of COVID‐19 diagnosis. There was evidence that beta‐blocker treatment was associated with greater odds of COVID‐19 diagnosis but additional adjustment for patients' underlying consultation frequency removed this association, which might be attributable to collider bias. After adjusting for covariates, including blood pressure, and consultation frequency, ACEIs, ARBs, calcium channel blockers and thiazide diuretics were associated with lower odds of COVID‐19 diagnosis. This might suggest that at a given level of blood pressure, patients treated with antihypertensive drugs may be at lower risk, but this pattern of association does not appear to be specific to any class of antihypertensive drug. Associations of AHT with COVID‐19 diagnosis may be modified by age, but the effects of adjusting for covariates, including comorbidities, suggest this may be attributable to confounding. In this cohort analysis of patients diagnosed in UK primary care with suspected or confirmed COVID‐19, recorded mortality was considerably higher than the overall reported infection fatality ratio for this condition but there was no evidence that any class of antihypertensive drug might be associated with increased mortality risk, and the adjusted hazard for drugs acting on the RAAS was similar to that for patients treated with other classes of AHT drugs or patients not receiving AHT.
This study drew on a large, longitudinal population‐based data resource that enabled us to conduct a matched case‐control analysis of risk factors for clinical COVID‐19 diagnosis, as well as a cohort study of risk factors for COVID‐19 mortality. As noted above, most cases were diagnosed clinically as suspected cases because of the limited capacity for testing in the early stage of the pandemic. General practice systems capture comprehensive data for all prescriptions issued by the general practice, so we can be confident that this exposure was accurately recorded. However, prescription utilisation may not be universal and nonadherence to prescribed medicines may be widespread. Data for covariates were not always completely recorded. Data on ethnicity were missing for almost half the sample as reported by others 25 ; an important limitation given the disproportionate impact COVID‐19 has had on ethnic minority populations in the United States and the UK. 14 , 30 , 31 COVID‐19 susceptibility and severity have also been associated with measures of deprivation. 14 Matching on practice may have accounted for differences in area‐level deprivation to an extent, but deprivation based on participants' home postcodes or individual‐level deprivation measures might improve precision. It is known that observational studies on COVID‐19 outcomes may be susceptible to collider bias. 22 , 23 This can lead to spurious associations if both antihypertensive therapy and COVID‐19 are associated with greater likelihood of general practice consultations. Selection pressures may have biased the sample toward those with increased symptom severity 24 or who are otherwise more likely to recognise COVID‐19 symptoms and make contact with health services. We accounted for the effects of health‐seeking behaviour by introducing into the analysis the rate of events in each patient's clinical record in the year preceding the index date. There were substantial differences in consultation frequency between cases and controls, and adjusting for this metric nullified an apparent association between beta‐blocker use and COVID‐19 diagnosis. However, randomisation allocation will be preferred to provide a higher level of evidence. We were able to match our COVID‐19 cases to five controls on age, gender, index date and general practice. The age and gender distribution is indicative of the increased severity of disease among males and the elderly. 14 , 32 Our adjusted models included multiple variables as confounders, including BMI, blood pressure and comorbidities, which we found to be associated with the outcomes of interest in unadjusted analyses. We emphasise that results should be interpreted cautiously as the causal mechanisms of COVID‐19 infection are still not fully understood, and it is possible that one or more of these variables might lie on the causal pathway between exposure and outcome.
A substantial number of observational studies have previously evaluated whether drugs acting on the RAAS, including ACEIs and ARBs, might be associated with susceptibility, severity and mortality from COVID‐19, but few studies have considered the effects of beta‐blockers, calcium‐channel blockers and other classes of antihypertensive medications. Several systematic reviews have summarised the main findings, which generally find no evidence for worse COVID‐19 outcomes in patients treated with these medications. 33 , 34 , 35 , 36 , 37 , 38 Some studies suggest ACEIs and ARBs could be associated with reduced risks of indicators of severe COVID‐19 disease. 21 , 39 However, systematic reviews also highlight limitations of the evidence presented to date. 36 A high proportion of studies has been based on data from patients admitted to hospital with COVID‐19, sometimes with small samples. Many studies failed to include adequate adjustment for confounding. Few studies have directly addressed the question of collider bias, which distorts associations when data are gathered from patients conditional on their attendance for healthcare. COVID‐19 has been evaluated in an ongoing randomised controlled trial of ramipril treatment. 40 The analysis found no evidence for an effect of ramipril on COVID‐19 incidence or severity but the analysis comprised 102 patients with 11 cases of COVID‐19. 40 A larger trial in Brazil recruited patients who were hospitalised with mild to moderate COVID‐19 and taking ACEIs or ARBs and randomly allocated them to either discontinuation (n = 334) or continuation (n = 325) of their AHT. There was no difference in the mean number of days patients were alive and out of the hospital between the two groups (21.9 days versus 22.9 days) and other secondary outcomes measuring disease severity. 41 Further trials are ongoing. 15
5. CONCLUSION
Drawing on data for a large population‐based sample and using rigorous analytical methods, this study adds to the evidence that antihypertensive therapy may be safely continued during the SARS‐CoV‐2 pandemic. While previous studies have largely evaluated drugs acting on the RAAS, the study found no evidence that any class of antihypertensive therapy might be associated with greater risk of COVID‐19 diagnosis or mortality. There was evidence that, after adjusting for covariates including blood pressure, several classes of AHT might be associated with lower risk of a clinical COVID‐19 diagnosis, but this pattern of association was not apparent for COVID‐19 mortality. While this might be interpreted as evidence of a protective effect, in this observational study it is not possible to exclude the possibility that this pattern of association may be caused by bias.
DATA SOURCES
The study is based in part on data from the Clinical Practice Research Datalink obtained under license from the UK Medicines and Healthcare products Regulatory Agency. However, the interpretation and conclusions contained in this report are those of the authors alone.
COMPETING INTERESTS
All authors have completed the International Committee of Medical Journal Editors (ICMJE) uniform disclosure form at www.icmje.org/coi_disclosure.pdf and declare no conflicts of interest.
CONTRIBUTORS
E.R.P. designed the study with advice from M.G., A.D. and P.C. E.R.P. analysed the data and drafted the paper. M.G., A.D. and P.C. reviewed the analysis and contributed to drafts of the paper. All authors approved the final draft. The corresponding author attests that all listed authors meet authorship criteria and that no others meeting the criteria have been omitted.
Supporting information
SUPPORTING INFORMATION FIGURE S1 COVID‐19 cases by date of diagnosis (blue) and deaths by date of death (black) from 29 January to 25 June 2020.
SUPPORTING INFORMATION FIGURE S2 Age and gender distribution of COVID‐19 cases (left) and deaths (right).
SUPPORTING INFORMATION FIGURE S3 Subgroup case‐control analysis for confirmed COVID‐19 diagnoses showing unadjusted odds ratios (grey) and model adjusted for body mass index, blood pressure, smoking status, frailty level and comorbidities (blue) (n = 21 933).
SUPPORTING INFORMATION TABLE S1 Unadjusted and covariate adjusted relative odds of COVID‐19 diagnosis.
SUPPORTING INFORMATION TABLE S2 Unadjusted and covariate adjusted hazard ratios of COVID‐19 mortality.
SUPPORTING INFORMATION TABLE S3 Adjusted and fully adjusted odds of COVID‐19 diagnosis for subgroups by age category (years).
SUPPORTING INFORMATION TABLE S4 Adjusted and fully adjusted odds of COVID‐19 diagnosis for subgroups by body mass index (BMI) category.
SUPPORTING INFORMATION TABLE S5 Adjusted and fully adjusted odds of COVID‐19 diagnosis for subgroups by frailty category.
ACKNOWLEDGEMENTS
This study was supported by the National Institute for Health Research Biomedical Research Centre at Guy's and St Thomas' Hospitals. The views expressed are those of the authors and not necessarily those of the NHS, the NIHR or the Department of Health. The funder of the study had no role in study design, data collection, data analysis, data interpretation or writing of the report. The authors had full access to all the data in the study and all authors shared final responsibility for the decision to submit for publication.
Rezel‐Potts E, Douiri A, Chowienczyk PJ, Gulliford MC. Antihypertensive medications and COVID‐19 diagnosis and mortality: Population‐based case‐control analysis in the United Kingdom. Br J Clin Pharmacol. 2021;87(12):4598–4607. 10.1111/bcp.14873
Funding information NIHR Biomedical Research Centre at Guy’s and St Thomas’ Hospitals
DATA AVAILABILITY STATEMENT
Requests for access to data from the study should be addressed to emma.rezel‐potts@kcl.ac.uk. All proposals requesting data access will need to specify planned uses with approval of the study team and CPRD before data release.
REFERENCES
- 1. World Health Organization . COVID‐19 Weekly Epidemiological Update 9th February 2021. https://www.who.int/publications/m/item/weekly-epidemiological-update---9-february-2021 (accessed 15 February 2021).
- 2. Department of Health and Social Care, Public Health England . Coronavirus (COVID‐19) in the UK, Deaths in the United Kingdom, 2021. https://coronavirus.data.gov.uk/deaths (accessed 15 February 2021).
- 3. Lu R, Zhao X, Li J, et al. Genomic characterisation and epidemiology of 2019 novel coronavirus: implications for virus origins and receptor binding. Lancet. 2020;395(10224):565‐574. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Hoffmann M, Kleine‐Weber H, Schroeder S, et al. SARS‐CoV‐2 cell entry depends on ACE2 and TMPRSS2 and is blocked by a clinically proven protease inhibitor. Cell. 2020;181(2):271‐80.e8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Wan Y, Shang J, Graham R, et al. Receptor recognition by the novel coronavirus from Wuhan: an analysis based on decade‐long structural studies of SARS coronavirus. J Virol. 2020;94(7):e00127‐e00120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Li B, Yang J, Zhao F, et al. Prevalence and impact of cardiovascular metabolic diseases on COVID‐19 in China. Clin Res Cardiol. 2020;109(5):531‐538. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Bavishi C, Maddox TM, Messerli FH. Coronavirus disease 2019 (COVID‐19) infection and renin angiotensin system blockers. JAMA Cardiol. 2020;5(7):745‐747. [DOI] [PubMed] [Google Scholar]
- 8. Liu Y, Yang Y, Zhang C, et al. Clinical and biochemical indexes from 2019‐nCoV infected patients linked to viral loads and lung injury. Sci China Life Sci. 2020;63(3):364‐374. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Batlle D, Wysocki J, Satchell K. Soluble angiotensin‐converting enzyme 2: a potential approach for coronavirus infection therapy? Clin Sci. 2020;134(5):543‐545. 10.1042/cs20200163 [Epub ahead of print] [DOI] [PubMed] [Google Scholar]
- 10. NHS Digital . Health Survey for England 2016 Prescribed medicines, 2017. https://files.digital.nhs.uk/pdf/3/c/hse2016-pres-med.pdf (accessed 28 August 2020).
- 11. Yang J, Zheng Y, Gou X, et al. Prevalence of comorbidities and its effects in patients infected with SARS‐CoV‐2: a systematic review and meta‐analysis. Int J Infect Dis. 2020;94:91‐95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Wu Z, McGoogan JM. Characteristics of and important lessons from the coronavirus disease 2019 (COVID‐19) outbreak in China: Summary of a report of 72 314 cases from the Chinese Center for Disease Control and Prevention. JAMA. 2020;323(13):1239‐1242. [DOI] [PubMed] [Google Scholar]
- 13. Team CC‐R . Preliminary estimates of the prevalence of selected underlying health conditions among patients with coronavirus disease 2019 – United States, February 12‐March 28, 2020. MMWR Morb Mortal Wkly Rep. 2020;69(13):382‐386. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Williamson EJ, Walker AJ, Bhaskaran K, et al. Factors associated with COVID‐19‐related death using OpenSAFELY. Nature. 2020;584(7821):430‐436. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Mackey K, King VJ, Gurley S, et al. Risks and impact of angiotensin‐converting enzyme inhibitors or angiotensin‐receptor blockers on SARS‐CoV‐2 infection in adults: A living systematic review. Ann Intern Med. 2020;173(3):195‐203. 10.7326/M20-1515 [Epub ahead of print] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Baral R, White M, Vassiliou VS. Effect of renin‐angiotensin‐aldosterone system inhibitors in patients with COVID‐19: a systematic review and meta‐analysis of 28,872 patients. Curr Atheroscler Rep. 2020;22(10):61‐69. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Reynolds HR, Adhikari S, Pulgarin C, et al. Renin‐angiotensin‐aldosterone system inhibitors and risk of covid‐19. N Engl J Med. 2020;382(25):2441‐2448. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Rentsch CT, Kidwai‐Khan F, Tate JP, et al. Covid‐19 testing, hospital admission, and intensive care among 2,026,227 United States veterans aged 54‐75 years. MedRxiv 2020.04.09.20059964. 10.1101/2020.04.09.20059964 [DOI] [Google Scholar]
- 19. Mancia G, Rea F, Ludergnani M, Apolone G, Corrao G. Renin‐angiotensin‐aldosterone system blockers and the risk of covid‐19. N Engl J Med. 2020;382(25):2431‐2440. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Fosbøl EL, Butt JH, Østergaard L, et al. Association of angiotensin‐converting enzyme inhibitor or angiotensin receptor blocker use with COVID‐19 diagnosis and mortality. JAMA. 2020;324(2):168‐177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Hippisley‐Cox J, Young D, Coupland C, et al. Risk of severe COVID‐19 disease with ACE inhibitors and angiotensin receptor blockers: cohort study including 8.3 million people. Heart. 2020;106(19):1503‐1511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Griffith G, Morris TT, Tudball M, et al. Collider bias undermines our understanding of COVID‐19 disease risk and severity. medRxiv. 2020. 2020.05.04.20090506 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Herbert A, Griffith G, Hemani G, Zuccolo L. The spectre of Berkson's paradox: Collider bias in Covid‐19 research. Signif. 2020;17(4):6‐7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Boëlle P‐Y, Souty C, Launay T, et al. Excess cases of influenza‐like illnesses synchronous with coronavirus disease (COVID‐19) epidemic, France, March 2020. Euro Surveill. 2020;25(14):1‐4, 2000326. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Herrett E, Gallagher AM, Bhaskaran K, et al. Data Resource Profile: Clinical Practice Research Datalink (CPRD). Int J Epidemiol. 2015;44(3):827‐836. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Herrett E, Thomas SL, Schoonen WM, Smeeth L, Hall AJ. Validation and validity of diagnoses in the General Practice Research Database: a systematic review. Br J Clin Pharmacol. 2010;69(1):4‐14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Clegg A, Bates C, Young J, et al. Development and validation of an electronic frailty index using routine primary care electronic health record data. Age Ageing. 2016;45(3):353‐360. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Charlson ME, Pompei P, Ales KL, et al. A new method of classifying prognostic comorbidity in longitudinal studies: development and validation. J Clin Epidemiol. 1987;40(5):373‐383. [DOI] [PubMed] [Google Scholar]
- 29. Khan NF, Perera R, Harper S, Rose PW. Adaptation and validation of the Charlson Index for Read/OXMIS coded databases. BMC Fam Pract. 2010;11(1):1‐7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Khunti K, Singh AK, Pareek M, et al. Is ethnicity linked to incidence or outcomes of covid‐19? BMJ. 2020;369:137, m1548. [DOI] [PubMed] [Google Scholar]
- 31. Raisi‐Estabragh Z, McCracken C, Bethell MS, et al. Greater risk of severe COVID‐19 in Black, Asian and minority ethnic populations is not explained by cardiometabolic, socioeconomic or behavioural factors, or by 25 (OH)‐vitamin D status: study of 1326 cases from the UK Biobank. J Public Health. 2020;42(3):451‐460. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Goodacre S, Thomas B, Lee E, et al. Characterisation of 22445 patients attending UK emergency departments with suspected COVID‐19 infection: Observational cohort study. PLoS One. 2020;15(11):e0240206. 10.1371/journal.pone.0240206 [eCollection 2020] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Zhang X, Yu J, Pan LY, Jiang HY. ACEI/ARB use and risk of infection or severity or mortality of COVID‐19: A systematic review and meta‐analysis. Pharmacol Res. 2020;158:104927. 10.1016/j.phrs.2020.104927 [EPub ahead of print] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Chan CK, Huang YS, Liao HW, et al. Renin‐angiotensin‐aldosterone system inhibitors and risks of SARS‐CoV‐2 infection: A systematic review and meta‐analysis. Hypertension. 2020;76(5):1563‐1571. 10.1161/hypertensionaha.120.15989 [EPub ahead of print] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Dambha‐Miller H, Albasri A, Hodgson S, et al. Currently prescribed drugs in the UK that could upregulate or downregulate ACE2 in COVID‐19 disease: a systematic review. BMJ Open. 2020;10(9):e040644. [EPub ahead of print] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Hasan SS, Kow CS, Hadi MA, Zaidi STR, Merchant HA. Mortality and disease severity among COVID‐19 patients receiving renin‐angiotensin system inhibitors: A systematic review and meta‐analysis. Am J Cardiovasc Drugs. 2020;20(6):571‐590. 10.1007/s40256-020-00439-5 [EPub ahead of print] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Lee HW, Yoon C‐H, Jang EJ, Lee C‐H. Renin‐angiotensin system blocker and outcomes of COVID‐19: a systematic review and meta‐analysis. Thorax. 2021;76(5):479‐486. [DOI] [PubMed] [Google Scholar]
- 38. Volpe M, Battistoni A. Systematic review of the role of renin‐angiotensin system inhibitors in late studies on Covid‐19: A new challenge overcome? Int J Cardiol. 2020;321:150‐154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Wang Y, Chen B, Li Y, et al. The use of renin‐angiotensin‐aldosterone system (RAAS) inhibitors is associated with a lower risk of mortality in hypertensive COVID‐19 patients: A systematic review and meta‐analysis. J Med Virol. 2021;93(3):1370‐1377. [DOI] [PubMed] [Google Scholar]
- 40. Amat‐Santos IJ, Santos‐Martinez S, López‐Otero D, et al. Ramipril in high‐risk patients with COVID‐19. J Am Coll Cardiol. 2020;76(3):268‐276. 10.1016/j.jacc.2020.05.040 [EPub ahead of print] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Lopes RD, Macedo AVS, de Barros E Silva PGM, et al. Effect of discontinuing vs continuing angiotensin‐converting enzyme inhibitors and angiotensin II receptor blockers on days alive and out of the hospital in patients admitted with COVID‐19: A randomized clinical trial. JAMA. 2021;325(3):254‐264. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
SUPPORTING INFORMATION FIGURE S1 COVID‐19 cases by date of diagnosis (blue) and deaths by date of death (black) from 29 January to 25 June 2020.
SUPPORTING INFORMATION FIGURE S2 Age and gender distribution of COVID‐19 cases (left) and deaths (right).
SUPPORTING INFORMATION FIGURE S3 Subgroup case‐control analysis for confirmed COVID‐19 diagnoses showing unadjusted odds ratios (grey) and model adjusted for body mass index, blood pressure, smoking status, frailty level and comorbidities (blue) (n = 21 933).
SUPPORTING INFORMATION TABLE S1 Unadjusted and covariate adjusted relative odds of COVID‐19 diagnosis.
SUPPORTING INFORMATION TABLE S2 Unadjusted and covariate adjusted hazard ratios of COVID‐19 mortality.
SUPPORTING INFORMATION TABLE S3 Adjusted and fully adjusted odds of COVID‐19 diagnosis for subgroups by age category (years).
SUPPORTING INFORMATION TABLE S4 Adjusted and fully adjusted odds of COVID‐19 diagnosis for subgroups by body mass index (BMI) category.
SUPPORTING INFORMATION TABLE S5 Adjusted and fully adjusted odds of COVID‐19 diagnosis for subgroups by frailty category.
Data Availability Statement
Requests for access to data from the study should be addressed to emma.rezel‐potts@kcl.ac.uk. All proposals requesting data access will need to specify planned uses with approval of the study team and CPRD before data release.


