Abstract
Herein, we are reporting a case of Stevens–Johnson syndrome due to COVID‐19 vaccine, which has been proved by history taking, clinical examination and histopathology. To the best of our knowledge we are reporting the first case of COVID‐19 vaccine‐induced Stevens–Johnson syndrome.
Dear Editor,
Stevens–Johnson syndrome (SJS) is a severe cutaneous adverse drug reaction. There is scant information about its occurrence following vaccine.1 We report a case of SJS induced by a COVID‐19 vaccine in an adult.
A 60‐year‐old man presented with complaints of fever, oral ulceration and skin rash, which had started 3 days after he had received his first dose of the recombinant ChAdOx1 nCoV‐19 (Covishield, which is a patent product of AstraZeneca, manufactured by Serum Institute of India) COVID‐19 vaccine. He had consulted a local physician who had prescribed paracetamol and levocetrizine; however, the symptoms were not controlled and gradually the rashes became generalized in distribution. The patient presented to the emergency department 3 days following the first onset of the lesions (10 days after the vaccine administration) throughout which period the fever had persisted.
Physical examination revealed multiple purpuric macules present all over the body with perilesional erythema. The lesions had coalesced to form large sheets of necrosed skin over the front and back of the patient’s trunk, with a few areas showing bullae. Mucosal involvement was present in the form of oral erosions, haemorrhagic crusting over the lips, redness of and slight discharge from the eyes, and erosions on the glans (Fig. 1).
Figure 1.
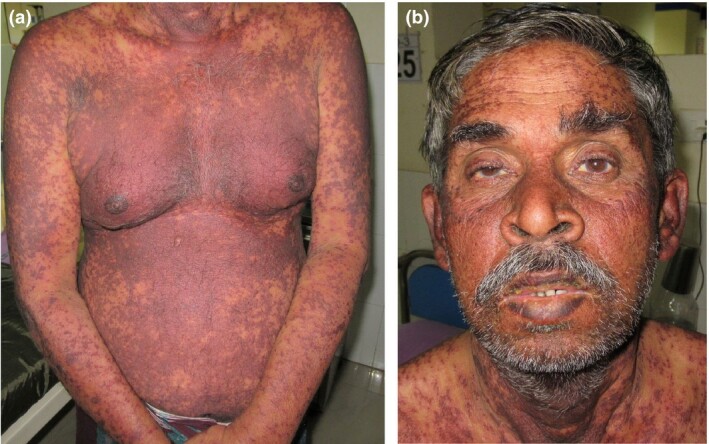
(a) Large sheets of necrosed skin in front of trunk, with a few areas showing bullae; (b) involvement of the face with erosions in palpebral conjunctiva and necrotic crusting over lips.
Based on the disease course and morphology, SJS was suspected and a detailed drug history was elicited, which revealed that for the past 6 months the patient had been taking teneligliptin and metformin for diabetes and amlodipine for hypertension. His other medications had been prescribed after he had developed fever and skin rash, and he denied any other drug intake before the development of his symptoms. The SCORE of Toxic Epidermal Necrosis was 1 on the day of admission, and the Naranjo algorithm revealed a causal association of 2 (possible association) between the vaccine and the adverse drug reaction.
Histopathological examination from the erythematous lesion revealed orthokeratosis with epidermal atrophy, moderate intraepidermal infiltration of lymphocytes and neutrophils with moderate spongiosis, scattered degenerated apoptotic keratinocytes, patchy areas of basal cell degeneration and interface dermatitis, perivascular and periadnexal inflammatory cell infiltrate, and extravasation of erythrocytes in the dermis (Fig. 2).
Figure 2.
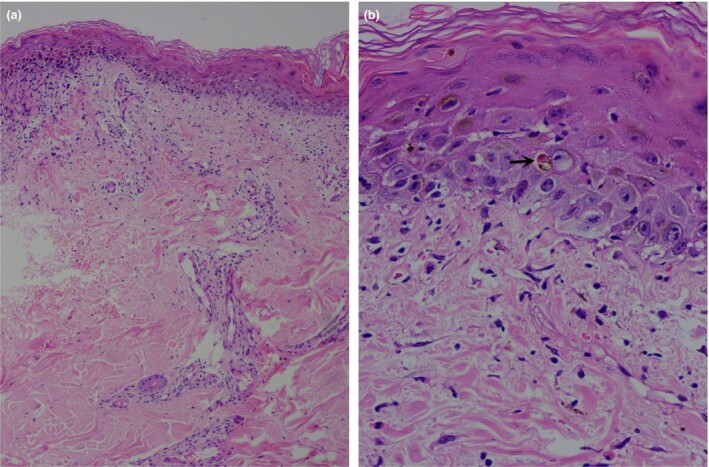
(a) Orthokeratosis with epidermal atrophy, scattered degenerated apoptotic keratinocytes, patchy areas of basal cell degeneration and interface dermatitis, perivascular and periadnexal inflammatory cell infiltrate, and extravasation of erythrocytes in the dermis; (b) apoptotic keratinocytes (arrow), upper dermal oedema and extravasation of erythrocytes. Haematoxylin and eosin, original magnification (a) × 50; (b) × 400.
The diagnosis of SJS was thus confirmed and the patient was started on oral ciclosporin 300 mg, which led to complete resolution after 7 days (Fig. S1). The patient was issued a drug card and advised to defer the second dose of vaccine.
Diagnosis of SJS is made on the basis of clinical suspicion and histological findings. In this case, suspicion of SJS was based on the sudden appearance of erythematous, reticulate patches on the skin, the mucosal ulceration and the constitutional symptoms. The diagnosis was confirmed by the presence of epidermal keratinocyte necrosis. Chahal et al. adopted a similar diagnostic approach to SJS, which included clinical findings, corroborative history and histopathological findings.1 The Naranjo algorithm score is widely used for assessing causal association in drug reaction.2 Our patient was known to have diabetes and hypertension, and was on teneligliptin, metformin and amlodipine. He had taken the antihypertensive drug and received the vaccine prior to development of SJS but the continued intake of the antihypertensive drug did not aggravate the condition. The Naranjo scale score of 2 suggested possible association of vaccine in the development of SJS.
All COVID‐19 vaccines have two components (virotopes and excipients) and both can cause severe drug reaction.3 We believe that in our patient, it was the virotopes that caused the SJS, as we did not find severe delayed‐type hypersensitivity to other vaccine ingredients. In a previous study,1 Chahal et al. hypothesized that expression of the virotopes on the surface of keratinocytes leads to a CD8+ T‐lymphocyte response against epidermal cells and causes apoptosis of keratinocytes and detachment at the dermoepidermal junction, leading to SJS in genetically susceptible individuals. This is further supported by the ability of the ChAdOx1 nCoV‐19 vaccine to induce a T‐cell‐specific response, which is predominantly T helper 1‐based, which may have induced an immune response with consequent keratinocyte cell damage.4 Further, in an extensive review we did not find any evidence of any of the excipients (L‐histidine, L‐histidine hydrochloride, sucrose, sodium chloride, magnesium chloride, polysorbate 80, edetate disodium, ethanol and water) causing severe delayed type hypersensitivity reactions such as SJS.3
In conclusion, we report the first case, to our knowledge, of COVID‐19 vaccine‐induced SJS. This case illustrates an exceedingly rare complication of the vaccine. As the benefits far outweigh the risk of the vaccine in the current pandemic, such rare reactions should not deter people from receiving the vaccine.
Acknowledgement
The patient provided written informed consent to publication of the case details and photographs.
Supplementary Material
Figure S1 (a,b) Healed lesions after treatment.
Contributor Information
S. Dash, Department of Dermatology and Venereology All India Institute of Medical Sciences (AIIMS) Bhubaneswar India
C. S. Sirka, Department of Dermatology and Venereology All India Institute of Medical Sciences (AIIMS) Bhubaneswar India
S. Mishra, Department of Dermatology and Venereology All India Institute of Medical Sciences (AIIMS) Bhubaneswar India
P. Viswan, Department of Dermatology and Venereology All India Institute of Medical Sciences (AIIMS) Bhubaneswar India
References
- Chahal D, Aleshin M, Turegano M et al. Vaccine‐induced toxic epidermal necrolysis: a case and systematic review. Dermatol Online J 2018; 24. 10.5070/D3241037941 [DOI] [PubMed] [Google Scholar]
- Belhekar MN, Taur SR, Munshi RP. A study of agreement between the Naranjo algorithm and WHO‐UMC criteria for causality assessment of adverse drug reactions. Indian J Pharmacol 2014; 46: 117–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stone CA Jr, Rukasin CRF, Beachkofsky TM, Phillips EJ. Immune‐mediated adverse reactions to vaccines. Br J Clin Pharmacol 2019; 85: 2694–706. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ramasamy MN, Minassian AM, Ewer KJ et al. Safety and immunogenicity of ChAdOx1 nCoV‐19 vaccine administered in a prime‐boost regimen in young and old adults (COV002): a single‐blind, randomised, controlled, phase 2/3 trial. Lancet 2021; 396: 1979–93. (pu blished corrections appear in Lancet 2021; 396: 1978 and Lancet 2021; 397: 1350). [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Figure S1 (a,b) Healed lesions after treatment.


