Data on the dynamics and duration of humoral immune responses against SARS‐CoV‐2 in hematological patients are still lacking. Preliminary studies in non‐immunocompromised subjects with COVID‐19 reported seroconversion 7 to 14 days following symptom onset, with increased IgM and IgG titers observed during the first month. IgM levels, after peaking by day 30, gradually decreased and were undetectable by day 180. The long‐lasting persistence of IgG has not been clearly demonstrated. 1 , 2 , 3 , 4 , 5
We evaluated the humoral response and kinetics of IgM and IgG against SARS‐CoV‐2 in 25 hematologic patients, receiving anticancer therapy, who were followed after real‐time quantitative polymerase chain reaction (RT‐qPCR) confirmation of SARS‐CoV‐2 infection. The patient demographics and clinical characteristics are shown in Table 1. The median age was 59 years (range 21–85). The underling hematologic diseases were: lymphoma (10/25), myeloma (7/25), chronic lymphoproliferative diseases (5/25) and acute leukemia (3/25). SARS‐CoV‐2 infection was mild symptomatic in 5/25 (20%) cases and symptomatic in 20/25 (80%), the most frequent symptoms being fever, sore throat, anosmia, cough, shortness of breathing, and fatigue. Four of the 20 symptomatic patients had pneumonia requiring hospitalization. None of these 25 patients died from COVID‐19.
TABLE 1.
Characteristics of 25 Hematologic patients with COVID‐19
| No. of cases | 25 |
| Sex (M/F) | 12/13 |
| Median age‐years (range) | 59 (21–85) |
| Hematologic malignancies | |
|
10/25 (40%) |
|
7/25 (28%) |
|
5/25 (20%) |
|
3/25 (12%) |
| Concomitant therapy | |
|
10/25 (40%) |
|
5/25 (20%) |
|
4/25 (16%) |
|
4/25 (16%) |
|
3/25 (12%) |
|
2/25 (8%) |
| Immunoglobulins, b mg/dl‐ median (range) | |
|
832 (167–2210) |
|
54,5 (6–2510) |
|
54 (8–605) |
| Lymphocytes b , N/mmc‐median (range) | 1100 (250–3300) |
1 Nivolumab; 1 Ponatinib and prednisone.
Median values at SARS‐CoV‐2 infection onset.
The median IgG, IgM, and IgA values at SARS‐CoV‐2 infection onset were 832 mg/dl (167–2210 mg/dl), 54.5 mg/dl (6–2510 mg/dl), and 54 mg/dl (8–605 mg/dl), respectively. The median lymphocyte count was 1100/mmc (250–3300/mmc).
The IgM and IgG antibodies against SARS‐CoV‐2 spike protein (subunity S1 and S2) were tested by chemiluminescence immunoassay (CLIA) with a positive cut‐off value of 12 UA/ml for both IgG and IgM. The specificity and sensibility of this assay was 98,5% and 97,4%, respectively. 6 All patients signed written informed consent for the serological test. To assess the kinetics of antibody titers, in our convalescent COVID‐19 patients, serum IgM and IgG levels were longitudinally measured at established time points: 1 month (T1) 2 months (T2), 3 months (T3), 4 months (T4) and 6 months (T6) after their first positive nasopharyngeal swab test. None of these cases received anti SARS‐CoV‐2 vaccination during the study period.
Among these 25 confirmed COVID‐19 cases, 21/25 (84%) developed specific anti SARS‐CoV‐2 antibodies with a titer > 12 UA/ml in almost one of the specific time points. However, as reported in Figure 1(A) and 1(B), after a peak of the IgG and an overall mild increase of IgM, the antibody titer declined from 4 months after the disease onset under the positive cut‐off value, although variation between patients was detected. The mean and median titers were detailed in Figure 1(A) and 1(B).
FIGURE 1.
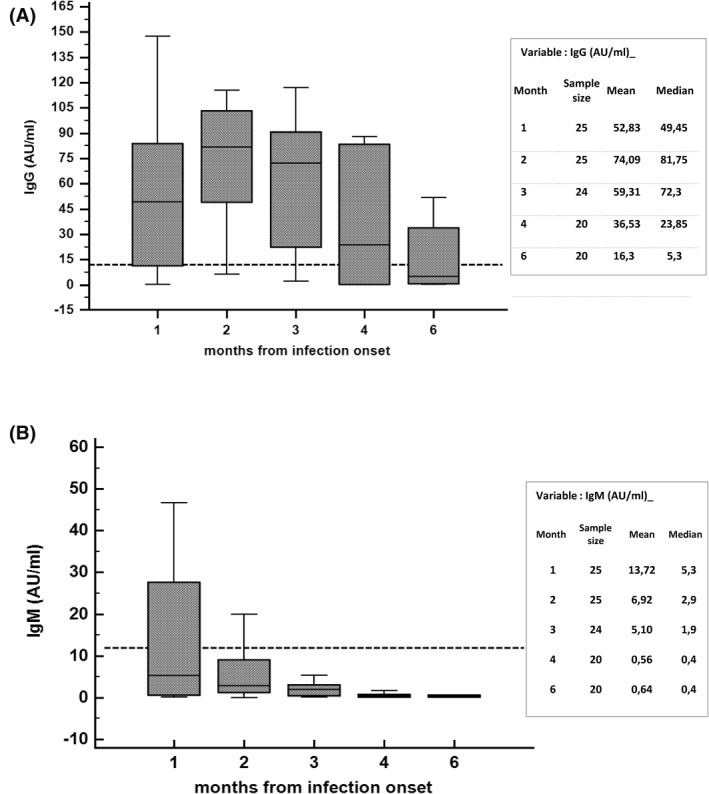
Determination of IgG (A) and IgM (1B) antibody levels against SARS‐CoV‐2 at different time points from SARS‐CoV‐2 infection onset. Horizontal dot line represents positive cut‐off value (12 UA/ml)
In summary, although we analyzed a limited number of cases, patients with hematological malignancy appear to have an antibody response to SARS‐CoV‐2 and a high rate of seroconversion (84%). However, the kinetic of antibody levels may suggest that the duration of antibody‐mediated protection against re‐infection with SARS‐CoV‐2 may be short‐lasting. 7 , 8 If confirmed in a larger number of cases, these findings would suggest that stringent infection prevention and control measures must be maintained in hematological patients who have recovered from COVID‐19. In addition, our results would suggest that hematological patients could require a periodic re‐vaccination. Obviously, additional studies of both humoral and cellular immunity (T and memory B cells) will be necessary to better understand the dynamics, duration, and intensity of the overall immunological response to SARS‐CoV‐2 infection in hematological malignancy patients.
CONFLICT OF INTEREST
The authors declare no conflicts of interest.
AUTHOR CONTRIBUTIONS
Anna Candoni performed the research, collected the data and wrote the letter. Umberto Pizzano collected the data, Martina Fabris performed the quantitative serological test. Francesco Curcio and Renato Fanin revised the letter. All the authors approved the final version.
PEER REVIEW
The peer review history for this article is available at https://publons.com/publon/10.1002/hon.2872.
DATA AVAILABILITY STATEMENT
The data that support the findings of this study are available from the corresponding author upon reasonable request.
REFERENCES
- 1. Long QX, Liu BZ, Deng HJ, et al. Antibody responses to SARS‐CoV‐2 in patients with COVID‐19. Nat Med 2020;26:845‐848. [DOI] [PubMed] [Google Scholar]
- 2. Zhao J, Yuan Q, Wang H, et al. Antibody responses to SARS‐CoV‐2 in patients of novel coronavirus disease 2019. Clin Infect Dis. 2020;71(16):2027–2034. 10.1093/cid/ciaa344 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Perreault J, Tremblay T, Fournier MJ, et al. Waning of SARS‐CoV‐2 RBD antibodies in longitudinal convalescent plasma samples within 4 months after symptom onset. Blood 2020;136(22):2588–2591. 10.1182/blood.2020008367 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Ibarrondo FJ, Fulcher JA, Goodman‐Meza D, et al. Rapid decay of anti‐SARS‐CoV‐2 antibodies in persons with mild COVID‐19. N Engl J Med 2020;383:1085‐1087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Seow J, Graham C, Merrick B, et al. Longitudinal evaluation and decline of antibody responses in SARS‐CoV‐2 infection. Nat Microbiol. 2020;5:1598–1607. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Soleimani R, Khourssaji M, Gruson D, et al. Clinical usefulness of fully automated chemiluminescent immunoassay for quantitative antibody measurements in COVID‐19 patients. J Med Virol. 2021;93(3):1465‐1477. 10.1002/jmv.26430. Epub 2020 Aug 24. PMID: 32797641; PMCID: PMC7436871. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Bongiovanni M, Basile F. Re‐infection by COVID‐19: a real threat for the future management of pandemia? Infectious Diseases 2020, 52, 581‐582. [DOI] [PubMed] [Google Scholar]
- 8. Hoang VT, Dao TL, Gautret P. Recurrence of positive SARS‐CoV‐2 in patients recovered from COVID‐19. J Med Virol 2020, 92, 2366‐2367 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The data that support the findings of this study are available from the corresponding author upon reasonable request.


