Despite unprecedented achievements in vaccine development, coronavirus disease 2019 (COVID‐19) is likely to continue to be a threat to humans. Although our knowledge of the lung pathology continues to accumulate, the functional impact remains poorly understood. Cohort studies with longitudinal clinic and home‐based monitoring of patients are needed. The most informative respiratory system assessments include: pulmonary function testing, oxygen saturation, and exercise challenge tests. The derived data will inform intervention trials and treatment guidelines for healthcare providers.
INTRODUCTION
Despite unprecedented achievements in vaccine development, the emergence of novel severe acute respiratory syndrome‐coronavirus 2 (SARS‐CoV‐2) variants with increased transmissibility and “immune escape” properties, coupled with vaccine hesitancy and less than optimal compliance with public health measures, suggests that COVID‐19 is likely to continue to be a threat to humans, at least in the near term. Clinically, these observations translate into the possibility that a significant number of COVID‐19 illnesses are likely to remain an unfortunate reality. 1 Recent increasing infection trends in a number of countries worldwide remind us of this potential risk.
This unfortunate possibility requires us to continue to expand our understanding of this disease. We must widen our lenses to understand now more fully both the natural history and the long‐term consequences of COVID‐19 illness, especially severe illness, on survivors of this infection. Neither of these has been well‐studied thus far. The long‐term sequelae of COVID‐19 is multifaceted and involves both neurological and neuropsychiatric symptoms, including depression and impaired cognitive function, 2 as well as altered lung function. 3 Both sets of conditions present different but formidable challenges in terms of long‐term follow‐up and patient care. The focus of this commentary is monitoring for impaired lung function.
Presently available data suggests that significant numbers of patients surviving acute, severe infection have persistent respiratory symptoms and signs requiring appropriate monitoring. 4 Because the natural history of these symptomatic patients has not been well‐established, the long‐term consequences of respiratory damage has not been adequately described. What is clear is that virus infection results in injury to the alveolar tissues (including blood vessels) resulting in interstitial tissue inflammation, ventilation perfusion (V/Q) mismatching, and impaired oxygenation. 5 Available information suggests that these injuries, in many cases, can result in a form of pulmonary interstitial fibrosis potentially leading to persistent symptoms of dyspnea, cough, and fatigue. 6
Although radiologic documentation of these pathologic effects in the lung continues to accumulate, 7 our understanding of the long‐term functional impact remains limited. Barriers to a more robust understanding of the course of this disease include larger and more widespread community‐based cohort studies that allow for both sufficient longitudinal clinic and home‐based monitoring of patients. These efforts will provide data to support adequate intervention trials, and subsequent diagnostic and treatment guidelines for healthcare providers. The need for these community‐based trials requires that home‐based monitoring takes an important role in monitoring and data collection. A comprehensive approach for patient remote monitoring in clinical trials was described by Dockendorf et al., 8 which includes outpatient specimen collection, use of digital devices to collect and measure physiological and behavioral data, as well as integration of digital data streams and data visualization. Transitioning of clinic measurements to remote settings has been in a spotlight during the pandemic; however, many assessments are still under development and require validation studies. 9
A recent review of studies assessing lung function in acutely ill patients with COVID‐19 indicated that the most pronounced change is the diminished diffusion capacity of the lungs for carbon monoxide (DLCO), followed by changes in mechanical parameters, such as forced vital capacity (FVC) and forced expiratory volume in one second (FEV1). The data from the studies included in the review did not reach a firm conclusion about obstructive versus restrictive patterns in lung tissue changes, due to variability in study design and relatively small numbers of patients included. 3 These preliminary data highlight the need for close longitudinal follow‐up of these patients to understand natural history, disease progression/resolution, and subsequent appropriate intervention and treatment.
The practical aspects of clinical measurements are of paramount importance for selection and deployment for research and clinical decision‐making. For example, DLCO is an informative parameter, but it requires sophisticated equipment and can be done only during clinic visits, whereas other pulmonary function test (PFT) parameters, like FVC and FEV1, can be performed remotely by means of mobile spirometry, which were demonstrated to be equivalent to clinic measures in a pilot study. 10 Recent guidance from the American Thoracic Society indicates that at‐home spirometry collection (FEV1 and FVC) can be a viable alternative to in‐clinic spirometry tests, although caution remains about remote test accuracy as data continues to accumulate about its measurement properties, of which initial reports appear to be favorable. 11
Consistent with the DLCO data, impaired arterial oxygenation, as measured by pulse oximetry, has been shown to be an important prognostic factor for worse outcomes during the acute phase of COVID‐19 illness. 12 Based on these observations, the use of oximetry is suggested for follow‐ups during the recovery period. Additionally, because it is known that V/Q mismatch can result in arterial desaturation (drop in arterial pO2) with exercise, and evidence suggests that normal pulse oximetry values taken at rest may deteriorate with physical exertion in the presence V/Q abnormalities, which are present in COVID illness, exertion‐induced desaturation may be a marker of persistent disease. 13 Several exertional tests for measuring oxygen desaturation in patients with post‐COVID‐19 are safe and easy to perform at home, such as the 1‐min sit‐to‐stand test and the 40‐step test. These tests are less well‐characterized compared to the more conventional 6‐min walk test (6MWT) but are very practical for in‐home conditions and in frail patients. 13
Therefore, available data suggests that the three categories of assessments most useful in characterizing the natural history of COVID‐19 illness in the respiratory system include: PFT, capillary blood oxygen saturation (SpO2), and exercise challenge tests. These measurements are all capable of being measured in the community setting and at home.
From a clinical point of view, the utility of wearable devices and home based “remote” patient monitoring was recognized in the early days of the coronavirus pandemic. In the absence of readily available SARS‐CoV‐2 polymerase chain reaction (PCR) tests, monitoring for vital signs via devices, such as digital thermometers and pulse oximeters, provided a viable opportunity for monitoring patients and triaging the need for immediate intervention.
Today, remote monitoring of vital signs and symptoms during the acute phase of COVID‐19 is commonplace and well‐characterized. The combination of self‐reported symptoms and data from wearable monitors provide a relatively accurate method of detecting symptomatic infections. Notably, the DETECT study, run by the Scripps Research Institute, provides an algorithm to predict COVID‐19 based on data collected by fitness trackers. 14
Considering the large number of symptomatic survivors of COVID‐19 illness, identifying tools and instruments that can be deployed remotely, conveniently, and with the patient at home (Table 1) may help monitor patients suffering from a slow or incomplete recovery or those worsening over time. 3 However, several barriers need to be removed to make remote patient monitoring a reality. These barriers include technological challenges: not all clinic assessments can be transitioned to a remote mode and a comprehensive monitoring package should include both clinic and at home measures (Figure 1). The above‐mentioned example of DLCO requires an in‐clinic assessment whereas other pulmonary function parameters, like FVC and FEV1, can be collected remotely 10 (Table 1). The biotech/pharmaceutical industry as well as the healthcare and regulatory communities need to get comfortable with this approach. These measurements need to be validated in remote settings prior to a wide deployment to understand its measurement properties. 9 Moreover, healthcare providers may need the demonstration of the outcome benefits of this approach. Changes for remote monitoring reimbursement were introduced during the pandemic but remain authorized only for the duration of COVID‐19 health emergency. 15 Remote patient monitoring might give an answer to the numerous questions about the natural history of this illness, choice of sensitive and specific measurement tools, and frequency of monitoring. In addition to defining the natural history of this illness, these remote measurement tools can be put to work monitoring disease and evaluating therapeutic interventions to mitigate the morbidity and mortality effects of this ongoing virus pandemic.
TABLE 1.
A comparison of clinic and remote measurements to assess COVID‐19 long‐term impact on lung function
| Assessment type | Clinic measurements | Remote measurements | Considerations |
|---|---|---|---|
| Lung X‐ray or CT scan | Standard assessment for detecting lung tissue abnormalities | Not applicable | |
| Spirometry | DLCO, FVC, FEV1 | FVC, FEV1 | Comparability between FEV1 clinic and remote measures has been demonstrated 10 ; data may be more variable due to an unsupervised test procedure |
| Pulse oximetry | Heart rate, SpO2 | Heart rate, SpO2 | Pulse oximeters with regulatory clearance ensure higher quality of data; validation on people with different skin tones is lacking. |
| Exercise challenge tests | Supervised CPET, 6MWT, 1‐min sit‐to‐stand test and the 40‐step test | Unsupervised 6MWT, 1‐min sit‐to‐stand test and the 40‐step test conducted by means of wearable sensors | Feasibility and patient safety should be considered carefully when deploying these tests at home |
Abbreviations: 6MWT, 6‐minute walk test; COVID‐19, coronavirus disease 2019; CPET, cardiopulmonary exercise test; CT, computed tomography; DLCO, diffusion capacity for carbon monoxide; FEV1, forced expiratory volume in one second; FVC, forced vital capacity; SpO2, capillary blood oxygenation.
FIGURE 1.
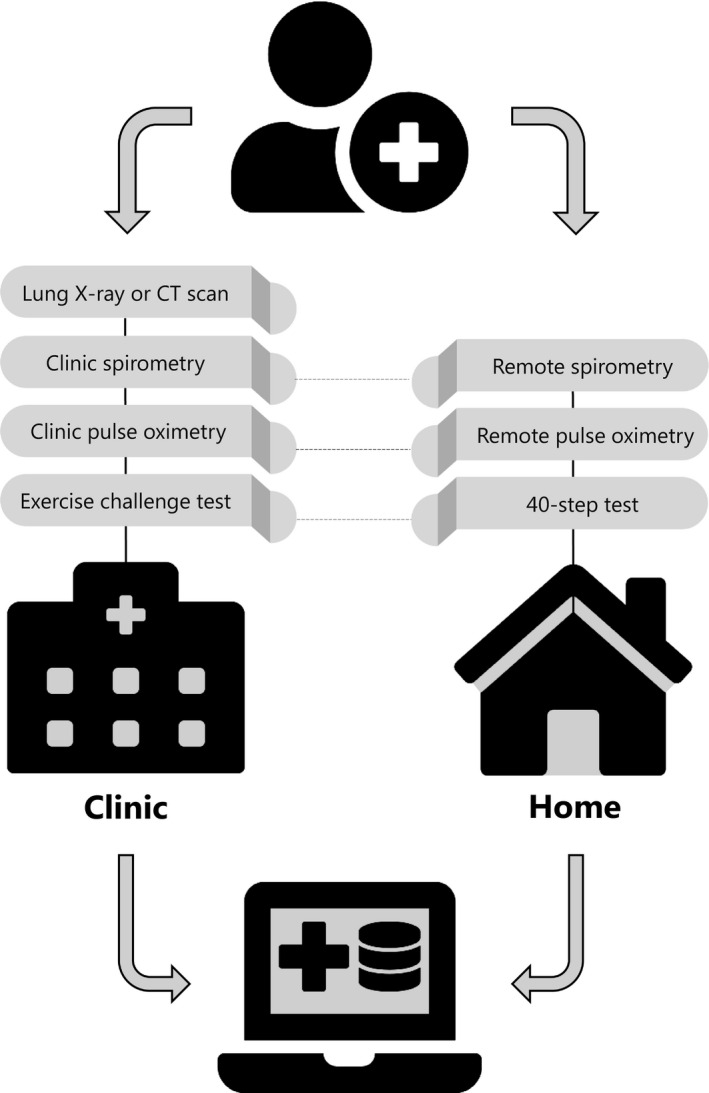
Tests performed in the clinic and at home. Both data sets can be integrated into a single database linked to patients’ medical records. Although not all tests can be performed remotely (computed tomography [CT] scan), remote data monitoring is more convenient for patients, and creates more comprehensive data sets
CONFLICTS OF INTEREST
E.S.I. is an employee of Koneksa Health and may own company stock. T.F.R. is on the Advisory Board of Koneska Health, and received stock options from the company. He is a member of the Board of Directors of the American Thoracic Society, the FDA Science Board, and has been an advisor to other pre‐commercial bio‐pharma companies. He was previously employed by Merck Research Labs, Novartis, Celgene, Covance, and Vanderbilt University.
ACKNOWLEDGEMENTS
The authors thank Stephen Golubieski for help with a graphic design.
Izmailova ES, Reiss TF. Closer to the patient means better decisions: Wearable remote monitoring of patients with COVID‐19 lung disease. Clin Transl Sci. 2021;14:2091–2094. 10.1111/cts.13085
Funding information
No funding was received for this work.
REFERENCES
- 1. John Hopkins University coronavirus resource Center . https://coronavirus.jhu.edu/us‐map. Accessed June 3, 2021.
- 2. Varatharaj A, Thomas N, Ellul MA, et al. Neurological and neuropsychiatric complications of COVID‐19 in 153 patients: a UK‐wide surveillance study. Lancet Psychiatry. 2020;7:875‐882. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Torres‐Castro R, Vasconcello‐Castillo L, Alsina‐Restoy X, et al. Respiratory function in patients post‐infection by COVID‐19: a systematic review and meta‐analysis [published online ahead of print November 25, 2020]. Pulmonology. 10.1016/j.pulmoe.2020.10.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Hull JH, Lloyd JK, Cooper BG. Lung function testing in the COVID‐19 endemic. Lancet Respir Med. 2020;8:666‐667. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Dhont S, Derom E, Van Braeckel E, Depuydt P, Lambrecht BN. The pathophysiology of ‘happy’ hypoxemia in COVID‐19. Respir Res. 2020;21:198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Lutchmansingh DD, Knauert MP, Antin‐Ozerkis DE, et al. A clinic blueprint for post‐coronavirus disease 2019 recovery: learning from the past, looking to the future. Chest. 2021;159(3):949‐958. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. van Gassel RJJ , Bels JLM, Raafs A, et al. High prevalence of pulmonary sequelae at 3 months after hospital discharge in mechanically ventilated survivors of COVID‐19. Am J Respir Crit Care Med. 2020;203:371‐374. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Dockendorf MF, Hansen BJ, Bateman KP, et al. Digitally enabled, patient‐centric clinical trials: shifting the drug development paradigm. Clin Transl Sci. 2021;14:445‐459. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Izmailova ES, Ellis R, Benko C. Remote monitoring in clinical trials during the COVID‐19 pandemic. Clin Transl Sci. 2020;13:838‐841. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Huang C, Izmailova ES, Jackson N, et al. Remote FEV1 monitoring in asthma patients: a pilot study. Clin Transl Sci. 2021;14:529‐535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Wilson KC, Kaminsky DA, Michaud G, et al. Restoring pulmonary and sleep services as the COVID‐19 pandemic lessens. from an association of pulmonary, critical care, and sleep division directors and American Thoracic Society–coordinated Task Force. Ann Am Thorac Soc. 2020;17:1343‐1351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Petrilli CM, Jones SA, Yang J, et al. Factors associated with hospitalization and critical illness among 4,103 patients with Covid‐19 disease in New York City [published online ahead of print April 11, 2020]. medRxiv Preprint. 10.1101/2020.04.08.20057794. [DOI] [Google Scholar]
- 13. University of Oxford the center for evidence‐based medicine . https://www.cebm.net/covid‐19/what‐is‐the‐efficacy‐and‐safety‐of‐rapid‐exercise‐tests‐for‐exertional‐desaturation‐in‐covid‐19/. March 4, 2021.
- 14. Quer G, Radin JM, Gadaleta M, et al. Wearable sensor data and self‐reported symptoms for COVID‐19 detection. Nat Med. 2021;27:73‐77. [DOI] [PubMed] [Google Scholar]
- 15. How to make remote monitoring Part of everyday healthcare, Harvard business review. https://hbr.org/2020/07/how‐to‐make‐remote‐monitoring‐tech‐part‐of‐everyday‐health‐care. May 3, 2021.


