Abstract
Retiform purpura has been described as a relatively frequent cutaneous finding in patients with coronavirus disease 2019 (COVID‐19). The etiology is hypothesized to be related to thrombotic vasculopathy based on lesional biopsy specimen findings, but the pathogenesis of the vasculopathy is not completely understood. Here, we present a case of a retiform purpuric patch on the sacrum/buttocks in a hospitalized patient prior to subsequent diagnosis of COVID‐19 and an eventual fatal disease course. Two lesional biopsy specimens at different time points in the disease course revealed thrombotic vasculopathy, despite therapeutic anticoagulation. Detailed histopathologic evaluation using immunohistochemical markers suggest the etiology of the vasculopathy involves both persistent complement activation and platelet aggregation, which possibly promote ongoing thrombus formation. This case highlights that sacral/buttock retiform purpuric patches may be a presenting sign of infection with SARS‐CoV‐2 virus and may represent an ominous sign supporting a future severe disease course. In addition, biopsy specimen findings at separate time points demonstrate that cutaneous vasculopathy may persist despite adequate systemic anticoagulation, possibly due to the combination of persistent complement and platelet activation. Finally, occlusive thrombi in sacral/buttock retiform purpuric patches may contribute to future ulceration and significant cutaneous morbidity in patients who survive COVID‐19.
Keywords: complement, COVID‐19, platelets, retiform purpura, thrombotic vasculopathy
1. INTRODUCTION
Coronavirus disease 2019 (COVID‐19) is a syndrome caused by the novel coronavirus, SARS‐CoV‐2, which presents with a wide variety of clinical manifestations affecting numerous organ systems. As COVID‐19‐related hospitalizations continue around the world, identifying dermatologic signs that predict current infection and/or portend poor prognosis are critical for limiting transmission and adequate treatment. Retiform purpura and livedo reticularis have been reported in patients with COVID‐19. 1 , 2 To our knowledge, however, these dermatologic findings have been exclusively described in patients following confirmation of COVID‐19 diagnosis and at a single time point during the disease course. Here, we present a patient who developed a retiform purpuric patch on the sacrum/buttocks during hospitalization for another condition and prior to other COVID‐19‐related symptoms and diagnosis.
2. CASE REPORT
A 66‐year‐old woman with hypertension, diabetes, end‐stage renal disease requiring hemodialysis, chronic obstructive pulmonary disease (COPD) and coronary artery disease was hospitalized at our institution for an ST‐elevation myocardial infarction. She had successful coronary artery stent placement, but developed ulnar artery occlusion secondary to balloon angioplasty and was anticoagulated with clopidogrel 75 mg daily, aspirin 81 mg daily, and a continuous heparin infusion (500 units/h).
On Day 5 of hospitalization, she complained of buttock pain and was noted to have a new, nonindurated, retiform purpuric patch involving her buttocks (Figure 1B). On Day 6 of hospitalization, the dermatology team was consulted due to concern for calciphylaxis. During examination, the patient was comfortable, talkative, and able to easily move herself. A lesional punch biopsy of the purpuric patch was performed. The same day, the patient underwent a nasal swab test for COVID‐19, as she developed a low‐grade fever and had recently been in close contact with two sons newly diagnosed with COVID‐19. On Day 7, the patient's COVID‐19 polymerase chain reaction test resulted positive. Within 24 hours of COVID‐19 diagnosis, she developed acute respiratory distress requiring transfer to the intensive care unit and mechanical ventilation.
FIGURE 1.
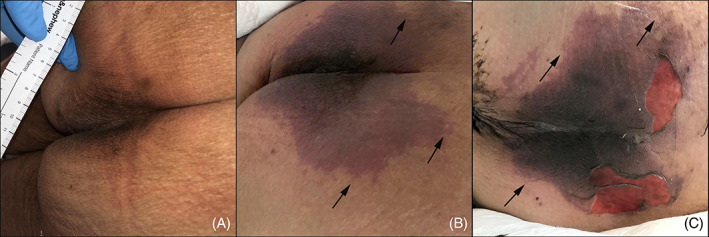
Evolution of the patient's sacral/buttocks retiform purpuric patch during her COVID‐19 disease course. A, Baseline sacral skin photograph documented per standard protocol by the wound care team after consultation for intergluteal hyperpigmentation. The wound care team assessed that the hyperpigmentation was normal and there was no evidence of pressure injury or purpura. B, Retiform purpuric patch on Day 6 of hospitalization at time of initial dermatology evaluation; C, worsening of the purpuric patch with superficial ulceration on Day 12 of hospitalization at time of second dermatology evaluation. Arrows in panels B and C indicate branching retiform purpuric areas at the periphery of the wound edge, indicative of a thromboembolic process
Histopathologic sections of the buttock biopsy specimen revealed full‐thickness epidermal necrosis with minimal underlying inflammation (Figure 2A). Widespread dermal thrombotic vasculopathy was noted, despite the patient being fully anticoagulated at time of biopsy (Figure 2A,B). To better understand the thrombotic process, phosphotungstic acid hematoxylin (PTAH), CD41, CD61, and C4d immunohistochemical stains were performed (Figures 2C‐F). The PTAH stain revealed intravascular thromboses composed largely of fibrin. CD41 and CD61 stains revealed numerous vessels with peripheral luminal platelet aggregations, with some vessels being completely occluded by platelets. Vessel lumina stained positive for C4d, an important downstream effector of the complement cascade. 3 A von Kossa stain was performed and did not reveal intravascular or extravascular calcium deposition suggestive of calciphylaxis (not shown). Serologic evaluation was negative for the presence of anti‐platelet factor 4 (PF4)/heparin antibodies, arguing against heparin‐induced thrombocytopenia. The dermatology team relayed their opinion that the purpuric patch was consistent with a cutaneous manifestation of COVID‐19.
FIGURE 2.
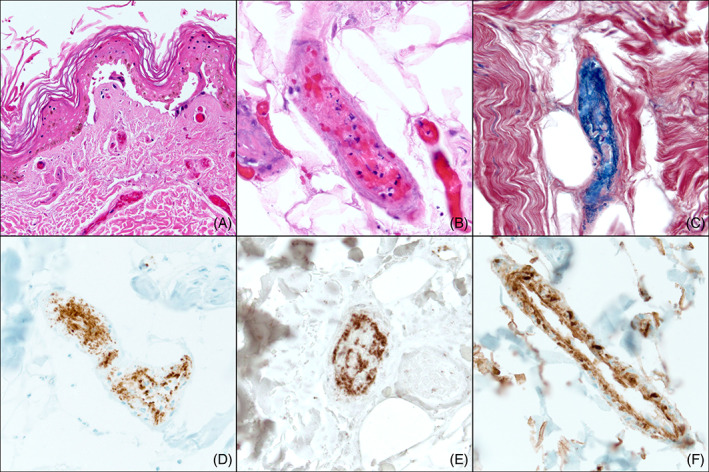
Histopathologic findings of a lesional biopsy specimen on hospitalization Day 6. Of note, biopsies from Days 6 and 12 revealed the same features: A, H&E sections reveal epidermal necrosis and papillary dermal vessels with intraluminal thrombi despite current therapeutic anticoagulation (×200). B, A deep dermal/subcutaneous vessel with extensive thrombosis admixed with inflammatory cell debris (×400). C, Phosphotungstic acid hematoxylin (PTAH) stain highlights fibrin‐rich thrombi staining blue within the vessels (×400). Platelet markers, D, CD61 (×400) and, E, CD41 (×400) reveal platelet aggregation within vessel lumina, with predominant aggregation at the vessel periphery. F, Complement split product C4d is diffusely present along the walls of vessel lumina (×400)
Over the next few days, the patient remained intubated and the purpuric sacral/buttocks patch worsened. Because of this progression, the dermatology team was again consulted on Day 12 to evaluate for possible calciphylaxis. The patient's d‐dimer levels increased from 5140 ng/mL (normal <500 ng/mL fibrinogen‐equivalent units on the day of biopsy to 11 740 ng/mL 3 days later (on Day 10 of hospitalization) (Table 1). On examination, the purpuric patch was notably more violaceous and had progressed to superficial ulceration in some areas (Figure 1C). A second lesional punch biopsy was performed and revealed histopathologic findings similar to the first biopsy, including persistent thrombotic vasculopathy with fibrin‐rich thromboses highlighted by PTAH stain, occlusive or peripheral luminal platelet aggregations in numerous vessels highlighted by CD41 and CD61 stains, and vessel lumina positive for C4d (Figure 3). A von Kossa stain again demonstrated the absence of calcium deposition suggestive of calciphylaxis. The patient remained on anticoagulation therapy, with an activated partial thromboplastin time (aPTT) >139 seconds (target on anticoagulation ≥100 seconds) and normal platelet levels (Table 1). On Day 14, after failing extubation, the patient required reintubation and vasopressor support. On Day 15, the patient's d‐dimer levels increased to 34 100 ng/mL, she developed bradycardia and hypotension unresponsive to vasopressors, and expired.
TABLE 1.
Hematologic laboratory data throughout patient's hospital course
| Biopsy 1 | Biopsy 2 a | Expired | ||||
|---|---|---|---|---|---|---|
| Lab (reference range) | Admission date | Day 4 | Day 7 | Day 10 | Day 13 | Day 15 |
| aPTT (2.3‐32.4 s) | 38.2 | 120.9 | 93.8 | 38.3 | >139 | 50 |
| PT (9.7‐13 s) | 15.1 | 14.9 | 27.4 | 12.1 | NR | NR |
| INR (0.9‐1.3) | 1.4 | 1.4 | 2.6 | 1.1 | NR | NR |
| Platelets (150‐400k/μL) | 86 | 114 | 84 | 149 | 175 | 241 |
| d‐dimer (<500 ng/mL) | NR | NR | 5140 | 11 740 | NR | 34 100 |
| Hgb (11.5‐15.5k/μL) | 8.5 | 6.9 | 7.5 | 8.0 | 7.9 | 8.6 |
| WBC (3.7‐11k/μL | 4.3 | 4.8 | 5.8 | 16.2 | 12.9 | 22.9 |
Abbreviations: aPPT, activated partial thromboplastin time; Hgb, hemoglobin; INR, international normalized ratio; PT, prothrombin time; WBC, white blood cells.
Biopsy 2 was performed on Day 12 of hospitalization; however, data were not reported on Day 12.
FIGURE 3.
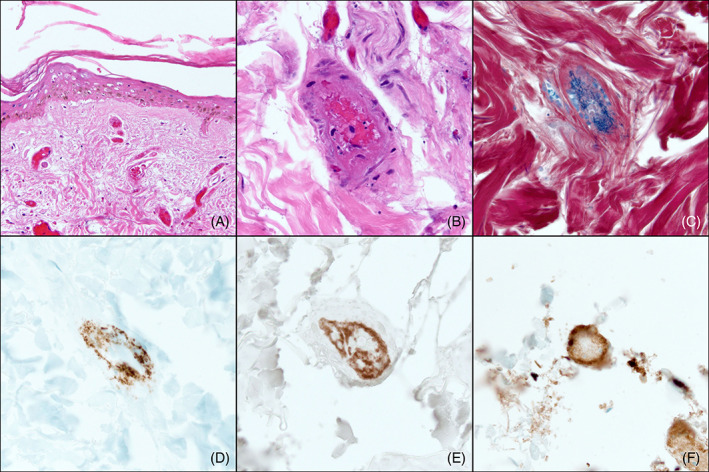
Histopathologic findings of second lesional biopsy specimen on hospitalization Day 12. The biopsy displays features similar to those seen on the patient's initial biopsy: A, H&E sections reveal epidermal necrosis and dilated papillary dermal vessels with intraluminal thrombi despite current therapeutic anticoagulation (×100). B, A deep dermal vessel with thrombosis admixed with inflammatory cell debris (×400). C, Phosphotungstic acid hematoxylin (PTAH) stain highlights fibrin‐rich thrombi staining blue within a vessel (×400). Platelet markers, D, CD61 (×400) and, E, CD41 (×400) reveal platelet aggregation within vessel lumina with predominant aggregation at the vessel periphery. F, Complement split product C4d is diffusely present along the walls of vessel lumina (×400)
3. DISCUSSION
COVID‐19 has been associated with numerous dermatologic manifestations, including purpuric skin lesions such as pernio‐like changes of the fingers/toes, macular purpuric rashes on the extremities, palpable purpura, and retiform purpura. 4 , 5 , 6 Our patient presented with a new purpuric patch on the sacrum/buttocks prior to development of other symptoms or diagnosis of COVID‐19. Retiform purpura on the buttocks has been reported in patients with known COVID‐19, but to our knowledge this case is the first suggesting retiform purpura can be a presenting sign of COVID‐19. Development of retiform purpura, including purpuric patches on the buttocks, has mainly been reported in critically ill COVID‐19 patients, supporting it is a poor prognostic indicator. 4 , 5 Underscoring this, cutaneous thrombotic purpura is thought to be a risk factor for thrombotic events in other organs. 2
Histopathologic features suggest our patient's purpuric patch resulted from thrombotic vasculopathy that involves complement activation and platelet aggregation resistant to standard anticoagulation. COVID‐19‐associated vasculopathy in critically ill patients appears to be associated with a minimal type‐I interferon immunologic response, excessive viral replication, and extensive complement activation. 5 An adequate type‐I interferon immunologic response has been recognized as important in patients with COVID‐19, with patients developing severe respiratory distress syndrome typically having minimal type‐I interferon activity. 7 Its importance is further underscored by the finding of inborn immunologic errors impairing type‐I interferon responses in some patients with severe COVID‐19. 8 With respect to skin manifestations, a robust type‐I interferon response has been suggested to be present in patients with perniosis‐like lesions, who reportedly have minimal clinical symptoms of SARS‐CoV‐2 infection. Alternatively, those with retiform purpura are suggested to have minimal type‐I interferon responses, leading to severe COVID‐19 symptoms. 5
Complement activation may also be involved in COVID‐19‐associated vasculopathy. Although there is still limited evidence supporting systemic complement activation occurs early in COVID‐19 patients, it is hypothesized that SARS‐CoV‐2 spike protein may be able to directly activate complement, causing endothelial cell injury and thrombosis. 3 , 5 , 9 Once initiated, complement activation can promote coagulation, resulting in platelet activation, intravascular platelet aggregation, and fibrin thrombi formation. 10
C4d is a complement product representative of the classical/lectin pathway. 11 In addition, elevated C4d levels have been associated with increased oxygen demand in COVID‐19 patients. 11 Magro et al previously demonstrated C5b‐9 and C4d in lung and cutaneous microvasculature of five COVID‐19 patients. 3 As C4d is a sensitive indicator of complement activation, its persistent deposition on endothelial cells in our patient's biopsies may indicate continued complement activity that amplifies thrombus formation. 12 This could explain why thromboses persisted in our patient's biopsies over time and despite therapeutic anticoagulation. Furthermore, the presence of SARS‐CoV‐2 RNA in platelets has been previously documented. 13 Although we were not able to evaluate for SARS‐CoV‐2 RNA in our patient's platelets, if present it may have contributed to persistent thromboses in the face of therapeutic anticoagulation.
In contrast to reports highlighting fibrin‐rich clots without significant platelet aggregation, our patient's vasculopathy was associated with both intravascular fibrin‐rich thrombi and platelet aggregations. 4 To our knowledge, platelet aggregations within thrombosed cutaneous vessels in COVID‐19 patients have not yet been reported. Although complement activation has been a focus of study, there is accumulating evidence that platelet dysfunction also plays a role in COVID‐19 coagulopathy. 14 Platelets in patients with severe COVID‐19 are hyperactivated, contribute to the degree of systemic inflammation, and have increased pro‐thrombotic potential. 12 Also, platelet aggregation appears to occur at lower thrombin levels than expected in COVID‐19 patients. 12 Furthermore, elevated d‐dimer levels are seen as a by‐product of the coagulation cascade in essentially all COVID‐19 patients with thrombotic complications, including our case. 15 Thus, both complement activation and platelet dysfunction likely contributed to our patient's persistent vasculopathy despite therapeutic anticoagulation.
Notably, our patient's normal platelet levels throughout most of her COVID‐19 course are in contrast to what would be expected in disseminated intravascular coagulation (DIC) and are more reminiscent of complement‐mediated thrombotic microangiopathy (TMA) syndromes, as may be seen in severe systemic lupus erythematosus (SLE) and catastrophic antiphospholid antibody syndrome (APS). 16 , 17 Coagulopathy in APS results from the presence of autoantibodies that recognize phospholipid‐binding proteins (such as beta‐2‐glycoprotein) alone or in combination with anionic phospholipids (such as cardiolipin). 17 This antibody‐antigen interaction is thought to activate platelets and endothelial cells via mechanisms similarly proposed in patients with severe COVID‐19. 16 , 17 In both APS and severe COVID‐19‐related thrombosis, tissue factor is elevated and thought to play an important role in promoting the extrinsic arm of the coagulation cascade. 16 , 17 In patients similar to ours, it may be valuable in the future to assess whether von Willebrand factor and thrombomodulin expression is altered, as mortality in COVID‐19 patients has been found to significantly correlate with levels of both. 18
It is also noteworthy that our patient developed thromboses despite being fully anticoagulated. The development of microvascular and macrovascular thromboses in COVID‐19 patients is well established, leading to efforts in developing optimal prophylactic antithrombotic regimens. 19 In the INSPIRATION clinical trial, patients were randomized to standard (40 mg daily) and intermediate (1 mg/kg daily) doses of enoxaparin to determine if more aggressive anticoagulation could decrease thrombotic events. Unfortunately, intermediate enoxaparin doses failed to significantly decrease thrombotic events. 20 Although our patient received unfractionated heparin infusions, its use as antithrombotic prophylaxis in COVID‐19 patients must be balanced against risk of bleeding. Nevertheless, a clinical trial exploring its use for this purpose is planned. 21 Currently, however, our patient's persistent thromboses despite full anticoagulation, in addition to failure of various anticoagulation strategies to decrease thrombotic events, support that a refractory pro‐thrombotic state develops in COVID‐19 patients. Furthermore, our patient's African‐American ethnicity and multiple medical comorbidities, including cardiovascular disease, renal disease, COPD, and diabetes, are known to promote a prothrombotic state and may also have contributed to a refractory vasculopathy. 22
Finally, persistent and refractory thrombotic vasculopathy and clinical progression of our patient's purpuric patch with initial signs of skin breakdown suggest these lesions may evolve to sacral ulcerations over time. 23 Although our patient's purpuric patch had only superficial ulceration at time of death, diffuse thrombotic occlusion of deep dermal blood vessels on both biopsies suggest cutaneous necrosis could have become more significant. Indeed, we and others have seen numerous COVID‐19 patients who have developed large sacral ulcers in the setting of severe COVID‐19 and signs of significant hypercoagulability. 23 , 24 Recognizing that these lesions may contribute to sacral/buttocks ulceration is important, as these ulcers can lead to significant morbidity or even mortality as portals of infection entry. Addressing such patches with either improved systemic treatments, supportive maneuvers, or pressure offloading devices may help minimize risk of significant cutaneous necrosis.
Although, in our patient, the first biopsy specimen was obtained before her COVID‐19 diagnosis and while she was still mobile, it is possible that clinicians could assume purpuric sacral/buttocks patches first observed after patients have already been bedbound for prolonged periods with severe COVID‐19 are secondary to pressure injury and not COVID‐19‐associated thrombotic events. Although the histopathologic features of decubitus/pressure ulcers have not been extensively studied, a small body of literature does exist. In one of the largest studies, biopsies performed from pressure injuries in 59 patients were examined. 25 Histopathologic features in stage I ulcers (intact skin, nonblanchable erythema localized over bony prominences), which would correlate with our patient's initial presentation, included tissue eosinophilia with epidermal erosions and superficial crusts. 25 Subepidermal bulla formation and/or epidermal necrosis were present in some sections, while others displayed follicular degeneration and hemorrhage. 25 Features in stage II ulcers (partial thickness loss of dermis and pink wound beds, without slough), which would be consistent with our patient's clinical findings at time of her second biopsy, included profound loss of dermal papillae, changes in superficial dermal collagen fibers, necrosis of adnexal structures, and abundant dermal and subcutaneous inflammation. 25 , 26
While major histopathologic features reported in biopsy specimens from pressure ulcers do not correlate with features seen in our patients' biopsies, it is important to note that some studies support that microthrombi play an important role in pressure‐induced injuries and can be seen as a histopathologic feature in pressure ulcer biopsy specimens. 27 , 28 , 29 Thus, in COVID‐19 patients who have been bedbound by the time they are initially evaluated for sacral/buttocks purpura, it is unclear whether histopathologic evaluation of lesional biopsy specimens can distinguish between COVID‐19‐associated vasculopathy and pressure‐induced injury. With this in mind, we cannot entirely rule out the possibility that pressure‐induced changes contributed to the persistence of vascular thromboses seen on our patient's second biopsy and their refractoriness to anticoagulation. We are currently comparing histopathologic features in biopsy specimens taken from a cohort of COVID‐19 patients with sacral/buttocks purpura to those from biopsy specimens from a control cohort with pressure ulcers to explore whether unique features exist that may reliably distinguish between potential etiologies. We hope to report results of this study in the near future.
In summary, we report development of sacral/buttock retiform purpura on the buttocks as an ominous presenting sign of severe COVID‐19. Clinicians should be highly suspicious of COVID‐19 if this cutaneous abnormality is observed. Our case further supports retiform purpuric patches are secondary to thrombotic vasculopathy that is resistant to therapeutic anticoagulation. Immunohistochemical findings suggest this vasculopathy is reminiscent of complement‐mediated TMA syndromes, as may be seen in severe SLE and APS, as opposed to DIC. Aligning with our patient's clinical course, anticoagulation is known to be insufficient in TMA syndromes related to SLE and APS. 30 Identifying sacral/buttocks retiform purpura and its possible association with severe COVID‐19 course may allow aggressive early treatment that minimizes end‐organ damage and death. Additional work to better understand the contributions of complement activation and platelet dysfunction in the pathogenesis of COVID‐19‐associated coagulopathy is needed before optimally effective treatments can be developed. Furthermore, research is needed to determine the role of pressure‐induced injury in sacral/buttocks purpura that arises after COVID‐19 diagnosis and a prolonged bedbound state.
ACKNOWLEDGEMENTS
We would like to thank Janine Sot, MBA, for her expertise in preparing the figures accompanying this manuscript. The work has no formal funding sources.
McBride JD, Narang J, Simonds R, et al. Development of sacral/buttock retiform purpura as an ominous presenting sign of COVID‐19 and clinical and histopathologic evolution during severe disease course. J Cutan Pathol. 2021;48(9):1166–1172. 10.1111/cup.14038
DATA AVAILABILITY STATEMENT
The data that support the findings of this study are available from the corresponding author upon reasonable request.
REFERENCES
- 1. Bosch‐Amate X, Giavedoni P, Podlipnik S, et al. Retiform purpura as a dermatological sign of coronavirus disease 2019 (COVID‐19) coagulopathy. J Eur Acad Dermatol Venereol. 2020;34(10):e548‐e549. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Freeman EE, McMahon DE, Lipoff JB, et al. The spectrum of COVID‐19‐associated dermatologic manifestations: an international registry of 716 patients from 31 countries. J Am Acad Dermatol. 2020;83(4):1118‐1129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Magro C, Mulvey JJ, Berlin D, et al. Complement associated microvascular injury and thrombosis in the pathogenesis of severe COVID‐19 infection: a report of five cases. Transl Res. 2020;220:1‐13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Galvan Casas C, Catala A, Carretero Hernandez G, et al. Classification of the cutaneous manifestations of COVID‐19: a rapid prospective nationwide consensus study in Spain with 375 cases. Br J Dermatol. 2020;183(1):71‐77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Magro C, Mulvey JJ, Laurence J, et al. The differing pathophysiologies that underlie COVID‐19 associated perniosis and thrombotic retiform purpura: a case series. Br J Dermatol. 2021;184(1):141‐150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Garrido Ruiz MC, Santos‐Briz A, Santos‐Briz A, et al. Spectrum of clinicopathologic findings in COVID‐19‐induced skin lesions: demonstration of direct viral infection of the endothelial cells. Am J Surg Pathol. 2021;45(3):293‐303. [DOI] [PubMed] [Google Scholar]
- 7. Sa Ribero M, Jouvenet N, Dreux M, Nisole S. Interplay between SARS‐CoV‐2 and the type I interferon response. PLoS Pathog. 2020;16(7):e1008737. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Zhang Q, Bastard P, Liu Z, et al. Inborn errors of type I IFN immunity in patients with life‐threatening COVID‐19. Science. 2020;370(6515):eabd4570. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Risitano AM, Mastellos DC, Huber‐Lang M, et al. Complement as a target in COVID‐19? Nat Rev Immunol. 2020;20(6):343‐344. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Fletcher‐Sandersjoo A, Bellander BM. Is COVID‐19 associated thrombosis caused by overactivation of the complement cascade? A literature review. Thromb Res. 2020;194:36‐41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Holter JC, Pischke SE, de Boer E, et al. Systemic complement activation is associated with respiratory failure in COVID‐19 hospitalized patients. Proc Natl Acad Sci U S A. 2020;117(40):25018‐25025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Cohen D, Colvin RB, Daha MR, et al. Pros and cons for C4d as a biomarker. Kidney Int. 2012;81(7):628‐639. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Zaid Y, Puhm F, Allaeys I, et al. Platelets can associate with SARS‐Cov‐2 RNA and are hyperactivated in COVID‐19. Circ Res. 2020;127(11):1404‐1418. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Koupenova M, Freedman JE. Platelets and COVID‐19: inflammation, hyperactivation and additional questions. Circ Res. 2020;127(11):1419‐1421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Piazza G, Morrow DA. Diagnosis, management, and pathophysiology of arterial and venous thrombosis in COVID‐19. JAMA. 2020;324(24):2548‐2549. [DOI] [PubMed] [Google Scholar]
- 16. Canzano P, Brambilla M, Porro B, et al. Platelet and endothelial activation as potential mechanisms behind the thrombotic complications of COVID‐19 patients. JACC Basic Transl Sci. 2021;6(3):202‐218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Mackworth‐Young CG. Antiphospholipid syndrome: multiple mechanisms. Clin Exp Immunol. 2004;136(3):393‐401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Goshua G, Pine AB, Meizlish ML, et al. Endotheliopathy in COVID‐19‐associated coagulopathy: evidence from a single‐centre, cross‐sectional study. Lancet Haematol. 2020;7(8):e575‐e582. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Bikdeli B, Madhavan MV, Jimenez D, et al. COVID‐19 and thrombotic or thromboembolic disease: implications for prevention, antithrombotic therapy, and follow‐up: JACC state‐of‐the‐art review. J Am Coll Cardiol. 2020;75(23):2950‐2973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Investigators I, Sadeghipour P, Talasaz AH, et al. Effect of intermediate‐dose vs standard‐dose prophylactic anticoagulation on thrombotic events, extracorporeal membrane oxygenation treatment, or mortality among patients with COVID‐19 admitted to the intensive care unit: the INSPIRATION randomized clinical trial. JAMA. 2021;325(16):1620‐1630. 10.1001/jama.2021.4152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Busani S, Tosi M, Mighali P, et al. Multi‐centre, three arm, randomized controlled trial on the use of methylprednisolone and unfractionated heparin in critically ill ventilated patients with pneumonia from SARS‐CoV‐2 infection: a structured summary of a study protocol for a randomised controlled trial. Trials. 2020;21(1):724. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Frydman GH, Boyer EW, Nazarian RM, Van Cott EM, Piazza G. Coagulation status and venous thromboembolism risk in African Americans: a potential risk factor in COVID‐19. Clin Appl Thromb Hemost. 2020;26:1076029620943671. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Young S, Narang J, Kumar S, et al. Large sacral/buttocks ulcerations in the setting of coagulopathy: a case series establishing the skin as a target organ of significant damage and potential morbidity in patients with severe COVID‐19. Int Wound J. 2020;17(6):2033‐2037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Chand S, Rrapi R, Lo JA, et al. Purpuric ulcers associated with COVID‐19 infection: a case‐series. JAAD Case Rep. 2021;11:13‐19. 10.1016/j.jdcr.2021.01.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Witkowski JA, Parish LC. Histopathology of the decubitus ulcer. J Am Acad Dermatol. 1982;6(6):1014‐1021. [DOI] [PubMed] [Google Scholar]
- 26. Arao H, Obata M, Shimada T, Hagisawa S. Morphological characteristics of the dermal papillae in the development of pressure sores. J Tissue Viability. 1998;8(3):17‐23. [DOI] [PubMed] [Google Scholar]
- 27. Parish LC, Lowthian P, Witkowski JA. The decubitus ulcer: many questions but few definitive answers. Clin Dermatol. 2007;25(1):101‐108. [DOI] [PubMed] [Google Scholar]
- 28. Edsberg LE. Pressure ulcer tissue histology: an appraisal of current knowledge. Ostomy Wound Manag. 2007;53(10):40‐49. [PubMed] [Google Scholar]
- 29. Vande Berg JS, Rudolph R. Pressure (decubitus) ulcer: variation in histopathology‐a light and electron microscope study. Hum Pathol. 1995;26(2):195‐200. [DOI] [PubMed] [Google Scholar]
- 30. Merrill JT, Erkan D, Winakur J, James JA. Emerging evidence of a COVID‐19 thrombotic syndrome has treatment implications. Nat Rev Rheumatol. 2020;16(10):581‐589. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The data that support the findings of this study are available from the corresponding author upon reasonable request.


