Abstract
Objective
Rheumatoid arthritis (RA) and its treatments are associated with an increased risk of infection, but it remains unclear whether these factors have an impact on the risk or severity of COVID‐19. The present study was undertaken to assess the risk and severity of COVID‐19 in a US Department of Veterans Affairs (VA) cohort of patients with RA and those without RA.
Methods
A matched cohort study using national VA data was conducted. Patients diagnosed as having RA were identified among nondeceased individuals who were active in the VA health care system as of January 1, 2020 and who had received care in a VA medical center in 2019; patients for whom no RA diagnostic code was indicated were matched to the RA patients (1:1) by age, sex, and VA site (non‐RA controls). Patients diagnosed as having COVID‐19 and those with severe COVID‐19 (defined as requiring hospitalization or leading to death) were ascertained from a national VA COVID‐19 surveillance database through December 10, 2020. Multivariable Cox models were used to compare the risk of COVID‐19 and COVID‐19 hospitalization or death between RA patients and non‐RA controls, after adjusting for demographic characteristics, comorbidities, health care utilization and access, and county‐level COVID‐19 incidence rates.
Results
This VA cohort of RA patients and non‐RA controls (n = 33,886 subjects per group) predominantly comprised male patients (84.5%), and the mean age was 67.8 years. During follow‐up, 1,503 patients in the cohort were diagnosed as having COVID‐19; among them, 388 patients had severe COVID‐19 (hospitalization or death), while in 228 patients, the deaths were not related to COVID‐19. In the multivariable model, RA was associated with a higher risk of COVID‐19 (adjusted hazard ratio [HR] 1.25 [95% confidence interval (95% CI) 1.13–1.39]) and a higher risk of COVID‐19 hospitalization or death (adjusted HR 1.35 [95% CI 1.10–1.66]) as compared to non‐RA controls. Use of disease‐modifying antirheumatic drugs and prednisone, as well as self‐reported Black race, self‐reported Hispanic ethnicity, and presence of several chronic conditions, but not seropositivity for RA autoantibodies, were each associated with risk of COVID‐19 and severe COVID‐19 (hospitalization or death).
Conclusion
Patients with RA are at higher risk of developing COVID‐19 and severe COVID‐19 (leading to hospitalization or death) compared to those without RA. With a risk of COVID‐19 that approaches that of other recognized chronic conditions, these findings suggest that RA patients should be prioritized for COVID‐19 prevention and management strategies.
INTRODUCTION
Despite marked improvements in the long‐term outcomes observed in patients with rheumatoid arthritis (RA) (1), infections frequently complicate the natural course of the disease and are likely overrepresented in this patient population; this could be attributable to several mechanisms. An increased risk of infection or serious infection in patients with RA is associated with the immune dysregulation inherent to RA itself, but also could be associated with RA treatments, such as disease‐modifying antirheumatic drugs (DMARDs) and glucocorticoids, and with chronic conditions that often develop in conjunction with RA (2, 3). However, most of the research in RA has focused on bacterial, and not viral, etiologies.
COVID‐19 appears to yield a disproportionate impact among vulnerable populations, particularly among the elderly and those with chronic diseases (4, 5). With the rapid development of effective vaccines for COVID‐19, individuals with select chronic conditions that predispose the individual to a more severe COVID‐19 disease course have been prioritized for vaccine administration (6). High‐risk chronic conditions and lifestyle factors specified in these recommendations include cancer, chronic kidney disease, cardiovascular disease, prior solid organ transplantation, sickle cell disease, type 2 diabetes mellitus, obesity, smoking, and pregnancy (6). RA and other rheumatic diseases that require immunosuppressive therapies for management have not been prioritized. While the vaccine supply is increasing in the US, the need, timing, and prioritization of subsequent booster vaccination is unknown.
Few observational studies have evaluated whether rheumatic diseases and related immunosuppressive therapies are associated with COVID‐19 outcomes. Findings from an international registry of rheumatic disease patients identified links between the severity of rheumatic disease, use of prednisone, and use of select immunosuppressive therapies with a higher risk of mortality, specifically among those with RA (7). Results of subsequent analyses have suggested that confounding by indication may explain the association between prednisone treatment and worse COVID‐19 outcomes (8). Moreover, these observations have also been noted in the general population, including an association of certain ethnic groups and comorbidities with the development of severe COVID‐19 or COVID‐19–related death (7, 9). In a multicenter study utilizing electronic health records, patients with COVID‐19 and systemic autoimmune rheumatic diseases had a higher risk of severe outcomes compared with matched comparators, although this appeared largely related to accompanying comorbidities (10). Similarly, a separate study in a multicenter health care system found that the association of rheumatic diseases with mechanical ventilation in COVID‐19 was attenuated by the presence of comorbidities (11). In a study using a national sample of primary care patients from the UK, patients diagnosed as having RA, lupus, or psoriasis were found to have a 19% higher risk of COVID‐19–related death (12). Although these findings, along with evidence from other investigators, are beginning to shed light on the outcomes experienced by patients with COVID‐19, the aforementioned studies are prone to selection bias, which could be related to the approach taken for patient enrollment and/or to the fact that patient selection may be conditioned on a positive SARS–CoV‐2 test finding. Moreover, prior studies have focused on heterogeneous populations, comprising patients with a number of different rheumatic conditions and treatments; this is likely to reduce the precision of the risk estimates generated.
Recognizing these gaps in our understanding and the significant limitations of prior study designs, the objective of the present study was to compare the risk of SARS–CoV‐2 infection and the development of severe COVID‐19 between patients with RA and matched comparator patients in an at‐risk population. We hypothesized that patients with RA would have a higher risk of acquiring a SARS–CoV‐2 infection, and would be more likely to require hospitalization or to die after having been diagnosed with COVID‐19.
PATIENTS AND METHODS
Study design
We conducted a retrospective, matched cohort study within the US Department of Veterans Affairs (VA) Health Administration database. Patients who were active in the VA system as of January 1, 2020 were identified as having RA based on administrative algorithms, which searched for a record of multiple RA diagnostic codes, a rheumatologist’s diagnosis of RA, treatment with a DMARD, or a positive test finding for RA autoantibodies (rheumatoid factor [RF] and/or anti–cyclic citrullinated peptide [anti‐CCP] antibodies). Such algorithms have a >90% positive predictive value for the diagnosis of RA (13). Patients who had received care (outpatient or inpatient encounter) during the 2019 calendar year at the same VA medical center and for whom no RA diagnostic code was assigned were matched 1:1 to the RA patients by age, sex, and site (non‐RA controls). Patients with other autoimmune conditions (rheumatic or nonrheumatic diseases) and those who were receiving immunosuppressant drugs were not excluded from the non‐RA control group; this ensured that the control group was fully reflective of the VA non‐RA population. For study eligibility, both RA patients and non‐RA controls had to be alive as of January 1, 2020. Patients were subsequently followed up from January 1, 2020 to the first diagnosis of COVID‐19, occurrence of death, or end of the study period (December 10, 2020). This study received institutional review board approval.
Identification of COVID‐19
Among this VA cohort of RA patients and non‐RA controls, a diagnosis of COVID‐19 and information on related outcomes were obtained through the VA COVID‐19 shared data resource. This is a national VA COVID‐19 surveillance database that captures testing for COVID‐19, clinical results, severity of the disease, and patient outcomes, for the purposes of secondary research studies (14, 15, 16). In addition to capturing molecular SARS–CoV‐2 test results and COVID‐19 diagnoses within the VA health care system, natural language processing and medical record validation are performed to identify COVID‐19 cases that are diagnosed outside the VA system (17). However, a negative SARS–CoV‐2 test result that is determined outside the VA system is not captured in this resource.
The primary definition of COVID‐19 included positive test results within or outside the VA health care system. We also identified patients with severe COVID‐19, defined as COVID‐19 that required hospitalization or that resulted in death within 30 days of infection. In sensitivity analyses, respiratory illnesses that could be attributed to SARS–CoV‐2 infection but which lacked confirmation of the diagnosis (according to the VA COVID‐19 shared data resource case definition) were also classified as COVID‐19 (17). Data on death were collected from the VA COVID‐19 shared data resource and from vital status records maintained by the VA.
Covariates
Data on covariates were obtained from the VA Corporate Data Warehouse. The covariates included race, ethnicity, body mass index (BMI), urban versus rural residence, presence of a VA service–connected condition (i.e., a condition directly related to military service for which the individual receives VA benefits), private insurance status, smoking status (current, former, or never), comorbidities, number of hospitalizations in the prior year, and county‐level COVID‐19 rates as of November 16, 2020. Demographic characteristics (race, ethnicity) were obtained from administrative VA data collected at the time of enrollment in the VA. BMI was calculated from the nearest visit preceding January 1, 2020, using vital signs data obtained from VA clinic encounters, as previously described (18, 19). Smoking status was assessed by selecting and coding health factors recorded in the VA electronic medical record (20). Comorbid conditions were assessed using scores on the Elixhauser Comorbidity Index (21) (excluding RA and collagen vascular diseases). In addition, we identified specific conditions within the Elixhauser Comorbidity Index that are recognized to portend a higher risk of COVID‐19, including heart failure, chronic lung disease, diabetes, hypertension, cancer, chronic kidney disease, and liver disease (21). We required a record of at least 1 diagnostic code from outpatient or inpatient encounters during 2019 for one of these comorbid conditions to be considered present. International Classification of Diseases, Tenth Revision codes for each of the comorbidities were obtained from the Healthcare Cost and Utilization Project Elixhauser Comorbidity Software database (https://www.hcup‐us.ahrq.gov/toolssoftware/comorbidityicd10/comorbidity_icd10.jsp). Cumulative county‐level incidence rates of COVID‐19 since the start of the pandemic were obtained from the COVID‐19 Data Repository by the Center for Systems Science and Engineering at Johns Hopkins University (https://github.com/cssegisanddata/covid‐19; accessed November 16, 2020) (22).
Medications and RA autoantibody status
We assessed recent treatments based on medical records noting a dispensing or infusion of DMARDs and prednisone in the 180 days prior to and including January 1, 2020, except for rituximab, which we assessed during an infusion period up to 365 days prior to January 1, 2020. The noted medications included conventional synthetic DMARDs (csDMARDs) (methotrexate, hydroxychloroquine, sulfasalazine, and leflunomide), and biologic or targeted synthetic DMARDs (bDMARDs/tsDMARDs) (tumor necrosis factor inhibitors [etanercept, adalimumab, certolizumab, golimumab, and infliximab], abatacept and interleukin‐6 inhibitors [tocilizumab and sarilumab], rituximab, and JAK inhibitors [tofacitinib, baricitinib, and upadacitinib]). Prior work has shown that RA patients in the VA system rarely obtain DMARDs from non‐VA sources (23). RA autoantibody status was determined based on laboratory data from the VA Corporate Data Warehouse database, with patients classified as either seronegative or seropositive for RF and anti‐CCP antibodies.
Statistical analysis
Descriptive statistics were used to compare demographic and clinical characteristics between the RA patients and non‐RA controls. We used multivariable Cox regression models to assess the incidence and risk of COVID‐19 in RA patients compared to non‐RA controls, censoring for non–COVID‐19–related death or end of the study period. Models were clustered by matched pair, and included all of the aforementioned covariates. Similar models and covariates were used to compare the risk of severe COVID‐19 (hospitalization or death) between RA patients and non‐RA controls.
Sensitivity analyses were performed to 1) additionally include in the COVID‐19 case definition respiratory illnesses that may have been related to SARS–CoV‐2 infection but which lacked testing confirmation as evidence of COVID‐19, and 2) model the effect of individual comorbidities, rather than the overall Elixhauser Comorbidity Index, on the risk of COVID‐19. Secondary analyses included those that stratified RA patients and non‐RA controls according to RA‐specific autoantibody status (seropositive, seronegative, and unknown), recent DMARD use (none, csDMARDs, and bDMARDs/tsDMARDs), prednisone use (yes versus no), and use of combination treatment with DMARDs and prednisone. To address missing covariate data, we used multiple imputation with 10 imputations. Each imputed covariate (race, ethnicity, smoking status, BMI, urban/rural residence, and insurance status) was missing for <7% of patients.
Recognizing that COVID‐19 outcomes began to improve later in the pandemic among both patients with rheumatic diseases and those without rheumatic diseases (24, 25), we assessed time‐dependent differences in the risk of COVID‐19 through proportional hazards testing. Schoenfeld residuals and interactions between RA status and log(time) were not significant (all P > 0.3). Similarly, log–log survival rates and Schoenfeld residual plots indicated that there was no violation of the proportional hazards assumption (data not shown). All analyses were completed using Stata MP software, version 15.1 (StataCorp).
RESULTS
Characteristics of the study patients
In this study cohort, we identified 33,886 patients with RA and 33,886 non‐RA controls who were age‐, sex‐, and site‐matched to each RA patient. Compared to controls, RA patients were more likely to be current smokers and to have a higher BMI, greater comorbidity burden according to the Elixhauser Comorbidity Index, and more hospitalizations in the prior year (Table 1). The majority of patients with RA were seropositive for RF or anti‐CCP antibodies (60.5%). DMARDs were dispensed within the prior 180 days (i.e., recent DMARD treatment) in 73% of the patients with RA, with 34.2% having received a bDMARD or tsDMARD. The frequency of recent use of each individual DMARD is provided in Supplementary Table 1 (available on the Arthritis & Rheumatology website at http://onlinelibrary.wiley.com/doi/10.1002/art.41800/abstract).
Table 1.
Baseline characteristics of the RA patients and non‐RA controls*
| RA patients (n = 33,886) | Non‐RA controls (n = 33,886) | P | |
|---|---|---|---|
| Age, years | 67.8 ± 11.1 | 67.8 ± 11.1 | ND |
| Male sex, % | 84.5 | 84.5 | ND |
| Race, % | 0.01 | ||
| White | 74.4 | 73.7 | |
| Black | 17.3 | 17.2 | |
| Other | 3.3 | 2.9 | |
| Unknown | 5.1 | 6.2 | |
| Ethnicity, % | <0.001 | ||
| Non‐Hispanic | 90.7 | 91.0 | |
| Hispanic | 6.1 | 5.0 | |
| Unknown | 3.2 | 3.9 | |
| Smoking status, % | <0.001 | ||
| Current | 50.0 | 40.8 | |
| Former | 31.8 | 31.3 | |
| Never | 15.7 | 21.4 | |
| Unknown | 2.5 | 6.6 | |
| BMI category, % | <0.001 | ||
| <18.5 kg/m2 | 0.7 | 0.6 | |
| 18.5–25 kg/m2 | 8.4 | 9.0 | |
| 25–30 kg/m2 | 28.8 | 30.8 | |
| 30–35 kg/m2 | 31.1 | 31.1 | |
| >35 kg/m2 | 31.0 | 27.9 | |
| Unknown | 0.1 | 0.7 | |
| Elixhauser Comorbidity Index | 3.0 ± 2.2 | 2.5 ± 2.0 | <0.001 |
| Type of residence, % | 0.33 | ||
| Urban | 63.5 | 64.0 | |
| Rural | 34.9 | 34.4 | |
| Highly rural | 1.5 | 1.5 | |
| Unknown | 0.1 | 0.1 | |
| RF or anti‐CCP antibody status, % | <0.001 | ||
| Seropositive | 60.5 | 0.9 | |
| Seronegative | 25.7 | 3.8 | |
| Unknown | 13.9 | 95.4 | |
| DMARDs in prior 180 days, %† | <0.001 | ||
| None | 27.4 | 98.9 | |
| csDMARDs | 37.6 | 0.5 | |
| bDMARDs/tsDMARDs | 34.2 | 0.5 | |
| Prednisone in prior 180 days, % | 24.7 | 3.6 | <0.001 |
| Number of hospitalizations in prior year | 0.2 ± 0.8 | 0.1 ± 0.6 | <0.001 |
| Insurance beneficiary, % | 80.9 | 78.7 | <0.001 |
| Service‐connected condition, % | 62.6 | 58.7 | <0.001 |
Patients who were not assigned a diagnostic code for rheumatoid arthritis (RA) were matched to each RA patient by age, sex, and site (non‐RA controls). Except where indicated otherwise, values are the mean ± SD. ND = no difference; BMI = body mass index; RF = rheumatoid factor; anti‐CCP = anti–cyclic citrullinated peptide.
Disease‐modifying antirheumatic drugs (DMARDs), which included conventional synthetic DMARDs (csDMARDs) and biologic or targeted synthetic DMARDs (bDMARDs/tsDMARDs), were assessed 180 days prior to and including January 1, 2020, except for rituximab, which was assessed during an infusion period up to 365 days prior to January 1, 2020.
Incidence rates of COVID‐19
In analyses spanning 62,894 patient‐years of follow‐up among patients assessed according to the primary case definition of COVID‐19 (i.e., confirmed SARS–CoV‐2 infection), we identified 1,503 patients with COVID‐19. Among these, the diagnosis was categorized as severe in 388 cases, of which 345 required hospitalization and 84 resulted in death. During the same period of observation, there were 288 non–COVID‐related deaths.
When a more sensitive case definition of COVID‐19 was applied to the cohort (i.e., additional inclusion of patients with respiratory illnesses possibly attributable to COVID‐19 but lacking testing confirmation), we identified 2,037 patients with COVID‐19, with 468 cases resulting in either hospitalization or death. Crude incidence rates of COVID‐19 and severe COVID‐19 (hospitalization or death), both in the primary analysis and in the sensitivity analysis, were higher in RA patients compared to matched non‐RA controls (Table 2).
Table 2.
Crude incidence rates of COVID‐19 and severe COVID‐19 in patients with RA and non‐RA controls*
| Number of events | Person‐years of follow‐up | Incidence rate per 1,000 person‐years (95% CI) | |
|---|---|---|---|
| Primary analysis | |||
| All COVID‐19 | |||
| Non‐RA | 647 | 31,552 | 20.5 (19.0–22.1) |
| RA | 856 | 31,342 | 27.3 (25.5–29.2) |
| Severe COVID‐19† | |||
| Non‐RA | 153 | 31,552 | 4.8 (4.1–5.7) |
| RA | 235 | 31,342 | 7.5 (6.6–8.5) |
| Sensitivity analysis‡ | |||
| All COVID‐19 | |||
| Non‐RA | 863 | 31,465 | 27.4 (25.7–29.3) |
| RA | 1,174 | 31,217 | 37.6 (35.5–39.8) |
| Severe COVID‐19† | |||
| Non‐RA | 181 | 31,465 | 5.8 (5.0–6.7) |
| RA | 287 | 31,217 | 9.2 (8.2–10.3) |
RA = rheumatoid arthritis; 95% CI = 95% confidence interval.
Severe COVID‐19 was defined as COVID‐19 requiring hospitalization or resulting in death.
Sensitivity analyses additionally included patients with respiratory illnesses that were suspected to be attributable to COVID‐19 but lacked confirmation.
Risk of COVID‐19 in RA patients
In unadjusted multivariable Cox regression models assessing RA patients compared to non‐RA matched controls, as well as adjusted models that further accounted for demographic characteristics, comorbidities, health care utilization and access, and county‐level COVID‐19 incidence rates, the risk of COVID‐19 was found to be significantly higher in RA patients compared to non‐RA controls. Specifically, the risk of COVID‐19 in RA patients was 25% higher (adjusted hazard ratio [HR] 1.25 [95% confidence interval (95% CI) 1.13–1.39]) and the risk of COVID‐19 hospitalization or death was 35% higher (adjusted HR 1.35 [95% CI 1.10–1.66]) when compared to non‐RA controls (Table 3). Results of the sensitivity analysis, in which possible COVID‐19 cases were included, were consistent with those of the primary analysis (Table 3).
Table 3.
Risk of COVID‐19 and severe COVID‐19 in RA patients relative to non‐RA controls*
| Unadjusted HR (95% CI) | Adjusted HR (95% CI) | |
|---|---|---|
| Primary analysis | ||
| All COVID‐19 | ||
| Non‐RA | 1 (Referent) | 1 (Referent) |
| RA | 1.34 (1.21–1.48) | 1.25 (1.13–1.39) |
| Severe COVID‐19† | ||
| Non‐RA | 1 (Referent) | 1 (Referent) |
| RA | 1.55 (1.26–1.90) | 1.35 (1.10–1.66) |
| Sensitivity analysis‡ | ||
| All COVID‐19 | ||
| Non‐RA | 1 (Referent) | 1 (Referent) |
| RA | 1.38 (1.26–1.50) | 1.29 (1.18–1.41) |
| Severe COVID‐19† | ||
| Non‐RA | 1 (Referent) | 1 (Referent) |
| RA | 1.60 (1.33–1.93) | 1.39 (1.15–1.68) |
Results are the hazard ratio (HR) with 95% confidence interval (95% CI) for the risk of developing COVID‐19 or severe COVID‐19 in patients with rheumatoid arthritis (RA) relative to matched non‐RA controls, as determined in unadjusted models and models adjusted for race, ethnicity, smoking status, Elixhauser Comorbidity Index, private insurance status, urban/rural residence, number of hospitalizations in prior year, military service–connected condition, and county‐level COVID‐19 incidence rates.
Severe COVID‐19 was defined as COVID‐19 requiring hospitalization or resulting in death.
Sensitivity analyses additionally included patients with respiratory illnesses that were suspected to be attributable to COVID‐19 but lacked confirmation.
In addition to the association with RA, other factors that were significantly associated with a higher risk of COVID‐19 were self‐reported Black race, self‐reported Hispanic ethnicity, a higher Elixhauser Comorbidity Index, lack of insurance, presence of a military service–connected condition, a BMI categorized as either underweight or obese, a greater number of hospitalizations in the prior year, and higher county‐level incidence rates of COVID‐19 (Table 4). Fewer factors were significantly associated with a higher risk of severe COVID‐19 (resulting in hospitalization or death). These factors included a higher Elixhauser Comorbidity Index, lack of insurance, a greater number of hospitalizations in the prior year, and higher county‐level COVID‐19 incidence rates.
Table 4.
Fully adjusted models evaluating potential risk factors for COVID‐19 and severe COVID‐19 in the study cohort*
| All COVID‐19 | Severe COVID‐19 | |
|---|---|---|
| RA, vs. non‐RA | 1.25 (1.13–1.39)† | 1.35 (1.10–1.66)† |
| Race | ||
| White | 1 (Referent) | 1 (Referent) |
| Black | 1.22 (1.07–1.39)† | 1.25 (0.97–1.60) |
| Other | 1.29 (0.99–1.68) | 1.23 (0.71–2.11) |
| Ethnicity | ||
| White | 1 (Referent) | 1 (Referent) |
| Hispanic | 1.48 (1.23–1.78)† | 1.25 (0.85–1.86) |
| Smoking status | ||
| Current | 0.78 (0.68–0.90) | 1.09 (0.80–1.50) |
| Former | 0.90 (0.78–1.04) | 1.31 (0.94–1.82) |
| Never | 1 (Referent) | 1 (Referent) |
| Elixhauser Comorbidity Index, per 1‐unit increase | 1.12 (1.09–1.15)† | 1.24 (1.20–1.30)† |
| Insurance (no, vs. yes) | 1.40 (1.24–1.58)† | 1.88 (1.49–2.37)† |
| Service‐connected condition | 1.22 (1.10–1.37)† | 1.08 (0.87–1.34) |
| BMI category | ||
| <18.5 kg/m2 | 1.78 (1.01–3.15)† | 1.30 (0.38–4.47) |
| 18.5–25 kg/m2 | 1 (Referent) | 1 (Referent) |
| 25–30 kg/m2 | 1.21 (0.96–1.54) | 1.29 (0.79–2.11) |
| 30–35 kg/m2 | 1.36 (1.08–1.72)† | 1.42 (0.88–2.31) |
| >35 kg/m2 | 1.51 (1.19–1.90)† | 1.59 (0.98–2.57) |
| Urban/rural status | ||
| Urban | 1 (Referent) | 1 (Referent) |
| Rural | 0.90 (0.80–1.01) | 0.98 (0.78–1.22) |
| Highly rural | 1.06 (0.70–1.61) | 0.79 (0.29–2.14) |
| No. of hospitalizations in prior year | 1.11 (1.05–1.16)† | 1.13 (1.06–1.21)† |
| County COVID‐19 incidence rate per 100,000 | 1.00 (1.00–1.00)† | 1.00 (1.00–1.00)† |
Values are the hazard ratio (95% confidence interval) for the risk of developing COVID‐19 or severe COVID‐19 (requiring hospitalization or resulting in death) based on each factor assessed. RA = rheumatoid arthritis; BMI = body mass index.
P < 0.05.
In sensitivity analyses that incorporated individual comorbidities rather than the overall Elixhauser Comorbidity Index, both the risk of COVID‐19 and the risk of severe COVID‐19 (hospitalization or death) were elevated to a similar extent as that seen in patients with RA in patients with individual comorbidities, specifically among patients with heart failure (adjusted HRs for COVID‐19 and severe COVID‐19 of 1.30 and 1.70, respectively), those with chronic lung disease (adjusted HRs of 1.28 and 1.32, respectively), those with diabetes (adjusted HRs of 1.29 and 1.85, respectively), those with liver disease (adjusted HRs of 1.35 and 1.52, respectively), and those with chronic kidney disease (adjusted HRs of 1.14 and 1.76, respectively) (Table 5).
Table 5.
Sensitivity analysis of fully adjusted models evaluating potential risk factors for COVID‐19 and severe COVID‐19 in the study cohort*
| All COVID‐19 | Severe COVID‐19 | |
|---|---|---|
| RA, vs. non‐RA | 1.27 (1.14–1.41)† | 1.39 (1.13–1.71)† |
| Race | ||
| White | 1 (Referent) | 1 (Referent) |
| Black | 1.25 (1.09–1.42)† | 1.24 (0.97–1.59) |
| Other | 1.26 (0.97–1.65) | 1.18 (0.67–2.08) |
| Ethnicity | ||
| White | 1 (Referent) | 1 (Referent) |
| Hispanic | 1.50 (1.24–1.80)† | 1.30 (0.88–1.92) |
| Smoking status | ||
| Current | 0.78 (0.68–0.90) | 1.15 (0.84–1.57) |
| Former | 0.90 (0.78–1.04) | 1.30 (0.93–1.80) |
| Never | 1 (Referent) | 1 (Referent) |
| Chronic condition | ||
| Heart failure | 1.30 (1.09–1.15)† | 1.70 (1.25–2.32)† |
| Chronic lung disease | 1.28 (1.13–1.44)† | 1.32 (1.04–1.67)† |
| Diabetes mellitus | 1.29 (1.16–1.45)† | 1.85 (1.47–2.33)† |
| Hypertension | 0.96 (0.86–1.08) | 1.18 (0.91–1.52) |
| Liver disease | 1.35 (1.11–1.63)† | 1.52 (1.09–2.12)† |
| Cancer | 1.16 (0.98–1.37) | 1.35 (1.00–1.82)† |
| Renal disease | 1.14 (0.97–1.34) | 1.76 (1.34–2.32)† |
| Insurance (no, vs. yes) | 1.40 (1.24–1.59)† | 2.01 (1.59–2.56)† |
| Service‐connected condition | 1.24 (1.11–1.38)† | 1.12 (0.90–1.38) |
| BMI category | ||
| <18.5 kg/m2 | 1.85 (1.04–3.27)† | 1.32 (0.39–4.52) |
| 18.5–25 kg/m2 | 1 (Referent) | 1 (Referent) |
| 25–30 kg/m2 | 1.21 (0.96–1.54) | 1.22 (0.74–2.00) |
| 30–35 kg/m2 | 1.38 (1.10–1.74)† | 1.32 (0.81–2.15) |
| >35 kg/m2 | 1.57 (1.24–1.98)† | 1.49 (0.91–2.45) |
| Urban/rural status | ||
| Urban | 1 (Referent) | 1 (Referent) |
| Rural | 0.89 (0.80–1.00) | 0.95 (0.76–1.18) |
| Highly urban | 1.04 (0.68–1.58) | 0.72 (0.26–2.00) |
| Number of hospitalizations in prior year | 1.17 (1.12–1.22)† | 1.23 (1.16–1.30)† |
| County COVID‐19 incidence rate per 100,000 | 1.00 (1.00–1.00)† | 1.00 (1.00–1.00)† |
Values are the hazard ratio (95% confidence interval) for the risk of developing COVID‐19 or severe COVID‐19 (requiring hospitalization or resulting in death) in sensitivity analyses of fully adjusted models, in which individual comorbidities, rather than the overall Elixhauser Comorbidity Index, were incorporated in the model. RA = rheumatoid arthritis; BMI = body mass index.
P < 0.05.
Risk of COVID‐19 by RA autoantibody status and medication usage
In secondary analyses, we compared the risk of COVID‐19 and severe COVID‐19 (resulting in hospitalization or death) between RA patients and non‐RA controls based on RA autoantibody status and use of medications. The risk of COVID‐19 and of severe COVID‐19 (hospitalization or death) was similar between patients with seronegativity for RA autoantibodies and those with a seropositive status, independent of potential confounders (Figures 1A and B).
Figure 1.
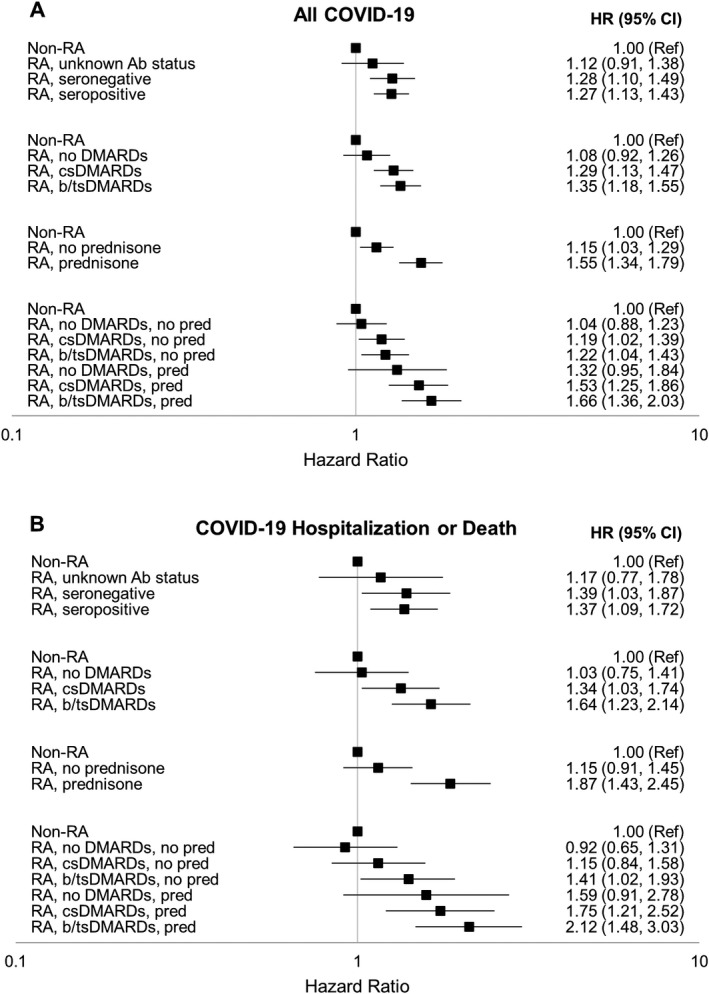
Risk of COVID‐19 (A) and severe COVID‐19 (requiring hospitalization or leading to death) (B) in subgroups of patients with rheumatoid arthritis (RA) and age‐, sex‐, and site‐matched non‐RA controls. RA patients were stratified according to antibody (Ab) status, treatment with disease‐modifying antirheumatic drugs (DMARDs), treatment with prednisone (pred), and combination treatment with DMARDs and prednisone. Values are the hazard ratio (HR) with 95% confidence interval (95% CI) for the risk of COVID‐19 or severe COVID‐19, adjusted for race, ethnicity, smoking status, body mass index, Elixhauser Comorbidity Index, insurance status, urban/rural residence, number of hospitalizations in prior year, presence of a military service–connected condition, and county‐level COVID‐19 incidence rates. Ref = referent; csDMARDs = conventional synthetic DMARDs; b/tsDMARDs = biologic/targeted synthetic DMARDs.
Moreover, compared to non‐RA controls, a higher risk of COVID‐19 and of severe COVID‐19 (hospitalization or death) was observed among RA patients who had received treatment with csDMARDs, bDMARDs/tsDMARDs, and prednisone (Figures 1A and B). RA patients treated with both bDMARDs/tsDMARDs and prednisone had the highest risk of COVID‐19 (adjusted HR 1.66 [95% CI 1.36–2.03]) and the highest risk of COVID‐19 hospitalization or death (adjusted HR 2.12 [95% CI 1.48–3.03]), relative to non‐RA controls.
DISCUSSION
In this large, national VA at‐risk cohort of RA patients and matched non‐RA controls, we found that patients with RA were at a significantly higher risk of developing SARS–CoV‐2 infection and having severe COVID‐19 leading to hospitalization or death. Those treated with DMARDs and prednisone were at the highest risk of COVID‐19 and more severe disease. This was independent of potential confounders, including demographic characteristics, comorbidities, health care utilization and access, and county‐level COVID‐19 incidence rates. Strikingly, the heightened risk of COVID‐19 related to RA was consistent with the risk posed by other chronic conditions that receive priority vaccination status. Thus, the immediate implication of our findings is the suggestion that similar consideration for vaccine prioritization should be given to patients with RA receiving immunosuppressive therapies.
After adjusting for potential confounders, we estimated that RA was associated with a 25% increased risk of COVID‐19 and a 35% increased risk of COVID‐19 hospitalization or death. These are among the first data to link RA with a higher risk of COVID‐19. Moreover, our results linking RA with a higher risk of viral infection is an important contribution to our understanding of how RA may affect the risk of developing other viral infections, since prior literature has focused on bacterial infections (3). Our findings of a higher risk of a more severe COVID‐19 disease course in RA are consistent with results from a UK study of at‐risk patients with RA, psoriasis, or lupus, in whom a 19% higher risk of COVID‐19–associated death was observed (12), and also consistent with results from another study of COVID‐19 patients in which a 14% higher risk of hospitalization in those with a rheumatic disease was observed (10). Our findings are also in line with prior estimates of the risk of serious infection (bacterial or nonbacterial) in RA patients relative to those without RA, with one study of patients in the FORWARD registry showing a 50% increased risk of serious infection (26).
In our study, RA patients receiving csDMARDs, those receiving bDMARDs/tsDMARDs, and those receiving prednisone had a higher risk of COVID‐19 and higher risk of a severe COVID‐19 disease course. Although we are not aware of prior literature describing a link between these therapies and COVID‐19 risk in RA, others have similarly found that select immunosuppressive therapies, specifically rituximab and prednisone, were associated with a more severe disease course among patients with COVID‐19, including those with RA (7, 10, 27). The highest‐risk RA subgroup in our study was those who were receiving both bDMARDs/tsDMARDs and prednisone. These individuals had a >2‐fold higher risk of COVID‐19 hospitalization or death compared to non‐RA controls. Similar risks have been identified in RA patients who were receiving bDMARDs/tsDMARDs and prednisone for non–COVID‐19–related severe infections (28, 29). While there has been hope that some of these therapies might suppress the hyperinflammatory features of COVID‐19 and improve outcomes, findings in this study highlight the notion that the timing of immunosuppressive therapies (preceding or at the time of exposure, early infection, or severe infection stages) may be crucial for measuring their potential impact on disease outcomes. Use of immunosuppressive medications may predispose an individual to COVID‐19 infection or a more severe disease course early, but during the hyperinflammatory phase of severe COVID‐19 (30), select immunosuppressive therapies may become beneficial. Our findings, and those of others, also raise concerns that patients with other medical conditions receiving these immunosuppressive therapies may be at higher risk of COVID‐19 and severe COVID‐19. Importantly, confounding by indication is recognized as a complicating factor in such observational studies of therapies and COVID‐19, and therefore causal conclusions cannot be drawn (8). Further study is warranted.
Our findings have clear relevance to health policy surrounding COVID‐19 prevention and management. Risk stratification for vaccination, for example, has primarily been established on the basis of age, occupational risks, and the presence of several chronic conditions. RA or the use of immunosuppressive therapies outside the setting of prior organ transplantation are not considered chronic conditions that would receive priority vaccination status (6). Our results suggest that RA patients receiving DMARDs and/or prednisone should be considered for priority status in these prevention efforts, including priority for receiving initial and booster vaccination. The 35% increased risk of COVID‐19 hospitalization or death related to RA falls within the range we estimated for other conditions that receive priority status (adjusted HR ranging from 1.18 to 1.85). With <1.0% of the population having RA (31), the inclusion of RA as a priority group is unlikely to drastically impact vaccine or treatment supplies for other individuals, and the vulnerable population would remain protected. Moreover, since treatment with DMARDs and/or prednisone was linked to more severe COVID‐19 in both our study (RA only) and other studies (including those with other rheumatic diseases [7,10]), prioritization may be warranted for any medical conditions requiring long‐term immunosuppressive medications.
Consistent with other studies in the general population and in patients with rheumatic diseases, we found that race and ethnicity were major determinants of SARS–CoV‐2 infection and severe COVID‐19 (9, 32). Black patients had a 22% higher risk of SARS–CoV‐2 infection and a 25% higher risk of COVID‐19 hospitalization or death. Similarly, patients with Hispanic ethnicity had a 48% higher risk of SARS–CoV‐2 infection and a 25% increased risk of COVID‐19 hospitalization or death, though the latter did not reach statistical significance. Importantly, the associations of race and ethnicity with COVID‐19 were independent of other factors, including demographic characteristics, comorbidity burden and individual chronic conditions, health care utilization and insurance, and county‐level COVID‐19 incidence rates, all of which may mediate or confound such associations. Our findings therefore suggest that additional factors may contribute to the racial and ethnic disparities in the incidence and severity of COVID‐19, such as differences in the severity of comorbid chronic conditions, community or occupational risks, time to receiving care, access during surges, or immune responses to SARS–CoV‐2 (32); these factors will require further study.
There are limitations to this study. There is a potential for misclassification of RA status with the use of administrative algorithms. This is most likely to occur in RA patients who were not receiving DMARDs. Misclassification of non‐RA patients as having RA should only bias the findings toward the null, resulting in an underestimation of the risk of COVID‐19 in RA. Our study was designed to compare the risk of COVID‐19 between RA patients and non‐RA controls, rather than comparing the risk between specific medications or medication doses. To generate valid data for such comparisons and avoid the misinformation that has plagued the pandemic (33), alternative study designs would be required. DMARD and prednisone doses were not available for these analyses. In addition, misclassification of COVID‐19 may have occurred, and the sensitivity of the standardized process utilized in the VA system for capturing non‐VA COVID‐19 cases has not been established. Our study population was composed primarily of older male patients (although >10,000 female patients were included), consistent with the demographics of the VA health care system, but findings may not be generalizable to other populations. While male sex has been associated with more severe COVID‐19 (34), it is not expected that the impact of RA and RA therapies on COVID‐19 risk would be differential between men and women. Finally, because of the observational nature of our study, unmeasured confounding may be present.
In conclusion, we observed that patients with RA in this VA cohort had a 25% increased risk of COVID‐19 and a 35% increased risk of severe COVID‐19 (leading to hospitalization or death), independent of several potential confounders. RA patients who had recently received DMARDs and prednisone were at the highest risk of COVID‐19 and more severe COVID‐19, risks that rivaled those accompanying other chronic conditions. Consideration should be given to the establishment of RA, and potentially other conditions that require treatment with similar immunosuppressive medications, as a chronic condition that should receive prioritization for COVID‐19 prevention and management strategies.
AUTHOR CONTRIBUTIONS
All authors were involved in drafting the article or revising it critically for important intellectual content, and all authors approved the final version to be published. Dr. England had full access to all of the data in the study and takes responsibility for the integrity of the data and the accuracy of the data analysis.
Study conception and design
England, Mikuls.
Acquisition of data
England, Roul, Yang, Sauer, Baker, Mikuls.
Analysis and interpretation of data
England, Roul, Yang, Kalil, Michaud, Thiele, Sauer, Baker, Mikuls.
Supporting information
Table S1
ACKNOWLEDGMENTS
This study was supported by access to data from the VA COVID‐19 Shared Data Resource, and by the resources and facilities of the VA Informatics and Computing Infrastructure (VA HSR RES 13‐457).
The views expressed in this article are those of the authors and do not necessarily reflect the position or policy of the US Department of Veterans Affairs or the US government.
Supported by the University of Nebraska Medical Center, College of Medicine. Drs. England and Mikuls’ work was supported by the US Department of Veterans Affairs (grant IK2‐CX‐002203 to Dr. England and grant I01‐BX‐004660 to Dr. Mikuls), the Rheumatology Research Foundation (Scientist Development Award to Dr. England and Innovative Research Award to Dr. Mikuls), and the National Institute of General Medical Sciences, NIH (grant U54‐GM‐115458 to Dr. England and grant U54‐GM‐115458 to Dr. Mikuls). Dr. Baker’s work was supported by the US Department of Veterans Affairs (grant I01‐CX‐001703).
Dr. England has received consulting fees from Boehringer Ingelheim (less than $10,000). Dr. Thiele has received consulting fees, speaking fees, and/or honoraria from Sanofi (less than $10,000). Dr. Baker has received consulting fees from Bristol Myers Squibb, Pfizer, and Gilead (less than $10,000 each). Dr. Mikuls has received consulting fees from Pfizer, Sanofi, Gilead, and Horizon (less than $10,000 each) and research support from Bristol Myers Squib and Horizon. No other disclosures relevant to this article were reported.
REFERENCES
- 1. Sparks JA. Rheumatoid arthritis. Ann Intern Med 2019;170:ITC1–16. [DOI] [PubMed] [Google Scholar]
- 2. Jani M, Barton A, Hyrich K. Prediction of infection risk in rheumatoid arthritis patients treated with biologics: are we any closer to risk stratification? [review]. Curr Opin Rheumatol 2019;31:285–92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Listing J, Gerhold K, Zink A. The risk of infections associated with rheumatoid arthritis, with its comorbidity and treatment [review]. Rheumatology (Oxford) 2013;52:53–61. [DOI] [PubMed] [Google Scholar]
- 4. Wu C, Chen X, Cai Y, Xia J, Zhou X, Xu S, et al. Risk factors associated with acute respiratory distress syndrome and death in patients with coronavirus disease 2019 pneumonia in Wuhan, China. JAMA Intern Med 2020;180:934–43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Zhou F, Yu T, Du R, Fan G, Liu Y, Liu Z, et al. Clinical course and risk factors for mortality of adult inpatients with COVID‐19 in Wuhan, China: a retrospective cohort study. Lancet 2020;395:1054–62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Oliver SE, Gargano JW, Marin M, Wallace M, Curran KG, Chamberland M, et al. The Advisory Committee on Immunization Practices’ updated interim recommendation for allocation of COVID‐19 vaccine—United States, December 2020. MMWR Morb Mortal Wkly Rep 2021;69:1657–60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Strangfeld A, Schafer M, Gianfrancesco MA, Lawson‐Tovey S, Liew JW, Ljung L, et al. Factors associated with COVID‐19‐related death in people with rheumatic diseases: results from the COVID‐19 Global Rheumatology Alliance physician‐reported registry. Ann Rheum Dis 2021;80:930–42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Schafer M, Strangfeld A, Hyrich KL, Carmona L, Gianfrancesco M, Lawson‐Tovey S, et al. Response to: 'Correspondence on 'Factors associated with COVID‐19‐related death in people with rheumatic diseases: results from the COVID‐19 Global Rheumatology Alliance physician reported registry'' by Mulhearn et al. Ann Rheum Dis 2021. doi: 10.1136/annrheumdis-2021-220134. E‐pub ahead of print. [DOI] [PubMed] [Google Scholar]
- 9. Gianfrancesco MA, Leykina LA, Izadi Z, Taylor T, Sparks JA, Harrison C, et al. Association of race and ethnicity with COVID‐19 outcomes in rheumatic disease: data from the COVID‐19 Global Rheumatology Alliance physician registry. Arthritis Rheumatol 2021;73:374–80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. D'Silva KM, Jorge A, Cohen A, McCormick N, Zhang Y, Wallace ZS, et al. COVID‐19 outcomes in patients with systemic autoimmune rheumatic diseases compared to the general population: a US multicenter, comparative cohort study. Arthritis Rheumatol 2021;73:914–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Serling‐Boyd N, D'Silva KM, Hsu TY, Wallwork R, Fu X, Gravallese EM, et al. Coronavirus disease 2019 outcomes among patients with rheumatic diseases 6 months into the pandemic. Ann Rheum Dis 2021;80:660–66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Williamson EJ, Walker AJ, Bhaskaran K, Bacon S, Bates C, Morton CE, et al. Factors associated with COVID‐19‐related death using OpenSAFELY. Nature 2020;584:430–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Chung CP, Rohan P, Krishnaswami S, McPheeters ML. A systematic review of validated methods for identifying patients with rheumatoid arthritis using administrative or claims data. Vaccine 2013;31 Suppl 10:K41–61. [DOI] [PubMed] [Google Scholar]
- 14. Donnelly JP, Wang XQ, Iwashyna TJ, Prescott HC. Readmission and death after initial hospital discharge among patients with COVID‐19 in a large multihospital system. JAMA 2021;325:304–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Bowe B, Cai M, Xie Y, Gibson AK, Maddukuri G, Al‐Aly Z. Acute kidney injury in a national cohort of hospitalized US veterans with COVID‐19. Clin J Am Soc Nephrol 2020;16:14–25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Luo J, Jeyapalina S, Stoddard GJ, Kwok AC, Agarwal JP. Coronavirus disease 2019 in veterans receiving care at Veterans Health Administration facilities. Ann Epidemiol 2021;55:10–4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. US Department of Veterans Affairs . VA informatics and computing infrastructure: updates to the VA COVID‐19 Shared Data Resource and its use for research. June 2020. URL: https://www.hsrd.research.va.gov/for_researchers/cyber_seminars/archives/video_archive.cfm?SessionID=3834.
- 18. Baker JF, Billig E, Michaud K, Ibrahim S, Caplan L, Cannon GW, et al. Weight loss, the obesity paradox, and the risk of death in rheumatoid arthritis. Arthritis Rheumatol 2015;67:1711–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. England BR, Baker JF, Sayles H, Michaud K, Caplan L, Davis LA, et al. Body mass index, weight loss, and cause‐specific mortality in rheumatoid arthritis. Arthritis Care Res (Hoboken) 2018;70:11–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. McGinnis KA, Brandt CA, Skanderson M, Justice AC, Shahrir S, Butt AA, et al. Validating smoking data from the Veteran's Affairs Health Factors dataset, an electronic data source. Nicotine Tob Res 2011;13:1233–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Elixhauser A, Steiner C, Harris DR, Coffey RM. Comorbidity measures for use with administrative data. Med Care 1998;36:8–27. [DOI] [PubMed] [Google Scholar]
- 22. Dong E, Du H, Gardner L. An interactive web‐based dashboard to track COVID‐19 in real time [letter]. Lancet Infect Dis 2020;20:533–4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Schwab P, Sayles H, Bergman D, Cannon GW, Michaud K, Mikuls TR, et al. Utilization of care outside the Veterans Affairs health care system by US veterans with rheumatoid arthritis. Arthritis Care Res (Hoboken) 2017;69:776–82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Dennis JM, McGovern AP, Vollmer SJ, Mateen BA. Improving survival of critical care patients with coronavirus disease 2019 in England: a national cohort study, March to June 2020. Crit Care Med 2021;49:209–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Jorge A, D'Silva KM, Cohen A, Wallace ZS, McCormick N, Zhang Y, et al. Temporal trends in severe COVID‐19 outcomes in patients with rheumatic disease: a cohort study. Lancet Rheumatol 2021;3:e131–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Mehta B, Pedro S, Ozen G, Kalil A, Wolfe F, Mikuls T, et al. Serious infection risk in rheumatoid arthritis compared with non‐inflammatory rheumatic and musculoskeletal diseases: a US national cohort study. RMD Open 2019;5:e000935. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Ungaro RC, Agrawal M, Park S, Hirten R, Colombel JF, Twyman K, et al. Autoimmune and chronic inflammatory disease patients with COVID‐19. ACR Open Rheumatol 2021;3:111–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Singh JA, Cameron C, Noorbaloochi S, Cullis T, Tucker M, Christensen R, et al. Risk of serious infection in biological treatment of patients with rheumatoid arthritis: a systematic review and meta‐analysis. Lancet 2015;386:258–65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. George MD, Baker JF, Winthrop K, Hsu JY, Wu Q, Chen L, et al. Risk for serious infection with low‐dose glucocorticoids in patients with rheumatoid arthritis: a cohort study. Ann Intern Med 2020;173:870–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Cao X. COVID‐19: immunopathology and its implications for therapy [review]. Nat Rev Immunol 2020;20:269–70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Cross M, Smith E, Hoy D, Carmona L, Wolfe F, Vos T, et al. The global burden of rheumatoid arthritis: estimates from the Global Burden of Disease 2010 study. Ann Rheum Dis 2014;73: 1316–22. [DOI] [PubMed] [Google Scholar]
- 32. Price‐Haywood EG, Burton J, Fort D, Seoane L. Hospitalization and mortality among black patients and white patients with Covid‐19. N Engl J Med 2020;382:2534–43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Kim AH, Sparks JA, Liew JW, Putman MS, Berenbaum F, Duarte‐Garcia A, et al. A rush to judgment? Rapid reporting and dissemination of results and its consequences regarding the use of hydroxychloroquine for COVID‐19 [editorial]. Ann Intern Med 2020;172:819–21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Peckham H, de Gruijter NM, Raine C, Radziszewska A, Ciurtin C, Wedderburn LR, et al. Male sex identified by global COVID‐19 meta‐analysis as a risk factor for death and ITU admission. Nat Commun 2020;11:6317. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Table S1


