Abstract
Objective
To study the mRNA and protein expression of GRP78 in tumor and serum of the RCC patients and compare with the controls and to correlate the expression with the grade and stage of RCC.
Materials and methods
A prospective cohort study involving 60 patients planned for radical/partial nephrectomy for primary RCC between July 2017 to June 2019. The RCC and adjacent non-tumorous renal tissues (Control) along with serum samples of patients were collected. Control for the serum samples is from the patients undergoing simple nephrectomy for non-functioning kidney due to benign etiology. The GRP78 expression was studied using RT-PCR for mRNA expression, Western blot analysis and immunohistochemistry (IHC) for protein expression and using ELISA in serum for both the subjects and controls.
Results
Mean age of patients was 50.3 years. The mRNA and protein expression of GRP78 in tissue samples were significantly higher in RCC patients as compared to controls (p < 0.001). IHC also demonstrated significantly higher expression in tumour samples as compared to controls (p < 0.001). Circulatory levels of GRP78 in serum samples were also significantly increased (p < 0.0001) in RCC patients in comparison to control subjects. The expression of GRP78 in circulation significantly correlated with the pathological tumor stage (p = 0.03), grade of disease (p < 0.001).
Conclusion
The GRP78 in RCC is significantly upregulated both at molecular and protein level expression. The overexpression of GRP78 correlates with the stage and grade of disease, thereby, highlighting its prognostic ability.
Keywords: RCC, Glucose regulated protein 78, Endoplasmic reticulum chaperone protein, Binding immunoglobulin protein (BiP)
RCC; Glucose regulated protein 78; Endoplasmic reticulum chaperone protein; Binding immunoglobulin protein (BiP).
1. Introduction
Renal Cell Carcinoma (RCC) constitutes around 3% of all cancers with an overall 50% mortality at 5 years and less than 10% 5-year survival among those with metastatic disease [1, 2]. Targeted therapy has resulted in improved survival in metastatic RCC and anti-angiogenic therapies targeting the vascular endothelial growth factor (VEGF) signaling pathway are now first-line therapies in its management [3]. However, anti-angiogenic therapy is generally cytostatic, not cytotoxic, and the tumor may survive and grow following an interval of stability [4]. Hence, the development of newer molecular targets is indispensable.
Uncontrolled proliferation of cancer cells results in local hypoxia and the tumor cells are exposed to limited oxygen and nutrient supply [5]. This produces an unfolded protein response (UPR) which is a stressor response to limit the synthesis of non-essential global protein. UPR proteins in the endoplasmic reticulum (ER) of cancer cells are among the potential therapeutic targets in oncology and one such protein is the chaperone glucose-regulated protein 78 (GRP78) [6].
GRP78 is a 78 kDa protein mainly expressed in ER and is also called binding immunoglobulin protein (BiP) or heat shock 70kDa protein 5(HSPA5). During hypoxic stress, cells activate the UPR, and GRP78 acts as a master regulator of ER stress signaling and helps to handle the increasing amounts of abnormal proteins to maintain homeostasis. GRP78 controls protein folding and assembly, preventing protein aggregation, and regulating UPR [7]. In tumor cells, because of glucose deprivation, hypoxia and, lactic acidosis, levels of GRP78 get upregulated and it further promotes cancer cell proliferation [8]. GRP 78 translocate to the surface of cancer cells and acts as an important regulator of oncogenic signaling [9]. In malignant cells, levels of GRP78 are upregulated both at the RNA and protein level, but not in benign cells. Further, monoclonal antibodies against GRP78 may have clinical application as a therapeutic drug in various cancers [10]. Thus, the expression of GRP78 may serve as a potential biomarker for predicting tumor behavior and treatment response, and it may act as a potential target for new therapies.
GRP78 is highly expressed in RCC and its expression has been proposed to be a poor prognostic factor [6]. However, the role of GRP78 remains unclear as a potential biomarker [4, 11, 12]. GRP78 can be detected in the serum and any correlation with disease stage or prognosis would be useful as a biomarker in patients with RCC. We aimed to study the mRNA and protein expression of GRP78 in tumor and serum of patients with RCC and controls and correlate the expression with the grade and stage of RCC. To our knowledge, this is the first study evaluating the role of serum GRP78 in RCC as a prognostic biomarker.
2. Materials and methods
This prospective cohort study was approved by the institutional ethics committee of All India Institute of Medical Sciences, AIIMS, Delhi, India (IEC -162/7.4.2017), and all subjects provided written informed consent. The inclusion criterion was patients with age >18 years with a provisional diagnosis of RCC. Patients in whom, there was concomitant second malignancy were excluded.
The sample size is calculated by using the following formula
Z is the value from the standard normal distribution (e.g., Z = 1.96 for 95%)
σ is the standard deviation of the outcome variable
E is the desired margin of error.
In a study (Weijin FU et al) the ratio of GRP78/GAPDH mRNA was 0.88 ± 0.34 in subjects vs. 0.44 ± 0.15 in controls [6]. Using this data and assuming 80% power, 5% significance level with 95% confidence interval, the minimum number of subjects required is 57. Thus, a sample size of 60 subjects is planned with 60 controls.
Between July 2017 and June 2019, 60 patients scheduled to undergo radical or partial nephrectomy for suspected primary RCC and 60 patients scheduled for simple nephrectomy (control for serum) for benign conditions were enrolled.
Clinical and demographic details of the patients including radiological features were entered in a pre-designed study proforma. The diagnosis of renal cell carcinoma was confirmed on histopathology by a single expert pathologist. The pathological staging was done using the TNM classification of the 7th edition of the American Joint Committee for Cancer Staging and grading was done according to the Fuhrman grading scheme by a single pathologist blinded to clinical and demographic details.
The RCC and adjacent non-tumorous renal tissues (located 3–5cm from the tumor site) which serve as the control for the tissue of patients undergoing radical nephrectomy were prospectively collected and stored. Each tissue (tumor and control) was cut into two pieces-one was stored in RNA Later solution for RNA isolation and the other part was stored for lysate preparation in -80 °C. The serum samples of these patients were also collected in 5ml sterile vials in the preop period. The serum samples of patients undergoing simple nephrectomy for non-functioning or poorly functioning kidney due to benign etiology, similar in age and gender were also collected to serve as controls for serum expression of GRP78.
2.1. Tissue samples
-
1.
RNA extraction, cDNA preparation, and expression of GRP78 in tumor and control tissue
RNA was isolated from the tissue samples using the Tri-reagent (sigma). The stored tissue samples were crushed by the snap-freeze method using liquid nitrogen and then, resuspended in Tri-reagent. The suspension was then allowed to stand for 5 min at room temperature followed by the addition of chloroform (200 μl/ml of the tri reagent). The mixture was then vortexed and incubated for 15 min followed by centrifugation at 12000 g at 2–8 °C. The aqueous phase containing the RNA was transferred to another tube and 0.5 ml of isopropyl alcohol was added. The mixture was incubated at room temperature for 10 min followed by centrifugation at 12000 g for 10 min at 2–8 °C. The supernatant was removed and the RNA pellet was thoroughly washed once with 1 ml of 75% ethanol. The RNA pellet was then briefly air-dried at room temperature, dissolved in Nuclease free water, and then quantified using a Nanodrop spectrophotometer.
The isolated RNA was given DNase treatment to remove DNA content, if any, from the sample. For this, 1U DNase and 1X DNase buffer were mixed with 1.5μg RNA in a total of 10.0ul reaction. The reaction was then incubated at 37 °C for 30min followed by the addition of 1μl EDTA to stop the reaction by heating at 65 °C for 10min. The reverse transcriptase reactions for cDNA preparation were done by heating a 12μl reaction mixture containing 1.0 μg total RNA and 0.5μg random hexamer at 70 °C for 10 min. The mixture was cooled and 20 U ribonuclease inhibitor and 200 U Moloney's murine leukemia virus ribonuclease reverse transcriptase was added in a final 20μl reaction mixture containing 10 mmol deoxy-NTP and 5 μl Moloney's murine leukemia virus reaction buffer, incubated for 1 h at 42 °C and heated 10 min at 70 °C. The cDNA was then used as a template for PCR to determine the transcript expression of GRP78.
For PCR analysis, primers for the GRP78 gene were designed using the Primer3 software. The annealing temperature of the primers was standardized by gradient PCR. Followed by this, the mRNA expression of GRP78 was determined by Real-time PCR using SYBR green chemistry. An aliquot containing total cDNA (100–200 ng) was subjected to PCR using specific primers for GRP78. 18 S was used as an internal control for normalization. The relative mRNA expression was then evaluated by 2−ðCt method where Ct represents the threshold cycle.
-
2.
Immunoblot (Western Blot) analysis
Tissues were crushed by snap-freezing method using liquid nitrogen followed by addition of ice-cold lysis buffer (1X PBS, 1% Nonidet P-40, 0.5% sodium deoxycholate, 0.1% SDS) supplemented with protease inhibitors (1 μg/ml leupeptin, 0.5 μg/ml aprotinin, 1.5 μg/ml pepstatin, 0.1 μg/ml phenylmethylsulphonyl fluoride) for lysis. The tissues were then sonicated three times at a pulse of 10s each after the gap of the 50s. Then, the samples were centrifuged at 10,000g for 20 min at 4 °C, supernatants were collected and used for protein concentration by Bradford assay. 30 μg protein was denatured at 95 °C for 10 min and then separated in SDS-PAGE followed by transfer onto the nitrocellulose membrane. The non-specific binding sites were blocked in 5% BSA followed by washing in Tris-buffer saline (TBS). Then the blot was incubated with GRP78 primary antibody (St John's Laboratory Ltd, London) (1:1000 dilution) overnight at room temperature followed by washing in TBS with 0.05% tween. Then incubation was done with anti-mouse HRP-conjugated secondary antibody for 2 h at RT. Finally, the blot was washed with TBS with 0.1% tween, and detection of proteins was done by chemiluminescence method.
-
3.
Immunohistochemistry
The immunohistochemical assay involved deparaffinization/rehydration of tissue specimens, antigen retrieval, and staining. The tissue slides were heated in an oven at 65 °C for 1 h. The tissue was deparaffinized and hydrated as follows: 2 Xylene washes for 5 min each, followed by two 100% ethanol rinse for 5 min each, followed by 95% ethanol, 70% ethanol, 50% ethanol, 30% ethanol, followed by H2O and a TBST wash for 5 min on a shaker. The retrieval of antigen was done as per the product manual guidelines with the immersion of slides into Antigen Retrieval Solution. After antigen retrieval, the slides were washed with TBST for 5 min on a shaker three times (3 min each on a shaker). A Blocking solution was applied for 1 h. As per recommendation in the product manual the primary antibody was diluted in the blocking buffer. The primary antibody is applied to each section and kept overnight at 4 °C in the humidified chamber. The slides were then washed three times with TBST (3 min each on a shaker) and secondary HRP-conjugated anti-rabbit antibody diluted in the blocking solution per manufacturer's recommendation was applied and incubated for 1 h at room temperature. The slides were then washed again three times with TBST (3 min each on a shaker). A freshly prepared DAB substrate was added to the sections and incubated at room temperature until suitable staining develops (generally 2–5 min). The sections were then rinsed with and counterstained with Hematoxylin. The samples were then dehydrated using 2 rinses with 100% Ethanol (Twenty dips per rinse) followed by 2 rinses with xylene (Thirty dips per rinse).
2.2. Serum samples
-
1.
Enzyme-Linked Immunosorbent Assay (ELISA) for serum expression of GRP78
Serum was collected by centrifuging the blood at 3000 rpm for 10 min. The serum was stored at -80 °C until use. A Commercially available highly sensitive ELISA kit (Uscn Life Science Inc., Cloud-Clone Corp., USA) was used for determining the levels of GRP78 in serum samples of the subjects. The wells of the microtiter strips provided were precoated with a monoclonal antibody against the GRP78 antigen. The antigens present in the serum sample or standard were incubated. After that primary monoclonal anti- GRP78 antibody respectively conjugated to biotin in respective microtiter plates was added. The washing step was done to remove the unbound antigen following incubation. An avidin-HRP conjugated antibody specific for the primary antibody was then added to the wells. Then, a TMB one-step substrate reagent reactive with HRP was added to the wells after incubation and following a wash. By adding acid, the color development was terminated and absorbance was measured at 450 nm. By plotting the different concentrations of standard samples versus absorbance a reference curve was obtained and levels of the antigens in samples tested were calculated by its standard plot.
2.3. Statistical analysis
Continuous variables were expressed as mean ± standard deviation (SD) or median (Interquartile range [IQR]) as appropriate. The normality of the data was checked and categorical variables were compared using the chi-square test and continuous variables were compared using the Mann Whitney test or Kruskal–Wallis test as appropriate. The correlation between two variables was assessed using Spearman's Rank Correlation or Pearson's coefficient as appropriate. p < 0.05 was taken as statistical significant. Data were analyzed using IBM SPSS Statistics software (version 20.0, Chicago. IL, USA).
3. Results
Sixty patients with clear cell carcinoma of the kidney undergoing radical or partial nephrectomy and 60 control patients undergoing simple nephrectomy for benign disease were included in the study. Among these, 32 patients (53.3%) had pT1b disease while 13 patients (21.7%) had pT3a disease. Forty four patients (73.3%) underwent a radical nephrectomy while 8 patients (13.3%) underwent a partial nephrectomy. The demographic and tumor details are provided in Tables 1 and 2. Based on Fuhrman grading, 39 patients (65%) had grade 2 disease while grade 3 and grade 4 disease was present in 8 patients (13.3%) and 5 patients (8.4%) respectively.
Table 1.
Characteristics of patients suffering from RCC included in the study (n = 60) eGFR-estimated Glomerular Filtration Rate.
| Study Parameter | ||
| Mean Age± SD, years | 50.3 ± 12.4 | |
| Gender (M/F) | 44/16 | |
| Side of the tumor (Right/Left) | 29/31 | |
| Maximum Radiological size, Mean ± SD, centimeters | 7.1 ± 3.8 | |
| Comorbidities | ||
| Diabetes (n), (%) | 12 (20.0%) | |
| Hypertension (n), (%) | 16 (12.7%) | |
| Coronary artery disease (n), (%) | 4 (6.7%) | |
| Chronic kidney Disease (eGFR<60 ml/min/m2) | 3 (5.0%) | |
| Mode of detection | Incidental (n), (%) | 21 (35.0%) |
| Pain (n), (%) | 14 (23.3%) | |
| Hematuria (n), (%) | 12 (20.0%) | |
| Pain & hematuria (n), (%) | 8 (13.4%) | |
| Others (n), (%) | 5 (8.3%) | |
| Surgical approach | Open (n), (%) | 37 (61.7%) |
| Laparoscopic (n), (%) | 22 (36.7%) | |
| Robotic (n), (%) | 1 (1.7%) | |
| Surgical procedure | Radical nephrectomy (n), (%) | 44 (73.3%) |
| Partial nephrectomy (n), (%) | 8 (13.3%) | |
| Cytoreductive nephrectomy (n), (%) | 8 (13.3%) | |
| Blood parameters | ||
| Mean Haemoglobin± SD, g/dl | 12.3 ± 1.7 | |
| Mean Platelets, x103 ± SD, /mm3 | 226 ± 104 | |
| Mean serum Creatinine ±SD, mg/dl | 0.9 ± 0.3 | |
Table 2.
Tumor characteristics of the RCC patients included in the study (n = 60).
| Parameter | Number (%) | ||
|---|---|---|---|
| Location of tumor | Upper pole, n (%) | 31 (51.7%) | |
| Mid pole, n (%) | 14 (23.3%) | ||
| Lower pole, n(%) | 15 (25.6%) | ||
| Renal vein and IVC involvement, n(%) | 10 (16.7%) | ||
| Metastasis, n(%) | 8 (13.3%) | ||
| pT stage of tumor | T1a | 1 (1.7%) | |
| T1b, n(%) | 32 (53.3%) | ||
| T2a, n(%) | 4 (6.7%) | ||
| T2b, n(%) | 3 (5.6%) | ||
| T3a, n(%) | 13 (21.7%) | ||
| T3b, n(%) | 7 (11.7%) | ||
| Clear cell carcinoma on histopathology | 60 (100%) | ||
| Fuhrman's Grade | Grade 1 | 8 (13.3%) | |
| Grade 2 | 39 (65%) | ||
| Grade 3 | 8 (13.3%) | ||
| Grade 4 | 5 (8.3%) | ||
3.1. Expression of GRP78 in tissue samples
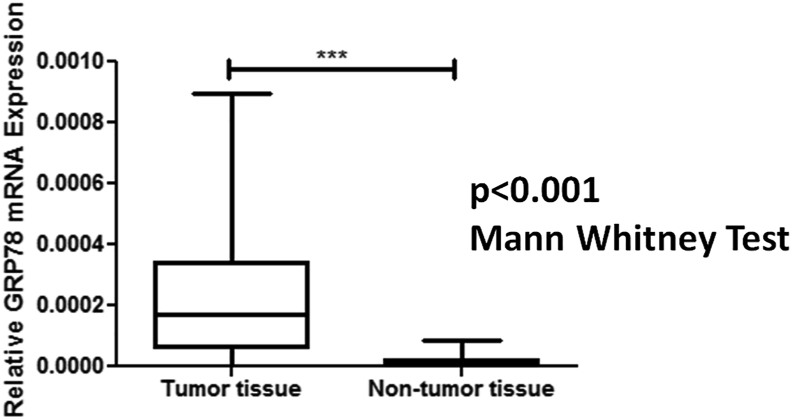
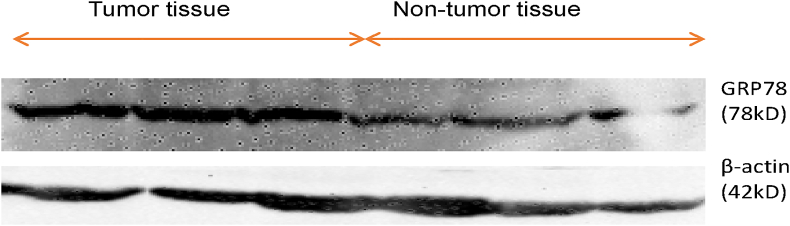
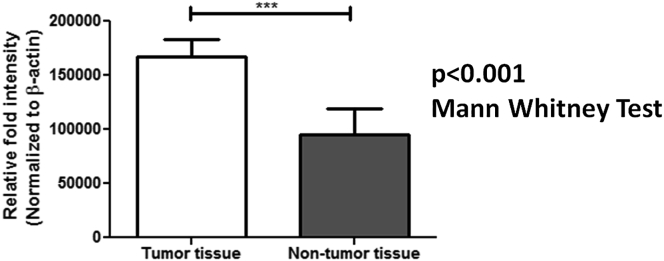
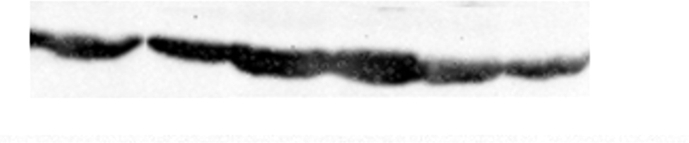
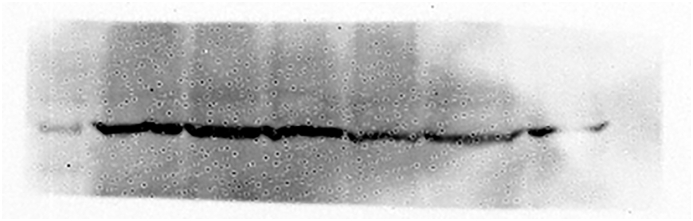
The median relative mRNA expression of GRP78 was significantly higher in RCC as compared to normal adjacent non-tumor renal tissue (control for the tissue) (p < 0.001) (Figure 1). Besides, the protein expression studied in the tissue samples as determined using Western blotting and quantified using ImageJ software was significantly higher in renal cell carcinoma patients compared to the controls (p < 0.001) as shown in Table 3. Figures 2 and 3 depict the Western blot image of protein expression of GRP78 and a comparison of its expression in tumor and adjacent normal tissues.
Figure 1.
Box whisker plot showing relative mRNA expression of GRP78 in tumor tissue and adjacent non-tumor tissue of RCC patients. 18S was used as an internal control for normalization. Mann Whitney U Test was applied to determine significance. ∗∗∗p < 0.001.
Table 3.
Comparative expression of Glucose Related Protein 78 (GRP78) in RCC patients and controls.
| Parameter | RCC (n = 60) |
Control population (n = 60) |
|
|---|---|---|---|
| Expression of GRP78 in tissue samples | |||
| Relative mRNA expression, median (range) |
0.00017 (2.34 × 10−6 - 0.00089) |
7.709 × 10−6 (1.53 × 10−6 - 8.66 × 10−5) |
p < 0.001 |
| Relative fold intensity for protein expression, median (range) | 1.20 (0.91–1.27) |
0.69 (0.44–0.92) |
p < 0.001 |
| Circulatory levels of GRP78 in serum samples | |||
| Concentration (ng/ml) Median (range) |
2.01 (0.42–11.44) |
0.98 (0.29–3.13) |
p < 0.001 |
Figure 2.
Western blot image showing GRP78 protein expression in tumor tissue and adjacent non-tumor tissue of RCC patients. The representative image includes the information of three different tumor tissues and their respective adjacent non-tumor tissues of the stage 1(Fuhrman grade 2), stage 2(Fuhrman grade 3) and stage 3(Fuhrman grade 3) of clear cell RCC patients. β-actin was used as an internal control for normalization. (Supplementary Material, Western blot of GRP78 expression in tumor and adjacent non tumor tissue of RCC patients.)
Figure 3.
Graph depicting relative fold intensity of GRP78 expression using Western blot assay in tumor tissue and adjacent non-tumor tissue of RCC patients. β-actin was used as an internal control for normalization. Mann Whitney Test was applied to determine significance. ∗∗∗p < 0.001.
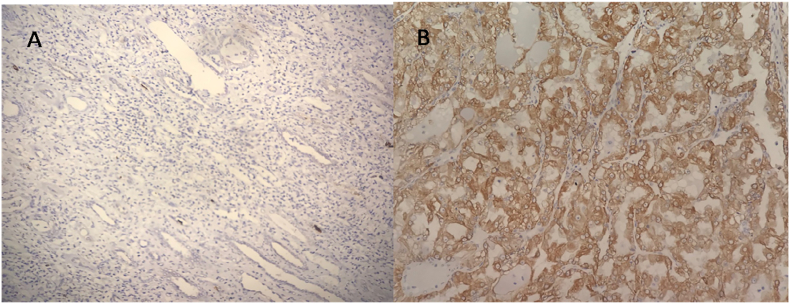
The tissue localization of GRP78 was also determined in study subjects using immunohistochemistry and the expression of GRP78 was found significantly elevated in tumor tissues as compared to normal adjacent non-tumor renal tissues (Figure 4).
Figure 4.
Representative images of GRP78 expression in adjacent non tumor tissue (A) and tumor tissue (B) of stage 3 (Fuhrman grade 3) of clear cell RCC patient studied using immunohistochemistry. All images were taken at 200 X magnification.
3.2. Expression of GRP78 in serum samples
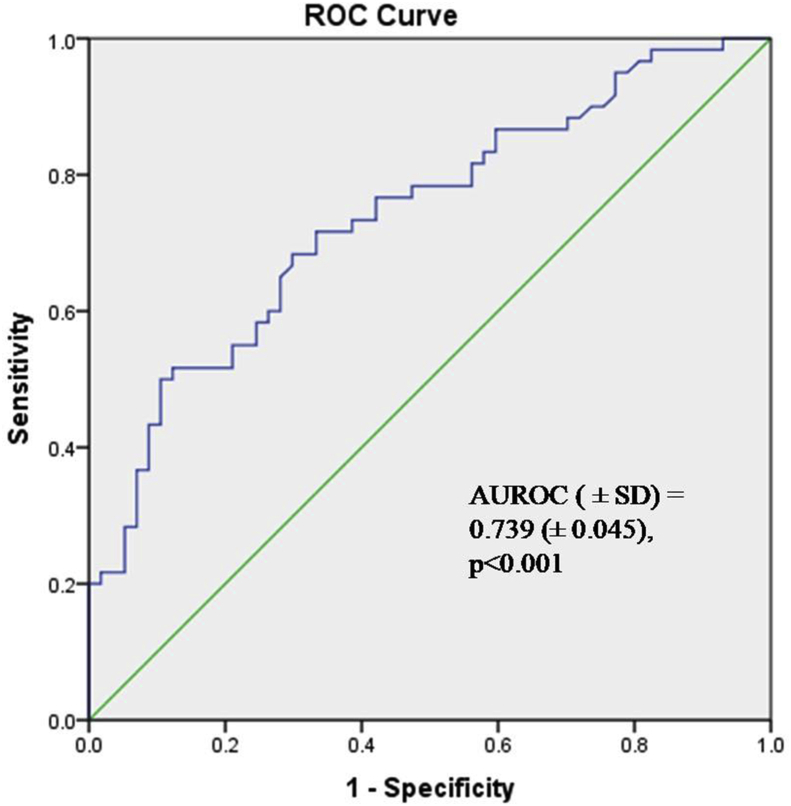
The median (range) circulatory levels of GRP78 in renal cell carcinoma patients was 2.007 (0.4153–11.44) ng/ml while the median (range) circulatory levels in control patients undergoing nephrectomy for benign diseases was 0.9757 (0.2873–3.133) ng/ml (p < 0.0001) (Table 3). Figure 5 demonstrates the median serum expression of GRP78 in cancer and control populations. Using ROC analysis, a cut-off of 1.203 ng/ml was able to differentiate between renal cell carcinoma and benign patients with a sensitivity and specificity of 71.7% and 66.7% respectively [AUC (±SD) = 0.739 (±0.045), p < 0.001].
Figure 5.
Receiver Operating curve of serum GRP78 expression to discriminate between the presence or absence of RCC.
3.3. Correlation of expression of serum GRP78 with stage, grade, and presence of metastasis
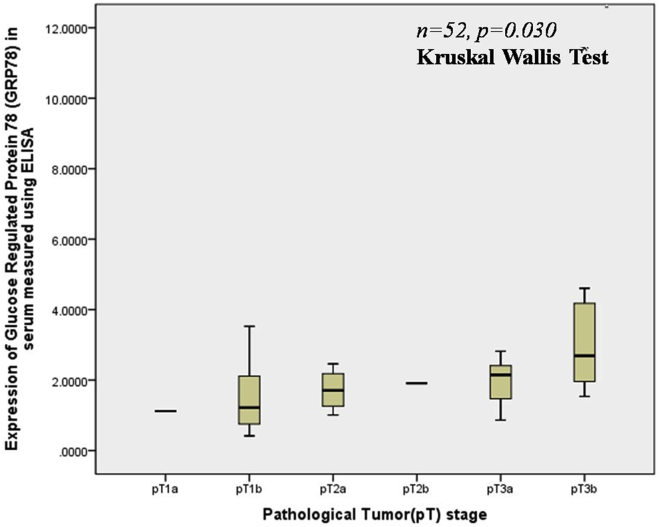
Table 3 and Figure 6 highlight the median expression of serum GRP78 in various tumor stages in non-metastatic RCC. The expression of serum GRP78 correlated significantly with the pathological tumor stage (p = 0.030). The upregulation of serum GRP78 also had a significant correlation with the presence of renal vein or inferior vena cava thrombus (p = 0.02).
Figure 6.
Box-whisker plot showing serum levels of GRP78 according to various pathological Tumor stage in RCC patients.
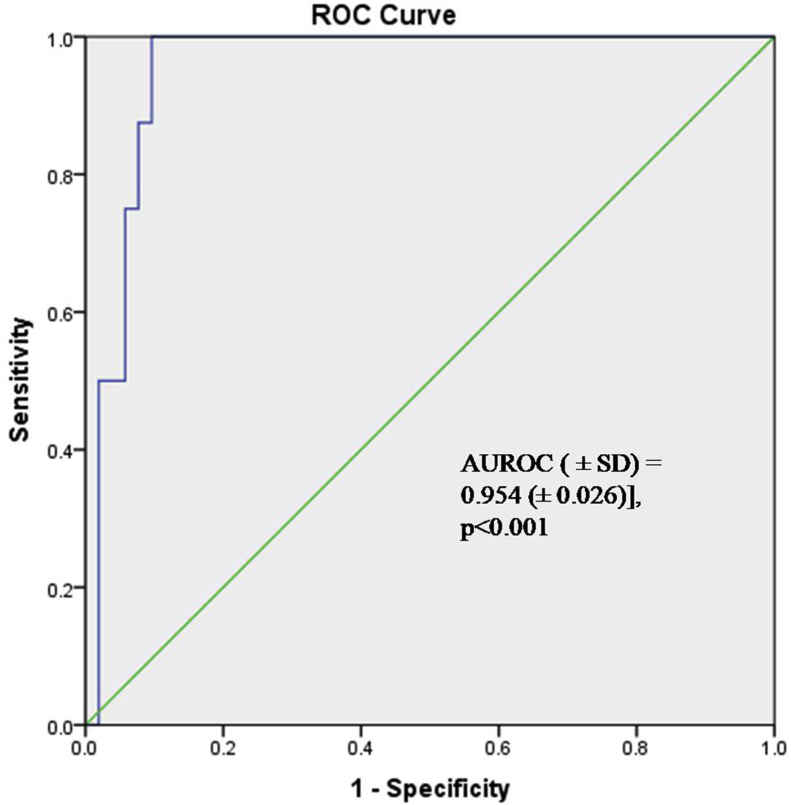
GRP78 expression was also significantly higher in patients with metastatic disease at presentation as compared to non-metastatic disease (p < 0.001). Using ROC analysis, a cut-off of 2.90 ng/ml could predict the presence or absence of metastasis with a sensitivity and specificity of 100% and 90.4% respectively [AUROC (±SD) = 0.954 (±0.026)] (Figure 7). Table 4 depicts the median serum expression of GRP78 with various pathological stages, grades, and metastasis.
Figure 7.
Receiver Operating curve of serum GRP78 expression to discriminate between the presence or absence of metastasis.
Table 4.
Comparative expression of serum Glucose Regulated Protein 78(GRP78) in patients with clear cell RCC (RCC). ∗Significant at p < 0.05
| Parameter | Number of patients, n(%) | Median Expression (IQR) of serum GRP78 ng/ml |
∗p-value |
|---|---|---|---|
| Renal vein and IVC involvement | 60 (100%) | p = 0.02 | |
| Present | 10 (16.7%) | 2.545 (1.997) | |
| Absent | 50 (83.3%) | 1.494 (1.651) | |
| Metastasis, n(%) | 60 (100%) | p = 0.001 | |
| Present | 8 (13.3%) | 4.822 (6.115) | |
| Absent | 52 (86.7%) | 1.525 (1.458) | |
| Pathological Tumor stage (excluding patients with metastases) | 52 (86.7%) | p = 0.030 | |
| pT1a | 1 (1.9%) | 1.120 | |
| pT1b | 30 (57.7%) | 1.218 (1.375) | |
| pT2a | 4 (7.7%) | 1.708 (1.186) | |
| pT2b | 1 (1.9%) | 1.909 | |
| pT3a | 9 (17.3%) | 2.146 (1.188) | |
| pT3b | 7 (13.5%) | 2.687 (2.691) | |
| Fuhrman Grade on histopathology | 60 (100%) | p < 0.001 | |
| Grade 1 | 8 (13.3%) | 1.256 (0.920) | |
| Grade 2 | 39 (65%) | 1.474 (1.359) | |
| Grade 3 | 8 (13.3%) | 3.974 (2.031) | |
| Grade 4 | 5 (8.4%) | 10.478 (4.904) | |
| Fuhrman Grade category on histopathology | 60 (100%) | p < 0.001 | |
| Low grade (Grade 1/Grade 2) | 47 (78.3%) | 1.468 (1.220) | |
| High grade (Grade 3/Grade 4) | 13 (21.7%) | 4.376 (6.252) |
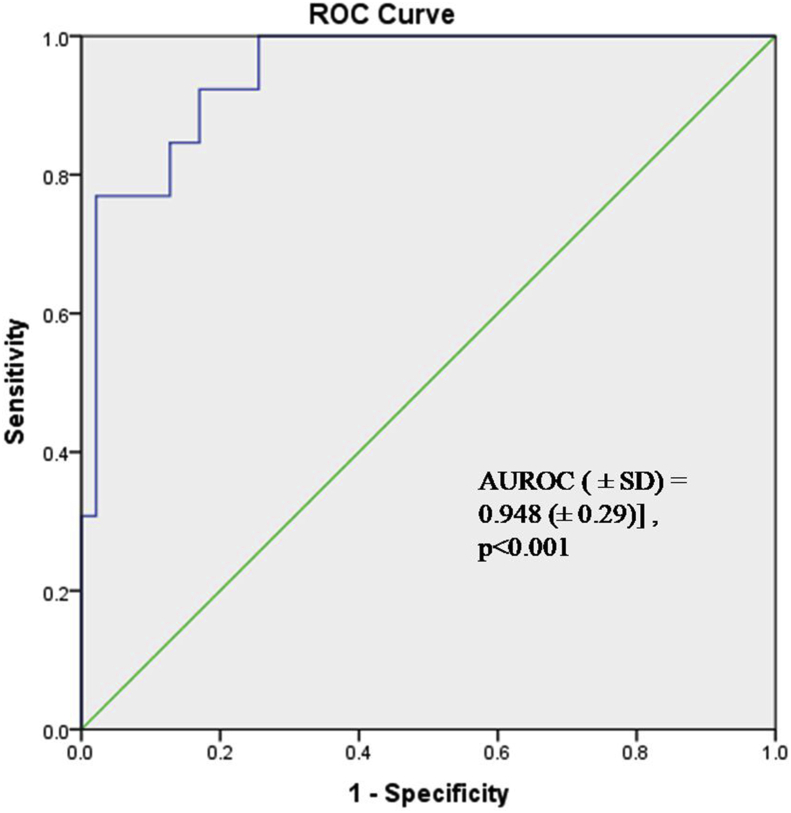
The median expression of serum GRP78 significantly increased with the grade in RCC (p < 0.001). The median (IQR) serum GRP 78 expression in high-grade disease (Grade 3 and Grade 4) was 4.376 (6.252) ng/ml compared to 1.468 (1.220)ng/ml in low-grade disease (Grade 1 and Grade 2). Using ROC analysis, a cut-off of 2.43 ng/ml could predict the high or low-grade disease with a sensitivity and specificity of 92% and 83% respectively [AUROC (±SD) = 0.948 (±0.29)] (Figure 8).
Figure 8.
Receiver Operating curve of serum GRP78 expression to discriminate between low grade (Grade 1/Grade 2) versus High Grade (Grade 3/Grade 4) disease in RCC patients.
4. Discussion
Our study demonstrated the potential role of GRP78 as a prognostic biomarker in the management of RCC. The significant upregulation of GRP78 in RCC studied both at the molecular level (using RT-PCR and Western blot assay) and tissue level (using immunohistochemical assay) as compared to controls (p < 0.001) highlights its potential role as a biomarker. This study is amongst the first to study the expression of serum GRP78 as a prognostic marker in RCC and its correlation with the stage, grade, and presence of metastasis. Using ROC analysis, the serum expression of GRP78 appeared to discriminate between high versus low-grade disease [AUROC (±SD) = 0.948 (±0.29)] and the presence or absence of metastasis [AUROC (±SD) = 0.954 (±0.026)].
GRP78 acts as the central regulator of ER-mediated response to stress. The upregulation of GRP78 has been reported in epithelial ovarian tumors, diffuse large B cell lymphomas, endometrial carcinoma, gastric carcinoma, and prostate cancer [10,13] while it is downregulated in esophageal and colorectal adenocarcinoma and oral squamous cell carcinoma [14, 15, 16]. Thus, its effect on the outcome of cancer might involve different signaling pathways and may be tissue-type specific [17]. There are three branches in the UPR signaling pathway, which are mediated by three ER stress sensors: Protein Kinase RNA like endoplasmic reticulum kinase (PERK), inositol requiring enzyme-1 (IRE1), and activating transcription factor 6(ATF 6). GRP 78 acts as a master regulator of all three sensors and keeps them in inactive form in the non-stressed situation. It has been demonstrated that elevated expression of GRP78 in various tumors leads to its translocation to the cancer cell surface and is released on activation in serum.
The role of GRP78 in RCC has been inadequately studied. Fu et al first proposed the potential role and clinical significance of GRP78 expression in RCC [6]. They demonstrated significant upregulation of GRP78 at molecular and tissue level using RT-PCR and immunohistochemistry in 42 patients undergoing radical nephrectomy for primary RCC (p < 0.001). They also reported significantly higher expression of GRP78 in higher stage (p < 0.001) and tumor size more than 7cm (p < 0.001). In another study, GRP78 expression was studied in 128 patients of localized RCC and 9 patients of metastatic RCC using immunohistochemical assays [11]. The authors found significant upregulation of GRP78 in RCC and the expression of GRP78 correlated significantly with tumor grade (p < 0.001), advanced pathological tumor stage (p = 0.0002), lymphovascular invasion (p < 0.001), nodal involvement (p = 0.008), and presence of metastases (p = 0.001). They also studied the disease-specific survival and progression-free survival and concluded GRP78 upregulation to be an independent predictor of shorter disease-specific and progression-free survival [12]. On the contrary, in a retrospective study, Shen et al studied the expression of GRP78 in 267 RCC patients undergoing radical nephrectomy using immunohistochemistry [12]. They showed no significant upregulation of GRP78 in renal tumor tissue as compared to adjacent non-neoplastic renal tissue and perinephric adipose tissue. Moreover, they concluded GRP78 as a poor prognostic marker of RCC.
This disparity could be explained by the basic methodologic differences in the approach to address tissue heterogeneity. Though tumor tissue is heterogeneous, the normal nephron also possesses greater tissue heterogeneity due to the presence of glomeruli rich in erythrocytes and tubular lumen of varying sizes. The tubules remain unstained on immunohistochemistry and glomeruli stain weakly as erythrocytes lack ER. Moreover, quantification of chromogen-stained intensity using immunohistochemistry has inherent limitations [18]. Our study also demonstrated similar results with significant upregulation of GRP78 in RCC and its correlation with grade and stage of the disease. However, due to the short duration of the study and limited follow-up, disease-specific survival and progression-free survival were not studied. Moreover, no previous study has reported the role of serum GRP78, measured using ELISA, as a prognostic biomarker for RCC.
Apart from the role of GRP78 as a prognostic marker of disease, it may also play a role in therapeutics. Lin et al studied 78 patients of clear cell RCC and demonstrated significant upregulation of GRP78 expression in both Von-Hippel Lindau (VHL) intact and VHL–null cell lines of RCC. They also demonstrated inhibition of GRP78 using siRNA (small interfering RNA) significantly suppressed the RCC growth and colony formation by inducing G1cell cycle arrest [19]. Wang et al also studied the role of tumor suppressor gene, microRNA-30a-Sp, in RCC and reported GRP78 as a direct target gene of miR-30a-Sp. The knock-down of GRP78 significantly correlated with tumor cell apoptosis and decreased tumor proliferation [20]. Han et al assessed the role of GRP78 in tumor cell proliferation and survival under stress conditions [4]. They observed the upregulation of ER-induced stress response by conventional anti-angiogenic therapy and induction of GRP78 appeared to protect the renal tumor cells from apoptosis via PERK/IF2α signaling. The knockdown of GRP78 expression in tumor cells suppressed tumor proliferation by inducing apoptosis. These studies highlight the potential role of anti-GRP78 therapy in patients with RCC and its potential in patients not responding to conventional anti-angiogenic therapy.
Thus, the GRP78 appears to be both a prognostic and therapeutic marker of RCC. This study has several strengths. The molecular expression (studied using both RT-PCR and Western blot assay) and tissue expression (studied using immunohistochemical assay) of GRP78 in tumor tissue specimens was compared to normal adjacent non-tumor tissue. Moreover, the pathologist and the biochemist were blinded to the demographic details, stage, and grade of the disease. This study is the first to report the role of serum GRP78 and its upregulation in RCC as compared to control serum samples of patients undergoing nephrectomy for benign renal disease. Moreover, we were able to propose the possible cut-off for serum expression of GRP78 to discriminate between a high or low grade of disease and the presence or absence of metastasis.
However, this study has a few limitations. The small sample size comes with the inherent bias. The study did not include follow-up of patients owing to the short duration of the study period and hence, the expression of GRP78 was not studied in correlation with tumor recurrence or progression-free survival. The study did not include control cell lines of RCC and the potential effect of GRP78 silencing on tumor proliferation and survival.
5. Conclusion
GRP78 expression is significantly upregulated in RCC, both in serum and at tissue levels as compared to controls. Serum GRP78 expression appeared to be a significant prognostic marker of RCC with a significant correlation with the stage of the disease, the grade of disease, and presence or absence of metastasis. Future studies assessing the role of GRP78 not only in prognostics but also in therapeutics, particularly in patients not responding to conventional anti-angiogenic therapy, are required.
Declarations
Author contribution statement
Manoj Kumar: Conceived and designed the experiments; Analyzed and interpreted the data; Contributed reagents, materials, analysis tools or data; Wrote the paper.
Harshit Garg: Analyzed and interpreted the data; Wrote the paper.
Nidhi Gupta: Conceived and designed the experiments; Performed the experiments; Wrote the paper.
Alpana Sharma: Conceived and designed the experiments; Analyzed and interpreted the data; Contributed reagents, materials, analysis tools or data.
Seema Kaushal: Conceived and designed the experiments; Performed the experiments.
Rajeev Kumar, Amit Kumar Dinda: Analyzed and interpreted the data; Contributed reagents, materials, analysis tools or data.
Funding statement
This work was supported by AIIMS Institute Research Grant (Code: A-518).
Data availability statement
Data associated with this study has been deposited at CTRI under the clinical trial registration number 2018/01/011291.
Declaration of interests statement
The authors declare no conflict of interest.
Additional information
No additional information is available for this paper.
Appendix A. Supplementary data
The following is the supplementary data related to this article:
Supplementary Material_beta-actin_8bit.
Supplementary material_Grp78_8bit.
References
- 1.Cohen H.T., McGovern F.J. Renal-cell carcinoma. N. Engl. J. Med. 2005 Dec 8;353(23):2477–2490. doi: 10.1056/NEJMra043172. [DOI] [PubMed] [Google Scholar]
- 2.Linehan W.M., Vasselli J., Srinivasan R., Walther M.M., Merino M., Choyke P. Genetic basis of cancer of the kidney: disease-specific approaches to therapy. Clin. Cancer Res. Off. J. Am. Assoc. Cancer Res. 2004 Sep 15;10(18 Pt 2):6282S–6289S. doi: 10.1158/1078-0432.CCR-050013. [DOI] [PubMed] [Google Scholar]
- 3.Motzer R.J., Russo P. Systemic therapy for RCC. J. Urol. 2000 Feb 1;163(2):408–417. [PubMed] [Google Scholar]
- 4.Han K.S., Li N., Raven P.A., Fazli L., Frees S., Ettinger S. Inhibition of endoplasmic reticulum chaperone protein glucose-regulated protein 78 potentiates anti-angiogenic therapy in RCC through inactivation of the PERK/eIF2α pathway. Oncotarget. 2015 Oct 27;6(33):34818–34830. doi: 10.18632/oncotarget.5397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Schröder M., Kaufman R.J. The mammalian unfolded protein response. Annu. Rev. Biochem. 2005;74:739–789. doi: 10.1146/annurev.biochem.73.011303.074134. [DOI] [PubMed] [Google Scholar]
- 6.Fu W., Wu X., Li J., Mo Z., Yang Z., Huang W. Upregulation of GRP78 in RCC and its significance. Urology. 2010 Mar 1;75(3):603–607. doi: 10.1016/j.urology.2009.05.007. [DOI] [PubMed] [Google Scholar]
- 7.Banhegyi G., Baumeister P., Benedetti A., Dong D., Fu Y., Lee A.S. Endoplasmic reticulum stress. Ann. N. Y. Acad. Sci. 2007 May 18;1113(1):58–71. doi: 10.1196/annals.1391.007. [DOI] [PubMed] [Google Scholar]
- 8.Li Z., Li Z. Glucose regulated protein 78: a critical link between tumor microenvironment and cancer hallmarks. Biochim. Biophys. Acta BBA - Rev. Cancer. 2012 Aug;1826(1):13–22. doi: 10.1016/j.bbcan.2012.02.001. [DOI] [PubMed] [Google Scholar]
- 9.Zhang Y., Tseng C.-C., Tsai Y.-L., Fu X., Schiff R., Lee A.S. Cancer cells resistant to therapy promote cell surface relocalization of GRP78 which complexes with PI3K and enhances PI(3,4,5)P3 production. Pizzo SV. PloS One. 2013 Nov 11;8(11) doi: 10.1371/journal.pone.0080071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Mozos A., Roué G., López-Guillermo A., Jares P., Campo E., Colomer D. The expression of the endoplasmic reticulum stress sensor BiP/GRP78 predicts response to chemotherapy and determines the efficacy of proteasome inhibitors in diffuse large B-cell lymphoma. Am. J. Pathol. 2011 Nov;179(5):2601–2610. doi: 10.1016/j.ajpath.2011.07.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Kuroda K., Horiguchi A., Asano T., Ito K., Asakuma J., Sato A. Glucose-regulated protein 78 positivity as a predictor of poor survival in patients with RCC. Urol. Int. 2011;87(4):450–456. doi: 10.1159/000330883. [DOI] [PubMed] [Google Scholar]
- 12.Shen K., Vesey D.A., Ellis R.J., Del Vecchio S.J., Cho Y., Teixeira-Pinto A. GRP78 expression in tumor and perinephric adipose tissue is not an optimal risk stratification marker for clear cell RCC. PloS One. 2019 Jan 17;14(1) doi: 10.1371/journal.pone.0210246. https://www.ncbi.nlm.nih.gov/pmc/articles/PMC6336240/ [Internet] [cited 2019 Jul 27]. Available from: [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Wu C.-T., Wang W.-C., Chen M.-F., Su H.-Y., Chen W.-Y., Wu C.-H. Glucose-regulated protein 78 mediates hormone-independent prostate cancer progression and metastasis through maspin and COX-2 expression. Tumour Biol. J. Int. Soc. Oncodevelopmental Biol. Med. 2014 Jan;35(1):195–204. doi: 10.1007/s13277-013-1024-4. [DOI] [PubMed] [Google Scholar]
- 14.Thornton M., Aslam M.A., Tweedle E.M., Ang C., Campbell F., Jackson R. The unfolded protein response regulator GRP78 is a novel predictive biomarker in colorectal cancer. Int. J. Cancer. 2013 Sep 15;133(6):1408–1418. doi: 10.1002/ijc.28137. [DOI] [PubMed] [Google Scholar]
- 15.Huang T.-T., Chen J.Y.-F., Tseng C.-E., Su Y.-C., Ho H.-C., Lee M.-S. Decreased GRP78 protein expression is a potential prognostic marker of oral squamous cell carcinoma in Taiwan. J. Formos. Med. Assoc. 2010 May;109(5):326–337. doi: 10.1016/S0929-6646(10)60060-5. [DOI] [PubMed] [Google Scholar]
- 16.Langer R., Feith M., Siewert J.R., Wester H.-J., Hoefler H. Expression and clinical significance of glucose regulated proteins GRP78 (BiP) and GRP94 (GP96) in human adenocarcinomas of the esophagus. BMC Cancer. 2008 Mar 10;8:70. doi: 10.1186/1471-2407-8-70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Luo B., Lee A.S. The critical roles of endoplasmic reticulum chaperones and unfolded protein response in tumorigenesis and anticancer therapies. Oncogene. 2013 Feb;32(7):805–818. doi: 10.1038/onc.2012.130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Samali A., FitzGerald U., Deegan S., Gupta S. Methods for monitoring endoplasmic reticulum stress and the unfolded protein response. Int. J. Cell Biol. 2010 doi: 10.1155/2010/830307. https://www.hindawi.com/journals/ijcb/2010/830307/ [Internet] [cited 2019 Jul 28]. Available from: [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Lin J.-A., Fang S.-U., Su C.-L., Hsiao C.-J., Chang C.-C., Lin Y.-F. Silencing glucose-regulated protein 78 induced RCC cell line G1 cell-cycle arrest and resistance to conventional chemotherapy. Urol. Oncol. 2014 Jan;32(1):29.e1–29.e11. doi: 10.1016/j.urolonc.2012.10.006. [DOI] [PubMed] [Google Scholar]
- 20.Wang C., Cai L., Liu J., Wang G., Li H., Wang X. MicroRNA-30a-5p inhibits the growth of RCC by modulating GRP78 expression. Cell. Physiol. Biochem. 2017;43(6):2405–2419. doi: 10.1159/000484394. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Data associated with this study has been deposited at CTRI under the clinical trial registration number 2018/01/011291.