Abstract
New Findings
-
What is the central question of this study?
We sought to investigate whether carotid stiffness, carotid intima–media thickness and the aortic augmentation index are altered in young adults 3–4 weeks after contraction of severe acute respiratory syndrome coronavirus 2 (SARS‐CoV‐2) compared with young healthy adults.
-
What is the main finding and its importance?
We found that carotid stiffness, Young's modulus and the aortic augmentation index were greater in young adults who tested positive for SARS‐CoV‐2 compared with healthy young adults. These findings provide additional evidence for detrimental effects of SARS‐CoV‐2 on young adult vasculature, which might have implications for cardiovascular health.
Abstract
Contracting severe acute respiratory syndrome coronavirus 2 (SARS‐CoV‐2) has been observed to cause decrements in vascular function of young adults. However, less is known about the impact of SARS‐CoV‐2 on arterial stiffness and structure, which might have additional implications for cardiovascular health. The purpose of this study was to assess the carotid artery stiffness and structure using ultrasound and the aortic augmentation index (AIx) using applanation tonometry in young adults after they tested positive for SARS‐CoV‐2. We hypothesized that carotid artery stiffness, carotid intima–media thickness (cIMT) and aortic AIx would be elevated in young adults with SARS‐CoV‐2 compared with healthy young adults. We evaluated 15 young adults (six male and nine female; 20 ± 1 years of age; body mass index, 24 ± 3 kg m−2) 3–4 weeks after a positive SARS‐CoV‐2 test result compared with young healthy adults (five male and 10 female; 23 ± 1 years of age; body mass index, 22 ± 2 kg m−2) who were evaluated before the coronavirus 2019 pandemic. Carotid stiffness, Young's modulus and cIMT were assessed using ultrasound, whereas aortic AIx and aortic AIx standardized to 75 beats min−1 (AIx@HR75) were assessed from carotid pulse wave analysis using SphygmoCor. Group differences were observed for carotid stiffness (control, 5 ± 1 m s−1; SARS‐CoV‐2, 6 ± 1 m s−1), Young's modulus (control, 396 ± 120 kPa; SARS‐CoV‐2, 576 ± 224 kPa), aortic AIx (control, 3 ± 13%; SARS‐CoV‐2, 13 ± 9%) and aortic AIx@HR75 (control, −3 ± 16%; SARS‐CoV‐2, 10 ± 7%; P < 0.05). However, cIMT was similar between groups (control, 0.42 ± 0.06 mm; SARS‐CoV‐2, 0.44 ± 0.08 mm; P > 0.05). This cross‐sectional analysis revealed higher carotid artery stiffness and aortic stiffness among young adults with SARS‐CoV‐2. These results provide further evidence of cardiovascular impairments among young adults recovering from SARS‐CoV‐2 infection, which should be considered for cardiovascular complications associated with SARS‐CoV‐2.
Keywords: aortic augmentation index, carotid stiffness, COVID‐19, intima–media thickness, SARS‐CoV‐2
1. INTRODUCTION
In March of 2020, transmission of the severe acute respiratory syndrome coronavirus 2 (SARS‐CoV‐2) was declared a pandemic by the World Health Organization. Given that SARS‐CoV‐2 initially attacks cells within the nasal cavity, upper and conducting airways and the pulmonary parenchyma, clinical presentations include mild symptoms akin to an upper respiratory tract infection, pneumonia and/or acute respiratory distress syndrome (Pirzada et al., 2020). However, consequences are not limited only to the respiratory system. In fact, decrements abound in the downstream physiological systems, including the nervous system, kidneys and the vasculature (Clerkin et al., 2020; Montalvan et al., 2020; Ratchford et al., 2020).
SARS‐CoV‐2 binds to the angiotensin‐converting enzyme 2 (ACE2) receptor, downregulating ACE2 expression and prohibiting the protective effects of the enzyme within several tissues, including the heart and blood vessels (Monteil et al., 2020). SARS‐CoV‐2 infection can, therefore, lead to damage of the myocardium and vascular endothelium (Evans et al., 2020; Pirzada et al., 2020; Siripanthong et al., 2020; Tadic et al., 2020). In addition to ACE2‐related impairments in the vascular endothelium, the systemic cytokine‐induced inflammatory response (Channappanavar & Perlman, 2017; Coperchini et al., 2020) caused by viral detection and propagation could also have further prolonged, deleterious, effects downstream of the initial viral parasitism of SARS‐CoV‐2 in the lung (Li, Hao et al., 2020). The combination of ACE2 inhibition along with heightened inflammation and oxidative stress can evoke alterations to vascular function, and potential structural alterations might be seen in the form of arterial stiffness. This acute inflammatory response can cause alterations to carotid stiffness and carotid intima–media thickness (cIMT), akin to those typically observed after acute bacterial and viral infections (Çiftel et al., 2014; Ibrahim et al., 2016; Wu et al., 2014), in addition to vaccination treatments (Vlachopoulos et al., 2005) and various inflammatory states (Roman et al., 2005; Zanoli, Boutouyrie et al., 2017; Zanoli, Granata et al., 2017; Zanoli et al., 2020). These observational studies from acute infectious agents highlight the potential for cIMT and other structural indices throughout the arterial tree to change acutely among individuals experiencing heightened infection‐related inflammation. Indeed, several infectious agents, including Chlamydia pneumoniae, Porphyromonas gingivalis, Helicobacter pylori, cytomegalovirus, Epstein–Barr virus, human immunodeficiency virus, herpes simplex virus‐1 and hepatitis C virus, have been implicated in promoting endothelial cell inflammation and macrophage formation and inhibiting smooth muscle cell apoptosis (Li, Xia et al., 2020). Together, these mechanisms for vascular alterations have potential implications for vascular health, progression of atherosclerosis, and cardiovascular event risk (Tobayama et al., 2013), which might be impacted by SARS‐CoV‐2.
Our group has previously provided evidence of vascular dysfunction after SARS‐CoV‐2 infection, as evidenced by lower brachial artery flow‐mediated dilatation and lower hyperaemic response to passive limb movement, in addition to an elevated peripheral carotid–femoral pulse wave velocity among young adults with SARS‐CoV‐2 compared with healthy control subjects (Ratchford et al., 2020). Likewise, a recent study using the rudimentary measure of pulse pressure as an arterial stiffness index has revealed an association between pulse pressure and all‐cause mortality in patients with SARS‐CoV‐2 (Rodilla et al., 2021). These data suggest an impairment in vascular regulation, which might predispose individuals to a greater risk of cardiovascular disorders, such as myocardial injury, arrhythmias, acute coronary syndrome or thromboembolism (Driggin et al., 2020). Indeed, cardiovascular symptoms can manifest without typical flu‐like symptoms of SARS‐CoV‐2 infection (Deng et al., 2020; Stefanini et al., 2020), with myocardial injury independently being associated with high mortality during the course of the disease (Shi et al., 2020). Although cardiovascular disease ranks among the top comorbid conditions associated with SARS‐CoV‐2‐related morbidity (Wu & McGoogan, 2020), little is known regarding how SARS‐CoV‐2 might alter other arterial factors associated with premature atherosclerosis, a heightened cardiovascular event risk and all‐cause mortality (Vlachopoulos et al., 2010), specifically carotid artery stiffness, carotid distensibility, cIMT and aortic augmentation index (AIx), which typically reflect changes to arterial structure compared with previously observed vascular alterations.
Therefore, the primary aim of this investigation was to examine whether carotid artery stiffness, carotid distensibility, cIMT and aortic AIx are altered in young adults after contraction of SARS‐CoV‐2 while using a cross‐sectional study design for comparison with young healthy adults. We hypothesized that carotid stiffness indices would be greater in the SARS‐CoV‐2 group, as reflected by changes in stiffness, distensibility, compliance and Young's modulus (Butlin & Qasem, 2017; Kim & Kim, 2019). We also hypothesized that cIMT would be greater in the SARS‐CoV‐2 group, providing a preclinical index of atherogenesis. Furthermore, we hypothesized greater indices of aortic augmentation in the SARS‐CoV‐2 group, indicated by the central haemodynamic measures derived from carotid pulse wave analysis (PWA), including aortic AIx and aortic AIx standardized for a heart rate for 75 beats min−1 (AIx@HR75). As a secondary aim, we sought to identify whether indices for myocardial oxygen demand and perfusion were altered in young adults after contraction of SARS‐CoV‐2 compared with young healthy adults. We hypothesized the SARS‐CoV‐2 group would have a greater myocardial stress response, derived from PWA, as indicated by the diastolic to systolic pressure–time integral as indices of myocardial oxygen demand and perfusion, further suggesting that these vascular alterations might have implications for overall cardiovascular health.
2. METHODS
2.1. Ethical approval
All procedures were approved by the Appalachian State University Institutional Review Board (IRB_20‐0304 and IRB_20‐0151). The study conformed to the standards set by the Declaration of Helsinki, except for registration in a database. Before testing, experimental procedures were explained to participants, both in writing and verbally, and subjects provided written informed consent.
2.2. Subjects
The inclusion criteria for subjects included relatively healthy young adults free from chronic metabolic, cardiovascular and pulmonary disease. Female subjects were premenopausal and not pregnant or trying to become pregnant. Potential subjects were excluded if they were smokers, had any orthopaedic limitations and were taking any medications known to alter vascular function, such as ACE inhibitors or diuretics. Potential subjects were also excluded if they were hospitalized owing to the virus. On 4–6 February 2020, which was before the first confirmed case of coronavirus disease 2019 (COVID‐19) in North Carolina, USA on 3 March 2020 and before the World Health Organization declared COVID‐19 a pandemic (NC Department of Health and Human Services, 2020), subjects in the control group were studied. Control subjects did not report any flu‐like symptoms. Subjects included in the SARS‐CoV‐2 group tested positive for SARS‐CoV‐2 using a nasopharyngeal swab PCR assay 3–4 weeks before study testing.
2.3. Study procedures
Before testing, subjects completed a health history questionnaire, detailing personal and family medical history, the frequency and duration of physical activity and any medication usage. Subjects arrived for testing in a fasted state having abstained from food and caffeine for 12 h and from exercise and alcohol for 24 h before testing. All study procedures were performed in a quiet, thermoneutral environment after subjects had lain supine for 20 min. For standardization purposes, study procedures were ordered in a similar manner between subjects.
2.4. COVID‐19 symptom severity survey
Subjects who tested positive for SARS‐CoV‐2 were asked to rank their COVID‐19 symptoms on the day of study testing. On a scale of 0–100 of increasing severity, subjects ranked their symptoms of chest pain, chills, diarrhoea, dizziness or vertigo, dry cough, dry eyes, dry mouth, fatigue, fever >37.9°C, headache, lack of appetite, loss of smell or taste (anosmia), muscle or body aches, nasal congestion or runny nose, nausea or vomiting, shortness of breath, difficulty breathing, dyspnoea, sore joints or sore throat. The values for all symptoms were totalled and averaged for total symptom severity. Mild symptoms were categorized as a symptom severity score of 0–33, moderate 34–66 and severe 67–100.
2.5. Experimental measurements
After 20 min of supine rest, an automated brachial artery cuff was used to determine supine blood pressures (EVOLV, Omron Healthcare, Kyoto, Japan) before carotid stiffness, cIMT and PWA measurements. All measurements were taken on the right side of the body among all subjects.
2.6. Carotid stiffness
Subsequent to supine blood pressure measurements, subjects remained supine for assessment of carotid stiffness using an ultrasound system (12L‐RS Linear Array Probe, GE Logiq eR7; GE Medical Systems, Milwaukee, WI, USA) over the common carotid artery, ≥1–2 cm proximal to the bifurcation, with an imaging frequency between 4 and 12 MHz in B‐mode. Between 10 and 15 cardiac cycles were recorded and analysed by a single operator with carotid studio (Cardiovascular Suite, Quipu srl, Pisa, Italy) to obtain measurements for stiffness using the Bramwell–Hill equation, distensibility, compliance and Young's modulus (Bruno et al., 2017), where D s is the external systolic diameter, D i is the internal diastolic diameter, and D d is the external diastolic diameter between the media–adventitia interfaces. Systolic and diastolic diameters were determined as the largest and smallest diameters, respectively, obtained during real‐time playback of the ultrasound recording. The D i is the internal diastolic diameter between the lumen–intima interfaces, P s is the brachial systolic pressure, P d is the brachial diastolic pressure, and ρ is the blood density constant of 1.05 g cm−3:
Carotid stiffness is considered an important index positively associated with the pathogenesis of cardiovascular disease. Carotid distensibility measures the carotid artery elasticity, which is negatively associated with cardiovascular disease (Godia et al., 2007). Carotid compliance is the reciprocal of stiffness and is negatively associated with cardiovascular disease (Van Merode et al., 1990). Young's modulus differs from compliance and distensibility in that the wall thickness is incorporated for its determination, thus providing a more integrated assessment of arterial circumferential stretching, which has been positively associated with age‐related atherosclerosis (Riley et al., 1992).
2.7. Carotid diameters and intima–media thickness
Using the same 10–15 cardiac cycles used to measure carotid stiffness, the systolic, diastolic and mean carotid diameters and cIMT were measured and analysed using carotid studio. Systolic, diastolic and mean carotid diameters were determined as the largest, smallest and average diameters obtained, respectively, of the interior, luminal diameters during each cardiac cycle measured from the ultrasound recordings. The cIMT was measured as the distance from the lumen–intima interface to the media–adventitia interface using the deep wall of the carotid artery to calculate the mean cIMT over the recorded 10–15 cardiac cycles. Once the media–adventitia borders were validated by the operator, the lumen–intima boundaries were detected automatically by the software and adjusted by the operator as necessary.
2.8. Carotid pulse wave analysis
A SphygmoCor CPv (AtCor Medical, Sydney, Australia) device was used to record carotid PWA via applanation tonometry. The probe was positioned over the common carotid artery. After 20 sequential waveforms were recorded, the intrinsic transfer function of the SphygmoCor generated average peripheral waveforms (Wilkinson et al., 1998). From these waveforms, central haemodynamic parameters were estimated using the general transfer function, including the aortic augmentation index (AIx), aortic augmentation pressure (AP), central pressures (systolic, diastolic, pulse and mean arterial blood pressures), heart rate period, duration of the waveform to reach peak pressure (aortic T2), pressure obtained at the first peak/shoulder (P1 height), end‐systolic pressure, mean systolic pressure and mean diastolic pressure. Systolic blood pressure was identified as the height of the first peak in the waveform, whereas diastolic blood pressure was identified as the value at the foot of the waveform. End‐systolic pressure was identified as the pressure at the end of the first peak in the waveform. The pulse pressure was calculated as the difference between systolic and diastolic pressures. Mean systolic pressure was defined as the average pressure between the beginning of the waveform and the end of the first peak, whereas mean diastolic pressure was defined as the average pressure from the end of the first peak to the end of the waveform. The AIx was defined as augmentation pressure relative to pulse pressure expressed as a percentage, where aortic AP was calculated as the difference between the first and second systolic pressure peaks of the waveform (Wilkinson et al., 2000, 2002). To standardize AIx for a heart rate of 75 beats min−1, the following equation was used:
2.9. Indices of myocardial oxygen demand and perfusion
During the PWA, the Buckberg subendocardial viability ratio (SERV) was determined as the ratio between diastolic pressure–time index (DPTI) and the systolic pressure–time index (SPTI), defined as the areas under the pulse pressure during diastole and systole (Scandale et al., 2018). Ejection duration was measured as the period of time (in milliseconds) from the beginning of the waveform to the incisura (Wilkinson et al., 2000).
2.10. Statistical analysis
Statistics were performed using commercially available software (IBM SPSS Statistics v.26, Armonk, NY, USA). Student's two‐tailed t‐tests for two samples of equal variance were performed between the control and SARS‐CoV‐2 groups. Data were checked for normality using the Shapiro–Wilk test and boxplots. Statistical significance was specified at P < 0.05. Hedge's g was measured as an indicator of effect size, because a value of one indicates that the two groups differ by one standard deviation, or a value of two indicates that the groups differ by two standard deviations. Subject characteristics and outcome measures are expressed as the mean ± SD.
3. RESULTS
3.1. Subject characteristics
The control group consisted of five male and 10 female adults, whereas the experimental group consisted of six male and nine female adults who tested positive for SARS‐CoV‐2. Subject characteristics are detailed in Table 1. In general, subjects were well matched for height, weight, physical activity and contraceptive use. Both groups were predominately Caucasian, except for one female subject in the control group whose ethnicity was Hispanic, Latino and one female subject in the SARS‐CoV‐2 group who was Asian. Subjects were otherwise free from medication usage. Although one female subject with SARS‐CoV‐2 was asymptomatic, subjects who tested positive for SARS‐CoV‐2 were tested 25 ± 5 days after symptom onset (n = 6 male and 8 female) and 24 ± 6 days after a positive SARS‐CoV‐2 PCR test result (n = 6 male and 9 female).
TABLE 1.
Subject characteristics
| Characteristics | Control (5 male, 10 female) | SARS‐CoV‐2 (6 male, 9 female) | P‐value | Hedge's g |
|---|---|---|---|---|
| Subjects (n) | 15 | 15 | – | – |
| Age (years) | 23 ± 1 | 20 ± 1 | <0.01 | 2.01 |
| Height (cm) | 168 ± 10 | 172 ± 11 | 0.48 | −0.38 |
| Weight (kg) | 63 ± 7 | 71 ± 13 | 0.11 | −0.71 |
| Body mass index (kg m−2) | 22 ± 2 | 24 ± 3 | 0.23 | −0.51 |
| Physical activity | ||||
| Frequency (days week−1) | 4 ± 2 | 4 ± 1 | 0.72 | 0.12 |
| Duration (min day−1) | 44 ± 15 | 41 ± 12 | 0.77 | 0.25 |
| Female contraceptive use (n) | 4 | 5 | 0.44 | −0.54 |
Data are the mean ± SD. Student's two‐tailed t‐tests for two samples of equal variance were performed between control and severe acute respiratory syndrome coronavirus 2 (SARS‐CoV‐2) groups.
3.2. SARS‐CoV‐2 symptom severity
Among the subjects who tested positive for SARS‐CoV‐2, 14 subjects (6 male and 8 female) had mild symptoms at the time of testing (Figure 1). Seven subjects had fatigue; six subjects had a loss of smell, nasal congestion or shortness of breath; four subjects had dry cough or dry mouth; three subjects had dizziness or vertigo; and two subjects had dry eyes, a headache, muscle or body aches or a sore throat. Overall, subjects had 3.2 ± 2.1 symptoms among the 18 symptoms surveyed, and the severity of these symptoms averaged 19 ± 13 out of 100 of increasing severity.
FIGURE 1.
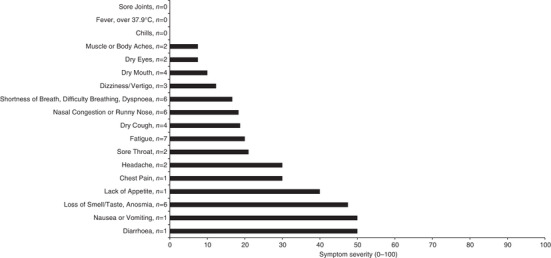
Severity of coronavirus disease 2019 symptoms. Subjects with severe acute respiratory syndrome coronavirus 2 (SARS‐CoV‐2) self‐reported symptom severity on a scale of 0–100, with increasing severity, on the day of testing (n = 6 male and 8 female). One female subject with SARS‐CoV‐2 was asymptomatic and excluded from the figure. Data are the mean among individuals with SARS‐CoV‐2 symptoms
3.3. Carotid stiffness
Carotid stiffness was greater in the SARS‐CoV‐2 group (6 ± 1 m s−1) compared with the control group (5 ± 1 m s−1; P = 0.02, Hedge's g = −1.08; Figure 2a). Carotid distensibility was lower in the SARS‐CoV‐2 group (30 × 10−3 ± 12 × 10−3 kPa−1) compared with the control group (40 × 10−3 ± 11 × 10−3 kPa−1; P = 0.01, Hedge's g = 0.97; Figure 2b). Carotid compliance was lower in the SARS‐CoV‐2 group (0.97 ± 0.33 mm2 kPa−1) compared with the control group (1.20 ± 0.31 mm2 kPa−1; P = 0.03, Hedge's g = 0.80; Figure 2c). Young's modulus of elasticity in the carotid artery was greater in the SARS‐CoV‐2 group (0.59 ± 0.24 kPa) compared with the control group (0.40 ± 0.12 kPa; P = 0.01, Hedge's g = −0.98; Figure 2d).
FIGURE 2.
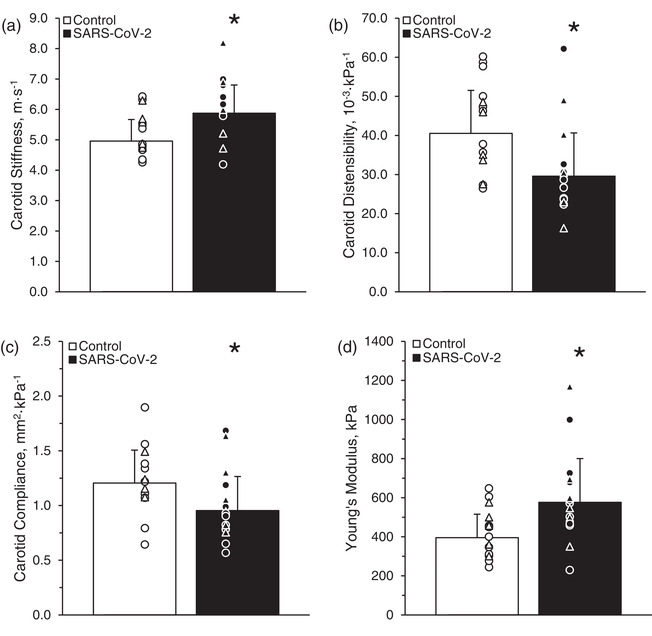
Carotid stiffness (a), distensibility (b), compliance (c) and carotid Young's modulus of elasticity (d) in control subjects and subjects who tested positive for severe acute respiratory syndrome coronavirus 2 (SARS‐CoV‐2). Student's two‐tailed t‐tests for two samples of equal variance were performed between the control (n = 5 male and 10 female) and SARS‐CoV‐2 (n = 6 male and 9 female) groups. * P < 0.05, between groups. Individual data are presented as open triangles (male subjects) and circles (female subjects) for the control group and filled triangles and circles for the SARS‐CoV‐2 group. Data are the mean ± SD
3.4. Carotid diameters and intima–media thickness
Measurements of carotid diameters and cIMT are detailed in Table 2. Generally, carotid diameters and cIMT were unaltered among adults with SARS‐CoV‐2 compared with healthy adults, although carotid systolic diameter was greater among adults with SARS‐CoV‐2 compared with healthy adults (P = 0.049).
TABLE 2.
Carotid diameters and intima–media thickness
| Control | SARS‐CoV‐2 | |||
|---|---|---|---|---|
| Parameter | (5 male, 10 female) | (6 male, 9 female) | P‐value | Hedge's g |
| Mean diameter (mm) | 6.48 ± 0.49 | 6.78 ± 0.57 | 0.13 | −0.55 |
| Diastolic diameter (mm) | 6.31 ± 0.53 | 6.46 ± 0.55 | 0.45 | −0.27 |
| Systolic diameter (mm) | 6.64 ± 0.57 | 7.07 ± 0.59 | <0.05 | −0.73 |
| cIMT (mm) | 0.42 ± 0.06 | 0.44 ± 0.08 | 0.59 | −0.19 |
Data are the mean ± SD. Student's two‐tailed t‐tests for two samples of equal variance were performed between the control and SARS‐CoV‐2 groups. Abbreviations: cIMT, carotid intima–media thickness; SARS‐CoV‐2, severe acute respiratory syndrome coronavirus 2.
3.5. Carotid pulse wave analysis
Peripheral and central blood pressures and carotid PWA measures (heart rate period, aortic T2, P1 height, aortic AP and aortic AIx) are detailed in Table 3. Peripheral carotid mean arterial pressure along with the central aortic systolic, pulse and mean arterial pressures were greater in the SARS‐CoV‐2 group compared with the control group. Aortic augmentation pressure was greater in the SARS‐CoV‐2 group compared with the control group. Mean systolic and diastolic pressures were greater in the SARS‐CoV‐2 group compared with the control group (Table 3). Aortic AIx was greater in the SARS‐CoV‐2 group (12.7 ± 9.1%) compared with the control group (3.3 ± 12.6%; P = 0.03, Hedge's g = −0.82; Figure 3a). Aortic AIx@HR75 was greater in the SARS‐CoV‐2 group (9 ± 7%) compared with the control group (−3 ± 17%; P = 0.01, Hedge's g = −1.00; Figure 3b).
TABLE 3.
Central pressures and carotid pulse wave analysis measures
| Parameter | Control (5 male and 10 female) | SARS‐CoV‐2 (6 male and 9 female) | P‐value | Hedge's g |
|---|---|---|---|---|
| Peripheral carotid pressures | ||||
| Peripheral systolic pressure (mmHg) | 121 ± 12 | 129 ± 13 | 0.09 | −0.63 |
| Peripheral diastolic pressure (mmHg) | 70 ± 8 | 74 ± 7 | 0.20 | −0.47 |
| Peripheral pulse pressure (mmHg) | 51 ± 9 | 55 ± 10 | 0.20 | −0.47 |
| Peripheral mean arterial pressure (mmHg) | 87 ± 10 | 99 ± 8 | <0.01 | −1.17 |
| Central aortic pressures | ||||
| Central systolic pressure (mmHg) | 109 ± 14 | 124 ± 11 | <0.01 | −1.20 |
| Central diastolic pressure (mmHg) | 70 ± 9 | 74 ± 7 | 0.22 | −0.45 |
| Central pulse pressure (mmHg) | 38 ± 12 | 50 ± 7 | <0.01 | −1.63 |
| Central mean arterial pressure (mmHg) | 85 ± 11 | 99 ± 8 | <0.01 | −1.39 |
| Pulse wave analysis | ||||
| Heart rate period (ms) | 1,004 ± 235 | 898 ± 150 | 0.15 | 0.52 |
| Ejection duration (ms) | 333 ± 14 | 316 ± 17 | 0.01 | 1.07 |
| Ejection duration (%) | 35 ± 7 | 36 ± 4 | 0.55 | −0.22 |
| Aortic T2 | 219 ± 22 | 217 ± 44 | 0.82 | 0.08 |
| P1 Height (P1 − DP) | 34 ± 12 | 44 ± 8 | 0.02 | −0.90 |
| Aortic augmentation pressure (mmHg) | 1.0 ± 5.7 | 6.2 ± 4.7 | 0.01 | −0.97 |
| Aortic AIx (P2/P1; %) | 108 ± 13 | 116 ± 12 | 0.07 | −0.67 |
| Buckberg SEVR (%) | 162 ± 44 | 146 ± 27 | 0.25 | −0.41 |
| SPTI (mmHg s min−1) | 2,099 ± 447 | 2,429 ± 368 | 0.04 | −0.78 |
| DPTI (mmHg s min−1) | 3,239 ± 453 | 3,476 ± 333 | 0.12 | −0.58 |
| End systolic pressure (mmHg) | 98 ± 12 | 107 ± 9 | 0.03 | −0.82 |
| Mean systolic pressure (mmHg) | 101 ± 10 | 113 ± 10 | <0.01 | −1.12 |
| Mean diastolic pressure (mmHg) | 83 ± 9 | 90 ± 7 | 0.02 | −0.87 |
Data are the mean ± SD. Student's two‐tailed t‐tests for two samples of equal variance were performed between the control (and severe acute respiratory syndrome coronavirus 2 (SARS‐CoV‐2) groups. Abbreviations: AIx, augmentation index; DP, diastolic pressure; DPTI, diastolic pressure–time index; P1, first systolic peak; P2, second systolic peak; SEVR, subendocardial viability ratio; STPI, systolic pressure–time index; T2, duration from the start of the pulse to the second systolic peak.
FIGURE 3.
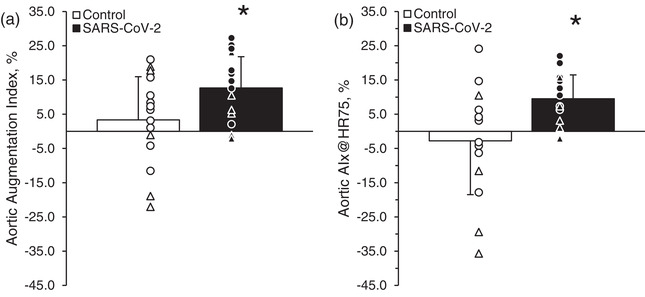
Aortic augmentation index (a) and aortic augmentation index corrected for a heart rate of 75 beats min−1 (AIx@HR75; b) in control subjects and subjects who tested positive for severe acute respiratory syndrome coronavirus 2 (SARS‐CoV‐2). Student's two‐tailed t‐tests for two samples of equal variance were performed between the control (n = 5 male and 10 female) and SARS‐CoV‐2 (n = 6 male and 9 female) groups. * P < 0.05, between groups. Individual data are presented as open triangles (male subjects) and circles (female subjects) for the control group and filled triangles and circles for the SARS‐CoV‐2 group. Data are the mean ± SD
3.6. Indices of myocardial oxygen demand and perfusion
Indices of myocardial oxygen demand and perfusion, including Buckberg SEVR, SPTI, DPTI and ejection duration, are detailed in Table 3. The SPTI was ∼16% greater (P = 0.04) and the ejection duration 17 ms faster (P = 0.01) in the SARS‐CoV‐2 group compared with the control group.
4. DISCUSSION
The aim of this study was to investigate whether carotid artery stiffness, carotid distensibility, cIMT and aortic AIx are altered in young adults after contraction of SARS‐CoV‐2 compared with young healthy adults using a cross‐sectional design. In support of our hypotheses, we observed less carotid compliance and distensibility among young adults who recently contracted SARS‐CoV‐2 compared with healthy control adults. Furthermore, we observed greater carotid stiffness and Young's modulus among individuals with SARS‐CoV‐2 compared with healthy controls. We observed a greater aortic AIx and AIx@HR75, which also suggests aortic stiffening and potential atherosclerotic risk progression. However, we did not observe any difference in cIMT. As a secondary objective, we determined the potential change among indices of myocardial oxygen demand and perfusion. In partial support of our secondary hypothesis, SPTI was elevated and ejection duration lower among individuals with SARS‐CoV‐2, suggesting minor elevations in myocardial oxygen demand and further evidence of arterial stiffness among adults recovering from SARS‐CoV‐2. Ultimately, these data suggest altered arterial stiffness among young adults who have recently contracted SARS‐CoV‐2, which might negatively influence cardiovascular health. Furthermore, these findings might have potential implications for future cardiovascular complications caused by SARS‐CoV‐2.
4.1. Carotid stiffness with SARS‐CoV‐2
Subclinical atherosclerosis and myocardial dysfunction have been observed in patients with increased arterial stiffness (Fernandes et al., 2008). For example, Kawasaki disease is characteristic of a cytokine storm in children and has been implicated in heightened arterial stiffness 1 month after symptom onset (Oguri et al., 2014), which can persist for years even after symptom restoration of the disease (Cheung et al., 2008) and could account for long‐term orthostatic and haemodynamic challenges later in life (Nakamura et al., 2020). Indeed, reports of Kawasaki disease‐like symptoms associated with SARS‐CoV‐2 infection among children (Verdoni et al., 2020) highlight the potential implications of the virally induced inflammation on the vasculature. Similar findings of heightened carotid stiffness have been observed in children with acute rheumatic fever, a persistent disease characterized by heightened oxidative stress and inflammation that can last for years (Chiu‐Braga et al., 2006; Çiftel et al., 2014) and be related independently to an increase in aortic stiffness and atherosclerosis (Nakhai‐Pour et al., 2007). In the present investigation, the SARS‐CoV‐2 group had increased carotid artery stiffness (Figure 2a), which increases risk of both cardiovascular events and all‐cause mortality, independently of other cardiovascular disease risk factors (van Sloten et al., 2014; Zieman et al., 2005). Moreover, a decrease in distensibility is an independent predictor of future cardiovascular events (Zieman et al., 2005). Our present findings of ∼27% lower carotid distensibility and ∼22% lower carotid compliance among adults with SARS‐CoV‐2 compared with healthy adults (Figures 2b,c) could worsen underlying cardiovascular event risk. Heightened arterial stiffness and lower distensibility might, therefore, pose a risk to the cardiovascular health of young adults with SARS‐CoV‐2, warranting longitudinal analyses to determine whether the decrements to the vasculature are prolonged.
Young's modulus is a critical variable to understand the arterial wall composition and structure as it relates to cardiovascular disease risk (Blankenhorn & Kramsch, 1989). In the present investigation, the SARS‐CoV‐2 subjects had a greater Young's modulus compared with the control subjects (Figure 2d). Furthermore, our present findings of a >1 SD in Young's modulus among the SARS‐CoV‐2 subjects compared with control subjects might represent a >15% increase in the risk of developing hypertension over the next 3 years, independent of established risk factors for hypertension and baseline blood pressures, if elasticity does not improve (Liao et al., 1999). These alterations in arterial stiffness and structure among young adults with SARS‐CoV‐2 suggest that these deleterious effects might be placing those with SARS‐CoV‐2 at greater risk for cardiovascular events, which is a concern, especially among populations susceptible to cardiovascular complications.
4.2. Carotid diameters and intima–media thickness with SARS‐CoV‐2
Injury to vessel walls attributable to acute inflammation, monocyte infiltration and macrophage differentiation is generally accepted as an essential component of atherogenesis (Ross, 1999) and increased cardiovascular mortality rate (Epstein et al., 2000; Espinola‐Klein et al., 2002). Arterial remodelling encompasses structural properties that can change over time in response to atherogenic or adverse haemodynamic alterations within the arterial environment, which often manifests in enlarged carotid diameters (Ward et al., 2000). In the present investigation, it is interesting to note the common carotid diameter was statistically greater among young adults with SARS‐CoV‐2 only during systole compared with healthy controls (Table 2). Whether these alterations persist in the long term remains unknown. However, chronically larger carotid diameters attributable to either atherosclerosis or pressure‐related media thickening are associated with greater incidence of stroke, cardiovascular disease and mortality (Sedaghat et al., 2018), which should be considered among those with incessant complications from SARS‐CoV‐2.
Furthermore, cIMT is an index and independent predictor of future atherosclerosis. Vessel wall inflammation precedes morphological changes caused by inflammatory diseases, as evidenced by heightened cIMT in cross‐sectional comparisons of children with acute diagnosis of Kawasaki disease (Wu et al., 2014), Mediterranean fever (Bilginer et al., 2008) or acute rheumatic fever (Çiftel et al., 2014) and in adults with Takayasu disease (Seth et al., 2006) or Behçet's disease (Alan et al., 2004), suggesting that cIMT might change acutely among individuals experiencing heightened infection‐related inflammation. However, results from these investigations suggest the acute manifestation of changes in cIMT to be indicative of endothelial dysfunction and inflammation of the vascular wall rather than inherent fibrotic changes and increased atherosclerotic risk. Furthermore, some studies have suggested an association between C. pneumoniae, H. pylori, cytomegalovirus or herpes simplex virus infection and carotid atherosclerosis (Espinola‐Klein et al., 2000; Melnick et al., 1993; Prager et al., 2002), whereas others have not yielded conclusive associations between infection and cIMT (Hirashima et al., 2002; Maeda et al., 2002). Variations in virulence and invasiveness of infection might be responsible for equivocal findings among a variety of disease states and those with various symptom severity. Given the mild cases of SARS‐CoV‐2 in the present investigation, it should not be too surprising that cIMT was unaltered by acute SARS‐CoV‐2 infection in young adults (Table 2). However, persistent symptoms over the course of months might influence cIMT and will be of interest in the future.
4.3. Carotid pulse wave analysis with SARS‐CoV‐2
Aortic AIx is a multifaceted marker of ventricular–vasculature coupling typically measured from wave reflections (Laurent et al., 2006). Moreover, cross‐sectional analyses of children with Kawasaki disease have revealed several accounts of vascular inflammation, including subclinical arterial stiffness (Oguri et al., 2014) and heightened radial artery AIx (Zheng et al., 2019). Although these short‐term manifestations can relate to increased risk of atherosclerotic progression (McCrindle et al., 2007; Tobayama et al., 2013), vascular impairments can persist for years after symptom amelioration of diseases associated with vascular inflammation (Pinto et al., 2013, 2017), including long‐term changes to arterial stiffness and AIx (Tobayama et al., 2013). The greater aortic AIx and AIx@HR75 among adults with SARS‐CoV‐2 in the present study (Figure 3), although arguably indicative of the viscoelastic properties of the aorta and large arteries, might therefore be corroborating our previous accounts of arterial stiffness, as evidenced by greater carotid–femoral pulse wave velocity, among young adults who recently contracted SARS‐CoV‐2 (Ratchford et al., 2020). Additionally, several studies have reported a rise in AIx with age (Fantin et al., 2007; McEniery et al., 2005), and AIx might ultimately be a sensitive index of aortic stiffness in younger adults ≤60 years old (Namasivayam et al., 2010). Our present results from young healthy adults are similar to previously reported data from ∼20‐year‐old adults, whereas our AIx data from ∼20‐year‐old adults with SARS‐CoV‐2 might be more reflective of adults 10 years older (Wojciechowska et al., 2006). Although this cross‐sectional analysis does not indicate an irreversible effect of these aortic alterations, more work is needed to track young adults with SARS‐CoV‐2 longitudinally to determine whether these effects are long lasting.
4.4. Indices of myocardial oxygen demand and perfusion with SARS‐CoV‐2
Using morphological analyses of the central arterial pressure wave recorded by tonometry, PWA can also provide a non‐invasive index of cardiac ischaemia risk using the Buckberg SEVR index (Buckberg et al., 1972; Hoffman & Buckberg, 1978). The SPTI, also known as the tension–time integral, is often a surrogate for myocardial oxygen demand. In the present investigation, we observed greater SPTI among young adults who recently contracted SARS‐CoV‐2, which might be indicative of a mild hypermetabolic (Yu et al., 2020) or hyperdynamic myocardium (Mahjoub et al., 2021), previously observed among those critically ill with SARS‐CoV‐2. Despite a potential for an acute myocardial stress response among young adults with SARS‐CoV‐2, the Buckberg SEVR index, a diastolic to systolic pressure–time integral ratio and an index of myocardial oxygen supply (DPTI) to demand (SPTI), appears similar between our SARS‐CoV‐2 and control groups (Table 3). Although the myocardium might be undergoing a stress response owing to this novel infection, the oxygen supply might, therefore, be maintained. Although these are indirect measures of cardiac oxygen supply and demand, they might provide evidence of a systemic cardiovascular stress response caused by SARS‐CoV‐2. However, more work is certainly warranted to assess more direct measures of cardiac abnormalities caused by SARS‐CoV‐2 in young adults.
Ejection duration is an indicator of arterial stiffness, because increases in arterial stiffness result in a shorter ejection duration period (Böcskei et al., 2019). An elevated resting heart rate is also associated with a decrease in ejection duration (Pieringer et al., 2014; Wilkinson et al., 2000) and an increased risk for cardiovascular disease among patients with hypertension, in addition to the general population (Diaz et al., 2005; Dyer et al., 1980). Combined, this evidence of increased arterial stiffening and chronic ejection into stiffer arteries might induce cardiac hypertrophy, even at similar arterial pressures, and might even alter the manner in which the heart is perfused (Lartaud‐Idjouadiene et al., 1999). The shorter ejection duration observed in young adults recovering from SARS‐CoV‐2 (Table 3), therefore, provides additional evidence for increased arterial stiffness that might exacerbate underlying cardiovascular event risk.
4.5. Study limitations
Although common among studies investigating physiological function in acute infectious diseases, we acknowledge the limitations of a cross‐sectional comparison such as this and recognize that some of the underlying group differences might be inherent to intersubject variability along with continued mild symptoms at the time of testing. The nature of this investigation was not appropriately powered to evaluate sex‐specific effects caused by SARS‐CoV‐2. Furthermore, previous findings suggest little evidence for a sex‐based difference in vascular alterations attributable to SARS‐CoV‐2 (Ratchford et al., 2020). Although the menstrual cycle of the female subject population was not controlled for and variations in contraceptive use between female subjects were observed, contraceptive use and menstrual cycle fluctuations among young healthy females have not be observed to influence carotid distensibility or PWA (Priest et al., 2018; Wilkinson et al., 1998; Willekes et al., 1999). Additional studies have also suggested no impacts of menstrual cycle or type of contraceptive on central arterial stiffness (Priest et al., 2018). Although future studies should certainly differentiate severity of symptoms and asymptomatic individuals among various age groups, our present results with only one asymptomatic individual failed to show any significant difference between symptomatic and asymptotic cases of SARS‐CoV‐2. Longitudinal studies are certainly needed to understand fully the implications of SARS‐CoV‐2 on arterial health and the potential recovery from this disease. However, the initial results indicating increased arterial stiffness are clinically relevant and warrant attention.
4.6. Conclusion
Initial evidence suggests an increase in carotid stiffness and aortic augmentation in young adults with SARS‐CoV‐2. These alterations to the vasculature are clinically relevant because they indicate unfavourable vascular remodelling, mild vascular ageing and potential exacerbation of underlying cardiovascular event risk. Future longitudinal studies with a larger, cardiovascular compromised cohort are warranted to determine whether the observed diminution to cardiovascular health is long lasting among susceptible groups.
AUTHOR CONTRIBUTIONS
J.L.S., A.S.L. and S.M.R. conceived and designed the research. V.M.P., N.L.S., M.A.A., L.K.K., J.L.S., A.S.L.S. and S.M.R. performed the experiments. All authors analysed the data and interpreted the results of experiments. R.E.S. and S.M.R. prepared the figures. R.E.S., J.L.S., A.S.L.S. and S.M.R. drafted the manuscript. All authors edited and revised manuscript, approved the final version of the manuscript and agree to be accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. All persons designated as authors qualify for authorship, and all those who qualify for authorship are listed.
COMPETING INTERESTS
None declared.
ACKNOWLEDGEMENTS
We would like to thank Rebecca Kappus for sharing her equipment and technical knowledge with us, without which this study could not have happened. This study was partially supported by an internal COVID‐19 Research Cluster Award at Appalachian State University.
Szeghy, R. E. , Province, V. M. , Stute, N. L. , Augenreich, M. A. , Koontz, L. K. , Stickford, J. L. , Stickford, A. S. L. , & Ratchford, S. M. (2022). Carotid stiffness, intima–media thickness and aortic augmentation index among adults with SARS‐CoV‐2. Experimental Physiology, 107, 694–707. 10.1113/EP089481
Edited by: Robert Brothers
DATA AVAILABILITY STATEMENT
The data that support the findings of this study are available upon request from the corresponding author. The data are not publicly available due to privacy or ethical restrictions.
REFERENCES
- Alan, S. , Ulgen, M. S. , Akdeniz, S. , Alan, B. , & Toprak, N. (2004). Intima‐media thickness and arterial distensibility in Behcet's disease. Angiology, 55, 413–419. 10.1177/000331970405500408 [DOI] [PubMed] [Google Scholar]
- Bilginer, Y. , Ozaltin, F. , Basaran, C. , Duzova, A. , Besbas, N. , Topaloglu, R. , Ozen, S. , & Bakkaloglu, A. (2008). Evaluation of intima media thickness of the common and internal carotid arteries with inflammatory markers in familial Mediterranean fever as possible predictors for atherosclerosis. Rheumatology International, 28, 1211–1216. 10.1007/s00296-008-0605-9 [DOI] [PubMed] [Google Scholar]
- Blankenhorn, D. H. , & Kramsch, D. M. (1989). Reversal of atherosis and sclerosis. The two components of atherosclerosis. Circulation, 79, 1–7. 10.1161/01.CIR.79.1.1 [DOI] [PubMed] [Google Scholar]
- Böcskei, R. M. , Benczúr, B. , Losonczy, G. , Illyés, M. , Cziráki, A. , Müller, V. , Bohács, A. , & Bikov, A. (2019). Soluble urokinase‐type plasminogen activator receptor and arterial stiffness in patients with COPD. Lung, 197, 189–197. 10.1007/s00408-019-00211-w [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bruno, R. M. , Cartoni, G. , Stea, F. , Armenia, S. , Bianchini, E. , Buralli, S. , Giannarelli, C. , Taddei, S. , & Ghiadoni, L. (2017). Carotid and aortic stiffness in essential hypertension and their relation with target organ damage: The CATOD study. Journal of Hypertension, 35, 310–318. 10.1097/HJH.0000000000001167 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buckberg, G. D. , Fixler, D. E. , Archie, J. P. , & Hoffman, J. I. (1972). Experimental subendocardial ischemia in dogs with normal coronary arteries. Circulation Research, 30, 67–81. 10.1161/01.RES.30.1.67 [DOI] [PubMed] [Google Scholar]
- Butlin, M. , & Qasem, A. (2017). Large artery stiffness assessment using SphygmoCor technology. Pulse (Basel), 4, 180–192. 10.1159/000452448 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Channappanavar, R. , & Perlman, S. (2017). Pathogenic human coronavirus infections: Causes and consequences of cytokine storm and immunopathology. Seminars in Immunopathology, 39, 529–539. 10.1007/s00281-017-0629-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cheung, Y.‐f. , O, K. , Woo, C. W. H. , Armstrong, S. , Siow, Y. L. , Chow, P.‐c. , & Cheung, E. W. Y. (2008). Oxidative stress in children late after Kawasaki disease: Relationship with carotid atherosclerosis and stiffness. BMC Pediatrics, 8, 20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chiu‐Braga, Y. Y. , Hayashi, S. Y. , Schafranski, M. , & Messias‐Reason, I. J. (2006). Further evidence of inflammation in chronic rheumatic valve disease (CRVD): High levels of advanced oxidation protein products (AOPP) and high sensitive C‐reactive protein (hs‐CRP). International Journal of Cardiology, 109, 275–276. 10.1016/j.ijcard.2005.04.030 [DOI] [PubMed] [Google Scholar]
- Çiftel, M. , Yılmaz, O. , Kardelen, F. , & Kocabaş, A. (2014). Carotid intima media thickness and arterial stiffness in children with acute rheumatic fever. Pediatric Cardiology, 35, 16–21. 10.1007/s00246-013-0732-2 [DOI] [PubMed] [Google Scholar]
- Clerkin, K. J. , Fried, J. A. , Raikhelkar, J. , Sayer, G. , Griffin, J. M. , Masoumi, A. , Jain, S. S. , Burkhoff, D. , Kumaraiah, D. , Rabbani, L. , Schwartz, A. , & Uriel, N. (2020). COVID‐19 and cardiovascular disease. Circulation, 141, 1648–1655. 10.1161/CIRCULATIONAHA.120.046941 [DOI] [PubMed] [Google Scholar]
- Coperchini, F. , Chiovato, L. , Croce, L. , Magri, F. , & Rotondi, M. (2020). The cytokine storm in COVID‐19: An overview of the involvement of the chemokine/chemokine‐receptor system. Cytokine & Growth Factor Reviews, 53, 25–32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Deng, Q. , Hu, B. , Zhang, Y. , Wang, H. , Zhou, X. , Hu, W. , Cheng, Y. , Yan, J. , Ping, H. , & Zhou, Q. (2020). Suspected myocardial injury in patients with COVID‐19: Evidence from front‐line clinical observation in Wuhan, China. International Journal of Cardiology, 311, 116–121. 10.1016/j.ijcard.2020.03.087 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Diaz, A. , Bourassa, M. G. , Guertin, M. C. , & Tardif, J. C. (2005). Long‐term prognostic value of resting heart rate in patients with suspected or proven coronary artery disease. European Heart Journal, 26, 967–974. 10.1093/eurheartj/ehi190 [DOI] [PubMed] [Google Scholar]
- Driggin, E. , Madhavan, M. V. , Bikdeli, B. , Chuich, T. , Laracy, J. , Biondi‐Zoccai, G. , Brown, T. S. , Der Nigoghossian, C. , Zidar, D. A. , Haythe, J. , Brodie, D. , Beckman, J. A. , Kirtane, A. J. , Stone, G. W. , Krumholz, H. M. , & Parikh, S. A. (2020). Cardiovascular considerations for patients, health care workers, and health systems during the COVID‐19 pandemic. Journal of the American College of Cardiology, 75, 2352–2371. 10.1016/j.jacc.2020.03.031 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dyer, A. R. , Persky, V. , Stamler, J. , Paul, O. , Shekelle, R. B. , Berkson, D. M. , Lepper, M. , Schoenberger, J. A. , & Lindberg, H. A. (1980). Heart rate as a prognostic factor for coronary heart disease and mortality: Findings in three Chicago epidemiologic studies. American Journal of Epidemiology, 112, 736–749. 10.1093/oxfordjournals.aje.a113046 [DOI] [PubMed] [Google Scholar]
- Epstein, S. E. , Zhu, J. , Burnett, M. S. , Zhou, Y. F. , Vercellotti, G. , & Hajjar, D. (2000). Infection and atherosclerosis: Potential roles of pathogen burden and molecular mimicry. Arteriosclerosis, Thrombosis, and Vascular Biology, 20, 1417–1420. 10.1161/01.ATV.20.6.1417 [DOI] [PubMed] [Google Scholar]
- Espinola‐Klein, C. , Rupprecht, H. J. , Blankenberg, S. , Bickel, C. , Kopp, H. , Rippin, G. , Hafner, G. , Pfeifer, U. , & Meyer, J. (2000). Are morphological or functional changes in the carotid artery wall associated with Chlamydia pneumoniae, Helicobacter pylori, cytomegalovirus, or herpes simplex virus infection? Stroke, 31, 2127–2133. 10.1161/01.STR.31.9.2127 [DOI] [PubMed] [Google Scholar]
- Espinola‐Klein, C. , Rupprecht, H. J. , Blankenberg, S. , Bickel, C. , Kopp, H. , Rippin, G. , Victor, A. , Hafner, G. , Schlumberger, W. , Meyer, J. , & AtheroGene Investigators (2002). Impact of infectious burden on extent and long‐term prognosis of atherosclerosis. Circulation, 105, 15–21. 10.1161/hc0102.101362 [DOI] [PubMed] [Google Scholar]
- Evans, P. C. , Rainger, G. E. , Mason, J. C. , Guzik, T. J. , Osto, E. , Stamataki, Z. , Neil, D. , Hoefer, I. E. , Fragiadaki, M. , Waltenberger, J. , Weber, C. , Bochaton‐Piallat, M.‐L. , & Bäck, M. (2020). Endothelial dysfunction in COVID‐19: A position paper of the ESC Working Group for Atherosclerosis and Vascular Biology, and the ESC Council of Basic Cardiovascular Science. Cardiovascular Research, 116, 2177–2184. 10.1093/cvr/cvaa230 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fantin, F. , Mattocks, A. , Bulpitt, C. J. , Banya, W. , & Rajkumar, C. (2007). Is augmentation index a good measure of vascular stiffness in the elderly? Age and Ageing, 36, 43–48. 10.1093/ageing/afl115 [DOI] [PubMed] [Google Scholar]
- Fernandes, V. R. S. , Polak, J. F. , Cheng, S. , Rosen, B. D. , Carvalho, B. , Nasir, K. , McClelland, R. , Hundley, G. , Pearson, G. , O'Leary, D. H. , Bluemke, D. A. , & Lima, J. A. C. (2008). Arterial stiffness is associated with regional ventricular systolic and diastolic dysfunction: The Multi‐Ethnic Study of Atherosclerosis. Arteriosclerosis, Thrombosis, and Vascular Biology, 28, 194–201. 10.1161/ATVBAHA.107.156950 [DOI] [PubMed] [Google Scholar]
- Godia, E. C. , Madhok, R. , Pittman, J. , Trocio, S. , Ramas, R. , Cabral, D. , Sacco, R. L. , & Rundek, T. (2007). Carotid artery distensibility: A reliability study. Journal of Ultrasound in Medicine, 26, 1157–1165. 10.7863/jum.2007.26.9.1157 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hirashima, Y. , Kuwayama, N. , Kubo, M. , Origasa, H. , & Endo, S. (2002). Chlamydia pneumoniae infection is not involved in carotid artery stenosis. Atherosclerosis, 163, 165–168. 10.1016/S0021-9150(01)00767-5 [DOI] [PubMed] [Google Scholar]
- Hoffman, J. I. E. , & Buckberg, G. D. (1978). The myocardial supply:demand ratio—A critical review. American Journal of Cardiology, 41, 327–332. 10.1016/0002-9149(78)90174-1 [DOI] [PubMed] [Google Scholar]
- Ibrahim, N. N. I. N. , Jaafar, H. , Rasool, A. H. , & Wong, A. R. (2016). Arterial stiffness during acute and recovery phases of children with rheumatic fever. Medical Journal of Malaysia, 71, 23–25. [PubMed] [Google Scholar]
- Kim, H.‐L. , & Kim, S.‐H. (2019). Pulse wave velocity in atherosclerosis. Frontiers in Cardiovascular Medicine, 6, 41. 10.3389/fcvm.2019.00041 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lartaud‐Idjouadiene, I. , Lompré, A. M. , Kieffer, P. , Colas, T. , & Atkinson, J. (1999). Cardiac consequences of prolonged exposure to an isolated increase in aortic stiffness. Hypertension, 34, 63–69. 10.1161/01.HYP.34.1.63 [DOI] [PubMed] [Google Scholar]
- Laurent, S. , Cockcroft, J. , Van Bortel, L. , Boutouyrie, P. , Giannattasio, C. , Hayoz, D. , Pannier, B. , Vlachopoulos, C. , Wilkinson, I. , & Struijker‐Boudier, H. ; European Network for Non‐invasive Investigation of Large Arteries (2006). Expert consensus document on arterial stiffness: Methodological issues and clinical applications. European Heart Journal, 27, 2588–2605. 10.1093/eurheartj/ehl254 [DOI] [PubMed] [Google Scholar]
- Li, B. , Xia, Y. , & Hu, B. (2020). Infection and atherosclerosis: TLR‐dependent pathways. Cellular and Molecular Life Sciences, 77, 2751–2769. 10.1007/s00018-020-03453-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li, K. , Hao, Z. , Zhao, X. , Du, J. , & Zhou, Y. (2020). SARS‐CoV‐2 infection‐induced immune responses: Friends or foes? Scandinavian Journal of Immunology, 92, e12895. 10.1111/sji.12895 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liao, D. , Arnett, D. K. , Tyroler, H. A. , Riley, W. A. , Chambless, L. E. , Szklo, M. , & Heiss, G. (1999). Arterial stiffness and the development of hypertension. The ARIC study. Hypertension, 34, 201–206. 10.1161/01.HYP.34.2.201 [DOI] [PubMed] [Google Scholar]
- Maeda, N. , Sawayama, Y. , Tatsukawa, M. , Shimizu, C. , Kashiwagi, S. , & Hayashi, J. (2002). Chlamydia pneumoniae seropositivity and early carotid atherosclerosis in a suburban Japanese population. Atherosclerosis, 164, 313–319. 10.1016/S0021-9150(02)00104-1 [DOI] [PubMed] [Google Scholar]
- Mahjoub, Y. , Rodenstein, D. O. , & Jounieaux, V. (2021). The hyperdynamic circulatory profile of patients with COVID‐19‐related AVDS. Letter regarding the article ‘Haemodynamic characteristics of COVID‐19 patients with acute respiratory distress syndrome requiring mechanical ventilation. An invasive assessment using right heart catheterization’. European Journal of Heart Failure, 23, 493. [DOI] [PubMed] [Google Scholar]
- McCrindle, B. W. , McIntyre, S. , Kim, C. , Lin, T. , & Adeli, K. (2007). Are patients after Kawasaki disease at increased risk for accelerated atherosclerosis? Journal of Pediatrics, 151, 244–248, 248.e1. 10.1016/j.jpeds.2007.03.056 [DOI] [PubMed] [Google Scholar]
- McEniery, C. M. , Yasmin, I. R. H. , Qasem, A. , Wilkinson, I. B. , Cockcroft, J. R. , & ACCT Investigators . (2005). Normal vascular aging: Differential effects on wave reflection and aortic pulse wave velocity: The Anglo‐Cardiff Collaborative Trial (ACCT). Journal of the American College of Cardiology, 46, 1753–1760. 10.1016/j.jacc.2005.07.037 [DOI] [PubMed] [Google Scholar]
- Melnick, S. L. , Shahar, E. , Folsom, A. R. , Grayston, J. T. , Sorlie, P. D. , Wang, S. P. , & Szklo, M. (1993). Past infection by Chlamydia pneumoniae strain TWAR and asymptomatic carotid atherosclerosis. Atherosclerosis Risk in Communities (ARIC) Study Investigators. American Journal of Medicine, 95, 499–504. 10.1016/0002-9343(93)90332-J [DOI] [PubMed] [Google Scholar]
- Montalvan, V. , Lee, J. , Bueso, T. , De Toledo, J. , & Rivas, K. (2020). Neurological manifestations of COVID‐19 and other coronavirus infections: A systematic review. Clinical Neurology and Neurosurgery, 194, 105921. 10.1016/j.clineuro.2020.105921 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Monteil, V. , Kwon, H. , Prado, P. , Hagelkrüys, A. , Wimmer, R. A. , Stahl, M. , Leopoldi, A. , Garreta, E. , Hurtado Del Pozo, C. , Prosper, F. , Romero, J. P. , Wirnsberger, G. , Zhang, H. , Slutsky, A. S. , Conder, R. , Montserrat, N. , Mirazimi, A. , & Penninger, J. M. (2020). Inhibition of SARS‐CoV‐2 infections in engineered human tissues using clinical‐grade soluble human ACE2. Cell, 181, 905–913.e7. 10.1016/j.cell.2020.04.004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nakamura, Y. , Hama, T. , Nakamura, Y. , Tsukada, H. , Oda, Y. , & Awa, S. (2020). Orthostatic and exercise effects in children years after Kawasaki disease. Pediatric Cardiology, 41, 24–30. 10.1007/s00246-019-02216-x [DOI] [PubMed] [Google Scholar]
- Nakhai‐Pour, H. R. , Grobbee, D. E. , Bots, M. L. , Muller, M. , & van der Schouw, Y. T. (2007). C‐reactive protein and aortic stiffness and wave reflection in middle‐aged and elderly men from the community. Journal of Human Hypertension, 21, 949–955. 10.1038/sj.jhh.1002255 [DOI] [PubMed] [Google Scholar]
- Namasivayam, M. , Adji, A. , & O'Rourke, M. F. (2010). Aortic augmentation index and aging: Mathematical resolution of a physiological dilemma? Hypertension, 56, e9–e10. [DOI] [PubMed] [Google Scholar]
- NC Department of Health and Human Services (2020). North Carolina identifies first case of COVID‐19, vol. 2020. NC Department of Health and Human Services, online. https://www.ncdhhs.gov/news/press-releases/north-carolina-identifies-first-case-covid-19 [Google Scholar]
- Oguri, M. , Nakamura, T. , Tamanuki, K. , Akita, C. , Kitaoka, C. , Saikawa, Y. , & Takahashi, M. (2014). Subclinical arterial stiffness in young children after Kawasaki disease. Cardiology in the Young, 24, 87–94. 10.1017/S1047951112002302 [DOI] [PubMed] [Google Scholar]
- Pieringer, H. , Brummaier, T. , Schmid, M. , Pichler, M. , Hayat‐Khayyati, A. , Ebner, S. , Biesenbach, G. , & Pohanka, E. (2014). Heart rate, ejection duration and subendocardial viability ratio in patients with rheumatoid arthritis as compared to controls. International Journal of Rheumatic Diseases, 17, 39–43. 10.1111/1756-185X.12046 [DOI] [PubMed] [Google Scholar]
- Pinto, F. F. , Gomes, I. , Loureiro, P. , Laranjo, S. , Timóteo, A. T. , & Carmo, M. M. (2017). Vascular function long term after Kawasaki disease: Another piece of the puzzle? Cardiology in the Young, 27, 488–497. 10.1017/S1047951116000780 [DOI] [PubMed] [Google Scholar]
- Pinto, F. F. , Laranjo, S. , Paramés, F. , Freitas, I. , & Mota‐Carmo, M. (2013). Long‐term evaluation of endothelial function in Kawasaki disease patients. Cardiology in the Young, 23, 517–522. 10.1017/S1047951112001357 [DOI] [PubMed] [Google Scholar]
- Pirzada, A. , Mokhtar, A. T. , & Moeller, A. D. (2020). COVID‐19 and myocarditis: What do we know so far? CJC Open, 2, 278–285. 10.1016/j.cjco.2020.05.005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Prager, M. , Türel, Z. , Speidl, W. S. , Zorn, G. , Kaun, C. , Niessner, A. , Heinze, G. , Huk, I. , Maurer, G. , Huber, K. , & Wojta, J. (2002). Chlamydia pneumoniae in carotid artery atherosclerosis: A comparison of its presence in atherosclerotic plaque, healthy vessels, and circulating leukocytes from the same individuals. Stroke, 33, 2756–2761. 10.1161/01.STR.0000039322.66575.77 [DOI] [PubMed] [Google Scholar]
- Priest, S. E. , Shenouda, N. , & MacDonald, M. J. (2018). Effect of sex, menstrual cycle phase, and monophasic oral contraceptive pill use on local and central arterial stiffness in young adults. American Journal of Physiology. Heart and Circulatory Physiology, 315, H357–H365. 10.1152/ajpheart.00039.2018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ratchford, S. M. , Stickford, J. L. , Province, V. M. , Stute, N. , Augenreich, M. A. , Koontz, L. K. , Bobo, L. K. , & Stickford, A. S. L. (2020). Vascular alterations among young adults with SARS‐CoV‐2. American Journal of Physiology. Heart and Circulatory Physiology, 320, H404–H410. 10.1152/ajpheart.00897.2020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Riley, W. A. , Barnes, R. W. , Evans, G. W. , & Burke, G. L. (1992). Ultrasonic measurement of the elastic modulus of the common carotid artery. The Atherosclerosis Risk in Communities (ARIC) Study. Stroke, 23, 952–956. 10.1161/01.STR.23.7.952 [DOI] [PubMed] [Google Scholar]
- Rodilla, E. , López‐Carmona, M. D. , Cortes, X. , Cobos‐Palacios, L. , Canales, S. , Sáez, M. C. , Campos Escudero, S. , Rubio‐Rivas, M. , Díez Manglano, J. , Freire Castro, S. J. , Vázquez Piqueras, N. , Mateo Sanchis, E. , Pesqueira Fontan, P. M. , Magallanes Gamboa, J. O. , González García, A. , Madrid Romero, V. , Tamargo Chamorro, L. , González Moraleja, J. , … SEMI‐COVID‐19 Network . (2021). Impact of arterial stiffness on all‐cause mortality in patients hospitalized with COVID‐19 in Spain. Hypertension, 77, 856–867. 10.1161/HYPERTENSIONAHA.120.16563 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roman, M. J. , Devereux, R. B. , Schwartz, J. E. , Lockshin, M. D. , Paget, S. A. , Davis, A. , Crow, M. K. , Sammaritano, L. , Levine, D. M. , Shankar, B. A. , Moeller, E. , & Salmon, J. E. (2005). Arterial stiffness in chronic inflammatory diseases. Hypertension, 46, 194–199. 10.1161/01.HYP.0000168055.89955.db [DOI] [PubMed] [Google Scholar]
- Ross, R. (1999). Atherosclerosis — An inflammatory disease. New England Journal of Medicine, 340, 115–126. 10.1056/NEJM199901143400207 [DOI] [PubMed] [Google Scholar]
- Scandale, G. , Dimitrov, G. , Recchia, M. , Carzaniga, G. , Minola, M. , Perilli, E. , Carotta, M. , & Catalano, M. (2018). Arterial stiffness and subendocardial viability ratio in patients with peripheral arterial disease. Journal of Clinical Hypertension, 20, 478–484. 10.1111/jch.13213 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sedaghat, S. , van Sloten, T. T. , Laurent, S. , London, G. M. , Pannier, B. , Kavousi, M. , Mattace‐Raso, F. , Franco, O. H. , Boutouyrie, P. , Ikram, M. A. , & Stehouwer, C. D. A. (2018). Common carotid artery diameter and risk of cardiovascular events and mortality: pooled analyses of four cohort studies. Hypertension, 72, 85–92. 10.1161/HYPERTENSIONAHA.118.11253 [DOI] [PubMed] [Google Scholar]
- Seth, S. , Goyal, N. K. , Jagia, P. , Gulati, G. , Karthikeyan, G. , Sharma, S. , & Talwar, K. K. (2006). Carotid intima‐medial thickness as a marker of disease activity in Takayasu's arteritis. International Journal of Cardiology, 108, 385–390. 10.1016/j.ijcard.2005.05.033 [DOI] [PubMed] [Google Scholar]
- Shi, S. , Qin, M. , Shen, B. , Cai, Y. , Liu, T. , Yang, F. , Gong, W. , Liu, X. , Liang, J. , Zhao, Q. , Huang, H. , Yang, B. , & Huang, C. (2020). Association of cardiac injury with mortality in hospitalized patients with COVID‐19 in Wuhan, China. JAMA Cardiology, 5, 802–810. 10.1001/jamacardio.2020.0950 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Siripanthong, B. , Nazarian, S. , Muser, D. , Deo, R. , Santangeli, P. , Khanji, M. Y. , Cooper, L. T., Jr. , & Chahal, C. A. A. (2020). Recognizing COVID‐19‐related myocarditis: The possible pathophysiology and proposed guideline for diagnosis and management. Heart Rhythm, 17, 1463–1471. 10.1016/j.hrthm.2020.05.001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stefanini, G. G. , Montorfano, M. , Trabattoni, D. , Andreini, D. , Ferrante, G. , Ancona, M. , Metra, M. , Curello, S. , Maffeo, D. , Pero, G. , Cacucci, M. , Assanelli, E. , Bellini, B. , Russo, F. , Ielasi, A. , Tespili, M. , Danzi, G. B. , Vandoni, P. , Bollati, M. , & Chieffo, A. (2020). ST‐Elevation myocardial infarction in patients with COVID‐19: Clinical and angiographic outcomes. Circulation, 141, 2113–2116. 10.1161/CIRCULATIONAHA.120.047525 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tadic, M. , Cuspidi, C. , Mancia, G. , Dell'Oro, R. , & Grassi, G. (2020). COVID‐19, hypertension and cardiovascular diseases: Should we change the therapy? Pharmacological Research, 158, 104906. 10.1016/j.phrs.2020.104906 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tobayama, H. , Takahashi, K. , Fukunaga, H. , Matsui, K. , Tanaka, N. , Harada, M. , Furukawa, T. , Oda, H. , Akimoto, K. , Kishiro, M. , & Shimizu, T. (2013). Analysis of arterial function in adults with a history of Kawasaki disease. Journal of Cardiology, 61, 330–335. 10.1016/j.jjcc.2012.12.007 [DOI] [PubMed] [Google Scholar]
- Van Merode, T. , Van Bortel, L. , Smeets, F. A. , Böhm, R. , Mooij, J. , Rahn, K. H. , & Reneman, R. S. (1990). The effect of verapamil on carotid artery distensibility and cross‐sectional compliance in hypertensive patients. Journal of Cardiovascular Pharmacology, 15, 109–113. 10.1097/00005344-199001000-00017 [DOI] [PubMed] [Google Scholar]
- van Sloten, T. T. , Schram, M. T. , van den Hurk, K. , Dekker, J. M. , Nijpels, G. , Henry, R. M. , & Stehouwer, C. D. (2014). Local stiffness of the carotid and femoral artery is associated with incident cardiovascular events and all‐cause mortality: The Hoorn study. Journal of the American College of Cardiology, 63, 1739–1747. 10.1016/j.jacc.2013.12.041 [DOI] [PubMed] [Google Scholar]
- Verdoni, L. , Mazza, A. , Gervasoni, A. , Martelli, L. , Ruggeri, M. , Ciuffreda, M. , Bonanomi, E. , & D'Antiga, L. (2020). An outbreak of severe Kawasaki‐like disease at the Italian epicentre of the SARS‐CoV‐2 epidemic: An observational cohort study. Lancet, 395, 1771–1778. 10.1016/S0140-6736(20)31103-X [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vlachopoulos, C. , Aznaouridis, K. , & Stefanadis, C. (2010). Prediction of cardiovascular events and all‐cause mortality with arterial stiffness: A systematic review and meta‐analysis. Journal of the American College of Cardiology, 55, 1318–1327. 10.1016/j.jacc.2009.10.061 [DOI] [PubMed] [Google Scholar]
- Vlachopoulos, C. , Dima, I. , Aznaouridis, K. , Vasiliadou, C. , Ioakeimidis, N. , Aggeli, C. , Toutouza, M. , & Stefanadis, C. (2005). Acute systemic inflammation increases arterial stiffness and decreases wave reflections in healthy individuals. Circulation, 112, 2193–2200. 10.1161/CIRCULATIONAHA.105.535435 [DOI] [PubMed] [Google Scholar]
- Ward, M. R. , Pasterkamp, G. , Yeung, A. C. , & Borst, C. (2000). Arterial remodeling. Mechanisms and clinical implications. Circulation, 102, 1186–1191. 10.1161/01.CIR.102.10.1186 [DOI] [PubMed] [Google Scholar]
- Wilkinson, I. B. , Fuchs, S. A. , Jansen, I. M. , Spratt, J. C. , Murray, G. D. , Cockcroft, J. R. , & Webb, D. J. (1998). Reproducibility of pulse wave velocity and augmentation index measured by pulse wave analysis. Journal of Hypertension, 16, 2079–2084. 10.1097/00004872-199816121-00033 [DOI] [PubMed] [Google Scholar]
- Wilkinson, I. B. , MacCallum, H. , Flint, L. , Cockcroft, J. R. , Newby, D. E. , & Webb, D. J. (2000). The influence of heart rate on augmentation index and central arterial pressure in humans. The Journal of Physiology, 525, 263–270. 10.1111/j.1469-7793.2000.t01-1-00263.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilkinson, I. B. , Prasad, K. , Hall, I. R. , Thomas, A. , MacCallum, H. , Webb, D. J. , Frenneaux, M. P. , & Cockcroft, J. R. (2002). Increased central pulse pressure and augmentation index in subjects with hypercholesterolemia. Journal of the American College of Cardiology, 39, 1005–1011. 10.1016/S0735-1097(02)01723-0 [DOI] [PubMed] [Google Scholar]
- Willekes, C. , Hoogland, H. J. , Keizer, H. A. , Hoeks, A. P. , & Reneman, R. S. (1999). Three months use of third‐generation oral contraceptives does not affect artery wall properties. Ultrasound in Medicine & Biology, 25, 723–728. [DOI] [PubMed] [Google Scholar]
- Wojciechowska, W. , Staessen, J. A. , Nawrot, T. , Cwynar, M. , Seidlerová, J. , Stolarz, K. , Gasowski, J. , Tichá, M. , Richart, T. , Thijs, L. , Grodzicki, T. , Kawecka‐Jaszcz, K. , & Filipovský, J. , & European Project on Genes in Hypertension (EPOGH) Investigators (2006). Reference values in white Europeans for the arterial pulse wave recorded by means of the SphygmoCor device. Hypertension Research, 29, 475–483. 10.1291/hypres.29.475 [DOI] [PubMed] [Google Scholar]
- Wu, T.‐H. , Kuo, H.‐C. , Tain, Y.‐L. , Lin, K.‐M. , Kuo, H.‐C. , & Chien, S.‐J. (2014). Common carotid artery intima‐media thickness is useful for diagnosis of the acute stage of Kawasaki disease. BMC Pediatrics, 14, 98. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu, Z. , & McGoogan, J. M. (2020). Characteristics of and important lessons from the coronavirus disease 2019 (COVID‐19) outbreak in China: Summary of a report of 72314 cases from the Chinese Center for Disease Control and Prevention. JAMA, 323, 1239–1242. 10.1001/jama.2020.2648 [DOI] [PubMed] [Google Scholar]
- Yu, P. J. , Cassiere, H. , DeRosa, S. , Bocchieri, K. , Yar, S. , & Hartman, A. (2020). Hypermetabolism and coronavirus disease 2019. JPEN. Journal of Parenteral and Enteral Nutrition, 44, 1234–1236. 10.1002/jpen.1948 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zanoli, L. , Boutouyrie, P. , Fatuzzo, P. , Granata, A. , Lentini, P. , Ozturk, K. , Cappello, M. , Theocharidou, E. , Tuttolomondo, A. , Pinto, A. , Camma, C. , Licata, A. , Blanco, J. , Rastelli, S. , Inserra, G. , Castellino, P. , & Laurent, S. (2017). Inflammation and aortic stiffness: An individual participant data meta‐analysis in patients with inflammatory bowel disease. Journal of the American Heart Association, 6, e007003. 10.1161/JAHA.117.007003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zanoli, L. , Briet, M. , Empana, J. P. , Cunha, P. G. , Mäki‐Petäjä, K. M. , Protogerou, A. D. , Tedgui, A. , Touyz, R. M. , Schiffrin, E. L. , Spronck, B. , Bouchard, P. , Vlachopoulos, C. , Bruno, R. M. , & Boutouyrie, P. ; Association for Research into Arterial Structure, Physiology (ARTERY) Society, the European Society of Hypertension (ESH) Working Group on Vascular Structure and Function, and the European Network for Noninvasive Investigation of Large Arteries (2020). Vascular consequences of inflammation: A position statement from the ESH Working Group on Vascular Structure and Function and the ARTERY Society. Journal of Hypertension, 38, 1682–1698. 10.1097/HJH.0000000000002508 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zanoli, L. , Granata, A. , Lentini, P. , Gaudio, A. , & Castellino, P. (2017). Augmentation index is increased in patients with inflammatory bowel disease, a meta‐analysis. European Journal of Internal Medicine, 39, e31–e32. 10.1016/j.ejim.2016.12.012 [DOI] [PubMed] [Google Scholar]
- Zheng, J. , Nakamura, T. , Lu, N. , Hori, K. , Oguri, M. , Sakurai, M. , & Ishizaki, M. (2019). The radial augmentation index in children with Kawasaki disease without acute coronary artery lesions during the convalescent period. Therapeutics and Clinical Risk Management, 15, 701–709. 10.2147/TCRM.S208632 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zieman, S. J. , Melenovsky, V. , & Kass, D. A. (2005). Mechanisms, pathophysiology, and therapy of arterial stiffness. Arteriosclerosis, Thrombosis, and Vascular Biology, 25, 932–943. 10.1161/01.ATV.0000160548.78317.29 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The data that support the findings of this study are available upon request from the corresponding author. The data are not publicly available due to privacy or ethical restrictions.


