Dear Editor,
Two messenger ribonucleic acid (mRNA) vaccines were authorized for emergency use by the United States Food and Drug Administration (U.S. FDA) against the Coronavirus Disease 2019 (COVID‐19) at the end of 2020. 1 Immunization is a significant milestone in fighting this pandemic; however, safety regarding special populations has yet to be determined. We report a case of a patient who suffered from vasculitis after COVID‐19 vaccination.
A 46‐year‐old female presented to the Department of Dermatology at Tufts Medical Center with a past medical history of psoriasis, psoriatic arthritis, irritable bowel syndrome, and leukocytoclastic vasculitis. The diagnosis of her vasculitis was biopsy‐proven (Fig. 1) with unremarkable laboratory exams including complete blood count, liver function test, hepatitis B and C serologies, and QuantiFERON‐TB Gold. The vasculitis was successfully treated with a prednisone taper. The patient denied any flares for 2 years until she received the BNT162b2 mRNA COVID‐19 vaccine. Within 2 days, she noted a mild exacerbation of her palpable purpuric papules on the bilateral lower legs (Fig. 2a). Clinical differential diagnosis includes small vessel vasculitis such as IgA vasculitis. The vasculitis stabilized, and 2 days after the second dose of the vaccine, it exacerbated again with significant exquisitely tender palpable purpuric papules distributed bilaterally on the lower legs, feet, upper extremities, lower back, and abdomen (Fig. 2b,c). No systemic involvement or manifestation was perceived. After discussing risks and benefits, particularly of potential lowered vaccine efficacy, the patient was prescribed topical steroids and a prednisone taper.
Figure 1.
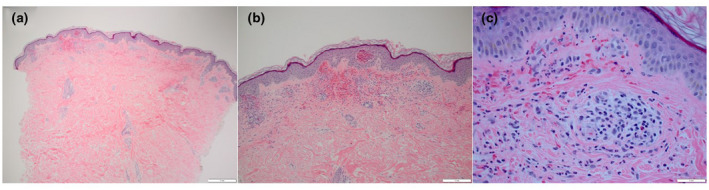
Histopathologic photomicrographs, H&E stain: Sections show a perivascular mixed inflammatory infiltrate with numerous neutrophils, lymphocytes, and occasional eosinophils. There is leukocytoclasia and erythrocyte extravasation, but fibrinoid necrosis of vessels is not observed. (a) Magnification at ×4, (b) magnification at ×10, and (c) magnification at ×40
Figure 2.
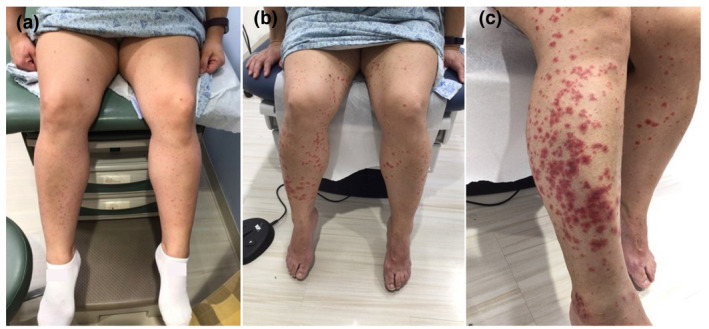
(a) Leukocytoclastic vasculitis after first dose of COVID‐19 vaccine. Palpable purpuric papules distributed on bilateral lower legs. (b) Leukocytoclastic vasculitis after second dose of COVID‐19 vaccine. Widespread palpable purpuric papules and plaques distributed on bilateral legs and feet. (c) Leukocytoclastic vasculitis after second dose of COVID‐19 vaccine. Closer look to widespread palpable purpuric papules and plaques located on the right lateral anterior lower leg
Vasculitis precipitation or exacerbation has been reported secondary to multiple vaccines such as the influenza virus, hepatitis B virus (HBV), Bacille Calmette‐Guerin (BCG), and human papillomavirus (HPV). However, this is rare and the relationship between immunizations and vasculitis has yet to be determined. 2
A possible mechanism for the exacerbation may be similar to the one hypothesized about the influenza vaccine, which can cause vessel damage likely secondary to abnormal immunological activation with vaccine‐related antigens promoting antibody development and immune complex deposition. The COVID‐19 virus may provoke hyperactivation of the immune system secondary to cross‐reactivity and molecular mimicry between the virus and self‐antigens, consequently triggering autoimmune disorders such as vasculitis, antiphospholipid syndrome, immune‐mediated myositis, and myocarditis. 3 Before the COVID‐19 vaccine became available, it was suspected that a similar scenario might happen following its administration. It is notable that despite a diagnosis of psoriasis and psoriatic arthritis, the patient had no exacerbation in these T helper 17 (Th17)‐mediated diseases.
Our patient was prescribed a prednisone taper. There is concern regarding prescribing such immunomodulatory agents shortly after vaccines. However, it has been reported that systemic steroids cause variable effects on immunity, which is dose‐dependent. Up to 20 mg/day of prednisone seems to not have any effect on patients' immune response to vaccines. For the COVID‐19 vaccine, it is speculated that regardless of the type of vaccine, systemic corticosteroids have “no or minimal risk” in patients' immune response. 4
It is important for healthcare providers and patients to be aware that the COVID‐19 vaccine can potentially precipitate or exacerbate cutaneous inflammation. When it comes to therapeutic options of such events, it will depend on a case‐by‐case basis to decide what is in the best interest of the patient.
Conflict of interest: Dr. David Rosmarin has received honoraria as a consultant for AbbVie, Celgene, Dermavant, Dermira, Janssen, Lilly, Novartis, Pfizer, and Regeneron Pharmaceuticals Inc.; has received research support from AbbVie, Bristol Meyers Squibb, Celgene, Dermira, Incyte, Janssen, Lilly, Merck, Novartis, Pfizer, and Regeneron Pharmaceuticals Inc.; and has served as a paid speaker for AbbVie, Celgene, Janssen, Lilly, Novartis, Pfizer, Regeneron Pharmaceuticals Inc., and Sanofi. Dr. Stephanie R. Cohen, Dr. Lisa Prussick, Jared S. Khan, David X. Gao, and Dr. Arash Radfar have no conflicts of interest to declare.
Funding source: Janssen Scientific Affairs, LLC. Title: Tufts Medical Center Psoriasis Research Fellowship. Grant ID: FS18343.
References
- 1. U.S. Food and Drug Administration . Infectious Disease Coronavirus. 2021. Pfizer‐BioNTech COVID‐19 Vaccine. https://www.fda.gov/emergency‐preparedness‐and‐response/coronavirus‐disease‐2019‐covid‐19/pfizer‐biontech‐covid‐19‐vaccine. Accessed on 16 January 2021.
- 2. Bonetto C, Trotta F, Felicetti P, et al. Vasculitis as an adverse event following immunization – systematic literature review. Vaccine 2016; 34: 6641–6651. [DOI] [PubMed] [Google Scholar]
- 3. Vojdani A, Kharrazian D. Potential antigenic cross‐reactivity between SARS‐CoV‐2 and human tissue with a possible link to an increase in autoimmune diseases. Clin Immunol 2020; 217: 108480. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Gresham LM, Marzario B, Dutz J, et al. An evidence‐based guide to SARS‐CoV‐2 vaccination of patients on immunotherapies in dermatology. J Am Acad Dermatol 2021; S0190‐9622(21)00196‐1. 10.1016/j.jaad.2021.01.047 [DOI] [PMC free article] [PubMed] [Google Scholar]


