Abstract
The mucocutaneous manifestations of Corona Virus Disease 2019 (COVID‐19) logically may reflect systemic visceral involvements. These findings are visible and easy to approach like biopsies for exact histopathologic evaluations. This systematic review was conducted to collect the mucocutaneous histopathologic data of COVID‐19 patients for future investigations and interpretations. The COVID‐19 dermatology resource of the Centre of Evidence‐Based Dermatology (CEBD) at the University of Nottingham, PubMed, Scopus, Google Scholar and Medscape was searched for relevant English articles published by June 3, 2020. This review included 31 articles, involving 459 patients. The common primary virus‐related mucocutaneous manifestations are easy to approach in the course of COVID‐19. The authors of this study supposed dermatopathological findings as the predictors of the nature of potential systemic involvements and outcomes of COVID‐19. Scrutinizing these findings can help with adopting more effective therapeutic and management strategies; nevertheless, this review found the severity and time of onset of symptoms not to be associated with the laboratory and histopathological findings. Deterioration of clinical conditions and laboratory tests was also not related to the histopathological findings. It is recommended that meta‐analyses be conducted in the future to detail on these data for having more comprehensive and better conclusion.
Keywords: biopsy, coronavirus, COVID‐19, cutaneous, dermatology, histopathology, mucocutaneous, pathology, SARS‐CoV‐2, skin, systematic review
Abbreviations
- ARDS
Acute Respiratory Distress Syndrome
- CEBD
Centre of Evidence‐Based Dermatology
- COVID‐19
Corona Virus Disease 2019
- RBC
Red Blood Cell
1. INTRODUCTION
COVID‐19 has just appeared in the world and has caused various symptoms in patients. These symptoms include skin manifestations. These skin symptoms may be caused by viruses or drug reactions taken by the patient during the illness. COVID‐19 was found to cause storms in viral‐host immune interactions and affect the skin and visceral compartments. Mucocutaneous manifestations can reflect pathologic events such as internal organ involvements during infection with SARS‐CoV‐2 as in the case of many other systemic disorders. As in the case of other viral disorders, SARS‐CoV‐2 can frequently cause morbilliform, exanthematous, maculopapular or urticarial eruption, which is quite expectable in adults, 1 , 2 , 3 , 4 , 5 , 6 , 7 , 8 infants and children. 9 , 10 The other skin manifestations included dermatitis herpetiformis, varicella‐like exanthem, pityriasis rosea and petechiae 11 , 12 , 13 , 14 as well as mucosal involvements such as aphthous ulcers or conjunctivitis. 1 , 15 The abnormal primary cutaneous manifestations in viral disorders frequently reported in COVID‐19 patients include “COVID toes” as acral vasculopathic rashes that clinically resemble chilblains or perniosis, 16 , 17 , 18 , 19 acral and digital ischaemia, 20 , 21 , 22 papulosquamous eruption, 23 petechiae 24 and livedo reticularis. 25 These manifestations suggest a coronavirus pathomechanism that differs from that of similar respiratory‐associated viruses, as this virus is not limited to few organs and the virus‐related consequences can involve almost all vital organs. The potentially exclusive skin manifestations of COVID‐19 such as thrombotic vasculopathy or vasculitis 19 , 20 , 21 , 22 can involve visceral tissues and cause acute respiratory distress syndrome (ARDS) and organ failure. Also, the drugs used to treat COVID‐19 could have several side effects, such as mucocutaneous drug reactions; morbilliform/exanthematous maculopapular rashes, urticarial eruptions and AGEP are most types of skin drug reactions that are usually managed by steroids during few days. 26 Hydroxychloroquine and lopinavir/ritonavir are the most prevalent used drugs with the highest skin adverse reaction. 26 So, mucocutaneous drug reactions should be considered in any prescription treatment.
Therefore, skin biopsies play a key role in better understanding the events during COVID‐19. Despite its great value, the skin biopsy cannot be performed in all COVID‐19 patients with the primary cutaneous eruption. 27
Mucocutaneous eruptions can help clinically predict the main histopathologic patterns of the rashes, not in all patients with SARS‐CoV‐2. Broadening knowledge about mucocutaneous manifestations in COVID‐19 patients is crucial given the reported skin rashes in approximately 20% of these patients before, during or after their infection. 20 The histopathologic features of the cutaneous manifestations can predict the nature of systemic involvements and COVID‐19 outcomes. The early detection of these symptoms can also help early diagnose COVID‐19 and increase the survival rate.
This systematic review was conducted to determine the correlations of clinical manifestations with courses of COVID‐19 and skin eruption in patients with COVID‐19 and primary skin eruptions by collecting their biopsies and histopathologic data. This study was intended to collect previous studies in this field to gain a clearer understanding of general and individual pathologic pictures during the infection. This systematic review was performed according to the PRISMA (Preferred Reporting Items for Systematic Reviews and Meta‐Analyses) statement. And, the PICO of this study was as follows: Population: humans with COVID‐19, Intervention and comparator: Histopathologic evaluations and skin biopsy of COVID‐19 patients who had primary cutaneous and mucosal eruptions, Outcomes: Early recognition of COVID‐19 patients to better treatment of COVID‐19 patients.
2. ELIGIBILITY CRITERIA
The inclusion criteria comprised all English articles on primary cutaneous and mucosal eruptions induced by COVID‐19 in patients undergoing histopathologic evaluations and skin biopsy. The population‐intervention‐comparator‐outcomes‐study design (PICOS) framework was used to identify eligible cases in this systematic review. Details of the criteria established a priori were as follows: Population: humans with COVID‐19, (there were no restrictions on age, gender or other demographics), Intervention and comparator: Histopathologic evaluations and skin biopsy of COVID‐19 patients who had primary cutaneous and mucosal eruptions, Outcomes: Early recognition of COVID‐19 patients to better treatment of COVID‐19 patients.
Exclusion criteria were reviews, animal studies, COVID‐19 articles without mucocutaneous manifestations, COVID‐19 articles with mucocutaneous manifestation without a histopathological biopsy.
3. DATABASES AND SEARCH STRATEGY
PubMed, Scopus, Google Scholar, Medscape and the COVID‐19 dermatology resource of the CEBD at the University of Nottingham (https://www.nottingham.ac.uk/), that is the link of skin manifestations of coronavirus, were searched for the articles published by June 3, 2020 using the following terms: "COVID‐19" OR "severe acute respiratory syndrome coronavirus 2" AND "Skin “ OR "Mucosa" OR "Cutaneous" OR "Skin Manifestations" OR "Dermatology" OR "Pathology" OR "Histopathology" OR "Histopathologic" OR "Histology" OR "Biopsy."
4. DATA EXTRACTION PROCESS
Endnote® X9 (Clarivate Analytics, Philadelphia, USA) was used for study screening and data extraction. Reviewers assigned each study to the inclusion and exclusion groups. They read the titles and abstracts, and if doubted, have evaluated the full text. Then they read the full text for the inclusion process. Disagreement situations regarding the inclusion process resolved through dialogue, and no necessity for a third‐party involvement occurred. In Figure 1, PRISMA flow diagram was shown.
FIGURE 1.
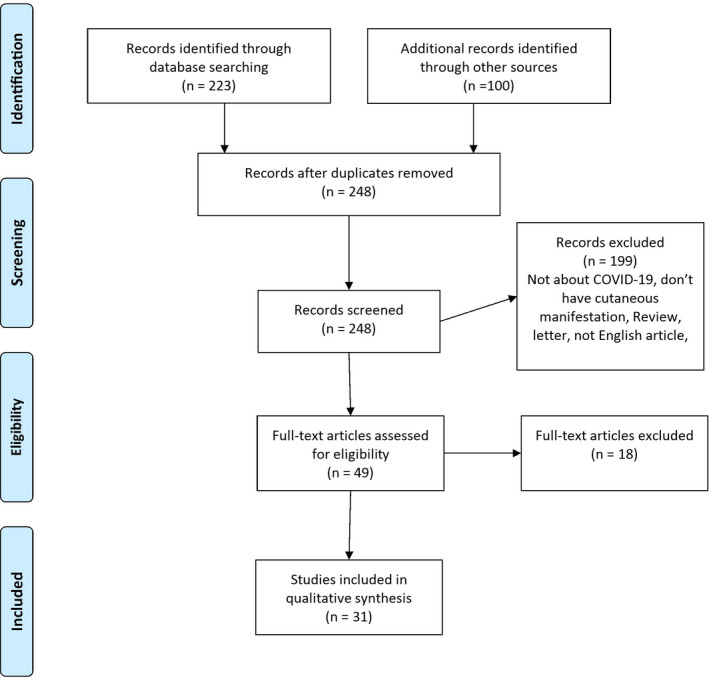
PRISMA flow diagram
Finally, the interpretation of data is the experts’ interpretation, which is achieved through discussion with each other. The experts were professors of dermatology and dermatopathology who have published numerous articles on COVID‐19 and have worked together for many years.
5. DATA EXTRACTION
Reviewers extracted data, filled a pre‐designed spreadsheet containing the following information for each study: the article title, Case characteristics, COVID‐19 signs and symptoms, Laboratory tests, COVID‐19 PCR, Cutaneous manifestations, Cutaneous symptoms, Distribution, Time of onset the cutaneous symptoms, New drugs during previous 2 weeks, Time of resolution of the skin symptoms and the result of the Skin biopsy.
6. QUALITY ASSESSMENT
MD and FS used two risk assessment tools for the studies that include in this study. The first risk bias assessment tool used in this study was the Risk of Bias in Non‐randomized Studies of Interventions (ROBINS‐I), 28 which is a list of seven domains. The first two domains of ROBINS‐1 are about the selection of participants. The third domain is about classification. The other four domains address issues after the start of interventions: biases due to deviations from intended interventions, missing data, measurement of outcomes and selection of the reported result. The second tool that we used for the methodological quality and synthesis of the case reports and case series was a new bias assessment tool that was suggested by Murad et al. 29
The bias assessment tool has four domains of Selection, Ascertainment, Causality and Reporting. The tool also has eight questions that each question has one score. MD and FS separately examined 31 articles that were included in the systematic review study. Disagreement situations regarding the inclusion process resolved through dialogue and no necessity for a third‐party involvement occurred.
7. STUDY SELECTION
Out of 248 articles screened based on the inclusion criteria, 49 were found eligible and their titles and abstracts reviewed by two dermatologists. 31 articles, including 459 patients, were ultimately selected and their full texts investigated for the sake of data entry in the systematic review. A study by Carreras‐Presas, Carmen et al, entitled “Oral vesiculobullous lesions associated with SARS‐CoV‐2 infection” and by Angelo Valerio Marzano et al, entitled “Varicella‐like exanthem as a specific COVID‐19‐associated skin manifestation: multicenter case series of 22 patients,” which included the data of skin biopsies and nonspecific viral infection patterns, did not describe microscopic histopathologic features.
8. STUDY CHARACTERISTICS
Details of the 459 patients presented in “Table 1” include their clinical presentation of mucocutaneous involvements and other COVID‐19‐related signs and symptoms and the histopathologic features of their biopsies. 19 , 23 , 24 , 27 , 30 , 31 , 32 , 33 , 34 , 35 , 36 , 37 , 38 , 39 , 40 , 41 , 42 , 43 , 44 , 45 , 46 , 47 , 48 , 49 , 50 , 51 , 52 , 53 , 54 , 55 , 56 Of the 459 patients in this study, 215 were male, 221 were female and 23 genders were not reported. Patients’ age ranged from two to 100 years old. In a 69‐year‐old woman of “A clinicopathological study of eight patients with COVID‐19 pneumonia and a late‐onset exanthema,” Coalescent erythematous maculopapules, pustules, desquamation were recorded 33 days after other symptoms, and in the case of “Chilblains in children in the setting of COVID‐19 pandemic,” skin symptoms were recorded 28 days before other symptoms. These two cases are the fastest and latest skin symptoms in the 459 cases studied in this systematic review.
TABLE 1.
Histopathologic findings in COVID‐19 patients with mucocutaneous manifestations
| Reference | Title | Case characteristics | COVID−19 signs and symptoms | Lab tests | COVID−19 PCR | Cutaneous manifestations | Cutaneous symptoms | Distribution | Time of onset the cutaneous symptoms (Compared to other symptoms) | New drugs during previous 2 weeks | Time of the lesion resolution | Skin biopsy |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 1 | A clinicopathological study of 8 patients with COVID−19 pneumonia and a late‐onset exanthema | 58‐year‐old male | NM | Lymphopenia, neutrophilia, eosinophilia elevated D‐dimer and CRP and liver enzymes | Positive | Coalescent, erythematous‐violaceous, maculopapules | NM | Generalized | 29 days after | None | 12 days | Subcorneal pustules, spongiosis, papillary oedema, dense perivascular and interstitial neutrophilic infiltrate with moderate presence of eosinophils, erythrocyte extravasation, fibrin thrombi, melanophages |
| 84‐year‐old female | NM | Lymphopenia and elevated D‐dimer and CRP | Negative | Coalescent erythematous, maculopapules | NM | Trunk, flexures | 12 days after | Hydroxychloroquine, lopinavir/ritonavir, ceftriaxone | 11 days | Subcorneal pustules, spongiosis, papillary oedema, moderate perivascular and interstitial neutrophilic infiltrate with discrete presence of eosinophils, erythrocyte extravasation, focal fibrin thrombi | ||
| 82‐year‐old female | NM | Lymphopenia and elevated D‐dimer and CRP | Positive | Ill‐defined erythematous patches | NM | Trunk, flexures | 29 days after | Fosfomycin | 16 days ongoing | Intraepidermal pustules, spongiosis, discrete perivascular and interstitial neutrophilic infiltrate with scarce presence of eosinophils | ||
| 68‐year‐old female | NM | Lymphopenia and elevated D‐dimer and C‐reactive protein | Positive | Ill‐defined erythematous patches | NM | Trunk, flexures | 28 days after | Metamizole, linezolid, piperallicin‐tazobactam, amiodarone | 9 days | Subcorneal pustules, spongiosis, papillary oedema, discrete perivascular and interstitial neutrophilic infiltrate with scarce presence of eosinophils | ||
| 51‐year‐old male | NM | Lymphopenia and elevated D‐dimer and CRP | Positive | Coalescent erythematous macules | NM | Trunk, proximal extremities | 29 days after | None | 10 days | Focal spongiosis, exocytosis of neutrophils, discrete, perivascular and interstitial neutrophilic infiltrate with discrete presence of eosinophils, focal fibrin thrombi, focal basal layer vacuolar degeneration | ||
| 88‐year‐old male | NM | Lymphopenia and elevated D‐dimer and CRP | Positive | Coalescent erythematous maculopapules | NM | Trunk, extremities, face, | 31 days after | Furosemide | 12 days | Subcorneal pustules, spongiosis, presence of necrotic keratinocytes, papillary oedema, discrete perivascular and interstitial neutrophilic infiltrate with scarce presence of eosinophils, melanophages | ||
| 69‐year‐old female | NM | Lymphopenia and elevated D‐dimer and CRP | Positive | Coalescent erythematous maculopapules, pustules, desquamation | NM | Trunk, flexures, face face | 33 days after | None | 15 days ongoing | Subcorneal pustules, spongiosis, papillary oedema, moderate perivascular and interstitial neutrophilic infiltrate with discrete presence of eosinophils | ||
| 78‐year‐old male | NM | Lymphopenia and elevated D‐dimer and CRP | Positive | Ill‐defined erythematous patches | NM | Trunk | 30 days after | Piperacillin‐tazobactam, meropenem, linezolid | 8 days ongoing | Spongiosis, discrete perivascular and interstitial neutrophilic infiltrate with scarce presence of eosinophils | ||
| 2 | Acral cutaneous lesions in the Time of COVID−19 | 14 cases: 11 children and 3 young adults, 6 males and 8 females | Only in three cases cough and fever preceded the onset of the lesions 3 weeks before | Normal | Negative | Acral eruption of erythemato‐violaceous papules and macules, with possible bullous evolution, or digital swelling. Two children developed erythemato‐papular targetoid lesions on the hands and elbows after few days | Mild pruritus | 8 cases on the feet,4 cases on the hand, and 2 cases on both sites | 3 weeks before in three cases | None | 14–28 days | Acral lesions: diffuse dense lymphoid infiltrate of the superficial and deep dermis, as well as hypodermis, with a prevalent perivascular pattern, and signs of endothelial activation, targetoid lesions of the elbows: mild superficial perivascular dermatitis. |
| 3 | Acral purpuric lesions (Erythema multiforme type) associated with thrombotic vasculopathy in a child during the COVID−19 pandemic | 12‐year‐old boy | None | Normal | Negative | Haemorrhagic purpuric eruption and vesicular blisters | Pruritus | Heels of both feet | 4 days after | None | NM | Partial epidermal necrosis and perivascular lymphoid infiltrate in superficial and deep dermis. In addition, some capillaries in papillary dermis showed images of microthrombi, with extravasation of red blood cells. Vasculitic changes were present in relation to the lymphoid component but not in the thrombotic one. |
| 4 | Acro‐ischaemia in hospitalized COVID−19 patients | Three cases | Atypical bilateral pneumonia | Elevated D‐dimer in all patients, elevated fibrinogen in two patients | Positive | Rounded reddish‐purple plaques, measuring between 0.5–1 cm, sharply defined, with no retiform borders | NM | Toes, soles | At the same time | NM | 14 days | Ischaemic necrosis affecting the epidermis and dermis with signs of re‐epithelialization with no evidence of vasculitis or microthrombi |
| 5 | Cutaneous lesions in a patient with COVID−19: are they related? | 57‐year‐old female | Fever (39°C) lasting for 4 days, and dry cough | NM | Positive | Diffuse fixed erythematous blanching maculopapular lesion with burning sensation over the palms | NM | Limbs and trunk | 2 days before | Paracetamol | 9 days | Slight spongiosis, basal cell vacuolation and mild perivascular lymphocytic infiltrate (c). PCR on whole‐skin biopsy specimen was negative for SARS‐CoV−2. |
| 6 | Chilblains is a common cutaneous finding during the COVID−19 pandemic: a retrospective nationwide study from France | 277 patients, 129 men and 130 women | Fever (n = 48), Respiratory symptoms (n = 44), Anosmia/ageusia (n = 18), Digestive symptoms (n = 16) in total 103 patients | NM | 25 cases were positive | Morbilliform lesions (n = 25), Acral lesions (n = 142), Chilblains (n = 106), Dyshidrosis‐like (n = 20), other (n = 16), Vesicular lesions (n = 41): Vesicles/Varicella‐like, Acral dyshidrosis‐like, Livedo reticularis (n = 4), Urticarial lesions (n = 26), Petechial lesions (n = 7), eczema‐like, angiomatous, annular lesions (n = 41) | NM NM | Trunk, limbs, face, Feet, Hands, Diffuse, Acral | NM | NM | NM | Biopsy of 3 chilblain‐like lesions showed a lichenoid dermatitis with a perivascular and eccrine mononuclear infiltrate, and vascular microthrombi in 2 cases. |
| 7 | Chilblains in children in the setting of COVID−19 pandemic | 22 cases, 13 men and 9 women | Mild respiratory symptoms (cough, rhinorrhea) (n = 9), gastrointestinal complaints (abdominal pain and diarrhoea) (n = 2) | Coagulation studies (n = 18), haemogram (n = 10), serum chemistry (n = 4), lupus anticoagulant (n = 1) was normal. D‐dimer level (n = 16) was elevated in one case. | Positive in one case | Erythematous to purpuric macules and violaceous swellings, dark ischaemic areas with superficial blisters, concomitant erythema multiforme in 4 cases | Pruritus (n = 9), pain or tenderness (n = 7) | Toes, feet, fingers and hands | 1 to 28 days before | Oral analgesics, oral antihistamines. For associated erythema multiforme: Topical corticosteroids and a short course of oral steroids | 3–5 weeks after their onset | Acral lesions (4 from the feet, 2 from the toes) in 6 patients: superficial and deep angiocentric and eccrinotropic lymphocytic infiltrate, papillary dermal oedema, vacuolar degeneration of the basal layer and lymphocytic exocytosis to the epidermis and acrosyringia, lymphocytic vasculopathy, mild dermal and perieccrine mucinosis, lymphocytic eccrine hidradenitis, vascular ectasia, red cell extravasation and focal thrombosis in papillary and reticular dermis capillaries |
| 8 | Chilblain‐ like lesions in children following suspected COVID−19 infection | : 11‐year‐old girl | Intermittent fever | Blood tests were normal | Negative | Erythematous and dusky 5–15 mm plaques | Pain, swelling | Left foot and toes | 20 days before | NM | Until now | Dense lymphocytic perivascular cuffing and periadnexal infiltration, vasculitis in small‐ to medium‐sized vessels with endothelial cell swelling and red blood cell extravasation, fibrin thrombus in superficial capillary vessels |
| 9 | Clinical and histopathological study of skin dermatoses in patients affected by COVID−19 infection in the Northern part of Italy | A hospitalized patient | Fever, sore throat, and cough | NM | NM | Exanthema | NM | Trunk and limbs | NM | Same time | NM | Perivascular spongiotic dermatitis with exocytosis along with a large nest of Langerhans cells and a dense perivascular lymphocytic infiltration eosinophilic rich around the swollen blood vessels with extravasated erythrocytes |
| old male | Fever, sore throat, and cough | NM | NM | Papular erythematous exanthema | NM | Trunk | NM | Same time | NM | oedematous dermis with many eosinophils, Cuffs of lymphocytes around blood vessels in a lymphocytic vasculitis histopathological pattern were observed | ||
| 10 | Cutaneous Clinico‐Pathological Findings in Three COVID−19‐Positive Patients Observed in the Metropolitan Area of Milan, Italy | 59‐year‐old female | Bilateral interstitial pneumonia | Elevated CRP | Positive | Widespread erythematous macules | None | Arms, trunk and lower limbs | Three days after admission | Lopinavir‐ritonavir, heparin and levofloxacin | 5 days | Superficial perivascular dermatitis with slight lymphocytic exocytosis, small thrombus in a vessel of mid dermis. Swollen thrombosed vessels with neutrophils, eosinophils and nuclear debris were patchy distributed in the dermis. |
| 89‐year‐old female | Fever and cough | A mild increase in fibrinogen and transaminases | Positive | Exanthem | NM | Trunk and arms | NM | Ceftriaxone and azithromycin | 8 days | Superficial and deep perivascular dermatitis with cuffs of lymphocytes surrounding blood vessels in a vasculitic pattern, extravasated red blood cells from damaged vessels in the mid dermis | ||
| 57‐year‐old male | Fever, headache, cough and arthralgia | NM | Positive | Widespread pruritic eruption of erythematous macules and papules | NM | Widespread | 2 days after systemic symptoms | Levofloxacin and hydroxychloroquine | 10 days | Superficial perivascular vesicular dermatitis, focal acantholytic suprabasal clefts, dyskeratotic and ballooning herpes‐like keratinocytes, patchy band‐like infiltration with occasional necrotic keratinocytes and minimal lymphocytic satellitosis. In the dermis, the vessels were swollen, with dense lymphocyte infiltration, mixed with rare eosinophils. Within the epidermis, a nest of Langerhans cells was also observed. | ||
| 11 | Clustered Cases of Acral Perniosis: Clinical Features, Histopathology and Relationship to COVID−19 | 6 cases; 3 boys and 3 girls (under 18 years old) | Rhinorrhea, congestion, sore throat and fever (n = 2) | Normal | Negative | Violaceous macules and dusky, purpuric plaques, superficial bullae and focal haemorrhagic crust and livedo reticularis (reticulated erythema) | Pruritus, tenderness and swelling | Toes, heels, soles and feet and flexor surfaces of the forearms and hands | NM | NM | NM | Superficial and deep lymphocytic infiltrate that also abuts the junctional zone, with vacuolar change and purpura, haemorrhagic parakeratosis in the stratum corneum. Dense infiltration of perivascular and perieccrine and intramural lymphocytes intramural lymphocytes, no evidence of thrombosis in the vessels. Direct immunofluorescence was negative |
| 12 | Complement associated microvascular injury and thrombosis in the pathogenesis of severe COVID−19 infection: A report of five cases | 32 years old male | Fever and cough and dyspnoea, acute respiratory failure | Elevated D‐dimer, INR, CH50, C3, C4 | NM | Retiform purpura with extensive surrounding inflammation | Mentioned | Buttocks | 4 days after intubation | Hydroxychloroquine, azithromycin, remdesivir | NM | Thrombogenic vasculopathy accompanied by extensive necrosis of the epidermis and adnexal structures, including the eccrine coil, interstitial and perivascular neutrophilia with prominent leukocytoclasia, extensive deposition of C5b−9 within the microvasculature |
| 66‐year‐old female | Fever, cough, diarrhoea, chest pain | Thrombocytopenia, elevated D‐dimer | NM | Dusky purpuric patches | NM | Palms and soles bilaterally | 1 day after intubation | Hydroxychloroquine, enoxaparin | NM | Superficial vascular ectasia and an occlusive arterial thrombus within the deeper reticular dermis in the absence of inflammation. Extensive vascular deposits of C5b−9, C3d and C4d. A biopsy of normal‐appearing deltoid skin also showed conspicuous microvascular deposits of C5b−9. | ||
| 40‐year‐old female | Dry cough, fever, myalgia, diarrhoea, and progressive dyspnoea | Elevated D‐dimer and INR | Positive | Purpuric reticulated eruptions consistent with livedo racemosa | NM | Chest, legs and arms | NM | NM | NM | Modest perivascular lymphocytic infiltrate in the superficial dermis along with deeper‐seated small thrombi within rare venules of the deep dermis, no vasculitis. Significant vascular deposits of C5b−9 and C4d. A biopsy of normal deltoid skin showed microvascular deposits of C5b−9 throughout the dermis. | ||
| 13 | Dermatologic findings in two patients with COVID−19 | 60‐year‐old male | Low‐grade fever, myalgia, fatigue and a mild cough | NM | Positive | Scattered erythematous maculescoalescing into papules. One week after recovery of systemic symptoms, small round purpuric macules were seen in the formerly involved areas | None | Back, flanks, groyne, lower extremities | 3 days before | NM | NM | Mild perivascular infiltrate of predominantly mononuclear cells surrounding the superficial blood vessels. The epidermis showed scattered foci of hydropic changes along with minimal acanthosis, slight spongiosis and foci of parakeratosis. |
| 14 | Digitate Papulosquamous Eruption Associated with Severe Acute Respiratory Syndrome Coronavirus2 Infection | An elderly patient | Fatigue, fever and dyspnoea | NM | Positive | Squamous and erythematous papules and patches | NM | Trunk and thighs, upper arms, shoulders | One day after hospital admission | NM | 7 days | Foci of spongiosis with focal parakeratosis in the epidermis and a few rounded spongiotic vesicles containing aggregates of lymphocytes and Langerhans cells, moderate lymphohistiocytic infiltrate was present in the superficial dermis and papillary dermal oedema. |
| 15 | Erythema multiforme‐like eruption in patients with COVID−19 infection: clinical and histological findings | 4 females | NM | Elevated CRP and D‐dimer, Decreased lymphocyte count | NM | Erythemato‐violaceous patches with a dusky centre, and a pseudo‐vesicle in the middle, palatal macules and petechiae | NM | Upper trunk, face, limbs, oral mucosa | 19.5 days after | Systemic corticosteroids | 2–3 weeks | Normal basket‐weave stratum corneum, and mild to moderate spongiosis in epidermis, dilated vessels filled with neutrophils, extravasation of red blood cells, and lymphocytic perivascular and interstitial infiltrate in the dermis. Basal vacuolar changes with interface dermatitis and lymphocytic exocytosis. |
| 16 | Cutaneous manifestations in COVID−19: a first perspective. Safety concerns of clinical images and skin biopsies | 32‐years‐old female | NM | NM | NM | Urticariform rash | NM | NM | 6 days after the onset of other symptoms | Hydroxychloroquine, azithromycin and oral antihistamines | 5 days | Perivascular infiltrate of lymphocytes, some eosinophils and upper dermal oedema |
| 17 | Coronavirus (COVID−19) infection‐induced chilblains: a case report with histopathological findings | 23‐year‐old male | Low‐grade fever and a dry cough | All lab tests were normal | Positive | Violaceous and infiltrated plaques | Painful | Toes and lateral feet | 3 days before | NM | NM | Superficial and deep lichenoid, perivascular and perieccrine infiltrate of lymphocytes with occasional plasma cells, vacuolar alteration along the basal layer of the epidermis with scattered singly necrotic (apoptotic) keratinocytes in the superficial layers of the epidermis. The basement membrane zone was smudged with papillary dermal fibrin. The infiltrate was dense and lichenoid in the papillary and superficial reticular dermis, and the deeper dermis had a tightly cuffed, perivascular and perieccrine distribution. Some nuclear debris was present, but no neutrophils were identified. The venules surrounded by the lymphoplasmacytic infiltrate had plump endothelial cells. Notably no intraluminal fibrin thrombi were identified, and no fibrin was identified within venule walls. Direct immunofluorescence was negative. |
| 18 | A late‐onset widespread skin rash in a previous COVID−19 infected patient:viral or multidrug effect? | 47‐year‐old male | Syncope | Leukocytosis | Positive | Multiple, raised erythematous wheals, alone or in cluster, some of them with central purple hyperpigmentation | Pruritus | Head, trunk and upper arms | 4 days after hospitalization | Ceftriaxone, lopinavir/ritonavir,hydroxychloroquine, enoxaparin, intravenous steroid and antihistamine agent | 7 days | Orthokeratotic hyperkeratosis, spongiosis, focal vacuolar degeneration of basal keratinocytes and focal lymphocytic exocytosis. Slight inflammatory lymphomorphonuclear infiltrate of superficial dermis with minimal perivascular neutrophilic component was observed, with occasional aspects of vessel wall damage. |
| 19 | Histologic features of long‐lasting chilblain‐like lesions in a paediatric COVID−19 patient | 16‐year‐old boy | Dysgeusia and mild diarrhoea | Normal | Positive | Erythematooedematous, partially eroded, macules and plaques | Asymptomatic | Dorsal aspects of the finger and toe | 20 days before | NM | Several weeks after the first symptoms | Oedema of the papillary dermis, superficial and deep lymphocytic infiltrate in a perivascular and strong perieccrine pattern; no signs of endothelial damage. |
| 20 | Novel outbreak of acral lesions in times of COVID−19: A description of 74 cases from a tertiary university hospital in Spain | 74 patients, 42 men and 32 women | Cough Fever Asthenia, myalgia Diarrhoea, nausea, vomiting Dyspnoea Anosmia, ageusia | NM | NM | Erythematous papules (76.4%), Purpuric macules (40.54%), Both (16.21%), Erosion (10.8%), Swelling (16.21%) | Pruritus (32.4%) Pain (27%) | Hands and Feet | NM | NM | NM | Lymphocytic perivascular and perieccrine infiltrate with no vascular occlusion or intravascular thrombi. Direct immunofluorescence study was negative. |
| 21 |
Petechial Skin Rash Associated with Severe Acute Respiratory Syndrome Coronavirus 2 Infection |
48‐year‐old male | Fever, pleuritic chest pain and shortness of breath | Lymphopenia, elevated level of CRP and D‐dimer | Positive | Confluent erythematous macules, papules and petechiae | Pruritus | Buttocks, popliteal fossae, proximal thighs, abdomen | 3 days after | Hydroxychloroquine, lopinavir‐ritonavir and azithromycin, loratadine and topical steroid | 5 days | Superficial perivascular lymphocytic infiltrate with abundant red cell extravasation and focal papillary oedema, along with focal parakeratosis and isolated dyskeratotic cells. No features of thrombotic vasculopathy |
| 22 | Acute urticaria with pyrexia as the first manifestations of a COVID−19 infection | 60‐year‐old female | Fever and dry cough | Mild lymphopenia and increased liver enzymes (SGOT, SGPT, LDH, GGT three times normal) | NM | Urticarial eruption | NM | Anterior and posterior trunk | 5 days after | None | NM | Slight vacuolar‐type interface dermatitis with occasional necrotic keratinocytes. No eosinophils were encountered. These histological alterations were compatible with an erythema multiforme‐like pattern |
| 23 | SARS‐CoV−2 infection presenting as a febrile rash | 39‐year‐old male | High grade fever | Normal | Positive | Erythematous and oedematous non‐pruritic annular fixed plaques | None | NM | Concomitant with fever | Hydroxychloroquine | One week after symptoms | Superficial perivascular infiltrate of lymphocytes without eosinophils, papillary dermal oedema, subtle epidermal spongiosis, mild lymphocyte exocytosis, lichenoid and vacuolar interface dermatitis with occasional dyskeratotic keratinocytes in the basal layer, no virally induced cytopathic alterations or intranuclear inclusions, negative DIF |
| 24 | Skin manifestations of COVID−19 | 68‐year‐old male | NM | NM | NM | Morbiliform rash, purpura, ulcerated, purpuric plaque with retiform livedoid borders | NM | Trunk, acral, buttocks | NM | NM | NM | Groups of apoptotic keratinocytes in the epidermis, suggestive of a viral exanthem and thrombotic vasculopathy |
| 25 | Cutaneous small‐vessel vasculitis secondary to COVID−19 infection: A case report | 83‐year‐old female | Sore throat, malaise and nausea one month ago | Elevated level of CRP, and LDH | PCR was negative but serological qualitative rapid testing for SARS‐COV−2 was positive for IgM and IgG antibodies | Purple palpable papules and serohaematic blisters | NM | Lower legs, feet and toes | 5 days before | Prednisone | 10 days later | Leukocytoclastic vasculitis (LCV) affecting dermal vessels, accompanied by extravasation of red cells, basal epidermal layer necrosis, dermal perivascular neutrophil infiltration and fibrin deposition. |
| 26 | Thrombotic occlusive vasculopathy in skin biopsy from a livedoid lesion of a COVID−19 patient | 61‐year‐old male | Severe bilateral pneumonia complicated with diabetic ketoacidosis | Increased fibrinogen and D‐dimer levels and leucopenia | Negative | Livedoid purplish retiform and roundish patches and purple ischaemic sites | NM | Fingertips and in both, volar and dorsal areas of both feet and hands | Same time | Low molecular weight heparin | 17 days | dilated blood vessels In the papillary dermis, most of them filled with hyaline thrombi and few with a mild neutrophilic component surrounding them. In some areas, larger arterial vessels located in the dermohypodermal interface showed focal fibrinoid necrosis surrounded by a scarce neutrophilic infiltrate. Orcein staining demonstrated that the larger vessel was an artery. Sweat gland necrosis, secretory portion of the eccrine sweat coil, with preserved eccrine ducts. |
| 27 | Unique skin manifestations of COVID−19: Is drug eruption specific to COVID−19? | 52‐year‐old female | Fever, cough, chills, fatigue, and shortness of breath | High white blood cell count with lymphocytopenia and increased neutrophils, high C‐reactive protein, and normal LDH | Positive | Well‐demarcated infiltrated erythema lesions and erosions | Pruritus | Trunk, limbs and oral mucosa (lips and buccal mucosa) | 2 days after | Cefcapene pivoxil hydrochloride hydrate, loxoprofen sodium hydrate, oral prednisolone, ampicillin/sulbactam,clarithromycin, levofloxacin | NM | First biopsy before hospital admission: slight liquefaction with perivascular and periadnexal mixed cell infiltrations from the papillary dermis to the deep subcutaneous tissue. Deep lymphocytic infiltrations are not typical for drug eruptions, second biopsy after admission:interface changes with liquefaction and perivascular mixed cell infiltrations including histiocytes and neutrophils in the papillary dermis |
| 28 | Clinical and histological characterization of vesicular COVID−19 rashes: A prospective study in a tertiary care hospital | 24 patients, 6 men and 18 women | 10 patients (41.7%) | NM | Positive | Diffuse (n = 18)small papules, vesicles and pustules, with varying sizes, Although clustered at some points | NM | Head, trunk, arm, leg, palms/soles | 11.1% before, 16.6% same time, 72.2% 13 day after | Lopinavir/ritonavir (n = 5), hydroxychloroquine (n = 6), and azithromycin (n = 2) (for all patients) | NM | Intraepidermal vesicle containing scattered multinucleated and ballooned keratinocytes, with mild acantholysis. A deeper section of the vesicle reveals more extensive damage, with epidermal detachment and confluent keratinocyte necrosis. The vesicle contains fibrinoid material with acute inflammation. |
| Localized (n = 6) monomorphic vesicles and pustules | NM | Trunk | 13–14 day after | NM | NM | NM | ||||||
| 29 | Histological pattern in Covid−19 induced viral rash | 67‐year‐old female | Progressive dyspnoea and fever | NM | Positive | Erythematous confluent rash, with undefined margins, bleaching | Pruritus |
Neck, trunk, back, and proximal portions of upper and lower limbs |
30 days after | Hydroxychloroquine, omeprazole, piperacillin/tazobactam, remdesevir and enoxaparine | NM | Slight superficial perivascular lymphocytic infiltrate, extremely dilated vessel in the papillary and middermis. |
| 30 | Acute Generalized Exanthematous Pustulosis with Erythema Multiforme‐Like lesions in a COVID−19 woman | 70‐year‐old female | Pneumonia | NM | NM | Eruption on an erythematous‐oedematous base, with scattered pinhead‐sized pustules and scales, targetoid lesions studded with small pustules. | NM | Face, trunk and upper limbs, buttocks, thighs and legs | 3 days after | Lopinavir/ritonavir and hydroxychloroquine, oral prednisone | NM | Subcorneal pustule with mild focal acanthosis and spongiosis, neutrophilic exocytosis, sparse keratinocyte necrosis, and a perivascular lymphocytic infiltrate with rare neutrophils and eosinophils, consistent with AGEP |
| 31 | Drug‐induced vasculitis in a patient with COVID−19 | 57‐year‐old female | Nonproductive cough and intermittent | Elevated D‐dimer level | Positive | Pink tored maculopapular exanthema | Pruritic and painful | Trunk and extremities | 2 days before | Amoxicillin, ibuprofen and metamizole, intravenous bolus of prednisolone, antihistamines | 9 days | Vasculitis |
CRP, C‐reactive protein; NM, Not mentioned.
9. RISK OF BIAS AND APPLICABILITY
The study mostly had a high/unclear risk of bias in each domain. In the selection section, most case report studies that include in the systematic review have few cases, and it does not provide scoring capability in this section. But on the other hand, in the report section, the vast majority of studies had good results. And they got a good score.
In general, the risk of bias of articles was generally unclear. So, more research and article in this field will help to have better conclusions.
10. RESULTS OF INDIVIDUAL STUDIES
Based on studies, atypical features were reported in the histopathologic examinations of the patients with morbilliform or exanthematous rashes. 57 The histopathological similarity between viral and drug‐induced exanthems lies in observing superficial vacuolar interface lichenoid dermatitis and superficial perivascular lymphocyte‐rich infiltrates without epidermal changes except for mild spongiosis in both cases. Although dyskeratosis is more in favour of the drugs, it was reported in COVID‐19 induced morbilliform eruption. 57 Morbilliform eruption in COVID‐19 patients with ballooning multinucleated cells and dyskeratotic cells can therefore mimic eczema herpeticum or Grover's disease. 27 Purpuric maculopapular and vesicular rashes also suggest spongiotic dermatitis and dense perivascular lymphoeosinophilic infiltrates, swollen blood vessels, RBC extravasation, exocytosis in inflammatory cells and a large nest of Langerhans cells. 27 Moreover, lymphocytic cuffs around blood vessels and lymphocytic vasculitis were reported in papular erythematous exanthema. 27 In addition, histopathologic examinations revealed intravascular microthrombosis and superficial perivascular spongiotic dermatitis with intraepidermal nests of Langerhans cells in severe macular haemorrhagic eruptions. 27 The virus interactions with immune‐induced histopathologic changes can therefore cause these cytopathic effects and create unique features in skin biopsies.
The findings obtained from the skin biopsies are as follows:
Similar histopathologic findings, including maculopapular, purpuric, urticarial and Gianotti‐like exanthems, as lymphocytic perivascular and eosinophilic infiltrates and papillary dermal oedema. 58
Pauci‐inflammatory thrombotic vasculopathy with extensive epidermal and adnexal necrolysis, for example, eccrine gland coils, significant interstitial and perivascular neutrophilic and leukocytoclastic (secondary vasculitis) and purpuric lesions were observed in patients with severe COVID‐19 using immunohistochemical staining resulting in C5b‐9 deposition in the microvasculature. Alternative, lectin and MBL‐associated serine protease pathways were therefore recommended for complement activation, and narsoplimab and eculizumab can be beneficial in the patients. 19
Livedoid and necrotic lesions suggested histopathologic alterations such as ketoacidotic coma bullae with necrosis of eccrine sweat glands or eccrine coil sparing the duct, extensive thrombosis and fibrinoid necrosis. 51
Superficial perivascular lymphocytic infiltrates with frequent RBC extravasation, papillary dermal oedema, focal parakeratosis and scattered dyskeratotic cells and no evidence of thrombotic vasculopathy were observed in petechiae. 24
Pernio/chilblain‐like rashes suggested superficial and deep lichenoid and perivascular infiltrates and perieccrine lymphocytic involvements with few plasma cells and scattered single keratinocytes without thrombotic vasculopathy and papillary dermal oedema. 51 Marked papillary dermal oedema was observed in COVID‐19 patients with chilblains in contrast to those with chilblain‐like lesions 64. The result of the direct immunofluorescence test was also negative in these lesions. 59
Papulosquamous eruption is associated with diffuse mild epidermal spongiosis and spongiotic vesicles; aggregation of lymphocytes and Langerhans cells, mild papillary dermal oedema with dermal lymphohistiocytic infiltrates. 23
It is thoroughly recommended that further studies be conducted using the coagulation profiles of retiform purpura, cyanosis, skin bullae, livedoid vasculopathy and necrotic skin ulceration. 19 , 51 , 57
In autopsies, usually inflammatory immune‐mediated or iatrogenic histologic findings are more prominent than viral cytopathic effects and to somehow comparable with skin biopsies that are the confirmatory data regarding focussing more on the immunomodulatory strategies for disease control. 60 , 61
11. DISCUSSION
The multi‐organ manifestations of COVID‐19 include skin involvements as a major one, which is caused by inflammation and vascular damage in most cases. Research suggests skin lesions are mainly caused by viraemia 1 , 2 , 8 , 27 , 57 and drug‐associated reactions, 44 , 56 , 62 , 63 , 64 and the occurrence, severity and type of skin lesions are not significantly related to the symptoms, severity and course of COVID‐19. They can, however, reflect events in the invisible parts of the body and constitute a diagnostic and predictive tool for previous, concomitant and future consequences, which were discussed in this systematic review. In case of emergence of skin lesions without routine clinical symptoms such as fever and cough, the diagnosis can only be performed based on a positive PCR test or a history of contact with suspected/infected individuals. Furthermore, skin manifestations may occur several weeks after the improvement of clinical symptoms. Skin biopsies should be therefore performed in suspected patients with mucocutaneous manifestations to rule out improbable causes. 1 , 57 COVID‐related specific comorbidities especially its mucocutaneous manifestations and major dermatologic considerations are of among most important hot topics in pandemic area and in the field of dermatology, 65 , 66 , 67 , 68 in our best knowledge although up to our search finished there were few systematic reviews about dermatology and COVID‐19 but not any relapsed systematic review in the field of dermatopathology and COVID‐19, existed. 69 , 70 , 71 , 72 , 73 , 74 , 75 Laboratory tests showed lymphopenia and elevated CRP and D‐dimer levels to be the most frequent findings in the patients. The other laboratory findings in peripheral blood counts included leukocytosis, eosinophilia and thrombocytopenia. Slight increases were also observed in liver enzymes and blood fibrinogen levels in some patients, while laboratory findings were normal or unreported in a small group of patients. Moreover, most patients were positive for PCR and only a few were negative.
The most common skin manifestations in the COVID‐19 patients, though highly varying, included maculopapular lesions, persistent erythematous plaques and erythematous patches with indistinct margins. Maculopapular lesions and erythematous or purple plaques were reported in the urethra, especially in children and adolescents, sometimes with blood blisters or swollen fingers. Chilblains and dyshidrosis were also reported in the urethra, and thyroid nodules were targeted in children. The other skin findings included morbilliform rashes, livedo reticularis and urticarial, and the skin lesions were associated with burning, itching and pain in some cases.
The skin lesions mostly lay on the upper extremity and end and acral areas, respectively. Generalized involvement of the body, head, face and buttocks was also observed in these patients.
Although the skin lesions mostly emerged several days to one month after the onset of COVID‐19, their emergence coincided with the onset of COVID‐19 in a small number of patients and preceded it by a few days/months in some others (a few weeks on average).
After the onset of COVID‐19 or skin symptoms, the patients were treated with medications such as antihistamines, painkillers, antibiotics, hydroxychloroquine and other antiviral drugs.
Despite the self‐healing of most of the skin lesions within 2–3 weeks, they persisted in a small number of patients until later in follow‐up periods.
The most frequent histopathological findings included dense perivascular and interstitial neutrophilic infiltrates with the moderate presence of eosinophils, subcorneal pustules, spongiosis, papillary dermal oedema, focal vacuolar degeneration of the basal layer, necrotic keratinocytes, fibrin thrombi, vasculitis and RBC extravasation.
No relationships were observed among the time of onset of symptoms, the severity of symptoms, laboratory findings and histopathological findings in the patients. The pathological findings in the patients with mild clinical manifestations resembled those in the patients with severe skin involvements. It is noteworthy at the time of skin lesions and pathological findings that histological findings in early biopsies at the time of skin lesions show more evidence of spongiosis, nonspecific infiltration around the dermal arteries or subcorneal posture if in prolonged biopsies show more evidence of lichenoid changes associated with vasculitis or vasculopathy. The histopathological findings were not associated with laboratory disorders and deterioration of clinical conditions in the patients. Paying attention to the emergence of unjustified skin lesions during the COVID‐19 pandemic is crucial given the occurrence of skin lesions before the onset of other symptoms of COVID‐19 in a large number of patients.
The authors of this study in overall focussed on the disease pathomechanism, the effects of multi‐potential drugs on COVID‐19 19 and COVID‐related specific comorbidities especially its mucocutaneous manifestations and significant dermatologic considerations in the pandemic area. 66 , 67 , 68 In conclusion, the visible skin rashes which about 20% of COVID‐19 patients may break out before, during or after their infection are feasible to approach. Gaining more knowledge about the symptoms and the associated pathomechanisms can help increase the survival rate in the patients. Differentiating viraemia from medications as the potential causes of cutaneous manifestations is crucial in managing the patients. Skin lesions can occur from before the onset of the disease to three weeks after. The results of the present review, which was performed to use cutaneous histopathologic findings as predictors of the nature of comorbid systemic involvements and COVID‐19 outcomes, are summarized as follows:
Fever, cough and respiratory symptoms constituted the most frequent clinical manifestations in the COVID patients with skin rashes.
Laboratory tests showed lymphopenia and elevated CRP and D‐dimer levels to be the most frequent findings in the patients.
The most common skin manifestations in the patients included maculopapular lesions, persistent erythematous plaques and erythematous patches with indistinct margins. The skin lesions mostly lay on the upper extremity and end and acral areas, respectively.
The lesions emerged in most of the patients several days to one month after the onset of COVID‐19 symptoms.
The medications used for treating the skin lesions mainly included antihistamines, painkillers, antibiotics, hydroxychloroquine and other antiviral drugs, and their majority healed within 2–3 weeks.
The most frequent histopathological findings included dense perivascular and interstitial neutrophilic infiltrates with moderate presence of eosinophils, subcorneal pustules, spongiosis, papillary dermal oedema, focal vacuolar degeneration of the basal layer, necrotic keratinocytes, fibrin thrombi, vasculitis and RBC extravasation.
No relationships were observed among the time of onset of symptoms, severity of symptoms, laboratory findings and histopathological findings, and deterioration of clinical condition and laboratory tests were not associated with the histopathological findings in the patients. It is recommended that meta‐analyses be conducted in the future to clarify these data for having more comprehensive and better conclusion.
Skin biopsy in the cutaneous manifestations of COVID‐19 was found essential for investigating their causes and different dimensions of COVID‐19. It is recommended that infection of patients with acute cutaneous manifestations with COVID‐19 be investigated in areas affected by the pandemic.
In this review, we had several limitations. One of the limitations of this study is just the inclusion of English studies in it, and the reviews exclusively based on English‐language reports are at higher risk of bias. The second limitation of this study is the limitation of numbers of previous studies. For a better conclusion, it is better to publish more researches in this field.
CONFLICT OF INTEREST
None declared.
AUTHORS’ CONTRIBUTIONS
NNN and AG performed the research; NNN, AG and FS designed, searched and wrote the study; ASB, EB and SM edited the paper; AG, MD and FS revised the paper; MD and FS implemented the quality assessment. AG submitted the paper.
All authors have read and approved the final manuscript and guarantee the accuracy of the manuscript. All members of this research team reviewed the manuscript and data and assume full responsibility for the content.
ACKNOWLEDGEMENTS
The authors would like to express their gratitude to the authorities of Rasool Akram Medical Complex Clinical Research Development Center (RCRDC), Iran University of Medical Sciences, for its technical and editorial assist.
Niloufar Najar Nobari and Farnoosh Seirafianpour are co‐first authors in this article.
DATA AVAILABILITY STATEMENT
Data is available in the text and table. As this paper is a review article.
REFERENCES
- 1. Galván Casas C, Catala A, Carretero Hernández G, et al. Classification of the cutaneous manifestations of COVID‐19: a rapid prospective nationwide consensus study in Spain with 375 cases. Br J Dermatol. 2020;183(1):71‐77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Mahe A, Birckel E, Krieger S, Merklen C, Bottlaender L. A distinctive skin rash associated with Coronavirus Disease 2019. J Eur Acad Dermatol Venereol. 2020;34:e246‐e247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Hunt M, Koziatek C. A Case of COVID‐19 pneumonia in a young male with full body rash as a presenting symptom. Clin Pract Cases Emerg Med. 2020;4(2):219‐221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Najarian DJ. Morbilliform exanthem associated with COVID‐19. JAAD Case Rep. 2020;6(6):493‐494. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Hoenig LJ, Pereira FA. Eruption as a clinical manifestation of COVID‐19: Photographs of a patient. Clin in Dermatol. 2020;38:502‐505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Amatore F, Macagno N, Mailhe M, et al. SARS‐CoV‐2 infection presenting as a febrile rash. J Eur Acad Dermatol Venererol. 2020;34(7):e304‐e306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Henry D, Ackerman M, Sancelme E, Finon A, Esteve E. Urticarial eruption in COVID‐19 infection. J Eur Acad Dermatol Venererol. 2020;34(6):e244‐e245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Quintana‐Castanedo L, Feito‐Rodríguez M, Valero‐López I, Chiloeches‐Fernández C, Sendagorta‐Cudós E, Herranz‐Pinto P. Urticarial exanthem as early diagnostic clue for COVID‐19 infection. JAAD Case Rep. 2020;6(6):498‐499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Chen Y, Peng H, Wang L, et al. Infants born to mothers with a new coronavirus (COVID‐19). Front Pediatr. 2020;8:104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Morey‐Olivé M, Espiau M, Mercadal‐Hally M, Lera‐Carballo E, García‐Patos V. Cutaneous manifestations in the current pandemic of coronavirus infection disease (COVID 2019). An Pediat. 2020;92(6):374‐375. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Marzano AV, Genovese G, Fabbrocini G, et al. Varicella‐like exanthem as a specific COVID‐19‐associated skin manifestation: Multicenter case series of 22 patients. J Am Acad Dermatol. 2020;83(1):280‐285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Genovese G, Colonna C, Marzano AV. Varicella‐like exanthem associated with COVID‐19 in an 8‐year‐old girl: a diagnostic clue? Pediatr Dermatol. 2020;37(3):435‐436. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Jimenez‐Cauhe J, Ortega‐Quijano D, Prieto‐Barrios M, Moreno‐Arrones OM, Fernandez‐Nieto D. Reply to "COVID‐19 can present with a rash and be mistaken for dengue": petechial rash in a patient with COVID‐19 infection. J Am Acad Dermatol. 2020;83(2):e141‐e142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Joob B, Wiwanitkit V. COVID‐19 can present with a rash and be mistaken for dengue. J Am Acad Dermatol. 2020;82(5):e177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Wu P, Liang L, Chen C, Nie S. A child confirmed COVID‐19 with only symptoms of conjunctivitis and eyelid dermatitis. Clin Exp Ophthalmol. 2020;258(7):1565‐1566. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Alramthan A, Aldaraji W. Two cases of COVID‐19 presenting with a clinical picture resembling chilblains: first report from the Middle East. Clin Exp Ophthalmol. 2020;45(6):746‐748. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Landa N, Mendieta‐Eckert M, Fonda‐Pascual P, Aguirre T. Chilblain‐like lesions on feet and hands during the COVID‐19 pandemic. Int J Dermatol. 2020;59(6):739‐743. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Piccolo V, Neri I, Filippeschi C, et al. Chilblain‐like lesions during COVID‐19 epidemic: a preliminary study on 63 patients. J Eur Acad Dermatol Venererol. 2020;34(7):e291‐e293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Magro C, Mulvey JJ, Berlin D, et al. Complement associated microvascular injury and thrombosis in the pathogenesis of severe COVID‐19 infection: a report of five cases. Transl Res. 2020;220:1‐13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Recalcati S, Barbagallo T, Frasin LA, et al. Acral cutaneous lesions in the time of COVID‐19. J Eur Acad Dermatol Venererol. 2020;34(8):e346‐e347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Mazzotta F, Troccoli T. Acute acro‐ischemia in the child at the time of COVID‐19. Eur. J Pediatr Dermatol. 2020;30(2):71‐74. [Google Scholar]
- 22. Zhang Y, Xiao M, Zhang S, et al. Coagulopathy and antiphospholipid antibodies in patients with Covid‐19. New Engl J Med. 2020;382(17):e38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Sanchez A, Sohier P, Benghanem S, et al. Digitate papulosquamous eruption associated with severe acute respiratory syndrome coronavirus 2 infection. JAMA Dermatol. 2020;156(7):819‐820. [DOI] [PubMed] [Google Scholar]
- 24. Diaz‐Guimaraens B, Dominguez‐Santas M, Suarez‐Valle A, et al. Petechial skin rash associated with severe acute respiratory syndrome coronavirus 2 infection. JAMA Dermatol. 2020;156:820‐822. [DOI] [PubMed] [Google Scholar]
- 25. Manalo IF, Smith MK, Cheeley J, Jacobs R. A dermatologic manifestation of COVID‐19: transient livedo reticularis. J Am Acad Dermatol. 2020;83(2):700. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Najar Nobari N, Seirafianpour F, Mashayekhi F, Goodarzi A. A systematic review on treatment‐related mucocutaneous reactions in COVID‐19 patients. Dermatol Ther. 2021;34(1):e14662. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Gianotti R, Zerbi P, Dodiuk‐Gad RP. Clinical and histopathological study of skin dermatoses in patients affected by COVID‐19 infection in the Northern part of Italy. J Dermatol Sci. 2020;98(2):141‐143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Sterne JA, Hernán MA, Reeves BC, et al. ROBINS‐I: a tool for assessing risk of bias in non‐randomised studies of interventions. Br Med J. 2016;355:i4919. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Murad MH, Sultan S, Haffar S, Bazerbachi F. Methodological quality and synthesis of case series and case reports. BMJ Evid Based Med. 2018;23(2):60‐63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Herrero‐Moyano M, Capusan T, Andreu‐Barasoain M, et al. A clinicopathological study of 8 patients with COVID‐19 pneumonia and a late‐onset exanthema. J Eur Acad Dermatol Venereol. 2020;34(9):e460‐e464. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Recalcati S, Barbagallo T, Frasin LA, et al. Acral cutaneous lesions in the time of COVID‐19. J Eur Acad Dermatol Venereol. 2020;34(8):e346‐e347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. García‐Gil MF, García García M, Monte Serrano J, Prieto‐Torres L, Ara‐Martín M. Acral purpuric lesions (erythema multiforme type) associated with thrombotic vasculopathy in a child during the COVID‐19 pandemic. J Eur Acad Dermatol Venereol. 2020;34(9):e443‐e445. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Suarez‐Valle A, Fernandez‐Nieto D, Diaz‐Guimaraens B, Dominguez‐Santas M, Carretero I, Perez‐Garcia B. Acro‐ischaemia in hospitalized COVID‐19 patients. J Eur Acad Dermatol Venereol. 2020;34(9):e455‐e457. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Ahouach B, Harent S, Ullmer A, et al. Cutaneous lesions in a patient with COVID‐19: are they related? Br J Dermatol. 2020;183(2):e31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. de Masson A, Bouaziz JD, Sulimovic L, et al. Chilblains is a common cutaneous finding during the COVID‐19 pandemic: a retrospective nationwide study from France. J Am Acad Dermatol. 2020;83(2):667‐670. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Andina D, Noguera‐Morel L, Bascuas‐Arribas M, et al. Chilblains in children in the setting of COVID‐19 pandemic. Pediatr Dermatol. 2020;37(3):406‐411. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Colonna C, Monzani NA, Rocchi A, Gianotti R, Boggio F, Gelmetti C. Chilblain‐like lesions in children following suspected COVID‐19 infection. Pediatr Dermatol. 2020;37(3):437‐440. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Gianotti R, Veraldi S, Recalcati S, et al. Cutaneous clinico‐pathological findings in three COVID‐19‐positive patients observed in the metropolitan area of Milan, Italy. Acta Derm Venereol. 2020;100(8):adv00124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Cordoro KM, Reynolds SD, Wattier R, McCalmont TH. Clustered cases of acral perniosis: clinical features, histopathology, and relationship to COVID‐19. Pediatric Dermatol. 2020;37(3):419‐423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Rivera‐Oyola R, Koschitzky M, Printy R, et al. Dermatologic findings in 2 patients with COVID‐19. JAAD Case Rep. 2020;6(6):537‐539. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Jimenez‐Cauhe J, Ortega‐Quijano D, Carretero‐Barrio I, et al. Erythema multiforme‐like eruption in patients with COVID‐19 infection: clinical and histological findings. Clin Exp Dermatol. 2020;45(7):892‐895. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Recalcati S. Cutaneous manifestations in COVID‐19: a first perspective. J Eur Acad Dermatol Venereol. 2020;34(5):e212‐e213. [DOI] [PubMed] [Google Scholar]
- 43. Kolivras A, Dehavay F, Delplace D, et al. Coronavirus (COVID‐19) infection‐induced chilblains: a case report with histopathologic findings. JAAD Case Rep. 2020;6(6):489‐492. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Skroza N, Bernardini N, Balduzzi V, et al. A late‐onset widespread skin rash in a previous COVID‐19‐infected patient: viral or multidrug effect? J Eur Acad Dermatol Venereol. 2020;34(9):e438‐e439. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Locatelli AG, Robustelli Test E, Vezzoli P, et al. Histologic features of long‐lasting chilblain‐like lesions in a paediatric COVID‐19 patient. J Eur Acad Dermatol Venereol. 2020;34(8):e365‐e368. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Saenz Aguirre A, De la Torre Gomar FJ, Rosés‐Gibert P, et al. Novel outbreak of acral lesions in times of COVID‐19: a description of 74 cases from a tertiary university hospital in Spain. Clin Expl Dermatol. 2020;45(8):1065‐1067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Van Damme C, Berlingin E, Saussez S, Accaputo O. Acute urticaria with pyrexia as the first manifestations of a COVID‐19 infection. J Eur Acad Dermatol Venereol. 2020;34(7):e300‐e301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Amatore F, Macagno N, Mailhe M, et al. SARS‐CoV‐2 infection presenting as a febrile rash. J Eur Acad Dermatol Venereol. 2020;34(7):e304‐e306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Young S, Fernandez AP. Skin manifestations of COVID‐19. Cleve Clin J Med. 2020. 10.3949/ccjm.87a.ccc031. Online ahead of print. [DOI] [PubMed] [Google Scholar]
- 50. Mayor‐Ibarguren A, Feito‐Rodriguez M, Quintana Castanedo L, et al. Cutaneous small vessel vasculitis secondary to COVID‐19 infection: a case report. J Eur Acad Dermatol Venereol. 2020;34(10):e541‐e542. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Llamas‐Velasco M, Muñoz‐Hernández P, Lázaro‐González J, et al. Thrombotic occlusive vasculopathy in a skin biopsy from a livedoid lesion of a patient with COVID‐19. Br J Dermatol. 2020;183(3):591‐593. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Sakaida T, Tanimoto I, Matsubara A, Nakamura M, Morita A. Unique skin manifestations of COVID‐19: is drug eruption specific to COVID‐19? J Dermatol Sci. 2020;99(1):62‐64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Fernandez‐Nieto D, Ortega‐Quijano D, Jimenez‐Cauhe J, et al. Clinical and histological characterization of vesicular COVID‐19 rashes: a prospective study in a tertiary care hospital. ClinExp Dermatol. 2020;45(7):872‐875. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Zengarini C, Orioni G, Cascavilla A, et al. Histological pattern in COVID‐19‐induced viral rash. J Eur Acad Dermatol Venereol. 2020;34(9):e453‐e454. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. Robustelli Test E, Vezzoli P, Carugno A, et al. Acute generalized exanthematous pustulosis with erythema multiforme‐like lesions in a COVID‐19 woman. J Eur Acad Dermatol Venereol. 2020;34(9):e457‐e459. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. Vanegas Ramirez A, Efe D, Fischer M. Drug‐induced vasculitis in a patient with COVID‐19. J Eur Acad Dermatol Venereol. 2020;34(8):e361‐e362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57. Young S, Fernandez AP. Skin manifestations of COVID‐19. Clev Clin J Med. 2020. Online ahead of print. [DOI] [PubMed] [Google Scholar]
- 58. Estébanez A, Pérez‐Santiago L, Silva E, Guillen‐Climent S, García‐Vázquez A, Ramón MD. Cutaneous manifestations in COVID‐19: a new contribution. J Eur Acad Dermatol Venererol. 2020;34:e250‐e251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59. Gianotti R, Recalcati S, Fantini F, et al. Histopathological study of a broad spectrum of skin dermatoses in patients affected or highly suspected of infection by COVID‐19 in the Northern Part of Italy: analysis of the many faces of the viral‐induced skin diseases in previous and new reported cases. Am J dermatopathol. 2020;42(8):564‐570. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60. Hanley B, Lucas SB, Youd E, Swift B, Osborn M. Autopsy in suspected COVID‐19 cases. J Clin Pathol. 2020;73(5):239‐242. [DOI] [PubMed] [Google Scholar]
- 61. Xu Z, Shi L, Wang Y, et al. Pathological findings of COVID‐19 associated with acute respiratory distress syndrome. Lancet Respir Med. 2020;8(4):420‐422. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62. Robustelli Test E, Vezzoli P, Carugno A, et al. Acute generalized exanthematous pustulosis with erythema multiforme‐like lesions induced by Hydroxychloroquine in a woman with coronavirus disease 2019 (COVID‐19). J Eur Acad Dermatol Venereol. 2019;2020:e457‐e459. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63. Türsen Ü, Türsen B, Lotti T. Cutaneous side‐effects of the potential COVID‐19 drugs. Dermatol Ther. 2020;33(4):e13476. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64. Litaiem N, Hajlaoui K, Karray M, Slouma M, Zeglaoui F. Acute generalized exanthematous pustulosis after COVID‐19 treatment with hydroxychloroquine. Dermatol Ther. 2020;33(4):e13565. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65. Seirafianpour F, Mozafarpoor S, Fattahi N, Sadeghzadeh‐Bazargan A, Hanifiha M, Goodarzi A. Treatment of COVID‐19 with pentoxifylline: Could it be a potential adjuvant therapy? Dermatol Ther. 2020;33(4):e13733. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66. Seirafianpour F, Sodagar S, Pour Mohammad A, et al. Cutaneous manifestations and considerations in COVID‐19 pandemic: a systematic review. Dermatol Ther. 2020;33:e13986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67. Nobari NN, Goodarzi A. Patients with specific skin disorders who are affected by COVID‐19: what do experiences say about management strategies? A systematic review. Dermatol Ther. 2020;33:e13867. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68. Mohamadi MM, Goodarzi A, Aryannejad A, et al. Geriatric challenges in the new coronavirus disease‐19 (COVID‐19) pandemic: a systematic review. Med J Islam Repub Iran. 2020;34(1):841‐848. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69. Kooranifar S, Sadeghipour A, Riahi T, Goodarzi A, Tabrizi S, Davoody N. Histopathologic survey on lung necropsy specimens of 15 patients who died from COVID‐19: a large study from Iran with a high rate of anthracosis. Med J Islam Repub Iran. 2021;35(1):481‐490. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70. Mohamadi M, Fattahi N, Goodarzi A, et al. A comprehensive review on COVID‐19 infection and comorbidities of various organs. Acta Med Iran. 2021;59(1):4‐14. [Google Scholar]
- 71. Najar Nobari N, Montazer F, Seirafianpour F, Nikkhah F, Aryanian Z, Goodarzi Z. Histopathologic changes and cellular events of organs systems in COVID‐19. J Cell Mol Anesth. 2021;6(1):81‐88. [Google Scholar]
- 72. Atefi NS, Behrangi E, Mozafarpoor S, Seirafianpour F, Peighambari S, Goodarzi A. N‐acetylcysteine and coronavirus disease 2019: May it work as a beneficial preventive and adjuvant therapy? A comprehensive review study. J Res Med Sci. 2020;25:109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73. Sadeghzadeh‐Bazargan A, Behrangi E, Goodarzi A. Systemic retinoids in the COVID‐19 era – are they helpful, safe, or harmful? A comprehensive systematized review. Iran. J Dermatol. 2020; 23(Suppl. 1 (COVID‐19)):9‐12. [Google Scholar]
- 74. Sadeghzadeh‐Bazargan A, Behrangi E, Goodarzi A. Cytokine storm and probable role of immunoregulatory drugs in COVID‐19: a comprehensive review. Iranian. J Dermatol. 2020;23(Suppl:1 (COVID‐19)):13‐18. [Google Scholar]
- 75. Sadeghzadeh‐Bazargan A, Rezai M, Najar Nobari N, Mozafarpoor S, Goodarzi A. Skin manifestations as potential symptoms of diffuse vascular injury in critical COVID‐19 patients. J Cutan Pathol. 2021. 10.1111/cup.14059. Online ahead of print. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Data is available in the text and table. As this paper is a review article.


