Abstract
Background and purpose
Although COVID‐19 predominantly affects the respiratory system, recent studies have reported the occurrence of neurological disorders such as stroke in relation to COVID‐19 infection. Encephalitis is an inflammatory condition of the brain that has been described as a severe neurological complication of COVID‐19. Despite a growing number of reported cases, encephalitis related to COVID‐19 infection has not been adequately characterised. To address this gap, this systematic review and meta‐analysis aims to describe the incidence, clinical course, and outcomes of patients who suffer from encephalitis as a complication of COVID‐19.
Methods
All studies published between 1 November 2019 and 24 October 2020 that reported on patients who developed encephalitis as a complication of COVID‐19 were included. Only cases with radiological and/or biochemical evidence of encephalitis were included.
Results
In this study, 610 studies were screened and 23 studies reporting findings from 129,008 patients, including 138 with encephalitis, were included. The average time from diagnosis of COVID‐19 to onset of encephalitis was 14.5 days (range = 10.8–18.2 days). The average incidence of encephalitis as a complication of COVID‐19 was 0.215% (95% confidence interval [CI] = 0.056%–0.441%). The average mortality rate of encephalitis in COVID‐19 patients was 13.4% (95% CI = 3.8%–25.9%). These patients also had deranged clinical parameters, including raised serum inflammatory markers and cerebrospinal fluid pleocytosis.
Conclusions
Although encephalitis is an uncommon complication of COVID‐19, when present, it results in significant morbidity and mortality. Severely ill COVID‐19 patients are at higher risk of suffering from encephalitis as a complication of the infection.
Keywords: complication, coronavirus, COVID‐19, encephalitis
INTRODUCTION
The coronavirus disease 2019 (COVID‐19) pandemic has caused an unprecedented burden on both economic and health care systems globally. Although COVID‐19 primarily affects the respiratory system, recent studies have found that it has severe impact on the neurological system as well [1]. Work has begun in the characterisation of some of these neurological complications of COVID‐19, such as stroke and anosmia [2]. However, less common neurological complications of COVID‐19 such as encephalitis [3], Guillain–Barré syndrome [4] and myelitis [5] are still not adequately explored.
There have been increasing reports of the development of encephalitis in severely ill COVID‐19 patients [6, 7, 8]. These patients fare poorly, with significant morbidity and mortality rates [6, 9]. The association of encephalitis with severe patient outcomes suggests that systematic studies are required to determine the risk factors predisposing to its development. Currently, systematic reviews that consolidate findings on encephalitis as a complication of COVID‐19 are scarce, providing limited information on this condition [10, 11, 12, 13]. To address this important gap in the literature, a systematic review was conducted to more comprehensively evaluate the epidemiology, clinical course, risk factors, and outcomes of patients who suffer from encephalitis as a complication of COVID‐19.
MATERIALS AND METHODS
This review was conducted in accordance with the Preferred Reporting Items for Systematic Reviews and Meta‐Analyses (PRISMA) guidelines [14]. A search string was developed to identify original research studies reporting clinical features and treatment outcomes of patients with encephalitis as a complication of COVID‐19 (Table S1). Encephalitis was defined as an inflammation of the brain [15]. For this analysis, studies reporting on any type of encephalitis were included, such as autoimmune encephalitis, acute disseminated encephalomyelitis (ADEM), and necrotising encephalitis. All cases of encephalitis were diagnosed radiologically, such as by computed tomography (CT) or magnetic resonance imaging (MRI) scans of the brain or by cerebrospinal fluid (CSF) analysis. The search was applied to the following three electronic databases: PubMed, Embase, and CENTRAL (Cochrane Central Register of Controlled Trials). Searches were performed for each database on 24 October 2020. Limits were applied to the search to identify studies published after 1 November 2019, as the first case of novel coronavirus was only reported in December 2019. This study was registered on PROSPERO (registration number CRD42020224776). All titles and abstracts were screened independently by two reviewers (I.S. and K.S.L.) against a set of predefined eligibility criteria. Potentially eligible studies were selected for full‐text analysis. Disagreements were resolved by consensus or appeal to a third senior reviewer (A.N.).
All original studies reporting the clinical characteristics (symptoms and signs, laboratory investigations, and radiological findings) and treatment outcomes of COVID‐19 patients with encephalitis complications were included in our systematic review. Case reports and studies of small sample sizes (<3) were excluded per recommendations in accordance with methodologies of previously published meta‐analyses [2, 16]. Other exclusion criteria included non‐English articles, non‐original research papers, laboratory‐based and epidemiological studies with no clinical characteristics reported, and nonhuman research subjects (Table S2). The PRISMA chart is detailed in Figure 1.
FIGURE 1.
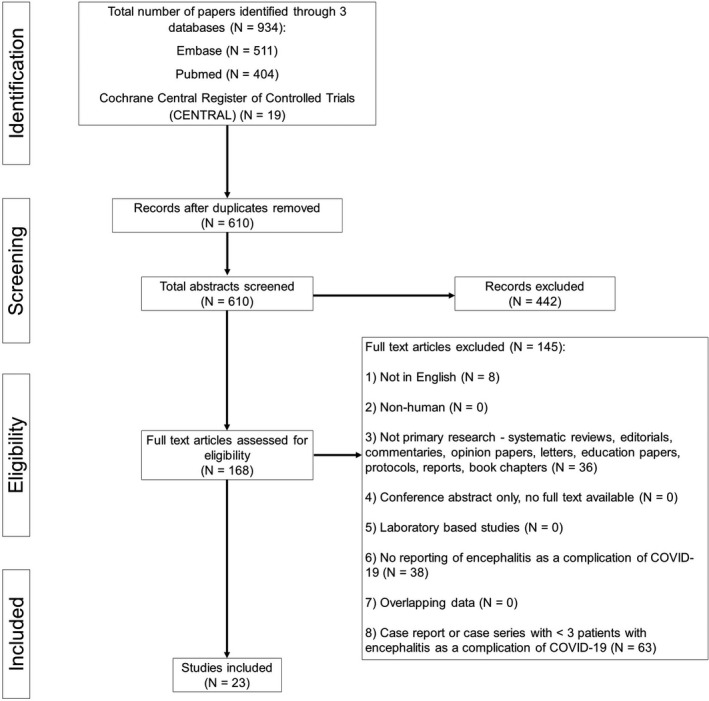
Preferred Reporting Items for Systematic Reviews and Meta‐Analyses study design
The quality of included studies was assessed using the Joanna Briggs Institute (JBI) checklist for prevalence studies and the JBI checklist for case series [17]. These tools rated the quality of selection, measurement, and comparability for all studies and gave a score for cross‐sectional studies and case series. Two researchers assessed the quality of all included studies and discussed discrepancies until consensus was reached. Data were extracted on the following variables: study details, sample size of study, method of diagnosis, age, gender, coexisting medical conditions, clinical symptoms, laboratory investigations, time from COVID‐19 infection to onset of encephalitis, treatment details, and patient outcomes. The outcome measure was mortality in hospital.
Random effects meta‐analyses were performed on variables and end points due to observed estimates and sampling variability across studies. Pooled proportions were computed with the inverse variance method using the variance‐stabilising Freeman–Tukey double arcsine transformation [18]. Confidence intervals (CIs) for individual studies were calculated using the Clopper–Pearson interval method. The I 2 statistics was used to present between‐study heterogeneity, where I 2 of 30% or less, between 30% and 50%, between 50% and 75%, and 75% or greater was considered to indicate low, moderate, substantial, and considerable heterogeneity, respectively [19]. Probability values for the I 2 statistics were computed by chi‐squared distribution of Cochran Q test. Missing values for mean were imputed using median. Statistical analysis was performed using R (R Foundation for Statistical Computing, Vienna, Austria; https://www.R‐project.org/) [20]. The significance level was set at p < 0.05, and 95% CIs were reported.
RESULTS
The search strategy yielded 610 unique publications after removal of duplicates. After screening of titles and abstracts, 168 publications were reviewed in full text. A total of 23 original studies [3, 6, 7, 8, 9, 21, 22, 23, 24, 25, 26, 27, 28, 29, 30, 31, 32, 33, 34, 35, 36, 37, 38] were eventually included in our systematic review with a combined population of 129,008 patients, including 138 who developed encephalitis [Table 1].
TABLE 1.
Summary of studies
| Study | Country | Study design | Encephalitis patients, n | Age, mean | Male, n (%) |
|---|---|---|---|---|---|
| Abenza‐Abildúa et al. | Spain | Case series | 4 | ||
| Benameur et al. | United States | Case series | 3 | 43.0 | 2 (66.7) |
| Cao et al. | France | Case series | 5 | 56.0 | 4 (80.0) |
| Dogan et al. | Turkey | Cross‐sectional | 3 | 43.3 | 3 (100.0) |
| Guilmot et al. | Belgium | Cross‐sectional | 1 | 80.0 | |
| Helms et al. | France | Cross‐sectional | 8 | ||
| Iltaf et al. | Pakistan | Cross‐sectional | 3 | >50 | |
| Kihira et al. | United States | Case series | 2 | 53.5 | 1 (50.0) |
| Koh et al. | Singapore | Cross‐sectional | 4 | ||
| Kremer et al. | France | Cross‐sectional | 8 | 60.8 | 7 (87.5) |
| Liotta et al. | United States | Cross‐sectional | 1 | ||
| Luigetti et al. | Italy | Cross‐sectional | 1 | ||
| Miró et al. | Spain | Cross‐sectional | 16 | ||
| Paterson et al. | United Kingdom | Case series | 12 | 53 | 4 (33.3) |
| Pilotto et al. (a) | Italy | Cross‐sectional | 14 | ||
| Pilotto et al. (b) | Italy | Case series | 25 | 65.9 | 15 (60.0) |
| Radmard et al. | United States | Cross‐sectional | 1 | 74 | 0 (0.0) |
| Rifino et al. | Italy | Cross‐sectional | 5 | 60 | 4 (80.0) |
| Romero‐Sánchez et al. | Spain | Cross‐sectional | 1 | 57 | 0 (0.0) |
| Shah et al. | United States | Cross‐sectional | 9 | ||
| Umapathi et al. | Singapore | Case series | 3 | 57 | 3 (100.0) |
| Varatharaj et al. | United Kingdom | Cross‐sectional | 7 | ||
| Zuccon et al. | Italy | Cross‐sectional | 2 | ||
| Overall | 138 | 59.4 | 68 (49.3) |
Of the 23 studies, five studies each originated from the United States and Italy. Three studies each originated from Spain and France, two studies each originated from Singapore and the United Kingdom, and one study each originated from Belgium, Pakistan, and Turkey (Table 1). Sixteen studies were cross‐sectional in nature (69.6%), and seven (30.4%) were case series. Of the 16 cross‐sectional studies, four studies attained a full score of 8 on the JBI checklist for cross‐sectional studies, eight studies attained a score of 7, and four studies attained a score of 6 (Table S3). Of the seven case series, four studies attained a full score of 10 on the JBI checklist for case series and three studies attained a score of 9 (Table S4).
General analysis
The two broad categories of encephalitis as a complication of COVID‐19 were autoimmune encephalitis and infectious encephalitis. Fourteen studies reported on the incidence of encephalitis as a complication of COVID‐19. Combining results, the pooled incidence of encephalitis as a complication of COVID‐19 was 0.215% (95% CI = 0.056%–0.441%). Notably, 10 of the 14 studies reported incidence of less than 1% (Figure 2). However, among severely ill patients with COVID‐19, the pooled incidence of encephalitis as a complication of COVID‐19 was higher at 6.7% (95% CI = 4.3%–9.4%; Figure S1). Ten studies reported the time from diagnosis of COVID‐19 to onset of encephalitis symptoms. Patients developed encephalitis an average of 14.5 days (SD = 10.8 ‐ 18.2 days) after onset of COVID‐19 symptoms (Table 2).
FIGURE 2.
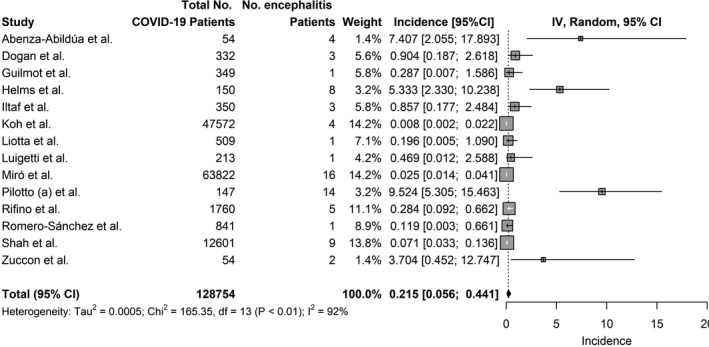
Incidence of encephalitis in COVID‐19. Inverse Variance (IV)
TABLE 2.
Time from COVID‐19 infection to encephalitis onset
| Study | Encephalitis patients, n | Days from COVID‐19 infection to encephalitis onset, mean (SD) |
|---|---|---|
| Benameur et al. | 3 | 11.7 (2.5) |
| Cao et al. | 5 | 20.0 (6.1) |
| Dogan et al. | 3 | 16.0 (2.2) |
| Guilmot et al. | 1 | 21.0 (0.0) |
| Kihira et al. | 2 | 10.5 (3.5) |
| Koh et al. | 4 | 24.0 (15–65) |
| Kremer et al. | 8 | 19.1 (8.3) |
| Pilotto et al. (b) | 25 | 6.8 (10.1) |
| Rifino et al. | 5 | 16.4 (19.7) |
| Umapathi et al. | 3 | 45.3 (24.4) |
| Overall | 59 | 14.5 (10.8–18.2) |
Severity of COVID‐19 illness
Twelve studies reported on the severity of COVID‐19 illness in patients who subsequently suffered from the complication of encephalitis, whether severe of mild. Severe illness was defined as patients who required intensive care unit (ICU) or high‐dependence unit (HDU) care. Mild illness included patients who were managed in the general ward with mild or no respiratory symptoms during their stay. Most patients had severe COVID‐19 illness prior to developing the complication of encephalitis, amounting to 83.8% (95% CI = 62.0%–98.6%; Figure S2).
Demographics
Demographic information of patients who suffered from encephalitis as a complication of COVID‐19 was analysed. The mean age of patients who suffered from encephalitis as a complication of COVID‐19 was 59.4 years (range = 43.0–80.0 years). A similar proportion of males and females suffered from encephalitis as a complication of COVID‐19, with 49.3% of such patients being male (Table 1). Most of these patients had at least one comorbidity, amounting to 71.7%. The most common comorbidities were hypertension (45.5% of patients), hyperlipidaemia (24.0%), and diabetes mellitus (16.0%). Less commonly encountered comorbidities included chronic kidney disease, chronic liver disease, and congestive cardiac failure (Table 3).
TABLE 3.
Comorbidities
| Study | Encephalitis patients, n | No comorbidities, n (%) | At least 1 comorbidity, n (%) | Diabetes mellitus, n (%) | Hypertension, n (%) | Hyperlipidaemia, n (%) |
|---|---|---|---|---|---|---|
| Benameur et al. | 3 | 0 (0.0) | 3 (100.0) | 2 (66.7) | ||
| Cao et al. | 5 | 1 (20.0) | 4 (80.0) | 2 (40.0) | ||
| Dogan et al. | 3 | 2 (66.7) | 1 (33.3) | 1 (33.3) | ||
| Kihira et al. | 2 | 2 (100.0) | 0 (0.0) | |||
| Pilotto et al. (b) | 25 | 3 (12.0) | 22 (88.0) | 4 (16.0) | 12 (48.0) | 6 (24.0) |
| Rifino et al. | 5 | 3 (60.0) | 2 (40.0) | 2 (40.0) | ||
| Umapathi et al. | 3 | 2 (66.7) | 1 (33.3) | 1 (33.3) | ||
| Overall | 46 | 13 (28.3) | 33 (71.7) | 4 (16.0) | 20 (45.5) | 6 (24.0) |
Symptoms
Thirteen studies reported on the clinical symptoms of patients, such as COVID‐19 symptoms and encephalitis symptoms. Of the eight studies that reported on COVID‐19 symptoms, shortness of breath (84.6% of patients) and fever (63.6%) were the most common symptoms experienced by patients. Cough (60.0%) and fatigue (50.0%) were less common. Additionally, 23.8% of patients were asymptomatic, that is, did not experience any COVID‐19 symptoms. Of the 11 studies that reported on symptoms of encephalitis, all reported that all the patients in the study experienced at least one symptom of encephalitis. Common symptoms of encephalitis included loss or decreased level of consciousness (77.1%), altered mental state (72.3%), seizures (38.2%), headaches (27.3%), and weakness (15.4%). Other less common symptoms that patients with encephalitis as a complication of COVID‐19 suffered from were aphasia, ataxia, and myoclonus (Table 4).
TABLE 4.
Clinical symptoms
| Study | Encephalitis patients, n | COVID‐19 symptoms | Encephalitis symptoms | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Asymptomatic, n (%) |
Fever, n (%) |
Cough, n (%) |
SOB, n (%) |
Fatigue, n (%) |
Headache, n (%) | Loss/decreased level of consciousness, n (%) |
Altered mental state, n (%) |
Seizure, n (%) |
Weakness, n (%) | ||
| Benameur et al. | 3 | 0 (0.0) | 2 (66.7) | 2 (66.7) | 3 (100.0) | 3 (100.0) | 1 (33.3) | ||||
| Cao et al. | 5 | 0 (0.0) | 4 (80.0) | 3 (60.0) | 4 (80.0) | 3 (60.0) | 3 (60.0) | 1 (20.0) | |||
| Guilmot et al. | 1 | 1 (100.0) | 1 (100.0) | 1 (100.0) | |||||||
| Kihira et al. | 2 | 0 (0.0) | 1 (50.0) | 1 (50.0) | 2 (100.0) | 1 (50.0) | |||||
| Koh et al. | 4 | 2 (50.0) | 4 (100.0) | 3 (75.0) | 2 (50.0) | ||||||
| Kremer et al. | 7 | 1 (14.3) | 6 (85.7) | 3 (42.9) | |||||||
| Paterson et al. | 12 | 7 (58.3) | |||||||||
| Pilotto et al. (b) | 25 | 19 (76.0) | 9 (36.0) | 1 (4.0) | |||||||
| Radmard et al. | 1 | 1 (100.0) | 1 (100.0) | ||||||||
| Rifino et al. | 5 | 2 (40.0) | 5 (100.0) | 2 (40.0) | |||||||
| Romero‐Sánchez et al. | 1 | 1 (100.0) | |||||||||
| Shah et al. | 9 | ||||||||||
| Umapathi et al. | 3 | 0 (0.0) | 1 (33.3) | 3 (100.0) | 1 (33.3) | 3 (100.0) | 1 (33.3) | 1 (33.3) | |||
| Overall | 78 | 5 (23.8) | 7 (63.6) | 6 (60.0) | 11 (84.6) | 4 (50.0) | 3 (27.3) | 27 (77.1) | 34 (72.3) | 13 (38.2) | 6 (15.4) |
Abbreviation: SOB, shortness of breath.
Clinical parameters
Clinical parameters of COVID‐19 patients who suffered from encephalitis as a complication were also analysed. Six studies reported results of serum analyses. D‐dimer levels were raised, with an average of 13.4 mg/L (range = 6.9–15.0 mg/L). Lactate dehydrogenase levels were raised, with an average of 358.7 U/L (range = 322.8–658.0 U/L). C‐reactive protein levels were raised, with an average of 58.8 mg/L (range = 39.9–216.6 mg/L). Interleukin 6 (IL‐6) levels were also raised, with an average of 1327.9 pg/ml (range = 88.1–3394.3 pg/ml; reference value < 6.5 pg/ml). Thirteen studies reported on CSF analyses. Protein levels were raised, with an average of 64.8 mg/dl (range = 38.0–115.0 mg/dl). Glucose levels were raised, with an average of 81.7 mg/dl (range = 59.0–130.0 mg/dl). Cellularity was increased, with a red blood cell level of 328.8 cells/µl (range = 11.5–1154.0 cells/µl) and white blood cell level of 14.8 cells/µl (range = 6.0–38.7 cells/µl). IgG levels were raised, with an average of 83.2 mg/L (range = 5.0–112.5 mg/L). Of the four studies that reported on oligoclonal bands, three studies found that they were absent in eight patients, whereas one study reported its presence in one patient (Table 5).
TABLE 5.
Clinical parameters
| Study | Encephalitis patients, n | Serum | CSF | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| D‐dimer, mg/L | LDH, U/L | CRP, mg/L | IL−6, pg/ml a | Protein, mg/dl | Glucose, mg/dl | Cells/mm3 | RBC, cells/µl | WBC, cells/µl | IgG, mg/L | Oligoclonal bands | ||
| Benameur et al. | 3 | 86.0 | 79.7 | 1154.0 | 38.7 | High | ||||||
| Cao et al. | 5 | 88.1 | ||||||||||
| Dogan et al. | 3 | 6.9 | 658.0 | 216.6 | 3394.3 | 55.9 | 130.0 | 0.0 | 5.0 | Absent | ||
| Guilmot et al. | 1 | 46.0 | ||||||||||
| Kihira et al. | 2 | 132.0 | 105.0 | 0.0 | Absent | |||||||
| Koh et al. | 4 | High | High | High | 56.0 | 22.0 | 6.0 | |||||
| Kremer et al. | 8 | 79.1 | Normal | 7.9 | 112.5 | Present | ||||||
| Luigetti et al. | 1 | 115.0 | Normal | |||||||||
| Paterson et al. | 12 | 15.0 | High | |||||||||
| Pilotto et al. (b) | 25 | 322.8 | 39.9 | 60.1 | 76.2 | 6.4 | ||||||
| Radmard et al. | 1 | High | 38.0 | 59.0 | 32.0 | 12.0 | ||||||
| Rifino et al. | 5 | High | High | High | 45.0 | 9.8 | High | High | ||||
| Romero‐Sánchez et al. | 1 | Normal | Normal | Normal | Normal | |||||||
| Umapathi et al. | 3 | 52.5 | 73.0 | 11.5 | 3.5 | Absent | ||||||
| Overall | 69 | 13.4 | 358.7 | 58.8 | 1327.9 | 64.8 | 81.7 | 6.3 | 328.8 | 14.8 | 83.2 | |
Reference value < 6.5 pg/ml.
Outcomes
Thirteen studies reported on the mortality rate of patients who suffered from encephalitis as a complication of COVID‐19. The pooled mortality rate of patients who suffered from encephalitis as a complication of COVID‐19 was 13.4% (95% CI = 3.8%–25.9%; Figure 3).
FIGURE 3.
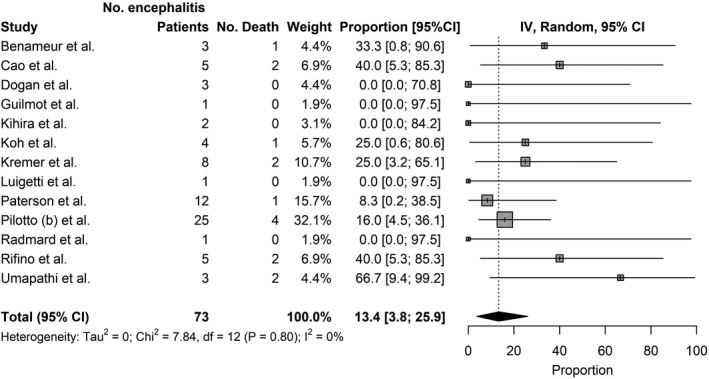
Mortality of patients with encephalitis as a complication of COVID‐19. Inverse Variance (IV)
DISCUSSION
A comprehensive evaluation of the epidemiology and clinical outcomes of patients who suffered from encephalitis as a complication of COVID‐19 was performed. A key finding is that the incidence of encephalitis in COVID‐19 patients is relatively low (<1%), but increases significantly to up to 6.7% in severely ill patients, defined as patients requiring ICU or HDU care. Patients who suffer from encephalitis as a complication of COVID‐19 have much poorer outcomes compared to the general population of COVID‐19 patients, including admission to intensive care facilities, use of ventilators, and high mortality rate. The mortality rate of patients with encephalitis as a complication of COVID‐19 is 13.4%, almost quadruple the 3.4% in the general population of COVID‐19 patients [39]. It might thus be helpful to be vigilant of encephalitis as a complication of COVID‐19 as, although uncommon, it can have severe consequences [6].
Several risk factors for encephalitis as a complication of COVID‐19 were elucidated. Demographic risk factors such as old age and underlying comorbidities may confer increased risk of complications from COVID‐19 infection, including the development of encephalitis [9, 21, 32]. Additionally, patients who are severely ill with COVID‐19 are at a much‐increased risk of suffering from the complication of encephalitis [6, 8, 22]. The incidence of encephalitis as a complication of COVID‐19 is less than 1% in the general population of COVID‐19 patients, but rises greatly to 6.7% in those who are severely ill. The physiological reserves theory might explain this phenomenon. The elderly with multiple comorbidities, or those who are severely ill with COVID‐19, are less able to compensate for physiological derangements, increasing vulnerability to severe complications such as encephalitis [40].
Common symptoms of COVID‐19 include mild symptoms such as cough, malaise, and fever as well as more severe symptoms such as shortness of breath [10]. Symptoms of encephalitis include loss or decreased level of consciousness and altered mental state, focal neurological signs such as weakness, and seizures [12]. Although there are case reports of several patients developing encephalitis weeks after initial infection with COVID‐19 [37], most patients develop both COVID‐19 symptoms and encephalitis symptoms during the same period [30, 32]. Notably, encephalitis is rarely the presenting symptom of COVID‐19. Most often, patients present with respiratory symptoms and develop encephalitis an average of 14.5 days later, during their hospital stay [7, 21, 25]. Therefore, it could be beneficial for physicians to monitor severely ill COVID‐19 patients more closely to mitigate the development of encephalitis as a complication of COVID‐19 should it occur [11]. However, a few COVID‐19 patients who suffer from encephalitis as a complication are asymptomatic carriers of COVID‐19 and only experience encephalitis symptoms [33, 34, 35]. In a case series by Radmard et al., a 74‐year‐old female presenting with altered mental status and no upper respiratory tract symptoms developed encephalitis and was subsequently found to be infected with COVID‐19 [33]. This brings into question whether there is a need for COVID‐19 testing in encephalitis patients with no COVID‐19 respiratory symptoms, as the management of such patients in close proximity to other ill patients might result in viral transmission.
Common MRI brain findings seen in these cases include diffuse white matter hyperintensities and haemorrhagic lesions on fluid‐attenuated inversion recovery and T2 sequences [24, 25, 33, 35, 37]. Other less common MRI findings include cerebral oedema and venous thrombosis [3, 9]. CT head findings were generally unremarkable [24]. Notably, some of these patients suffered from pre‐existing chronic medical conditions such as diabetes mellitus and hypertension [24, 37] and it is possible that some degree of imaging findings such as subcortical white matter hyperintensities and microbleeds in the deep grey nuclei could have resulted from chronic conditions such as hypertension. However, there are also a few reports of patients with encephalitis as a complication of COVID‐19 with normal brain imaging results [6]. This could be due to milder encephalitis or imaging conducted prematurely before brain changes developed. Neuroimaging findings in patients with encephalitis as a complication of COVID‐19 resemble those in the previous severe acute respiratory syndrome coronavirus 1 (SARS‐CoV‐1) and Middle East respiratory syndrome outbreaks, suggesting possible similarities in pathophysiology of central nervous system (CNS) involvement across these three coronaviruses [13]. Electroencephalography (EEG) in some patients showed patterns of general slowing [24]. Although some patients developed seizures during their clinical course [32, 34], sharp waves and epileptiform activity were uncommon findings [24, 33].
Interestingly, there have been increasing reports of severe encephalitis subtypes occurring even among young COVID‐19 patients, such as ADEM [41, 42]. ADEM is a postinfectious inflammatory demyelinating neurological condition that is more common in children than adults [43]. It is a serious form of encephalitis with much poorer outcomes compared to those in other subtypes of encephalitis, including poor functional recovery and a high mortality rate [43]. Worryingly, preliminary reports show that ADEM is not exclusive to COVID‐19 patients with demographic risk factors, as young COVID‐19 patients and those with no comorbidities also develop this complication [41, 42]. These patients also often develop new white matter lesions seen on contrast‐enhancement MRI [44]. Further surveillance is warranted to improve management of these lethal subtypes of encephalitis.
Efficacious management protocols for encephalitis in COVID‐19 are still being studied. Modes of management that have yielded positive patient outcomes include corticosteroids [21], intravenous immunoglobulin [30], plasmapheresis [9] and monoclonal antibodies such as rituximab [45]. In a case series of five patients by Cao et al., administration of corticosteroids (1 g/day intravenous methylprednisolone for 5–10 days) resulted in marked improvement of three patients, who showed dramatic improvement of neurologic status within 1 week and were subsequently discharged [21]. A combination of intravenous immunoglobulin and corticosteroids was successful in the treatment of COVID‐19 patients with inflammatory CNS conditions such as encephalitis, where 11 of the 12 patients studied recovered [30] Plasmapheresis was also shown to be effective in a case series of six critically ill COVID‐19 patients with encephalitis, where five of them recovered enough after commencement of plasmapheresis to be discharged from the ICU to a normal ward [9]. Rituximab was also effective in the treatment of an elderly gentleman with COVID‐19 encephalitis, causing marked improvement in neuropsychiatric symptoms and mental status after administration [45]. However, the preliminary success of these treatment modalities needs to be considered cautiously, as no large‐scale randomised control trials regarding their efficacy have been published to date. More work needs to be carried out in this area to determine their suitability as treatment modalities for encephalitis as a complication of COVID‐19.
There are three proposed mechanisms of the pathophysiology of encephalitis as a complication of COVID‐19: direct invasion of the nervous system, systemic inflammation, and molecular mimicry. First, direct invasion of the SARS‐CoV‐2 virus into the brain parenchyma could cause the development of encephalitis. SARS‐CoV‐2 could enter the brain parenchyma via a transsynaptic propagation or via haematogenous invasion. In transsynaptic propagation, SARS‐CoV‐2 binds to the angiotensin II (ACE‐II) receptor on the cell membrane of peripheral nerve cells and enters cells via receptor‐mediated endocytosis. It then uses active axonal machinery to travel retrogradely to the CNS [46]. One such route is via the olfactory epithelium, where SARS‐CoV‐2 invades the olfactory primary sensory neurons and travels to the cribriform plate of the ethmoidal bone. From there, it crosses into the anterior cranial fossa and may later spread throughout brain parenchyma to cause encephalitis [1]. In haematogenous invasion, SARS‐CoV‐2 crosses the blood–brain barrier (BBB) to enter the brain parenchyma. SARS‐CoV‐2 first invades vascular endothelial cells that express the ACE‐II receptor. It then interacts with ACE‐II on surrounding neurons, glial cells, and other vascular cells, beginning a cycle of viral budding [1]. This causes damage to both vascular and neuronal tissue, compromising the BBB and allowing the SARS‐CoV‐2 virus to enter the CNS [1]. Alternatively, haematogenous invasion could also occur through the infection of leukocytes [46]. Lymphocytes, monocytes, and granulocytes all express the ACE‐II receptor, making infection with SARS‐CoV‐2 possible. Once infected in blood vessels, these leukocytes cross the BBB, entering the CNS and taking the SARS‐CoV‐2 virus with them, where they can infect other cell types within the CNS to cause encephalitis [46]. However, it has been suggested that direct invasion of SARS‐CoV‐2 virus into the CNS may be less likely to be the main mechanism causing encephalitis in COVID‐19 [12, 13], as most patients with encephalitis in COVID‐19 have had negative CSF polymerase chain reaction against SARS‐CoV‐2, and symptoms of direct CNS involvement such as anosmia and ageusia have been very uncommon [30, 32, 33, 34].
Another proposed mechanism for the pathophysiology of encephalitis as a complication of COVID‐19 is the systemic inflammation caused by the SARS‐CoV‐2 virus [47]. SARS‐CoV‐2 infection causes activation of the innate immune system, causing release of large amounts of inflammatory cytokines (interferon [IFN] α, IFNγ, IL‐1β, IL‐6, IL‐12, etc.). This causes the phenomenon known as "cytokine storm," which results in systemic inflammatory response syndrome [47]. These inflammatory molecules are transported throughout the body, attacking all organ systems, including the nervous system. Resultant dysfunction of the nervous system could result in encephalitis [1]. Supportive evidence for this theory includes CSF analysis and serology, which show a proinflammatory state [24, 28, 30]. Additionally, EEG results reveal diffuse patterns indicative of extensive inflammation [13]. Further studies reporting CSF and/or serum levels of such inflammatory cytokines may be useful for elucidating underlying pathophysiological mechanisms.
A third proposed mechanism for encephalitis as a complication of COVID‐19 is molecular mimicry [1]. In response to infection with the SARS‐CoV‐2 virus, there is an expansion of host antibodies and lymphocytes. Although these immune molecules are supposed to be specific for SARS‐CoV‐2 viral antigens, some of them are cross‐reactive and can attack self‐antigens [1]. When cells in the vascular endothelium and brain parenchyma are affected, diffuse damage to CNS results, which may cause the development of encephalitis [1]. Notably, we found that the most common form of encephalitis in COVID‐19 is autoimmune encephalitis [9, 21, 24, 30, 33]. There have also been reports of acute haemorrhagic necrotising encephalopathy [48] and Guillain–Barré syndrome [49] which are known to develop via molecular mimicry, further supporting the theory of molecular mimicry as the pathophysiology of encephalitis as a complication of COVID‐19. Overall, primary data from COVID‐19 patients with encephalitis remains limited, hence conclusions regarding the mechanistic properties and pathophysiology of encephalitis in COVID‐19 cannot be drawn at present.
This study entails some limitations. First, as there are few robust cross‐sectional studies, we also included case series, which held significant publication bias. Second, some studies did not report all our variables of interest, leading to incomplete data. However, as there is currently limited information on encephalitis as a complication of COVID‐19, our detailed analysis of all available data presents good preliminary insight.
CONCLUSION
This systematic review and meta‐analysis evaluated the epidemiology, clinical course, and outcomes of patients who suffered from encephalitis as a complication of COVID‐19. Although the incidence of encephalitis in the general population of hospitalised COVID‐19 patients was low at 0.215%, the mortality rate of patients who suffered from encephalitis as a complication of COVID‐19 was high at 13.4%. Severely ill COVID‐19 patients were much more likely to suffer from encephalitis as a complication. Further research through collaborative international registries would help to comprehensively decipher the pathophysiology and prognosis of encephalitis in COVID‐19, improving the effectiveness of care.
CONFLICT OF INTEREST
The authors declare no financial or other conflicts of interest.
AUTHOR CONTRIBUTIONS
Isabel Siow: Conceptualisation (lead), methodology (lead), visualisation (lead), writing–original draft (lead), writing–review & editing (lead). Keng Siang Lee: Writing–original draft (supporting), writing–review & editing (supporting). John J. Y. Zhang: Writing–original draft (supporting), writing–review & editing (supporting). Seyed Ehsan Saffari: Data curation (lead), formal analysis (lead), software (lead). Adeline Ng: Supervision (lead).
ETHICAL APPROVAL
Research ethics approval was not applicable, as this submission did not involve human participants. All information was obtained from publicly available, published articles.
DISCLAIMERS
The views expressed in this article are entirely our own and not an official position of our institutions.
Supporting information
Fig S1
Fig S2
Supplementary Material
ACKNOWLEDGEMENTS
We thank Toh Kim Kee for her assistance in designing the initial search strategy.
Siow I, Lee KS, Zhang JJY, Saffari SE, Ng A. Encephalitis as a neurological complication of COVID‐19: A systematic review and meta‐analysis of incidence, outcomes, and predictors. Eur J Neurol. 2021;28:3491–3502. 10.1111/ene.14913
Contributor Information
Isabel Siow, Email: isabel.siow@yahoo.com.sg.
Adeline Ng, Email: adeline.ng.s.l@singhealth.com.sg.
DATA AVAILABILITY STATEMENT
The data that support the findings of this study are openly available in PubMed. Additionally, the data that support the findings of this study are available from the corresponding author upon reasonable request.
REFERENCES
- 1. Scoppettuolo P, Borrelli S, Naeije G. Neurological involvement in SARS‐CoV‐2 infection: a clinical systematic review. Brain Behav Immun Health. 2020;5:100094. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Siow I, Lee KS, Zhang JJ, Saffari SE, Ng A, Young B. Stroke as a neurological complication of COVID‐19: a systematic review and meta‐analysis of incidence, outcomes and predictors. J Stroke Cerebrovasc Dis. 2021;30(3):105549. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Benameur K, Agarwal A, Auld SC, et al. Early release‐encephalopathy and encephalitis associated with cerebrospinal fluid cytokine alterations and coronavirus disease, Atlanta, Georgia, USA, 2020. 2020. [DOI] [PMC free article] [PubMed]
- 4. Sedaghat Z, Karimi N. Guillain Barre syndrome associated with COVID‐19 infection: a case report. J Clin Neurosci. 2020;76:233‐235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Chakraborty U, Chandra A, Ray AK, Biswas P. COVID‐19–associated acute transverse myelitis: a rare entity. BMJ Case Reports CP. 2020;13(8):e238668. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Abenza‐Abildúa M, Ramírez‐Prieto M, Moreno‐Zabaleta R, et al. Neurological complications in critical patients with COVID‐19. Neurología (English Edition). 2020;35(9):621‐627. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Guilmot A, Slootjes SM, Sellimi A, et al. Immune‐mediated neurological syndromes in SARS‐CoV‐2‐infected patients. J Neurol. 2021;268(3):751‐757. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Zuccon W, Comassi P, Adriani L, et al. Intensive care for seriously ill patients affected by novel coronavirus sars‐CoV–2: experience of the Crema Hospital, Italy. Am J Emerg Med. 2020;45: 156–161. 10.1016/j.ajem.2020.08.005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Dogan L, Kaya D, Sarikaya T, et al. Plasmapheresis treatment in COVID‐19–related autoimmune meningoencephalitis: case series. Brain Behav Immun. 2020;87:155‐158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Garg RK, Paliwal VK, Gupta A. Encephalopathy in patients with COVID‐19: a review. J Med Virol. 2021;93(1):206‐222. [DOI] [PubMed] [Google Scholar]
- 11. Mondal R, Ganguly U, Deb S, et al. Meningoencephalitis associated with COVID‐19: a systematic review. J Neuro Virol. 2020;27:12‐25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Pouga L. Encephalitic syndrome and anosmia in COVID‐19: do these clinical presentations really reflect SARS‐CoV‐2 neurotropism? A theory based on the review of 25 COVID‐19 cases. J Med Virol. 2021;93(1):550‐558. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Siahaan YM, Puspitasari V, Pangestu AR. COVID‐19‐associated encephalitis: systematic review of case reports findings on cytokine‐immune‐mediated inflammation as an Underlying Mechanism. 2020; online ahead of print 10.21203/rs.3.rs-65579/v1 [DOI]
- 14. Moher D, Liberati A, Tetzlaff J, Altman DG, Group P . Preferred reporting items for systematic reviews and meta‐analyses: the PRISMA statement. PLoS Med. 2009;6(7):e1000097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Organisation WH. The ICD‐10 classification of mental and behavioural disorders clinical: Descriptions and Diagnostic Guidelines. 2019.
- 16. Zhang JJ, Ong JA‐H, Syn NL, et al. Extracorporeal membrane oxygenation in pregnant and postpartum women: a systematic review and meta‐regression analysis. J Intensive Care Med. 2019;36(2):220–228. [DOI] [PubMed] [Google Scholar]
- 17. Zeng X, Zhang Y, Kwong JS, et al. The methodological quality assessment tools for preclinical and clinical studies, systematic review and meta‐analysis, and clinical practice guideline: a systematic review. J Evid Based Med. 2015;8(1):2‐10. [DOI] [PubMed] [Google Scholar]
- 18. Nyaga VN, Arbyn M, Aerts M. Metaprop: a Stata command to perform meta‐analysis of binomial data. Arch Public Health. 2014;72(1):39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Higgins JP, Thomas J, Chandler J, et al. Cochrane Handbook for Systematic Reviews of Interventions. Hoboken, NJ: John Wiley & Sons; 2019. [Google Scholar]
- 20. Team RC. R . A Language and Environment for Statistical Computing. In. Vienna, Austria: R Core Team; 2013. [Google Scholar]
- 21. Cao A, Rohaut B, Le Guennec L, et al. Severe COVID‐19‐related encephalitis can respond to immunotherapy. 2020. [DOI] [PMC free article] [PubMed]
- 22. Helms J, Kremer S, Merdji H, et al. Delirium and encephalopathy in severe COVID‐19: a cohort analysis of ICU patients. Crit Care. 2020;24(1):1‐11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Iltaf S Sr, Fatima M, Salman S Sr, Salam J‐U, Abbas S. Frequency of neurological presentations of coronavirus disease in patients presenting to a tertiary care hospital during the 2019 coronavirus disease pandemic. Cureus. 2020;12(8). 10.7759/cureus.9846 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Kihira S, Delman B, Belani P, et al. Imaging features of acute encephalopathy in patients with COVID‐19: a case series. Am J Neuroradiol. 2020;41(10):1804‐1808. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Koh JS, De Silva DA, Quek AML, et al. Neurology of COVID‐19 in Singapore. J Neurol Sci. 2020;418:117118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Kremer S, Lersy F, Anheim M, et al. Neurologic and neuroimaging findings in patients with COVID‐19: a retrospective multicenter study. Neurology. 2020;95(13):e1868‐e1882. [DOI] [PubMed] [Google Scholar]
- 27. Liotta EM, Batra A, Clark JR, et al. Frequent neurologic manifestations and encephalopathy‐associated morbidity in Covid‐19 patients. Ann Clin Transl Neurol. 2020;7(11):2221‐2230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Luigetti M, Iorio R, Bentivoglio AR, et al. Assessment of neurological manifestations in hospitalized patients with COVID‐19. Eur J Neurol. 2020;27(11):2322‐2328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Miró Ò, Llorens P, Jiménez S, et al. Frequency of five unusual presentations in patients with COVID‐19: results of the UMC‐19‐S1. Epidemiol Infect. 2020;148:e189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Paterson RW, Brown RL, Benjamin L, et al. The emerging spectrum of COVID‐19 neurology: clinical, radiological and laboratory findings. Brain. 2020;143(10):3104‐3120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Pilotto A, Benussi A, Libri I, et al. COVID‐19 impact on consecutive neurological patients admitted to the emergency department. J Neurol Neurosurg Psychiatry. 2020;92(2):218–220. [DOI] [PubMed] [Google Scholar]
- 32. Pilotto A, Masciocchi S, Volonghi I, et al. The clinical spectrum of encephalitis in COVID‐19 disease: the ENCOVID multicentre study. Medrxiv. 2020. 10.3389/fneur.2020.00805 [DOI] [Google Scholar]
- 33. Radmard S, Epstein SE, Roeder HJ, et al. Inpatient neurology consultations during the onset of the SARS‐CoV‐2 New York City pandemic: a single center case series. Front Neurol. 2020;11: 805. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Rifino N, Censori B, Agazzi E, et al. Neurologic manifestations in 1760 COVID‐19 patients admitted to Papa Giovanni XXIII Hospital, Bergamo, Italy. J Neurol. 2020;1‐8. 10.1007/s00415-020-10251-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Romero‐Sánchez CM, Díaz‐Maroto I, Fernández‐Díaz E, et al. Neurologic manifestations in hospitalized patients with COVID‐19: The ALBACOVID registry. Neurology. 2020;95(8):e1060‐e1070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Shah VA, Nalleballe K, Zaghlouleh ME, Onteddu S. Acute encephalopathy is associated with worse outcomes in COVID‐19 patients. Brain Behav Immun Health. 2020;8:100136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Umapathi T, Quek WMJ, Yen JM, et al. Encephalopathy in COVID‐19 patients; viral, parainfectious, or both? Eneurologicalsci. 2020;21:100275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Varatharaj A, Thomas N, Ellul MA, et al. Neurological and neuropsychiatric complications of COVID‐19 in 153 patients: a UK‐wide surveillance study. Lancet Psychiatry. 2020;7(10):875‐882. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Baud D, Qi X, Nielsen‐Saines K, Musso D, Pomar L, Favre G. Real estimates of mortality following COVID‐19 infection. Lancet Infect Dis. 2020;20(7):773. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Esme M, Topeli A, Yavuz BB, Akova M. Infections in the elderly critically‐ill patients. Front Med. 2019;6:118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Langley L, Zeicu C, Whitton L, Pauls M. Acute disseminated encephalomyelitis (ADEM) associated with COVID‐19. BMJ Case Rep CP. 2020;13(12):e239597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Zhang T, Rodricks MB, Hirsh E. COVID‐19‐associated acute disseminated encephalomyelitis: a case report. MedRxiv. 2020. 10.1101/2020.04.16.20068148 [DOI] [Google Scholar]
- 43. Sonneville R, Klein I, De Broucker T, Wolff M. Post‐infectious encephalitis in adults: diagnosis and management. J Infect. 2009;58(5):321‐328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Granerod J, Davies N, Mukonoweshuro W, et al. Neuroimaging in encephalitis: analysis of imaging findings and interobserver agreement. Clin Radiol. 2016;71(10):1050‐1058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Haider A, Siddiqa A, Ali N, Dhallu M. COVID‐19 and the brain: acute encephalitis as a clinical manifestation. Cureus. 2020;12(10):e10784. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Pennisi M, Lanza G, Falzone L, Fisicaro F, Ferri R, Bella R. SARS‐CoV‐2 and the nervous system: from clinical features to molecular mechanisms. Int J Mol Sci. 2020;21(15):5475. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Pezzini A, Padovani A. Lifting the mask on neurological manifestations of COVID‐19. Nat Rev Neurol. 2020;16(11):636‐644. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Ghosh R, Dubey S, Finsterer J, Chatterjee S, Ray BK. SARS‐CoV‐2‐associated acute hemorrhagic, necrotizing encephalitis (AHNE) presenting with cognitive impairment in a 44‐year‐old woman without comorbidities: a case report. Am J Case Rep. 2020;21:e925641‐925641. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Unnithan AKA. A brief review of the neurological manifestations of the coronavirus disease. Egypt J Neurol Psychiatr Neurosurg. 2020;56(1):1‐7. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Fig S1
Fig S2
Supplementary Material
Data Availability Statement
The data that support the findings of this study are openly available in PubMed. Additionally, the data that support the findings of this study are available from the corresponding author upon reasonable request.


