Abstract
Background
Coronavirus disease 2019 (COVID‐19) is an emerging, rapidly evolving pandemic, hypertension is one of the most common co‐existing chronic conditions and a risk factor for mortality. Nearly one‐third of the adult population is hypertensive worldwide, it is urgent to identify the factors that determine the clinical course and outcomes of COVID‐19 patients with hypertension.
Methods and results
148 COVID‐19 patients with pre‐existing hypertension with clarified outcomes (discharge or deceased) from a national cohort in China were included in this study, of whom 103 were discharged and 45 died in hospital. Multivariate regression showed higher odds of in‐hospital death associated with high‐sensitivity cardiac troponin (hs‐cTn) > 28 pg/ml (hazard ratio [HR]: 3.27, 95% confidence interval [CI]: 1.55–6.91) and interleukin‐6 (IL‐6) > 7 pg/ml (HR: 3.63, 95% CI:1.54–8.55) at admission. Patients with uncontrolled blood pressure (BP) (n = 52) which were defined as systolic BP ≥140 mm Hg or diastolic BP ≥90 mm Hg for more than once (≥2 times) during hospitalization, were more likely to have ICU admission (p = 0.037), invasive mechanical ventilation (p = 0.028), and renal injury (p = 0.005). A stricter BP control with the threshold of 130/80 mm Hg was associated with lower mortality. Treatment with renin‐angiotensin‐aldosterone system (RAAS) suppressors, including angiotensin‐converting enzyme (ACE) inhibitors, angiotensin II receptor blockers (ARB), and spironolactone, was associated with a lower rate of ICU admission compared to other types of anti‐hypertensive medications (8 (22.9%) vs. 25 (43.1%), p = 0.048).
Conclusion
Among COVID‐19 patients with pre‐existing hypertension, elevated hs‐cTn and IL‐6 could help clinicians to identify patients with fatal outcomes at an early stage, blood pressure control is associated with better clinical outcomes, and RAAS suppressors do not increase mortality and may decrease the need for ICU admission.
Keywords: blood pressure control, cardiac injury, COVID‐19, hypertension, renin‐angiotensin‐aldosterone system suppressors
1. BACKGROUND
The outbreak of coronavirus disease 2019 (COVID‐19) has infected more than 122 million people and caused more than 2.7 million deaths. Hypertension is the most common co‐existing chronic disease among COVID‐19 patients and has consistently been reported as a risk factor for higher mortality. 1 , 2 , 3 Hypertension is a common cardiovascular condition that affects about 31.2% of adults worldwide. 4 As the COVID‐19 pandemic is becoming an emerging, rapidly evolving situation, it is urgent to identify the factors associated with clinical outcomes in such a huge hypertensive population with high risk after COVID‐19 infection, so that earlier surveillance and optimal treatment could be administered to save lives.
Angiotensin‐converting enzyme (ACE) inhibitors and angiotensin receptor blockers (ARB) are the first‐line medications for the management of hypertension, which have been used in a large population of hypertension patients. Previous reports demonstrated that ACEIs and ARBs treatment is associated with a lower risk of mortality, 5 , 6 and reduced the level of systemic inflammation in hypertensive COVID‐19 patients. 7 However, more recent clinical trial with 152 COVID‐19 patients with hypertension demonstrated that discontinuation and continuation of ACEIs and ARBs did not the change severity or duration of hospitalization. COVID‐19 patients with hypertension have been found to be more susceptible to cardiac injury, which may lead to poorer clinical outcomes. 1 , 6 , 8 However, there is a lack of panoramic picture; therefore, based on a national cohort, this study attempts to investigate the potential factors including laboratory findings, blood pressure control, and usage of renin‐angiotensin‐aldosterone system (RAAS) inhibitors that may affect the outcomes in COVID‐19 patients with pre‐existing hypertension, which may provide potential therapeutic targets for the future clinical trials.
2. METHODS
2.1. Study subjects
Led by the China National Health Commission, a retrospective cohort to study the laboratory‐confirmed COVID‐19 admitted cases from 575 hospitals throughout China was established as previously described. 3 By the time of March 22nd, 2020, a retrospective cohort of 548 hospitalized COVID‐19 cases with known prognosis (deceased or discharged) was extracted for this study. From this cohort, blood pressure was tracked from admission, all the cases who had pre‐existing hypertension were included in this study. Medical history, clinical, epidemiological, and laboratory data were reviewed and extracted by experienced respiratory clinicians from electronic medical records, patients with incomplete medical records or still in the hospitals were excluded. The diagnosis of all the cases was confirmed by positive results to high‐throughput sequencing or real‐time reverse‐transcriptase polymerase chain reaction (RT‐PCR) assay for nasal or pharyngeal swab specimens. The study was approved by the ethics commission of the First Affiliated Hospital of Guangzhou Medical University (IRB:202051). In light of the urgent need to collect data on this emerging pandemic, written informed consent was waived.
Pre‐existing hypertension was determined based on patient's medical history and was classified as Grade 1 (systolic blood pressure (SBP) 140 to 159 mm Hg or diastolic blood pressure (DBP) 90 to 99 mm Hg), Grade 2 (SBP 160 to 179 mm Hg or DBP 100 to 109 mm Hg), and Grade 3 (SBP ≥180 mm Hg or DBP ≥110 mm Hg) according to 2018 Guidelines of the European Society of Hypertension (ESH). 9 Patients showed SBP ≥140 mm Hg or DBP ≥90 mm Hg for more than once (≥ 2 times) based on blood pressure records during hospitalization were defined as uncontrolled blood pressure. In addition, a stricter criteria of blood pressure control (SBP <130 and DBP <80 mm Hg) were also included according to the 2017 American College of Cardiology/American Heart Association High Blood Pressure Guideline. 10
The primary clinical outcomes of this study were all cause mortality during hospitalization. Cardiac injury was defined as the serum high‐sensitivity cardiac troponin (hs‐cTn) concentration was above the upper limit of the reference range (>28 pg/ml). Renal injury was identified on serum creatinine level above 111 μmmol/L.
2.2. Statistical analysis
Statistical analyses were performed using SPSS software version 23.0. Kolmogorov–Smirnov test was used to test the normality of the data. Continuous variables were expressed as medians and interquartile ranges (IQR), and the categorical variables were presented as counts and percentages. Wilcoxon rank‐sum tests were applied to continuous variables, and chi‐square tests or Fisher's exact test was applied to categorical variables as appropriate. To explore the risk factors associated with clinical outcomes including in‐hospital death, variables including baseline characteristics, laboratory findings, and treatments were analyzed by univariate COX regression analyses, significant variables were further analyzed in multivariate COX regression to control for potential confounder, with the adjusted hazards ratio (HR) and 95% confidence interval (95% CI) being expressed. In addition to significant variables in the univariate Cox regression, factors considered as potential confounders in our analysis also included age and sex. The Kaplan‐Meier survivor curve was used to estimate time‐dependent hazards categorized by statistically significant risk factors. A P value less than 0.05 was considered statistically significant.
3. RESULTS
3.1. Characteristics of study participants
As shown in Table S1, of these included COVID‐19 cases with pre‐existing hypertension, 86 (58.1%) were male, 93 (62.8%) were taking anti‐hypertensive drugs, the most common co‐morbidities were diabetes (23.6%) and coronary heart disease (17.6%), and the most common symptoms were fever (91.9%), cough (77.7%), dyspnea (54.1%), fatigue (33.1%), and sputum (29.7%).
3.2. Outcomes
For the primary outcome, 45 (30.4%) patients had died during hospitalization. Compared with survivors, non‐survivors were older and more likely to receive invasive mechanical ventilation (IMV), an inhaled corticosteroid, to be admitted to ICU, and have dyspnea (Table S1). On admission, abnormalities of laboratory findings were more prominent in the fatal cases compared to survivors: lymphocytopenia, thrombocytopenia, and elevated levels of leukocytes, lactose dehydrogenase (LDH), high‐sensitivity cardiac troponin (hs‐cTn), prothrombin time (PT), D‐dimer, ferritin, interleukin‐6 (IL‐6), procalcitonin (PCT), C‐reactive protein (CRP), direct bilirubin (DBIL), total bilirubin (TBIL), creatine kinase‐MB (CK‐MB) and B‐type natriuretic peptide (BNP) (Table S1). The multivariate COX regression analysis demonstrated some independent predicted factors at the time of admission for in‐hospital death including an elevated level of hs‐cTn (HR: 3.98, 95% CI: 1.95–8.16) and IL‐6 (HR: 3.31, 95% CI: 1.42–7.72) (Figure 1A). Kaplan‐Meier survival plots for these prognostic factors are shown in Figure 1B,C.
FIGURE 1.
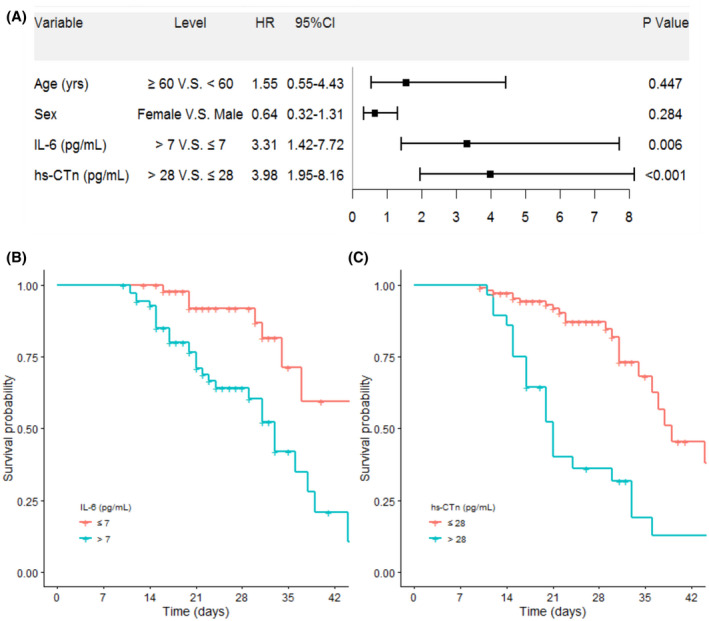
Risk factor of mortality in the proportional hazards model. (A) The hazards ratio (HR) and the 95% confidence interval (95%CI) are associated with the endpoint. (B‐C) The adjusted survival curves for time dependent hazards of different variables by multivariate Cox regression. CI, confidential interval; HR, hazard ratio; hs‐CTn, hypersensitive cardiac troponin; IL‐6, interleukin 6
3.3. Blood pressure control was associated with better clinical outcomes
Blood pressure was tracked from admission, and SBP was higher in non‐survivors compared to survivors most of the time (Figure 2); statistical analysis showed that baseline SBP on admission was significantly higher (Table S1), these implied that lower blood pressure may result in better outcomes. We thus analyzed the clinical characteristics between patients with and without blood pressure control. 52 (35.1%) patients showed SBP ≥140 mm Hg or DBP ≥90 mm Hg for more than once (≥2 times) during hospitalization, these cases had a higher rate of ICU admission, invasive mechanical ventilation, and renal injury and higher level of hs‐cTn, D‐dimer, ferritin and procalcitonin based on laboratory findings. We further conducted an analysis of the influence of blood pressure control with a stricter criterion (130/80 mm Hg), 105 (70.9%) patients showed elevated SBP or DBP for more than once (≥2 times) during hospitalization, and these cases had higher mortality rate (Table 1). Among the patients who underwent laboratory tests on admission, higher rate of abnormal findings (hs‐cTn, PCT, ferritin) were found in patients with uncontrolled blood pressure using cut‐off of 130/80 mm Hg (Table 1).
FIGURE 2.
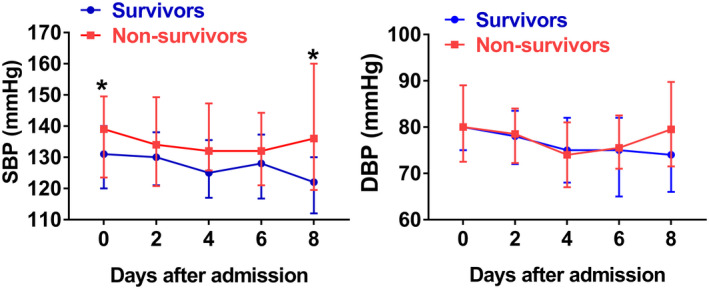
Temporal changes in systolic blood pressure (SBP) (A) and diastolic blood pressure (DBP) (B) from admission in hospitalized COVID‐19 patients with pre‐existing hypertension. * P <.05 between survivors and non‐survivors
TABLE 1.
Clinical characteristics, laboratory findings, treatments, and outcomes of 148 COVID‐19 patients with hypertension
| Blood pressure control (140/90) | Blood pressure control (130/80) | |||||
|---|---|---|---|---|---|---|
| Controlled (n = 96) | Uncontrolled (n = 52) | p value | Controlled (n = 43) | Uncontrolled (n = 105) | p value | |
| Clinical characteristics | ||||||
| Age (years old) | 66 (57–70) | 67 (59–74) | 0.171 | 64 (57–70) | 66 (58–74) | 0.280 |
| ≥60 years old, No. (%) | 62 (64.6) | 39 (75.0) | 0.194 | 28 (65.1) | 73 (69.5) | 0.601 |
| Male, No. (%) | 52 (54.2) | 34 (65.4) | 0.187 | 23 (53.5) | 63 (60.0) | 0.466 |
| Respiratory rate (times/minute) | 21 (20–23) | 21 (20–26) | 0.468 | 20 (20–23) | 22 (20–24) | 0.153 |
| >24 times/minute, No. (%) | 17 (17.7) | 16 (30.8) | 0.068 | 9 (20.9) | 24 (22.9) | 0.798 |
| Heart rate (times/minute) | 84 (80–93) | 85 (80–98) | 0.616 | 82 (80–91) | 84 (80–94) | 0.647 |
| ≥100 times/minute, No. (%) | 14 (14.6) | 12 (23.1) | 0.195 | 6 (14.0) | 20 (19.0) | 0.460 |
| SBP (mm Hg) | 125 (118–135) | 146 (139–160) | <0.001 | 120 (114–130) | 136 (128–152) | <0.001 |
| DBP (mm Hg) | 78 (72–83) | 90 (81–98) | <0.001 | 76 (68–80) | 83 (76–92) | <0.001 |
| Hypertension duration (years) | 10 (5–14) | 10 (4–20) | 0.996 | 10 (5–14) | 10 (5–20) | 0.377 |
| Laboratory findings | ||||||
| Lymphocyte count (×109/L) | 0.81 (0.56–1.12) | 0.71 (0.45–0.92) | 0.073 | 0.84 (0.63–1.11) | 0.74 (0.50–1.06) | 0.083 |
| <0.8 × 109/L, No. (%) | 44 (45.8) | 31 (59.6) | 0.109 | 18 (45.0) | 57 (59.4) | 0.125 |
| Platelet count (×109/L) | 203 (161–267) | 187 (129–249) | 0.177 | 204 (160–285) | 194 (139–241) | 0.282 |
| <100 × 109/L | 8 (9.0) | 4 (8.7) | 1.000 | 4 (9.8) | 8 (8.5) | 1.000 |
| LDH (U/L) | 338.5 (271.8–442.0) | 349.5 (288.8–491.0) | 0.373 | 320.0 (231.8–419.0) | 355.5 (285.8–492.3) | 0.067 |
| >245 U/L, No. (%) | 74 (80.4) | 41 (82) | 0.820 | 30 (75.0) | 85 (83.3) | 0.255 |
| Procalcitonin (ng/ml) | 0.05 (0.05–0.09) | 0.08 (0.05–0.24) | 0.005 | 0.05 (0.05–0.08) | 0.06 (0.05–0.13) | 0.035 |
| ≥0.1 ng/ml, No. (%) | 18 (22.0) | 19 (44.2) | 0.010 | 5 (13.5) | 32 (36.4) | 0.011 |
| Ferritin (g/ml) | 758.7 (403.3–1887.8) | 1796.6 (755.9–2000.0) | 0.041 | 452.4 (334.0–840.7) | 808.4 (424.2–1501.2) | 0.006 |
| >500 g/ml, No. (%) | 48 (58.5) | 26 (63.4) | 0.602 | 15 (41.7) | 59 (67.8) | 0.007 |
| BNP (pg/ml) | 61.0 (24.3–119.2) | 56.0 (32.5–146.8) | 0.418 | 60.5 (27.9–95.0) | 57.7 (28.2–148.1) | 0.460 |
| >400 pg/ml, No. (%) | 2 (3.4) | 3 (8.1) | 0.603 | 1 (4.3) | 4 (5.6) | 1.000 |
| Creatinine (μmol/L) | 72.5 (59.2–86.1) | 74.6 (57.7–106.0) | 0.259 | 67.6 (53.8–86.2) | 73.6 (60.2–90.9) | 0.186 |
| >111 μmol/L, No. (%) | 5 (5.6) | 10 (21.3) | 0.005 | 2 (5.0) | 13 (13.4) | 0.258 |
| hs‐cTn, (pg/ml) | 5.8 (2.6–17.4) | 10.1 (3.7–35.3) | 0.024 | 5.0 (2.1–14.3) | 8.6 (3.5–27.7) | 0.044 |
| >28 pg/ml, No. (%) | 15 (16.1) | 15 (30) | 0.052 | 5 (11.9) | 25 (24.8) | 0.086 |
| D‐dimer (ug/ml) | 0.94 (0.53–2.73) | 1.96 (0.81–12.95) | 0.015 | 0.89 (0.51–3.96) | 1.58 (0.65–4.61) | 0.120 |
| >1.0 ug/ml, No. (%) | 42 (49.4) | 28 (68.3) | 0.046 | 16 (43.2) | 54 (60.7) | 0.073 |
| IL‐6 (pg/ml) | 7.43 (5.80–9.74) | 8.08 (6.29–11.30) | 0.423 | 7.64 (6.90–10.62) | 7.36 (5.79–9.79) | 0.211 |
| >7 pg/ml, No. (%) | 48 (60.8) | 24 (57.1) | 0.700 | 23 (74.2) | 49 (54.4) | 0.053 |
| Treatments and outcomes | ||||||
| ICU admission, No. (%) | 23 (24.0) | 21 (40.4) | 0.037 | 8 (18.6) | 36 (34.3) | 0.058 |
| IMV, No. (%) | 12 (12.5) | 14 (26.9) | 0.028 | 5 (11.6) | 21 (20.0) | 0.224 |
| Death, No. (%) | 25 (26.0) | 20 (38.5) | 0.117 | 8 (18.6) | 37 (35.2) | 0.046 |
| Duration of hospitalization (days), No. (%) | 13 (9–18) | 14 (8–18) | 0.429 | 13 (9–17) | 14 (9–18) | 0.742 |
All the categorical variables are presented as number (percentage), and all the continuous variables were expressed by median and interquartile range (IQR). Wilcoxon rank‐sum tests weas applied to continuous variables, and chi‐square tests or Fisher's exact test were applied to categorical variables as appropriate.
Abbreviations: DBP, diastolic blood pressure at admission; hs‐cTn, high‐sensitivity cardiac troponin; ICU, intensive care unit; IL‐6, interleukin‐6; IMV, invasive mechanical ventilation; LDH, lactose dehydrogenase; BNP, B‐type natriuretic peptide; SBP, systolic blood pressure at admission.
3.4. Renin‐angiotensin‐aldosterone system (RAAS) suppressors was associated with a lower rate of severe illness
RAAS plays an important role in regulating blood pressure, and RAAS suppressors, including ACE inhibitors, ARBs, and spironolactone, are widely used in hypertensive population. 4 In our study, there were 35 (23.6%) patients taking RAAS suppressor including ACE inhibitors (n = 7, of whom 3 also took spironolactone), ARBs (n = 22, of whom 1 also took spironolactone), and spironolactone (n = 10); and 58 (39.2%) cases taking other types of antihypertensive medications. There was no modification of anti‐hypertensive medications in most of the patients, and the usage of RAAS suppressors remained the same in all the patients before and after admission. As shown in Table 2, compared to other types of antihypertensives (non‐RAAS suppressors), patients with RAAS suppressors treatment were less likely to be admitted to ICU and had a significantly higher creatinine level. However, the creatinine levels from most of the patients were under normal range, and RAAS suppressors did not change the chance of renal injury in COVID‐19 patients with pre‐existing hypertension.
TABLE 2.
Clinical characteristics, laboratory findings, and outcomes of 93 COVID‐19 patients with antihypertensive medications
| Variables | Other antihypertensives a (n = 58) | RAAS suppressors (n = 35) | p value |
|---|---|---|---|
| Clinical characteristics | |||
| Age (years old) | 66 (59–71) | 69 (59–76) | 0.256 |
| ≥60 years old, No. (%) | 43 (74.1) | 26 (74.3) | 0.987 |
| Male, No. (%) | 32 (55.2) | 19 (54.3) | 0.934 |
| Respiratory rate (times/minute) | 22 (20–25) | 20 (20–22) | 0.013 |
| >24 times/minute, No. (%) | 15 (25.9) | 5 (14.3) | 0.188 |
| Heart rate (times/minute) | 86 (79–98) | 82 (80–94) | 0.472 |
| ≥100 times/minute, No. (%) | 12 (20.7) | 3 (8.6) | 0.124 |
| SBP (mm Hg) | 132 (120–141) | 138 (122–152) | 0.074 |
| DBP (mm Hg) | 78 (74–87) | 82 (75–91) | 0.154 |
| Hypertension duration (years) | 10 (6–20) | 10 (4.8–20) | 0.867 |
| Laboratory findings | |||
| Lymphocyte count (×109/L) | 0.75 (0.53–1.04) | 0.77 (0.57–1.14) | 0.658 |
| <0.8 × 109/L, No. (%) | 30 (60.0) | 18 (54.5) | 0.622 |
| Platelet count (×109/L) | 203 (161–263) | 203 (118–297) | 0.462 |
| <100 × 109/L | 3 (6.0) | 5 (15.2) | 0.316 |
| Lactose dehydrogenase (U/L) | 356.5 (295.5–531.0) | 324.5 (246.3–478.3) | 0.216 |
| >245 U/L, No. (%) | 44 (81.5) | 26 (76.5) | 0.570 |
| Procalcitonin (ng/ml) | 0.06 (0.05–0.14) | 0.05 (0.05–0.10) | 0.322 |
| ≥0.1 ng/ml, No. (%) | 19 (38.0) | 7 (23.3) | 0.175 |
| Ferritin (g/ml) | 511.6 (382.2–1192.7) | 790.2 (402.0–1107.1) | 0.724 |
| >500 g/ml, No. (%) | 25 (51.0) | 16 (59.3) | 0.490 |
| BNP (pg/ml) | 54.3 (30.8–110.7) | 102.5 (28.6–166.3) | 0.310 |
| >400 pg/ml, No. (%) | 3 (7.5) | 0 | 0.485 |
| Creatinine (μmol/L) | 67.4 (52.5–85.7) | 77.9 (65.6–103.0) | 0.035 |
| >111 μmol/L, No. (%) | 5 (9.8) | 5 (15.2) | 0.693 |
| High‐sensitivity cardiac troponin (pg/ml) | 9.1 (3.3–22.3) | 6.2 (2.9–26.3) | 0.648 |
| >28 pg/ml, No. (%) | 13 (23.6) | 7 (20.6) | 0.738 |
| D‐dimer (ug/ml) | 1.27 (0.66–4.52) | 1.37 (0.53–5.57) | 0.868 |
| >1.0 ug/ml, No. (%) | 30 (61.2) | 17 (56.7) | 0.689 |
| IL‐6 (pg/ml) | 7.50 (6.07–9.60) | 7.33 (5.84–12.69) | 0.946 |
| >7 pg/ml, No. (%) | 28 (58.3) | 16 (55.2) | 0.786 |
| Treatments and outcomes | |||
| ICU admission, No. (%) | 25 (43.1) | 8 (22.9) | 0.048 |
| Invasive mechanical ventilation, No. (%) | 16 (27.6) | 4 (11.4) | 0.066 |
| Death, No. (%) | 20 (34.5) | 13 (37.1) | 0.795 |
| Duration of hospitalization (days), No. (%) | 15 (10–19) | 14 (10–19) | 0.924 |
All the categorical variables are presented as number (percentage), and all the continuous variables were expressed by median and interquartile range (IQR). Wilcoxon rank‐sum tests was applied to continuous variables, and chi‐square tests or Fisher's exact test were applied to categorical variables as appropriate.
Abbreviations: BNP, B‐type natriuretic peptide; DBP, diastolic blood pressure at admission; ICU, intensive care unit; IL‐6, interleukin‐6; RAAS, renin‐angiotensin‐aldosterone system; SBP, systolic blood pressure at admission.
Other antihypertensives refers to antihypertensive medication other than RAAS suppressors (angiotensin‐converting enzyme inhibitors, angiotensinⅡblocker, spironolactone).
4. DISCUSSION
In this nationwide investigation on COVID‐19 patients with hypertension in China, we found that fatal outcomes are associated with hs‐cTn >28 pg/ml and IL‐6 > 7 pg/ml on admission, and poor blood pressure control during hospitalization; and RAAS suppressors do not increase mortality and may decrease the need of ICU admission.
Cardiac injuries are common in patients with coronavirus infection: myocardial damage has been found in patients infected with the Middle East respiratory syndrome‐related coronavirus (MERS‐CoV), severe acute respiratory syndrome coronavirus (SARS‐CoV), and SARS‐CoV‐2, especially in those with a critical illness. These findings are not surprising because ACE2, the receptor for the coronavirus, is expressed on myocytes and vascular endothelial cells, which may facilitate direct cardiovascular involvement of the coronavirus, and in the autopsy of SARS patients, SARS‐CoV RNA was found in the heart samples. Since ACE2 is a membrane‐bound aminopeptidase and could protect the heart from injury via converting angiotensin II (Ang II) into angiotensin‐ (1‐7) [Ang‐ (1‐7)] which is a negative regulator of RAAS, the coronavirus infection may exhaust the enzymatic activity of ACE2 and enhance susceptibility to cardiac injury. Interstitial mononuclear inflammatory infiltrates have been found in the heart tissue from fatal COVID‐19 patients, 11 which may suggest systemic pro‐inflammatory responses caused by the viral infection may contribute to myocardial damage. In addition, co‐existing hypertension may contribute to higher susceptibility COVID‐19‐induced cardiac injury, a recent study demonstrated that elevated hs‐cTn presents more frequently in COVID‐19 patients with hypertension. 12 In our study, more than 20% of the hypertensive COVID‐19 patients had a cardiac injury on admission as indicated by hs‐cTn >28 which acts as an independent risk factor of mortality. Similarly, in a recent single‐centered study of 416 hospitalized COVID‐19 patients, the cardiac injury appears to be associated with fatal outcomes. 12 Another study with dynamic measurement of high‐sensitive cardiac troponin I (Hs‐TnI) at different time points shows that more than half of the deceased COVID‐19 patients had increased Hs‐TnI during hospitalization. 13 Patients with COVID‐19 can develop rapidly after the virus infection, thus assessing cardiac injury regularly is essential, especially in those with hypertension. In this study, hs‐cTn data was obtained at the time of admission, which implied that hypertensive COVID‐19 patients with cardiac injury at an early stage need intensive surveillance to prevent the potential deterioration and fatal outcomes. 12
IL‐6 is a pleiotropic cytokine that acts as a strong regulator of inflammation, elevated IL‐6 is common in COVID‐19 patients, especially those with critical illness and fatal outcomes. 13 In our study, almost 60% of the COVID‐19 patients with pre‐existing hypertension had elevated circulating IL‐6 at admission, and elevated IL‐6 was also an independent factor of death. Blocking IL‐6 signaling using tocilizumab as a treatment represses the deterioration of severe COVID‐19 patients in a preliminary investigation with 21 patients. 14 As the rate of elevated IL‐6 is high in COVID‐19 patients with hypertension, blocking IL‐6 signaling could be a potential treatment, which may need further investigation.
Blood pressure control is the cornerstone for hypertension management. However, about 35% COVID‐19 patients with a history of hypertension showed uncontrolled blood pressure during hospitalization in our study; and these patients tend to be more severely ill as indicated by a higher rate of ICU admission and IMV treatment, and to be more likely to have a cardiac injury and renal injury. Under the 2017 American College of Cardiology/American Heart Association guideline with a cut‐off of 130/80 mm Hg, the number of patients with uncontrolled blood pressure would be double in our study, and a higher rate of in‐hospital death and cardiac injury were found in these patients. Consistently, another group found that blood pressure was significantly higher in COVID‐19 patients in ICU compared to those not in ICU. 15 Given the fact that the blood pressure is under control in only 15.3% of hypertensive population in China, 16 our data may suggest that blood pressure control is important for COVID‐19 management. As viral infection may change the blood pressure, 15 the antihypertensive medications may need to be adjusted for some of the patients.
RAAS suppressors are commonly used in hypertensive patients; however, it remains debatable whether these medications should be continued during the COVID‐19 pandemic as these drugs have been reported to increase ACE2 expression. 17 , 18 As ACE2 is required for SARS‐CoV‐2 entry and propagation in host cells, 19 it is worried that upregulated ACE2 caused by RAAS suppressors may increase viral replication and thus deteriorates SARS‐CoV‐2 infection. However, recent retrospective studies among hypertensive COVID‐19 patients reported that ACE inhibitors/ARBs treatment is associated with lower or at least no higher rate of fatal outcomes. 5 , 6 , 7 , 20 In our study, RAAS suppressors including ACE inhibitors, ARBs, and spironolactone treatment in COVID‐19 patients with pre‐existing hypertension did not change in hospital death but decreased the rate of ICU admission. It is of note that RAAS suppressors were associated with increased serum creatinine level but no change in renal injury in our study, because creatinine level from most of the patients was under normal range even with the increment caused by RAAS suppressors, this finding is consistent with the previous report that ACE inhibitors and ARBs increase creatinine level in the population without COVID‐19. Together these may suggest that RAAS suppressors treatment is safe for hypertensive COVID‐19 patients, but should be cautious in patients with renal impairment.
Another worry of RAAS suppressors treatment during COVID‐19 outbreak is that these medications may increase the chance of infection via upregulating ACE2 expression. However, a study in New York with 12 594 patients indicated that ACE inhibitors and ARBs treatment in hypertensive patients does not change the rate of SARS‐CoV‐2 infection based on a study. 21 Previous study found that ACE inhibitors and ARBs increase ACE2 level in intestine, blood and urine, 22 but whether RAAS suppressors modulate the level of lung‐specific ACE2 is not known. Lung is the main target of COVID‐19, a recent study indicated that chronic obstructive pulmonary disease (COPD) patients have a higher level of ACE2 in respiratory tract epithelia, 23 these may lead to a higher rate of COVID‐19 infection in COPD patients speculatively. However, on the contrary, the actual rate of COVID‐19 patients with pre‐existing COPD is less than 1.5% based on two nationwide study in China, 1 , 3 which is much lower than the percentage of COPD among the whole adult population in the Nation (8%). These may suggest that increased ACE2 levels may not necessarily lead to a higher chance of SARS‐CoV‐2 infection. However, these data are based on retrospective study, therefore, prospective study with a large sample size and randomized controlled clinical trials are needed to further evaluate the effects of RAAS suppressors in hypertensive population under the emerging COVID‐19 pandemic.
5. LIMITATIONS
Our study has several limitations. First, a main limitation was the self‐report of hypertension history on admission, as less than half of the hypertensive individuals are aware of their conditions according to a recent survey in China, we may have excluded some true hypertensive COVID‐19 patients from our study. Second, due to the retrospective nature of this study, some parameters were not available in all the patients and their role might be underestimated, future prospective studies that include randomized clinical trial would be desirable.
6. CONCLUSION
Among COVID‐19 patients with pre‐existing hypertension, elevated hs‐cTn and IL‐6 at admission were independent risk factors for mortality and could possibly be used as predictors for fatal outcomes; blood pressure control with a target of <130/80 mm Hg during hospitalization is associated with fewer adverse clinical events; and RAAS suppressors do not change mortality and may decrease the need of ICU admission, which support the current guideline of continuous RAAS usage for antihypertensive treatment in COVID‐19 patients.
CONFLICT OF INTEREST
The authors declare that they have no conflict of interests.
CONSENT FOR PUBLICATION
Not applicable.
AUTHOR CONTRIBUTIONS
Tao Wang, Ruidi Tang, Honglian Ruan, Ruchong Chen, Zili Zhang, Nuofu Zhang, Nanshan Zhong and Shiyue Li participated in study design and study conception; Tao Wang, Ruchong Chen, Honglian Ruan, Ruidi Tang, Zili Zhang, Xi Su, Shuting Yi and Shiyue Li performed data analysis; Ling Sang, Zhengyi Ni, Yu Hu, Lei Liu, Hong Shan, Chunliang Lei, Yixiang Peng, Chunli Liu, Jing Li, Cheng Hong, Nuofu Zhang, Nanshan Zhong and Shiyue Li recruited patients; Tao Wang, Nuofu Zhang, Nanshan Zhong and Shiyue Li drafted the manuscript; all authors provided critical review of the manuscript and approved the final draft for publication.
ETHICS APPROVAL AND CONSENT TO PARTICIPATE
This study is approved by the ethics committee of the First Affiliated Hospital of Guangzhou Medical University (IRB:202051). Written informed consent was waived in light of the urgent need to collect data and the family members of the patients were in quarantine.
Supporting information
Table S1
ACKNOWLEDGMENTS
We thank all medical staff fighting against COVID‐19, and all the patients involved in the study. We also thank the hospital staffs for their efforts in collecting the data. We express sincere sympathies and deep condolences to the victims and bereaved families.
Wang T, Tang R, Ruan H, et al; China Medical Treatment Expert Group for COVID‐19 . Predictors of fatal outcomes among hospitalized COVID‐19 patients with pre‐existing hypertension in China. Clin Respir J. 2021;15:915–924. 10.1111/crj.13382
Tao Wang, Ruidi Tang, Honglain Ruan, Ruchong Chen and Zili Zhang contributed equally as co‐first authors.
Funding information
This study was supported by the National Science Foundation of China (Nos. 81770017, 81700426, & 81970046), Clinical Innovation Research Program of Guangzhou Regenerative Medicine and Health Guangdong Laboratory (2018GZR0201002)
Contributor Information
Nuofu Zhang, Email: nuofuzhanggmu@163.com.
Nanshan Zhong, Email: nanshan@vip.163.com.
Shiyue Li, Email: lishiyue@188.com.
DATA AVAILABILITY STATEMENT
SL had full access to all data in the study and take responsibility for the integrity of the data and the accuracy of the data analysis. Any additional data not included in this report and its supplementary files are available on request. All queries should be submitted to the corresponding author.
REFERENCES
- 1. Guan WJ, Liang WH, Zhao Y, et al. Comorbidity and its impact on 1590 patients with COVID‐19 in China: a nationwide analysis. Eur Respir J. 2020;55 (5):200547. 10.1183/13993003.13900547-13992020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Richardson S, Hirsch JS, Narasimhan M, et al. Presenting characteristics, comorbidities, and outcomes among 5700 patients hospitalized with COVID‐19 in the New York City area. JAMA. 2020;323 (20):2052‐2059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Guan WJ, Ni ZY, Hu Y, et al. Clinical characteristics of coronavirus disease 2019 in China. N Engl J Med. 2020;382 (18):1708‐1720. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Mills KT, Stefanescu A, He J. The global epidemiology of hypertension. Nat Rev Nephrol. 2020;16 (4):223‐237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Li J, Wang X, Chen J, Zhang H, Deng A. Association of renin‐angiotensin system inhibitors with severity or risk of death in patients with hypertension hospitalized for coronavirus disease 2019 (COVID‐19) infection in Wuhan, China. JAMA Cardiol. 2020;5 (7):825‐830. 10.1001/jamacardio.2020.1624 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Zhang P, Zhu L, Cai J, et al. Association of inpatient use of angiotensin converting enzyme inhibitors and angiotensin II receptor blockers with mortality among patients with hypertension hospitalized With COVID‐19. Circ Res. 2020;126:1671‐1681. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Meng J, Xiao G, Zhang J, et al. Renin‐angiotensin system inhibitors improve the clinical outcomes of COVID‐19 patients with hypertension. Emerg Microbes Infect. 2020;9 (1):757‐760. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Weiss P, Murdoch DR. Clinical course and mortality risk of severe COVID‐19. Lancet. 2020;395 (10229):1014‐1015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Williams B, Mancia G, Spiering W, et al. 2018 ESC/ESH guidelines for the management of arterial hypertension. Eur Heart J. 2018;39 (33):3021‐3104. [DOI] [PubMed] [Google Scholar]
- 10. Whelton PK, Carey RM, Aronow WS, et al. 2017 ACC/AHA/AAPA/ABC/ACPM/AGS/APhA/ASH/ASPC/NMA/PCNA guideline for the prevention, detection, evaluation, and management of high blood pressure in adults: executive summary: a report of the American College of Cardiology/American Heart Association Task Force on clinical practice guidelines. Circulation. 2018;138 (17):e426‐e483. [DOI] [PubMed] [Google Scholar]
- 11. Xu Z, Shi L, Wang Y, et al. Pathological findings of COVID‐19 associated with acute respiratory distress syndrome. Lancet Respir Med. 2020;8 (4):420‐422. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Shi S, Qin M, Shen B, et al. Association of cardiac injury with mortality in hospitalized patients with COVID‐19 in Wuhan, China. JAMA Cardiol. 2020;5 (7):802‐810. 10.1001/jamacardio.2020.095 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Zhou F, Yu T, Du R, et al. Clinical course and risk factors for mortality of adult inpatients with COVID‐19 in Wuhan, China: a retrospective cohort study. Lancet. 2020;395 (10229):1054‐1062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Xu X, Han M, Li T, et al. Effective treatment of severe COVID‐19 patients with tocilizumab. Proc Natl Acad Sci U S A. 2020;117 (20):10970–10975. 10.1073/pnas.2005615117 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Huang C, Wang Y, Li X, et al. Clinical features of patients infected with 2019 novel coronavirus in Wuhan, China. Lancet. 2020;395 (10223):497‐506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Wang Z, Chen Z, Zhang L, et al. Status of hypertension in China: results from the china hypertension survey, 2012–2015. Circulation. 2018;137 (22):2344‐2356. [DOI] [PubMed] [Google Scholar]
- 17. Ferrario CM, Jessup J, Chappell MC, et al. Effect of angiotensin‐converting enzyme inhibition and angiotensin II receptor blockers on cardiac angiotensin‐converting enzyme 2. Circulation. 2005;111 (20):2605‐2610. [DOI] [PubMed] [Google Scholar]
- 18. Keidar S, Gamliel‐Lazarovich A, Kaplan M, et al. Mineralocorticoid receptor blocker increases angiotensin‐converting enzyme 2 activity in congestive heart failure patients. Circ Res. 2005;97 (9):946‐953. [DOI] [PubMed] [Google Scholar]
- 19. Hoffmann M, Kleine‐Weber H, Schroeder S, et al. SARS‐CoV‐2 cell entry depends on ACE2 and TMPRSS2 and is blocked by a clinically proven protease inhibitor. Cell. 2020;181 (2):271‐280 e278. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Yang G, Tan Z, Zhou L, et al. Effects of ARBs and ACEIs on virus infection, inflammatory status and clinical outcomes in COVID‐19 patients with hypertension: a single center retrospective study. Hypertension. 2020;76:51‐58. [DOI] [PubMed] [Google Scholar]
- 21. Reynolds HR, Adhikari S, Pulgarin C, et al. Renin‐angiotensin‐aldosterone system inhibitors and risk of Covid‐19. N Engl J Med. 2020;382 (25):2441‐2448. 10.1056/NEJMoa2008975 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Vaduganathan M, Vardeny O, Michel T, et al. Renin‐angiotensin‐aldosterone system inhibitors in patients with Covid‐19. N Engl J Med. 2020;382 (17):1653‐1659. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Leung JM, Yang CX, Tam A, et al. ACE‐2 expression in the small airway epithelia of smokers and COPD patients: implications for COVID‐19. Eur Respir J. 2020;55 (5): 2000688. 10.1183/13993003.13900688-13992020 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Table S1
Data Availability Statement
SL had full access to all data in the study and take responsibility for the integrity of the data and the accuracy of the data analysis. Any additional data not included in this report and its supplementary files are available on request. All queries should be submitted to the corresponding author.


