Abstract
Severe acute respiratory syndrome coronavirus‐2 (SARS‐CoV‐2) is a novel coronavirus that causes coronavirus disease 19 (COVID‐19), which has infected millions of people worldwide in only a few months. A minority, but significant number, of infected individuals require hospitalization and intensive care. From the start of this new virus pandemic, it was apparent that obese and/or diabetic individuals had a bad prognosis for COVID‐19 progression, strongly suggesting an association between liver disease and severe COVID‐19. Because chronic liver disease (CLD) is associated with immune dysregulation and inflammation, it is unsurprising that patients with CLD may carry a greater risk of adverse outcomes following SARS‐CoV‐2 infection. Initial COVID‐19 data have also indicated that healthy infected individuals display abnormal liver function tests, suggesting a possible direct implication of SARS‐CoV‐2 in liver damage. Here we show that COVID‐19 affects the liver metabolism and increases the morbidity and mortality of individuals with underlying CLD.
Chronic liver disease is associated with COVID‐19 severity and mortality
Clinical indicators of liver disease can be prognostic markers of COVID‐19 severity
Long‐term liver impact of COVID‐19 must not be neglected and warrants further investigation

Abbreviations
- ACE2
angiotensin‐converting enzyme 2
- ALD
alcohol‐associated liver disease
- ALT
alanine transferase
- AST
aspartate transferase
- CI
confidence interval
- CLD
chronic liver disease
- COVID‐19
coronavirus disease 2019
- FIB‐4
fibrosis‐4
- GGT
gamma‐glutamyltransferase
- HCC
hepatocellular carcinoma
- HBV
hepatitis B virus
- HCV
hepatitis C virus
- HDL
high‐density lipoprotein
- IFN
interferon
- IL
interleukin
- NAFLD
nonalcoholic fatty liver disease
- NASH
nonalcoholic steatohepatitis
- OR
odds ratio
- SARS‐CoV‐2
severe acute respiratory syndrome coronavirus 2
- TIM‐3
T‐cell immunoglobulin and mucin‐domain containing 3
- TMPRSS2
transmembrane serine protease 2
- TNF
tumor necrosis factor
The severe acute respiratory syndrome coronavirus 2 (SARS‐CoV‐2) pandemic clearly highlights the importance of an effective host immune response.( 1 ) SARS‐CoV‐2 causes coronavirus disease 2019 (COVID‐19), which can involve a number of diverse symptoms, including immune dysregulation and cytokine storm. In COVID‐19, elevated cytokine levels have been implicated in lung injury and multi‐organ failure,( 2 ) and a more severe cytokine storm may contribute to COVID‐19 pathogenesis.( 3 , 4 ) Cytokines that are elevated in patients with COVID‐19 include interleukin (IL)‐1β, interleukin‐6, interferon (IFN)‐γ–induced protein 10, tumor necrosis factor (TNF), IFN‐γ, macrophage inflammatory protein 1α and 1β, and vascular endothelial growth factor.( 5 ) In particular, high IL‐6 levels have been associated with COVID‐19 severity and mortality.( 6 ) Although patients with COVID‐19 exhibit highly variable immune responses and immune‐related symptoms, those who are unable to control SARS‐CoV‐2 replication are more likely to show pathogenesis‐associated immune dysregulation (Fig. 1).
FIG. 1.
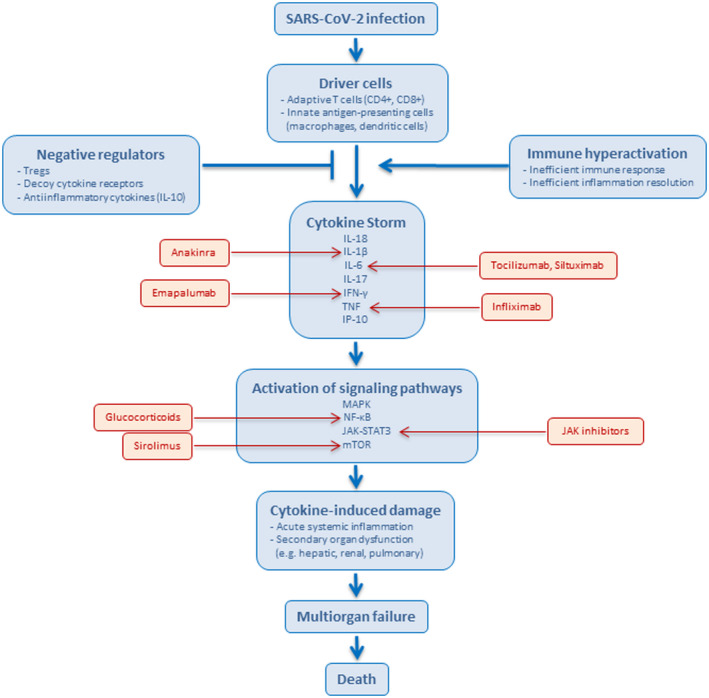
Pathophysiological features of SARS‐CoV‐2 infection. Following SARS‐CoV‐2 infection, cytokine storm may occur due to inappropriate or ineffective pathogen recognition, with immune evasion that generates an inappropriate response, including an exaggerated effector response, cytokine production, and/or failure to return homeostasis. The described pathways are based on a report by Fajgenbaum and June.( 1 ) Examples of drugs that can inhibit signaling pathways are shown in red. Abbreviations: JAK‐STAT3: Janus kinase–signal transducer and activator of transcription 3; MAPK, mitogen‐activated protein kinase; mTOR, mammalian target of rapamycin; NF‐κB, nuclear factor kappa B; Treg, regulatory T cell.
There is also an ongoing worldwide obesity pandemic that has led to insulin resistance, diabetes, and chronic liver disease (CLD), becoming a major public health burden.( 7 , 8 ) CLD is most commonly caused by chronic hepatitis B and C, alcohol‐associated liver disease (ALD), and nonalcoholic fatty liver disease (NAFLD). CLD can further progress to inflammation (nonalcoholic steatohepatitis [NASH]), fibrosis, and finally cirrhosis and hepatocellular carcinoma (HCC) as end‐stage diseases. Cirrhosis, viral hepatitis, and HCC account for approximately 2 million deaths per year worldwide.( 7 , 8 ) Liver cells, primarily hepatocytes, constitute a major source of proteins involved in innate and adaptive immune responses. The liver regulates immune homeostasis by preventing the systemic spread of microbial and dietary antigens that arrive from the gut, and through the synthesis of soluble molecules that are essential for effective immune responses.( 9 ) Thus, liver injury can lead to compromised immune surveillance by reducing the hepatic synthesis of proteins involved in innate immunity and pathogen‐associated molecular pattern recognition.
Both CLD and cirrhosis are characterized by immune dysregulation.( 10 ) CLD causes impairment of the liver’s homeostatic role in the systemic immune response. The molecular patterns from damaged liver cells prompt circulating immune cells to induce systemic inflammation, in the form of activated circulating immune cells and increased serum levels of pro‐inflammatory cytokines (e.g., TNF and IL‐6). Importantly, liver‐associated immune dysfunction can increase susceptibility to infections. In this context, it is unsurprising that patients with CLD, especially those with decompensated cirrhosis, are at higher risk of COVID‐19‐related morbidity and mortality.( 11 ) The combination of SARS‐CoV‐2 infection and cirrhosis appears to be a lethal duo, likely due to the combination of biological processes characterized by immune dysregulation (Fig. 2). Importantly, liver transplantation restores hepatic function in patients with decompensated cirrhosis, thus reducing the risk of COVID‐19 mortality to that of the general population.( 12 )
FIG. 2.
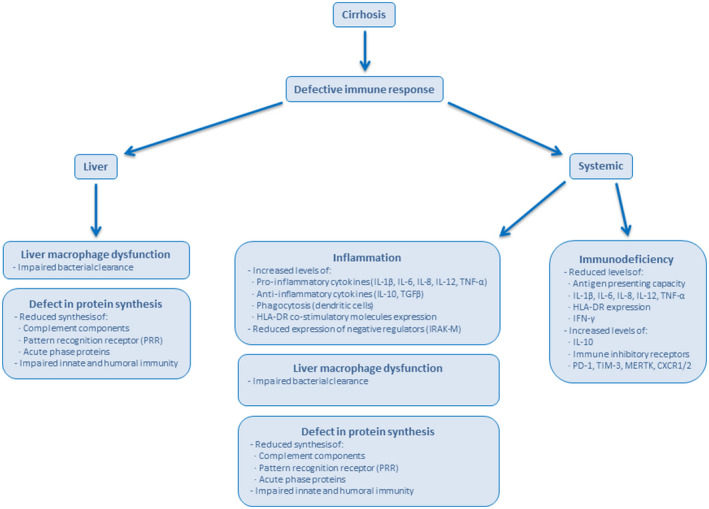
Features of immunodeficiency commonly found in patients with cirrhosis. Cirrhosis disrupts the liver architecture and cellular organization, and diminishes the hepatic ability to synthesize proteins. Cirrhosis affects the functions of circulating and intestinal populations of immune cells, resulting in a number of abnormalities that affect cellular and soluble components of the immune response both at the liver and systemically.( 10 ) Abbreviations: CXCR1/2, chemokine receptor 1/2; HLA‐DR, human leukocyte antigen‐DR; IRAK‐M, interleukin receptor‐associated kinase 3; MERTK, c‐mer proto‐oncogene tyrosine kinase; PD‐1, programmed cell death‐1; TGF‐β, transforming growth factor β; TIM‐3, T‐cell immunoglobulin and mucin‐domain containing 3.
In addition to the bad prognostic impact of CLD in patients with COVID‐19, SARS‐CoV‐2 infection may also negatively affect liver health in previously healthy individuals. The available clinical data indicate that patients with COVID‐19 commonly show abnormal liver function tests, such as aspartate transferase (AST) and alanine transferase (ALT).( 13 ) However, the mechanisms underlying the impact of COVID‐19 on liver function are not fully understood and may be multifactorial, and it has not been clearly demonstrated whether SARS‐CoV‐2 can infect hepatocytes. Because acute liver injury or cholestasis can develop in severe cases of cytokine storm,( 1 , 14 ) not related to COVID‐19, it is possible that SARS‐CoV‐2‐originated immune dysregulation may play a role in COVID‐19‐associated liver pathology.
We aim here to review and discuss the implications of COVID‐19 in healthy liver individuals as well as the risks of severe COVID‐19 in patients with fatty liver disease, viral hepatitis, cirrhosis, HCC, ALD, and liver‐transplantation recipients. The effect of COVID‐19 antiviral and anti‐inflammatory therapies on the liver will be also scrutinized and reviewed.
COVID‐19 Impact in Individuals With Healthy Liver
As mentioned previously, patients with COVID‐19 commonly exhibit elevated markers associated with liver injury, such as AST, ALT, alkaline phosphatase, and gamma‐glutamyltransferase (GGT).( 13 , 15 , 16 , 17 ) The prognostic value of elevated liver injury markers in patients with COVID‐19 remains controversial, as reviewed by Bertolini et al.,( 13 ) with some studies showing that elevated AST or ALT values are correlated with a bad prognosis, while others find no association with COVID‐19 progression or mortality.
One possible mechanism for the liver injury observed in patients with COVID‐19 is direct hepatic infection by SARS‐CoV‐2. The SARS‐CoV‐2 host cell receptor is angiotensin‐converting enzyme 2 (ACE2),( 18 ) and SARS‐CoV‐2 cellular entry also involves transmembrane serine protease 2 (TMPRSS2).( 18 ) Sequencing studies have revealed ACE2 messenger RNA (mRNA) expression in a subpopulation of cholangiocytes, but no or minimal expression in hepatocytes, and no expression in other liver cell types.( 19 , 20 , 21 , 22 ) TMPRSS2 mRNA expression has been found in a subset of hepatocytes and cholangiocytes.( 23 , 24 ) ACE2 protein has not been detected in hepatocytes.( 25 ) These findings strongly suggest that SARS‐CoV‐2 does not directly cause cytopathic liver damage. Interestingly, SARS‐CoV‐2 can replicate in the HCC cell line Huh‐7.( 26 ) Additionally, SARS‐CoV‐2 particles without membrane‐bound vesicles have been detected in the cytoplasm of hepatocytes from 2 patients with COVID‐19 with elevated liver injury markers( 27 ); however, these samples were not assessed for the presence of viral RNA to exclude another origin of these particles.( 28 ) Notably, SARS‐CoV‐2 can infect intestine‐related tissue,( 29 , 30 ) and some patients with COVID‐19 (<15%) exhibit viral RNA in their blood.( 31 , 32 ) Therefore, it has been suggested that viruses may enter the portal circulation, thus reaching the liver, and Kupffer cells might attempt to clear the virus and produce local inflammation.( 13 )
The liver could also be affected by the hypoxia and cytokine storm that follows SARS‐CoV‐2 infection, which is associated with multi‐organ failure in some patients with COVID‐19.( 1 , 33 ) The cytokine storm may also be implicated in the disseminated intravascular coagulation (DIC) and thrombocytopenia, which can worsen the DIC complications, as observed in COVID‐19.( 34 ) Patients with COVID‐19 have exhibited endotheliitis in the liver,( 35 ) fibrin microthrombi in liver sinusoids,( 36 , 37 ) and activation of the complement system.( 38 ) One report demonstrated that, in contrast to patients with mild COVID‐19, patients with non‐COVID‐19 pneumonia did not exhibit elevated levels of AST, ALT, or GGT,( 39 ) suggesting that patients with mild COVID‐19 may have elevated liver damage markers independently of the presence of inflammation.
The pattern of abnormal liver biochemistries at admission and over the course of illness in hospitalized patients with COVID‐19 is associated with AST‐predominant aminotransferase elevations.( 40 ) This finding suggests a virus‐specific mediated mechanism of liver injury. This result is in contrast to that observed with viral hepatitis, which classically leads to an ALT‐predominant liver injury. AST is produced in the muscle, and patients with COVID‐19 have markers of muscle injury. AST‐predominant elevations are also associated with ALD, ischemia and cirrhosis, revealing the impact of COVID‐19 on the liver. AST and GGT elevations have been also associated with influenza virus infection and the associated cytokine‐mediated injury.( 41 )
Liver biochemistries have been associated with COVID‐19 severity and clinical outcomes. However, the impact of COVID‐19 on a healthy liver appears to be negligible, and limited to patients with severe infection and multi‐organ failure.( 42 , 43 )
Hepatotoxicity of COVID‐19 Antiviral and Anti‐inflammatory Therapies
Another possible source of liver damage in patients with COVID‐19 is drug‐induced liver injury associated with COVID‐19 therapies. One study reports that among patients with COVID‐19 exhibiting normal liver injury markers on admission, nearly half had developed abnormal liver marker levels after 1 week following admission.( 44 ) Lopinavir‐ritonavir treatment has been associated with elevated levels of AST, ALT, and bilirubin( 45 ); remdesevir reportedly induces AST and ALT elevations( 46 , 47 ); and acetaminophen and hydroxychloroquine are associated with liver marker abnormalities.( 48 , 49 ) The liver toxicity of remdesivir has been widely debated. Randomized trials have shown that equivalent liver enzyme elevations between remdesivir‐treated and control groups.( 47 ) However, the WHO safety report database indicates a statistically significant odds ratio for liver injury with the use of remdesivir.( 50 ) Angiotensin II receptor blockers and ACE inhibitors have been also explored as possible COVID‐19 treatment. These drugs also caused elevated liver enzyme levels.( 45 ) Unfortunately, the former repurposed drugs do not appear to have an impact on COVID‐19.( 51 ) On the other hand, immunomodulators appear to be a more effective therapeutic strategy for treating COVID‐19 morbidity and mortality—with corticosteroids, such as dexamethasone, significantly increasing survival and reducing morbidity among hospitalized patients with COVID‐19( 52 ) and in patients with moderate or severe COVID‐19.( 53 ) Because corticosteroids are derived from cholesterol metabolism, which can interfere with multiple aspects of glucose homeostasis, attention should be paid to the possible effects of these compounds on liver metabolism and hepatic steatosis.( 52 ). Indeed, corticosteroid treatment in severe COVID‐19 has been associated with significant liver injury.( 54 ) Tocilizumab, a humanized monoclonal antibody against the IL‐6 receptor, used to treat rheumatoid arthritis and now profusely used to counter the cytokine‐mediated injury in severe COVID‐19, is also known to induce a significant elevation of ALT.( 55 ) This retrospective, observational cohort study reported that the tocilizumab group had a significantly higher elevation of ALT.( 55 ) Finally, antibiotics, which are one of the most common causes of drug‐induced liver injury, can also contribute to liver damage in patients with COVID‐19.
Recently, another retrospective study that included 1,040 patients with COVID‐19 found elevated levels of ALT/AST in 22.6% of the study individuals.( 56 ) In this cohort, the use of lopinavir–ritonavir, with or without ribavirin, IFN‐β and/or corticosteroids, was independently associated with ALT/AST elevations. New specific SARS‐CoV‐2 antivirals are now in phase 3 clinical trials (e.g., molnupiravir),( 57 ) and they may enter the clinic soon. Future studies would be worth conducting in determining the possible effects of new SARS‐CoV‐2 drugs on liver function in patients with COVID‐19. Specifically, individuals with CLD could be included in these studies. Favipiravir is an oral broad‐spectrum inhibitor of viral RNA‐dependent RNA polymerase that is approved for treatment of influenza in Japan and displays a modest effect against COVID‐19.( 58 ) Favipiravir appeared to be well tolerated.( 58 ) However, a case of cholestatic liver injury induced by favipiravir has been described in an individual with previous liver injury due to ALD. This favipiravir case report reveals the relevance of baseline CLD on future antiviral therapies.
Fatty Liver and COVID‐19 Progression
The high worldwide prevalence of NAFLD and NASH, which affect approximately 25% of the global population, makes it inevitable that NAFLD/NASH will overlap with COVID‐19. NAFLD is associated with obesity and other lifestyle‐related metabolic disorders (e.g., type 2 diabetes). Since the start of the COVID‐19 pandemic, obesity and diabetes have been linked with a bad disease prognosis.( 17 , 59 ) Elevated levels of the liver enzymes AST/ALT (≥2× the upper limit of normal [i.e., 80 U/L]) are common, and are independently associated with adverse clinical outcomes among patients with COVID‐19.( 60 , 61 , 62 ) In one study, AST/ALT elevations were observed in 235 patients classified as having severe COVID‐19.( 56 ) Another study included 31,461 adults with COVID‐19, and found that moderate/severe liver disease was significantly associated with mortality (odds ratio [OR], 2.62; 95% confidence interval [CI], 1.53 to 4.47).( 63 ) In a similar investigation of 2,780 patients with COVID‐19, liver disease was again significantly associated with mortality (OR, 2.8; 95% CI, 1.9 to 4.0).( 64 ) Compared to patients without NAFLD, patients with NAFLD reportedly show a higher risk of disease progression (6.6% vs. 44.7%), higher likelihood of abnormal liver function from admission to discharge (70% vs. 11.1%), and longer viral shedding time (17.5 ± 5.2 days vs. 12.1 ± 4.4 days).( 65 ) Thus, the high global prevalence of NAFLD suggests that a large proportion of the population is at risk of severe COVID‐19.
Because NAFLD prognosis is determined based on liver fibrosis severity, it has been considered that patients with NAFLD with higher noninvasive liver fibrosis scores may carry a greater risk of severe COVID‐19. Indeed, the risk of severe COVID‐19 is significantly higher in patients with NAFLD who have been diagnosed with hepatic steatosis based on computed tomography (OR, 4.32; 95% CI, 1.94 to 9.59) or with an intermediate or high fibrosis‐4 (FIB‐4) index (OR, 5.73; 95% CI, 1.84 to 17.9), regardless of metabolic comorbidities.( 66 , 67 ) Compared to patients with NAFLD with low FIB‐4 scores or individuals without NAFLD, patients with NAFLD with intermediate or high FIB‐4 scores are more likely to be older and obese; to have diabetes, higher liver enzymes, and higher C‐reactive protein; and to exhibit lower levels of lymphocytes, platelets, triglycerides, and high‐density lipoprotein cholesterol.( 7 , 8 ) Moreover, among patients with COVID‐19, the need for mechanical ventilation is independently associated with obesity (OR 4.5), diabetes mellitus (OR 2.55) and FIB‐4 ≥ 2.67 (OR 3.09), and FIB‐4 ≥ 2.67 is also associated with increased 30‐day mortality (OR, 8.4; 95% CI, 2.23 to 31.7).( 68 )
The association between NAFLD/NASH and a severe course of COVID‐19 suggests the possibility that a genetic risk score for NAFLD, based on the weighted effect of risk variants in PNPLA3 (patatin‐like phospholipase domain containing 3)–TM6SF2 (transmembrane 6 superfamily member 2)–MBOAT7 (membrane‐bound O‐acyltransferase domain‐containing protein 7)–GCKR (glucokinase regulator) on hepatic fat in the general population,( 69 ) may affect COVID‐19 risk.( 70 ) In an analysis of 1,460 individuals, of whom 526 were positive and 934 were negative for SARS‐CoV‐2, the genetic risk score for NAFLD was not associated with an increased risk of COVID‐19. However, this investigation revealed a trend of protection against COVID‐19 conferred by the single‐nucleotide polymorphism rs738409, which encodes the p.I148M PNPLA3 variant.( 70 ) It has been also suggested that obese individuals and patients with NAFLD/NASH may have higher hepatic expression of the SARS‐CoV‐2 receptors ACE2 and TMPRSS2,( 71 , 72 ) which could explain the worse clinical course of these patients. One study found that NAFLD/NASH did not influence the liver expression of the ACE2 and TMPRSS2 genes.( 72 ) However, another investigation revealed that obese patients with NASH showed markedly higher expressions of these genes, suggesting that advanced stages of NAFLD might predispose to COVID‐19.( 73 ) Gut microbiota and incomplete fatty acid oxidation products were also associated with NAFLD/NASH and severe COVID‐19,( 74 , 75 , 76 ) possibly by modulating host immune response.( 77 )
Within the context of COVID‐19, it has been clearly demonstrated that the presence of NAFLD/NASH predisposes patients to severe COVID‐19 morbidity and mortality. Because there are no approved therapies for NAFLD/NASH, numerous trials are being conducted in this clinical research field. However, many such trials have been disrupted due to the COVID‐19 pandemic. The high global prevalence of CLD warrants the continuation of ongoing NAFLD/NASH trials.( 78 )
Alcohol‐Associated Liver Disease
ALD is independently associated with a 1.8‐fold increased risk for mortality in patients with COVID‐19.( 11 ) Furthermore, patients with both ALD and COVID‐19 appear to exhibit more severe liver injury, as the proportion of patients without cirrhosis is only 6% among patients with ALD compared to 62% among those with NAFLD.( 11 ) A case report describes 2 patients who were diagnosed with nosocomial COVID‐19 infections and severe COVID‐19 pneumonia while hospitalized during treatment for ALD.( 79 ) Both patients developed multiple‐organ failure and died over a short period of time. An additional study indicated that the risk of severe COVID‐19 was significantly associated with alcoholic liver damage (OR, 7.05; 95% CI, 6.30 to 7.88) and alcoholic liver cirrhosis (OR, 7.00; 95% CI, 6.15 to 7.97).( 80 ) Nevertheless, few studies have explored the impact of alcohol during the COVID‐19 pandemic, nor the mechanisms through which alcohol consumption and ALD may impact COVID‐19 pathogenesis.( 81 )
Excessive alcohol consumption may have immune‐modulating effects and predispose individuals to viral and bacterial infections.( 82 , 83 ) It is estimated that alcohol causes 3.3 million deaths annually.( 84 ) Several pathologies are linked to alcohol consumption, with CLD and cirrhosis being the main alcohol‐associated health problems. Unfortunately, alcohol consumption is showing annual increases nearly worldwide.( 84 ) Moreover, social distancing and lockdown measures are resulting in increased alcohol abuse, which is aggravating alcohol‐associated CLD and liver injury.( 85 )
COVID‐19 in Patients With Cirrhosis and Patients With Liver Cancer
Cirrhosis is a late stage of fibrosis of the liver, caused primarily by NAFLD/NASH, ALD, and viral hepatitis. As discussed previously, cirrhosis is associated with immune dysfunction( 10 ) (Fig. 2), which is related to a bad COVID‐19 prognosis.( 1 ) In patients with severely decompensated cirrhosis, cirrhosis‐associated immune dysfunction can switch from predominantly pro‐inflammatory to predominantly immunodeficient.( 10 ) Systemic inflammation can affect the functions of tissue somatic cells and modify the clinical manifestation of cirrhosis.( 10 ) An analysis of 745 patients with CLD infected with SARS‐CoV‐2 from 29 countries revealed that cirrhosis was strongly associated with death (OR, 9.32; 95% CI, 4.80 to 18.08).( 11 ) Among the 745 patients, 150 (20%) died, of whom 123 had cirrhosis. Importantly, the main cause of death was lung injury, with only 19% of the patients dying due to cirrhosis‐related complications. This finding strongly suggests that cirrhosis is a driving force in lung injury development, and this relationship could be due to cirrhosis‐associated immune dysfunction.( 1 ) Cirrhosis has been associated with impaired responses to pneumococcus and hepatitis B virus (HBV) vaccines,( 86 , 87 ) suggesting a possible poor response to SARS‐CoV‐2 vaccination. Potential mechanisms of disease linking cirrhosis with severe COVID‐19 are increased systemic inflammation, cirrhosis‐associated immune dysfunction, coagulopathy, and intestinal dysbiosis.( 88 ) Because gut microbiota composition plays a role in regulating the magnitude of COVID‐19 severity, possibly by modulating host immune responses, and cirrhosis is characterized by changes in gut microbiota composition, we can also speculate about the link among microbiota, cirrhosis, and COVID‐19 severity. An interesting aspect of the former study is that patients with cirrhosis were less likely to receive targeted antiviral therapy due to safety concerns, highlighting the importance of evaluating hepatotoxicity during COVID‐19 clinical trials, and allowing patients with cirrhosis to enter these studies.
Intriguingly, patients with cirrhosis have a reduced risk of SARS‐CoV‐2 infection.( 89 ) Analysis of 88,747 COVID‐19‐positive individuals revealed that patients with cirrhosis were less likely to test positive than patients without cirrhosis (8.5% vs. 11.5%; OR, 0.83; 95% CI, 0.69 to 0.99).( 89 ) As previously described, patients with cirrhosis exhibited increased 30‐day mortality and ventilation rates (17.1% and 13.0%, respectively), being 4.1 times more likely to undergo mechanical ventilation and 3.5 more likely to die. In another retrospective study, patients with cirrhosis and SARS‐CoV‐2 infection had a 30‐day mortality rate of 34%.( 90 ) This increased mortality rate could be related to the median age of the study population (67 years; interquartile range, 61‐74).( 91 ) This study showed that the severity of lung and liver diseases independently predicted mortality, and that patients with cirrhosis and COVID‐19 were significantly more likely to die than those hospitalized with bacterial infections.
HCC is the sixth‐most common cancer in the world, accounting for nearly 6% of all cancer occurrences.( 92 ) Based on the immune dysfunction generated by SARS‐CoV‐2 infection, it can be hypothesized that patients with HCC may be more susceptible to the effects of COVID‐19 than patients with other cancers.( 93 ) Overall, cancer patients with COVID‐19 appear to have a higher chance of requiring invasive ventilation or of death (39%) compared to patients with COVID‐19 without cancer (8%).( 94 ) Furthermore, cancer treatment is associated with a higher risk of COVID‐19 adverse events (hazard ratio = 4.1).( 95 ) HCC is underrepresented in the available studies of cancer and COVID‐19, making it difficult to extract a robust conclusion regarding the impact of COVID‐19 on patients with HCC. Nevertheless, the data obtained from patients with cirrhosis suggests that COVID‐19 likely takes a high toll on patients with HCC. One important aspect regarding patients with HCC is their management during the current pandemic. It is expected that a higher proportion of patients will suffer advanced HCC as a consequence of interruptions of surveillance programs among patients with cirrhosis and high‐risk patients.( 93 )
Concerns have been raised that patients with liver transplants might have an increased risk of adverse outcomes after SARS‐CoV‐2 infection. However, in an international cohort of 151 adult transplant recipients with COVID‐19, liver transplantation was not independently associated with death.( 12 ) On the other hand, another study found that liver injury during COVID‐19 in liver‐transplantation recipients was significantly associated with mortality (OR, 6.91; 95% CI, 1.68 to 28.48).( 96 ) Importantly, reduction of immunosuppression during COVID‐19 in these liver‐transplantation recipients did not increase the risk for mortality or graft failure.( 96 ) Unfortunately, in some countries, the COVID‐19 pandemic has reduced (from 25% to 80%) organ donation and liver transplantations.( 97 )
Alterations of Lipid Metabolism Induced by SARS‐CoV‐2 Infection
Lipids as cell membrane and exosome components, and energy storage elements, are closely related to virus life cycle. Virus infection usually affects cell lipid metabolism and circulating lipids. Viruses manipulate lipid metabolism to benefit their replication, including the expression and activity of essential enzymes in lipid biosynthesis. Lipid metabolism changes could also be attributed to host response to infection. SARS‐CoV‐2 is not an exception, and following infection generates important changes in lipid metabolism.( 98 ) In particular, SARS‐CoV‐2 infection induces an overall down‐regulation of over 100 serum lipids, comprising sphingolipids, glycerophospholipids, and fatty acids.( 98 ) Lipids are not only altered in COVID‐19 but are also implicated in pathogenesis and disease progression. The observed down‐regulation of lipids after SARS‐CoV‐2 infection is linked to liver injury, which is supported by changes in bilirubin and bile acids. Many of the observed lipid and lipoproteins alterations in COVID‐19 are associated with hepatic functions.( 99 , 100 ) Plasma lipidomic analysis was investigated during the course of COVID‐19.( 101 ) Most of the significantly changed lipids were up‐regulated levels of sphingomyelins and monosialodihexosyl gangliosides, and reduced amounts of diacylglycerols. Increased levels of monosialodihexosyl gangliosides positively correlated with disease severity. Again, inflammation and infection could be involved in disrupting circulating normal lipid profiles. A cytokine storm can induce the release of unsaturated fatty acids as a defense mechanism. Pro‐inflammatory lipids and lipid mediators could modulate the immune response during COVID‐19 disease.( 98 )
COVID‐19 is also characterized by a decrease in serum total cholesterol. Individuals with inflammatory diseases, including sepsis and lower respiratory tract infections, displayed significant decreases in high‐density lipoprotein (HDL) cholesterol and increases serum triglyceride levels.( 102 , 103 ) Similarly, patients with COVID‐19 showed hypolipidemia in association with disease progression and severity.( 104 , 105 ) Compared with the healthy controls, patients with COVID‐19 had decreased concentrations of serum total cholesterol, HDL‐cholesterol, and low‐density lipoprotein cholesterol. Decreased serum HDL cholesterol was associated with the severity of COVID‐19 infection.( 104 , 105 ) The molecular mechanism explaining the functional role of lipids during SARS‐CoV‐2 infection is still unknown. Previous work described HLD cholesterol as an essential part in host defense and anti‐inflammatory responses.( 98 , 106 ) Notably, HDL cholesterol has been proposed as a predictive marker of an increased risk of infection‐related hospitalizations.( 107 , 108 ) An interesting finding was that patients on statins had a better COVID‐19 course.( 109 ) It could be hypothesized that reduction in membrane‐associated cholesterol may affect the interaction of ACE2 and virus spike protein. Lipid‐lowering therapies such as statins have been suggested as a possible therapeutic approach to reduce the clinical complications of COVID‐19.( 110 )
Impact of COVID‐19 on Viral Hepatitis
HBV and hepatitis C virus (HCV) cause chronic hepatic infection and disease, and constitute two of the main sources of liver disease, infecting 300 million and 70 million people worldwide, respectively.( 111 , 112 ) HBV and HCV, respectively, account for nearly 12% and 11% of the underlying causes of CLD. It remains unclear whether HBV and/or HCV per se can impact susceptibility to SARS‐CoV‐2.( 113 ) It appears that HBV and HCV show low prevalence rates among patients hospitalized with COVID‐19( 11 , 17 ); however, few studies have analyzed viral hepatitis incidence. Similarly, the scarcity of available data makes it difficult to estimate the influence of HBV and/or HCV infection on COVID‐19 severity. Liver fibrosis and liver injury status are presently the best prognostic factors for SARS‐CoV‐2‐infected individuals with viral hepatitis.
A Chinese study detected HBV infection in 12.2% (15 of 123) of patients with COVID‐19 and found that HBV infection was associated with a more severe course and higher mortality rate (13.3% vs. 2.8%).( 114 ) However, two other retrospective studies from China (where there is a high incidence of HBV infection) found no evidence that SARS‐CoV‐2/HBV co‐infection could aggravate liver injury or extend duration of hospitalization.( 115 , 116 ) Lymphopenia induced by SARS‐CoV‐2 or by the use of immunosuppressive therapies (e.g., corticosteroids) may constitute a risk for patients with active or past HBV infection. A prospective cohort study reported a low risk of HBV reactivation in patients with severe COVID‐19 and resolved HBV infection who were undergoing immunosuppressive therapy.( 117 ) However, the authors still suggested that a short course of antiviral prophylaxis may be a safe option.
Viral hepatitis is an example of how the effects of the SARS‐CoV‐2 pandemic extend beyond the direct morbidity and mortality associated with exposure and infection. Disruptions to hepatitis programming across the continuum of care have already been documented. The SARS‐CoV‐2 pandemic may have profound and unexpected short‐term and long‐term effects on mortality from liver disease, through the limitation of access to health care services for HCV treatment. Mathematical models estimate that a 1‐year delay of HCV treatment administration will yield an excess mortality of 44,800 for HCC and 72,300 for liver‐related deaths.( 118 ) These models emphasize the necessity of mitigating the impact of COVID‐19 on viral hepatitis programing and treatment.
Conclusions
Liver injury and CLD are associated with COVID‐19 severity and mortality, such that determination of indicators of liver disease—including liver enzymes, liver fibrosis, and liver steatosis—could be prioritized as prognostic markers of COVID‐19 severity. The immune inflammatory status of individuals with liver disease is particularly consequential in other infectious diseases. This is particularly relevant considering the current co‐occurring worldwide pandemics of NAFLD/NASH and COVID‐19. Special attention must be also paid to the screening and treatment of individuals with CLD (HCC, viral hepatitis, NAFLD/NASH, and ALD). Patients with CLD, particularly those with cirrhosis or advanced liver injury, should be prioritized for SARS‐CoV‐2 vaccination. Follow‐up of vaccinated patients with CLD may reveal further clues regarding the impaired immune response of these individuals. During the COVID‐19 pandemic, the management of patients infected with SARS‐CoV‐2 has reduced and postponed services for other medical pathologies. Such policies are inevitably having collateral downstream effects on patients with CLD. These collateral effects include delayed diagnosis and treatment of diverse liver diseases that may increase the morbidity and mortality associated with CLD. The COVID‐19 pandemic has led to a significant contraction in organ donation and liver transplantations. COVID‐19 is also impacting the efforts to eradicate viral hepatitis worldwide. In addition to the negative effect of the pandemic on liver services, patient unhealthy behaviors can increase the global burden of liver disease in the near future. On the other hand, it appears that the direct liver damage induced by SARS‐CoV‐2 infection in individuals with healthy livers is limited. Nevertheless, the long‐term liver impact of COVID‐19 must not be neglected and warrants further investigation.
Supported by the Spanish Ministry of Science and Innovation (PID2019‐103955RB‐100).
Potential conflict of interest: Nothing to report.
References
Author names in bold designate shared co‐first authorship.
- 1. Fajgenbaum DC, June CH. Cytokine storm. N Engl J Med 2020;383:2255‐2273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Sinha P, Matthay MA, Calfee CS. Is a “cytokine storm” relevant to COVID‐19? JAMA Intern Med 2020;180:1152‐1154. [DOI] [PubMed] [Google Scholar]
- 3.. Dexamethasone in hospitalized patients with Covid‐19—preliminary report. N Engl J Med 2021;384:693‐704. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Moore JB, June CH. Cytokine release syndrome in severe COVID‐19. Science 2020;368:473‐474. [DOI] [PubMed] [Google Scholar]
- 5. Huang C, Wang Y, Li X, Ren L, Zhao J, Hu YI, et al. Clinical features of patients infected with 2019 novel coronavirus in Wuhan. China Lancet 2020;395:497‐506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Del Valle DM, Kim‐Schulze S, Huang HH, Beckmann ND, Nirenberg S, Wang B, et al. An inflammatory cytokine signature predicts COVID‐19 severity and survival. Nat Med 2020;26:1636‐1643. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Sepanlou SG, Safiri S, Bisignano C, Ikuta KS, Merat S, Saberifiroozi M, et al. The global, regional, and national burden of cirrhosis by cause in 195 countries and territories, 1990‐2017: a systematic analysis for the Global Burden of Disease Study 2017. Lancet Gastroenterol Hepatol 2020;5:245‐266. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Asrani SK, Devarbhavi H, Eaton J, Kamath PS. Burden of liver diseases in the world. J Hepatol 2019;70:151‐171. [DOI] [PubMed] [Google Scholar]
- 9. Racanelli V, Rehermann B. The liver as an immunological organ. Hepatology 2006;43:S54‐S62. [DOI] [PubMed] [Google Scholar]
- 10. Albillos A, Lario M, Álvarez‐Mon M. Cirrhosis‐associated immune dysfunction: distinctive features and clinical relevance. J Hepatol 2014;61:1385‐1396. [DOI] [PubMed] [Google Scholar]
- 11. Marjot T, Moon AM, Cook JA, Abd‐Elsalam S, Aloman C, Armstrong MJ, et al. Outcomes following SARS‐CoV‐2 infection in patients with chronic liver disease: an international registry study. J Hepatol 2020;74:567‐577. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Webb GJ, Marjot T, Cook JA, Aloman C, Armstrong MJ, Brenner EJ, et al. Outcomes following SARS‐CoV‐2 infection in liver transplant recipients: an international registry study. Lancet Gastroenterol Hepatol 2020;5:1008‐1016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Bertolini A, van de Peppel IP, Bodewes FAJA, Moshage H, Fantin A, Farinati F, et al. Abnormal liver function tests in patients with COVID‐19: relevance and potential pathogenesis. Hepatology 2020;72:1864‐1872. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Templin C, Ghadri JR, Diekmann J, Napp LC, Bataiosu DR, Jaguszewski M, et al. Clinical features and outcomes of Takotsubo (Stress) cardiomyopathy. N Engl J Med 2015;373:929‐938. [DOI] [PubMed] [Google Scholar]
- 15. Phipps MM, Barraza LH, LaSota ED, Sobieszczyk ME, Pereira MR, Zheng EX, et al. Acute liver injury in COVID‐19: prevalence and association with clinical outcomes in a large U.S. cohort. Hepatology 2020;72:807‐817. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Goyal P, Choi JJ, Pinheiro LC, Schenck EJ, Chen R, Jabri A, et al. Clinical characteristics of Covid‐19 in New York City. N Engl J Med 2020;382:2372‐2374. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Richardson S, Hirsch JS, Narasimhan M, Crawford JM, McGinn T, Davidson KW, et al. Presenting characteristics, comorbidities, and outcomes among 5700 patients hospitalized with COVID‐19 in the New York City area. JAMA 2020;323:2052‐2059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Hoffmann M, Kleine‐Weber H, Schroeder S, Krüger N, Herrler T, Erichsen S, et al. SARS‐CoV‐2 cell entry depends on ACE2 and TMPRSS2 and is blocked by a clinically proven protease inhibitor. Cell 2020;181:e8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. De Smet V, Verhulst S, van Grunsven LA. Single cell RNA sequencing analysis did not predict hepatocyte infection by SARS‐CoV‐2. J Hepatol 2020;73:993‐995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Zou X, Chen K, Zou J, Han P, Hao J, Han Z. Single‐cell RNA‐seq data analysis on the receptor ACE2 expression reveals the potential risk of different human organs vulnerable to 2019‐nCoV infection. Front Med 2020;14:185‐192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Qi F, Qian S, Zhang S, Zhang Z. Single cell RNA sequencing of 13 human tissues identify cell types and receptors of human coronaviruses. Biochem Biophys Res Commun 2020;526:135‐140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Chai X, Hu L, Zhang Y, Han W, Lu Z, Ke A, et al. Specific ACE2 expression in cholangiocytes may cause liver damage after 2019‐nCoV infection. bioRxiv 2020;2020.02.03.931766. [Google Scholar]
- 23. Hamming I, Timens W, Bulthuis MLC, Lely AT, Navis GJ, van Goor H. Tissue distribution of ACE2 protein, the functional receptor for SARS coronavirus. A first step in understanding SARS pathogenesis. J Pathol 2004;203:631‐637. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Hikmet F, Méar L, Edvinsson Å, Micke P, Uhlén M, Lindskog C. The protein expression profile of ACE2 in human tissues. Mol Syst Biol 2020;16:e9610. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Pirola CJ, Sookoian S. SARS‐CoV‐2 virus and liver expression of host receptors: putative mechanisms of liver involvement in COVID‐19. Liver Int 2020;40:2038‐2040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Chu H, Chan J‐W, Yuen T‐T, Shuai H, Yuan S, Wang Y, et al. Comparative tropism, replication kinetics, and cell damage profiling of SARS‐CoV‐2 and SARS‐CoV with implications for clinical manifestations, transmissibility, and laboratory studies of COVID‐19: an observational study. Lancet Microbe 2020;1:e14‐e23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Wang Y, Liu S, Liu H, Li W, Lin F, Jiang L, et al. SARS‐CoV‐2 infection of the liver directly contributes to hepatic impairment in patients with COVID‐19. J Hepatol 2020;73:807‐816. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Philips CA, Ahamed R, Augustine P. SARS‐CoV‐2 related liver impairment—perception may not be the reality. J Hepatol 2020;73:991‐992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Redd WD, Zhou JC, Hathorn KE, McCarty TR, Bazarbashi AN, Thompson CC, et al. Prevalence and characteristics of gastrointestinal symptoms in patients with severe acute respiratory syndrome coronavirus 2 infection in the United States: a multicenter cohort study. Gastroenterology 2020;159:765‐767.e2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Lamers MM, Beumer J, Van Der VJ, Knoops K, Puschhof J, Breugem TI, et al. SARS‐CoV‐2 productively infects human gut enterocytes. Science 2020;369:50‐54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Wang W, Xu Y, Gao R, Lu R, Han K, Wu G, et al. Detection of SARS‐CoV‐2 in different types of clinical specimens. JAMA 2020;323:1843‐1844. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Zhang W, Du R‐H, Li B, Zheng X‐S, Yang X‐L, Hu B, et al. Molecular and serological investigation of 2019‐nCoV infected patients: implication of multiple shedding routes. Emerg Microbes Infect 2020;9:386‐389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Wang H, Ma S. The cytokine storm and factors determining the sequence and severity of organ dysfunction in multiple organ dysfunction syndrome. Am J Emerg Med 2008;26:711‐715. [DOI] [PubMed] [Google Scholar]
- 34. Sahu KK, Cerny J. A review on how to do hematology consults during COVID‐19 pandemic. Blood Rev 2021;47:100777. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Varga Z, Flammer AJ, Steiger P, Haberecker M, Andermatt R, Zinkernagel AS, et al. Endothelial cell infection and endotheliitis in COVID‐19. Lancet 2020;395:1417‐1418. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Hooper JE, Padera RF, Dolhnikoff M, da Silva LFF, Duarte‐Neto AN, Kapp ME, et al. A postmortem portrait of the coronavirus disease 2019 (COVID‐19) pandemic: a large multiinstitutional autopsy survey study. Arch Pathol Lab Med 2021;145;529‐535. [DOI] [PubMed] [Google Scholar]
- 37. Duarte‐Neto AN, Monteiro RAA, Silva LFF, Malheiros DMAC, Oliveira EP, Theodoro‐Filho J, et al. Pulmonary and systemic involvement in COVID‐19 patients assessed with ultrasound‐guided minimally invasive autopsy. Histopathology 2020;77:186‐197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Wang X, Sahu KK, Cerny J. Coagulopathy, endothelial dysfunction, thrombotic microangiopathy and complement activation: potential role of complement system inhibition in COVID‐19. J Thromb Thrombolysis 2020;15:1‐6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Zhao D, Yao F, Wang L, Zheng L, Gao Y, Ye J, et al. A comparative study on the clinical features of coronavirus 2019 (COVID‐19) pneumonia with other pneumonias. Clin Infect Dis 2020;71:756‐761. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Bloom PP, Meyerowitz EA, Reinus Z, Daidone M, Gustafson J, Kim AY, et al. Liver biochemistries in hospitalized patients with COVID‐19. Hepatology 2020;73:890‐900. [DOI] [PubMed] [Google Scholar]
- 41. Papic N, Pangercic A, Vargovic M, Barsic B, Vince A, Kuzman I. Liver involvement during influenza infection: perspective on the 2009 influenza pandemic. Influenza Other Respir Viruses 2012;6:e2‐e5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Cabibbo G, Rizzo GEM, Stornello C, Craxì A. SARS‐CoV‐2 infection in patients with a normal or abnormal liver. J Viral Hepat 2021;28:4‐11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Pawlotsky JM. COVID‐19 and the liver‐related deaths to come. Nat Rev Gastroenterol Hepatol 2020;17:523‐525. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Fan Z, Chen L, Li J, Cheng X, Yang J, Tian C, et al. Clinical features of COVID‐19‐related liver functional abnormality. Clin Gastroenterol Hepatol 2020;18:1561‐1566. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Cai Q, Huang D, Yu H, Zhu Z, Xia Z, Su Y, et al. COVID‐19: abnormal liver function tests. J Hepatol 2020;73:566‐574. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Goldman JD, Lye DCB, Hui DS, Marks KM, Bruno R, Montejano R, et al. Remdesivir for 5 or 10 days in patients with severe Covid‐19. N Engl J Med 2020;383:1827‐1837. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Beigel JH, Tomashek KM, Dodd LE, Mehta AK, Zingman BS, Kalil AC, et al. Remdesivir for the treatment of Covid‐19—final report. N Engl J Med 2020;383:1813‐1826. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Hernandez AV, Roman YM, Pasupuleti V, Barboza JJ, White CM. Hydroxychloroquine or chloroquine for treatment or prophylaxis of COVID‐19: a living systematic review. Ann Intern Med 2020;173:287‐296. [DOI] [PubMed] [Google Scholar]
- 49. National Instite of Diabetes en Digestive and Kidney Diseases . Acetaminophen; Livertox. National Institute of Diabetes and Digestive and Kidney Diseases; 2020. https://www.ncbi.nlm.nih.gov/books/NBK547852/. [Google Scholar]
- 50. Montastruc F, Thuriot S, Durrieu G. Hepatic disorders with the use of remdesivir for coronavirus 2019. Clin Gastroenterol Hepatol 2020;18:2835‐2836. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Martinez MA. Clinical trials of repurposed antivirals for SARS‐CoV‐2. Antimicrob Agents Chemother 2020;64:e01101‐e1120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Alessi J, De Oliveira GB, Schaan BD, Telo GH. Dexamethasone in the era of COVID‐19: friend or foe? An essay on the effects of dexamethasone and the potential risks of its inadvertent use in patients with diabetes. Diabetol Metab Syndr 2020;12:80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Tomazini BM, Maia IS, Cavalcanti AB, Berwanger O, Rosa RG, Veiga VC, et al. Effect of dexamethasone on days alive and ventilator‐free in patients with moderate or severe acute respiratory distress syndrome and COVID‐19: the CoDEX Randomized Clinical Trial. JAMA 2020;324:1307‐1316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Liu J, Zhang S, Dong X, Li Z, Xu Q, Feng H, et al. Corticosteroid treatment in severe COVID‐19 patients with acute respiratory distress syndrome. J Clin Invest 2020;130:6417‐6428. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. Guaraldi G, Meschiari M, Cozzi‐Lepri A, Milic J, Tonelli R, Menozzi M, et al. Tocilizumab in patients with severe COVID‐19: a retrospective cohort study. Lancet Rheumatol 2020;2:e474‐e484. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. Yip T‐F, Lui G‐Y, Wong V‐S, Chow V‐Y, Ho T‐Y, Li T‐M, et al. Liver injury is independently associated with adverse clinical outcomes in patients with COVID‐19. Gut 2020;70:733‐742. [DOI] [PubMed] [Google Scholar]
- 57. Wahl A, Gralinski LE, Johnson CE, Yao W, Kovarova M, Dinnon KH, et al. SARS‐CoV‐2 infection is effectively treated and prevented by EIDD‐2801. Nature 2021;591:451‐457. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58. Doi Y, Hibino M, Hase R, Yamamoto M, Kasamatsu YU, Hirose M, et al. A prospective, randomized, open‐label trial of early versus late favipiravir therapy in hospitalized patients with COVID‐19. Antimicrob Agents Chemother 2020;64:e1897‐e1920. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59. Guan W, Ni Z, Hu Y, Liang W, Ou C, He J, et al. Clinical characteristics of coronavirus disease 2019 in China. N Engl J Med 2020;382:1708‐1720. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60. Higuera‐de la Tijera F, Servín‐Caamaño A, Reyes‐Herrera D, Flores‐López A, Robiou‐Vivero EJA, Martínez‐Rivera F, et al. Impact of liver enzymes on SARS‐CoV‐2 infection and the severity of clinical course of COVID‐19. Liver Res 2021;5:21‐27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61. Khan A, Bilal M, Morrow V, Cooper G, Thakkar S, Singh S, et al. Impact of COVID‐19 pandemic on gastrointestinal procedures and cancers in the United States. A Multicenter Research Network Study. Gastroenterology 2021;S0016‐5085(21)00460‐1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62. Shehab M, Alrashed F, Shuaibi S, Alajmi D, Barkun A. Gastroenterological and hepatic manifestations of patients with COVID‐19, prevalence, mortality by country, and intensive care admission rate: systematic review and meta‐analysis. BMJ Open Gastroenterol 2021;8:e000571. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63. Harrison SL, Fazio‐Eynullayeva E, Lane DA, Underhill P, Lip GYH. Comorbidities associated with mortality in 31,461 adults with COVID‐19 in the United States: a federated electronic medical record analysis. PLoS Med 2020;17:e1003321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64. Singh S, Khan A. Clinical characteristics and outcomes of coronavirus disease 2019 among patients with preexisting liver disease in the United States: a Multicenter Research Network Study. Gastroenterology 2020;159:e3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65. Ji D, Qin E, Xu J, Zhang D, Cheng G, Wang Y, et al. Non‐alcoholic fatty liver diseases in patients with COVID‐19: a retrospective study. J Hepatol 2020;73:451‐453. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66. Targher G, Mantovani A, Byrne CD, Wang X‐B, Yan H‐D, Sun Q‐F, et al. Risk of severe illness from COVID‐19 in patients with metabolic dysfunction‐associated fatty liver disease and increased fibrosis scores. Gut 2020;69:1545‐1547. [DOI] [PubMed] [Google Scholar]
- 67. Davidov‐Derevynko Y, Ben Yakov G, Wieder A, Segal G, Naveh L, Orlova N, et al. The liver in severe acute respiratory syndrome coronavirus 2 (SARS‐CoV‐2) infection. Eur J Gastroenterol Hepatol 2021. Feb 26. 10.1097/MEG.0000000000002048. [DOI] [PubMed] [Google Scholar]
- 68. Sterling RK, Oakes T, Gal TS, Stevens MP, deWit M, Sanyal AJ. The fibrosis‐4 index is associated with need for mechanical ventilation and 30‐day mortality in patients admitted with coronavirus disease 2019. J Infect Dis 2020;222:1794‐1797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69. Dongiovanni P, Stender S, Pietrelli A, Mancina RM, Cespiati A, Petta S, et al. Causal relationship of hepatic fat with liver damage and insulin resistance in nonalcoholic fatty liver. J Intern Med 2018;283:356‐370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70. Valenti L, Jamialahmadi O, Romeo S. Lack of genetic evidence that fatty liver disease predisposes to COVID‐19. J Hepatol 2020;73:709‐711. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71. Meijnikman AS, Bruin S, Groen AK, Nieuwdorp M, Herrema H. Increased expression of key SARS‐CoV‐2 entry points in multiple tissues in individuals with NAFLD. J Hepatol 2021;74:748‐749. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72. Biquard L, Valla D, Rautou PE. No evidence for an increased liver uptake of SARS‐CoV‐2 in metabolic‐associated fatty liver disease. J Hepatol 2020;73:717‐718. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73. Fondevila MF, Mercado‐Gómez M, Rodríguez A, Gonzalez‐Rellan MJ, Iruzubieta P, Valentí V, et al. Obese patients with NASH have increased hepatic expression of SARS‐CoV‐2 critical entry points. J Hepatol 2021;74:469‐471. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74. Qi X, Yang M, Stenberg J, Dey R, Fogwe L, Alam MS, et al. Gut microbiota mediated molecular events and therapy in liver diseases. World J Gastroenterol 2020;26:7603‐7618. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75. Wu J, Zhao M, Li C, Zhang Y, Wang DW. The SARS‐CoV‐2 induced targeted amino acid profiling in patients at hospitalized and convalescent stage. Biosci Rep 2021;41:BSR20204201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76. Zulhendri F, Ravalia M, Kripal K, Chandrasekaran K, Fearnley J, Perera CO. Propolis in metabolic syndrome and its associated chronic diseases: a narrative review. Antioxidants 2021;10:348. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77. Yeoh YK, Zuo T, Lui G‐Y, Zhang F, Liu Q, Li AYL, et al. Gut microbiota composition reflects disease severity and dysfunctional immune responses in patients with COVID‐19. Gut 2021;70:698‐706. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78. Alkhouri N, Kohli A, Loomba R, Harrison SA. Maintaining patient safety and data integrity of nonalcoholic steatohepatitis clinical trials during the severe acute respiratory syndrome‐coronavirus 2 pandemic. Hepatology 2020;72:1509‐1513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79. Wiśniewska H, Skowron M, Bander D, Hornung M, Jurczyk K, Karpińska E, et al. Nosocomial COVID‐19 infection and severe covid‐19 pneumonia in patients hospitalized for alcoholic liver disease: a case report. Am J Case Rep 2020;21:1‐8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80. Wang QQ, Davis PB, Xu R. COVID‐19 risk, disparities and outcomes in patients with chronic liver disease in the United States. EClinicalMedicine 2021;31:100688. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81. Poynard T, Deckmyn O, Rudler M, Peta V, Ngo Y, Vautier M, et al. Performance of serum apolipoprotein‐A1 as a sentinel of Covid‐19. PLoS One 2020;15:e0242306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82. Pasala S, Barr T, Messaoudi I. Impact of alcohol abuse on the adaptive immune system. Alcohol Res 2015;37:185‐197. [PMC free article] [PubMed] [Google Scholar]
- 83. Szabo G, Saha B. Alcohol’s effect on host defense. Alcohol Res Curr Rev 2015;37:159‐170. [PMC free article] [PubMed] [Google Scholar]
- 84. WHO . GLOBAL status report on noncommunicable diseases [cited December 24, 2020]. https://scholar.google.es/scholar?q=WHO.+GLOBAL+STATUS+REPORT+on+noncommunicable+diseases+2014.+n.d.&hl=es&as_sdt=0&as_vis=1&oi=scholart. Accessed 2014.
- 85. Da BL, Im GY, Schiano TD. Coronavirus disease 2019 hangover: a rising tide of alcohol use disorder and alcohol‐associated liver disease. Hepatology 2020;72:1102‐1108. [DOI] [PubMed] [Google Scholar]
- 86. Aggeletopoulou I, Davoulou P, Konstantakis C, Thomopoulos K, Triantos C. Response to hepatitis B vaccination in patients with liver cirrhosis. Rev Med Virol 2017;27:e1942. [DOI] [PubMed] [Google Scholar]
- 87. McCashland TM, Preheim LC, Gentry‐Nielsen MJ. Pneumococcal vaccine response in cirrhosis and liver transplantation. J Infect Dis 2000;181:757‐760. [DOI] [PubMed] [Google Scholar]
- 88. Marjot T, Webb GJ, Barritt AS, Moon AM, Stamataki Z, Wong VW, et al. COVID‐19 and liver disease: mechanistic and clinical perspectives. Nat Rev Gastroenterol Hepatol 2021;10:1‐17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89. Ioannou GN, Liang PS, Locke E, Green P, Berry K, O’Hare AM, et al. Cirrhosis and SARS‐CoV‐2 infection in US Veterans: risk of infection, hospitalization, ventilation and mortality. Hepatology 2020. Nov 21. 10.1002/hep.31649. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90. Iavarone M, D'Ambrosio R, Soria A, Triolo M, Pugliese N, Del Poggio P, et al. High rates of 30‐day mortality in patients with cirrhosis and COVID‐19. J Hepatol 2020;73:1063‐1071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91. Medhat MA, El Kassas M. Letter regarding “High rates of 30‐day mortality in patients with cirrhosis and COVID‐19”. J Hepatol 2020;73:1569‐1570. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92. Siegel RL, Miller KD, Jemal A. Cancer statistics, 2020. CA: Cancer J Clin 2020;70:7‐30. [DOI] [PubMed] [Google Scholar]
- 93. Chan SL, Kudo M. Impacts of COVID‐19 on liver cancers: during and after the pandemic. Liver Cancer 2020;9:491‐502. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94. Liang W, Guan W, Chen R, Wang W, Li J, Xu KE, et al. Cancer patients in SARS‐CoV‐2 infection: a nationwide analysis in China. Lancet Oncol 2020;21:335‐337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95. Zhang L, Zhu F, Xie L, Wang C, Wang J, Chen R, et al. Clinical characteristics of COVID‐19‐infected cancer patients: a retrospective case study in three hospitals within Wuhan. China Ann Oncol 2020;31:894‐901. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96. Rabiee A, Sadowski B, Adeniji N, Perumalswami PV, Nguyen V, Moghe A, et al. Liver injury in liver transplant recipients with coronavirus disease 2019 (COVID‐19): U.S. multicenter experience. Hepatology 2020;72:1900‐1911. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97. Reddy MS, Hakeem AR, Klair T, Marcon F, Mathur A, Samstein B, et al. Trinational study exploring the early impact of the COVID‐19 pandemic on organ donation and liver transplantation at national and unit levels. Transplantation 2020;104:2234‐2243. [DOI] [PubMed] [Google Scholar]
- 98. Casari I, Manfredi M, Metharom P, Falasca M. Dissecting lipid metabolism alterations in SARS‐CoV‐2. Prog Lipid Res 2021;82:101092. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99. Wu Z, Shon JC, Liu K‐H. Mass spectrometry‐based lipidomics and its application to biomedical research. J Lifestyle Med 2014;4:17‐33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100. Bruzzone C, Bizkarguenaga M, Gil‐Redondo R, Diercks T, Arana E, García de Vicuña A, et al. SARS‐CoV‐2 infection dysregulates the metabolomic and lipidomic profiles of serum. iScience 2020;23:101645. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101. Song J‐W, Lam SM, Fan X, Cao W‐J, Wang S‐Y, Tian HE, et al. Omics‐driven systems interrogation of metabolic dysregulation in COVID‐19 pathogenesis. Cell Metab 2020;32:188‐202.e5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102. Cirstea M, Walley KR, Russell JA, Brunham LR, Genga KR, Boyd JH. Decreased high‐density lipoprotein cholesterol level is an early prognostic marker for organ dysfunction and death in patients with suspected sepsis. J Crit Care 2017;38:289‐294. [DOI] [PubMed] [Google Scholar]
- 103. Gruber M, Christ‐Crain M, Stolz D, Keller U, Müller C, Bingisser R, et al. Prognostic impact of plasma lipids in patients with lower respiratory tract infections—an observational study. Swiss Med Wkly 2009;139:166‐172. [DOI] [PubMed] [Google Scholar]
- 104. Wei X, Zeng W, Su J, Wan H, Yu X, Cao X, et al. Hypolipidemia is associated with the severity of COVID‐19. J Clin Lipidol 2020;14:297‐304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105. Hu X, Chen D, Wu L, He G, Ye W. Declined serum high density lipoprotein cholesterol is associated with the severity of COVID‐19 infection. Clin Chim Acta 2020;510:105‐110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106. Sumbria D, Berber E, Mathayan M, Rouse BT. Virus infections and host metabolism—can we manage the interactions? Front Immunol 2020;11:594963. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107. Madsen CM, Varbo A, Tybjærg‐Hansen A, Frikke‐Schmidt R, Nordestgaard BG. U‐shaped relationship of HDL and risk of infectious disease: two prospective population‐based cohort studies. Eur Heart J 2018;39:1181‐1190. [DOI] [PubMed] [Google Scholar]
- 108. Trinder M, Walley KR, Boyd JH, Brunham LR. Causal inference for genetically determined levels of high‐density lipoprotein cholesterol and risk of infectious disease. Arterioscler Thromb Vasc Biol 2020;40:267‐278. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109. Zhang X‐J, Qin J‐J, Cheng XU, Shen L, Zhao Y‐C, Yuan Y, et al. In‐hospital use of statins is associated with a reduced risk of mortality among individuals with COVID‐19. Cell Metab 2020;32:176‐187.e4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110. Morawietz H, Julius U, Bornstein SR. Cardiovascular diseases, lipid‐lowering therapies and European registries in the Covid‐19 pandemic. Cardiovasc Res 2020;116:E122‐E125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111. Tong S, Revill P. Overview of hepatitis B viral replication and genetic variability. J Hepatol 2016;64:S4‐S16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112. World Health Organization . Global Hepatitis Report. 2017. http://apps.who.int/iris/bitstream/10665/255016/1/9789241565455‐ eng.pdf?ua=1. Accessed 2017.
- 113. Lens S, Miquel M, Mateos‐Muñoz B, García‐Samaniego J, Forns X. SARS‐CoV‐2 in patients on antiviral HBV and HCV therapy in Spain. J Hepatol 2020;73:1262‐1263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114. Chen X, Jiang Q, Ma Z, Ling J, Hu W, Cao Q, et al. Clinical characteristics of hospitalized patients with SARS‐CoV‐2 and hepatitis B virus co‐infection. Virol Sin 2020;35:842‐845. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115. Liu R, Zhao LI, Cheng X, Han H, Li C, Li D, et al. Clinical characteristics of COVID‐19 patients with hepatitis B virus infection—a retrospective study. Liver Int 2021;41:720‐730. [DOI] [PubMed] [Google Scholar]
- 116. Chen L, Huang S, Yang J, Cheng X, Shang Z, Lu H, et al. Clinical characteristics in patients with SARS‐CoV‐2/HBV co‐infection. J Viral Hepat 2020;27:1504‐1507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117. Rodríguez‐Tajes S, Miralpeix A, Costa J, López‐Suñé E, Laguno M, Pocurull A, et al. Low risk of hepatitis B reactivation in patients with severe COVID‐19 who receive immunosuppressive therapy. J Viral Hepat 2021;28:89‐94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118. Blach S, Kondili LA, Aghemo A, Cai Z, Dugan E, Estes C, et al. Impact of COVID‐19 on global HCV elimination efforts. J Hepatol 2021;74:31‐36. [DOI] [PMC free article] [PubMed] [Google Scholar]


