Abstract
December 2019 will never be forgotten in the history of medicine when an outbreak of pneumonia of unknown etiology in Wuhan, China sooner or later prompted the World Health Organization to issue a public health warning emergency. This is not the first nor will it be the last time that a member of β‐coronaviruses (CoVs) is waging a full‐scale war against human health. Notwithstanding the fact that pneumonia is the primary symptom of the novel coronavirus (2019nCoV; designated as SARS‐CoV‐2), the emergence of severe disease mainly due to the injury of nonpulmonary organs at the shadow of coagulopathy leaves no choice, in some cases, rather than a dreadful death. Multiple casual factors such as inflammation, endothelial dysfunction, platelet and complement activation, renin‐angiotensin‐aldosterone system derangement, and hypoxemia play a major role in the pathogenesis of coagulopathy in coronavirus disease 2019 (COVID‐19) patients. Due to the undeniable role of coagulation dysfunction in the initiation of several complications, assessment of coagulation parameters and the platelet count would be beneficial in early diagnosis and also timely prediction of disease severity. Although low‐molecular‐weight heparin is considered as the first‐line of treatment in COVID‐19‐associated coagulopathy, several possible therapeutic options have also been proposed for better management of the disease. In conclusion, this review would help us to gain insight into the pathogenesis, clinical manifestation, and laboratory findings associated with COVID‐19 coagulopathy and would summarize management strategies to alleviate coagulopathy‐related complications.
Keywords: coagulopathy, coronavirus, COVID‐19, SARS‐CoV‐2, thromboembolism
1. INTRODUCTION
Since early 2020, almost all people around the world are tracking the statistics of SARS‐CoV‐2 and offering the scary number of death tolls which is increasing day after day. Actually, life is on hold as death and disease have cast a sinister shadow on people's lives. It is absolutely heartbreaking for the generation in their last decades dying alone and also painful for families losing their loved ones. While SARS‐CoV1 and MERS‐CoV infections infected about 10,000 cases with mortality rates of 10% (Organization, 2003) and 37% (O'Keefe, 2016) respectively, the situation is quite eerie in the recent case recalling that the novel coronavirus might only be the tip of the iceberg with potentially more secretive characteristics to be discovered. Clinically, coronavirus disease 2019 (COVID‐19) is a mysterious respiratory syndrome with a wide spectrum of symptoms; while some cases will present no or mild symptoms, others will develop more serious complications entailing specialized management in the intensive care units (ICU) (Guan et al., 2020).
The first step of SARS‐CoV‐2 infection happens when the virus enters target cells by the interaction between the viral surface S (spike) protein and Type I integral membrane receptor, angiotensin‐converting enzyme 2 (ACE‐2) that is well expressed in various tissues including lungs, kidney, heart, and gastrointestinal tract (Wang et al., 2020e). The next step is the cleavage of the viral S protein which is done by host cellular proteases. This proteolytic process is primarily promoted by TMPRSS2, the cellular serine protease which is expressed abundantly on the human airway epithelial cells (Sathler, 2020). In presence of this plasma membrane protease, the viral S protein endures irreversible structural changes that facilitate virus entry via the merge of the virus to the host cell membrane. However, in the absence of the exogenous or membrane‐bound protease, SARS‐CoV‐2 probably goes through a “late pathway” and is internalized via either clathrin‐ or nonclathrin‐mediated endocytosis. Following the virus entry, the pH in the endosome decreases, and then, low pH activates endosomal protease such as cathepsins which leads to the fusion pathway and release of the SARS‐CoV‐2 genome (Tang et al., 2020e). Both mechanisms will drive the release of the viral RNA genome in the host cell and the subsequent start of the viral replication cycle (Matsuyama et al., 2010; Sathler, 2020). The virus is also targeting endothelial cells, one of the largest organs in the human body that widely express ACE‐2. Endothelial cells represent a major role in several physiologic processes (Sardu et al., 2020). They manage the innate immune responses, increase tissue permeability, set up inflammation, and therefore, may contribute to the severity of the disease (Pons et al., 2020). Hence, the dysfunction of the endothelium may lead to systemic damage with abnormal coagulation, kidney disorders, pulmonary embolism (PE), and sepsis (Varga et al., 2020). Although hypoxemia secondary to acute respiratory distress syndrome (ARDS) plays a major role in COVID‐19 mortality, there is growing evidence representing that thrombotic complexity and coagulation disorders emerge as a critical issue in COVID‐19 patients. Coagulation disorders often occur in severe cases with poor prognosis but the nature of this abnormality is not clear yet (Al‐Ani et al., 2020). Abnormal hematological findings related to coagulopathy including thrombocytopenia, prolonged prothrombin time (PT) and activated partial thromboplastin time (aPTT), increased fibrin degradation product (FDP) levels and, in particular, elevated D‐dimer have been associated with poor prognosis and also an increased rate of mortality in COVID‐19 patients (Giannis et al., 2020). Besides, data extracted from recent studies consolidates that the risk of developing disseminated intravascular coagulation (DIC) is higher in patients infected by SARS‐CoV2 (Wang et al., 2020g). Therefore, a deeper understanding of new strategies to manage and treat the disease will help to better control the complications attributed to the hypercoagulable state. In the present review, we are focusing on the association between the pathophysiology of coagulation and COVID‐19 and also gathering strategies that help the management and treatment of COVID‐19‐associated coagulopathy.
2. PATHOPHYSIOLOGY OF COVID‐19‐ASSOCIATED COAGULOPATHY
Pathophysiology of coagulation disorders related to COVID‐19 is mostly relying on a wide variety of complicated interactions between the secretion of proinflammatory cytokines/factors, platelets hyper‐activation, and damage to the endothelial cells (Becker, 2020). Viral infection gives rise to the release of proinflammatory cytokines by activating inflammatory responses of the innate immune system. Following this inflammation and upregulated immune response, platelets are activated and natural mechanisms of anti‐coagulant are downregulated (Esmon, 2005). Endothelial dysfunction, on the other hand, is another risk factor responsible for coagulopathy in COVID‐19. In addition to the indicated abnormalities, other pathogenic mechanisms including increased secretion of von‐Willebrand factor (vWF) from damaged endothelium, activation of TLRs, and complement activation are involved in COVID‐19‐associated coagulopathy. In the next section, we have a glance at the underlying mechanisms proposed for the hypercoagulable state in these patients.
2.1. Inflammation
Following the viral infection, high levels of inflammatory cytokines in a systemic circulation stimulate macrophages and other leukocytes and promote local recruitment of these inflammatory cells especially to the lung (Liu et al., 2020e). In turn, infiltrated macrophages and polymorphonuclear neutrophils (PMNs) produce a higher level of inflammatory cytokines and chemokines which subsequently exacerbate the recruitment of other immune cells in parenchyma; an event which subsequently leads to hyperinflammation and cytokine storm in severe cases of COVID‐19 (Channappanavar et al., 2016). As there is a large amount of proinflammatory cytokines released from activated macrophages and leukocytes, some clinicians and researchers suggest hemophagocytic lymphohistiocytosis‐like syndrome that is a combination of its protean nature, secondary infections and end‐organ failure (Zuckier et al., 2020).
Generally speaking, RNA viruses are recognized by pattern recognition receptors, an event which in turn leads to the production of Type I interferons (IFNs) (Jensen & Thomsen, 2012). However, SARS‐CoV2, as well as SARS‐CoV1, impair antiviral responses by inhibiting signaling pathways of TLR3 and TLR7 which end to Type I IFNs production (Li et al., 2016). Subsequently, it gives rise to an increased viral replication and induction of a direct cytopathic effect which together with cytokines and DAMPs released from infected cells negatively exacerbate macrophages and neutrophils infiltration. Due to the direct cytopathic effects of SARS‐CoV2, not only apoptotic deaths of CD4+ T cells are increased but also the production level of IFN‐γ is suppressed. Since CD4+ lymphocytes are critical for regulating the balance of the inflammatory responses, a decreased number of these cells could undoubtedly result in a hyperinflammatory state through compromised suppression of inflammation (Chen et al., 2010). A study of a mouse model of SARS confirmed the association between Type I IFN response and the severity of the disease. They reported that the later IFN Type 1 was produced, the greater extent of lung injury and pulmonary edema would be observed (Channappanavar et al., 2016).
It has been reported that the levels of interleukin (IL)‐1β, IL‐6, tumor necrosis factor alpha (TNF‐α), G‐CSF, and ferritin are significantly elevated in patients infected with SARS‐CoV2 (Henry et al., 2020a). Yang et al. (2020c) investigated the plasma level of 48 cytokines in 53 COVID‐19 patients and indicated that IL‐1ra, IP‐10 (IFN‐γ induced protein 10), and monocyte chemotactic protein‐3 are three independent predictors of the development of disease. Transcriptome sequencing of RNAs also confirmed the relationship between COVID‐19 pathogenesis and extreme release of cytokines such as CCL2/MCP‐1, CXCL10/IP‐10, CCL3/MIP‐1A, and CCL4/MIP1B (Xiong et al., 2020b). The results of a nonrandomized clinical study performed by Xu et al. (2020c) revealed that the suppression of the IL‐6 network using tocilizumab improved the disease outcome, as the need for oxygen was significantly reduced in 75% of patients within 5 days of treatment. The data extracted from this study clearly highlighted the importance of excessive production of proinflammatory cytokines in the pathogenesis of COVID‐19.
Evidence of abnormal coagulation parameters and increased biomarkers of inflammation has been reported in several studies. Ranucci reported elevated levels of fibrinogen, D‐dimer, and IL‐6 in COVID‐19 patients with ARDS (Ranucci et al., 2020). In agreement, Zhou et al. (2020c) suggested that the elevated D‐dimer (more than 1.2 µg/ml on admission), increased PT and elevated IL‐6 are important factors associated with COVID‐19 mortality. The results of this study showed that 50% of nonsurvivors had evidence of coagulopathy and infection. In another study conducted on 183 COVID‐19 patients, 15 of 21 nonsurvivors were diagnosed with overt DIC with a median beginning at 4 days after admission. During their hospitalization, they underwent DIC with decreased fibrinogen, elevated D‐dimer, and increased PT. Antithrombin levels were also decreased over hospitalization but it was not below the normal ranges in the majority (Tang et al., 2020d).
The relationship between inflammation and activation of the procoagulant pathways has been identified while the nature of this relation is not yet well‐established (Risitano et al., 2012). Tissue factor (TF), as a primary initiator of the coagulation cascade, is highly induced by inflammatory cytokines like TNF‐α on the cell surface of leukocyte and endothelial cells. So, inducible expression of TF by inflammatory mediators may strongly contribute to a hypercoagulable state (Witkowski et al., 2016). Upon stimulation with inflammatory mediators, neutrophils release neutrophil extracellular traps (NETs) in a process named NETosis. Actually, NETs are evolved as a defense mechanism; however, this network, on one hand, could activate platelets, endothelial cells, and the complement system, and on the other hand, may trigger the intrinsic pathway through stimulation of FXII (Keragala et al., 2018). Apart from these mechanisms, polyphosphates derived from microorganisms could activate platelets and FXII and amplify the procoagulant response of the intrinsic coagulation pathway. In a vicious cycle, the activated platelets provoke NETosis in the recruited neutrophils (Zucoloto & Jenne, 2019). To provide a better outlook, a summary of the mechanisms involved in SARS‐CoV‐2‐induced inflammation was represented in Figure 1.
Figure 1.
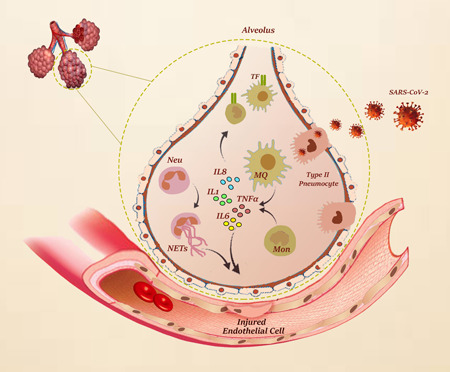
SARS‐CoV‐2‐induced inflammation. In viral infections, immune cells such as monocyte/macrophage and other leukocytes are activated by pathogen‐associated molecular patterns (PAMPs) and host‐derived damage‐associated molecular patterns (DAMPs). In COVID‐19, this stimulation leads to increased infiltration of activated immune cells to the lung and other organs and intensifies the production of proinflammatory cytokines. Proinflammatory cytokines induce the expression of tissue factor (TF), the primary initiator of the extrinsic coagulation cascade, which strongly contributes to a hypercoagulable state. Activated neutrophils and neutrophil extracellular traps (NETs) are also involved in endothelial dysfunction, which eventually leads to coagulation disorders in COVID‐19
2.2. Endothelial cells dysfunction/activation
Being a transmembrane receptor of factor VII (FVII), TF is an early beginner of the coagulation cascade. Where there is no need for the activation of the clotting cascade, the endothelium prevents the binding of TF to circulating FVII. However, upon the endothelial damage and in the presence of the inflammatory cytokines TF is recruited on the surface of the cells and subsequently leads to the induction of a hypercoagulable state (Witkowski et al., 2016). In addition, inflammatory cytokines such as IL‐6, IL‐8, and TNF‐α increase the secretion of large vWF fragments from injured endothelium which intensifies platelets aggregation. Normally, these ultra‐large fragments are broken down into small thrombotic forms by the enzymatic activity of ADAMTS13, but this action is inhibited by IL‐6 in an acute inflammatory state (Levi & Poll, 2015).
Considering the important role of the endothelium in regulating fibrinolysis, endothelial dysfunction may act as a trigger for immunothrombosis, resulting in hypercoagulopathy in COVID‐19 patients (Varga et al., 2020). Impairing or inactivation of the endogenous fibrinolytic system in the acute inflammatory state gives rise to antithrombin consumption and downregulation of the protein C pathway which is highly sensitive to inflammatory mediators (Levi & Poll, 2015). Apart from the stimulatory effects of inflammation on endothelial cell activation, SARS‐CoV‐2 may directly activate/impair these cells (Kolaczkowska et al., 2015). In turn, it leads to the excess generation of thrombin that could later activate platelets, increase the permeability of vessels, and suppress fibrinolysis. Additionally, reduced fibrinolysis (hypofibrinolysis) was observed due to an increased level of fibrinolytic inhibitor plasminogen activator inhibitor 1 (PAI‐1, the main inhibitor of fibrinolysis) in COVID‐19‐related ARDS (Whyte et al., 2020; Wright et al., 2020). Notably, inflammation can also promote PAI‐1 release from endothelial cells, an event which in turn suppresses urokinase‐plasminogen activator and tissue‐type plasminogen activator (tPA) and ultimately leads to reduced fibrin degradation (Loo et al., 2021). It is also worth noting that there is a firm connection between the fibrinolytic system and the renin‐angiotensin system (RAA) and ACE‐2. After attaching the virus, ACE2 is competitively consumed and Angiotensin II (AngII) remains in excess, therefore, freely acting as a potent stimulator of PAI‐1. On the other hand, simultaneous activation of factor XII increases bradykinin which stimulates tPA (Kwaan, 2020b). Fibrinolysis may thus be affected by tPA and/or PAI‐I, inducing a prothrombotic or prohemorrhagic state depending on the sites and severity of the biologic process. Complications such as intra‐alveolar bleeding may be due to increased tPA, while persistence or worsening of microthrombosis and evolution towards pulmonary fibrosis may root in escalated PAI‐1. To sum up, the impaired balance between activation and inhibition of fibrinolysis may elucidate the coexistence of thrombotic and hemorrhagic features in lungs, and also in other organs such as kidneys in COVID‐19 patients (Hirsch et al., 2020).
2.3. Platelets activation
Within the viral infection, platelets and innate immune effector cells such as PMNs, monocytes/macrophages, and complement components interact via surface receptors to form clots. This interaction causes activation of platelets which results in shape changes and the release of granules that contain costimulatory molecules with the ability to activate PMNs and macrophages. CXCL4, CXCL12, and P‐selectin are upregulated in the storing granules of the activated platelets that intensify interaction with other immune cells (Jensen & Thomsen, 2012, Li et al., 2016). It has been also suggested that increased expression of TLR9 and its engagement by novel endogenous ligands may promote platelet hyperreactivity and thrombosis through activating the protein kinase B (Akt/PKB) and IL‐1 receptor‐associated kinase 1 pathways (Panigrahi et al., 2013). For the first time, Manne et al. (2020) showed that there is a significant alteration in the expression profile of genes that are involved in the hyper‐activation of the platelets in patients with COVID‐19. Inflammatory mediators may also increase the activity of platelets which ultimately triggers the coagulant response and disturb fibrinolysis. In addition to being activated during inflammation, activated platelets exert proinflammatory effects as they act as important sources of proinflammatory cytokines like IL‐1β (Rondina and GUO, 2019).
The imbalance of endothelial function and hyperinflammation in systemic viral infection can alter hemostasis through reduced production or action of mediators like nitric oxide essential for the platelets activation regulation (Canzano et al., 2021). Also, the interaction of platelets with extracellular matrix (ECM) proteins can facilitate platelets adhesion to endothelial surface, and subsequently, trigger signaling events that activate platelets. Production of these ECM proteins is increased in lung fibrosis and endothelial inflammation, which may serve as a complementary mechanism to activate platelets in COVID‐19 patients (Hottz et al., 2020). Another key mediator of platelet activation is proteinase‐activated receptor 1, which is, in turn, mediated by thrombin (Ulanowska & Olas, 2021). Activated platelets express an active TF, an essential trigger of coagulation and thrombosis in a P‐selectin‐ and αⅡb/β3‐dependent manner (Hottz et al., 2020). A study conducted by Zhang et al. (2020c) showed that platelets are hyperactive in COVID‐19 patients. The authors demonstrated for the first time that platelets express ACE2 and TMPRSS2, and the spike protein of SARS‐CoV‐2 directly binds to platelet ACE2 and increases platelet activation in vitro. The Spike also enhances thrombus formation in wild‐type mice transfused with hACE2 transgenic platelets. All in all, Zhang et al. (2020c) uncovered a novel function of SARS‐CoV‐2 on platelet activation via binding of Spike to ACE2 which may participate in thrombus formation and inflammatory responses in COVID‐19 patients. Last but not least, the crosstalk between platelets and coagulation factors provides a proper platform for substrate and enzyme/cofactor to make a procoagulant complex (Walsh, 2004). Taken together, activated platelets trigger coagulation proteins and provide a fertile ground to amplify COVID‐19‐induced coagulopathy.
2.4. Complement activation
The complement system is a part of the innate immune system composed of several circulating proteins. Three pathways, including classical, alternative, and lectin were introduced through which the complement system is activated to produce C3a and C5a. Apart from C5a, the cleavage of C5 produce another component named C5b, which participates deeply in the production of the lytic membrane attack complex and bacterial opsonization (Bajic et al., 2015). The crosstalk between complement components and coagulation leads to thrombotic microangiopathy, hypercoagulable state, and also inflammation in COVID‐19. Complement proteins C3 and C5 exert proinflammatory and prothrombotic effects. In turn, it gives rise to mast cell degranulation and activation of endothelial cells leading to TF expression and vWF secretion, respectively (Keragala et al., 2018). In a mouse model of SARS, Gralinski et al. (2018) showed that the dysregulation of complement activation in C3 knocked‐out mice (C3−/−) contributed to milder lung damage as compared to wild‐type counterparts; shedding light on the contributory role of the complement system in SARS‐induced lung damage (Gralinski et al., 2018). Other components of the complement system like MASP‐1 and MASP‐2 proteins also accelerate coagulopathy by augmenting clot formation through converting fibrinogen to fibrin and prothrombin to thrombin. Examination of skin and lung tissue of severe COVID‐19 cases demonstrated significant deposits of terminal complement components in the microvasculature, which further highlighted the constant stimulation of the complement system in this infection (Magro et al., 2020).
Given the critical role of complement in the regulation of innate immune‐mediated consequences of severe SARS‐CoV‐2 infections, it seems that the application of novel strategies to harness this system may bring valuable therapeutic effects for patients (Campbell & Kahwash, 2020). In a study of anticomplement C5 therapy, Diurno et al. (2020) reported that Eculizumab has an acceptable efficacy in the treatment of COVID‐19 patients with severe pneumonia or ARDS. In agreement, Mastaglio et al. examined C3 inhibitor AMY‐101 for the first time to treat a patient with severe ARDS due to COVID‐19 pneumonia. Besides being safe, they indicated that the application of AMY‐101 was coupled with favorable results (Mastaglio et al., 2020). All in all, these studies further emphasized the significant pathophysiologic role of complement activation in COVID‐19 and suggested that the inhibition of this pathway may bring outstanding outcomes in this infection, in particular for those with a severe condition. A schematic representation of COVID‐19‐induced coagulopathy due to the complex interconnections between inflammation and activation of endothelial cells, platelets, and complement was represented in Figure 2.
Figure 2.
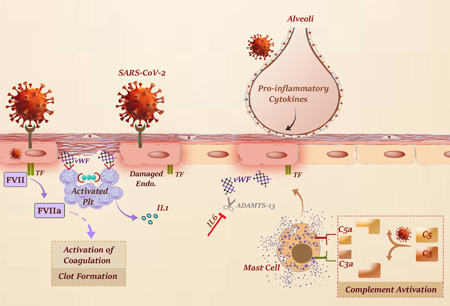
Interconnections between inflammation and activation of endothelial cells, platelets, and complement in COVID‐19‐induced coagulopathy. Endothelial cell activation/damage is triggered through multiple pathways, including proinflammatory cytokines, NETosis, hypoxia, and complement activation. Injured endothelial cells release ultra‐large vWF multimers which in turn stimulate platelets adhesion and activation. Not only do activated platelets intensify inflammation through secretion of proinflammatory cytokines but also provide an exposed surface for assembly of enzyme‐cofactor‐substrate complexes throughout the coagulation cascade. Cleavage of C3 and C5 also gives rise to mast cell degranulation which results in an increased expression of TF and endothelial cell damage. COVID‐19, coronavirus disease 2019; TF, tissue factor
2.5. Renin‐angiotensin‐aldosterone system (RAAS) derangement
RAAS derangement is observed in the pathophysiology of COVID‐19; an event that is associated with the promotion of coagulation cascade and further microthrombi formation (Zhang & Baker, 2017). To be more in detail, ACE2 (as one of the crucial members of RAAS) converts AngII to Ang1–7. Upon binding to its receptor (AngII receptor Type 1), AngII could induce vasoconstriction, inflammation, and even fibrosis (5). Impairing the balance between ACE/ACE2 levels followed by a decrease in ACE2 activation within SARS‐CoV2/ACE2 interaction leads to an elevated concentration of AngII in plasma. Increased AngII eventually causes pulmonary vasoconstriction, inflammation, and organ damage. Moreover, AngII‐mediated pulmonary vasoconstriction could induce hypoxemia, which in turn, stimulates the hyper‐coagulation state (Henry et al., 2020b). Khan et al. (2017) conducted a Phase II trial examining the safety and efficacy of a recombinant human ACE2 in patients with ARDS. The result of this study indicated the administration of GSK2586881 is well‐tolerated and safe, leading to a rapid decrease in the plasma level of Ang II and an increase in Ang1–7, as well (Khan et al., 2017).
2.6. Hypoxemia
Hypoxemia is another probable mechanism that is associated with an increased risk of thrombosis in COVID‐19 patients (Gupta et al., 2019). In a cohort study, Xie et al. (2020) showed that hypoxemia could independently elevate the risk of in‐hospital mortality of COVID‐19 cases. Gattinoni et al. (2020) also discovered that in 20%–30% of COVID‐19 patients admitted to ICU, severe hypoxemia is associated with compliance values less than 40 ml/cmH2O, indicating severe ARDS. The endothelial damage and hypercoagulable state due to COVID‐19 are exacerbated by hypoxemia via increasing the viscosity of blood and activating the hypoxia‐inducible factor (HIF). This occurrence intensified the risk of both PE and microthrombosis in pulmonary small vessels, two phenomena that are widely reported in COVID‐19 patients. The HIF‐dependent signaling pathways intensify thrombosis and consequently impair pulmonary gas exchange. It causes severe lung inflammation and contributes to worsen hypercoagulability (Marchandot et al., 2020). For the time being, there is no enough evidence yet to support the idea, and further studies are required to prospect whether there is a crosstalk between pulmonary and endothelial ACE2 receptors localization, hypoxemia, and pulmonary microthrombi in COVID‐19.
2.7. Changes in gene expression
The results of previous epidemic outbreaks indicated a number of changes in the gene expression profile of procoagulant‐related genes. Infected mononuclear cells in SARS‐CoV infection highly expressed TLR9, fibrinogen, factor II, factor III, factor X, and thromboxane (TBXAS) (Ng et al., 2004). It should be noted that the thromboxane production and TLR‐9 expression in platelets could promote platelet activation, aggregation, and degranulation. Han et al. (2008) suggested that nucleocapsid protein (N protein) of SARS‐CoV‐1 induced human fibrinogen‐like protein‐2 prothrombinase gene transcription, which has been identified as one of the significant factors contributing to a prothrombic state in this infection. Dysregulation of the urokinase signaling pathway and also downregulation of the genes involved in this pathway can play a key role in the pathogenesis of SARS‐CoV‐related coagulation, leading to a fatal infection (Gralinski & Baric, 2015). Given the great similarity in the genome of SARS‐CoV and SARS‐CoV‐2 (Xu et al., 2020a), it is expected that their clinical features should be somehow the same. However, several differences should be considered when data from these infections are comparing with each other.
3. CLINICAL MANIFESTATIONS AND COMPLICATIONS OF COVID‐19 COAGULOPATHY
The emergence of thromboembolic complications and subsequent organ dysfunction is common in COVID‐19 (Zhou et al., 2020a). In fact, over‐inflammatory responses due to systemic infection are strictly linked to endothelial dysfunction and mainly affect both the venous and the arterial vascular system in COVID‐19 patients (Fischetti & Tedesco, 2006). The venous thromboembolism (VTE) risk in COVID‐19 is an emerging issue. The previous history of VTE and genetic thrombophilia could potentially increase the risk of this complication in hospitalized COVID‐19 patients (Terpos et al., 2020). The combination of these risk factors alongside inflammatory mediators may also lead to deep vein thrombosis (DVT) or even PE due to thrombosis migration.
In agreement with previous studies from SARS experiments, a recent study conducted on 1026 COVID‐19 patients showed that 40% of cases were at the risk of VTE when they were referred to the hospital (Wang et al., 2020f). Another recent study also reported a 25% (20 of 81) incidence rate of VTE using systematic assessment in severe COVID‐19 cases of which 8 patients died (Cui et al., 2020). Klok et al. (2020) conducted a comprehensive study about the incidence of venous and arterial thrombosis in 184 COVID‐19 patients who were hospitalized in the ICU. The results of this study demonstrated that 31% of the studied population experienced a thrombotic complication, indicating a remarkably high incidence of coagulation disorders in severe COVID‐19 cases who were admitted to the ICU. In a similar study, Lodigiani et al. (2020) evaluated 388 patients who required intensive care. They found that 28 (7.7%) of closed cases (defined as patients discharged or died of the disease) experienced thromboembolic events. Furthermore, the results of VTE imaging tests were positive in 16 out of 44 (36%) patients.
Given the existence of microthrombotic disorders in pulmonary arteries of COVID‐19 patients, it was reasonable to assume PE as an etiology of acute respiratory deterioration in this infection. First reports from European countries imply the significantly high incidence of PE and thrombosis in the most severe and fatal cases of the disease (Ciceri et al., 2020, Danzi et al., 2020, Klok et al., 2020). PE was diagnosed in 32/106 (30%) of COVID‐19 patients undergoing pulmonary CT angiograms (Léonard‐Lorant et al., 2020). Notably, this rate of pulmonary embolus is remarkably higher than the incidence rate usually encountered in either critically ill patients without SARS‐CoV‐2 infection (1.3%) (Lim et al., 2015) or those who were hospitalized in the emergency department (3%–10%) (Corrigan et al., 2016). In a case report study of a COVID‐19 patient, PE was verified with no VTE risk factors. Other case series of autopsies of postmortem also represent that 7 of 12 (58%) of patients developed VTE while PE was the direct cause of death in 4 (33%) patients (Wichmann et al., 2020). The results of a recent study conducted on 328 SARS‐CoV‐2 infected patients also noted that 72 patients had PE. Of particular importance, patients with Body mass index (BMI) of more than 30 kg/m2 were mainly classified into the PE group (Danzi, et al., 2020).
It has been reported that the accompaniment of low‐grade DIC with localized pulmonary thrombotic microangiopathy may lead to organ dysfunction in severe cases of the disease. Considering the results of the first four autopsies from New Orleans which proposed thrombotic microangiopathy within the alveolar capillaries as a fatal mechanism in severe COVID‐19 patients (Fox et al., 2020), it may be concluded that damage to the small blood vessels of vital tissues, eventually followed by intravascular coagulopathy, will probably be in charge of multiorgan failure (MOF). Postmortem findings also showed typical microvascular thrombosis in small vessels of the lungs and other organs. Coagulation activation and endothelial dysfunction also led to elevated levels of cardiac biomarkers which may serve as a predictor of death (Ma et al., 2020). In a study of 388 COVID‐19 patients, the rate of ischemic stroke, acute coronary syndrome/myocardial infarction, and overt DIC was 2.5%, 1.1%, and 2.2%, respectively (Lodigiani et al., 2020).
Due to an imbalance in platelet production and consumption, some patients with COVID‐19 are assumed to have an increased risk of bleeding (Magdi & Rahil, 2019). However, similar to SARS‐CoV‐1 infection, hemorrhagic complications are uncommon in this infection (Iba et al., 2020) even in those with severe coagulopathy (Tang et al., 2020b). But, it is still a need to further investigate whether bleeding complications might happen at the specific stage of the disease or in a specific group of patients. Taken together, although the incidence of VTE and PE are frequent in severe cases of COVID‐19 patients, still the information about the mechanisms through which these complications happen remained unclear.
4. DIAGNOSTIC AND PROGNOSTIC FACTORS IN COVID‐19 COAGULOPATHY
Coagulation‐related laboratory findings can deliver several valuable diagnostic and prognostic markers. Although both the sensitivity and specificity of these markers should be examined in additional studies, it has been declared that they may reliably help to better stratify COVID‐19 patients. In the following section, we take a look at the main coagulation‐related features in COVID‐19 patients.
4.1. Platelet count
Since thrombocytopenia is among the main hematological manifestations in viral infections (Assinger, 2014), it comes as no surprise to find that patients with rapidly evolving β‐coronaviruses may experience low platelet count (Giannis et al., 2020), and COVID‐19 shall not be considered as an exception. Although the accurate explanation for the occurrence of thrombocytopenia in COVID‐19 is still ambiguous, direct invasion of bone marrow cells by the virus, platelet destruction by the triggered immune system, and platelet aggregation in the lungs were suspected as the possible mechanisms (Xu et al., 2020b). Due to the platelet release from fully mature megakaryocytes settled in the lung, a decrease in the pulmonary capillary bed or any structural change may also lead to an unfortunate defragmentation of platelets (Yang et al., 2005). A study including 1099 patients from 31 provinces in China noted that 36.2% had thrombocytopenia (Guan et al., 2020). Thrombocytopenia has also been reported in 37 out of 198 COVID‐19 patients tested from Jinyintan Hospital in China (Wu et al., 2020a). A retrospective multicenter study of 28 COVID‐19 cases in Korea demonstrated that 53.6% of patients had thrombocytopenia on admission (Kim et al., 2020). Other studies also reported a decreased number of platelets as a significant hematologic finding in this infection (Chen et al., 2020b; Huang et al., 2020; Pourbagheri‐Sigaroodi et al., 2020). As a result, it can be concluded from the mentioned studies that platelet count is a valuable parameter in the diagnosis of COVID‐19.
In scoring systems like the Acute Physiology, Simplified Acute Physiology Score II, Multiple Organ Dysfunction Score, and Chronic Health Evaluation II thrombocytopenia acts as an indicator of severe disease. As a result, the platelet count is used by these systems to determine the severity (Lippi, Plebani & Henry, 2020). Severe COVID‐19 cases, as compare to nonsevere counterparts, more frequently experience decreased numbers of platelets (Xu, Zhou, 2020b). The higher incidence of thrombocytopenia in severe COVID‐19 patients has also been confirmed in the recent two meta‐analyses (Bashash et al., 2020a, Lippi & Plebani, 2020). Other studies regarding the SARS‐CoV outbreak also suggested that thrombocytopenia could increase the risk of the disease progression (He et al., 2003, Yang et al., 2005). Thrombocytopenia was identified in up to 57.7% of severe cases of COVID‐19 patients in comparison with 31.6% of patients with the milder form of the disease (odds ratio: 2.96; 95% confidence interval [CI]: 2.07–4.22) (Lippi & Plebani, 2020). According to a recent study, thrombocytopenia was also reported in 36.2% of the total COVID‐19 cases and 57.7% (90/156) in those with severe illness (Guan et al., 2020). It should be noted that the combination of platelet count together with other prognostic factors could make a good team for predicting the outcome of the patients. In agreement, it has been claimed that platelet count along with hypoxemia could anticipate severe cases of COVID‐19 with an accuracy of 96.2% (Yang, et al., 2005; Zou et al., 2004). A recent study conducted on 383 COVID‐19 patients, of whom 49 (12.8%) faced death and 334 (87.2%) were discharged from the hospital, reported thrombocytopenia as a prognostic factor of in‐hospital mortality (p < .05) and noted that the elevation in platelet counts per 50 × 109/L reduced the chance of death by 40% (hazard ratio: 0.60; 95% CI: 0.43, 0.84) (Liu et al., 2020c). Finally, while most of the reports concerning the platelet count revealed that COVID‐19 cases experienced thrombocytopenia, Chen et al. (2020b) identified thrombocytosis in a minority of patients. Although it is not yet clear how thrombocytosis may occur in this infection, one of the major suspects is proinflammatory cytokines such as IL‐1β and IL‐6 (Conti et al., 2020).
4.2. Coagulation‐based laboratory tests
On the report of Chinese published articles, viral sepsis, MOF, and DIC were found in a group of severe COVID‐19 cases (Tang et al., 2020d; Yin et al., 2020b) and were considered among the leading causes of death in these patients (Zhou et al., 2020c). In a study of 183 COVID‐19 patients, PT, aPTT, antithrombin, fibrinogen, D‐dimer, FDP were sequentially quantified during 2‐week hospitalization. While PT and aPTT were significantly longer in the nonsurvivor group compared to the survivors, the fibrinogen and antithrombin levels were sharply decreased on admission. By the late hospitalization in all deceased individuals, D‐dimer and FDP—which are the manifestation of coagulation activation, disrupted thrombin generation, defective natural anticoagulants, and fibrinolysis—were significantly increased (Tang et al., 2020d). According to recent reports, prolonged PT, aPTT, and elevated D‐Dimer levels likely occur in moderate‐to‐severe COVID‐19 patients with poorer outcomes (Henry et al., 2020a, Terpos et al., 2020). Notably, the results of a recent meta‐analysis by Bashash et al. declared that while the mean value of D‐dimer was significantly higher in severe patients as compared to nonsevere cases (χ 2 = 6.34, p = .01), alteration of PT and aPTT were not statistically significant (χ 2 = 2.69, p = .1; χ 2 = 0.01, p = .94, respectively) (Bashash et al., 2020b).
In a study performed on 5700 COVID‐19 hospitalized patients in New York City, the median level of D‐dimer was 438 ng/ml which was significantly above the normal range (0–229 ng/ml) (Richardson et al., 2020). Guan et al. (2020) also reported that 46.4% of 560 hospitalized patients with COVID‐19 showed D‐Dimer level above 500 ng/ml. In another study on 140 COVID‐19 patients with 58 severe cases, Zhang et al. (2020b) declared that increased levels of D‐dimer may be a proper marker to distinguish severe and nonsevere cases. Age and elevated D‐dimer levels (>1 mg/L) were also suggested to be two independent indicators of poor prognosis for COVID‐19 survival (Tang et al., 2020d, Wu et al., 2020a, Zhou et al., 2020c). Elevated levels of FDPs and D‐dimer were also detected mainly in patients with severe disease (Chen et al., 2020b, Guan et al., 2020, Huang et al., 2020; Wang et al., 2020b; Xu et al., 2020d). These findings are aligned with the comparability analysis of risk factors including 17 studies that reported D‐dimer, age, C‐reactive protein, albumin, body temperature, sequential organ failure assessment score, and diabetes as the most related factors to COVID‐19 severity (Rod et al., 2020). In a recent study, although increased D‐dimer was reported as the strongest distinguisher of status in 14 adult ICU COVID‐19 patients, it was not associated with death. In the mentioned study, Juneja et al. (2021) reported that D‐dimer lacks prognostic power to characterize the clinical course of patients with COVID‐19. The data obtained from several studies reporting the values of platelets and coagulation‐related laboratory tests in severe and mild COVID‐19 patients were summarized in Table 1.
Table 1.
Values of platelets and coagulation‐related laboratory tests in severe and nonsevere COVID‐19 patients
| Platelet | Prothrombin time (PT) | Partial thromboplastin time (PTT) | D‐dimer | |||||
|---|---|---|---|---|---|---|---|---|
| Nonsevere | Severe | Nonsevere | Severe | Nonsevere | Severe | Nonsevere | Severe | |
| Huang et al. (2020) | 149 (131–263) | 196 (165–263) | 10.7 (9.8–12.1) | 12.2 (11.2–13.4) | 27.7 (24.8–34.1) | 26.2 (22.5–33.9) | 0.5 (0.3–0.8) | 2.4 (0.6–14.4) |
| Chen et al. (2000a, 2000b) | 161 (±44.2) | 164 (±45.8) | 13.4 (±1.0) | 14.1 (±0.9) | 44.6 (±3.7) | 36.2 (±5.8) | 0.4 (±0.3) | 8.2 (±9.0) |
| Tang et al. (2020c) | 231 (±99) | 178 (±92) | 13.6 (13.0–14.3) | 15.5 (14.4‐16.3) | 41.2 (36.9–44) | 44.8 (40.2–51) | 0.61 (0.35–1.29) | 2.12 (0.77–5.27) |
| Wu et al. (2020c) | 10.6 (10.1–11.5) | 11.7 (11.1–12.45) | 29.7 (25.5–32.8) | 26 (22.5–35) | 0.52 (0.33–0.93) | 1.16 (0.46–5.37) | ||
| Wang et al (2020c) | 186.9 (±79.3) | 174.8 (±90.97) | 14.1 (13.7–14.6) | 14.5 (13.5–17.5) | 40.7 (±7.5) | 39.0 (±4.6) | 0.8 (0.3–1.4) | 11.3 (2.6–21) |
| Bao et al. (2020) | 251 (202–317) | 186 (103.5–249) | 12.7 (12.15–13.59) | 14.55 (13.4–16.53) | 25.9(25.11–29.33) | 29.2(26.95–32.84) | 0.42 (0.28–0.79) | 1.05 (0.68–5.90) |
| Xiong et al. (2020a) | 235 (165–266) | 188 (128–234) | 11.4 (10.8–11.8) | 11.5 (10.9–11.9) | 28.3 (24.8–31.1) | 27.8 (25.2–33.4) | 0.28 (0.18–0.38) | 0.94 (0.57–1.89) |
| Yang et al. (2020a) | 155 (125–192) | 145 (120.5–177) | 11 (10.5–11.4) | 11.4 (10.6–12.0) | 33.3 (30.1–36.2) | 36.7 (34.0–41.0) | 0.49 (0.3–0.74) | 1.10 (0.63–1.82) |
| Cao et al. (2020) | 177 (143–220) | 147 (120–179) | 13.3 (12.9–13.7) | 13.8 (13.3–14.7) | 39.1 (36.7–42.15) | 42.4 (38.2–49.5) | 0.365 (0.26–0.56) | 0.77 (0.43–1.23) |
| Zhang et al. (2020a) | 175 (136–213) | 169 (111–202) | 12.7 (12.1–13.4) | 13.4 (12.3–14.8) | 31.1 (29.1–33.0) | 31.1 (29–34.9) | 0.18 (0.11–0.32) | 0.31 (0.29–0.33) |
| Wan et al. (2020) | 170 (136–234) | 147 (118–213) | 10.8 (10.4–11.3) | 11.3 (10.7–11.8) | 26.6 (24.5–28.8) | 29.7 (26.2–39.4) | 0.3 (0.2–0.5) | 0.6 (0.4–1.1) |
| Wang et al. (2020a) | 165 (125–188) | 142 (119–202) | 12.9 (12.3–13.4) | 13.2 (12.3–14.5) | 31.7 (29.6–33.5) | 30.4 (28–33.5) | 0.16 (0.1–0.28) | 0.41 (0.19–1.32) |
| Gao et al. (2020) | 12.03 (±1.21) | 11.26 (±1.42) | 30.41 (±5.31) | 27.29 (±6.09) | 0.21 (0.19–0.27) | 0.49 (0.29–0.91) | ||
| Zheng et al. (2020) | 12.91 (±0.63) | 13.49 (±0.96) | 0.78 (±0.76) | 2.65 (±3.93) | ||||
| Zhou et al. (2020b) | 220 (168–271) | 165.5 (107–229) | 11.4 (10.4–12.6) | 12.1 (11.2–13.7) | 0.6 (0.3–1.0) | 5.2 (1.5–21.1) | ||
| Tang et al. (2020a) | 14.6 (±2.1) | 16.5 (±8.4) | 1.47 (0.78–4.16) | 4.70 (1.42–21) | ||||
| Gong et al. (2020) | 180 (147, 221) | 167 (139.5, 200) | 39.1 (±4.4) | 40 (±5.4) | 0.99 (0.6–1.38) | 1.22 (0.66–1.72) | ||
| Peng et al. (2020a) | 13 (12.5–14.1) | 13.9 (12.6–14.7) | 35.75 (31.6–40) | 36.4 (33.1–44) | ||||
| Marchandot et al. (2020) | 12.20 (±0.88) | 12.65 (±1.13) | 28.56 (±2.66) | 29.53 (±3.48) | ||||
| Yang et al. (2020b) | 164 (±74) | 191 (±63) | 10.9 (±2.7) | 12.9 (±2.9) | ||||
| Lei et al. (2020) | 192 (139–237) | 150 (116–225) | 0.28 (0.18–0.46) | 0.6 (0.28–1.4) | ||||
| Liu et al. (2020a) | 173.20 (±55.37) | 143.90 (±64.81) | 0.39 (0.2–1.07) | 0.56 (0.21–6.84) | ||||
| Zou et al. (2020) | 13.4 (13.0–13.8) | 13.8 (13.4–14.8) | 39.2 (36.3–42.4) | 43.2 (41.0–49.7) | 0.43 (0.31–0.77) | 1.04 (0.73–1.72) | ||
| Mao et al. (2020) | 219 (42–583) | 204.5 (18–576) | 0.4 (0.2–8.7) | 0.9 (0.1–20) | ||||
| Peng et al. (2020b) | 13.0 (12.5–14.1) | 13.90 (12.6–14.7) | 35.75 (31.6–40.0) | 36.45 (33.1–44.0) | ||||
| Lu et al. (2020) | 13.42 (±0.95) | 13.94 (±1.15) | 41.98 (±9.26) | 40.08 (±6.37) | 1.01 (±2.98) | 4.89 (±6.65) | ||
| Zhang et al. (2020d) | 11.5 (10.9–11.83) | 12.0 (11.75–12.8) | 26.1 (24.1–28.43) | 30.4 (24.3–34.65) | 0.44 (0.25–1.19) | 5.95 (1.23–20.08) | ||
| Long et al. (2020) | 12.34 (±1.91) | 12.14 (±1.16) | 34.9 (±9.17) | 36.47 (±9.29) | 0.85 (±1.68) | 1.78 (±4.40) | ||
| Qian et al. (2020) | 198 (144–248) | 152 (127–208) | 0.3 (0.1–0.4) | 0.45 (0.16–0.48) | ||||
Abbreviation: COVID‐19, coronavirus disease 2019.
Apart from coagulation‐based tests, Escher et al. (2020) identified anticardiolipin and anti‐β2‐GPI antibodies (immunoglobulin M) in a patient with COVID‐19 pneumonia and ARDS. They also found a noticeable elevation in both the antigen and the activity of vWF (~fourfold above the upper limit of normal) as well as an elevated level of factor VIII in a patient with COVID‐19 pneumonia and ARDS. In agreement, a number of patients in critical states have been reported to develop antiphospholipid antibodies, coagulopathy, and elevated venous and arterial thrombotic complications such as cerebral infarction (Zhang et al., 2020e). Taken together, planning of comprehensive studies aiming to determine the prognostic value of laboratory tests reflecting SARS‐CoV‐2‐induced coagulopathy, foremost D‐dimer, will definitively be beneficial to predict the severity of the disease.
5. MANAGEMENT AND TREATMENT OF COAGULATION DISORDERS IN COVID‐19
Coagulopathy is a leading cause of mortality in severe cases of COVID‐19 (Wu et al., 2020a), and DIC may occur in the majority of COVID‐19‐related deaths (Tang et al., 2020d). A shred of evidence also suggested that dysfunction in blood coagulation and thrombosis are coupled with poor prognosis and high mortality in this infection. Hence, using the active application of anticoagulants (such as heparin), at least for patients with no contraindications, seems to be essential. A summary of the most appropriate therapeutic strategies used in the treatment of COVID‐19‐related coagulopathy was represented in Figure 3 and reviewed in the next sections.
Figure 3.
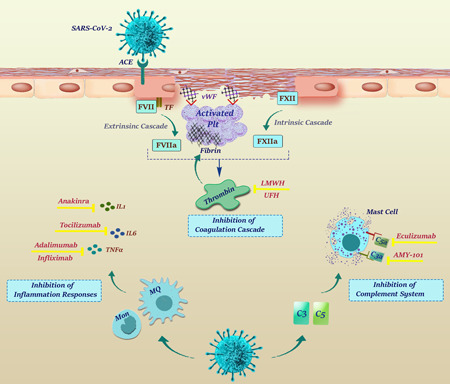
Therapeutic options for treatment of COVID‐19‐related coagulopathy. Low‐molecular‐weight heparin (LMWH) and unfractionated heparin which are considered as the first line of treatment, inhibit both extrinsic and extrinsic coagulation cascades mainly through inhibition of thrombin function. Due to the interaction of inflammation and complement activation with the coagulation pathway, anticomplement and antiinflammatory agents may be also considered as drugs of choice. Anakinra, Tocilizumab, Infliximab and Adalimumab inhibit IL‐1, IL‐6, and TNF pathways, respectively, while AMY‐101 and Eculizumab function through inhibiting C3 and C5, respectively. COVID‐19, coronavirus disease 2019; IL, interleukin; TNF, tumor necrosis factor
5.1. Heparin
The International Society of Thrombosis and Hemostasis guidelines introduced interim guidelines for coagulopathy management in COVID‐19 (Thachil et al., 2020b). They suggested that every hospitalized COVID‐19 patient would receive a prophylactic dose of low‐molecular‐weight heparin (LMWH) if there are no contraindications, such as active bleeding or platelet count lower than 25 × 109/L (Thachil et al., 2020b). Apart from anticoagulation, LMWH has antiinflammatory activities which may be useful in COVID‐19 infection where proinflammatory cytokines playing a key role in disease development (Guan et al., 2020). The British Society of Hematology has also recommended prophylactic doses of either LMWH or unfractionated heparin (UFH) in COVID‐19 (Hematology, 2020). The American Society of Hematology has suggested LMWH or fondaparinux unless the probability of hemorrhage exceeds thrombotic events. In patients with a contraindication for anticoagulation, pneumatic compression devices—that are used to hinder the formation of blood clots in the deep veins of the legs—should be applied. The followings are some of the studies performed to assess the function and the challenges of choosing the proper dose of heparin in the treatment of coagulopathy in COVID‐19.
A retrospective analysis published by Tang et al. (2020b) examined the 28‐day mortality rate in 449 COVID‐19 patients. Notably, this study revealed that the consumption of therapeutic doses of heparin for a week or more would improve the prognosis of the patients with sepsis‐induced coagulopathy, severe infection, or those with significantly elevated D‐dimer levels. In another retrospective study, Yin et al. (2020a) compared severe pneumonia‐induced coagulopathy by SARS‐CoV‐2 and non‐SARS‐CoV‐2. Although the 28‐day mortality rate in both groups was the same, SARS‐CoV2 patients with a sixfold elevation in D‐dimer achieved promising results from anticoagulation therapy (Yin et al., 2020a). Multiple studies suggested LMWH for the treatment of DIC and VTE to diminish the mortality rate in COVID‐19 (Barrett et al., 2020, Casini et al., 2020a, Cattaneo et al., 2020, Song et al., 2020, Vivas et al., 2020). Another recent study reported that prophylactic doses of LMWH are also beneficial for COVID‐19 patients who do not require ventilation (Grandone et al., 2021). In addition, guidelines from Britain recommended LMWH and VTE prophylaxis for all high‐risk patients. In an interesting publication, Barrett et al. (2020) raised awareness about patients who continue to clot even with the utilization of prophylactic doses of heparin. Although the elevated level of D‐dimer and fibrinogen have been considered to be a marker of hypercoagulable state, still low level of antithrombin which could confer resistance against heparin has not been addressed. Generally, Barrett et al. (2020) reported that UFH is a better choice for hospitalized patients if there are no contraindications. Their results indicated that the risk of bleeding would increase in patients who developed PE if LMWH is prescribed in combination with tissue plasminogen activator (tPA), further highlighting that the administration of UFH would be a better approach in these patients. The high frequency of renal failure in severe cases of COVID‐19 is another reason that they recommend UFH.
Taken together, heparin is one of the best therapeutic options for the management and treatment of coagulopathy in COVID‐19; however, the failure in the treatment of some cases suggested that the determination of the proper dose is essential. Many healthcare centers increased the therapeutic dose of LMWH to “intermediate intensity” doses such as administrating enoxaparin at the dose of 0.5 mg/kg twice a day (Connors & Levy, 2020). Based on the results of a study conducted by Bikdeli et al., (2020) while 31.6% and 5.2% responded properly to the intermediate intensity and therapeutic dose respectively, the rest of the patients achieved better results with the standard dose of VTE prophylaxis that was used for patients with moderate‐to‐severe COVID‐19 without DIC. In another cohort study, the incidences of PE and DVT in hospitalized COVID‐19 individuals being treated with LMWH were investigated. PE was reported in 12% and 1.6% of patients hospitalized in the ICU and non‐ICU ward, respectively. DVT occurred in 27% of the former and 1.6% of the latter group. In patients struggling with PE or DVT, the mortality rate was 3.3 times higher. After the release of the final results, the dose of LMWH increased to double in all patients in ICU (Middeldorp et al., 2020). In agreement, Klok et al. (2020) strongly suggested an increasing dose of LMWH, even in the absence of randomized evidence, due to the remarkably high incidence of thrombotic complications in ICU patients (31%). Thachil et al. (2020) also published an article concerning the possible need for a higher dose of LMWH in patients with extremely high D‐dimers, high BMI, ARDS, or increasing oxygen requirements. Additionally, Savla et al. (2021) recommended a prophylactic dose of 5000–7500 units of LMWH for hospitalized COVID‐19 patients to prevent VTE. Inline, recent recommendations published by the Swiss Society of Hematology declared that some factors such as the patients' BMI, D‐dimer levels along with the establishment of respiratory, hepatic, or renal failure should be considered for dose adjustment (Casini et al., 2020b). They reported that the elevated dose of UFH should be administrated for patients with the creatinine clearance less than 30 ml/min. If the patient has a creatinine clearance more than 30 ml/min weighted more than 100 kg, the dose of LMWH should be escalated, as well. According to their recommendations, the dosage of anticoagulation should be increased for patients with elevated D‐dimer levels or those who are at risk of respiratory, hepatic, or renal failure (Casini et al., 2020b). Finally, a recent study conducted by Chistolini et al. (2020) pointed out the advantageous use of a higher dose of LMWH. In this study which included 27 patients admitted to ICU, 14 patients (51.9%) were treated with low‐dose LMWH (100 IU/kg/day) and 13 (48.1%) were treated with high‐dose LMWH (100 IU/kg/twice daily). The results demonstrated that the use of a higher dose of LMWH, as thromboembolic prophylaxis, reduced the incidence of thrombotic complications without an increase in bleeding events. Although heparin is considered as the first‐line treatment of coagulopathy‐related complications in several studies, its prescription should be done with caution as multiple factors play a crucial role in designating the proper dose.
5.2. Other therapeutic options
Thachil et al. (2020a) studied the administration of oral anticoagulants and their implementation as a treatment strategy in COVID‐19. The results noted that it would be beneficial to transfer patients currently using vitamin K antagonists (VKA) to a direct‐acting oral anticoagulant (DOAC) since they do not need laboratory monitoring. Generally speaking, DOAC is safer than VKA in terms of the incidence of intracranial bleeding; however, a detailed caution should be taken into account for hospitalized patients on a DOAC due to the possible intervention of the agent with anti‐retroviral drugs and the necessity of close monitoring in patients with kidney malfunction (Testa et al., 2020a). DOACs are partially metabolized through the Cytochrome P450 and P‐glycoprotein metabolic pathways that make them a target for multiple drug interactions (Aly et al., 2021). Indeed, antiviral drugs such as remedesvir are the substrate of CYP 3A4, CYP 2D6, and CYP 2C8, and dexamethasone is also an inducer of CYP3A4. These drugs cause drug–drug interactions, and subsequently, alter the pharmacodynamics and pharmacokinetic profile of DOAC. Indeed, it leads to unstable and unpredictable DOAC anticoagulant effects and exposes patients to the risk of uncontrolled bleeding and thrombotic complications (Schutgens, 2020). In agreement, Testa et al. (2020b) believe that consumption of DOAC should be withheld in COVID‐19 patients treated with antiviral agents (lopinavir, ritonavir, or darunavir) due to an increase of C‐trough DOAC level.
Thrombomodulin (TM) acts as a cofactor in the thrombin‐induced activation of proteins C and S in the anticoagulant pathway thus inhibiting blood coagulation via inhibition of activated factors V and VIII. In this regard, the results of three clinical trials indicated that administration of the intravenous recombinant human TM was successful in diminishing the mortality of COVID‐19 patients with DIC (Valeriani et al., 2020). Another potential therapy reported by Asakura & Ogawa, (2020) is the combination of heparin with a serine protease inhibitor named Nafamostat which suppresses proteolytic enzymes including plasmin, trypsin, or thrombin. They showed that this agent may potentiate the efficacy of heparin in COVID‐19 patients with coagulopathy.
Another promising option for the treatment of severely ill COVID‐19 patients is the antiplatelet agent dipyridamole which acts as a phosphodiesterase inhibitor and elevates intracellular cyclic adenosine monophosphate (cAMP)/cyclic guanosine monophosphate (cGMP). Notably, it can also bind to SARS‐CoV‐2 protease Mpro, thereby suppressing viral replication in vitro. The result of a clinical trial involving 31 patients showed a remarkable decrease in D‐dimers levels, increased lymphocytes, platelet recovery in the circulation, and significantly improved clinical outcomes after receiving dipyridamole. Seven out of eight critically ill patients (87.5%) successfully responded to the remedy and were discharged from the hospitals (Liu et al., 2020b). In a study performed by Viecca et al., (2020) the efficacy of antiplatelet therapy has been also evaluated on the outcome of severe COVID‐19 patients who suffered from hypercoagulability. Acetylsalicylic acid and oral clopidogrel were given to five patients for 30 days and then patients underwent the treatment with 25 μg/kg/body weight tirofiban followed by a continuous infusion of 0.15 μg/kg/body weight per minute for 48 h. During hospitalization, subcutaneous Fondaparinux (2.5 mg/day) was given to patients. Prophylactic or therapeutic doses of heparin also were given to the control group. After receiving the antiplatelet agents, the admission of continuous positive airway pressure was stopped in all patients after 3 days except one. This was not true for the control group, showing that the antiplatelet therapy might be effective in alleviating respiratory complications in COVID‐19 patients with severe respiratory failure.
One of the raised hypotheses about the cause of lung injury and adverse symptomology of breathing is probably the devastating effects of SARS‐CoV‐2 on multiple fibrinolysis pathways (Kwaan, 2020a). As a result of the coagulation system activation, local fibrins may accumulate due to the repression of the fibrinolysis system (Bastarache et al., 2006). Therefore, the administration of tPA may be beneficial in patients with COVID‐19 who suffered from ARDS (Wang et al., 2020d); however, the bleeding risk in patients is an important issue that should be taken seriously when tPA is administrated. Compromised immune system due to the reduction of T and B cells together with acute thrombocytopenic disorders including heparin‐induced thrombocytopenia or immune thrombocytopenic purpura are frequent findings in COVID‐19. Hence, intravenous immunoglobulin G can be considered as a possible therapeutic option for the treatment of COVID‐19‐induced coagulation disorders. Lin et al. (2020) reported that the combination of 0.5 g/kg/body weight immunoglobulin G with LMWH given to patients for 5 days led to a constant decrease in the D‐dimer level. Taken together, since both inflammation and complement activation could result in the initiation of the coagulation cascade, administration of antiinflammatory agents and complement inhibitors—in addition to anticoagulants as the main therapeutic options—seems promising for the treatment of COVID‐19‐related coagulopathy (Table 2).
Table 2.
Therapeutic options for the treatment of COVID‐19‐induced coagulopathy
| Type of drug | Name of drug | Description | Reference |
|---|---|---|---|
| Main therapy | |||
| Heparin | Dalteparin, Enoxaparin, Nadroparin, Tinzaparin, UFH | Main treatment for coagulation complications such as DIC, VTE, and PE. | (Barrett et al., 2020, C., 2020, Casini et al., 2020a, Cattaneo, et al., 2020, Song et al., 2020, Vivas et al., 2020) |
| Alternative therapies | |||
| Direct‐acting oral anticoagulant | Apixaban, Dabigatran, Rivaroxaban, Edoxaban | Excluding the laboratory tests for monitoring. | (Testa et al., 2020a; Thachil et al., 2020a) |
| Immunoglobulin | IVIg | In combination with LMWH decreases D‐dimers as well as B and T cell counts. | (Lin et al., 2020) |
| Activator of fibrinolysis | tPA | Functional in treatment of ARDS in COVID‐19 patients. | (Wang, et al., 2020d) |
| Thrombomodulin | rhsTM | Reducing mortality rate in COVID‐19 patients with DIC. | (Valeriani et al., 2020) |
| Serine protease inhibitor | Nafamosat Mesylate | Potentiating the efficacy of heparin in COVID‐19 patients with coagulopathy. | (Asakura & Ogawa, 2020) |
| Nucleoside transport & PDE3 inhibitor | Dipyridamole (DIP) | Suppressing viral replication and act as an antiplatelet inhibitor. | (Liu et al., 2020b) |
| Antiplatelet agents | Tirofiban, Clopidogrel | Alleviating complications in COVID‐19 cases with severe respiratory failure. | (Viecca et al., 2020) |
| Immunosuppressive agents | Hydroxychloroquine | Exerts antithrombotic properties, especially against antiphospholipid antibodies. | (Bikdeli et al., 2020) |
| Immunomodulating agents | Fingolimod | Improving outcomes in patients suffering from acute ischemic stroke. | (Zhu et al., 2015) |
| Antiinflammatory agents | Tocilizumab | Inhibiting IL‐6 pathway and complement cascade activation. | (Xu et al., 2020c) |
| Anakinra (IL‐1Ra) | Inhibiting IL‐1, thereby reversing cytokine storm in patients with COVID‐19. | (Monteagudo et al., 2020) | |
| Infliximab, Adalimumab | Inhibiting TNF, thereby reducing inflammation in COVID‐19 patients. | (Feldmann et al., 2020) | |
| Eculizumab | Preventing coagulation and hyper‐inflammation via inhibiting C5 complement. | (Diurno et al., 2020) | |
| AMY‐101 | Treating severe ARDS due to COVID‐19 pneumonia by inhibiting C3. | (Mastaglio et al., 2020) | |
| Platelet aggregation inhibitor | Ticagrelor | Preventing SIC in COVID‐19 via inhibiting P2Y12 receptor. | (Omarjee et al., 2020) |
| Corticosteroid | Methylprednisolone | Reducing the risk of death in COVID‐19 patients with ARDS. | (Wu et al., 2020b) |
Abbreviations: ARDS, acute respiratory distress syndrome; COVID‐19, coronavirus disease 2019; IL, interleukin.
6. CONCLUSION AND FUTURE PERSPECTIVE
The systemic inflammatory response, platelet activation, and endothelial dysfunction all and all go hand in hand to disturb the tune balance between the pro‐ and anticoagulant pathways and orchestrate a catastrophic event that makes the disease more complicated. Indeed, the participation of a wide range of molecules in the propagation of coagulation complications and our minimal information about the molecular mechanisms evolving this process put serious obstacles in the way of successful management and treatment of COVID‐19‐associated coagulopathy. Thus far, laboratory monitoring of coagulopathy parameters (i.e., D‐dimer, fibrinogen, platelet count, and coagulation‐based tests) undeniably assists clinicians to better stratify COVID‐19 patients due to the risk of thrombosis. Concerning the therapeutic approaches, thromboprophylaxis with LMWH is one of the main treatment strategies that is widely prescribed for patients with a high risk of thrombosis; however, like other kinds of thrombotic disorders, this anticoagulant prophylaxis should also be personalized on the authority of patients' profile. While many unanswered questions remain, clinical and laboratory data arising from ongoing clinical trials will definitively aid us to better optimize the management of coagulopathy in COVID‐19.
CONFLICT OF INTERESTS
The authors declare that there are no conflict of interests.
ACKNOWLEDGMENTS
The authors would like to express their gratitude to Shahid Beheshti University of Medical Sciences (Tehran, Iran) for supporting this study.
Kohansal Vajari, M. , Shirin, M. , Pourbagheri‐Sigaroodi, A. , Akbari, M. E. , Abolghasemi, H. , & Bashash, D. (2021). COVID‐19‐related coagulopathy: A review of pathophysiology and pharmaceutical management. Cell Biol Int, 45, 1832–1850. 10.1002/cbin.11623
DATA AVAILABILITY STATEMENT
Data sharing not applicable to this article as no datasets were generated or analyzed during the current study.
REFERENCES
- Bashash, D. , Hosseini‐Baharanchi, F. S. , Rezaie‐Tavirani, M. , Safa, M. , Akbari Dilmaghani, N. , Faranoush, M. , & Abolghasemi, H. (2020a). The Prognostic value of thrombocytopenia in COVID‐19 patients: A systematic review and meta‐analysis. Archives of Academic Emergency Medicine, 8(1), e75. [PMC free article] [PubMed] [Google Scholar]
- Bashash, D. , Abolghasemi, H. , Salari, S. , Olfatifar, M. , Eshghi, P. , & Akbari, M. E. (2020b). Elevation of D‐Dimer, But Not PT and aPTT, Reflects the Progression of COVID‐19 Toward an Unfavorable Outcome: A Meta‐Analysis. Iranian Journal of Blood and Cancer, 12(2), 47‐53. [Google Scholar]
- Al‐Ani, F. , Chehade, S. , & Lazo‐Langner, A. (2020). Thrombosis risk associated with COVID‐19 infection. A scoping review. Thrombosis Research, 192, 152–160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aly, R. , Gupta, S. , Singh, B. , Kaur, P. , Kim, K. , & Gupta, S. (2021). The use of direct acting oral anticoagulants in patients with COVID‐19 infection. Journal of Community Hospital Internal Medicine Perspectives, 11, 184–186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Asakura, H. , & Ogawa, H. (2020). Potential of heparin and nafamostat combination therapy for COVID‐19. Journal of thrombosis and haemostasis: JTH, 18, 1521–1522. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Assinger, A. (2014). Platelets and infection–an emerging role of platelets in viral infection. Frontiers in Immunology, 5, 649. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bajic, G. , Degn, S. E. , Thiel, S. , & Andersen, G. R. (2015). Complement activation, regulation, and molecular basis for complement‐related diseases. The EMBO Journal, 34, 2735–2757. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bao, C. , Tao, X. , Cui, W. , Yi, B. , Pan, T. , Young, K. H. , & Qian, W. (2020). SARS‐CoV‐2 induced thrombocytopenia as an important biomarker significantly correlated with abnormal coagulation function, increased intravascular blood clot risk and mortality in COVID‐19 patients. Experimental Hematology & Oncology, 9, 16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barrett, C. D. , Moore, H. B. , Yaffe, M. B. , & Moore, E. E. (2020). ISTH interim guidance on recognition and management of coagulopathy in COVID‐19: A comment. Journal of Thrombosis and Haemostasis: JTH, 18, 2060–2063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bastarache, J. A. , Ware, L. B. , & Bernard, G. R. (2006). The role of the coagulation cascade in the continuum of sepsis and acute lung injury and acute respiratory distress syndrome. Seminars in respiratory and critical care medicine, 27, 365–376. [DOI] [PubMed] [Google Scholar]
- Becker, R. C. (2020). COVID‐19 update: Covid‐19‐associated coagulopathy. Journal of Thrombosis and Thrombolysis, 50, 1–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bikdeli, B. , Madhavan, M. V. , Jimenez, D. , Chuich, T. , Dreyfus, I. , Driggin, E. , Nigoghossian, C. , Ageno, W. , Madjid, M. , Guo, Y. , Tang, L. V. , Hu, Y. , Giri, J. , Cushman, M. , Quéré, I. , Dimakakos, E. P. , Gibson, C. M. , Lippi, G. , Favaloro, E. J. Global COVID‐ Thrombosis Collaborative Group, Endorsed by the ISTH, NATF, ESVM, and the IUA, Supported by the ESC Working Group on Pulmonary Circulation and Right Ventricular, F. (2020). COVID‐19 and thrombotic or thromboembolic disease: Implications for prevention, antithrombotic therapy, and follow‐up: JACC state‐of‐the‐art review. Journal of the American College of Cardiology, 75, 2950–2973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Campbell, C. M. , & Kahwash, R. (2020). Will complement inhibition be the new target in treating COVID‐19‐related systemic thrombosis? Circulation, 141, 1739–1741. [DOI] [PubMed] [Google Scholar]
- Canzano, P. , Brambilla, M. , Porro, B. , Cosentino, N. , Tortorici, E. , Vicini, S. , Poggio, P. , Cascella, A. , Pengo, M. F. , Veglia, F. , Fiorelli, S. , Bonomi, A. , Cavalca, V. , Trabattoni, D. , Andreini, D. , Omodeo Salè, E. , Parati, G. , Tremoli, E. , & Camera, M. (2021). Platelet and endothelial activation as potential mechanisms behind the thrombotic complications of COVID‐19 patients. JACC Basic to translational science, 6, 202–218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cao, M. , Zhang, D. , Wang, Y. , Lu, Y. , Zhu, X. , Li, Y. , Xue, H. , Lin, Y. , Zhang, M. , Sun, Y. , Yang, Z. , Shi, J. , Wang, Y. , Zhou, C. , Dong, Y. , Liu, P. , Dudek, S. M. , Xiao, Z. , Lu, H. , & Peng, L. (2020). Clinical features of patients infected with the 2019 novel coronavirus (COVID‐19) in Shanghai. China. MedRxiv. [Google Scholar]
- Casini, A. , Alberio, L. , Angelillo‐Scherrer, A. , Fontana, P. , Gerber, B. , Graf, L. , et al. (2020b). Suggestions for thromboprophylaxis and laboratory monitoring for in‐hospital patients with COVID‐19. Swiss Medical Weekly, 150, w20247. [DOI] [PubMed] [Google Scholar]
- Casini, A. , Alberio, L. , Angelillo‐Scherrer, A. , Fontana, P. , Gerber, B. , Graf, L. , Hegemann, I. , Korte, W. , Kremer Hovinga, J. , Lecompte, T. , Martinez, M. , Nagler, M. , Studt, J. D. , Tsakiris, D. , Wuillemin, W. , & Asmis, L. (2020a). Thromboprophylaxis and laboratory monitoring for in‐hospital patients with COVID‐19—A Swiss consensus statement by the Working Party Hemostasis. Swiss Medical Weekly, 150, w20247. [DOI] [PubMed] [Google Scholar]
- Cattaneo, M. , Bertinato, E. M. , Birocchi, S. , Brizio, C. , Malavolta, D. , Manzoni, M. , Muscarella, G. , & Orlandi, M. (2020). Pulmonary embolism or pulmonary thrombosis in COVID‐19? Is the recommendation to use high‐dose heparin for thromboprophylaxis justified? Thrombosis and Haemostasis, 120, 1230–1232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Channappanavar, R. , Fehr, A. R. , Vijay, R. , Mack, M. , Zhao, J. , Meyerholz, D. K. , & Perlman, S. (2016). Dysregulated Type I interferon and inflammatory monocyte‐macrophage responses cause lethal pneumonia in SARS‐CoV‐infected mice. Cell Host & Microbe, 19, 181–193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen, G. , Wu, D. , & Guo, W. (2020a). Clinical and immunologic features in severe and moderate forms of Coronavirus Disease 2019. Journal of Clinical Investigation, 130(5), 2620‐2629. 10.1172/JCI137244 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen, J. , Lau, Y. F. , Lamirande, E. W. , Paddock, C. D. , Bartlett, J. H. , Zaki, S. R. , & Subbarao, K. (2010). Cellular immune responses to severe acute respiratory syndrome coronavirus (SARS‐CoV) infection in senescent BALB/c mice: CD4+ T cells are important in control of SARS‐CoV infection. Journal of Virology, 84, 1289–1301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen, N. , Zhou, M. , Dong, X. , Qu, J. , Gong, F. , Han, Y. , Qiu, Y. , Wang, J. , Liu, Y. , Wei, Y. , Xia, J. , Yu, T. , Zhang, X. , & Zhang, L. (2020b). Epidemiological and clinical characteristics of 99 cases of 2019 novel coronavirus pneumonia in Wuhan, China: A descriptive study. Lancet (London, England), 395, 507–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chistolini, A. , Ruberto, F. , Alessandri, F. , Santoro, C. , Barone, F. , Cristina Puzzolo, M. , Ceccarelli, G. , De Luca, M. L. , Mancone, M. , Alvaro, D. , Pulcinelli, F. M. , Martelli, M. , Foà, R. , Pugliese, F. , & Policlinico Umberto I COVID‐, G. (2020). Effect of low or high doses of low‐molecular‐weight heparin on thrombin generation and other haemostasis parameters in critically ill patients with COVID‐19. British Journal of Haematology, 190, e214‐e218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ciceri, F. , Beretta, L. , Scandroglio, A. M. , Colombo, S. , Landoni, G. , Ruggeri, A. , Peccatori, J. , D'Angelo, A. , De Cobelli, F. , Rovere‐Querini, P. , Tresoldi, M. , Dagna, L. , & Zangrillo, A. (2020). Microvascular COVID‐19 lung vessels obstructive thromboinflammatory syndrome (MicroCLOTS): An atypical acute respiratory distress syndrome working hypothesis. Critical Care and Resuscitation: Journal of the Australasian Academy of Critical Care Medicine, 22, 95–97. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Connors, J. M. , & Levy, J. H. (2020). COVID‐19 and its implications for thrombosis and anticoagulation. Blood, 135, 2033–2040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Conti, P. , Ronconi, G. , Caraffa, A. , Gallenga, C. E. , Ross, R. , Frydas, I. , & Kritas, S. K. (2020). Induction of pro‐inflammatory cytokines (IL‐1 and IL‐6) and lung inflammation by Coronavirus‐19 (COVI‐19 or SARS‐CoV‐2): Anti‐inflammatory strategies. Journal of Biological Regulators and Homeostatic Agents, 34, 34–331. [DOI] [PubMed] [Google Scholar]
- Corrigan, D. , Prucnal, C. , & Kabrhel, C. (2016). Pulmonary embolism: The diagnosis, risk‐stratification, treatment and disposition of emergency department patients. Clinical and Experimental Emergency Medicine, 3, 117–25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cui, S. , Chen, S. , Li, X. , Liu, S. , & Wang, F. (2020). Prevalence of venous thromboembolism in patients with severe novel coronavirus pneumonia. Journal of Thrombosis and Haemostasis: JTH, 18, 1421–1424. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Danzi, G. B. , Loffi, M. , Galeazzi, G. , & Gherbesi, E. (2020). Acute pulmonary embolism and COVID‐19 pneumonia: a random association? European Heart Journal, 41, 1858. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Diurno, F. , Numis, F. G. , Porta, G. , Cirillo, F. , Maddaluno, S. , Ragozzino, A. , De Negri, P. , Di Gennaro, C. , Pagano, A. , Allegorico, E. , Bressy, L. , Bosso, G. , Ferrara, A. , Serra, C. , Montisci, A. , D'Amico, M. , Schiano Lo Morello, S. , Di Costanzo, G. , Tucci, A. G. , … Facchini, G. (2020). Eculizumab treatment in patients with COVID‐19: Preliminary results from real life ASL Napoli 2 Nord experience. European Review for Medical and Pharmacological Sciences, 24, 4040–4047. [DOI] [PubMed] [Google Scholar]
- Escher, R. , Breakey, N. , & Lämmle, B. (2020). Severe COVID‐19 infection associated with endothelial activation. Thrombosis Research, 190, 62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Esmon, C. T. (2005). The interactions between inflammation and coagulation. British Journal of Haematology, 131, 417–430. [DOI] [PubMed] [Google Scholar]
- Feldmann, M. , Maini, R. N. , Woody, J. N. , Holgate, S. T. , Winter, G. , Rowland, M. , Richards, D. , & Hussell, T. (2020). Trials of anti‐tumour necrosis factor therapy for COVID‐19 are urgently needed. Lancet (London, England), 395, 1407–1409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fischetti, F. , & Tedesco, F. (2006). Cross‐talk between the complement system and endothelial cells in physiologic conditions and in vascular diseases. Autoimmunity, 39, 417–428. [DOI] [PubMed] [Google Scholar]
- Fox, S. E. , Akmatbekov, A. , Harbert, J. L. , Li, G. , Brown, J. Q. , Vander, & Heide, R. S. (2020). Pulmonary and cardiac pathology in African American patients with COVID‐19: An autopsy series from New Orleans. The Lancet Respiratory Medicine, 8, 681–686. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gao, Y. , Li, T. , Han, M. , Li, X. , Wu, D. , Xu, Y. , Zhu, Y. , Liu, Y. , Wang, X. , & Wang, L. (2020). Diagnostic utility of clinical laboratory data determinations for patients with the severe COVID‐19. Journal of Medical Virology, 92, 791–796. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gattinoni, L. , Chiumello, D. , & Rossi, S. (2020). COVID‐19 pneumonia: ARDS or not? Critical care (London, England), 24, 154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Giannis, D. , Ziogas, I. A. , & Gianni, P. (2020). Coagulation disorders in coronavirus infected patients: COVID‐19, SARS‐CoV‐1, MERS‐CoV and lessons from the past. Journal of Clinical Virology: The Official Publication of the Pan American Society for Clinical Virology, 127, 104362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gong, J. , Ou, J. , Qiu, X. , Jie, Y. , Chen, Y. , Yuan, L. , Cao, J. , Tan, M. , Xu, W. , Zheng, F. , Shi, Y. , & Hu, B. (2020). A tool to early predict severe corona virus disease 2019 (COVID‐19): A multicenter study using the risk nomogram in Wuhan and Guangdong, China. Clinical Infectious Diseases, 71, 833–840. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gralinski, L. E. , & Baric, R. S. (2015). Molecular pathology of emerging coronavirus infections. The Journal of Pathology, 235, 185–195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gralinski, L. E. , Sheahan, T. P. , Morrison, T. E. , Menachery, V. D. , Jensen, K. , Leist, S. R. , Whitmore, A. , Heise, M. T. , & Baric, R. S. (2018). Complement activation contributes to severe acute respiratory syndrome coronavirus pathogenesis. mBio, 9, 9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grandone, E. , Tiscia, G. , Pesavento, R. , De Laurenzo, A. , Ceccato, D. , Sartori, M. T. , Mirabella, L. , Cinnella, G. , Mastroianno, M. , Dalfino, L. , Colaizzo, D. , Vettor, R. , Intrieri, M. , Ostuni, A. , & Margaglione, M. , CSS‐COVID . (2021). Use of low‐molecular weight heparin, transfusion and mortality in COVID‐19 patients not requiring ventilation. Journal of Thrombosis and Thrombolysis, 1–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guan, W. J. , Ni, Z. Y. , Hu, Y. , Liang, W. H. , Ou, C. Q. , He, J. X. , Liu, L. , Shan, H. , Lei, C. L. , Hui, D. , Du, B. , Li, L. J. , Zeng, G. , Yuen, K. Y. , Chen, R. C. , Tang, C. L. , Wang, T. , Chen, P. Y. , & Xiang, J. , China Medical Treatment Expert Group for Covid‐19 . (2020). Clinical characteristics of coronavirus disease 2019 in China. The New England Journal of Medicine, 382, 1708–1720. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gupta, N. , Zhao, Y. Y. , & Evans, C. E. (2019). The stimulation of thrombosis by hypoxia. Thrombosis Research, 181, 77–83. [DOI] [PubMed] [Google Scholar]
- Han, M. , Yan, W. , Huang, Y. , Yao, H. , Wang, Z. , Xi, D. , Li, W. , Zhou, Y. , Hou, J. , Luo, X. , & Ning, Q. (2008). The nucleocapsid protein of SARS‐CoV induces transcription of hfgl2 prothrombinase gene dependent on C/EBP alpha. Journal of Biochemistry, 144, 51–62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- He, W. Q. , Chen, S. B. , Liu, X. Q. , Li, Y. M. , Xiao, Z. L. , & Zhong, N. S. (2003). [Death risk factors of severe acute respiratory syndrome with acute respiratory distress syndrome]. Zhongguo wei zhong bing ji jiu yi xue = Chinese critical care medicine = Zhongguo weizhongbing jijiuyixue, 15, 336–337. [PubMed] [Google Scholar]
- Henry, B. M. , de Oliveira, M. H. S. , Benoit, S. , Plebani, M. , & Lippi, G. (2020a). Hematologic, biochemical and immune biomarker abnormalities associated with severe illness and mortality in coronavirus disease 2019 (COVID‐19): a meta‐analysis. Clinical Chemistry and Laboratory Medicine, 58, 1021–1028. [DOI] [PubMed] [Google Scholar]
- Henry, B. M. , Vikse, J. , Benoit, S. , Favaloro, E. J. , & Lippi, G. (2020b). Hyperinflammation and derangement of renin‐angiotensin‐aldosterone system in COVID‐19: A novel hypothesis for clinically suspected hypercoagulopathy and microvascular immunothrombosis. Clinica Chimica Acta; International Journal of Clinical Chemistry, 507, 167–173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hirsch, J. S. , Ng, J. H. , Ross, D. W. , Sharma, P. , Shah, H. H. , Barnett, R. L. , Hazzan, A. D. , Fishbane, S. , Jhaveri, K. D. , Northwell COVID‐ Research, C. , & Northwell Nephrology COVID‐ Research, C. (2020). Acute kidney injury in patients hospitalized with COVID‐19. Kidney International, 98, 209–218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hottz, E. D. , Azevedo‐Quintanilha, I. G. , Palhinha, L. , Teixeira, L. , Barreto, E. A. , Pão, C. R. R. , Righy, C. , Franco, S. , Souza, T. , Kurtz, P. , Bozza, F. A. , & Bozza, P. T. (2020). Platelet activation and platelet‐monocyte aggregate formation trigger tissue factor expression in patients with severe COVID‐19. Blood, 136, 1330–1341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang, C. , Wang, Y. , Li, X. , Ren, L. , Zhao, J. , Hu, Y. , Zhang, L. , Fan, G. , Xu, J. , Gu, X. , Cheng, Z. , Yu, T. , Xia, J. , Wei, Y. , Wu, W. , Xie, X. , Yin, W. , Li, H. , Liu, M. , … Cao, B. (2020). Clinical features of patients infected with 2019 novel coronavirus in Wuhan, China. Lancet (London, England), 395, 497–506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Iba, T. , Levy, J. H. , Levi, M. , & Thachil, J. (2020). Coagulopathy in COVID‐19. Journal of Thrombosis and Haemostasis, 18, 2103–2109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jensen, S. , & Thomsen, A. R. (2012). Sensing of RNA viruses: A review of innate immune receptors involved in recognizing RNA virus invasion. Journal of Virology, 86, 2900–2910. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Juneja, G. K. , Castelo, M. , Yeh, C. H. , Cerroni, S. E. , Hansen, B. E. , Chessum, J. E. , Abraham, J. , Cani, E. , Dwivedi, D. J. , Fraser, D. D. , Slessarev, M. , Martin, C. , McGilvray, S. , Gross, P. L. , Liaw, P. C. , Weitz, J. I. , Kim, P. Y. (2021). Biomarkers of coagulation, endothelial function, and fibrinolysis in critically‐ill patients with COVID‐19: A single‐centre prospective longitudinal study. Journal of thrombosis and haemostasis. 10.1111/jth.15327 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Keragala, C. B. , Draxler, D. F. , McQuilten, Z. K. , & Medcalf, R. L. (2018). Haemostasis and innate immunity—A complementary relationship: A review of the intricate relationship between coagulation and complement pathways. British Journal of Haematology, 180, 782–798. [DOI] [PubMed] [Google Scholar]
- Khan, A. , Benthin, C. , Zeno, B. , Albertson, T. E. , Boyd, J. , Christie, J. D. , Hall, R. , Poirier, G. , Ronco, J. J. , Tidswell, M. , Hardes, K. , Powley, W. M. , Wright, T. J. , Siederer, S. K. , Fairman, D. A. , Lipson, D. A. , Bayliffe, A. I. , & Lazaar, A. L. (2017). A pilot clinical trial of recombinant human angiotensin‐converting enzyme 2 in acute respiratory distress syndrome. Critical care (London, England), 21, 234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim, E. S. , Chin, B. S. , Kang, C. K. , Kim, N. J. , Kang, Y. M. , Choi, J. P. , Oh, D. H. , Kim, J. H. , Koh, B. , Kim, S. E. , Yun, N. R. , Lee, J. H. , Kim, J. Y. , Kim, Y. , Bang, J. H. , Song, K. H. , Kim, H. B. , Chung, K. H. , Oh, M. D. Korea National Committee for Clinical Management of, C. (2020). Clinical course and outcomes of patients with severe acute respiratory syndrome coronavirus 2 infection: a preliminary report of the first 28 patients from the korean cohort study on COVID‐19. Journal of Korean Medical Science, 35, e142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klok, F. A. , Kruip, M. , van der Meer, N. J. M. , Arbous, M. S. , Gommers, D. , Kant, K. M. , Kaptein, F. , van Paassen, J. , Stals, M. , Huisman, M. V. , & Endeman, H. (2020). Incidence of thrombotic complications in critically ill ICU patients with COVID‐19. Thrombosis Research, 191, 145–147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kolaczkowska, E. , Jenne, C. N. , Surewaard, B. G. , Thanabalasuriar, A. , Lee, W. Y. , Sanz, M. J. , Mowen, K. , Opdenakker, G. , & Kubes, P. (2015). Molecular mechanisms of NET formation and degradation revealed by intravital imaging in the liver vasculature. Nature Communications, 6, 6673. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kwaan, H. C. (2020a). Coronavirus disease 2019: The role of the fibrinolytic system from transmission to organ injury and sequelae. Seminars in Thrombosis and Hemostasis: Thieme Medical Publishers, 46, 841–844. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kwaan, H. C. (2020b). Coronavirus disease 2019: The role of the fibrinolytic system from transmission to organ injury and sequelae. Seminars in Thrombosis and Hemostasis, 46, 841–844. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lei, L. , Jian‐Ya, G. , Wan‐mei, H. , Xian‐xiang, Z. , Lian, G. , Chun‐qiu, L. , Yue‐wu, T. , Chun‐hui, L. , Fang‐zheng, M. , Fang‐zheng, M. , Zheng‐jun, Y. , Qin‐qin, P. , Kai, S. , Jiang‐lin, X. , & Jiang‐feng, X. (2020). Clinical characteristics of 51 patients discharged from hospital with COVID‐19 in Chongqing. China. medRxiv. 20025536. 10.1101/2020.02.20.20025536 [DOI] [Google Scholar]
- Levi, M. , & Poll, T. (2015). Coagulation in patients with severe sepsis. Seminars in Thrombosis and Hemostasis, 41, 9–15. [DOI] [PubMed] [Google Scholar]
- Li, S. W. , Wang, C. Y. , Jou, Y. J. , Huang, S. H. , Hsiao, L. H. , Wan, L. , Lin, Y. J. , Kung, S. H. , & Lin, C. W. (2016). SARS coronavirus papain‐like protease inhibits the TLR7 signaling pathway through removing Lys63‐linked polyubiquitination of TRAF3 and TRAF6. International Journal of Molecular Sciences, 17, 17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lim, W. , Meade, M. , Lauzier, F. , Zarychanski, R. , Mehta, S. , Lamontagne, F. , Dodek, P. , McIntyre, L. , Hall, R. , Heels‐Ansdell, D. , Fowler, R. , Pai, M. , Guyatt, G. , Crowther, M. A. , Warkentin, T. E. , Devereaux, P. J. , Walter, S. D. , Muscedere, J. , Herridge, M. , … Cook, D. J. , PROphylaxis for ThromboEmbolism in Critical Care Trial Investigators . (2015). Failure of anticoagulant thromboprophylaxis: Risk factors in medical‐surgical critically ill patients*. Critical Care Medicine, 43, 401–410. [DOI] [PubMed] [Google Scholar]
- Lin, L. , Lu, L. , Cao, W. , & Li, T. (2020). Hypothesis for potential pathogenesis of SARS‐CoV‐2 infection‐a review of immune changes in patients with viral pneumonia. Emerging Microbes & Infections, 9, 727–732. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lippi, G. , & Plebani, M. (2020). The critical role of laboratory medicine during coronavirus disease 2019 (COVID‐19) and other viral outbreaks. Clinical Chemistry and Laboratory Medicine, 58, 1063–1069. [DOI] [PubMed] [Google Scholar]
- Lippi, G. , Plebani, M. , & Henry, B. M. (2020). Thrombocytopenia is associated with severe coronavirus disease 2019 (COVID‐19) infections: A meta‐analysis. Clinica Chimica Acta; International Journal of Clinical Chemistry, 506, 145–148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu, W. , Tao, Z.‐W. , Wang, L. , Yuan, M. L. , Liu, K. , Zhou, L. , Wei, S. , Deng, Y. , Liu, J. , Liu, H. G. , Yang, M. , & Hu, Y. (2020a). Analysis of factors associated with disease outcomes in hospitalized patients with 2019 novel coronavirus disease. Chinese Medical Journal, 133, 1032–1038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu, X. , Li, Z. , Liu, S. , Sun, J. , Chen, Z. , Jiang, M. , Zhang, Q. , Wei, Y. , Wang, X. , Huang, Y. Y. , Shi, Y. , Xu, Y. , Xian, H. , Bai, F. , Ou, C. , Xiong, B. , Lew, A. M. , Cui, J. , Fang, R. , … Luo, H. B. (2020b). Potential therapeutic effects of dipyridamole in the severely ill patients with COVID‐19. Acta Pharmaceutica Sinica B, 10, 1205–1215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu, Y. , Sun, W. , Guo, Y. , Chen, L. , Zhang, L. , Zhao, S. , et al. (2020c). Association between platelet parameters and mortality in coronavirus disease 2019: Retrospective cohort study. Platelets, 31, 490–496. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu, Y. , Yang, Y. , Zhang, C. , Huang, F. , Wang, F. , Yuan, J. , et al. (2020e). Clinical and biochemical indexes from 2019‐nCoV infected patients linked to viral loads and lung injury. Science China Life Sciences, 63, 364–374. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lodigiani, C. , Iapichino, G. , Carenzo, L. , Cecconi, M. , Ferrazzi, P. , Sebastian, T. , Kucher, N. , Studt, J. D. , Sacco, C. , Bertuzzi, A. , Sandri, M. T. , Barco, S. , & Humanitas COVID‐ Task, F. (2020). Venous and arterial thromboembolic complications in COVID‐19 patients admitted to an academic hospital in Milan, Italy. Thrombosis Research, 191, 9–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Long, H. , Nie, L. , Xiang, X. , Li, H. , Zhang, X. , Fu, X. , Ren, H. , Liu, W. , Wang, Q. , & Wu, Q. (2020). D‐Dimer and prothrombin time are the significant indicators of severe COVID‐19 and poor prognosis. BioMed Research International, 2020, 6159720. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Loo, J. , Spittle, D. A. , & Newnham, M. (2021). COVID‐19, immunothrombosis and venous thromboembolism: Biological mechanisms. Thorax, 76(4), 412–420. 10.1136/thoraxjnl-2020-216243 [DOI] [PubMed] [Google Scholar]
- Lu, Y. , Sun, K. , Guo, S. , Wang, J. , Li, A. , & Rong, X. , et al (2020). Early warning indicators of severe COVID‐19: A single‐center study of cases from Shanghai. China Frontiers in Medicine, 7, 432. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Léonard‐Lorant, I. , Delabranche, X. , Séverac, F. , Helms, J. , Pauzet, C. , Collange, O. , Schneider, F. , Labani, A. , Bilbault, P. , Molière, S. , Leyendecker, P. , Roy, C. , & Ohana, M. (2020). Acute pulmonary embolism in COVID‐19 patients on CT angiography and relationship to D‐Dimer Levels. Radiology, 296, 201561–E191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ma, L. , Song, K. , & Huang, Y. (2020). Coronavirus disease 2019 (COVID‐19) and cardiovascular complications. Journal of Cardiothoracic and Vascular Anesthesia, 35, 1860–1865. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Magdi, M. , & Rahil, A. (2019). Severe immune thrombocytopenia complicated by intracerebral haemorrhage associated with coronavirus infection: A case report and literature review. European journal of case reports in internal medicine, 6, 001155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Magro, C. , Mulvey, J. J. , Berlin, D. , Nuovo, G. , Salvatore, S. , Harp, J. , Baxter‐Stoltzfus, A. , & Laurence, J. (2020). Complement associated microvascular injury and thrombosis in the pathogenesis of severe COVID‐19 infection: A report of five cases. Translational Research, 220, 1–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Manne, B. K. , Denorme, F. , Middleton, E. A. , Portier, I. , Rowley, J. W. , Stubben, C. , Petrey, A. C. , Tolley, N. D. , Guo, L. , Cody, M. , Weyrich, A. S. , Yost, C. C. , Rondina, M. T. , & Campbell, R. A. (2020). Platelet gene expression and function in COVID‐19 patients. Blood, 136, 1317–1329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mao, L. , Jin, H. , Wang, M. , Hu, Y. , Chen, S. , He, Q. , Chang, J. , Hong, C. , Zhou, Y. , Wang, D. , Miao, X. , Li, Y. , & Hu, B. (2020). Neurologic Manifestations of hospitalized patients with coronavirus disease 2019 in Wuhan, China. JAMA Neurology, 77, 683–690. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marchandot, B. , Sattler, L. , Jesel, L. , Matsushita, K. , Schini‐Kerth, V. , Grunebaum, L. , & Morel, O. (2020). COVID‐19 related coagulopathy: A distinct entity. Journal of Clinical Medicine, 9, 1651. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mastaglio, S. , Ruggeri, A. , Risitano, A. M. , Angelillo, P. , Yancopoulou, D. , Mastellos, D. C. , Huber‐Lang, M. , Piemontese, S. , Assanelli, A. , Garlanda, C. , Lambris, J. D. , & Ciceri, F. (2020). The first case of COVID‐19 treated with the complement C3 inhibitor AMY‐101. Clinical Immunology, 215, 108450. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matsuyama, S. , Nagata, N. , Shirato, K. , Kawase, M. , Takeda, M. , & Taguchi, F. (2010). Efficient activation of the severe acute respiratory syndrome coronavirus spike protein by the transmembrane protease TMPRSS2. Journal of Virology, 84, 12658–12664. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Middeldorp, S. , Coppens, M. , van Haaps, T. F. , Foppen, M. , Vlaar, A. P. , Müller, M. C. A. , Bouman, C. , Beenen, L. , Kootte, R. S. , Heijmans, J. , Smits, L. P. , Bonta, P. I. , & van Es, N. (2020). Incidence of venous thromboembolism in hospitalized patients with COVID‐19. Journal of Thrombosis and Haemostasis: JTH, 18, 1995–2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Monteagudo, L. A. , Boothby, A. , & Gertner, E. (2020). Continuous Intravenous Anakinra infusion to calm the cytokine storm in macrophage activation syndrome. ACR Open Rheumatology, 2, 276–282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ng, L. F. , Hibberd, M. L. , Ooi, E.‐E. , Tang, K.‐F. , Neo, S.‐Y. , Tan, J. , Murthy, K. R. , Vega, V. B. , Chia, J. M. , Liu, E. T. , & Ren, E. C. (2004). A human in vitro model system for investigating genome‐wide host responses to SARS coronavirus infection. BMC Infectious Diseases, 4, 34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- O'Keefe, L. C. (2016). Middle East respiratory syndrome coronavirus. Workplace Health & Safety, 64, 184–186. [DOI] [PubMed] [Google Scholar]
- Omarjee, L. , Meilhac, O. , Perrot, F. , Janin, A. , & Mahe, G. (2020). Can Ticagrelor be used to prevent sepsis‐induced coagulopathy in COVID‐19? Clinical immunology (Orlando, Fla), 216, 108468. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Organization, W. H. Summary of probable SARS cases with onset of illness from 1 November 2002 to 31 July 2003. http://www.who.int/csr/sars/country/table2004_04_21/en/index.html
- Panigrahi, S. , Ma, Y. , Hong, L. , Gao, D. , West, X. Z. , Salomon, R. G. , Byzova, T. V. , & Podrez, E. A. (2013). Engagement of platelet toll‐like receptor 9 by novel endogenous ligands promotes platelet hyperreactivity and thrombosis. Circulation Research, 112, 103–112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peng, Y. , Meng, K. , Guan, H. , Leng, L. , Zhu, R. , Wang, B. , et al. (2020a). Clinical characteristics and outcomes of 112 cardiovascular disease patients infected by 2019‐nCoV. Zhonghua Xin Xue Guan Bing Za Zhi, 48, E004. [DOI] [PubMed] [Google Scholar]
- Peng, Y. D. , Meng, K. , Guan, H. Q. , Leng, L. , Zhu, R. R. , Wang, B. Y. , et al. (2020b). [Clinical characteristics and outcomes of 112 cardiovascular disease patients infected by 2019‐nCoV]. Zhonghua Xin Xue Guan Bing Za Zhi, 48, E004. [DOI] [PubMed] [Google Scholar]
- Pons, S. , Fodil, S. , Azoulay, E. , & Zafrani, L. (2020). The vascular endothelium: The cornerstone of organ dysfunction in severe SARS‐CoV‐2 infection. Critical Care, 24, 1–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pourbagheri‐Sigaroodi, A. , Bashash, D. , Fateh, F. , & Abolghasemi, H. (2020). Laboratory findings in COVID‐19 diagnosis and prognosis. Clinica Chimica Acta; International Journal of Clinical Chemistry, 510, 475–82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Qian, G.‐Q. , Yang, N.‐B. , Ding, F. , Ma, A. H. Y. , Wang, Z.‐Y. , Shen, Y.‐F. , Shi, C. W. , Lian, X. , Chu, J. G. , Chen, L. , Wang, Z. Y. , Ren, D. W. , Li, G. X. , Chen, X. Q. , Shen, H. J. , & Chen, X. M. (2020). Epidemiologic and clinical characteristics of 91 hospitalized patients with COVID‐19 in Zhejiang, China: A retrospective, multi‐centre case series. QJM: An International Journal of Medicine, 113, 474–481. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ranucci, M. , Ballotta, A. , Di Dedda, U. , Bayshnikova, E. , Dei Poli, M. , Resta, M. , Falco, M. , Albano, G. , & Menicanti, L. (2020). The procoagulant pattern of patients with COVID‐19 acute respiratory distress syndrome. Journal of Thrombosis And Haemostasis: JTH, 18, 1747–1751. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Richardson, S. , Hirsch, J. S. , Narasimhan, M. , Crawford, J. M. , McGinn, T. , Davidson, K. W. , the Northwell COVID‐ Research, C. , Barnaby, D. P. , Becker, L. B. , Chelico, J. D. , Cohen, S. L. , Cookingham, J. , Coppa, K. , Diefenbach, M. A. , Dominello, A. J. , Duer‐Hefele, J. , Falzon, L. , Gitlin, J. , Hajizadeh, N. , … Zanos, T. P. (2020). Presenting characteristics, comorbidities, and outcomes among 5700 patients hospitalized with COVID‐19 in the New York City area. Journal of the American Medical Association, 323, 2052–2059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Risitano, A. , Beaulieu, L. M. , Vitseva, O. , & Freedman, J. E. (2012). Platelets and platelet‐like particles mediate intercellular RNA transfer. Blood. The Journal of the American Society of Hematology, 119, 6288–6295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rod, J. E. , Oviedo‐Trespalacios, O. , & Cortes‐Ramirez, J. (2020). A brief‐review of the risk factors for covid‐19 severity. Revista de Saude Publica, 54, 60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rondina, M. T. , & Guo, L. (2019). The era of thromboinflammation: Platelets are dynamic sensors and effector cells during infectious diseases. Frontiers in Immunology, 10, 2204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sardu, C. , Gambardella, J. , Morelli, M. B. , Wang, X. , Marfella, R. , & Santulli, G. (2020). Hypertension, thrombosis, kidney failure, and diabetes: Is COVID‐19 an endothelial disease? A comprehensive evaluation of clinical and basic evidence. Journal of Clinical Medicine, 9, 1417. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sathler, P. C. (2020). Hemostatic abnormalities in COVID‐19: A guided review. Anais da Academia Brasileira de Ciencias, 92, e20200834. [DOI] [PubMed] [Google Scholar]
- Savla, S. R. , Prabhavalkar, K. S. , & Bhatt, L. K. (2021). Cytokine storm associated coagulation complications in COVID‐19 patients: Pathogenesis and Management. Expert Review of Anti‐Infective Therapy, 1–17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schutgens, R. E. (2020). DOAC in COVID‐19: Yes or No? Hemasphere, 5, 526. e526‐‐52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Song, J. C. , Wang, G. , Zhang, W. , Zhang, Y. , Li, W. Q. , & Zhou, Z. (2020). Chinese expert consensus on diagnosis and treatment of coagulation dysfunction in COVID‐19. Military Medical Research, 7, 19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tang, N. , Bai, H. , Chen, X. , Gong, J. , Li, D. , & Sun, Z. (2020a). Anticoagulant treatment is associated with decreased mortality in severe coronavirus disease 2019 patients with coagulopathy. Journal of Thrombosis and Haemostasis, 18, 1094–1099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tang, N. , Bai, H. , Chen, X. , Gong, J. , Li, D. , & Sun, Z. (2020b). Anticoagulant treatment is associated with decreased mortality in severe coronavirus disease 2019 patients with coagulopathy. Journal of thrombosis and haemostasis: JTH, 18, 1094–1099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tang, N. , Li, D. , Wang, X. , & Sun, Z. (2020c). Abnormal coagulation parameters are associated with poor prognosis in patients with novel coronavirus pneumonia. Journal of Thrombosis and Haemostasis, 18, 844–847. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tang, N. , Li, D. , Wang, X. , & Sun, Z. (2020d). Abnormal coagulation parameters are associated with poor prognosis in patients with novel coronavirus pneumonia. Journal of Thrombosis and Haemostasis: JTH, 18, 844–847. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tang, T. , Bidon, M. , Jaimes, J. A. , Whittaker, G. R. , & Daniel, S. (2020e). Coronavirus membrane fusion mechanism offers a potential target for antiviral development. Antiviral Research, 178, 104792. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Terpos, E. , Ntanasis‐Stathopoulos, I. , Elalamy, I. , Kastritis, E. , Sergentanis, T. N. , Politou, M. , Psaltopoulou, T. , Gerotziafas, G. , & Dimopoulos, M. A. (2020). Hematological findings and complications of COVID‐19. American Journal of Hematology, 95, 834–847. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Testa, S. , Paoletti, O. , Giorgi‐Pierfranceschi, M. , & Pan, A. (2020a). Switch from oral anticoagulants to parenteral heparin in SARS‐CoV‐2 hospitalized patients. Internal and Emergency Medicine, 15, 1–3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Testa, S. , Prandoni, P. , Paoletti, O. , Morandini, R. , Tala, M. , Dellanoce, C. , Giorgi‐Pierfranceschi, M. , Betti, M. , Danzi, G. B. , Pan, A. , & Palareti, G. (2020b). Direct oral anticoagulant plasma levels' striking increase in severe COVID‐19 respiratory syndrome patients treated with antiviral agents: The Cremona experience. Journal of Thrombosis and Haemostasis: JTH, 18, 1320–1323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thachil, J. (2020). The versatile heparin in COVID‐19. Journal of Thrombosis and Haemostasis: JTH, 18, 1020–1022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thachil, J. , Tang, N. , Gando, S. , Falanga, A. , Cattaneo, M. , Levi, M. , Clark, C. , & Iba, T. (2020a). DOACs and “newer” hemophilia therapies in COVID‐19: Reply. Journal of Thrombosis and Haemostasis: JTH, 18, 1795–1796. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thachil, J. , Tang, N. , Gando, S. , Falanga, A. , Cattaneo, M. , Levi, M. , Clark, C. , & Iba, T. (2020b). ISTH interim guidance on recognition and management of coagulopathy in COVID‐19. Journal of Thrombosis and Haemostasis, 18, 1023–1026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ulanowska, M. , & Olas, B. (2021). Modulation of hemostasis in COVID‐19; blood platelets may be important pieces in the COVID‐19 Puzzle. Pathogens, 10, 370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Valeriani, E. , Squizzato, A. , Gallo, A. , Porreca, E. , Vincent, J. L. , Iba, T. , Hagiwara, A. , & Di Nisio, M. (2020). Efficacy and safety of recombinant human soluble thrombomodulin in patients with sepsis‐associated coagulopathy: A systematic review and meta‐analysis. Journal of Thrombosis and Haemostasis: JTH, 18, 1618–1625. [DOI] [PubMed] [Google Scholar]
- Varga, Z. , Flammer, A. J. , Steiger, P. , Haberecker, M. , Andermatt, R. , Zinkernagel, A. S. , Mehra, M. R. , Schuepbach, R. A. , Ruschitzka, F. , & Moch, H. (2020). Endothelial cell infection and endotheliitis in COVID‐19. Lancet (London, England), 395, 1417–1418. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Viecca, M. , Radovanovic, D. , Forleo, G. B. , & Santus, P. (2020). Enhanced platelet inhibition treatment improves hypoxemia in patients with severe Covid‐19 and hypercoagulability. A case control, proof of concept study. Pharmacological Research, 158, 104950. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vivas, D. , Roldán, V. , Esteve‐Pastor, M. A. , Roldán, I. , Tello‐Montoliu, A. , Ruiz‐Nodar, J. M. , Cosín‐Sales, J. , Gámez, J. M. , Consuegra, L. , Ferreiro, J. L. , Marín, F. , Arrarte, V. , Anguita, M. , Cequier, Á. , & Pérez‐Villacastín, J. (2020). Recommendations on antithrombotic treatment during the COVID‐19 pandemic. Position statement of the Working Group on Cardiovascular Thrombosis of the Spanish Society of Cardiology. Revista Espanola de Cardiologia, 73, 749–757. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walsh, P. N. (2004). Platelet coagulation‐protein interactions. Seminars in Thrombosis and Hemostasis, 30, 461–471. [DOI] [PubMed] [Google Scholar]
- Wan, S. , Xiang, Y. , Fang, W. , Zheng, Y. , Li, B. , Hu, Y. , Lang, C. , Huang, D. , Sun, Q. , Xiong, Y. , Huang, X. , Lv, J. , Luo, Y. , Shen, L. , Yang, H. , Huang, G. , & Yang, R. (2020). Clinical features and treatment of COVID‐19 patients in northeast Chongqing. Journal of Medical Virology, 92, 797–806. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang, D. , Hu, B. , Hu, C. , Zhu, F. , Liu, X. , Zhang, J. , et al. (2020a). Clinical characteristics of 138 hospitalized patients with 2019 novel coronavirus–infected pneumonia in Wuhan, China. Journal of the American Medical Association. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang, D. , Hu, B. , Hu, C. , Zhu, F. , Liu, X. , Zhang, J. , Wang, B. , Xiang, H. , Cheng, Z. , Xiong, Y. , Zhao, Y. , Li, Y. , Wang, X. , & Peng, Z. (2020b). Clinical characteristics of 138 hospitalized patients with 2019 novel coronavirus‐infected pneumonia in Wuhan, China. Journal of the American Medical Association, 323, 1061–1069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang, F. , Yang, Y. , Dong, K. , Yan, Y. , Zhang, S. , Ren, H. , Yu, X. , & Shi, X. (2020c). Clinical characteristics of 28 patients with diabetes and COVID‐19 in Wuhan, China. Endocrine Practice: Official Journal of the American College of Endocrinology and the American Association of Clinical Endocrinologists, 26, 668–674. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang, J. , Hajizadeh, N. , Moore, E. E. , McIntyre, R. C. , Moore, P. K. , Veress, L. A. , Yaffe, M. B. , Moore, H. B. , & Barrett, C. D. (2020d). Tissue plasminogen activator (tPA) treatment for COVID‐19 associated acute respiratory distress syndrome (ARDS): A case series. Journal of Thrombosis and Haemostasis: JTH, 18, 1752–1755. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang, J. , Saguner, A. M. , An, J. , Ning, Y. , Yan, Y. , & Li, G. (2020e). Dysfunctional coagulation in COVID‐19: From cell to bedside. Advances in Therapy, 37, 3033–3039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang, T. , Chen, R. , Liu, C. , Liang, W. , Guan, W. , Tang, R. , Tang, C. , Zhang, N. , Zhong, N. , & Li, S. (2020f). Attention should be paid to venous thromboembolism prophylaxis in the management of COVID‐19. The Lancet Haematology, 7, e362‐e363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang, Y. D. , Zhang, S. P. , Wei, Q. Z. , Zhao, M. M. , Mei, H. , Zhang, Z. L. , et al. (2020g). [COVID‐19 complicated with DIC: 2 cases report and literatures review]. Zhonghua xue ye xue za zhi =. Zhonghua xueyexue zazhi, 41, 245–247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Whyte, C. S. , Morrow, G. B. , Mitchell, J. L. , Chowdary, P. , & Mutch, N. J. (2020). Fibrinolytic abnormalities in acute respiratory distress syndrome (ARDS) and versatility of thrombolytic drugs to treat COVID‐19. Journal of Thrombosis and Haemostasis: JTH, 18, 1548–1555. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wichmann, D. , Sperhake, J.‐P. , Lütgehetmann, M. , Steurer, S. , Edler, C. , Heinemann, A. , Heinrich, F. , Mushumba, H. , Kniep, I. , Schröder, A. S. , Burdelski, C. , de Heer, G. , Nierhaus, A. , Frings, D. , Pfefferle, S. , Becker, H. , Bredereke‐Wiedling, H. , de Weerth, A. , Paschen, H. R. , … Kluge, S. (2020). Autopsy findings and venous thromboembolism in patients with COVID‐19: A prospective cohort study. Annals of Internal Medicine, 173, 268–277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Witkowski, M. , Landmesser, U. , & Rauch, U. (2016). Tissue factor as a link between inflammation and coagulation. Trends in Cardiovascular Medicine, 26, 297–303. [DOI] [PubMed] [Google Scholar]
- Wright, F. L. , Vogler, T. O. , Moore, E. E. , Moore, H. B. , Wohlauer, M. V. , Urban, S. , Nydam, T. L. , Moore, P. K. , & McIntyre RC, J.r (2020). Fibrinolysis shutdown correlation with thromboembolic events in severe COVID‐19 infection. Journal of the American College of Surgeons, 231, 193–203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu, C. , Chen, X. , Cai, Y. , Xia, J. , Zhou, X. , Xu, S. , Huang, H. , Zhang, L. , Zhou, X. , Du, C. , Zhang, Y. , Song, J. , Wang, S. , Chao, Y. , Yang, Z. , Xu, J. , Zhou, X. , Chen, D. , Xiong, W. , … Song, Y. (2020b). Risk factors associated with acute respiratory distress syndrome and death in patients with coronavirus disease 2019 pneumonia in Wuhan, China. JAMA Internal Medicine, 180, 934–943. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu, C. , Chen, X. , Cai, Y. , Xia, J. , Zhou, X. , Xu, S. , Huang, H. , Zhang, L. , Zhou, X. , Du, C. , Zhang, Y. , Song, J. , Wang, S. , Chao, Y. , Yang, Z. , Xu, J. , Zhou, X. , Chen, D. , Xiong, W. , … Song, Y. (2020a). Risk factors associated with acute respiratory distress syndrome and death in patients with coronavirus disease 2019 pneumonia in Wuhan. China. JAMA Internal Medicine, 180, 934. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu, C. , Chen, X. , Cai, Y. , Xia, J. , Zhou, X. , Xu, S. , Huang, H. , Zhang, L. , Zhou, X. , Du, C. , Zhang, Y. , Song, J. , Wang, S. , Chao, Y. , Yang, Z. , Xu, J. , Zhou, X. , Chen, D. , Xiong, W. , … Song, Y. (2020c). Risk factors associated with acute respiratory distress syndrome and death in patients with coronavirus disease 2019 pneumonia in Wuhan, China. JAMA Internal Medicine, 180, 934. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xie, J. , Covassin, N. , Fan, Z. , Singh, P. , Gao, W. , Li, G. , et al. (2020). Association between hypoxemia and mortality in patients with COVID‐19, Mayo Clinic Proceedings. Elsevier; [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xiong, B. , Liu, T. , Luo, P. , Wei, Y. , Zhou, Y. , Liu, M. , Zhang, Y. , Wang, H. , Zhang, X. , Wang, X. , & Zhou, F. (2020a). Prominent hypercoagulability associated with inflammatory state among cancer patients with SARS‐CoV‐2 infection. Frontiers in Oncology, 10, 1345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xiong, Y. , Liu, Y. , Cao, L. , Wang, D. , Guo, M. , Jiang, A. , Guo, D. , Hu, W. , Yang, J. , Tang, Z. , Wu, H. , Lin, Y. , Zhang, M. , Zhang, Q. , Shi, M. , Liu, Y. , Zhou, Y. , Lan, K. , & Chen, Y. (2020b). Transcriptomic characteristics of bronchoalveolar lavage fluid and peripheral blood mononuclear cells in COVID‐19 patients. Emerging Microbes & Infections, 9, 761–770. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu, J. , Zhao, S. , Teng, T. , Abdalla, A. E. , Zhu, W. , Xie, L. , Wang, Y. , & Guo, X. (2020a). Systematic comparison of two animal‐to‐human transmitted human coronaviruses: SARS‐CoV‐2 and SARS‐CoV. Viruses, 12, 12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu, P. , Zhou, Q. , & Xu, J. (2020b). Mechanism of thrombocytopenia in COVID‐19 patients. Annals of Hematology, 99, 1–4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu, X. , Han, M. , Li, T. , Sun, W. , Wang, D. , Fu, B. , et al. (2020c). Effective treatment of severe COVID‐19 patients with tocilizumab. Proceedings of the National Academy of Sciences, 117, 10970–10975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu, Z. , Shi, L. , Wang, Y. , Zhang, J. , Huang, L. , Zhang, C. , et al. (2020d). Pathological findings of COVID‐19 associated with acute respiratory distress syndrome. The Lancet. Respiratory Medicine, 8, 420–422. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang, L. , Liu, J. , Zhang, R. , Li, M. , Li, Z. , Zhou, X. , Hu, C. , Tian, F. , Zhou, F. , & Lei, Y. (2020a). Epidemiological and clinical features of 200 hospitalized patients with corona virus disease 2019 outside Wuhan, China: A descriptive study. Journal of Clinical Virology: The Official Publication of the Pan American Society for Clinical Virology, 129, 104475. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang, M. , Ng, M. H. , & Li, C. K. (2005). Thrombocytopenia in patients with severe acute respiratory syndrome. Hematology, 10, 101–105. [DOI] [PubMed] [Google Scholar]
- Yang, X. , Yu, Y. , Xu, J. , Shu, H. , Xia, J. , Liu, H. , Wu, Y. , Zhang, L. , Yu, Z. , Fang, M. , Yu, T. , Wang, Y. , Pan, S. , Zou, X. , Yuan, S. , & Shang, Y. (2020b). Clinical course and outcomes of critically ill patients with SARS‐CoV‐2 pneumonia in Wuhan, China: A single‐centered, retrospective, observational study. The Lancet Respiratory Medicine, 8, 475–481. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang, Y. , Shen, C. , Li, J. , Yuan, J. , Wei, J. , Huang, F. , Wang, F. , Li, G. , Li, Y. , Xing, L. , Peng, L. , Yang, M. , Cao, M. , Zheng, H. , Wu, W. , Zou, R. , Li, D. , Xu, Z. , Wang, H. , … Liu, Y. (2020c). Plasma IP‐10 and MCP‐3 levels are highly associated with disease severity and predict the progression of COVID‐19. Journal of Allergy and Clinical Immunology, 146, 119–127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yin, S. , Huang, M. , Li, D. , & Tang, N. (2020a). Difference of coagulation features between severe pneumonia induced by SARS‐CoV2 and non‐SARS‐CoV2. Journal of Thrombosis and Thrombolysis, 51, 1–4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yin, S. , Huang, M. , Li, D. , & Tang, N. (2020b). Difference of coagulation features between severe pneumonia induced by SARS‐CoV2 and non‐SARS‐CoV2. Journal of Thrombosis and Thrombolysis, 51, 1107–1110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang, G.‐.Q. , Hu, C. , Luo, L.‐.J. , Fang, F. , Chen, Y.‐.F. , & Li, J.‐.G. , et al (2020a). Clinical features and treatment of 221 patients with COVID‐19 in Wuhan, China. Journal of Clinical Virology, 127, 104364. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang, H. , & Baker, A. (2017). Recombinant human ACE2: Acing out angiotensin II in ARDS therapy. Critical care (London, England), 21, 305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang, J. , Dong, X. , Cao, Y. , Yuan, Y. , Yang, Y. , Yan, Y. , Akdis, C. A. , & Gao, Y. (2020b). Clinical characteristics of 140 patients infected with SARS‐CoV‐2 in Wuhan, China. Allergy, 75, 1730–1741. [DOI] [PubMed] [Google Scholar]
- Zhang, S. , Liu, Y. , Wang, X. , Yang, L. , Li, H. , Wang, Y. , Liu, M. , Zhao, X. , Xie, Y. , Yang, Y. , Zhang, S. , Fan, Z. , Dong, J. , Yuan, Z. , Ding, Z. , Zhang, Y. , & Hu, L. (2020c). SARS‐CoV‐2 binds platelet ACE2 to enhance thrombosis in COVID‐19. Journal of Hematology & Oncology, 13, 1–22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang, Y. , He, L. , Chen, H. , Lu, S. , Xiong, Y. , Liu, J. , Zheng, Y. , Wang, S. , Liu, L. (2020d). Manifestations of blood coagulation and its relation to clinical outcomes in severe COVID‐19 patients: Retrospective analysis. International Journal of Laboratory Hematology, 42, 766–772. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang, Y. , Xiao, M. , Zhang, S. , Xia, P. , Cao, W. , Jiang, W. , Chen, H. , Ding, X. , Zhao, H. , Zhang, H. , Wang, C. , Zhao, J. , Sun, X. , Tian, R. , Wu, W. , Wu, D. , Ma, J. , Chen, Y. , Zhang, D. , … Zhang, S. (2020e). Coagulopathy and antiphospholipid antibodies in patients with covid‐19. The. New England Journal of Medicine, 382, e38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zheng, Y. , Xu, H. , Yang, M. , Zeng, Y. , Chen, H. , Liu, R. , Li, Q. , Zhang, N. , & Wang, D. (2020). Epidemiological characteristics and clinical features of 32 critical and 67 noncritical cases of COVID‐19 in Chengdu. Journal of Clinical Virology, 127, 104366. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou, B. , She, J. , Wang, Y. , & Ma, X. (2020a). Venous thrombosis and arteriosclerosis obliterans of lower extremities in a very severe patient with 2019 novel coronavirus disease: A case report. Journal of Thrombosis and Thrombolysis, 50, 229–232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou, F. , Yu, T. , Du, R. , Fan, G. , Liu, Y. , Liu, Z. , Xiang, J. , Wang, Y. , Song, B. , Gu, X. , Guan, L. , Wei, Y. , Li, H. , Wu, X. , Xu, J. , Tu, S. , Zhang, Y. , Chen, H. , & Cao, B. (2020b). Clinical course and risk factors for mortality of adult inpatients with COVID‐19 in Wuhan, China: A retrospective cohort study. The Lancet, 395, 1054–1062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou, F. , Yu, T. , Du, R. , Fan, G. , Liu, Y. , Liu, Z. , Xiang, J. , Wang, Y. , Song, B. , Gu, X. , Guan, L. , Wei, Y. , Li, H. , Wu, X. , Xu, J. , Tu, S. , Zhang, Y. , Chen, H. , & Cao, B. (2020c). Clinical course and risk factors for mortality of adult inpatients with COVID‐19 in Wuhan, China: A retrospective cohort study. Lancet (London, England), 395, 1054–1062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhu, Z. , Fu, Y. , Tian, D. , Sun, N. , Han, W. , Chang, G. , Dong, Y. , Xu, X. , Liu, Q. , Huang, D. , & Shi, F. D. (2015). Combination of the immune modulator fingolimod with alteplase in acute ischemic stroke: A pilot trial. Circulation, 132, 1104–1112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zou, Y. , Guo, H. , Zhang, Y. , Zhang, Z. , Liu, Y. , Wang, J. , Lu, H. , & Qian, Z. (2020). Analysis of coagulation parameters in patients with COVID‐19 in Shanghai. China Bioscience Trends. 14, 285–289. [DOI] [PubMed] [Google Scholar]
- Zou, Z. , Yang, Y. , Chen, J. , Xin, S. , Zhang, W. , Zhou, X. , Mao, Y. , Hu, L. , Liu, D. , Chang, B. , Chang, W. , Liu, Y. , Ma, X. , Wang, Y. , & Liu, X. (2004). Prognostic factors for severe acute respiratory syndrome: A clinical analysis of 165 cases. Clinical Infectious Diseases, 38, 483–489. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zuckier, L. S. , Moadel, R. M. , Haramati, L. B. , & Freeman, L. M. (2020). Diagnostic evaluation of pulmonary embolism during the COVID‐19 pandemic. Journal of Nuclear Medicine, 61, 630–631. [DOI] [PubMed] [Google Scholar]
- Zucoloto, A. Z. , & Jenne, C. N. (2019). Platelet‐neutrophil interplay: insights into neutrophil extracellular trap (NET)‐driven coagulation. Frontiers in Cardiovascular Medicine, 6, 85. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Data sharing not applicable to this article as no datasets were generated or analyzed during the current study.


