Key Messages.
Daily aspirin does not change nasal expression of ACE2 or TMPRSS2 or alter levels of angiotensin‐derived peptides in patients with AERD or aspirin‐tolerant asthma, suggesting that its use is unlikely to increase risk of severe SARS‐CoV‐2 infection.
Patients with AERD or aspirin‐tolerant asthma do not have differences in plasma levels of angiotensin‐derived peptides compared with non‐asthmatic controls.
In March 2020, the French Minister of Health warned against the use of non‐steroidal anti‐inflammatory drugs (NSAIDs) like aspirin and ibuprofen, suggesting that their use might be associated with more severe disease presentation in patients with COVID‐19. 1 This advice was apparently based on unpublished anecdotal reports that patients who had been exposed to NSAIDs developed more severe symptoms of COVID‐19. At the time, there was limited indirect data suggesting that NSAIDs may up‐regulate angiotensin‐converting enzyme 2 (ACE2) expression, 2 which is the target receptor for cell entry of severe acute respiratory syndrome coronavirus 2 (SARS‐CoV‐2), and some speculated that an NSAID‐induced up‐regulation of ACE2 could explain the French minister's observations. Though more recent clinical reports have not found any association between NSAID use and a more severe course of COVID‐19, 3 , 4 there is still remaining concern about possible mechanistic links between NSAID use and ACE2 expression. Specifically for our patients with aspirin‐exacerbated respiratory disease (AERD), for whom high‐dose aspirin therapy is often an efficacious treatment to help reduce the regrowth of nasal polyps and decrease respiratory inflammation, 5 it is important that we are able to provide them with evidence‐based management regarding the safety of their aspirin therapy during this pandemic.
SARS‐CoV‐2 cell entry requires ACE2, along with transmembrane serine protease 2 (TMPRSS2), to bind to host epithelial cells, and the highest expression of ACE2 is found in the secretory cells of nasal epithelium. 6 , 7 , 8 Nasal epithelial cells express both ACE2 and TMPRSS2 and their increased expression in the upper airway may allow for increased host susceptibility to infection. However, once SARS‐CoV‐2 binds to ACE2, receptor‐mediated internalization leads to decreased ACE2 activity. ACE2 can generate the biologically active fragments Ang (1–9) and Ang (1–7), which block Angiotensin‐II and inhibit ACE, so that the ACE2 axis negatively regulates ACE. 9
In patients with acute respiratory distress syndrome and lung injury, plasma Ang‐I levels are significantly higher in non‐survivors, whereas survival is associated with higher plasma Ang (1–9) and Ang (1–7). This suggests that high ACE2 activity systemically is beneficial for survival of acute lung injury, 10 which has become a major cause of morbidity and mortality during this COVID‐19 pandemic. Therefore, although ACE2 expression in the nasal epithelium is a required entry point for viral infection, continued high levels of systemic ACE2 activity may paradoxically be beneficial post‐infection. Consequently, internalization of ACE2 during SARS‐CoV‐2 infection could reduce ACE2 activity that is important for survival. The host factors that regulate ACE2 expression and activity are not fully known and are missing pieces required to expand our understanding of the pathogenesis of this disease. The influence of aspirin on nasal ACE2 expression and the subsequent production of angiotensin peptides has never been studied. A single human study showed that plasma ACE2 levels were potentially higher in patients taking aspirin, suggesting a possible protective effect of NSAIDs. 2 In this study, we investigated the effect of 8 weeks of high‐dose aspirin therapy on nasal ACE2 and TMPSSR2 expression and production of downstream SARS‐CoV‐2‐related angiotensin peptides for patients with aspirin‐tolerant asthma (ATA) and AERD.
We analysed plasma from 41 asthmatics (11 with ATA and 30 with AERD), at baseline and again after 8 weeks of twice‐daily aspirin 650 mg, and from 11 healthy subjects not on NSAIDs. 5 No subjects were on oral corticosteroids or on any biologic medication. For a subset of the AERD subjects, nasal fluid and nasal inferior turbinate epithelial cell scrapings had also been collected. The Mass General Brigham Institutional Review Board approved the study and subjects provided informed consent; Protocol 2013P001659.
Peripheral blood was drawn into 3.2% sodium citrate tubes, and centrifuged twice—first at 200 g × 15 minutes and then at 4700 g × 30 minutes at room temperature. The resulting platelet‐depleted plasma was stored at −80°C until further analysis. Nasal secretion fluid was sampled from the nare with Nasosorption™ FX (Hunt Developments, Midhurst, England), a non‐invasive upper airway sampling method that uses a synthetic absorptive matrix to collect nasal lining fluid directly from the nasal mucosal surface. Nasal fluid was placed in either 0.5% bovine serum albumin and stored at −80°C until further analysis. Nasal epithelial tissue was collected from the inferior turbinate using the Rhino‐Pro Curette, a sterile, disposable, mucosal collection device. One sample was taken from the right or left mid‐inferior portion of the inferior turbinate using a gentle scraping motion and was placed directly in RNAprotect Tissue Reagent (Qiagen, Germantown, MD).
Nasal fluid and plasma were measured for levels of ACE2 (RayBiotech, Norcross, GA), Ang‐I (Enzo Life Sciences, Farmingdale, NY), Ang‐II (Sigma‐Aldrich, St. Louis, MO), Ang(1–7) and Ang(1–9) (MyBioSource, San Diego, CA) by ELISA. RNA from the nasal epithelial scrapings was extracted and analysed with SYBR® Green‐based quantitative real‐time PCR methodologies for ACE2 and TMPRSS2 expression levels, normalized to GAPDH (all reagents and primers from Qiagen).
Two‐sided Student's t test assessed paired differences before and after aspirin, and ANOVA assessed unpaired differences between baselines of the three patient groups. Analysis was performed using GraphPad Prism version 7.0d (GraphPad, La Jolla, CA).
1. RESULTS AND DISCUSSION
Patient demographics are summarized in Table 1. Plasma levels of ACE2, Ang‐I, Ang‐II, Ang(1–7) and Ang(1–9) were detectable in most samples (Figure 1, A‐F), though only Ang(1–7) was consistently detectable in nasal fluid (Figure 1, E). There were no significant baseline differences in any angiotensin peptides across the three patient groups, nor were there significant aspirin‐induced differences in asthmatic subjects. There were some AERD subjects with an aspirin‐induced increase in plasma ACE2, but this was not statistically significant (1.79 ±1.21ng/mL to 5.87 ±3.27ng/mL, p=0.156). ACE2 transcript was detectable in most nasal scraping samples, and TMPRSS2 was detectable in all samples. There was no significant aspirin‐induced difference in either ACE2 or TMPRSS2 transcript expression (Figure 1, G‐H).
TABLE 1.
Patient demographics and clinical characteristics
|
Healthy (n = 11) |
ATA (n = 11) |
AERD (n = 30) |
|
|---|---|---|---|
| Mean age, y (STDEV) | 30 (8) | 32 (16) | 47 (11) |
| Sex, Female, N (%) | 9 (82%) | 9 (82%) | 17 (57%) |
| Race, N (%) | |||
| White | 9 (82%) | 9 (82%) | 29 (97%) |
| Black | 0 | 1 (9%) | 0 |
| Asian | 1 (9%) | 0 | 1 (3%) |
| Other | 1 (9%) | 1 (9%) | 0 |
| Ethnicity, N (%) | |||
| Hispanic | 2 (18%) | 1 (9%) | 2 (7%) |
| Mean FEV1, L (STDEV) | 3.51 (0.78) | 2.93 (0.48) | 3.14 (0.79) |
| Mean FEV1% predicted, % (STDEV) | 99.9 (9.1) | 86.9 (11.5) | 92.5 (12.2%) |
| Mean FVC, L (STDEV) | 4.10 (0.96) | 3.91 (0.64) | 4.15 (1.08) |
| Mean ACQ−7 (STDEV) | 0.06 (0.10) | 1.10 (0.55) | 0.87 (0.91) |
| Fluticasone Proprionate equivalent ICS Dose (≤ 200 mcg—low), N (%) | 6 (55%) | 10 (33%) | |
| Fluticasone Proprionate equivalent ICS Dose (≤ 500 mcg—medium), N (%) | 2 (20%) | 9 (30%) | |
| Fluticasone Proprionate equivalent ICS Dose (≥1000 mcg—high), N (%) | 0 | 6 (20%) | |
Abbreviations: ACQ‐7, Asthma control questionnaire; AERD, Aspirin‐exacerbated respiratory disease; ATA, Aspirin Tolerant Asthma; FEV1, Forced expiratory volume in one second; FVC, Forced vital capacity; STDEV, Standard deviation.
This article is being made freely available through PubMed Central as part of the COVID-19 public health emergency response. It can be used for unrestricted research re-use and analysis in any form or by any means with acknowledgement of the original source, for the duration of the public health emergency.
As patients with AERD are often treated with daily high‐dose aspirin therapy, we aimed to understand the immunologic influence of daily aspirin on SARS‐CoV‐2 susceptibility. Eight weeks of daily aspirin treatment in patients with asthma or AERD did not significantly change nasal epithelial transcript expression of ACE2 or TMPRSS2 or alter levels of angiotensin‐derived peptides potentially relevant to survival in acute respiratory distress syndrome. Furthermore, consistent with studies showing asthma is generally not a risk factor for severe COVID‐19, our data show that asthmatic subjects did not have any difference in angiotensin‐derived peptide levels compared with controls. Although we are not able to extrapolate these results out for patients who are on aspirin for more than eight weeks, our data suggest that there is no immunologic indication that daily aspirin therapy would lead to an increased susceptibility to more severe COVID‐19 and suspect that the early anecdotal reports of worsened COVID‐19 with NSAIDs were likely due to indication bias. Any recommendations for medication usage and treatment changes should be made on a case‐by‐case basis with patient input and shared decision‐making—we are hopeful that these additional immunologic data will help to further inform the safety of aspirin usage for patients with AERD.
CONFLICT OF INTEREST
T Laidlaw has served on scientific advisory boards for GlaxoSmithKline, Sanofi‐Genzyme and Regeneron. K Buchheit has served on scientific advisory boards for AstraZeneca and GlaxoSmithKline. D Gakpo, J Hacker, J Mullur and A Sohail have no conflicts of interest to disclose.
AUTHOR CONTRIBUTIONS
K.B. and T.L designed and directed the project; J.H. and A.S. performed the experiments; C.D. K.B, J.H., D.G., J.M., A.S. and T.L. contributed to the analysis of the results and to the writing of the manuscript.
2.
FIGURE 1.
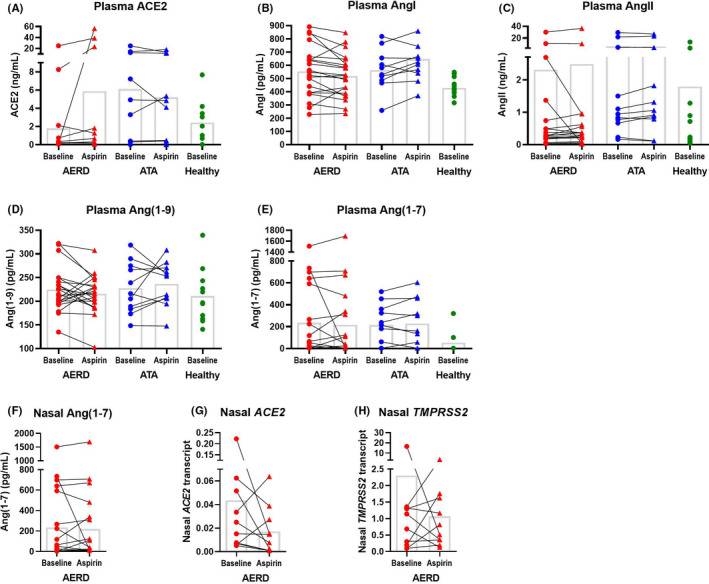
ACE2 and angiotensin‐derived peptide levels and nasal inferior turbinate cell expression of ACE2 and TMPRSS2. Plasma from healthy control subjects at baseline and from subjects with aspirin‐exacerbated respiratory disease (AERD) and aspirin‐tolerant asthma (ATA) at baseline and after 8 weeks of 650mg aspirin twice daily was measured for ACE2, AngI, AngII, Ang(1‐9), and Ang(1‐7) (A‐E). Nasal fluid from AERD subjects was measured for Ang(1‐7) (F). Epithelial cell inferior turbinate scrapings from AERD patients at a baseline visit and again after 8 weeks of treatment with 650mg aspirin twice daily was measured for ACE2 and TMPRSS2 expression, normalized to GAPDH (G‐H). Means shown in columns
Funding information
This work was supported by the National Institutes of Health (NIH grant nos U19AI095219, K23AI139352) and by generous contributions from the Vinik and Kaye Families.
REFERENCES
- 1. Le ministre de la santé déconseille l’ibuprofène contre le coronavirus. Le Monde. 2020 March 14.
- 2. Rice GI, Jones AL, Grant PJ, Carter AM, Turner AJ, Hooper NM. Circulating activities of angiotensin‐converting enzyme, its homolog, angiotensin‐converting enzyme 2, and neprilysin in a family study. Hypertension. 2006;48(5):914‐920. [DOI] [PubMed] [Google Scholar]
- 3. Lund LC, Kristensen KB, Reilev M, et al. Adverse outcomes and mortality in users of non‐steroidal anti‐inflammatory drugs who tested positive for SARS‐CoV‐2: A Danish nationwide cohort study. PLoS Med. 2020;17(9):e1003308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Rinott E, Kozer E, Shapira Y, Bar‐Haim A, Youngster I. Ibuprofen use and clinical outcomes in COVID‐19 patients. Clin Microbiol Infect. 2020;26(9):1259.e5‐1259.e7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Laidlaw TM. Aspirin desensitization vs biologics for patients with aspirin‐exacerbated respiratory disease. Ann Allergy Asthma Immunol. 2021;126(2):118‐119. [DOI] [PubMed] [Google Scholar]
- 6. Chen J, Jiang Q, Liu K, et al. Individual Variation of the SARS‐CoV2 Receptor ACE2 Gene Expression and Regulation. PrePrints. 2020;19(7):2020030191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Ziegler C, Allon SJ, Nyquist SK, et al. SARS‐CoV‐2 Receptor ACE2 is an Interferon‐Stimulated Gene in Human Airway Epithelial Cells and Is Enriched in Specific Cell Subsets Across Tissues. SSRN Electron J. 2020;20:00767. 10.2139/ssrn.3555145 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Hoffmann M, Kleine‐Weber H, Schroeder S, et al. SARS‐CoV‐2 Cell Entry Depends on ACE2 and TMPRSS2 and Is Blocked by a Clinically Proven Protease Inhibitor. Cell. 2020;181(2):271‐80 e8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Wevers B, van der Hoek L. Renin–angiotensin system in human coronavirus pathogenesis. Fut Virol. 2010;5(2):145‐161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Reddy R, Asante I, Liu S, et al. Circulating angiotensin peptides levels in Acute Respiratory Distress Syndrome correlate with clinical outcomes: A pilot study. PLoS One. 2019;14(3):e0213096. [DOI] [PMC free article] [PubMed] [Google Scholar]


