Abstract
Background
A high prevalence of pulmonary embolism (PE) has been described during COVID‐19. Our aim was to identify predictive factors of PE in non‐ICU hospitalized COVID‐19 patients.
Methods
Data and outcomes were collected upon admission during a French multicenter retrospective study, including patients hospitalized for COVID‐19, with a CT pulmonary angiography (CTPA) performed in the emergency department for suspected PE. Predictive factors significantly associated with PE were identified through a multivariate regression model.
Results
A total of 88 patients (median [IQR] age of 68 years [60‐78]) were analyzed. Based on CTPA, 47 (53.4%) patients were diagnosed with PE, and 41 were not. D‐dimer ≥3000 ng/mL (OR 8.2 [95% CI] 1.3‐74.2, sensitivity (Se) 0.84, specificity (Sp) 0.78, P = .03), white blood count (WBC) ≥12.0 G/L (29.5 [2.3‐1221.2], Se 0.47, Sp 0.92, P = .02), and ferritin ≥480 µg/L (17.0 [1.7‐553.3], Se 0.96, Sp 0.44, P = .03) were independently associated with the PE diagnosis. The presence of the double criterion D‐dimer ≥3000 ng/mL and WBC ≥12.0 G/L was greatly associated with PE (OR 21.4 [4.0‐397.9], P = .004).
Conclusion
The white blood count, the D‐dimer and ferritin levels could be used as an indication for CTPA to confirm PE on admission in non‐ICU COVID‐19 patients.
Keywords: COVID‐19, D‐dimer, ferritin, predictive factor, pulmonary embolism, SARS‐CoV‐2, white blood count
Highlights.
A high prevalence of pulmonary embolism (PE) occurs during COVID‐19 infection.
D‐dimer ≥3000 ng/mL or white blood count ≥12 G/L or ferritin ≥480 µg/L is associated of PE diagnosis.
These predictive factors might allow a rapid diagnosis of COVID‐19‐associated PE when positive, and a reduction of unnecessary computed tomography pulmonary angiography by their absence.
1. INTRODUCTION
The severity of COVID‐19 infection is highly variable, with approximately 15% of patients developing severe respiratory complications and requiring oxygen therapy and hospitalization. In approximately 5% of patients, these respiratory complications are delayed and occur within 7 to 10 days after the onset of symptoms, requiring an increase in oxygen supply or mechanical ventilation. 1 , 2 , 3 While COVID‐19 alone can cause severe pneumonia, some studies have reported a high prevalence of thrombotic events that may be responsible for delayed COVID‐19‐associated respiratory complications. 4 , 5 , 6 , 7 , 8
The high prevalence of thrombotic events would be related to the combination of hypoxia with an immune‐triggered thrombo‐inflammation responsible for endothelial injuries and coagulability disorders. 9 , 10 , 11 , 12 Many patients during COVID‐19 infection often present with an increased plasma D‐dimer concentration that reflects, in that context, both the coagulation disorder and inflammatory state. Such an increase has been previously identified as one of the predictors of intensive care unit (ICU) admission and death. 1 , 4 , 11 , 13 , 14 , 15 Moreover, some authors have proposed plasma D‐dimer thresholds for predicting the diagnosis of pulmonary embolism (PE) during COVID‐19 infection. 15 , 16 However, most of these studies did not differentiate ICU and non‐ICU patients. While the prevalence of PE in ICU COVID‐19 patients ranges from 13.6% to 23.1%, the exact prevalence of PE in COVID‐19 patients hospitalized in non‐critical medicine departments (non‐ICU patients) remains between 1.6% and 8%. 17 , 18 , 19 , 20 , 21 , 22 Improved prediction of PE in this population would allow a better targeting of patients at high risk of PE and could help guide the prescription of anticoagulant therapy. It would decrease PE‐related transfer in the ICU and mortality. 23
The objective of our study was to identify clinical, laboratory, and CT‐scan predictive factors of PE in hospitalized non‐ICU COVID‐19 patients upon admission.
2. PATIENTS AND METHODS
2.1. Setting
The CLOTVID study is a multicenter retrospective study conducted from April 6 to April 28, 2020, in 18 participating French hospitals located in 12 cities (five university hospitals and seven non‐university hospitals).
2.2. Patients
All adults (≥18 years old) with laboratory‐confirmed COVID‐19 infection (positive RT‐PCR) and in whom a CT pulmonary angiography (CTPA) were performed for clinical suspicion of PE on admission to the emergency department (ED), and before being hospitalized in one of the participating medicine ward, were included. We excluded patients with clinical suspicion of PE and/or patients diagnosed with deep vein thrombosis (DVT) for whom CTPA was not performed on ED admission. We also excluded patients directly admitted to the ICU and then transferred to the medicine ward.
2.3. Data collection
For each patient, the data collected included demographics (age, gender and body mass index (BMI)), medical background (comorbidities and previous long‐term curative/prophylactic anticoagulation therapy), COVID‐19 infection history (date of the first symptoms, date of hospital admission, and date of positive RT‐PCR), symptoms, and vital parameters on admission. A radiologist blinded to the hypothesis locally reviewed each CTPA, analyzing the type of lesion (ground glass and/or condensation) and their extension (absent (<10%), minimal (10%‐25%), moderate (25%‐50%), extensive (50%‐75%), and severe (>75%)). 24 We also collected laboratory tests on admission (white blood count (WBC), serum creatinine level, C‐reactive protein (CRP), serum ferritin, prothrombin time ratio, fibrinogen, plasma D‐dimers, brain natriuretic peptide (BNP), and troponin), both nonspecific (oxygen and ventilator support) and possibly specific treatment of the COVID‐19 infection (antiviral therapy, hydroxychloroquine, steroids, and biotherapies) and outcome during the follow‐up (death/ICU admission or recovery).
2.4. Statistics
Continuous variables are expressed as median (interquartile range) and categorical variables as number (percentage).
Univariate analysis was performed using Mann‐Whitney‐U, t‐Student, chi2 and Fisher's exact tests, as appropriate. Significant variables with P‐value ≤.2 in the univariate regression were selected as candidates for the multivariate logistic regression model. Biomarkers were considered both directly and after conversion into a binary variable using a threshold determined by a receiver operating characteristic (ROC) curve analysis. We chose the threshold with the best specificity‐sensitivity ratio to discriminate between patients with and without a PE diagnosis. Results are expressed as odds ratios (OR) with their 95% confidence intervals (CI). We also tested by a logistic regression the association between PE and the presence of double criterion associating D‐dimers ≥3000 ng/mL and WBC ≥12 G/L, based on their respective thresholds. The discriminative capacity of the predictive factors identified was illustrated via their sensitivity, their specificity, based on a prevalence fixed at 5%, their positive (PPV) and negative predictive value (NPV). In addition, the Wells’ score modified for PE alone and the Wells’ score associated with D‐dimer level adjusted on age were retrospectively calculated for each patient. 25 Finally, the ROC curves and area under curve (AUC) between each biomarkers identified and wells’ score were compared with DeLong's test. 26
A P‐value of <.05 was considered statistically significant. No imputation method was used for missing unrecoverable data. Data were analyzed using R software, v3.6.1 (R Foundation for Statistical Computing; http://www.R‐project.org/). The study was developed, and the results are reported according to the guidelines on the Strengthening the Reporting of Observational Studies in Epidemiology (STROBE). 27
2.5. Ethics
This non‐interventional study was based on medical records, which were in strict compliance with the French reference methodology MR‐004, established by the French National Commission on Informatics and Liberties (CNIL) (reference 2217565 v 0), and was approved by the Institutional Data Protection Authority of Assistance Publique – Hôpitaux de Paris (University Hospital of Paris). Data collection was conducted with the consent of the patient or his/her guardian by a medical investigator in one of the participating hospitals. Anonymized patient data were collected by local investigators by means of a standardized clinical report form (CRF) and then centralized by the three principal investigators (JG, BT, and DS).
3. RESULTS
Overall 191 patients were included from the 18 French COVID‐19 units and 103 patients were excluded (Figure 1). Finally, 88 patients (sex ratio M/F: 7/3; median [IQR] age: 68 years [60‐78]) were analyzed (follow‐up period of 19 [14‐25] days) specifically about suspicion of PE in the emergency department (Tables 1a and 1b). Based on CTPA, 47 (53.4%) patients were diagnosed with PE (PE group), and 41 (46.6%) were not (non‐PE group).
FIGURE 1.
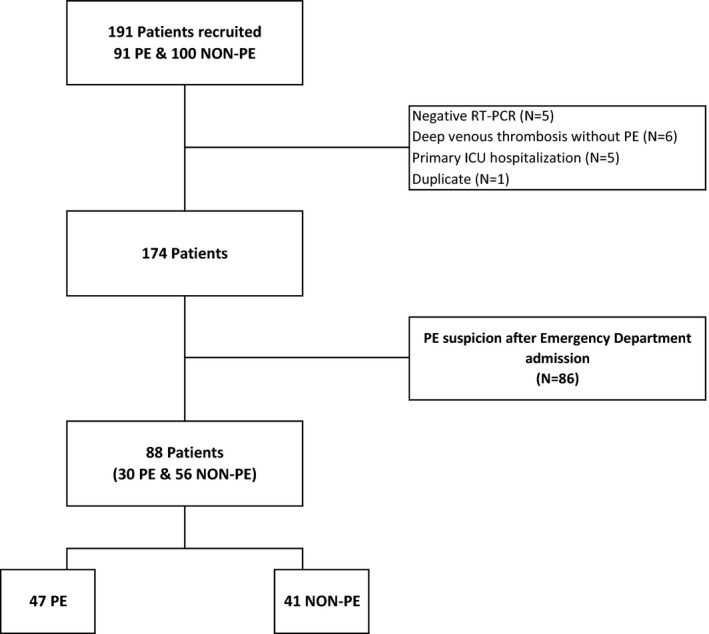
Flowchart of the CLOTVID study. ICU, Intensive care unit; PE, pulmonary embolism; NON‐PE, absence of PE; RT‐PCR, reverse transcriptase‐polymerase chain reaction
TABLE 1a.
Baseline characteristics of all patients
|
All patients N = 88 |
PE N = 47 |
NON‐PE N = 41 |
P‐value | |
|---|---|---|---|---|
| Baseline characteristics | ||||
| Age [years] | 68 [60‐78] | 69 [61‐78] | 68 [58‐76] | .4488 |
| Age ≥65 years, n (%) | 54 (61.4) | 31 (66.0) | 23 (56.1) | .3433 |
| Sex gender [male], n (%) | 62 (70.5) | 35 (74.5) | 27 (65.9) | .3769 |
| Body mass index [kg/m2] | 25.6 [23.5‐28.6] | 25.2 [23.1‐26.8] | 26.8 [24.2‐30.1] | .0967 |
| Comorbidities | ||||
| Number of comorbidities by each patients | 1 [1‐2] | 1 [0‐1] | 2 [1‐3] | .0004 |
| Respiratory disease, n (%) | 15 (17.0) | 8 (17.0) | 7 (17.1) | .9948 |
| Obesity (BMI > 30 kg/m2), n (%) | 14 (15.9) | 3 (6.4) | 11 (26.8) | .0170 |
| Arterial hypertension, n (%) | 43 (48.9) | 19 (40.4) | 24 (58.5) | .0899 |
| Diabetes mellitus, n (%) | 21 (10.9) | 5 (10.6) | 16 (39.5) | .0024 |
| Cardiovascular disease, n (%) | 16 (18.2) | 7 (14.9) | 9 (22.0) | .3918 |
| Active smoking, n (%) | 7 (8.0) | 3 (6.4) | 4 (9.8) | .7004 |
| Immunodeficiency, n (%) | 3 (3.4) | 1 (2.1) | 2 (4.9) | .5961 |
| CTD or systemic vasculitis, n (%) | 3 (3.4) | 2 (4.3) | 1 (2.4) | 1 |
| Thrombo‐embolic risk factor | ||||
| Presence of at least one risk factor, n (%) | 12 (13.6) | 6 (12.8) | 6 (14.6) | 1 |
| Estrogen use, n (%) | 0 | 0 | 0 | NE |
| Active cancer, n (%) | 6 (6.8) | 5 (10.6) | 1 (2.4) | .2091 |
| Prolonged immobilization, n (%) | 6 (6.8) | 1 (2.1) | 5 (12.2) | .0931 |
| Recent surgery (<3 mo), n (%) | 2 (2.3) | 0 | 2 (4.9) | .2142 |
| Long‐term curative anticoagulant therapy, n (%) | 3 (3.4) | 3 (6.4) | 0 | .2449 |
Data are presented in total (percentage) for categorical variables and median (interquartile range [IQR]) for continuous variables. Follow‐up period represented the time between COVID‐19 first symptom and last medical visit. Cardiovascular diseases (myocardial ischemia, cardiac injury, stroke). Immunodeficiency (primitive or secondary immunodeficiency [CD4+ T‐cell <0.2 G/L, ongoing chemotherapy, long‐term steroid therapy or immunosuppressive therapy]).
Abbreviations: BMI, body mass index; CTD, connective tissue disease.
P‐value: PE group vs NON‐PE group. P‐value < .05 is illustrated by bold value.
TABLE 1b.
COVID‐19 history of all patients
|
All patients N = 88 |
PE N = 47 |
NON‐PE N = 41 |
P‐value | |
|---|---|---|---|---|
| COVID‐19 history | ||||
| Symptoms at admission | ||||
| Fever >38°C, n (%) | 65 (73.9) | 38 (80.9) | 27 (65.9) | .1102 |
| Cough, n (%) | 53 (60.2) | 26 (55.3) | 27 (65.9) | .3138 |
| Dyspnea, n (%) | 68 (77.3) | 37 (78.7) | 31 (75.6) | .7280 |
| COVID‐19 pneumonia, HRCT: n (%) | ||||
| Ground glass | 78 (88.6) | 40 (85.1) | 38 (92.7) | .3542 |
| Consolidation | 56 (63.6) | 28 (59.6) | 28 (68.3) | .6351 |
| Lung lesional extension | ||||
| Absent or minimal extension | 28 (31.8) | 20 (42.6) | 8 (19.5) | .0834 |
| Moderate extension | 37 (42.0) | 18 (38.3) | 19 (46.3) | |
| Extensive and Severe extension | 17 (19.3) | 7 (14.9) | 10 (24.4) | |
| Time from COVID‐19 first symptoms to CTPA [days] | 9 [5‐14] | 10 [6‐16] | 8 [5‐11] | .1308 |
| Vital parameters at the time of CTPA | ||||
| Heart rate/min | 98 [88‐105] | 98 [84‐105] | 98 [88‐108] | .9023 |
| Respiratory frequency [/min] | 27 [22‐31] | 28 [20‐31] | 27 [24‐31] | .8228 |
| Respiratory frequency >22/min, n (%) | 45 (51.1) | 29 (61.7) | 16 (39.0) | .4398 |
| SpO2 [%] | 95 [93‐97] | 95 [93‐97] | 95 [93‐96] | .4889 |
| SpO2 < 96%, n (%) | 41 (46.6) | 24 (51.1) | 17 (41.5) | .8970 |
| Oxygen [L/min], mean ± SD | 4 ± 5 | 4 ± 5 | 4 ± 5 | .5469 |
| Oxygen flow ≤2 L/min, n (%) | 40 (45.5) | 24 (51.1) | 16 (39.0) | .8167 |
| Oxygen flow ≥6 L/min, n (%) | 19 (21.6) | 10 (21.3) | 9 (22.0) | .6034 |
| Laboratory parameters | ||||
| Platelets [G/L] | 247 [192‐331] | 248 [190‐332] | 247 [201‐332] | .8452 |
| White blood count (WBC) [G/L] | 8.2 [5.9‐12.5] | 11.5 [6.3‐15.0] | 7.2 [5.5‐8.9] | .0007 |
| White blood count >10 G/L, n (%) | 46 (52.3) | 17 (36.2) | 7 (17.1) | .0003 |
| Lymphocytes [G/L] | 1.0 [0.7‐1.3] | 0.9 [0.7‐1.4] | 1.0 [0.7‐1.2] | .6425 |
| Neutrophils [G/L] | 6.1 [4.4‐10.3] | 8.8 [4.5‐11.7] | 5.0 [4.0‐7.0] | .0218 |
| Neutrophils/Lymphocytes ratio | 6.7 [3.9‐11.6] | 7.0 [4.4‐11.7] | 6.1 [3.1‐9.8] | .3420 |
| C‐Reactive Protein, [mg/L] | 109 [63‐193] | 119 [60‐196] | 103 [66‐183] | .9739 |
| BNP [pg/mL] | 75 [25‐524] | 112 [39‐647] | 57 [13‐336] | .1862 |
| BNP level >1500 pg/mL, n (%) | 8 (9.1) | 5 (10.6) | 3 (7.3) | .7108 |
| Troponin [ng/L] | 12.0 [5.0‐27.0] | 10.0 [4.3‐36.4] | 18.0 [8.0‐24.5] | .3104 |
| Serum creatinine [µM] | 81 [71‐103] | 82 [71‐98] | 81 [66‐109] | .8397 |
| Ferritin [µg/L] | 876 [476‐1342] | 1055 [729‐2193] | 561 [338‐1148] | .0157 |
| PT ratio | 1.00 [1.02‐1.17] | 1.12 [1.07‐1.18] | 1.04 [1.02‐1.12] | .0116 |
| Fibrinogen [g/L] | 6.3 [4.9‐7.3] | 6.3 [4.2‐7.8] | 6.4 [5.4‐6.9] | .5556 |
| D‐dimer [ng/mL] | 3752 [1192‐10 000] | 7830 [3856‐15 070] | 1225 [748‐2378] | <.0001 |
| D‐dimer level ≥3000 ng/mL, n (%) | 45 (51.1) | 37 (78.7) | 8 (19.5) | <.0001 |
| D‐dimer ≥3000 ng/mL and WBC ≥12 G/L, n (%) | 18 (20.5) | 17 (36.2) | 1 (2.4) | .0002 |
| Standard of care | ||||
| Oxygenotherapy, n (%) | 73 (83.0) | 39 (83.0) | 34 (82.9) | 1 |
| Ventilation | ||||
| Optiflow, n (%) | 2 (2.3) | 1 (2.1) | 1 (2.4) | 1 |
| CPAP, n (%) | 2 (2.3) | 1 (2.1) | 1 (2.1) | 1 |
| Outcome | ||||
| Follow‐up period [days] | 19 [14‐25] | 19 [14‐25] | 17 [13‐19] | .7984 |
| Length of hospital stay [days] | 7 [4‐12] | 7 [4‐11] | 9 [3‐14] | .9853 |
| Death or ICU transfer, n (%) | 17 (19.3) | 9 (19.1) | 8 (19.5) | .9656 |
| ICU transfer, n (%) | 11 (12.5) | 7 (14.9) | 4 (9.8) | .5330 |
| Death, n (%) | 7 (8.0) | 2 (4.3) | 5 (12.2) | .2436 |
| Hospital discharge, n (%) | 52 (59.1) | 28 (59.6) | 24 (58.5) | .9213 |
Data are presented in total (percentage) for categorical variables and median (interquartile range [IQR]) for continuous variables. Follow‐up period represented the time between COVID‐19 first symptom and last medical visit. Length of hospital stay was calculated on patients alive and not transfer in ICU department.
Abbreviations: BNP, brain natriuretic peptide; CPAP, continuous positive airway pressure; ICU, intensive care unit; PE, pulmonary embolism; Respiratory disease (asthma, chronic obstructive pneumonia disease [COPD], chronic infiltrative pneumonia…); CTPA, computed tomography pulmonary angiography; HRCT, high‐resolution computed tomography; PT, prothrombin time; SARS‐CoV‐2, severe acute respiratory syndrome coronavirus 2; µM, µmol/L; NE, not estimable.
P‐value: PE group vs NON‐PE group. P‐value < .05 is illustrated by bold value.
3.1. Comparison of PE and non‐PE patients on admission
Patients diagnosed with PE less frequently had diabetes mellitus and were less frequently obese (BMI > 30 kg/m2, P < .05 for both). Three patients (3.4%) had previous long‐term full‐dose anticoagulation on admission, all in the PE group (P = .2). PE and non‐PE patients did not differ significantly regarding both COVID‐19 infection history and COVID‐19 infection associated severity (Tables 1a and 1b): the median time from COVID‐19 infection first symptoms to CTPA (9 days [5‐14]), COVID‐19‐related symptoms, vital parameters, and oxygen requirement on admission were not significantly different between the two groups.
Blinded analysis of CTPA found similar proportions of ground glass and consolidation between the two groups (P > .05), and a similar extension of lung lesions between the two groups (P = .08). PE was segmental in half of the cases (N = 23‐48.9%) and proximal in 44.7% of the cases. The remaining cases were sub‐segmental (N = 3‐6.4%) (Table S1). PE was bilateral in 44.7% of patients.
Laboratory parameters on admission differed between the two groups. WBC and the ferritin level were higher in the PE group (Figure 2; P < .001 and P = .01, respectively) while C‐reactive protein, elevated in 93% of patients, did not significantly differ (P = .9). The prothrombin time ratio was lower and D‐dimer levels were 6‐fold higher in the PE group than in the non‐PE group (P = .01 and P < .0001, respectively). Platelets and fibrinogen were not different (P = .8 and .5, respectively). Cardiac and renal biomarkers were similar between the two groups. No association was found between WBC, or the D‐dimer level, or the ferritin level or the prothrombin time ratio and the extension of lung lesions on the HRCT (all P > .05, Figure S1).
FIGURE 2.
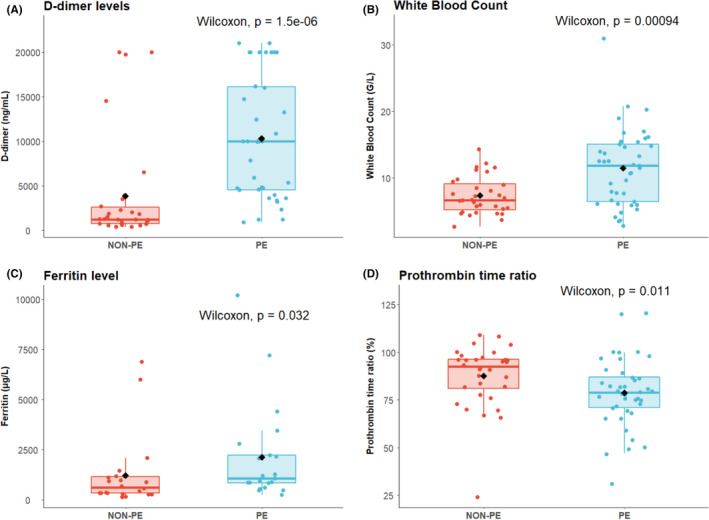
Biomarker levels according to pulmonary embolism status. Comparison of biomarkers according to PE status for D‐dimer level (Panel A), White Blood Count (Panel B), Ferritin (Panel C), and Prothrombin Time ratio (Panel D)
3.2. Patient management and outcome
There was no difference in the use of antibiotics, antiviral therapies, corticosteroids or biotherapies between the groups (P > .05 for all) during the hospitalization in medicine wards. All patients diagnosed with PE received anticoagulation therapy including therapeutic doses of low‐molecular‐weight heparin (LMWH; N = 33‐70.2%), unfractionated heparin (N = 9‐19.1%), or direct‐acting oral anticoagulant (N = 4‐8.5%) (Table S1). The follow‐up period (19 days [14‐25]) was similar in the PE and non‐PE groups. At the end of the inclusion, 17 (19.3%) patients died or were transferred to the ICU, 52 (59.1%) were discharge from the hospital, and 19 (21.6%) remain hospitalized with no difference between the two groups.
3.3. Risk factors associated with PE events
We interpreted biomarkers as binary variables based on the best specificity‐sensitivity ratio using ROC curves (Figure 3). Univariate analysis showed that D‐dimer level ≥3000 ng/mL, WBC ≥12.0 G/L, prothrombin time ratio >1.05 (P < .001 for all), and ferritin level ≥480 µg/L (P < .01) were associated with an increased risk of PE, whereas the presence obesity and diabetes mellitus were not associated with an increased risk of PE (Table 2).
FIGURE 3.
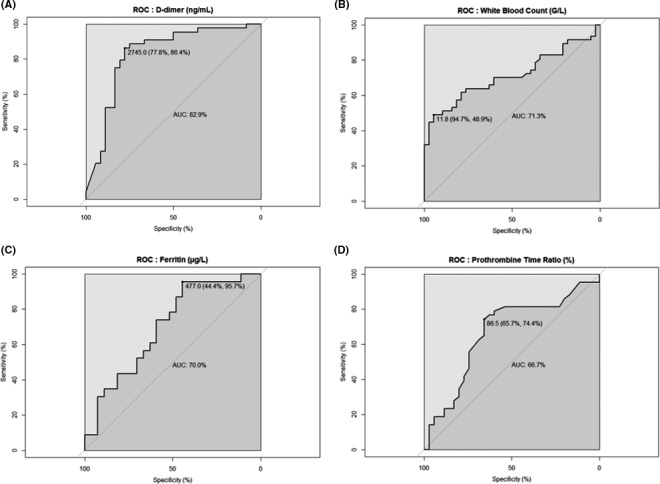
Receiver operating characteristic for biomarkers. ROC curves for D‐dimer level (Panel A), White Blood Count (Panel B), Ferritin (Panel C), and Prothrombin Time ratio (Panel D)
TABLE 2.
Factors associated with risk of pulmonary embolism at admission
| Variable | Univariate analysis | Multivariate analysis | ||||
|---|---|---|---|---|---|---|
| OR | 95% CI | P‐value | Adjusted OR | 95% CI | P‐value | |
| Obesity | 0.2 | 0.04‐0.7 | .0152 | ‐ | ‐ | ‐ |
| Diabetes mellitus | 0.2 | 0.05‐0.5 | .0032 | ‐ | ‐ | ‐ |
| D‐dimer ≥3000 ng/mL | 18.5 | 6.4‐61.5 | <.0001 | 8.2 | 1.3‐74.2 | .0344 |
| WBC ≥12 G/L | 15.8 | 4.16‐104.6 | .0004 | 29.5 | 2.3‐1221.2 | .0256 |
| Ferritin ≥480 µg/L | 17.6 | 3.0‐338.1 | .0087 | 17.0 | 1.7‐553.3 | .0361 |
| Prothrombin time ratio >1.05 | 5.7 | 2.1‐16.0 | .0006 | ‐ | ‐ | ‐ |
No obesity corresponded to BMI < 30 kg/m2.
Abbreviation: WBC, white blood count.
Multivariate analysis showed that only D‐dimer ≥3000 ng/mL (OR 8.2 [95% CI] 1.3‐74.2, sensitivity (Se) 0.84, specificity (Sp) 0.78, P = .03), WBC ≥12.0 G/L (29.5 [2.3‐1221.2], Se 0.47, Sp 0.92, P = .02), and ferritin levels ≥480 µg/L (17.0 [1.7‐553.3], Se 0.96, Sp 0.44, P = .03) were independently associated with the risk of PE (Table 2; Table S2). The presence of the double criterion D‐dimer ≥3000 ng/mL and WBC ≥12.0 G/L was greatly associated with PE (36.2% vs 2.4%, OR 21.4 [4.0‐397.9], P = .004). Expressed in continuous values, D‐dimer level (P = .01) and WBC (P = .006) were confirmed as independent predictive factors of PE in multivariate analysis based with biomarkers as continuous variables, while the ferritin level does not reach the threshold of significance (P = .06) (Table S3).
In comparison, no CTPA was indicated from the predictive guidance based on the Wells’ score modified for PE. Finally, the wells’ score associated with D‐dimer level adjusted on age was not better, with a CTPA indicated in only 3/47 (ie, true positive rate, 6.4%) of patients with PE and 5/41 (ie false positive rate, 12.2%) of patients without PE, with a Se [95% CI] of 0.06 [0.01‐0.18], and a Sp of 0.88 [0.74‐0.96]. The D‐dimer level and WBC were more accurate than Wells’ score in predicting the diagnosis of PE (Delong's test P < .0001 and P = .01, respectively), in contrast of the ferritin level (P > .05) (Figure S2).
4. DISCUSSION
In this series of 88 non‐ICU COVID‐19‐infected patients undergoing CTPA, we compared the clinical, laboratory, and radiological characteristics of patients diagnosed with PE and identify three simple and easily available biomarkers to help the clinician guide the management of COVID‐19 patients upon admission. Several suspected COVID‐19 patients had chest CT scans to detect specific lesions related to the virus upon admission to the ED. However, no consensus exists regarding the indication for CTPA specifically for the COVID‐19. 8 , 16 , 28 D‐dimer level ≥3000 ng/mL, WBC ≥12 G/L, and ferritin level ≥480 µg/L represented independent predictive factors of PE during COVID‐19.
Initially, we recruited 174 non‐ICU COVID‐19 patients with CTPA available for suspected PE, either in the ED or during a regular ward hospitalization. We decided to exclude this subset of patients, called “postadmission” patients (diagnosis of PE >24 hours after admission) and analyzed separately 29 , and focus only on the “upon admission” population, that is, COVID‐19‐infected patients who underwent CTPA in the ED (≤24 hours after admission). The reasons were as follows: (a) we wanted to avoid confounding factors linked to hospitalization and treatment (decrease in D‐dimer levels due to curative or prophylactic dose of heparin, variation in WBC linked to steroid or antibiotic use, etc), and (b) we wanted to propose predictive factors for emergency physicians to define the indication for CTPA upon admission.
Regarding the radiological data, we did not find a relationship between the occurrence of PE and the severity of lung extension lesions in accordance with other reports, 16 , 28 , 30 which are two distinct characteristics during COVID‐19 infection. Then, we identified pre‐radiological predictive factors to reduce the number of scans requested, improving the availability of imaging machines during a pandemic, and reducing patient irradiation and the risk of acute kidney injury and intrahospital transport during hospitalization.
Multivariate analyses found significant and independent differences between the two groups only on laboratory parameters data: WBC, D‐dimer, and ferritin levels. These predictive factors differ from those based only on clinical data and used in the Wells’ score and the revised Geneva score for excluding PE. 31 , 32 The Wells’ score assessed in this series displayed a very low predictive ability to PE. Herein, we report easily available three laboratory markers routinely measured upon patient admission, with a greater impact than clinical and radiological data in the early triage of COVID‐19 patients. These laboratory results were strongly and independently associated with PE. All these findings support the hypothesis that the sensitivity of the conventional strategies for PE suspicion might be insufficient.
The plasma D‐dimer threshold for excluding thromboembolism is not comparable to that of a non‐COVID‐19 population (D‐dimers: 3000 ng/mL in a COVID‐19 population vs 500 ng/mL in a non‐COVID‐19 population). 31 , 32 Several observational studies have shown an elevated D‐dimer concentration in patients with COVID‐19, including non‐PE patients. 4 , 14 , 15 , 16 , 33 Guan et al found elevated D‐dimer concentrations (>500 ng/mL) in 260 (46%) of 560 patients with COVID‐19. 4 Currently, the concentration of D‐dimer is well identified as one of the predictors of severity and mortality. 1 , 4 , 11 , 13 , 14 , 15 Tang et al described 183 COVID‐19 patients and found a mean D‐dimer concentration of 2120 ng/mL (range 770‐5270) in nonsurvivor patients vs a concentration of 610 ng/mL (350‐1290) in survivors. 15 Zhou et al showed that ICU patients had higher median [IQR] D‐dimer concentrations than non‐ICU patients did (2400 ng/mL [600‐14 400] vs 500 [300‐800], respectively), and the mortality rate was 18 times higher with D‐dimer concentration >1000 ng/mL upon admission. 14 In addition to the elevated levels of D‐dimer, we reported a high level of white blood count (especially neutrophils) with a significant difference in PE patients in multivariate analysis. This significant increase in thrombo‐embolic risk proportional to the concentration of WBC, and more specifically neutrophils, is not described in the current literature. One hypothesis could be the NETosis phenomenon: to trap and disarm microbes in the extracellular environment, neutrophils produce the “Neutrophil Extracellular Trap” (NET), a kind of fibrillar network made up of nuclear materials (histones, DNA, etc). This highly charged material (negatively charged (DNA) and positively charged (histones)) attracts and activates platelets, von Willebrand factor, and tissue factor and is a pathway for the induction of thrombogenesis. This thrombogenic process is involved in venous and arterial macrovascular thrombotic events. 34 , 35 Although the increased WBC was poorly described in the COVID‐19 literature, the cutoff D‐dimer value (3000 ng/mL) was well documented as an excellent prognostic and therapeutic factor. Tang et al and Yin et al, in a cohort of 449 COVID patients in Wuhan, described an increased risk of 28‐day mortality beyond this value (32.8% if D‐dimer <3000 ng/mL vs 52.4%, P = .01) and the indication for anticoagulant treatment beyond this threshold. 7 , 23 In the same way, the increase of ferritin levels was reported in many other studies in COVID‐19, 36 , 37 as being the consequence of the cytokine storm. 38 An elevated ferritin level was reported to be associated with COVID‐19‐related thrombosis compared to thrombosis without COVID‐19. 39 The systemic inflammatory response against the SARS‐CoV‐2 leads to a hypercoagulability state, reflected by the increase of some inflammatory markers, including elevated circulating IL‐6, D‐dimer, ferritin, elevated WBC or the presence of NET in thrombi, and leading to vascular endothelial injury. 5 , 14 Analyses on lungs obtained during autopsy from patients who died from COVID‐19 highlighted the presence of pulmonary vascular endothelialitis, thrombosis and microangiopathy, and features of angiogenesis. 40 Nevertheless, in a recent publication, the International Society on Thrombosis and Haemostasis (ISTH) did not recommend to screen venous thromboembolism based only on elevated D‐dimer levels and maintained the diagnose VTE based on clinical index of suspicion. 41
The CLOTVID study is multicentric; the prevalence of PE in non‐ICU patients upon admission is estimated to be between 3% and 5% over all hospitals that included patients. The prevalence was specifically 5% at Lariboisière Hospital, Paris, France, the most important center of the study. The prevalence of PE in patients with COVID‐19 infection is estimated to be between 5% and 30%, depending on the series. 6 , 16 , 17 , 20 , 21 , 22 , 42 However, many studies have not distinguished between patients hospitalized in the ICU or not and, consequently, have overestimated the real prevalence in the medical ward. Some studies have specifically described the prevalence of PE in non‐ICU patients between 1.6% and 8%. 20 , 21 , 22
Our study has limitations: limited numbers of patients, descriptive study, and enrollment of patients with PE excluding other arterial or DVT. Nevertheless, these predictive factors may help quickly to determine the risk of PE upon admission, thus allowing for the rapid prescription of CTPA and anticoagulant treatment in EDs, and they seem to be more accurate than the currently used scores (eg, the Wells’ score). The number of COVID‐19‐infected patients is still growing around the world, with an increasing number of fatal cases, and in European countries, after a break in the pandemic between May and July 2020, we are now facing new successive waves without consensual therapeutic algorithms. Taking into account our experience during the first wave of the pandemic, we believe that the biological predictive factors would be helpful for physicians in the ED in identifying the best indication of CPTA and for patients in limiting unnecessary imaging and CPTA side effects. In addition, it could save time on management because access to CTPA could be prolonged during the COVID‐19 pandemic or unavailable in many cities. In practice, a D‐dimer level ≥3000 ng/mL, WBC ≥12 G/L or ferritin level ≥480 µg/L, and even more the combination of D‐dimer ≥3000 ng/mL and WBC ≥12 G/L should classify the noncritically COVID‐19 patients to high risk of venous thromboembolism, and should consider a CTPA imaging and a first therapeutic dose of anticoagulant.
5. CONCLUSION
The white blood count, and the D‐dimer and ferritin levels are simple and easy to use, and could be used upon admission in non‐ICU COVID‐19 patients by an emergency physician to triage, guide therapeutic management and validate the prescription of a CTPA, reduce unnecessary transport for patients (sometimes unstable), and permit rapid to adapt anticoagulant regimen if needed. Currently, there does not seem to be any respite from the COVID‐19 pandemic worldwide, and the infection could persist until a global vaccination campaign. A significant number of deaths could be prevented with the proper screening of PE and the early prescription of anticoagulants. However, a prospective study is necessary to validate these predictive factors and build a diagnostic score in a prospective manner with a higher number of unselected patients being included.
CONFLICT OF INTEREST
None.
AUTHOR CONTRIBUTION
Dr Thoreau, Dr Galland, and Prof. Sene had full access to all of the data in the study and take responsibility for the integrity of the data and the accuracy of the data analysis. Dr Thoreau, Dr Galland, Dr Vodovar, Dr Kevorkian, Dr Mangin, Dr Delrue, Dr, Chauvin, Prof. Siguret, Prof. Moulis, and Prof. Sene contributed to the initial study design, analysis and interpretation of data, drafting of the submitted article, critical revisions for intellectual content, and final approval of the version to be published. Dr Thoreau provided statistical analysis. Dr Thoreau, Dr Galland, Dr Vodovar, Dr Delrue, Dr Neuwirth, Dr Stepanian, Dr Chauvin, Dr Dellal, Dr Nallet, Dr Roriz, D Devaux, Dr London, Dr Martin‐Lecamp, Dr Froissart, Dr Arab, Dr Ferron, Dr Groff, Dr Queyrel, Dr Lorut, Dr Regard, Dr Berthoud, Dr Bayer, Dr Comarmond, Dr Lioger, Prof. Mekinian, Dr Szwebel, Dr Sené, Dr Amador‐Boreiro, Dr Mangin, Prof. Sellier and Prof. Sene contributed to data collection, critical revisions for intellectual content, and final approval of the version to be published.
Supporting information
Figure S1‐S2
Table S1‐S3
ACKNOWLEDGEMENTS
All coauthors would like to thank all the medical, paramedical, and administrative staff involved in the management of the COVID‐19 pandemic.
Lariboisière COVID Group members: Albertini Mathieu, Bouajila Sara, Britany Kimbimbi, Burlacu Ruxandra, Cacoub Léa, Champion Karine, Delcey Véronique, Dillinger Jean‐Guillaume, Feron Florine, Frazier Aline, Thomas Funck‐Bretano, Gauthier Diane‐Cecile, Gautier Jean‐François, Henry Patrick, Huscenot Tessa, Sarah Izabel, Mathilde Jaulerry, Jouabli Moenes, Julla Jean‐Baptiste, Laloi Michelin Marie, Leroy Pierre, Lopes Amanda, Megarbane Bruno, Michon Maxime, Munier Anne‐Lise, Nahmani Yoram, Nicol Martin, Nicolas Eroan, Poulat Audrey, Revue Eric, Richette Pascal, Riveline Jean‐Pierre, Rubenstein Emma, Zanin Adrien, Aveneau Clément, Bastard Paul, Beauvais Diane, Boghez Loredana, Borderiou Alix, Conway Paul, Cosma Lavignia, Davy Vincent, Desjardin Clément, Devatine Sandra, Ducroz Gerardin Christel, Dupe Charlotte, Gobert Chloé, Gros Clotilde, Kadiri Soumaya, Khan Enmat, Ongnessek Sandrine, Rhmari Fatima, Sacco Isabelle, Saptefrat Natalia, Schaupp Pauline, Serre Justine, Sideris Georgios, Smati Sonia, Tournier Marine, Treca Pauline, Truong Tony, Tuffier Mathilde, Arcelli Mattéo, Boue Yvonnick, Copie Alban, Deye Nicolas, Ekherian Jean‐Michel, Errabih Zaccaria, Gonde Antoine, Grant Caroline, Guerin Emmanuelle, Magalhaes Adèle, Malissin Isabelle, Meurisse Edouard, Mrad Aymen, Naim Giulia, Nguyen Philippe, Nitenberg Kiyoko, Pepin‐Lehalleur Adrien, Perault Arthur, Perrin Lucile, Renaud Maxime, Sutterlin Laetitia, Delrue Maxime, Siguret Virginie, Stepanian Alain.
Galland J, Thoreau B, Delrue M, et al; Lariboisière COVID Group . White blood count, D‐dimers, and ferritin levels as predictive factors of pulmonary embolism suspected upon admission in noncritically ill COVID‐19 patients: The French multicenter CLOTVID retrospective study. Eur J Haematol. 2021;107:190–201. 10.1111/ejh.13638
J Galland and B Thoreau contributed equally to this work.
Contributor Information
Benjamin Thoreau, Email: benjamin.thoreau@aphp.fr.
Lariboisière COVID Group:
Albertini Mathieu, Bouajila Sara, Britany Kimbimbi, Burlacu Ruxandra, Cacoub Léa, Champion Karine, Delcey Véronique, Dillinger Jean‐Guillaume, Feron Florine, Frazier Aline, Thomas Funck‐Bretano, Gauthier Diane‐Cecile, Gautier Jean‐François, Henry Patrick, Huscenot Tessa, Sarah Izabel, Mathilde Jaulerry, Jouabli Moenes, Julla Jean‐Baptiste, Laloi Michelin Marie, Leroy Pierre, Lopes Amanda, Megarbane Bruno, Michon Maxime, Munier Anne‐Lise, Nahmani Yoram, Nicol Martin, Nicolas Eroan, Poulat Audrey, Revue Eric, Richette Pascal, Riveline Jean‐Pierre, Rubenstein Emma, Zanin Adrien, Aveneau Clément, Bastard Paul, Beauvais Diane, Boghez Loredana, Borderiou Alix, Conway Paul, Cosma Lavignia, Davy Vincent, Desjardin Clément, Devatine Sandra, Ducroz Gerardin Christel, Dupe Charlotte, Gobert Chloé, Gros Clotilde, Kadiri Soumaya, Khan Enmat, Ongnessek Sandrine, Rhmari Fatima, Sacco Isabelle, Saptefrat Natalia, Schaupp Pauline, Serre Justine, Sideris Georgios, Smati Sonia, Tournier Marine, Treca Pauline, Truong Tony, Tuffier Mathilde, Arcelli Mattéo, Boue Yvonnick, Copie Alban, Deye Nicolas, Ekherian Jean‐Michel, Errabih Zaccaria, Gonde Antoine, Grant Caroline, Guerin Emmanuelle, Magalhaes Adèle, Malissin Isabelle, Meurisse Edouard, Mrad Aymen, Naim Giulia, Nguyen Philippe, Nitenberg Kiyoko, Pepin‐Lehalleur Adrien, Perault Arthur, Perrin Lucile, Renaud Maxime, Sutterlin Laetitia, Delrue Maxime, Siguret Virginie, and Stepanian Alain
DATA AVAILABILITY STATEMENT
This article adheres to community standards for full data disclosure.
REFERENCES
- 1. Huang C, Wang Y, Li X, et al. Clinical features of patients infected with 2019 novel coronavirus in Wuhan, China. Lancet Lond Engl. 2020;395(10223):497‐506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Petrilli CM, Jones SA, Yang J, et al. Factors associated with hospital admission and critical illness among 5279 people with coronavirus disease 2019 in New York City: prospective cohort study. BMJ. 2020;m1966. 10.1136/bmj.m1966 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Wu Z, McGoogan JM. Characteristics of and important lessons from the coronavirus disease 2019 (COVID‐19) outbreak in China: summary of a report of 72 314 cases from the Chinese center for disease control and prevention. JAMA. 2020;323(13):1239‐1242. [DOI] [PubMed] [Google Scholar]
- 4. Guan W‐J, Ni Z‐Y, Hu YU, et al. Clinical characteristics of coronavirus disease 2019 in China. N Engl J Med. 2020;382(18):1708‐1720. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Connors JM, Levy JH. COVID‐19 and its implications for thrombosis and anticoagulation. Blood. 2020;135(23):2033‐2040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Bikdeli B, Madhavan MV, Jimenez D, et al. COVID‐19 and thrombotic or thromboembolic disease: implications for prevention, antithrombotic therapy, and follow‐up. J Am Coll Cardiol. 2020;75(23):2950‐2973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Tang N, Bai H, Chen X, Gong J, Li D, Sun Z. Anticoagulant treatment is associated with decreased mortality in severe coronavirus disease 2019 patients with coagulopathy. J Thromb Haemost. 2020;18(5):1094‐1099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Thachil J, Tang N, Gando S, et al. ISTH interim guidance on recognition and management of coagulopathy in COVID‐19. J Thromb Haemost. 2020;18(5):1023‐1026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. McGonagle D, O’Donnell JS, Sharif K, Emery P, Bridgewood C. Immune mechanisms of pulmonary intravascular coagulopathy in COVID‐19 pneumonia. Lancet Rheumatol. 2020;2:e437‐e445. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Copin M‐C, Parmentier E, Duburcq T, Poissy J, Mathieu D. Time to consider histologic pattern of lung injury to treat critically ill patients with COVID‐19 infection. Intensive Care Med. 2020;23:1‐3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Chen T, Wu DI, Chen H, et al. Clinical characteristics of 113 deceased patients with coronavirus disease 2019: retrospective study. BMJ. 2020;368:m1091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Diehl J‐L, Peron N, Chocron R, et al. Respiratory mechanics and gas exchanges in the early course of COVID‐19 ARDS: a hypothesis‐generating study. Ann Intensive Care. 2020;10(1):95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Wang D, Hu BO, Hu C, et al. Clinical characteristics of 138 hospitalized patients with 2019 novel coronavirus‐infected pneumonia in Wuhan, China. JAMA. 2020;323(11):1061‐1069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Zhou F, Yu T, Du R, et al. Clinical course and risk factors for mortality of adult inpatients with COVID‐19 in Wuhan, China: a retrospective cohort study. Lancet. 2020;395(10229):1054‐1062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Tang N, Li D, Wang X, Sun Z. Abnormal coagulation parameters are associated with poor prognosis in patients with novel coronavirus pneumonia. J Thromb Haemost. 2020;18(4):844‐847. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Leonard‐Lorant I, Delabranche X, Severac F, et al. Acute pulmonary embolism in COVID‐19 patients on CT angiography and relationship to D‐dimer levels. Radiology. 2020;296:201561. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Klok FA, Kruip M, van der Meer N, et al. Incidence of thrombotic complications in critically ill ICU patients with COVID‐19. Thromb Res. 2020;191:145‐147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Poissy J, Goutay J, Caplan M, et al. Pulmonary Embolism in Patients With COVID‐19. Circulation. 2020;142(2):184–186. 10.1161/circulationaha.120.047430 [DOI] [PubMed] [Google Scholar]
- 19. Llitjos J‐F, Leclerc M, Chochois C, et al. High incidence of venous thromboembolic events in anticoagulated severe COVID‐19 patients. J Thromb Haemost. 2020;18:1743‐1746. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Bilaloglu S, Aphinyanaphongs Y, Jones S, Iturrate E, Hochman J, Berger JS. Thrombosis in hospitalized patients with COVID‐19 in a New York City health system. JAMA. 2020;324:799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Middeldorp S, Coppens M, Haaps TF, et al. Incidence of venous thromboembolism in hospitalized patients with COVID‐19. J Thromb Haemost. 2020;18(8):1995–2002. 10.1111/jth.14888 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Poyiadji N, Cormier P, Patel PY, et al. Acute pulmonary embolism and COVID‐19. Radiology. 2020;297:E335‐E338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Yin S, Huang M, Li D, Tang N. Difference of coagulation features between severe pneumonia induced by SARS‐CoV2 and non‐SARS‐CoV2. J Thromb Haemost. 2021;51(4):1107–1110. 10.1007/s11239-020-02105-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Revel M‐P, Parkar AP, Prosch H, et al. COVID‐19 patients and the radiology department – advice from the European Society of Radiology (ESR) and the European Society of Thoracic Imaging (ESTI). Eur Radiol. 2020;30(9):4903‐4909. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Gibson NS, Sohne M, Kruip MJHA, et al. Further validation and simplification of the Wells clinical decision rule in pulmonary embolism. Thromb Haemost. 2008;99(01):229‐234. [DOI] [PubMed] [Google Scholar]
- 26. DeLong ER, DeLong DM, Clarke‐Pearson DL. Comparing the areas under two or more correlated receiver operating characteristic curves: a nonparametric approach. Biometrics. 1988;44(3):837‐845. [PubMed] [Google Scholar]
- 27. Vandenbroucke JP, von Elm E, Altman DG, et al. Strengthening the reporting of observational studies in epidemiology (STROBE): explanation and elaboration. Ann Intern Med. 2007;147(8):W163‐W194. [DOI] [PubMed] [Google Scholar]
- 28. Grillet F, Behr J, Calame P, Aubry S, Delabrousse E. Acute Pulmonary Embolism Associated with COVID‐19 Pneumonia Detected with Pulmonary CT Angiography. Radiology. 2020;296(3):E186–E188. 10.1148/radiol.2020201544 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Thoreau B, Galland J, Delrue M, et al. D‐Dimer Level and Neutrophils Count as Predictive and Prognostic Factors of Pulmonary Embolism in Severe Non‐ICU COVID‐19 Patients. Viruses. 2021;13(5):758. 10.3390/v13050758 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Chen J, Wang X, Zhang S, et al. Characteristics of acute pulmonary embolism in patients with COVID‐19 associated pneumonia from the city of Wuhan. Clin Appl Thromb Hemost. 2020;26:1076029620936772. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Wells P, Anderson D, Rodger M, et al. Derivation of a simple clinical model to categorize patients probability of pulmonary embolism: increasing the models utility with the SimpliRED D‐dimer. Thromb Haemost. 2000;83(3):416‐420. [PubMed] [Google Scholar]
- 32. Le Gal G, Righini M, Roy P‐M, et al. Prediction of pulmonary embolism in the emergency department: the revised Geneva score. Ann Intern Med. 2006;144(3):165‐171. [DOI] [PubMed] [Google Scholar]
- 33. Helms J, Tacquard C, Severac F, et al. High risk of thrombosis in patients with severe SARS‐CoV‐2 infection: a multicenter prospective cohort study. Intensive Care Med. 2020;46:1089‐1098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. de Boer O, Li X, Teeling P, et al. Neutrophils, neutrophil extracellular traps and interleukin‐17 associate with the organisation of thrombi in acute myocardial infarction. Thromb Haemost. 2013;109(2):290‐297. [DOI] [PubMed] [Google Scholar]
- 35. Fuchs TA, Brill A, Wagner DD. Neutrophil extracellular trap (NET) impact on deep vein thrombosis. Arterioscler Thromb Vasc Biol. 2012;32(8):1777‐1783. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Chen G, Wu DI, Guo W, et al. Clinical and immunological features of severe and moderate coronavirus disease 2019. J Clin Invest. 2020;130(5):2620‐2629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Qin C, Zhou L, Hu Z, et al. Dysregulation of immune response in patients with coronavirus 2019 (COVID‐19) in Wuhan, China. Clin Infect Dis. 2020;71(15):762‐768. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. de la Rica R, Borges M, Gonzalez‐Freire M. COVID‐19: in the eye of the cytokine storm. Front Immunol. 2020;11:558898. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Brosnahan SB, Smilowitz NR, Amoroso NE, et al. Thrombosis at hospital presentation in patients with and without coronavirus disease 2019. J Vasc Surg Venous Lymphat Disord. 2020. 10.1016/j.jvsv.2020.11.004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Ackermann M, Verleden SE, Kuehnel M, et al. Pulmonary vascular endothelialitis, thrombosis, and angiogenesis in Covid‐19. N Engl J Med. 2020;383(2):120‐128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Spyropoulos AC, Levy JH, Ageno W, et al. Scientific and standardization committee communication: clinical guidance on the diagnosis, prevention, and treatment of venous thromboembolism in hospitalized patients with COVID‐19. J Thromb Haemost. 2020;18(8):1859‐1865. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Voicu S, Bonnin P, Stépanian A, et al. High prevalence of deep vein thrombosis in mechanically ventilated COVID‐19 patients. J Am Coll Cardiol. 2020;76:480‐482. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Figure S1‐S2
Table S1‐S3
Data Availability Statement
This article adheres to community standards for full data disclosure.


