Abstract
Aims:
To identify factors associated with vascular events in patients with giant cell arteritis (GCA).
Methods:
We performed a retrospective study of GCA patients diagnosed over a 20-year-period, who all underwent vascular imaging evaluation at diagnosis. Symptomatic vascular events were defined as the occurrence of any aortic event (aortic dissection or symptomatic aortic aneurysm), stroke, myocardial infarction, limb or mesenteric ischemia and de novo lower limbs arteritis stage 3 or 4. Patients with symptomatic vascular event (VE+) and without were compared, and risk factors were identified in a multivariable analysis.
Results:
Thirty-nine (15.4%) of the 254 included patients experienced at least one symptomatic vascular event during follow-up, with a median time of 21.5 months. Arterial hypertension, diabetes, lower limbs arteritis or vascular complication at diagnosis were more frequent in VE+ patients (p < 0.05), as an abnormal computed tomography (CT)-scan at diagnosis (p = 0.04), aortitis (p = 0.01), particularly of the descending thoracic aorta (p = 0.03) and atheroma (p = 0.03). Deaths were more frequent in the VE+ group (37.1 versus 10.3%, p = 0.0003). In multivariable analysis, aortic surgery [hazard ratio (HR): 10.46 (1.41–77.80), p = 0.02], stroke [HR: 22.32 (3.69–135.05), p < 0.001], upper limb ischemia [HR: 20.27 (2.05–200.12), p = 0.01], lower limb ischemia [HR: 76.57 (2.89–2027.69), p = 0.009], aortic atheroma [HR: 3.06 (1.06–8.82), p = 0.04] and aortitis of the descending thoracic aorta on CT-scan at diagnosis [HR: 4.64 (1.56–13.75), p = 0.006] were independent predictive factors of a vascular event.
Conclusion:
In this study on GCA cases with large vessels imaging at diagnosis, aortic surgery, stroke, upper or lower limb ischemia, aortic atheroma and aortitis of the descending thoracic aorta on CT-scan, at GCA diagnosis, were independent predictive factors of a vascular event.
Plain language summary
Risk factors for symptomatic vascular events in giant cell arteritis
This study was performed to identify the risk factors for developing symptomatic vascular event during giant cell arteritis (GCA) because these are poorly known.
We performed a retrospective study of GCA patients diagnosed over a 20-year-period, who all underwent vascular imaging evaluation at diagnosis.
Patients with symptomatic vascular event (VE+) and without (VE-) were compared, and risk factors were identified in a multivariable analysis.
Thirty-nine patients experienced at least one symptomatic vascular event during follow-up, with a median time of 21.5 months.
Arterial hypertension, diabetes, lower limbs arteritis or vascular complication at diagnosis were significantly more frequent in VE+ patients, as an abnormal CT-scan at diagnosis, aortitis, particularly of the descending thoracic aorta and atheroma. Deaths were more frequent in the VE+ group.
Among 254 GCA patients, 39 experienced at least one vascular event during follow-up.
Aortic surgery, stroke, upper and lower limb ischemia were vascular event risk factors.
Aortic atheroma and descending thoracic aorta aortitis on CT-scan were vascular event risk factors.
This study on GCA cases with large vessels imaging at diagnosis, showed that aortic surgery, stroke, upper or lower limb ischemia, aortic atheroma and aortitis of the descending thoracic aorta on CT-scan, at GCA diagnosis, were independent predictive factors of a vascular event.
Keywords: aortitis, giant cell arteritis, large-vessel imaging, risk factors, vascular event
Introduction
Giant cell arteritis (GCA) is a form of vasculitis primarily affecting people over age 50 years, affecting cranial arteries and also extracranial large vessels, mainly aorta and supra-aortic trunks. In this affection, parietal vascular inflammation may favor arterial structural lesions such as luminal occlusions causing ischemic complications, or arterial dilatations/aneurysms, especially on the aorta, leading to potential lethal aortic dissection. Cardiovascular and cerebrovascular mortality are 1.5–2 times greater than in the general population, 1 with increased risk for myocardial infarction, stroke and peripheral vascular ischemia.2,3 In addition, traditional cardiovascular risk factors are frequent in GCA patients, and also promote ischemic complications. 4 The risk of an aortic aneurysm occurrence in GCA patients, particularly a thoracic aneurysm, is 2–17.3 times5,6 higher than in the general population.
Moreover, aortic dissection, which is one complication of these aneurysms, is associated with an increased mortality.7,8 Very few and controversial studies have been conducted so far trying to identify predictive factors of large-artery complications in GCA patients.7,9–11
The improvement of imaging techniques has made it possible to better describe vascular abnormalities, particularly aortic involvement, present from the time of diagnosis, and this has led to recommendations for carrying out these examinations.12,13
The aim of this study was to identify factors associated with subsequent vascular events in a cohort of GCA patients who underwent systematic large-vessel imaging assessment at diagnosis.
Methods
Patients and definitions
In this monocentric retrospective cohort study, GCA patients, diagnosed between April 1998 and April 2018, were included.
Patients fulfilled the GCA American College of Rheumatology (ACR) 1990 criteria or they had age over 50 years, C-reactive protein >10 mg/L and one or more GCA clinical signs and involvement of large vessels with wall inflammation on imaging.12–14
Each patient had an imaging assessment of large vessels at diagnosis of GCA, by CT-scan, and/or positron emission tomography (PET)-scan and/or angio-magnetic resonance imaging (MRI). Patients had to have a minimum follow-up of 6 months. Clinical, biological and radiological data were collected in a standardized anonymized electronic form.
The choice of treatment was at the discretion of the physician and respected current recommendations.12,15,16 In this monocentric study, the tapering in corticosteroid treatment was standardized with an initial dose between 0.7 and 1 mg/kg per day with a decrease of 10 mg every 2– 3 weeks initially. In the case of cortisone sparing or relapse requiring immunosuppressive treatments, methotrexate was used as first-line treatment at 0.3 mg/kg per week in the absence of contraindications. Three investigators reviewed medical records. When performed, Doppler ultrasound data were also recorded.
GCA large vessel involvement at CT-scan was defined by: an inflammatory involvement of the vessel wall described by the radiologist (with a circumferential parietal thickening), aortic ectasia, aneurysm, dissection, stenosis and thrombus, atheroma excepted.
An aortic involvement at diagnosis was defined by aortitis (circumferential aortic parietal thickening >2.2 mm on CT-scan/MRI, and/or a grade 2 or 3 parietal aortic hypermetabolism on PET-scan), aneurysm (a thoracic aortic diameter > 4 cm or abdominal aorta diameter >3 cm) or by aortic dissection.
The 18F-fluorodeoxyglucose (FDG) uptakes were analyzed with visual grading. FDG uptake in aorta and its main branches was graded according to liver FDG uptake: grade 0, no vascular uptake; grade 1, vascular uptake less than liver uptake; grade 2, vascular uptake equal to liver uptake; grade 3, vascular uptake greater than liver uptake. 17
On CT-scan and Doppler ultrasound, inflammatory involvement of the peripheral arteries was defined by long circumferential hypoechoic thickening greater than 1 mm without atherosclerotic lesion (calcification or irregular soft atheroma). Atheroma was evaluated for each patient in iliac, common femoral, subclavian and axillary arteries for atheromatous lesions according to Berthod et al. 18 on CT-scan or Tatsumi et al. 19 if patients had only PET-scan. In addition, for patients with peripheral CT-scan, the same methodology was applied. Moreover, ultrasound scans were performed by OE, JC, MA and CD, who are physicians with extensive experience in large vessel vasculitis (LVV).
Symptomatic vascular events were defined as the occurrence of any symptomatic aortic event (dissection, aneurysm rupture, or development of a symptomatic aneurysm with dyspnea, thoracic or abdominal pain, requiring emergency surgery or an increase in specific treatment like immunosuppressive drugs), stroke, myocardial infarction, limb or digestive ischemia and de novo lower limbs arteritis stage 3 or 4. Vascular events occurring at GCA diagnosis were not taken into account to form groups with (VE+) or without (VE−) symptomatic vascular events; only vascular events occurring during follow-up were taken into account to classify patients in VE+ or VE− groups.
This study has been conducted in compliance with the Declaration of Helsinki principles and has received ethics board approval by GNEDS (Groupe Nantais d’Ethique et de Soin), the local ethics committee of the University Hospital of Nantes (20200108). Each patient included in this study received written information and no patient objected to this study. The need for written informed consent was waived by the ethics committee because of the retrospective study design (French public health code article: L 1121-1).
Statistical analysis
Quantitative variables were described as median (Quartile 1–Quartile 3) and mean (±SD). Qualitative variables were described as number (%). The two patient groups with symptomatic vascular events (VE+) and without (VE−) were compared using the Fisher exact test for categorical variables and Student tests (Mann–Whitney test when n < 30) for quantitative variables. Univariable Cox model was used to identify factors associated with a vascular event. All baseline variables with a p-value <0.20 in univariable analysis and cardiovascular risk factors, statin and antiplatelet drug at diagnosis, 20 variables already known to be confounding factors of vascular events, were included in multivariable Cox model. The hazard ratio (HR) and the confidence intervals are presented in the tables. No correction for multiple testing was performed. All p-values were two-sided and p-values <0.05 were considered statistically significant.
The multivariable analysis has been performed on 229 patients who had a CT-scan, for quality and statistical consistency. The statistical analysis was performed with SPSS v.26 (IBM Corp.).
Results
Cohort description
This study included 254 patients (Figure 1) [186 women (73.2%)], with a median follow-up of 32.5 months. Median age at diagnosis was 72 years. In terms of cardiovascular risk factors, obesity was present in 8% of patients, arterial hypertension in 42.5%, diabetes mellitus in 11% and hypercholesterolemia in 23.2%.
Figure 1.
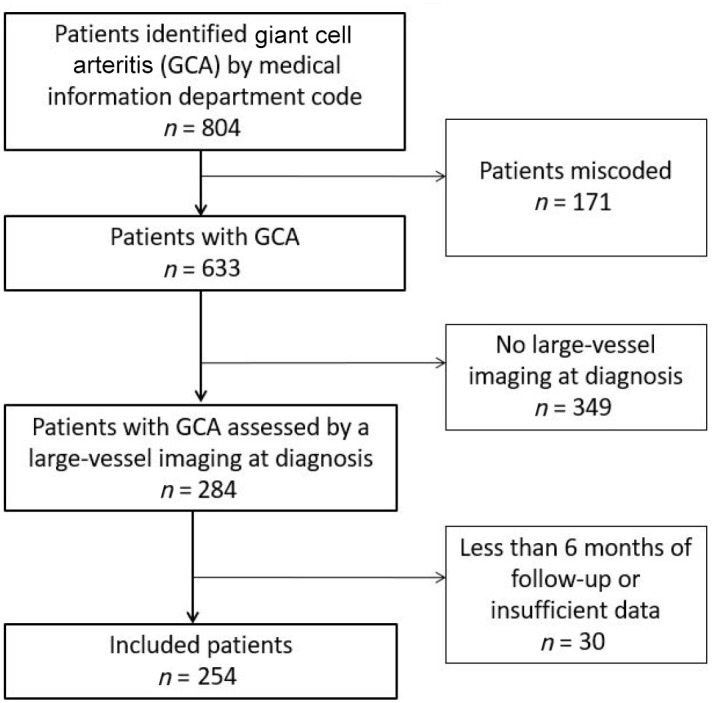
Flow-chart of GCA patients selection.
One hundred and sixty patients (63%) had a large-vessel involvement, all types of imaging included; 229 patients had a CT-scan at diagnosis. On CT-scan, 110 patients had aortitis, with a mean aorta wall thickening of 4.2 mm ± 1.9 and 40 patients had aortic aneurysm or ectasia.
Among the 79 patients who had a PET-scan, 56 (70.9%) had an inflammatory involvement, as 69.2% of the 13 patients who had an angio-MRI, 43.4% of the 122 patients who had a supra-aortic trunks Doppler ultrasound and 36.1% of the 97 patients who had a lower limbs and aortic Doppler ultrasound.
Vascular events
Thirty-nine patients (15.4%) experienced at least one vascular event during follow-up, with a median time to event of 21.5 months. Aortic complication occurred in 7.1% of patients (n = 18), limb ischemia in 5.5% (n = 14), myocardial ischemia in 13 patients, stroke in 12 and de novo lower limbs arteritis stage 3 or 4 in nine. Mesenteric ischemia happened in two patients.
At GCA diagnosis, 10 patients (4%) had aortic surgery, six (2.4%) had stroke, six (2.4%) upper limb ischemia, four (1.6%) lower limb ischemia, one (0.4%) myocardial infarction and one (0.4%) mesenteric ischemia. Patients who had a vascular event during follow-up had significantly more aortic surgery, stroke, and upper and lower limb ischemia at GCA diagnosis.
Among the 10 patients who had aortic surgery at diagnosis, five experienced a new aortic event during follow-up; one of them had also stroke and another one myocardial ischemia. Moreover, among the five patients who did not experience a new aortic event, one experienced myocardial ischemia and limb ischemia.
Three of the six patients who had stroke at diagnosis experienced a new stroke during follow-up. Three (50%) of the patients with an upper limb ischemia at diagnosis had a new limb ischemia during follow-up, as three (75%) of the patients who had limb ischemia at diagnosis.
Comparison of the groups
The comparison of clinical (including vascular events at the start of the study) and biological characteristics at diagnosis is summarized in Table 1: arterial hypertension (p = 0.03), diabetes (p = 0.02), lower limb arteritis (p = 0.001), aortic surgery (p = 0.001), stroke (p = 0.049), upper limb ischemia (p = 0.049) and lower limb ischemia (p = 0.01) at diagnosis were significantly more frequent in VE+ patients, as follow-up duration was longer (p = 0.03).
Table 1.
Comparison of clinical and biological characteristics at diagnosis in giant cell arteritis patients with or without vascular event during follow-up.
| Without vascular event n = 215 n/available data (%) |
With vascular event n = 39 n/available data (%) |
p-value | |
|---|---|---|---|
| Clinical characteristics | |||
| • Mean age at diagnosis | 71.9 ± 8.7 | 72.3 ± 8.2 | 0.79 |
| • Male | 58/215 (27.0) | 10/39 (25.6) | >0.99 |
| • Arterial hypertension | 85/215 (39.5) | 23/39 (59.0) | 0.03 |
| • Hypercholesterolemia | 46/215 (21.4) | 13/39 (33.3) | 0.14 |
| • Smoking | 32/215 (14.9) | 5/39 (12.8) | >0.99 |
| • Diabetes mellitus | 19/215 (8.8) | 9/39 (23.1) | 0.02 |
| • Obesity, BMI >30 kg/m2 | 14/212 (6.6) | 6/39 (15.4) | 0.10 |
| • Positive temporal artery biopsy | 140/210 (66.7) | 27/38 (71.1) | 0.70 |
| • Temporal artery tenderness or swelling | 60/202 (29.7) | 5/34 (14.7) | 0.09 |
| • Weight loss | 100/212 (47.2) | 15/39 (38.5) | 0.38 |
| • Mean weight loss, kg | 2.9 ± 3.5 | 3.0 ± 4.4 | 0.90 |
| • Fever | 68/214 (31.8) | 14/38 (36.8) | 0.57 |
| • Headaches at diagnosis | 131/214 (61.2) | 19/39 (48.7) | 0.15 |
| • Jaw claudication | 60/214 (28.0) | 8/39 (20.5) | 0.43 |
| • Scalp tenderness | 50/213 (23.5) | 8/39 (20.5) | 0.83 |
| • Ophthalmological signs | 40/214 (18.7) | 6/39 (15.4) | 0.82 |
| • Polymyalgia rheumatica | 49/213 (23.0) | 7/38 (18.4) | 0.67 |
| • Peripheral musculoskeletal manifestation | 55/204 (27.0) | 9/38 (23.7) | 0.84 |
| • Cough | 40/211 (19.0) | 8/38 (21.1) | 0.82 |
| • Chest pain | 17/213 (8.0) | 4/39 (10.3) | 0.54 |
| • Abdominal pain | 12/209 (5.7) | 1/38 (2.6) | 0.69 |
| Vascular events at diagnosis | |||
| • Aortic surgery | 4/212 (1.9) | 6/39 (15.4) | 0.001 |
| • Stroke | 3/213 (1.4) | 3/39 (7.7) | 0.049 |
| • Upper limb ischemia | 3/212 (1.4) | 3/39 (7.7) | 0.049 |
| • Lower limb ischemia | 1/211 (0.5) | 3/39 (7.7) | 0.01 |
| • Myocardial infarction | 1/212 (0.5) | 0/39 (0.0) | >0.99 |
| • Mesenteric ischemia | 0/212 (0.0) | 1/39 (2.6) | 0.15 |
| • Lower limbs arteritis stage II/III/IV | 11/213 (5.2) | 9/39 (23.1) | 0.001 |
| Biological characteristics | |||
| • ESR, mm/h, mean ± σ (median) | 78.5 ± 33.8 (80) | 80.3 ± 25.8 (79) | 0.90 |
| • CRP, mg/L, mean ± σ (median) | 105.3 ± 79.1 (85) | 112.1 ± 73.7 (95) | 0.62 |
| • Hb, g/dL, mean ± σ (median) | 11.2 ± 1.6 (11.3) | 11.0 ± 1.7 (10.5) | 0.37 |
| • Platelets, G/L, mean ± σ (median) | 412.1 ± 133.7 (401) | 413.8 ± 169.0 (406.5) | 0.94 |
| • Follow-up duration, months, mean ± σ (median) | 49.7 ± 99.2 (28) | 85.4 ± 61.9 (65) | 0.03 |
Data are expressed as mean ± σ (median) and number (percentage), n (%). Bold p value are <0.05.
BMI, body mass index; CRP, C-reactive protein; ESR, erythrocyte sedimentation rate; Hb, hemoglobin.
Median time between diagnosis and initiation of corticosteroids did not differ between the two groups (0 days, p > 0.99), with an initial dose of corticosteroids at 0.8 ± 0.2 (median 0.7 mg/kg per day) in the VE− group and 0.8 ± 0.2 (median 0.9 mg/kg per day) in the VE+ group (p = 0.36). VE− patients received methylprednisolone infusions at diagnosis in 16.4% of cases versus 12.8% for the VE+ (p = 0.81) and 60.5% of VE+ patients received an antiplatelet drug at diagnosis versus 57.9% (p = 0.85) of VE− patients (Table 2). An immunosuppressive drug was used during follow-up in 46 patients [26.5% of VE+ versus 24.5% of VE− (p = 0.82)], in the context of a first relapse in 26 patients, a second relapse in 13 patients, a third relapse in three patients and early corticoresistance in one patient.
Table 2.
GCA treatments at diagnosis and during follow-up.
| Without vascular event n = 215 n/available data (%) |
With vascular event n = 39 n/available data (%) |
p-value | |
|---|---|---|---|
| Delay between diagnosis and onset of corticotherapy, days | 11.8 ± 54.2 (0) | 11.8 ± 23.6 (0) | >0.99 |
| Initial dose/weight of prednisone, mg/kg per day) | 0.8 ± 0.2 (0.7) | 0.8 ± 0.2 (0.9) | 0.36 |
| Methylprednisolone infusion at diagnosis | 35/213 (16.4%) | 5/39 (12.8%) | 0.81 |
| Immunosuppressive drug at diagnosis | 8/208 (3.8%) | 0/38 (0.0%) | 0.61 |
| Platelet antiaggregant at diagnosis | 121/209 (57.9%) | 23/38 (60.5%) | 0.85 |
| Statin at diagnosis | 40/208 (19.2%) | 13/38 (34.2%) | 0.05 |
| Conversion enzyme inhibitor at diagnosis | 23/201 (11.4%) | 7/37 (18.9%) | 0.27 |
| Prednisone dose at month 3, mg/day | 21.4 ± 10.8 (20) | 25.3 ± 16.0 (20) | 0.07 |
| Prednisone dose at month 6, mg/day | 11.0 ± 6.8 (10) | 15.4 ± 12.6 (10) | 0.06 |
| Prednisone dose at month 12, mg/day | 6.1 ± 5.5 (5) | 6.6 ± 4.2 (6) | 0.14 |
| Prednisone dose at month 18, mg/day | 4.3 ± 5.9 (5) | 5.6 ± 4.1 (5) | 0.02 |
| Prednisone dose at month 24, mg/day | 4.0 ± 6.9 (3) | 4.8 ± 4.9 (5) | 0.18 |
| Prednisone dose >5 mg/day in the latest news | 33/174 (19.0%) | 4/30 (13.3%) | 0.61 |
| Prednisone dose = 0 mg/day in the latest news | 77/173 (44.5%) | 16/31 (51.6%) | 0.55 |
| Use of an immunosuppressive drug during follow-up | 45/184 (24.5%) | 9/34 (26.5%) | 0.82 |
| Delay between diagnosis and the use of an immunosuppressive drug, months | 11.8 ± 14.1 (5) | 22.6 ± 24.6 (12.5) | 0.04 |
Data are expressed as mean ± σ (median) and number (percentage), n (%). Bold p value are <0.05.
Among the 54 patients who received an immunosuppressive drug, 39 received methotrexate, seven hydroxychloroquine, six azathioprine, five tocilizumab, two cyclophosphamide and one leflunomide.
CT-scan characteristics at diagnosis are shown in Table 3: GCA large vessel involvement at diagnosis (p = 0.04), aortitis (p = 0.01), particularly of the descending thoracic aorta (p = 0.03) and atheroma (p = 0.03) were more frequent in VE+ patients.
Table 3.
CT-scan data at diagnosis.
| Without vascular event n = 215 n/available data (%) |
With vascular event n = 39 n/available data (%) |
p-value | |
|---|---|---|---|
| GCA large vessel involvement at CT-scan a | 97/193 (50.3) | 25/36 (69.4) | 0.04 |
| Atheroma | 38/145 (26.2) | 14/30 (46.7) | 0.03 |
| Aortitis | 86/175 (49.1) | 24/32 (75.0) | 0.01 |
| Descending thoracic aorta | 67/175 (38.3) | 18/30 (60.0) | 0.03 |
| Ascending thoracic aorta | 59/173 (34.1) | 9/29 (31.0) | 0.83 |
| Abdominal aorta | 57/167 (34.1) | 14/30 (46.7) | 0.21 |
| Aortic ectasia | 11/176 (6.3) | 3/32 (9.4) | 0.45 |
| Aortic aneurysm | 23/177 (13.0) | 8/32 (25.0) | 0.10 |
| Aortic dissection | 5/176 (2.8) | 3/32 (9.4) | 0.10 |
| Aortic stenosis | 2/174 (1.1) | 1/32 (3.1) | 0.39 |
| Aortic thrombus | 5/175 (2.9) | 2/32 (6.3) | 0.29 |
| Inflammation of carotids | 13/67 (19.4) | 3/12 (25.0) | 0.70 |
| Inflammation of the brachiocephalic trunk | 29/141 (20.6) | 2/13 (15.4) | 0.99 |
| Inflammation of arm arteries | 33/147 (22.4) | 8/18 (44.4) | 0.08 |
| Inflammation of lower limbs arteries | 29/149 (19.5) | 5/20 (25.0) | 0.55 |
GCA large vessel involvement at CT-scan: inflammation of the vessel wall, aortic ectasia, aneurysm, dissection, stenosis and thrombus, atheroma excepted. Bold p value are <0.05.
CT, computed tomography; GCA, giant cell arteritis.
The comparison of vascular involvement in the two groups, for patients who had PET-scan and/or angio-MRI, did not show any difference. Concerning Doppler ultrasound (Supplemental material Table 1 online), we found more inflammatory involvement in the VE+ group at diagnosis in popliteal arteries (33.3% versus 8.6%, p = 0.02).
The characteristics of the groups according to the presence of at least three ACR criteria for GCA or LVV without ACR criteria are presented in the Supplemental Table 2. The occurrence of vascular events during follow-up was not significantly different between these groups.
Predictors of symptomatic vascular event
In multivariable Cox analysis (Table 4), aortic surgery, upper limb ischemia, lower limb ischemia, aortic atheroma and aortitis of the descending thoracic aorta on CT-scan at diagnosis were associated with the occurrence of vascular complication during follow-up. Baseline variables assessed in the model are shown in Supplemental Table 3.
Table 4.
Variables associated with a vascular event in giant-cell arteritis in a Cox proportional hazard model.
| Variables at diagnosis | Univariate HR (95% CI) | p | Multivariate HR (95% CI) | p-value |
|---|---|---|---|---|
| Arterial hypertension | 1.68 (0.84–3.33) | 0.14 | 1.32 (0.44-3.90) | 0.62 |
| Hypercholesterolemia | 1.98 (0.96–4.09) | 0.07 | 2.12 (0.65–6.94) | 0.21 |
| Diabetes mellitus | 2.14 (0.92–4.95) | 0.07 | 2.77 (0.86–8.96) | 0.09 |
| Aortic surgery | 7.27 (2.94–17.97) | <0.0001 | 10.46 (1.41–77.80) | 0.02 |
| Stroke | 7.76 (2.30–26.22) | 0.001 | 22.32 (3.69–135.05) | <0.001 |
| Upper limb ischemia | 8.91 (2.61–30.44) | <0.0005 | 20.27 (2.05–200.12) | 0.01 |
| Lower limb ischemia | 5.28 (1.24–22.52) | 0.02 | 76.57 (2.89–2027.69) | 0.009 |
| Lower limbs arteritis stage II/III/IV | 3.80 (1.70–8.48) | 0.001 | 3.18 (0.85–11.91) | 0.086 |
| Atheroma at CT-scan | 1.95 (0.91–4.18) | 0.09 | 3.06 (1.06–8.82) | 0.04 |
| Aortic aneurysm | 1.93 (0.82–4.57) | 0.13 | 2.09 (0.48–9.01) | 0.32 |
| Aortic dissection | 2.84 (0.67–12.08) | 0.16 | 2.74 (0.22–34.69) | 0.44 |
| Aortitis of descending thoracic aorta | 2.06 (0.95–4.44) | 0.07 | 4.64 (1.56–13.75) | 0.006 |
| Aortitis of abdominal aorta | 2.00 (0.92–4.34) | 0.08 | 0.64 (0.20–2.03) | 0.45 |
| Statin at diagnosis | 2.56 (1.20–5.46) | 0.01 | 0.72 (0.13–4.08) | 0.71 |
| Antiplatelet drug at diagnosis | 1.47 (0.71–3.08) | 0.30 | 3.27 (0.84–12.70) | 0.09 |
CI, confidence interval; CT, computed tomography; HR, hazard ratio. Bold p value are <0.05.
Follow-up and deaths
One hundred and seventeen of our 254 patients relapsed at least once during follow-up, with 59% (23/39) of VE+ patients versus 43.7% (94/215) of VE− patients (p = 0.08). Among relapsing patients, 30.4% of VE+ patients relapsed more than two times, versus 15.9% of VE− (p = 0.14).
At the end of the follow-up, the number of deaths in the VE+ group was higher (37.1% versus 10.3%, p = 0.0003), with vascular deaths in 50% of cases in the VE+ group versus none in the VE− (p = 0.08).
Discussion
To our knowledge, our study is the first to assess the factors associated with vascular complications during GCA in a large cohort of patients who all had a detailed arterial evaluation at diagnosis.
First, in arterial evaluation, the interpretation of the images is sometimes complex. There are typical images of aortitis or atheromatous lesion on CT-scan, PET-scan and angio-MRI, but sometimes some soft atheromatous lesions are difficult to distinguish from inflammatory lesions.19,21–24 Moreover, calcifications can be found in atheromatous plaques as in old inflammatory lesions too.25,26
Interestingly, where our results showed that 15% of cases experienced at least one vascular event during follow-up, with a median time to event of 21.5 months, a retrospective cohort of 168 patients from 1950 to 1998 found 27% of vascular complications. 9 These included 18% aortic complications and 13% stenosis of other large vessels, with a longer median follow-up (7.6 years versus 32.5 months here). However, in this study, complications detected within 1 year prior to the diagnosis of GCA or thereafter were taken into account: this difference in the protocol might also explain the greater number of complications.
Nuenninghoff et al. 9 found cranial symptoms (headache, scalp hypersensitivity, abnormal temporal arteries) to be protective factors for large-artery stenosis (HR: 0.10), as well as an increased erythrocyte sedimentation rate (HR: 0.80). Hyperlipidemia and coronary artery disease were associated with aortic aneurysm and/or dissection (p < 0.05 for both). However, in that study, 96.1% of the patients had a positive cranial artery biopsy at diagnosis (so more cranial forms) versus 67.3% in ours.
In our study, hypertension, diabetes, lower limb arteritis, and a vascular event at diagnosis were significantly more frequent in VE+ patients. This is consistent with the work of Gonzalez-Gay et al., in which the presence of traditional cardiovascular risk factors at the time of diagnosis of GCA significantly increased the risk of developing at least one severe ischemic complication (odds ratio = 1.79). 4
In our study, in multivariate analysis, aortic surgery (HR: 10.5), stroke (HR: 22.3), upper limb ischemia (HR: 20.3) and lower limb ischemia (HR: 76.6) at diagnosis were risk factors for vascular events during follow-up. The presence of a prior vascular disease, favored by cardiovascular risk factors, leading to atheroma lesions, could promote the emergence of large-vessel complications. A synergistic effect of atheromatous and inflammatory arteritis, with a more severe and earlier involvement (vascular events at diagnosis) in patients who will present a vascular event during follow-up, may even be suggested, 27 hence the importance of atheroma and therefore the control of cardiovascular risk factors. Thus, associated with a specific treatment of GCA, it seems very important to handle carefully cardiovascular risk factors, especially diabetes and hypertension, in order to limit the occurrence of aneurysmal evolution 28 and vascular events.
CT-scan at diagnosis was more often abnormal in VE+ patients, with an aortitis and in particular of the descending thoracic part of the artery. Multivariate analysis confirmed the aortitis of the descending thoracic aorta on CT-scan at diagnosis as a risk factor for vascular event during follow-up (HR: 4.6). The presence of aortitis at diagnosis may indicate an extensive extracranial involvement of the disease and therefore a particular vascular phenotype may be thought to be inherently different (with more structural abnormalities such as aneurysms, ectasia, thickening). This phenotype would be more prone to dissections, aortic aneurysm ruptures and ischemic complications of large vessels.
Preferential involvement of the aorta with significant parietal thickening in newly diagnosed GCA patients has originally been demonstrated by our group, 29 compared with control patients. It preferentially involved the ascending thoracic aorta, which is also the preferential site of aneurysm in patients with GCA and abdominal aorta.
In the present study, VE+ patients had more frequently atheroma on CT-scan at diagnosis (HR: 3.1). It can sometimes be very challenging to distinguish an authentic atheromatous lesion from an old post-inflammatory lesion with calcification using current imaging techniques. Thus, atheroma could be a stigma of a long-standing disease in a pauci-symptomatic form, or could mark a particular phenotype.
On Doppler evaluation, VE+ patients had a significantly more frequent involvement of the popliteal and digestive arteries, suggesting that the Doppler exploration of these vascular axes, especially since popliteal arteries are not visualized on the aortic CT-scan, is of particular interest. These data are also consistent with our findings: extensive involvement of GCA at diagnosis would then be associated with more vascular events.
Missing data, associated with the retrospective nature of the study, are its main limitation. The choice of treatment was at the discretion of the physician, hence a lack of standardization of treatment tapering modalities, although our results show that the median corticosteroid doses from 3 to 24 months are fully consistent with current recommendations.12,14–16,30,31
An additional limitation is inherent in the presence of cardiovascular risk factors in patients, whose control could not be assessed. Recommendations for the management of these factors have also evolved over time.
Conclusion
In a large cohort of patients who all had a large-vessel imaging assessment at GCA diagnosis, we show that the presence of aortic atheroma, aortitis of descending thoracic aorta at CT-scan and a history of aortic surgery, stroke, or upper or lower limb ischemia at diagnosis are associated with the development of symptomatic vascular complication. These results must be confirmed on a prospective cohort, but they support the importance for prognosis of assessing large vessels at diagnosis, and implementing strict control of GCA and cardiovascular risk factors, especially diabetes and hypertension.
Supplemental Material
Supplemental material, sj-docx-1-tab-10.1177_1759720X211006967 for Risk factors for symptomatic vascular events in giant cell arteritis: a study of 254 patients with large-vessel imaging at diagnosis by Donatienne de Mornac, Christian Agard, Jean-Benoit Hardouin, Mohamed Hamidou, Jérôme Connault, Agathe Masseau, Alexandra Espitia-Thibault, Mathieu Artifoni, Chan Ngohou, François Perrin, Julie Graveleau, Cécile Durant, Pierre Pottier, Antoine Néel and Olivier Espitia in Therapeutic Advances in Musculoskeletal Disease
Footnotes
Author contributions: Donatienne de Mornac: study concept and design; acquisition of data; analysis and interpretation of data; drafting of the manuscript. Christian Agard: study concept and design; acquisition of data; analysis and interpretation of data; drafting of the manuscript. Jean-Benoit Hardouin: methodology, formal analysis. Mohamed Hamidou: critical review, commentary and revision. Jérôme Connault: critical review, commentary and revision. Agathe Masseau: critical review, commentary and revision. Alexandra Espitia-Thibault: critical review, commentary and revision. Mathieu Artifoni: critical review, commentary and revision. Chan Ngohou: methodology. François Perrin: critical review, commentary and revision. Julie Graveleau: critical review, commentary and revision. Cécile Durant: critical review, commentary and revision. Pierre Pottier: critical review, commentary and revision. Antoine Néel: critical review, commentary and revision. Olivier Espitia: study concept and design; acquisition of data; analysis and interpretation of data; drafting of the manuscript; supervision.
Conflict of interest statement: The authors declare that there is no conflict of interest.
Funding: This research received no specific grant from any funding agency in the public, commercial, or not-for-profit sectors.
ORCID iDs: Donatienne de Mornac  https://orcid.org/0000-0003-4133-1474
https://orcid.org/0000-0003-4133-1474
Olivier Espitia  https://orcid.org/0000-0003-0821-9990
https://orcid.org/0000-0003-0821-9990
Supplemental material: Supplemental material for this article is available online.
Contributor Information
Donatienne de Mornac, Department of Internal Medicine, CHU Nantes, Nantes, France.
Christian Agard, Department of Internal Medicine, CHU Nantes, Nantes, France.
Jean-Benoit Hardouin, INSERM UMR 1246-SPHERE, Nantes University, Tours University, France; Methodology and Biostatistics Platform, Nantes University Hospital, Nantes, France.
Mohamed Hamidou, Department of Internal Medicine, CHU Nantes, Nantes, France.
Jérôme Connault, Department of Internal Medicine, CHU Nantes, Nantes, France.
Agathe Masseau, Department of Internal Medicine, CHU Nantes, Nantes, France.
Alexandra Espitia-Thibault, Department of Internal Medicine, CHU Nantes, Nantes, France.
Mathieu Artifoni, Department of Internal Medicine, CHU Nantes, Nantes, France.
Chan Ngohou, Department of Medical Information, Nantes University Hospital, Nantes, France.
François Perrin, Department of Internal Medicine, Saint-Nazaire Hospital, France.
Julie Graveleau, Department of Internal Medicine, Saint-Nazaire Hospital, France.
Cécile Durant, Department of Internal Medicine, CHU Nantes, Nantes, France.
Pierre Pottier, Department of Internal Medicine, CHU Nantes, Nantes, France.
Antoine Néel, Department of Internal Medicine, CHU Nantes, Nantes, France.
Olivier Espitia, Department of Internal Medicine, CHU Nantes, 1 place Alexis Ricordeau, Nantes, 44093, France.
References
- 1. Uddhammar A, Eriksson A-L, Nyström L, et al. Increased mortality due to cardiovascular disease in patients with giant cell arteritis in northern Sweden. J Rheumatol 2002; 29: 737–742. [PubMed] [Google Scholar]
- 2. Amiri N, De Vera M, Choi HK, et al. Increased risk of cardiovascular disease in giant cell arteritis: a general population-based study. Rheumatology 2016; 55: 33–40. [DOI] [PubMed] [Google Scholar]
- 3. Tomasson G, Peloquin C, Mohammad A, et al. Risk for cardiovascular disease early and late after a diagnosis of giant-cell arteritis: a cohort study. Ann Intern Med 2014; 160: 73–80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Gonzalez-Gay MA, Piñeiro A, Gomez-Gigirey A, et al. Influence of traditional risk factors of atherosclerosis in the development of severe ischemic complications in giant cell arteritis. Medicine 2004; 83: 342–327. [DOI] [PubMed] [Google Scholar]
- 5. Robson JC, Kiran A, Maskell J, et al. The relative risk of aortic aneurysm in patients with giant cell arteritis compared with the general population of the UK. Ann Rheum Dis 2015; 74: 129–135. [DOI] [PubMed] [Google Scholar]
- 6. Evans JM, O’Fallon WM, Hunder GG. Increased incidence of aortic aneurysm and dissection in giant cell (temporal) arteritis. A population-based study. Ann Intern Med 1995; 122: 502–507. [DOI] [PubMed] [Google Scholar]
- 7. Espitia O, Blonz G, Urbanski G, et al. Symptomatic aortitis at giant cell arteritis diagnosis: a prognostic factor of aortic event. Arthritis Res Ther 2021; 23: 14. 10.1186/s13075-020-02396-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Nuenninghoff DM, Hunder GG, Christianson TJH, et al. Mortality of large-artery complication (aortic aneurysm, aortic dissection, and/or large-artery stenosis) in patients with giant cell arteritis: a population-based study over 50 years. Arthritis Rheum 2003; 48: 3532–3537. [DOI] [PubMed] [Google Scholar]
- 9. Nuenninghoff DM, Hunder GG, Christianson TJH, et al. Incidence and predictors of large-artery complication (aortic aneurysm, aortic dissection, and/or large-artery stenosis) in patients with giant cell arteritis: a population-based study over 50 years. Arthritis Rheum 2003; 48: 3522–3531. [DOI] [PubMed] [Google Scholar]
- 10. Espitia O, Néel A, Leux C, et al. Giant cell arteritis with or without aortitis at diagnosis. A retrospective study of 22 patients with longterm followup. J Rheumatol 2012; 39: 2157–2162. [DOI] [PubMed] [Google Scholar]
- 11. Mackie SL, Dasgupta B, Hordon L, et al. Ischaemic manifestations in giant cell arteritis are associated with area level socio-economic deprivation, but not cardiovascular risk factors. Rheumatology 2011; 50: 2014–2022. [DOI] [PubMed] [Google Scholar]
- 12. Bienvenu B, Ly KH, Lambert M, et al. Management of giant cell arteritis: recommendations of the French Study Group for Large Vessel Vasculitis (GEFA). Rev Med Interne 2016; 37: 154–165. [DOI] [PubMed] [Google Scholar]
- 13. Dejaco C, Ramiro S, Duftner C, et al. EULAR recommendations for the use of imaging in large vessel vasculitis in clinical practice. Ann Rheum Dis 2018; 77: 636–643. [DOI] [PubMed] [Google Scholar]
- 14. Stone JH, Tuckwell K, Dimonaco S, et al. Trial of tocilizumab in giant-cell arteritis. N Engl J Med 2017; 377: 317–328. [DOI] [PubMed] [Google Scholar]
- 15. Mackie SL, Dejaco C, Appenzeller S, et al. British Society for Rheumatology guideline on diagnosis and treatment of giant cell arteritis: executive summary. Rheumatology 2020; 59: 487–494. [DOI] [PubMed] [Google Scholar]
- 16. Hellmich B, Agueda A, Monti S, et al. 2018 Update of the EULAR recommendations for the management of large vessel vasculitis. Ann Rheum Dis 2020; 79: 19–30. [DOI] [PubMed] [Google Scholar]
- 17. Slart RHJA, Writing Group, Reviewer Group, Members of EANM Cardiovascular; et al. FDG-PET/CT(A) imaging in large vessel vasculitis and polymyalgia rheumatica: joint procedural recommendation of the EANM, SNMMI, and the PET Interest Group (PIG), and endorsed by the ASNC. Eur J Nucl Med Mol Imaging 2018; 45: 1250–1269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Berthod PE, Aho-Glélé S, Ornetti P, et al. CT analysis of the aorta in giant-cell arteritis: a case-control study. Eur Radiol 2018; 28: 3676–3684. [DOI] [PubMed] [Google Scholar]
- 19. Tatsumi M, Cohade C, Nakamoto Y, et al. Fluorodeoxyglucose uptake in the aortic wall at PET/CT: possible finding for active atherosclerosis. Radiology 2003; 229: 831–837. [DOI] [PubMed] [Google Scholar]
- 20. Antithrombotic Trialists’ (ATT) Collaboration; Colin Baigent, Lisa Blackwell, et al. Aspirin in the primary and secondary prevention of vascular disease: collaborative meta-analysis of individual participant data from randomised trials. Lancet 2009; 373: 1849–1860. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Fernández-Friera L, Fuster V, López-Melgar B, et al. Vascular inflammation in subclinical atherosclerosis detected by hybrid PET/MRI. J Am Coll Cardiol 2019; 73: 1371–1382. [DOI] [PubMed] [Google Scholar]
- 22. Blomberg BA, Thomassen A, Takx RAP, et al. Delayed 18F-fluorodeoxyglucose PET/CT imaging improves quantitation of atherosclerotic plaque inflammation: results from the CAMONA study. J Nucl Cardiol 2014; 21: 588–597. [DOI] [PubMed] [Google Scholar]
- 23. Figueroa AL, Subramanian SS, Cury RC, et al. Distribution of inflammation within carotid atherosclerotic plaques with high-risk morphological features: a comparison between positron emission tomography activity, plaque morphology, and histopathology. Circ Cardiovasc Imaging 2012; 5: 69–77. [DOI] [PubMed] [Google Scholar]
- 24. Journeau L, de la, Chapelle M, Guimard T, et al. A strobe multicenter descriptive study of 55 infectious aortitis. Medicine 2020; 99: e22422. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Craiem D, Chironi DG, Casciaro ME, et al. Association of thoracic aorta calcium and non cardiac vascular events in cardiac disease-free individuals. Atherosclerosis 2016; 245: 22–27. [DOI] [PubMed] [Google Scholar]
- 26. Banerjee S, Bagheri M, Sandfort V, et al. Vascular calcification in patients with large-vessel vasculitis compared to patients with hyperlipidemia. Semin Arthritis Rheum 2019; 48: 1068–1073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. de Boysson H, Liozon E, Espitia O, et al. Different patterns and specific outcomes of large-vessel involvements in giant cell arteritis. J Autoimmun 2019; 103: 102283. [DOI] [PubMed] [Google Scholar]
- 28. Muratore F, Crescentini F, Spaggiari L, et al. Aortic dilatation in patients with large vessel vasculitis: a longitudinal case control study using PET/CT. Semin Arthritis Rheum 2019; 48: 1074–1082. [DOI] [PubMed] [Google Scholar]
- 29. Agard C, Barrier J-H, Dupas B, et al. Aortic involvement in recent-onset giant cell (temporal) arteritis: a case-control prospective study using helical aortic computed tomodensitometric scan. Arthritis Rheum 2008; 59: 670–676. [DOI] [PubMed] [Google Scholar]
- 30. Hoffman GS, Cid MC, Hellmann DB, et al. A multicenter, randomized, double-blind, placebo-controlled trial of adjuvant methotrexate treatment for giant cell arteritis. Arthritis Rheum 2002; 46: 1309–1318. [DOI] [PubMed] [Google Scholar]
- 31. Mahr AD, Jover JA, Spiera RF, et al. Adjunctive methotrexate for treatment of giant cell arteritis: an individual patient data meta-analysis. Arthritis Rheum 2007; 56: 2789–2797. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplemental material, sj-docx-1-tab-10.1177_1759720X211006967 for Risk factors for symptomatic vascular events in giant cell arteritis: a study of 254 patients with large-vessel imaging at diagnosis by Donatienne de Mornac, Christian Agard, Jean-Benoit Hardouin, Mohamed Hamidou, Jérôme Connault, Agathe Masseau, Alexandra Espitia-Thibault, Mathieu Artifoni, Chan Ngohou, François Perrin, Julie Graveleau, Cécile Durant, Pierre Pottier, Antoine Néel and Olivier Espitia in Therapeutic Advances in Musculoskeletal Disease


