Abstract
Background:
Hip arthroscopy has frequently been shown to produce successful outcomes as a treatment for femoroacetabular impingement (FAI) and labral tears. However, there is less literature on whether the favorable results of hip arthroscopy can justify the costs, especially when compared with a nonoperative treatment.
Purpose:
To systematically review the cost-effectiveness of hip arthroscopy for treating FAI and labral tears.
Study Design:
Systematic review; Level of evidence, 3.
Methods:
PubMed/MEDLINE, Embase, and Cochrane Library databases, and the Tufts University Cost-Effectiveness Analysis Registry were searched to identify articles that reported the cost per quality-adjusted life-year (QALY) generated by hip arthroscopy. The key terms used were “hip arthroscopy,” “cost,” “utility,” and “economic evaluation.” The threshold for cost-effectiveness was set at $50,000/QALY. The Methodological Index for Non-Randomized Studies instrument and Quality of Health Economic Studies (QHES) score were used to determine the quality of the studies. This study was prospectively registered on PROSPERO (CRD42020172991).
Results:
Six studies that reported the cost-effectiveness of hip arthroscopy were identified, and 5 of these studies compared hip arthroscopy to a nonoperative comparator. These studies were found to have a mean QHES score of 85.2 and a mean cohort age that ranged from 33-37 years. From both a health care system perspective and a societal perspective, 4 studies reported that hip arthroscopy was more costly but resulted in far greater gains than did nonoperative treatment. The preferred treatment strategy was most sensitive to duration of benefit, preoperative osteoarthritis, cost of the arthroscopy, and the improvement in QALYs with hip arthroscopy.
Conclusion:
In the majority of the studies, hip arthroscopy had a higher initial cost but provided greater gain in QALYs than did a nonoperative treatment. In certain cases, hip arthroscopy can be cost-effective given a long enough duration of benefit and appropriate patient selection. However, there is further need for literature to analyze willingness-to-pay thresholds.
Keywords: hip arthroscopy, cost-effectiveness, femoroacetabular impingement
Hip arthroscopy has emerged as a highly popular, minimally invasive treatment of femoroacetabular impingement (FAI) and labral tears by correcting the femoral and acetabular morphology and addressing the chondrolabral pathology. 13 Hip arthroscopy has been shown to lead to significant improvements of patient-reported outcomes (PROs) 12,15,16,18 with minimal complications at short-, mid-, and long-term follow-up times 8,10,24,43 and low rates of secondary surgery. 5,9,17,35 As a result, rates of hip arthroscopy have increased dramatically, both in the United States 3,8,23,38 and around the world. 19,32
Despite the favorable outcomes of hip arthroscopy, the limited resources of today’s health care environment dictate the necessity of determining whether the procedure is truly cost-effective. An economic analysis provides a useful method for assessing the value of an intervention by assessing the cost associated with a health outcome (eg, reduced rate of total hip arthroplasty [THA] conversion). 1 The 2 most frequently used full economic evaluations are cost-effectiveness analysis (CEA) and cost-utility analysis (CUA). In a CEA, the health outcomes generated by the intervention and comparator are assumed to be different. A CUA is closely related to a CEA, but the health outcomes are quantified as quality-adjusted life-years (QALYs), a scale that ranges from 1 (equivalent to a year of perfect health) to 0 (equivalent to death). QALYs are often calculated from a functional outcome score (eg, EuroQol-5D). In CEAs and CUAs, the primary outcome typically determined is the incremental cost-effectiveness ratio (ICER), which is the difference in net cost between an intervention and its comparator divided by the difference in net benefit. This figure is assessed against a predetermined willingness-to-pay threshold to determine whether the treatment is cost-effective.
Although reviews 7,27,30,34 have been conducted on the cost-effectiveness of other orthopaedic procedures, none have specifically focused on hip arthroscopy. Thus, the purpose of this study was to systematically review the cost-effectiveness of hip arthroscopy for treating FAI and labral tears. We hypothesized that hip arthroscopy would have an ICER <$50,000/QALY, making it cost-effective when compared with a nonoperative treatment.
Methods
Search Strategy
A systematic review of the electronic databases PubMed/MEDLINE, Embase, and Cochrane Library was performed according to the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) guidelines to identify articles that reported on CEA of hip arthroscopy for FAI and labral tears. Databases were searched between time of inception and June 2020. The key search terms used were “hip arthroscopy” paired with “cost,” “utility,” “quality,” and “economic evaluation.” The Tufts University CEA Registry was searched as well. 34 This systematic review was prospectively registered on PROSPERO (CRD42020172991).
Two reviewers (C.C.G. and C.K.) independently reviewed titles and abstracts to select studies for full-text review. The references of selected articles were searched for relevant articles. Studies were included if (1) the patient population was treated using hip arthroscopy for a diagnosis of FAI and labral tears, (2) hip arthroscopy was the patient’s index procedure, and (3) they contained an economic evaluation (either partial or full) of hip arthroscopy. Studies were excluded if they were ongoing; were written in a language other than English; or were review articles, opinion articles, case reports, or technique articles. Any disagreement on article selection was resolved by discussion with a third author (D.R.M.) until a consensus was reached.
Data Extraction and Assessment
The following data were extracted from each selected study: year of publication, location where the study was conducted, type of economic evaluation, study population, comparator, time horizons, payment perspective, procedural cost, QALY gained, ICER, and main results. If the level of evidence of the study was not mentioned, it was determined using the criteria of Spindler et al. 41 Economic evaluations were classified as full economic evaluations only if they had costs, consequences, and a comparator. If they were missing any of the 3, they were classified as a partial economic evaluation. 21 When ICERs were not directly reported, they were calculated based on the formula of the difference in cost divided by the difference in QALYs between the intervention and comparator. For example, if the cost for hip arthroscopy was $20,000 and for nonoperative treatment was $10,000 and the QALYs for hip arthroscopy and nonoperative treatment were 2 QALYs and 1 QALY, respectively, then the ICER was calculated as ($20,000 – $10,000)/(2 QALYs – 1 QALY), equating to $10,000/QALY.
Hip arthroscopy was deemed to be cost-effective if the resulting ICER was less than the willingness-to-pay threshold. If the authors stated a preferred willingness-to-pay threshold, that number was used to determine cost-effectiveness. Otherwise, a figure of $50,000/QALY was chosen, as it is currently the most commonly used figure. 28 Although $100,000/QALY and $150,000/QALY 26,28 have also been proposed as potential alternatives, $50,000/QALY was ultimately selected in order to provide a more conservative threshold. Thus, an example of hip arthroscopy being cost-effective is when the willingness-to-pay threshold is $50,000/QALY and the ICER for a study is $20,000/QALY. The lower the ICER, the more cost-effective hip arthroscopy becomes. If hip arthroscopy was cost-effective, it was deemed the preferred treatment strategy. Otherwise, if hip arthroscopy was not cost-effective, the nonoperative intervention became the treatment strategy of choice. If sensitivity analyses were conducted, a parameter was deemed sensitive if varying it within the authors’ decided range changed the preferred treatment strategy. Examples of parameters included, but were not limited to, initial costs of treatments, improvements in QALYs, and duration of benefit.
To allow for comparisons among different countries and different years, foreign currency was converted to US dollars at their historical rate using the Treasury reporting rates of exchange. 42 All costs were then adjusted for inflation to the 2019 US dollar using the US Department of Labor’s Consumer Price Index inflation calculator. 6 When the date the cost analysis was run was not explicitly stated, the publication date was used.
Quality Assessment
Two authors (C.C.G. and C.K.) quantitively assessed the methodological quality of studies using the Methodological Index for Non-Randomized Studies (MINORS) criteria. 39 This validated instrument is composed of 8 questions for noncomparative studies with 4 additional questions asked for comparative studies. Each question receives a score between 0 and 2, with a maximum of 24 possible points for comparative studies. A higher score indicates higher methodological quality. The quality of the studies from an economic consideration was then quantitively assessed using the Quality of Health Economic Studies (QHES) criteria. 30,31 The QHES scale is a validated list of 16 questions, each with a weighted point value adding up to 100. Responses are a binary yes or no, with the full allotment of points assigned for a positive answer. The QHES score assesses factors such as sources of data, time horizons, and data analyses. A study with a QHES score >75 can be considered to be high quality, 34 while a QHES score >90 is excellent quality. 27 If there were any disagreements, 2 authors (C.C.G. and C.K.) discussed them until a consensus was reached.
Results
Eligible Studies and Study Characteristics
The literature search yielded 593 articles, with 393 unique articles remaining once duplicates were removed (Figure 1). After title and abstract review, the full texts of 15 articles were assessed for eligibility. Nine of these articles were excluded for the following reasons: 7 of the studies reported only utilities, 1 study was still ongoing, and 1 study reported only on costs prior to diagnosis. Six studies were ultimately included. When the quality of the economic evaluations was assessed using QHES, the mean score was 85.2. The majority of the studies (67%) were determined to be high quality, 14,20,22,37 with a QHES score >75. 34 Two 20,37 of the CEAs had a QHES score >90 and thus were considered excellent quality. 27 The MINORS score ranged from 18 to 23, out of a maximum of 24 potential points, for the comparative studies. 14,20,22,36,37 The MINORS score for Clement et al, 4 a noncomparative study, was 14 out of 16. The overall characteristics of each study have been included in Table 1.
Figure 1.
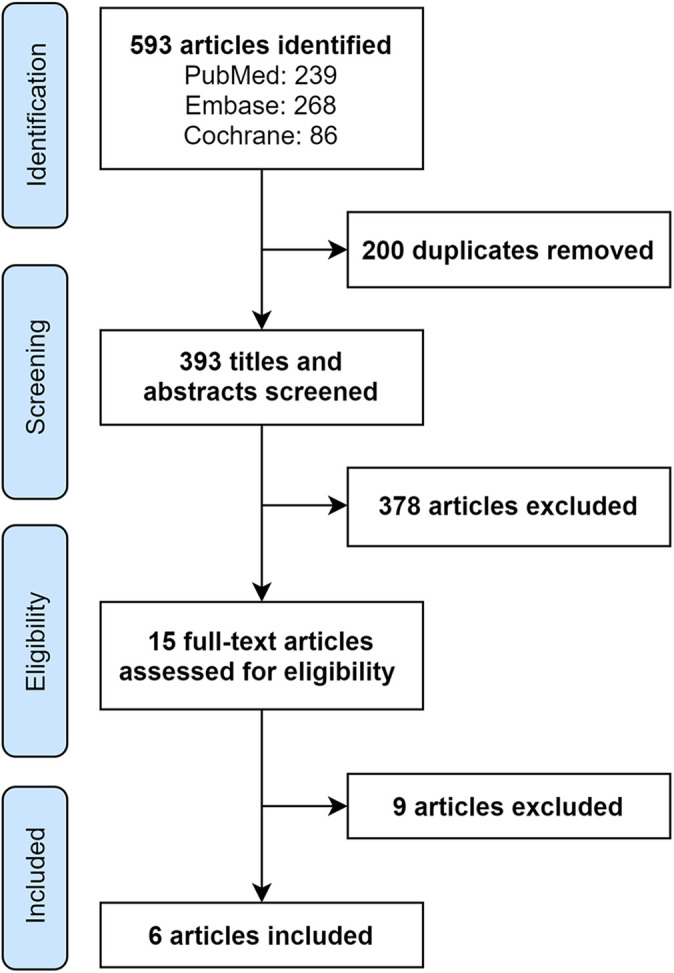
PRISMA (Preferred Reporting Items for Systematic Reviews and Meta-Analyses) flow diagram.
TABLE 1.
Study Characteristics a
| Lead Author (Year) | LOE | N | MINORS/QHES Scores | Study Type | Time Horizon | Study Design | Study Population | Conclusion |
|---|---|---|---|---|---|---|---|---|
| Clement (2014) 4 | 3 | 58 | 14
b
/72 |
Cost outcome description | 1, 2, and 10 y | Prospectively collected registry | Patients with FAI (mean age, 34 y) | Hip arthroscopy is cost-effective 1-10 y after surgery |
| Griffin (2018) 14 | 1 | 348 | 23/89 |
Cost-utility analysis | 1 y | Randomized controlled trial | Patients with FAI (mean age, 35 y) | Hip arthroscopy is not cost-effective in the first 12 mo |
| Lodhia (2016) 20 | 2 | Registry: NR | 20/95 |
Cost-utility analysis | Lifetime | Markov model (literature and own registry) | Patients with an acetabular labral tear without OA (age range, 20-80 y) | Hip arthroscopic surgery is cost-effective, resulting in a lower incidence of OA for patients without preexisting OA |
| Mather (2018) 22 | 2 | Registry: 102 | 19/88 |
Cost-utility analysis | 10 y | Markov model (literature and own registry) | Patients with no OA and mild to no hip dysplasia (mean age, 33 y) | Hip arthroscopy leads to substantial indirect savings (eg, lost wages) |
| Scott (2020) 36 | 3 | 864 | 20/72 |
Cost-effectiveness analysis | NR | Humana claims database | National database of patients with a labral tear | Hip arthroscopy does not lower conversion rate to THA and has a higher cost of care |
| Shearer (2012) 37 | 3 | NA | 18/95 |
Cost-utility analysis | Lifetime | Markov model (literature) | Patients with FAI (mean age, 36 y) | OA progression affects the cost-effectiveness of hip arthroscopy |
a FAI, femoroacetabular impingement; LOE, level of evidence; MINORS, Methodological Index for Non-Randomized Studies; NA, not applicable; NR, not reported; OA, osteoarthritis; QHES, Quality of Health Economic Studies; THA, total hip arthroplasty.
b Clement et al is not a comparative study, so the maximum score is 16.
Articles in this systematic review were published between 2012 and 2020, and the studies were conducted in either the United States 20,22,36,37 or the United Kingdom. 4,14 Five of the studies 14,20,22,36,37 were full economic evaluations that compared the cost-effectiveness of hip arthroscopy with a nonoperative intervention. In 4 of the 5 comparative studies, 14,20,36,37 hip arthroscopy was more costly than was the comparator, but Griffin et al, 14 Lodhia et al, 20 and Mather et al 22 reported that arthroscopy generated greater QALYs. As a cost-outcome description study that lacked a comparator, Clement et al 4 was the only partial economic evaluation (Table 1). All studies except for one assessed costs and measured outcomes in the form of QALYs, while Scott et al 36 examined rates of conversion to THA. QALYs were typically derived from PRO measurements, such as the Harris Hip Score, 12-Item Short Form Health Survey 6 Dimensions, and EuroQol-5D. Information concerning costs and utility was derived from a patient registry only, 4 literature only, 37 both literature and a patient registry, 20,22 a national claims database, 36 and a randomized controlled trial. 14
Shearer et al 37 was a model-based evaluation that relied solely on the best possible evidence available from the literature, while Lodhia et al 20 and Mather et al 22 used a combination of literature and data from their own registry. Clement et al 4 and Griffin et al 14 used clinical data from a prospective patient cohort and randomized controlled trial, respectively, while Scott et al 36 used the Humana claims database. The mean age across studies ranged from 33 to 37 years. The time horizon varied from 1 year to lifetime.
Costs
Two of the studies 20,22 adopted a societal perspective, 3 of the studies 4,36,37 adopted a health care perspective, and Griffin et al 14 analyzed from both. A discounting rate, which is applied to account for the decreased value of future QALYs and costs, of either 3% or 5% was carried out in all applicable studies. Studies differed in their methods of determining direct and indirect costs, but most used claims made to a national database. Clement et al 4 obtained cost of hip arthroscopy from the Scottish National Tariff, while Lodhia et al 20 calculated total societal costs using the national average Medicare reimbursements. Scott et al 36 queried the PearlDriver Research Database, which includes all claims covered by Humana. For hip arthroscopy, they determined direct costs by summing all encounters within 6 months of hip arthroscopy for the operative group and within 6 months of diagnosis for the nonoperative group. Similar to Scott et al, 36 Mather et al 22 calculated reimbursement for hip arthroscopy by querying the PearlDriver Patient Records Database. All reimbursements within 7 days of arthroscopy were included in the direct costs. Direct costs for rehabilitation were calculated by tracking treatments of patients in the year before surgery. Indirect costs were determined by conducting a regression analysis to estimate the relationship between functional status and productivity. Estimated loss in productivity was converted to indirect costs using data from the National Center for Health Statistics.
In contrast, Griffin et al 14 and Shearer et al 37 evaluated costs through tracking cases performed at their institution. Griffin et al followed a subsample of trial participants to determine costs resulting from staff time, surgical theater use in hours, disposable surgical equipment, anesthetic drugs, and inpatient stay. Societal costs were determined by assessing further reimbursements across a 12-month follow-up period. Shearer et al 37 estimated direct costs of hip arthroscopy through reviewing the 10 most recent cases performed at their institution and applying a cost-to-charge ratio to total charges, including facility and anesthesia fees. Indirect costs, such as clinic visits and loss of productivity, were not included in their assessment. Shearer et al had the lowest determined costs for nonoperative care but did not report how this was assessed.
Cost-effectiveness of Hip Arthroscopy
Clement et al, 4 as the only partial economic evaluation, did not compare the results of hip arthroscopy with those of a nonoperative treatment. Instead, one of its primary purposes was to report the correlation between cost per QALY and the time from hip arthroscopy. The cost per QALY at 1 year was $35,000, 2 years was $18,000/QALY, and 10 years was $5000/QALY (Table 2). At all 3 time points examined, the cost per QALY was less than the threshold of $50,000, leading Clement et al to conclude that hip arthroscopy was overall cost-effective.
TABLE 2.
Characteristics Related to Cost-effectiveness Analysis a
| Lead Author (Year) | Comparator | Payment Perspective | Procedural Cost | Time Until Hip Arthroscopy Is Cost-effective | ICER, $/QALY | QALY Gained | Arthroscopy Cost per QALY, $/QALY |
|---|---|---|---|---|---|---|---|
| Clement (2014) 4 | None | HCS | $6840 | <1 | NA |
|
|
| Griffin (2018) 14 | Personalized hip therapy | HCS | A: $3957 C: $872 | Never | $592,500 | A: 0.62 ± 0.25 C: 0.58 ± 0.24 |
NR |
| Personalized hip therapy | Societal | A: $3944 C: $1311 | Never | Surgery is both more expensive and less effective than rehabilitation | NR | NA | |
| Lodhia (2016) 20 | Rehabilitation | Societal | A: $27,816 ± $8518 C: $25,104 ± $5572 | NR | $997 | A: 21.8 ± 4.9 C:17.8 ± 3.2 |
$1276 |
| Mather (2018) 22 | Nonoperative care | Societal | A: $24,626 ± $10,949 C: $97,570 ± $15,631 | 1.87 y | Surgery is both cheaper and more effective than nonoperative care | A: 8.5 ± 0.5 C: 6.5 ± 0.4 |
$2897 |
| Scott (2020) 36 | Nonoperative care | HCS | A: $14,267 ± $7188 C: $29,412 ± $2664 | NR | NR | NR | NR |
| Shearer (2012) 37 | Nonoperative care | HCS | A: $13,817 C: $292 | 1.1 y | $25,302 without OA; $92,697 with OA | 0.2 ± 0.05 | NR |
a Data are presented as mean ± SD. All costs are reported as 2019 US dollar. A, hip arthroscopy; C, comparator; HCS, health care system; ICER, incremental cost-effectiveness ratio; QALY, quality-adjusted life-year; OA, osteoarthritis; NA, not applicable; NR, not reported.
The 5 full economic evaluations 14,20,22,36,37 compared hip arthroscopy with a nonoperative intervention (Table 2). Two of the studies 22,37 concluded that hip arthroscopy was not initially cost-effective at 1 year after surgery but became cost-effective at >2 years after surgery in a patient population without osteoarthritis (OA). Shearer et al 37 reported that the ICER was <$50,000/QALY if the duration of benefit was >13 months. Increasing the duration of benefit led to a lower amount of QALYs that needed to be to gained to have the same ICER. For example, if hip arthroscopy had a duration of benefit of 1 year, it would have to provide an improvement of utility of 0.2 QALYs, whereas if the duration of benefit lasted 10 years, arthroscopy would only have to improve utility by 0.04 QALYs. 37 For a general patient population, Mather et al 22 concluded that the threshold for cost was 1.87 years, or approximately 22 months, when indirect costs (i.e., loss of productivity due to decreased functional status) were considered. In contrast, Griffin et al 14 concluded that arthroscopy was never cost-effective compared with personalized hip therapy, as arthroscopy was both more expensive and led to fewer overall QALYs gained. This study had a time horizon of 1 year, which was notably shorter than that of the other CEAs. Lodhia et al 20 did not comment on the amount of time it took for hip arthroscopy to become cost-effective, but instead concluded that with a lifetime horizon, hip arthroscopy was cost-effective in 94.5% of trials.
Sensitivity Analyses
Sensitivity analyses revealed that the cost-effectiveness of hip arthroscopy was highly dependent on OA (Table 3). Shearer et al 37 indicated that the ICER decreased from $21,700/QALY to $19,200/QALY if hip arthroscopy delayed the progression of OA, defined as an increase in Tönnis grade, for 3 years. If the procedure delayed OA for >5 years, then the ICER decreased to <$10,000/QALY. On the other hand, for patients with preoperative arthritis, hip arthroscopy only became cost-effective at 8 years after surgery. Subsequent THA contributed to much of the increased costs associated with OA; Lodhia et al 20 reported that primary THA alone accounted for an additional $3893 in costs. In contrast, Scott et al 36 reported that there were similar rates of conversion to THA between operative and nonoperative groups.
TABLE 3.
Sensitivity Analysis a
| Lead Author (Year) | Payment Perspective | Probability That Surgery Is Cost-effective | Sensitive Parameters for ICER |
|---|---|---|---|
| Clement (2014) 4 | Health care system | NR | Preoperative SF12-6D score |
| Griffin (2018) 14 | Health care system | 0.002 | None |
| Societal | Never | None | |
| Lodhia (2016) 20 | Societal | 0.945 | Utility of an asymptomatic hip after arthroscopy |
| Mather (2018) 22 | Societal | 0.99 | None |
| Scott (2020) 36 | Health care system | NR | NR |
| Shearer (2012) 37 | Health care system | 0.85 without OA 0.23 with OA |
Arthroscopy duration of benefit, improvement in utility postoperatively |
a NR, not reported; ICER, incremental cost-effectiveness ratio; OA, osteoarthritis; SF12-6D, 12-Item Short Form Health Survey 6 Dimensions.
In addition to OA, the initial cost of hip arthroscopy and postoperative improvement in utility were sensitive parameters; that is, they most affected the preferred treatment based on the ICER of hip arthroscopy. Lodhia et al 20 reported that the preferred treatment based on cost alone was sensitive to the discount rate, probability of recurring pain after surgery, cost of arthroscopic surgery and rehabilitation, probability of retear after rehabilitation, age, and relative risk of OA. In contrast, the treatment strategy based on ICER favored arthroscopy over rehabilitation when arthroscopy generated a QALY of 0.75 or greater. Likewise, Mather et al 22 concluded that the time horizon, utility improvement, and cost of surgery were most influential on the Markov model, although these parameters were deemed individually robust and did not change the preferred treatment strategy.
Discussion
The systematic review identified 6 studies that examined the cost-effectiveness of hip arthroscopy. When compared with a nonoperative treatment, hip arthroscopy had a higher initial cost but led to increased QALYs. The cost per QALY of hip arthroscopy was found to be most influenced by duration of benefit, the presence of OA, cost of arthroscopy, and QALY gain produced by arthroscopy.
Given that the number of CEA studies has risen in the past few years, assessing the quality of these studies has become increasingly important for determining policy. Overall, the quality of CEA focusing on hip arthroscopy can be rated as high, with a mean QHES score of 85.2 (range, 72-95), which is similar to that reported in other orthopaedic cost-effectiveness systematic reviews. A systematic review of hand and upper extremity surgical CEA reported a mean QHES score of 82. 34 More generally, when Nwachukwu et al 27 conducted a systematic review of orthopaedic sports medicine in 2015, they identified 12 CEAs with an average QHES score of 81.8 (range, 70-94).
The majority of the studies in this systematic review suggested that hip arthroscopy can be a cost-effective intervention for the treatment of FAI and labral tears when compared with a nonoperative treatment. These results echo those of other orthopaedic CEA reviews, which have reported that surgical interventions in orthopaedics are often cost-effective. In a review of 8 CUA articles on orthopaedic trauma, Nwachukwu et al 30 reported that open reduction and internal fixation is more cost-effective than is cast immobilization or nonoperative observation for the treatment of fractures of the clavicle, distal radius, and scaphoid. Coyle et al 7 concluded that surgical treatment of ankle and calcaneal fractures was cost-effective compared with conservative management.
The cost-effectiveness of hip arthroscopy is highly dependent on the duration of benefit, with a greater time associated with a lower ICER. Clement et al, 4 Shearer et al, 37 and Mather et al 22 indicated that hip arthroscopy became cost-effective at <1, 1.1, and 1.87 years, respectively. In contrast, Griffin et al 14 reported that hip arthroscopy was not effective, but their results were constrained by their time horizon of 1 year. The need for a longer duration of benefit is consistent with that reported in the current hip arthroscopy literature, which has described how improvements in patient outcomes can occur even up to 2 years postoperatively. Wolfson et al 44 reported that the number of patients achieving the minimal clinically important difference was 78%, 88%, 90%, and 93% at 3 months, 6 months, 1 year, and 2 years, respectively. Similarly, 43%, 63%, 66%, and 73% of patients achieved the patient acceptable symptomatic state at 3 months, 6 months, 1 year, and 2 years, respectively. In a cohort study of 719 patients undergoing primary hip arthroscopy, Nwachukwu et al 29 reported that 84.8% of patients achieved the minimal clinically important difference for the 33-Item International Hip Outcome Tool at 1 year, which improved to 93.6% of the patients at 2 years.
The presence of preexisting OA can further increase the duration of benefit required for cost-effectiveness, as several of the studies identified OA as a major factor influencing both the ICER and the cost per QALY. 20,22,37 OA has been shown to negatively affect patient outcomes while increasing conversion rate to THA. 40 A systematic review by Piuzzi et al 33 reported that in patients with preoperative OA, the overall conversion rate to THA ranged from 9.5% to 50%, with the highest rates of conversion present in patients with the most advanced OA at the time of arthroscopy. They showed a mean time for conversion to THA of 13.5 months. Likewise, Domb et al 11 reported that patients without arthritis and those with arthritis had conversion rates to THA of 8.3% and 23%, respectively, and mean conversion times of 26.1 and 17.1 months, respectively. Thus, the increase in ICER associated with patients with OA may be attributed to either a decreased QALY gain or the limited window that hip arthroscopy has to provide an improvement in utility, especially given that several studies have highlighted that hip arthroscopy is only cost-effective with a longer duration of benefit. As patients with preexisting OA may experience fewer benefits from arthroscopy, these results serve to highlight the importance of appropriate patient selection in providing cost-effective care.
Although the sensitivity analyses did not indicate that initial costs affected preferred treatment strategy, costs for hip arthroscopy and rehabilitation varied among studies, as each had its own unique methodology. The lowest costs for hip arthroscopy were seen in Clement et al 4 and Griffin et al, 14 which were both conducted in the United Kingdom. Studies conducted in the United States had higher costs for hip arthroscopy. However, Mather et al 22 estimated the highest cost for nonoperative intervention compared with other studies, as it was the only study to incorporate indirect costs stemming from loss of productivity.
As hip arthroscopy was more expensive than was conservative management in the majority of the comparative studies, the cost-effectiveness of hip arthroscopy can also be improved by decreasing initial costs associated with hip arthroscopy. One such method includes mandated bundled payments, which has been mandated by Medicare for joint replacements. An observational study observed significant reductions in both internal hospital costs ($675.12 or 21.7% of savings per episode) and postacute care spending ($2443.12 or 78.4% of savings per episode). 25 Barnett et al 2 reported a 3.1% decrease in costs in areas mandated to participate in bundled payments for hip and knee replacements compared with control areas. A similar approach in hip arthroscopy could likewise produce significant cost savings.
Strengths
To the best of our knowledge, this is one of the few systematic reviews examining the cost-effectiveness of hip arthroscopy compared with nonoperative treatment. Next, all costs were converted to 2019 US dollar purchasing power, which allowed for greater comparability among studies and eliminated differences due to purchasing power and inflation. Moreover, this systematic review also assessed both the quality of studies using the validated QHES scoring tool and the economic findings. Previous systematic reviews have largely limited themselves to investigating the quality of the economic evaluations. 27 Furthermore, the validated PRISMA method was used to conduct a comprehensive search of the available literature relevant to the subject.
Limitations
This systematic review has several limitations that must be acknowledged. Although all studies had a high level of evidence, there was a limited number of studies and a lack of homogeneity. Differing comparators, countries, time horizons, and payment perspectives limited comparison among different studies and precluded the use of pooled analyses or meta-analysis. These factors also served to limit the generalizability of the findings. Next, the only type of full economic evaluation conducted was the CUA, which has several inherent limitations. Unlike a cost-benefit analysis, a CUA does not examine patient willingness to pay. Instead, the ICERs were compared against $50,000/QALY, the most common figure used for the willingness-to-pay threshold. Finally, most of the studies included in the present systematic review were conducted by a single orthopaedist at a high-volume hip arthroscopy center, which may limit generalizability. Although findings may vary if looking at larger databases with multiple surgeons, 3 of the studies 20,22,37 supplemented their claims by basing their primary analysis on data generated primarily from the literature, while Scott et al 36 used a national claims database as their primary source of data.
Conclusion
In a majority of the studies, hip arthroscopy had a higher initial cost but provided greater gain in QALYs than did a nonoperative treatment. In certain cases, hip arthroscopy can be cost-effective given a long enough duration of benefit and appropriate patient selection. However, there is further need for literature to analyze willingness-to-pay thresholds.
Footnotes
Final revision submitted September 8, 2020; accepted October 2, 2020.
One or more of the authors has declared the following potential conflict of interest or source of funding: B.G.D. has had ownership interests in Hinsdale Orthopaedics, the American Hip Institute SCD#3, North Shore Surgical Suites, and Munster Specialty Surgery Center and has received research support from Arthrex, ATI, the Kauffman Foundation, Stryker, and Pacira Pharmaceuticals; consulting fees from Adventist Hinsdale Hospital, Arthrex, MAKO Surgical, Medacta, Pacira Pharmaceuticals, and Stryker; educational support from Arthrex, Breg, and Medwest; speaking fees from Arthrex and Pacira Pharmaceuticals; and royalties from Arthrex, DJO Global, MAKO Surgical, Stryker, and Orthomerica. D.R.M. has received hospitality payments from Arthrex, Stryker, and Smith & Nephew. AOSSM checks author disclosures against the Open Payments Database (OPD). AOSSM has not conducted an independent investigation on the OPD and disclaims any liability or responsibility relating thereto.
References
- 1. Angevine P, Berven S. Health economic studies: an introduction to cost-benefit, cost-effectiveness, and cost-utility analyses. Spine (Phila Pa 1976). 2014;39(22)(Suppl 1):S9–S15. [DOI] [PubMed] [Google Scholar]
- 2. Barnett ML, Wilcock A, McWilliams JM, et al. Two-year evaluation of mandatory bundled payments for joint replacement. N Engl J Med. 2019;380(3):252–262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Bozic KJ, Chan V, Valone FH, Feeley BT, Vail TP. Trends in hip arthroscopy utilization in the United States. J Arthroplasty. 2013;28(8):140–143. [DOI] [PubMed] [Google Scholar]
- 4. Clement ND, MacDonald D, Gaston P. Hip arthroscopy for femoroacetabular impingement: a health economic analysis. Hip Int. 2014;24(5):457–464. [DOI] [PubMed] [Google Scholar]
- 5. Comba F, Yacuzzi C, Ali PJ, Zanotti G, Buttaro M, Piccaluga F. Joint preservation after hip arthroscopy in patients with FAI: prospective analysis with a minimum follow-up of seven years. Muscles Ligaments Tendons J. 2016;6(3):317–323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Consumer Price Index inflation calculator. US Department of Labor. Accessed May 9, 2019. https://www.bls.gov/data/inflation_calculator.htm
- 7. Coyle S, Kinsella S, Lenehan B, Queally JM. Cost-utility analysis in orthopaedic trauma: what pays? A systematic review. Injury. 2018;49(3):575–584. [DOI] [PubMed] [Google Scholar]
- 8. Cvetanovich GL, Chalmers PN, Levy DM, et al. Hip arthroscopy surgical volume trends and 30-day postoperative complications. Arthroscopy. 2016;32(7):1286–1292. [DOI] [PubMed] [Google Scholar]
- 9. Cvetanovich GL, Harris JD, Erickson BJ, Bach BR, Bush-Joseph CA, Nho SJ. Revision hip arthroscopy: a systematic review of diagnoses, operative findings, and outcomes. Arthroscopy. 2015;31(7):1382–1390. [DOI] [PubMed] [Google Scholar]
- 10. Degen RM, Bernard JA, Pan TJ, et al. Hip arthroscopy utilization and associated complications: a population-based analysis. J Hip Preserv Surg. 2017;4(3):240–249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Domb BG, Gui C, Lodhia P. How much arthritis is too much for hip arthroscopy: a systematic review. Arthroscopy. 2015;31(3):520–529. [DOI] [PubMed] [Google Scholar]
- 12. Domb BG, Martin TJ, Gui C, Chandrasekaran S, Suarez-Ahedo C, Lodhia P. Predictors of clinical outcomes after hip arthroscopy: a prospective analysis of 1038 patients with 2-year follow-up. Am J Sports Med. 2018;46(6):1324–1330. [DOI] [PubMed] [Google Scholar]
- 13. Griffin DR, Dickenson EJ, O’Donnell J, et al. The Warwick Agreement on femoroacetabular impingement syndrome (FAI syndrome): an international consensus statement. Br J Sports Med. 2016;50(19):1169–1176. [DOI] [PubMed] [Google Scholar]
- 14. Griffin DR, Dickenson EJ, Wall PDH, et al. Hip arthroscopy versus best conservative care for the treatment of femoroacetabular impingement syndrome (UK FASHIoN): a multicentre randomised controlled trial. Lancet. 2018;391(10136):2225–2235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Griffin DW, Kinnard MJ, Formby PM, McCabe MP, Anderson TD. Outcomes of hip arthroscopy in the older adult: a systematic review of the literature. Am J Sports Med. 2017;45(8):1928–1936. [DOI] [PubMed] [Google Scholar]
- 16. Hevesi M, Krych AJ, Johnson NR, et al. Multicenter analysis of midterm clinical outcomes of arthroscopic labral repair in the hip: minimum 5-year follow-up. Am J Sports Med. 2018;46(2):280–287. [DOI] [PubMed] [Google Scholar]
- 17. Kester BS, Capogna B, Mahure SA, Ryan MK, Mollon B, Youm T. Independent risk factors for revision surgery or conversion to total hip arthroplasty after hip arthroscopy: a review of a large statewide database from 2011 to 2012. Arthroscopy. 2018;34(2):464–470. [DOI] [PubMed] [Google Scholar]
- 18. Khan M, Habib A, de Sa D, et al. Arthroscopy up to date: hip femoroacetabular impingement. Arthroscopy. 2016;32(1):177–189. [DOI] [PubMed] [Google Scholar]
- 19. Lee Y-K, Ha Y-C, Yoon B-H, Koo K-H. National trends of hip arthroscopy in Korea. J Korean Med Sci. 2014;29(2):277–280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Lodhia P, Gui C, Chandrasekaran S, Suarez-Ahedo C, Dirschl DR, Domb BG. The economic impact of acetabular labral tears: a cost-effectiveness analysis comparing hip arthroscopic surgery and structured rehabilitation alone in patients without osteoarthritis. Am J Sports Med. 2016;44(7):1771–1780. [DOI] [PubMed] [Google Scholar]
- 21. Martelli N, Devaux C, van den Brink H, Pineau J, Prognon P, Borget I. A systematic review of the level of evidence in economic evaluations of medical devices: the example of vertebroplasty and kyphoplasty. PLoS One. 2015;10(12):e0144892. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Mather RC, Nho SJ, Federer A, et al. Effects of arthroscopy for femoroacetabular impingement syndrome on quality of life and economic outcomes. Am J Sports Med. 2018;46(5):1205–1213. [DOI] [PubMed] [Google Scholar]
- 23. Montgomery SR, Ngo SS, Hobson T, et al. Trends and demographics in hip arthroscopy in the United States. Arthroscopy. 2013;29(4):661–665. [DOI] [PubMed] [Google Scholar]
- 24. Nakano N, Lisenda L, Jones TL, Loveday DT, Khanduja V. Complications following arthroscopic surgery of the hip: a systematic review of 36 761 cases. Bone Joint J. 2017;99(12):1577–1583. [DOI] [PubMed] [Google Scholar]
- 25. Navathe AS, Troxel AB, Liao JM, et al. Cost of joint replacement using bundled payment models. JAMA Intern Med. 2017;177(2):214–222. [DOI] [PubMed] [Google Scholar]
- 26. Neumann PJ, Cohen JT, Weinstein MC. Updating cost-effectiveness—the curious resilience of the $50,000-per-QALY threshold. N Engl J Med. 2014;371(9):796–797. [DOI] [PubMed] [Google Scholar]
- 27. Nwachukwu B, Schairer W, Bernstein J, Dodwell E, Marx R, Allen A. Cost-effectiveness analyses in orthopaedic sports medicine: a systematic review. Am J Sports Med. 2015;43(6):1530–1537. [DOI] [PubMed] [Google Scholar]
- 28. Nwachukwu BU, Bozic KJ. Updating cost effectiveness analyses in orthopedic surgery: resilience of the $50,000 per QALY threshold. J Arthroplasty. 2015;30(7):1118–1120. [DOI] [PubMed] [Google Scholar]
- 29. Nwachukwu BU, Chang B, Adjei J, et al. Time required to achieve minimal clinically important difference and substantial clinical benefit after arthroscopic treatment of femoroacetabular impingement. Am J Sports Med. 2018;46(11):2601–2606. [DOI] [PubMed] [Google Scholar]
- 30. Nwachukwu BU, Schairer WW, O’Dea E, McCormick F, Lane JM. The quality of cost-utility analyses in orthopedic trauma. Orthopedics. 2015;38(8):e673–e680. [DOI] [PubMed] [Google Scholar]
- 31. Ofman JJ, Sullivan SD, Neumann PJ, et al. Examining the value and quality of health economic analyses: implications of utilizing the QHES. J Manag Care Pharm. 2003;9(1):53–61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Palmer AJR, Malak TT, Broomfield J, et al. Past and projected temporal trends in arthroscopic hip surgery in England between 2002 and 2013. BMJ Open Sport Exerc Med. 2016;2(1):e000082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Piuzzi NS, Slullitel PAI, Bertona A, et al. Hip arthroscopy in osteoarthritis: a systematic review of the literature. Hip Int. 2016;26(1):8–14. [DOI] [PubMed] [Google Scholar]
- 34. Rajan PV, Qudsi RA, Dyer GSM, Losina E. Cost-utility studies in upper limb orthopaedic surgery: a systematic review of published literature. Bone Joint J. 2018;100(11):1416–1423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Rosinsky PJ, Go CC, Shapira J, Maldonado DR, Lall AC, Domb BG. Validation of a risk calculator for conversion of hip arthroscopy to total hip arthroplasty in a consecutive series of 1400 patients. J Arthroplasty. 2019;34(8):1700–1706. [DOI] [PubMed] [Google Scholar]
- 36. Scott BL, Lee CS, Shi LL, Lee MJ, Athiviraham A. Nonoperative management of hip labral tears yields similar total hip arthroplasty conversion rate to arthroscopic treatment. J Arthroplasty. 2020;35(1):23–27.e1. [DOI] [PubMed] [Google Scholar]
- 37. Shearer DW, Kramer J, Bozic KJ, Feeley BT. Is hip arthroscopy cost-effective for femoroacetabular impingement? Clin Orthop Relat Res. 2012;470(4):1079–1089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Sing DC, Feeley BT, Tay B, Vail TP, Zhang AL. Age-related trends in hip arthroscopy: a large cross-sectional analysis. Arthroscopy. 2015;31(12):2307–2313.e2. [DOI] [PubMed] [Google Scholar]
- 39. Slim K, Nini E, Forestier D, Kwiatkowski F, Panis Y, Chipponi J. Methodological Index for Non-Randomized Studies (MINORS): development and validation of a new instrument. ANZ J Surg. 2003;73(9):712–716. [DOI] [PubMed] [Google Scholar]
- 40. Sogbein OA, Shah A, Kay J, et al. Predictors of outcomes after hip arthroscopic surgery for femoroacetabular impingement: a systematic review. Orthop J Sports Med. 2019;7(6):2325967119848982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Spindler KP, Kuhn JE, Dunn W, Matthews CE, Harrell FE, Jr, Dittus RS. Reading and reviewing the orthopaedic literature: a systematic, evidence-based medicine approach. J Am Acad Orthop Surg. 2005;13(4):220–229. [DOI] [PubMed] [Google Scholar]
- 42. US Treasury reporting rates of exchange—historical rates. US Department of the Treasury. Accessed May 9, 2019. https://www.fiscal.treasury.gov/reports-statements/treasury-reporting-rates-exchange/historical.html
- 43. Weber AE, Harris JD, Nho SJ. Complications in hip arthroscopy: a systematic review and strategies for prevention. Sports Med Arthrosc Rev. 2015;23(4):187–193. [DOI] [PubMed] [Google Scholar]
- 44. Wolfson TS, Ryan MK, Begly JP, Youm T. Outcome trends after hip arthroscopy for femoroacetabular impingement: when do patients improve? Arthroscopy. 2019;35(12):3261–3270. [DOI] [PubMed] [Google Scholar]


