Key Points
Question
What is the 24-hour urinary excretion of N-nitrosodimethylamine (NDMA), a probable human carcinogen, after oral ranitidine (300 mg) compared with placebo?
Findings
In this crossover, randomized clinical trial that included 18 healthy participants, oral ranitidine (300 mg), compared with placebo, did not significantly increase 24-hour urinary excretion of NDMA when administered with a noncured-meats diet or a cured-meats diet (median of the paired differences, 0 [interquartile range, −6.9 to 0] ng and −1.1 [interquartile range, −9.1 to 11.5] ng, respectively).
Meaning
The findings do not support that ranitidine is converted to NDMA in a general, healthy population.
Abstract
Importance
In 2019, the US Food and Drug Administration (FDA) received a citizen petition indicating that ranitidine contained the probable human carcinogen N-nitrosodimethylamine (NDMA). In addition, the petitioner proposed that ranitidine could convert to NDMA in humans; however, this was primarily based on a small clinical study that detected an increase in urinary excretion of NDMA after oral ranitidine consumption.
Objective
To evaluate the 24-hour urinary excretion of NDMA after oral administration of ranitidine compared with placebo.
Design, Setting, and Participants
Randomized, double-blind, placebo-controlled, crossover clinical trial at a clinical pharmacology unit (West Bend, Wisconsin) conducted in 18 healthy participants. The study began in June 2020, and the end of participant follow-up was July 1, 2020.
Interventions
Participants were randomized to 1 of 4 treatment sequences and over 4 periods received ranitidine (300 mg) and placebo (randomized order) with a noncured-meats diet and then a cured-meats diet. The cured-meats diet was designed to have higher nitrites, nitrates (nitrate-reducing bacteria can convert nitrates to nitrites), and NDMA.
Main Outcome and Measure
Twenty-four–hour urinary excretion of NDMA.
Results
Among 18 randomized participants (median age, 33.0 [interquartile range {IQR}, 28.3 to 42.8] years; 9 women [50%]; 7 White [39%], 11 African American [61%]; and 3 Hispanic or Latino ethnicity [17%]), 17 (94%) completed the trial. The median 24-hour NDMA urinary excretion values for ranitidine and placebo were 0.6 ng (IQR, 0 to 29.7) and 10.5 ng (IQR, 0 to 17.8), respectively, with a noncured-meats diet and 11.9 ng (IQR, 5.6 to 48.6) and 23.4 ng (IQR, 8.6 to 36.7), respectively, with a cured-meats diet. There was no statistically significant difference between ranitidine and placebo in 24-hour urinary excretion of NDMA with a noncured-meats diet (median of the paired differences, 0 [IQR, −6.9 to 0] ng; P = .54) or a cured-meats diet (median of the paired differences, −1.1 [IQR, −9.1 to 11.5] ng; P = .71). No drug-related serious adverse events were reported.
Conclusions and Relevance
In this trial that included 18 healthy participants, oral ranitidine (300 mg), compared with placebo, did not significantly increase 24-hour urinary excretion of NDMA when participants consumed noncured-meats or cured-meats diets. The findings do not support that ranitidine is converted to NDMA in a general, healthy population.
Trial Registration
ClinicalTrials.gov Identifier: NCT04397445
This phase 1 randomized crossover clinical trial evaluates the 24-hour urinary excretion of N-nitrosodimethylamine (NDMA) after oral administration of ranitidine compared with placebo.
Introduction
Ranitidine, a histamine 2 (H2) receptor blocker, was approved in the US in 1983 and became widely used as an over-the-counter drug for heartburn. In September 2019, the US Food and Drug Administration (FDA) received a citizen petition indicating that high levels of N-nitrosodimethylamine (NDMA), a probable human carcinogen,1 had been detected in specific lots of ranitidine.2 The petitioner also proposed that ranitidine could convert to NDMA in vivo by ranitidine releasing its dimethylamine (DMA) group, which could then be nitrosated to NDMA in the presence of nitrite.2,3 In response, FDA immediately alerted the public and initiated an investigation.4,5
The FDA found that the analytical procedure previously used to quantify NDMA could convert ranitidine to NDMA under the high temperatures used. Thus, a new method using lower temperatures was developed and validated,6 which resulted in approximately 3000-fold lower NDMA amounts; however, many ranitidine products still contained NDMA at levels above the acceptable daily intake limit (96 ng/d).7
Regarding potential in vivo conversion of ranitidine to NDMA, the primary evidence came from a 10-participant study that reported an approximately 400-fold increase in NDMA and an approximately 2.5-fold increase in DMA excreted in urine after oral ranitidine (150 mg).3 However, there were general study limitations (eg, no randomization); no information on controlling for environmental or dietary exposure to nitrites, nitrates, or NDMA8,9,10,11; and limited details on sample handling and validation of analytical methods, which used elevated temperatures.
Considering these limitations, the current study was designed as a randomized, double-blind, placebo-controlled, crossover study with 18 participants and ranitidine (300 mg), with detailed sample handling procedures and validated bioanalytical methods using low temperatures. In addition, participants received ranitidine and placebo with 2 different diets, the second designed to contain higher nitrites, nitrates, and NDMA.9,10,11 The primary objective of this study was to compare 24-hour urinary excretion of NDMA after ranitidine vs placebo.
Methods
Study Setting and Dates
A randomized clinical trial with healthy participants was performed in a clinical pharmacology unit (West Bend, Wisconsin). This study was approved by the local institutional review board (Advarra [https://www.advarra.com]). All participants provided written informed consent. The protocol and statistical analysis plan are available in Supplement 1.
Recruitment
The study was conducted in June and July of 2020. Participants were recruited by standard approaches for a phase 1 healthy volunteer study (ie, online advertising and emails/texts to individuals in the site’s database of potential participants for healthy volunteer studies). Self-identified race and ethnicity were collected in an open-ended format by clinical staff as recommended by the FDA’s guidance document, Collection of Race and Ethnicity Data in Clinical Trials.12 Participants remained in the clinic for 10 days. Key inclusion criteria were age 18 to 50 years, nonsmoking, and negative test results for alcohol or drugs of abuse. Key exclusion criteria were a positive Helicobacter pylori test at screening or a history of H pylori or ulcer disease.
Randomization
Study participants were randomized to 1 of 4 treatment sequences using a random number generator in R statistical software and received 4 different combinations of study drug (ranitidine [300 mg] or placebo, randomized sequence) and diet (2 diets, fixed sequence) over 4 study periods (Figure 1). Randomization was conducted in block sizes of 4 for the first 16 participants enrolled, and the remaining 2 participants were randomly placed in 2 of the 4 treatment sequences. The study enrolled equal numbers of men and women, but randomization did not account for sex and treatment sequence interactions.
Figure 1. Flow of Participants in Study, Interventions, and Overall Study Design.
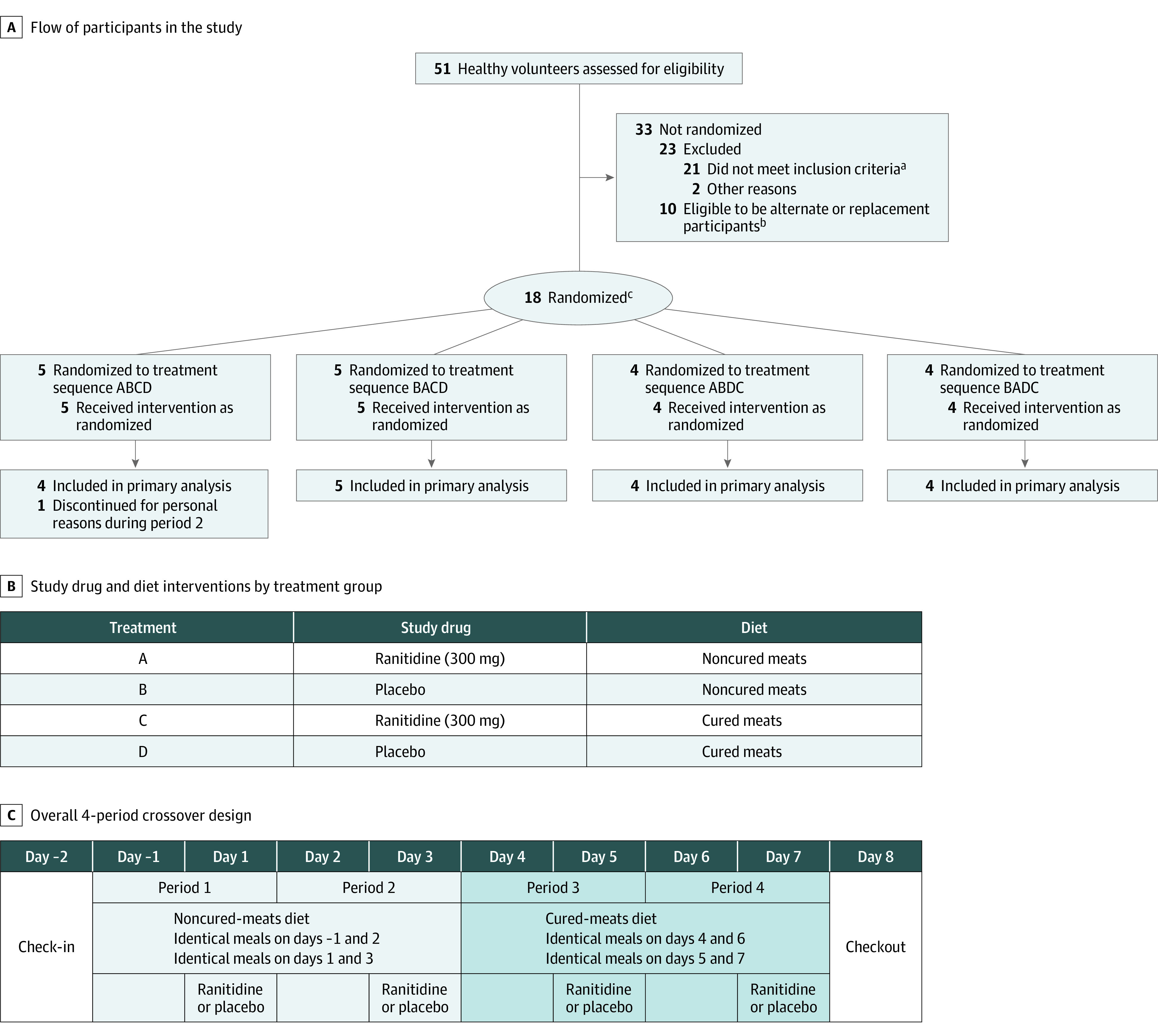
aThe most common reason for participant exclusion was a history of Helicobacter pylori infection or ulcer disease or a positive H pylori breath test result (n = 12). Additional reasons for exclusion: abnormal medical history findings, clinical laboratory results, vital sign measurements, 12-lead electrocardiogram results, or physical examination findings at screening (n = 5); having signs or symptoms consistent with COVID-19 (n = 3); nicotine use within 6 weeks of screening (n = 1); history of clinically significant disorder, condition, or disease (n = 1); or clinically significant laboratory results (n = 1).
bA sample size of 14 participants was determined to have greater than 90% power to detect an increase in 24-hour urinary excretion of N-nitrosodimethylamine (NDMA). Eighteen participants were randomized in a single cohort to account for potential dropouts without needing to enroll an additional cohort. This was done so all participants would be served identical meals with the same sourcing of fresh foods to avoid potential variability in nitrite, nitrate, or NDMA content. Because there was a delay between screening and determining final eligibility for the study (eg, receiving all laboratory tests), 10 additional people met all inclusion criteria and were eligible to be alternates (eg, if someone had to cancel or did not show up at check-in) or replacements (eg, if there had been fewer than 14 completers and a second cohort was enrolled).
cRandomization was conducted in block sizes of 4. The remaining 2 participants were randomly placed in 2 of the 4 treatment sequences.
Interventions
The ranitidine lot was tested on 3 occasions and confirmed to have NDMA below the acceptable daily limit (6.3 and 7.5 ng prior to study start and 10.5 ng after study completion) (eTable 1 in Supplement 2). Each participant received a diet with noncured meats, organic vegetables/fruits and distilled water (noncured-meats diet) for 4 days and then cured meats, conventional vegetables/fruits, and tap water (cured-meats diet) for 4 days (Figure 1). The second diet was designed based on the literature to have higher nitrite, nitrate, and NDMA levels (eMethods 1 in Supplement 2).9,10,11
After consuming the same diet on the pretreatment day as the treatment day, participants received ranitidine or placebo at 0 hours (after overnight fast) on treatment days (days 1, 3, 5, and 7) and then began eating breakfast 1 minute afterward. This was done because the formation of NDMA is dependent on having nitrite and acidic conditions.13 Because gastric acidity is highest in the fasting state and then decreases after eating,13 this sequence was selected to maximize the 2 factors. Additional meals were provided at 4, 7.5, and 11.5 hours after dosing. Participants were required to finish meals within 25 minutes (eTable 2 in Supplement 2). Participants were not allowed to take any prescription or nonprescription drugs, excluding contraceptives or acetaminophen, during the study unless prescribed by the study physician.
All urine voided over the 24 hours after drug administration was collected, weighed, and aliquoted to containers on ice with sodium hydroxide within 15 minutes and frozen on dry ice within 30 minutes (Urine Procedure Plan in Supplement 1). Void collection times were scheduled for 0- (predose), 3-, 6-, 9-, 12-, 15-, and 24-hours; unscheduled voids were also collected. Thirteen blood samples were collected on treatment days at 0 (predose), 0.5, 1, 1.5, 2, 3, 4, 5, 6, 9, 11, 14, and 24 hours after treatment. NDMA, DMA, and ranitidine concentrations in urine and plasma were measured by validated liquid chromatographic tandem mass spectrometric methods (eMethod 2 in Supplement 2) per the FDA’s Bioanalytical Method Validation Guidance for Industry.14 Deidentified participant data are available in Supplement 3 (eDictionary in Supplement 2).
Outcomes
The primary outcome was 24-hour urinary excretion of NDMA. The exploratory outcomes included 24-hour urinary excretion of DMA and ranitidine; plasma pharmacokinetic parameters for NDMA, DMA, and ranitidine (including area under the plasma concentration vs time curve [AUC] and maximum concentration); and comparisons between cured-meats and noncured-meats diets. Adverse events were recorded by clinic staff and adjudicated by the principal investigator.
Statistical Analysis
A sample size of 14 participants was determined to have greater than 90% power to detect an increase in 24-hour urinary excretion of NDMA. The analysis assumed a 100% coefficient of variability, at least a 2-fold increase in NDMA, and 1-sided α of .05 because the primary objective was to determine if ranitidine increased urinary excretion of NDMA. A 2-fold difference was selected because it is a typical convention for pharmacokinetic studies, but it does not imply that a 2-fold increase in NDMA is clinically meaningful. Eighteen participants were randomized to account for dropouts without needing to enroll an additional cohort.
The analysis population included all participants who completed 2 paired treatment periods (ie, ranitidine and placebo with the same diet). Concentrations below the lower limit of quantitation (LLOQ) were considered zero. Missing data were not imputed. Because NDMA urine and plasma data were not normally distributed, a Wilcoxon signed-rank test was used for analyses per the statistical analysis plan (Supplement 1) without accounting for period or sequence. Because DMA and ranitidine data were log-normally distributed, analyses were based on a mixed-effects analysis of geometric means with terms for treatment, period, and sequence. A 1-sided lower P value <.05 was considered significant for the primary and exploratory analyses of ranitidine vs placebo because the study aim was to evaluate if ranitidine increased NDMA or DMA exposure. Comparisons were performed separately with each diet without adjustment for multiplicity. Exploratory analyses on the effect of diet used a 1-sided lower P value <.05 for NDMA because the cured-meats diet was designed to have higher NDMA, but a 2-sided P value <.05 for DMA and ranitidine because the diet was not designed to affect these outcomes. All analyses except for the primary outcome should be interpreted as exploratory because of the potential for type I error due to multiple comparisons. Pharmacokinetic and statistical analyses were performed in R version 3.6.3 (R Project for Statistical Computing).
Demographics are reported with standard descriptive statistics. NDMA results are reported as median (interquartile range [IQR]). DMA and ranitidine results are reported as geometric means (coefficient of variation percent [CV %]). Comparisons between groups are reported as median (IQR) of the paired differences for NDMA (calculated by first determining the difference between 2 treatments for each individual participant and then determining the median and IQR of those values) and geometric mean ratios for DMA and ranitidine (with 90% CIs for DMA comparisons between ranitidine and placebo and 95% CIs for all other comparisons).
Two post hoc sensitivity analyses were performed for the primary outcome: (1) removing 1 participant who was an outlier for NDMA urinary excretion in 3 of the 4 treatment periods and (2) performing different imputations for NDMA urine values below the LLOQ (eg, assign all samples below the LLOQ of 0.0156 ng/mL to a value of 0.0156 ng/mL).
Results
Eighteen participants (median age, 33.0 [IQR, 28.3 to 42.8] years; 9 women [50%]; 7 White [39%], 11 African American [61%]; and 3 Hispanic or Latino ethnicity [17%]) (Table 1) were randomized to 4 different treatment sequences (Figure 1). One participant discontinued during period 2 (day 3) for personal reasons. The data from that participant were not included in summary tables or comparisons between treatment groups. Multiple samples were below the LLOQ for urine (NDMA [LLOQ, 0.0156 ng/mL], 73% of samples; DMA [LLOQ, 0.50 μg/mL], <1% of samples; and ranitidine [LLOQ, 15.6 ng/mL], <1% of samples) and plasma (NDMA [LLOQ, 0.0156 ng/mL], 89% of samples; DMA [LLOQ, 0.50 μg/mL], 5% of samples; and ranitidine [LLOQ, 15.6 ng/mL], 11% of samples) (eTable 3 in Supplement 2).
Table 1. Study Participant Demographic Characteristics.
| Characteristica | No. (%) |
|---|---|
| No. | 18 |
| Age, median (IQR), y | 33 (28.3-42.8) |
| Sex | |
| Men | 9 (50) |
| Women | 9 (50) |
| Raceb | |
| Black or African American | 11 (61) |
| White | 7 (39) |
| Hispanic or Latino ethnicityb | 3 (17) |
| Body weight, median (IQR), kg | 76.9 (62.6-83.7) |
| State (area of residence) | |
| Wisconsin (Milwaukee area) | 7 (39) |
| Illinois (Chicago area) | 5 (28) |
| Michigan (Detroit area) | 3 (17) |
| Other US states | 3 (17) |
Abbreviation: IQR, interquartile range.
Based on study screening requirements, all participants were between 18 and 50 years; none smoked; all had unremarkable medical histories, screening laboratory values, vital signs, and electrocardiograms; all tested negative for alcohol and drugs of abuse; none used recent prescription, nonprescription, or alternative medications other than contraceptives or acetaminophen; none had a history of Helicobacter pylori infection or ulcerative disease; none were pregnant; and none had signs, symptoms, or diagnostic testing results suggestive of COVID-19. Full inclusion and exclusion criteria are reported in Supplement 1.
Self-identified race and ethnicity were collected in an open-ended format by clinical staff.
Primary Outcome—Urinary Excretion of NDMA
Multiple participants had no quantifiable NDMA in urine (Figure 2; eFigure 1 in Supplement 2) when receiving the noncured-meats diet (8 with ranitidine and 7 with placebo) or the cured-meats diet (3 with ranitidine and 3 with placebo). The median 24-hour NDMA urinary excretion values for ranitidine and placebo were 0.6 ng (IQR, 0 to 29.7) and 10.5 ng (IQR, 0 to 17.8), respectively, with the noncured-meats diet and 11.9 ng (IQR, 5.6 to 48.6) and 23.4 ng (IQR, 8.6 to 36.7), respectively, with the cured-meats diet (Table 2 and Figure 2). There was no statistically significant difference between ranitidine and placebo in 24-hour urinary excretion of NDMA with a noncured-meats diet (median of the paired differences, 0 [IQR, −6.9 to 0] ng; P = .54) or a cured-meats diet (median of the paired differences, −1.1 [IQR, −9.1 to 11.5] ng; P = .71) (Table 2). Figure 3 shows these differences between treatment groups over 24 hours.
Figure 2. Twenty-Four–Hour Urinary Excretion and Maximum Plasma Concentration of NDMA and DMA by Treatment.
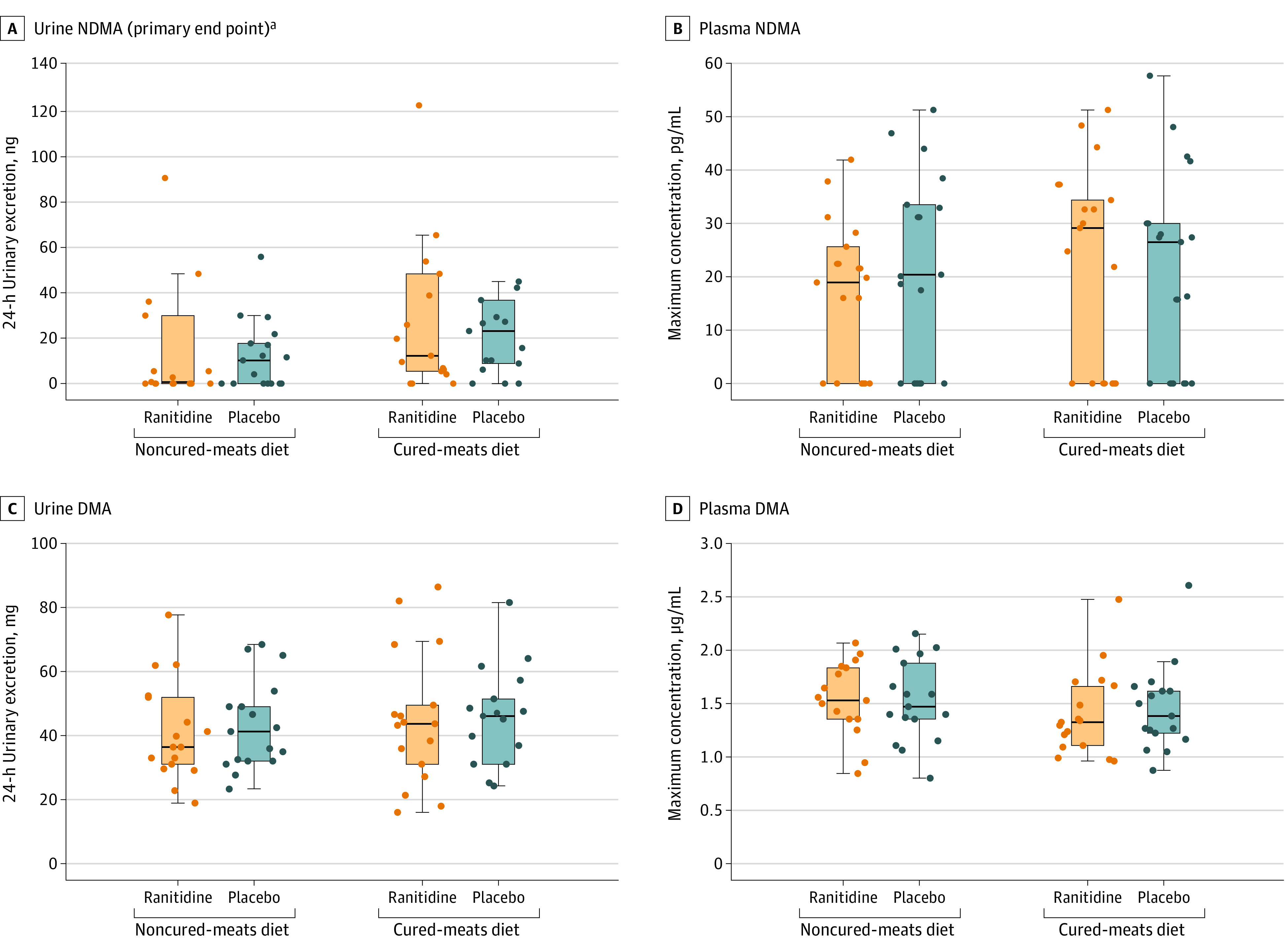
Individual participant (n = 17) observed data and box-and-whisker plot summaries. The line through each box represents the median. The lower and upper borders of the box represent the 25th and 75th percentile, respectively. The whisker extends from the box border to the last observation within 1.5 times the interquartile range. Twenty-four–hour urinary excretion and maximum plasma concentration of ranitidine by treatment are shown in eFigure 7 in Supplement 2. DMA indicates dimethylamine; NDMA, N-nitrosodimethylamine.
aTwo participants had 24-hour urinary excretion of NDMA in 1 or more periods that are outside the scale of the graph in panel A. Participant 12 had a cumulative NDMA excretion of 512 ng with the ranitidine/noncured-meats diet, 649 ng with the ranitidine/cured-meats diet, and 746 ng with the placebo/cured-meats diet (see post hoc assessments section of the Results and eTable 6 in Supplement 2 for additional information on this participant). Participant 03 had a cumulative NDMA excretion of 271 ng with the placebo/cured-meats diet.
Table 2. Effect of Ranitidine on NDMA and DMA 24-Hour Urinary Excretion and Plasma Measurements (Primary and Exploratory Outcomes).
| Outcome | Diet | Ranitidine, median (IQR) | Placebo, median (IQR) | Paired differences, median (IQR)a | P valueb |
|---|---|---|---|---|---|
| No. | 17 | ||||
| Primary outcome | |||||
| NDMA 24-h urinary excretion, ng | Noncured meats | 0.6 (0-29.7) | 10.5 (0-17.8) | 0 (−6.9 to 0) | .54 |
| Cured meats | 11.9 (5.6-48.6) | 23.4 (8.6-36.7) | −1.1 (−9.1 to 11.5) | .71 | |
| Exploratory outcomes | |||||
| Maximum NDMA plasma concentration, pg/mL | Noncured meats | 18.9 (0-25.7) | 20.3 (0-33.5) | 0 (−20.5 to 13.6) | .79 |
| Cured meats | 29.0 (0-34.5) | 16.2 (0-27.4) | 2.2 (−9.4 to 9.1) | .23 | |
| Ranitidine, geometric mean (CV %) | Placebo, geometric mean (CV %) | Geometric mean ratio (90% CI) | |||
| DMA 24-h urinary excretion, mg | Noncured meats | 38.8 (38) | 41.1 (33) | 0.94 (0.87 to 1.01) | .92 |
| Cured meats | 40.7 (52) | 43.1 (34) | 0.95 (0.81 to 1.10) | .74 | |
| Maximum DMA plasma concentration, μg/mL | Noncured meats | 1.51 (25) | 1.48 (27) | 1.02 (0.97 to 1.08) | .26 |
| Cured meats | 1.36 (27) | 1.41 (26) | 0.97 (0.91 to 1.03) | .82 | |
| 24-h plasma DMA area under the curve, h∙μg/mL | Noncured meats | 26.3 (34) | 26.2 (35) | 1.00 (0.91 to 1.11) | .46 |
| Cured meats | 20.9 (53) | 20.9 (46) | 1.00 (0.80 to 1.25) | .49 |
Abbreviations: CV, coefficient of variation; DMA, dimethylamine; IQR, interquartile range; NDMA, N-nitrosodimethylamine.
Calculated by first determining the difference between the 2 treatments for each individual participant and then determining the median of those values. The Wilcoxon signed-rank test used for NDMA analyses is also based on first determining the difference between the treatment groups for each individual participant.
A 1-sided lower P value <.05 was considered significant for the primary and exploratory analyses of ranitidine vs placebo because the study aim was to evaluate if ranitidine increased NDMA or DMA exposure.
Figure 3. Effect of Ranitidine and Diet on Cumulative Excretion of NDMA in Urine Over 24 Hours.
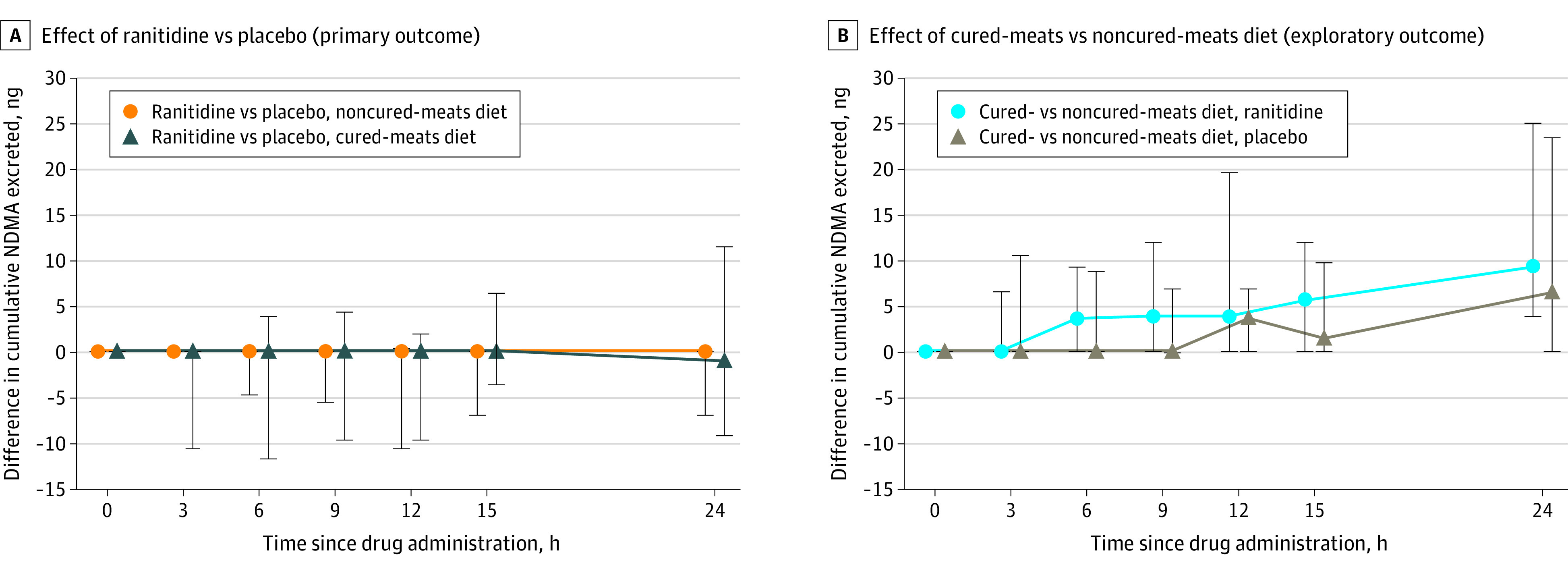
Paired differences in cumulative excretion of N-nitrosodimethylamine (NDMA) in urine over 24 hours. At each listed time point on the x-axis since study drug administration (ie, 0 [pre-dose], 3, 6, 9, 12, 15, and 24 hours), the y-axis shows the median (point) and interquartile range (lines) of the paired differences in cumulative NDMA excretion between the 2 specific treatment groups up until that time point. Note that the median and interquartile range of the paired differences is calculated by first determining the difference between 2 treatments for each individual participant and then determining the median and interquartile range of those values. Ranitidine or placebo was administered at 0 hours. Meals were administered at 0, 4, 7.5, and 11.5 hours. Individual participant profiles for each treatment group are shown in eFigure 1 in Supplement 2.
Exploratory Outcomes
Plasma NDMA
Multiple participants had no quantifiable NDMA in plasma when receiving the noncured-meats diet (6 with ranitidine and 6 with placebo) or the cured-meats diet (6 with ranitidine and 7 with placebo) (Figure 2). NDMA plasma profiles (eFigures 2 and 3 in Supplement 2) did not show sustained quantifiable amounts of NDMA and thus did not support calculation of an AUC. Maximum plasma concentration data by group is shown in Figure 2 and Table 2 (additional pharmacokinetic parameters are reported in eTable 4 in Supplement 2). There were no statistically significant differences between ranitidine and placebo with the noncured-meats diet (median of the paired differences, 0 [IQR, −20.5 to 13.6] ng; P = .79) or the cured-meats diet (median of the paired differences, 2.2 [IQR, −9.4 to 9.1] ng; P = .23) (Table 2).
DMA
All participants had quantifiable DMA in plasma and urine across all treatment groups (Figure 2; eFigures 4-6 in Supplement 2). Across the 4 treatment groups, the geometric mean maximum plasma concentration range was 1.36 to 1.51 μg/mL (CV, 25%-27%), the geometric mean AUC range was 20.9 to 26.3∙h·μg/mL (CV, 34%-53%), and the geometric mean amount excreted in urine range was 38.8 to 43.1 mg (CV, 33%-52%) (Table 2; additional pharmacokinetic parameters are reported in eTable 4 in Supplement 2). All comparisons between ranitidine and placebo had geometric mean ratio 90% CIs crossing 1.
Ranitidine
Geometric mean ranitidine 24-hour urinary excretion was 91.7 mg (CV, 29%) for the noncured-meats diet and 74.1 mg (CV, 39%) for the cured-meats diet. Additional urine and plasma data are reported in eTable 5 and eFigures 7-11 in Supplement 2.
Comparisons Between Diets for Ranitidine and Placebo
The cured-meats diet compared with the noncured-meats diet was associated with significantly higher 24-hour urinary excretion of NDMA with placebo (median of the paired differences, 6.4 [IQR, 0 to 23.4]; P = .007) and ranitidine (median of the paired differences, 9.3 [IQR, 3.8 to 25.0]; P = .005) (Figure 3 and Table 3). In addition, the cured-meats diet, compared with the noncured-meats diet, was associated with significantly lower plasma DMA AUC geometric mean ratios with both placebo and ranitidine (0.80 [95% CI, 0.65 to 0.98]; P = .03 with placebo and 0.80 [95% CI, 0.67 to 0.95]; P = .02 with ranitidine) and maximum plasma concentration with ranitidine (0.90 [95% CI, 0.84 to 0.97]; P = .009) but not placebo (0.95 [95% CI, 0.86 to 1.06]; P = .33) (Table 3). Comparisons between diets for 24-hour DMA urinary excretion had 95% CIs crossing 1 (Table 3).
Table 3. Effect of Diet on NDMA and DMA 24-Hour Urinary Excretion and Plasma Measurements (Exploratory Outcomes).
| Study drug | Cured-meats diet, median (IQR) | Noncured-meats diet, median (IQR) | Paired differences, median (IQR)a | P valueb | |
|---|---|---|---|---|---|
| No. | 17 | ||||
| NDMA 24-h urinary excretion, ng | Ranitidine | 11.9 (5.6-48.6) | 0.6 (0-29.7) | 9.3 (3.8 to 25.0) | .005 |
| Placebo | 23.4 (8.6-36.7) | 10.5 (0-17.8) | 6.4 (0 to 23.4) | .007 | |
| Maximum NDMA plasma concentration, pg/mL | Ranitidine | 29.0 (0-34.5) | 18.9 (0-25.7) | 0.4 (−5.1 to 32.6) | .15 |
| Placebo | 16.2 (0-27.4) | 20.3 (0-33.5) | 0 (−18.8 to 8.1) | .74 | |
| Cured-meats diet, geometric mean (CV %) | Noncured-meats diet, geometric mean (CV %) | Geometric mean ratio (95% CI) | |||
| DMA 24-h urinary excretion, mg | Ranitidine | 40.7 (52) | 38.8 (38) | 1.05 (0.90 to 1.23) | .52 |
| Placebo | 43.1 (34) | 41.1 (33) | 1.05 (0.93 to 1.19) | .42 | |
| Maximum DMA plasma concentration, μg/mL | Ranitidine | 1.36 (27) | 1.51 (25) | 0.90 (0.84 to 0.97) | .009 |
| Placebo | 1.41 (26) | 1.48 (27) | 0.95 (0.86 to 1.06) | .33 | |
| 24-h plasma DMA area under the curve, h∙μg/mL | Ranitidine | 20.9 (53) | 26.3 (34) | 0.80 (0.67 to 0.95) | .02 |
| Placebo | 20.9 (46) | 26.2 (35) | 0.80 (0.65 to 0.98) | .03 |
Abbreviations: CV, coefficient of variation; DMA, dimethylamine; IQR, interquartile range; NDMA, N-nitrosodimethylamine.
Calculated by first determining the difference between the 2 treatments for each individual participant and then determining the median of those values. The Wilcoxon signed-rank test used for NDMA analyses is also based on first determining the difference between the treatment groups for each individual participant.
Exploratory analyses on the effect of diet used a 1-sided lower P value <.05 for NDMA, as the cured-meats diet was designed to have higher NDMA but used P < .05 (2-sided) for DMA, as the effect of diet on these outcomes was not known.
Post Hoc Assessments
One participant’s 24-hour NDMA urinary excretion was an outlier in periods 2 through 4 (ranitidine/noncured-meats diet, 512 ng; ranitidine/cured-meats diet, 649 ng; and placebo/cured-meats diet, 746 ng) but not in period 1 (placebo/noncured-meats diet, 29 ng). The participant’s maximum plasma concentration for NDMA did not follow this pattern; however, the NDMA amount in predose urine samples followed the same pattern (eTable 6 in Supplement 2). Clinical staff noted that the participant began menstruating between study periods 1 and 2, continuing through study period 4, and blood was detected and visible in urine samples. When removing this participant from the analysis, ranitidine, compared with placebo, still did not significantly increase 24-hour NDMA urinary excretion (eTable 7 in Supplement 2). In addition, when performing the second set of sensitivity analyses (imputations for samples below the LLOQ), there was still no statistically significant difference in 24-hour urinary excretion of NDMA (eTable 8 in Supplement 2).
Adverse Events
No serious drug-related adverse events were reported. The most common adverse event was vessel puncture site pain, which developed in 2 participants (eTable 9 in Supplement 2).
Discussion
In this randomized, placebo-controlled study in healthy participants, oral administration of ranitidine (300 mg) did not significantly increase 24-hour urinary excretion of NDMA. The lack of a significant increase occurred even when participants were served a diet designed to be higher in nitrites, which in vitro studies have suggested can potentiate NDMA formation. Furthermore, exploratory analyses did not reveal any significant difference between ranitidine and placebo for NDMA in plasma or DMA in urine or plasma.
This study was designed and conducted to further investigate the findings from a prior clinical study that observed an approximately 400-fold increase in 24-hour urinary excretion of NDMA and an approximately 2.5-fold increase in DMA level after ranitidine administration.3 After completion of this study and submission of this report for publication, the prior study was retracted at the request of the authors, citing that an analytical artifact could have contributed to the levels of NDMA measured.15 The analytical artifact was likely the primary factor; however, other factors could have contributed to falsely high NDMA levels in the prior study (summarized in eTable 10 in Supplement 2). Furthermore, the absence of a significant increase in NDMA levels in the present study occurred despite using a higher ranitidine dose (300 mg vs 150 mg) and having participants consume a diet with higher nitrite and nitrate content for at least 24 hours before and after administration of ranitidine or placebo.
The 24-hour urinary excretion of NDMA with placebo in this study was within the range of previously reported results.3,16,17,18,19 While ranitidine did not have a significant effect on NDMA or DMA values, the cured-meats diet was associated with a significant increase in NDMA urinary excretion, suggesting that the study design and methods were sufficiently sensitive to detect the relatively small effect of diet on NDMA urinary excretion. Regarding DMA, the cured-meats diet was associated with significantly lower DMA plasma exposure. DMA content in foods is known to differ.20 There was a consistent level of DMA in plasma throughout the day, with approximately 40 mg excreted in urine daily across all treatment groups. This demonstrated that the human body is constantly exposed to large amounts of DMA.
A recent study by Braunstein et al21 reported that ranitidine converted to NDMA under simulated physiologic conditions in gastric fluid. However, in an article by Gao et al,13 comparison to clinical studies measuring human gastric nitrite concentrations and pH suggested that in the study by Braunstein et al the tested gastric fluid conditions did not represent physiologic nitrite concentrations. Gao et al included physiologic nitrite concentrations in simulated gastric fluid experiments and found that NDMA did not form until nitrite concentrations were approximately 50-fold higher than the upper range of physiologic nitrite at pH less than 6.13 Furthermore, at more acidic pH, where NDMA formation is favored, the difference compared with the 95th percentile of the study population was approximately 9000-fold for fasting patients undergoing upper endoscopy and 600-fold from a study with 24-hour gastric nitrite and pH measurements (fed and fasting) with patients with precancerous conditions.13,22,23
The importance of assessing physiologic relevance extends to other settings. A rodent study24 has been cited as preliminary evidence of ranitidine being associated with cancer.2,25 While that study found that co-dosing ranitidine and sodium nitrite to 2-month old rats could be genotoxic, it involved extremely high ranitidine (175 or 350 mg/kg) and sodium nitrite (80 mg/kg) doses while fasting animals for 60 hours to reduce gastric pH.24 This vastly exceeds human ranitidine doses (≈2-4 mg/kg), and the sodium nitrite dose was close to the median lethal dose in rats.24 Regarding human studies, a recent commentary25 also referenced an observational study26 as preliminary evidence of ranitidine being associated with cancer. The study found that current ranitidine users had an increased risk of ductal carcinoma (breast cancer)26; however, the study did not control for cancer risk factors and performed more than 40 comparisons without adjustment for multiple comparisons, and 4 other studies found no relationship between ranitidine and breast cancer.27,28,29,30 When considering all cancer types,26,27,28,29,30,31,32 while each study had limitations (eg, confounding by indication, residual confounding, multiple comparisons), no consistent signals emerged across studies, and studies with comparison to active controls found no association between ranitidine and overall or specific cancer risk.27,28,29,31
Ranitidine products were withdrawn from the US market in April 2020 because of higher than acceptable NDMA amounts being detected in the drug product that could increase over time during storage.33 However, the approvals were not withdrawn, and the FDA may consider allowing ranitidine product back on the market if a specific product is stable and NDMA amounts do not increase to unsafe levels over time during storage.34 When considering the potential risk to patients who may have taken ranitidine with NDMA amounts above the acceptable daily limit, it is important to note that this is the amount predicted to result in a cancer risk of 1 in 100 000 if taken daily for a lifetime (ie, 70 years of 96 ng/d of NDMA for a 50-kg person).35
Limitations
This study has several limitations. First, 1 participant was an outlier for 24-hour NDMA urinary excretion in 3 treatment periods; however, the data suggest that this was not due to ranitidine or an in vivo effect because (1) the outlier values occurred when the participant was menstruating, (2) blood was visible and detected in urine samples, and (3) predose urine sample NDMA amounts followed the same pattern while maximum plasma concentration of NDMA did not. Because red blood cells contain high intracellular nitrite concentration,36,37 it is possible that ex vivo lysis of red blood cells in acidic urine samples facilitated conversion of DMA to NDMA. Second, the study included many urine samples with NDMA levels below the LLOQ. However, the NDMA assay was very sensitive (LLOQ, 0.0156 ng/mL) and there was no notable difference between ranitidine and placebo in the number of participants with all sample below the LLOQ. Sensitivity analyses for each of the first 2 limitations did not change the study results. Third, the exact dietary amount of nitrite, nitrate, and NDMA is not known. However, ranitidine did not significantly increase NDMA values with either diet, including the cured-meats diet designed to have high nitrite content. Fourth, this study only included healthy participants and did not exclude formation of NDMA in the gastrointestinal tract that was not absorbed and detected in plasma or urine. However, the results support that ranitidine did not significantly increase levels of NDMA or DMA detected in plasma or urine using highly sensitive assays, and the accompanying in vitro gastric fluid study13 indicated that NDMA was not formed until nitrite concentrations were substantially higher than those seen in patients.13,22,23
Conclusions
In this trial that included 18 healthy participants, oral ranitidine (300 mg), compared with placebo, did not significantly increase 24-hour urinary excretion of NDMA when participants consumed noncured-meats or cured-meats diets. These findings do not support that ranitidine is converted to NDMA in a general, healthy population.
Trial Protocol
eTable 1. NDMA testing results from the drug product used in the study
eTable 2. Listing of participants, treatment groups, and diets where all items were not consumed on treatment days
eTable 3. Samples below the lower limit of quantification by analyte and biospecimen
eTable 4. Summary table of additional NDMA and DMA 24-hr pharmacokinetic parameters
eTable 5. Summary table of 24-hr urinary excretion and pharmacokinetic parameters for ranitidine
eTable 6. Listing of pre-dose and 24-hour cumulative NDMA urine levels and maximum concentration for participant 12a
eTable 7. Comparison of 24-hour urinary excretion of NDMA with participant 12 removed
eTable 8. Comparison of 24-hour urinary excretion of NDMA with different LLOQ and limit of detection imputations
eTable 9. Incidence and number of adverse events by treatment group
eTable 10. Comparison of the current and prior clinical studies on ranitidine and urinary excretion of NDMA
eFigure 1. Individual participant cumulative 24-hr urine excretion profiles of NDMA by group
eFigure 2. Arithmetic mean NDMA plasma concentration over time by group
eFigure 3. Individual participant NDMA plasma concentration profiles by group
eFigure 4. Cumulative DMA excreted in urine over 24-hours on a linear scale and DMA plasma concentration on a log scale by group
eFigure 5. Individual participant cumulative 24-hr urine excretion profiles of DMA by group
eFigure 6. Individual participant DMA plasma concentration profiles by group
eFigure 7. 24-hour urinary excretion and maximum plasma concentration of ranitidine by group
eFigure 8. Geometric mean ranitidine 24-hour urinary excretion by diet
eFigure 9. Geometric mean ranitidine plasma concentration over time by diet
eFigure 10. Individual participant cumulative 24-hr urine excretion profiles of ranitidine by diet
eFigure 11. Individual participant ranitidine plasma concentration profiles by diet
eMethods 1. Selection of Meals for Study and Full Menu Listing
eMethods 2. Bioanalytical Methods for the Analytes and Biospecimens in the Clinical Study
eDictionary. Data Dictionary for Participant Data Listings
eReferences
Individual Participant Urine Data
Individual Participant Plasma Data
Data Sharing Statement
References
- 1.Liteplo RG, Meek ME, Windle W. Concise International Chemical Assessment Document 38: N-nitrosodimethylamine. World Health Organization. Published 2002. Accessed March 21, 2021. https://www.who.int/ipcs/publications/cicad/en/cicad38.pdf
- 2.Valisure citizen petition on ranitidine. Regulations.gov. Published 2019. Accessed March 19, 2020. http://www.regulations.gov/document/FDA-2019-P-4281-0001
- 3.Zeng T, Mitch WA. Oral intake of ranitidine increases urinary excretion of N-nitrosodimethylamine. Carcinogenesis. 2016;37(6):625-634. Retracted in: Carcinogenesis. May 4, 2021. doi: 10.1093/carcin/bgw034 [DOI] [PubMed] [Google Scholar]
- 4.Statement alerting patients and health care professionals of NDMA found in samples of ranitidine. US Food and Drug Administration. Published 2019. Accessed March 23, 2021. https://www.fda.gov/news-events/press-announcements/statement-alerting-patients-and-health-care-professionals-ndma-found-samples-ranitidine
- 5.Final response letter from FDA CDER to Valisure, LLC. US Food and Drug Administration. Published 2020. Accessed March 19, 2021. http://www.regulations.gov/document/FDA-2019-P-4281-0008
- 6.FDA Updates and Press Announcements on NDMA in Ranitidine (Zantac): FDA-published testing method to provide an option for regulators and industry to detect NDMA impurities. US Food and Drug Administration. Published 2020. Accessed May 11, 2021. https://www.fda.gov/drugs/drug-safety-and-availability/fda-updates-and-press-announcements-ndma-ranitidine-zantac
- 7.Laboratory tests: ranitidine. US Food and Drug Administration. Published 2019. Accessed March 19, 2021. https://www.fda.gov/drugs/drug-safety-and-availability/laboratory-tests-ranitidine
- 8.Public Health Goals for Chemicals in Drinking Water: N-Nitrosodimethylamine. California Environmental Protection Agency. Published 2006. Accessed March 31, 2021. https://oehha.ca.gov/media/downloads/water/chemicals/phg/122206ndmaphg.pdf
- 9.Hord NG, Tang Y, Bryan NS. Food sources of nitrates and nitrites: the physiologic context for potential health benefits. Am J Clin Nutr. 2009;90(1):1-10. doi: 10.3945/ajcn.2008.27131 [DOI] [PubMed] [Google Scholar]
- 10.Hsu J. AJ, Lee N.A.. Nitrate and nitrite quantification from cured meat and vegetables and their estimated dietary intake in Australians. Food Chemistry. 2009;115(1):334-339. doi: 10.1016/j.foodchem.2008.11.081 [DOI] [Google Scholar]
- 11.Park JE, Seo JE, Lee JY, Kwon H. Distribution of seven N-nitrosamines in food. Toxicol Res. 2015;31(3):279-288. doi: 10.5487/TR.2015.31.3.279 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Collection of race and ethnicity data in clinical trials: guidance for industry and Food and Drug Administration staff. US Food and Drug Administration. Published 2016. Accessed March 19, 2021. https://www.fda.gov/media/75453/download
- 13.Gao Z, Karfunkle M, Ye W, et al. In vitro analysis of N-nitrosodimethylamine (NDMA) formation from ranitidine under simulated gastrointestinal conditions. JAMA Netw Open. Published online June 28, 2021. doi: 10.1001/jamanetworkopen.2021.18253 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Bioanalytical method validation guidance for industry. US Food and Drug Administration. Published 2018. Accessed March 21, 2021. https://www.fda.gov/regulatory-information/search-fda-guidance-documents/bioanalytical-method-validation-guidance-industry
- 15.Notice of retraction: Zeng T, Mitch WA. Oral intake of ranitidine increases urinary excretion of N-nitrosodimethylamine. Carcinogenesis. 2016;37(6):625-634. Published online May 4, 2021. doi: 10.1093/carcin/bgab029 [DOI] [PubMed] [Google Scholar]
- 16.Garland WA, Kuenzig W, Rubio F, Kornychuk H, Norkus EP, Conney AH. Urinary excretion of nitrosodimethylamine and nitrosoproline in humans: interindividual and intraindividual differences and the effect of administered ascorbic acid and alpha-tocopherol. Cancer Res. 1986;46(10):5392-5400. [PubMed] [Google Scholar]
- 17.van Maanen JM, Pachen DM, Dallinga JW, Kleinjans JC. Formation of nitrosamines during consumption of nitrate- and amine-rich foods, and the influence of the use of mouthwashes. Cancer Detect Prev. 1998;22(3):204-212. [DOI] [PubMed] [Google Scholar]
- 18.van Maanen JM, Welle IJ, Hageman G, Dallinga JW, Mertens PL, Kleinjans JC. Nitrate contamination of drinking water: relationship with HPRT variant frequency in lymphocyte DNA and urinary excretion of N-nitrosamines. Environ Health Perspect. 1996;104(5):522-528. doi: 10.1289/ehp.96104522 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Vermeer IT, Pachen DM, Dallinga JW, Kleinjans JC, van Maanen JM. Volatile N-nitrosamine formation after intake of nitrate at the ADI level in combination with an amine-rich diet. Environ Health Perspect. 1998;106(8):459-463. doi: 10.1289/ehp.106-1533225 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Mitchell SC, Zhang AQ, Smith RL. Dimethylamine and diet. Food Chem Toxicol. 2008;46(5):1734-1738. doi: 10.1016/j.fct.2008.01.010 [DOI] [PubMed] [Google Scholar]
- 21.Braunstein LZ, Kantor ED, O’Connell K, et al. Analysis of ranitidine-associated N-nitrosodimethylamine production under simulated physiologic conditions. JAMA Netw Open. 2021;4(1):e2034766. doi: 10.1001/jamanetworkopen.2020.34766 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Xu GP, Reed PI. N-nitroso compounds in fresh gastric juice and their relation to intragastric pH and nitrite employing an improved analytical method. Carcinogenesis. 1993;14(12):2547-2551. doi: 10.1093/carcin/14.12.2547 [DOI] [PubMed] [Google Scholar]
- 23.Hall CN, Darkin D, Brimblecombe R, Cook AJ, Kirkham JS, Northfield TC. Evaluation of the nitrosamine hypothesis of gastric carcinogenesis in precancerous conditions. Gut. 1986;27(5):491-498. doi: 10.1136/gut.27.5.491 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Brambilla G, Cavanna M, Faggin P, et al. Genotoxic effects in rodents given high oral doses of ranitidine and sodium nitrite. Carcinogenesis. 1983;4(10):1281-1285. doi: 10.1093/carcin/4.10.1281 [DOI] [PubMed] [Google Scholar]
- 25.White CM. Ranitidine’s N-nitrosodimethylamine problem may be tip of the iceberg. JAMA Netw Open. 2021;4(1):e2035158. doi: 10.1001/jamanetworkopen.2020.35158 [DOI] [PubMed] [Google Scholar]
- 26.Mathes RW, Malone KE, Daling JR, Porter PL, Li CI. Relationship between histamine2-receptor antagonist medications and risk of invasive breast cancer. Cancer Epidemiol Biomarkers Prev. 2008;17(1):67-72. doi: 10.1158/1055-9965.EPI-07-0765 [DOI] [PubMed] [Google Scholar]
- 27.Yoon HJ, Kim JH, Seo GH, Park H. Risk of cancer following the use of N-nitrosodimethylamine (NDMA) contaminated ranitidine products: a nationwide cohort study in South Korea. J Clin Med. 2021;10(1):153. doi: 10.3390/jcm10010153 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Iwagami M, Kumazawa R, Miyamoto Y, et al. Risk of cancer in association with ranitidine and nizatidine vs other H2 blockers: analysis of the Japan medical data center claims database 2005-2018. Drug Saf. 2021;44(3):361-371. doi: 10.1007/s40264-020-01024-0 [DOI] [PubMed] [Google Scholar]
- 29.Kantor ED, O’Connell K, Du M, Mendelsohn RB, Liang PS, Braunstein LZ. Ranitidine use and cancer risk: results from UK biobank. Gastroenterology. 2021;160(5):1856-1859.e5. doi: 10.1053/j.gastro.2020.12.037 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Habel LA, Levin TR, Friedman GD. Cimetidine use and risk of breast, prostate, and other cancers. Pharmacoepidemiol Drug Saf. 2000;9(2):149-155. doi: [DOI] [PubMed] [Google Scholar]
- 31.Kumar S, Goldberg DS, Kaplan DE. Ranitidine use and gastric cancer among persons with Helicobacter pylori. Dig Dis Sci. Published online April 15, 2021. doi: 10.1007/s10620-021-06972-w [DOI] [PubMed] [Google Scholar]
- 32.Tran KT, McMenamin UC, Hicks B, et al. Proton pump inhibitor and histamine-2 receptor antagonist use and risk of liver cancer in two population-based studies. Aliment Pharmacol Ther. 2018;48(1):55-64. doi: 10.1111/apt.14796 [DOI] [PubMed] [Google Scholar]
- 33.FDA Requests Removal of All Ranitidine Products (Zantac) from the Market. US Food and Drug Administration. Published April 1, 2021. Accessed March 23, 2020. https://www.fda.gov/news-events/press-announcements/fda-requests-removal-all-ranitidine-products-zantac-market
- 34.Questions and answers: NDMA impurities in ranitidine (commonly known as Zantac). US Food and Drug Administration. Updated April 1, 2020. Accessed March 19, 2021. https://www.fda.gov/drugs/drug-safety-and-availability/questions-and-answers-ndma-impurities-ranitidine-commonly-known-zantac
- 35.Control of nitrosamine impurities in human drugs: guidance for industry. US Food and Drug Administration. Published 2020. Accessed March 19, 2021. https://www.fda.gov/media/141720/download
- 36.Wang X, Bryan NS, MacArthur PH, Rodriguez J, Gladwin MT, Feelisch M. Measurement of nitric oxide levels in the red cell: validation of tri-iodide-based chemiluminescence with acid-sulfanilamide pretreatment. J Biol Chem. 2006;281(37):26994-27002. doi: 10.1074/jbc.M603953200 [DOI] [PubMed] [Google Scholar]
- 37.Dejam A, Hunter CJ, Pelletier MM, et al. Erythrocytes are the major intravascular storage sites of nitrite in human blood. Blood. 2005;106(2):734-739. doi: 10.1182/blood-2005-02-0567 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Trial Protocol
eTable 1. NDMA testing results from the drug product used in the study
eTable 2. Listing of participants, treatment groups, and diets where all items were not consumed on treatment days
eTable 3. Samples below the lower limit of quantification by analyte and biospecimen
eTable 4. Summary table of additional NDMA and DMA 24-hr pharmacokinetic parameters
eTable 5. Summary table of 24-hr urinary excretion and pharmacokinetic parameters for ranitidine
eTable 6. Listing of pre-dose and 24-hour cumulative NDMA urine levels and maximum concentration for participant 12a
eTable 7. Comparison of 24-hour urinary excretion of NDMA with participant 12 removed
eTable 8. Comparison of 24-hour urinary excretion of NDMA with different LLOQ and limit of detection imputations
eTable 9. Incidence and number of adverse events by treatment group
eTable 10. Comparison of the current and prior clinical studies on ranitidine and urinary excretion of NDMA
eFigure 1. Individual participant cumulative 24-hr urine excretion profiles of NDMA by group
eFigure 2. Arithmetic mean NDMA plasma concentration over time by group
eFigure 3. Individual participant NDMA plasma concentration profiles by group
eFigure 4. Cumulative DMA excreted in urine over 24-hours on a linear scale and DMA plasma concentration on a log scale by group
eFigure 5. Individual participant cumulative 24-hr urine excretion profiles of DMA by group
eFigure 6. Individual participant DMA plasma concentration profiles by group
eFigure 7. 24-hour urinary excretion and maximum plasma concentration of ranitidine by group
eFigure 8. Geometric mean ranitidine 24-hour urinary excretion by diet
eFigure 9. Geometric mean ranitidine plasma concentration over time by diet
eFigure 10. Individual participant cumulative 24-hr urine excretion profiles of ranitidine by diet
eFigure 11. Individual participant ranitidine plasma concentration profiles by diet
eMethods 1. Selection of Meals for Study and Full Menu Listing
eMethods 2. Bioanalytical Methods for the Analytes and Biospecimens in the Clinical Study
eDictionary. Data Dictionary for Participant Data Listings
eReferences
Individual Participant Urine Data
Individual Participant Plasma Data
Data Sharing Statement


