Abstract
Background
Urinary catheterisation is a common procedure, with approximately 15% to 25% of all people admitted to hospital receiving short‐term (14 days or less) indwelling urethral catheterisation at some point during their care. However, the use of urinary catheters is associated with an increased risk of developing urinary tract infection. Catheter‐associated urinary tract infection (CAUTI) is one of the most common hospital‐acquired infections. It is estimated that around 20% of hospital‐acquired bacteraemias arise from the urinary tract and are associated with mortality of around 10%.
This is an update of a Cochrane Review first published in 2005 and last published in 2007.
Objectives
To assess the effects of strategies for removing short‐term (14 days or less) indwelling catheters in adults.
Search methods
We searched the Cochrane Incontinence Specialised Register, which contains trials identified from CENTRAL, MEDLINE, MEDLINE In‐Process, MEDLINE Epub Ahead of Print, CINAHL, ClinicalTrials.gov, WHO ICTRP, and handsearching of journals and conference proceedings (searched 17 March 2020), and reference lists of relevant articles.
Selection criteria
We included all randomised controlled trials (RCTs) and quasi‐RCTs that evaluated the effectiveness of practices undertaken for the removal of short‐term indwelling urethral catheters in adults for any reason in any setting.
Data collection and analysis
Two review authors performed abstract and full‐text screening of all relevant articles. At least two review authors independently performed risk of bias assessment, data abstraction and GRADE assessment.
Main results
We included 99 trials involving 12,241 participants. We judged the majority of trials to be at low or unclear risk of selection and detection bias, with a high risk of performance bias. We also deemed most trials to be at low risk of attrition and reporting bias. None of the trials reported on quality of life. The majority of participants across the trials had undergone some form of surgical procedure.
Thirteen trials involving 1506 participants compared the removal of short‐term indwelling urethral catheters at one time of day (early morning removal group between 6 am to 7 am) versus another (late night removal group between 10 pm to midnight). Catheter removal late at night may slightly reduce the risk of requiring recatheterisation compared with early morning (RR 0.71, 95% CI 0.53 to 0.96; 10 RCTs, 1920 participants; low‐certainty evidence). We are uncertain if there is any difference between early morning and late night removal in the risk of developing symptomatic CAUTI (RR 1.00, 95% CI 0.61 to 1.63; 1 RCT, 41 participants; very low‐certainty evidence). We are uncertain whether the time of day makes a difference to the risk of dysuria (RR 2.20; 95% CI 0.70 to 6.86; 1 RCT, 170 participants; low‐certainty evidence).
Sixty‐eight trials involving 9247 participants compared shorter versus longer durations of catheterisation. Shorter durations may increase the risk of requiring recatheterisation compared with longer durations (RR 1.81, 95% CI 1.35 to 2.41; 44 trials, 5870 participants; low‐certainty evidence), but probably reduce the risk of symptomatic CAUTI (RR 0.52, 95% CI 0.45 to 0.61; 41 RCTs, 5759 participants; moderate‐certainty evidence) and may reduce the risk of dysuria (RR 0.42, 95% CI 0.20 to 0.88; 7 RCTs; 1398 participants; low‐certainty evidence).
Seven trials involving 714 participants compared policies of clamping catheters versus free drainage. There may be little to no difference between clamping and free drainage in terms of the risk of requiring recatheterisation (RR 0.82, 95% CI 0.55 to 1.21; 5 RCTs; 569 participants; low‐certainty evidence). We are uncertain if there is any difference in the risk of symptomatic CAUTI (RR 0.99, 95% CI 0.60 to 1.63; 2 RCTs, 267 participants; very low‐certainty evidence) or dysuria (RR 0.84, 95% CI 0.46 to 1.54; 1 trial, 79 participants; very low‐certainty evidence).
Three trials involving 402 participants compared the use of prophylactic alpha blockers versus no intervention or placebo. We are uncertain if prophylactic alpha blockers before catheter removal has any effect on the risk of requiring recatheterisation (RR 1.18, 95% CI 0.58 to 2.42; 2 RCTs, 184 participants; very low‐certainty evidence) or risk of symptomatic CAUTI (RR 0.20, 95% CI 0.01 to 4.06; 1 trial, 94 participants; very low‐certainty evidence). None of the included trials investigating prophylactic alpha blockers reported the number of participants with dysuria.
Authors' conclusions
There is some evidence to suggest the removal of indwelling urethral catheters late at night rather than early in the morning may reduce the number of people who require recatheterisation. It appears that catheter removal after shorter compared to longer durations probably reduces the risk of symptomatic CAUTI and may reduce the risk of dysuria. However, it may lead to more people requiring recatheterisation. The other evidence relating to the risk of symptomatic CAUTI and dysuria is too uncertain to allow us to draw any conclusions.
Due to the low certainty of the majority of the evidence presented here, the results of further research are likely to change our findings and to have a further impact on clinical practice. This systematic review has highlighted the need for a standardised set of core outcomes, which should be measured and reported by all future trials comparing strategies for the removal of short‐term urinary catheters. Future trials should also study the effects of short‐term indwelling urethral catheter removal on non‐surgical patients.
Plain language summary
What are the best strategies for removing drainage tubes (urinary catheters) from the urinary bladder after 14 days or less?
Key messages
• Removing drainage tubes late at night instead of early in the morning might reduce the number of people who need to have the drainage tube reinserted.
• Removing drainage tubes sooner rather than later probably reduces the risk of infection caused by the drainage tube and painful urination. However, it may lead to more people needing to have the tube reinserted.
• We need future studies to research the effects of drainage tube removal for people who did not have surgery.
What are urinary catheters?
Urinary catheters are flexible, hollow tubes that are used to empty the urinary bladder and collect urine in a bag. They are often used for short periods of time for people who cannot pass urine themselves, for example during or after surgery, or when healthcare staff need to measure someone’s urine. One harmful effect of catheters is the risk of developing urinary tract infections (UTIs). If catheters are removed quickly, the risk of infection is reduced, but if they are removed too soon, they may need to be reinserted.
What did we want to find out?
We wanted to investigate the effects of different strategies on the risk of:
• needing to have the catheter reinserted; • developing a urinary tract infection (UTI); • experiencing pain when urinating.
What did we do?
We searched for studies that looked at the use of short‐term urinary catheters in adults. We defined ‘short‐term’ as 14 days or less. Studies could take place anywhere and participants could have any condition or illness.
We compared and summarised the results of the studies and rated our confidence in the evidence, based on factors such as study methods and sizes.
What did we find?
We found 99 studies with 12,241 participants. Most participants were surgical patients and many of the studies (50) assessed women only.
The studies investigated:
• removing the catheter early in the morning compared with late at night (13 studies); • retaining the catheter for shorter or longer times (68 studies); • clamping catheters or allowing them to drain freely (7 studies); and • giving men treatment (alpha blockers) to relax the prostate compared to no treatment before removing the catheter (3 studies). The prostate is a small gland located between the penis and the bladder.
Early‐morning compared to late‐night removal
Late‐night catheter removal might reduce the risk of needing to have the catheter reinserted compared with early‐morning removal. We are uncertain if there is any difference between early‐morning and late‐night removal for developing UTI or painful urination.
Shorter compared to longer use of catheters
People who have their catheters removed after a shorter length of time are probably less likely to develop UTIs and may be less likely to experience painful urination compared with those who have their catheters for longer. However, we also found that people may be more likely to need the catheter reinserting if they have the catheter for a shorter compared with a longer time.
Clamping
There may be little to no difference between clamping and free drainage on the risk of needing the catheter to be reinserted. We are uncertain if there is any difference in the risk of UTIs or painful urination.
Treatment to relax the prostate
We are uncertain whether giving alpha‐blockers before the catheter is removed has any effect on the need to have catheters reinserted or the risk of developing UTIs. There was no evidence about the risk of experiencing painful urination.
What are the limitations of the evidence?
Many of the included trials had design flaws, did not recruit enough people, or did not report enough information about their results. This means our confidence in the evidence is limited.
How up‐to‐date is this evidence?
The evidence is current up to 17 March 2020.
Summary of findings
Background
This is an update of a Cochrane Review first published in 2005 and last published in 2007. See Appendix 1 for a glossary of medical terms.
Description of the condition
Catheterisation is an important and common clinical procedure. Approximately 15% to 25% of all people who are hospitalised will be catheterised at some point during their management (CDC 2016). However, it should be noted that not every patient who has a urethral catheter inserted requires one. Trials have shown that it is common for catheters to be placed in patients without an appropriate indication (Loeb 2008; Meddings 2014). Catheterisation could be for the short term (up to 14 days) or long term (14 days or longer). The indications for short‐term catheterisation include monitoring of urine output during the perioperative stage or in acutely unwell patients, as part of a urological procedure, or the treatment of patients with acute urinary retention. Short‐term catheterisation could also be used for investigative purposes, such as imaging of the urinary tract and urodynamic trials (Dunn 2000a). Long‐term catheterisation is usually a last resort option in people with recurrent urinary retention, reduced bladder contractility or urinary incontinence.
Retention of urine has been reported as a common problem following the removal of indwelling urethral catheters, particularly following surgery and anaesthesia, where post‐operative urinary retention has a reported incidence between 5% and 70% (Baldini 2009). The risk factors associated with an increase the risk of developing post‐operative urinary retention have been thoroughly researched and include age (over 50 years), sex (male), type of surgery, duration of surgery, type of anaesthesia (general or regional, e.g. epidural), analgesia (use of opiates) and the amount of intravenous fluids used. Post‐operative urinary retention may lead to urinary tract infections, abnormal autonomic responses (e.g. cardiac arrhythmias) as well as over‐distension of the bladder resulting in permanent detrusor muscle damage (Baldini 2009; Madersbacher 2012; Rosseland 2002; Zaouter 2009). For hospital inpatients, the duration of catheterisation in the peri‐operative period remains controversial and is one that is ultimately down to the preference of the surgeon or anaesthetist responsible for the patient. Removal too early, however, may result in the patient developing urinary retention again and thus risking requiring recatheterisation alongside the complications associated with it (Baldini 2009).
The procedure of indwelling urethral catheterisation is associated with complications such as catheter‐associated urinary tract infection (CAUTI), bacteruria, stricture formation, structural damage to the urinary tract, bleeding, cystitis or prostatitis, and patient discomfort (Igawa 2008; Fisher 2017). CAUTI is the most common cause of hospital‐acquired infections with some 70% to 80% of these associated with the use of indwelling urethral catheters (Lo 2014; Nicolle 2014). CAUTI arise from the formation of a biofilm on both the extraluminal and intraluminal portal surfaces of the catheter. This biofilm mainly consists of extraluminal organisms, which adhere to the surfaces of the catheter as soon as it has been inserted. It has the ability to defend microbes from the host's defences as well as antimicrobials (Haque 2018; Nicolle 2014). It is estimated that around 20% of hospital‐acquired bacteraemias arise from the urinary tract and are associated with a mortality of around 10%. The incidence of bacteraemia following a single catheterisation episode has been shown to be as high as 8%, with the duration of catheterisation being the most important risk factor (EAU 2020). Development of symptomatic CAUTI can have serious consequences in some patients and has been shown to increase the length of hospital stay, worsen patient renal function, increase patient mortality and lead to increased costs for healthcare providers. However, with aseptic technique during placement of the catheter, the risk of CAUTI can be reduced (Baldini 2009; EAU 2020; Fisher 2017; Gould 2009; Lo 2014; NICE 2012).
Indwelling urethral catheters are prone to various other complications that prevent effective drainage of urine. The most common non‐infective cause is due to urethral stricture formation. Urethral strictures can develop after repeated urethral catheterisation with long‐term urinary catheter use, as well as after urethral trauma. The most common infective cause is the development of encrustation within the catheter. This is when crystalline compounds (such as calcium phosphate and struvite) precipitate in the alkaline conditions of urine to form solid deposits in the catheter lumen. This process is accelerated in the presence of micro‐organisms such as Proteus mirabilis which resides in the body's own bowel flora. These micro‐organisms produce the enzyme urease, allowing the production of ammonia, which causes further alkalinisation of the urine and catalyses the encrustation process. Catheter encrustation and blockage is thought to be experienced by roughly 50% of patients with long‐term catheters (Stickler 2010). Once a urethral catheter is failing to drain properly, flushing them with saline can often help in trying to relieve the obstruction. However, if this fails it is likely that the urethral catheter will need to be removed and the patient's need for a urinary catheter reassessed (Cravens 2000).
There are two routes of infection through which symptomatic urinary tract infections (UTIs) occur: endogenous and exogenous. Endogenous infections are due to bacteria naturally present in the human body. Typical routes of infection of the urinary tract are rectal, vaginal and meatal (bodily passages). Exogenous sources of infection include contamination by healthcare workers or non‐sterile equipment.
Pathogens typically gain access to the urinary tract either by migrating alongside the exterior surface of the lumen, or by movement alongside the inner lumen of the catheter via contaminated urine collection bags. Thus, maintenance of a sterile, closed urinary drainage system is key to prevent symptomatic CAUTIs. Clinical features of symptomatic UTIs include dysuria, urinary frequency or urgency, haematuria, suprapubic pain or tenderness, loin or flank pain, rigors, fever, altered mental status (e.g. confusion, particularly in the elderly), and nausea and vomiting (CDC 2016; Gould 2009; Grabe 2015; Hollingsworth 2013).
The Centers for Disease Control and Prevention (CDC) has defined symptomatic CAUTI as a UTI in the presence of an indwelling catheter which is in place for two or more calendar days on the date of the UTI, where day one was the date upon which the catheter was placed; or, the catheter was in place on the date of the UTI or the day before and then removed. The patient’s urine culture (from a mid‐stream or catheter bag sample) must also contain no more than two species of organisms, where one of which has a bacterial colony count of ≥ 105 colony forming unit (cfu)/mL. The CDC criteria for symptomatic UTI must also be met, which states that the patient must also have at least one of the following signs or symptoms: fever (> 38 °C); suprapubic tenderness; urinary urgency; increased urinary frequency or dysuria (pain during voiding) (CDC 2016; Gould 2009).
The Infectious Disease Society of America (IDSA) definition differs slightly according to their published guidelines. The IDSA considers symptomatic CAUTI as any UTI associated with a catheter in the presence of clinical features consistent with UTI, with no other identified sources of infection and a bacterial count of ≥ 103 cfu/mL of ≥ 1 bacterial species in a single midstream urine (MSU) or catheter specimen. The IDSA definition of symptomatic CAUTI covers patients with indwelling, intermittent and suprapubic catheters, unlike the CDC definition, which excludes intermittent catheterisation (Hooton 2010).
Patients who do not meet this criterion may still meet the various criteria for asymptomatic bacteraemic urinary tract infections (ABUTI), which is defined by the CDC as people who are asymptomatic but have a urine culture of at least 105 cfu/mL of a bacterial species in their urine sample. Between 75% to 90% of people who have ABUTIs have been shown not to produce a systemic inflammatory response or other indications, which would indicate infection (Gould 2009). The decision on how to monitor and treat these individuals is still undecided and varies amongst health providers. The CDC guidelines on symptomatic CAUTI state that the treatment of ABUTI has not been shown to provide any clinical benefit.
Description of the intervention
For the purpose of this review, we only considered short‐term indwelling urethral catheterisation. We defined short term as an intended duration of urethral catheterisation of 14 days or less. While there is extensive literature on the type, maintenance and techniques for insertion of urinary catheters, limited attention has been given to the policies and procedures for their removal. Although the insertion, removal and management of the catheter are usually undertaken by nurses, decisions about the removal of the catheter often remain with the medical practitioner. While the importance of short‐term urethral catheter management is recognised, there is no consensus among clinicians about the optimal time and method for removal of indwelling urethral catheters. Policies are likely to be based on personal preference and established practices rather than on research evidence (Irani 1995). While clinicians have established policies, there has been no objective and systematic examination of the effect of the time of day the catheter is removed, the length of time the catheter is left in place or if clamping the catheter prior to removal influences patient outcomes.
Indwelling urethral catheters are catheters that are inserted into the bladder, via the urethra, to allow continuous drainage of urine into a closed urine collection system. In some clinical contexts, valves may also be used as an alternative to continuous drainage. The urethral route is most commonly used by health professionals. Other routes of urinary catheterisation include intermittent urethral and suprapubic urinary catheterisation. However, these routes of urinary catheterisation are outwith the scope of this systematic review. Urethral catheterisation usually requires the use of a lubricant gel, which often contains a local anaesthetic, and can be used both in short‐term and long‐term catheterisation. The length of duration of urethral catheterisation is commonly associated with the development of complications, the most common being UTI (Nicolle 2014). Around 60% to 80% of hospitalised patients with indwelling catheters will require antibiotics at some stage of their care, although this is usually for reasons other than UTI (Durojaiye 2015; Foxman 2003). A recent prevalence survey published in The New England Journal of Medicine found that urinary catheters are the most common indwelling device in hospitals, used in 23.6% of patients in 183 hospitals in the USA and roughly 17.5% of patients in 66 European hospitals (Magil 2014).
As a result, the bacteria present in urine are continuously exposed to antimicrobials, thus aiding the development of antimicrobial‐resistant organisms. This rise in antimicrobial‐resistant organisms has proven to be a huge burden for healthcare providers from both an economic and medical standpoint, with many providers struggling to control devastating outbreaks. There is limited evidence for the use of antibiotic prophylaxis in short‐term indwelling urethral catheters (Lusardi 2013).
How the intervention might work
Some investigators have hypothesised the potential advantage of morning or midnight removal of catheters. One argument for the removal of urethral catheters early in the morning is that reduced staff at night might fail to respond to complications, such as urinary retention, that can develop following the removal of the catheter (Blandy 1989; Crowe 1993; Ganta 2005; Kelleher 2002; Webster 2006). Other suggested benefits of removing the catheter early in the morning include allowing the patient to rest through the night and then to adjust back to their normal voiding pattern during the day (Gross 2007).
Researchers have also reported that patients whose catheters were removed in the night had larger volumes at first void compared to other people whose catheters were removed in the morning (Chillington 1992; Noble 1990; Webster 2006). It has been suggested that the timing of catheter removal may affect a patient's length of stay in hospital with consequent resource implications. In one trial it was found that removal of catheters at midnight resulted in patients being discharged a mean of 14 hours earlier than patients whose catheters were removed in the morning (Chillington 1992), thus resulting in economic benefits related to a shorter length of hospitalisation and efficient discharge planning (Kelleher 2002).
There has been some debate about whether flexible policies are better than relatively fixed policies for catheter removal (Wyman 1987). However, practice is known to vary. For example, local clinical audits for catheter removal have indicated that 49% of catheters are removed either at the discretion of the nurse or at the time of the medical rounds and only 34% were removed at midnight (Watt 1998). Of those indwelling urethral catheters that were scheduled for removal in the morning, only 70% were removed on time (Noble 1990; Watt 1998).
Practice also varies with respect to the length of time the catheter is left in situ and the procedure for its removal. The factors that influence this decision include: the condition/reason for which the patient is catheterised; clinician/surgeon preference; patient tolerance; and hospital policy (EAUN 2012). Various international guideline panels agree that indwelling urethral catheters should be removed as soon as they are no longer necessary (CDC 2016; EAU 2020; Grabe 2015; Hooton 2010; NICE 2012). The removal of indwelling urethral catheters after shorter durations may prove to be beneficial, as it has the potential to reduce hospital stays and the number of patients developing symptomatic CAUTIs, thus saving healthcare costs and improving patient outcomes (Baldini 2009; Lo 2014).
Bladder dysfunction and post‐operative voiding impairment has been documented following catheterisation and can lead to infections of the urinary tract. The intermittent clamping of the indwelling urethral catheter draining tube prior to withdrawal has been suggested on the basis that this simulates normal filling and emptying of the bladder (EAUN 2012). While clamping catheters might minimise post‐operative neurogenic urinary dysfunction, it could also result in bladder infection or distension if the clamps are not released as scheduled (Roe 1990; Wang 2016).
Another strategy practised prior to removal of urethral catheters is the use of alpha adrenergic blocker drugs. It is thought that post‐operative urinary retention is potentially linked to the stress‐induced, high sympathetic activity occurring around the peri‐operative period. Counteracting its activity with the inhibition of alpha receptors located in the bladder and urethra may potentially reduce the risk of acute urinary retention (Ghuman 2018; Madani 2014; Patel 2018). It has also been reported that alpha blockers are effective in the treatment of voiding dysfunction by enhancing detrusor contractibility and lowering urethral resistance in patients with underactive bladder (Yamanishi 2004). Thus, prophylactic usage of alpha blockers in people with indwelling urethral catheters could reduce the episodes of developing voiding dysfunction after catheter removal.
Why it is important to do this review
This systematic review summarises the evidence from randomised controlled trials (RCTs) related to alternative approaches to the removal of short‐term indwelling urethral catheters. The findings of this review will help determine the safest method of short‐term catheter removal as well as potentially help reduce the risks associated with catheterisation for patients. Since the last version of this review was published (Griffiths 2007), the evidence base has grown substantially and it is important to incorporate findings from new trials into the review in a manner that will enable clinicians to develop evidence‐based policies for practice.
Objectives
To assess the effects of strategies for removing short‐term (14 days or less) indwelling catheters in adults.
Methods
Criteria for considering studies for this review
Types of studies
We included RCTs and quasi‐RCTs that evaluated the effects of strategies for removing short‐term indwelling urethral catheters.
For the purposes of this review, we defined 'indwelling catheterisation' in accordance with the European Association of Urology (EAU), which states that it is the passage of a urinary catheter into the bladder via the urethra and held in place by an inflatable balloon (EAU 2020; Grabe 2015; Tenke 2008). We defined as 'short‐term' cases where the intended duration of catheterisation was 14 days or less (Dunn 2000a; Kidd 2015; Lam 2014).
Types of participants
We included trials of adults requiring short‐term indwelling urethral catheterisation in any setting (hospital, community, nursing home) for any reason. These included individuals who were acutely unwell, required surgery, had urinary retention or women during childbirth.
Types of interventions
We included all interventions involving short‐term indwelling urethral catheterisation and made the following comparisons.
Removal of indwelling urethral catheters at one specified time of day (6 am to 7 am) versus another specified time of day (10 pm to midnight)
Shorter durations of indwelling urethral catheterisation versus longer durations of indwelling urethral catheterisation e.g. immediate/early removal versus removal of the indwelling urethral catheter one day post‐surgery
Flexible durations of indwelling urethral catheterisation versus fixed duration of indwelling urethral catheterisation
Clamping of indwelling urethral catheterisation versus free drainage of indwelling urethral catheterisation prior to removal
Prophylactic use of alpha blocker prior to indwelling urethral catheter removal versus no intervention or placebo
We defined early removal of catheters as the removal of an indwelling urethral catheter up to eight hours post‐operatively.
We have not considered the following interventions as they are either covered in separate Cochrane Reviews or do not meet the objectives of this review:
Suprapubic or intermittent urethral catheterisation (Kidd 2015)
Long‐term catheterisation (Cooper 2016)
Differing catheter insertion techniques (e.g. use of aseptic liquid/cream based agents or topical antibiotic creams)
Meatal care management techniques
Types of catheter materials for short‐term catheters (e.g. latex, silicone) (Lam 2014)
Types of catheter coatings for short‐term catheters (e.g. antibiotic coating, silver) (Lam 2014)
Types of drainage container
Treatment of drainage bag with antiseptic/antibiotic
The use of antibiotic prophylaxis as a primary or secondary outcome (Foon 2012; Lusardi 2013)
The use of reminders or protocols for catheter removal, for example, stop‐orders
It should be noted that the use of alpha blockers prior to urethral catheter removal in acute urinary retention (AUR) is covered by another Cochrane Review (Fisher 2014). Our review only looks at the use of prophylactic alpha blockers in short‐term indwelling urethral catheters in instances other than AUR. We excluded trials that looked at the use of antibiotic prophylaxis as a primary or secondary outcome on the basis that this is covered by another Cochrane Review and is not related to the intervention of interest of this review (Lusardi 2013). We did not exclude trials that used antibiotic prophylaxis for both intervention and control groups as part of their hospital policy.
Types of outcome measures
We analysed the following outcomes in this review. It should be noted that we did not use them as a basis for including or excluding trials.
Primary outcomes
Number of participants who required recatheterisation following removal of indwelling urethral catheter
Secondary outcomes
-
Complications/adverse effects
-
Incidence of UTI
symptomatic CAUTI
asymptomatic bacteriuria
Incidence of urinary retention
Other complications of catheterisation (or recatheterisation), for example, haemorrhage, stricture formation, fever
-
-
Patient‐reported
Patient pain or discomfort
Patient satisfaction
Urinary incontinence
Number of patients reporting dysuria
-
Clinician‐reported
Volume of first void (mL)
Time to first void (hours)
Post‐void residual volume (mL)
Length of hospitalisation (days)
Time between removal of catheter to discharge (days)
-
Health status/quality of life
Condition‐specific or generic quality‐of‐life measures (e.g. Short Form 36 (Ware 1992))
Psychological outcome measures (e.g. Hospital Anxiety and Depression Scale (Zigmond 1983))
Main outcomes for summary of findings tables
Number of participants requiring recatheterisation
Symptomatic CAUTI
Dysuria
Condition‐specific or generic quality‐of‐life measures (e.g. Short Form 36)
Search methods for identification of studies
We did not impose any language or other restrictions on any of the searches described below.
Electronic searches
We identified relevant trials from the Cochrane Incontinence Specialised Register. For more details of the search methods used to build the Specialised Register, please see the Group's webpages where details of the Register's development (from inception) and the most recent searches performed to populate the Register can be found. The Register contains trials identified from the Cochrane Central Register of Controlled Trials (CENTRAL), MEDLINE, MEDLINE In‐Process, MEDLINE Epub Ahead of Print, CINAHL, ClinicalTrials.gov, WHO ICTRP, Be Part of Research and handsearching of journals and conference proceedings. Many of the trials in the Cochrane Incontinence Specialised Register are also contained in CENTRAL.
The date of the last search was: 17 March 2020.
The terms used to search the Cochrane Incontinence Specialised Register are given in Appendix 2.
For an earlier version of this review update we searched CINAHL (on EBSCO), covering December 1981 to 11 May 2016 (searched on 12 May 2016). For the most recent update of the search (17 March 2020) only the Cochrane Incontinence Specialised Register was searched, as this now incorporates the CINAHL search. The search strategy used in CINAHL is given in Appendix 3.
The search strategies used to search for the previous version of this review (Griffiths 2007) are given in Appendix 4.
Searching other resources
We also searched the reference lists of all relevant articles.
Data collection and analysis
For this update, we used the following methods to assess the new reports that were identified as a result of the updated search. For methods used in the previous version of this review, see Griffiths 2007.
Selection of studies
Two review authors (AE and IO) independently screened the titles and abstracts of each trial using Covidence before obtaining the full text for all potentially eligible trials. If the title and abstract were inconclusive, we obtained the full text for further assessment. We attempted to obtain any missing trial data by contacting the trial authors for further information. Duplicate trials that had been reported in more than one publication were included only once. We reached decisions about trial eligibility by a discussion between the author team and resolved any disagreements by consulting an independent third party.
Data extraction and management
Four review authors (AE, FS, EK, IO) extracted data independently using a standardised form and AE compared their results. If the data in trials had not been fully reported, we attempted to contact the trial authors for further classification. We entered the extracted data into Review Manager 5 software (Review Manager 2020).
We have only reported those outcomes that were pre‐specified in the Types of outcome measures. However, there were occasions where the outcomes reported were worded differently despite belonging to the same underlying theme ‐ for example, asymptomatic bacteriuria was also reported as positive urine culture. As these are the same underlying concepts, omitting this information was not appropriate. We therefore chose to collate all data from trials that reported positive urine culture with asymptomatic bacteriuria if they met the CDC definition for asymptomatic bacteriuria.
Assessment of risk of bias in included studies
Four review authors (AE, FS, EK, IO) assessed the included trials for risk of bias using Cochrane's 'Risk of bias' tool (Higgins 2011). We assessed the following domains: random sequence generation (selection bias); allocation concealment (selection bias); blinding of participants and personnel (performance bias); blinding of outcome assessment (detection bias); blinding of microbiological outcome (detection bias); incomplete outcome data (attrition bias); selective reporting of outcomes (reporting bias); and other potential sources of bias.
Two of the review authors (AE and one of either IO, FS or EK) independently assessed each of the trials and rated each as 'low risk', 'unclear risk' or 'high risk'. We resolved any difference in opinion by discussion or by consulting an independent third party.
Measures of treatment effect
We processed all trial data as outlined in the Cochrane Handbook for Systematic Reviews of Interventions (Li 2021). Where appropriate, we undertook meta‐analysis. We combined outcome data by using a fixed‐effect model to calculate pooled estimates and their 95% confidence intervals (CI). We considered the random‐effects model only when there were concerns about heterogeneity affecting the analysis. For categorical outcomes, we related the numbers reporting an outcome to the numbers at risk in each group to calculate a risk ratio (RR) with 95% CI. For continuous variables, we used means and standard deviations to derive the mean difference (MD) with 95% CI.
Unit of analysis issues
In parallel‐group trials, the primary analysis was per participant randomised. Where there were trials that involved a variation of this type of randomisation, for example, cross‐over trials or cluster‐randomised trials, we performed analysis as outlined by the Cochrane Handbook for Systematic Reviews of Interventions (Higgins 2021).
Dealing with missing data
We analysed the data on an intention‐to‐treat (ITT) basis where possible, meaning that all participants were analysed according to the group they were randomised in irrespective of whether they received their assigned intervention.
Where participants were excluded after allocation or withdrew from the trial, we reported any details provided in full. If there were data missing, we attempted to contact the original trial authors to obtain the missing trial data. If there was evidence of differential dropout between the groups, the review authors imputed data for the missing results once we had contacted the trial authors. Where trials reported mean values without standard deviations (SDs) but with P values or 95% CI, we used a conversion Excel document designed by a statistician to obtain the SDs. In cases of missing SDs with no P values or 95% CIs, we estimated the SD from another trial in the same meta‐analysis.
Assessment of heterogeneity
We only combined trials if there was evidence that they were clinically similar. We assessed heterogeneity by visual inspection of forest plots, the Chi² test for heterogeneity and the I² statistic (Higgins 2003). If significant heterogeneity existed, we used a random‐effects model. We considered statistical heterogeneity significant if either the P value for the Chi² test was low (P < 0.10) or if the I² statistic suggested heterogeneity. We used the following thresholds for interpreting the I² statistic (Deeks 2021):
0% to 40%: heterogeneity might not be that important
30% to 60%: moderate heterogeneity
50% to 90%: substantial heterogeneity
75% to 100%: considerable heterogeneity
Assessment of reporting biases
In view of the difficulties associated with the detection and correction of publication bias, as well as various other reporting biases, we employed a comprehensive search strategy involving multiple databases and sources. We assessed the likelihood of any potential publication bias by using funnel plots.
Data synthesis
We combined trials for analysis if the interventions were considered to be clinically similar and used a fixed‐effect approach to carry out meta‐analysis. We considered using a random‐effects model if there was substantial statistical heterogeneity (as judged by the Chi² test or I² statistic).
For illustrative purposes, we displayed data in subgroups in the meta‐analysis to help identify the different types of surgery and catheter durations participants were undergoing.
Subgroup analysis and investigation of heterogeneity
We performed the following subgroup analyses for the primary outcome for each comparison.
The type of surgery (urological versus non‐urological) that participants underwent is likely to have an impact with regard to infection, dysuria, haemorrhage and stricture formation etc. If a participant was to be admitted for surgery involving the urological tract (e.g. transurethral resection of the prostate (TURP)), it is likely that the passage of a urethral catheter in these participants would have a potentially worsening impact than those participants with urethral catheters who did not have any urological surgery. This is because the urological tract is likely to have sustained some damage as a result of the trauma involved during the surgery.
The sex of an individual can impact the intervention being studied. Women are more prone to urinary tract infections due to their shorter urethra when compared to the anatomy of men. However, the passage of urethral catheters in women is likely to be less challenging than men. Many men in this review were hospitalised for TURP, implying that passing a urethral catheter is likely to be more technically difficult in men.
Antibiotic prophylaxis: the use of antibiotic prophylaxis for participants with short‐term indwelling urethral catheters is likely to impact outcomes looking at infections (e.g. the number of participants developing symptomatic CAUTI and asymptomatic bacteriuria). Attitudes towards antibiotic prophylaxis in short‐term urethral catheterisation vary, as their use is also associated with an increased risk of developing a hospital‐acquired infection by Clostridium difficile.
Where data were available, we performed post hoc subgroup analysis to assess the impact of prophylactic antibiotics on the number of participants developing symptomatic CAUTI. The use of prophylactic antibiotics is a confounding factor in the number of participants developing CAUTI. We also conducted post hoc subgroup analysis for the outcome of length of hospitalisation to explore the effect of type of surgery as a possible explanation for very high heterogeneity in the meta‐analysis.
For outcomes other than the primary outcome and CAUTI, we used the subgroup function for illustrative purposes only to show the different types of surgery that participants underwent and the different catheter durations. It should be noted that, in these cases, we did not report any results of subgroup analysis in relation to the statistical test for subgroup differences.
Sensitivity analysis
Where data were available, we conducted sensitivity analyses for our primary outcome by excluding trials we judged as high risk of bias for the domains relating to random sequence generation and allocation concealment.
Summary of findings and assessment of the certainty of the evidence
We prepared summary of findings tables for our main comparisons and presented the results for the outcomes prespecified in the Types of outcome measures.
We assessed the certainty of the body of evidence using the GRADE approach. When choosing which outcomes to select, we looked at previous Cochrane Reviews involving urethral catheterisation, the review teams for which had conducted group discussions with people who had undergone short‐term indwelling urethral catheterisation to assist with the selection of appropriate outcomes for inclusion in the summary of findings tables (Kidd 2015; Lam 2014; Omar 2013). We classified the primary and secondary outcomes as critical, important or not important from the patients' perspective for decision‐making.
Results
Description of studies
Results of the search
We screened 1583 records, which were identified by the literature search for this review, and retrieved the full texts of 223 reports of trials to assess their eligibility for inclusion. We included 124 reports of 99 trials in this review, and excluded 89 reports of 85 trials from the review. There are nine reports of eight ongoing trials, details of which can be located in the Characteristics of ongoing studies. One trial is still awaiting classification after we obtained further information regarding the trial during the final stages of this review (NCT02602132). Please see the Characteristics of studies awaiting classification for more details. The flow of literature through the assessment process is shown in the PRISMA diagram (Page 2020; Figure 1).
1.
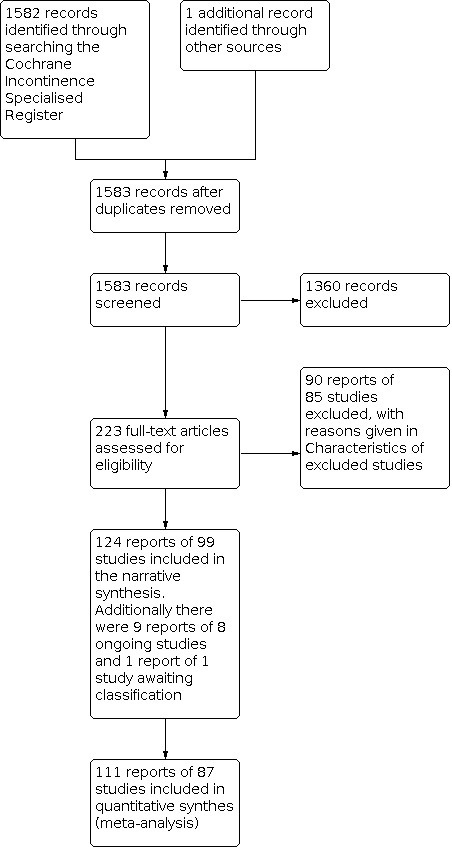
PRISMA flow diagram
Newly included trials
In this update, we re‐assessed the 26 trials included in the previous version of this review and re‐extracted their data (Griffiths 2007). We also evaluated their risk of bias. After performing a new search, we identified a further 73 eligible trials.
Included studies
The trials are detailed in the Characteristics of included studies. We were unable to include 12 trials (13 reports) in the meta‐analysis because they reported data in insufficient detail (Azarkish 2005; Bristoll 1989; Dunn 1999; Dunn 2000b; Iversen Hansen 1984; Nguyen 2012; Ruminjo 2015; Talreja 2016; Wilson 2000; Yee 2015), or they were single trials reporting an outcome for a particular comparison (Liu 2015; Williamson 1982), or reported zero events for a particular outcome and so the result was not estimable (Liu 2015). We contacted the trial authors by email to request further data.
Design
Ninety‐four trials included in the review were RCTs and five trials were quasi‐RCTs (Li 2014; Liu 2015; Noble 1990; Valero Puerta 1998; Zhou 2012).
Sample sizes
The number of participants randomised in the included trials ranged from eight (Williamson 1982), to 501 (Barone 2015). In total, the 99 trials randomised 12,241 participants.
Reason for hospitalisation/catheterisation
The reasons for catheterisation varied between the trials (see Table 5).
1. Types of participants.
| Trial ID | Reason for hospitalisation | Type of surgery/reason for being admitted | Gender |
| Ahmed 2014 | Elective gynaecological surgery | Total abdominal hysterectomy with or without bilateral salpingo‐oophorectomy | Female |
| Alessandri 2006 | Elective gynaecological surgery | Vaginal hysterectomy | Female |
| Allen 2016 | Patients undergoing cardiothoracic surgery | General thoracic surgical procedure, in whom an epidural catheter was placed for analgesia | Mixed |
| Alonzo‐Sosa 1997 | Elective gynaecological surgery | Anterior colporrhapy, anterior and posterior colporrhaphy with or without vaginal hysterectomy | Female |
| Aref 2020 | Elective CS | Participants admitted for elective CS | Female |
| Aslam 2019 | Elective gynaecological surgery | Participants undergoing minimally invasive pelvic organ prolapse surgery | Female |
| Azarkish 2003 | Elective CS | Participants admitted for elective CS | Female |
| Azarkish 2005 | Emergency CS | Participants admitted for emergency CS | Female |
| Barone 2015 | Elective gynaecological surgery | Participants admitted for vaginal fistula repair | Female |
| Basbug 2020 | Elective CS | Participants admitted for elective CS | Female |
| Benoist 1999 | Elective GI surgery | Extensive rectal resection (total or subtotal proctectomy) | Mixed |
| Bristoll 1989 | Not reported | Not reported | Unknown |
| Carpiniello 1988 | Elective orthopaedic surgery | Total joint replacement (hip or knee) | Female |
| Carter‐Brooks 2018 | Elective gynaecological surgery | Participants undergoing pelvic organ prolapse surgery | Female |
| Chai 2011 | Elective gynaecological surgery | Total abdominal hysterectomy with or bilateral salpingo‐oophorectomy for various benign gynaecological diseases | Female |
| Chen 2013 | Admitted to ICU | Patients requiring mechanical ventilation for respiratory failure | Mixed |
| Chia 2009 | Elective cardiothoracic surgery | Thoracotomy | Mixed |
| Chillington 1992 | Elective urological surgery | TURP | Male |
| Cornia 2003 | Admitted to medicine and cardiology services | Patients admitted to the medicine and cardiology services | Mixed |
| Coyle 2015 | Elective GI surgery | Elective transabdominal colectomy, proctectomy or coloproctectomy | Mixed |
| Crowe 1993 | Admitted to urology ward | Patients admitted to the urology ward with IUCs or who were catheterised during their inpatient stay | Mixed |
| Dunn 1999 | Elective obstetric and gynaecological surgery | Patients undergoing elective obstetric or gynaecological surgery | Female |
| Dunn 2000b | Elective gynaecological surgery or CS | Patients undergoing hysterectomy or CS who do not require bladder suspension or strict fluid management | Female |
| Dunn 2003 | Elective gynaecological surgery | Women undergoing hysterectomy for various benign diseases (e.g. fibroid tumours, abnormal uterine bleeding, chronic pain, and persistent cervical dysplasia or micro invasive cancer | Female |
| Durrani 2014 | Elective urological surgery | Patients with bladder outflow obstruction due to benign prostatic enlargement undergoing TURP | Male |
| El‐Mazny 2014 | Primary or elective CS | Patients admitted to the prenatal wards for primary or repeat elective CS | Female |
| Ganta 2005 | Elective urological surgery | TURP | Male |
| Glavind 2007 | Elective gynaecological surgery | Patients undergoing any type of vaginal prolapse surgery | Female |
| Gong 2017 | Elective gynaecological surgery | Patients undergoing radical hysterectomy for cervical cancer FIGO stage IB‐IIB | Female |
| Gross 2007 | Admitted to stroke ward | Patients with a stroke admitted to the ward | Mixed |
| Gungor 2014 | Elective gynaecological surgery | Patients with pelvic organ prolapse and/or urinary incontinence undergoing anterior colporrhaphy | Female |
| Guzman 1994 | Elective gynaecological surgery | Patients undergoing vaginal surgery | Female |
| Hakvoort 2004 | Elective gynaecological surgery | Patients undergoing anterior colporrhaphy for vaginal prolapse surgery | Female |
| Hall 1998 | Elective general surgery | Patients admitted to the general surgery wards | Mixed |
| Han 1997 | Elective urological surgery | Patients with benign prostatic enlargement undergoing TURP | Male |
| Hewitt 2001 | Elective urological surgery | Patients requiring radical perineal prostatectomy | Male |
| Huang 2011 | Elective gynaecological surgery | Patients with cystocele of at least stage II, who were symptomatic and desired operative treatment with anterior vaginal repair with or without other concomitant pelvic surgeries | Female |
| Ind 1993 | Elective hysterectomy, posterior exenteration, colposuspension, anterior colporrhaphy, total/radical vulvectomy, radical oophorectomy, ovarian cystectomy, adhesiolysis myomectomy | Patients which were admitted for any of the following operations: hysterectomy, posterior exenteration, colposuspension, anterior colporrhaphy, total/radical vulvectomy, radical oophorectomy, ovarian cystectomy, adhesiolysis myomectomy | Female |
| Irani 1995 | Elective transurethral prostatic surgery | Patients admitted for transurethral prostatic surgery due to benign hyperplasia | Male |
| Iversen Hansen 1984 | Urethral strictures | Patients with urethral strictures | Not reported |
| Jang 2012 | Surgery for rectal cancer | Patients undergoing elective rectal surgery for cancer | Mixed |
| Jeong 2014 | Robot‐assisted laparoscopic radical prostatectomy | Patients with localised or advanced prostate cancer | Men |
| Joshi 2014 | Elective hysterectomy with salpingo‐oophorectomy | Patients undergoing uneventful hysterectomy with salpingo‐oophorectomy | Female |
| Jun 2011 | Elective TURP | Patients admitted for TURP | Male |
| Kamilya 2010 | Vaginal prolapse surgery | Patients undergoing vaginal prolapse surgery | Female |
| Kelleher 2002 | Urological surgery | Patients admitted to urology or renal unit | Not reported |
| Kim 2012 | Radical prostatectomy | Patients undergoing extraperitoneal laparoscopic radical prostatectomy | Men |
| Koh 1994 | Elective TURP | Patients admitted for TURP | Men |
| Kokabi 2009 | Anterior colporrhaphy for pelvic organ prolapse | Patients undergoing anterior colporrhaphy due to pelvic organ prolapse and stress incontinence | Female |
| Lang 2020 | Elective gynaecological surgery | Patients admitted for elective benign gynaecological surgery | Female |
| Lau 2004 | Elective general surgery | Patients admitted for elective general surgery | Mixed |
| Li 2014 | Elective TURP | Patients admitted for TURP | Men |
| Liang 2009 | Laparoscopic vaginal hysterectomy | Patients admitted for laparoscopic vaginal hysterectomy | Female |
| Lista 2020 | Elective urological surgery | Patients admitted for robot‐assisted radical prostatectomy for localised prostate cancer | Male |
| Liu 2015 | Neurosurgery | Patients undergoing neurosurgery | Mixed |
| Lyth 1997 | TURP or bladder neck incision | Patients undergoing TURP or bladder neck incision | Unclear |
| Mao 1994 | Elective gynaecological surgery | Patients undergoing surgery for total hysterectomy or salpingo‐oophorectomy | Female |
| Matsushima 2015 | Surgery for prostate cancer removal (unclear what operation was done) | Patients with prostate cancer | Male |
| McDonald 1999 | TURP | Patients undergoing TURP | Male |
| Naguimbing‐Cuaresma 2007 | Elective CS | Participants admitted for elective CS | Female |
| Nathan 2001 | Elective gynaecological surgery | Patients undergoing surgery for benign gynaecological conditions | Female |
| Nguyen 2012 | Elective urological surgery for urethral strictures | Patients undergoing surgery for urethral strictures | Unclear |
| Nielson 1985 | Elective urological surgery for urethral strictures | Patients undergoing surgery for urethral strictures | Unclear |
| Noble 1990 | Elective urological surgery and procedures | Patients admitted to the urological unit | Mixed |
| Nyman 2010 | Orthopaedic surgery | Patients admitted with hip fractures in need of surgery | Mixed |
| Oberst 1981 | Elective general surgery | Patients undergoing surgery for bowel cancer; low anterior bowel resection or abdominoperineal resection | Mixed |
| Onile 2008 | Elective CS | Patients admitted for elective CS | Female |
| Ouladsahebmadarek 2012 | Elective gynaecological surgery | Patienst admitted for elective abdominal hysterectomy or laparotomy for being pathology (fibroma, AUB, chronic pelvic pain, ovarian cysts etc.) | Female |
| Pervaiz 2019 | Elective urological surgery | Patients undergoing TURP | Male |
| Popiel 2017 | Elective gynaecological surgery | Patients undergoing robotic sacrocolpopexy for vaginal prolapse | Female |
| Rajan 2017 | Elective gynaecological surgery | Patients undergoing surgery for Ward Mayo operation; Manchester repair; vaginal hysterectomy and amputation of cervix | Female |
| Ruminjo 2015 | Elective gynaecological surgery | Patients undergoing fistula repair surgery | Female |
| Sahin 2011 | Elective urological surgery | Patients admitted for TURP due to benign prostate hypertrophy | Male |
| Sandberg 2019 | Elective gynaecological surgery | Patients undergoing laparoscopic hysterectomy | Female |
| Schiotz 1995 | Elective gynaecological surgery | Patients admitted for vaginal plastic surgery (anterior colporrhaphy, anterior plus posterior colporrhaphy or a full Manchester repair) | Female |
| Schiotz 1996 | Elective urogynaecological surgery | Patients admitted for elective retro‐pubic surgery for stress incontinence | Female |
| Sekhavat 2008 | Elective gynaecological surgery | Patients undergoing anterior colporrhaphy | Female |
| Shahnaz 2016 | Elective gynaecological surgery | Patients undergoing surgery for pelvic organ prolapse | Female |
| Shrestha 2013 | Elective gynaecological surgery | Patients admitted for vaginal hysterectomy, anterior colporrhaphy or Manchester operations | Female |
| Souto 2004 | Elective urological surgery | Patients admitted for retropubic radical prostatectomy | Male |
| Sun 2004 | Elective urogynaecological surgery | Patients admitted for Burch's colposuspension | Female |
| Tahmin 2011 | Elective gynaecological surgery | Patients with genital prolapses admitted for vaginal hysterectomy and or pelvic floor repair | Female |
| Talreja 2016 | Elective urological surgery | Patients with benign prostatic enlargement undergoing TURP | Male |
| Taube 1989 | AUR | Patients admitted to the hospital with AUR | Male |
| Toscano 2001 | Elective urological surgery | Patients with benign prostatic enlargement undergoing TURP | Male |
| Valero Puerta 1998 | Elective urological surgery | Patients with benign prostatic enlargement undergoing TURP | Male |
| Vallabh‐Patel 2020 | Elective gynaecological surgery | Patients undergoing robotic sacrocolpopexy for pelvic organ prolapse | Female |
| Webster 2006 | General surgery and medical patients | Patients who required IUC on general surgery and medical wards | Mixed |
| Weemhoff 2011 | Elective gynaecological surgery | Patients admitted for anterior colporrhaphy | Female |
| Williamson 1982 | Elective surgery (unspecific) | Patients undergoing surgery (not specified by trial) | Female |
| Wilson 2000 | Elective urological surgery | Patients with benign prostatic enlargement undergoing TURP | Male |
| Wu 2015 | Elective gallbladder or biliary tree surgery | Pateints undergoing gallbladder or biliary tree surgery | Mixed |
| Wyman 1987 | Elective urological surgery | Patients with benign prostatic enlargement undergoing TURP | Male |
| Yaghmaei 2017 | Elective CS | Patients who underwent CS | Female |
| Yee 2015 | Elective CS | Patients who underwent CS under spinal anaesthesia | Female |
| Zaouter 2009 | Elective major abdominal and thoracic surgery | Patients admitted for elective major abdominal and thoracic surgery | Mixed |
| Zhou 2012 | Elective CS | Patients who underwent CS | Female |
| Zmora 2010 | Elective colon and rectal surgery with pelvic dissection | Patients admitted for elective colon and rectal surgery | Mixed |
| Zomorrodi 2018 | Elective renal transplant surgery | Patients with end‐stage renal failure undergoing renal transplant surgery | Mixed |
AUB: abnormal uterine bleeding; AUR: acute urinary retention; CS: cesarean section; GI: gastrointestinal; FIGO: International Federation of Gynecology and Obstetrics; ICU: intensive care unit; IUC: indwelling urethral catheter; TURP: transurethral resection of the prostate
Urological or urogenital surgery (Chillington 1992; Durrani 2014; Ganta 2005; Han 1997; Hewitt 2001; Irani 1995; Jeong 2014; Jun 2011; Kelleher 2002; Kim 2012; Koh 1994; Li 2014; Lista 2020; Lyth 1997; Matsushima 2015; McDonald 1999; Noble 1990; Pervaiz 2019; Sahin 2011; Souto 2004; Talreja 2016; Toscano 2001; Valero Puerta 1998; Wilson 2000; Wyman 1987)
Urethrotomy and urethral strictures (Iversen Hansen 1984; Nguyen 2012; Nielson 1985)
Obstetric and gynaecological surgery (Ahmed 2014; Alessandri 2006; Alonzo‐Sosa 1997; Aref 2020; Aslam 2019; Azarkish 2003; Azarkish 2005; Barone 2015; Basbug 2020; Carter‐Brooks 2018; Chai 2011; Dunn 1999; Dunn 2000b; Dunn 2003; El‐Mazny 2014; Glavind 2007; Gong 2017; Gungor 2014; Guzman 1994; Hakvoort 2004; Huang 2011; Ind 1993; Joshi 2014; Kamilya 2010; Kokabi 2009; Lang 2020; Liang 2009; Mao 1994; Naguimbing‐Cuaresma 2007; Onile 2008; Ouladsahebmadarek 2012; Popiel 2017; Rajan 2017; Ruminjo 2015; Nathan 2001; Sandberg 2019; Schiotz 1995; Schiotz 1996; Sekhavat 2008; Shahnaz 2016; Shrestha 2013; Sun 2004; Tahmin 2011; Vallabh‐Patel 2020; Weemhoff 2011; Yaghmaei 2017; Yee 2015
Management of acute urinary retention (Lau 2004; Taube 1989; Wu 2015)
Major abdominal or thoracic surgery, or both (Allen 2016; Chia 2009; Zaouter 2009)
Colon or rectal surgery (Benoist 1999; Coyle 2015; Jang 2012; Lau 2004; Oberst 1981; Zmora 2010)
Women undergoing any surgery (Williamson 1982)
Stroke (Gross 2007)
Orthopaedic surgery (Carpiniello 1988; Nyman 2010)
Urology ward (Crowe 1993)
Intensive care unit (Chen 2013; Zomorrodi 2018)
Medicine and cardiology patients (Cornia 2003)
General medical or surgery ward (Hall 1998; Webster 2006)
Neurosurgery (Liu 2015)
Sex
Fifty trials included women only (Ahmed 2014; Alessandri 2006; Alonzo‐Sosa 1997; Aref 2020; Aslam 2019; Azarkish 2003; Azarkish 2005; Barone 2015; Basbug 2020; Carpiniello 1988; Carter‐Brooks 2018; Chai 2011; Dunn 1999; Dunn 2000b; Dunn 2003; El‐Mazny 2014; Glavind 2007; Gong 2017; Gungor 2014; Guzman 1994; Hakvoort 2004; Huang 2011; Ind 1993; Joshi 2014; Kamilya 2010; Kokabi 2009; Lang 2020; Liang 2009; Mao 1994; Naguimbing‐Cuaresma 2007; Nathan 2001; Onile 2008; Ouladsahebmadarek 2012; Popiel 2017; Rajan 2017; Ruminjo 2015; Sandberg 2019; Schiotz 1995; Schiotz 1996; Sekhavat 2008; Shahnaz 2016; Shrestha 2013; Sun 2004; Tahmin 2011; Vallabh‐Patel 2020; Weemhoff 2011; Williamson 1982; Yaghmaei 2017; Yee 2015; Zhou 2012).
Twenty‐two trials included men only (Chillington 1992; Durrani 2014; Ganta 2005; Han 1997; Hewitt 2001; Irani 1995; Jeong 2014; Kim 2012; Koh 1994; Li 2014; Lista 2020; Matsushima 2015; McDonald 1999; Pervaiz 2019; Sahin 2011; Souto 2004; Talreja 2016; Taube 1989; Toscano 2001; Valero Puerta 1998; Wilson 2000; Wyman 1987).
Twenty‐one trials included participants of both sexes (Allen 2016; Benoist 1999; Chen 2013; Chia 2009; Cornia 2003; Coyle 2015; Crowe 1993; Gross 2007; Hall 1998; Jang 2012; Jun 2011; Lau 2004; Liu 2015; Noble 1990; Nyman 2010; Oberst 1981; Webster 2006; Wu 2015; Zaouter 2009; Zmora 2010; Zomorrodi 2018).
Six trials did not report participants' sex (Bristoll 1989; Iversen Hansen 1984; Kelleher 2002; Lyth 1997; Nguyen 2012; Nielson 1985).
Age
A wide range of ages was reported in the included trials (see Table 6). Twenty‐three trials did not report the age of participants (Aslam 2019; Azarkish 2005; Bristoll 1989; Chillington 1992; Cornia 2003; Crowe 1993; Dunn 1999; Dunn 2000b; Dunn 2003; Hall 1998; Hewitt 2001; Kelleher 2002; Kim 2012; Kokabi 2009; Lyth 1997; Mao 1994; Naguimbing‐Cuaresma 2007; Nguyen 2012; Noble 1990; Popiel 2017; Ruminjo 2015; Wilson 2000; Yee 2015). In trials that did report age of participants, reported it for each trial arm, overall or both.
2. Interventions and age of participants.
| TrialID | InterventionA | Intervention B |
Age (A), years Mean (SD) |
Age (B), years Mean (SD) |
Age (overall), years |
| Ahmed 2014 | IUC removal immediately post‐op | IUC removal 24 h post‐op | 59.1(8.3) | 61.3 (10.5) | Not reported |
| Alessandri 2006 | IUC removal immediately post‐op | IUC removal 12 h post‐op | 51 (4.3) | 47 (5) | Not reported |
| Allen 2016 | IUC removed within 48 h post‐op | IUC removed within 6 h after epidural removal | 61.1 (range 31–85) | 61.7 (range 21–87) | 61.5 (range 21‐87) |
| Alonzo‐Sosa 1997 | IUC removal 1 day post‐op | IUC removal 3 days post‐op | 53.5 (range 37‐63) | 47.1 (range 37‐67) | Not reported |
| Aref 2020 | IUC removal 6 h post‐op | IUC removal 24 h post‐op | 25.3 (2) | 25.6 (3) | Not reported |
| Aslam 2019 | IUC removal immediately post‐op | IUC removal 1‐day post‐op | Not reported | Not reported | Not reported |
| Azarkish 2003 | IUC removal 2‐3 h after surgery | IUC removal the morning after surgery | 24.96 (4.88) | 27.06 (5.56) | Not reported |
| Azarkish 2005 | IUC removal 2‐3 h after surgery | IUC removal 24 h after surgery | Not reported | Not reported | Not reported |
| Barone 2015 | IUC removal 7 days after surgery | IUC removal 14 days after surgery | 31.9 (11.5) | 30.6 (11.7) |
Not reported |
| Basbug 2020 | IUC removal 2 h after surgery | IUC removal 12 h after surgery | 30.13 (5.83) | 29.96 (4.71) | Not reported |
| Benoist 1999 | IUC removal 1 day post‐op | IUC removal 5 days post‐op | 55 (18) | 56 (17) | Not reported |
| Bristoll 1989 | threshold clamping | complete drainage | Not reported | Not reported | Not reported |
| Carpiniello 1988 | IUC removal immediately post‐op | IUC removal 1‐day post‐op | 73 (6.6) | 70 (8.6) | Not reported |
| Carter‐Brooks 2018 | IUC removal 4 h after surgery | IUC removal 6 am on post‐op day 1 | 64.9 (11.5) | 65.2 (10.3) | Not reported |
| Chai 2011 | IUC removal immediately post‐op | IUC removal 1 day post‐op | 46.4 (3.9) | 46.4 (4.0) | Not reported |
| Chen 2013 | IUC removal ≤ 7 days | IUC removal > 7 days | 77 (12.7) | 78 (10.5) | Not reported |
| Chia 2009 | IUC removal 1 day post‐op | IUC removal 3 days post‐op | 54.7 (11.2) | 55.7 (10.3) | Not reported |
| Chillington 1992 | IUC removal at midnight | IUC removal at 6 am the next morning | Not reported | Not reported | Not reported |
| Cornia 2003 | A computer study order was used to remind staff to remove the IUC after 3 days | A computer study order was not used to remind staff to remove the IUC after 3 days | Not reported | Not reported | Not reported |
| Coyle 2015 | IUC removal 2 days post‐op | IUC removal within 12 h of withdrawal of epidural anaesthesia | 63.5 (SD not reported) | 62 (SD not reported) | Not reported |
| Crowe 1993 | IUC removal at 6 am | IUC removal at midnight | Not reported | Not reported | Not reported |
| Dunn 1999 | IUC removal immediately post‐op | Delayed IUC removal post‐op | Not reported | Not reported | Not reported |
| Dunn 2000b | IUC removal immediately post‐op | IUC removal 1 day post‐op | Not reported | Not reported | Not reported |
| Dunn 2003 | IUC removal immediately post‐op | IUC removal 1 day post‐op | Not reported | Not reported | Not reported |
| Durrani 2014 | IUC removal 1 day post‐op | IUC removal 4 or 5 days post‐op | Not reported | Not reported | 71.32 (5.94) |
| El‐Mazny 2014 | IUC removal immediately post‐op | IUC removal 12 h post‐op | 24.5 (4.2) | 23.8 (3.9) | Not Reported |
| Ganta 2005 | IUC removal at midnight | IUC removal at 6 am | 69.9 (SD not reported) | 68.2 (SD not reported) | 68.9 (SD not reported) |
| Glavind 2007 | IUC removal 3 h post‐op | IUC removal the next morning | Not reported | Not reported | 61 (range 31‐88) |
| Gong 2017 | IUC for 48 h with intermittent clamping | IUC for 48 h without intermittent clamping | 46.14 (8.33) | 45.70 (9.63) | Not Reported |
| Gross 2007 | IUC removal at 10 pm the day the order for removal was written | IUC removal at 7 am the day after the order for removal was written | Not reported | Not reported | 70.3 (11.7) |
| Gungor 2014 | IUC removal 2 days post‐op | IUC removal 3 or 4 days post‐op | 55.7 (8.8) | 3 days: 58.5 (10.1) 4 days: 55.8 (9.0) |
Not reported |
| Guzman 1994 | IUC removal 1 day post‐op | IUC removal 3 days post‐op (with and without bladder‐clamping) | 56 (range 40‐75) | No clamping: 58 (range 8‐79) Clamping: 57 (range 36‐75) |
Not reported |
| Hakvoort 2004 | IUC removal on the morning after surgery | IUC removal 5 days post‐op | 67 (range 36 ‐ 86) | 66 (range 33‐87) | Not reported |
| Hall 1998 | IUC removal between 7 am and 9 am | IUC removal between 9 pm and 11 pm | Not reported | Not reported | Not reported |
| Han 1997 | IUC removal 2 days post‐op | IUC removal ≥ 3 days post‐op | 64.6 (range 50‐86) | 68.2 (range 50‐90) | Not reported |
| Hewitt 2001 | IUC removal 4‐6 days post‐op | IUC removal at 14 days post‐op | Not reported | Not reported | Not reported |
| Huang 2011 | IUC removal 2 days post‐op | IUC removal 3 or 4 days post‐op | 61.21, (10.17) | 3 days: 63.93 (10.43) 4 days: 63.7 (12.5) |
62.9 (10.93) |
| Ind 1993 | IUC removal at 6 am | IUC removal at midnight | 49.59 (14.2) | 49.84 (16.6) | Not reported |
| Irani 1995 | IUC removal within 48 h | IUC removal at surgeon's discretion | 70.7 (range 42‐88) | 70 (range 58‐85) | Not reported |
| Iversen Hansen 1984 | IUC removal 1 day post‐op | IUC removal 14 days post‐op | Not reported | Not reported | 70 (range 24‐85) |
| Jang 2012 | No alpha blockers given | Prophylactic alpha blockers given | 54 (range 48‐62) | 59 (range 54‐66) | Not reported |
| Jeong 2014 | Prophylactic alpha blockers given | No alpha blockers given | 63.6 (6.6) | 63.4 (8) | Not reported |
| Joshi 2014 | IUC removal immediately post‐op | IUC removal 1 day post‐op | 46.8 (6.9) | 45.09 (6.44) | Not reported |
| Jun 2011 | Prophylactic alpha blockers given | No alpha blockers given | 68.71 (7.6) | 71.4 (7.85) | Not reported |
| Kamilya 2010 | IUC removal 1 day post‐op | IUC removal 4 days post‐op | 46.9 (12.02) | 47.9 (12.78) | Not reported |
| Kelleher 2002 | IUC removal at 6 am | IUC removal at midnight | Not reported | Not reported | Not reported |
| Kim 2012 | IUC removal on post‐op day 3/4 | IUC removal on post‐op day 7/8 | Not reported | Not reported | Not reported |
| Koh 1994 | IUC removal 1 day post‐op | IUC removal 2 days post‐op | 68.8, 7.3 (mean, SD) | 73, 7.6 (mean, SD) | Not reported |
| Kokabi 2009 | IUC removal 1 day post‐op | IUC removal 2 days post‐op OR 4 days post‐op (3‐arm trial) | Not reported | Not reported | Not reported |
| Lang 2020 | IUC removal 4 h post‐op | IUC removal day 1 post‐op | Not reported | Not reported | 44.4 (8.8) |
| Lau 2004 | "In out" catheterisation | IUC overnight | Not reported | Not reported | 63.3 (4.9) |
| Li 2014 | IUC removal on day 1‐2 post‐op | IUC removal on day 5‐7 post‐op | Not reported | Not reported | Range 56 ‐ 92 |
| Liang 2009 | IUC removal immediately | IUC removal 1 day post‐op OR 2 days post‐op (3‐arm trial) |
43.7 (3.9) | B) 45.7 ( 3.5) C) 45.7 ( 5.8) |
Not reported |
| Lista 2020 | IUC removal on day 3 post‐op | IUC removal on day 5 post‐op | 63 (range 48 ‐ 75) | 64 (range 45 – 75) | Not reported |
| Liu 2015 | Clamping of IUC | No clamping of IUC i.e. free drainage | 51 (13.2) | 52 (16.4 SD) | Not reported |
| Lyth 1997 | IUC removal at 6 am | IUC removal at midnight | Not reported | Not reported | Not reported |
| Mao 1994 | IUC duration 7 am to 8 pm (same day) | IUC duration 7 am to 6 am (next day) | Not reported | Not reported | Not reported |
| Matsushima 2015 | IUC removal 2 days post‐op | IUC removal 4 days post‐op | Not reported | Not reported | 65.9 (5.5) |
| McDonald 1999 | IUC removal at midnight | IUC removal at 6 am | 66.7 (range 51‐81) | 68.7 (range 57‐89) | 67.8 (range 51‐89) |
| Naguimbing‐Cuaresma 2007 | IUC removal 4 h post‐op | IUC removal day 1 post‐op | Not reported | Not reported | Not reported |
| Nathan 2001 | IUC removal at 6 am | IUC removal at midnight | 46.5 (5.6) | 45.7 (5.4) | Not reported |
| Nguyen 2012 | IUC removal 2 days post‐op | IUC removal 10 days post‐op | Not reported | Not reported | Not reported |
| Nielson 1985 | IUC removal 3 days post‐op | IUC removal 28 days post‐op | 64 (range 21‐81) | 64 (range 16‐78) | Not reported |
| Noble 1990 | IUC removal at 6 am | IUC removal at midnight | Not reported | Not reported | Not reported |
| Nyman 2010 | Clamping of IUC | No clamping of IUC | 79 (11) | 80 (11.2) | Not reported |
| Oberst 1981 | Clamping of IUC | No clamping of IUC | 64.5 (10.26) | 59 (11.92) | Not reported |
| Onile 2008 | IUC removal 1 day post‐op | IUC removed immediately post‐op | 31.67 (6.042) | 32.72 (5.96) | Not reported |
| Ouladsahebmadarek 2012 | IUC removed immediately post‐op | IUC removal 1 day post‐op | 37.48 (8.85) | 39.48 (9.54) | Not reported |
| Pervaiz 2019 | IUC removal on day 1 post‐op | IUC removal on day 4 post‐op | 67.00 (9.11) | 65.56 (9.25) | Not reported |
| Popiel 2017 | IUC removal within 6 h of operation completion | IUC removal on day 1 post‐op | Not reported | Not reported | Not reported |
| Rajan 2017 | IUC removal within 3 h of operation completion | IUC removal on day 1 post‐op | 50 (18) | 48 (2.4) | Not reported |
| Ruminjo 2015 | IUC removal on day 7 post‐op | IUC removal on day 14 post‐op | Not reported | Not reported | Not reported |
| Sahin 2011 | IUC removal 1 day post‐op | IUC removal 2 days post‐op AND 3 days post‐op (3‐arm trial) |
62.5 (SD not reported) | B: 61.5, C: 62 (SD not reported) | 62 (range 48‐77) |
| Sandberg 2019 | IUC removal immediately post‐op | IUC removal 18‐24 h post‐op | 49.3 (10.5) | 51.5 (11.9) | Not reported |
| Schiotz 1995 | IUC removal 1 day post‐op | IUC removal 3 days post‐op | Not reported | Not reported | 65.9 (range 29.9‐95.2) |
| Schiotz 1996 | IUC removal 1 day post‐op | IUC removal 1 day post‐op | Not reported | Not reported | 50.3 (range 26.9‐72.6) |
| Sekhavat 2008 | IUC removed immediately post‐op | IUC removal 1 day post‐op | 38.9 (2.9) | 39 (3.8) | Not reported |
| Shahnaz 2016 | IUC removal 24 h post‐op | IUC removal 72 h post‐op | 39.4 (3.2) | 38.8 (2.8) | Not reported |
| Shrestha 2013 | IUC removal 1 day post‐op | IUC removal 3 days post‐op | Not reported | Not reported | 53.35 (10.94) |
| Souto 2004 | IUC removal 7 days post‐op | IUC removal 14 days post‐op | 64 (7.3) | 61 (7.3) | 62 (range 50‐73) |
| Sun 2004 | IUC removal on the next morning post‐op | IUC removal 5 days post‐op | 46.7 (6.7) | 48.3 (8.3) | Not reported |
| Tahmin 2011 | IUC removal 2 days post‐op | IUC removal 5 days post‐op | 51.75 (10.8) | 53.95 (12.8) | Not reported |
| Talreja 2016 | Clamping of IUC | No clamping of IUC i.e. free drainage | 63.05 (4.69) | 64.21 (5.36) | Not reported |
| Taube 1989 | IUC removal immediately after emptying of bladder | IUC removal 1 day post‐op AND 2 days post‐op (3‐arm trial) |
Not reported | Not reported | Not reported |
| Toscano 2001 | IUC removal 1 day post‐op | IUC removal 2 days post‐op | Not reported | Not reported | Not reported |
| Valero Puerta 1998 | IUC removal on day 2 post‐op | IUC removal according to usual care | 70 (range 53‐83) | 69 (range 50‐87) | Not reported |
| Vallabh‐Patel 2020 | IUC removal 6 h post‐op | IUC removal 1 day post‐op | 59.52 (8.5) | 59.57 (11.2) | Not reported |
| Webster 2006 | IUC removal at 6 am | IUC removal at 10 pm | 55.02 (19.97) | 55.05 (18.99) | Not reported |
| Weemhoff 2011 | IUC removal 2 days post‐op | IUC removal 5 days post‐op | 59.9 (10.2) | 60.7 (11.1) | Not reported |
| Williamson 1982 | Clamping of IUC | No clamping of IUC i.e. free drainage | Not reported | Not reported | Range 22‐40 |
| Wilson 2000 | Bladder infusion with normal saline by gravity until bladder was full | IUC removal at 6 am | Not reported | Not reported | Not reported |
| Wu 2015 | Catheter clamped when patient woke up post‐op. On Day 1 morning post‐op, when the patient felt urge to pass urine, the urinary catheter balloon was deflated and the catheter allowed to be self‐dislodged during urination | On the morning of Day1 post‐op, after the patient passed urine (through the catheter), saline was used to wash the bladder and the catheter clamped. 10 min after clamping, the balloon was deflated and the catheter allowed to be self‐dislodged during urination | 45.6 (7.2) | 46.1 (7) | Not reported |
| Wyman 1987 | IUC removal between 6 am and 7 am | IUC removal between 10 pm and 11 pm | Not reported | Not reported | 70.8 (range 50‐89) |
| Yaghmaei 2017 | IUC removal 6 h post‐op | IUC removal 12‐24 h post‐op | 28.19 (5.80) | 28.01 (5.83) | Not reported |
| Yee 2015 | IUC removal 8 h post‐op | IUC removal 1 day post‐op | Not reported | Not reported | Not reported |
| Zaouter 2009 | IUC removal on the same morning as the surgery | IUC removal when the epidural anaesthesia was removed | 57 (15) | 63 (11) | Not reported |
| Zhou 2012 | IUC removal 6‐8 h post‐op | IUC removal 24 h post‐op | 25.11(4.88) | 26.33 (5.08) | Not reported |
| Zmora 2010 | IUC removal 1 day post‐op | IUC removal 3 days post‐op AND 5 days post‐op (3‐arm trial) |
57.4 (range 18‐85) | B: 54.6 (range 25‐81) C: 54.2 (range 22‐78) |
Not reported |
| Zomorrodi 2018 | IUC removal 3 days post‐op | IUC removal 7 days post‐op | 43.52 (13.6) | 43.20 (14.39) | Not reported |
IUC: indwelling urethral catheter
In eight trials participants were less than 35 years old (Aref 2020; Azarkish 2003; Barone 2015; Basbug 2020; El‐Mazny 2014; Onile 2008; Yaghmaei 2017; Zhou 2012). In 49 trials, participants were 35 to 65 years old (Ahmed 2014; Alessandri 2006; Allen 2016; Alonzo‐Sosa 1997; Benoist 1999; Carter‐Brooks 2018; Chai 2011; Chia 2009; Coyle 2015; Dunn 2003; Glavind 2007; Gong 2017; Gungor 2014; Guzman 1994; Huang 2011; Ind 1993; Jang 2012; Jeong 2014; Joshi 2014; Kamilya 2010; Lang 2020; Lau 2004; Liang 2009; Lista 2020; Liu 2015; Nathan 2001; Nielson 1985; Oberst 1981; Ouladsahebmadarek 2012; Rajan 2017; Sahin 2011; Sandberg 2019; Schiotz 1996; Sekhavat 2008; Shahnaz 2016; Shrestha 2013; Souto 2004; Sun 2004; Tahmin 2011; Talreja 2016; Valero Puerta 1998; Vallabh‐Patel 2020; Webster 2006; Weemhoff 2011; Williamson 1982; Wu 2015; Zaouter 2009; Zmora 2010; Zomorrodi 2018). Nineteen trials had participants between 65 to 75 years old (Carpiniello 1988; Chen 2013; Durrani 2014; Ganta 2005; Gross 2007; Hakvoort 2004; Han 1997; Irani 1995; Iversen Hansen 1984; Jun 2011; Koh 1994; Li 2014; Matsushima 2015; McDonald 1999; Pervaiz 2019; Schiotz 1995; Taube 1989; Toscano 2001; Wyman 1987). The participants of one trial were more than 75 years old (Nyman 2010).
Participants who received antibiotics during hospitalisation
There was considerable variation between trials in participants receiving antibiotic prophylactic therapy (see Table 7). We think this is most likely due to the reasons for hospitalisation.
3. Use of antibiotic prophylaxis.
| TrialID | Comparison | Antibiotic prophylaxis used | Details |
| Ahmed 2014; | 2 | Yes | Prophylaxis was given to all patients on the morning of surgery in the form of 1 g of ceftriaxone IM |
| Alessandri 2006 | 2 | Yes | Prophylaxis was given as a single dose before operation |
| Allen 2016 | 2 | No | N/A |
| Alonzo‐Sosa 1997 | 2 | No | N/A |
| Aref 2020 | 2 | Yes | Single dose of prophylactic antibiotic in the form of ceftriaxone 1 g IM |
| Aslam 2019 | 2 | Not reported | Not reported |
| Azarkish 2003 | 2 | Not reported | Not reported |
| Azarkish 2005 | 2 | Not reported | Perineum wash by povidone iodine 10% before catheter insertion |
| Barone 2015 | 2 | No | N/A |
| Basbug 2020 | 2 | Yes | All participants received 1 g IV cefazolin as prophylaxis |
| Benoist 1999 | 2 | Yes | All participants received IV antibiotics as a single dose at the induction of anaesthesia |
| Bristoll 1989 | 3 | Not reported | N/A |
| Carpiniello 1988 | 2 | Yes | Prophylactic cefazolin sodium or clindamycin was given on post‐op day 3 |
| Carter‐Brooks 2018 | 2 | Not reported | N/A |
| Chai 2011 | 2 | No | N/A |
| Chen 2013 | 2 | No | Routine prophylaxis was not given. Antibiotics were only used in symptomatic participants. |
| Chia 2009 | 2 | Yes | Single dose of prophylactic antibiotic was given IV in all participants |
| Chillington 1992 | 1 | Not reported | Not reported |
| Cornia 2003 | 2 | Not reported | Not reported |
| Coyle 2015 | 2 | Not reported | Not reported |
| Crowe 1993 | 1 | Not reported | Not reported |
| Dunn 1999 | N/A | Not reported | Not reported |
| Dunn 2000b | N/A | Not reported | Not reported |
| Dunn 2003 | 2 | Yes | Single dose of antibiotic prophylaxis before operation |
| Durrani 2014 | 2 | Yes | Cephalosporin 1 g was administered IV at the time of induction of anaesthesia |
| El‐Mazny 2014 | 2 | Yes | Cefazolin 2 g IV single dose 30 min before surgery |
| Ganta 2005 | 1 | Not reported | Not reported |
| Glavind 2007 | 2 | Yes | Participants who had vaginal hysterectomy or high uterosacral suspension received 1 pre‐op injection of cefuroxime. No antibiotic prophylaxis was used in the remaining participants. |
| Gong 2017 | 3 | Not reported | Not reported |
| Gross 2007 | 1 | Not reported | Not reported |
| Gungor 2014 | 2 | Not reported | Not reported |
| Guzman 1994 | 2 and 3 | Yes | All participants received Quemicetina as prophylaxis |
| Hakvoort 2004 | 2 | Not reported | Not reported |
| Hall 1998 | 1 | Not reported | Not reported |
| Han 1997 | 2 | Not reported | Not reported |
| Hewitt 2001 | 2 | Not reported | Not reported |
| Huang 2011 | 2 | Yes | Ciprofloxacin used during all days of hospitalisation in all 3 groups |
| Ind 1993 | 1 | Not reported | Not reported |
| Irani 1995 | 2 | Yes | Antibiotics (quinolones) were given from the day of operation until the participant was discharged home |
| Iversen Hansen 1984 | 2 | Yes | Antibiotics were not administered routinely but participants with urinary infections pre‐ or post‐op were treated with antibiotics according to urine culture results. |
| Jang 2012 | 4 | Yes | All participants were given an IV dose of antibiotic during anaesthesia induction before operation |
| Jeong 2014 | 4 | Not reported | Not reported |
| Joshi 2014 | 2 | Yes | All participants received 1 dose of antibiotic prophylaxis at the time of surgery and continued post‐op as per department protocol |
| Jun 2011 | 4 | Not reported | Not reported |
| Kamilya 2010 | 2 | Yes | All participants received 2 doses of antibiotic injection ceftriaxone 1 g, one just before the operation and another 12 h after the first dose |
| Kelleher 2002 | 1 | Not reported | Not reported |
| Kim 2012 | 2 | Not reported | Not reported |
| Koh 1994 | 2 | Yes | Antibiotics were given at induction to participants with IUCs or proven urinary tract infections |
| Kokabi 2009 | 2 | Not reported | Not reported |
| Lang 2020 | 2 | Yes | All participants received pre‐op antibiotics with either American College of Obstetricians and Gynecologists approved dosing of cefazolin (78%) or a combination of gentamicin and clindamycin (22%) with no difference between fast‐track or conventional Foley management groups |
| Lau 2004 | 2 | Yes | Single dose of parenteral antibiotic was given upon induction of general anaesthesia in most cholecystectomies, hernia repairs, gastrointestinal and anorectal operations |
| Li 2014 | 2 | Not reported | Not reported |
| Liang 2009 | 2 | Yes | IV antibiotics consisting of cefazolin 500 mg after induction of general anaesthesia |
| Lista 2020 | 2 | Not reported | Not reported |
| Liu 2015 | 3 | Not reported | Not reported |
| Lyth 1997 | 1 | Not reported | Not reported |
| Mao 1994 | 2 | Not reported | Not reported |
| Matsushima 2015 | 2 | Not reported | Not reported |
| McDonald 1999 | 1 | Not reported | Not reported |
| Naguimbing‐Cuaresma 2007 | 2 | Not reported | Not reported |
| Nathan 2001 | 1 | Not reported | Not reported |
| Nguyen 2012 | 2 | Not reported | Not reported |
| Nielson 1985 | 2 | Not reported | Not reported |
| Noble 1990 | 1 | Not reported | Not reported |
| Nyman 2010 | 3 | Not reported | Not reported |
| Oberst 1981 | 3 | Not reported | Not reported |
| Onile 2008 | 2 | Not reported | Not reported |
| Ouladsahebmadarek 2012 | 2 | Yes | Cephazoline 1 g IV half an hour before surgery started and continued every 6 h for another 2 doses |
| Pervaiz 2019 | 2 | Not reported | Not reported |
| Popiel 2017 | 2 | Not reported | Not reported |
| Rajan 2017 | 2 | Not reported | Not reported |
| Ruminjo 2015 | 2 | Not reported | Not reported |
| Sahin 2011 | 2 | Not reported | Not reported |
| Sandberg 2019 | 2 | Not reported | Not reported |
| Schiotz 1995 | 2 | Not reported | Not reported |
| Schiotz 1996 | 2 | Not reported | Not reported |
| Sekhavat 2008 | 2 | Not reported | Not reported |
| Shahnaz 2016 | 2 | No | "antibiotic was not regularly given except for patients who had abnormal urinary symptoms and unusual urinary analysis in urinary sample 48 h after the surgery" |
| Shrestha 2013 | 2 | Yes | Antibiotics given for 7 days |
| Souto 2004 | 2 | Not reported | Not reported |
| Sun 2004 | 2 | Yes | All participants were given prophylactic antibiotics for 2 days (1 g cefazolin IV 3 times daily) |
| Tahmin 2011 | 2 | Not reported | Not reported |
| Talreja 2016 | 3 | Yes | Participants were given 1 dose of third‐generation cephalosporin in pre‐op period |
| Taube 1989 | 2 | Not reported | Not reported |
| Toscano 2001 | 2 | Yes | Antiobiotic prophylaxis with first generation cephalosporin was given at the induction of anaesthesia for up to 7 days after the operation |
| Valero Puerta 1998 | 2 | Yes | 1 g of ceftriaxone every 24 h for 2 days |
| Vallabh‐Patel 2020 | 2 | Yes | All participants received appropriate perioperative antibiotics per American College of Obstetricians and Gynecologists guidelines |
| Webster 2006 | 1 | Not reported | Not reported |
| Weemhoff 2011 | 2 | Yes | All participants received antibiotic prophylaxis at the beginning of the operation. |
| Williamson 1982 | 3 | Not reported | Not reported |
| Wilson 2000 | 1 | Not reported | Not reported |
| Wu 2015 | 3 | Not reported | Not reported |
| Wyman 1987 | 1 | Not reported | Not reported |
| Yaghmaei 2017 | 2 | Yes | Cefazolin 1 g |
| Yee 2015 | 2 | Not reported | Not reported |
| Zaouter 2009 | 2 | Yes | 2 g cefazolin with or without 500 mg of metronidazole was given IV |
| Zhou 2012 | 2 | Not reported | Not reported |
| Zmora 2010 | 2 | Yes | Prophylactic antibiotics were given 24 h in the perioperative period according to department protocol |
| Zomorrodi 2018 | 2 | Not reported | Not reported |
IM: intramuscular(ly); IUC: indwelling urethral catheter; IV: intravenous(ly); N/A: not applicable
Sixty trials did not report whether antibiotic prophylaxis was given to participants or not (Allen 2016; Aslam 2019; Azarkish 2003; Azarkish 2005; Bristoll 1989; Carter‐Brooks 2018; Chillington 1992; Cornia 2003; Coyle 2015; Crowe 1993; Dunn 1999; Dunn 2000b; Ganta 2005; Gong 2017; Gross 2007; Gungor 2014; Hakvoort 2004; Hall 1998; Han 1997; Hewitt 2001; Ind 1993; Jeong 2014; Jun 2011; Kelleher 2002; Kim 2012; Kokabi 2009; Li 2014; Lista 2020; Liu 2015; Lyth 1997; Mao 1994; Matsushima 2015; McDonald 1999; Naguimbing‐Cuaresma 2007; Nathan 2001; Nguyen 2012; Nielson 1985; Noble 1990; Nyman 2010; Oberst 1981; Onile 2008; Pervaiz 2019; Popiel 2017; Rajan 2017; Ruminjo 2015; Sahin 2011; Sandberg 2019; Schiotz 1995; Schiotz 1996; Souto 2004; Tahmin 2011; Taube 1989; Webster 2006; Williamson 1982; Wilson 2000; Wu 2015; Wyman 1987; Yee 2015; Zhou 2012; Zomorrodi 2018).
Participants received antibiotic therapy in 33 trials (Ahmed 2014; Alessandri 2006; Aref 2020; Basbug 2020; Benoist 1999; Carpiniello 1988; Chia 2009; Dunn 2003; Durrani 2014; El‐Mazny 2014; Glavind 2007; Guzman 1994; Huang 2011; Irani 1995; Jang 2012; Joshi 2014; Kamilya 2010; Koh 1994; Lang 2020; Lau 2004; Liang 2009; Ouladsahebmadarek 2012; Sekhavat 2008; Shrestha 2013; Sun 2004; Talreja 2016; Toscano 2001; Valero Puerta 1998; Vallabh‐Patel 2020; Weemhoff 2011; Yaghmaei 2017; Zaouter 2009; Zmora 2010).
Participants did not receive routine prophylactic antibiotic therapy in five trials (Alonzo‐Sosa 1997; Barone 2015; Chai 2011; Chen 2013; Shahnaz 2016). Some participants received antibiotic therapy when others did not (Iversen Hansen 1984).
Interventions
We split the trials into five different interventions with the following comparisons for the removal of indwelling urethral catheters:
Thirteen trials (1506 participants) compared the removal of indwelling urethral catheters at one specified time of day (6 am to 7 am) versus another specified time of day (10 pm to midnight) (Chillington 1992; Crowe 1993; Ganta 2005; Gross 2007; Hall 1998; Ind 1993; Kelleher 2002; Lyth 1997; McDonald 1999; Nathan 2001; Noble 1990; Webster 2006; Wyman 1987);
Sixty‐eight trials (9247 participants) compared shorter durations of indwelling urethral catheterisation versus longer durations of indwelling urethral catheterisation (Ahmed 2014; Alessandri 2006; Allen 2016; Alonzo‐Sosa 1997; Aref 2020; Aslam 2019; Azarkish 2003; Barone 2015; Basbug 2020; Benoist 1999; Carpiniello 1988; Carter‐Brooks 2018; Chai 2011; Chen 2013; Chia 2009; Cornia 2003; Coyle 2015; Dunn 2003; Durrani 2014; El‐Mazny 2014; Glavind 2007; Gungor 2014; Guzman 1994; Hakvoort 2004; Han 1997; Hewitt 2001; Huang 2011; Irani 1995; Joshi 2014; Kamilya 2010; Kim 2012; Koh 1994; Kokabi 2009; Lang 2020; Lau 2004; Li 2014; Liang 2009; Lista 2020; Mao 1994; Matsushima 2015; Naguimbing‐Cuaresma 2007; Nguyen 2012; Nielson 1985; Onile 2008; Ouladsahebmadarek 2012; Pervaiz 2019; Popiel 2017; Rajan 2017; Sahin 2011; Sandberg 2019; Schiotz 1995; Schiotz 1996; Sekhavat 2008; Shahnaz 2016; Shrestha 2013; Souto 2004; Sun 2004; Tahmin 2011; Taube 1989; Toscano 2001; Valero Puerta 1998; Vallabh‐Patel 2020; Weemhoff 2011; Yaghmaei 2017; Zaouter 2009; Zhou 2012; Zmora 2010; Zomorrodi 2018);
No trials compared flexible durations of indwelling urethral catheterisation versus fixed duration of indwelling urethral catheterisation;
Seven trials (714 participants) compared clamping of indwelling urethral catheterisation versus free drainage of indwelling urethral catheterisation prior to removal (Gong 2017; Guzman 1994; Liu 2015; Nyman 2010; Oberst 1981; Williamson 1982; Wu 2015);
Three trials (402 participants) compared prophylactic use of alpha blocker prior to indwelling urethral catheter removal versus no intervention or placebo (Jang 2012; Jeong 2014; Jun 2011).
Guzman 1994 reported data for both clamping regimes as well as shorter versus longer durations of catheters and is therefore included in both comparisons.
Outcome measures
Thirty‐five of the 99 included trials did not report our primary outcome of number of participants who required recatheterisation (Azarkish 2003; Azarkish 2005; Barone 2015; Benoist 1999; Bristoll 1989; Coyle 2015; Crowe 1993; El‐Mazny 2014; Gross 2007; Gungor 2014; Han 1997; Iversen Hansen 1984; Jeong 2014; Lang 2020; Li 2014; Liang 2009; Mao 1994; McDonald 1999; Nguyen 2012; Nielson 1985; Noble 1990; Popiel 2017; Ruminjo 2015; Souto 2004; Sun 2004; Taube 1989; Toscano 2001; Valero Puerta 1998; Williamson 1982; Wilson 2000; Wu 2015; Yaghmaei 2017; Yee 2015; Zhou 2012; Zomorrodi 2018).
Forty‐four trials reported symptomatic CAUTI (Ahmed 2014; Alessandri 2006; Allen 2016; Alonzo‐Sosa 1997; Aref 2020; Aslam 2019; Azarkish 2003; Barone 2015; Benoist 1999; Carter‐Brooks 2018; Chai 2011; Chen 2013; Chia 2009; Cornia 2003; Coyle 2015; Dunn 2003; Durrani 2014; Gong 2017; Gross 2007; Guzman 1994; Huang 2011; Jang 2012; Kamilya 2010; Koh 1994; Kokabi 2009; Lang 2020; Lau 2004; Li 2014; Liang 2009; Lista 2020; Ouladsahebmadarek 2012; Pervaiz 2019; Popiel 2017; Rajan 2017; Sandberg 2019; Schiotz 1995; Schiotz 1996; Sekhavat 2008; Sun 2004; Vallabh‐Patel 2020; Weemhoff 2011; Zaouter 2009; Zmora 2010; Zomorrodi 2018).
Nineteen trials reported asymptomatic bacteriuria (Ahmed 2014; Aref 2020; Basbug 2020; Carpiniello 1988; Chai 2011; Chen 2013; El‐Mazny 2014; Glavind 2007; Hakvoort 2004; Irani 1995; Joshi 2014; Kamilya 2010; Nathan 2001; Onile 2008; Sandberg 2019; Shahnaz 2016; Shrestha 2013; Tahmin 2011; Zmora 2010).
Twenty‐four trials reported incidence of urinary retention (Barone 2015; Benoist 1999; Coyle 2015; El‐Mazny 2014; Guzman 1994; Han 1997; Irani 1995 ; Jeong 2014; Jun 2011; Kim 2012; Lista 2020; Mao 1994; Nielson 1985; Popiel 2017; Rajan 2017; Schiotz 1995; Sekhavat 2008; Shahnaz 2016; Taube 1989; Toscano 2001; Valero Puerta 1998; Webster 2006; Wu 2015: Zhou 2012).
Four trials reported data on complications of catheterisation (Dunn 2003; Nielson 1985; Webster 2006; Yaghmaei 2017).
Twelve trials reported data on patient pain or discomfort (Carter‐Brooks 2018; Chai 2011; Chia 2009; Dunn 2003; Joshi 2014; Naguimbing‐Cuaresma 2007; Nielson 1985; Ouladsahebmadarek 2012; Sandberg 2019; Sekhavat 2008; Webster 2006; Zaouter 2009).
Four trials reported data on patient satisfaction (Chillington 1992; Lyth 1997; Noble 1990; Yaghmaei 2017).
Eight trials reported data on urinary incontinence (Ahmed 2014; Barone 2015; Gungor 2014; Han 1997; Kim 2012; Onile 2008; Souto 2004; Webster 2006).
Nine trials reported dysuria (Ahmed 2014; Aref 2020; Basbug 2020; El‐Mazny 2014; Liu 2015; Onile 2008; Ouladsahebmadarek 2012; Webster 2006; Yaghmaei 2017).
Seventeen trials reported volume of first void (Chillington 1992; Crowe 1993; Ganta 2005; Gross 2007; Gungor 2014; Hall 1998; Huang 2011; Ind 1993; Kelleher 2002; Liu 2015; Lyth 1997; Mao 1994; McDonald 1999; Nathan 2001; Noble 1990; Webster 2006; Yaghmaei 2017).
Sixteen trials reported time to first void (Carter‐Brooks 2018; Chillington 1992; Crowe 1993; Ganta 2005; Gross 2007; Hall 1998; Ind 1993; Kelleher 2002; McDonald 1999; Naguimbing‐Cuaresma 2007; Nathan 2001; Noble 1990; Oberst 1981; Webster 2006; Williamson 1982; Yaghmaei 2017).
Six trials reported post‐void residual volume (Gross 2007; Gungor 2014; Huang 2011; Jang 2012; Jeong 2014; Nguyen 2012).
Forty trials reported length of hospitalisation (Ahmed 2014; Alessandri 2006; Allen 2016; Alonzo‐Sosa 1997; Aref 2020; Aslam 2019; Basbug 2020; Carter‐Brooks 2018; Chillington 1992; Durrani 2014; El‐Mazny 2014; Guzman 1994; Hakvoort 2004; Han 1997; Ind 1993; Irani 1995; Jang 2012; Jun 2011; Kamilya 2010; Kim 2012; Koh 1994; Lau 2004; Li 2014; Lista 2020; Naguimbing‐Cuaresma 2007; Nathan 2001; Nyman 2010; Onile 2008; Ouladsahebmadarek 2012; Sandberg 2019; Schiotz 1996; Sekhavat 2008; Shahnaz 2016; Shrestha 2013; Sun 2004; Tahmin 2011; Valero Puerta 1998; Weemhoff 2011; Yaghmaei 2017; Zaouter 2009).
Two trials reported time between removal of catheter and discharge (Lyth 1997; Webster 2006).
We did not identify any trials that reported condition‐specific or generic quality of life measures or psychological outcome measures.
The included trials used a number of different ways to define microbiological outcomes. Sixteen trials reported symptomatic UTI defined in one of the following ways:
105 cfu/mL or higher and at least one other symptom of UTI (e.g. fever, suprapubic tenderness, dysuria; Ahmed 2014; Aref 2020; Alonzo‐Sosa 1997; Chai 2011; Joshi 2014; Kamilya 2010; Onile 2008; Schiotz 1995; Schiotz 1996; Zmora 2010);
107 cfu/mL or higher, symptoms of UTI (e.g. dysuria, frequency/urgency, suprapubic tenderness) and fever of 38°C or higher (Zaouter 2009);
CDC criteria for symptomatic UTI (Chen 2013; Gong 2017; Gross 2007; Liang 2009; Vallabh‐Patel 2020).
Six trials reported UTI as significant bacteriuria (≥ 105 cfu/mL) with or without symptoms of UTI (Benoist 1999; Carter‐Brooks 2018; Cornia 2003; Gong 2017; Kamilya 2010; Sekhavat 2008), whilst there were 13 trials that reported UTI as a urine culture of ≥ 105 cfu/mL regardless of clinical features of UTI (Alessandri 2006; Basbug 2020; Carpiniello 1988; El‐Mazny 2014; Glavind 2007; Guzman 1994; Hakvoort 2004; Pervaiz 2019; Shahnaz 2016; Sun 2004; Tahmin 2011; Weemhoff 2011; Zhou 2012). By following the EAU criteria, these outcomes were classified as asymptomatic bacteriuria.
Four trials defined asymptomatic bacteriuria:
105 cfu/mL or higher on urine culture with the absence of symptoms (Alonzo‐Sosa 1997; Schiotz 1995; Schiotz 1996)
pus cells greater than 5 per high‐power field in routine examination of urine and bacterial culture positive (Shrestha 2013)
Sixty‐five trials did not report any clear definitions for symptomatic UTI or asymptomatic bacteriuria (Allen 2016; Aslam 2019; Azarkish 2003; Azarkish 2005; Barone 2015; Bristoll 1989; Chia 2009; Chillington 1992; Coyle 2015; Crowe 1993; Dunn 1999; Dunn 2000b; Dunn 2003; Durrani 2014; Ganta 2005; Gungor 2014; Hall 1998; Han 1997; Hewitt 2001; Huang 2011; Ind 1993; Irani 1995; Iversen Hansen 1984; Jang 2012; Jeong 2014; Jun 2011; Kelleher 2002; Kim 2012; Koh 1994; Kokabi 2009; Lang 2020; Lau 2004; Li 2014; Lista 2020; Liu 2015; Lyth 1997; Mao 1994; Matsushima 2015; McDonald 1999; Naguimbing‐Cuaresma 2007; Nathan 2001; Nguyen 2012; Nielson 1985; Noble 1990; Nyman 2010; Oberst 1981; Ouladsahebmadarek 2012; Popiel 2017; Rajan 2017; Ruminjo 2015; Sahin 2011; Sandberg 2019; Souto 2004; Talreja 2016; Taube 1989; Toscano 2001; Valero Puerta 1998; Webster 2006; Williamson 1982; Wilson 2000; Wu 2015; Wyman 1987; Yaghmaei 2017; Yee 2015; Zomorrodi 2018).
While there was some consistency in the choice of outcome measures amongst trials, the differences in the measures or the way the data were reported limited the possibilities for combining results from individual trials.
Excluded studies
We excluded 89 reports of 85 trials from the review for a variety of reasons, including inappropriate trial design (i.e. not RCTs or quasi‐RCTs), or because the intervention was not relevant, as the trial focused on suprapubic or intermittent catheterisation or centred on long‐term catheterisation (i.e. intended catheterisation of more than 14 days).
Further details regarding the excluded trials can be found in the Characteristics of excluded studies.
Ongoing studies
We identified eight ongoing trials, details of which can be found in the Characteristics of ongoing studies.
Risk of bias in included studies
We give the details of the risk of bias of each trial included in the review in the Characteristics of included studies. The 'Risk of bias' graph and summary figures also provide further information regarding the included trials (see Figure 2 and Figure 3).
2.

Risk of bias graph: review authors' judgements about each risk of bias item presented as percentages across all included trials
3.

Risk of bias summary: review authors' judgements about each risk of bias item for each included trial
Allocation
Random sequence generation
We judged random sequence generation to be adequate and deemed to be low risk of bias in 39 trials (Ahmed 2014; Alessandri 2006; Allen 2016; Aref 2020; Barone 2015; Basbug 2020; Benoist 1999; Carter‐Brooks 2018; Chai 2011; Chen 2013; Chia 2009; Coyle 2015; Dunn 2003; Durrani 2014; El‐Mazny 2014; Gong 2017; Gungor 2014; Huang 2011; Irani 1995; Jang 2012; Joshi 2014; Kamilya 2010; Lang 2020; Matsushima 2015; Naguimbing‐Cuaresma 2007; Nyman 2010; Ouladsahebmadarek 2012; Pervaiz 2019; Rajan 2017; Sandberg 2019; Sekhavat 2008; Shahnaz 2016; Tahmin 2011; Vallabh‐Patel 2020; Webster 2006; Weemhoff 2011; Wu 2015; Zaouter 2009; Zmora 2010).
There were eight trials that we judged to have inadequate methods of random sequence generation and deemed to be at high risk of bias (Cornia 2003; Ind 1993; Lau 2004; Li 2014; Liu 2015; Noble 1990; Valero Puerta 1998; Zhou 2012). Two of these trials used quasi‐randomisation (Liu 2015; Noble 1990).
The remaining 52 trials provided insufficient information regarding the method of random sequence generation so we judged them to be at unclear risk of bias (Alonzo‐Sosa 1997; Aslam 2019; Azarkish 2003; Azarkish 2005; Bristoll 1989; Carpiniello 1988; Chillington 1992; Crowe 1993; Dunn 1999; Dunn 2000b; Ganta 2005; Glavind 2007; Gross 2007; Guzman 1994; Hakvoort 2004; Hall 1998; Han 1997; Hewitt 2001; Iversen Hansen 1984; Jeong 2014; Jun 2011; Kelleher 2002; Kim 2012; Koh 1994; Kokabi 2009; Liang 2009; Lista 2020; Lyth 1997; Mao 1994; McDonald 1999; Nathan 2001; Nguyen 2012; Nielson 1985; Oberst 1981; Onile 2008; Popiel 2017; Ruminjo 2015; Sahin 2011; Schiotz 1995; Schiotz 1996; Shrestha 2013; Souto 2004; Sun 2004; Talreja 2016; Taube 1989; Toscano 2001; Williamson 1982; Wilson 2000; Wyman 1987; Yaghmaei 2017; Yee 2015; Zomorrodi 2018).
Allocation concealment
We judged 22 trials to have used adequate allocation concealment methods and so were at low risk of bias (Alessandri 2006; Allen 2016; Barone 2015; Carter‐Brooks 2018; Chai 2011; Coyle 2015; Dunn 2003; Durrani 2014; Glavind 2007; Gross 2007; Huang 2011; Joshi 2014; Kamilya 2010; Lang 2020; Liang 2009; Nyman 2010; Schiotz 1995; Schiotz 1996; Webster 2006; Weemhoff 2011; Wu 2015; Zmora 2010).
We judged 10 trials to have inadequate allocation concealment methods and therefore were at high risk of bias (Cornia 2003; El‐Mazny 2014; Ind 1993; Kokabi 2009; Lau 2004; Liu 2015; Noble 1990; Sandberg 2019; Zaouter 2009; Zhou 2012).
The remaining 66 trials had insufficient information to judge allocation concealment so we judged them to be at unclear risk of bias (Ahmed 2014; Alonzo‐Sosa 1997; Aref 2020; Aslam 2019; Azarkish 2003; Azarkish 2005; Basbug 2020; Benoist 1999; Bristoll 1989; Carpiniello 1988; Chen 2013; Chia 2009; Chillington 1992; Crowe 1993; Dunn 1999; Dunn 2000b; Ganta 2005; Gong 2017; Gungor 2014; Guzman 1994; Hakvoort 2004; Hall 1998; Han 1997; Hewitt 2001; Irani 1995; Iversen Hansen 1984; Jang 2012; Jeong 2014; Jun 2011; Kelleher 2002; Kim 2012; Koh 1994; Li 2014; Lista 2020; Lyth 1997; Mao 1994; Matsushima 2015; McDonald 1999; Naguimbing‐Cuaresma 2007; Nathan 2001; Nguyen 2012; Nielson 1985; Oberst 1981; Onile 2008; Ouladsahebmadarek 2012; Pervaiz 2019; Popiel 2017; Rajan 2017; Ruminjo 2015; Sahin 2011; Sekhavat 2008; Shahnaz 2016; Shrestha 2013; Souto 2004; Sun 2004; Talreja 2016; Tahmin 2011; Taube 1989; Toscano 2001; Valero Puerta 1998; Vallabh‐Patel 2020; Williamson 1982; Wilson 2000; Wyman 1987; Yaghmaei 2017; Yee 2015; Zomorrodi 2018).
Blinding
Blinding of participants and personnel
We judged one trial to have used adequate blinding methods of participants and personnel, which we therefore assessed as being at low risk of bias (Chen 2013). We judged the remaining 98 trials to have used inadequate methods of blinding of participants and personnel and we therefore assessed them as being at high risk of bias.
Blinding of outcome assessment
Eight trials reported no blinding of outcome assessment, so we deemed them to be at high risk of bias (Alessandri 2006; Barone 2015; Bristoll 1989; Chen 2013; Gong 2017; Ind 1993; Joshi 2014; Liu 2015).
Five trials did report blinding of the principal investigator or the health professional who conducted the outcome assessment on participants (Chai 2011; Durrani 2014; Hall 1998; Naguimbing‐Cuaresma 2007; Nyman 2010). As a result, we deemed them to be at low risk of bias.
The remaining 86 trials did not report blinding of the outcome assessors. Thus, we decided to assign them to unclear risk of bias (Ahmed 2014; Allen 2016; Alonzo‐Sosa 1997; Aref 2020; Aslam 2019; Azarkish 2003; Azarkish 2005; Basbug 2020; Benoist 1999; Carpiniello 1988; Carter‐Brooks 2018; Chia 2009; Chillington 1992; Cornia 2003; Coyle 2015; Crowe 1993; Dunn 1999; Dunn 2000b; Dunn 2003; El‐Mazny 2014; Ganta 2005; Glavind 2007; Gross 2007; Gungor 2014; Guzman 1994; Hakvoort 2004; Han 1997; Hewitt 2001; Huang 2011; Irani 1995; Iversen Hansen 1984; Jang 2012; Jeong 2014; Jun 2011; Kamilya 2010; Kelleher 2002; Kim 2012; Koh 1994; Kokabi 2009; Lang 2020; Lau 2004; Li 2014; Liang 2009; Lista 2020; Lyth 1997; Mao 1994; Matsushima 2015; McDonald 1999; Nathan 2001; Nguyen 2012; Nielson 1985; Noble 1990; Oberst 1981; Onile 2008; Ouladsahebmadarek 2012; Pervaiz 2019; Popiel 2017; Rajan 2017; Ruminjo 2015; Sahin 2011; Sandberg 2019; Schiotz 1995; Schiotz 1996; Sekhavat 2008; Shahnaz 2016; Shrestha 2013; Souto 2004; Sun 2004; Tahmin 2011; Talreja 2016; Taube 1989; Toscano 2001; Valero Puerta 1998; Vallabh‐Patel 2020; Webster 2006; Weemhoff 2011; Williamson 1982; Wilson 2000; Wu 2015; Wyman 1987; Yaghmaei 2017; Yee 2015; Zaouter 2009; Zhou 2012; Zmora 2010; Zomorrodi 2018).
Blinding of assessment of microbiological outcomes
We assumed that microbiological outcomes were assessed by a microbiologist who would not be aware of the catheter duration or the fact that participants were involved in a trial. We rated all 99 included trials as being low risk of bias for microbiological outcomes.
Incomplete outcome data
We deemed 68 trials to be at low risk of attrition bias (Alessandri 2006; Alonzo‐Sosa 1997; Aref 2020; Aslam 2019; Azarkish 2003; Barone 2015; Basbug 2020; Carpiniello 1988; Carter‐Brooks 2018; Chai 2011; Chia 2009; Cornia 2003; Crowe 1993; Dunn 2003; Durrani 2014; El‐Mazny 2014; Ganta 2005; Gong 2017; Guzman 1994; Hakvoort 2004; Ind 1993; Jang 2012; Joshi 2014; Jun 2011; Kamilya 2010; Kelleher 2002; Koh 1994; Lau 2004; Li 2014; Liang 2009; Liu 2015; Mao 1994; Naguimbing‐Cuaresma 2007; Nathan 2001; Nguyen 2012; Nielson 1985; Noble 1990; Nyman 2010; Oberst 1981; Onile 2008; Ouladsahebmadarek 2012; Pervaiz 2019; Rajan 2017; Sandberg 2019; Sahin 2011; Schiotz 1995; Schiotz 1996; Sekhavat 2008; Shahnaz 2016; Shrestha 2013; Souto 2004; Sun 2004; Tahmin 2011; Talreja 2016; Taube 1989; Toscano 2001; Valero Puerta 1998; Vallabh‐Patel 2020; Webster 2006; Weemhoff 2011; Williamson 1982; Wilson 2000; Wu 2015; Wyman 1987; Zaouter 2009; Zhou 2012; Zmora 2010; Zomorrodi 2018). These trials either had no dropouts or no differential dropouts.
Nine trials had incomplete outcome data as well as having a differential loss to follow‐up (Ahmed 2014; Azarkish 2005; Gross 2007; Huang 2011; Irani 1995; Lang 2020; Lyth 1997; Yaghmaei 2017; Yee 2015). As a result of this, we deemed them to be at high risk of attrition bias.
The remaining 22 trials had insufficient information to make a decision and therefore we judged them to be at unclear risk of attrition bias (Allen 2016; Benoist 1999; Bristoll 1989; Chen 2013; Chillington 1992; Coyle 2015; Dunn 1999; Dunn 2000b; Glavind 2007; Gungor 2014; Hall 1998; Han 1997; Hewitt 2001; Iversen Hansen 1984; Jeong 2014; Kim 2012; Kokabi 2009; Lista 2020; Matsushima 2015; McDonald 1999; Popiel 2017; Ruminjo 2015).
Selective reporting
We assessed selective reporting based on the outcomes mentioned in the Methods section (Types of outcome measures), and the results that were reported, as well as whether the trials reported all the expected outcomes in accordance with their objectives. We did not conduct a search for the protocols for each trial due to time constraints.
We deemed 72 trials to be low risk of bias (Ahmed 2014; Alessandri 2006; Allen 2016; Alonzo‐Sosa 1997; Aref 2020; Aslam 2019; Barone 2015; Basbug 2020; Benoist 1999; Carter‐Brooks 2018; Chai 2011; Chen 2013; Coyle 2015; Dunn 2003; Durrani 2014; El‐Mazny 2014; Ganta 2005; Glavind 2007; Gong 2017; Gungor 2014; Guzman 1994; Hakvoort 2004; Hall 1998; Ind 1993; Irani 1995; Jang 2012; Jeong 2014; Kamilya 2010; Kelleher 2002; Kim 2012; Koh 1994; Lang 2020; Lau 2004; Li 2014; Liang 2009; Lista 2020; Liu 2015; Lyth 1997; Mao 1994; Matsushima 2015; McDonald 1999; Nathan 2001; Nielson 1985; Noble 1990; Nyman 2010; Oberst 1981; Onile 2008; Ouladsahebmadarek 2012; Pervaiz 2019; Rajan 2017; Sandberg 2019; Schiotz 1995; Schiotz 1996; Sekhavat 2008; Shahnaz 2016; Shrestha 2013; Souto 2004; Sun 2004; Tahmin 2011; Talreja 2016; Toscano 2001; Valero Puerta 1998; Vallabh‐Patel 2020; Webster 2006; Weemhoff 2011; Wilson 2000; Wu 2015; Yaghmaei 2017; Zaouter 2009; Zhou 2012; Zmora 2010; Zomorrodi 2018).
We deemed 14 trials to be at high risk of bias for selective reporting (Azarkish 2003; Azarkish 2005; Bristoll 1989; Carpiniello 1988; Cornia 2003; Dunn 2000b; Gross 2007; Hewitt 2001; Huang 2011; Iversen Hansen 1984; Naguimbing‐Cuaresma 2007; Popiel 2017; Sahin 2011; Yee 2015).
We assigned the remaining 13 trials to unclear risk of bias for selective reporting (Chia 2009; Chillington 1992; Crowe 1993; Dunn 1999; Han 1997; Joshi 2014; Jun 2011; Kokabi 2009; Nguyen 2012; Ruminjo 2015; Taube 1989; Williamson 1982; Wyman 1987).
Other potential sources of bias
We judged one trial to be at high risk of bias (Williamson 1982). This trial included just eight participants and, as a result, we deemed this trial to be underpowered. The remaining 98 trials included in this review appeared to be free from other sources of bias and we therefore judged them to be at low risk of bias.
Effects of interventions
See: Table 1; Table 2; Table 3; Table 4
Summary of findings 1. Removal of short‐term indwelling urethral catheters in adults at one time of day (6 am to 7 am) versus another time of day (10 pm to midnight).
| Removal of short‐term indwelling urethral catheters in adults at one time of day (6 am to 7 am) versus another time of day (10 pm to midnight) | ||||||
| Patient or population: adults with short‐term indwelling urethral catheters that need to be removed Setting: secondary care Intervention: removal of indwelling urethral catheters at 10 pm to midnight Comparison: removal of indwelling urethral catheters at 6 am to 7am | ||||||
| Outcomes | Anticipated absolute effects* (95% CI) | Relative effect (95% CI) | № of participants (trials) | Certainty of the evidence (GRADE) | Comments | |
| Risk with removal of IUC at 6 am to 7 am | Risk with removal of IUC at 10 pm to midnight | |||||
| Number of participants requiring recatheterisation | Trial population | RR 0.70 (0.52 to 0.94) | 1920 (10 RCTs) | ⊕⊕⊝⊝ Lowa | ||
| 94 per 1000 | 66 per 1000 (50 to 90) | |||||
| Symptomatic catheter‐associated urinary tract infection (CAUTI) | Trial population | RR 1.00 (0.61 to 1.63) | 41 (1 RCT) | ⊕⊝⊝⊝ Very lowb,c | ||
| 611 per 1000 | 611 per 1000 (373 to 996) | |||||
| Dysuria | Trial population |
RR 2.20 (0.70 to 6.86) |
170 (1 RCT) | ⊕⊕⊝⊝ Lowc | ||
| 48 per 1000 | 105 per 1000 (33 to 327) | |||||
| Condition‐specific QoL or generic QoL measure | ‐ | ‐ | ‐ | ‐ | ‐ | Not reported |
| *The risk in the intervention group (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). CI: confidence interval; IUC: indwelling urethral catheter; QoL: quality of life; RCT: randomised controlled trial; RR: risk ratio | ||||||
| GRADE Working Group grades of evidence High certainty: we are very confident that the true effect lies close to that of the estimate of the effect. Moderate certainty: we are moderately confident in the effect estimate: the true effect is likely to be close to the estimate of the effect, but there is a possibility that it is substantially different. Low certainty: our confidence in the effect estimate is limited: the true effect may be substantially different from the estimate of the effect. Very low certainty: we have very little confidence in the effect estimate: the true effect is likely to be substantially different from the estimate of effect. | ||||||
aDowngraded two levels for risk of bias (random sequence generation, allocation concealment and blinding of outcome assessors are all unclear). bDowngraded one level for risk of bias (random sequence generation and blinding of outcome assessors are unclear). cDowngraded two levels for imprecision: few participants and 95% confidence interval is consistent with possible benefit and possible harm.
Summary of findings 2. Removal of short‐term indwelling urethral catheters in adults after shorter versus longer durations.
| Removal of short‐term indwelling urethral catheters in adults after shorter versus longer durations | ||||||
| Patient or population: adults with short‐term indwelling urethral catheters that need to be removed Setting: secondary care Intervention: shorter durations of IUC Comparison: longer durations of IUC | ||||||
| Outcomes | Anticipated absolute effects* (95% CI) | Relative effect (95% CI) | № of participants (trials) | Certainty of the evidence (GRADE) | Comments | |
| Risk with longer durations of catheterisation | Risk with shorter durations of catheterisation | |||||
| Number of participants requiring recatheterisation | Trial population | RR 1.81 (1.35 to 2.41) | 5870 (44 RCTs) | ⊕⊕⊝⊝ Lowa,b | ||
| 75 per 1000 | 136 per 1000 (102 to 182) | |||||
|
Symptomatic catheter associated urinary tract infection (CAUTI) |
Trial population | RR 0.52 (0.45 to 0.61) | 5759 (41 RCTs) | ⊕⊕⊕⊝ Moderatea | ||
| 126 per 1000 | 66 per 1000 (57 to 77) | |||||
| Dysuria | Trial population | RR 0.42 (0.20 to 0.88) | 1398 (7 RCTs) | ⊕⊕⊝⊝ Lowa,b | ||
| 118 per 1000 | 50 per 1000 (24 to 104) | |||||
| Condition‐specific QoL or generic QoL measure | ‐ | ‐ | ‐ | ‐ | ‐ | Not reported |
| *The risk in the intervention group (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). CI: confidence interval; IUC: indwelling urethral catheter; QoL: quality of life; RCT: randomised controlled trial; RR: risk ratio | ||||||
| GRADE Working Group grades of evidence High certainty: we are very confident that the true effect lies close to that of the estimate of the effect. Moderate certainty: we are moderately confident in the effect estimate: the true effect is likely to be close to the estimate of the effect, but there is a possibility that it is substantially different. Low certainty: our confidence in the effect estimate is limited: the true effect may be substantially different from the estimate of the effect. Very low certainty: we have very little confidence in the effect estimate: the true effect is likely to be substantially different from the estimate of effect. | ||||||
aDowngraded one level for risk of bias (unclear risk of selection bias and detection bias). bDowngraded one level for inconsistency (heterogeneity in direction and size of effect).
Summary of findings 3. Removal of short‐term indwelling urethral catheters in adults: clamping compared to free drainage.
| Removal of short‐term indwelling urethral catheters in adults: clamping compared to free drainage | ||||||
|
Patient or population: adults with short‐term indwelling urethral catheters that need to be removed
Settings: secondary care Intervention: clamping of indwelling urethral catheter Comparison: free drainage of indwelling urethral catheter | ||||||
| Outcomes | Anticipated absolute effects* (95% CI) | Relative effect (95% CI) | № of participants (trials) | Certainty of the evidence (GRADE) | Comments | |
| Assumed risk | Corresponding risk | |||||
| Risk with free drainage | Risk with clamping regimes | |||||
| Number of participants requiring recatheterisation | Trial population | RR 0.82 (0.55 to 1.21) | 569 (5 RCTs) | ⊕⊕⊝⊝ Lowa,b | ||
| 160 per 1000 | 131 per 1000 (88 to 193) | |||||
| Symptomatic catheter associated urinary tract infection (CAUTI) | Trial population | RR 0.99 (0.60 to 1.63) | 267 (2 RCTs) | ⊕⊝⊝⊝ Very lowc,d | ||
| 195 per 1000 |
193 per 1000 (117 to 318) |
|||||
| Dysuria | Trial population | RR 0.84 (0.46 to 1.54) | 79 (1 RCT) | ⊕⊝⊝⊝ Very lowd,e | ||
| 385 per 1000 |
323 per 1000 (177 to 592) |
|||||
| Condition‐specific QoL or generic QoL measure | ‐ | ‐ | ‐ | ‐ | ‐ | Not reported |
| *The risk in the intervention group (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). CI: confidence interval; IUC: indwelling urethral catheter; QoL: quality of life; RCT: randomised controlled trial; RR: risk ratio | ||||||
| GRADE Working Group grades of evidence High certainty: we are very confident that the true effect lies close to that of the estimate of the effect. Moderate certainty: we are moderately confident in the effect estimate: the true effect is likely to be close to the estimate of the effect, but there is a possibility that it is substantially different. Low certainty: our confidence in the effect estimate is limited: the true effect may be substantially different from the estimate of the effect. Very low certainty: we have very little confidence in the effect estimate: the true effect is likely to be substantially different from the estimate of effect. | ||||||
aDowngraded one level for risk of bias (unclear random sequence generation, allocation concealment and blinding of outcome assessors). bDowngraded one level for imprecision (95% CI is consistent with possible benefit and possible harm). cDowngraded one level for risk of bias (unclear random sequence generation and high risk due to lack of blinding of outcome assessors. dDowngraded two levels for imprecision (few participants and 95% CI is consistent with possible benefit and possible harm). eDowngraded one level for risk of bias (high risk for randomisation and allocation concealment).
Summary of findings 4. Removal of short‐term indwelling urethral catheters in adults: prophylactic use of alpha blocker versus no drug or intervention.
| Removal of short‐term indwelling urethral catheters in adults: prophylactic use of alpha blocker versus no drug or intervention | ||||||
|
Patient or population: adults with short‐term indwelling urethral catheters that need to be removed
Settings: secondary care Intervention: prophylactic use of alpha blocker Comparison: no drug or intervention | ||||||
| Outcomes | Anticipated absolute effects* (95% CI) | Relative effect (95% CI) | № of participants (trials) | Certainty of the evidence (GRADE) | Comments | |
| Assumed risk | Corresponding risk | |||||
| Risk with no alpha blocker | Risk with prophylactic alpha blocker | |||||
| Number of participants requiring recatheterisation | Trial population | RR 1.18 (0.58 to 2.42) | 184 (2 RCTs) | ⊕⊝⊝⊝ Very lowa,b | ||
| 120 per 1000 | 141 per 1000 (69 to 289) | |||||
| Symptomatic catheter associated urinary tract infection | Trial population |
RR 0.20 (0.01 to 4.06) |
94 (1 RCT) | ⊕⊝⊝⊝ Very lowa,b | ||
| 43 per 1000 |
9 per 1000 (0 to 173) |
|||||
| Dysuria | ‐ | ‐ | ‐ | ‐ | ‐ | Not reported |
| Condition‐specific QoL or generic QoL measure | ‐ | ‐ | ‐ | ‐ | ‐ | Not reported |
| *The risk in the intervention group (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). CI: confidence interval; IUC: indwelling urethral catheter; QoL: quality of life; RCT: randomised controlled trial; RR: risk ratio | ||||||
| GRADE Working Group grades of evidence High certainty: we are very confident that the true effect lies close to that of the estimate of the effect. Moderate certainty: we are moderately confident in the effect estimate: the true effect is likely to be close to the estimate of the effect, but there is a possibility that it is substantially different. Low certainty: our confidence in the effect estimate is limited: the true effect may be substantially different from the estimate of the effect. Very low certainty: we have very little confidence in the effect estimate: the true effect is likely to be substantially different from the estimate of effect. | ||||||
aDowngraded one level for risk of bias (unclear random sequence generation, allocation concealment and blinding of outcome assessors). bDowngraded two levels for imprecision: few participants and wide 95% confidence interval that is consistent with possible benefit and possible harm.
Comparison 1: removal of indwelling urethral catheter at one specified time of day (6 am to 7 am) versus another specified time of day (10 pm to midnight)
Thirteen trials compared catheter removal at different times of the day (Chillington 1992; Crowe 1993; Ganta 2005; Gross 2007; Hall 1998; Ind 1993; Kelleher 2002; Lyth 1997; McDonald 1999; Nathan 2001; Noble 1990; Webster 2006; Wyman 1987). We were not always able to perform meta‐analysis due to either a lack of included trials reporting the same outcome or the presence of considerable clinical heterogeneity between included trials.
Primary outcomes
Number of participants who required recatheterisation following removal of indwelling urethral catheter
Ten trials reported the number of participants who required recatheterisation following removal of indwelling urethral catheters (Chillington 1992; Crowe 1993; Ganta 2005; Hall 1998; Ind 1993; Kelleher 2002; Lyth 1997; Nathan 2001; Webster 2006; Wyman 1987). Removal of indwelling urethral catheters at midnight may slightly reduce the risk of requiring recatheterisation compared with early morning removal (RR 0.70, 95% CI 0.52 to 0.94; I² = 0%; 10 trials, 1920 participants; low‐certainty evidence; Analysis 1.1; Table 1).
1.1. Analysis.

Comparison 1: Removal of indwelling urethral catheter at one specified time of day (10 pm to midnight) versus another specified time of day (6 am to 7 am), Outcome 1: Number needing to be recatheterised
The asymmetry in the funnel plot could be indicative of bias due to missing results (Figure 4). However, with only ten trials contributing to the analysis, we cannot rule out the play of chance as the source of asymmetry.
4.

Funnel plot of comparison 1. Removal of indwelling urethral catheter at one time of day (6 am to 7 am) versus another time of day (10 pm to midnight). Outcome 1.1. number needing to be recatheterised
In the sensitivity analysis, we removed the one trial that we judged to be high risk of bias in the randomisation and allocation concealment domains (Ind 1993). This changed the effect estimate and 95% confidence interval slightly (RR 0.75, 95% CI 0.54 to 1.03; I2 = 0%), which indicates that the overall effect estimate with all trials included may need to be interpreted with caution.
Subgroup analysis based on type of surgery did not suggest evidence that the effect of removing indwelling catheters at midnight versus early in the morning is different in groups of people undergoing different types of surgery (test for subgroup differences: P = 0.07, overlapping confidence intervals; Analysis 1.1).
Subgroup analysis based on sex also did not suggest that the effect of removing indwelling catheters at midnight versus early in the morning on risk of requiring recatheterisation is different between men and women (test for subgroup differences: P = 0.25, overlapping confidence intervals; Analysis 1.2).
1.2. Analysis.

Comparison 1: Removal of indwelling urethral catheter at one specified time of day (10 pm to midnight) versus another specified time of day (6 am to 7 am), Outcome 2: Number needing to be recatheterised: subgroup analysis based on sex
No trials reported the use of antibiotic prophylaxis for this outcome.
Secondary outcomes
Complications/adverse events
Incidence of urinary tract infection
Symptomatic catheter‐associated urinary tract infections (CAUTI): one trial reported the number of participants with symptomatic CAUTIs (Gross 2007). We are uncertain if removing the indwelling urethral catheter at midnight compared with early morning removal has any effect on the risk of symptomatic CAUTI (RR 1.00, 95% CI 0.61 to 1.63; very low‐certainty evidence; Analysis 1.3; Table 1).
Asymptomatic bacteriuria: one trial (107 participants) reported the number of participants undergoing gynaecological surgery who had asymptomatic bacteriuria as a result of indwelling urethral catheterisation (Nathan 2001). There was insufficient evidence to suggest whether removal of the indwelling urethral catheter at midnight or in the morning affected the number of participants developing this (RR 0.74, 95% CI 0.37 to 1.49; Analysis 1.4).
1.3. Analysis.

Comparison 1: Removal of indwelling urethral catheter at one specified time of day (10 pm to midnight) versus another specified time of day (6 am to 7 am), Outcome 3: Symptomatic catheter‐associated urinary tract infection (number of participants)
1.4. Analysis.

Comparison 1: Removal of indwelling urethral catheter at one specified time of day (10 pm to midnight) versus another specified time of day (6 am to 7 am), Outcome 4: Asymptomatic bacteriuria (number of participants)
Incidence of urinary retention
One trial, Webster 2006, reported on the development of urinary retention following discharge and indicated that eight participants in each group (10%) developed this complication (RR 0.98; 95% CI 0.38 to 2.48; 170 participants; Analysis 1.5).
1.5. Analysis.

Comparison 1: Removal of indwelling urethral catheter at one specified time of day (10 pm to midnight) versus another specified time of day (6 am to 7 am), Outcome 5: Incidence of urinary retention
There was insufficient evidence to suggest any difference between the two groups in terms of difficulty passing urine post‐discharge (9/86 versus 8/84; RR 1.10; 95% CI 0.45 to 2.71; 170 participants; Analysis 1.6; Webster 2006).
1.6. Analysis.

Comparison 1: Removal of indwelling urethral catheter at one specified time of day (10 pm to midnight) versus another specified time of day (6 am to 7 am), Outcome 6: Difficulty in passing urine
Other complications of catheterisation (or recatheterisation)
Not reported.
Patient‐reported
Patient pain or discomfort
Loin pain: in Webster 2006, four out of 86 participants whose indwelling urethral catheters were removed in the morning experienced loin pain following discharge compared with one out of 84 participants whose catheter was removed in the morning (RR 3.91, 95% CI 0.45 to 34.24; Analysis 1.7).
Fever: Webster 2006 reported the number of participants who developed urinary‐related fever post‐discharge (7/86 versus 4/84; RR 1.71, 95% CI 0.52 to 5.62; Analysis 1.8). It should be noted that, although the post‐discharge fever was indicated as urinary‐related in the trial, Webster 2006 did not specify whether this was likely to be a direct result of urethral catheterisation or the procedure that the participant underwent.
1.7. Analysis.

Comparison 1: Removal of indwelling urethral catheter at one specified time of day (10 pm to midnight) versus another specified time of day (6 am to 7 am), Outcome 7: Loin pain
1.8. Analysis.

Comparison 1: Removal of indwelling urethral catheter at one specified time of day (10 pm to midnight) versus another specified time of day (6 am to 7 am), Outcome 8: Fever
Patient satisfaction
One trial reported participant satisfaction and indicated that late night removal of the indwelling urethral catheter was associated with more sleep disturbances (P = 0.004; Ganta 2005). Another trial reported that participants whose indwelling urethral catheters were removed late at night had "disturbed sleep, were tired and confused in the morning and had a delayed establishment of voiding pattern" (Lyth 1997). Five other trials in this review contrasted with Lyth 1997 and reported that late night removal of indwelling urethral catheters did not interrupt the participants' sleep (Chillington 1992; Crowe 1993; Ind 1993; Kelleher 2002; Noble 1990). Some participants went back to sleep immediately after the indwelling urethral catheter was removed, whilst others slept through the removal process. This could be due to the anaesthesia or other medications given to the participants.
When recatheterisation was required, one trial reported that two of the three participants who had their indwelling urethral catheters removed in the morning were recatheterised at "unsocial hours" (8.30 pm and 3 am; Chillington 1992). This was reported to not only be distressing for the participant but also resulted in recatheterisation being performed by a doctor who was on call and not familiar with the case.
Urinary incontinence
In Webster 2006, seven out of 86 participants whose indwelling urethral catheters were removed at night developed urinary incontinence after discharge compared with 11 out of 84 in the morning group (RR 0.62, 95% CI 0.25 to 1.53; Analysis 1.9). Webster 2006 included participants on both medical and surgical wards. The participants on surgical wards were hospitalised for either bladder‐related surgery, non‐bladder related surgery, gynaecological surgery, general surgery or orthopaedic surgery. The trial also included participants on medical wards. Thus, we found it difficult to ascertain whether the urinary incontinence was due to the urethral catheter or due to another medical or surgical intervention for which the participants were hospitalised.
1.9. Analysis.

Comparison 1: Removal of indwelling urethral catheter at one specified time of day (10 pm to midnight) versus another specified time of day (6 am to 7 am), Outcome 9: Incontinence
Number of patients reporting dysuria
One trial reported that fewer participants whose indwelling urethral catheters were removed in the morning developed pain following discharge (9/86 versus 4/84; Webster 2006). We are uncertain if indwelling urethral catheter removal at 10 pm increases the risk of dysuria compared with removal at 6 am because the quality of evidence is low and the 95% CI is consistent with possible benefit and possible harm (RR 2.20, 95% CI 0.70 to 6.86; 1 trial, 170 participants; low‐certainty evidence; Analysis 1.10; Table 1).
1.10. Analysis.

Comparison 1: Removal of indwelling urethral catheter at one specified time of day (10 pm to midnight) versus another specified time of day (6 am to 7 am), Outcome 10: Dysuria (number of participants)
Clinician‐reported
Volume of first void (mL)
Twelve trials reported data on the volume of the first void following the removal of the indwelling urethral catheter (Chillington 1992; Crowe 1993; Ganta 2005; Gross 2007; Hall 1998; Ind 1993; Kelleher 2002; Lyth 1997; McDonald 1999; Nathan 2001; Noble 1990; Webster 2006). Ind 1993 reported the median volume of first void (Analysis 1.12), and therefore was not included in the meta‐analysis (Analysis 1.11); the difference between medians was 175 mL more in the group who had their catheter removed late at night (P > 0.0001). The remaining nine trials were included in the meta‐analysis (Chillington 1992; Crowe 1993; Gross 2007; Hall 1998; Kelleher 2002; McDonald 1999; Nathan 2001; Noble 1990; Webster 2006), along with two trials that reported means but no SDs (Ganta 2005; Lyth 1997).
1.12. Analysis.
Comparison 1: Removal of indwelling urethral catheter at one specified time of day (10 pm to midnight) versus another specified time of day (6 am to 7 am), Outcome 12: Volume of first void (median and range)
| Volume of first void (median and range) | |||
| Study | Midnight removal | Morning removal | Significance |
| Following gynaecological surgery | |||
| Ind 1993 | 275 ml (10 to 600 ml) 49 participants |
100 ml (5 to 450 ml) 46 participants |
P < 0.0001 (95% CI 124.9 to 225.5) |
1.11. Analysis.

Comparison 1: Removal of indwelling urethral catheter at one specified time of day (10 pm to midnight) versus another specified time of day (6 am to 7 am), Outcome 11: Volume of the first void (mL)
Participants who had their catheter removed late at night passed larger volumes at first void when compared to those participants who had their catheters removed in the morning (MD 21.98 mL, 95% CI 3.04 to 40.92; I² = 80%; 11 trials, 1198 participants; Analysis 1.11). It should be noted that although this result indicates statistical significance, the increase in volume of first void is not likely to be of any clinical importance.
Time to first void (hours)
Eleven trials reported data on the time to first void (Chillington 1992; Crowe 1993; Ganta 2005; Gross 2007; Hall 1998; Ind 1993; Kelleher 2002; McDonald 1999; Nathan 2001; Noble 1990; Webster 2006). Ind 1993 reported the median time to first void (see Analysis 1.14); the difference between medians was 1 hour 40 minutes less in the group who had their catheter removed late at night (P = 0.012). The remaining eight trials were included in the meta‐analysis (Chillington 1992; Crowe 1993; Gross 2007; Kelleher 2002; McDonald 1999; Nathan 2001; Noble 1990; Webster 2006), along with two trials that reported means but no SDs (Ganta 2005; Hall 1998).
1.14. Analysis.
Comparison 1: Removal of indwelling urethral catheter at one specified time of day (10 pm to midnight) versus another specified time of day (6 am to 7 am), Outcome 14: Time to first void (median)
| Time to first void (median) | |||
| Study | Midnight removal | Morning removal | Significance |
| Following gynaecological surgery | |||
| Ind 1993 | Median time
3 hours 20 minutes 49 participants |
Median time
5 hours 46 participants |
P = 0.012 (95% CI 0.33 to 2.58) |
Those participants who had their catheters removed late at night were found to have a longer time to first void when compared to morning removal (MD 0.71, 95% CI 0.41 to 1.01; I² = 0%; 10 trials, 1140 participants; Analysis 1.13).
1.13. Analysis.

Comparison 1: Removal of indwelling urethral catheter at one specified time of day (10 pm to midnight) versus another specified time of day (6 am to 7 am), Outcome 13: Time to first void (hours)
Post‐void residual volume (mL)
One trial (48 participants) reported post‐void residual volume in participants hospitalised to general medical and surgical wards (Gross 2007). There was insufficient evidence to suggest that the removal of an indwelling catheter late at night or in the early morning had any effect on post‐void residual volume (MD −25.50, 95% CI −214.40 to 163.40; Analysis 1.15).
1.15. Analysis.

Comparison 1: Removal of indwelling urethral catheter at one specified time of day (10 pm to midnight) versus another specified time of day (6 am to 7 am), Outcome 15: Post‐void residual volume
Length of hospitalisation (days)
Three trials provided data on the length of hospitalisation of participants (Chillington 1992; Ind 1993; Nathan 2001). Only one trial reported means and SDs, which favoured late night catheter removal as it reduced participant hospital stay (MD −0.60, 95% CI −1.13 to −0.07; 107 participants; Nathan 2001; Analysis 1.16). The remaining trials reported their data in a format unsuitable for meta‐analysis (Analysis 1.17). One trial reported the mean but no SDs (Chillington 1992), while the other reported median values only (Ind 1993).
1.16. Analysis.

Comparison 1: Removal of indwelling urethral catheter at one specified time of day (10 pm to midnight) versus another specified time of day (6 am to 7 am), Outcome 16: Length of hospitalisation in days
1.17. Analysis.
Comparison 1: Removal of indwelling urethral catheter at one specified time of day (10 pm to midnight) versus another specified time of day (6 am to 7 am), Outcome 17: Length of hospitalisation in days
| Length of hospitalisation in days | |||
| Study | Midnight removal | Morning removal | significance |
| Urological surgery and procedures (mean, total) | |||
| Chillington 1992 | 4.7 (35) | 5.4 (48) | |
| Gynaecological surgery involving the bladder /urethra (median, range) | |||
| Ind 1993 | 9 days (4 to 17 days) | 12 days (5 to 20 days) | p=0.043 |
| Gynaecological surgery not involving the bladder/urethra (median, range) | |||
| Ind 1993 | 6 days (1 to 14 days) | 7 days (2 to 18 days) | |
Time between removal of catheter to discharge (days)
Two trials reported the time between removal of catheter to discharge (Lyth 1997; Webster 2006). There was insufficient evidence to suggest that late night or early morning removal of catheters affected the time between catheter removal to discharge (MD 0.08, 95% CI −5.96 to 6.12; I² = 0%; 2 trials 272 participants; Analysis 1.18).
1.18. Analysis.

Comparison 1: Removal of indwelling urethral catheter at one specified time of day (10 pm to midnight) versus another specified time of day (6 am to 7 am), Outcome 18: Time between removal of catheter to discharge
Health status/quality of life
Condition‐specific or generic quality‐of‐life measures
Not reported
Psychological outcome measures
Not reported
Comparison 2: shorter versus longer duration of indwelling urethral catheterisation
Sixty‐eight trials included in this review investigated the effects of shorter versus longer durations of indwelling urethral catheterisation (Ahmed 2014; Alessandri 2006; Allen 2016; Alonzo‐Sosa 1997; Aref 2020; Aslam 2019; Azarkish 2003; Barone 2015; Basbug 2020; Benoist 1999; Carpiniello 1988; Carter‐Brooks 2018; Chai 2011; Chen 2013; Chia 2009; Cornia 2003; Coyle 2015; Dunn 2003; Durrani 2014; El‐Mazny 2014; Glavind 2007; Gungor 2014; Guzman 1994; Hakvoort 2004; Han 1997; Hewitt 2001; Huang 2011; Irani 1995; Joshi 2014; Kamilya 2010; Kim 2012; Koh 1994; Kokabi 2009; Lang 2020; Lau 2004; Li 2014; Liang 2009; Lista 2020; Mao 1994; Matsushima 2015; Naguimbing‐Cuaresma 2007; Nguyen 2012; Nielson 1985; Onile 2008; Ouladsahebmadarek 2012; Pervaiz 2019; Popiel 2017; Rajan 2017; Sahin 2011; Sandberg 2019; Schiotz 1995; Schiotz 1996; Sekhavat 2008; Shahnaz 2016; Shrestha 2013; Souto 2004; Sun 2004; Tahmin 2011; Taube 1989; Toscano 2001; Valero Puerta 1998; Vallabh‐Patel 2020; Weemhoff 2011; Yaghmaei 2017; Zaouter 2009; Zhou 2012; Zmora 2010; Zomorrodi 2018).
We have not yet incorporated data from three trials into the results for outcomes because the trials did not clearly report numbers per group (Dunn 1999; Dunn 2000b; Ruminjo 2015). We have contacted the authors and we are awaiting clarification before we can use the data. We have not incorporated data from Yee 2015 into the results for outcomes as the conference abstract only reported P values. We have contacted the author to provide further information and we are currently awaiting a reply. Iversen Hansen 1984 and Azarkish 2005 reported data in insufficient detail for us to use them for the meta‐analysis. We have contacted the author to provide further information and we await their reply.
Outcomes for this comparison reported by trials that were not mentioned in the Types of outcome measures are reported in Appendix 5.
We have used subgrouping for illustrative purposes only according to the following: early removal of urinary catheter versus later; one‐day policy versus later; and two to seven‐day policy versus later removal.
Primary outcomes
Number of participants who required recatheterisation following removal of indwelling urethral catheter
Forty‐four trials reported incidence of recatheterisation in participants undergoing either a shorter duration of indwelling urethral catheterisation or longer duration (Ahmed 2014; Alessandri 2006; Allen 2016; Alonzo‐Sosa 1997; Aref 2020; Aslam 2019; Basbug 2020; Carpiniello 1988; Carter‐Brooks 2018; Chai 2011; Chen 2013; Chia 2009; Dunn 2003; Durrani 2014; Glavind 2007; Guzman 1994; Hakvoort 2004; Hewitt 2001; Huang 2011; Irani 1995; Joshi 2014; Kamilya 2010; Kim 2012; Koh 1994; Kokabi 2009; Lau 2004; Lista 2020; Matsushima 2015; Naguimbing‐Cuaresma 2007; Onile 2008; Pervaiz 2019; Rajan 2017; Sahin 2011; Sandberg 2019; Schiotz 1995; Schiotz 1996; Sekhavat 2008; Shahnaz 2016; Shrestha 2013; Tahmin 2011; Vallabh‐Patel 2020; Weemhoff 2011; Zaouter 2009; Zmora 2010), with one trial comparing three different intervention groups (Irani 1995).
Shorter durations of catheterisation may increase the risk of requiring recatheterisation (RR 1.81, 95% CI 1.35 to 2.41; I² = 56%; 44 trials, 5870 participants; low‐certainty evidence; Analysis 2.1; Table 2).
2.1. Analysis.

Comparison 2: Shorter versus longer duration of catheter, Outcome 1: Number needing to be recatheterised
There was evidence of clinical heterogeneity between the trials and so we decided to compare the fixed‐effect (RR 1.75, 95% CI 1.51 to 2.04; I² = 56%) and random‐effects (RR 1.81, 95% CI 1.35 to 2.41; I² = 56%) models. We decided to use the random‐effects model due to the presence of heterogeneity. The presence of heterogeneity was factored in when we assessed the certainty of evidence.
The symmetry in the funnel plot did not suggest any bias due to missing results or small study effects (Figure 5).
5.

Funnel plot of comparison 2. Shorter versus longer duration of catheter. Outcome: 2.1 number needing to be recatheterised
The sensitivity analysis, in which we removed the one trial that we judged to be high risk of bias in the randomisation and allocation concealment domains (Lau 2004), did not substantially change the effect estimate (RR 1.84, 95% CI 1.37 to 2.46).
The test for subgroup differences based on type of surgery indicated heterogeneity between subgroups (P = 0.03, I² = 72.4%). The 95% confidence intervals of the summary effect estimate in the urological surgery subgroup do not substantially overlap with those of the gynaecological or obstetric surgery subgroups, which suggests that the effect of shorter versus longer duration of catheterisation may be different in people undergoing urological surgery in terms of the risk of requiring recatheterisation (Analysis 2.2). For people undergoing urological surgery, it is not certain whether there is a difference between shorter and longer indwelling urethral catheter durations in terms of the risk of requiring recatheterisation (RR 0.91, 95% CI 0.50 to 1.67; 9 trials, 1104 participants).
2.2. Analysis.

Comparison 2: Shorter versus longer duration of catheter, Outcome 2: Number needing to be recatheterised: subgroup analysis based on type of surgery
Subgroup analysis based on sex also suggested that the effect of shorter versus longer duration of catheterisation may be different in men and women (test for subgroup differences: P = 0.009, I² = 85%, 95% CIs do not substantially overlap; Analysis 2.3). For men, it is not certain if there is a difference between shorter and longer indwelling urethral catheter durations in terms of the risk of requiring recatheterisation (RR 0.91, 95% CI 0.50 to 1.67; 9 trials, 1104 participants).
2.3. Analysis.

Comparison 2: Shorter versus longer duration of catheter, Outcome 3: Number needing to be recatheterised: subgroup analysis based on sex
Subgroup analysis based on the use of antibiotic prophylaxis did not reveal heterogeneity between the subgroups (test for subgroup differences: P = 0.92, I² = 0%, overlapping 95% CIs; Analysis 2.4).
2.4. Analysis.

Comparison 2: Shorter versus longer duration of catheter, Outcome 4: Number needing to be recatheterised: subgroup analysis based on antibiotic prophylaxis
Not all trials could participate in the subgroup analysis by surgery type, either because participants did not undergo surgery or because the type of surgery was too unique to meet the subgroup definitions (Allen 2016; Carpiniello 1988; Chen 2013; Lau 2004; Zmora 2010). The following trials did not mention whether they used antibiotic prophylaxis or not (Aslam 2019; Carter‐Brooks 2018; Hakvoort 2004; Hewitt 2001; Kim 2012; Kokabi 2009; Lista 2020; Matsushima 2015; Naguimbing‐Cuaresma 2007; Onile 2008; Pervaiz 2019; Rajan 2017; Sahin 2011; Sandberg 2019; Schiotz 1995; Schiotz 1996; Tahmin 2011).
Secondary outcomes
Complications/adverse events
Incidence of urinary tract infection
Symptomatic catheter‐associated urinary tract infections (CAUTI): 41 trials reported CAUTI (Ahmed 2014; Alessandri 2006; Allen 2016; Alonzo‐Sosa 1997; Aref 2020; Aslam 2019; Azarkish 2003; Barone 2015; Benoist 1999; Carter‐Brooks 2018; Chai 2011; Chen 2013; Chia 2009; Cornia 2003; Coyle 2015; Dunn 2003; Durrani 2014; Guzman 1994; Huang 2011; Kamilya 2010; Koh 1994; Kokabi 2009; Lang 2020; Lau 2004; Li 2014; Liang 2009; Lista 2020; Ouladsahebmadarek 2012; Pervaiz 2019; Popiel 2017; Rajan 2017; Sandberg 2019; Schiotz 1995; Schiotz 1996; Sekhavat 2008; Sun 2004; Vallabh‐Patel 2020; Weemhoff 2011; Zaouter 2009; Zmora 2010; Zomorrodi 2018). One trial had two sets of data for CAUTI, as participants underwent either total mesorectum excision or rectal excision (Benoist 1999). Further details regarding each trial's definition of CAUTI can be found in Table 8.
Shorter durations of catheter probably reduce the risk of developing symptomatic CAUTI compared to later removal (RR 0.52, 95% CI 0.45 to 0.61; I² = 31%; 41 trials, 5759 participants; moderate‐certainty evidence; Analysis 2.5; Table 2). The shape of the funnel plot indicates there may be studies missing in areas that would be favourable to the experimental intervention therefore we judged that the asymmetry was not due to non‐reporting biases and we did not downgrade the certainty of evidence for suspected publication bias (Figure 6).
Post‐hoc subgroup analysis based on antibiotic prophylaxis did not reveal any heterogeneity between the subgroups (test for subgroup differences: P = 0.26, I² = 21%, overlapping 95% CIs; Analysis 2.6).
The following 16 trials did not report whether they gave antibiotic prophylaxis or not and so we did not include them in the post‐hoc subgroup analysis (Aslam 2019; Azarkish 2003; Carter‐Brooks 2018; Cornia 2003; Coyle 2015; Kokabi 2009; Li 2014; Lista 2020; Pervaiz 2019; Popiel 2017; Rajan 2017; Sandberg 2019; Schiotz 1995; Schiotz 1996; Sekhavat 2008; Zomorrodi 2018).
Asymptomatic bacteriuria: 18 trials had data related to asymptomatic bacteriuria (Ahmed 2014; Aref 2020; Basbug 2020; Carpiniello 1988; Chai 2011; Chen 2013; El‐Mazny 2014; Glavind 2007; Hakvoort 2004; Irani 1995; Joshi 2014; Kamilya 2010; Onile 2008; Sandberg 2019; Shahnaz 2016; Shrestha 2013; Tahmin 2011; Zmora 2010). Irani 1995 compared three different intervention groups. Participants who had indwelling urethral catheterisation for a shorter duration were less likely to develop asymptomatic bacteriuria (RR 0.47, 95% CI 0.38 to 0.58; I² = 56%; 18 trials, 2611 participants; Analysis 2.7). One trial reported the total number of participants with asymptomatic bacteriuria (23 participants out of 96) however did not specify the numbers in each group (Schiotz 1996). We attempted to contact the trial authors and await their response.
Further details regarding asymptomatic bacteriuria definitions from the CDC, ISDA and EAU can be found in Table 9 and Table 10. Heterogeneity amongst how this outcome was reported existed across the trials with some trials choosing to report 'positive urine culture' despite meeting the CDC definition for asymptomatic bacteriuria (Table 11).
4. Measurement of symptomatic urinary tract infection.
| TrialID | Outcome as defined by trial authors | Trial definition | Relevant definition outlined by International Guideline Panel |
| Ahmed 2014 | Symptomatic UTI | Significant bacteriuria with at least one of the following symptoms: dysuria, frequency of micturition, urgency, suprapubic pain ir burning sensation at micturition |
CDC |
| Alessandri 2006 | UTI | Significant bacteria which is determined by: urine culture and defined as at least 105 cfu/mL | EAU: symptomatic bacteriuria |
| Allen 2016 | UTI | Not reported | N/A |
| Alonzo‐Sosa 1997 | UTI | Defined as a positive urine sample associated with: dysuria, polyuria, incomplete emptying, pain, fever or sepsis. A positive urine sample was defined as the presence of > 105 cfu/mL if MSU and 104 cfu/mL in a catheter sample. |
CDC |
| Aref 2020 | Symptomatic UTI | “The diagnosis of symptomatic urinary tract infection was based on the following criteria: significant bacteriuria with at least one of the following symptoms; dysuria, frequency of micturition, urgency, supra pubic pain, or burning sensation at micturition.” | CDC |
| Aslam 2019 | UTI | Not reported | N/A |
| Azarkish 2003 | UTI | Not reported | N/A |
| Azarkish 2005 | Not reported | Not reported | N/A |
| Barone 2015 | UTI | Not reported | N/A |
| Basbug 2020 | Significant bacteruria | Significant microscopic bacteriuria was defined as ≥ 100,000 bacteria/ mL MSU | EAU: asymptomatic bacteriuria |
| Benoist 1999 | UTI | Culture yield of > 105 cfu/mL with or without symptoms | With symptoms: CDC Without symptoms: EAU definition for "Asymptomatic bacteriuria" |
| Bristoll 1989 | Not reported | Not reported | N/A |
| Carpiniello 1988 | UTI | Culture yield of 105 cfu/mL | EAU: asymptomatic bacteriuria |
| Carter‐Brooks 2018 | UTI | Defined as a positive culture or symptoms and antibiotic treatment | N/A |
| Chai 2011 | Symptomatic UTI | Positive urine culture: > 105 cfu/mL of an identified single uropathogen/mL of urine Symptomatic UTI: fever (> 38 °C) and dysuria with a positive urine culture |
CDC |
| Chen 2013 | CAUTI | CDC criteria used to define symptomatic UTI and asymptomatic bacteriuria | CDC |
| Chia 2009 | CAUTI | Not reported | N/A |
| Chillington 1992 | Not reported | N/A | N/A |
| Cornia 2003 | CAUTI | Growth from a urine specimen aseptically aspirated from the catheter of ≥ 100 cfu of a predominant pathogen OR ≥ 10 leukocytes per high‐power field on urinalysis in a patient with a clinical diagnosis of UTI | N/A |
| Coyle 2015 | Bacteriuria | Symptomatic or asymptomatic bacteriuria used. No definition given | N/A |
| Crowe 1993 | Not reported | N/A | N/A |
| Dunn 1999 | Not reported | N/A | N/A |
| Dunn 2000b | UTI | Not reported | N/A |
| Dunn 2003 | UTI | Determined by either microscopic abnormality or any patient symptoms | N/A |
| Durrani 2014 | UTI | Not reported | N/A |
| El‐Mazny 2014 | Significant bacteriuria | Significant bacteriuria: > 105 cfu/mL of urine in a MSU sample collected 24 h post‐op | EAU: asymptomatic bacteriuria |
| Ganta 2005 | Not reported | N/A | N/A |
| Glavind 2007 | Positive urine culture | Defined as the presence of ≥ 105 cfu/mL | EAU: asymptomatic bacteriuria |
| Gong 2017 | Symptomatic UTI | Defined as bacteriuria with fever, frequent or painful urination or burning on urination | CDC |
| Gross 2007 | UTI | Used CDC criteria | CDC |
| Gungor 2014 | Not reported | N/A | N/A |
| Guzman 1994 | UTI | Urine culture of > 105 cfu/mL reported as outcome. Definition is not provided | EAU: asymptomatic bacteriuria |
| Hakvoort 2004 | UTI | Signs of UTI: having > 10 WBC/high‐powered field and significant microscopic bacteriuria (1/high‐powered field) in the urine sediment UTI: presence of > 105 cfu/mL in urine culture |
EAU: asymptomatic bacteriuria |
| Hall 1998 | Not reported | N/A | N/A |
| Han 1997 | Not reported | N/A | N/A |
| Hewitt 2001 | Not reported | N/A | N/A |
| Huang 2011 | UTI | Not reported | N/A |
| Ind 1993 | Not reported | N/A | N/A |
| Irani 1995 | UTI | Not reported | N/A |
| Iversen Hansen 1984 | Not reported | N/A | N/A |
| Jang 2012 | Not reported | N/A | N/A |
| Jeong 2014 | Not reported | N/A | N/A |
| Joshi 2014 | Symptomatic UTI | Symptomatic UTI: based on the presence of significant bacteriuria accompanied by at least 1 of the following symptoms: fever, dysuria, increased frequency of micturition, urinary urgency, suprapubic pain and dysuria | CDC |
| Jun 2011 | Not reported | N/A | N/A |
| Kamilya 2010 | Symptomatic UTI | Symptomatic UTI: positive urine culture of > 105 cfu/mL plus 1 of the following symptoms: dysuria, fever (> 38 °C) or rigors | CDC |
| Kelleher 2002 | Not reported | N/A | N/A |
| Kim 2012 | Not reported | N/A | N/A |
| Koh 1994 | UTI | Not reported | N/A |
| Kokabi 2009 | UTI | Not reported | N/A |
| Lang 2020 | UTI | Not reported | N/A |
| Lau 2004 | Positive urine culture | Not reported | N/A |
| Li 2014 | Infection | Not reported | N/A |
| Liang 2009 | UTI | UTI: positive urine culture of > 105 cfu/mL. However, treatment was only given for positive urine cultures if participant had adverse urinary symptoms or post‐op pyrexia (> 38 °C) | CDC |
| Lista 2020 | UTI | Not reported | N/A |
| Liu 2015 | Not reported | N/A | N/A |
| Lyth 1997 | Not reported | N/A | N/A |
| Mao 1994 | Not reported | N/A | N/A |
| Matsushima 2015 | Not reported | N/A | N/A |
| McDonald 1999 | Not reported | N/A | N/A |
| Naguimbing‐Cuaresma 2007 | Not reported | N/A | N/A |
| Nathan 2001 | Positive catheter specimen urine (CSU) | Not reported | N/A |
| Nguyen 2012 | Not reported | N/A | N/A |
| Nielson 1985 | Not reported | N/A | N/A |
| Noble 1990 | Not reported | N/A | N/A |
| Nyman 2010 | Not reported | N/A | N/A |
| Oberst 1981 | Not reported | N/A | N/A |
| Onile 2008 | Significant bacteriuria | Significant bacteriuria: positive urine culture of > 105 cfu/mL in a sample of MSU collected 72 h post‐op with signs of a fever ( a temperature of > 38 °C on 2 occasions within 10 days of the procedure, excluding the first 24 h) | CDC |
| Ouladsahebmadarek 2012 | Symptomatic UTI | Not reported | N/A |
| Pervaiz 2019 | UTI | Urine sample was obtained to assess UTI (bacterial colony count > 105 cfu/mL on urine culture after removal of catheter assessed on day 7) | EAU: catheter‐associated asymptomatic bacteriuria |
| Popiel 2017 | UTI | Not reported | N/A |
| Rajan 2017 | UTI | Urinary infections defined as when microscopic examination of the urine revealed pus cells or when urine culture showed growth of pathogenic organisms | N/A |
| Ruminjo 2015 | Not reported | N/A | N/A |
| Sahin 2011 | Not reported | N/A | N/A |
| Sandberg 2019 | UTI | Standard urine test for nitrite and leucocytes in combination with clinical symptoms | Not clear whether test is dipstick only or whether it involves microscopy/culture |
| Schiotz 1995 | UTI | Positive cultures: culture of > 105 cfu/mL in a sample of MSU or CSU culture of > 104 cfu/mL UTI: positive urine culture in the absence of symptoms. Patients were defined as having UTI if there was any doubt |
EAU: asymptomatic bacteriuria |
| Schiotz 1996 | UTI | Positive cultures: culture of > 105 cfu/mL in a sample of MSU or CSU culture of > 104 cfu/mL UTI: positive urine culture in the absence of symptoms. Participants were defined as having UTI if there was any doubt |
EAU: asymptomatic bacteriuria |
| Sekhavat 2008 | Positive urine culture | Positive urine culture: prevalence of symptomatic UTI was confirmed through a positive urine culture OR through signs of UTI such as: frequency, urgency, dysuria, suprapubic pain or fever | Does not fully meet the criteria for CDC. Must be positive cultures AND clinical features |
| Shahnaz 2016 | Positive urine culture | The presence of positive urinary culture or > 100,000 colony counts in each mL of urine or > 10 pieces of leukocyte in each microscopy field was considered as a urinary infection. | EAU: asymptomatic bacteriuria |
| Shrestha 2013 | Asymptomatic bacteriuria | Asymptomatic bacteriuria: pus cells of > 5 per high‐power field in routine examination of urine and bacterial culture positive | EAU: asymptomatic bacteriuria |
| Souto 2004 | Not reported | N/A | N/A |
| Sun 2004 | UTI | UTI: positive urine culture of > 105 cfu/mL or WBC > 5/high‐power field in urine analysis | EAU: asymptomatic bacteriuria |
| Tahmin 2011 | UTI | UTI: positive urine culture of > 105 cfu/mL | EAU: asymptomatic bacteriuria |
| Talreja 2016 | Not reported | N/A | N/A |
| Taube 1989 | Not reported | N/A | N/A |
| Toscano 2001 | Not reported | N/A | N/A |
| Valero Puerta 1998 | Not reported | N/A | N/A |
| Vallabh‐Patel 2020 | UTI | For the purpose of this trial, participants were considered positive for a UTI if they had (1) positive urine cultures per CDC guidelines or (2) treated empirically over the phone for symptoms of UTI, even in the absence of a urine culture | CDC |
| Webster 2006 | Not reported | N/A | N/A |
| Weemhoff 2011 | UTI | UTI: > 25 WBC/high‐power field, nitrate production, > 20 bacteria/high‐power field, positive urine culture of > 105 cfu/mL | EAU: asymptomatic bacteriuria |
| Williamson 1982 | Not reported | N/A | N/A |
| Wilson 2000 | Not reported | N/A | N/A |
| Wu 2015 | Not reported | N/A | N/A |
| Wyman 1987 | Not reported | N/A | N/A |
| Yaghmaei 2017 | Not reported | N/A | N/A |
| Yee 2015 | Not reported | N/A | N/A |
| Zaouter 2009 | UTI | UTI: pyrexia of > 38 °C, clinical features of UTI (dysuria, frequency, urgency, suprapubic pain, urinary incontinence) and a positive urine culture (107 bacterial colonies of micro‐organism‐forming units/L within 2 weeks after the removal of bladder catheter) | CDC EAU: complicated UTI |
| Zhou 2012 | Not reported | Defined as post‐catheter removal MSU clean catch culture of ≥ 104 cfu/mL for Gram positive organisms or ≥ 105 cfu/mL for Gram negative organisms | EUA: asymptomatic bacteriuria |
| Zmora 2010 | UTI Asymptomatic bacteriuria |
UTI: positive urine culture and symptoms suggestive of UTI | CDC |
| Zomorrodi 2018 | UTI | Not reported | N/A |
CAUTI: catheter‐associated urinary tract infection; CDC: Centers for Disease Control and Prevention; cfu: colony forming unit; CSU: catheter specimen urine; EAU: European Association of Urology; MSU: midstream urine; N/A: not applicable; UTI: urinary tract infection; WBC: white blood count
2.5. Analysis.

Comparison 2: Shorter versus longer duration of catheter, Outcome 5: Symptomatic catheter‐associated urinary tract infection (number of participants)
6.

Funnel plot of comparison 2. Shorter versus longer duration of catheter. Outcome: 2.2. symptomatic catheter‐associated urinary tract infection (number of participants)
2.6. Analysis.

Comparison 2: Shorter versus longer duration of catheter, Outcome 6: Symptomatic catheter‐associated urinary tract infection: post‐hoc subgroup analysis by antibiotic prophylaxis
2.7. Analysis.

Comparison 2: Shorter versus longer duration of catheter, Outcome 7: Asymptomatic bacteruria (number of participants)
5. Definitions for urinary tract infection.
| Guideline Committee | Population | Clinical features | Microbiological findings |
|
Centres for Disease Control and Prevention (CDC) (CDC 2016; Gould 2009) |
CAUTI ‐ UTI in patients who have IUCs that have been in place for > 2 days (day 1 being when the catheter was placed) | At least one of the following:
|
AND a urine culture of at least ≥ 105 cfu/mL with no more than 2 species of organisms |
|
Infectious Diseases Society of America (IDSA) (Hooton 2010) |
UTI in patients with urethral (indwelling or intermittent) or suprapubic catheters that are inserted at the time or removed in the previous 48 h | Patient must have clinical features compatible with UTI (not specified) | AND a MSUor CSU with a urine culture of ≥ 103 cfu/mL of ≥ 1 species of bacterial organism (single‐catheter specimen or MSU) |
|
European Association of Urology (EAU) (EAU 2020; Grabe 2015) |
Asymptomatic bacteriuria | No clinical features |
|
| Uncomplicated UTI (see Table 10 for definition) |
|
|
|
| Complicated UTI (see Table 10 for definition) |
No urinary symptoms 4 weeks before |
|
CAUTI: catheter‐associated urinary tract infection; CDC: Centers for Disease Control and Prevention; cfu: colony forming unit; CSU: catheter specimen urine; EAU: European Association of Urology; MSU: midstream urine; UTI: urinary tract infection; WBC: white blood count
6. European Association of Urology classification of urinary tract infection.
| Uncomplicated UTIs | Acute, sporadic or recurrent lower (uncomplicated cystitis) and/or upper (uncomplicated pyelonephritis) UTI, limited to non‐pregnant, premenopausal women with no known relevant anatomical and functional abnormalities within the urinary tract or co‐morbidities |
| Complicated UTIs | All UTIs that are not defined as uncomplicated. Meaning in a narrower sense UTIs in a patient with an increased chance of a complicated course: i.e. all men, pregnant women, patients with relevant anatomical or functional abnormalities of the urinary tract, IUCs, renal diseases, and/or with other concomitant immunocompromising diseases for example, diabetes |
| Recurrent UTIs | Recurrences of uncomplicated and/or complicated UTIs, with a frequency of at least 3 UTIs/year or 2 UTIs in the last 6 months |
| Catheter associated UTIs (CAUTI) | Catheter‐associated urinary tract infection (CAUTI) refers to UTIs occurring in a person whose urinary tract is currently catheterised or has had a catheter in place within the past 48 h |
| Urosepsis | Urosepsis is defined as life‐threatening organ dysfunction caused by a disregulated host response to infection originating from the urinary tract and/or male genital organs |
CAUTI: catheter‐associated urinary tract infection; IUC: indwelling urethral catheter; UTI: urinary tract infection
Table obtained from EAU Guidelines on Urological Infections (EAU 2020).
7. Heterogeneity of reported outcomes.
| Reported outcomes in this review | Similar outcomes reported by trials |
| Asymptomatic bacteriuria |
|
| Incidence of urinary retention |
|
| Loin pain |
|
| Fever |
|
| Dysuria |
|
| Difficulty in passing urine |
|
| Incontinence |
|
Incidence of urinary retention
Fifteen trials reported data on short‐term urinary retention (Barone 2015; Benoist 1999; Coyle 2015; El‐Mazny 2014; Han 1997; Kim 2012; Mao 1994; Nielson 1985; Popiel 2017; Rajan 2017; Sekhavat 2008; Taube 1989; Toscano 2001; Valero Puerta 1998; Zhou 2012). One trial had three different intervention groups (Taube 1989), while another trial had participants who had different types of surgery (Benoist 1999). We decided not to pool the results as doing so would involve double counting of Taube 1989 (see Analysis 2.8). We decided to use the random‐effects model due to the presence of heterogeneity.
2.8. Analysis.

Comparison 2: Shorter versus longer duration of catheter, Outcome 8: Incidence of urinary retention
It is uncertain if early catheter removal versus later catheter removal has any effect on incidence of urinary retention (RR 1.07, 95% CI 0.57 to 2.00; I² = 70%; 7 trials; 1108 participants; Analysis 2.8.1). Participants who received catheter removal policies involving removal the day after surgery were more likely to develop short‐term urinary retention then those whose catheters were removed after longer durations (RR 1.36, 95% CI 1.03 to 1.81; I² = 6%; 7 trials; 680 participants; Analysis 2.8.2). It is uncertain if there is any difference in incidence of urinary retention between catheter removal at two days or seven days (RR 1.37, 95% CI 0.88 to 2.12; I² = 0%; 6 trials, 881 participants).
Two trials addressed delayed voiding after catheter removal (Schiotz 1996; Sun 2004), with both comparing the removal of indwelling urethral catheters on post‐operative day 1 to a longer duration. Schiotz 1996 compared urethral catheter removal on day 1 and day 3, whereas Sun 2004 compared catheter removal on day 1 and day 5 post‐operatively. There was no evidence to suggest that shorter or longer durations of catheterisation caused delayed voiding after catheter removal (RR 1.02, 95% CI 0.53 to 1.97; I² = 53%; 2 trials, 176 participants; Analysis 2.9). Both trials involved procedures for the treatment of stress urinary incontinence. Sun 2004 used a bladder retraining programme on the third post‐operative day, which involved clamping the catheter for 1 hour and 45 minutes. We think that this could likely be the cause of heterogeneity between the two trials.
2.9. Analysis.

Comparison 2: Shorter versus longer duration of catheter, Outcome 9: Delayed voiding after catheter removal
Two trials reported chronic urinary retention (Benoist 1999; Irani 1995). Irani 1995 reported two sets of results, as participants received either TURP or transurethral incision of prostate (TUIP). From the evidence available, we are unable to ascertain whether earlier or later removal of the indwelling urinary catheter has an effect on the development of chronic urinary retention (RR 0.84, 95% CI 0.29 to 2.44; I² = 0%; 2 trials; 339 participants; Analysis 2.10).
2.10. Analysis.

Comparison 2: Shorter versus longer duration of catheter, Outcome 10: Chronic urinary retention
Other complications of catheterisation (or recatheterisation)
It is uncertain whether shorter or longer durations of catheterisation has any effect on the risk of fever (RR 1.17, 95% CI 0.40 to 3.40; I2 = 0%; 2 trials; 470 participants; Analysis 2.11). Dunn 2003 compared immediate removal of IUC and removal on day 1 post‐op in patients undergoing hysterectomy and the other, Yaghmaei 2017, compared IUC removal 6 hours post‐op and 12‐24 hours post‐op in participants undergoing caesarean section. Another trial, which compared immediate removal of IUC and removal on day one post‐op in patients undergoing abdominal hysterectomy or laparotomy (Ouladsahebmadarek 2012), reported more fever in the later removal group but the data were not presented in useable form (OR 3.97, 95% CI 1.62 to 9.75).
2.11. Analysis.

Comparison 2: Shorter versus longer duration of catheter, Outcome 11: Other complications of catheterisation: fever
One trial reported data on epididymitis (Nielson 1985). Of the 20 participants whose catheters were removed 28 days after urethrotomy, two developed epididymitis compared with none of 20 in the three‐day removal group (RR 0.20, 95% CI 0.01 to 3.92; Analysis 2.12). There were insufficient data to suggest there was any evidence that early or later removal of urethral catheters affected the incidence of epididymitis.
2.12. Analysis.

Comparison 2: Shorter versus longer duration of catheter, Outcome 12: Other complications of catheterisation: epididymitis
Patient‐reported
Patient pain or discomfort
Eleven trials reported data on pain or discomfort (Carter‐Brooks 2018; Chai 2011; Chia 2009; Dunn 2003; Joshi 2014; Naguimbing‐Cuaresma 2007; Nielson 1985; Ouladsahebmadarek 2012; Sandberg 2019; Sekhavat 2008; Zaouter 2009). Five trials used a visual analogue scale (VAS) to assess pain (Carter‐Brooks 2018; Chai 2011; Chia 2009; Ouladsahebmadarek 2012; Zaouter 2009), whilst the other five trials measured pain as a dichotomous variable (Chia 2009; Joshi 2014; Naguimbing‐Cuaresma 2007; Nielson 1985; Sekhavat 2008). Dunn 2003 reported data on pain as a percentage but did not report the number of participants in each group. The authors were contacted for more information.
It is uncertain if early removal has any effect on pain or discomfort measured as a dichotomous outcome (presence/absence of pain or discomfort) (RR 0.52 95% CI 0.21 1.27; I2 = 82%; 5 trials; 510 participants; Analysis 2.13). Pain scores measured on a 0‐10 visual analogue scale (higher score = greater pain) may be reduced with early removal compared with later removal (MD ‐0.34, 95% CI ‐0.47 to ‐0.20; I2 = 28%; 5 trials; 695 participants; Analysis 2.14). However, the difference may not be clinically meaningful.
2.13. Analysis.

Comparison 2: Shorter versus longer duration of catheter, Outcome 13: Pain or discomfort (dichotomous)
2.14. Analysis.

Comparison 2: Shorter versus longer duration of catheter, Outcome 14: Pain or discomfort: 0‐10 VAS (higher score = greater pain)
Patient satisfaction
One trial reported data on patient satisfaction using a questionnaire and compared IUC removal 6 hours post‐op compared to 12‐24 hours post‐op in females undergoing caesarean section (Yaghmaei 2017). This trial was originally written in Persian and, after being translated, it is unclear when their participants were asked to complete this questionnaire. More women were satisfied or very satisfied in the early removal group than in the later removal group (RR 3.27, 95% CI 2.30 to 4.64; 220 women; Analysis 2.15).
2.15. Analysis.

Comparison 2: Shorter versus longer duration of catheter, Outcome 15: Patient satisfaction
Urinary incontinence
Seven trials addressed this outcome (Ahmed 2014; Barone 2015; Gungor 2014; Han 1997; Kim 2012; Onile 2008; Souto 2004). Fewer participants developed urinary incontinence when their catheter was removed earlier (RR 0.55, 95% CI 0.35 to 0.86; I2 = 45%; 7 trials, 1195 participants; Analysis 2.16).
2.16. Analysis.

Comparison 2: Shorter versus longer duration of catheter, Outcome 16: Urinary incontinence
Number of patients reporting dysuria
Seven trials reported data on dysuria (Ahmed 2014; Aref 2020; Basbug 2020; El‐Mazny 2014; Onile 2008; Ouladsahebmadarek 2012; Yaghmaei 2017). Low‐certainty evidence suggests participants may be less likely to report dysuria when their catheters were removed early post‐operatively compared to later (RR 0.42, 95% CI 0.20 to 0.88; I² = 61%; 7 trials, 1398 participants; Analysis 2.17; Table 2).
2.17. Analysis.

Comparison 2: Shorter versus longer duration of catheter, Outcome 17: Dysuria
Clinician‐reported
Volume of first void (mL)
Three trials reported the volume of the first void (Gungor 2014; Huang 2011; Mao 1994). There was insufficient evidence to suggest that participants who had their catheters removed after a shorter duration of catheterisation tended to have larger volumes of first void (MD 27.02, 95% CI 1.00 to 53.04; I² = 31%; 3 trials, 364 participants; Analysis 2.18). Although this result was not statistically significant, the mean volume is not likely to be of any clinical significance.
2.18. Analysis.

Comparison 2: Shorter versus longer duration of catheter, Outcome 18: Volume of first void (mL)
Time to first void (hours)
Two trials reported time to first void (Carter‐Brooks 2018; Yaghmaei 2017). We decided to use the random‐effects model due to the presence of heterogeneity. Those participants who had their catheters removed earlier were found to have a shorter time to first void when compared to later removal (MD ‐5.52, 95% CI ‐6.08 to ‐4.95; I2 = 98%; 2 trials, 277 participants; Analysis 2.19). The heterogeneity may be explained by variations in the type of surgery and level of anaesthesia which are likely to have a substantial impact on an individual’s ability to control their bladder.
2.19. Analysis.

Comparison 2: Shorter versus longer duration of catheter, Outcome 19: Time to first void (hours)
Post‐void residual volume (mL)
Three trials reported data on post‐void residual volume (Gungor 2014; Huang 2011; Nguyen 2012). There were insufficient data to suggest post‐void residual volume was affected by shorter or longer durations of catheterisation in participants undergoing indwelling urethral catheterisation for two to seven days compared to longer durations (MD 6.37, 95% CI −9.14 to 21.88; I2 = 0%; 2 trials, 137 participants; Analysis 2.20). No trials included participants having catheters removed early or after a one‐day removal policy. Nguyen 2012 reported median and range without SDs and, as a result, could not be incorporated into the meta‐analysis (Analysis 2.21).
2.20. Analysis.

Comparison 2: Shorter versus longer duration of catheter, Outcome 20: Post‐void residual volume (mL)
2.21. Analysis.
Comparison 2: Shorter versus longer duration of catheter, Outcome 21: Post‐void residual volume (median and range) (mL)
| Post‐void residual volume (median and range) (mL) | |||
| Study | Outcome | IUC for 2 days | IUC for 10 days |
| Nguyen 2012 | Median (range) post‐void residual volume (mls) | Pre‐op: 100 (20 ‐ 400) 3 months post‐op: 35 (30 ‐ 200) |
Pre‐op: 50 (0 ‐ 180) 3 months post‐op: 20 (0 ‐ 180) 6 months post‐op: 20 (0 ‐ 65) 12 months post‐op: 30 (0 ‐ 100) |
Length of hospitalisation (days)
Twenty‐six trials reported data on length of hospitalisation (Ahmed 2014; Alessandri 2006; Aref 2020; Aslam 2019; Basbug 2020; Carter‐Brooks 2018; Durrani 2014; El‐Mazny 2014; Hakvoort 2004; Han 1997; Irani 1995; Kamilya 2010; Kim 2012; Koh 1994; Lau 2004; Li 2014; Naguimbing‐Cuaresma 2007; Onile 2008; Ouladsahebmadarek 2012; Schiotz 1996; Sekhavat 2008; Shahnaz 2016; Shrestha 2013; Sun 2004; Yaghmaei 2017). Six trials did not report SDs (Han 1997; Irani 1995; Koh 1994; Shrestha 2013; Tahmin 2011; Valero Puerta 1998), while six trials reported the median and range values (Allen 2016; Alonzo‐Sosa 1997; Lista 2020; Sandberg 2019; Weemhoff 2011; Zaouter 2009; Analysis 2.22; Analysis 2.24). We calculated SDs for two trials by using their reported P values and inserting them into a conversion Excel document designed by a statistician (Hakvoort 2004; Schiotz 1996).
2.22. Analysis.

Comparison 2: Shorter versus longer duration of catheter, Outcome 22: Length of hospitalisation in days
2.24. Analysis.
Comparison 2: Shorter versus longer duration of catheter, Outcome 24: Length of hospitalisation in days (median and range)
| Length of hospitalisation in days (median and range) | ||
| Study | Early Removal; median and range | Later Removal; median and range |
| Allen 2016 | 5 (4‐42) | 5 (3‐24) |
| Alonzo‐Sosa 1997 | 2 (range not reported) | 3 (range not reported) |
| Lista 2020 | 4 (3‐7) | 6 (4‐8) |
| Sandberg 2019 | 1.5 (0‐4) | 1 (1‐4) |
| Weemhoff 2011 | 3 (2‐42) | 5 (1‐59) |
| Zaouter 2009 | 7 (5‐11) | 9 (6‐14) |
Early removal may reduce hospital stay compared with later removal (MD ‐1.13 days, 95% CI ‐1.42 to ‐0.83; I2 = 98%; 3917 participants; Analysis 2.22). The substantial statistical heterogeneity in this analysis is most likely due to the variation between studies in type of surgery, which in turn has an impact on length of hospital stay. The test for subgroup differences suggests that the type of surgery that the participants underwent could be an effect modifier (P = 0.0006, I² = 82.6%; Analysis 2.23).
2.23. Analysis.

Comparison 2: Shorter versus longer duration of catheter, Outcome 23: Length of hospitalisation in days: subgrouping based on type of surgery
Time between removal of catheter to discharge (days)
Not reported
Health status/quality of life
Condition‐specific or generic quality‐of‐life measures
Not reported
Psychological outcome measures
Not reported
Comparison 3: flexible versus fixed duration of indwelling urethral catheterisation
We did not find any trials that addressed this comparison.
Comparison 4: clamping versus free drainage before catheter removal
Seven trials involving 714 participants investigated the practices of clamping and release polices versus free drainage of indwelling urethral catheters (Gong 2017; Guzman 1994; Liu 2015; Nyman 2010; Oberst 1981; Williamson 1982; Wilson 2000). All seven trials used different clamping regimes. We used subgrouping to present the analysis according to the following: clamping versus removal (of catheter) at 24 hours; clamping versus removal (of catheter) at 48 hours; and clamping versus removal (of catheter) at 72 hours.
We were unable to include three trials in the meta‐analysis (Bristoll 1989; Talreja 2016; Wilson 2000). We do not know the duration of catheterisation in Talreja 2016. We have contacted the author and we are currently awaiting a reply. Two trials reported data that were not relevant to the outcomes measured by this review (Bristoll 1989; Wilson 2000).
Outcomes for this comparison not pre‐stated in the Types of outcome measures are reported in Appendix 6.
Primary outcomes
Number of participants who required recatheterisation following removal of indwelling urethral catheter
Five trials addressed this outcome (Gong 2017; Guzman 1994; Liu 2015; Nyman 2010; Oberst 1981). There may be little to no difference between using a clamping regimen and free drainage in terms of the risk of requiring recatheterisation (RR 0.82, 95% CI 0.55 to 1.21; I² = 0%; 5 trials, 569 participants; low‐certainty evidence; Analysis 3.1; Table 3).
3.1. Analysis.

Comparison 3: Clamping versus free drainage, Outcome 1: Number needing to be recatheterised
The test for subgroup differences did not suggest a difference in effect between trials with women only and trials with a mixed population of men and women, or between urological surgery and non‐urological surgery (P = 0.64, overlapping confidence intervals; Analysis 3.2). We could not perform subgroup analysis based on antibiotic prophylaxis as only one trial reported it (Guzman 1994).
3.2. Analysis.

Comparison 3: Clamping versus free drainage, Outcome 2: Number needing to be recatheterised: subgroup analysis based on type of surgery and sex
The sensitivity analysis, in which we removed the one trial that we judged to be high risk of bias in the randomisation and allocation concealment domains (Liu 2015), did not change the effect estimate (RR 0.82, 95% CI 0.55 to 1.21; I² = 0%; 5 trials, 490 participants).
Secondary outcomes
Complications/adverse events
Incidence of urinary tract infection
Symptomatic catheter associated urinary tract infections (CAUTI): two trials reported data on symptomatic CAUTI (Gong 2017; Guzman 1994). We are uncertain if there is any difference between clamping regimes and free drainage effects in terms of the risk of symptomatic CAUTI (RR 0.99, 95% CI 0.60 to 1.63; I² = 1%; 2 trials, 267 participants; very low‐certainty evidence; Analysis 3.3; Table 3).
Asymptomatic bacteriuria: not reported
3.3. Analysis.

Comparison 3: Clamping versus free drainage, Outcome 3: Symptomatic catheter‐associated urinary tract infection (number of participants)
Incidence of urinary retention
Two trials reported data on urinary retention (Guzman 1994; Wu 2015). There was insufficient evidence to suggest that the use of clamping regimes versus free drainage affects the incidence of urinary retention in participants (RR 1.18, 95% CI 0.69 to 2.02; I² = 0%; 2 trials, 169 participants; Analysis 3.4).
3.4. Analysis.

Comparison 3: Clamping versus free drainage, Outcome 4: Incidence of urinary retention (number of participants)
Other complications of catheterisation (or recatheterisation)
Not reported
Patient‐reported
Patient pain or discomfort
Not reported
Patient satisfaction
Not reported
Urinary incontinence
Not reported
Number of patients reporting dysuria
One trial reported data on dysuria (Liu 2015). It is uncertain if there is any difference between clamping regimes and free drainage in terms of the risk of dysuria for (RR 0.84, 95% CI 0.46 to 1.54; 1 trial, 79 participants; very low‐certainty evidence; Analysis 3.5; Table 3).
3.5. Analysis.

Comparison 3: Clamping versus free drainage, Outcome 5: Dysuria (number of participants)
Clinician‐reported
Volume of first void (mL)
One trial reported data on the volume of first void (Liu 2015). For participants who had their catheters removed at 72 hours, participants with free drainage catheters tended to have larger volumes of first void when compared to those with clamped catheters (MD 39.60, 95% CI 2.23 to 76.97; 1 trial, 79 participants; Analysis 3.6). Although this result was statistically significant in this trial, the increase in mean volume is unlikely to be of any clinical significance.
3.6. Analysis.

Comparison 3: Clamping versus free drainage, Outcome 6: Volume of first void (mL)
Time to first void (minutes)
Two trials addressed this outcome (Oberst 1981; Williamson 1982). One trial did not report SDs (Williamson 1982). As a result, we could not perform meta‐analysis. One trial found that, on average, there was a shorter duration of time to first void in participants receiving the clamping regime when compared to those with free drainage (MD −118, 95% CI −190.54 to −45.46; Analysis 3.7).
3.7. Analysis.

Comparison 3: Clamping versus free drainage, Outcome 7: Time to first void (minutes)
Post‐void residual volume (mL)
Not reported
Length of hospitalisation (days)
Two trials reported data on the length of hospitalisation (Guzman 1994; Nyman 2010). One trial presented their data with medians and no SDs (Guzman 1994; Analysis 3.8). This left one trial (Nyman 2010), so we could not perform meta‐analysis. There was insufficient evidence to suggest that the use of clamping regimes over free drainage affected the length of hospital stay of participants (Analysis 3.9).
3.8. Analysis.
Comparison 3: Clamping versus free drainage, Outcome 8: Length of hospitalisation (median days)
| Length of hospitalisation (median days) | ||
| Study | Catheter Removal at 72 hours without bladder re‐training (median) | Catheter Removal at 72 hours with bladder re‐training i.e. clamping (median) |
| Guzman 1994 | 6.9 | 6.9 |
3.9. Analysis.

Comparison 3: Clamping versus free drainage, Outcome 9: Length of hospitalisation (days)
Time between removal of catheter to discharge (days)
Not reported
Health status/quality of life
Condition‐specific or generic quality‐of‐life measures
Not reported
Psychological outcome measures
Not reported
Comparison 5: Removal using prophylactic alpha blocker drugs versus other methods
Three trials investigated the effects of the use of prophylactic alpha blockers in participants undergoing indwelling urethral catheterisation (Jang 2012; Jeong 2014; Jun 2011). All trials differed in the dosage of alpha blocker and the time when alpha blockers were given to participants. Tamsulosin was the alpha blocker of choice across the three trials, with two trials opting to use 0.2 mg (Jang 2012; Jun 2011), and one trial using 0.4 mg (Jeong 2014).
Primary outcomes
Number of participants who required recatheterisation following removal of indwelling urethral catheter
Two trials reported this outcome (Jang 2012; Jun 2011). We are uncertain if prophylactic alpha blockers have any effect on the risk of requiring recatheterisation (RR 1.18, 95% CI 0.58 to 2.42; I² = 0%; 2 trials, 184 participants; very low‐certainty evidence; Analysis 4.1; Table 4). We did not perform subgroup analysis due to only two trials reporting this outcome.
4.1. Analysis.

Comparison 4: Prophylactic use of alpha blocker versus no drug or intervention, Outcome 1: Number of participants needing to be recatheterised
Secondary outcomes
Complications/adverse events
Incidence of urinary tract infection
Symptomatic catheter associated urinary tract infections (CAUTI): one trial addressed the incidence of symptomatic CAUTI (Jang 2012). We are uncertain if prophylactic alpha blockers have any effect on the risk of symptomatic CAUTI (0/47 versus 2/47; RR 0.20, 95% CI 0.01 to 4.06; 1 trial, 94 participants; very low‐certainty evidence; Analysis 4.2; Table 4).
Asymptomatic bacteriuria: not reported
4.2. Analysis.

Comparison 4: Prophylactic use of alpha blocker versus no drug or intervention, Outcome 2: Symptomatic catheter‐associated urinary tract infection (number of participants)
Incidence of urinary retention
Two trials reported data on acute urinary retention (Jeong 2014; Jun 2011). Fewer participants developed acute urinary retention in the prophylactic alpha blocker group than in the control group (RR 0.38, 95% CI 0.20 to 0.73; I² = 0% ; 2 trials, 308 participants; Analysis 4.3).
4.3. Analysis.

Comparison 4: Prophylactic use of alpha blocker versus no drug or intervention, Outcome 3: Incidence of urinary retention (number of participants)
Other complications of catheterisation (or recatheterisation)
Not reported
Patient‐reported
Patient pain or discomfort
Not reported
Patient satisfaction
Not reported
Incidence of urinary incontinence
Not reported
Number of patients reporting dysuria
Not reported
Clinician‐reported
Volume of first void (mL)
Not reported
Time to first void (hours)
Not reported
Post‐void residual volume (mL)
Two trials addressed this outcome (Jang 2012; Jeong 2014). There was insufficient evidence to suggest that the use of prophylactic alpha blockers affected post‐void residual volumes in participants receiving indwelling urethral catheterisation (MD −2.00, 95% CI −11.42 to 7.42; I² = 55%; 2 trials, 301 participants; Analysis 4.4). It should be noted that one trial measured post‐void residual volume on post‐operative day seven (Jang 2012), whereas the other trial measured post‐void residual volume two weeks post‐operatively (Jeong 2014).
4.4. Analysis.

Comparison 4: Prophylactic use of alpha blocker versus no drug or intervention, Outcome 4: Post‐void residual volume
Length of hospitalisation (days)
Two trials addressed this outcome (Jang 2012; Jun 2011). One trial reported data in a format that we could not use for statistical analysis (Jang 2012; Analysis 4.6). Participants who received prophylactic alpha blockers tended to have shorter stays in hospital when compared to those participants who did not (MD −1.22, 95% CI −1.54 to −0.90; Analysis 4.5).
4.6. Analysis.
Comparison 4: Prophylactic use of alpha blocker versus no drug or intervention, Outcome 6: Length of hospitalisation in days (median, range, N)
| Length of hospitalisation in days (median, range, N) | ||
| Study | Alpha Blocker | No Intervention |
| Jang 2012 | 9, (7.0‐12.0), 47 | 9, (8.0‐11.0), 47 |
4.5. Analysis.

Comparison 4: Prophylactic use of alpha blocker versus no drug or intervention, Outcome 5: Length of hospitalisation in days
Time between removal of catheter to discharge (days)
Not reported
Health status/quality of life
Condition‐specific or generic quality‐of‐life measures
Not reported
Psychological outcome measures
Not reported
Discussion
Summary of main results
This review includes 99 eligible trials that addressed 14 outcome measures (see Appendix 5 and Appendix 6 for a list of additional outcomes reported by trials).
Removal of indwelling urethral catheters at one specified time of day (6 am to 7 am) versus another specified time of day (10 pm to midnight)
Based on summary data from 13 trials, removal of indwelling urethral catheters late at night may slightly reduce the risk of requiring recatheterisation compared with early morning removal (low‐certainty evidence; Table 1). It is uncertain if there is any difference between late night or early morning removal of indwelling urethral catheters in terms of the number of people developing symptomatic CAUTI (very low‐certainty evidence; Table 1) or dysuria (low certainty‐evidence; Table 1). None of the trials that compared late night to early morning removal of indwelling urethral catheters reported data relating to quality of life.
Shorter versus longer durations of indwelling urethral catheterisation
Based on summary data from 68 trials, shorter durations of catheterisation may increase the risk of requiring recatheterisation compared with longer durations (low‐certainty evidence; Table 2). However, shorter durations of catheterisation probably reduce the risk of symptomatic CAUTI (moderate‐certainty evidence; Table 2) and may reduce the risk of dysuria (low‐certainty evidence; Table 2).
Subgroup analysis suggested that the effect of shorter versus longer indwelling urethral catheterisation duration may be more uncertain in men undergoing urological surgery compared with women or with people undergoing other types of surgery.
None of the trials comparing shorter to longer indwelling urethral catheterisation duration reported data relating to quality of life.
Clamping regimes compared to free drainage
Summary data from seven trials revealed there may be little to no difference between clamping regimes and free drainage in terms of the number of participants who required recatheterisation (low‐certainty evidence; Table 3). Two trials reported data on the number of participants with symptomatic CAUTI. We are very uncertain whether the use of clamping regimes compared with free drainage has any effect on the risk of symptomatic CAUTI or dysuria (both very low‐certainty evidence; Table 3). Condition‐specific or generic quality of life measures were not reported for this comparison.
Use of prophylactic alpha blocker therapy versus no drug or intervention before catheter removal
Based on summary data from three trials, we are uncertain if the use of prophylactic alpha blockers has any effect on the risk of requiring recatheterisation, risk of symptomatic CAUTI (both very low‐certainty evidence) or risk of dysuria (Table 4). Trials did not report dysuria and condition‐specific or generic quality of life measures for this comparison.
Overall completeness and applicability of evidence
The comprehensive search strategy, along with the increased efforts made to obtain unpublished data, means that we can be confident that the evidence presented in this review is as complete as possible. We did not conduct a search for the protocols for each trial due to time constraints. We found that the population in each of the included trials tended to vary considerably due to participants being catheterised for a variety of different indications. The majority of participants included in this systematic review had some form of surgical procedure. Additionally, the type of surgery that participants underwent varied significantly across the trials, with the most common being gynaecological surgery. It is likely that this heterogeneity between the trial populations had an impact during the analysis of the trials.
Despite the large number of trials identified, uncertainties still remain regarding the effects different indwelling urethral catheter removal strategies. Two of our most important participant‐centred outcomes (recatheterisation and CAUTI) were generally well reported but dysuria was less commonly reported and no trials at all reported any quality‐of‐life data.
Ten trials included in this review did not provide data that could contribute to meta‐analysis. We contacted their authors for further information and we await their reply. It should be noted that we identified very few trials that involved non‐surgical populations.
Diagnostic criteria for symptomatic UTI and recatheterisation
For this review, we chose to use the definition of symptomatic UTI outlined by the CDC. The reasoning for using this definition was that various international guideline committees such as the AUA and EAU also use this definition (Gould 2009; Trautner 2010). The IDSA has also outlined definitions for symptomatic UTI in their guidelines. However, these recommendations are tailored for other types of catheterisation, such as suprapubic or intermittent catheterisation and, as a result, we did not use it (Hooton 2010). The current definitions for symptomatic UTI and asymptomatic bacteriuria outlined by various guideline committees can be found in Table 9 and Table 10.
The definition for symptomatic UTI used in each trial was down to the trial authors' preference, as no international agreement exists as to which definition should be used in trials assessing symptomatic UTI. However, this is an important outcome and future trials should use standardised definitions of CAUTI (see Table 9). Only seven trials in this review stated that symptomatic CAUTI was defined using the CDC guidelines (Ahmed 2014; Aref 2020; Chai 2011; Chen 2013; Gong 2017; Joshi 2014; Kamilya 2010). Six trials reported UTI that met the definition for symptomatic UTI by the CDC (Alonzo‐Sosa 1997; Gross 2007; Liang 2009; Vallabh‐Patel 2020; Zaouter 2009; Zmora 2010). Similar issues have been encountered by the EAU guideline committee, who have found assessing the urinary catheter literature problematic due to this lack of definition by trials (EAU 2020).
As the primary outcome of this review was the number of participants requiring recatheterisation, it was noted that very few trials provided a definition for recatheterisation or information regarding the circumstances that led participants to be recatheterised. Given that the insertion of catheters is associated with its own complications and risks (urethral trauma, urethral stricture formation, increased patient pain or discomfort, and bladder perforation (Hollingsworth 2013; Igawa 2008; Fisher 2017)), it may be useful for future trials to report whether any of these complications occurred in participants who were recatheterised. Future trials should aim to improve the reporting of complications of catheterisation (or recatheterisation) as this will help improve future recommendations for recatheterisation as an intervention.
Other strategies to prevent CAUTI
Emerging literature has shown that other strategies can be devised in an attempt to reduce both the placement and the duration of urethral catheters.
Meddings 2014 has explored the use of various other strategies to help reduce unnecessary indwelling urethral catheter use. One of these methods includes stop‐orders, which prompt healthcare workers to remove an indwelling urethral catheter after a certain time has elapsed or a specific condition has occurred. Stop‐order protocols tended to be similar in that they would all generally include a list of appropriate circumstances for which patients should be catheterised, as well as state a default time period before the catheter had to be removed. Meddings 2014 argued that the use of protocols designed to reduce inappropriate catheter placement or prompting their removal can result in reduced catheter usage and rates of CAUTI. By reminding clinicians and nurses of the catheter's existence, stop‐orders and other strategies could potentially help reduce the number of patients developing problems associated with prolonged or unnecessary urinary catheterisation. Although the use of stop‐orders does not meet the inclusion criteria for this review, it should be noted that other methods are available to help not only reduce the duration of catheterisation but also potentially reduce the need for them in the first place.
Quality of the evidence
Despite a large number of trials in this review, we found the certainty of evidence for most outcomes to be low or very low. This was primarily due to many of the included trials suffering from methodological flaws as well as insufficient reporting. This subsequently affected the risk of bias domains of trials, resulting in them being assigned to unclear risk of bias and consequently downgrading the certainty of evidence. We judged the risk of selection bias through randomisation and allocation concealment to be unclear due to inadequate reporting. We generally deemed the risk of performance and detection bias to be high for most trials as it became clear that it was not possible in many instances for the outcome assessor or healthcare professionals to be adequately blinded due to the nature of the intervention. As a result, we downgraded the certainty of evidence due to serious concerns about risk of bias.
In addition to downgrading for risk of bias, we also downgraded the certainty of evidence for some outcomes for imprecision due to the low numbers of participants in the included trials. Higher numbers of participants give the trials more power and consequently, the effect estimate is more precise and more likely to be closer to the true effect of the intervention.
Potential biases in the review process
We searched all relevant databases during our search process without imposing any language restrictions. This allowed our search to identify as many relevant trials as possible. The search also included ongoing trials, which are registered in trial registries. However, even with this rigorous search strategy, it is possible that we did not identify all eligible trials. Although challenging for older trials, we contacted trial authors when more data were required, with no replies received to our emails. We did not conduct a search for the protocols for each trial due to time constraints.
To reduce the risk of bias in the review process, two or more review authors independently undertook study selection, data extraction, risk of bias assessment and GRADE assessments. Another potential source of bias may have occurred during the process of determining the certainty of evidence when we chose the critical GRADE outcomes. We attempted to reduce the risk of bias in the selection of outcomes for inclusion in the GRADE evidence profile. We took into account patients' views obtained through focus groups, as well as advice from clinical experts.
Agreements and disagreements with other studies or reviews
We found the following reviews or guidelines, or both, to be related to this systematic review. We noted that the GRADE certainty of evidence framework was not performed by any other review. Some overlap was found to exist between this update and another Cochrane Review (Phipps 2006). Their review evaluated the use of urinary catheters after urogenital surgery and looked at various outcomes to establish the optimal use of urinary catheters post‐surgery.
Comparison 1: removal of indwelling urethral catheter at one time of day (6 am to 7 am) versus another time of day (10 pm to midnight)
Number of participants requiring recatheterisation
One systematic review conducted by Fernandez 2003a found that the removal of indwelling urethral catheters late at night had no effect on recatheterisation rates in participants undergoing TURP or urological surgery. These findings are similar to the findings in this review.
An earlier Cochrane Review looked at short‐term indwelling urethral catheterisation policies and found that removing the indwelling urethral catheter late at night resulted in fewer participants requiring recatheterisation (Phipps 2006).
The CDC acknowledged that further research is required into the removal of indwelling urethral catheters at different times of the day in their guidelines on symptomatic CAUTI (Gould 2009).
Comparison 2: shorter versus longer duration of indwelling urethral catheterisation
Number of participants requiring recatheterisation
Zhang 2015 found similar results in their meta‐analysis when comparing early versus delayed catheter removal in women following uncomplicated hysterectomy. Removal of the catheter early resulted in a significant increase in recatheterisation in participants (RR 3.32, 95% CI 1.48 to 7.46).
Phipps 2006 also looked at shorter post‐operative catheter durations compared to longer durations. However, their review only involved 12 trials, whereas this systematic review involved 98. Phipps and colleagues reported that there was insufficient evidence to conclude whether shorter durations of catheterisation affected recatheterisation rates in participants. Phipps 2006 did not perform an analysis of the quality of this evidence.
The CDC guidelines acknowledge that there is an increased risk of recatheterisation in shorter durations of catheterisation compared to longer durations (Gould 2009).
Number of participants with symptomatic CAUTI
Zhang 2015 found that early removal of the indwelling urethral catheters resulted in a significant reduction in symptomatic UTI (RR 0.23, 95% CI 0.10 to 0.52). Although this result is similar to this review, we found a larger effect due to more trials being included in our meta‐analysis. A reduction in asymptomatic bacteriuria was also found by Zhang 2015 in the early removal group, which we also saw in our review (RR 0.60, 95% CI 0.40 to 0.88).
Phipps 2006 also reported a reduction in UTI when catheters were removed after a shorter duration versus longer duration (RR 0.50, 95% CI 0.29 to 0.87), which agrees with the findings of this review.
Our findings relating to shorter compared with longer indwelling urethral catheterisation duration and the risk of CAUTI are consistent with existing literature on catheter duration and the risk of developing symptomatic CAUTI (CDC 2016; EAU 2020; Gould 2009; Grabe 2015; Hooton 2010; NICE 2012; Tenke 2008; Tiguert 2004).
Comparison 3: clamping regimes compared to free drainage
Number of participants requiring recatheterisation
The CDC guidelines report that there is no benefit from clamping short‐term indwelling urethral catheters before removal (CDC 2016; Gould 2009). This was classified as a weak recommendation based on evidence reported by two Cochrane Reviews, one of which is the previous version of this review (Fernandez 2003b), the other being Phipps 2006.
A systematic review conducted by Fernandez 2005 found that there was no statistically significant difference in the number of patients requiring recatheterisation between both the clamped and unclamped groups. The results of this trial are similar to the findings in this review.
A systematic review and meta‐analysis conducted by Wang 2016 found that there were no significant differences between clamping and unclamping groups in reference to risk of recatheterisation, urinary retention, rate of UTI or subjective symptoms related to voiding.
Number of participants with symptomatic CAUTI
The CDC guidelines have outlined that clamping policies should not be used in short‐term catheterisation as it has been shown that clamping policies do not provide any benefit with regards to bacteriuria (CDC 2016; Gould 2009).
The EAU has made a slightly different recommendation, however. Upon their evaluation of the evidence, they concluded that the literature was of poor methodological quality and, as a result, no clinical recommendations could be made as to whether or not there is any benefit from the use of clamping policies. It concludes by stating that further research is required to fully determine the value of clamping regimes in short‐term catheterisation (EAUN 2012; Grabe 2015).
Fernandez 2005 found no statistically significant difference between the clamped and free drainage groups with regards to the number of patients developing UTIs at 72 hours (RR 0.55, 95% CI 0.15 to 2.01).
Comparison 4: use of prophylactic alpha blocker therapy versus no drug or intervention before catheter removal
Number of participants requiring recatheterisation
Another Cochrane Review has evaluated the use of alpha blockers in short‐term indwelling urethral catheters in men with AUR (Fisher 2014). However, their prophylactic use has not been studied in a Cochrane Review.
A RCT conducted by Patel 2018 looked at the use of alpha blockers in participants undergoing colorectal surgery below the peritoneal reflection. Their trial compared indwelling urethral catheter removal on day 1 post‐operatively in combination with an alpha blocker versus standard removal of the catheter at 3 days post‐operatively with no alpha blocker. There was no significant difference in the number of participants requiring recatheterisation between both groups. There was significant reduction in symptomatic CAUTI and length of stay in the early catheter removal group. Their trial was excluded from this systematic review as the trial compared both shorter versus longer durations of catheterisation (comparison 2) and the use of alpha blockers in their participants (comparison 5).
A systematic review and meta‐analysis performed by Ghuman 2018 assessed the use of prophylactic alpha blockers on the prevention of post‐operative urinary retention. Their systematic review did not define duration of catheterisation (short or long‐term indwelling urethral catheterisation) and also included intermittent catheterisation. The administration of prophylactic alpha blockers varied across their trials from one week before surgery to four to six hours post‐operation. Two of the trials in their review were also included in our meta‐analysis. The use of prophylactic alpha‐1 adrenergic blockers resulted in a significant reduction in the risk of post‐operative urinary retention (RR 0.48, 95% CI 0.33 to 0.70; P = 0.001; I² = 65.49%); however, their results showed substantial heterogeneity. Further subgroup analysis revealed a strong risk reduction in men (RR 0.33, 95% CI 0.23 to 0.47; P < 0.001, I² = 10.58%) and participants receiving spinal anaesthesia (RR 0.26, 95% CI 0.14 to 0.46; P < 0.0001, I2 = 0%).
Number of participants with symptomatic CAUTI
Ghuman 2018 found no evidence to suggest the use of alpha blockers had any effect on the number of participants with symptomatic CAUTI (RR 0.64, 95% CI 0.30 to 1.37; P = 0.25).
Authors' conclusions
Implications for practice.
The available evidence suggests that the removal of short‐term indwelling urethral catheters late at night, in comparison to early in the morning, may reduce the risk of requiring recatheterisation and the risk of dysuria. The same evidence was uncertain about the effect on the risk of symptomatic catheter‐associated urinary tract infections (CAUTI).
In addition, using a catheter for a shorter length of time may increase the risk of requiring recatheterisation compared with longer durations, but probably reduces the risk of symptomatic CAUTI. It may reduce the risk of dysuria.
Current evidence remains uncertain about the effect of clamping compared to free drainage or the use of prophylactic alpha blockers. We did not identify any trials comparing flexible duration versus fixed duration of catheter use and so we could not draw any conclusions.
Due to the low certainty of the majority of the evidence presented here, the results of further research are likely to change our findings and to have a further impact on clinical practice.
More research is needed to study the effects of short‐term indwelling urethral catheterisation removal on non‐surgical patients.
Implications for research.
This review highlights the need for adequately powered, well‐designed and well‐reported trials, which should measure the following important outcomes: the number of participants requiring recatheterisation; the number of participants developing symptomatic CAUTI; dysuria; and quality of life.
Future trials should ensure that the CONSORT statement is followed and that clinically relevant outcomes are measured. The development of a clearly defined core outcome set, such as those facilitated by the Core Outcome Measures in Effectiveness Trials initiative (COMET), for research relating to short‐term catheterisation would assist trialists in identifying and investigating clinically important questions. This would allow systematic reviewers more scope for the meaningful synthesis of the evidence and, in turn, lead to more robust clinical recommendations made by guideline panels and decision makers.
In addition, patients consider clinically important outcomes important for decision‐making. By measuring these outcomes, improved recommendations can be made on the basis of higher quality evidence, which could improve the overall care of patients.
Future trials should aim to report the size of the catheter used as well as the use of antibiotic prophylaxis. This review highlighted how poorly both these outcomes were reported across the included trials and will allow future subgroup analysis to be more informed.
With regard to how data should be collected and measured, the complications associated with recatheterisation should be reported in more detail. When measuring outcomes such as symptomatic urinary tract infection and asymptomatic bacteriuria, future trials should adopt a standard definition, which has been outlined by a well‐recognised international guideline panel (Table 9; Table 10; Table 11). The reporting of the critical GRADE outcomes was particularly lacking across the RCTs. This resulted in a lack of evidence for dysuria and quality of life. Trials should use a standardised form that assesses the domain of dysuria in patients with short‐term catheters and report this in a systematic format. Quality of life should be measured by using a validated health questionnaire that is universally recognised (for example, SF‐36). All continuous data should also be measured and reported as means and standard deviations so the statistical significance of the results can be established.
Future trials should also aim to have adequate allocation concealment and blinding methods, as well as improve on their reporting of random sequence generation. More trials that investigate each of the comparisons discussed in this review are also needed as, more often than not, the cause for insufficient evidence was a lack of trials. The most common surgical procedures in this review were transurethral resection of prostate and vaginal hysterectomy. Future research should aim to include trial populations who have not undergone a surgical procedure or involve types of surgeries that are not already seen in this review. This will allow a better understanding of the effects of short‐term catheter removal in both surgical and non‐surgical patients. Future considerations should also involve mixed populations to help ascertain whether the strategies discussed benefit both sexes equally, or whether it favours one sex over another.
What's new
| Date | Event | Description |
|---|---|---|
| 21 June 2021 | New search has been performed | This update, published in 2021, includes the following changes.
1. The search was updated to March 2020 and a further 73 trials have been included (taking the total of included trials to 99): Ahmed 2014; Alessandri 2006; Allen 2016; Alonzo‐Sosa 1997; Aref 2020; Aslam 2019; Azarkish 2003; Azarkish 2005; Barone 2015; Basbug 2020; Bristoll 1989; Carpiniello 1988; Carter‐Brooks 2018; Chai 2011; Chen 2013; Chia 2009; Cornia 2003; Coyle 2015; Dunn 1999; Dunn 2000b; Durrani 2014; El‐Mazny 2014; Glavind 2007; Gong 2017; Gross 2007; Gungor 2014; Hall 1998; Han 1997; Hewitt 2001; Huang 2011; Jang 2012; Jeong 2014; Joshi 2014; Jun 2011; Kamilya 2010; Kim 2012; Kokabi 2009; Lang 2020; Li 2014; Liang 2009; Lista 2020; Liu 2015; Mao 1994; Matsushima 2015; Naguimbing‐Cuaresma 2007; Nathan 2001; Nguyen 2012; Nyman 2010; Onile 2008; Ouladsahebmadarek 2012; Pervaiz 2019; Popiel 2017; Rajan 2017; Ruminjo 2015; Sahin 2011; Sandberg 2019; Schiotz 1995; Sekhavat 2008; Shahnaz 2016; Shrestha 2013; Souto 2004; Tahmin 2011; Talreja 2016; Valero Puerta 1998; Vallabh‐Patel 2020; Weemhoff 2011; Wu 2015; Yaghmaei 2017; Yee 2015; Zaouter 2009; Zhou 2012; Zmora 2010; Zomorrodi 2018. 2. We revised the outcomes in line with the GRADE recommendations in order to include outcomes deemed important for clinical and patient decision‐making. 3. We performed post‐hoc subgroup analysis for or one outcome in one comparison to assess whether the use of prophylactic antibiotics would impact the number of participants developing symptomatic catheter‐associated urinary tract infection. We also performed post hoc subgroup analysis for length of hospitalisation in one comparison to explore possible reasons for very high heterogeneity. 4. The review was substantially updated in accordance with current Cochrane methodology, including performing a risk of bias assessment on all 99 included trials and adopting the GRADE approach for assessing the certainty of evidence. |
| 21 June 2021 | New citation required but conclusions have not changed | The review has been updated; however, the conclusions did not change. |
History
Protocol first published: Issue 1, 2003 Review first published: Issue 1, 2005
| Date | Event | Description |
|---|---|---|
| 13 October 2008 | Amended | Converted to new review format. |
| 21 February 2007 | New citation required and conclusions have changed | Substantive amendment. Update Issue 2, 2007. Twenty‐six trials (eight new) involving a total of 2933 participants were included in this first update of the review. One trial (Guzman 1994) included three treatment groups. Eleven (three new) compared late night versus early morning removal of catheters (Chillington 1992; Crowe 1994; Ganta 2005; Ind 1993; Kelleher 2002; Lyth 1997; McDonald 1999; Noble 1990; Webster 2006; Wilson 2000; Wyman 1987); thirteen (five new) compared various durations of catheterisation (Benoist 1999; Dunn 2003; Guzman 1994; Hakvoort 2004; Hansen 1984; Irani 1995; Koh 1994; Lau 2004; Nielson 1985; Schiotz 1996; Sun 2004; Taube 1989; Toscano 2001); and three (Guzman 1994; Oberst 1981; Williamson 1982) compared clamping to free drainage. |
Acknowledgements
For this version, published in 2021, the review authors would like to acknowledge valuable comments from Dwayne Boyers, Suzanne Hagen, Esther Martin, Cathy Murphy, Robert Pickard, Jacqui Prieto, Marilyn Walsh and Luke Vale. We are also grateful to Sheila Wallace for running searches for the review and to Euan Fisher and A/Prof Jae Hung Jung for their assistance in the translation of review papers. We are also grateful to all translators who contributed to the review.
The review authors would like to acknowledge the contributions of Rhonda Griffiths and Ritin Fernandez to the previous versions of the review (Fernandez 2003b; Griffiths 2005; Griffiths 2007).
Appendices
Appendix 1. Plain language medical glossary
Abscess: a collection of pus
Alpha‐blocker: medication used to relax muscle or blood vessels
Antimicrobials: a substance that kills or stops the growth of potentially harmful tiny organisms that can only be seen under a microscope
Bacteruria: bacteria in the urine
Cystitis: inflammation of the bladder wall
Detrusor: the outer muscular structure of the bladder wall
Dysuria: difficult or painful passage of urine
Flank: the fleshy part of the body between the ribs and hip bone
Haematuria: blood in the urine
Haemorrhage: excessive bleeding
Incontinence: involuntary leakage of urine
In situ: in place
Loin: the part of the body on either side of the spine between the ribs and hip bone
Lumen: the walls of a urethral catheter
Meatal: relating to a body passage (in this case the urethra or passage to the bladder)
Morbidity: the rate of sickness
Perioperative: occurring around the time of surgery
Prostatitis: inflammation of the prostate
Radical prostatectomy: complete surgical removal of the prostate
Rigors: shivering from the chills
Stricture: the narrowing of a bodily structure
Suprapubic: above the pelvic area
Urodynamic trials: tests assessing the bladder and urethra’s ability to store and pass urine
Urological: relating to the organs responsible for making and passing urine
Appendix 2. Search strategies for the 2021 update of the review
Cochrane Incontinence Specialised Register
We searched the Cochrane Incontinence Specialised Register using the Group's own keyword system. The date of the last search was: 17 March 2020. The search terms used were:
(design.rct* or design.cct*) AND (intvent.mech.cath* OR intvent.mech.device* OR intvent.mech.sheaths. OR intvent.prevent.antibiotics* OR intvent.prevent.antinfect.* OR intvent.prevent.cath* OR intvent.prevent.cleaning fluids* OR intvent.prevent.surg* OR intvent.surg.intraoperativemanagement* OR intvent.surg.postsurgman* OR intvent.surg.presurgman*. OR intvent.surg.urethrotomy.)
All searches were of the keywords field of EndNote 2018.
Appendix 3. CINAHL search strategy used for an earlier update of this version of the review
CINAHL (on EBSCO) covering December 1981 to 11 May 2016 (searched on 12 May 2016). For the 2020 update of the search, this search was incorporated into the search for the Cochrane Incontinence Specialised Register and was not searched separately. The search strategy used is given below:
| # | Query |
| S29 | (S23 AND S28) |
| S28 | S24 OR S25 OR S26 OR S27 |
| S27 | TI urin* N6 catheter* OR AB urin* N6 catheter* |
| S26 | (MH "Catheter Removal") OR (MH "Sheath Removal") OR (MH "Urinary Catheter Care (Saba CCC)") OR (MH "Urinary Catheter Insertion (Saba CCC)") OR (MH "Urinary Catheter Irrigation (Saba CCC)") OR (MH "Urinary Tract Infections, Catheter‐Related") OR (MH "Urinary Catheterization+") OR (MH "Catheters, Urinary+") |
| S25 | (MH "Catheter Occlusion") |
| S24 | (MH "Catheter Care, Urinary+") |
| S23 | S1 or S2 or S3 or S4 or S5 or S6 or S7 or S8 or S9 or S10 or S11 or S12 or S13 or S14 or S15 or S16 or S17 or S18 or S19 or S20 or S21 or S22 |
| S22 | TI ( singl* N25 blind* OR singl* N25 mask* OR doubl* N25 blind* or doubl* N25 mask* OR trebl* N25 blind* OR trebl* N25 mask*OR tripl* N25 blind* OR tripl* N25 mask* ) or AB ( singl* N25 blind* OR singl* N25 mask* OR doubl* N25 blind* or doubl* N25 mask* OR trebl* N25 blind* OR trebl* N25 mask*OR tripl* N25 blind* OR tripl* N25 mask* ) |
| S21 | (MH "Comparative Studies") |
| S20 | (MH "Clinical Research+") |
| S19 | (MH "Static Group Comparison") |
| S18 | (MH "Quantitative Studies") |
| S17 | (MH "Crossover Design") or (MH "Solomon Four‐Group Design") |
| S16 | (MH "Factorial Design") |
| S15 | (MH "Community Trials") |
| S14 | (MH "Random Sample") |
| S13 | TI balance* N2 block* or AB balance* N2 block* |
| S12 | TI "latin square" or AB "latin square" |
| S11 | TI factorial or AB factorial |
| S10 | TI clin* N25 trial* or AB clin* N25 trial* |
| S9 | (MH "Study Design") |
| S8 | (AB random*) OR (TI random*) |
| S7 | (AB placebo*) OR (TI placebo*) |
| S6 | (MH "Placebos") |
| S5 | (PT Clinical Trial) OR (PT "randomized controlled trial") |
| S4 | (MH "Clinical Trials+") |
| S3 | MH (random assignment) OR (crossover design) |
| S2 | cross‐over |
| S1 | crossover |
Key: * = truncation; AB = abstract TI = title; PT = publication type; MH = major subject heading; N = near (adjacency) eg N6 means within 6 words, in any order.
Appendix 4. Details of the additional searches conducted for previous versions of this review
Please find below details of the searches for the previous version of this review (Griffiths 2007).
Cochrane Incontinence Specialised Register
The terms used to search the Cochrane Incontinence Specialised Register are given below:
({design.cct*} OR {design.rct*}) AND {topic.mech.cath*}
All searches were of the keyword field of Reference Manager 2002. Searched: 7 December 2005.
Electronic bibliographic databases
The following electronic bibliographic databases were searched.
Cochrane Central Register of Controlled Trials (CENTRAL; 2006, Issue 2), (on web, Update Software site, via OVID in July 2006) using the following search strategy:
1. Urin* 2. Ureth* 3. (1 or 2) 4. Cath* 5. (3 and 4) 6. Time 7. Morn* 8. Night 9. Dawn 10. Dusk 11. Evening 12. Afternoon 13. Noon 14. Day 15. 6AM 16. (6 or 7 or 8 or 9 or 10 or 11 or 12 or 13 or 14 or 15) 17. (5 and 16) 18. Suprapubic 19. (17 not 18) 20. Removal 21. (19 and 20) Key: * = truncation symbol.
MEDLINE (via Ovid) (years searched: January 1966 to 12 July 2006) using the following search terms:
1. urinary catheterization/ or catheter, urinary/ 2. (catheter$ and (urin$ or urethra$)).mp. 3. 1 or 2 4. (remov$ or withdraw$).mp. 5. Time Factors/ 6. (time or timing or morning$ or afternoon$ or evening$ or night$ or day$).mp. 7. 5 or 6 8. 3 and 4 and 7 Key: / = MeSH term with all subheadings; $ = truncation symbol; mp = map, searches a number of fields including MeSH terms and textwords in titles and abstracts
EMBASE (via Ovid) (years searched: January 1980 to 12 July 2006) using the following search terms:
1. urinary catheterization/ or catheter, urinary/ 2. (catheter$ and (urin$ or urethra$)).mp. 3. 1 or 2 4. (remov$ or withdraw$).mp. 5. Time Factors/ 6. (time or timing or morning$ or afternoon$ or evening$ or night$ or day$).mp. 7. 5 or 6 8. 3 and 4 and 7 Key: / = MeSH term with all subheadings; $ = truncation symbol; mp = map, searches a number of fields including EMTREE terms and textwords in titles and abstracts
CINAHL (via Ovid) (years searched: January 1982 to 12 July 2006) using the following search terms:
1. urinary catheterization/ or catheter, urinary/ 2. (catheter$ and (urin$ or urethra$)).mp. 3. 1 or 2 4. (remov$ or withdraw$).mp. 5. Time Factors/ 6. (time or timing or morning$ or afternoon$ or evening$ or night$ or day$).mp. 7. 5 or 6 8. 3 and 4 and 7 Key: / = MeSH term with all subheadings; $ = truncation symbol; mp = map, searches a number of fields including CINAHL subject terms and textwords in titles and abstracts
Nursing Collection Journals @ OVID (years searched: January 1995 to January 2002) using the following search terms:
1. urinary catheterization/ or catheter, urinary/ 2. (catheter$ and (urin$ or urethra$)).mp. 3. 1 or 2 4. (remov$ or withdraw$).mp. 5. Time Factors/ 6. (time or timing or morning$ or afternoon$ or evening$ or night$ or day$).mp. 7. 5 or 6 8. 3 and 4 and 7 Key: / = MeSH term with all subheadings; $ = truncation symbol; mp = map, searches a number of fields including textwords in titles and abstracts
Conference proceedings
The following conference proceedings were scanned briefly:
International Continence Society (ICS), Annual Meeting (1995 to 2000 inclusive);
International Urogynecological Association (IUGA), Annual Meeting (2000 and 2001);
Hong Kong Urological Association, Annual Meeting (1995 to 2001 inclusive).
Other sources
The reference lists of relevant articles were searched for other possible relevant trials. Manufacturers, researchers and experts in the field were contacted to ask for other possibly relevant trials, published or unpublished.
We did not impose any language or other limits on any of the searches described above.
Appendix 5. Shorter duration versus longer duration of catheter use
Outcomes not mentioned in Types of outcome measures
Frequency of micturition
Two trials reported data on the frequency of micturition (Ahmed 2014; El‐Mazny 2014). Participants who had their catheters removed immediately tended to micturate more frequently than those who had their catheters removed later (RR 0.18, 95% CI 0.06 to 0.53; I² = 0%; 2 trials; 521 participants; Analysis 2.25).
2.25. Analysis.

Comparison 2: Shorter versus longer duration of catheter, Outcome 25: Frequency of micturition
Time to first ambulation (hours)
Nine trials provided data on time to first ambulation (Ahmed 2014; Alessandri 2006; Aref 2020; El‐Mazny 2014; Naguimbing‐Cuaresma 2007; Onile 2008; Ouladsahebmadarek 2012; Sekhavat 2008; Yaghmaei 2017). Immediate removal of the indwelling urethral catheters compared to later resulted in a shorter time to first ambulation of participants (MD −5.06, 95% CI −5.24 to −4.88; I2 = 99%; 9 trials, 1688 participants; Analysis 2.26). There was significant heterogeneity between the trials, which we think is most likely due to the differing types of surgeries the participants received. As a result, we would recommend these data to be interpreted with caution.
2.26. Analysis.

Comparison 2: Shorter versus longer duration of catheter, Outcome 26: Time to first ambulation (hours)
Appendix 6. Clamping versus free drainage before catheter removal
Outcomes not mentioned in Types of outcome measures
Time required to return to normal bladder function
One trial reported this outcome (Nyman 2010). The data were presented in medians and interquartile range and therefore we could not perform meta‐analysis (Analysis 3.10).
3.10. Analysis.
Comparison 3: Clamping versus free drainage, Outcome 10: Time required to return to normal bladder function (hours)
| Time required to return to normal bladder function (hours) | ||
| Study | Clamping | Free Drainage |
| Nyman 2010 | 6 (4‐8); median (quartiles) | 4 (3‐7.25); median (quartiles) |
Data and analyses
Comparison 1. Removal of indwelling urethral catheter at one specified time of day (10 pm to midnight) versus another specified time of day (6 am to 7 am).
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1.1 Number needing to be recatheterised | 10 | 1920 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.70 [0.52, 0.94] |
| 1.1.1 Urological surgery and procedures | 6 | 1400 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.87 [0.60, 1.27] |
| 1.1.2 Gynaecological surgery | 2 | 202 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.33 [0.15, 0.69] |
| 1.1.3 General medical and surgical patients | 2 | 318 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.73 [0.36, 1.46] |
| 1.2 Number needing to be recatheterised: subgroup analysis based on sex | 6 | 1200 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.44 [0.25, 0.76] |
| 1.2.1 Men only | 4 | 998 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.63 [0.28, 1.44] |
| 1.2.2 Women only | 2 | 202 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.33 [0.15, 0.69] |
| 1.3 Symptomatic catheter‐associated urinary tract infection (number of participants) | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Totals not selected | |
| 1.3.1 General medical and surgical patients | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Totals not selected | |
| 1.4 Asymptomatic bacteriuria (number of participants) | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Totals not selected | |
| 1.4.1 Gynaecological surgery | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Totals not selected | |
| 1.5 Incidence of urinary retention | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Totals not selected | |
| 1.5.1 General medical and surgical patients | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Totals not selected | |
| 1.6 Difficulty in passing urine | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Totals not selected | |
| 1.6.1 General medical and surgical patients | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Totals not selected | |
| 1.7 Loin pain | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Totals not selected | |
| 1.7.1 General medical and surgical patients | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Totals not selected | |
| 1.8 Fever | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Totals not selected | |
| 1.8.1 General medical and surgical patients | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Totals not selected | |
| 1.9 Incontinence | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Totals not selected | |
| 1.9.1 General medical and surgical patients | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Totals not selected | |
| 1.10 Dysuria (number of participants) | 1 | 170 | Risk Ratio (M‐H, Fixed, 95% CI) | 2.20 [0.70, 6.86] |
| 1.10.1 General medical and surgical patients | 1 | 170 | Risk Ratio (M‐H, Fixed, 95% CI) | 2.20 [0.70, 6.86] |
| 1.11 Volume of the first void (mL) | 11 | 1198 | Mean Difference (IV, Fixed, 95% CI) | 21.98 [3.04, 40.92] |
| 1.11.1 Urological surgery and procedures | 8 | 923 | Mean Difference (IV, Fixed, 95% CI) | 11.63 [‐10.65, 33.91] |
| 1.11.2 Gynaecological surgery | 1 | 107 | Mean Difference (IV, Fixed, 95% CI) | 34.00 [‐11.30, 79.30] |
| 1.11.3 General medical and surgical patients | 2 | 168 | Mean Difference (IV, Fixed, 95% CI) | 74.54 [15.35, 133.73] |
| 1.12 Volume of first void (median and range) | 1 | Other data | No numeric data | |
| 1.12.1 Following gynaecological surgery | 1 | Other data | No numeric data | |
| 1.13 Time to first void (hours) | 10 | 1140 | Mean Difference (IV, Fixed, 95% CI) | 0.71 [0.41, 1.01] |
| 1.13.1 Urological surgery and procedures | 6 | 703 | Mean Difference (IV, Fixed, 95% CI) | 0.72 [0.39, 1.06] |
| 1.13.2 Gynaecological surgery | 1 | 107 | Mean Difference (IV, Fixed, 95% CI) | 0.10 [‐1.46, 1.66] |
| 1.13.3 General medical and surgical patients | 3 | 330 | Mean Difference (IV, Fixed, 95% CI) | 0.79 [0.02, 1.57] |
| 1.14 Time to first void (median) | 1 | Other data | No numeric data | |
| 1.14.1 Following gynaecological surgery | 1 | Other data | No numeric data | |
| 1.15 Post‐void residual volume | 1 | Mean Difference (IV, Fixed, 95% CI) | Totals not selected | |
| 1.15.1 General medical and surgical patients | 1 | Mean Difference (IV, Fixed, 95% CI) | Totals not selected | |
| 1.16 Length of hospitalisation in days | 1 | Mean Difference (IV, Fixed, 95% CI) | Totals not selected | |
| 1.16.1 Gynaecological surgery | 1 | Mean Difference (IV, Fixed, 95% CI) | Totals not selected | |
| 1.17 Length of hospitalisation in days | 2 | Other data | No numeric data | |
| 1.17.1 Urological surgery and procedures (mean, total) | 1 | Other data | No numeric data | |
| 1.17.2 Gynaecological surgery involving the bladder /urethra (median, range) | 1 | Other data | No numeric data | |
| 1.17.3 Gynaecological surgery not involving the bladder/urethra (median, range) | 1 | Other data | No numeric data | |
| 1.18 Time between removal of catheter to discharge | 2 | 272 | Mean Difference (IV, Fixed, 95% CI) | 0.08 [‐5.96, 6.12] |
| 1.18.1 Urological surgery and procedures | 1 | 72 | Mean Difference (IV, Fixed, 95% CI) | 0.00 [‐6.06, 6.06] |
| 1.18.2 General medical and surgical patients | 1 | 200 | Mean Difference (IV, Fixed, 95% CI) | 15.50 [‐67.34, 98.34] |
Comparison 2. Shorter versus longer duration of catheter.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 2.1 Number needing to be recatheterised | 44 | 5870 | Risk Ratio (M‐H, Random, 95% CI) | 1.81 [1.35, 2.41] |
| 2.1.1 Early removal versus later | 19 | 2528 | Risk Ratio (M‐H, Random, 95% CI) | 2.59 [1.47, 4.57] |
| 2.1.2 1‐day policy versus later | 16 | 1874 | Risk Ratio (M‐H, Random, 95% CI) | 1.45 [0.93, 2.25] |
| 2.1.3 2‐day to 7‐day policy versus later | 10 | 1468 | Risk Ratio (M‐H, Random, 95% CI) | 1.67 [0.93, 2.99] |
| 2.2 Number needing to be recatheterised: subgroup analysis based on type of surgery | 37 | 4736 | Risk Ratio (M‐H, Random, 95% CI) | 1.85 [1.36, 2.51] |
| 2.2.1 Urological surgery | 9 | 1104 | Risk Ratio (M‐H, Random, 95% CI) | 0.91 [0.50, 1.67] |
| 2.2.2 Gynaecological surgery | 24 | 2935 | Risk Ratio (M‐H, Random, 95% CI) | 2.25 [1.58, 3.22] |
| 2.2.3 Obstetric surgery | 4 | 697 | Risk Ratio (M‐H, Random, 95% CI) | 3.36 [0.93, 12.15] |
| 2.3 Number needing to be recatheterised: subgroup analysis based on sex | 37 | 4736 | Risk Ratio (M‐H, Random, 95% CI) | 1.85 [1.36, 2.51] |
| 2.3.1 Men only | 9 | 1104 | Risk Ratio (M‐H, Random, 95% CI) | 0.91 [0.50, 1.67] |
| 2.3.2 Women only | 28 | 3632 | Risk Ratio (M‐H, Random, 95% CI) | 2.29 [1.64, 3.18] |
| 2.4 Number needing to be recatheterised: subgroup analysis based on antibiotic prophylaxis | 27 | 3839 | Risk Ratio (M‐H, Random, 95% CI) | 1.72 [1.11, 2.65] |
| 2.4.1 Antibiotic prophylaxis given | 22 | 3040 | Risk Ratio (M‐H, Random, 95% CI) | 1.73 [1.04, 2.89] |
| 2.4.2 No antibiotic prophylaxis | 5 | 799 | Risk Ratio (M‐H, Random, 95% CI) | 1.65 [0.70, 3.86] |
| 2.5 Symptomatic catheter‐associated urinary tract infection (number of participants) | 41 | 5759 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.52 [0.45, 0.61] |
| 2.5.1 Early versus later | 17 | 2220 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.55 [0.43, 0.71] |
| 2.5.2 1 day versus later | 15 | 1879 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.48 [0.37, 0.62] |
| 2.5.3 2 to 7 days versus later | 9 | 1660 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.55 [0.39, 0.78] |
| 2.6 Symptomatic catheter‐associated urinary tract infection: post‐hoc subgroup analysis by antibiotic prophylaxis | 24 | 3516 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.47 [0.38, 0.59] |
| 2.6.1 Antibiotic prophylaxis | 20 | 2871 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.49 [0.39, 0.62] |
| 2.6.2 No antibiotic prophylaxis given | 4 | 645 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.28 [0.11, 0.72] |
| 2.7 Asymptomatic bacteruria (number of participants) | 18 | 2611 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.47 [0.38, 0.58] |
| 2.7.1 Early versus later | 10 | 1461 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.59 [0.45, 0.77] |
| 2.7.2 1 day versus later | 6 | 683 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.37 [0.26, 0.54] |
| 2.7.3 2 to 7 days versus later | 3 | 467 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.32 [0.18, 0.59] |
| 2.8 Incidence of urinary retention | 19 | Risk Ratio (M‐H, Random, 95% CI) | Subtotals only | |
| 2.8.1 Early versus later | 7 | 1108 | Risk Ratio (M‐H, Random, 95% CI) | 1.07 [0.57, 2.00] |
| 2.8.2 1 day versus later | 7 | 680 | Risk Ratio (M‐H, Random, 95% CI) | 1.36 [1.03, 1.81] |
| 2.8.3 2 to 7 days versus later | 6 | 881 | Risk Ratio (M‐H, Random, 95% CI) | 1.37 [0.88, 2.12] |
| 2.9 Delayed voiding after catheter removal | 2 | 176 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.02 [0.53, 1.97] |
| 2.9.1 1 day versus later | 2 | 176 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.02 [0.53, 1.97] |
| 2.10 Chronic urinary retention | 2 | 339 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.84 [0.29, 2.44] |
| 2.10.1 1‐day policy versus later | 2 | 230 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.81 [0.26, 2.59] |
| 2.10.2 2 to 7 days versus later | 1 | 109 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.02 [0.07, 15.87] |
| 2.11 Other complications of catheterisation: fever | 2 | 470 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.17 [0.40, 3.40] |
| 2.11.1 Early versus later | 2 | 470 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.17 [0.40, 3.40] |
| 2.12 Other complications of catheterisation: epididymitis | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Totals not selected | |
| 2.12.1 2 to 7 days versus later | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Totals not selected | |
| 2.13 Pain or discomfort (dichotomous) | 5 | 510 | Risk Ratio (M‐H, Random, 95% CI) | 0.52 [0.21, 1.27] |
| 2.13.1 Immediate post‐op removal versus removal 24 hours post‐op | 3 | 230 | Risk Ratio (M‐H, Random, 95% CI) | 0.31 [0.04, 2.64] |
| 2.13.2 Removal 4 hours post‐op versus removal 24 hours post‐op | 1 | 240 | Risk Ratio (M‐H, Random, 95% CI) | 1.00 [0.78, 1.29] |
| 2.13.3 Removal 3 days post‐op versus removal 28 days post‐op | 1 | 40 | Risk Ratio (M‐H, Random, 95% CI) | 0.20 [0.01, 3.92] |
| 2.14 Pain or discomfort: 0‐10 VAS (higher score = greater pain) | 5 | 695 | Mean Difference (IV, Fixed, 95% CI) | ‐0.34 [‐0.47, ‐0.20] |
| 2.14.1 Removal 4 hours post‐op versus removal at 6am 1 day post‐op | 1 | 57 | Mean Difference (IV, Fixed, 95% CI) | ‐0.60 [‐1.65, 0.45] |
| 2.14.2 Immediate post‐op removal versus removal 24 hours post‐op | 3 | 433 | Mean Difference (IV, Fixed, 95% CI) | ‐0.37 [‐0.52, ‐0.23] |
| 2.14.3 Immediate removal post‐op versus removal 3‐5 days post‐op | 1 | 205 | Mean Difference (IV, Fixed, 95% CI) | 0.10 [‐0.37, 0.56] |
| 2.15 Patient satisfaction | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Totals not selected | |
| 2.16 Urinary incontinence | 7 | 1195 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.55 [0.35, 0.86] |
| 2.16.1 Early versus later | 2 | 396 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.13 [0.03, 0.55] |
| 2.16.2 2 to 7 days versus later | 5 | 799 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.82 [0.52, 1.32] |
| 2.17 Dysuria | 7 | 1398 | Risk Ratio (M‐H, Random, 95% CI) | 0.42 [0.20, 0.88] |
| 2.17.1 Early versus later | 7 | 1398 | Risk Ratio (M‐H, Random, 95% CI) | 0.42 [0.20, 0.88] |
| 2.18 Volume of first void (mL) | 3 | 364 | Mean Difference (IV, Fixed, 95% CI) | 27.02 [1.00, 53.04] |
| 2.18.1 Early removal versus later | 1 | 227 | Mean Difference (IV, Fixed, 95% CI) | 12.00 [‐21.97, 45.97] |
| 2.18.2 2‐day to 7‐day policy versus later | 2 | 137 | Mean Difference (IV, Fixed, 95% CI) | 48.36 [7.88, 88.84] |
| 2.19 Time to first void (hours) | 2 | 277 | Mean Difference (IV, Random, 95% CI) | ‐8.59 [‐16.16, ‐1.01] |
| 2.19.1 Early removal versus later | 2 | 277 | Mean Difference (IV, Random, 95% CI) | ‐8.59 [‐16.16, ‐1.01] |
| 2.20 Post‐void residual volume (mL) | 2 | 137 | Mean Difference (IV, Fixed, 95% CI) | 6.37 [‐9.14, 21.88] |
| 2.20.1 2‐day to 7‐day policy versus later | 2 | 137 | Mean Difference (IV, Fixed, 95% CI) | 6.37 [‐9.14, 21.88] |
| 2.21 Post‐void residual volume (median and range) (mL) | 1 | Other data | No numeric data | |
| 2.22 Length of hospitalisation in days | 27 | Mean Difference (IV, Random, 95% CI) | Subtotals only | |
| 2.22.1 Early removal versus later | 13 | 2012 | Mean Difference (IV, Random, 95% CI) | ‐0.54 [‐0.82, ‐0.27] |
| 2.22.2 1‐day policy versus later | 10 | 1249 | Mean Difference (IV, Random, 95% CI) | ‐1.66 [‐2.25, ‐1.07] |
| 2.22.3 2‐day to 7‐day policy versus later | 5 | 474 | Mean Difference (IV, Random, 95% CI) | ‐5.00 [‐5.89, ‐4.11] |
| 2.23 Length of hospitalisation in days: subgrouping based on type of surgery | 27 | 3735 | Mean Difference (IV, Random, 95% CI) | ‐1.13 [‐1.42, ‐0.83] |
| 2.23.1 Urological procedures | 7 | 1005 | Mean Difference (IV, Random, 95% CI) | ‐3.40 [‐4.75, ‐2.05] |
| 2.23.2 Gynaecological procedures | 13 | 1453 | Mean Difference (IV, Random, 95% CI) | ‐0.92 [‐1.33, ‐0.51] |
| 2.23.3 Obstetric procedures | 6 | 1217 | Mean Difference (IV, Random, 95% CI) | ‐0.50 [‐0.87, ‐0.13] |
| 2.23.4 General surgical procedures | 1 | 60 | Mean Difference (IV, Random, 95% CI) | ‐1.10 [‐2.77, 0.57] |
| 2.24 Length of hospitalisation in days (median and range) | 6 | Other data | No numeric data | |
| 2.25 Frequency of micturition | 2 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 2.25.1 Early versus later | 2 | 521 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.18 [0.06, 0.53] |
| 2.26 Time to first ambulation (hours) | 9 | 1688 | Mean Difference (IV, Fixed, 95% CI) | ‐5.06 [‐5.24, ‐4.88] |
| 2.26.1 Early versus later | 9 | 1688 | Mean Difference (IV, Fixed, 95% CI) | ‐5.06 [‐5.24, ‐4.88] |
Comparison 3. Clamping versus free drainage.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 3.1 Number needing to be recatheterised | 5 | 569 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.82 [0.55, 1.21] |
| 3.1.1 Clamping versus removal at 48 hours | 2 | 311 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.94 [0.51, 1.71] |
| 3.1.2 Clamping versus removal at 72 hours or longer | 3 | 258 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.73 [0.44, 1.21] |
| 3.2 Number needing to be recatheterised: subgroup analysis based on type of surgery and sex | 5 | 569 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.82 [0.55, 1.21] |
| 3.2.1 Gynaecological surgery (women) | 2 | 267 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.92 [0.48, 1.77] |
| 3.2.2 Non‐gynaecological surgery (men and women) | 3 | 302 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.76 [0.47, 1.23] |
| 3.3 Symptomatic catheter‐associated urinary tract infection (number of participants) | 2 | 267 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.99 [0.60, 1.63] |
| 3.3.1 Clamping versus removal at 48 hours | 1 | 198 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.13 [0.65, 1.95] |
| 3.3.2 Clamping versus removal at 72 hours or longer | 1 | 69 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.55 [0.15, 2.01] |
| 3.4 Incidence of urinary retention (number of participants) | 2 | 169 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.18 [0.69, 2.02] |
| 3.4.1 Clamping versus removal at 24 hours | 1 | 100 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.86 [0.31, 2.37] |
| 3.4.2 Clamping versus removal at 72 hours or longer | 1 | 69 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.39 [0.74, 2.61] |
| 3.5 Dysuria (number of participants) | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Totals not selected | |
| 3.5.1 Clamping versus removal at 72 hours or longer | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Totals not selected | |
| 3.6 Volume of first void (mL) | 1 | Mean Difference (IV, Fixed, 95% CI) | Totals not selected | |
| 3.6.1 Clamping versus removal at 72 hours or longer | 1 | Mean Difference (IV, Fixed, 95% CI) | Totals not selected | |
| 3.7 Time to first void (minutes) | 2 | Mean Difference (IV, Fixed, 95% CI) | Totals not selected | |
| 3.7.1 Clamping versus removal at 24 hours | 1 | Mean Difference (IV, Fixed, 95% CI) | Totals not selected | |
| 3.7.2 Clamping versus removal at 72 hours or longer | 1 | Mean Difference (IV, Fixed, 95% CI) | Totals not selected | |
| 3.8 Length of hospitalisation (median days) | 1 | Other data | No numeric data | |
| 3.9 Length of hospitalisation (days) | 1 | Mean Difference (IV, Fixed, 95% CI) | Totals not selected | |
| 3.9.1 Clamping versus removal at 48 hours | 1 | Mean Difference (IV, Fixed, 95% CI) | Totals not selected | |
| 3.10 Time required to return to normal bladder function (hours) | 1 | Other data | No numeric data |
Comparison 4. Prophylactic use of alpha blocker versus no drug or intervention.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 4.1 Number of participants needing to be recatheterised | 2 | 184 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.18 [0.58, 2.42] |
| 4.2 Symptomatic catheter‐associated urinary tract infection (number of participants) | 1 | 94 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.20 [0.01, 4.06] |
| 4.3 Incidence of urinary retention (number of participants) | 2 | 308 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.38 [0.20, 0.73] |
| 4.4 Post‐void residual volume | 2 | 301 | Mean Difference (IV, Fixed, 95% CI) | ‐2.00 [‐11.42, 7.42] |
| 4.5 Length of hospitalisation in days | 1 | Mean Difference (IV, Fixed, 95% CI) | Totals not selected | |
| 4.6 Length of hospitalisation in days (median, range, N) | 1 | Other data | No numeric data |
Characteristics of studies
Characteristics of included studies [ordered by study ID]
Ahmed 2014.
| Study characteristics | ||
| Methods |
Study design: RCT Dates study conducted: April 2010‐December 2012 |
|
| Participants |
Number of participants: 233 eligible; 221 randomised; 221 reported Setting: Ismailia Country: Egypt Population: women Age (mean (SD)): A 59.1 (8.3); B 58.3 (6.9); C 61.3 (10.5) Inclusion criteria: women undergoing total abdominal hysterectomy with or without bilateral salpingo‐oophorectomy for various benign gynaecological diseases (uterine fibroids, abnormal uterine bleeding) Condition for hospitalisation: hysterectomy Exclusion criteria: known history of neurological disorders, women who had UTI pre‐operatively confirmed by urine analysis ± culture and sensitivity, women for whom a complicated procedure was encountered during hysterectomy so that an IUC had to be kept post‐operatively on surgeon’s decision, women had spinal anaesthesia by choice or when general anaesthesia was contraindicated, women who had urge incontinence, women who refused to participate in study Use of antibiotic prophylaxis: on the morning of surgery, all participants received a single dose of prophylactic antibiotic in the form of ceftriaxone 1 g IM |
|
| Interventions |
A (n = 73): IUC removed immediately after surgery B (n = 81): IUC removed 6 h post‐operatively C (n = 67): IUC removed 24 h post‐operatively Size and type (e.g. silver‐coated/PTFE) of catheter used: 12F Foley catheter, latex Study definition of short‐term catheterisation (days): not reported Intended duration of catheterisation for each group: A: immediately after surgery; B: 6 h post‐operatively; C: 24 h post‐operatively |
|
| Outcomes | Urine retention and re‐catheterisation (%) Symptomatic UTI (%) Post‐operative urine culture First ambulation Hospital stay Urinary symptoms 1 week post‐operatively Fever Dysuria Frequency Urgency Loin pain Positive urine culture 1 week post‐operatively |
|
| Definition of CAUTI or bacteriuria | The diagnosis of symptomatic UTI was based on the following criteria: significant bacteriuria with at least one of the following symptoms; dysuria, frequency of micturition, urgency, suprapubic pain or burning sensation at micturition | |
| Sponsorship/funding | Not reported | |
| Ethical approval | The study was carried out in accordance to the ethical principles for medical research involving human subjects included in Helsinki declaration and was approved by the Suez Canal University (SCU) Ethical Committee. | |
| Notes | All participants had continuous bladder drainage during the surgery The time to ambulation was defined as the period between the end of surgery and the time when the patient first walked supported by a nurse or relative. The length of hospital stay was defined as the time between the end of surgery and hospital discharge Patients were recatheterised with a disposable female catheter if they were not able to empty their bladders 6 h after catheter removal. If unable to empty bladder 12 h after catheter removal, an indwelling catheter was inserted |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: “The remaining 221 women were divided into three groups by simple randomization using computer‐generated random numbers” Comment: adequate method of randomisation used |
| Allocation concealment (selection bias) | Unclear risk | Not reported Comment: probably not done |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Not reported. Comment: unlikely blinding was possible due to the intervention |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Not reported Comment: no information given. Outcomes such as urinary symptoms could be affected by detection bias |
| Blinding of microbiological outcome (detection bias) | Low risk | Not reported Comment: likely urine samples were sent to a laboratory where the microbiologist would not know which patients were in the trial |
| Incomplete outcome data (attrition bias) All outcomes | High risk | Quote: “Twelve patients were finally excluded from the study; five patients had intra‐operative complications (iatrogenic bladder injury)… while seven did not complete the postoperative follow‐up…” Comment: patients should have been analysed according to ITT analysis of patients lost to follow‐up. |
| Selective reporting (reporting bias) | Low risk | All pre‐specified outcomes have been accounted for in both the methods and results |
| Other bias | Low risk | Appears to be free from other sources of bias |
Alessandri 2006.
| Study characteristics | ||
| Methods |
Study design: RCT Dates study conducted: September 2003‐March 2004 |
|
| Participants |
Number of participants: 96 eligible; 96 randomised; 94 reported Setting: Genova Country: Italy Population: women Age (mean (SD), N): A 51 (4.3), 32; B 49 (3.7), 30; C 47 (5.0), 32 Inclusion criteria: women having hysterectomy for benign diseases (fibroids, abnormal uterine bleeding, and persistent cervical dysplasia) Condition for hospitalisation: vaginal hysterectomy Exclusion criteria: anticipated complicated surgical procedure (e.g. uterine prolapse, bladder suspension or colporrhaphy, diagnosis suspicious for malignant disease or severe endometriosis); recurrent UTIs (significant bacteriuria, determined by urine culture and defined as at least 105 cfu/mL of urine) and/or urinary incontinence; neurological disorders Use of antibiotic prophylaxis: women received a single dose of antibiotic prophylaxis before operation |
|
| Interventions |
A (n = 32): immediate removal of IUC in the operating room B (n = 30): removal of IUC at 6 h after the operation C (n = 32): removal of IUC at 12 h after the operation Size and type (e.g. silver‐coated/PTFE) of catheter used: 16F latex catheters with a 10 mL balloon Study definition of short‐term catheterisation (days): not reported Intended duration of catheterisation for each group (h): A: Duration of surgical procedure B: Duration of surgical procedure + 6 h C: Duration of surgical procedure + 12 h |
|
| Outcomes | Number of women requiring re‐catheterisation after operation Number of women with symptomatic UTIs Time to first ambulation Length of hospital stay |
|
| Definition of CAUTI or bacteriuria | Significant bacteriuria, determined by urine culture and defined as at least 105 cfu/mL of urine | |
| Sponsorship/funding | Not reported | |
| Ethical approval | Informed consent obtained. Protocol approved by hospital’s ethics committee | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: "Randomization was performed by using a computer‐generated randomization list drawn up by a statistician and concealed by keeping it with the nurse." Comment: adequate method of randomisation |
| Allocation concealment (selection bias) | Low risk | Quote: "Before entering the operating room, the surgeon received from the theatre nurse a sealed opaque envelope that contained the randomization assignment. In all cases, the envelope was opened at the end of the surgical procedure." Comment: adequate method of concealment |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Quote: "Not possible ‐ Although our findings are strengthened by the fact the surgeon was made aware of the randomization only at the end of the operation, a limitation of our study may consists in the fact that the observers of outcome were not blinded to the randomization" Comment: blinding of participants was not possible due to the intervention |
| Blinding of outcome assessment (detection bias) All outcomes | High risk | Quote: "the observers of outcome were not blinded to the randomization" Comment: blinding of outcome assessment not possible |
| Blinding of microbiological outcome (detection bias) | Low risk | Not reported Comment: likely samples were sent to a laboratory and thus unlikely that microbiologist knew which patient was in the study |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Quote: "Two patients in group B were excluded from the study because of the necessity of an unanticipated complicated surgical procedure (bladder suspension during VH)" Comment: low attrition and not differential |
| Selective reporting (reporting bias) | Low risk | All outcomes reported in methods and reported in full in the results section. However, protocol was not available for assessment |
| Other bias | Low risk | Appears to be free from other sources of bias |
Allen 2016.
| Study characteristics | ||
| Methods |
Study design: RCT Dates study conducted: February 2012‐August 2014 |
|
| Participants |
Number of participants: 374 eligible; 374 randomised (217 (58%) men and 157 (42%) women), 247 reported Setting: Minnesota Country: USA Population: mixed Age (median and range): median age 61.5 (21‐87); A 61.1 (31–85); B 61.7 (21–87) Inclusion criteria: patients undergoing a general thoracic surgical procedure, in whom an epidural catheter was placed for analgesia Condition for hospitalisation: general thoracic surgical procedure, in whom an epidural catheter was placed for analgesia Exclusion criteria: patients aged < 18 years, those who died in the hospital within 30 days of the operation, length of stay was < 48 h, epidural catheter was removed before post‐operative day 2, with suprapubic catheter or no bladder, required a urologist or a urologic technician to insert the IUC at the time of the operation, intermittently catheterised pre‐operatively, known UTI pre‐operatively, and who required the IUC to be kept in place because of the need for close monitoring of urinary output Use of antibiotic prophylaxis: not reported |
|
| Interventions |
Group A (n = 121): IUC removed within 48 h post‐op Group B (n = 126): IUC removed within 6 h after epidural removal Size and type and type of catheter used (e.g. Foley 16F): not reported Study definition of short‐term catheterisation (days): not reported Intended duration of catheterisation for each group: Group A: IUC removal within 48 h post‐op Group B: IUC removal 6 h after epidural removed |
|
| Outcomes | Number of participants requiring recatheterisation Incidence of UTI Length of hospitalisation |
|
| Definition of CAUTI or bacteriuria | Not reported | |
| Sponsorship/funding | Not reported | |
| Ethical approval | Not reported | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: “A computerized random number generator and double‐blinded envelope system were used to randomize patients 1:1 to removal of the urinary catheter within 48 hours of leaving the operating room or to removal 6 ± 4 hours after the epidural catheter was removed." Comment: computer randomisation used |
| Allocation concealment (selection bias) | Low risk | Quote: “A computerized random number generator and double‐blinded envelope system were used to randomize patients 1:1 to removal of the urinary catheter within 48 hours of leaving the operating room or to removal 6 ± 4 hours after the epidural catheter was removed." Comment: double‐blinded envelope system used |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Not reported. Unlikely this is possible |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Not reported |
| Blinding of microbiological outcome (detection bias) | Low risk | Not reported Comment: likely samples were sent to a laboratory and thus unlikely that microbiologist knew which patient was in the study |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | No ITT analysis, explanations given for data deemed ineligible for analysis but numbers not reported per randomised group |
| Selective reporting (reporting bias) | Low risk | Outcomes seem to be reported in full |
| Other bias | Low risk | Appears to be free from other sources of bias |
Alonzo‐Sosa 1997.
| Study characteristics | ||
| Methods |
Study design: RCT Dates study conducted: March‐November 1994 |
|
| Participants |
Number of participants: eligible, not reported; 50 randomised; 50 reported Country: Mexico Population: women Age (mean (range)): A 53.5 (37‐63); B 47.1 (37‐67) Inclusion criteria: women booked for elective corrective surgery of the pelvic floor (anterior colporrhaphy, anterior and posterior colporrhaphy with or without vaginal hysterectomy) Condition for hospitalisation: pelvic floor surgery with or without vaginal hysterectomy Exclusion criteria: patients with UTI were not included Use of antibiotic prophylaxis: "Antibiotic prophylaxis was not given" |
|
| Interventions |
Group A (n = 25): IUC for 1 day post‐op Group B (n = 25): IUC for 3 days post‐op Size of catheter used: 16F catheter Type of indwelling catheter: not reported Study definition of short‐term catheterisation (days): not reported Intended duration of catheterisation for each group (h): Group A: 1 day Group B: 3 days |
|
| Outcomes | AUR/number needing re‐catheterisation (%) UTI (%) Duration of catheterisation Length of hospital stay |
|
| Definition of CAUTI or bacteriuria | A positive urine sample was defined as the presence of > 100,000 cfu/mL if taken mid‐stream and 10,000 cfu/mL in a sample taken by catheterisation. UTI was defined as positive sample associated with dysuria, polyuria, incomplete emptying, pain, fever or sepsis. Asymptompatic bacteriuria was defined as a positive sample in the absence of symptoms. |
|
| Sponsorship/funding | Not reported | |
| Ethical approval | Not reported | |
| Notes | "After removing the catheter, the volume of residual urine was measured and if greater than 50mL was considered urinary retention and another foley catheter was inserted, with patients removed from the study and brought to restoration of normal bladder function" | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Quote: "Randomised controlled blinded clinical trial ..." Comment: unclear as to how randomisation was performed |
| Allocation concealment (selection bias) | Unclear risk | Not reported Comment: unclear as to whether allocation concealment was performed |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Quote: "Randomised controlled blinded clinical trial ..." Comment: unclear as to who was blinded and how blinding was performed. Unlikely blinding was possible due to the type of intervention |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Not reported |
| Blinding of microbiological outcome (detection bias) | Low risk | Not reported Comment: assume microbiologist would be blinded as samples would be sent to a laboratory where the microbiologist would not know which patient belonged to the trial. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | No evidence of incomplete outcome data bias |
| Selective reporting (reporting bias) | Low risk | No evidence of selective reporting bias |
| Other bias | Low risk | No evidence of other bias identified |
Aref 2020.
| Study characteristics | ||
| Methods |
Study design: RCT Dates study conducted: September 2016–April 2018 |
|
| Participants |
Population: women Setting: Taif, Saudi Arabia Inclusion criteria: pregnant women with term singleton pregnancy prepared for term elective CS either primary or repeated Condition for hospitalisation: elective CS Exclusion criteria: women who had UTIs pre‐operatively, confirmed by urine analysis ± culture and sensitivity, women with iatrogenic bladder injury so that IUC had to be kept post‐operatively on the surgeon’s decision, women with severe pre‐eclampsia or eclampsia and/or any other conditions requiring post‐operative monitoring of urinary output, and women who had spinal anaesthesia by choice or contraindicated for general anaesthesia Number of participants: 238 eligible; 221 randomised; 221 reported Age (mean and SD): A 26.1 ± 4, B 25.3 ± 2, C 25.6 ± 3 Use of antibiotic prophylaxis: all participants received a single dose of prophylactic antibiotic in the form of ceftriaxone 1 g IM |
|
| Interventions |
Group A (n = 73): IUC removal immediately after surgery Group B (n= 81): IUC removal 6 h post‐op Group C (n = 67): IUC removal 24 h after operation Size and type of catheter used: size 12 silicone, 2‐way Foley’s catheter Study definition of short‐term catheterisation (days): up to 24 h Intended duration of catheterisation for each group: Group A: immediate removal after surgery (n = 73) Group B: removal 6 h post‐op (n = 81) Group C: removal 24 h post‐op (n = 67) |
|
| Outcomes | Number of participants requiring recatheterisation Symptomatic UTI (number of participants) Time to first ambulation (h; mean ± SD) Length of hospital stay (days; mean ± SD) Positive urine culture Fever Dysuria |
|
| Definition of CAUTI or bacteriuria | “The diagnosis of symptomatic UTI was based on the following criteria: significant bacteriuria with at least one of the following symptoms; dysuria, frequency of micturition, urgency, supra pubic pain, or burning sensation at micturition.” | |
| Sponsorship/funding | Not reported | |
| Ethical approval | “This study was carried out in accordance with the ethical principles for medical research involving human subjects included in Helsinki declaration and was approved by Ethical Committee.” | |
| Notes | “Urinary retention defined as: inability for spontaneous micturition within 6 h after the removal of urinary catheter” “Seventeen patients were finally excluded from the study; five patients had intraoperative complications (iatrogenic bladder injury) and therefore an indwelling catheter had to be kept postoperatively on the surgeon’s request while 12 did not complete the postoperative follow‐up.” |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: “were divided into three groups by simple randomization using computer‐generated random numbers.” Comment: adequate randomisation method |
| Allocation concealment (selection bias) | Unclear risk | Not reported. |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Not reported. Unlikely given nature of intervention |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Not reported. Unlikely outcomes are affected by non‐blinding however. |
| Blinding of microbiological outcome (detection bias) | Low risk | Not reported. Assume microbiologist was blinded to participants of the study |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | No incomplete outcome data |
| Selective reporting (reporting bias) | Low risk | Appears to be free form reporting bias |
| Other bias | Low risk | Appears to be free from other sources of bias |
Aslam 2019.
| Study characteristics | ||
| Methods |
Study design: RCT (multicenter trial) Dates study conducted: not reported |
|
| Participants |
Population: women Setting: USA Inclusion criteria: participants undergoing minimally invasive pelvic organ prolapse surgery Condition for hospitalisation: pelvic organ prolapse Exclusion criteria: not reported Number of participants: 73 eligible (planned to recruit 100); 73 randomised; 73 reported Age (mean and SD): not reported Use of antibiotic prophylaxis: not reported |
|
| Interventions |
Group A (n = 32): IUC removal immediately after surgery Group B (n = 41): IUC removal day 1 post‐op Size and type of catheter used: not reported Study definition of short‐term catheterisation (days): not reported Intended duration of catheterisation for each group: Group A: immediate removal Group B: day 1 post‐op |
|
| Outcomes | Length of hospital stay (h ± SD) Number of participants requiring recatheterisation UTI Patient satisfaction Pain scores Patient responses on whether they would use the same treatment |
|
| Definition of CAUTI or bacteriuria | Not reported | |
| Sponsorship/funding | Not reported | |
| Ethical approval | Not reported | |
| Notes | Conference abstract | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Not reported |
| Allocation concealment (selection bias) | Unclear risk | Not reported |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Not reported. Given nature of intervention, unlikely to be possible |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Not reported |
| Blinding of microbiological outcome (detection bias) | Low risk | Not reported. Assume microbiologist blinded to trial participants |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | No incomplete outcome data |
| Selective reporting (reporting bias) | Low risk | Appears to be free from reporting bias |
| Other bias | High risk | Trial stopped early due to poor recruitment resulting in an imbalance across the 2 groups |
Azarkish 2003.
| Study characteristics | ||
| Methods |
Study design: RCT Setting: Iran Dates study conducted: not reported |
|
| Participants |
Population: women Inclusion criteria: pregnant women age 18‐35, gravid 0‐4, gestational age 37‐42 with normal urine culture, colony count, gram staining) before surgery and taken 3 litres IV fluid during 24 h after surgery Exclusion criteria: women with urinary incontinence, frequent urination and dysuria more than twice, diabetes. History of kidney stone, fever/chills, secondary kidney disorder over the past year, history of smoking, rupture of chorionic membranes for > 6 h during labour, stimulation or acceleration of labour over 6 h, and oral temperature > 38 C in labour. In addition, mothers who have had a bladder injury during CS, uterine incision expansion, longitudinal uterine incision, prolonged operation for > 60 min, or haematocrit < 33% after surgery, acute bleeding, re‐catheterisation more than twice Condition for hospitalisation: CS Number of participants: 60 eligible; 60 randomised; 60 reported Age (mean and SD): A 24.96 ± 4.88; B 27.06 ± 5.56 Use of antibiotic prophylaxis: not reported |
|
| Interventions |
Group A (n = 30): IUC removal 2‐3 h after surgery Group B (n = 30): IUC removal morning after surgery Size and type of catheter used: Foley catheter 14 gauge (5‐10 cc balloon) Study definition of short‐term catheterisation (days): not reported Intended duration of catheterisation for each group: Group A: 2‐3 h after surgery Group B: morning after surgery |
|
| Outcomes | UTI Microscopic pyuria |
|
| Definition of CAUTI or bacteriuria | Not reported | |
| Sponsorship/funding | Not reported | |
| Ethical approval | Not reported | |
| Notes | Original trial report in Farsi. Data extraction performed by Persian translator | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Not reported |
| Allocation concealment (selection bias) | Unclear risk | Not reported |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Not reported |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Not reported. Unlikely given nature of intervention |
| Blinding of microbiological outcome (detection bias) | Low risk | Not reported. Assume urine samples were sent to a laboratory where the microbiologist would not know which patients were in the trial |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Data reported in full |
| Selective reporting (reporting bias) | High risk | Baseline data collected however not reported by authors in results section |
| Other bias | Unclear risk | Appears to be free from other sources of bias |
Azarkish 2005.
| Study characteristics | ||
| Methods |
Study design: RCT Dates study conducted: May 2001‐September 2001 |
|
| Participants |
Population: women Setting: Mashahd, Iran Inclusion criteria: emergency CS, age 18‐35 years old, pregnancy 1‐4, pregnancy period 37‐42 weeks, no UTIs Exclusion criteria: diabetic mothers, women with fever and trembling 24 h before surgery Condition for hospitalisation: emergency CS Number of participants: 333 eligible; 60 randomised; 56 reported Age (mean and SD): not reported Use of antibiotic prophylaxis: perineum wash by povidone iodine 10% before catheter insertion |
|
| Interventions |
Group A (n = 30): IUC removal 2‐3 h post‐op Group B (n = 30): IUC removal 24 h post‐op Size and type of catheter used: size 14 Study definition of short‐term catheterisation (days): not reported Intended duration of catheterisation for each group: Group A: IUC removal 2‐3 h post‐op Group B: IUC removal 24 h post‐op |
|
| Outcomes | Average pain severity of IUC insertion on pain VAS (mean ± SD) Average pain severity of IUC removal on pain VAS (mean ± SD) |
|
| Definition of CAUTI or bacteriuria | Not reported | |
| Sponsorship/funding | Dr. Fazli Bazzaz (Research vice chancellor at Mashad Medical University) | |
| Ethical approval | Not reported | |
| Notes | Paper written in Farsi. Translation provided by a translator | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Quote from translator: “2 groups, 30 persons each, randomised totally by chance” Comment: unclear method of randomisation |
| Allocation concealment (selection bias) | Unclear risk | Not reported |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Not reported. Unlikely possible given nature of intervention |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Not reported |
| Blinding of microbiological outcome (detection bias) | Low risk | No microbiological outcomes reported |
| Incomplete outcome data (attrition bias) All outcomes | High risk | Quote from translator: “number of participants is 56 person, but there is no explanation for that in the paper” Comment: translator could not identify reason for missing pain data for four participants in the pain on removal of catheter group |
| Selective reporting (reporting bias) | High risk | No baseline data reported despite authors mentioning data was collected |
| Other bias | Low risk | Appears to be free from other sources of bias |
Barone 2015.
| Study characteristics | ||
| Methods |
Study design: RCT Dates study conducted: March 2012‐May 2013 |
|
| Participants |
Countries: Democratic Republic of the Congo, Ethiopia, Guinea, Kenya, Niger, Nigeria, Sierra Leone, and Uganda Population: women Condition for hospitalisation: vaginal fistula repair Number of participants: 1007 eligible; 524 randomised; 501 reported Age (mean and SD): A 31.9 ± 11.5; B 30.6 ± 11.7 Use of antibiotic prophylaxis: “…did not receive prophylactic antibiotics” Inclusion criteria: women who had a simple fistula, established by the surgeon after repair surgery; had a closed fistula at completion of surgery and up to 7 days after surgery on the basis of negative dye test results; understood study procedures and requirements; agreed to return for 1 follow‐up 3 months after surgery; and provided informed consent for study participation Exclusion criteria: women were excluded if their fistula was deemed not simple, radiation‐induced, associated with cancer, or due to lymphogranuloma venereum, or if they were pregnant. Women with multiple fistulas were later excluded |
|
| Interventions |
Group A (n = 261): IUC removal at day 7 post‐op Group B (n =262): IUC removal at day 14 post‐op Size and type of catheter used: not reported Study definition of short‐term catheterisation (days): 7 days Intended duration of catheterisation for each group: Group A: 7 days post‐op; IUC removed on the day of randomisation, which was 7 days after surgery Group B: 14 days post‐op; IUC removed after an additional 7 days (i.e. IUC is in place for 14 days in total) Both groups: “We scheduled participants to stay at the facility for 7 days after catheter removal” |
|
| Outcomes | Breakdown between 8 days after IUC removal and 3 months after surgery Breakdown between IUC removal and 3 months after surgery Urinary retention during hospital stay UTI Febrile episode Extended hospital stay Catheter blockage Residual urinary incontinence at 3 months |
|
| Definition of CAUTI or bacteriuria | Not reported | |
| Sponsorship/funding | “This study was funded by the Office of Health, Infectious Diseases and Nutrition, and the Office of Population and Reproduction Health, at the US Agency for International Development under the terms of associate cooperative agreement GHS‐A‐00–07–00021–00 to Engender Health and grant GHA‐G‐00–09–00003 to WHO.” | |
| Ethical approval | “The protocol received technical and ethical approval from the WHO Research Project Review Panel (RP2) and Research Ethics Review Committee, respectively” | |
| Notes | “We declare no competing interests” | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: “The allocation sequence was computer generated centrally at WHO and enrolment and randomisation was done by a research assistant based at each study site. Randomisation was in a 1:1 ratio, stratified by country, and restricted with randomly varying block sizes of 4–6.” Comment: adequate randomisation method |
| Allocation concealment (selection bias) | Low risk | Quote: “We concealed allocation through sealed opaque envelopes.” Comment: adequate allocation concealment |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Quote: “Because of the nature of fistula repair services and low availability of clinical staff at study sites, we could not mask participants, coinvestigators, those assessing outcomes, or other study staff to treatment allocation” Comment: blinding not performed. Lack of blinding can influence outcomes. |
| Blinding of outcome assessment (detection bias) All outcomes | High risk | Quote: “Because of the nature of fistula repair services and low availability of clinical staff at study sites, we could not mask participants, coinvestigators, those assessing outcomes, or other study staff to treatment allocation” Comment: blinding not performed. Lack of blinding can influence outcomes. |
| Blinding of microbiological outcome (detection bias) | Low risk | Assume microbiologist was blinded to the participants of the trial |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Low attrition and not differential: 11/261 and 11/263 lost to follow‐up. ITT analysis was done for our outcomes of interest. |
| Selective reporting (reporting bias) | Low risk | Outcomes prespecified in published protocol are reported in full. |
| Other bias | Low risk | Appears to be free from other sources of bias |
Basbug 2020.
| Study characteristics | ||
| Methods |
Study design: RCT Dates study conducted: December 2015‐December 2016 |
|
| Participants |
Population: women Setting: Duzce, Turkey Inclusion criteria: women who were accepted for primary or recurrent elective CS Condition for hospitalisation: elective CS Exclusion criteria: patients with UTI (evaluated by urine examination), severe vaginal bleeding, severe pre‐eclampsia, eclampsia, and any other conditions requiring post‐operative monitoring of urinary output were excluded from the trial. Number of participants: 172 eligible; 136 randomised; 136 reported Age (mean and SD): A 30.13 ± 5.83; B 29.96 ± 4.71 Use of antibiotic prophylaxis: “All patients received 1 g IV cefazolin as prophylaxis” |
|
| Interventions |
Group A (n = 62): IUC removal 2 h after procedure Group B (n = 72): IUC removal 12 h after procedure Size and type of catheter used: French size 16 silicone‐covered latex Foley catheters Study definition of short‐term catheterisation (days): not reported Intended duration of catheterisation for each group: Group A: 2 h Group B: 12 h |
|
| Outcomes | Number of participants requiring recatheterisation Dysuria Asyptomatic bacteriuria Urinary frequency Urgency Length of hospitalisation stay (h) Fever Time to first void (h; mean ± SD) |
|
| Definition of CAUTI or bacteriuria | Significant microscopic bacteriuria was defined as ≥ 100,000‐bacteria/mL urine in a midstream sample | |
| Sponsorship/funding | Not reported | |
| Ethical approval | The study was approved by the Ethics Committee of the Duzce Medical Faculty (IRB No. 000021705, Approval No. 2015/174) | |
| Notes | Recatheterisation was performed if spontaneous micturition was not possible or urinary retention was detected in the suprapubic region by either abdominal examination or measurement of post‐voiding residual (PVR) volume by ultrasound The definition of urinary retention was, lack of spontaneous micturition 6 h after the removal of catheter or PVR volume > 200 mL measured by transabdominal ultrasound |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: “Patients were randomly allocated by a computer program in a 1:1 ratio to the early or delayed catheter removal groups” Comment: adequate randomisation method |
| Allocation concealment (selection bias) | Unclear risk | Quote: “Patients were randomly allocated by a computer program in a 1:1 ratio to the early or delayed catheter removal groups.” Comment: not reported |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Likely blinding not possible for this outcome |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Not reported |
| Blinding of microbiological outcome (detection bias) | Low risk | Not reported. Assume urine samples were sent to a laboratory where the microbiologist would not know which patients were in the trial |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | ‘As‐treated’ analysis carried out. 4/68 participants in 1 group were analysed in the other group but unlikely to have substantial impact |
| Selective reporting (reporting bias) | Low risk | Outcomes seem to be reported in full |
| Other bias | Low risk | Appears to be free from other sources of bias |
Benoist 1999.
| Study characteristics | ||
| Methods |
Study design: RCT Dates study conducted: January 1994‐June 1997 |
|
| Participants |
Number of participants: eligible: not reported; 132 randomised; 126 reported Setting: Paris Country: France Population: mixed Age (mean (SD)): A 55 (18); B 56 (17) Inclusion criteria: patients undergoing extensive rectal resection (total or subtotal proctectomy) Condition for hospitalisation: rectal resection Exclusion criteria: patients receiving pre‐operative therapeutic antibiotics; suspected bladder tumour or urinary tract malignancies; previous IUC that ended < 48 h before insertion of the current catheter Use of antibiotic prophylaxis: as prophylaxis for bowel surgery, all patients were injected IV with a single dose of antibiotics on the induction of anaesthesia. |
|
| Interventions |
Group A (n = 64): IUC removal 1 day post‐op Group B (n = 62): IUC removal 5 days post‐op Size of catheter used: 14F catheter Type of indwelling catheter: not reported Study definition of short‐term catheterisation (days): not reported Intended duration of catheterisation for each group: A: removal of IUC 1 day after surgery B: removal of IUC 5 days after surgery |
|
| Outcomes | AUR Chronic urinary retention UTI Long‐term urinary complications Patients undergoing total mesorectum excision |
|
| Definition of CAUTI or bacteriuria | Urinary infection was diagnosed if a culture yielded > 105 cfu/mL, with or without clinical symptoms | |
| Sponsorship/funding | Not reported | |
| Ethical approval | “…which was approved by the hospitals ethics committee” | |
| Notes | AUR defined as absence of spontaneous micturition 12 h after catheter removal or after single intermittent catheterisation. Catheters were never clamped and were maintained on a closed drainage system. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: “… patients were randomized into 1‐day or 5‐day urinary drainage groups according to the following computer‐generated randomization sequence.” Comment: adequate method of randomisation |
| Allocation concealment (selection bias) | Unclear risk | Not reported |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Not reported Comment: unlikely blinding was possible |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Not reported |
| Blinding of microbiological outcome (detection bias) | Low risk | Quote: “… urine samples from both groups were sent to a laboratory for culture” Comment: cultures were sent to a laboratory so it is unlikely that the microbiologist knew which samples corresponded to patients in the trial. |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | Quote: "...1 patient died postoperatively, 2 had postoperative complications requiring early reoperation, 2 inadvertently removed catheters, 1 required prolonged urine output monitoring because of transient respiratory failure requiring prolonged artificial ventilation." Comment: unclear what effect this has an the outcome of interest |
| Selective reporting (reporting bias) | Low risk | All outcomes reported in methods was reported in results. Protocol not available |
| Other bias | Low risk | Appears to be free from other sources of bias |
Bristoll 1989.
| Study characteristics | ||
| Methods |
Study design: not reported. Described as “small multiple single case study” and “single case experimental design” Dates study conducted: not reported |
|
| Participants |
Setting: St Joseph’s Hospital, Milwaukee Country: USA Number of participants: eligible – not reported; 6 randomised; 6 reported Population: adults (no further information given) Condition for hospitalisation: not reported Age (mean and SD): not reported Use of antibiotic prophylaxis: not reported Inclusion criteria: “Patients who had not voided for 6 hours or more, appears to have more than 1000mLs of urine in their bladder, requiring catheterisation for retention according to physicians request” Exclusion criteria: “Obstetric patients, spinal cord injuries, patients undergone urological procedures in the past 6 months” |
|
| Interventions |
Group A (n = 3): threshold clamping Group B (n = 3): complete drainage Size and type of catheter used: Foley catheter Study definition of short‐term catheterisation (days): not reported Intended duration of catheterisation for each group: not reported |
|
| Outcomes | Blood pressure Pulse rate Haematuria “Patients were monitored for any untoward reactions to the procedure, such as pain, diaphoresis, or frank bleeding, which would be expected to occur within 30 min of catheterization. Each patient’s urine was also cultured for the presence of infection, which might explain any hematuria |
|
| Definition of CAUTI or bacteriuria | Not reported | |
| Sponsorship/funding | Not reported | |
| Ethical approval | Not reported | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Quote: “Participants were randomised” Comment: no description of how randomisation was performed |
| Allocation concealment (selection bias) | Unclear risk | Not reported |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Blinding not possible. Lack of blinding could have an impact on the outcome |
| Blinding of outcome assessment (detection bias) All outcomes | High risk | Quote: “While one investigator took each patient's blood pressure and pulse at predetermined intervals, the nurse caring for the patient inserted a Foley catheter, and a second investigator took urine samples at one‐minute intervals. Each sample was tested for blood using a Hemastix reagent strip. One investigator and the patient’s nurse verified the results” Comment: outcome assessors do not appear to be blinded |
| Blinding of microbiological outcome (detection bias) | Low risk | Not reported but likely that the urine samples were sent to a laboratory where the microbiologist would not know which patients were in the trial |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | No data reported |
| Selective reporting (reporting bias) | High risk | No data reported for any outcomes |
| Other bias | Low risk | Appears to be free from other sources of bias |
Carpiniello 1988.
| Study characteristics | ||
| Methods |
Study design: RCT Dates study conducted: November 1985‐March 1986 |
|
| Participants |
Number of participants: 218 eligible; 77 randomised; 77 reported Setting: Philadelphia, Pennsylvania Country: USA Population: women Age (mean and SD): A 73 (6.6); B 70 (8.3); C 70 (8.6) Inclusion criteria: consenting elderly women who underwent treatment for urinary complications post‐total joint replacement Condition for hospitalisation: total joint replacement (hip or knee) Exclusion criteria: men excluded to avoid confusion of influence of prostatic disease; non‐primary total joint replacement; positive pre‐operative urine cultures; general anaesthesia and on bed rest post‐operatively Use of antibiotic prophylaxis: prophylactic cefazolin sodium or clindamycin on post‐operative day 3 |
|
| Interventions |
Group A (n = 31): IUC in recovery room Group B (n = 23): no IUC Group C (n = 23): IUC inserted immediately pre‐operatively and removed 24 h post‐operative Size and type of catheter used: not reported Type of indwelling catheter: not reported Study definition of short‐term catheterisation (days): not reported Intended duration of catheterisation for each group: A: not clear. Catheterisation in recovery room only B: no catheterisation C: Foley catheter inserted immediate pre‐operatively and removed 24 h post‐operatively |
|
| Outcomes | UTIs Time catheter in place Recatheterisation post‐catheter removal Postive urine culture |
|
| Definition of CAUTI or bacteriuria | “100,000 colonies/millimetre” | |
| Sponsorship/funding | Not reported | |
| Ethical approval | Not reported | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Quote: “… patients were randomly assigned to one of three groups” Comment: randomisation method unclear |
| Allocation concealment (selection bias) | Unclear risk | Not reported |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Not reported Comment: unlikely blinding was possible |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Not reported |
| Blinding of microbiological outcome (detection bias) | Low risk | Not reported Comment: the midstream clean‐catch urine cultures would be sent to a laboratory where the microbiologist would not know which patient belonged to the trial |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | All participants completed the study i.e. no withdrawals |
| Selective reporting (reporting bias) | High risk | Group C seems to be missing catheterisation volume in recovery room and after recovery room. |
| Other bias | Low risk | Appears to be free from other sources of bias |
Carter‐Brooks 2018.
| Study characteristics | ||
| Methods |
Study design: RCT Dates study conducted: February 2016‐March 2017 |
|
| Participants |
Number of participants: eligible not reported, 57 reported Setting: Connecticut Country: USA Population: women Age (mean and SD): A 64.9 ± 11.5; B 65.2 ± 10.3 Inclusion criteria: surgical management of pelvic organ prolapse requiring an overnight hospital admission Condition for hospitalisation: pelvic organ prolapse Exclusion criteria: same‐day surgery, non‐ambulatory (allowed to use an assistive device), inability to provide informed consent, age < 21 years, pregnancy or desire for future pregnancy, systematic disease known to affect bladder function (Parkinson's disease, multiple sclerosis, spina bifida, spinal cord injury or trauma and neurogenic bladder), known pre‐operative urinary retention (defined as a post‐void residual > 100 mL), an untreated UTI at the time of surgery, treatment at the time of surgery for UTI, symptoms of UTI on the day of surgery Use of antibiotic prophylaxis: not reported |
|
| Interventions |
Group A (n = 27): IUC removal 4 h post‐op Group B (n = 30): IUC removal 6 am on post‐op day 1 Size and type of catheter used (e.g. Foley 16F): not reported Study definition of short‐term catheterisation (days): not reported Intended duration of catheterisation for each group: Group A: voiding trial 4 h post‐operatively Group B: voiding trial at 6 am day 1 post‐operative |
|
| Outcomes | Number of participants requiring recatheterisation* Incidence of UTI Patient comfort or discomfort VAS pain scores** Time to first void (h) Length of hospitalisation (h) Psychological outcome measures e.g. Hospital Anxiety and Depression Scale. Anxiety measured by State‐Trait Anxiety Inventory state subscale (STAI‐S) |
|
| Definition of CAUTI or bacteriuria | “Defined as a positive culture or symptoms and antibiotic treatment” | |
| Sponsorship/funding | “This project was supported by the Clinical Research Trainee Award from Magee‐Womens Research Institute and the National Institutes of Health through grant number UL1‐TR‐000005.” | |
| Ethical approval | “This study was approved by the Institutional Review Board of the University of Pittsburgh (PRO15100653 approved 1/14/16)” | |
| Notes | *Derived from outcome “Spontaneous void after 1st voiding trial attempt” **Pain scores were measured using the VAS, a continuous scale comprising a horizontal line 10 cm in length, anchored by the verbal descriptors “no pain” and “worst imaginable pain”. |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: “Randomization was computer generated with 1:1 group allocation to an early or standard voiding trial in fixed blocks of 6.” Comment: adequate method of randomisation |
| Allocation concealment (selection bias) | Low risk | Quote: “Randomization was concealed by a research assistant not involved in trial enrolment using consecutively numbered opaque envelopes” Comment: adequate method of concealment |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Blinding not possible |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Not reported |
| Blinding of microbiological outcome (detection bias) | Low risk | Not reported. Assume urine samples were sent to a laboratory where the microbiologist would not know which patients were in the trial |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | ITT analysis and per‐protocol analysis reported. No withdrawals |
| Selective reporting (reporting bias) | Low risk | Outcomes appear to be reported in full |
| Other bias | Low risk | Appears to be free from other sources of bias |
Chai 2011.
| Study characteristics | ||
| Methods |
Study design: RCT Dates study conducted: November 2007‐September 2009 |
|
| Participants |
Number of participants: 112 eligible; 70 randomised; 70 reported Setting: Hong Kong, China Population: women Age (mean (SD)): A 46.4 (3.9); B 46.4 (4.0) Inclusion criteria: women undergoing total abdominal hysterectomy + bilateral salpingo‐oophorectomy for various benign gynaecological diseases Condition for hospitalisation: hysterectomy Exclusion criteria: known history of neurological disorder; known history of urinary incontinence; women who had recurrent UTI or positive urine culture (> 105 cfu/mL) pre‐operatively; women for whom a complicated procedure was encountered during hysterectomy so that an indwelling catheter had to be kept in post‐operatively on surgeon’s decision; women who had spinal anaesthesia by choice or received patient‐controlled analgesia as post‐operative pain relief Use of antibiotic prophylaxis: routine prophylactic antibiotics were not given |
|
| Interventions |
Group A (n = 35): immediate removal of IUC post‐op Group B (n = 35): removal of IUC 24 h post‐op on day 1 Size and type of catheter used: 12F with a 10 mL balloon Foley catheter, latex Study definition of short‐term catheterisation (days): not reported Intended duration of catheterisation for each group: A: 0 h (duration of operation) B: 24 h after the end of the operation |
|
| Outcomes | Pain at urethral site (pain assessment was performed using a VAS (0 to 100) to assess the level of pain at the urethral site: nil to 33 on the scale was categorised as mild pain; 34‐66 as moderate, and 67‐100 as severe pain. Patients were asked to complete the scale on post‐operative day 1) Post‐operative positive urine culture Symptomatic UTI Recatheterisation rate |
|
| Definition of CAUTI or bacteriuria | Positive urine culture (> 105 cfu of an identified single uropathogen per mL of urine) Symptomatic UTI: fever (> 38) and dysuria + positive urine culture |
|
| Sponsorship/funding | Department of Obstetrics and Gynaecology, University of Hong Kong | |
| Ethical approval | Institutional Review Board of the University of Hong Kong/Hospital Authority Hong Kong West Cluster | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | “The randomization schedule for each surgeon was generated from the computer in a block of four.” Comment: adequate method of randomisation |
| Allocation concealment (selection bias) | Low risk | “…sealed, opaque envelopes. The randomization envelope was opened and the patient had the catheter removed according to the randomization allocation” Comment: adequate method of concealment |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Not reported; not possible |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | “A reviewer who was blinded to the study assignment evaluated the pain assessment.” Comment: blinding used so pain scores were not likely to be affected. |
| Blinding of microbiological outcome (detection bias) | Low risk | “… urine sample for culture and microscopy.” Comment: urine samples were sent to a laboratory where the microbiologist is unlikely to know which patient belongs to the study. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | “42 women excluded from final analysis; 39 women had patient‐controlled analgesia after operation and three women postoperatively required an in‐dwelling catheter at the surgeon’s request” – patients excluded prior to randomization, no patients lost after randomization.” Comment: all withdrawals and exclusions are accounted for |
| Selective reporting (reporting bias) | Low risk | All outcomes measured in results was the same as was mentioned in the methods section |
| Other bias | Low risk | Appears to be free from other sources of bias |
Chen 2013.
| Study characteristics | ||
| Methods |
Study design: RCT Dates study conducted: April‐November 2008 |
|
| Participants |
Number of participants: 509 eligible; 278 randomised; 278 reported Setting: Taiwan Population: mixed Age (mean (SD): A 77 (12.7); B 78 (10.5) Inclusion criteria: all adult patients admitted to respiratory ICU Condition for hospitalisation: multiple. Most of the patients in the study had respiratory failure and were being treated with mechanical ventilation. Exclusion criteria: had not had IUC; did not stay in respiratory ICU for > 2 days Use of antibiotic prophylaxis: not used. Antibiotics were only given to symptomatic patients |
|
| Interventions |
A: Intervention group – use of IUC removal reminder protocol (n = 147) Group 1 (n = 86): IUC removed ≤ 7 days Group 2 (n = 61): IUC removed > 7 days B: Control group i.e. no IUC removal reminder policy (n = 131) Group 1 (n = 48): IUC removed ≤ 7 days Group 2 (n = 83): IUC removed > 7 days Size and type of catheter used: not reported Study definition of short‐term catheterisation (days): not reported Intended duration of catheterisation for each group: A: intervention group – use of catheter removal reminder protocol Group 1 (catheter removed ≤ 7 days) Group 2 (catheter removed > 7days as clinically indicated) B: control group i.e. no catheter removal reminder policy Group 1 (catheter removed ≤ 7 days) Group 2 (catheter removed > 7days as clinically indicated) |
|
| Outcomes | Total CAUTIs Asymptomatic bacteriuria Symptomatic UTI Catheter‐associated asymptomatic bacteriuria Catheter‐associated symptomatic UTI Duration of catheterisation (mean, SD) Recatheterisation |
|
| Definition of CAUTI or bacteriuria | Determination of CAUTI was performed in accordance with criteria of the CDC and the National Healthcare Safety Network, including symptomatic UTI and asymptomatic bacteriuria. A CAUTI is a UTI that occurs in a patient who had an IUC in place within the 48 h before the onset of the UTI. | |
| Sponsorship/funding | “This study was supported in part by a research grant from Taipei Veterans General Hospital (Taipei VGH‐V97A‐055)” | |
| Ethical approval | The study was approved by the appropriate institutional review board before implementation. | |
| Notes | Patients whose IUCs were removed later than planned were excluded from the per‐protocol analysis and were moved to a treatment‐contamination group. Protocol was not followed | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: “Computer‐generated random numbers were used …” Comment: adequate randomisation method |
| Allocation concealment (selection bias) | Unclear risk | Not reported |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Quote: “These professionals had no knowledge of which group (control or intervention) a patient was assigned to ... most patients had respiratory failure and were being treated with mechanical ventilation.” Comment: adequate method of blinding. Unlikely participants knew due to being in ICU |
| Blinding of outcome assessment (detection bias) All outcomes | High risk | Quote: “The investigator responsible for the daily identification and assessment of all patients with indwelling urinary catheters, however, knew which group each patient was assigned to” Comment: outcome assessment was not blinded |
| Blinding of microbiological outcome (detection bias) | Low risk | Quote: “… all samples were sent to the laboratory…” Comment: unlikely laboratory staff knew which patients were in the trial |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | Unclear. 278 patients were randomised but in table 3 there are 180 in group A and 181 in group B |
| Selective reporting (reporting bias) | Low risk | All outcomes are reported in both methods and results sections |
| Other bias | Low risk | Appears to be free from other sources of bias |
Chia 2009.
| Study characteristics | ||
| Methods |
Study design: RCT Dates study conducted: not reported |
|
| Participants |
Number of participants: eligible – not reported; 80 randomised (40 in each group); 78 reported Setting: Taiwan Population: mixed Age (e.g. mean and SD): A 54.7 ± 11.2; B 55.7 ± 10.3 Inclusion criteria: patients of ASA physical status I−III undergoing thoracotomy Condition for hospitalisation: thoracotomy Exclusion criteria: urological/spinal/cardiopulmonary/neurological diseases; coagulopathy and/or any medication that might interfere with the sympathetic nervous system or micturition were excluded from this study Use of antibiotic prophylaxis: a single dose of prophylactic antibiotic was given IV in all participants |
|
| Interventions |
Group A: IUC removed on the 1st post‐operative day (n = 38) Group B: IUC removed after discontinuation of PCEA (3rd post‐operative day) (n = 40) Size and type and type of catheter used: 14F Foley catheter Study definition of short‐term catheterisation (days): not reported Intended duration of catheterisation for each group: A: IUC removed on the 1st post‐operative day B: IUC removed on the 3rd post‐operative day |
|
| Outcomes | Recatheterisation for urinary retention CAUTI Average duration of bladder drainage Pain intensity at rest (VAS) Urethral pain intensity (VAS) |
|
| Definition of CAUTI or bacteriuria | Not reported | |
| Sponsorship/funding | Not reported | |
| Ethical approval | “After obtaining approval from the Human Investigation Committee at Kaohsiung Veterans General Hospital and written informed consent from all patients.” | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: “…the eligible patients were randomly assigned into two groups according to a table of random numbers generated by a computer.” Comment: adequate method of randomisation |
| Allocation concealment (selection bias) | Unclear risk | Not reported |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Not reported. Unlikely, blinding was not possible |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Not reported |
| Blinding of microbiological outcome (detection bias) | Low risk | No information given, but can assume urine sample was assessed by microbiologist who would not know allocation of participants |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Quote: “two patients in group 1 were excluded due to inadequate pain relief by postoperative PCEA” Comment: low number of participants excluded |
| Selective reporting (reporting bias) | Unclear risk | Data reported graphically in way that did not allow precise data extraction – VAS scores were reported as significant without P values or the mean VAS scores presented in the figures |
| Other bias | Low risk | Appears to be free from other sources of bias |
Chillington 1992.
| Study characteristics | ||
| Methods |
Study design: RCT Dates study conducted: not reported |
|
| Participants |
Setting: Hereford Country: UK Population: men Age (mean (SD)): not reported Inclusion criteria: patients undergoing TURP Condition for hospitalisation: TURP Exclusion criteria: not reported Number of participants: eligible, not reported; randomised, not reported; 100 reported Use of antibiotic prophylaxis: not reported |
|
| Interventions |
Group A (n = 35): removal of IUC at midnight Group B (n = 48): removal of IUC at 6 am Size and type of catheter used: not reported Study definition of short‐term catheterisation (days): not reported Intended duration of catheterisation for each group: A: IUC removed at midnight B: IUC removed at 6 am |
|
| Outcomes | Time to first void Volume of first void Number needing to be recatheterised Length of hospital stay Percentage of participants achieving acceptable voiding within 24 h of catheter removal |
|
| Definition of CAUTI or bacteriuria | Not reported | |
| Sponsorship/funding | Not reported | |
| Ethical approval | Not reported | |
| Notes | Information from a letter in British Journal of Urology accessible. Full text not available | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Not reported |
| Allocation concealment (selection bias) | Unclear risk | Not reported |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Not reported Comment: unlikely blinding was possible |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Comment: not much information reported in letter, difficult to ascertain whether outcome assessor was blinded |
| Blinding of microbiological outcome (detection bias) | Low risk | Not reported Comment: urine samples would be assessed in a laboratory, who would not know allocation of participant |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | No information provided |
| Selective reporting (reporting bias) | Unclear risk | No information on outcomes intended to be measured was given. Protocol not available |
| Other bias | Low risk | Appears to be free from other sources of bias |
Cornia 2003.
| Study characteristics | ||
| Methods |
Study design: RCT Dates study conducted: 15 November 2000‐6 March 2001 |
|
| Participants |
Setting: Washington Country: USA Population: mixed Age: not reported Inclusion criteria: all patients admitted to the medicine and cardiology services Condition for hospitalisation: mixed Exclusion criteria: patients who were transferred to a non‐medicine or non‐cardiology service, or to a ward other than the second or fourth floor, were removed from the study. Number of participants: 742 eligible; 648 randomised (70 patients received IUCs); 70 reported Use of antibiotic prophylaxis: not reported |
|
| Interventions |
Group A: had the choice to use a designated computer study order, enter standard written order or not enter an order for IUC Group B: did not have a designated computer study order Size and type of catheter used: not reported Study definition of short‐term catheterisation (days): not reported Intended duration of catheterisation for each group: A: computer study order had default stop date of 72 h after placement (either renew or discontinue catheter order) B: not reported |
|
| Outcomes | Mean duration of catheterisation CAUTI Catheter reinsertion |
|
| Definition of CAUTI or bacteriuria | CAUTI: growth from a urine specimen aseptically aspirated from the catheter of ≥ 100 cfu of a predominant pathogen or ≥ 10 leukocytes per high‐power field on urinalysis in a patient with a clinical diagnosis of UTI | |
| Sponsorship/funding | Not reported | |
| Ethical approval | Human Subjects Committee of the University of Washington and the Research and Development Committee of the VA Puget Sound Health Care System | |
| Notes | “Written informed consent was not required from patients or providers as the standard of care was to not use computerized urinary catheter orders” | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | High risk | Quote: “During the first 8 weeks of the study, the fourth floor served as the study ward and the second floor served as the control ward; during the second 8 weeks, the second floor became the study ward and the fourth floor became the control” Comment: method of randomisation not truly random |
| Allocation concealment (selection bias) | High risk | Comment: method of randomisation means that allocation concealment would not be possible |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Not reported Comment: unlikely that blinding was possible due to intervention |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Not reported |
| Blinding of microbiological outcome (detection bias) | Low risk | Urine samples were sent away to a laboratory, where the allocation of the participant would not be known |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Quote: “94 patients excluded as moved wards or the treating specialty not included in study” Comment: adequate reason for exclusion |
| Selective reporting (reporting bias) | High risk | Quote: “Of the 5 patients who required reinsertion of a urinary catheter, only 1 had received an automatic computer order to remove the previous catheter” Comment: not reported based on allocation, cannot interpret significance of results |
| Other bias | Low risk | Appears to be free from other sources of bias |
Coyle 2015.
| Study characteristics | ||
| Methods |
Study design: RCT Dates study conducted: January 2012‐July 2013 |
|
| Participants |
Number of participants: 46 eligible; 44 randomised (22 in each arm); 35 reported (7 participants in Group A did not receive intervention, 1 participant from Group A excluded from analysis (catheter reinserted); 2 participants from Group B excluded from analysis due to medical necessity) Setting: Galway Country: Ireland Population: mixed (30 male, 14 female) Age (mean): A 63.5; B 62 Inclusion criteria: 18 years old; competent to consent for research purposes; plan to undergo elective transabdominal colectomy, proctectomy or coloproctectomy with post‐operative epidural analgesia Condition for hospitalisation: elective transabdominal colectomy, proctectomy or coloproctectomy Exclusion criteria: Pre‐operative: prior surgery to lower urinary tract; pre‐existing lower urinary tract disease; intermittent self‐catheterisation; neurogenic bladder; pregnancy; prior transabdominal pelvic surgery; known enterovesical fistula; planned; synchronous urinary tract surgery; anticholinergic therapy; IPSS ≥ 20; urethral catheter indwelling > 24 h prior to surgery Post‐operative: epidural analgesia withdrawn ≤ 12 h post‐operatively; surgical instrumentation of or dissection involving the urinary tract; delay in removal of IUC due to medical necessity; pelvic sepsis at surgery; unexpected finding of entero‐ or rectovesical fistula at surgery; premature dislodgement of urethral catheter; failed epidural catheterisation Use of antibiotic prophylaxis: not reported |
|
| Interventions |
Group A: urethral catheter removal 48 h post‐op (n = 13) Group B: urethral catheter removal within 12 h of withdrawal of epidural analgesia (n = 20) Size and type of catheter used: not reported Study definition of short‐term catheterisation (days): not reported Intended duration of catheterisation for each group: A: 48 h B: 12 h after withdrawal of epidural analgesia (median duration of catheterisation was 85.5 h) |
|
| Outcomes | Development of post‐operative urinary retention (total) Bacteriuria (UTI) |
|
| Definition of CAUTI or bacteriuria | Symptomatic or asymptomatic bacteriuria used. Unclear which definition is used however | |
| Sponsorship/funding | None | |
| Ethical approval | “Ethical approval given by the local Clinical Research Ethics Committee (CA 661)” | |
| Notes | In total, 9 participants (20.5%) were excluded from analysis during the post‐operative period. In SG1, 7 patients were excluded due to the following reasons: premature accidental dislodgement of IUC (n = 2); epidural catheter dislodgement < 24 h post‐operatively (n = 2), unplanned instrumentation of the urinary tract at surgery (n = 1); IUC re‐inserted post‐operatively due to oliguria (n = 1); withdrawal of consent for patient participation (n = 1). In SG2, 2 participants were excluded due to IUC removal being delayed as a result of medical necessity. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: “… were randomised using a computer generated randomisation system” Comment: adequate method of randomisation |
| Allocation concealment (selection bias) | Low risk | Quote: “The operator was blinded as to the allocated arm, which was contained in a sealed envelope, at the time of catheter insertion.” Comment: adequate method of allocation concealment |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Not reported – unlikely it was possible |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Not reported |
| Blinding of microbiological outcome (detection bias) | Low risk | Not reported. Likely however that samples were sent to a laboratory where the microbiologist would be unaware of the study |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | Flowchart indicates that group A reported on 14 patients and group B on 20 patients – but in table there are only 13 patients in group A |
| Selective reporting (reporting bias) | Low risk | All outcomes in methods are reported in full in results. |
| Other bias | Low risk | Appears to be free from other sources of bias |
Crowe 1993.
| Study characteristics | ||
| Methods |
Study design: RCT Dates study conducted: October 1990‐November 1991 |
|
| Participants |
Number of participants: eligible, not reported; 242 randomised; 242 reported Setting: Melbourne Country: Australia Population: mixed Age (mean (SD)): not reported Inclusion criteria: patients admitted to the urology ward with IUCs or who were catheterised during their inpatient stay Condition for hospitalisation: patients admitted to the urology ward Exclusion criteria: patients with permanent indwelling catheters; self‐catheterisation; functioning urinary diversions e.g. nephrostomy tube or suprapubic catheter Use of antibiotic prophylaxis: not reported |
|
| Interventions |
Group A (n = 127): removal of IUC at 6 am Group B (n = 115): removal of IUC at midnight Size and type of catheter used: not reported Study definition of short‐term catheterisation (days): not reported Intended duration of catheterisation for each group: not reported |
|
| Outcomes | Number needing to be recatheterised (derived from failed trial of void) Failed trial of void requiring recatheterisation Mean volume of first void (mL) Mean time to first void (min) Discharged same day as catheter removed |
|
| Definition of CAUTI or bacteriuria | Not reported | |
| Sponsorship/funding | Not reported | |
| Ethical approval | Not reported | |
| Notes | Another trial published in 1993 | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Quote: “… patients were randomized…” Comment: method of randomisation unclear |
| Allocation concealment (selection bias) | Unclear risk | Not reported |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Not reported. Comment: unlikely that blinding was possible |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Not reported |
| Blinding of microbiological outcome (detection bias) | Low risk | No microbiological outcomes reported |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | No patients lost to follow‐up, none withdrew |
| Selective reporting (reporting bias) | Unclear risk | Outcomes measured are the same in methods and results section. Recatheterisation rates and UTI rates were not measured. |
| Other bias | Low risk | Appears to be free from other sources of bias |
Dunn 1999.
| Study characteristics | ||
| Methods |
Study design: RCT Dates study conducted: 1 July1996‐1 July 1997 |
|
| Participants |
Number of participants: not reported how many were eligible or analysed, states "100 women entered the trial", no further information Setting: Denver Country: USA Population: women Age: not reported Inclusion criteria: patients undergoing Obs‐Gynae surgery Condition for hospitalisation: obstetric and gynaecological surgery Exclusion criteria: pre‐eclampsia; bladder injury; surgery for incontinence; vaginal vault prolapse; anterior/posterior colporrhaphy Use of antibiotic prophylaxis: not reported |
|
| Interventions |
Group A: immediate removal of IUC post‐operatively (n = not reported) Group B: delayed IUC removal post‐operatively (n = not reported) Size and type of catheter used: not reported Study definition of short‐term catheterisation (days): not reported Intended duration of catheterisation for each group: |
|
| Outcomes | Pain post‐op Recatheterisation |
|
| Definition of CAUTI or bacteriuria | Not reported | |
| Sponsorship/funding | Not reported | |
| Ethical approval | Not reported | |
| Notes | Foley catheterisation increases the amount of pain without a clear benefit. Further subgroup analysis by type of surgery, hospital length and UTI is still underway All information obtained from a conference abstract |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Not reported |
| Allocation concealment (selection bias) | Unclear risk | Not reported |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | No information given. Unlikely blinding was possible |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Not reported |
| Blinding of microbiological outcome (detection bias) | Low risk | Not reported |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | No data given, only preliminary results |
| Selective reporting (reporting bias) | Unclear risk | Not enough information in abstract to assess for elective reporting |
| Other bias | Low risk | Appears to be free from other sources of bias |
Dunn 2000b.
| Study characteristics | ||
| Methods |
Study design: RCT Dates study conducted: not reported |
|
| Participants |
Number of participants: eligible, not reported; randomised, not reported; 78 reported Setting: Denver Country: USA Population: women Age (mean (SD)): not reported Inclusion criteria: all patients undergoing hysterectomy or CS, not requiring bladder suspension or strict fluid management Condition for hospitalisation: hysterectomy or CS Exclusion criteria: requiring bladder suspension or strict fluid management Use of antibiotic prophylaxis: not reported |
|
| Interventions |
Group A: Foley catheter removed immediately (n= not reported) Group B: Foley catheter removed post‐operatively (n= not reported) Group C: Foley catheter removed on the first post‐operative day (n= not reported) Size and type of catheter used: not reported Study definition of short‐term catheterisation (days): not reported Intended duration of catheterisation for each group: A: immediate removal B: post‐operative removal C: first operative day |
|
| Outcomes | Fever Infection (UTI) Recatheterisation Level of pain (measured by using a standardised scale split into mild, moderate and severe) |
|
| Definition of CAUTI or bacteriuria | Not reported | |
| Sponsorship/funding | Not reported | |
| Ethical approval | Not reported | |
| Notes | Abstract, data not presented in a format which is compatible for meta‐analysis | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | No information given |
| Allocation concealment (selection bias) | Unclear risk | No information given |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Not reported. Unlikely that blinding was possible |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Not reported |
| Blinding of microbiological outcome (detection bias) | Low risk | Not reported. Likely that urine samples were sent to a laboratory |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | No data are presented in tables. Very brief sets of data in results section. Unclear which outcome related to which group |
| Selective reporting (reporting bias) | High risk | Outcomes reported in way that data could not be extracted – unclear if due to unsatisfactory results or because an abstract |
| Other bias | Low risk | Appears to be free from other sources of bias |
Dunn 2003.
| Study characteristics | ||
| Methods |
Study design: RCT Setting: Denver, Colorado, USA Dates study conducted: January 1998‐December 2001 |
|
| Participants |
Number of participants: 323 eligible; 250 randomised; 250 reported Setting: Denver, Colorado Country: USA Population: women Age (median, range): 47 (25‐72) Inclusion criteria: consenting women undergoing hysterectomy for various benign diseases (e.g. fibroid tumours, abnormal uterine bleeding, chronic pain, and persistent cervical dysplasia or micro invasive cervical cancer) Condition for hospitalisation (e.g. hysterectomy or TURP): hysterectomy Exclusion criteria: women for whom a complicated surgical procedure was anticipated (i.e. patients who underwent bladder suspension or colporrhaphy, diagnosis suspicious for severe endometriosis or for whom strict fluid treatment was required) Use of antibiotic prophylaxis: single dose of antibiotic prophylaxis before operation |
|
| Interventions |
Group A (n = 125): immediate removal of the IUC in the operating room Group B (n = 125): removal of IUC on post‐operative day 1 Size and type of catheter used (e.g. Foley 16F): 16F with 10 cc balloon, latex Study definition of short‐term catheterisation (days): not reported Intended duration of catheterisation for each group: Group A: duration of operation, removal in the operating room after operation finished Group B: post‐op day 1 |
|
| Outcomes | Post‐operative fever UTI Recatheterisation Pain (data cannot be incorporated as trial reports percentages without information on the number of participants in each group) |
|
| Definition of CAUTI or bacteriuria | Determined by either microscopic abnormality or any patient symptoms | |
| Sponsorship/funding | Not reported | |
| Ethical approval | Institutional Review Board of the University of Colorado | |
| Notes | Pain was assessed with a pictorial questionnaire Post‐operative fever = temperature > 38.5 °C |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: “… computer generated randomisation” Comment: adequate randomisation method |
| Allocation concealment (selection bias) | Low risk | Quote: “… sealed opaque envelopes” Comment: adequate concealment method |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Not reported, can assume blinding was not performed/possible |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Not reported, VAS may be prone to bias and misinterpretation |
| Blinding of microbiological outcome (detection bias) | Low risk | UTI was diagnosed with either microscopic abnormality or any patient symptoms. If only microscopic abnormality was used, would be low risk of bias |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | No withdrawals or exclusions/dropouts |
| Selective reporting (reporting bias) | Low risk | All outcomes in methods are reported in results. Protocol not available |
| Other bias | Low risk | Appears to be free from other sources of bias |
Durrani 2014.
| Study characteristics | ||
| Methods |
Study design: RCT Dates study conducted: 1 September 2009‐31 July 2011 |
|
| Participants |
Number of participants: eligible, not reported; 320 randomised; 320 reported Setting: Peshawar Country: Pakistan Population: men Age (mean and SD): 71.32 ± 5.94 Inclusion criteria: patients with bladder outflow obstruction due to benign prostatic enlargement undergoing TURP Condition for hospitalisation: TURP Exclusion criteria: large post‐void urine volume; urethral stricture; patients undergoing simultaneous internal urethrotomy and TURP; comorbidities such as uncontrolled diabetes mellitus, spinal cord problem, cerebro‐vascular accident or any condition that might result in neurogenic urinary bladder; intra‐operative complications like capsular or bladder perforation, severe haemorrhage during or immediately after surgery Use of antibiotic prophylaxis: cephalosporin 1 gm was administered IV at the time of induction of anaesthesia |
|
| Interventions |
Group A (n = 163): delayed IUC removal (conventional) Group B (n = 157): early IUC removal Size and type of catheter used: 22 Fr catheter Study definition of short‐term catheterisation (days): not reported Intended duration of catheterisation for each group: Group A: removal of IUC after > 1 day post‐op (usually 4th or 5th day) Group B: removal on the 1st day post‐op |
|
| Outcomes | Mean catheter removal day Mean length of hospital stay in group Recatheteristaion Mild dilutional hyponatraemia Emergency re‐admission Reoperation, clot evacuation and diathermy of bleeding/oozing points UTI |
|
| Definition of CAUTI or bacteriuria | Not reported | |
| Sponsorship/funding | Not reported | |
| Ethical approval | “Written informed consent was taken from all the patients before including them in the study.” | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: “Patients were divided into the two groups by randomly selecting from a pile of sealed opaque envelopes containing assignment as A or B group as the patients came and were included in the study.” Comment: adequate method of randomisation |
| Allocation concealment (selection bias) | Low risk | Quote: “Sealed opaque envelopes were kept in a box in equal proportion and patients were asked to select one sealed envelope. Fifty envelopes with 25 for each group, A and B, were kept in the box initially and when 10 would remain, another 50 with the same proportions would be added.” Comment: adequate method of concealment |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Not reported. Unlikely participants were not blinded as to when their catheter was removed. |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Quote: “The box was kept locked all the time and under the supervision of the principal investigator … a doctor who did not know the actual grouping of patients collected all the data.” Comment: adequate method of blinding of outcome assessment |
| Blinding of microbiological outcome (detection bias) | Low risk | Not reported. Assumed microbiologist was blinded |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | No report number of patients excluded or number of dropouts. All participants who were included in the study completed the study. |
| Selective reporting (reporting bias) | Low risk | Outcomes mentioned in methods are reported in results section. Protocol was not available for assessment |
| Other bias | Low risk | No other sources of bias apparent |
El‐Mazny 2014.
| Study characteristics | ||
| Methods |
Study design: RCT Dates study conducted: November 2012‐March 2014 |
|
| Participants |
Number of participants: 335 eligible; 300 randomised; 300 reported Setting: Cairo Country: Egypt Population: women Age (mean and SD): Group A: 24.5 ± 4.2; Group B: 23.8 ± 3.9 Inclusion criteria: women admitted to the prenatal wards for primary or repeat elective CS were screened to determine eligibility for inclusion. Condition for hospitalisation: primary or elective CS Exclusion criteria: urinary infection (assessed clinically and by midstream urinalysis); significant vaginal bleeding; severe pre‐eclampsia or eclampsia; and/or any other conditions requiring post‐operative monitoring of urinary output; contraindications for general anaesthesia Use of antibiotic prophylaxis: cefazolin 2 g IV single dose 30 min before surgery |
|
| Interventions |
Group A (n = 150): IUC removed immediately after the procedure Group B (n = 150): IUC removed 12 h post‐operatively Size and type of catheter used: 16F Study definition of short‐term catheterisation (days): not reported Intended duration of catheterisation for each group: Group A: IUC removed immediately post‐op Group B: IUC removed 12 h post‐op |
|
| Outcomes | Significant bacteriuria Urinary retention Dysuria Urinary frequency Urgency Time to post‐op ambulation (h) Time to first void post‐op (h) Hospital stay (h) |
|
| Definition of CAUTI or bacteriuria | Significant bacteriuria = > 105 bacteria per mL urine in a midstream sample collected 24 h post‐operatively | |
| Sponsorship/funding | Not reported | |
| Ethical approval | The study protocol was approved by the Scientific Research Committee, and informed consent was obtained from all participants. | |
| Notes | If patient still had difficulty in passing urine after 6 h and/or if abdominal examination showed palpable urinary bladder, recatheterisation was done | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: “A total of 300 women were allocated into two groups in a 1:1 ratio by block randomisation using computer‐generated random numbers.” Comment: adequate method of randomisation |
| Allocation concealment (selection bias) | High risk | Not reported. Unlikely that allocation concealment was performed |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Not reported. Unlikely blinding was possible |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Not reported |
| Blinding of microbiological outcome (detection bias) | Low risk | Not reported. It is likely that urine samples were sent to a laboratory where the microbiologist would be unlikely to know if patients were in a trial or not |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | No withdrawals reported |
| Selective reporting (reporting bias) | Low risk | All outcomes are reported in both the methods and results section and are fully accounted for |
| Other bias | Low risk | Nothing to indicate any other source of bias |
Ganta 2005.
| Study characteristics | ||
| Methods |
Study design: RCT Dates study conducted: not reported |
|
| Participants |
Setting: West Bromwich Country: UK Population: men Age (mean and SD): overall: 68.9; Group A: 68.2, Group B: 69.9 Inclusion criteria: patients undergoing TURP Condition for hospitalisation: TURP Exclusion criteria: not reported Number of participants: eligible, not reported; 84 randomised; 84 reported Use of antibiotic prophylaxis: not reported |
|
| Interventions |
Group A (n = 40): removal of catheter at 6 am Group B (n = 44): removal of catheter at midnight Size and type of catheter used: not reported Study definition of short‐term catheterisation (days): not reported Intended duration of catheterisation for each group: Group A: removal of catheter at 6 am Group B: removal of catheter at midnight |
|
| Outcomes | Mean volume of first void (mL) Mean time to first void (min) Incidence of recatheterisations Discharged same day as IUC removal Comfort rated on 0‐5 scale |
|
| Definition of CAUTI or bacteriuria | Not reported | |
| Sponsorship/funding | Not reported | |
| Ethical approval | Not reported | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Quote: “… were randomised” Comment: randomisation method unclear |
| Allocation concealment (selection bias) | Unclear risk | Not reported |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | No information given, unlikely that participants and personnel were blinded |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Not reported |
| Blinding of microbiological outcome (detection bias) | Low risk | No microbiological outcomes reported in study |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | No patients lost to follow‐up/dropouts |
| Selective reporting (reporting bias) | Low risk | All outcomes reported in the methods section are accounted for in the results section |
| Other bias | Low risk | Appears to be free from other sources |
Glavind 2007.
| Study characteristics | ||
| Methods |
Study design: RCT Dates study conducted: December 2004‐April 2006 |
|
| Participants |
Number of participants: eligible, not reported; 140 randomised; 134 reported Setting: Aalborg Country: Denmark Population: women Age (mean and range): 61 years (31‐88) Inclusion criteria: women consenting to undergo any type of vaginal prolapse surgery Condition for hospitalisation (e.g. hysterectomy or TURP): vaginal surgery for genital prolapse Exclusion criteria: 1 patient due to bladder perforation during procedure Use of antibiotic prophylaxis: all participants who had a vaginal hysterectomy or high uterosacral suspension operation performed received 1 pre‐operative injection of Cefuroxime. No antibiotic prophylaxis was used in the remaining participants |
|
| Interventions |
Group A (n = 66): IUC removed after 3 h post‐operatively Group B (n = 68): IUC removed next morning Size and type of catheter used (e.g. Foley 16F): not reported Study definition of short‐term catheterisation (days): not reported Intended duration of catheterisation for each group: Group A: 3 h post‐op Group B: IUC removed the next morning after operation |
|
| Outcomes | Minimal bleeding Menstrual bleed Heavier bleed Haematoma Recathetersation Positive urine culture |
|
| Definition of CAUTI or bacteriuria | A positive urine culture was defined as the presence of ≥ 105 cfu/mL. | |
| Sponsorship/funding | Not reported | |
| Ethical approval | All patients who underwent any kind of vaginal prolapse surgery were included in the study after informed consent | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Quote: “Randomisation was performed with sealed envelopes opened at the end of the operation.” Comment: method of randomisation unclear |
| Allocation concealment (selection bias) | Low risk | Quote: “… sealed enveloped opened at the end of the operation” Comment: adequate method of concealment |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Quote: “Patients were surveyed by the nurses in the department” Comment: does not report whether participants or nurses were blinded. Unlikely blinding was possible |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Not reported |
| Blinding of microbiological outcome (detection bias) | Low risk | Quote: “Patients were contacted by telephone after 3 weeks to be informed about the urine culture after 14 days, and questioned about bleeding and retention.” Comment: likely urine samples were sent to a laboratory where the microbiologist would be unaware which patient belonged to the trial |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | Quote: “One patient was excluded from the study because of bladder perforation during the operation. Five patients were excluded because of violation of the protocol, both because they had to have the vaginal pack and catheter removed before time due to pain or because, in error, they did not have the catheter and vaginal pack removed until the next day in spite of belonging to Group 1.” Comment: effect on relevant outcomes unclear |
| Selective reporting (reporting bias) | Low risk | All outcomes reported in methods are the same in the results |
| Other bias | Low risk | Appears to be free from other sources of bias |
Gong 2017.
| Study characteristics | ||
| Methods |
Study design: RCT Dates study conducted: February 2012‐April 2015 |
|
| Participants |
Number of participants: 210 eligible; 198 randomised; 198 reported Setting: First Affiliated Hospital of Chongqing Medical University (FAH‐CMU) Country: China Population: women Age (mean ± SD): A 46.14 ± 8.33; B 45.70 ± 9.63 Inclusion criteria: patients with cervical cancer FIGO stage IB‐IIB Condition for hospitalisation: radical hysterectomy Exclusion criteria: patients were excluded if they had urinary incontinence, interstitial cystitis, cognitive impairment or difficulties in completing the training sheet Use of antibiotic prophylaxis: not reported |
|
| Interventions |
Group A (n = 70): IUC for 48 h with intermittent clamping Group B (n = 128): IUC for 48 h without intermittent clamping Size and type of catheter used: not reported Study definition of short‐term catheterisation (days): not reported Intended duration of catheterisation for each group: A: 48 h B: 48 h |
|
| Outcomes | Recatheterisation Residual urine volume 24 h after removal CAUTI Duration of first catheterisation (days) |
|
| Definition of CAUTI or bacteriuria | Symptomatic UTI was defined as bacteriuria with fever, frequent or painful urination or burning on urination | |
| Sponsorship/funding | None | |
| Ethical approval | The Institutional Review Board of Chongqing Medical University approved the study (File No.: 2012045), and all patients provided written informed consent. | |
| Notes | In the clamping group, bladder reconditioning was performed 2 days before IUC removal. The participants did the intermittent clamping, while the nurses performed the catheter insertion and removal. A designed training sheet was handed to the participants, who were educated on how to clamp the IUC and finish the sheet. In detail, IUCs were clamped for 4 h or until participants had urination desire, followed by a 5‐min urinary drainage, a cycle repeated in the daytime for 2 days. The schedule was chosen because it appeared to mimic a normal pattern of bladder filling and emptying | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: "All patients were randomised on 2:1 using a computer‐generated list into two groups, the clamping group and the control group" Comment: adequate method of randomisation |
| Allocation concealment (selection bias) | Unclear risk | Not reported |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Quote: "Both the researchers and the patients were not blind to the group assignment due to the procedure of the study" Comment: not possible to blind participants due to type of intervention |
| Blinding of outcome assessment (detection bias) All outcomes | High risk | Quote: "Outcome assessors were not blinded in the study..." Comment: outcome assessors not blinded |
| Blinding of microbiological outcome (detection bias) | Low risk | Urine samples would be sent to a laboratory where the allocation of a participant would not be known |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Quote: "Five patients were excluded from the clamping group (three failed to record the training sheet, two had severe urine leakage during clamping because of the unfitted catheters and the catheters were removed without training). Seven patients in the control group dropped out because the catheters were removed in other hospitals and the data were missing.” Comment: adequate reasons for exclusion |
| Selective reporting (reporting bias) | Low risk | Outcomes mentioned under Methods section are reported in Results. All outcomes expected from the objective of this trial are reported. |
| Other bias | Low risk | Appears to be free from other sources of bias |
Gross 2007.
| Study characteristics | ||
| Methods |
Study design: RCT Dates study conducted: not reported |
|
| Participants |
Setting: Kentucky Country: USA Population: mixed Age (mean (SD)): 70.3 (11.7) Inclusion criteria: presence of IUC on admission or inserted during rehabilitation programme; age ≥ 18 years; medical order for catheter removal Condition for hospitalisation: stroke Exclusion criteria: not reported Number of participants: eligible, not reported; 45 randomised; 45 reported Use of antibiotic prophylaxis: not reported |
|
| Interventions |
Group A (n = 26): IUC removal at 10 pm the day the order for removal was written Group B (n = 19): IUC removal at 7 am the day after the order form for removal was written Size and type of catheter used: not reported Study definition of short‐term catheterisation (days): not reported Intended duration of catheterisation for each group: Group A: IUC removal at 10 pm Group B: IUC removal at 7 am |
|
| Outcomes | Time to first void Post‐voided residual urine Volume of first void UTI |
|
| Definition of CAUTI or bacteriuria | The CCD criteria for UTI provided the defining characteristics to determine the presence of infection on admission to rehabilitation | |
| Sponsorship/funding | Not reported | |
| Ethical approval | “Institutional review board approval was obtained.” | |
| Notes | IUCs had been in place an average of 18.2 days (SD = 19.3), a time interval closely corresponding to the length of time since stroke onset (mean 20.5 days, SD 21.3) | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Quote: “Subjects were randomized to groups by drawing sealed envelopes indicating group designation.” Comment: unclear as to how randomisation was done |
| Allocation concealment (selection bias) | Low risk | Quote: “…by drawing sealed envelopes …” Comment: adequate method of allocation concealment |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Not reported, unlikely to have been blinded as to which participant belonged to which intervention. Not possible |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Not reported |
| Blinding of microbiological outcome (detection bias) | Low risk | Urine samples would be sent to a laboratory where the allocation of a participant would not be known |
| Incomplete outcome data (attrition bias) All outcomes | High risk | Recatheterisation data are not presented in the results table 2 (summary of outcomes) |
| Selective reporting (reporting bias) | High risk | Recatheterisation is mentioned as an outcome in the methods section but it is not represented in the results section. |
| Other bias | Low risk | Appears to be free from other sources of bias |
Gungor 2014.
| Study characteristics | ||
| Methods |
Study design: RCT Dates study conducted: January 2012‐January 2014 |
|
| Participants |
Number of participants: eligible, not reported; 58 randomised; 58 reported Setting: Istanbul Country: Turkey Population: women Age (mean ±SD): A 55.7 ± 8.8; B 58.8 ± 10.1; C 55.8 ± 9.0 Inclusion criteria: patients who had applied with the complaints of pelvic organ prolapse and/or urinary incontinence and had undergone anterior colporrhaphy Condition for hospitalisation (e.g. hysterectomy or TURP): colporrhaphy Exclusion criteria: not reported Use of antibiotic prophylaxis: not reported |
|
| Interventions |
Group A (n = 21): IUC removal 2 days post‐op Group B (n = 17): IUC removal 3 days post‐op Group C (n = 20): IUC removal 4 days post‐op Size and type of catheter used (e.g. Foley 16F): not reported Study definition of short‐term catheterisation (days): not reported Intended duration of catheterisation for each group: A: 2 days post‐op B: 3 days post‐op C: 4 days post‐op |
|
| Outcomes | Urinary incontinence (stress and mixed combined) Micturition volume (mL) Residual urine volume (mL) |
|
| Definition of CAUTI or bacteriuria | Not reported | |
| Sponsorship/funding | No funding or grant was used | |
| Ethical approval | Ethics approval was obtained from the Istanbul University Ethics Committee | |
| Notes | Residual urine volume was measured with 10Fr catheter after micturition | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: “Patients were randomized using www.randomization.com programme.” Comment: adequate method of randomisation |
| Allocation concealment (selection bias) | Unclear risk | Not reported |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Not reported. Unlikely that this was possible |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Not reported |
| Blinding of microbiological outcome (detection bias) | Low risk | Urine samples would be sent to a laboratory where the allocation of the participant would not be known. |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | As only an abstract, inadequate information to know about attrition bias |
| Selective reporting (reporting bias) | Low risk | Outcomes in methods are reported in results also |
| Other bias | Low risk | Appears to be free from other sources of bias |
Guzman 1994.
| Study characteristics | ||
| Methods |
Study design: RCT Dates study conducted: January 1990‐December 1991 |
|
| Participants |
Number of participants: eligible, not reported; 106 randomised; 106 reported Setting: Gynaecology Unit of Valdivia Regional Hospital Country: Chile Population: women Age (mean and range): Group A 56 (40‐75); Group B 58 (8‐79); Group C 57 (36‐75) Inclusion criteria: women undergoing vaginal surgery Condition for hospitalisation (e.g. hysterectomy or TURP): vaginal conditions (74 had complete genital prolapse of grade 2 or 3 and 32 had grade 2 or 3 cystoceles) Use of antibiotic prophylaxis: all patients received Quemicetina as antibiotic prophylaxis |
|
| Interventions |
Group A (n = 37): removal of IUC within 24 h Group B (n = 36): removal of IUC at 72 h Group C (n = 33): removal of IUC at 72 h plus bladder re‐training, which involved intermittent clamping of the catheter Size and type of catheter used (e.g. Foley 16F): 14F with 10 mL balloon, latex Study definition of short‐term catheterisation (days): not reported Intended duration of catheterisation for each group: Group A: 1 day (no clamping regime) Group B: 3 days (no clamping regime) Group C: 3 days (with clamping regime) |
|
| Outcomes | Urinary retention Re‐installation of Foley catheter Urine culture > 100,000 cfu Average days spent in hospital (median) |
|
| Definition of CAUTI or bacteriuria | Not reported | |
| Sponsorship/funding | Not mentioned | |
| Ethical approval | Not specified | |
| Notes | Urinary retention defined as residual urine volume of > 100 mL for 2 consecutive micturitions UTI defined by urine cultures All participants were administered prophylactic antibiotics Size of urethral catheter 14F Foley |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Comment: method of randomisation not specified |
| Allocation concealment (selection bias) | Unclear risk | Not reported |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Not reported. Unlikely that blinding was possible due to the nature of the intervention |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Not reported |
| Blinding of microbiological outcome (detection bias) | Low risk | Not reported. Assume cultures were sent to a laboratory and so microbiologist would not know which patient belonged to the trial |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | No evidence to suggest incomplete data |
| Selective reporting (reporting bias) | Low risk | No evidence for selective reporting |
| Other bias | Low risk | No other source of bias identified |
Hakvoort 2004.
| Study characteristics | ||
| Methods |
Study design: RCT Dates study conducted: February 2000‐July 2001 |
|
| Participants |
Country: Netherlands Population: women Inclusion criteria: patients undergoing anterior colporrhaphy Condition for hospitalisation (e.g. hysterectomy or TURP): vaginal prolapse surgery (anterior colporrhaphy) Exclusion criteria: patients with UTI pre‐operatively Number of participants: 100 eligible; 100 randomised; 94 reported Age (median and range): Group A 66 (33‐87); Group B 67 (36‐86) Use of antibiotic prophylaxis: not reported |
|
| Interventions |
Group A (n = 46): IUC was removed on the 5th post‐operative day Group B (n = 48): IUC removed the morning after surgery Size and type of catheter used (e.g. Foley 16F): 14F Foley catheter Study definition of short‐term catheterisation (days): “short term catheterisation” – morning after surgery Intended duration of catheterisation for each group: Group A (standard prolonged) = catheter removal on the 5th post‐operative day Group B (not prolonged catheterisation) = catheter removed the morning after surgery |
|
| Outcomes | Repeated catheterisation Mean catheterisation days per participant Asymptomatic bacteruria Mean hospital stay (days) |
|
| Definition of CAUTI or bacteriuria | Signs of a UTI pre‐operatively was defined as having > 10 white blood cells per high‐power field and significant microscopic bacteriuria (one per high‐power field) in the urine sediment A UTI was defined as the presence of > 105 cfu/mL in the culture. |
|
| Sponsorship/funding | Not reported | |
| Ethical approval | The study design was approved by the institutional medical ethical committee. | |
| Notes | All participants with imminent overfilling, defined as a post‐voiding residual volume of 200 mL or more, had another transurethral catheter inserted for a period of 3 days (recatheterisation). | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Quote: “… patients were randomised by the use of closed non‐diaphane envelopes” Comment: unclear how the randomisation was done |
| Allocation concealment (selection bias) | Unclear risk | Quote: “… use of closed non‐diaphane envelopes” Comment: unclear what these envelopes are |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Not reported. Unlikely blinding was possible |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Not reported |
| Blinding of microbiological outcome (detection bias) | Low risk | Not reported. Suggestive that urine cultures were sent to laboratory and assessed by staff who would not know if patients were in a trial or not. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Quote: “Only 6 patients not included in analysis (4 in group A, 2 in group B). 4 patients excluded in group 1 (2 for UTI, 2 only having posterior colporraphy). 2 patients excluded in group 2 (UTI)” Comment: valid reasons for excluding patients from analysis |
| Selective reporting (reporting bias) | Low risk | All outcomes reported in methods are also reported in results section. Protocol is not available |
| Other bias | Low risk | Appears to be free from other sources of bias |
Hall 1998.
| Study characteristics | ||
| Methods |
Study design: RCT Dates study conducted: not reported |
|
| Participants |
Number of participants: eligible, not reported; 123 randomised; 123 reported Setting: Huddersfield Country: UK Population: mixed Age: not reported Inclusion criteria: all patients in the general surgery ward with short‐term IUC Condition for hospitalisation: hospitalisation for surgery (general) Exclusion criteria: not reported Use of antibiotic prophylaxis: not reported |
|
| Interventions |
Group A (n = 66): IUC removal between 7 am and 9 am Group B (n = 57): IUC removal between 9 pm and 11 pm Size and type of catheter used (e.g. Foley 16F): not reported Study definition of short‐term catheterisation (days): not reported Intended duration of catheterisation for each group: A: removed between 7 am and 9 am B: removed between 9 pm and 11 pm |
|
| Outcomes | Time to first void (mean; min) First void volume (mean; mL) Recatheterisation for retention |
|
| Definition of CAUTI or bacteriuria | Not reported | |
| Sponsorship/funding | Not reported | |
| Ethical approval | Not reported | |
| Notes | The majority of those in the late group had their first void before 6 am, and therefore had a disturbed night’s sleep. This is a conference abstract with limited information. Data were collected from a conference abstract |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Quote: "prospectively randomised" Comment: no further information given |
| Allocation concealment (selection bias) | Unclear risk | Not reported |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Not reported. Unlikely participants were blinded due to intervention |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Not reported. Outcomes are objective and unlikely to be affected by blinding |
| Blinding of microbiological outcome (detection bias) | Low risk | No microbiological outcomes reported |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | Only an abstract, unable to assess withdrawal rates and dropouts |
| Selective reporting (reporting bias) | Low risk | All outcomes reported in methods are reported in results |
| Other bias | Low risk | Appears to be free from other sources of bias |
Han 1997.
| Study characteristics | ||
| Methods |
Study design: RCT Dates study conducted: January 1994‐December 1995 |
|
| Participants |
Number of participants: eligible, not reported; 118 randomised; 101 reported Country: Korea Population: men Age (mean and range): A 64.6 (50‐86); B 68.2 (50‐90) Inclusion criteria: patients with benign prostatic hyperplasia Condition for hospitalisation: TURP Exclusion criteria: chronic urinary retention history; neurogenic bladder; urethral stricture Use of antibiotic prophylaxis: not reported |
|
| Interventions |
Group A (n = 48): IUC removed within 48 h following TURP Group B (n = 53): IUC removed on ≥ post‐op day 3 Size and type of catheter used (e.g. Foley 16F): not reported Study definition of short‐term catheterisation (days): not reported Intended duration of catheterisation for each group: A: < 48 h B: IUC removed after 3 days |
|
| Outcomes | Average catheter indwelling time (days) (range) Average length of hospital stay (days) (mean (range)) Failure to void Fever TUR syndrome Delayed bleeding Urethral stricture Incontinence (> 3 months) Pre‐op UTI |
|
| Definition of CAUTI or bacteriuria | Not reported | |
| Sponsorship/funding | None reported | |
| Ethical approval | Not reported | |
| Notes | Excluded: 17 (bladder injury during TURP, injury of prostatic capsule during TURP, chronic urinary retention history, patient with neurogenic bladder, patients with urethral stricture) Lost to follow‐up: none (they only did the research during hospitalisation) |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Trial authors just reported that the trial was a randomised trial, with no further details |
| Allocation concealment (selection bias) | Unclear risk | Trial authors did not report on allocation concealment |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Trial authors did not report on blinding. Unlikely blinding was possible in this study |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Trial authors did not report on blinding |
| Blinding of microbiological outcome (detection bias) | Low risk | Not reported. Likely samples were sent to a laboratory and so unlikely the microbiologist knew which patient was in the trial |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | Trial authors excluded the patients after surgery and individualised number of cases in each group was not reported |
| Selective reporting (reporting bias) | Unclear risk | Trial authors did not report some important characteristics in the results part that are in the methods, such as prostate volume and PSA. |
| Other bias | Low risk | Appears to be free from other sources of bias |
Hewitt 2001.
| Study characteristics | ||
| Methods |
Study design: RCT Dates study conducted: not reported |
|
| Participants |
Number of participants: eligible, not reported; 20 randomised; 20 reported Setting: Tauranga Country: New Zealand Population: men Age: not reported Inclusion criteria: men requiring radical perineal prostatectomy Condition for hospitalisation: radical perineal prostatectomy Exclusion criteria: not reported Use of antibiotic prophylaxis: not reported |
|
| Interventions |
Intervention for each group (e.g. catheter removal, bladder infusion) with times (e.g. midnight catheter removal): Group A: early IUC removal (n = 10), catheter removed 4‐6 days post‐op Group B: delayed IUC removal (n = 10), catheter removed at 14 days post‐op Size and type of catheter used (e.g. Foley 16F): not reported Study definition of short‐term catheterisation (days): not reported |
|
| Outcomes | Anastomotic leakage at time of urethrogram Recatheterisation Ureteral stenosis developed Use of medication following surgery Catheter‐related symptoms (none – unbearable) (reported for Group B only) |
|
| Definition of CAUTI or bacteriuria | Not reported | |
| Sponsorship/funding | Not reported | |
| Ethical approval | Not reported | |
| Notes | Patients in the early group had a retrograde urethrogram and voiding cystogram performed 4‐6 days post‐op to assess their anastomosis for extravasation. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Quote: “… twenty patients were randomised …” Comment: randomisation method not clear |
| Allocation concealment (selection bias) | Unclear risk | Not reported |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Not reported, unlikely that blinding was possible |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Not reported |
| Blinding of microbiological outcome (detection bias) | Low risk | No microbiological outcomes reported |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | Unclear as to whether there were any dropouts or exclusions |
| Selective reporting (reporting bias) | High risk | Catheter‐related symptoms only reported for group B participants. Protocol not available so not assessed |
| Other bias | Low risk | Appears to be free from other sources of bias |
Huang 2011.
| Study characteristics | ||
| Methods |
Study design: RCT Dates study conducted: not reported |
|
| Participants |
Number of participants: 90 eligible; 90 randomised; 79 reported Setting: London Country: UK Population: women Age (mean (SD)): A 61.21 (10.17); B 63.93 (10.43); C 63.7 (12.55); overall: 62.90 (10.93) Inclusion criteria: women with cystocele of at least stage II, who were symptomatic and desired operative treatment with anterior vaginal repair with or without other concomitant pelvic surgeries Condition for hospitalisation: anterior vaginal wall repair Exclusion criteria: diabetes mellitus; pre‐operative lower UTI; unpredicted complications occurred during the surgery; history of cervical cancer who had undergone radical hysterectomy or those who had ever received radiation therapy Use of antibiotic prophylaxis: ciprofloxacin used during all days of hospitalisation in all three groups |
|
| Interventions |
Group A (n = 30): 2‐day IUC Group B (n = 30): 3‐day IUC Group C (n = 30): 4‐day IUC Size and type of catheter used (e.g. Foley 16F): Foley catheter (no size reported) Study definition of short‐term catheterisation (days): not reported Intended duration of catheterisation for each group: A: 2 days B: 3 days C: 4 days |
|
| Outcomes | Recatheterisation Volume of first time voiding (morning) (mL) Post‐void residual urine UTI |
|
| Definition of CAUTI or bacteriuria | Not reported | |
| Sponsorship/funding | Not reported | |
| Ethical approval | The study design was approved by the Institutional Review Board of Changhua Christian Hospital. | |
| Notes | There was no significant difference in subjective urine frequency, overflow incontinence, and objective urine retention between the 3 groups of participants. Although our data suggest that post‐operative catheterisation for 4 days may carry more risk of discomfort than a shorter duration, 2 days of post‐operative catheterisation may potentially be even longer than necessary and may contribute to patient discomfort and bacteriuria | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: “Patients were randomly allocated to three groups at the day of admission and surgery by letting each participant choose one of 90 envelopes in a large box.” Comment: adequate method of randomisation |
| Allocation concealment (selection bias) | Low risk | Quote: “Each questionnaire was concealed in a white non‐transparent envelope.” Comment: adequate concealment method used |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Not reported. Likely that blinding was possible |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Not reported |
| Blinding of microbiological outcome (detection bias) | Low risk | Not reported. Likely that samples were sent to a laboratory where the microbiologist would not know which patient belonged to the study |
| Incomplete outcome data (attrition bias) All outcomes | High risk | 11/90 not included in analysis Group A: 2/30 (incomplete data collection) Group B: 2/30 (incomplete data collection) Group C: 7/30 (requested early termination from the study secondary to intolerance of catheter discomfort) Differential attrition, reason for withdrawal in 1 group directly related to catheter |
| Selective reporting (reporting bias) | High risk | Stated that aim was "to determine the optimal duration of indwelling urethral catheterization to minimize co‐morbidity" but comorbidities not defined or measured |
| Other bias | Low risk | Appears to be free from other sources of bias |
Ind 1993.
| Study characteristics | ||
| Methods |
Study design: RCT Dates study conducted: not reported |
|
| Participants |
Number of participants: eligible, not reported; 101 randomised; 95 reported Setting: London Country: UK Population: women Age (mean and SD): Group A 49.59 (14.2); Group B 49.84 (16.6) Inclusion criteria: patients who had a urethral Foley catheter inserted at operation Condition for hospitalisation (e.g. hysterectomy or TURP): hysterectomy, posterior exenteration, colposuspension, anterior colporrhaphy, total/radical vulvectomy, radical oophorectomy, ovarian cystectomy, adhesiolysis myomectomy Exclusion criteria: patients who had suprapubic catheters Use of antibiotic prophylaxis: not reported |
|
| Interventions |
Group A (n = 46): removal of IUC at 6 am Group B (n = 49): removal of IUC at midnight Size and type of catheter used (e.g. Foley 16F): Foley catheter Study definition of short‐term catheterisation (days): not reported Intended duration of catheterisation for each group: A: IUC removed at 6 am following operation B: IUC removed at midnight following operation |
|
| Outcomes | Median length of hospital stay Median time to first void Median volume of first void Urinary retention (number of participants who developed urinary retention and required recatheterisation following removal of the catheter) |
|
| Definition of CAUTI or bacteriuria | Not reported | |
| Sponsorship/funding | Not reported | |
| Ethical approval | Not reported | |
| Notes | 6 participants were excluded from the study: 5 for UTI and 1 for taking distigmine | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | High risk | Quote: “Catheter removal was randomized by hospital number …” Comment: method of randomisation not truly random |
| Allocation concealment (selection bias) | High risk | Quote: “Patients with odd hospital numbers had their catheter removed at 6:00 am (group A) and patients with even numbers had their catheters removed at midnight (group B).” Comment: patients and hospital staff can easily tell which patient belongs to which group |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Not reported. Unlikely to have occurred |
| Blinding of outcome assessment (detection bias) All outcomes | High risk | Quote: “age, use of night sedation, incidence of urinary retention, length of hospitalisation and incidence of UTIs were subsequently audited from the hospital notes” Comment: all information regarding each patient was easily available from the notes |
| Blinding of microbiological outcome (detection bias) | Low risk | Quote: “All patients had midstream, urine analysis before operation, and had catheter specimen urine culture on removal” Comment: likely that urine samples were sent to a laboratory for analysis |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Quote: “Six patients were not included in the analysis (5 with pre‐existing postoperative UTIs and 1 patient on distigmine).” Comment: reasons for exclusion of patients is relevant |
| Selective reporting (reporting bias) | Low risk | All outcomes reported in methods are reported in results. Protocol not available |
| Other bias | Low risk | Appears to be free from other sources of bias |
Irani 1995.
| Study characteristics | ||
| Methods |
Study design: RCT Dates study conducted: 1 August 1991‐15 December 1993 |
|
| Participants |
Number of participants: eligible, not reported; 213 randomised; 213 reported Setting: Poitiers Country: France Population: men Age (mean and range): A 70.7 (42‐88); B 70 (58‐85) Inclusion criteria: patients undergoing transurethral prostatic surgery for urinary outflow obstruction due to benign hyperplasia Condition for hospitalisation (e.g. hysterectomy or TURP): transurethral prostatic surgery for urinary flow obstruction due to benign prostate hyperplasia Exclusion criteria: simultaneous bladder neck resection or cystolithotripsy, patients with clinically apparent prostatic carcinoma Use of antibiotic prophylaxis: antibiotics (quinolones) were given from the day of operation until the patient was discharged home. |
|
| Interventions |
A: removal of IUC at 24 h who received TUIP Group 1 (n = 52): IUC removal at 24 h Group 2 (n = 52): IUC removal at surgeons' discretion B: removal of IUC at 48 h who received TURP Group 1 (n = 54): IUC removal at 48 h Group 2 (n = 55): removal of IUC according to surgeons' discretion Size and type of catheter used (e.g. Foley 16F): 20F, 3‐way irrigating latex catheter with a 30 cc balloon, latex with hydrophilic coating Study definition of short‐term catheterisation (days): not reported Intended duration of catheterisation for each group: A (TUIP) Group 1: 24 h, Group 2: at surgeons' discretion B (TURP) Group 1: 48 h, Group 2: at surgeons' discretion |
|
| Outcomes | Number of participants requiring recatheterisation after TUIP Number of participants requiring recatheterisation after TURP Mean length of hospital stay after TUIP Mean length of hospital stay after TURP Complete urinary retention at 3 months after TUIP Complete urinary retention at 3 months after TURP Mean flow at 3 months after TUIP Mean flow at 3 months after TURP Asymptomatic UTI 3 months after TUIP (done via urinalysis at 3‐month follow‐up) Asymptomatic UTI 3 months after TURP (done via urinalysis at 3‐month follow‐up) |
|
| Definition of CAUTI or bacteriuria | Not reported: “Urinary infections at 3 months were asymptomatic and discovered by urinalysis” | |
| Sponsorship/funding | Not reported | |
| Ethical approval | Not reported | |
| Notes | 4 participants lost to follow‐up UTI detected using urinalysis | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: “Immediately postoperatively the patients were randomly divided into 2 groups using a permutation table” Comment: adequate method of randomisation |
| Allocation concealment (selection bias) | Unclear risk | Not reported |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Quote: “The catheter was withdrawn according to the usual criteria of the surgeon …” Comment: unlikely that the participants or the personnel were blinded |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Not reported |
| Blinding of microbiological outcome (detection bias) | Low risk | Quote: “All patients were reviewed 3 months postoperatively with a PSA level and urine culture” Comment: likely that samples were sent to a laboratory and so unlikely that the microbiologist knew which patients would be in the trial |
| Incomplete outcome data (attrition bias) All outcomes | High risk | Quote: “Results excluding 24 patients whose hospitalisation was prolonged for social reasons” Comment: social reasons are not given, thus patients have been excluded for no valid reason. |
| Selective reporting (reporting bias) | Low risk | All outcomes reported in methods are also reported in results. |
| Other bias | Low risk | Appears free from other sources of bias |
Iversen Hansen 1984.
| Study characteristics | ||
| Methods |
Study design: RCT Dates study conducted: not reported |
|
| Participants |
Number of participants: 66 eligible; randomised, not reported; 43 reported Country: Denmark Population: unclear Age (median and range): 70 (24‐85) Inclusion criteria: patients with urethral strictures Condition for hospitalisation (e.g. hysterectomy or TURP): urethral strictures Exclusion criteria: not reported Use of antibiotic prophylaxis: antibiotics were not administered routinely but patients with urinary infections pre‐ or post‐operatively were treated with antibiotics according to urine culture. |
|
| Interventions |
Group A (n = 21): IUC treatment for 1 day Group B (n = 22): IUC treatment for 14 days Size and type of catheter used (e.g. Foley 16F): unclear. Retrograde urethrography was performed with a 10F Foley catheter with balloon Study definition of short‐term catheterisation (days): not reported Intended duration of catheterisation for each group: A: IUC treatment for 1 day post‐op B: IUC treatment for 14 days post‐op |
|
| Outcomes | Complication rate Recurrence of strictures using maximal flow rate ≤ 12 (mL/second) Recurrence of strictures using urethrography Restenosis Patient satisfaction |
|
| Definition of CAUTI or bacteriuria | Not reported | |
| Sponsorship/funding | Not reported | |
| Ethical approval | Not reported | |
| Notes | All participants had voiding interview, flowmetry and retrograde urethrography performed pre‐operatively as well as 3 and 6 months post‐operatively. A Disa flowmeter, type 517B was used for flowmetry Antibiotics were administered only to participants with UTI 23 participants did not complete the operative and post‐operative programme Information regarding reasons for withdrawals and losses to follow‐up provided |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Quote: “For the operation, patients were randomly allocated into two groups …” Comment: randomisation performed although method of randomisation is not stated |
| Allocation concealment (selection bias) | Unclear risk | Not reported |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Not reported. Blinding of participants unlikely to be possible in this situation |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Not reported |
| Blinding of microbiological outcome (detection bias) | Low risk | Quote: “Urinary infections pre‐ or postoperatively were treated with antibiotics according to urine culture …” Comment: suggests that urine samples were sent to a laboratory. Unlikely the microbiologists knew which patient belonged to the trial |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | Quote: “Of the 66 patients admitted to the study, 23 patients did not complete the operative and post‐operative programme.” Comment: large withdrawal numbers. Reasons for withdrawal given but not reported in relation to intervention group |
| Selective reporting (reporting bias) | High risk | Outcomes are not reported in methods and are reported in the results section only. Protocol not available for assessment |
| Other bias | Low risk | Appears to not be at risk of any other bias |
Jang 2012.
| Study characteristics | ||
| Methods |
Study design: RCT Dates study conducted: May 2007‐September 2010 |
|
| Participants |
Number of participants: 113 eligible; 94 randomised (abstract reports 105 randomised); 94 reported (abstract reports 105) Country: Korea Population: mixed Age (mean and range): A 54.0 (48.0‐62.0); B 59.0 (54.0‐66.0) Inclusion criteria: rectal cancer patients 20‐80 years old in general good health, willing to participate in the study, understand and accept to sign the informed consent form, receiving proctectomy for rectal cancer located ≤ 15 cm of the anal verge Condition for hospitalisation: surgery for rectal cancer Exclusion criteria: documented problem of pre‐operative urinary dysfunction, any post‐surgery change in patient condition that requires insertion of IUC after surgery, past history of recurrent UTI or malignancy of urinary system organs, past history of surgery for urinary system organs, current administration of Finasteride or Dutasteride Liver dysfunction (SGOT or SGPT ≥ 100 IU/L), kidney dysfunction (serum creatinine ≥ 3 mg/dL) Use of antibiotic prophylaxis: all patients were given IV injections of a single dose of antibiotic during anaesthesia induction and before the operation |
|
| Interventions |
Group A (n = 47 (abstract reports 51)): tamsulosin 0.2 mg/day orally from the day of the operation to post‐operative day 7 Group B (n = 47 (abstract reports 54)): no intervention Size and type of catheter used (e.g. Foley 16F): 16F or 18F Foley Study definition of short‐term catheterisation (days): not reported Intended duration of catheterisation for each group: Groups A and B: 3 days after operation. On post‐operative day 3, the maximum and average flow rates were checked after removing the IUC. Voided volume, residual urine volume, and IPSS* were checked on post‐op day 7. A IUC was reinserted if the patient failed to void successfully after removing the catheter. Unsuccessful voiding was defined as follows: (1) no voiding sensation for > 6 h after removing the catheter; (2) voided volume < 100 mL; or (3) residual urine volume < 200 mL |
|
| Outcomes | N requiring recatheterisation on post‐op day 3 Voided volume on post‐op day 7 (mL) Residual volume on post‐op day 7 (mL) Hospital stay (days) (median, IQR, N) Other complications (excluding acute voiding difficulty) Wound problem Chylous ascites Ileus Intraluminal bleeding UTI Rectovaginal fistula Anastomotic leakage IPSS (post‐op day 7) QoL due to urinary symptoms |
|
| Definition of CAUTI or bacteriuria | Not reported | |
| Sponsorship/funding | Not reported | |
| Ethical approval | Approved and overseen by the institutional review board of our hospital (approval no. B‐0702‐042‐006) (Seoul National University Bundang Hospital) | |
| Notes | *Scores for individual domains of IPSS also reported, if needed (0–35 scale, higher score = more severe symptoms. QoL component of IPSS (0‐6 scale, higher score = lower QoL) | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: “patients were randomized (1:1)… using computer generated numbers” Comment: adequate method of randomisation |
| Allocation concealment (selection bias) | Unclear risk | Not reported |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Control group gets no intervention at all. Protocol is available on Clinicaltrials.gov record states "double blind (Subject, Caregiver, Investigator)" but there is no description of placebo intervention. Lack of blinding or lack of placebo could influence the care provided or the perception of symptoms. |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Not reported in published version of the report. Protocol is available on Clinicaltrials.gov record states "double blind (Subject, Caregiver, Investigator)" |
| Blinding of microbiological outcome (detection bias) | Low risk | Not reported |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | No withdrawals reported |
| Selective reporting (reporting bias) | Low risk | Outcomes specified in clinicaltrials.gov record are reported |
| Other bias | Low risk | Nothing to indicate any other source of bias |
Jeong 2014.
| Study characteristics | ||
| Methods |
Study design: RCT Dates study conducted: April 2010‐July 2011 |
|
| Participants |
Number of participants: eligible, not stated; 236 randomised; 218 reported in primary analysis, 207 in secondary analysis Setting: Seoul Country: Korea Population: men Age (e.g. mean and SD): Group A (intervention) 63.6 (6.6); Group B (control) 63.4 (8.0) Inclusion criteria: localised or locally advanced prostate cancer; undergoing robot‐assisted laparoscopic radical prostatectomy (RARP); able to provide written informed consent Condition for hospitalisation: RARP Exclusion criteria: patients must not have a history of treatment with alpha blockers within 4 weeks; patients must not have previously undergone transurethral resection, laser therapy, or other surgery of the prostate; patients must not have previously been diagnosed with neurogenic bladder; patients must not have hypersensitivity to trial drug or other alpha‐blockers; patients must not have the participation of other clinical trial within the past 3 months Use of antibiotic prophylaxis: not reported |
|
| Interventions |
Group A (n = 118): treatment with 0.4 mg of tamsulosin from the day before RARP up until 14 days after surgery (tamsulosin group) Group B (n = 118): no tamsulosin treatment (control group) Size and type of catheter used (e.g. Foley 16F): 20 FR Foley catheter Study definition of short‐term catheterisation (days): not reported Intended duration of catheterisation for each group: IUC was removed on the 5th post‐op day for both groups |
|
| Outcomes | ICS male short‐form questionnaire 2 weeks after surgery: voiding sum, incontinence sum, frequency score, nocturia score, QoL item Postvoid residual volume, 2 weeks after surgery (mL) IPSS 2 weeks after surgery (including total score, storage subscale, voiding subscale, IPSS QoL item) AUR (participants with AUR on post‐op day 5 (defined as a painful, palpable or percussable bladder, with the patient unable to pass any urine) Adverse events |
|
| Definition of CAUTI or bacteriuria | Not reported | |
| Sponsorship/funding | This study was supported by Astellas Pharm, Co. | |
| Ethical approval | The study was approved by the local institutional review board and registered at the ClinicalTrial.gov website (ID: NCT01209988) | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Quote: “… randomly assigned” Comment: mentions randomised but does not specify method of randomisation |
| Allocation concealment (selection bias) | Unclear risk | Not reported |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Not possible to blind participants |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Not reported |
| Blinding of microbiological outcome (detection bias) | Low risk | No microbiological outcomes reported |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | No differential attrition. Per‐protocol analysis only |
| Selective reporting (reporting bias) | Low risk | Outcomes in methods also presented in results section |
| Other bias | Low risk | Appears to be free from other sources of bias |
Joshi 2014.
| Study characteristics | ||
| Methods |
Study design: RCT Dates study conducted: July 2008‐December 2009 |
|
| Participants |
Number of participants: eligible, not reported; 70 randomised; 70 reported Setting: Chandigarh Country: India Population: women Age (mean ±SD): A 46.80 ± 6.90; B 45.09 ± 6.44 Inclusion criteria: women undergoing uneventful abdominal hysterectomy with or without salpingo‐oophorectomy Condition for hospitalisation: abdominal hysterectomy with or without salpingo‐oophorectomy Exclusion criteria: anticipated complicated surgical procedure requiring strict fluid replacement post‐operatively; bladder suspension or colporrhaphy surgery; positive or unavailable pre‐operative urine culture report; comorbid illness requiring strict intake output monitoring Use of antibiotic prophylaxis: all patients received 1 dose of antibiotic prophylaxis at the time of surgery and continued post‐operatively as per department protocol |
|
| Interventions |
Group A (n = 35): immediate removal of IUC in the operating room Group B (n = 35): IUC removal after 24 h Size and type of catheter used (e.g. Foley 16F): standard 16F Foley’s catheter with 10 cc balloon Study definition of short‐term catheterisation (days): not reported Intended duration of catheterisation for each group: A: immediate removal of IUC in the operating room B: removal of IUC 24 h post‐operatively |
|
| Outcomes | Recatheterisation (defined as inability to pass urine at the end of 12 h, or failure to void after 2 attempts) Positive urine culture on day 2 post‐op Positive urine culture 2 weeks post‐op Febrile morbidity Pain perception |
|
| Definition of CAUTI or bacteriuria | “The diagnosis of symptomatic UTI was based on the presence of significant bacteriuria accompanied by at least one of the following symptoms: Fever, dysuria, increased frequency of urination, urinary urgency, suprapubic pain, and burning micturition.” | |
| Sponsorship/funding | Not reported | |
| Ethical approval | “Informed consent was obtained from enrolled patients and protocol was approved by the Institute’s Ethical Committee.” | |
| Notes | Pain was assessed with a pictorial questionnaire that assessed the level of pain and location of pain, that is, bladder or urethra versus surgical site. The questionnaires were site‐specific for the pain. All patients were given same analgesia in the post‐operative period. Febrile morbidity was defined as 2 consecutive oral temperatures of > 100.4 °F (37.78 °C) measured 6 h apart. Of 12 culture‐positive most common organism was Escherichia coli. None of these had repeat culture‐positive at 2 weeks. 3/9 culture‐positive cases in late removal group had symptoms of UTI and fever. |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: “Randomization was performed by using a computer generated randomization table” Comment: adequate randomisation method |
| Allocation concealment (selection bias) | Low risk | Quote: “Allocation group was kept in sealed envelope. The operating surgeon was made aware of randomization and accordingly the patient was assigned to one of the two groups. In all cases, the envelope was opened at the end of the surgical procedure” Comment: adequate concealment method |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Not reported. Blinding not possible due to intervention |
| Blinding of outcome assessment (detection bias) All outcomes | High risk | Quote: “a limitation of our study may exist in the fact that the observer of outcome was not blinded to the randomization” Comment: observer was not blinded to randomisation |
| Blinding of microbiological outcome (detection bias) | Low risk | Quote: “A clear voided midstream urine specimen was obtained on the second postoperative day for culture and sensitivity.” Comment: urine samples likely were sent to a laboratory and so microbiologist is unlikely to know which patients belong to the trial |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | No withdrawals reported, all participants who were randomised were included in analysis |
| Selective reporting (reporting bias) | Unclear risk | Symptomatic UTI does not seem to be reported |
| Other bias | Low risk | Appears to be free from other sources of bias |
Jun 2011.
| Study characteristics | ||
| Methods |
Study design: RCT Dates study conducted: from June 2008‐February 2010 |
|
| Participants |
Number of participants: 90 eligible; 90 randomised; 90 reported Setting: Shanghai Country: China Population: mixed Age (mean + SD): Group A 68.71 + 7.60; Group B 71.40 + 7.85 Inclusion criteria: lower urinary tract symptoms such as urinary tract stimulation or urinary tract obstruction; enlarged prostate gland diagnosed with rectal examination or B‐mode ultrasonography; aged between 55‐86 years Condition for hospitalisation: TURP Exclusion criteria: gastric retention; glaucoma; prostatic cancer; detrusor muscle weakness; diabetes; abnormal liver function; severe UTI Use of antibiotic prophylaxis: not reported |
|
| Interventions |
Group A: IUC until the urine turned clear in conjunction with 0.2 mg tamsulosin hydrochloride once a day and 200 mg celecoxib twice a day for a week. Group B: IUC for 5 days post‐op Size and type of catheter used (e.g. Foley 16F): not mentioned Study definition of short‐term catheterisation (days): not mentioned Intended duration of catheterisation for each group: Group A: 1 day Group B: 5 days |
|
| Outcomes | Success rate of the first time catheter removal i.e. participants not requiring recatheterisation Length of hospitalisation Incidence of urinary retention Cystospasm Haemorrhage |
|
| Definition of CAUTI or bacteriuria | Not reported | |
| Sponsorship/funding | Not reported | |
| Ethical approval | Not reported | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Not reported |
| Allocation concealment (selection bias) | Unclear risk | Not reported |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Not reported. Unlikely that blinding was possible |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Not reported |
| Blinding of microbiological outcome (detection bias) | Low risk | No microbiological outcomes reported |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Appears to be no withdrawals or dropouts. All participants were accounted for in the results section. |
| Selective reporting (reporting bias) | Unclear risk | Not reported |
| Other bias | Low risk | Not reported |
Kamilya 2010.
| Study characteristics | ||
| Methods |
Study design: RCT Dates study conducted: August 2005‐December 2007 |
|
| Participants |
Setting: Kolkata Country: India Population: women Inclusion criteria: patients undergoing vaginal prolapse surgery Condition for hospitalisation: vaginal prolapse surgery Exclusion criteria: women for whom complicated surgical procedure was anticipated (patient with long‐standing prolapse with severe fibrosis); prolapse surgery associated with plan of bladder or vault suspension or repair by mesh; only posterior colporrhaphy Number of participants: 200 eligible; 200 randomised; 197 reported Age (mean ± SD): A 46.9 ± 12.02; B 47.9 ± 12.78 Use of antibiotic prophylaxis: all participants received 2 doses of antibiotic injection ceftriaxone (1 g). 1 just before the operation and another dose 12 h after the 1st dose |
|
| Interventions |
Group A (n = 98): IUC removal on the 1st post‐op day Group B (n = 99): IUC removal in the 4th post‐op day Size and type of catheter used (e.g. Foley 16F): no.16 Foley catheter Study definition of short‐term catheterisation (days): not reported Intended duration of catheterisation for each group: A: 1 day after surgery, plus 3 days if not able to void or when there was no urge within 8 h after catheter removal, or plus 3 days if residual urine volume > 150 mL B: 4 days after surgery, plus 3 days if not able to void or when there was no urge within 8 h after catheter removal, or plus 3 days if residual urine volume > 150 mL |
|
| Outcomes | Mean catheter days Number of participants requiring recatheterisation (if not able to void or when there was no urge within 8 h after the catheter removal, or residual urine volume > 150 mL) (%) Mean hospital days (defined as the time interval between the completion of surgery and hospital discharge) Mean hospital days of recatheterised patients UTI UTI asymptomatic UTI symptomatic Post‐op fever Post‐op antibiotic treatment other than UTI |
|
| Definition of CAUTI or bacteriuria | The presence of UTI was defined as positive urine culture of > 105 cfu/mL. plus one of the following: dysuria, fever > 38.5°C or rigors | |
| Sponsorship/funding | Not reported | |
| Ethical approval | “Ethical approval for the study was obtained from hospital institutional review board. A written informed consent was obtained from all patients before the randomization process.” | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: “Randomization was performed by using a computer generated randomization list drawn up by a statistician.” Comment: adequate randomisation method |
| Allocation concealment (selection bias) | Low risk | Quote: “Assignments were placed in sealed serially numbered opaque envelopes and were revealed only after the end of operative procedure.” Comment: adequate concealment method |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Not reported. Unlikely blinding was possible due to intervention |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Not reported |
| Blinding of microbiological outcome (detection bias) | Low risk | Quote: “Sample of urine was sent for culture during catheter removal.” Comment: urine samples were sent to a laboratory for analysis. Unlikely that microbiologist knew which patient was in the trial and which was not |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | No differential attrition. Adequate explanation for withdrawals |
| Selective reporting (reporting bias) | Low risk | All outcomes mentioned in methods are identical to those presented in the results section |
| Other bias | Low risk | Appears to be free from other sources of bias |
Kelleher 2002.
| Study characteristics | ||
| Methods |
Study design: RCT Dates study conducted: not reported |
|
| Participants |
Number of participants: eligible, not reported; 160 randomised; 160 reported Country: Australia Population: not reported Age (mean and SD): not reported Inclusion criteria: patients admitted to urology or renal unit Condition for hospitalisation: urological surgery Exclusion criteria: patients with suprapubic catheters, those admitted for trial of void, undergone open prostatic or bladder surgery, with dementia or psychiatric illness Use of antibiotic prophylaxis: not reported |
|
| Interventions |
Group A (n = 80): removal of IUC at 6 am Group B (n = 80): removal of IUC at midnight Size and type of catheter used: not reported Study definition of short‐term catheterisation (days): not reported Intended duration of catheterisation for each group: A: removal of catheter at 0600 h the day after the surgery B: removal of catheter at midnight the same day as the surgery |
|
| Outcomes | Time to first void Volume of first void Discharge same day as catheter removal Patients requiring recatheterisation IUC not removed on time |
|
| Definition of CAUTI or bacteriuria | Not reported | |
| Sponsorship/funding | Not reported | |
| Ethical approval | Not reported | |
| Notes | Majority (61%) of the patients had TURP Patients in the midnight group were catheterised within 12 h of catheter removal while patients in the morning removal group were catheterised 24 h‐30 h after catheter removal |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Quote: “… randomly allocated to one of two groups using a computer generated random number table. The odd numbers were allocated to group 1 … the even numbers were allocated to group 2.” Comment: randomisation used however method of randomisation doesn't seem truly random |
| Allocation concealment (selection bias) | Unclear risk | Not reported |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Not reported. Unlikely it was possible to blind participants |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Not reported |
| Blinding of microbiological outcome (detection bias) | Low risk | No microbiological outcomes reported |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Outcomes are reported for all participants. No withdrawals or dropouts reported |
| Selective reporting (reporting bias) | Low risk | All outcomes are reported in both the methods section and the results section |
| Other bias | Low risk | Appears to be no other sources of bias. |
Kim 2012.
| Study characteristics | ||
| Methods |
Study design: RCT Dates study conducted: not reported |
|
| Participants |
Number of participants: eligible, not reported; 67 randomised; 67 reported Country: South Korea Population: men Age: not reported Inclusion criteria: patients who underwent extraperitoneal laparoscopic radical prostatectomy Condition for hospitalisation: radical prostatectomy Exclusion criteria: not reported Use of antibiotic prophylaxis: not reported |
|
| Interventions |
Group A (n = 30): IUC removed on post‐op day 3, 4 Group B (n = 37): IUC removed on post‐op day 7, 8 Size and type of catheter used: not reported Study definition of short‐term catheterisation (days): not reported Intended duration of catheterisation for each group: A: 3 or 4 days post‐op B: 7 or 8 days post‐op |
|
| Outcomes | Recatheterisation Continence at 3 months (defined as ≤ 1 pad per day) Time to acquisition of continence (months) Complications Hospital duration |
|
| Definition of CAUTI or bacteriuria | Not reported | |
| Sponsorship/funding | Not reported | |
| Ethical approval | Not reported | |
| Notes | All information is from a conference abstract | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Quote: “… randomly categorised…” Comment: mentions randomised but randomisation method is not defined |
| Allocation concealment (selection bias) | Unclear risk | Not reported |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Not reported. Unlikely it is possible to blind participants |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Not reported |
| Blinding of microbiological outcome (detection bias) | Low risk | Not reported |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | No withdrawals reported. As only abstract, full data check is not possible |
| Selective reporting (reporting bias) | Low risk | Outcomes seem to be reported in full. |
| Other bias | Low risk | Appears to be free from other sources of bias |
Koh 1994.
| Study characteristics | ||
| Methods |
Study design: RCT Dates study conducted: September 1992‐December 1992 |
|
| Participants |
Setting: Leeds Country: UK Population: men Inclusion criteria: patients undergoing TURP for bladder outflow obstruction Condition for hospitalisation: TURP Exclusion criteria: patients whose urine was still darkly blood‐stained or whose temperature was above 38 °C. In addition, 1 patient was excluded because he had sustained an iatrogenic injury and 5 others because they had chronic retention of urine and a longer period of catheterisation was considered to be beneficial Number of participants: 96 eligible; 59 randomised; 59 reported Age (mean (SD)): Group A 68.8 (7.3); Group B 73.0 (7.6) Use of antibiotic prophylaxis: “Antibiotics were given at induction to patients with indwelling catheters or proven urinary tract infections” |
|
| Interventions |
Group A (n = 29): IUC removed on 1st post‐op day Group B (n = 30): IUC removed on 2nd post‐op morning Size and type of catheter used: not reported Study definition of short‐term catheterisation (days): not reported Intended duration of catheterisation for each group: A: IUC removal on the 1st post‐op morning B: IUC removal on the 2nd post‐op morning |
|
| Outcomes | Average length of hospital stay Incidence of recatheterisation Incidence of UTI Incidence of secondary haemorrhage Incidence of DVT |
|
| Definition of CAUTI or bacteriuria | Not reported | |
| Sponsorship/funding | Not reported | |
| Ethical approval | Not reported | |
| Notes | 31 patients excluded prior to randomisation because urine was still darkly blood stained or had a temperature above 38 degrees centigrade 1 patient excluded because he had iatrogenic injury 5 others excluded because they had chronic retention of urine and required a longer period of catheterisation |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Quote: “Randomized into two groups …” Comment: unclear how randomisation was performed |
| Allocation concealment (selection bias) | Unclear risk | Not reported |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Not reported. Unlikely it was possible in this respect to blind participants |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Not reported |
| Blinding of microbiological outcome (detection bias) | Low risk | Quote: “… patients who were found to have positive urine cultures in specimens taken at the time of catheter removal: as they had already been discharged by the time the results were available this information was communicated to their general practitioners who treated them appropriately” Comment: suggests that microbiologist would have received the samples at the laboratory like every other patient |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Quote: “Excluded were 31 patients whose urine was still darkly blood‐stained or whose temperature was above 38°C …” Comment: no withdrawals reported. All those who were randomised went on to complete the trial. Any participant excluded was excluded with valid reason |
| Selective reporting (reporting bias) | Low risk | All outcomes in methods section accounted for in the results sections. Protocol not available. |
| Other bias | Low risk | No indications of other bias |
Kokabi 2009.
| Study characteristics | ||
| Methods |
Study design: RCT Dates study conducted: not reported |
|
| Participants |
Number of participants: eligible, not reported; 189 randomised; 189 reported Country: Iran Population: women Age: not reported Inclusion criteria: patients who had undergone anterior colporrhaphy due to pelvic organ prolapse and stress incontinence Condition for hospitalisation: anterior colporrhaphy for pelvic organ prolapse Exclusion criteria: > 1 surgery at the time of colporrhaphy were excluded Use of antibiotic prophylaxis: not reported |
|
| Interventions |
Group A (n = 62): removal of IUC after 1 day Group B (n = 64): removal of IUC after 2 days Group C (n = 63): removal of IUC after 4 days “In all three groups, the catheter Foley were clamped every 4 hrs for 3 times” Size and type of catheter used: not reported Study definition of short‐term catheterisation (days): not reported Intended duration of catheterisation for each group: A: 1 day B: 2 days C: 4 days |
|
| Outcomes | Number requiring recatheterisation Post‐void residual volume > 68% Post‐void residual volume < 33% Post‐void residual volume between 33% and 68% UTI |
|
| Definition of CAUTI or bacteriuria | Not reported | |
| Sponsorship/funding | Financial support from Department of Research and Education of Fasa Medical University for their financial supports | |
| Ethical approval | Not reported | |
| Notes | In all 3 groups, the catheter Foley was clamped 3 times every 4 h to keep bladder ready for urination. Finally, before opening the Foley clamp, the catheter was removed from the bladder and the participants were guided for immediate urinary evacuations. In the meantime, the residual urine was collected and measured. The ratio of the post‐void residual urine volume and the total urine volume of each participant were measured. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Quote: “… selected randomly and divided into three different groups … the patients were divided in three groups according to their post void residual volume of less than 33%, between 33 to 68% and more than 68%.” Comment: unclear how randomisation process occurred. It seems that patients were randomised into 3 groups and then further stratified after their post‐void residuals were obtained. |
| Allocation concealment (selection bias) | High risk | Quote: “The patients were divided in three groups according to their post void residual volume of less than 33%, between 33 to 68% and more than 68%.” Comment: concealment did not occur as investigator needs to know which participant belongs to which group in order to do this. |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Not reported. Unlikely blinding occurred due to the type of intervention |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Not reported |
| Blinding of microbiological outcome (detection bias) | Low risk | Not reported. Unlikely that microbiologist knew patients belonged to the trial |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | Quote: “The patients who had more than one surgery at the time of Colporrhaphy were omitted” Comment: unclear what this refers to e.g. were they excluded before or after randomisation? |
| Selective reporting (reporting bias) | Unclear risk | Some outcomes in methods not reported in results fully e.g. post‐void residual volume (mL) and total urine volume (mL) |
| Other bias | Low risk | Appears to be free from other sources of bias |
Lang 2020.
| Study characteristics | ||
| Methods |
Study design: RCT Setting: The Christ Hospital, Cincinnati, USA Dates study conducted: November 2014‐August 2017 |
|
| Participants |
Population: women Inclusion criteria: all women presenting to The Christ Hospital for gynaecologic surgery anticipated to require at least a 1‐night stay and who would be expected to have an IUC overnight Condition for hospitalisation: benign gynaecological surgery Exclusion criteria: patients with a current UTI being treated with antibiotic(s), or anticipated to undergo concomitant prolapse or incontinence surgery, or a pre‐operative diagnosis of gynaecologic malignancy, or a history of chronic IUC use, or a history of renal transplant or current dialysis use, or intraoperative lower urinary tract injury necessitating prolonged post‐op catheter use Number of participants: 200 eligible; 200 randomised; 164 reported Age (mean and SD): 44.4 ± 8.8 years Use of antibiotic prophylaxis: all participants received pre‐operative antibiotics with either The American College of Obstetricians and Gynecologists approved dosing of cefazolin (78%) or a combination of gentamicin and clindamycin (22%) with no difference between fast‐track or conventional Foley management groups (P = 0.54). |
|
| Interventions |
Group A (n = 81): IUC removal 4‐h post‐op (“fast track”) Group B (n = 83): IUC removal day 1 post‐op (“conventional”) Size and type of catheter used: not reported Study definition of short‐term catheterisation (days): 1 day Intended duration of catheterisation for each group: Group A: 4 h post‐op Group B: 1 day post‐op |
|
| Outcomes | Median dwell time for Foley catheters Voiding trial failure rate Incidence of UTI |
|
| Definition of CAUTI or bacteriuria | Not reported | |
| Sponsorship/funding | Not reported | |
| Ethical approval | The institutional review board at The Christ Hospital approved this trial investigating 2 catheter management strategies among postgynaecologic surgery patients | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: “Permuted block randomization was performed, via “Microsoft Excel,” with a block size of 4 used to ensure balanced enrolment.” Comment: adequate method of restricted randomisation |
| Allocation concealment (selection bias) | Low risk | Quote: “The allocation sequence was concealed from the researcher enrolling patients through the use of sequentially numbered, opaque sealed envelopes” Comment: adequate allocation method |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Not reported. Unlikely possible given intervention |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Not reported |
| Blinding of microbiological outcome (detection bias) | Low risk | Not reported. Assumed microbiologist was blinded |
| Incomplete outcome data (attrition bias) All outcomes | High risk | Quote: "In addition, our study had a dropout rate of 38%. This is likely due to the fact that postoperative follow‐up was obtained via phone calls and not in‐person at the time of a
clinic visit." Comment: 124 participants included in the final analysis from the original 200 participants who were randomised. Large dropout due to loss to follow‐up |
| Selective reporting (reporting bias) | Low risk | Outcomes mentioned in methods are reported in results section. Protocol not available for assessment |
| Other bias | Low risk | Appears to be free from other sources of bias |
Lau 2004.
| Study characteristics | ||
| Methods |
Study design: RCT Dates study conducted: January 2002‐June 2003 |
|
| Participants |
Number of participants: eligible, unclear; 60 randomised; 60 reported Setting: Hong Kong Population: mixed Age (mean and SD): overall mean 63.3 (14.9) Inclusion criteria: all patients who underwent inpatient elective general surgery Condition for hospitalisation: all elective patients in general surgery Exclusion criteria: ambulatory surgery, endoscopic procedures, procedures performed under local anaesthesia, urological procedures, as well as abdominal operations that required pre‐operative IUC Use of antibiotic prophylaxis: “In the present study a single dose of parenteral antibiotic was given upon induction of general anaesthesia in most cholecystectomies, hernia repairs, gastrointestinal and anorectal operations. This could account for the low incidence of urinary tract infection.” |
|
| Interventions |
Group A (n = 31): in‐out catheterisation Group B (n = 29): IUC overnight Size and type of catheter used: not reported Study definition of short‐term catheterisation (days): not reported Intended duration of catheterisation for each group: A: "in‐out catheterization" B: IUC until 24 h after surgery |
|
| Outcomes | Recatheterisation after removal of IUC Positive urine culture Mean length of hospital stay | |
| Definition of CAUTI or bacteriuria | Not reported | |
| Sponsorship/funding | This project was partly supported by The Tung Wah Group of Hospitals Research Fund | |
| Ethical approval | The research protocol was approved by the Hospital Ethics Committee of Tung Wah Hospital | |
| Notes | Urinary retention was defined as the requirement of IUC, which was performed only if the patient failed to pass urine and was found to have a palpable urinary bladder. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | High risk | Quote: “Randomization method was based on the patient’s hospital number. Patients whose hospital number was odd were assigned to in–out catheterization while the patients with even hospital numbers were randomized to have the catheter left indwelling until 24h after operation” Comment: randomisation process not truly random |
| Allocation concealment (selection bias) | High risk | Group allocation can be worked out due to odd or even number of patient’s hospital number |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Not reported. Not possible to blind participants |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Not reported |
| Blinding of microbiological outcome (detection bias) | Low risk | Quote: “Catheterized urine was sent for routine microscopy and culture.” Comment: unlikely microbiologist knew which patients were in the study |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | No withdrawals reported. All patients that were randomized had their outcomes reported. |
| Selective reporting (reporting bias) | Low risk | Outcomes are reported both in the methods section and results section in full. No protocol was available. |
| Other bias | Low risk | Appears to be free from other sources of bias |
Li 2014.
| Study characteristics | ||
| Methods |
Study design: quasi‐RCT, single‐centre study Dates study conducted: not reported |
|
| Participants |
Number of participants: 128 randomised Setting: not reported Country: Mongolia Population (men/women/mixed): patients in the hospital with benign prostatic hyperplasia Condition for hospitalisation (e.g. hysterectomy or TURP): benign prostatic hyperplasia Age (mean and SD): range 56–92. No further details reported Use of antibiotic prophylaxis: not reported |
|
| Interventions |
Group A (n = 64): removal of IUC on post‐op day 1‐2 Group B (n = 64): removal of IUC on post‐op days 5‐7 Size and type of catheter used (e.g. Foley 16F): not reported Study definition of short‐term catheterisation (days): not reported Intended duration of catheterisation for each group: Group A: removal of IUC on post‐op day 1‐2 Group B: removal of IUC on post‐op days 5‐7 |
|
| Outcomes | Length of hospital stay Residual urine Infection Complication (urethral strictures) |
|
| Definition of CAUTI or bacteriuria | Not reported | |
| Sponsorship/funding | None reported | |
| Ethical approval | None reported | |
| Notes | Paper translated | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | High risk | Patients were allocated to intervention and control group using a random number chart, based on the order they completed surgery |
| Allocation concealment (selection bias) | Unclear risk | Allocation concealment not reported |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Likely not possible given intervention |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Not reported in translation of trial |
| Blinding of microbiological outcome (detection bias) | Low risk | Not reported. Unlikely that microbiologist knew participants belonged to the trial |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | All data accounted for |
| Selective reporting (reporting bias) | Low risk | Appears to be free from reporting bias |
| Other bias | Low risk | Nothing to suggest any other source of bias from translation |
Liang 2009.
| Study characteristics | ||
| Methods |
Study design: RCT Dates study conducted: July 2007‐January 2008 |
|
| Participants |
Number of participants: 162 eligible; 150 randomised; 150 reported Setting: Taiwan Population: women Age (mean ± SD): A 43.7 ± 3.9; B 45.7 ± 3.5; C 45.7 ± 5.8 Inclusion criteria: consenting women undergoing laparoscopic‐assisted vaginal hysterectomy. Included uterine myoma, adenomyosis, tubo‐ovarian abscess, intra‐epithelial neoplasia of the cervix, grade 3 and intractable hemorrhagic Condition for hospitalisation: hysterectomy Exclusion criteria: patients that had pelvic organ prolapse or urodynamic stress incontinence or found with bacteriuria form pre‐operative urinalysis or clinically adverse urinary symptoms such as dysuria, frequency of micturition, urgency stress incontinence or obstructive voiding symptoms Use of antibiotic prophylaxis: IV prophylactic antibiotics consisting of cefazolin 500 mg after induction of general anaesthesia |
|
| Interventions |
Group A (n = 50): no IUC Group B (n = 50): IUC removed after 1 day Group C (n = 50): IUC removed after 2 days Size and type of catheter used: indwelling Foley catheter Study definition of short‐term catheterisation (days): not reported Intended duration of catheterisation for each group: A: no IUC use post‐op B: IUC removed 1 day post‐op (removal at 7 am‐8 am) C: IUCremoved 2 days post‐op (removal at 7 am‐8 am) |
|
| Outcomes | UTI Urinary retention Duration of catheter time |
|
| Definition of CAUTI or bacteriuria | UTI was defined as a positive urine culture with colonies of bacteria > 105 organisms/μL. However, treatment was instituted for positive urine cultures only if the patient had adverse urinary symptoms or post‐op pyrexia (> 38 °C). | |
| Sponsorship/funding | This work was supported by Medical Research Project Grant CMRPG 360291 and BMRP 412 from Chang Gung Memorial Hospital | |
| Ethical approval | The ethics committee of the hospital approved the study protocol (No. 95‐1179B). | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Quote: “Randomly allocated …” Comment: unclear as to how randomisation was actually performed |
| Allocation concealment (selection bias) | Low risk | Quote: “Randomization was achieved by selection of sealed envelopes, which were opened just before surgery. When patients’ number in each group reached 50, we ended the patient collection.” Comment: adequate method of concealment |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Not reported. Likely not possible to blind participants |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Not reported |
| Blinding of microbiological outcome (detection bias) | Low risk | Not reported. Unlikely that microbiologist knew participants belonged to the trial |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | No withdrawals reported |
| Selective reporting (reporting bias) | Low risk | All outcomes reported in full in methods and results sections |
| Other bias | Low risk | Appears to be free from other sources of bias |
Lista 2020.
| Study characteristics | ||
| Methods |
Study design: RCT Dates study conducted: September 2016‐May 2017 |
|
| Participants |
Population: men Setting: Milan Country: Italy Inclusion criteria: inclusion criteria were age ≤ 75 years, signed informed consent, and absence of contraindications to robotic surgery. Furthermore, only patients with a negative leakage test, performed intraoperatively with intravesical administration of 250 cc of diluted methylene blue, were included. Condition for hospitalisation: robot‐assisted radical prostatectomy for localised prostate cancer Exclusion criteria: previous prostatic or urethral surgery, previous pelvic radiation therapy, presence of urethral disease (e.g. urethral strictures and diverticulum), and pre‐existing urinary stress, urge, or mixed incontinence Number of participants: 206 eligible; 176 randomised; 146 reported Age (median and range): A 63 (48‐75); B 64 (45–75) Use of antibiotic prophylaxis: not reported |
|
| Interventions |
Group A (n = 72): IUC removal post‐op day 3 Group B (n = 74): IUC removal post‐op day 5 Size and type of catheter used: not reported Study definition of short‐term catheterisation (days): not reported Intended duration of catheterisation for each group: Group A: removal 3 days post‐op Group B: removal 5 days post‐op |
|
| Outcomes | AUR Length of hospital stay UTI at 30 days |
|
| Definition of CAUTI or bacteriuria | Not reported | |
| Sponsorship/funding | None | |
| Ethical approval | After Ethical Committee approval (internal protocol no. 1624) | |
| Notes | "In addition, the economic impact of this strategy has been evaluated. A significant reduction in costs was observed in group 1, with €296 saved per patient and with a total amount of approximately €80 000 saved yearly.Considering also the potential number of hospital beds gained, it has been estimated that almost €320 000 per year could be saved as an additional benefit". | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Quote: “were randomly allocated with a 1:1 ratio to the two study arms” Comment: method of randomisation unclear |
| Allocation concealment (selection bias) | Unclear risk | Not reported |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Not reported. Unlikely given nature of intervention |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Not reported |
| Blinding of microbiological outcome (detection bias) | Low risk | Not reported. It is likely that urine samples were sent to a laboratory where the microbiologist would be blinded to participants involved in the trial |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | Figure 1 illustrates 7 participants lost to follow‐up with no clear explanation |
| Selective reporting (reporting bias) | Low risk | All outcomes stated in methods section and protocol reported |
| Other bias | Low risk | Appears to be free from other sources of bias |
Liu 2015.
| Study characteristics | ||
| Methods |
Study design: quasi‐RCT Dates study conducted: February 2012‐June 2012 |
|
| Participants |
Number of participants: 89 eligible; 79 randomised; 79 reported Setting: Beijing Country: China Population: mixed Age (mean ± SD): A 51 ± 13.2; B 52 ± 16.4 Inclusion criteria: undergone neurosurgery; IUC in situ upon return from the operating theatre; planned IUC duration of 1‐14 days; aged 18‐85 years; willingness to participate in the study; pre‐operatively able to urinate without problem and express the intention to urinate Condition for hospitalisation: patients undergoing neurosurgery Exclusion criteria: IUC in situ pre‐operatively; history of UTI; prostatic hyperplasia; urologic problems or sensory disorders; unable to communicate; signs of cognitive impairment defined as: disorientation to place, time or person, disorganised thinking or agitation Use of antibiotic prophylaxis: not reported |
|
| Interventions |
Group A (n = 39): no clamping of participants' IUC i.e. control group Group B (n = 40): clamping of participants' IUC i.e. observation group Size and type of catheter used: not reported Study definition of short‐term catheterisation (days): not reported Intended duration of catheterisation for each group: A: no clamping of participants' IUC i.e. control group B: clamping of participants' IUC i.e. observation group |
|
| Outcomes | Time to first void Urinary retention requiring re‐catheterisation Abnormal micturition function Volume of first void Dysuria Incomplete voiding |
|
| Definition of CAUTI or bacteriuria | Not reported | |
| Sponsorship/funding | Not reported | |
| Ethical approval | “The study was approved by a University Ethics Review Board and Director of Nursing. The research protocol conformed with the provisions of the Declaration of Helsinki (1995).” | |
| Notes | “the IDC [indwelling catheter] was clamped immediately upon return from the operating theatre and unclamped at certain intervals. The intervals were adjusted by the bedside nurse depending on the patient’s input and output volumes, in order to avoid over distension of the bladder. If the patient was receiving intravenous fluids, the IDC was unclamped at 2–3 h intervals for 10 min at a time. If the patient was not receiving intravenous fluids, the IDC was unclamped at 3–4 h intervals. During catheter clamping periods, patients were told to notify the nurse when they felt the need to urinate and that the nurse would then unclamp the catheter. The duration of each unclamping period was 10 min to allow for complete bladder emptying. For removal, nurses clamped the catheter again and removed it clamped when the patient felt the need to urinate.” | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | High risk | Quote: “The neurosurgical ward has four structural divisions: A, B, C and D. Participants admitted to divisions A and B were in the observation group, and those admitted to C and D, the control group” Comment: used quasi‐randomisation |
| Allocation concealment (selection bias) | High risk | Quote: “The neurosurgical ward has four structural divisions: A, B, C and D. Participants admitted to divisions A and B were in the observation group, and those admitted to C and D, the control group” Comment: no allocation concealment as used quasi‐randomisation |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Quote: “The study was not blinded. This was not actually possible and might have increased the risk of observer bias.” |
| Blinding of outcome assessment (detection bias) All outcomes | High risk | Quote: “The study was not blinded.” Comment: blinding not performed |
| Blinding of microbiological outcome (detection bias) | Low risk | Not reported. Unlikely that microbiologist would know which patient belonged to the study when samples were sent to the laboratory |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | No withdrawals reported |
| Selective reporting (reporting bias) | Low risk | All outcomes are reported in full. |
| Other bias | Low risk | No other indications to other sources of bias |
Lyth 1997.
| Study characteristics | ||
| Methods |
Study design: RCT Dates study conducted: not reported |
|
| Participants |
Number of participants: eligible, not reported; 118 randomised; 107 reported Country: UK Population: unclear Age (mean and SD): not reported Inclusion criteria: TURP or bladder neck incision Condition for hospitalisation: TURP or bladder neck incision Exclusion criteria: not reported Use of antibiotic prophylaxis: not reported |
|
| Interventions |
Group A (n = 33): removal of IUC at 6 am Group B (n = 39): removal of IUC at midnight A third group of 35 participants were not included in our analysis because they received an intervention (infusion trial of micturition) that was outside the scope of this review. Size and type of catheter used: not reported Study definition of short‐term catheterisation (days): not reported Intended duration of catheterisation for each group: A: IUC removed at 6 am B: IUC removed at midnight C: infusion trial of micturition (infusion performed by nursing staff, infusing saline from a 500 mL bag of saline via a standard IV giving set attached to the catheter at a fast drip rate until the patient felt the bladder was full) |
|
| Outcomes | Mean volume of first void (mL) Removal of catheter to discharge decision (h; mean, SD) Incidence of urinary retention and recatheterisation Patient satisfaction |
|
| Definition of CAUTI or bacteriuria | Not reported | |
| Sponsorship/funding | Not reported | |
| Ethical approval | Not reported | |
| Notes | 96 participants had TURP and 22 participants had bladder neck incision 11 participants were excluded from the analysis as data on 5 participants were incomplete and 2 participants had to be recatheterised |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Quote: “… randomized trial …” Comment: unclear as to what the randomisation process involved |
| Allocation concealment (selection bias) | Unclear risk | Not reported |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Not reported. Unlikely that participants could have been blinded |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Not reported |
| Blinding of microbiological outcome (detection bias) | Low risk | No microbiological outcomes reported |
| Incomplete outcome data (attrition bias) All outcomes | High risk | 5/118 excluded due to missing data, 6/118 excluded because they "failed the trial and had to be re‐catheterised". Unclear which intervention group these belonged to |
| Selective reporting (reporting bias) | Low risk | Outcomes seem to be reported in full in methods and results sections |
| Other bias | Low risk | Appears to be free from other sources of bias |
Mao 1994.
| Study characteristics | ||
| Methods |
Study design: RCT Dates study conducted: February 1992‐November 1992 |
|
| Participants |
Population: obstetric ward patients who underwent abdominal surgery (total hysterectomy or salpingo‐oophorectomy). No previous urinary incontinence or infection. No urinary leakage or damage during surgery Country: China Condition for hospitalisation: abdominal surgery (total hysterectomy or salpingo‐oophorectomy) Exclusion criteria: ovarian or cervical conditions were exclusions Surgical wounds too severe Number of participants: 227 randomised; 227 reported Age (mean and SD): not reported Use of antibiotic prophylaxis: not reported |
|
| Interventions |
Group A (n = 114): IUC removal same day Group B (n = 113): IUC removal next day Size and type of catheter used (e.g. Foley 16F): not reported Study definition of short‐term catheterisation (days): not reported Intended duration of catheterisation for each group: Group A (intervention): catheter duration 7 am to 8 pm same day (114) Group B (control): catheter duration 7 am to 6 am next day (113) |
|
| Outcomes | Number of participants who passed urine spontaneously after removal (defined as passing spontaneously = able to pass without dribbling or sensation of incomplete urination, post‐void volume < 100 mL, passing more than a small amount. Any of the above present considered failure to pass spontaneously) Amount of urine passed for first voiding Time to first spontaneous passage of urine Total number of times passing urine within 12 h of removal |
|
| Definition of CAUTI or bacteriuria | Not reported | |
| Sponsorship/funding | Not reported | |
| Ethical approval | Not reported | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Randomisation process not reported |
| Allocation concealment (selection bias) | Unclear risk | Not reported |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Not reported. Unlikely possible given nature of intervention |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Not reported |
| Blinding of microbiological outcome (detection bias) | Low risk | Not reported. Can assume urine samples would have been sent to a lab where the microbiologist would have been blinded |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | No evidence to suggest any missing data |
| Selective reporting (reporting bias) | Low risk | No evidence to suggest selective reporting |
| Other bias | Low risk | No other sources of bias noted |
Matsushima 2015.
| Study characteristics | ||
| Methods |
Study design: RCT Dates study conducted: March 2012‐September 2014 |
|
| Participants |
Number of participants: 125 eligible; 119 randomised; 113 reported Country: Japan Population: men Age (mean ± SD): overall mean 65.9 ± 5.5 Inclusion criteria: localised prostate cancer without lymph node and distant metastasis and age < 75 years Condition for hospitalisation: prostate cancer Exclusion criteria: previous radiotherapy; previous prostatic; bladder neck; urethral, or pelvic surgery; presence of an IUC Use of antibiotic prophylaxis: not reported |
|
| Interventions |
Group A (n = 60): IUC removed on post‐op day 2 Group B (n = 59): IUC removed on post‐op day 4 Size and type of catheter used: 20‐Fr Foley catheter Study definition of short‐term catheterisation (days): not reported Intended duration of catheterisation for each group: A 2 days; B 4 days |
|
| Outcomes | AUR/recatheterisation Urinary incontinence (data related to treatment of cancer and not catheterisation); Continence (defined as a pad‐free status) Serious complications Intraoperative urine leakage |
|
| Definition of CAUTI or bacteriuria | Not reported | |
| Sponsorship/funding | Not reported | |
| Ethical approval | Ethical approval for the design of this study was granted by the Keio University Hospital Ethical Committee. Written informed consent was obtained from all patients prior to participation in this study | |
| Notes | This study was registered with the University Hospital Medical Information Network Clinical Trials Registry in Japan (UMIN000014944) on 12 March 2012 | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: “Randomization was carried out after consent using a computer generated random table by an independent researcher who was not directly involved with the study.” Comment: adequate method of randomisation |
| Allocation concealment (selection bias) | Unclear risk | Not reported |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Quote: “Blinding was not possible in this trial because the timing of catheter removal was different.” Comment: blinding was not possible |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Not reported |
| Blinding of microbiological outcome (detection bias) | Low risk | Not reported. No microbiological outcomes measured |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | 3/60 and 3/59 excluded from analysis because of “extravasation”. Comment: unclear how this will affect the outcome measures |
| Selective reporting (reporting bias) | Low risk | All outcomes mentioned in methods are reported in results |
| Other bias | Low risk | Appears free from other sources of bias |
McDonald 1999.
| Study characteristics | ||
| Methods |
Study design: RCT Dates study conducted: November 1995‐October 1996 |
|
| Participants |
Number of participants: eligible, unclear; 48 randomised; 48 reported Country: Australia Population: men Inclusion criteria: patients undergoing TURP Condition for hospitalisation: TURP Exclusion criteria: not reported Age (mean and range): A 66.7 (51‐81); B 68.7 (57‐89); overall: 67.8 (51‐89) Use of antibiotic prophylaxis: not reported |
|
| Interventions |
Group A (n = 20): removal of IUC at midnight Group B (n = 28): removal of IUC at 6 am Size and type of catheter used: not reported Study definition of short‐term catheterisation (days): not reported Intended duration of catheterisation for each group: A: IUC removed at midnight B: IUC removed at 6 am |
|
| Outcomes | Mean volume of first void Mean time to first void Discharged same day as IUC removal Discharged next day |
|
| Definition of CAUTI or bacteriuria | Not reported | |
| Sponsorship/funding | Not reported | |
| Ethical approval | “The study was approved by the research committee; verbal consent was judged adequate for participation in this investigation.” | |
| Notes | 3 participants were withdrawn from analysis as 1 passed urine in the toilet without informing the staff, the second experienced an extended length of stay due to superficial vein thrombosis and the third failed his trial of void for 10 h after catheter removal. There was no significant difference between the 2 groups with respect to tissue pathology. |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Quote: “A random‐digit chart was used to allocate patients” Comment: method of randomisation unclear |
| Allocation concealment (selection bias) | Unclear risk | Not reported |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Not reported. Unlikely participants were able to be blinded. |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Not reported |
| Blinding of microbiological outcome (detection bias) | Low risk | No microbiological outcomes reported |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | 3/48 excluded from analysis. Unclear which group these 3 belonged to. |
| Selective reporting (reporting bias) | Low risk | All outcomes seem to be reported in full in both the methods and results section. Protocol not available. |
| Other bias | Low risk | Appears to be free from other sources of bias |
Naguimbing‐Cuaresma 2007.
| Study characteristics | ||
| Methods |
Study design: RCT Dates study conducted: April 2004‐April 2005 |
|
| Participants |
Population: women Setting: Manila Country: Phillipines Inclusion criteria: women admitted for an elective repeat CS and those who underwent emergency CS for the following indications: malpresentation, multiple gestation, cord accidents, placenta praevia totalis, non‐reassuring fetal status, and previous CS in labour Condition for hospitalisation: CS Exclusion criteria: pregnant patients with concomitant hypertensive diseases, cardiovascular diseases, preeclampsia, eclampsia, gestational diabetes mellitus, bronchial asthma, thyroid disorders, connective tissue diseases and malignancy Number of participants: 240 eligible; 240 randomised; 240 reported Age (mean and SD): not reported Use of antibiotic prophylaxis: not reported |
|
| Interventions |
Group A (n = 120): IUC removal 4 h post‐op Group B (n = 120): IUC removal 24 h post‐op Size and type of catheter used: not reported Study definition of short‐term catheterisation (days): 24 h Intended duration of catheterisation for each group: Group A: 4 h post‐op Group B: 24 h post‐op |
|
| Outcomes | Time to first void Urinary discomfort Time to first ambulate Length of hospital stay Number of participants requiring recatheterisation |
|
| Definition of CAUTI or bacteriuria | Not reported | |
| Sponsorship/funding | Not reported | |
| Ethical approval | Not reported | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: “Subjects were randomly assigned using a table of random numbers into two groups” Comment: adequate randomisation method |
| Allocation concealment (selection bias) | Unclear risk | Not reported |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Quote: “The surgeons were blinded prior to the operation as to where the patient would be included and would only be informed immediately after the cesarean section to give the post‐operative order for urinary catheter removal” Comment: unlikely this was possible given intervention |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Quote: “Interview was done on day 1 post operation by a medical personnel blinded from the study and information as to the time of first void, level of discomfort, time of first ambulation were obtained from each subject” Comment: outcome assessor blinded |
| Blinding of microbiological outcome (detection bias) | Low risk | No microbiological outcomes reported |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | No dropouts or withdrawals |
| Selective reporting (reporting bias) | High risk | No baseline data reported. No data reported for discomfort measured by VAS |
| Other bias | Low risk | Appears to be free from other sources of bias |
Nathan 2001.
| Study characteristics | ||
| Methods |
Study design: RCT Dates study conducted: not reported |
|
| Participants |
Number of participants: eligible, not reported; 107 randomised; 107 reported Setting: Belfast Country: UK Population: women Age (mean ± SD): A 46.5 ± 5.6; B 45.7 ± 5.4 Inclusion criteria: women undergoing benign gynaecological surgery (morning lists) Condition for hospitalisation: benign gynaecological conditions Exclusion criteria: women with permanent indwelling catheters pre‐operatively and those requiring prolonged catheterisation post‐surgery e.g. operations for stress incontinence and gynaecological malignancies Use of antibiotic prophylaxis: not reported |
|
| Interventions |
Group A (n = 52): 6 am IUC removal on the second morning following surgery Group B (n = 55): 12 am catheter removal on the first day of surgery Size and type of catheter used: not reported Study definition of short‐term catheterisation (days): not reported Intended duration of catheterisation for each group: A: until 6 am on the second morning after surgery B: until midnight on first day after surgery |
|
| Outcomes | Volume of first void (mL) Positive catheter specimen urine culture (%) Time to first void (min) Recatheterisation (%) Length of hospitalisation (day of discharge) Requiring night sedation |
|
| Definition of CAUTI or bacteriuria | Not reported | |
| Sponsorship/funding | Not reported | |
| Ethical approval | Not reported | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Quote: “.. were prospectively randomised…” Comment: randomisation method unclear |
| Allocation concealment (selection bias) | Unclear risk | Not reported |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Unlikely that blinding was not possible |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Not reported |
| Blinding of microbiological outcome (detection bias) | Low risk | Not reported. Unlikely that microbiologist knew participants belonged to the trial |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | No withdrawals reported, all patients in the study were included in the analysis |
| Selective reporting (reporting bias) | Low risk | Outcomes are reported in full with no missing data |
| Other bias | Low risk | Appears to be free from any other sources of bias |
Nguyen 2012.
| Study characteristics | ||
| Methods |
Study design: RCT Dates study conducted: March 2009‐July 2011 |
|
| Participants |
Number of participants: eligible, not reported; 24 randomised; 24 reported Setting: Berne Country: Switzerland Population: unclear, potentially mixed Age: not reported Inclusion criteria: patients scheduled for internal urethrotomy Condition for hospitalisation: urethral strictures Exclusion criteria: not reported Use of antibiotic prophylaxis: not reported |
|
| Interventions |
Group A (n = 9): post‐op IUC for 2 days Group B (n = 15): post‐op IUC for 10 days Size and type of catheter used: not reported Study definition of short‐term catheterisation (days): not reported Intended duration of catheterisation for each group: A: 2 days post‐op B: 10 days post‐op |
|
| Outcomes | Recurrent stricture Median stricture length (mm) Post‐void residual volume: pre‐op; 3 months post‐op; 6 months post‐op; 12 months post‐op (no mean reported) IPSS IPSS – S (median (range)): pre‐op; 3 months post‐op; 6 months post‐op; 12 months post‐op IPSS – L (median (range)): pre‐op ; 3 months post‐op; 6 months post‐op; 12 months post‐op |
|
| Definition of CAUTI or bacteriuria | Not reported | |
| Sponsorship/funding | None | |
| Ethical approval | Not reported | |
| Notes | Data obtained from conference abstract and so limited information | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Quote: “Patients were randomised to postoperative …” Comment: randomisation was done but method not stated |
| Allocation concealment (selection bias) | Unclear risk | Not reported |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Not reported. Not possible to blind the participants |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Not reported |
| Blinding of microbiological outcome (detection bias) | Low risk | Not microbiological outcomes reported |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | No withdrawals or dropouts mentioned in the study. Assume all participants went on to complete the study |
| Selective reporting (reporting bias) | Unclear risk | As this is an abstract with limited information, selective reporting seems unclear |
| Other bias | Low risk | Appears to be free from other sources of bias |
Nielson 1985.
| Study characteristics | ||
| Methods |
Study design: RCT Dates study conducted: not reported |
|
| Participants |
Number of participants: 40 eligible; 40 randomised; 40 reported Country: Denmark Population: unclear Age (mean and range): A 64 (21‐81); B 64 (16‐78) Inclusion criteria: not reported Condition for hospitalisation: urethral stricture Exclusion criteria: not reported Use of antibiotic prophylaxis: not reported |
|
| Interventions |
Group A (n = 20): 3 days post‐op IUC Group B (n = 20): 28 days post‐op IUC Size and type of catheter used: 16 Foley silicone catheter Study definition of short‐term catheterisation (days): not reported Intended duration of catheterisation for each group: A: 3 days; B: 28 days |
|
| Outcomes | Incidence of epididymitis Urinary retention after removal of IUC Urethral pain and discharge Successful urethrotomy at 3 months Successful urethrotomy at 6 months |
|
| Definition of CAUTI or bacteriuria | Not reported | |
| Sponsorship/funding | Not reported | |
| Ethical approval | Not reported | |
| Notes | Criteria for assessing results were as follows. Successful: patient satisfied, maximum urinary flow ≥ 10 mL/second Unsuccessful: patient not satisfied and or maximal flow < 10 mL /second | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Quote: “… were randomly allocated …” Comment: method of randomisation not clear |
| Allocation concealment (selection bias) | Unclear risk | Not reported |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Not reported. Unlikely this was possible |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Not reported |
| Blinding of microbiological outcome (detection bias) | Low risk | No microbiological outcomes reported |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | No withdrawals reported |
| Selective reporting (reporting bias) | Low risk | Outcomes are reported in full in both the methods and results sections. Protocol not available |
| Other bias | Low risk | Appears to be free from other sources of bias |
Noble 1990.
| Study characteristics | ||
| Methods |
Study design: quasi‐RCT Dates study conducted: not reported |
|
| Participants |
Number of participants: 108 eligible; 108 randomised; 86 reported Setting: London Country: UK Population: mixed Age (mean and SD): not reported Inclusion criteria: patients requiring urethral catheterisation that were admitted to the urology unit Condition for hospitalisation: urological procedures and surgery Exclusion criteria: patients who had UTI prior to recruitment Use of antibiotic prophylaxis: not reported |
|
| Interventions |
Group A (n = 46): removal of IUC at 6 am Group B (n = 40): removal of IUC at midnight Size and type of catheter used: not reported Study definition of short‐term catheterisation (days): not reported |
|
| Outcomes | Volume of first void Time to first void Discharge same day as IUC removal IUC not removed on time |
|
| Definition of CAUTI or bacteriuria | Not reported | |
| Sponsorship/funding | Not reported | |
| Ethical approval | Not reported | |
| Notes | 22 participants excluded from study due to pre‐existing UTIs More men than women in each group |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | High risk | Quote: “… entered alternately into 1 of 2 groups …” Comment: quasi‐randomisation method |
| Allocation concealment (selection bias) | High risk | Quote: “… entered alternately ….” Comment: unlikely any concealment occurred. Participant group could easily be found |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Not reported. Not likely possible to blind participants |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Not reported |
| Blinding of microbiological outcome (detection bias) | Low risk | No microbiological outcomes reported |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | No withdrawals, all data reported in full |
| Selective reporting (reporting bias) | Low risk | All outcomes seem to be reported in full in both methods and results |
| Other bias | Low risk | Appears to be free from other sources of bias |
Nyman 2010.
| Study characteristics | ||
| Methods |
Study design: RCT Dates study conducted: April 2006‐March 2007 |
|
| Participants |
Number of participants: 348 eligible; 113 randomised; 113 reported Country: Sweden Population: mixed Age (mean and SD): A 79 ± 11.0; B 80 ± 11.2 Inclusion criteria: patients with a hip fracture in need of surgery Condition for hospitalisation: hip fracture; < 50 years Exclusion criteria: < 50 years, had a IUC at the time of admission, showed signs of cognitive impairment or had additional severe physical problems at admission. Use of antibiotic prophylaxis: not reported. However, skin disinfectant was used |
|
| Interventions |
Group A (n = 55): use of clamping in IUC Group B (n = 58): free drainage of IUC Size and type of catheter used: 14 FR Foley catheter Study definition of short‐term catheterisation (days): not reported Intended duration of catheterisation for each group: A: IUC clamped and removed at 6 am on post‐op day 2 B: free‐draining IUC removed at 6 am on post‐op day 2 |
|
| Outcomes | Time required to return to normal bladder function (median (quartiles)) Need for recatheterisation (%) Length of hospital stay, days (mean ± SD) |
|
| Definition of CAUTI or bacteriuria | Not reported | |
| Sponsorship/funding | This research was supported by grants from the Department of Orthopaedics Orebro University Hospital and Centre for Assessment of Medical Technology, Orebro County Council | |
| Ethical approval | Those who agreed to participate signed informed consent forms before data collection. Ethical approval was obtained from the regional ethical review board of Uppsala, Sweden. | |
| Notes | In the Cochrane Review (Griffiths 2007), two trials reported that clamping reduced the time patients needed to return to normal bladder function. However, this trial could not verify those findings. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: “The researcher carried out randomisation using sealed envelopes placed in a random order in two boxes, one for men and one for women” Comment: adequate method of randomisation |
| Allocation concealment (selection bias) | Low risk | Quote: “…through a concealed allocation to the clamped catheter group” Comment: adequate method of allocation concealment |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Quote: “Blinding of group assignment for nurses and patients was not possible in this study.” Comment: blinding was not possible in this study |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Quote: “The primary outcome in this study, return to normal bladder function, was measured with a bladder scan, which is an objective measure (Bent et al. 1997). The measurements were performed in a similar way by the nurses. However, the measurements were made by different persons, and a disadvantage in this study is that the reliability of the measurements was not confirmed.” Comment: unlikely that outcome measure was affected by blinding |
| Blinding of microbiological outcome (detection bias) | Low risk | No microbiological outcomes reported |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Quote: “Five patients did not receive the treatment they were initially randomised to, four patients removed their indwelling catheter themselves by mistake and three patients were transferred to other wards … Adherence to the randomization was 95%” Comment: reasons for withdrawals and exclusions are valid. |
| Selective reporting (reporting bias) | Low risk | All outcomes in methods were accounted for in the results section. Protocol not available |
| Other bias | Low risk | Appears to be free from other sources of bias |
Oberst 1981.
| Study characteristics | ||
| Methods |
Study design: RCT Dates study conducted: not reported |
|
| Participants |
Number of participants: eligible, unclear; 120 randomised; 110 reported Country: USA Population: mixed Age (mean (SD)): A 64.5 (10.26); B: 59 (11.92) Inclusion criteria: patients with IUC following either abdominoperineal resection (APR) or lower anterior resection (LAR) for cancer of the bowel and who had no evidence of existing urinary infection or kidney disease, no medical, problems precluding normal fluid intake, clear sensorium, spoke English and no surgical contradiction to bladder recompression Condition for hospitalisation: bowel cancer surgery – APR or LAR Exclusion criteria: not reported Use of antibiotic prophylaxis: not reported |
|
| Interventions |
Group A (n = 52): IUCs clamped Group B (n = 58): IUCs not clamped (gravity drainage) Size and type of catheter used: not reported Study definition of short‐term catheterisation (days): not reported Intended duration of catheterisation for each group: Group A: clamping. From 4th day post‐op catheter was clamped for increasingly longer periods beginning with 1‐h interval until max 4‐h interval on day 6. Clamping periods alternated with 5 min drainage. Catheter left to straight drainage during the night and on the final day the clamping continued for a full 24 h. Group B: straight drainage. Catheter remained in place until physician advised its removal, usually 10th day post‐op |
|
| Outcomes | Incidence of recatheterisation in patients following APR Incidence of recatheterisation in patients following LAR Time to first void |
|
| Definition of CAUTI or bacteriuria | Not reported | |
| Sponsorship/funding | Not reported | |
| Ethical approval | Not reported | |
| Notes | Clamping commenced on the 4th post‐op day. The IUC was clamped for increasingly longer periods beginning with a 1‐h interval until the maximum 4‐h interval was reached on day 6. Clamping periods were alternated with drainage periods of 5 min. On the first 5 study days, the IUC was left to straight gravity drainage during the night. On the final day the clamping continued for a full 24 h Reasons for withdrawals and dropouts: 3 participants had post‐op complications, 3 had their IUC removed erroneously, 1 was commenced on the trial in error and 3 were unable to follow the schedule |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Quote: “Eligible patients were stratified by sex and surgical procedure and randomly assigned to one of two study conditions.” Comment: method of randomisation unclear |
| Allocation concealment (selection bias) | Unclear risk | Not reported |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Not reported. Unlikely participants were blinded |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Not reported |
| Blinding of microbiological outcome (detection bias) | Low risk | Not reported. Unlikely that microbiologist knew participants belonged to the trial |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Quote: “In addition to the 110 patients in the final sample, 10 other patients were later dropped from the study …” Comment: withdrawal and exclusions from the study are accounted for and reasons provided. Unclear if it will have impact on measured outcomes |
| Selective reporting (reporting bias) | Low risk | All outcomes seem to be accounted for in both results and methods sections |
| Other bias | Low risk | Appears to be free from other sources of bias |
Onile 2008.
| Study characteristics | ||
| Methods |
Study design: RCT Dates study conducted: not reported |
|
| Participants |
Number of participants: eligible, unclear; 200 randomised; 175 reported Country: Nigeria Population: women Inclusion criteria: consenting women having elective CS Condition for hospitalisation: elective CS Exclusion criteria: women with severe pre‐eclampsia or eclampsia post‐op or any other conditions that needed to monitor urinary output. Women with significant growth of bacteria on pre‐operative urine culture Age (mean (SD)): A 31.67 (6.042); B 32.72 (5.96) Use of antibiotic prophylaxis: not reported |
|
| Interventions |
Group A (n = 89): IUC removed after 24 h Group B (n = 86): IUC removed immediately post‐op Size and type of catheter used: Foley 16F Study definition of short‐term catheterisation (days): not reported Intended duration of catheterisation for each group: A: IUC removed after 24 h B: immediate post‐op removal of IUC |
|
| Outcomes | Number needing to be recatheterised/urinary retention Dysuria Urinary incontinence Ambulation time h Hospital stay h 72 h post‐op + urine culture |
|
| Definition of CAUTI or bacteriuria | “… significant bacteriuria— defined as more than 100 000 bacteria of the same colony per milliliter of urine ‐ in a sample of midstream urine collected 72 hours postoperatively for MCS” “ …fever (defined as temperature of 38 °C or more on 2 occasions within 10 days of the procedure, excluding the first 24 hours” |
|
| Sponsorship/funding | Not reported | |
| Ethical approval | Ethical approval was obtained from the ethical clearance committee of the Obafemi Awolowo University Teaching Complex, Ile‐Ife | |
| Notes | No significant difference in post‐op ambulation time between groups A and B. Group A showed lower incidence of positive urine culture compared to group B. Recommend immediate removal of catheter after elective CS | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Quote: “ … were randomized into 2 groups (groups A and B), by block randomization using a random numbers table.” Comment: unclear as to how randomisation process was performed |
| Allocation concealment (selection bias) | Unclear risk | Not reported |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Not possible to blind participants, other measures of blinding are not reported |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Not reported |
| Blinding of microbiological outcome (detection bias) | Low risk | Quote: “Women with a significant growth of bacteria on preoperative urine microscopy, culture, and sensitivity (MCS) were excluded from other parts of the study …” Comment: suggests that samples were sent to a laboratory and so unlikely that microbiologist would know which patient belonged to the trial and which did not |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Withdrawal rates from both groups are similar. Adequate reasons given for withdrawals |
| Selective reporting (reporting bias) | Low risk | Outcomes seem to be reported in full in both methods and results sections. Protocol not available |
| Other bias | Low risk | Nothing to indicate any other sources of bias |
Ouladsahebmadarek 2012.
| Study characteristics | ||
| Methods |
Study design: RCT Dates study conducted: 2009‐2010 |
|
| Participants |
Number of participants: eligible, not reported; 200 randomised; 200 reported Country: Iran Population: women Age (e.g. mean and SD): A 37.48 ± 8.85; B 39.48 ± 9.54 Inclusion criteria: elective abdominal hysterectomy or laparotomy for benign pathology (e.g. fibroma, abnormal uterine bleeding, chronic pelvic pain, ovarian cysts) under general anaesthesia; written informed consent Condition for hospitalisation: abdominal hysterectomy or laparotomy Exclusion criteria: patients who had intraoperative bleeding > 1 L; operation length > 2 h, severe endometriosis; dense pelvic adhesions; bladder suspension; underlying medical problems were excluded from the study Use of antibiotic prophylaxis: cefazoline 1 g IV 30 min before surgery started and continued every 6 h until 2 doses |
|
| Interventions |
Group A (n = 100): Foley catheter removed immediately after surgery Group B (n = 100): Foley catheter removed 24 h after surgery Size and type of catheter used: 14 F Foley catheter with 15 cc balloon Study definition of short‐term catheterisation (days): not reported Intended duration of catheterisation for each group: A 0 h; B 24 h |
|
| Outcomes | Operation to discharge duration (days) Time to ambulation (h) Subjective measure of pain Fever (> 38.5 °C) Use of Nelaton catheter (for AUR) Re‐use of indwelling Foley catheter Urethral burn Urine analysis Symptomatic UTI Dysuria at the beginning of urination |
|
| Definition of CAUTI or bacteriuria | Mentions symptomatic UTIs but no definition given | |
| Sponsorship/funding | Vice Chancellor for Research, Tabriz University of Medical Sciences | |
| Ethical approval | The Ethics Committee of Tabriz University of Medical Sciences approved the study protocol | |
| Notes | We contacted the trial authors for missing data but no we received no reply. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: “The randomization procedure was password protected, web based, using permuted blocks and stratified by study centre and invasive procedure.” Comment: adequate method of randomisation. Randomisation was probably done |
| Allocation concealment (selection bias) | Unclear risk | Not reported |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Not reported. Unlikely that blinding is possible |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Not specified |
| Blinding of microbiological outcome (detection bias) | Low risk | Microbiologists were assumed to be blinded |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | All 200 participants completed the trial |
| Selective reporting (reporting bias) | Low risk | All outcomes are accounted for. Protocol was unavailable for assessment |
| Other bias | Low risk | Appears to be free form other sources of bias |
Pervaiz 2019.
| Study characteristics | ||
| Methods |
Study design: RCT Dates study conducted: January 2018‐June 2018 |
|
| Participants |
Population: men Setting: Lahore Country: Pakistan Inclusion criteria: men between 50‐80 years of age presenting with benign prostate enlargement (history of difficulty in micturition for at least 1 month) undergoing TURP Condition for hospitalisation: TURP Exclusion criteria: abnormal coagulation profile (prothrombin time (PT) > 15 sec; activated partial thromboplastin time (APTT) > 35 s), patients with systemic problems like BP > 140/90 mmHg, blood sugar range > 200 mg/dL, abnormal echocardiogram and ejection fraction < 55% on echocardiography, very large prostate (> 100 g) Number of participants: 100 eligible; 100 randomised; 100 reported Age (mean and SD): A 67.00 ± 9.11; B 65.56 ± 9.25 Use of antibiotic prophylaxis: not reported |
|
| Interventions |
Group A (n = 50): IUC removal day 1 post‐op Group B (n = 50): IUC removal day 4 post‐op Size and type of catheter used: 3‐way Foley catheter Study definition of short‐term catheterisation (days): not reported Intended duration of catheterisation for each group: Group A: removal on post‐op day 1 Group B: removal on post‐op day 4 |
|
| Outcomes | Number of participants requiring recatheterisation UTI |
|
| Definition of CAUTI or bacteriuria | Urine sample was obtained to assess UTI (bacterial colony count >105 cfu/mL on urine culture after removal of catheter assessed on day 7) | |
| Sponsorship/funding | Not reported | |
| Ethical approval | Not reported | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: “Then patients were randomly assigned in two sets by utilizing lottery technique.” Comment: adequate randomisation method |
| Allocation concealment (selection bias) | Unclear risk | Not reported |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Not reported. Unlikely possible given nature of intervention |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Not reported |
| Blinding of microbiological outcome (detection bias) | Low risk | Not reported. Assume lab technician was blinded to participants belonging to the trial |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | No exclusions or withdrawals |
| Selective reporting (reporting bias) | Low risk | All outcomes stated in methods reported in results. Protocol not available for assessment |
| Other bias | Low risk | Appears to be free from other sources of bias |
Popiel 2017.
| Study characteristics | ||
| Methods |
Study design: RCT Dates study conducted: not reported |
|
| Participants |
Number of participants: 75 randomised Setting: not reported Country: not reported Population: women Age (mean and SD): not reported Inclusion criteria: women who were scheduled for robotic sacrocolpopexy Condition for hospitalisation: vaginal prolapse Exclusion criteria: not reported Use of antibiotic prophylaxis: not reported |
|
| Interventions |
Group A (n = 39): Foley catheter removal within 6 h of operation completion (no Foley) Group B (n= 36): Foley catheter removal on day 1 post‐op (Foley) Size and type of catheter used (e.g. Foley 16F): not reported Study definition of short‐term catheterisation (days): not reported Intended duration of catheterisation for each group: Group A: within 6 h Group B: on post‐op day 1 |
|
| Outcomes | Number of participants with urinary retention UTI |
|
| Definition of CAUTI or bacteriuria | Not reported | |
| Sponsorship/funding | Not reported | |
| Ethical approval | Not reported | |
| Notes | Declarations of interest: “Disclosures: P. Popiel: nothing to disclose; V. Vallabh‐Patel: nothing to disclose; C. Salamon: Consultant: Intuitive Surgical, Inc.” | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Quote: “single blinded randomized study” Comment: method of randomisation unclear |
| Allocation concealment (selection bias) | Unclear risk | Not reported |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Not reported. Unlikely possible given nature of intervention |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Not reported |
| Blinding of microbiological outcome (detection bias) | Low risk | Not reported. Can assume specimens were sent to a lab where microbiologist would be unaware of trial participants |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | No details given regarding withdrawals/exclusions etc |
| Selective reporting (reporting bias) | High risk | All outcomes not reported in full |
| Other bias | Low risk | Appears to be free from other sources of bias |
Rajan 2017.
| Study characteristics | ||
| Methods |
Study design: RCT Dates study conducted: September 2008‐March 2010 |
|
| Participants |
Number of participants: 200 participants randomised into 2 groups Setting: tertiary teaching institute South India Country: India Population: women undergoing vaginal surgery Age (mean and SD): Group A: 50 ± 18; Group B: 48 ± 2.4 Inclusion criteria: all women undergoing vaginal surgery namely Ward Mayo operation; Manchester repair; vaginal hysterectomy and amputation of cervix Condition for hospitalisation: vaginal surgery Exclusion criteria: all women having pre‐operative positive urine cultures; elevated renal parameters (blood urea > 40 mg/dL, serum creatinine > 1 mg/dL); comorbid illness ‐diabetes; intra operative visceral injury; Kelly’s stitch and consent not given by patient Use of antibiotic prophylaxis: measured but not reported specifically |
|
| Interventions |
Group A (n = 100): removal of IUC and vaginal pack in 3 h Group B (n = 100): removal of IUC and vaginal pack in 24 h Size and type of catheter used (e.g. Foley 16F): not reported Study definition of short‐term catheterisation (days): not reported Intended duration of catheterisation for each group: Group A: IUC removal 3 h after surgery Group B: IUC removal 24 h after surgery |
|
| Outcomes | Number of participants requiring recatheterisation Incidence of UTI Incidence of urinary retention |
|
| Definition of CAUTI or bacteriuria | “urinary infections defined as when microscopic examination of the urine revealed pus cells or when urine culture showed growth of pathogenic organisms” | |
| Sponsorship/funding | “No external sources of funding” | |
| Ethical approval | “The study was approved by institutional research board (IRB) of Jawaharlal Institute of Post‐graduate Medical Education & Research (JIPMER), Puducherry, India (EC Ref # 5_ 2008)” | |
| Notes | Declarations of interest: “The authors declare that they have no competing interest” | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: “They were randomised into two groups based on a computer‐generated randomization table." Comment: adequate method of randomisation |
| Allocation concealment (selection bias) | Unclear risk | Not reported |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Not possible given intervention |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Not reported |
| Blinding of microbiological outcome (detection bias) | Low risk | Not reported. Likely microbiologist would have been blinded as to which samples were in the trial |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | No evidence of any incomplete data |
| Selective reporting (reporting bias) | Low risk | Outcomes reported in full |
| Other bias | Low risk | Appears to be free from other sources of bias |
Ruminjo 2015.
| Study characteristics | ||
| Methods |
Study Design: RCT Dates study conducted: not reported |
|
| Participants |
Number of participants: not reported Setting: 8 Sub‐Saharan Africa countries Population: women Age (mean and SD): not reported Inclusion criteria: women undergoing fistula repair surgery Condition for hospitalisation: fistula repair Exclusion criteria: not reported Use of antibiotic prophylaxis: not reported |
|
| Interventions |
Group A (n = unknown): IUC for 7 days post‐fistula repair Group B (n = unknown): IUC for 14 days post‐fistula repair Size and type of catheter used (e.g. Foley 16F): not reported Study definition of short‐term catheterisation (days): not reported Intended duration of catheterisation for each group: not reported Group A: 7 days Group B: 14 days |
|
| Outcomes | Urinary retention, catheter blockage and febrile episodes | |
| Definition of CAUTI or bacteriuria | Not reported | |
| Sponsorship/funding | “Randomized clinical trial conducted collaboratively by EngenderHealth’s Fistula Care Project and World Health Organization with key in‐country fistula researchers” | |
| Ethical approval | Not reported | |
| Notes | Abstract only. No usable data | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Not reported |
| Allocation concealment (selection bias) | Unclear risk | Not reported |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Blinding not possible |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Not reported |
| Blinding of microbiological outcome (detection bias) | Low risk | Not reported. Assume urine samples were sent to a laboratory where the microbiologist would not know which patients were in the trial |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | No information about numbers randomised or number of participants included in the analysis |
| Selective reporting (reporting bias) | Unclear risk | Abstract only. Outcomes not reported in full |
| Other bias | Low risk | Appears to be free from other sources of bias |
Sahin 2011.
| Study characteristics | ||
| Methods |
Study design: RCT Dates study conducted: February 2006‐ January 2008 |
|
| Participants |
Number of participants: eligible, not reported; 66 randomised; reported, not reported Setting: Istanbul Country: Turkey Population: men Age (mean): range: 48‐77 (average 62); A 62.5; B 61.5; C 62 Inclusion criteria: surgical candidates diagnosed with benign prostatic hyperplasia Condition for hospitalisation: TURP Exclusion criteria: cases with > 50 cc of residual urine, central and peripheric nervous system illnesses or diabetes were excluded from the study Use of antibiotic prophylaxis: not reported |
|
| Interventions |
Group A (n = 22): IUC removal on the 1st post‐op day Group B (n = 22): IUC removal on the 2nd post‐op day Group C (n = 22): IUC removal on the 3rd post‐op day Size and type of catheter used: 22F 3‐way Foley catheter Study definition of short‐term catheterisation (days): not reported Intended duration of catheterisation for each group: A: IUC removal on the 1st post‐op day B: IUC removal on the 2nd post‐op day C: IUC removal on the 3rd post‐op day Note: catheter removal criteria were defined as having clear or pinkish urine colour and the absence of haemorrhage. 2 cases from Group A and one case from Group B did not meet these criteria; hence their catheters were not removed on the designated day. |
|
| Outcomes | Recatheterisation | |
| Definition of CAUTI or bacteriuria | Not reported | |
| Sponsorship/funding | Not reported | |
| Ethical approval | Not reported | |
| Notes | We determined criteria for recatheterisation to be development of vesical globe, complaints of excessive irritation and the obstruction of urinary flow due to clotted or non‐clotted bleeding | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Quote: “Cases were randomised into three groups” Comment: method of randomisation unclear |
| Allocation concealment (selection bias) | Unclear risk | Not reported |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Not reported. Unlikely blinding was possible |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Not reported |
| Blinding of microbiological outcome (detection bias) | Low risk | No microbiological outcomes reported |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | No withdrawals or exclusions from the study |
| Selective reporting (reporting bias) | High risk | The methods section mentions that urine analysis was performed however no data on infection or bacteriuria were presented in the results |
| Other bias | Low risk | Appears to be free from other sources of bias |
Sandberg 2019.
| Study characteristics | ||
| Methods |
Study design: RCT Dates study conducted: 31 May 2016‐22 July 2017 |
|
| Participants |
Population: women Setting: Leiden Country: Netherlands Inclusion criteria: women > 18 years, scheduled for laparoscopic hysterectomy for benign indication or low‐grade malignancy (with or without salpingo‐oophorectomy) Exclusion criteria: women with concomitant procedures such as prolapse surgery, extensive endometriosis surgery or advanced oncological dissection including nodal dissection, were excluded, as well as those with stress and urge incontinence, or other systemic diseases potentially influencing their ability to void (e.g. multiple sclerosis) Condition for hospitalisation: laparoscopic hysterectomy Number of participants: 162 eligible; 162 randomised; 155 reported Age (mean and SD): A 49.3 ± 10.5; B 51.5 ± 11.9 Use of antibiotic prophylaxis: not reported |
|
| Interventions |
Group A (n = 74): immediate IUC removal post‐op Group B (n = 81): IUC removal 18‐24 h post‐op Size and type of catheter used: “Foley Catheter”, otherwise not specified Study definition of short‐term catheterisation (days): not reported Intended duration of catheterisation for each group: Group A: IUC removed directly in the operating room post‐op Group B: IUC removal 18‐24 h post‐op |
|
| Outcomes | Number needing to be recatheterised UTI Time to first ambulation Length of hospital stay Asymptomatic bacteriuria Number of patients not discharged on day of IUC removal Other complications of catheterisation (or recatheterisation) ‐ requested earlier catheter removal because of “complaints” Patient comfort or discomfort (0‐10 VAS for overall pain and discomfort 6 h after surgery) Patient comfort or discomfort (0‐10 VAS for overall pain and discomfort 24 h after surgery) Patient satisfaction (0‐10 VAS for satisfaction with treatment 6 weeks after surgery) Patient satisfaction (0‐10 VAS for satisfaction with hospitalisation 6 weeks after surgery) |
|
| Definition of CAUTI or bacteriuria | “standard urine test for nitrite and leucocytes in combination with clinical symptoms” | |
| Sponsorship/funding | “There was no patient or public involvement in this study and no core set outcomes were used” |
|
| Ethical approval | The protocol was approved by the Ethics Committee of Leiden University Medical Centre (LUMC) in Leiden, the Netherlands (P15.382/NL55504.058.15) and the boards of all participating hospitals | |
| Notes | Declarations of interest: “EM Sandberg reports receiving a research grant from Bronovo Hospital Fund (The Hague, the Netherlands). The funding source had no involvement during the conduction of the research and/or preparation of the article. The other authors report no conflict of interest. Completed disclosure of interest forms are available to view online as supporting information.” | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: “The randomisation procedure was performed by the operating gynaecologist through an online and secured program called PROMISE. The randomisation sequence was computer‐generated with variable blocks of two and four, stratified by centre” Comment: adequate randomisation method |
| Allocation concealment (selection bias) | Low risk | Quote: “In the operating room, at the end of the surgery, patients were randomised (1:1 ratio) to either ICR or DCR.The allocation code was disclosed directly on the website after entering patient identification number and confirming inclusion criteria." Comment: adequate allocation concealment |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Quote: "Neither the women nor the medical staff were blinded for the allocated treatment." Comment: no blinding of participants or personnel |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Not reported |
| Blinding of microbiological outcome (detection bias) | Low risk | Not reported. Likely specimens sent to a lab where it would not be known whether specimen belonged to a trial or not |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Quote: “Three women withdrew consent within 24 h after surgery and four women were randomised despite the fact that the gynaecologist decided immediately at the end of the surgery that prolonged catheterisation was necessary regardless of the randomisation result. These cases were considered dropouts and were not included in any further analyses” Comment: not all participants who were randomised are included in final analysis but numbers of participants withdrawing are low and balanced across groups. |
| Selective reporting (reporting bias) | Low risk | All outcomes reported in full |
| Other bias | Low risk | Appears to be free from other sources of bias |
Schiotz 1995.
| Study characteristics | ||
| Methods |
Study design: RCT Dates study conducted: November 1992‐April 1994 |
|
| Participants |
Number of participants: eligible, not reported; 165 randomised; 165 reported Country: Norway Population: women Age (mean and range): overall 65.9 (29.9‐85.2) Inclusion criteria: not reported Condition for hospitalisation: elective vaginal plastic repair surgery (anterior colporrhaphy, anterior plus posterior colporrhaphy or a full Manchester repair) Exclusion criteria: not reported Use of antibiotic prophylaxis: not reported |
|
| Interventions |
Group A (n = 82): 1 day IUC post‐op Group B (n = 83): 3 days’ post‐op IUC Size and type of catheter used: 12 or 14F Foley catheter, Teflon‐coated Study definition of short‐term catheterisation (days): not reported |
|
| Outcomes | UTI Urinary retention Number of patients needing to be recatheterised |
|
| Definition of CAUTI or bacteriuria | Cultures were defined as positive when a midstream urine specimen yielded > 100,000 cfu/mL of any organism or a catheter specimen yielded > 10,000 cfu/mL. UTI was defined as a positive culture associated with dysuria, pain, fever or sepsis. Asymptomatic bacteriuria was defined as positive culture in the absence of symptoms. When there was a doubt, participants were defined as having UTI. |
|
| Sponsorship/funding | This study was supported by a grant from, Anders Jahre’s Foundation, Oslo, Norway. | |
| Ethical approval | Not reported | |
| Notes | A size 12 or 14 Fr transurethral Teflon‐coated Foley catheter was used for both groups. Post‐catheter removal all participants were encouraged to void spontaneously, those that could not were recatherised. A least 3 urine cultures were taken. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Quote: “… randomized by means of a nurse drawing a closed opaque envelope” Comment: no other information reported |
| Allocation concealment (selection bias) | Low risk | Quote: “…closed opaque envelope” Comment: closed envelopes were used to conceal allocation |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Not reported. Unlikely that participants could be blinded |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Not reported |
| Blinding of microbiological outcome (detection bias) | Low risk | Not reported. Suggests that urine samples were sent to a laboratory for microscopy and culture. Unlikely that microbiologist knew which patients belonged to the trial |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | No withdrawals reported |
| Selective reporting (reporting bias) | Low risk | All outcomes reported in methods are reported in full in results |
| Other bias | Low risk | Appears to be free from other sources of bias |
Schiotz 1996.
| Study characteristics | ||
| Methods |
Study design: RCT Dates study conducted: November 199‐April 1994 |
|
| Participants |
Number of participants: eligible, not reported; 109 randomised; 91 reported Country: Norway Population: women Age (mean and range): overall 50.3 (26.9‐72.6) Inclusion criteria: women admitted for elective retropubic surgery for urinary stress continence Condition for hospitalisation: elective retropubic surgery for urinary stress incontinence Exclusion criteria: not reported Use of antibiotic prophylaxis: not reported |
|
| Interventions |
Group A (n = 45): IUC removal after 1 day Group B (n = 46): IUC removal after 3 days Size and type of catheter used: 12 or 14 Fr Foley catheter, Teflon‐coated Study definition of short‐term catheterisation (days): not reported Intended duration of catheterisation for each group: A: 1 day post‐op IUC B: 3 day post‐op IUC |
|
| Outcomes | UTI Delayed spontaneous voiding after catheter removal Recatheterisation Length of hospital stay Asymptomtic bacteriuria (cannot be incorporated as reported as total number without the numbers in each group) |
|
| Definition of CAUTI or bacteriuria | Cultures were defined as positive when an midstream urine specimen yielded > 100,000 cfu/mL of any organism, or a catheter specimen yielded > 10,000 cfu/mL. UTI was defined as a positive culture associated with dysuria, pain, fever or sepsis. Asymptomatic bacteriuria was defined as a positive culture in the absence of symptoms. If there was doubt, participants were defined as having UTI rather than asymptomatic bacteriuria. |
|
| Sponsorship/funding | This study was supported by a grant from Anders Jahre’s Foundation, Oslo, Norway. | |
| Ethical approval | Not reported | |
| Notes | 18 participants were excluded following randomisation; 15 participants were excluded as they were administered antibiotic prophylaxis and 3 had confounding post‐op antibiotic treatment Cultures were defined as positive when a midstream urine specimen yielded > 100,000 cfu/mL of any organism or a catheter specimen yielded > 10,000 cfu/mL UTI was defined as a positive culture associated with dysuria, pain, fever or sepsis Asymptomatic bacteriuria was defined as positive culture in the absence of symptoms |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Quote: “Patients were pre‐operatively randomized to …” Comment: randomisation method unclear |
| Allocation concealment (selection bias) | Low risk | Quote: “… by means of a nurse drawing a closed envelope.” Comment: envelopes were concealed |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Not reported. Unlikely that blinding of participants occurred |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Not reported |
| Blinding of microbiological outcome (detection bias) | Low risk | Urine cultures were taken from microscopy and culture. Suggests that microbiologist processed them at a laboratory and so unlikely to know which patients were part of the study. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Quote: “15 patients were excluded owing to …” Comment: reasons for withdrawals given. Participants who completed the study are reported in full |
| Selective reporting (reporting bias) | Low risk | All outcomes reported in full. However, protocol not available for assessment |
| Other bias | Low risk | Appears to be free from other sources of bias |
Sekhavat 2008.
| Study characteristics | ||
| Methods |
Study design: RCT Dates study conducted: December 2002‐November 2004 |
|
| Participants |
Number of participants: eligible, not reported; 90 randomised; 90 reported Country: Iran Population: women Age (mean and SD): A 38.9 ± 2.9; B 39 ± 3.8 Inclusion criteria: women who underwent anterior repair Condition for hospitalisation: anterior colporrhaphy (pelvic organ prolapse) Exclusion criteria: not reported Use of antibiotic prophylaxis: in addition, the first dose of 1 mg cephalexin was given immediately before the beginning of operation and the second, given 6 h after the initial dose. |
|
| Interventions |
Group A (n = 45): IUC removed straight after surgery Group B (n = 45): IUC removed 24 h after surgery Size and type of catheter used: 16F Foley catheter with 10 mL balloon, latex Study definition of short‐term catheterisation (days): not reported Intended duration of catheterisation for each group: A: IUC removed immediately post‐op B: IUC removed at least 24 h post‐op |
|
| Outcomes | UTI Urinary retention Voided spontaneously Number needing to be recatheterised (reported as in and out catheterisation) Ambulation time post‐op (h) Hospital stay (h) Urinary discomfort |
|
| Definition of CAUTI or bacteriuria | The prevalence of symptomatic UTI was confirmed, detected in the urine culture by a positive urine culture or through urinary signs such as burning urination, frequency, urgency, suprapubic pain and fever. | |
| Sponsorship/funding | Not reported | |
| Ethical approval | The adopted protocol was approved by the hospital research and ethics committee | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: “The patients were randomly (the randomisation schedules were prepared using a computer‐generated random number table)…” Comment: computer‐generated randomisation |
| Allocation concealment (selection bias) | Unclear risk | Not reported |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Not reported. Unlikely that this was possible. No blinding reported |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Not reported |
| Blinding of microbiological outcome (detection bias) | Low risk | Not reported. Assume microbiologist was blinded |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Quote: “All women enrolled in the study were included in the analysis” Comment: no withdrawals reported |
| Selective reporting (reporting bias) | Low risk | All outcomes outlined in methods are reported in full in results section. However, protocol was not available for analysis |
| Other bias | Low risk | Appears to be free from other sources of bias |
Shahnaz 2016.
| Study characteristics | ||
| Methods |
Study design: RCT Dates study conducted: 2013‐2015 |
|
| Participants |
Number of participants: 70 randomised; 70 reported Setting: Martyrs Hospital in the Persian Gulf Country: Iran Population: women Age (mean and SD): A 39.4 ± 3.2; B 38.8 ± 2.8 Inclusion criteria: the inclusion criteria included prolapse of vaginal anterior with grades 2 and 3, age between 25‐49 years old, and body mass index of 19‐24 kg/m2 Condition for hospitalisation: pelvic organ prolapse Exclusion criteria: vaginal anterior prolapse grade 1 and 4, diabetes, connective tissue diseases, different kinds of true urinary incontinence, having a history of hysterectomy Use of antibiotic prophylaxis: “After the surgery, the antibiotic was not regularly given except for patients who had abnormal urinary symptoms and unusual urinary analysis in urinary sample 48 h after the surgery” |
|
| Interventions |
Group A (n = 35): IUC removal 24 h after surgery Group B (n = 35): IUC removal 72 h after surgery Size and type of catheter used (e.g. Foley 16F): not reported Study definition of short‐term catheterisation (days): not reported Intended duration of catheterisation for each group: Group A: 24 h after surgery Group B: 72 h after surgery |
|
| Outcomes | Number of participants with urinary retention Number of participants requiring recatheterisation Positive urine culture Length of hospitalisation |
|
| Definition of CAUTI or bacteriuria | Urine analysis and culture examination was done prior to surgery in all participants. The presence of positive urinary culture or > 100,000 cfu/mL of urine or > 10 pieces of leukocyte in each microscopy field was considered as a urinary infection. | |
| Sponsorship/funding | Not reported | |
| Ethical approval | “approved by the Institutional Ethical Review Board” | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: “Patients were randomized into two groups using computer‐generated randomized schedules” Comment: adequate randomisation method |
| Allocation concealment (selection bias) | Unclear risk | Not reported |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Blinding not possible with this intervention |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Not reported |
| Blinding of microbiological outcome (detection bias) | Low risk | Not reported. Assume urine samples were sent to a laboratory where the microbiologist would not know which patients were in the trial |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | All data accounted for with no withdrawals/dropouts |
| Selective reporting (reporting bias) | Low risk | Outcomes seem to be reported in full |
| Other bias | Low risk | Appears to be free from other sources of bias |
Shrestha 2013.
| Study characteristics | ||
| Methods |
Study design: RCT Dates study conducted: January 2012‐January 2013 |
|
| Participants |
Number of participants: eligible, not reported; 100 randomised; 100 reported Setting: Kathmandu, Nepal Population: women Age (mean and SD): 53.35 ± 10.94 Inclusion criteria: vaginal hysterectomy; anterior colporrhaphy; Manchester operations Condition for hospitalisation: women who underwent vaginal hysterectomy, anterior colporrhaphy and Manchester operations (79 patients underwent vaginal hysterectomy with pelvic floor repair, 19 anterior colporrhaphy and 2 Manchester operation) Exclusion criteria: history of previous urine retention; pre‐operative urinary infection; bladder injury; other associated complication during operation Use of antibiotic prophylaxis: antibiotics are given for 7 days |
|
| Interventions |
Group A (n = 50): IUC removal 24 h post‐op Group B (n = 50): IUC removal 72 h post‐op Size of catheter used: Foley Study definition of short‐term catheterisation (days): not reported Intended duration of catheterisation for each group: A: IUC was removed after 24 h B: IUC was removed after 72 h |
|
| Outcomes | Recatheterisation Mean catheterisation time (days) Mean hospital stay (days) (mean) UTI: pus cells in urine > 5 per high‐power field; bacteria culture positive |
|
| Definition of CAUTI or bacteriuria | Asymptomatic bacteriuria = pus cells > 5 per high‐power field in routine examination of urine and bacterial culture positive | |
| Sponsorship/funding | Not reported | |
| Ethical approval | Protocol was approved by Ethical Committee of hospital and informed consent was obtained from each woman. | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Quote: “They were randomized into group A, which include the patients, whom Foley catheterization was kept for 24 hours and group B, which include the patients, whom Foley catheterization was kept for 72 hours” Comment: method of randomisation not stated clearly |
| Allocation concealment (selection bias) | Unclear risk | Not reported |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Not reported. Unlikely blinding was possible |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Not reported |
| Blinding of microbiological outcome (detection bias) | Low risk | Unlikely that the microbiologist knew which urine sample belonged to which patient |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | All outcome data is complete with no dropouts |
| Selective reporting (reporting bias) | Low risk | All outcomes mentioned in methods are accounted for in results section. However, protocol was not available for assessment |
| Other bias | Low risk | Appears to be free from other sources of bias |
Souto 2004.
| Study characteristics | ||
| Methods |
Study design: RCT Dates study conducted: January 2000‐July 2002 |
|
| Participants |
Number of participants: eligible, not reported; 73 randomised; 73 reported Country: Brazil Population: men Age (mean ± SD (range)): overall: 62 (50‐73); A 64 ± 7.3 (50‐77); B 61 ± 7.3 (49‐73) Inclusion criteria: no cystography evaluation performed Condition for hospitalisation: retropubic radical prostatectomy Exclusion criteria: not reported Use of antibiotic prophylaxis: not reported |
|
| Interventions |
Group A (n = 37): IUC removed 7 days after surgery Group B (n = 36): IUC removed 14 days after surgery Size and type of catheter used (e.g. Foley 16F): 2‐way 20Fr Foley catheter Study definition of short‐term catheterisation (days): not reported Intended duration of catheterisation for each group: A: IUC removal 7 days post‐op B: IUC removal 14 days post‐op |
|
| Outcomes | Urinary retention and haematuria Vesical neck stenosis Urinary incontinence Operating time |
|
| Definition of CAUTI or bacteriuria | Not reported | |
| Sponsorship/funding | Not reported | |
| Ethical approval | “… approved by the Institutional ethics committee” | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Quote: “The patients were randomized into 2 groups …” Comment: method of randomisation is unclear |
| Allocation concealment (selection bias) | Unclear risk | Not reported |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Not reported. Unlikely blinding was possible. No other types of blinding reported |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Not reported |
| Blinding of microbiological outcome (detection bias) | Low risk | No microbiological outcomes reported |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | All participants completed the trial. No dropouts/withdrawals |
| Selective reporting (reporting bias) | Low risk | All outcomes in methods and results reported in full. However, protocol was not available for assessment |
| Other bias | Low risk | Appears to be free from other sources of bias |
Sun 2004.
| Study characteristics | ||
| Methods |
Study design: RCT Dates study conducted: not reported |
|
| Participants |
Number of participants: eligible, unclear; 86 randomised; 85 reported Country: Taiwan Population: women Age (mean (SD)): A 46.7 (6.7); B 48.3 (8.3) Inclusion criteria: patients with proven genuine stress incontinence who underwent Burch's colposuspension Condition for hospitalisation: Burch colposuspension Exclusion criteria: not reported Use of antibiotic prophylaxis: all participants received prophylactic antibiotics for 2 days (1 g cefazolin IV, 3 times a day). No other antibiotic was administered thereafter unless a fever was noted and its origin was identified. A febrile episode was defined as a body temperature of 38 °C orally |
|
| Interventions |
Group A (n = 43): IUC removed post‐op the next morning Group B (n = 43): IUC were left in place until the 5th post‐op day Size and type of catheter used: Foley catheter Study definition of short‐term catheterisation (days): not reported Intended duration of catheterisation for each group: A: IUC removed post‐op the next morning after surgery B: IUC left in place until the 5th post‐op day. The catheter was clamped on the 3rd post‐op day so that participants could participate in a bladder training programme. The bladder training programme involved clamping the catheter for 1 h 45 min and unclamping the catheter for 15 min |
|
| Outcomes | Post‐op UTIs Immediate voiding difficulty Delayed voiding difficulty Incomplete emptying of the bladder De novo frequency and urgency syndrome Length of hospitalisation |
|
| Definition of CAUTI or bacteriuria | A UTI was defined as bacteriuria (> 105 cfu/mL urine) or white blood cell count > 5 /high‐power field in urine analysis | |
| Sponsorship/funding | Not reported | |
| Ethical approval | Not reported | |
| Notes | The participant was instructed to comply with a fluid intake of 200 mL‐250 mL every 2 h. All participants received prophylactic antibiotics for 2 days Post‐op voiding difficulty was classified as the participant experiencing hesitancy in voiding, a weak stream, or a discontinuous flow and/or residual urine of > 100 mL. |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Quote: “…were then randomly placed into two groups …” Comment: method of randomisation unclear |
| Allocation concealment (selection bias) | Unclear risk | Not reported |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Not reported. Unlikely that this was possible due to the intervention |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Not reported. No types of blinding reported |
| Blinding of microbiological outcome (detection bias) | Low risk | Quote: “The post void residual urine volume was checked and an urine analysis and culture were performed to detect any urinary tract infection” Comment: suggests that all urine samples were sent to a laboratory; unlikely the microbiologist knew which patients were in the trial and which were not |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | 86 participants randomised, 85 reported: “One patient in Group A was lost at follow‐up due to immigration”. |
| Selective reporting (reporting bias) | Low risk | All outcomes reported in full in methods and results sections |
| Other bias | Low risk | Appears to be free from other sources of bias |
Tahmin 2011.
| Study characteristics | ||
| Methods |
Study design: RCT Dates study conducted: July 2007‐June 2008 |
|
| Participants |
Number of participants: eligible, not reported; 80 randomised; 80 reported Country: Bangladesh Population: women Age (mean and SD): A 51.75 ± 10.8; B 53.95 ± 12.8 Inclusion criteria: after proper evaluation genital prolapse cases awaiting vaginal hysterectomy and or pelvic floor repair, were enrolled for the study Condition for hospitalisation: vaginal hysterectomy with pelvic floor repair Exclusion criteria: UTI, diabetes mellitus Use of antibiotic prophylaxis: not reported |
|
| Interventions |
Group A (n = 40): IUC removal on the 2nd post‐op day Group B (n = 40): IUC removal on the 5th post‐op day Note: recatheterisation was done for 3 more days if residual volume > 200 mL after removal of catheter Size and type of catheter used: not reported Study definition of short‐term catheterisation (days): not reported Intended duration of catheterisation for each group: A: IUC was removed on 2nd post‐op day B: IUC was removed on 5th post‐op day |
|
| Outcomes | Mean duration of catheterisation (h) Recatheterisation Asymptomatic bacteruria Mean hospital stay (days) |
|
| Definition of CAUTI or bacteriuria | “UTI was defined as the presence of >105 colony forming units/mL in the culture” | |
| Sponsorship/funding | Not reported | |
| Ethical approval | Informed consent was obtained from each woman, and protocol was approved by ethical committee | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: “To facilitate that process equal numbers of pre‐labelled pieces of papers (40 for short period and 40 for conventional period of catheterisation) were placed and mixed thoroughly in a box.” Comment: adequate randomisation method |
| Allocation concealment (selection bias) | Unclear risk | Not reported |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Not reported. Unlikely that blinding of participants was possible |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Not reported |
| Blinding of microbiological outcome (detection bias) | Low risk | Quote: “Urine samples were taken before removal of catheter for routine microscopic examination and culture sensitivity test.” Comment: unlikely the microbiologist knew which individual belonged to which group |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | No withdrawals from the study |
| Selective reporting (reporting bias) | Low risk | All outcomes reported in the methods section is accounted for in the results section. However, protocol was not available for assessment |
| Other bias | Low risk | Appears to be free from other sources of bias |
Talreja 2016.
| Study characteristics | ||
| Methods |
Study design: RCT Dates study conducted: January 2014‐July 2015 |
|
| Participants |
Population: men Setting: Karachi Country: Pakistan Inclusion criteria: all patients admitted for TURP during the period were recruited in the study Condition for hospitalisation: TURP Exclusion criteria: history of trauma to spinal cord and cerebrovascular accidents; patients having comorbid conditions like diabetes mellitus or any other urogenital problems such as urethral strictures Number of participants: eligible, not reported; 86 randomised; 86 reported Age (mean and SD): Group A 64.21 ± 5.36; Group B 63.05 ± 4.69 Use of antibiotic prophylaxis: participants were given 1 dose of 3rd‐generation cephalosporin in pre‐operative period |
|
| Interventions |
Intervention for each group with times: Group A (n = 43): IUC was not clamped prior to its removal Group B (n = 43): IUC was clamped prior to its removal Size and type of catheter used: not reported Study definition of short‐term catheterisation (days): not reported Intended duration of catheterisation for each group: Group A: IUC was not clamped prior to its removal Group B: clamping of the IUC was performed prior to its removal |
|
| Outcomes | AUR Recatheterisation UTI (resulting in recatheterisation) Bleeding (resulting in recatheterisation Length of hospitalisation Catheter removal successful |
|
| Definition of CAUTI or bacteriuria | Not reported | |
| Sponsorship/funding | Not reported | |
| Ethical approval | “Written and informed consent was taken, and confidentiality of the patients was taken into account” | |
| Notes | “Clamping refers to interrupting bladder flow by obstructing the drainage pipe of Foley catheter and releasing it intermittently as patient feels urge to void. Foley catheter was removed once patient got mobilized, passed stool, and had no active bleeding or infection. Foley catheter was removed in the early morning in all cases.” | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Quote: “Eighty‐six study participants who underwent TURP were randomly allocated into two groups.” Comment: mentions randomisation but methods are not reported |
| Allocation concealment (selection bias) | Unclear risk | Not reported |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Not reported; unlikely that this was possible due to the intervention |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Not reported |
| Blinding of microbiological outcome (detection bias) | Low risk | Not reported. Unlikely that microbiologist would know which patient belonged to a clinical trial |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | No withdrawals reported |
| Selective reporting (reporting bias) | Low risk | Outcomes reported in the methods are also accounted for in the results section |
| Other bias | Low risk | No other indications of other sources of bias |
Taube 1989.
| Study characteristics | ||
| Methods |
Study design: RCT Dates study conducted: 9‐month period (not specified) |
|
| Participants |
Number of participants: 83 eligible; 60 randomised; 60 reported Country: UK Population: male Age (mean and range): successful: 72 (57‐85); failed: 76.9 (53‐86) Inclusion criteria: male patients with AUR Condition for hospitalisation: AUR Exclusion criteria: patients with significant renal impairment or clot retention Use of antibiotic prophylaxis: not reported |
|
| Interventions |
Group A (n = 18): IUC removed immediately after emptying Group B (n = 20): IUC removed after 24 h Group C (n = 22): IUC removed after 48 h Size and type of catheter used (e.g. Foley 16F): 16F Foley catheter Study definition of short‐term catheterisation (days): not reported Intended duration of catheterisation for each group: A: IUC removed immediately after emptying B: IUC removed after 24 h C: IUC removed after 48 h |
|
| Outcomes | Successful remicturition after IUC removal | |
| Definition of CAUTI or bacteriuria | Not reported | |
| Sponsorship/funding | Not reported | |
| Ethical approval | Not reported | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Quote: “The patients were randomized into three groups …” Comment: unclear as to how randomisation was performed |
| Allocation concealment (selection bias) | Unclear risk | Not reported |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Not reported. Unlikely that blinding was possible. No other types of blinding reported |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Not reported |
| Blinding of microbiological outcome (detection bias) | Low risk | Quote: “A sample of urine was taken immediately for microscopy and culture …” Comment: unlikely microbiologist knew which sample belonged to the study when it was processed in the laboratory |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | No withdrawals or dropouts |
| Selective reporting (reporting bias) | Unclear risk | Report published before CONSORT guidelines. Not sure if this is selective reporting or poor reporting. Protocol not available |
| Other bias | Low risk | Appears to be free from other sources of bias |
Toscano 2001.
| Study characteristics | ||
| Methods |
Study design: RCT Dates study conducted: July 1997‐November 1998 |
|
| Participants |
Number of participants: eligible, not reported; 104 randomised; 104 reported Country: Brazil Population: men Age (mean and SD): A 68.6 ± 7.4; B 69.5 ± 6.4 Inclusion criteria: patients undergoing surgery for benign prostatic hyperplasia; no coagulation disorders; no use of anticoagulants (mainly acetylsalicylic acid) in the month before the operation Condition for hospitalisation: TURP Exclusion criteria: not reported Use of antibiotic prophylaxis: antibiotic therapy with first‐generation cephalosporin was given at induction of anaesthesia and for up to 7 days after the operation. |
|
| Interventions |
Group A (n = 54): removal of the IUC within 24 h Group B (n = 50): removal of the IUC within 48 h Size and type of catheter used: 22F Foley catheter Study definition of short‐term catheterisation (days): not reported Intended duration of catheterisation for each group: A removal of IUC within 24 h post‐op B removal of IUC within 48 h post‐op |
|
| Outcomes | Haematuria Urinary retention |
|
| Definition of CAUTI or bacteriuria | Not reported | |
| Sponsorship/funding | Not reported | |
| Ethical approval | Not reported | |
| Notes | 22 F Owens catheter used (similar to Foley but has a third route for irrigation) Surgery undertaken by residents under supervision. Patients had bladder irrigation for 24 h. Definition of urinary retention not stated | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Quote: “A seleção dos pacientes que teriam a sonda retirada com 24 ou 48 horas foi feita por sorteio ao término do procedimento.” Comment: method of randomisation unclear |
| Allocation concealment (selection bias) | Unclear risk | Not reported |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Not reported. Unlikely that it was possible due to the intervention. No other blinding mentioned |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Not reported |
| Blinding of microbiological outcome (detection bias) | Low risk | No microbiological outcomes reported |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | No withdrawals or dropouts |
| Selective reporting (reporting bias) | Low risk | All outcomes seem to be reported in methods and results in full. Published protocol not available but this was not common practice at the time of the study |
| Other bias | Low risk | Nothing to indicate any other source of bias |
Valero Puerta 1998.
| Study characteristics | ||
| Methods |
Study design: quasi‐RCT Dates study conducted: not reported |
|
| Participants |
Population: men with benign prostatic hyperplasia undergoing TURP Country: Spain Inclusion criteria: via clinic Condition for hospitalisation (e.g. hysterectomy or TURP): TURP Exclusion criteria: not reported Number of participants: 117 randomised; 117 reported Age (e.g. mean and SD; median, IQR): Group A mean 70 (53‐83); Group B mean 69 (50‐87) Use of antibiotic prophylaxis: Yes. 1 g of ceftriaxone every 24 h for 2 days |
|
| Interventions |
Group A (n = 55): IUC removal at 48 h Group B (n = 62): IUC removal according to usual care Intervention for each group (e.g. catheter removal, bladder infusion) with times (e.g. midnight catheter removal): Group A: IUC removal at 48 h Group B: IUC removal according to usual care (lack of haematuria) Size and type of catheter used (e.g. Foley 16F): not reported Study definition of short‐term catheterisation (days): not reported Intended duration of catheterisation for each group: A removal of catheter at 48 h B removal according to usual care |
|
| Outcomes | Duration of post‐op hospital stay Duration of total hospital stay Volume of dried tissue Number of men requiring transfusion Number of men with urinary retention Number of men readmitted to hospital |
|
| Definition of CAUTI or bacteriuria | Not reported | |
| Sponsorship/funding | Not reported | |
| Ethical approval | Not reported | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | High risk | Participants were assigned to the 2 groups according to the day of the week of their TURP operation |
| Allocation concealment (selection bias) | Unclear risk | Not reported |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Not reported. Unlikely possible given nature of intervention |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Not reported |
| Blinding of microbiological outcome (detection bias) | Low risk | Not reported. Can assume that samples were sent to a laboratory where the microbiologist is unlikely to know which patient belongs to the study |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | No evidence that there was incomplete data |
| Selective reporting (reporting bias) | Low risk | All outcomes measured in results were the same as was mentioned in the methods section |
| Other bias | Low risk | Appears to be free form other sources of bias |
Vallabh‐Patel 2020.
| Study characteristics | ||
| Methods |
Study design: RCT Dates study conducted: December 2015‐May 2017 |
|
| Participants |
Population: women Setting: New Jersey Country: USA Inclusion criteria: women undergoing robotic sacrocolpopexy for pelvic organ prolapse Exclusion criteria: a history of prior vaginal mesh, history of pre‐operative urinary retention or postvoid residual of > 200 mL, pregnancy or desire for future pregnancy, and intraoperative complications necessitating a post‐op IUC such as intraoperative cystotomy, bowel injury, or estimated blood loss > 500 mL Condition for hospitalisation: pelvic organ prolapse Number of participants: 94 eligible; 88 randomised; 88 reported Age (mean and SD): A 59.52 ± 8.5; B 59.57 ± 11.2 Use of antibiotic prophylaxis: “All participants received appropriate perioperative antibiotics per American College of Obstetricians and Gynecologists guidelines.” |
|
| Interventions |
Group A (n = 44): IUC removal 6 h post‐op Group B (n = 44): IUC removal post‐op day 1 Size and type of catheter used: not reported Study definition of short‐term catheterisation (days): not reported Intended duration of catheterisation for each group: Group A: IUC removal 6 h post‐op Group B: IUC removal day 1 post‐op |
|
| Outcomes | Incidence of UTI Number of participants requiring recatheterisation Complications |
|
| Definition of CAUTI or bacteriuria | “For the purpose of this study, patients were considered positive for a UTI if they had (1) positive urine cultures per CDC guidelines or (2) if a patient was treated empirically over the phone for symptoms of UTI, even in the absence of a urine culture” | |
| Sponsorship/funding | “Funding was provided through a grant from Morristown Medical Center Research Foundation” | |
| Ethical approval | "Approval for this study was obtained by the Atlantic Health System institutional review board (# 908398‐5)." | |
| Notes | Declarations of interest: “The authors declare that they have no conflict of interest” | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: "Randomization was performed in the immediate postoperative period utilizing REDCap (Research Electronic Data Capture) (Nashville, Tenn) using a random‐number generator for an overall allocation ratio of 1:1." Comment: adequate randomisation method |
| Allocation concealment (selection bias) | Unclear risk | Not reported |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | No blinding of participants or personnel |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Not reported |
| Blinding of microbiological outcome (detection bias) | Low risk | Not reported. Likely specimens sent to a lab blinded as to which specimens belonged to the trial |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | No missing data, no withdrawals |
| Selective reporting (reporting bias) | Low risk | All outcomes reported in full |
| Other bias | Low risk | Appears to be free from other sources of bias |
Webster 2006.
| Study characteristics | ||
| Methods |
Study design: RCT Dates study conducted: February 2001‐March 2003 |
|
| Participants |
Number of participants: 631 eligible; 210 randomised; 206 reported Setting: Brisbane Country: Australia Population: mixed Inclusion criteria: > 18 years of age; able to give written informed consent Condition for hospitalisation: general surgery and medical patients who require IUCs as part of their health care Exclusion criteria: patients with a suprapubic catheter or a long‐term IUC who were pregnant or newly diagnosed with gynaecologic cancer Use of antibiotic prophylaxis: not reported |
|
| Interventions |
Group A (n = 98): removal of IUC at 6 am Group B (n = 97): removal of IUC at 10 pm Size and type of catheter used (e.g. Foley 16F): not reported Study definition of short‐term catheterisation (days): not reported Intended duration of catheterisation for each group: A: IUC removal at 6 am B: IUC removal at 10 pm |
|
| Outcomes | Time between catheter removal and discharge (h) Duration of catheterisation (h) Time to first void (h) Mean volume of first void Recatheterisation/failed trial of void Post discharge urinary problems: retention; difficulty passing urine; pain when passing urine; loin pain; febrile; incontinent |
|
| Definition of CAUTI or bacteriuria | Not reported | |
| Sponsorship/funding | The Queensland Nursing Council and the Queensland University of Technology funded the study. | |
| Ethical approval | The hospital’s human research ethics committee approved the study, and the authors obtained informed consent from all participants | |
| Notes | Sample size calculation stated The ward or location in which the catheter was inserted and fluid intake in the previous 24 h were also recorded. |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: “Randomization was performed using a computer‐generated table of random numbers supplied by the hospital’s perinatal research centre.” Comment: adequate randomisation method |
| Allocation concealment (selection bias) | Low risk | Quote: “Individuals were allocated to either to 22:00‐hour catheter removal (intervention group), or to 06:00‐hour catheter removal (control group) by telephone call to a scientist who was independent of the recruitment process and blinded to baseline interview.” Comment: adequate concealment method |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Quote: “Neither the clinicians nor the patients were blinded to the intervention.” Comment: blinding of participants and staff is not possible |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Quote: “Ward staff, who were aware of group assignment but who were not part of the research team, recorded outcome data. Data were processed and coded by a researcher who was unconnected with treatment but who was not blind to randomization.” Comment: attempts were made to blind outcome assessment but still prone to detection bias |
| Blinding of microbiological outcome (detection bias) | Low risk | No microbiological outcome reported |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | All data reported in full. No exclusions or withdrawals |
| Selective reporting (reporting bias) | Low risk | All outcomes reported in full in methods and results sections |
| Other bias | Low risk | Appears to be free from other sources of bias |
Weemhoff 2011.
| Study characteristics | ||
| Methods |
Study design: RCT Dates study conducted: January 2006‐September 2008 |
|
| Participants |
Number of participants: 390 eligible; 246 randomised; 246 reported Setting: 3 different hospitals Country: Netherlands Population: women Age (mean and SD): A 59.9 ± 10.2; B 60.7 ± 11.1 Inclusion criteria: patients undergoing anterior colporrhaphy Condition for hospitalisation: anterior colporrhaphy Exclusion criteria: excluded were women who were performing self‐catheterisation because of voiding dysfunctions pre‐operatively, women < 18 years of age, and those who were not able to understand informed consent because of low IQ or a language barrier. Use of antibiotic prophylaxis: all patients received prophylactic antibiotics at the beginning of the operation. Post‐op prophylactic antibiotics were not given routinely. |
|
| Interventions |
Group A (n = 124): 2‐day IUC Group B (n = 122): 5‐day IUC Size and type of catheter used: not reported Study definition of short‐term catheterisation (days): not reported Intended duration of catheterisation for each group: A: IUC for 2 days (removed in the morning) B: IUC for 5 days (removed in the morning) |
|
| Outcomes | Participants needing temporary catheter replacement/recatheterisation (%) Participants with a UTI at the time of first catheter removal (%) Hospital stay (median (range)) Percentage of participants with uneventful post‐op period UTIs: post‐voiding residual > 200 mL; post‐voiding residual < 200 mL |
|
| Definition of CAUTI or bacteriuria | “Signs of urine tract infection were defined as having more than 25 white blood cells per high‐power field, nitrate production, or more than 20 bacteria per high‐power field. When urinary tract infection after the removal of the catheter was confirmed by a positive culture, patients were treated with antibiotics irrespective of complaints. A culture was scored positive when the sample contained more than 105 colony forming units per milliliter. For the outcome measure urinary tract infection, only the infections proven by a positive culture at the time of the first removal of the catheter were included. No other urinary cultures were taken on behalf of the study protocol.” | |
| Sponsorship/funding | “None” | |
| Ethical approval | After informed consent, participants were included at the outpatient clinic at the time the operation was planned. The protocol was approved by the medical ethical committees of the three participating hospitals. | |
| Notes | “Based on retrospectively collected data in one of the participating hospitals, the average percentage of patients needing repeated catheterization after removal of the catheter on the fifth day after an anterior colporrhaphy was 10%.” | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: “A randomization list was made by an independent statistician. Randomization was performed in blocks and was stratified for the different hospitals. According to the randomization list, opaque, numbered, and sealed envelopes were prepared by an independent person. At the start of the operation, urine was collected for sedimentation. After the operation was finished, the indwelling catheter was inserted; the envelope with study number was opened, and at that moment, the patient was randomized to temporary indwelling catheterization for either 2 or 5 days” Comment: adequate randomisation methods |
| Allocation concealment (selection bias) | Low risk | Quote: “According to the randomization list, opaque, numbered, and sealed envelopes were prepared by an independent person.” Comment: adequate concealment method |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Not reported. Unlikely blinding was possible due to intervention. |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Not reported |
| Blinding of microbiological outcome (detection bias) | Low risk | Quote: “After removal of the catheter, urine samples were taken for sedimentation and culture.” Comment: unlikely the microbiologist knew which patient belonged to which group as study implies routine cultures were taken |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Quote: “One patient, randomized to the 2‐day protocol, died of a heart attack on the first postoperative day with the catheter in situ. She died before she could participate in the study. Two patients allocated to the 5‐day protocol had their catheter removed on the third day because of miscommunication. The three patients were analysed in the allocated group.” Comment: patients analysed on an ITT basis |
| Selective reporting (reporting bias) | Low risk | All outcomes mentioned in the methods were reported in the results section fully |
| Other bias | Low risk | Appears to be free from other sources of bias |
Williamson 1982.
| Study characteristics | ||
| Methods |
Study design: RCT Dates study conducted: 14‐month period (not specified) |
|
| Participants |
Number of participants: eligible, unclear; 8 randomised; 8 reported Country: USA Population: female Age (range): 22‐40 years Inclusion criteria: IUC durations of at least 36 h Condition for hospitalisation: all female patients undergoing surgery Exclusion criteria: history of UTI or urinary incontinence in the preceding 12 months, patients whose urinalysis identified bacteriuria, and patients with spinal cord injuries and muscular degenerative disorders. Baseline residual urinary volume of > 25 mL were not considered. Patients who had taken medication known to cause bladder dystonia or urinary retention were not allowed to continue in the study Use of antibiotic prophylaxis: not reported |
|
| Interventions |
Group A (n = 4): bladder reconditioning Group B (n = 4): no reconditioning Size and type of catheter used: Foley catheter Study definition of short‐term catheterisation (days): Intended duration of catheterisation for each group: A: bladder reconditioning. Reconditioning included clamping to prevent drainage of urine for 3‐h cycles. At the end of 3 h the drainage tubing was unclamped for 5 min to allow complete emptying. Tubing was reclamped for 3 h followed by 5 min drainage period and a final 3 h followed by 5 min drainage. Reconditiong required a total of 9 h and 10 min. Reconditioning was conducted by the investigator. After catheter removal each participant in both groups maintained a minimum fluid intake of 100 mL/h B: control group (no reconditioning) |
|
| Outcomes | Mean time to first void Post‐IUC residual urine volume |
|
| Definition of CAUTI or bacteriuria | Not reported | |
| Sponsorship/funding | Not reported | |
| Ethical approval | Not reported | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Quote: “The four subjects randomly assigned…” Comment: randomisation method unclear |
| Allocation concealment (selection bias) | Unclear risk | Not reported |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Not reported. Unlikely that it was possible to blind participants and staff. Nursing staff needed to know which patient needed reconditioning and so would be aware which patient was in which group. |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Not reported |
| Blinding of microbiological outcome (detection bias) | Low risk | No microbiological outcomes reported |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | No withdrawals or dropouts. However, only 8 participants |
| Selective reporting (reporting bias) | Unclear risk | Very limited information. Published in 1982. Not sure if this is selective reporting or poor reporting |
| Other bias | High risk | Underpowered study with only 8 participants |
Wilson 2000.
| Study characteristics | ||
| Methods |
Study design: RCT Dates study conducted: not reported |
|
| Participants |
Number of participants: 75 eligible; 75 randomised; 75 reported Setting: Scotland Country: UK Population: men Age (mean and SD): not reported Inclusion criteria: patients undergoing TURP Condition for hospitalisation: TURP Exclusion criteria: inability of the patient to give consent Use of antibiotic prophylaxis: not reported |
|
| Interventions |
Group A (n = 37): bladder infusion with normal saline at 6 am by gravity from a 500 mL bag, until the participant felt that their bladder was full Group B (n = 38): IUC removed at 6 am and participant advised to drink fluids Size and type of catheter used: not reported Study definition of short‐term catheterisation (days): not reported Intended duration of catheterisation for each group: A: infusion of normal saline at 6 am by gravity from a 500 mL bag until participant felt bladder was full. IUC then removed B: IUC removed at 6 am with no infusion protocol |
|
| Outcomes | Ready for discharge same day as trial of voiding Discharged same day as trial of voiding | |
| Definition of CAUTI or bacteriuria | Not reported | |
| Sponsorship/funding | Not reported | |
| Ethical approval | Ethical committee approval was obtained for the trial | |
| Notes | A trial of voiding was carried out on the second day after TURP in all participants | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Quote: “The patients were randomized by opening marked, easily identifiable envelopes for each stratum with the allocation schedules enclosed.” Comment: unclear as to whether randomisation method was adequate |
| Allocation concealment (selection bias) | Unclear risk | Quote: “… easily identifiable envelopes for each stratum with the allocation schedules enclosed …” Comment: unclear as to whether these envelopes were sealed or opaque |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Quote: “…was not blinded.” Comment: blinding was not used. Likely blinding would not be possible with regards to the intervention |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Not reported |
| Blinding of microbiological outcome (detection bias) | Low risk | No microbiological outcomes reported |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | No withdrawals or dropouts reported |
| Selective reporting (reporting bias) | Low risk | All outcomes reported in methods are reported in results |
| Other bias | Low risk | Appears to be free from other sources of bias |
Wu 2015.
| Study characteristics | ||
| Methods |
Study design: RCT Dates study conducted: January 2011‐December 2013 |
|
| Participants |
Population: mixed Setting: not reported Inclusion criteria: hospital patients who had biliary surgery for gallstones (in the gallbladder or in the biliary tree) Condition for hospitalisation: cholecystectomy Exclusion criteria: renal insufficiency pre‐surgery, UTI, post‐surgery severe complication, clinically unstable Number of participants: eligible, not reported; 100 randomised; 100 reported “Group A and B patients gender, age and surgery types and liver function stages were similar at baseline, with no statistically significant differences at P>0.05” Age (e.g. mean and SD; median, IQR): Group A: 24‐77 years (min‐max), average 45.6 years, SD 7.2 years Group B: 23‐79 years, average 46.1, SD 7 years Use of antibiotic prophylaxis: not reported |
|
| Interventions |
Intervention for each group: Group A: catheter clamped when participant woke up after the surgery. On Day 1 morning after surgery, when the participant felt the urge to pass urine, the IUC balloon was deflated and the catheter allowed to be self‐dislodged during urination. [Translator Note, the catheter remains clamped] (n = 50) Group B: on the morning of Day1 post‐surgery, after the participant passes urine (through the catheter), saline used to wash the bladder and the catheter clamped. 10 min after clamping, the balloon was deflated and the catheter allowed to be self‐dislodged during urination (n = 50) Size and type of catheter used: not reported Study definition of short‐term catheterisation (days): not reported Intended duration of catheterisation for each group: Group A: catheter clamped when participant woke up after the surgery. On Day 1 morning after surgery, when the participant felt the urge to pass urine, the IUC balloon was deflated and the catheter allowed to be self‐dislodged during urination. Group B: on the morning Day 1 post surgery, after the participant passes urine (through the catheter), saline used to wash the bladder and the catheter clamped. 10 min after clamping, the balloon was deflated and the catheter allowed to be self‐dislodged during urination. |
|
| Outcomes | Percentage of participants able to pass urine spontaneously on their own (success defined as: when they feel the urge, and 30 min after deflation of the balloon, the participant is able to spontaneously pass urine and dislodge/push out the catheter in one urination. Failure is defined as: not dislodged/pushed out in one urination, time taken > 30 min, needing to keep the catheter for passing urine) | |
| Definition of CAUTI or bacteriuria | Not reported | |
| Sponsorship/funding | Not reported | |
| Ethical approval | Not reported | |
| Notes | Trial report translated by contact for Cochrane Incontinence Biliary surgery patients for: Group A: gallstones in the biliary tract (28 participants) and gallstones (22 participants). Liver function Child‐Pugh stage A (30), Stage B (20) Group B – gallstones in the biliary tract (29 participant) and gallstones (21 participant). Liver function Child‐Pugh stage A (32), Stage B (18) |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Statement of “patients were assigned using a random number chart” |
| Allocation concealment (selection bias) | Low risk | Allocation using a random number chart |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Not reported. Unlikely possible given nature of intervention |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Not reported |
| Blinding of microbiological outcome (detection bias) | Low risk | No microbiological outcomes reported |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | No data unaccounted for |
| Selective reporting (reporting bias) | Low risk | No evidence to report selective reporting |
| Other bias | Low risk | No other sources of bias identified |
Wyman 1987.
| Study characteristics | ||
| Methods |
Study design: RCT Dates study conducted: not reported |
|
| Participants |
Number of participants: eligible, not reported; 103 randomised; 103 reported Setting: Derby Country: UK Population: men Age (mean and range): 70.8 (50‐89) Inclusion criteria: men undergoing TURP Condition for hospitalisation: TURP Exclusion criteria: not reported Use of antibiotic prophylaxis: not reported |
|
| Interventions |
Group A (n = 51): removal of IUC between 6 am and 7 am Group B (n = 52): removal of IUC between 10 pm and 11 pm Size and type of catheter used: 20‐22 Fr 3‐way Foley catheter Study definition of short‐term catheterisation (days): not reported Intended duration of catheterisation for each group: A: IUC removal between 6 am and 7 am B: IUC removal between 10 pm and 11 pm |
|
| Outcomes | Urinary retention Time interval between IUC removal and recatheterisation |
|
| Definition of CAUTI or bacteriuria | Not reported | |
| Sponsorship/funding | Not reported | |
| Ethical approval | Not reported | |
| Notes | All participants were catheterised using a 3‐way Simplastic urethral catheter size 20 or 22 French gauge Higher incidence of post‐op retention in patients with pre‐operative retention |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Quote: “Patients were randomized into two groups …” Comment: method of randomisation unclear |
| Allocation concealment (selection bias) | Unclear risk | Not reported |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Not reported. Unlikely this was possible due to the intervention |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Not reported |
| Blinding of microbiological outcome (detection bias) | Low risk | No microbiological outcomes reported |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | No withdrawals or dropouts |
| Selective reporting (reporting bias) | Unclear risk | Difficult to judge as report is very short. All outcomes seem to be reported in methods and results section |
| Other bias | Low risk | Appears to be free from other sources of bias |
Yaghmaei 2017.
| Study characteristics | ||
| Methods |
Study design: RCT Dates study conducted: 2017 |
|
| Participants |
Number of participants: 110 randomised; 110 reported Setting: Zahedan, Sistan and Balouchestan Country: Iran Population: women Age (mean and SD): Group A 28.19 ± 5.80; Group B 28.01 ± 5.83 Inclusion criteria: caesarean volunteers in Imam Ali Hospital Exclusion criteria: haemorrhage > 1000 cc during surgery, pyuria before surgery, urinary bladder injury during or before surgery, special medication conditions such as: diabetes, drug addictions, pregnancy high blood pressure, urinary system problems signs and record, bladder synechiae, existence of > 5 leucocyte in patients’ blood test before surgery Condition for hospitalisation: CS Use of antibiotic prophylaxis: cefazolin 1 g |
|
| Interventions |
Group A (n =110): IUC removal 6 h post‐op Group B (n = 110): IUC removal 12‐24 h post‐op Size and type of catheter used (e.g. Foley 16F): not reported Study definition of short‐term catheterisation (days): not reported Intended duration of catheterisation for each group: Group A: 6 h post‐op Group B: 12‐24 h post‐op |
|
| Outcomes | Urinary urgency Urinary difficulty Urinary frequency Urinary irritation Pyuria after surgery Time to first ambulation Time to first void Length of hospitalisation Fever Patient satisfaction |
|
| Definition of CAUTI or bacteriuria | Not reported | |
| Sponsorship/funding | Not reported | |
| Ethical approval | Not reported | |
| Notes | Farsi paper. Translation provided by independent translator | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Not reported |
| Allocation concealment (selection bias) | Unclear risk | Not reported |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Not reported. Unlikely given the nature of intervention |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Not reported |
| Blinding of microbiological outcome (detection bias) | Low risk | Not reported. Assumed microbiologist were blinded to the intervention |
| Incomplete outcome data (attrition bias) All outcomes | High risk | Quote from translator: “Ready Samples for pyuria in 1st group (catheters were taken out 6 hours after surgery) was 91 and for 2nd group (was taken out 12,24 hours after) was 82. So totally it was 173 and there is no explanation regarding the missing data unfortunately.” Comment: unclear as to why some data were not available |
| Selective reporting (reporting bias) | Low risk | Appears to be free from reporting bias. Protocol not available for assessment, however |
| Other bias | Low risk | Appears to be free from other sources of bias |
Yee 2015.
| Study characteristics | ||
| Methods |
Study design: RCT Dates study conducted: not reported |
|
| Participants |
Number of participants: eligible, not reported; 112 randomised; 112 reported Setting: Penang General Hospital Country: Malaysia Population: women Age: not reported Inclusion criteria: women who underwent CS under spinal anaesthesia Condition for hospitalisation: elective CS under spinal anaesthesia Exclusion criteria: not reported Use of antibiotic prophylaxis: not reported |
|
| Interventions |
Group A (n = not reported by abstract): IUC removal at 8 h post‐op Group B (n = not reported by abstract): IUC removal at 24 h post‐op Size and type of catheter used: not reported Study definition of short‐term catheterisation (days): not reported Intended duration of catheterisation for each group: A: 8 h post‐op B: 24 h post‐op |
|
| Outcomes | Severe pain or discomfort CAUTI AUR |
|
| Definition of CAUTI or bacteriuria | Not reported | |
| Sponsorship/funding | Not reported | |
| Ethical approval | Not reported | |
| Notes | Conference abstract with limited information | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Not reported |
| Allocation concealment (selection bias) | Unclear risk | Not reported |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Not reported. Unlikely possible given nature of intervention |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Not reported |
| Blinding of microbiological outcome (detection bias) | Low risk | Not reported. Assumed microbiologists were blinded to the intervention |
| Incomplete outcome data (attrition bias) All outcomes | High risk | High risk of bias due to incomplete data being reported. Only P values are reported |
| Selective reporting (reporting bias) | High risk | Conference abstract. Reported with limited information with only P values |
| Other bias | Low risk | No other bias likely present |
Zaouter 2009.
| Study characteristics | ||
| Methods |
Study design: RCT Dates study conducted: 1 February‐31 October 2008 |
|
| Participants |
Number of participants: 321 eligible; 215 randomised; 215 reported Setting: Montreal Country: Canada Population: mixed Age (mean and SD): A 57 ± 15; B 63 ± 11 Inclusion criteria: patients scheduled for elective major abdominal and thoracic surgery Condition for hospitalisation: major elective abdominal and thoracic surgery Exclusion criteria: history of post‐op urinary retention and with medical conditions and surgical conditions recognised to be at risk for post‐op urinary retention. All patients completed a questionnaire on lower urinary tract flow obstruction, if positive they underwent uroflowmetry and if considered at risk of urinary retention and were excluded. Use of antibiotic prophylaxis: 20 min before skin incision, 2 g cefazolin with or without 500 mg metronidazole was administered IV. If the surgery lasted for > 5 h, a second dose of cefazolin (1 g) would be administered. Once the urine and blood samples were sent for culture and sensitivity, an empirical treatment with broad‐spectrum antibiotics based on local susceptibility patterns was started. Afterward, when the urine sample was positive, targeted antibiotic therapy was prescribed according to the urine culture results. |
|
| Interventions |
Group A (n = 110): IUC removed same morning as the surgery Group B (n = 105): IUC removed when epidural anaesthesia removed Size and type of catheter used: not reported Study definition of short‐term catheterisation (days): not reported Intended duration of catheterisation for each group: A: IUC removed the same morning as the surgery B: IUC up until the epidural removed (3‐5 days) |
|
| Outcomes | Contracted UTI Recatheterisation Length of hospital stay Duration of bladder catheterisation VAS pain score |
|
| Definition of CAUTI or bacteriuria | “Patients were diagnosed having in‐hospital UTI according to international guidelines based on the following characteristics: pyrexia to a temperature of 38‐C, urinary tract symptoms (dysuria, increased frequency of urination, urinary urgency, suprapubic pain, burning on micturition, or onset or aggravation of urinary incontinence), and positive urine culture (107 bacterial colonies of microorganism‐forming units per litre within 2 weeks after the removal of bladder catheter).” | |
| Sponsorship/funding | This work was supported by internal funds, Department of Anesthesia, McGill University Health Centre. | |
| Ethical approval | The trial was approved by the ethics board of the McGill University Health Centre and written informed consent was obtained from all participants. | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: “... allocated, using a computer‐generated block randomization schedule.” Comment: adequate randomisation method |
| Allocation concealment (selection bias) | High risk | Not reported |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Not reported. Unlikely blinding was possible |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Not reported |
| Blinding of microbiological outcome (detection bias) | Low risk | Quote: “Once the urine and blood samples were sent for culture and sensitivity” Comment: implies that samples were sent to a laboratory and so unlikely the microbiologist would know which patient belonged to the trial |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | There are no dropouts from the study |
| Selective reporting (reporting bias) | Low risk | Outcomes reported in methods are represented in the results section. However, the protocol is not available for viewing. |
| Other bias | Low risk | No other bias likely present |
Zhou 2012.
| Study characteristics | ||
| Methods |
Study design: quasi‐RCT Dates study conducted: January‐December 2011 |
|
| Participants |
Population: women undergoing CS for: cephalopelvic disproportionate; social reasons; stuck fetus; abnormal placenta; twins; overly large baby; scarred uterus; other reasons unstated Setting: Guangdong Hospital Inclusion criteria: obstetric patients undergoing CS Exclusion criteria: heart, liver, kidney, brain or other severe conditions, no obstetric complications or conditions Surgical or anaesthesia complications Condition for hospitalisation: CS Number of participants: eligible, not reported; 138 randomised; 138 reported Age (mean and SD): Group A: mean 25.11, SD 4.88, rRange 20‐33 Group B: mean 26.33, SD 5.08, range 19‐35 Use of antibiotic prophylaxis: not reported |
|
| Interventions |
Size and type of catheter used (e.g. Foley 16F): not reported Study definition of short‐term catheterisation (days): not reported Intended duration of catheterisation for each group: Group A: removal of IUC at 6 h post surgery (intervention) (n = 46) Group B: removal of IUC at 8 h post surgery (intervention) (n = 46) Group C: removal at 24 h (control) (n = 46) |
|
| Outcomes | Urinary retention Post‐op 24 h bleeding Post‐op comfort after removal (measuring using VAS and urinary symptoms. “Mild” – pain score 1‐3, “Moderate” 4‐7, “Severe” 8‐10) |
|
| Definition of CAUTI or bacteriuria | Defined as post‐catheter removal midstream clean catch culture of ≥ 104 cfu/mL for Gram‐positive organisms or ≥ 105 cfu/mL for Gram‐negative organisms | |
| Sponsorship/funding | Not reported | |
| Ethical approval | Not reported | |
| Notes | Translator note: there is no description given of how the intervention group is separated into 6‐ or 8‐hour removal | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | High risk | Patients were allocated based on timing of presentation (odd or even days) into either intervention (6 h or 8 h removal) or control (24 h) |
| Allocation concealment (selection bias) | High risk | Patients were allocated based on timing of presentation (odd or even days) into either intervention (6 h or 8 h removal) or control (24 h) |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Not reported. Unlikely possible given nature of intervention |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Not reported |
| Blinding of microbiological outcome (detection bias) | Low risk | Not reported, likely that specimens sent to a lab |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | All completed, none lost |
| Selective reporting (reporting bias) | Low risk | Appears free from selective bias |
| Other bias | Low risk | Nothing to indicate any other source of bias |
Zmora 2010.
| Study characteristics | ||
| Methods |
Study design: RCT Dates study conducted: 2005‐2008 |
|
| Participants |
Number of participants: eligible, not reported; 118 randomised; 118 reported Country: Israel and Egypt Population: mixed Age (mean and range): A 57.4 (18‐85); B 54.6 (25‐81); C 54.2 (22‐78) Inclusion criteria: age ≥ 18 years; pelvic colorectal surgery with dissection of the rectum below the level of the sacral promontory; elective surgery; ASA score 1‐3; the ability to understand the objectives of the study and give an informed consent Condition for hospitalisation: patients undergoing colon and rectal surgery with pelvic dissection via an abdominal approach Exclusion criteria: pre‐operative antibiotic treatment other than routine perioperative prophylaxis; past or current urinary tract malignancy; IUC inserted 48 h before surgery or longer; chronic IUC drainage; known renal failure with blood creatinine levels of 2.0 mg or higher, including end‐stage renal disease requiring dialysis; previous pelvic surgery via the abdominal approach, including rectal, gynaecologic, and lower urinary tract procedures; severe benign prostatic hyperplasia with an AUA symptom index of ≥ 20; chronic urinary diseases including chronic infections and urinary anomalies; daily intake of medications affecting urinary output or urinary bladder contraction; neurogenic bladder; chronic intermittent IUC; past or current enterovesicle fistula; pregnancy; known pelvic abscess; malnutrition with albumin levels of < 2.7 g; immunosuppression (after organ transplantation, HIV‐positive with a CD4 count of < 200, chemotherapy in the past 2 weeks) Use of antibiotic prophylaxis: all participants received prophylactic perioperative antibiotics for 24 h according to the participating department’s protocols, and antibiotic treatment was uniform across the groups. |
|
| Interventions |
Group A (n = 41): IUC removed post‐op day 1 Group B (n = 38): IUC removed post‐op day 3 Group C (n = 39): IUC removed post‐op day 5 Size and type of catheter used (e.g. Foley 16F): Foley catheter, size not specified Study definition of short‐term catheterisation (days): not reported Intended duration of catheterisation for each group: A: the Foley catheter was removed on post‐op day 1 B: the Foley catheter was removed on post‐op day 3 C: the Foley catheter was removed on post‐op day 5 |
|
| Outcomes | Urinary retention/recatheterisation UTI Asymptomatic bacteriuria Anastomotic leak (%) Overall surgical site infection Pulmonary complications Overall complications rate |
|
| Definition of CAUTI or bacteriuria | UTI diagnosed based on symptoms and positive urine culture, symptomatic bacteriuria based on cultures routinely taken on catheter removal | |
| Sponsorship/funding | Not reported | |
| Ethical approval | Not reported | |
| Notes | AUR was defined as the inability to pass urine despite significant urge and attempt for at least 30 min, or if the patient did not spontaneously pass urine within 8 h after removal of the Foley catheter. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: “Randomization was done by use of computer‐generated institutional randomisation tables with blocks of 15; that is, in each 15 patients from the same institution, 5 patients were randomly assigned to each of the groups.” Comment: adequate method of randomisation |
| Allocation concealment (selection bias) | Low risk | Quote: “Each patient’s allocation was revealed after completion of surgery.” Comment: adequate method of concealment |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Not reported. Unlikely that blinding of patients was possible |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Not reported |
| Blinding of microbiological outcome (detection bias) | Low risk | Quote: “… based on cultures routinely taken on catheter removal.” Comment: suggests that cultures were taken alongside routine cultures for other patients and not specifically for the trial. Thus unlikely that the microbiologist knew which patient belonged to the trial and which did not. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | “Sixteen protocol violations were recorded, including 6 patients in whom routine urinary cultures were not undertaken on removal of the catheter, 5 male patients without a reported history of BPH in whom AUA‐BPH symptom scores were not recorded, and 5 patients in whom intraoperative nerve identification was not recorded. All violations were considered minor, and did not require exclusion of these patients from the study.” No dropouts or withdrawals. All participants who were randomised were analysed according to their allocated intervention group. |
| Selective reporting (reporting bias) | Low risk | All outcomes reported in methods are accounted for. Protocol was not available to assess |
| Other bias | Low risk | Nothing to indicate any other source of bias |
Zomorrodi 2018.
| Study characteristics | ||
| Methods |
Study design: RCT Dates study conducted: April 2016‐September 2016 |
|
| Participants |
Population: mixed Setting: Tabriz Country: Iran Inclusion criteria: all patients suffered from end‐stage renal failure and had negative urinary culture and had been operated by the same team of surgery using 3 medications (tacrolimus, prednisolone and mycophenolate mofetil) Exclusion criteria: any patient with history of lower urinary tract disease and abnormality of lower urinary tract and also any patient who disagreed with the study was excluded Condition for hospitalisation: renal transplantation for end stage renal failure Number of participants: eligible; 88 randomised; 88 reported Age (mean and SD): A 43.52 ± 13.6; B 43.20 ± 14.39 Use of antibiotic prophylaxis: not reported |
|
| Interventions |
Group A (n = 44): IUC removal 3 days post‐op Group B (n = 44): IUC removal 7 days post‐op Size and type of catheter used: not reported Study definition of short‐term catheterisation (days): not reported Intended duration of catheterisation for each group: Group A: 3 days post‐op Group B: 7 days post‐op |
|
| Outcomes | UTI | |
| Definition of CAUTI or bacteriuria | Not reported | |
| Sponsorship/funding | This study was supported by Tabriz University of Medical Sciences, Tabriz, Iran. | |
| Ethical approval | The research followed the tenets of the Declaration of Helsinki. Consent for operation and study had been taken. The ethical committee of Tabriz University of Medical Sciences approved the research. All patients’ information remained confidential. | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Quote: “The patients were all divided into two groups randomly” Comment: unclear method of randomisation |
| Allocation concealment (selection bias) | Unclear risk | Not reported |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Not reported. Unlikely given nature of intervention |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Not reported |
| Blinding of microbiological outcome (detection bias) | Low risk | Not reported. Assume lab technician blinded to participants of the trial |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Appears to be free from attrition bias |
| Selective reporting (reporting bias) | Low risk | Appears to be free from reporting bias. Outcomes reported in protocol also reported in trial |
| Other bias | Low risk | Appears to be free from other sources of bias |
APR: abdominoperineal resection; ASA: American Society of Anesthesiologists; AUA: American Urological Association; AUB: abnormal uterine bleeding; AUR: acute urinary retention; BP: blood pressure; CAUTI: catheter‐associated urinary tract infection; CDC: Centers for Disease Control and Prevention; cfu: colony forming unit; CS: caesarian section; DVT: deep vein thrombosis; EAU: European Association of Urology; FIGO: International Federation of Gynecology and Obstetrics; ICU: intensive care unit; IM: intramuscular(ly); IPSS: International Prostate Symptom Score; IQR: interquartile range; ITT: intention‐to‐treat; IUC: indwelling urethral catheter; IV: intravenous; LAR: low anterior resection; PCEA: patient‐controlled epidural anaesthesia; Post‐op: post‐operative(ly); PSA: prostate‐specific antigen; PTFE: polytetrafluoroethylene; QoL: quality of life; RCT: randomised controlled trial; RUV: residual urine volume; SD: standard deviation; TUIP: transurethral incision of the prostate; TURP: transurethral resection of the prostate; UTI: urinary tract infection; VAS: visual analogue scale
Characteristics of excluded studies [ordered by study ID]
| Study | Reason for exclusion |
|---|---|
| 2004‐005138‐38 | Trial looked at the prophylactic usage of cefuroxime |
| ACTRN12617001191381 | Intervention not relevant |
| Agrawal 1993 | Not an RCT or quasi‐RCT |
| Airaksinen 1979 | Intervention was not relevant |
| Aunruean 2007 | Intervention was not relevant |
| Bach 1990 | Intervention was not relevant |
| Benjamin 2018 | Intervention not relevant |
| Bergqvist 1979 | The study compares catheter materials for long‐term usage |
| Boyd 2019 | Intervention not relevant |
| Christensen 1983 | Intervention was not relevant. Trial looks at intermittent drainage in long‐term IUC |
| Cleland 1971 | Comparative study of interventions to prevent infection |
| CTRI/2019/02/017836 | Participants of trial are children aged 1‐10 years |
| Dhariwal 2019 | Intervention not relevant |
| Downey 1997 | Not an RCT or quasi‐RCT |
| Efimenko 2004 | Intervention not relevant |
| Farag 2018 | Intervention not relevant |
| Farrell 1989 | Intervention not relevant. Involved suprapubic catheterisation |
| Fattah 2013 | Not an RCT or quasi‐RCT |
| Fernandez‐Gonzalez 2019 | Trial uses intermittent self‐catheterisation |
| Ghoreishi 2003 | Intervention not relevant. Trial compared catheterisation to no catheterisation in women undergoing cesarean deliveries |
| Gillespie 1962 | Intervention not relevant. Trial looked at catheter disinfection and also involved intermittent catheterisation |
| Gross 1990 | Intermittent catheterisation used |
| Halaska 1991 | Intervention not relevant. Trial involved suprapubic catheters |
| Hollingsworth 2013 | Not an RCT |
| Hu 1999 | Intervention not relevant |
| ISRCTN44339585 | Intervention was not relevant |
| ISRCTN48516968 | Intervention not relevant |
| Jankowska 1995 | Intervention not relevant. Trial compares no catheterisation to 24‐h catheterisation |
| Ledermair 1970 | Intervention not relevant |
| Loeb 2008 | Intervention not relevant. Trial uses stop orders for the removal of IUCs under specified criteria and does not compare durations of catheterisation. There were no fixed time points for when catheters were removed. |
| Mamo 1991 | Retrospective study ‐ not an RCT or quasi‐RCT |
| Mayer 1973 | Intervention not relevant |
| Medina 2005 | Intervention not relevant. Trial compares physiological and retrograde filling of the bladder to determine if one method would substantially shorten the evaluation of bladder emptying. |
| Menshawy 2020 | Not an RCT |
| Michelson 2005 | Intervention not relevant. Catheter protocols are not related to catheter removal |
| Miller 1960 | Intervention not relevant. Describes outcomes between closed and open drainage systems |
| Mills 2018 | Intervention not relevant |
| Moon 2012 | Study involves long‐term catheterisation |
| Mustafa 1968 | Study compares catheterisation and no catheterisation |
| Nadu 2001 | Not an RCT or quasi‐RCT |
| Nardos 2011 | Catheterisation is part of intervention (vesicovaginal fistula repair) |
| Nardos 2012 | Catheterisation is part of intervention (vesicovaginal fistula repair) |
| NCT00182832 | Intervention not relevant. Study compares two methods of measuring post‐void residual volume |
| NCT00392210 | Intervention not relevant. Study compares voiding techniques post‐surgery |
| NCT00446732 | Intervention not relevant. Study compares efficacy of Uroshield treatment with standard therapy |
| NCT00959920 | Study compared intermittent and indwelling catheterisation |
| NCT01067768 | Intervention not relevant. Study compared efficacy of daily nurse reviews of the IUC |
| NCT01108757 | Interention not relevant. Study compared efficacy of antibiotic prophylaxis |
| NCT01343784 | Intervention not relevant. Study compares various sling procedures |
| NCT01525498 | Study withdrawn prior to recruitment stages |
| NCT01646190 | Intervention not relevant. Study compares fast track programme to regular practice |
| NCT01797146 | Intervention not relevant. Study compares catheter reminder programmes |
| NCT01926756 | Study compares catheterisation to no catheterisation |
| NCT02054065 | Intervention not relevant. Study looks at catheter reminder systems |
| NCT02126813 | Intervention not relevant. Study looking at fast track programmes in surgery |
| NCT02357251 | Intervention not relevant. Study compares current perioperative care of the investigators' gynaecologic oncology patients with a standardised perioperative "enhanced recovery" pathway |
| NCT02996968 | Intervention not relevant. Uses intermittent catheterisation |
| NCT03646136 | Intervention not relevant |
| NCT03684941 | Trial registration. Uses intermittent catheterisation |
| NL2677 | Study compares indwelling and intermittent catheterisation |
| Norton 1987 | Study uses suprapubic catheterisation |
| Okrainec 2017 | Not an RCT. Prospective cohort study |
| Panknin 2007 | This is a commentary on a non‐randomised study |
| Patel 2018 | Intervention only partially meets criteria for review. Trial compares shorter durations of catheterisation and also includes the use of alpha blockers |
| Pellegrini 1995 | Intervention not relevant. Compares intermittent catheterisation |
| Peniakov 2004 | Intervention not relevant. Study involved intermittent catheterisation |
| Perera 2002 | Not an RCT or quasi‐RCT |
| Priefer 1982 | Long‐term catheterisation |
| Rabkin 1998 | Not an RCT or quasi‐RCT |
| Ratahi 2005 | Study compares catheterisation to no catheterisation |
| Rehm 1962 | Not an RCT or quasi‐RCT |
| Ross 1966 | This trial compares infection rates when IUCs were inserted with and without the application of topical antibiotics |
| Salem Mohamed 2018 | Intervention not relevant |
| Sandberg 2018 | Not an RCT |
| Souto 2000 | Group allocation determined on clinical criteria i.e. not an RCT or quasi‐RCT |
| Symonds 1967 | Intervention not relevant |
| Tomaszewski 2015 | Not an RCT or quasi‐RCT |
| Uberoi 2013 | Not a RCT or quasi‐RCT |
| UMIN000014474 | Intervention not relevant. Compares catheterisation to no catheterisation |
| UMIN000015289 | Intervention not relevant |
| Watt 1998 | Not an RCT or quasi‐RCT |
| Weitzel 2008 | Not an RCT or quasi‐RCT |
| Wilson 2013 | Not an RCT or quasi‐RCT |
| Zhang 1999 | Intervention not relevant |
| Zhao 1994 | Compares suprapubic versus urethral catheterisation |
IUC: indwelling urethral catheter; RCT: randomised controlled trial
Characteristics of studies awaiting classification [ordered by study ID]
NCT02602132.
| Methods | Study design: RCT |
| Participants |
Inclusion criteria: adult patients of both sexes, aged 18‐85 years who require IUC short‐term (1‐14 days) in the units of internal medicine at University Hospital Alcorcón Foundation. Patients who express a desire to participate in the study by signing the informed consent. Exclusion criteria: patients with permanent long‐term (≥ 15 days) urinary catheter; patients with recurrent episodes of UTI, which has submitted episodes of urinary retention in the last month, or who have urologic pathology; patients with cognitive impairments that hinder communication with the medical staff; disoriented patients in person, time and place; anatomical and physiological genito‐urinary system alterations; patients taking a drug that affects the bladder and kidney function the week prior to catheterisation; pregnant patients; patients with a known history of benign prostatic hyperplasia |
| Interventions | Group A: to clamp before the removal of short‐term IUC Group B: IUC is clamped before removal and unclamped when the patient expresses desire to urinate |
| Outcomes | Complications of IUC |
| Notes | This trial is currently listed as suspended. We contacted the trial author to provide further information and confirmed that this trial had been suspended due to poor recruitment. |
IUC: indwelling urethral catheter; RCT: randomised controlled trial; UTI: urinary tract infection
Characteristics of ongoing studies [ordered by study ID]
ACTRN12611000414910.
| Study name | Bladder care following laparoscopy for benign non‐hysterectomy gynaecological conditions – a randomised controlled trial (ACTRN12611000414910) |
| Methods | Study design: RCT |
| Participants | Patients undergoing laparoscopic surgery for benign non‐hysterectomy gynaecological conditions Inclusion criteria: elective laparoscopy for a benign gynaecological condition; patients to be aged ≥ 18 years at time of surgery; patients who understand the conditions of the study and are willing to participate for the length of the prescribed term of follow‐up; patients who are capable of, and have given written informed consent to their participation in the study; patients presenting with benign gynaecological conditions that require surgical intervention as agreed to by the patient and her attending medical team Exclusion criteria: concurrent involvement in other research studies; past history of incontinence surgery; surgery for urinary incontinence or prolapse; suspected or confirmed gynaecological malignancy; patients scheduled for hysterectomy as part of their surgical procedure; patients with long‐term bladder catheterisation (intermittent or permanent); suspected or confirmed pregnancy at the time of surgery; intermittent flow pattern on uroflowmetry (indicative of pre‐existing voiding dysfunction); pre‐operative PVR ≥ 150 mL |
| Interventions |
Group A: immediate removal of the IUC post‐laparoscopic surgery for benign non‐hysterectomy gynaecological conditions Group B: removal of the IUC at 6 am on the 1st post‐op day following laparoscopic surgery for benign non‐hysterectomy gynaecological conditions |
| Outcomes | Incidence of post‐op UTI Incidence and pattern of post‐op voiding dysfunction PVR urine volume in patients before surgery Duration of hospital stay Re‐admission to hospital (incidence and indication) Unscheduled presentation to general practitioner Emergency department or outpatient service (clinic/rooms) Economic analyses of the 2 modalities for care |
| Starting date | 1 March 2012 |
| Contact information | Associate Professor Jason Abbott Royal Hospital for Women Barker Street Randwick NSW 2031 Country: Australia Phone: +61 2 93826111 Email: j.abbott@unsw.edu.au |
| Notes | Recruitment status: not yet recruiting |
ChiCTR1800016149.
| Study name | Randomized control study of early extubation of indwelled urinary catheter after rectal cancer radical operation |
| Methods |
Study design: randomised controlled trial Setting: Sixth Affiliated Hospital of Sun Yat‐sen University, China |
| Participants | Inclusion criteria: aged 18‐75 years old; pre‐operative fibrosis colonoscopy and pathological biopsy confirmed colorectal cancer as the primary cancer; no difficulty in urination and no UTI before operation; provision of written informed consent Exclusion criteria: patients with colorectal cancer palliative surgery, Miles operation and emergency surgery; patients with obvious urinary diseases including urinary tract stone, tumour, prostatic hyperplasia; patients having a history of urinary enuresis within 72 h; patients with history of pelvic surgery or with severe systemic diseases such as heart, lung, kidney, etc.; patients required lateral lymph node dissection; patients with recurrent rectal cancer or multiple rectal cancer and with other tumour; patient unconscious and unable to express the intention of urination correctly; patients with dementia, stroke or mental illness. Antibiotic treatment was applied 1 week before surgery. |
| Interventions |
Group A: removal of IUC within 24 h directly after surgery Group B: removal of IUC after training bladder function on the 3rd day regularly after surgery |
| Outcomes | AUR UTI Urethral bleeding Residual volume Urinary incontinence Discomfort of lower urinary tract |
| Starting date | 14 May 2018 |
| Contact information | Tenghui Ma, austin_2004@163.com |
| Notes |
CTRI/2018/11/016299.
| Study name | A randomized controlled trial comparing early versus late catheter removal after radical hysterectomy |
| Methods | Study design: RCT |
| Participants | Effect of early catheter removal in those undergoing radical hysterectomy in early‐stage cervical cancer compared to the late removal group Inclusion criteria: women aged 18‐80, all early stages (IA2, IB1, IIA1) cervical cancer undergoing radical hysterectomy Exclusion criteria: non‐cervical cancer, previous pelvic irradiation, prior urinary dysfunction, bladder injury during surgery, other indications for prolonging bladder catheterisation |
| Interventions |
Group A: will be assigned for catheter removal on post‐op day 4+/‐1 day Group B: will be assigned for catheter removal on post‐op day 10+/‐ day |
| Outcomes | CAUTI |
| Starting date | 11 November 2018 |
| Contact information | Amy Jose, amy.jose@cmcvellore.ac.in |
| Notes |
IRCT20180208038670N1.
| Study name | The effect of urinary catheter removal time on the incidence of urinary infection and satisfaction level in patients undergoing lower extremity fracture surgery |
| Methods | Study design: RCT |
| Participants |
Inclusion criteria: age 18‐60 years; placement in the lower extremity surgery list Exclusion criteria: UTI; common chronic diseases (ِdiabetes, heart failure, renal failure, chronic obstructive pulmonary disease); multiple trauma; other infections Target sample size: 96 |
| Interventions |
Group A: people with IUC to 24 h after catheterisation Group B: people with IUC to 48 h after catheterisation Group C: people with IUC 72 h after catheterisation |
| Outcomes | Incidence of UTI |
| Starting date | 31 July 2018 |
| Contact information | Tahereh Haghparast, shirinehaghparast1389@yahoo.com |
| Notes |
NCT03539107.
| Study name | Voiding assessment based on minimum spontaneous void of 150 mL compared to retrograde fill method after female pelvic floor reconstructive surgery |
| Methods |
Study design: RCT This study will compare voiding assessment based on a minimum spontaneous voided volume of 150 cc with the standard retrograde fill approach in women after pelvic floor procedures. |
| Participants | Women undergoing pelvic floor procedures Inclusion criteria: all women ≥ 18 years who undergo surgery for urinary incontinence and/or pelvic organ prolapse (POP) Exclusion criteria: patients who require prolonged Foley catheter or suprapubic catheter |
| Interventions |
Group A: retrograde bladder fill ‐ participants will have their bladder retrograde filled with 300 mL of fluid prior to a voiding trial Group B: spontaneous void ‐ participants will not have retrograde fill of bladder, rather will be required to void 150 mL spontaneously prior to discharge |
| Outcomes | The percentage of participants who did not meet the required voiding assessment criteria and needed catheterisation |
| Starting date | 1 September 2019 |
| Contact information | Harmanli Oz, MD |
| Notes |
NCT03668535.
| Study name | Filling of the urinary bladder during difficult cesaerean section |
| Methods |
Allocation: randomised
Intervention model: parallel Assignment
Intervention model description: 2 groups of women with difficult CS at risk of urinary bladder injury. Group A will receive the intervention. Group B will not receive the intervention. Masking: double (participant, investigator) Masking description: closed envelope will be used for randomisation. The patient and the investigator will be blinded. Primary purpose: prevention |
| Participants |
Patients and Methods Inclusion criteria: pregnant women at gestation from 20‐ 41 weeks who have any of the following risk factors: previous CS ≥ 3 times; previous history of bladder injury during CS; operative report of extensive adhesions in the last CS; CS for placenta accreta spectrum No exclusion criteria reported Methods: this is a RCT done at the department of Obstetrics & Gynaecology unit, South Valley University from 1 August 2017‐30 August 2018. The research is approved by the Committee of Ethics for Biomedical Researches, South Valley University at June 2017. All cases have informed consent before inclusion in the research. Closed envelope is used to randomise patients to either group. Group A: are cases of CS who have the intervention. Group B: are cases of CS who do not have the intervention. |
| Interventions |
Group A: bladder filling: participants have a triple‐way IUC insertion before establishment of anaesthesia. Evaluation of the drained urine is done (including: amount, character, and culture and sensitivity). Instillation of 200 mL sterile saline is done by 50 mL syringe through the irrigation way. The irrigation way is closed temporarily by artery forceps. After laparotomy the bladder may be deflated by 50 mL or further inflated by 50 mL if needed to allow comfortable dissection. Group B: bladder deflation: participants have Foley's catheter inserted as usual. The catheter is connected freely to urinary bag |
| Outcomes | Intra‐operative rate of urinary bladder injury |
| Starting date | 1 August 2017 |
| Contact information | Mohammad AM Ahmed, MD |
| Notes |
NCT03835351.
| Study name | Urinary retention after laparoscopic inguinal hernia repair: comparing the use of the intraoperative urinary catheter |
| Methods |
Study design: RCT This will be a RCT that will compare the rate of post‐op urinary retention after laparoscopic inguinal hernia repair between patients who receive an intra‐operative IUC and those who do not. The primary aim of the study is to determine if the use of intra‐operative IUC reduces the incidence of post‐op urinary retention after laparoscopic inguinal hernia repair. Specific patient inclusion criteria include all patients aged ≥ 18 years presenting for an elective unilateral or bilateral inguinal hernia repair, who are able to tolerate general anaesthesia and are considered eligible to have a hernia repair through a laparoscopic approach. |
| Participants | Laparoscopic inguinal hernia repair between patients who receive an intra‐operative urinary catheter and those who do not Inclusion criteria: ≥ 18 years; able to give informed consent; unilateral or bilateral inguinal hernia; scheduled for elective inguinal hernia repair; eligible to tolerate general anaesthesia; eligible to undergo minimally invasive inguinal hernia repair Exclusion criteria: diagnosed with benign prostate hyperplasia (BPH); < 18 years old; unable to give informed consent Emergent inguinal hernia repairs ( acute incarceration or strangulation); unable to tolerate general anaesthesia; not eligible for minimally invasive inguinal hernia repair |
| Interventions |
Group A: intraoperative IIUC ‐ after induction of general anaesthesia, a standard catheterization kit available at the institution where the surgery is being performed will be used to place the urinary catheter using standard sterile technique. Intervention: device: IUC Group B: no intraoperative IUC. No intraoperative IUC will be used during the case |
| Outcomes | "The rate of post‐op urinary retention requiring insertion of a urinary catheter Intraoperative bladder injuries (time frame: measured from start to end of procedure.) This will be determined by comparing the rates of intraoperative bladder injuries between the 2 study groups Complications of intra‐operative urinary catheter (time frame: from the day of surgery until post‐op day 30). This will be accomplished by analysing the rates of urinary tract injury, infections and bladder injuries due to intraoperative catheter placement. IUC complications for patients who develop retention (time frame: from the day of surgery until post‐op day 30). This will be accomplished by analysing the rates of urinary tract injury, or infections and bladder injuries due to catheter placement after patients develop post operative urinary retention." |
| Starting date | 7 March 2019 |
| Contact information | Michael Rosen, MD, rosenm@ccf.org |
| Notes | Currently recruiting |
Xu 2019.
| Study name | A single‐centre, prospective, randomized clinical trial to investigate the optimal removal time of the urinary catheter after laparoscopic anterior resection of the rectum: study protocol for a randomized controlled trial |
| Methods |
Study design: RCT This study is a superiority trial and is designed as a prospective, single‐centre, randomized, parallel‐group, trial. It will be enrolled and divided into 2 groups: the early removal group (the intervention group) and the normal removal group (the control group). The flow diagram for this trial is shown in Fig. 1. The sample size was estimated as follows. According to the latest studies, the incidence of urinary retention after rectal surgery is 25% when the catheter is removed within 2 days after surgery and 10% when the catheter is removed after 7 days. To detect these outcomes with α = 0.05 and β = 0.2, we would need 100 patients per group (total 200). We decided to enrol 110 patients in each group (total 220), to allow for a possible 10% dropout rate. |
| Participants | The study participants will be rectal cancer patients requiring laparoscopic anterior resection of the rectum. Inclusion criteria: age 18–75 years; diagnosed with rectal cancer and posted for total or tumour‐specific mesorectal excision with colorectal or coloanal anastomosis; ASA classification of 1–3 Exclusion criteria: pre‐operatively diagnosed UTIs or urinary system diseases (including end‐stage renal disease, neurological bladder dysfunction, and malignancy); previous history of urinary retention or of having received drugs likely to affect bladder function; male patients with disease of the prostate (such as benign prostatic hyperplasia); patients receiving emergency surgery |
| Interventions | A total of 220 participants meeting the inclusion criteria will be randomly assigned to an experimental group or a control group. Group A: the experimental group will have their IUCs removed on post‐op day 2. Group B: control group will have their IUCs removed on post‐op day 7. In both groups, catheter removal will be performed when the bladder is full. |
| Outcomes |
Primary outcome: post‐operative urinary retention requiring recatheterisation following IUC removal Secondary outcome: UTI occurring following IUC removal |
| Starting date | July 2017 |
| Contact information | Correspondence: Xiaoy@pumch.cn; Xiaoy@pumcn.cn |
| Notes | "This trial protocol is approved by the Ethics Committee of Peking Union Medical College Hospital (reviewed in 2017 as ZS‐1269) and has been registered at ClinicalTrials.gov under the identifier NCT03065855, registered on February 23, 2017. All eligible participants and their legal surrogates will be fully informed of the potential risks and benefits of the interventions in each group." |
ASA: American Society of Anesthesiologists; AUR: acute urinary retention; CAUTI: catheter‐associated urinary tract infection; CS: caesarean section; IUC: indwelling urethral catheter; PVR: post‐void residual; RCT: randomised controlled trial; UTI: urinary tract infection
Differences between protocol and review
For this update, published in 2021, we made the following changes.
Changes to the search methods
The Cochrane Incontinence Specialised Register now also covers MEDLINE In‐Process, MEDLINE Epub Ahead of Print, ClinicalTrials.gov, WHO ICTRP, Be Part of Research and handsearching of journals and conference proceedings as well as MEDLINE and Cochrane Central Register of Controlled Trials (CENTRAL). Embase is now searched centrally for Cochrane and the search of CENTRAL for the Cochrane Incontinence Specialised Register will pick up these Embase records; a separate search of Embase was therefore not conducted. CINAHL was searched to ensure coverage of the nursing and allied health professions' literature. By the time of the last search run for this review, the CINAHL search had been incorporated into the search for the Cochrane Incontinence Specialised Register and was not run separately for this review; please see Appendix 2 for further details.
Changes to outcomes
After reviewing the original protocol for this review, we decided to add the following clinically important outcomes to bring the updated review in line with the GRADE recommendations to report outcomes deemed important for clinical decision making.
Patient pain or discomfort
Urinary incontinence
Number of patients reporting dysuria/difficulty passing urine
Symptomatic catheter‐associated urinary tract infection (CAUTI)
Post‐void residual volume
Asymptomatic bacteriuria
Other complications of catheterisation (or recatheterisation), for example, haemorrhage, stricture formation, fever
Number of patients not discharged on day of indwelling urethral catheter removal
Time between removal of catheter to discharge (days)
The following outcomes were removed from the previous update (in 2007) as they were deemed to be either not clinically relevant or not related to short‐term urethral catheterisation: indwelling urethral catheter not removed on time; deep vein thrombosis (DVT); secondary haemorrhage; recurrence of strictures; long‐term urinary complications (unspecified); post‐operative fever; number of patients not discharged on day of indwelling urethral catheter removal.
Changes to methods
The review authors re‐abstracted trial data for all previously and newly included trials, as well as assessing the risk of bias in all included trials. The GRADE approach for assessing the certainty of evidence was adopted for five critical outcomes which are included in the summary of findings tables.
Post‐hoc subgroup analysis
We performed post‐hoc subgroup analysis for one outcome in comparison 2 to assess whether the use of prophylactic antibiotics would impact the number of participants developing symptomatic CAUTI (Analysis 2.6). This was not stated in our protocols or methods section and was conducted after the results were obtained to assess the effect of prophylactic antibiotics on participants developing symptomatic CAUTI. It could not be performed for the other comparisons due to an insufficient number of trials reporting whether antibiotic prophylaxis was used.
Similarly, we performed post hoc subgroup analysis for length of hospitalisation in comparison 2 to explore possible reasons for very high heterogeneity.
Contributions of authors
AE: screened the abstracts and full‐text reports of all potentially eligible trials; performed data extraction and risk of bias assessment for all included trials; assessed the certainty of evidence and conducted data analysis; wrote the manuscript and responded to peer review comments. FS: performed some data extraction and risk of bias assessments for all the trials (shared with MIO and EAK); assessed the certainty of evidence and conducted data analysis. EAK: performed some data extraction and risk of bias assessments for all the trials (shared with FS and MIO). RG: contributed to drafts of this review and approved the final version. RF: contributed to drafts of this review and approved the final version. MIO: screened the abstracts and full‐text reports of all potentially eligible trials; performed some data extraction and risk of bias assessments for all the trials (shared with FS and EAK); assessed the certainty of evidence and conducted data analysis; assisted in responding to peer review comments.
Sources of support
Internal sources
-
University of Aberdeen, UK
Awaiss Ellahi and Emily Kidd were supported by the University of Aberdeen School of Medicine.
External sources
-
National Institute for Health Research, UK
This project was supported by the National Institute for Health Research, via Cochrane infrastructure funding to Cochrane Incontinence. The views and opinions expressed therein are those of the authors and do not necessarily reflect those of the Evidence Synthesis Programme, the NIHR, NHS or the Department of Health and Social Care.
Declarations of interest
In accordance with Cochrane's Commercial Sponsorship Policy, the following declarations have been made: AE: none known FS: none known EAK: none known RG: none known RF: none known MIO: none known
New search for studies and content updated (no change to conclusions)
References
References to studies included in this review
Ahmed 2014 {published data only}
- Ahmed MR, Sayed Ahmed WA, Atwa KA, Metwally L. Timing of urinary catheter removal after uncomplicated total abdominal hysterectomy: a prospective randomized trial. European Journal of Obstetrics Gynecology and Reproductive Biology 2014;176(1):60-3. [sr-incont61660] [DOI] [PubMed] [Google Scholar]
Alessandri 2006 {published data only}
- Alessandri F, Mistrangelo E, Lijoi D, Ferrero S, Ragni N. A prospective, randomized trial comparing immediate versus delayed catheter removal following hysterectomy. Acta Obstetricia et Gynecologica Scandinavica 2006;85(6):716-20. [sr-incont22108] [DOI] [PubMed] [Google Scholar]
Allen 2016 {published data only}
- Allen MS, Blackmon SH, Nichols FC 3rd, Cassivi SD, Harmsen WS, Lechtenberg B, et al. Optimal timing of urinary catheter removal after thoracic operations: a randomized controlled study. Annals of Thoracic Surgery 2016;102(3):925-30. [NCT01611519] [sr-incont74076] [DOI] [PubMed] [Google Scholar]
- NCT01611519. Early or late Foley removal after thoracotomy [Optimal timing of Foley catheter removal in patients undergoing thoracic surgery with epidural analgesia: a randomized, controlled trial]. clinicaltrials.gov/show/NCT01611519 (first received 5 June 2012). [NCT01611519] [sr-incont63825]
Alonzo‐Sosa 1997 {published data only}
- Alonzo-Sosa JE, Flores-Contreras JT, Paredes-Canul M. Method for transurethral catheterization for 1-3 days for pelvic floor relaxation in the postoperative period [Manejo del catéter transuretral por uno y por tres días en pacientes postoperadas por relajación del piso pélvico]. Ginecologia y Obstetricia de Mexico 1997;65:455-7. [sr-incont6791] [PMID: ] [PubMed] [Google Scholar]
Aref 2020 {published data only}
- Aref NK. Does timing of urinary catheter removal after elective cesarean section affects postoperative morbidity?: a prospective randomized trial. Journal of Maternal-Fetal and Neonatal Medicine 2020;33(18):3141-6. [DOI: 10.1080/14767058.2019.1569619] [sr-incont78674] [DOI] [PubMed] [Google Scholar]
Aslam 2019 {published data only}
- Aslam MF, Bazzi AA, Osmundsen BC, Hagglund K, Gregory WT. When to remove the indwelling catheter after minimally invasive sacrocolpopexy? CARESS (CAtheter REmoval after Sacrocolpopexy Surgery) (Abstract number: Poster 54). Female Pelvic Medicine & Reconstructive Surgery 2019;25(Suppl 1 5S):S203-4. [NCT02189291] [sr-incont78489] [DOI] [PubMed] [Google Scholar]
- NCT02189291. Study to assess duration of indwelling catheter after sacrocolpopexy (CARESS) [When to remove the indwelling catheter after minimally invasive sacrocolpopexy?]. clinicaltrials.gov/show/NCT02189291 (first received 14 July 2014). [NCT02189291] [sr-incont63843]
Azarkish 2003 {published data only}
- Azarkish F, Latifnejad R, Seeyyedi Alavi CH, Khajeh Karamoddin M, Esmaeeli H. The effect of early removal of indwelling urinary catheter after cesarean delivery on the urinary tract infections. Journal of Mashhad School of Nursing and Midwifery 2003;5(15):32. [sr-incont78676] [Google Scholar]
Azarkish 2005 {published data only}
- Azarkish F. The effect of removal of indwelling urinary catheter on the pain after cesarean section. Quarterly Scientific Journal of Kurdistan University of Medical Sciences 2005;10(1 (serial number 35)):23-7. [sr-incont78675] [Google Scholar]
Barone 2015 {published data only}
- Barone MA, Widmer M, Arrowsmith S, Ruminjo J, Seuc A, Landry E, et al. Breakdown of simple female genital fistula repair after 7 day versus 14 day postoperative bladder catheterisation: a randomised, controlled, open-label, non-inferiority trial. Lancet 2015;386(9988):55-62. [sr-incont63823] [DOI] [PubMed] [Google Scholar]
Basbug 2020 {published data only}
- Basbug A, Yuksel A, Ellibes Kaya A. Early versus delayed removal of indwelling catheters in patients after elective cesarean section: a prospective randomized trial. Journal of Maternal-Fetal and Neonatal Medicine 2020;33(1):68-72. [sr-incont78677] [DOI] [PubMed] [Google Scholar]
Benoist 1999 {published data only}
- Benoist S, Panis Y, Denet C, Mauvais F, Mariani P, Valleur P. Optimal duration of urinary drainage after rectal resection: a randomized controlled trial. Surgery 1999;125(2):135-41. [sr-incont8245] [PubMed] [Google Scholar]
Bristoll 1989 {published data only}
- Bristoll SL, Fadden T, Fehring RJ, Rohde L, Smith PK, Wohlitz BA. The mythical danger of rapid urinary drainage. American Journal of Nursing 1989;89(3):344-5. [sr-incont1169] [PubMed] [Google Scholar]
Carpiniello 1988 {published data only}
- Carpiniello VL, Cendron M, Altman HG, Malloy TR, Booth R. Treatment of urinary complications after total joint replacement in elderly females. Urology 1988;32(3):186-8. [sr-incont1179] [DOI] [PubMed] [Google Scholar]
Carter‐Brooks 2018 {published data only}
- Carter-Brooks CM, Zyczynski HM, Moalli PA, Brodeur PG, Shepherd JP. Early catheter removal after pelvic floor reconstructive surgery: a randomized trial. International Urogynecology Journal 2018;29(8):1203-12. [NCT02739256] [sr-incont77939] [DOI] [PMC free article] [PubMed] [Google Scholar]
Chai 2011 {published data only}
- Chai J, Pun TC. A prospective randomized trial to compare immediate and 24-hour delayed catheter removal following total abdominal hysterectomy. Acta Obstetricia et Gynecologica Scandinavica 2011;90(5):478-82. [NCT01182714] [sr-incont41657] [DOI] [PubMed] [Google Scholar]
- NCT01182714. A prospective randomized control trial comparing immediate and 24-hours delayed catheter removal following hysterectomy [A prospective randomized trial to compare immediate and 24-hours delayed catheter removal following total abdominal hysterectomy]. clinicaltrials.gov/show/NCT01182714 (first received 17 August 2010). [NCT01182714] [sr-incont63744]
Chen 2013 {published data only}
- Chen YY, Chi MM, Chen YC, Chan YJ, Chou SS, Wang FD. Using a criteria-based reminder to reduce use of indwelling urinary catheters and decrease urinary tract infections. American Journal of Critical Care 2013;22(2):105-14. [sr-incont49304] [DOI] [PubMed] [Google Scholar]
Chia 2009 {published data only}
- Chia YY, Wei RJ, Chang HC, Liu K. Optimal duration of urinary catheterization after thoracotomy in patients under postoperative patient-controlled epidural analgesia. Acta Anaesthesiologica Taiwanica 2009;47(4):173-9. [sr-incont39528] [DOI] [PubMed] [Google Scholar]
Chillington 1992 {published data only}
- Chillington B. Early removal advances discharge home. Comparison of midnight and early morning catheter removal following prostatectomy. Professional Nurse 1992;8(2):84-9. [sr-incont2346] [PubMed] [Google Scholar]
Cornia 2003 {published data only}
- Cornia PB, Amory JK, Fraser S, Saint S, Lipsky BA. Computer-based order entry decreases duration of indwelling urinary catheterization in hospitalized patients. American Journal of Medicine 2003;114(5):404-7. [sr-incont24018] [DOI] [PubMed] [Google Scholar]
Coyle 2015 {published data only}
- Coyle D, Joyce K, Garvin JT, Regan M, McAnena OJ, Neary P, et al. Early post-operative removal of urethral catheter in patients undergoing colorectal surgery with epidural analgesia - a single-centre prospective randomised controlled clinical trial (Abstract number 5). Irish Journal of Medical Science 2014;183(1 Suppl 1):S23. [NCT01508767] [sr-incont69511] [Google Scholar]
- Coyle D, Joyce KM, Garvin JT, Regan M, McAnena OJ, Neary PM, et al. Early post-operative removal of urethral catheter in patients undergoing colorectal surgery with epidural analgesia - a prospective pilot clinical study. International Journal Of Surgery 2015;16(Pt A):94-8. [NCT01508767] [sr-incont67111] [DOI] [PubMed] [Google Scholar]
- NCT01508767. Early post-operative removal of urethral catheter in patients undergoing colorectal surgery with epidural analgesia [Prospective randomized controlled trial of early post-operative removal of urethral catheter in patients undergoing colorectal surgery with epidural analgesia]. clinicaltrials.gov/show/NCT01508767 (first received 12 January 2012). [NCT01508767] [sr-incont63821]
Crowe 1993 {published data only}
- Crowe H, Clift R, Duggan G, Bolton D, Costello A. Randomized study of the effect of midnight removal of urinary catheters. Urologic Nursing 1994;14(1):18-20. [sr-incont43] [PubMed] [Google Scholar]
- Crowe H, Clift R, Duggan G, Bolton DM, Costello AJ. Randomised study of the effect of midnight versus 0600 removal of urinary catheters. British Journal of Urology 1993;71(3):306-8. [sr-incont133] [DOI] [PubMed] [Google Scholar]
Dunn 1999 {published data only}
- Dunn T, Stamm C, Forshner D. A randomized trial of Foley catheter use in obstetric and gynecological surgery (Abstract). In: 15th Annual Meeting of the International Society of Technology Assessment in Health Care; 1999 Jun 20-23; Edinburgh, UK. 1999:145. [sr-incont17221]
Dunn 2000b {published data only}
- Dunn TS, Forshner D, Stamm C. Foley catheterization in the postoperative patient. Obstetrics and Gynecology 2000;95(4 Suppl):S30. [sr-incont11861] [Google Scholar]
Dunn 2003 {published data only}
- Dunn TS, Shlay J, Forshner D. Are in-dwelling catheters necessary for 24 hours after hysterectomy? American Journal of Obstetrics and Gynecology 2003;189(2):435-7. [sr-incont16480] [DOI] [PubMed] [Google Scholar]
Durrani 2014 {published data only}
- Durrani SN, Khan S, Ur Rehman A. Transurethral resection of prostate: early versus delayed removal of catheter. Journal of Ayub Medical College Abbottabad 2014;26(1):38-41. [sr-incont71557] [PMID: ] [PubMed] [Google Scholar]
El‐Mazny 2014 {published data only}
- El-Mazny A, El-Sharkawy M, Hassan A. A prospective randomized clinical trial comparing immediate versus delayed removal of urinary catheter following elective cesarean section. European Journal of Obstetrics, Gynecology, and Reproductive Biology 2014;181:111-4. [sr-incont66168] [DOI] [PubMed] [Google Scholar]
Ganta 2005 {published data only}
- Ganta SB, Chakravarti A, Somani B, Jones MA, Kadow K. Removal of catheter at midnight versus early morning: the patients' perspective. Urologia Internationalis 2005;75(1):26-9. [sr-incont20807] [DOI] [PubMed] [Google Scholar]
Glavind 2007 {published data only}
- Glavind K, Morup L, Madsen H, Glavind J. A prospective, randomised, controlled trial comparing 3 hour and 24 hour postoperative removal of bladder catheter and vaginal pack following vaginal prolapse surgery. Acta Obstetricia et Gynecologica Scandinavica 2007;86(9):1122-5. [sr-incont24175] [DOI] [PubMed] [Google Scholar]
Gong 2017 {published data only}
- Gong Y, Zhao L, Wang L, Wang F. The effect of clamping the indwelling urinary catheter before removal in cervical cancer patients after radical hysterectomy. Journal of Clinical Nursing 2017;26(7-8):1131-6. [DOI: 10.1111/jocn.13579] [NCT02083614] [sr-incont73588] [DOI] [PubMed] [Google Scholar]
- NCT02083614. The effect of clamping the indwelling urethral catheter before removal from patients after type III radical hysterectomy. clinicaltrials.gov/show/NCT02083614 (first received 11 March 2014). [NCT02083614] [sr-incont66626]
Gross 2007 {published data only}
- Gross JC, Hardin-Fanning F, Kain M, Faulkner EA, Goodrich S. Effect of time of day for urinary catheter removal on voiding behaviors in stroke patients. Urologic Nursing 2007;27(3):231-5. [sr-incont23785] [PubMed] [Google Scholar]
Gungor 2014 {published data only}
- Gungor Ugurlucan F, Yasa C, Comba C, Dural O, Ayvacikli G, Yalcin O. Effect of duration of bladder catheterization on bladder function in patients undergoing anterior colporraphy: prospective randomized study (Abstract number 769). In: 44th Annual Meeting of the International Continence Society (ICS); 2014 Oct 20-4; Rio de Janeiro, Brazil. 2014. [sr-incont64739]
Guzman 1994 {published data only}
- Guzman S, Israel E, Puente R, Iglesias R, Rosa G, Ulloa C. Handling of Foley catheter regarding urinary retention syndrome following vaginal surgery. Revista Chilena de Obstetricia y Ginecologia 1994;59(4):280-3. [sr-incont3080] [PubMed] [Google Scholar]
Hakvoort 2004 {published data only}
- Hakvoort RA, Elberink R, Vollebregt A, Ploeg T, Emanuel MH. How long should urinary bladder catheterization be continued after vaginal prolapse surgery? A randomised controlled trial comparing short term versus long term catheterisation after vaginal prolapse surgery. BJOG 2004;111(8):828-30. [sr-incont19285] [DOI] [PubMed] [Google Scholar]
- Hakvoort RA, Elberink R, Vollebregt A, Van Der Ploeg T, Emanuel MH. How long should transurethral catheterization be applied after vaginal prolapse surgery? [Hoe lang moet transurethrale katheterisatie toegepast worden na vaginale prolapschirurgie?]. Nederlands Tijdschrift voor Obstetrie en Gynaecologie 2005;118(3):52-4. [sr-incont40772] [Google Scholar]
Hall 1998 {published data only}
- Hall NR, Clune C, Macdonald RC, Holdsworth PJ. What time of day should a urinary catheter be removed? (Abstract number: poster number 26). British Journal of Surgery 1998;85(Suppl 1):72. [sr-incont23731] [Google Scholar]
Han 1997 {published data only}
- Han SH, Yoo TK, Park RJ. Early catheter removal following transurethral prostatectomy: a prospective study of 101 consecutive patients. Korean Journal of Urology 1997;38(4):399-403. [sr-incont68724] [Google Scholar]
Hewitt 2001 {published data only}
- Hewitt A. Early catheter removal following radical perineal prostatectomy: a randomized clinical trial. Urologic Nursing 2001;21(1):37-8. [sr-incont15624] [PubMed] [Google Scholar]
Huang 2011 {published data only}
- Huang CC, Ou CS, Yeh GP, Der Tsai H, Sun MJ. Optimal duration of urinary catheterization after anterior colporrhaphy. International Urogynecology Journal 2011;22(4):485-91. [sr-incont41522] [DOI] [PubMed] [Google Scholar]
Ind 1993 {published data only}
- Ind TE, Brown R, Pyneeandee VM, Swanne M, Taylor G. Midnight removal of urinary catheters - improved outcome after gynecological surgery. International Urogynecology Journal 1993;4(6):342-5. [sr-incont7119] [Google Scholar]
Irani 1995 {published data only}
- Irani J, Fauchery A, Dore B, Bon D, Marroncle M, Aubert J. Systematic removal of catheter 48 hours following transurethral resection and 24 hours following transurethral incision of prostate: a prospective randomized analysis of 213 patients. Journal of Urology 1995;153(5):1537-9. [sr-incont6970] [PubMed] [Google Scholar]
Iversen Hansen 1984 {published data only}
- Iversen Hansen R, Reimer Jensen A. Recurrency after optical internal urethrotomy. A comparative study of long term and short term catheter treatment. Urologia Internationalis 1984;39(5):270-1. [sr-incont696] [DOI] [PubMed] [Google Scholar]
Jang 2012 {published data only}
- Jang J-H, Kang S-B, Lee S-M, Lee T-G, Park J-S, Kim D-W, et al. Randomized controlled trial of alpha blocker for prevention of acute voiding dysfunction after rectal cancer surgery (Abstract number F16). Colorectal Disease 2011;13(Suppl s6):5. [NCT00606983] [sr-incont63655] [Google Scholar]
- Jang J-H, Kang S-B, Lee S-M, Park J-S, Kim D-W, Ahn S. Randomized controlled trial of tamsulosin for prevention of acute voiding difficulty after rectal cancer surgery. World Journal of Surgery 2012;36(11):2730-7. [NCT00606983] [sr-incont47471] [DOI] [PubMed] [Google Scholar]
- NCT00606983. Prevention of acute voiding difficulty after radical proctectomy [Prevention of acute voiding difficulty after radical proctectomy for rectal cancer with tamsulosin]. clinicaltrials.gov/ct2/show/NCT00606983 (first received 5 February 2008). [NCT00606983] [sr-incont60942]
Jeong 2014 {published data only}
- Jeong IG, You D, Yoon JH, Hong S, Lim JH, Hong JH, et al. Impact of tamsulosin on urinary retention following early catheter removal after robot-assisted laparoscopic radical prostatectomy: a prospective randomized controlled trial. International Journal of Urology 2014;21(2):164-8. [NCT01209988] [sr-incont50420] [DOI] [PubMed] [Google Scholar]
- NCT01209988. Impact of tamsulosin on voiding patterns following early catheter removal after robot-assisted laparoscopic radical prostatectomy [A prospective randomized study examining the impact of tamsulosin on voiding patterns following early catheter removal after robot-assisted laparoscopic radical prostatectomy]. clinicaltrials.gov/show/NCT01209988 (first received 28 September 2010). [NCT01209988] [sr-incont63587]
Joshi 2014 {published data only}
- Joshi B, Aggarwal N, Chopra S, Taneja N. A prospective randomized controlled comparison of immediate versus late removal of urinary catheter after abdominal hysterectomy. Journal of Mid-life Health 2014;5(2):68-71. [sr-incont66193] [DOI] [PMC free article] [PubMed] [Google Scholar]
Jun 2011 {published data only}
- Jun L, Xu Z, Chang S. Study on the indwelling time of catheter after TURP. Chinese Journal of Andrology 2011;25(3):42-4, 51. [sr-incont45009] [Google Scholar]
Kamilya 2010 {published data only}
- Kamilya G, Seal SL, Mukherji J, Bhattacharyya SK, Hazra A. A randomized controlled trial comparing short versus long-term catheterization after uncomplicated vaginal prolapse surgery. Journal of Obstetrics and Gynaecology Research 2010;36(1):154-8. [sr-incont39454] [DOI] [PubMed] [Google Scholar]
Kelleher 2002 {published data only}
- Kelleher MM. Removal of urinary catheters: midnight vs 0600 hours. British Journal of Nursing 2002;11(2):84-90. [sr-incont14712] [DOI] [PubMed] [Google Scholar]
Kim 2012 {published data only}
- Kim TN, Ahn JH, Choi YH, Kim HW, Park SW, Lee W, et al. Early removal of urethral catheter after laparoscopic radical prostatectomy (Abstract number UP-107). Journal of Endourology / Endourological Society 2012;26 Suppl 1:A311. [sr-incont45620] [Google Scholar]
Koh 1994 {published data only}
- Koh KB, MacDermott JP, Smith PH, Whelan P. Early catheter removal following transurethral prostatectomy--impact on length of hospital stay. British Journal of Urology 1994;74(1):61-3. [sr-incont1340] [DOI] [PubMed] [Google Scholar]
Kokabi 2009 {published data only}
- Kokabi R, Fereidouni Z, Meshkibaf MH, Miladpoor B. Post operative voiding efficacy after anterior colporrhaphy. Acta Medica Iranica 2010;48(1):33-5. [sr-incont40871] [PubMed] [Google Scholar]
- Kokabi R, Fereidouni Z, Meshkibaf MH. Postoperative voiding efficacy after surgery for urine incontinency and uterine prolapse. Gazzetta Medica Italiana Archivio per le Scienze Mediche 2009;168(2):101-4. [sr-incont40240] [Google Scholar]
Lang 2020 {published data only}
- Lang P, Quezada Y, Whiteside J. Urinary catheter management approaches among women undergoing benign gynecologic surgery: a randomized trial. Female Pelvic Medicine & Reconstructive Surgery 2020;26(12):e73-7. [DOI: 10.1097/SPV.0000000000000781] [NCT03127280] [sr-incont78685] [DOI] [PubMed] [Google Scholar]
- Lang P, Quezada Y, Whiteside JL. A randomized trial comparing conventional and "fast track" indwelling urinary catheter management among women undergoing benign gynecologic surgery (Abstract number 20). American Journal of Obstetrics and Gynecology 2018;218(2 Suppl 2):S905. [sr-incont77938] [Google Scholar]
- NCT03127280. Can investigators reduce urinary catheter use and lower urinary tract infection among women undergoing benign gynecologic surgery? clinicaltrials.gov/show/NCT03127280 (first received 25 April 2017). [NCT03127280] [sr-incont76152]
Lau 2004 {published data only}
- Lau H, Lam B. Management of postoperative urinary retention: a randomized trial of in-out versus overnight catheterization. ANZ Journal of Surgery 2004;74(8):658-61. [sr-incont20270] [DOI] [PubMed] [Google Scholar]
Li 2014 {published data only}
- Li SX, Liu CX. Early removal of the urethral catheter after transurethral plasma kinetic resection of the prostate in the treatment of BPH. Zhonghua Nan Ke Xue [National Journal of Andrology] 2014;20(3):249-52. [sr-incont71057] [PubMed] [Google Scholar]
Liang 2009 {published data only}
- Liang CC, Lee CL, Chang TC, Chang YL, Wang CJ, Soong YK. Postoperative urinary outcomes in catheterized and non-catheterized patients undergoing laparoscopic-assisted vaginal hysterectomy—a randomized controlled trial. International Urogynecology Journal 2009;20(3):295-300. [NCT00564135] [sr-incont32151] [DOI] [PubMed] [Google Scholar]
- NCT00564135. Postoperative urinary retention and urinary track infection (UTI) after laparoscopic assisted vaginal hysterectomy (LAVH) for benign disease [Postoperative urinary retention and UTI after LAVH for benign disease]. clinicaltrials.gov/show/NCT00564135 (first received 27 November 2007). [NCT00564135] [sr-incont60917]
Lista 2020 {published data only}
- Lista G, Lughezzani G, Buffi NM, Saita A, Vanni E, Hurle R, et al. Early catheter removal after robot-assisted radical prostatectomy: results from a prospective single-institutional randomized trial (Ripreca Study). European Urology Focus 2020;6(2):259-66. [sr-incont78686] [DOI] [PubMed] [Google Scholar]
Liu 2015 {published data only}
- Liu YS, Wei S, Elliott M. The effects of a catheter clamping protocol on bladder function in neurosurgical patients: a controlled trial. International Journal of Nursing Practice 2015;21(1):29-36. [sr-incont69875] [DOI] [PubMed] [Google Scholar]
Lyth 1997 {published data only}
- Lyth DR, Braslis R, Iacovou JW. The infusion trial of micturition. British Journal of Urology 1997;79(1):94-5. [sr-incont6774] [DOI] [PubMed] [Google Scholar]
Mao 1994 {published data only}
- Mao XY, Li LZ, Shi SM, Yang L, Xia PL. The exploration on the lasting urinary catheter post abdominal gynecological surgery. Zhonghua Hu Li za Zhi [Chinese Journal of Nursing] 1994;29(1):6-8. [sr-incont18487] [PubMed] [Google Scholar]
Matsushima 2015 {published data only}
- Matsushima M, Miyajima A, Hattori S, Takeda T, Mizuno R, Kikuchi E, et al. Comparison of continence outcomes of early catheter removal on postoperative day 2 and 4 after laparoscopic radical prostatectomy: a randomized controlled trial. BMC Urology 2015;15:77. [UMIN000014944] [sr-incont68109] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matsushima M. A study to evaluate the efficacy of early removal of the catheter after laparoscopic radical prostatectomy. umin.ac.jp/ctr/index.htm (available at: upload.umin.ac.jp/cgi-open-bin/ctr/ctr.cgi?function=brows&action=brows&type=summary&language=E&recptno=R000017388) (first received 25 August 2014). [UMIN000014944] [sr-incont68895]
McDonald 1999 {published data only}
- McDonald CE, Thompson JM. A comparison of midnight versus early morning removal of urinary catheters after transurethral resection of the prostrate. Journal of Wound, Ostomy, and Continence Nursing 1999;26(2):94-7. [sr-incont10030] [DOI] [PubMed] [Google Scholar]
Naguimbing‐Cuaresma 2007 {published data only}
- Naguimbing-Cuaresma AE, Habana MA. Early removal of urinary catheter in cesarean delivery in a tertiary training hospital. Philippine Journal of Obstetrics & Gynecology 2007;31(2):69-74. [sr-incont78689] [Google Scholar]
Nathan 2001 {published data only}
- Nathan K, Hunter AJ, Nyheim T, McFaul P. Study of timing of urinary catheter removal and post operative morbidity in benign gynaecological surgery (Abstract number 241). In: 29th British Congress of Obstetrics and Gynaecology; 2001 Jul 10-13; Birmingham, UK. 2001. [sr-incont24025]
Nguyen 2012 {published data only}
- Nguyen DP, Giannarini G, Studer UE, Thalmann GN. How long should patients keep the transurethral catheter in situ after internal urethrotomy? Preliminary results of a prospective randomised study. European Urology Supplements 2012;11(1):e.611-e.611b. [sr-incont45647] [Google Scholar]
- Nguyen DP, Giannarini G, Studer UE, Thalmann GN. Optimal duration of postoperative catheter treatment after internal urethrotomy: a comparative, prospective randomized study (Abstract number 933). Journal of Urology 2012;187(4 Suppl 1):e379-80. [sr-incont45008] [Google Scholar]
Nielson 1985 {published data only}
- Nielsen HO, Christensen K, Larsen CF, Dyreborg U. Visual internal urethrotomy. A prospective controlled trial comparing the results after 3 versus 28 days of postoperative catheterisation. Annales Chirurgiae et Gynaecologiae 1985;74(5):244-6. [sr-incont608] [PubMed] [Google Scholar]
Noble 1990 {published data only}
- Noble JG, Menzies D, Cox PJ, Edwards L. Midnight removal: an improved approach to removal of catheters. British Journal of Urology 1990;65(6):615-7. [sr-incont5182] [DOI] [PubMed] [Google Scholar]
Nyman 2010 {published data only}
- Nyman MH, Johansson JE, Gustafsson M. A randomised controlled trial on the effect of clamping the indwelling urinary catheter in patients with hip fracture. Journal of Clinical Nursing 2010;19(3-4):405-13. [sr-incont40230] [DOI] [PubMed] [Google Scholar]
Oberst 1981 {published data only}
- Oberst MT, Graham D, Geller NL, Stearns MW Jr, Tiernan E. Catheter management programs and postoperative urinary dysfunction. Research in Nursing & Health 1981;4(1):175-81. [sr-incont753] [DOI] [PubMed] [Google Scholar]
Onile 2008 {published data only}
- Onile TG, Kuti O, Orji EO, Ogunniyi SO. A prospective randomised clinical trial of urethral catheter removal following elective cesarean delivery. International Journal of Gynaecology and Obstetrics 2008;102(3):267-70. [sr-incont32157] [DOI] [PubMed] [Google Scholar]
Ouladsahebmadarek 2012 {published data only}
- Ouladsahebmadarek E, Sayyah-Melli M, Jafari-Shobeiri M. A randomized clinical trial to compare immediate versus delayed removal of Foley catheter following abdominal hysterectomy and laparotomy. Pakistan Journal of Medical Sciences 2012;28(3):380-3. [sr-incont59710] [Google Scholar]
Pervaiz 2019 {published data only}
- Pervaiz A, Islam SU, Nasrullah F, Mahmood MT, Joshi A, Akhtar SM. Comparison of outcome of removal of three way Foley's catheter after transurethral resection of prostate on 1st versus 4th postoperative day. Pakistan Journal of Medical and Health Sciences 2019;13(2):350-2. [sr-incont78690] [Google Scholar]
Popiel 2017 {published data only}
- Popiel P, Vallabh-Patel V, Salamon C. Indwelling vs immediate removal of Foley catheter after robotic assisted laparoscopic sacrocolpopexy: a randomized prospective study (Abstract number: oral poster 96). Female Pelvic Medicine & Reconstructive Surgery 2017;23(5 Suppl 1):S62. [sr-incont77935] [DOI] [PubMed] [Google Scholar]
Rajan 2017 {published data only}
- Rajan P, Soundara Raghavan S, Sharma D. Study comparing 3 hour and 24 hour postoperative removal of bladder catheter and vaginal pack following vaginal surgery: a randomised controlled trial. BMC Women's Health 2017;17(1):Article number: 78. [sr-incont77934] [DOI] [PMC free article] [PubMed] [Google Scholar]
Ruminjo 2015 {published data only}
- Ruminjo J, Barone M, Arrowsmith S, Khisa W, Muleta M, Widmer M. Translating randomized clinical trial findings to practice: non-inferiority of short duration bladder catheterization after fistula repair (Abstract number FCS100.9). International Journal of Gynaecology and Obstetrics 2015;131(Suppl 5):E313. [0020-7292] [sr-incont77933] [Google Scholar]
Sahin 2011 {published data only}
- Sahin C, Kalkan M. The effect of catheter removal time following transurethral resection of the prostate on postoperative urinary retention. European Journal of General Medicine 2011;8(4):280-3. [sr-incont66224] [Google Scholar]
Sandberg 2019 {published data only}
- Jansen FW. When is the best moment to remove the urinary catheter after laparoscopic hysterectomy? MUCH study. trialregister.nl/trial/5496 (first received 1 April 2016). [NCT02742636] [NL5496] [NTR5818] [sr-incont73372]
- NCT02742636. When is the best moment to remove the urinary catheter after laparoscopic hysterectomy? (MUCH) [When is the best moment to remove the urinary catheter after laparoscopic hysterectomy: MUCH study]. clinicaltrials.gov/show/NCT02742636 (first received 19 April 2016). [NCT02742636] [NTR5818] [sr-incont72652]
- Sandberg EM, Twijnstra A, Van Meir CA, Kok HS, Van Geloven N, Gludovacz K, et al. Immediate versus delayed removal of urinary catheter after laparoscopic hysterectomy: a randomised controlled trial. BJOG 2019;126(6):804-13. [NCT02742636] [NTR5818] [sr-incont78692] [DOI] [PMC free article] [PubMed] [Google Scholar]
Schiotz 1995 {published data only}
- Schiotz HA. Comparison of 1 and 3 days' transurethral Foley cathererization after vaginal plastic surgery. International Urogynecology Journal and Pelvic Floor Dysfunction 1995;6(3):158-61. [sr-incont6919] [DOI] [PubMed] [Google Scholar]
- Schiotz HA. Comparison of 1 and 3 days transurethral Foley catheterization after vaginal plastic surgery (Abstract number 394). In: 25th Annual Meeting of the International Continence Society (ICS); 1995 Oct 17-20; Sydney, Australia. 1995:536-7. [sr-incont10904]
Schiotz 1996 {published data only}
- Schiotz HA. Comparison of 1 and 3 days' transurethral Foley catheterization after retropubic incontinence surgery. International Urogynecology Journal and Pelvic Floor Dysfunction 1996;7(2):98-101. [sr-incont5953] [DOI] [PubMed] [Google Scholar]
Sekhavat 2008 {published data only}
- Sekhavat L, Farajkhoda T, Davar R. The effect of early removal of indwelling urinary catheter on postoperative urinary complications in anterior colporrhaphy surgery. Australian & New Zealand Journal of Obstetrics & Gynaecology 2008;48(3):348-52. [sr-incont27813] [DOI] [PubMed] [Google Scholar]
Shahnaz 2016 {published data only}
- IRCT201506134339N11. Comparison of the effect of 1 versus 3 days urinary catheterization after gynecological operation on urinary complications [Comparison of the effect of 1 versus 3 days post operative urinary catheterization after anterior colporrhaphy on urinary complications]. en.irct.ir/trial/4625 (first received 26 July 2015). [IRCT201506134339N11] [sr-incont77941]
- Shahnaz A, Elham R, Kambiz A, Sara K. Comparison of 1 versus 3 days post-operative catheterization after anterior colporrhaphy. Asian Journal of Pharmaceutical Research and Health Care 2016;8(S1):1-6. [IRCT201506134339N11] [sr-incont75091] [Google Scholar]
Shrestha 2013 {published data only}
- Shrestha B, Marhatha R, Kayastha S, Jaishi S. Short-term versus long-term catheterization after vaginal prolapse surgery. Nepal Medical College Journal : NMCJ 2013;15(2):102-5. [sr-incont61652] [PubMed] [Google Scholar]
Souto 2004 {published data only}
- Souto CA, Rhoden EL, De Conti R, Chammas M Jr, Laste SE, Fornari A, et al. Urethral catheter removal 7 or 14 days after radical retropubic prostatectomy: clinical implications and complications in a randomised study. Revista do Hospital das Clinicas 2004;59(5):262-5. [sr-incont22166] [DOI] [PubMed] [Google Scholar]
Sun 2004 {published data only}
- Sun MJ, Chang SY, Lin KC, Chen GD. Is an indwelling catheter necessary for bladder drainage after modified Burch colposuspension? International Urogynecology Journal 2004;15(3):203-7. [sr-incont19279] [DOI] [PubMed] [Google Scholar]
Tahmin 2011 {published data only}
- Tahmin K, Begum SN. Short term versus conventional catheterisation after genital prolapse surgery. Bangladesh Journal of Obstetrics and Gynecology 2011;26(2):68-71. [sr-incont59769] [Google Scholar]
Talreja 2016 {published data only}
- Talreja V, Ali A, Saeed S, Rani K, Samnani SS, Farid FN, et al. Trail without catheter after transurethral resection of prostate: clamp it or not? Scientifica (Cairo) 2016;2016:1562153. [sr-incont72282] [DOI] [PMC free article] [PubMed] [Google Scholar]
Taube 1989 {published data only}
- Taube M, Gajraj H. Trial without catheter following acute retention of urine. British Journal of Urology 1989;63(2):180-2. [sr-incont1170] [DOI] [PubMed] [Google Scholar]
Toscano 2001 {published data only}
- Toscano IL Jr, Maciel LC, Martins FG, Fernandes AR, Mello LF, Glina S. Transurethral resection of the prostate: prospective randomized study of catheter removal after 24 or 48 hours following surgery. Brazilian Journal of Urology 2001;27(2):144-7. [sr-incont18095] [Google Scholar]
Valero Puerta 1998 {published data only}
- Valero Puerta JA, Sanchez Gonzalez M, Medina Perez M, Valpuesta Fernandez I, Guerrero Guerra JL. Reduction of hospital stay, because of the early removal of the bladder catheter in transurethral resection of the prostate [Reduccion de la estancia hospitalaria, por la retirada precoz de sonda vesical en la reseccion transuretral de prostata]. Archivos Espanoles de Urologia 1998;51(4):327-30. [sr-incont71940] [PubMed] [Google Scholar]
Vallabh‐Patel 2020 {published data only}
- NCT02765893. Indwelling vs immediate removal of Foley catheter after robotic assisted laparoscopic sacrocolpopexy: a prospective study. clinicaltrials.gov/show/NCT02765893 (first received 9 May 2016). [NCT02765893] [sr-incont72649]
- Vallabh-Patel V, Popiel P, Salamon C. Indwelling versus immediate removal of transurethral catheter after robotic sacrocolpopexy: a randomized clinical trial. Female Pelvic Medicine & Reconstructive Surgery 2020;26(10):617-21. [NCT02765893] [sr-incont78693] [DOI] [PubMed] [Google Scholar]
Webster 2006 {published data only}
- Webster J, Osborne S, Woolett K, Shearer J, Courtney M, Anderson D. Does evening removal of urinary catheters shorten hospital stay among general hospital patients? A randomized controlled trial. Journal of Wound, Ostomy, and Continence Nursing 2006;33(2):156-63. [sr-incont19148] [DOI] [PubMed] [Google Scholar]
Weemhoff 2011 {published data only}
- NCT00622076. Postoperative catheterization after anterior colporrhaphy [Randomised controled multicenter trial regarding the appropriate length of postoperative catheterization after anterior colporrhaphy for repair of cystocele]. clinicaltrials.gov/show/NCT00622076 (first received 22 February 2008). [sr-incont63850]
- Weemhoff M, Wassen MM, Korsten L, Serroyen J, Kampschoer PH, Roumen FJ. Postoperative catheterization after anterior colporrhaphy: 2 versus 5 days. A multicentre randomized controlled trial. International Urogynecology Journal 2011;22(4):477-83. [NCT00622076] [sr-incont41523] [DOI] [PMC free article] [PubMed] [Google Scholar]
Williamson 1982 {published data only}
- Williamson ML. Reducing post-catheterization bladder dysfunction by reconditioning. Nursing Research 1982;31(1):28-30. [sr-incont2612] [PubMed] [Google Scholar]
Wilson 2000 {published data only}
- Wilson ID, Bramwell SP, Hollins GW. A randomized trial comparing bladder infusion with standard catheter removal after transurethral resection of the prostate. BJU International 2000;86(9):993-5. [sr-incont15645] [DOI] [PubMed] [Google Scholar]
Wu 2015 {published data only}
- Wu C-M, Jiang X-J, Cheng X. Clinical effects of different methods of early catheter removal after biliary surgery. Shi jie hua ren xiao hua za zhi = World Chinese Journal of Digestology 2015;23(10):1653-5. [sr-incont69497] [Google Scholar]
Wyman 1987 {published data only}
- Wyman A. What time of day should a urethral catheter be removed? Journal of the Royal Society of Medicine 1987;80(12):755-6. [sr-incont1108] [DOI] [PMC free article] [PubMed] [Google Scholar]
Yaghmaei 2017 {published data only}
- Yaghmaei M, Mokhtari M, Tamizi A, Mohammadi M. Comparing the outcomes of urinary catheter removal 6 hour and 12 to 24 hours after cesarean delivery. Majallāh-i zanān-i mamāʼī va nāzāʼī-i Īrān = Iranian Journal of Obstetrics, Gynecology and Infertility 2017;20(9):1-7. [sr-incont77932] [Google Scholar]
Yee 2015 {published data only}
- Yee TS, Saaid R, Sivakumaran, Hairi FM, Yen KS. A prospective trial of timing of urethral catheter removal following elective caesarean delivery (Abstract number FC 9.07). Journal of Obstetrics and Gynaecology Research 2015;41(S1):50. [sr-incont72800] [Google Scholar]
Zaouter 2009 {published data only}
- Zaouter C, Kaneva P, Carli F. Less urinary tract infection by earlier removal of bladder catheter in surgical patients receiving thoracic epidural analgesia. Regional Anesthesia and Pain Medicine 2009;34(6):542-8. [sr-incont39560] [DOI] [PubMed] [Google Scholar]
- Zaouter C, Wuethrich P, Miccoli M, Carli F. Early removal of urinary catheter leads to greater post-void residuals in patients with thoracic epidural. Acta Anaesthesiologica Scandinavica 2012;56(8):1020-5. [sr-incont46768] [DOI] [PubMed] [Google Scholar]
Zhou 2012 {published data only}
- Zhou B, Lin Z, Huang Y. Effect of extubation time of indwelling urinary catheters on postoperative recovery after cesarean section. Nan Fang Yi Ke da Xue Xue Bao [Journal of Southern Medical University] 2012;32(8):1221-2. [sr-incont48747] [PubMed] [Google Scholar]
Zmora 2010 {published data only}
- Zmora O, Madbouly K, Tulchinsky H, Hussein A, Khaikin M. Urinary bladder catheter drainage following pelvic surgery--is it necessary for that long? Diseases of the Colon and Rectum 2010;53(3):321-6. [sr-incont39517] [DOI] [PubMed] [Google Scholar]
Zomorrodi 2018 {published data only}
- Matin S, Bageri A, Joiban A, Zomorrodi A. Early removal of Foley catheter on infection of recipient post renal transplantation [Early catheter removing effect on the reduced rate of urinary tract infection in renal transplant recipients of Tabriz Imam Reza Hospital]. en.irct.ir/trial/20086 (first received 31 December 2017). [IRCT20150808023559N14] [sr-incont77940]
- Zomorrodi A, Kakei F, Bagheri A, Zomorrodi S, Mohammadrahimi M. Does early removal of Foley's catheter have any influence on infection of recipient post renal transplantation? Is it safe? A randomized clinical trial. Journal of Nephropathology 2018;7(3):122-6. [IRCT20150808023559N14] [sr-incont78734] [Google Scholar]
References to studies excluded from this review
2004‐005138‐38 {published data only}
- 2004-005138-38. Antibiotikaprofylakse ved vaginalplastik. clinicaltrialsregister.eu/ctr-search/search?query=eudract_number:2004-005138-38 (first received 17 January 2005). [EUCTR2004-005138-38-DK] [sr-incont65511]
ACTRN12617001191381 {published data only}
- ACTRN12617001191381. Reducing urinary catheter use: a randomised controlled study on the efficacy of an electronic reminder system in hospitalised patients. anzctr.org.au/ACTRN12617001191381.aspx (first received 15 August 2017). [ACTRN12617001191381] [sr-incont77738]
Agrawal 1993 {published data only}
- Agrawal SK, Kumar AS. Early removal of catheter following transurethral resection of the prostate. British Journal of Urology 1993;72(6):928-9. [DOI] [PubMed] [Google Scholar]
Airaksinen 1979 {published data only}
- Airaksinen P, Sinkkonen S, Bolodin M, Raili Jauhiainen R. Clinical study of patients with indwelling catheters [Kestokatetrihoidon kliininen tutkimus]. Duodecim; Laaketieteellinen Aikakauskirja 1979;95(4):164-70. [sr-incont18782] [PubMed] [Google Scholar]
Aunruean 2007 {published data only}
- Aunruean W, Duangmun S, Pahomtup T. Effectiveness of standard nursing care for decreasing nosocomial urinary tract infection in surgical 3B ward, Srinagarind Hospital, Thailand (Abstract number CS1.4). Asian Journal of Nursing 2007;10(1):46-7. [sr-incont65846] [Google Scholar]
Bach 1990 {published data only}
- Bach D, Hesse A, Prange CH. Prevention of incrustations and urinary tract infections during transurethral continuous catheterization. Therapiewoche Urologie Nephrologie 1990;2(1):25-32. [sr-incont22992] [Google Scholar]
Benjamin 2018 {published data only}
- Benjamin A, Bhagavath B. Postoperative urinary retention is significantly decreased by backfilling urinary bladder-a RCT (Abstract number FCS436). International Journal of Gynaecology and Obstetrics 2018;143(S3):334-5. [sr-incont78678] [Google Scholar]
Bergqvist 1979 {published data only}
- Bergqvist D, Hedelin H, Stenstrom G, Stahl A. Clinical evaluation of Foley catheters. Lakartidningen 1979;76(15):1416-8. [sr-incont21105] [PubMed] [Google Scholar]
Boyd 2019 {published data only}
- Boyd SS, O'Sullivan DM, Tunitsky E. Predictive factors of short-term catheterization postoperatively after pelvic reconstructive surgery (Abstract number: Poster 39). Female Pelvic Medicine & Reconstructive Surgery 2019;25(Suppl 1 5S):S197. [sr-incont78491] [Google Scholar]
Christensen 1983 {published data only}
- Christensen MG, Thorup J, Walter S, Vejlsgaard R, Selmer J, Geerdsen JP. Intermittent drainage of the bladder in patients with indwelling catheters. A controlled study of the occurrence of bacteriuria following after Cystomat without irrigation. Ugeskrift for Laeger 1983;145(1):22-3. [sr-incont1049] [PubMed] [Google Scholar]
Cleland 1971 {published data only}
- Cleland V, Cox F, Berggren H, MacInnis MR. Prevention of bacteriuria in female patients with welling catheters. Nursing Research 1971;20(4):309-18. [PubMed] [Google Scholar]
CTRI/2019/02/017836 {published data only}
- CTRI/2019/02/017836. To study the effect of urinary catheter on surgery for abnormal urethral opening [To study the effect of duration of indwelling urinary catheter on surgical outcome following tubularised incised plate repair in patients with distal hypospadias]. ctri.nic.in/Clinicaltrials/pmaindet2.php?trialid=27487 (first received 26 February 2019). [CTRI/2019/02/017836] [sr-incont78464]
Dhariwal 2019 {published data only}
- Dhariwal L, Salamon C. A prospective randomized control trial comparing continuous urinary drainage to a urinary catheter valve in women being discharged with a Foley after urogynecologic surgery (Abstract number: short oral 87). Female Pelvic Medicine & Reconstructive Surgery 2019;25(5 Suppl 1):S70-1. [NCT03178734] [sr-incont78680] [Google Scholar]
- Dhariwal L, Salamon CG. A prospective randomized control trial comparing continuous urinary drainage to a urinary catheter valve in women being discharged with a Foley after urogynecologic surgery (Abstract). Journal of Minimally Invasive Gynecology 2019;26(7 Suppl):S11. [NCT03178734] [sr-incont78681] [Google Scholar]
Downey 1997 {published data only}
- Downey P, Dean M, Hayes J. Perfect timing. Nursing Times 1997;93(5):89-90. [PubMed] [Google Scholar]
Efimenko 2004 {published data only}
- Efimenko NA, Shaplygin LV. The prophylaxis and treatment of urination disorders in the early postoperative period. Voenno-Meditsinskii Zhurnal 2004;325(6):14-7, 80. [sr-incont20232] [PubMed] [Google Scholar]
Farag 2018 {published data only}
- Farag S, Frazzini Padilla P, Smith KA, Zimberg SE, Sprague ML. Post-operative urinary retention rates after autofill versus backfill void trial following total laparoscopic hysterectomy: a randomized controlled trial (Abstract number 359). Journal of Minimally Invasive Gynecology 2018;25(7 Suppl):S129. [sr-incont78682] [DOI] [PubMed] [Google Scholar]
Farrell 1989 {published data only}
- Farrell SA, Webster RD, Higgins LM, Steeves RA. Duration of postoperative catheterization: a randomized, double-blind trial comparing two catheter management protocols and the effect of bethanechol chloride (Abstract). In: 10th Annual Meeting of the American Urogynecology Society, 1989 Oct 5-7; Scottsdale, Arizona. 1989. [sr-incont14569]
Fattah 2013 {published data only}
- Fattah AN, Santoso BI. Urinary catheterization in gynecological surgery: when should it be removed? Medical Journal of Indonesia 2013;22(3):183-8. [sr-incont75938] [Google Scholar]
Fernandez‐Gonzalez 2019 {published data only}
- Fernandez-Gonzalez S, Martinez Franco E, Martinez-Cumplido R, Molinet Coll C, Ojeda Gonzalez F, Gomez Roig MD, et al. Reducing postoperative catheterisation after anterior colporrhaphy from 48 to 24 h: a randomised controlled trial. International Urogynecology Journal 2019;30(11):1897-902. [sr-incont78683] [DOI] [PubMed] [Google Scholar]
Ghoreishi 2003 {published data only}
- Ghoreishi J. Indwelling urinary catheters in cesarean delivery. International Journal of Gynaecology and Obstetrics 2003;83(3):267-70. [sr-incont17942] [DOI] [PubMed] [Google Scholar]
Gillespie 1962 {published data only}
- Gillespie WA, Lennon GG, Linton KB, Slade N. Prevention of catheter infection of urine in female patients. British Medical Journal 1962;2(5296):13-6. [sr-incont7723] [DOI] [PMC free article] [PubMed] [Google Scholar]
Gross 1990 {published data only}
- Gross JC. Bladder dysfunction after a stroke: it's not always inevitable. Journal of Gerontological Nursing 1990;16(4):20-25, 41-2. [sr-incont2464] [DOI] [PubMed] [Google Scholar]
Halaska 1991 {published data only}
- Halaska M, Martan A, Voigt R, Sindlar M, Koleska T. Interrupted drainage system after colposuspension. In: 21st Annual Meeting of the International Continence Society (ICS), 1991 Oct 10-12; Hannover, Federal Republic of Germany. 1991:233-4. [sr-incont9024]
Hollingsworth 2013 {published data only}
- Hollingsworth JM, Rogers MA, Krein SL, Hickner A, Kuhn L, Cheng A, et al. Determining the noninfectious complications of indwelling urethral catheters: a systematic review and meta-analysis. Annals of Internal Medicine 2013;159(6):401-10. [sr-incont48487] [DOI] [PubMed] [Google Scholar]
Hu 1999 {published data only}
- Hu YQ, Zheng MZ, Li XL, Tan ZH. Effects of extracting urethral catheter early plus glycerin enema on the health of mother and infant after cesarean section. Journal of Nursing Science 1999;14(6):323-4. [sr-incont18178] [Google Scholar]
ISRCTN44339585 {published data only}ISRCTN44339585
- ISRCTN44339585. Laparoscopic treatment for female urinary incontinence. isrctn.org/ISRCTN44339585 (first received 23 January 2004). [ISRCTN44339585] [sr-incont17206]
ISRCTN48516968 {published data only}ISRCTN48516968
- ISRCTN48516968. Conventional versus laparoscopic surgery for colorectal cancer within an Enhanced Recovery program EnROL. isrctn.org/ISRCTN48516968 (first received 23 July 2007). [ISRCTN48516968] [sr-incont62941]
Jankowska 1995 {published data only}
- Jankowska A, Stratton JF, Gordon H. Voiding difficulties after abdominal hysterectomy - the role of urinary catheterisation (Abstract number 421). In: 27th Annual Meeting of the British Congress of Obstetrics & Gynaecology, 1995 Jul 4-7; Dublin, Ireland. 1995. [sr-incont10979]
Ledermair 1970 {published data only}
- Ledermair O, Schurz AR. Prevention of urinary tract infection following gynecologic surgery. Wiener Medizinische Wochenschrift 1970;120(25):478-80. [sr-incont18816] [PubMed] [Google Scholar]
Loeb 2008 {published data only}
- Loeb M, Hunt D, O'Halloran K, Carusone SC, Dafoe N, Walter SD. Stop orders to reduce inappropriate urinary catheterization in hospitalized patients: a randomized controlled trial. Journal of General Internal Medicine 2008;23(6):816-20. [sr-incont27622] [DOI] [PMC free article] [PubMed] [Google Scholar]
- NCT00157625. Automatic stop orders for urinary catheters [A randomized controlled trial of automatic stop orders for urinary catheterization in hospitalized patients]. clinicaltrials.gov/show/NCT00157625 (first received 12 September 2005). [NCT00157625] [sr-incont60932]
Mamo 1991 {published data only}
- Mamo GJ, Cohen SP. Early catheter removal vs. conventional practice in patients undergoing transurethral resection of prostate. Urology 1991;37(6):519-22. [DOI] [PubMed] [Google Scholar]
Mayer 1973 {published data only}
- Mayer HG. Prevention of urinary tract infections following gynecologic surgery. Wiener Medizinische Wochenschrift 1973;123(12):188-90. [sr-incont32337] [PubMed] [Google Scholar]
Medina 2005 {published data only}
- Medina CA, Takacs P. Shortening the process of determining postvoid residual. International Journal of Gynaecology and Obstetrics 2005;91(3):266-7. [sr-incont21868] [DOI] [PubMed] [Google Scholar]
Menshawy 2020 {published data only}
- Menshawy A, Ghanem E, Menshawy E, Taher Masoud A, El-Sharkawy M, Taher A, et al. Early versus delayed removal of indwelling urinary catheter after elective caesarean delivery: systematic review and meta-analysis of randomized controlled trials. Journal of Maternal-fetal & Neonatal Medicine 2020;33(16):2818-25. [sr-incont78687] [DOI] [PubMed] [Google Scholar]
Michelson 2005 {published data only}
- Michelson J, Lotke PA. The utility of bladder catheterization in total hip arthroplasty. Clinical Orthopaedics and Related Research 2005;438:295; author reply 295-6. [sr-incont32195] [DOI] [PubMed] [Google Scholar]
Miller 1960 {published data only}
- Miller A, Linton KB, Gillespie WA, Slade N, Mitchell JP. Catheter drainage and infection in acute retention of urine. Lancet 1960;1(7119):310-2. [DOI] [PubMed] [Google Scholar]
Mills 2018 {published data only}
- Mills J, Shaw N, Hougen H, Agard H, Case R, McMurry T, et al. A randomized, controlled trial of active vs. passive voiding trials (Abstract number: poster #M2). Neurourology and Urodynamics 2018;37(Suppl 1):S577. [sr-incont78688] [Google Scholar]
Moon 2012 {published data only}
- Moon HJ, Chun MH, Lee SJ, Kim BR. The usefulness of bladder reconditioning before indwelling urethral catheter removal from stroke patients. American Journal of Physical Medicine & Rehabilitation 2012;91(8):681-8. [sr-incont45152] [DOI] [PubMed] [Google Scholar]
Mustafa 1968 {published data only}
- Mustafa MA, Pinkerton JH. Significant bacteriuria after major gynaecological surgery. Lancet 1968;1(7547):839-41. [sr-incont7703] [PubMed] [Google Scholar]
Nadu 2001 {published data only}
- Nadu A, Salomon L, Hoznek A, Olsson LE, Saint F, la Taille A, et al. Early removal of the catheter after laparoscopic radical prostatectomy. Journal of Urology 2001;166(5):1662-4. [PubMed] [Google Scholar]
Nardos 2011 {published data only}
- Nardos R, Member B, Browning A. Obstetric fistula repair outcome comparing 10 day vs. 14 day Foley catheterization: a prospective randomized study (Abstract number: paper 28). Female Pelvic Medicine & Reconstructive Surgery 2011;17(5 Suppl 1):S64. [sr-incont77937] [Google Scholar]
Nardos 2012 {published data only}
- Nardos R, Menber B, Browning A. Outcome of obstetric fistula repair after 10-day versus 14-day Foley catheterization. International Journal of Gynaecology and Obstetrics 2012;118(1):21-3. [sr-incont44649] [DOI] [PubMed] [Google Scholar]
NCT00182832 {published data only}
- NCT00182832. e-CHAMP: enhancing care for hospitalized older adults with memory problems [Enhancing care for hospitalized older adults with cognitive impairment]. clinicaltrials.gov/show/NCT00182832 (first received 16 September 2005). [NCT00182832] [sr-incont66298]
NCT00392210 {published data only}
- NCT00392210. Assessment of two postoperative techniques used to predict voiding efficiency after gynecologic surgery. clinicaltrials.gov/show/NCT00392210 (first received 25 October 2006). [NCT00392210] [sr-incont63582]
NCT00446732 {published data only}
- NCT00446732. The use of the UroShield device in patients with indwelling urinary catheters (CAUTI) [Catheter associated urinary tract infections]. clinicaltrials.gov/show/NCT00446732 (first received 13 March 2007). [NCT00446732] [sr-incont60931]
NCT00959920 {published data only}
- NCT00959920. Bladder drainage during labor: a randomized controlled Study of Obstetric Foley Therapy (SOFT) [Study of Obstetric Foley Therapy (SOFT Trial)]. clinicaltrials.gov/show/NCT00959920 (first received 17 August 2009). [NCT00959920] [sr-incont64492]
NCT01067768 {published data only}
- NCT01067768. Reduction of catheter-associated urinary tract infection with a daily nursing review of the indication [Reduction of catheter-associated urinary tract infection with a daily nursing review of the indication. Randomized controlled trial]. clinicaltrials.gov/show/NCT01067768 (first received 12 February 2010). [NCT01067768] [sr-incont60936]
NCT01108757 {published data only}
- NCT01108757. Prevention of catheter-associated urinary tract infection in incontinence and reconstructive pelvic surgery patients (PRECAUTION) [Prevention of catheter-associated urinary tract infections in patients undergoing incontinence and reconstructive pelvic surgery: a randomized controlled trial]. clinicaltrials.gov/show/NCT01108757 (first received 22 April 2010). [NCT01108757] [sr-incont66630]
NCT01343784 {published data only}
- NCT01343784. Assessment of Voiding After Sling (AVAS) [Assessment of Voiding After Sling (AVAS): a randomized trial of two methods of post-operative catheter management after midurethral sling for female stress urinary incontinence]. clinicaltrials.gov/show/NCT01343784 (first received 28 April 2011). [NCT01343784] [sr-incont61986]
NCT01525498 {published data only}
- NCT01525498. Foley catheterization following sacrocolpopexy [Urinary bladder catheterization following sacrocolpopexy]. clinicaltrials.gov/show/NCT01525498 (first received 3 February 2012). [NCT01525498] [sr-incont60953]
NCT01646190 {published data only}
- NCT01646190. Fast-track colorectal surgery in senior patients (FT-SS) [Fast-track perioperative care after elective colorectal surgery in senior patients. Randomized controlled trial]. clinicaltrials.gov/show/NCT01646190 (first received 20 July 2012). [NCT01646190] [sr-incont62949]
NCT01797146 {published data only}
- NCT01797146. Effectiveness of catheter reminder and evaluation program (CARE) [Effectiveness of catheter reminder and evaluation (CARE) program in reducing the catheter-day and prevention of catheter-associated infections]. clinicaltrials.gov/show/NCT01797146 (first received 22 February 2013). [NCT01797146] [sr-incont60938]
NCT01926756 {published data only}
- NCT01926756. Does straight catheterization in short gynecologic procedures cause bacteriuria? [Intraoperative one-time catheterization in short gynecologic procedures and its potential effect on symptomatic and asymptomatic bacteriuria]. clinicaltrials.gov/show/NCT01926756 (first received 21 August 2013). [NCT01926756] [sr-incont63847]
NCT02054065 {published data only}
- NCT02054065. Effect of CAUTI prevention alert - a randomized control trial. clinicaltrials.gov/show/NCT02054065 (first received 4 February 2014). [NCT02054065] [sr-incont60928]
NCT02126813 {published data only}
- NCT02126813. When to perform bladder catheterization in fast-track hip and knee arthroplasty (POUR-RCT) [Urinary bladder catheterization in fast-track hip and knee arthroplasty - what is the optimal bladder volume? A randomized, controlled study]. clinicaltrials.gov/show/NCT02126813 (first received 30 April 2014). [NCT02126813] [sr-incont63822]
NCT02357251 {published data only}
- NCT02357251. Enhanced recovery after surgery: a RCT of perioperative management of gynecologic patients [Enhanced recovery after surgery: a randomized control trial of perioperative management of gynecologic patients]. clinicaltrials.gov/show/NCT02357251 (first received 6 February 2015). [NCT02357251] [sr-incont66619]
NCT02996968 {published data only}
- NCT02996968. Self-discontinuation of a transurethral catheter [Is self-discontinuation of a transurethral catheter following pelvic reconstructive surgery as effective as office-based discontinuation? A randomized controlled trial]. clinicaltrials.gov/show/NCT02996968 (first received 19 December 2016). [NCT02996968] [sr-incont76145]
NCT03646136 {published data only}
- NCT03646136. Post hysterectomy benefits of retained cystoscopy fluid [Benefits of retained cystoscopy fluid following benign laparoscopic and robotic hysterectomy; a randomized controlled trial]. clinicaltrials.gov/show/NCT03646136 (first received 24 August 2018). [NCT03646136] [sr-incont78458]
NCT03684941 {published data only}
- NCT03684941. Evaluation of a new catheter coating process for urinary catheters used for intermittent catheterization [Evaluation of a new catheter coating process for urinary catheters used for intermittent catheterization - a prospective, blinded, randomized, cross-over, multicenter study]. clinicaltrials.gov/show/NCT03684941 (first received 26 September 2018). [NCT03684941] [sr-incont78158]
NL2677 {published data only}
- NL2677. Catheter management and complications for symptomatic postpartum urinary retention - CAMPUR [Catheter management bij symptomatische urineretentie na de bevalling]. trialregister.nl/trial/2677 (first received 14 March 2011). [NL2677 ] [NTR2806] [sr-incont66314]
Norton 1987 {published data only}
- Norton P. Urethral mini-catheter revisited. In: 17th Annual Meeting of the International Continence Society (ICS), 1987 Sep 2-5; Bristol, UK. 1987:146-7. [sr-incont9035]
Okrainec 2017 {published data only}
- Okrainec A, Aarts MA, Conn LG, McCluskey S, McKenzie M, Pearsall EA, et al. Compliance with urinary catheter removal guidelines leads to improved outcome in enhanced recovery after surgery patients. Journal of Gastrointestinal Surgery 2017;21(8):1309-17. [sr-incont77936] [DOI] [PubMed] [Google Scholar]
Panknin 2007 {published data only}
- Panknin HT. Reducing catheter associated urinary tract infections on the intensive care unit by early removal of urinary drainage. Kinderkrankenschwester 2007;26(4):143-4. [sr-incont32173] [PubMed] [Google Scholar]
Patel 2018 {published data only}
- NCT01923129. Optimal duration of indwelling urinary catheter following pelvic surgery [Prospective study investigating optimal duration of indwelling urinary catheter following infraperitoneal colorectal surgery and role of postoperative alpha blockade]. clinicaltrials.gov/show/NCT01923129 (first received 15 August 2013). [NCT01923129] [sr-incont62947]
- Patel DN, Felder SI, Luu M, Daskivich TJ, Zaghiyan K, Fleshner P. Early urinary catheter removal following pelvic colorectal surgery: a prospective, randomized, non-inferiority trial (Abstract number S36). Diseases of the Colon and Rectum 2018;61(5):e61. [NCT01923129] [sr-incont78060] [DOI] [PubMed]
- Patel DN, Felder SI, Luu M, Daskivich TJ, Zaghiyan KN, Fleshner P. Early urinary catheter removal following pelvic colorectal surgery: a prospective, randomized, noninferiority trial. Diseases of the Colon and Rectum 2018;61(10):1180-6. [NCT01923129] [sr-incont78057] [DOI] [PubMed] [Google Scholar]
Pellegrini 1995 {published data only}
- Pellegrini VD Jr, Knight R, Smith P. Bladder management after total joint replacement. Eastern Orthopaedic Association 1995;19(1):46. [Google Scholar]
Peniakov 2004 {published data only}
- Peniakov M, Antonelli J, Naor O, Miron D. Reduction in contamination of urine samples obtained by in-out catheterization by culturing the later urine stream. Pediatric Emergency Care 2004;20(6):418-9. [sr-incont24075] [DOI] [PubMed] [Google Scholar]
Perera 2002 {published data only}
- Perera ND, Nandasena AC. Early catheter removal after transurethral resection of the prostate. Ceylon Medical Journal 2002;47(1):11-2. [DOI] [PubMed] [Google Scholar]
Priefer 1982 {published data only}
- Priefer BA, Duthie EH, Gambert SR. Frequency of urinary catheter change and clinical urinary tract infection. Study in hospital-based, skilled nursing home. Urology 1982;20(2):141-2. [sr-incont7556] [DOI] [PubMed] [Google Scholar]
Rabkin 1998 {published data only}
- Rabkin DG, Stifelman MD, Birkhoff J, Richardson KA, Cohen D, Nowygrod R, et al. Early catheter removal decreases incidence of urinary tract infections in renal transplant recipients. Transplantation Proceedings 1998;30(8):4314-6. [DOI] [PubMed] [Google Scholar]
Ratahi 2005 {published data only}
- Ratahi E, Lynskey T. Preoperative urethral catheterisation in patients undergoing total hip and knee arthroplasty. A randomized controlled trial (Abstract). Journal of Bone and Joint Surgery. British Volume 2005;87-B(Suppl 1):21. [sr-incont27050] [Google Scholar]
Rehm 1962 {published data only}
- Rehm RA. Urinary infections following cystoscopy. Journal of Urology 1962;88(2):301-2. [sr-incont40812] [DOI] [PubMed] [Google Scholar]
Ross 1966 {published data only}
- Ross PW, Smylie HG. A comparison of two regimes of catheterisation in the prophylaxis of urinary tract infection in gynaecological patients. British Journal of Urology 1966;38(2):194-8. [DOI] [PubMed] [Google Scholar]
Salem Mohamed 2018 {published data only}
- Salem Mohamed S, El Ebiary M, Badr M. Early versus late trail of catheter removal in patients with urinary retention secondary to benign prostatic hyperplasia under tamsulosin treatment. Urological Science 2018;29(6):288-92. [sr-incont78691] [Google Scholar]
Sandberg 2018 {published data only}
- Sandberg EM, Leinweber FS, Herbschleb PJ, Berends-van der Meer DM, Jansen FW. Urinary catheterisation management after laparoscopic hysterectomy: a national overview and a nurse preference survey. Journal of Obstetrics and Gynaecology 2018;38(8):1115-20. [NCT02742636] [NTR5818] [sr-incont78080] [DOI] [PubMed] [Google Scholar]
Souto 2000 {published data only}
- Souto CA, Teloken C, Souto JC, Rhoden EL, Ting HY. Experience with early catheter removal after radical retropubic prostatectomy. Journal of Urology 2000;163(3):865-6. [sr-incont11981] [PubMed] [Google Scholar]
Symonds 1967 {published data only}
- Symonds EM. Role of trauma and catheterization in puerperal urinary tract infection. Journal of Obstetrics and Gynaecology of the British Commonwealth 1967;74(2):294-8. [sr-incont7708] [DOI] [PubMed] [Google Scholar]
Tomaszewski 2015 {published data only}
- Tomaszewski D, Bałkota M. Intramuscular administration of drotaverine hydrochloride decreases both incidence of urinary retention and time to micturition in orthopedic patients under spinal anesthesia: a single blinded randomized study. BioMed Research International 2015;2015:926953. [DOI: 10.1155/2015/926953] [DOI] [PMC free article] [PubMed] [Google Scholar]
Uberoi 2013 {published data only}
- Uberoi V, Calixte N, Coronel V, Furlong D, Orlando R, Lerner L. Reducing urinary catheter days. Nursing 2013;43(1):16-20. [sr-incont65708] [DOI] [PubMed] [Google Scholar]
UMIN000014474 {published data only}
- UMIN000014474. Randomized evaluation of the necessity of indwelling urethral catheter for patients with upper urinary tract calculi under retrograde intra renal surgery (RIRS). upload.umin.ac.jp/cgi-open-bin/ctr/ctr.cgi?function=brows&action=brows&type=summary&language=E&recptno=R000016829 (first received 4 July 2014). [JPRN-UMIN000014474] [sr-incont69709]
UMIN000015289 {published data only}
- UMIN000015289. Clinical trial using the urethra catheter having a function to operate on for infiltration anesthesia for urethra mucous membrane. upload.umin.ac.jp/cgi-open-bin/ctr/ctr.cgi?function=brows&action=brows&type=summary&language=E&recptno=R000009224 (first received 30 September 2014). [JPRN-UMIN000015289] [sr-incont69708]
Watt 1998 {published data only}
- Watt E, Lillibridge J. Time of day urinary catheters are removed: a study of current practices. Urologic Nursing 1998;18(1):23-5. [PubMed] [Google Scholar]
Weitzel 2008 {published data only}
- Weitzel T. To cath or not to cath? Nursing 2008;38(2):20-1. [sr-incont32164] [DOI] [PubMed] [Google Scholar]
Wilson 2013 {published data only}
- Wilson B, Phelps C. Identifying and applying a targeted evidence-based practice change in the maternal/child health inpatient setting. Nursing for Women's Health 2013;17(6):490-7. [sr-incont65833] [DOI] [PubMed] [Google Scholar]
Zhang 1999 {published data only}
- Zhang H, Xing HY, Yang SM, Chen Y. The influence of the selection of urinary catheterisation time on the heart rate and blood pressure of general anesthesetic patients. Journal of Nursing Science 1999;14(2):75-7. [sr-incont18223] [Google Scholar]
Zhao 1994 {published data only}
- Zhao WH, Zang YF. Research on two methods of urinary catheterization post radio frequency therapy for the patient with hypertrophy of the prostate. Zhonghua Hu Li za Zhi [Chinese Journal of Nursing] 1994;29(7):408-9. [sr-incont18484] [PubMed] [Google Scholar]
References to studies awaiting assessment
NCT02602132 {published data only}
- NCT02602132. Clamping vs. free drainage before the removal of short-term indwelling urethral catheters in internal medicine patients. clinicaltrials.gov/show/NCT02602132 (first received 11 November 2015). [NCT02602132] [sr-incont69919]
References to ongoing studies
ACTRN12611000414910 {published data only}
- ACTRN12611000414910. Bladder care following laparoscopy for benign non-hysterectomy gynaecological conditions – a randomised controlled trial [A randomised controlled trial of patients undergoing laparoscopic surgery for benign non-hysterectomy gynaecological conditions, randomising patients to either immediate removal of the urinary catheter or removal on the first post-operative day, comparing any difference in the rate of urinary tract infection and voiding dysfunction]. anzctr.org.au/ACTRN12611000414910.aspx (first received 19 April 2011). [ACTRN12611000414910] [sr-incont62938]
ChiCTR1800016149 {published data only}
- ChiCTR1800016149. Randomized control study of early extubation of indwelled urinary catheter after rectal cancer radical operation. www.chictr.org.cn/showproj.aspx?proj=27447. [ChiCTR1800016149] [sr-incont78679]
CTRI/2018/11/016299 {published data only}
- CTRI/2018/11/016299. Comparison of early versus late removal of urinary catheter after surgery for cervical cancer [A randomized controlled trial comparing early versus late catheter removal after radical hysterectomy]. ctri.nic.in/Clinicaltrials/pmaindet2.php?trialid=27086 (first received 9 November 2018). [CTRI/2018/11/016299] [sr-incont78466]
IRCT20180208038670N1 {published data only}
- IRCT20180208038670N1. The effect of urinary catheter removal time on the incidence of urinary infection [The effect of urinary catheter removal time on the incidence of urinary infection and satisfaction level in patients undergoing lower extremity fracture surgery]. en.irct.ir/trial/29727 (first received 22 April 2018). [IRCT20180208038670N1] [sr-incont78684]
NCT03539107 {published data only}
- NCT03539107. Voiding assessment based on minimum spontaneous void of 150 mL compared to retrograde fill method after female pelvic floor reconstructive surgery [Voiding assessment based on minimum spontaneous void of 150 mL compared to retrograde fill method after female pelvic floor reconstructive surgery: a randomized controlled trial]. clinicaltrials.gov/show/NCT03539107 (first received 29 May 2018). [NCT03539107] [sr-incont78444]
NCT03668535 {published data only}
- NCT03668535. Filling of the urinary bladder during difficult cesarean section. clinicaltrials.gov/show/NCT03668535 (first received 12 September 2018). [NCT03668535] [sr-incont78461]
NCT03835351 {published data only}
- NCT03835351. Urinary retention after laparoscopic inguinal hernia repair: comparing the use of the intraoperative urinary catheter [Urinary retention after laparoscopic inguinal hernia repair: a randomized controlled trial comparing intraoperative urinary catheter versus no catheter]. clinicaltrials.gov/show/NCT03835351 (first received 8 February 2019). [NCT03835351] [sr-incont78472]
Xu 2019 {published data only}
- NCT03065855. Removal time of urinary catheter after laparoscopic anterior resection of the rectum [Amonocenter, prospective, randomized clinical trial to investigate the removal time of urinary catheter after laparoscopic anterior resection of the rectum]. clinicaltrials.gov/show/NCT03065855 (first received 28 February 2017). [NCT03065855] [sr-incont76151]
- Xu L, Tao ZY, Lu JY, Zhang GN, Qiu HZ, Wu B, et al. A single-center, prospective, randomized clinical trial to investigate the optimal removal time of the urinary catheter after laparoscopic anterior resection of the rectum: study protocol for a randomized controlled trial. Trials 2019;20(1):133. [NCT03065855] [sr-incont78694] [DOI] [PMC free article] [PubMed] [Google Scholar]
Additional references
Baldini 2009
- Baldini G, Bagry H, Aprikian A, Carli F. Postoperative urinary retention: anesthetic and perioperative considerations. Anesthesiology 2009;110(5):1139-57. [DOI] [PubMed] [Google Scholar]
Blandy 1989
- Blandy JP, Moors J. Urology for Nurses. Oxford: Blackwell Scientific Publication, 1989. [Google Scholar]
CDC 2016
- National Healthcare Safety Network (NHSN) Training, Centers for Disease Control and Prevention (CDC). CDC NHSN Patient Safety Component (PSC) Manual. Urinary tract infection (Catheter-Associated Urinary Tract Infection [CAUTI] and non-catheter-associated urinary tract infection [UTI]) and other Urinary System Infection [USI]) events. Chapter 7: device-associated module; pages 1-13. Available at www.cdc.gov/nhsn/pdfs/pscmanual/7psccauticurrent.pdf (accessed 25 January 2021).
COMET
- Core Outcome Measures in Effectiveness Trials (COMET) Initiative. COMET Initiative. www.comet-initiative.org/ (accessed 5 March 2016).
Cooper 2016
- Cooper FPM, Alexander CE, Sinha S, Omar IM. Policies for replacing long‐term indwelling urinary catheters in adults. Cochrane Database of Systematic Reviews 26 July 2016, Issue 7. Art. No: CD011115. [DOI: 10.1002/14651858.CD011115.pub2] [DOI] [PMC free article] [PubMed] [Google Scholar]
Covidence [Computer program]
- Veritas Health Innovation, Melbourne, Australia Covidence. Version accessed 11 March 2016. Melbourne: Veritas Health Innovation, Melbourne, Australia, 13 March 2016. Available at covidence.org.
Cravens 2000
- Cravens DD, Zweig S. Urinary catheter management. American Family Physician 2000;61(2):369-76. [PubMed] [Google Scholar]
Deeks 2021
- Deeks JJ, Higgins JP, Altman DG (editors). Chapter 10: Analysing data and undertaking meta-analyses. In: Higgins JP, Thomas J, Chandler J, Cumpston M, Li T, Page MJ, Welch VA (editors). Cochrane Handbook for Systematic Reviews of Interventions version 6.2 (updated February 2021). Cochrane, 2021. Available from www.training.cochrane.org/handbook.
Dunn 2000a
- Dunn SP, Pretty L, Reid H, Evans D. Management of short term indwelling urethral catheters to prevent urinary tract infections: a systematic review. Adelaide: The Joanna Briggs Institute for Evidence Based Nursing and Midwifery, 2000. [Google Scholar]
Durojaiye 2015
- Durojaiye CO, Healy B. Urinary tract infections: diagnosis and management. Prescriber 2015;26(11):21-9. [Google Scholar]
EAU 2020
- Bonkat G, Bartoletti R, Bruyère F, Cai T, Geerlings SE, Köves B, et al. EAU Guidelines: Urological Infections 2020. Arnhem, The Netherlands: EAU Guidelines Office. Available at uroweb.org/guideline/urological-infections/ (accessed 25 January 2021).
EAUN 2012
- Geng V, Cobussen-Boekhorst H, Farrell J, Gea-Sánchez M, Pearce I, Schwennesen T, et al, European Association of Urology Nurses. Catheterisation: indwelling catheters in adults - urethral and suprapubic. Evidence-based guidelines for best practice in urological health care. Available at nurses.uroweb.org/guideline/catheterisation-indwelling-catheters-in-adults-urethral-and-suprapubic/ (accessed 25 January 2021). [ISBN: 978-90-79754-50-2]
EndNote 2018 [Computer program]
- EndNote. Version X8.2. Philadelphia (PA): Clarivate Analytics, 2018.
Fernandez 2003a
- Fernandez R, Griffiths R, Murie P. Comparison of late night and early morning removal of short-term urethral catheters. JBI Reports 2003;1(1):1-16. [PubMed] [Google Scholar]
Fernandez 2005
- Fernandez RS, Griffiths RD. Clamping short-term indwelling catheters: a systematic review of the evidence. Journal of Wound, Ostomy and Continence Nursing 2005;32(5):329-36. [DOI] [PubMed] [Google Scholar]
Fisher 2014
- Fisher E, Subramonian K, Omar MI. The role of alpha blockers prior to removal of urethral catheter for acute urinary retention in men. Cochrane Database of Systematic Reviews 2014, Issue 6. Art. No: CD006744. [DOI: 10.1002/14651858.CD006744.pub3] [DOI] [PMC free article] [PubMed] [Google Scholar]
Fisher 2017
- Fisher E, Dahm P, Omar MI. Challenges of catheter associated urinary tract infection: is prevention better than cure? Urology News UK 2017;22(1):1-5. [Google Scholar]
Foon 2012
- Foon R, Toozs‐Hobson P, Latthe P. Prophylactic antibiotics to reduce the risk of urinary tract infections after urodynamic studies.. Cochrane Database of Systematic Reviews 2012, Issue 12 Art. No.: CD008224.. Art. No: CD008224. [DOI: 10.1002/14651858.CD008224.pub2] [DOI] [PubMed] [Google Scholar]
Foxman 2003
- Foxman B. Epidemiology of urinary tract infections: incidence, morbidity, and economic costs. Disease-a-Month 2003;49(2):53-70. [DOI] [PubMed] [Google Scholar]
Ghuman 2018
- Ghuman A, De Jonge SW, Dryden SD, Feeney T, Buitrago DH, Phang PT. Prophylactic use of alpha-1 adrenergic blocking agents for prevention of postoperative urinary retention: a review & meta-analysis of randomized clinical trials. American Journal of Surgery 2018;215(5):973-9. [DOI] [PubMed] [Google Scholar]
Gould 2009
- Gould CV, Umscheid CA, Agarwal RK, Kuntz G, Pegues DA, Healthcare Infection Control Practices Advisory Committee (HICPAC). Guideline for prevention of catheter-associated urinary tract infections (2009). Available at www.cdc.gov/hicpac/pdf/CAUTI/CAUTIguideline2009final.pdf (accessed 25 November 2015). [DOI] [PubMed]
Grabe 2015
- Grabe M, Bartoletti R, Bjerklund Johansen TE, Cai T, Çek M, Köves B, et al. Guidelines on Urological Infections. European Association of Urology. 2015 Available at uroweb.org/guideline/urological-infections/ (accessed 25 January 2021).
Haque 2018
- Haque M, Sartelli M, McKimm J, Bakar MA. Health care-associated infections – an overview. Infection and Drug Resistance 2018;11:2321-33. [DOI] [PMC free article] [PubMed] [Google Scholar]
Higgins 2003
- Higgins JP, Thompson SG, Deeks JJ, Altman DG. Measuring inconsistency in meta-analyses. BMJ 2003;327:557-60. [DOI] [PMC free article] [PubMed] [Google Scholar]
Higgins 2011
- Higgins JP, Altman DG, Sterne JA (editors). Chapter 8: Assessing risk of bias in included studies. In: Higgins JP, Green S (editors). Cochrane Handbook for Systematic Reviews of Interventions Version 5.1.0 (updated March 2011). The Cochrane Collaboration, 2011. Available from training.cochrane.org/handbook/archive/v5.1/.
Higgins 2021
- Higgins JP, Li T, Deeks JJ (editors). Chapter 6: Choosing effect measures and computing estimates of effect. In: Higgins JP, Thomas J, Chandler J, Cumpston M, Li T, Page MJ, Welch VA (editors). Cochrane Handbook for Systematic Reviews of Interventions version 6.2 (updated February 2021). Cochrane, 2021. Available from www.training.cochrane.org/handbook.
Hooton 2010
- Hooton TM, Bradley SF, Cardenas DD, Colgan R, Geerlings SE, Rice JC, et al. Diagnosis, prevention, and treatment of catheter-associated urinary tract infection in adults: 2009 International Clinical Practice Guidelines from the Infectious Diseases Society of America. Clinical Infectious Diseases 2010;50(5):625-63. [DOI] [PubMed] [Google Scholar]
Igawa 2008
- Igawa Y, Wyndaele JJ, Nishizawa O. Catheterization: possible complications and their prevention and treatment. International Journal of Urology 2008;15(6):481-5. [DOI] [PubMed] [Google Scholar]
Kidd 2015
- Kidd EA, Stewart F, Kassis NC, Hom E, Omar MI. Urethral (indwelling or intermittent) or suprapubic routes for short-term catheterisation in hospitalised adults. Cochrane Database of Systematic Reviews 2015, Issue 12. Art. No: CD004203. [DOI: 10.1002/14651858.CD004203.pub3] [DOI] [PMC free article] [PubMed] [Google Scholar]
Lam 2014
- Lam TB, Omar MI, Fisher E, Gillies K, MacLennan S. Types of indwelling urethral catheters for short-term catheterisation in hospitalised adults. Cochrane Database of Systematic Reviews 2014, Issue 9. Art. No: CD004013. [DOI: 10.1002/14651858.CD004013.pub4] [DOI] [PMC free article] [PubMed] [Google Scholar]
Li 2021
- Li T, Higgins JP, Deeks JJ (editors). Chapter 5: Collecting data. In: Higgins JP, Thomas J, Chandler J, Cumpston M, Li T, Page MJ, Welch VA (editors). Cochrane Handbook for Systematic Reviews of Interventions version 6.2 (updated February 2021). Cochrane, 2021. Available from www.training.cochrane.org/handbook.
Lo 2014
- Lo E, Nicolle LE, Coffin SE, Gould C, Maragakis LL, Meddings J, et al. Strategies to prevent catheter-associated urinary tract infections in acute care hospitals: 2014 update. Infection Control and Hospital Epidemiology 2014;35(5):464-79. [DOI] [PubMed] [Google Scholar]
Lusardi 2013
- Lusardi G, Lipp A, Shaw C. Antibiotic prophylaxis for short-term catheter bladder drainage in adults. Cochrane Database of Systematic Reviews 2013, Issue 7. Art. No: CD005428. [DOI: 10.1002/14651858.CD005428.pub2] [DOI] [PMC free article] [PubMed] [Google Scholar]
Madani 2014
- Madani AH, Aval HB, Mokhtari G, Nasseh H, Esmaeili S, Shakiba M, et al. Effectiveness of tamsulosin in prevention of post-operative urinary retention: a randomized double-blind placebo-controlled study. International Braz J Urol 2014;40(1):30-6. [DOI] [PubMed] [Google Scholar]
Madersbacher 2012
- Madersbacher H, Cardozo L, Chapple C, Abrams P, Toozs‐Hobson P, Young JS, et al. What are the causes and consequences of bladder overdistension?: ICI-RS 2011. Neurourology and Urodynamics 2012;31(3):317-21. [DOI] [PubMed] [Google Scholar]
Magil 2014
- Magill SS, Edwards JR, Bamberg W, Beldavs ZG, Dumyati G, Kainer MA, et al. Multistate point-prevalence survey of health care–associated infections. New England Journal of Medicine 2014;370(13):1198-208. [DOI] [PMC free article] [PubMed] [Google Scholar]
Meddings 2014
- Meddings J, Rogers MA, Krein SL, Fakih MG, Olmsted RN, Saint S. Reducing unnecessary urinary catheter use and other strategies to prevent catheter-associated urinary tract infection: an integrative review. BMJ Quality & Safety 2014;23(4):277-89. [DOI] [PMC free article] [PubMed] [Google Scholar]
NICE 2012
- National Institute for Health and Care Excellence (NICE). Healthcare-associated infections: prevention and control in primary and community care. Clinical guideline [CG139]. Published: 28 March 2012; last updated: 15 February 2017. Available from www.nice.org.uk/guidance/cg139 (accessed 26 January 2021).
Nicolle 2014
- Nicolle LE. Catheter associated urinary tract infections. Antimicrobial Resistance and Infection Control 2014;3:23. [DOI: 10.1186/2047-2994-3-23] [DOI] [PMC free article] [PubMed] [Google Scholar]
Omar 2013
- Omar MI, Lam TB, MacLennan S. Critical outcomes in a Cochrane Systematic Review: patients' perspective [poster]. In: Abstracts of the 21st Cochrane Colloquium, 2013 Sept 19-23, Quebec City, Canada. Cochrane Database of Systematic Reviews 2013;(9 Suppl): P2.004. Available from doi.org/10.1002/14651858.CD201300. (accessed 26 January 2021).
Page 2020
- Page MJ, McKenzie JE, Bossuyt PM, Boutron I, Hoffmann TC, Mulrow CD, et al. The PRISMA 2020 statement: an updated guideline for reporting systematic reviews. BMJ 2021;372:n71. [DOI: 10.1136/bmj.n71] [DOI] [PMC free article] [PubMed] [Google Scholar]
Phipps 2006
- Phipps S, Lim YN, McClinton S, Barry C, Rane A, N'Dow JM. Short term urinary catheter policies following urogenital surgery in adults. Cochrane Database of Systematic Reviews 2006, Issue 2. Art. No: CD004374. [DOI: 10.1002/14651858.CD004374.pub2] [DOI] [PubMed] [Google Scholar]
Reference Manager 2002 [Computer program]
- ISI ResearchSoft Reference Manager. Version 9.5 N. Berkeley (CA): ISI ResearchSoft, 2002.
Review Manager 2020 [Computer program]
- The Cochrane Collaboration Review Manager 5 (RevMan 5). Version 5.4. Copenhagen: The Cochrane Collaboration, 2020.
Roe 1990
- Roe B. Do we need to clamp catheters? Nursing Times 1990;86(43):66-7. [PubMed] [Google Scholar]
Rosseland 2002
- Rosseland LA, Stubhaug A, Breivik H. Detecting postoperative urinary retention with an ultrasound scanner. Acta Anaesthesiologica Scandinavica 2002;46(3):279-82. [DOI] [PubMed] [Google Scholar]
Stickler 2010
- Stickler DJ, Feneley RC. The encrustation and blockage of long-term indwelling bladder catheters: a way forward in prevention and control. Spinal Cord 2010;48(11):784-90. [DOI] [PubMed] [Google Scholar]
Tenke 2008
- Tenke P, Kovacs B, Bjerklund Johansen TE, Matsumoto T, Tambyah PA, Naber KG. European and Asian guidelines on management and prevention of catheter-associated urinary tract infections. International Journal of Antimicrobial Agents 2008;31(Suppl 1):S68-78. [DOI] [PubMed] [Google Scholar]
Tiguert 2004
- Tiguert R, Rigaud J, Fradet Y. Safety and outcome of early catheter removal after radical retropubic prostatectomy. Urology 2004;63(3):513-7. [DOI] [PubMed] [Google Scholar]
Trautner 2010
- Trautner BW. Management of catheter-associated urinary tract infection (CAUTI). Current Opinion in Infectious Diseases 2010;23(1):76-82. [DOI] [PMC free article] [PubMed] [Google Scholar]
Wang 2016
- Wang L-H, Tsai M-F, Han C-Y, Huang Y-C, Liu H-E. Is bladder training by clamping before removal necessary for short-term indwelling urinary catheter inpatient? A systematic review and meta-analysis. Asian Nursing Research 2016;10(3):173-81. [DOI] [PubMed] [Google Scholar]
Ware 1992
- Ware JE Jr, Sherbourne CD. The MOS 36-item short-form health survey (SF-36). I. Conceptual framework and item selection. Medical Care 1992;30(6):473-83. [PubMed] [Google Scholar]
Yamanishi 2004
- Yamanishi T, Yasuda K, Kamai T, Tsujii T, Sakakibara R, Uchiyama T, et al. Combination of a cholinergic drug and an alpha-blocker is more effective than monotherapy for the treatment of voiding difficulty in patients with underactive detrusor. International Journal of Urology 2004;11(2):88-96. [DOI] [PubMed] [Google Scholar]
Zhang 2015
- Zhang P, Hu W-L, Cheng B, Cheng L, Xiong X-K, Zeng Y-J. A systematic review and meta-analysis comparing immediate and delayed catheter removal following uncomplicated hysterectomy. International Urogynecology Journal 2015;26(5):665-74. [sr-incont66908] [DOI] [PubMed] [Google Scholar]
Zigmond 1983
- Zigmond AS, Snaith RP. The hospital anxiety and depression scale. Acta Psychiatrica Scandinavica 1983;67(6):361-70. [DOI] [PubMed] [Google Scholar]
References to other published versions of this review
Fernandez 2003b
- Fernandez R, Griffiths R. Policies for removal of indwelling urethral catheters for short-term management of voiding in adults and children. Cochrane Database of Systematic Reviews 2003, Issue 1. Art. No: CD004011. [DOI: 10.1002/14651858.CD004011] [DOI] [PubMed] [Google Scholar]
Griffiths 2005
- Griffiths R, Fernandez R. Policies for the removal of short-term indwelling urethral catheters. Cochrane Database of Systematic Reviews 2005, Issue 1. Art. No: CD004011. [DOI: 10.1002/14651858.CD004011.pub2] [DOI] [PubMed] [Google Scholar]
Griffiths 2007
- Griffiths R, Fernandez R. Strategies for the removal of short-term indwelling urethral catheters in adults. Cochrane Database of Systematic Reviews 2007, Issue 2. Art. No: CD004011. [DOI: 10.1002/14651858.CD004011.pub3] [DOI] [PMC free article] [PubMed] [Google Scholar]


