Abstract
The adult mammalian heart is minimally regenerative after injury, whereas neonatal hearts fully recover even after major damage. New work from the Red-Horse and Woo labs (Das et al., 2019) shows that collateral artery formation is a key mechanism contributing to successful regeneration in newborn mice and provides insights into how collateral arteries form.
Zebrafish and newborn mice are able to repair their hearts even after a major injury. The adult mammalian heart, however, completely lacks this regenerative potential. This represents a problem many have tried to understand and address, resulting in numerous exciting recent discoveries (Leach et al., 2017; Bassat et al., 2017; Mohamed et al., 2018). One promising approach has been to dissect the mechanisms underlying successful heart regeneration and to translate these to the adult heart with the aim to restore its regenerative ability (Tzahor and Poss, 2017). With this strategy in mind, Das et al. (2019, this issue of Cell) have taken a closer look at the vascular system during regeneration and have asked whether and how the formation of coronary collateral arteries contributes to successful regeneration of the neonatal heart after myocardial infarction (Das et al., 2019).
The formation of collateral circulation occurs in multiple organs as a response to injury to restore blood flow to areas downstream of the damage site, and the presence of coronary collateral arteries is associated with improved prognosis after myocardial infarction (Habib et al., 1991). Rapid revascularization is further a key feature of zebrafish heart repair and is required for successful regeneration (Marín-Juez et al., 2016), suggesting that a similar mechanism may be at play during neonatal heart regeneration. Das et al. (2019) elegantly demonstrate that neonatal mice (P2) that have undergone induced myocardial infarction do indeed form an abundant network of collateral arteries in the watershed area of the heart and that this response is lost in hearts injured after the regenerative period (P7 or later) (Figures 1A and 1B). Notably, the authors’ powerful whole-organ imaging approach allows them to describe collateral arteries comprehensively and at high resolution. This enables a detailed characterization of a new model of vascularization used in collateral artery formation that is termed "artery reassembly". During artery reassembly, single arterial endothelial cells migrate along existing capillaries in the watershed area before proliferating and coalescing into collateral arteries. Intriguingly, this is different from normal, postnatal artery growth, which happens through arterialization of capillaries at vessel tips or via growth of pre-existing collateral arteries.
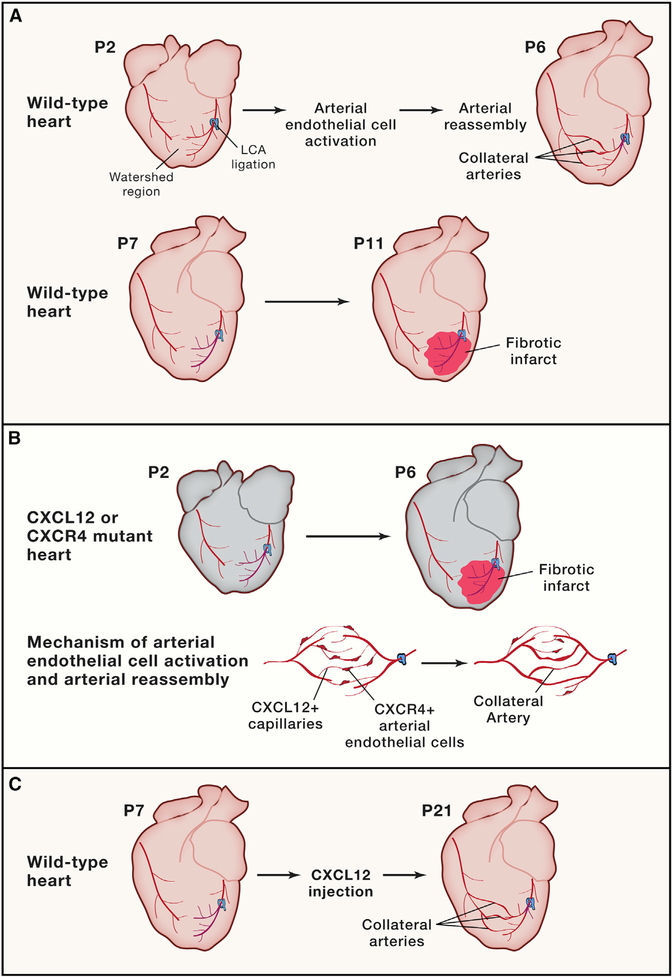
Figure 1. Cxcr4/Cxcl12 Signaling Activates Arterial Endothelial Cells to Guide Collateral Artery Formation in Response to Myocardial Infarction.
(A) Neonatal (P2) hearts subjected to left coronary artery ligation are able to restore blood flow to the injured region through the formation of collateral arteries by arterial reassembly. After the regenerative window (P7) hearts subjected to left coronary artery (LCA) ligation do not form collateral arteries.
(B) Neonatal hearts lacking Cxcr4 in the arterial endothelial cells or Cxcl12 in the capillary bed are unable to recruit arterial endothelial cells (AECs) for arterial reassembly and form fibrotic scars after LCA ligation. Collateral artery reassembly is triggered by CXCL12-expressing capillaries, which activate and recruit CXCR4-expressing AECs to migrate, proliferate, and form new collateral arteries.
(C)The injection of a high dose of CXCL12 rescues the formation of collateral arteries in P7 hearts.
Before the concept of collateral artery formation can be translated to enhance regeneration in the adult heart, two important questions remained to be answered. First, is collateral artery formation required for successful heart regeneration? And second, what are the underlying signaling pathways that induce the formation of collateral arteries upon injury, and will those be effective in the adult heart? To address these questions, Das et al. (2019) investigate the role of Cxcl12/Cxcr4 signaling, which has previously been shown to be involved in coronary endothelial cell migration during development. Developmental Cxcr4/ Cxcl12 signaling is not required to establish the primitive vasculature but rather to activate coronary endothelial cells to mature the vasculature. This maturation mechanism is mediated by signaling between Cxcl12 from the epicardium and Cxcr4-expressing coronary endothelial cells, and disruption of this signaling axis results in late-gestation lethality in mice (Cavallero et al., 2015). Similar mechanisms were found to regulate coronary vasculature development in zebrafish, hinting at the broad relevance of this pathway (Harrison et al., 2015).
The authors build upon these findings to show that the Cxcr4/Cxcl12 signaling axis is repurposed to encourage artery reassembly in injured neonatal hearts. Specifically, they show that the capillary endothelial cells of the watershed area upregulate Cxcl12 after myocardial infarction, encouraging the Cxcr4-expressing arterial endothelial cells to populate the capillary bed (Figure 1D). The ability of arterial endothelial cells to form collateral arteries is greatly diminished either if Cxcl12 is deleted from the capillary endothelial cells or if Cxcr4 is deleted from the arterial endothelial cells, demonstrating the spatiotemporal requirement of the ligand/receptor pair for revascularization (Figure 1C). This signaling dynamic is seemingly utilized in neonatal injured hearts to reactivate endogenous arterial endothelial cells, which then migrate along the existing capillary bed to assemble new collateral arteries in a manner reminiscent of the developmental maturation mechanism.
Most importantly, the ability for mice to recover after myocardial infarction is dependent on intact Cxcr4/Cxcl12 signaling. Loss of either Cxcl12 or Cxcr4 results in an inability to restore ejection fraction after injury, convincingly tying the ability to generate collateral arteries to the ability to recover heart function. Furthermore, the authors relate their findings to their previous observation that Cxcl12 injection increases recovery post myocardial infarction in the adult heart by stimulating the growth of coronary collateral arteries (Figure 1B) (Goldstone et al., 2018). This finding holds great therapeutic potential, and the additional mechanistic understanding provided here is an important step toward clinical application.
By leveraging whole-organ imaging techniques and strong genetic approaches, the present study has added important new insights to our understanding of the regenerative strategies in neonatal mice, proposes a new model of arterial growth during collateral artery formation, and provides mechanistic insight into a potential therapeutic approach for patients with myocardial infarction. The observation that interrupting the Cxcl12/ Cxcr4 signaling axis reduces the proliferative response of neonatal cardiomyocytes after injury ties other relevant cell populations such as the cardiomyocytes to the regeneration mechanism described here, opening exciting new avenues of research. Does Cxcl12/Cxcr4 signaling induce cardiomyocyte proliferation directly, or via growth of collateral arteries that bring other stimulatory factors to the site of injury? Is there an interplay between the myocardium and vasculature that goes beyond the restoration of blood flow to the injured site? Can the epicardium, which expresses Cxcl12 and is also damaged after myocardial infarction, be leveraged to amplify this mechanism after injury, as has been proposed previously? Importantly, how and why adult hearts lose the ability to grow coronary collateral arteries after injury remains an intriguing concept to elucidate. It is unclear whether the capillary beds lose the ability to express Cxcl12 in adulthood, nor is it known if arterial endothelial cells retain the capacity to become activated in response. Future research along these avenues promises to uncover strategies to enhance the currently very limited capacity of the adult mammalian heart to recover after injury.
ACKNOWLEDGMENTS
E.S.B. is supported by an NIH pre-doctoral research fellowship 1F31HL136216-01A1. N.C.D. is supported by NIH grant 1R01HL134956-01.
REFERENCES
- Bassat E, Mutlak YE, Genzelinakh A, Shadrin IY, Baruch Umansky K, Yifa O, Kain D, Rajchman D, Leach J, Riabov Bassat D, et al. (2017). The extracellular matrix protein agrin promotes heart regeneration in mice. Nature 547, 179–184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cavallero S, Shen H, Yi C, Lien CL, Kumar SR, and Sucov HM (2015). CXCL12 Signaling Is Essential for Maturation of the Ventricular Coronary Endothelial Plexus and Establishment of Functional Coronary Circulation. Dev. Cell 33, 469–477. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Das S, Goldstone AB, Wang H, Farry J, D’Amato G, Paulsen MJ, Eskandari A, Hironaka CE, Phansalkar R, Sharma B, et al. (2019). A Unique Collateral Artery Development Program Promotes Neonatal Heart Regeneration. Cell 176, this issue, 1128–1142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goldstone AB, Burnett CE, Cohen JE, Paulsen MJ, Eskandari A, Edwards BE, Ingason AB, Steele AN, Patel JB, MacArthur JW, et al. (2018). SDF 1-alpha Attenuates Myocardial Injury Without Altering the Direct Contribution of Circulating Cells. J. Cardiovasc. Transl. Res. 11, 274–284. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Habib GB, Heibig J, Forman SA, Brown BG, Roberts R, Terrin ML, and Bolli R; The TIMI Investigators (1991). Influence of coronary collateral vessels on myocardial infarct size in humans. Results of phase I thrombolysis in myocardial infarction (TIMI) trial. Circulation 83, 739–746. [DOI] [PubMed] [Google Scholar]
- Harrison MRM, Bussmann J, Huang Y, Zhao L, Osorio A, Burns CG, Burns CE, Sucov HM, Siekmann AF, and Lien CL (2015). Chemokine-guided angiogenesis directs coronary vasculature formation in zebrafish. Dev. Cell 33, 442–454. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leach JP, Heallen T, Zhang M, Rahmani M, Morikawa Y, Hill MC, Segura A, Willerson JT, and Martin JF (2017). Hippo pathway deficiency reverses systolic heart failure after infarction. Nature 550, 260–264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marín-Juez R, Marass M, Gauvrit S, Rossi A, Lai S-L, Materna SC, Black BL, and Stainier DYR (2016). Fast revascularization of the injured area is essential to support zebrafish heart regeneration. Proc. Natl. Acad. Sci. USA 113, 11237–11242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mohamed TMA, Ang YS, Radzinsky E, Zhou P, Huang Y, Elfenbein A, Foley A, Magnitsky S, and Srivastava D (2018). Regulation of Cell Cycle to Stimulate Adult Cardiomyocyte Proliferation and Cardiac Regeneration. Cell 173, 104–116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tzahor E, and Poss KD (2017). Cardiac regeneration strategies: Staying young at heart. Science 356, 1035–1039. [DOI] [PMC free article] [PubMed] [Google Scholar]