Abstract
Sleep is a vital part of our lives as it is required to maintain health and optimal cognition. In humans, sex differences are relatively well-established for many sleep phenotypes. However, precise differences in sleep phenotypes between male and female rodents are less documented. The main goal of this article is to review sex differences in sleep architecture and electroencephalographic (EEG) activity during wakefulness and sleep in rodents. The effects of acute sleep deprivation on sleep duration and EEG activity in male and female rodents will also be covered, in addition to sex differences in specific circadian phenotypes. When possible, the contribution of the female estrous cycle to the observed differences between males and females will be described. In general, male rodents spend more time in non-rapid eye movement sleep (NREMS) in comparison to females, while other differences between sexes in sleep phenotypes are species- and estrous cycle phase-dependent. Altogether, the review illustrates the need for a sex-based perspective in basic sleep and circadian research, including the consideration of sex chromosomes and gonadal hormones in sleep and circadian phenotypes.
Keywords: Sex differences, Sleep architecture, Electroencephalographic activity, Sleep deprivation, Circadian rhythm, Estrous cycle
Highlights
-
•
In rodents, males spend less time awake, and more time in NREMS than females.
-
•
The recovery from sleep deprivation is also dependent on biological sex.
-
•
Gonadal hormones modulate sleep and circadian phenotypes in rodents.
-
•
A more systematic comparison of sex in basic sleep/circadian research is needed.
List of abbreviations
- CA1
cornu ammonis area 1 of the hippocampus
- FCG
four core genotype
- GDX
gonadectomy
- E2
17β-estradiol
- EEG
electroencephalographic
- KO
knockout
- Npas2
neuronal PAS (Per-Arnt-Sim) domain protein 2 gene
- NREMS
non-rapid eye movement sleep
- OVX
ovariectomized
- P
progesterone
- PER2::LUC
Period 2::Luciferase
- PFC
prefrontal cortex
- Pcdh10
Protocadherin 10 gene
- REMS
rapid eye movement sleep
- SCN
suprachiasmatic nucleus
- Sry
sex-determining region of the Y chromosome
- SWA
slow wave activity
- WT
wild-type
- V2
secondary visual cortex
1. Introduction
Adequate sleep is required for health, metabolic function, and cognition (Durmer and Dinges, 2005; Klinzing et al., 2019; Spiegel et al., 1999). In mammals, two main sleep states, non-rapid eye movement sleep (NREMS) and rapid eye movement sleep (REMS), alternate with wakefulness, and both their relative amount and quality are generally acknowledged to be determined by the interactions of circadian and homeostatic regulatory processes (Borbély, 1982; Borbély et al., 2016; Daan et al., 1984; Dijk and Czeisler, 1995; Dijk et al., 1997). The circadian process is driven by an internal clock synchronizing the sleep-wake cycle to the external light-dark cycle, whereas the homeostatic process controls a pressure for sleep that is reflected by the dynamics of slow wave activity (SWA: ~0.75–4.5 Hz) measured from the NREMS electroencephalogram (EEG) (Borbély et al., 2016; Dijk et Czeisler, 1995; Dijk and Lockley, 2002).
In human adults, various sex differences in sleep have been noted (Carrier et al., 2017). For instance, women report having more frequent insomnia symptoms, reduced sleep quality, and difficulty staying asleep compared to men (Husby and Lingjaerde, 1990; Li et al., 2002; Lindberg et al., 1997; Mong et al., 2011). Paradoxically, objective sleep measures show that compared to men, women have longer total sleep time (Bixler et al., 2009; Redline et al., 2004; Ursin et al., 2005), and higher power in the high sigma range (14-16 Hz) during NREMS (Carrier et al., 2001; Mongrain et al., 2005). In fact, it has been shown that women have higher EEG power in most frequencies during both NREMS and REMS (Carrier et al., 2001; Dijk et al., 1989). Several sex differences in sleep phenotypes persist in older individuals, such as the higher amplitude and longer duration of NREMS spindles in women compared to men (Carrier et al., 2017). In addition, the litterature suggests that circadian functions may differ between sexes with, in particular, circadian phase generally occurring earlier relative to clocktime and endogenous period being shorter in women than in men (Cain et al., 2010; Campbell et al., 1989; Duffy et al., 2011; Mongrain et al., 2004). Lastly, sex differences in the consequences of chronic insufficient sleep, such as a higher risk of hypertension in women than in men (Cappuccio et al., 2007), have also been reported.
There is important support for the role of gonadal hormones in mediating sex differences in sleep phenotypes in humans. Indeed, in women, periods of hormonal fluctuations, including puberty, menstrual cycle, pregnancy and menopause, are associated with an increased prevalence of sleep disturbances and modifications in EEG-measured sleep (Baker et al., 1997, 2002; Brown and Gervais, 2020; Brunner et al., 1994; Erkkola et al., 1991; Gervais et al., 2017; Johnson et al., 2006; Mong et al., 2011). In view of this knowledge, sleep and circadian research in rodents have traditionally mainly excluded females, limiting our understanding of the mechanisms behind sex differences. Indeed, a rapid overview of PubMed literature suggests that around 25% of non-human sleep studies have considered females in the past 5 years. A similar search performed for rhythms has previously reported <20% of studies including females (Kuljis et al., 2013), and a recent meta-analysis found that <7% of rodent studies concerned with circadian phase shift have included females (Lee et al., 2021). Nevertheless, some specific sex-based analyses of sleep and circadian phenotypes in rodents have been applied, and will be reviewed in the present article. More precisely, we are synthesizing findings from mice and rats that compare males and females on wakefulness/sleep architecture and EEG activity. We also discuss the differential effect of sleep deprivation on EEG activity, with a particular focus on SWA, in males and females, and how specific variables associated to the functioning of the circadian system differ between sexes in different rodents species (including species other than mice and rats). Factors contributing to sex differences in sleep and circadian phenotypes in rodents, including circulating gonadal hormones, gonadal phenotype, and sex chromosomes will also be considered.
2. Wake/sleep architecture
2.1. Wakefulness amount
We found three studies in mice showing that males spend less time in wakefulness than females (Ehlen et al., 2013; Koehl et al., 2006; Paul et al., 2006). This observation was made for C57BL/6 mice when comparing sexes during the dark period, corresponding to the active period in mice (Koehl et al., 2006; Paul et al., 2006), during the light period (mostly rest in mice) and during a full 24-h period (Koehl et al., 2006). More precisely, Koehl et al. (2006) showed that across the nychthemeron, males spent 46.7% of their time awake, whereas females spent 52.5% of their time in wakefulness (i.e., ~83 min difference between sexes). However, we identified two other studies not specifically designed to investigate sex differences that found no sex difference in time spent awake in the same mouse strain (Grønli et al., 2016; Huitron-Resendiz et al., 2018). In rats, a shorter time spent awake in males was also found in comparison to females but this difference depends on the phase of the estrous cycle. Indeed, females spend more time awake than males during the light and early dark period when they are in proestrus (Swift et al., 2020), but generally not in other phases (Kostin et al., 2020; Swift et al., 2020), and not when the phase of the estrous cycle is not considered and only a part of the nychthemeron (i.e., 6 h during the middle of the light period) is analyzed (Garner et al., 2018). Given that females will generally spend 20-25% of their time in the proestrus phase, EEG monitoring not considering the estrous cycle is more likely to occur outside this phase, and therefore to report similar time spent awake in male and female rats. Overall, studies specifically designed to investigate sex differences in rodents have reported a generally lower wakefulness amount in males than females (Fig. 1), which predominates in the dark period in mice and when females are in proestrus in rats.
Fig. 1.
Summary of the main sex differences observed in the time spent in wakefulness, NREMS and REMS in mice (A) and rats (B). (A) Sex differences in mice have been compiled from light, dark and/or 24-h periods datasets of Franken et al. (2006), Koehl et al. (2006),Paul et al. (2006), Ehlen et al. (2013), Nichols et al. (2020), and Saré et al. (2020). Of note is that an absence of sex difference have been reported for wakefulness and NREMS by Grønli et al. (2016) and Huitron-Resendiz et al. (2018). (B) Sex differences in rats have been compiled from light, dark and/or 24-h periods datasets of Cusmano et al. (2014), Fang and Fishbein (1996), and Swift et al. (2020). Of note is that sex differences in opposite directions have been reported for NREMS and REMS when females rats are in the estrus phase (Kostin et al., 2020; Swift et al., 2020).
Importantly, mice and rats submitted to gonadectomy (GDX) show no sex difference in time spent in wakefulness (Cusmano et al., 2014; Paul et al., 2006), suggesting that circulating gonadal hormones modulate wake/sleep amount in both rodent species. In particular, findings by Paul et al. (2006) suggest a predominant effect of ovarian hormones on time spent awake given that GDX did not significantly alter wakefulness amount in males but significantly decreased it in females. This is supported by the observation of an impact of 17β-estradiol (E2) replacement on time spent in wakefulness. Indeed, E2 increases wakefulness in the dark period in ovariectomized (OVX) female rats (Cusmano et al., 2014; Deurveilher et al., 2011), but not in castrated males or females with masculinized brain organization (Cusmano et al., 2014). Thus, according to Cusmano et al. (2014), the effects of E2 are restricted to female-specific brain organization. As illustrated below, sex differences in wakefulness are mirrored by sex differences in NREMS.
2.2. NREMS amount
To our knowledge, there is only one study showing that male mice have an overall shorter resting period than females (Franken et al., 2006), and another in rats showing less NREMS in males when compared to females in the estrus phase (Kostin et al., 2020). An absence of sex difference in time spent in NREMS in mice was reported by one study using a piezoelectric assessment of sleep (no EEG quantification; Wang et al., 2020), two studies underpowered to assess sex differences (e.g., n = 3 males) (Brankack et al., 2010; Hellman et al., 2010), and two other studies not designed to primarily investigate sex differences (Grønli et al., 2016; Huitron-Resendiz et al., 2018). In contrast, we found seven studies having shown that male mice and rats spend more time in NREMS than females (Ehlen et al., 2013; Franken et al., 2006; Koehl et al., 2006; Nichols et al., 2020; Paul et al., 2006; Saré et al., 2020; Swift et al., 2020). In particular, compared to females, C57BL/6 male mice spend more time in NREMS during the dark period, which also brings their daily percentage of NREMS to a greater level (Paul et al., 2006). In line with this, Koehl et al. (2006) showed that male mice spend more time in NREMS during both the dark and light periods in comparison to females in diestrus, and Franken et al. (2006) reported more time spent in NREMS in males than females only when considering the full 24-h period (i.e., no significant sex difference for the light and dark periods analyzed separately). In rats, males spent more time in NREMS during the light period and first half of the dark period when compared to females in proestrus (Swift et al., 2020). In sum, rodent studies have generally reported higher NREMS amount in males than females (Fig. 1), which applies to females in proestrus in rats and seems to lack a strong predominance for a specific part of the nychthemeron in both mice and rats.
This difference has been specifically attributed to gonadal hormones. First, animals submitted to GDX show no sex difference in time spent in NREMS (Cusmano et al., 2014; Paul et al., 2006), which was proposed, similar to time spent awake, to be mostly driven by ovarian hormones rather than by androgens (Cusmano et al., 2014; Paul et al., 2006). To tease apart the effects of sex chromosomes (XY vs XX) from that of gonadal phenotype (testes vs. ovaries), the four core genotype (FCG) model, whose sex chromosomes (XY/XX) are independent of the gonadal phenotype (testes/ovaries; driven by a Sry gene dissociated from the Y chromosome), has been used (Ehlen et al., 2013; Nichols et al., 2020). Males with XX or XY chromosomes spent more time in NREMS than XX or XY females, confirming that gonadal phenotype/hormones regulate NREMS (Ehlen et al., 2013; Nichols et al., 2020). However, although GDX greatly reduced this difference in the first study (Ehlen et al., 2013), the difference persists in the latter (Nichols et al., 2020). Therefore, these results seem to indicate that male rodents have more NREMS than females, and that this sex difference is influenced by circulating gonadal hormones and Sry expression, and not by sex chromosomes. It seems interesting to point out that more time spent in NREMS in male compared to female rodents contrasts with the frequent reports of lower sleep quality and shorter time spent in deep NREMS in men than women when sleep is objectively measured in humans (Carrier et al., 2017).
2.3. REMS amount
In mice, we identified four studies showing that the time spent in REMS is equivalent in both sexes (Ehlen et al., 2013; Franken et al., 2006; Grønli et al., 2016; Paul et al., 2006). In this same line, sex chromosomes did not significantly impact REMS in mice with intact gonads (Ehlen et al., 2013). Nevertheless, observations reported by four other studies indicate that males spend more time in REMS particularly during the light period (Huitron-Resendiz et al., 2018; Koehl et al., 2006; Paul et al., 2009a) or the dark period (Nichols et al., 2020). Given the same number of mouse studies reporting a sex differences in REMS and an absence of such difference, the schematic representation of sex differences in Fig. 1A is not emphasizing any clear difference for this state. Interestingly, a genotype change in mice was shown to create a sex difference in time spent in REMS that was not observed in wild-type (WT) mice (Franken et al., 2006). More precisely, male mice knockout (KO) for the neuronal PAS domain protein 2 (Npas2−/−) spend less time in REMS than Npas2−/− females (Franken et al., 2006). Given that the Npas2 gene codes for a trancription factor implicated in the regulation of endogenous circadian timekeeping (DeBruyne et al., 2007), the finding by Franken et al. (2006) support that the relationship between molecular circadian clock components and sleep amount is modulated by sex. This research clearly underlines the importance of investigating the consequences of genetic modifications in both males and females since mutations can affect wake/sleep variables differently in the two sexes.
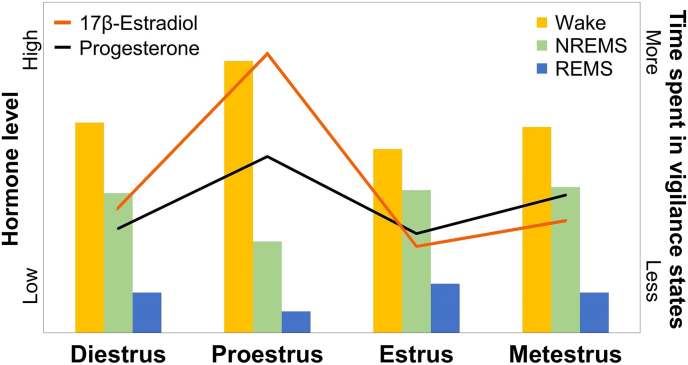
Unlike observations in mice, REMS data in rats seem more consistent in supporting a higher REMS amount in males compared to females (Fig. 1B). Indeed, male rats were found to spend more time in REMS than females, a difference that is observed during both the light and dark periods (Fang and Fishbein, 1996). A recent study also shows a predominance of REMS in males, when compared to females in proestrus, specifically during the second half of the light period and the first half of the dark period (Swift et al., 2020). Of importance is that the difference disappears (Kostin et al., 2020), or is reversed during the second half of the dark period (Swift et al., 2020), when females are in estrus (E2 levels at their lowest), or when the estrous cycle is not considered (Garner et al., 2018 but see Fang and Fishbein. 1996). These overall findings suggest that in female rats, higher levels of E2 suppress time spent in REMS. This is corroborated by comparisons of OVX females without E2 replacement to those with replacement or with intact ovaries (Cusmano et al., 2014; Deurveilher et al., 2011; Fang and Fishbein, 1996; see also Paul et al., 2009b for mice), and by effects of the estrous cycle (Hadjimarkou et al., 2008; Koehl et al., 2003; Schwierin et al., 1998; Swift et al., 2020). Fig. 2 depicts concurrent fluctuations in ovarian hormones and in approximate time spent in wakefulness and sleep states across the estrous cycle in female rats. Notably, E2 level is fluctuating together with progesterone (P) level, which could also contribute to the observed estrous cycle phase-dependent sex difference and effects of OVX in rats. This is notably supported by the observation of less time spent in REMS under P supplementation in comparison to control females (Deurveilher et al., 2011). Taken together, these findings indicate that sex differences in REMS are modulated by gonadal hormones, in particular ovarian hormones, and are possibly species dependent.
Fig. 2.
Time spent in wakefulness and sleep states represented together with fluctuations of 17β-estradiol (E2) and progesterone (P) in the course of the estrous cycle in rats. Levels of E2 and levels of P have been adapted from the review of Hussain et al. (2014). Histogram of time spent in wakefulness, non-rapid eye movement sleep (NREMS) and rapid eye movement sleep (REMS) have been compiled from Fang and Fishbein (1996), Schwierin et al. (1998), Swift et al. (2020), and Yamaoka et al. (1980). The figure shows that during proestrus in comparison to other phases of the estrous cycle, female rats have higher levels of E2 and P, spend more time awake and less time in NREMS and REMS.
2.4. Wakefulness and sleep consolidation/fragmentation
Another variable associated with wake/sleep architecture impacted by biological sex is the consolidation/fragmentation of wakefulness and sleep. In general, longer and fewer episodes of vigilance states reflect greater consolidation, while shorter and more frequent episodes indicate fragmentation. More fragmentation in male mice in comparison to females is supported by three main studies. First, shorter individual NREMS episodes during the dark period and more REMS episodes during the light period have been reported in males compared to females (Huitron-Resendiz et al., 2018). Second, Paul et al. (2006) found shorter wake episodes and higher number of transitions between wakefulness, NREMS and REMS during the dark period in males than females (Paul et al., 2006). These latter differences were no longer significant after GDX (Paul et al., 2006). Third, in the FCG model, male mice (XX and XY with Sry gene and born with testies) submitted to GDX showed more brief arousals, NREMS episodes, and state transitions during the dark period in comparisons to OVX females (XX and XY without Sry and born with ovaries) (Nichols et al., 2020). However, we found one study showing fewer NREMS-REMS transitions and NREMS episodes of less than 1 min in male mice in comparison to females (light and dark periods combined; Franken et al., 2006). It was also observed that male mice have fewer bouts of activity than females during the light period (Ruby et al., 2018), which could be indicative of more consolidated wakefulness and sleep alternations. Thus, sex differences in the consolidation/fragmentation of wakefulness and sleep states seem to exist in mice with, among articles using EEG measurements, three pointing to more fragmentation/less consolidation of wakefulness and sleep in males particularly during the dark period (Huitron-Resendiz et al., 2018; Nichols et al., 2020; Paul et al., 2006), and one showing indication of the opposite (Franken et al., 2006).
In rats, sex differences in wakefulness and sleep consolidation/fragmentation were shown to depend on the estrous cycle phase as was the case for time spent in each state. When compared to females in estrus, males show fewer NREMS episodes of relatively long duration; 2-5 min during the light period and >10 min during the dark period (Kostin et al., 2020); and less REMS episodes during the dark period (Swift et al., 2020). When compared to females in proestus, male rats show fewer episodes of wake during the light and more REMS episodes during the dark, shorter wake episodes during the dark, and globally longer episodes of NREMS and REMS (Swift et al., 2020). It is likely that the shorter wake episodes and longer NREMS/REMS episodes observed in male rats in comparison to females in proestrus is contributing to the sex differences in time spent in wakefulness (less in males) and sleep states (more in males) reported above (that are particularly noticeable when females are in proestrus). Of note is that sex differences in wake/sleep fragmentation/consolidation are also observed when comparing males to OVX females with hormone replacement (but not when the estrous cycle is not considered; Garner et al., 2018). Indeed, a higher number of brief awakenings and/or NREMS episodes was found in males in comparison to OVX female rats receiving low E2, high E2 or low E2 with high P (Deurveilher et al., 2011). Therefore, male rats could show indications of higher wake/sleep fragmentation than female rats (similar to mice), and this difference also seems to strongly depend on gonadal (in particular ovarian) hormones. Interestingly, it is also generally recognized from human sleep research that men show less sleep consolidation in comparison to women, as indicated by more wakefulness and light NREMS during their main sleep episode (Carrier et al., 2017).
3. EEG activity
EEG activity measured over different areas of the cerebral cortex (e.g., frontal, somatosensory, or visual cortex) is used to identify wakefulness and sleep states. In addition, EEG activity over different cortical areas within each vigilance state can reflect state quality and be indicative of the underlying neurophysiology/network connectivity. An earlier study has indicated that male rats express less delta and more theta (3.4–7.3 Hz) power than females during a 2-h EEG recording (Juarez et al., 1995). While it is unclear whether this difference occurred during wakefulness, NREMS or REMS, this demonstrates that biological sex modulates EEG-measured synchronized brain activity. It is interesting to note that sex differences in synchronized neuronal activity resembling NREMS were also reported for ex vivo slice recording of the mouse somatosensory cortex (Sigalas et al., 2017). We will next discuss studies that have reported differences in EEG activity in well-defined vigilance states, starting with wakefulness and REMS before presenting a more detailed perspective about NREMS, on which most rodent studies have focussed. For this section and the followings, SWA (generally 0.75–4.5 Hz) and delta (1-4 Hz) activity data will be collectively referred to as delta power given the major frequency overlap between the two.
Considering the light and dark periods of the nychthemeron combined, it has been shown that compared to females, male mice have more theta (5-10 Hz) power during wakefulness, and more delta and sigma (10-15 Hz) power during REMS (Franken et al., 2006). Given that wakefulness enriched with theta (and gamma [55-80 Hz]) activity has been linked to orexin signaling and sleep need (Vassalli and Franken, 2017), higher waking theta in male mice could contribute to their reduced time spent awake and increased time spent in NREMS reported in sections 2.1, 2.2 above. However, we identified three other mouse studies reporting no significant effect of sex on wakefulness and REMS power spectra computed up to 60 Hz also for 24 h (Grønli et al., 2016; Hellman et al., 2010-potentially underpowered to assess sex difference; Huitron-Resendiz et al., 2018). During the resting-state (comparable to quiet wakefulness), another study showed that male mice have less activity in gamma (60-100 Hz) frequencies than females, while no sex difference was reported for the other frequencies (Port et al., 2017). This last study also emphasizes the need to investigate genotype effects in both males and females, given that female mice heterozygous for Protocadherin-10 (Pcdh10+/−), coding for a cell surface molecule with tumor suppressor activity (Xu et al., 2015), were shown to have a stronger activity coupling between alpha and gamma frequencies when compared to WT females (and to Pcdh10+/− males), whereas no such genotype difference was found in males (Port et al., 2017). Since the coupling of alpha and gamma EEG activity was proposed to be indicative of the excitability of neuronal ensembles (Wagner et al., 2019), the finding by Port et al. (2017) may point to a sex-specific genotype effect in information processing.
In rats, a higher global EEG power between 0 and 100 Hz in the frontal cortex was recently reported for females (unspecified estrous cycle phase) in comparison to males during quiet wakefulness (Wong et al., 2020). Of note is that this global sex difference in waking EEG activity was reversed in animals KO for Fmr1 (Wong et al., 2020), a gene linked to Fragile X syndrome, morphological and neurological symptoms in humans. Concerning REMS, an absence of difference in EEG power spectrum (0.5-20 Hz) was reported between males and OVX females submitted to different gonadal hormone treatments (Deurveilher et al., 2011). This contrasts with the significant effect of the estrous cycle phase on REMS EEG activity previously reported (Schwierin et al., 1998), and recently described for the prefrontal cortex (PFC) and visual cortex (V2), showing, in particular, an increase in REMS theta activity during the dark period in proestrus (Swift et al., 2020). It overall seems that there is insufficient data concerning sex differences in EEG activity during wakefulness and REMS in rodents, which should be more systematically integrated in future studies.
3.1. NREMS EEG activity in mice
In mice, the general NREMS EEG power spectrum was shown to be similar between males and females (Grønli et al., 2016; Hellman et al., 2010-but underpowered), except specifically concerning delta and sigma power (Franken et al., 2006; Koehl et al., 2006; Paul et al., 2006). With regard to delta power, sex differences have been reported for total power (Franken et al., 2006), but were also shown to be specific to different parts of the nychthemeron (Koehl et al., 2006; Paul et al., 2006). First, considering NREMS delta power summed over the 24-h day (i.e., combined light and dark periods), male mice were shown to have less power than females in one study (Franken et al., 2006), whereas no sex difference was reported by four other studies (Koehl et al., 2006; Grønli et al., 2016; Paul et al., 2006; Nichols et al., 2020). Second, when considering the time course of NREMS delta power over the 24-h day (frequently used as a marker of homeostatic sleep pressure dynamics), males were shown to have higher power than females at the very beginning of the dark period and lower power for some precise intervals later in the dark period (Koehl et al., 2006; Paul et al., 2006). Furthermore, compared to females, male mice were shown to begin the light period with lower NREMS delta power (Paul et al., 2006). According to the known homeostatic regulation of NREMS delta power (Dijk and Czeisler, 1995; Dijk and Lockley, 2002), these time-dependent sex differences in delta power could be driven by the time-dependent differences in time spent in NREMS reported in section 2.2 (see also Fig. 1). Indeed, the greater time spent asleep in males during the dark period is likely resulting in less delta power during subsequent NREMS (easier to capture using a time course analysis in comparison to a full day average measurement).
Sex differences in the time course of delta power were shown to be eliminated by GDX in mice (Paul et al., 2006), which implies that gonadal hormones are contributing to time-dependent sex differences in NREMS delta activity. However, it should be noted that we found two studies reporting an absence of sex difference in the time course of delta power in mice (Franken et al., 2006; Grønli et al., 2016). Interestingly, one of these studies showed that a change in genotype creates a time-specific sex difference in delta power that was not observed in WT (Franken et al., 2006). In fact, Npas2−/− male mice have less NREMS delta power during some intervals of the mid and late light period when compared to Npas2−/− females (Franken et al., 2006). This emphasizes once more the need to consider sex as a regulating factor in assessing genotype effects on sleep phenotypes. Overall, we have described above three studies that have found a decreased NREMS delta power in male compared to female mice, either globally or at specific time intervals during the light-dark cycle (Franken et al., 2006; Koehl et al., 2006; Paul et al., 2006), and two studies reporting an absence of sex differences in total delta power and/or its time course (Grønli et al., 2016; Nichols et al., 2020).
In addition, a sex difference in the NREMS EEG was reported for sigma frequencies in mice (Franken et al., 2006), although another study did not detect such a difference (Grønli et al., 2016). Franken et al. (2006) specifically showed that male mice have less NREMS sigma (10-15 Hz) power than females, a difference that predominates during the light period and that may be indicative of differences in circadian or homeostatic sleep regulation (Dijk and Czeisler, 1995). Nevertheless, more research is definitely required before clear conclusions can be made regarding the impact of sex on the activity in EEG frequencies outside of delta during NREMS in mice. In summary, male mice may express less NREMS sigma and delta power than females, which is generally time of day-dependent, and influenced by genotype and the presence of gonadal hormones in the case of delta. It is interesting to note that these sex differences are in the same direction as the differences in EEG activity observed in delta and sigma frequencies between men and women (Carrier et al., 2001; Dijk et al., 1989; Mongrain et al., 2005), although the EEG is recorded above the skull in humans and generally directly on the cortical surface in rodents.
3.2. NREMS EEG activity in rats
In rats, no significant sex difference was reported for NREMS delta activity or its time course using a 6-h recording centered on the middle of the light period (Garner et al., 2018). Similarly, no difference was found in the NREMS EEG power spectrum (0.5-50 Hz) between males and OVX females supplemented or not with gonadal hormones (Deurveilher et al., 2011). However, NREMS EEG activity was shown to be altered by the estrous cycle in females (Schwierin et al., 1998; Swift et al., 2020). For instance, when compared to the other phases, rats in proestrus showed a decreased delta power and increased activity in 10-25 Hz frequencies (Schwierin et al., 1998). A more recent study also shows higher NREMS delta power during proestrus, which was restricted to the dark period and continued into the beginning of the light period of the estrus phase (Swift et al., 2020). Accordingly, the phase of the estrous cycle in females influences whether sex differences in NREMS EEG activity are observed in different brain regions in rats. Indeed, Swift et al. (2020) recorded EEG activity in three regions (CA1, medial PFC, V2) across the estrous cycle of females and in males over the same time frame, and observed sex differences that were sensitive to region, time of day, and estrous cycle phase. For instance, at the very end of the dark period, males were shown to have significantly more NREMS delta power than females in estrus in the secondary visual cortex (V2) and CA1 region, but not in the medial PFC (Swift et al., 2020). Furthermore, NREMS slow gamma (31-60 Hz) activity in the medial PFC and V2 was observed to be lower in males than in females in proestrus during the early dark period, which was not statistically significant in CA1 (Swift et al., 2020). Taken together, sex differences in NREMS EEG activity are detectable when female rats are stratified by the estrous cycle phase. The region-specific effects are particularly novel and warrant further investigation, as it will help elucidate sex differences in sleep regulation and eventually improve the specificity of treatments regarding sleep pathologies.
4. Response to sleep deprivation
Rodent sleep research has also explored whether males and females respond to sleep deprivation differently. Our review will focus on sex differences in sleep architecture and EEG activity following acute (total) sleep deprivation of 6-8 h. While some studies have examined sex differences in memory performance following acute sleep deprivation (e.g., Baratta et al., 2018) or in sleep phenotypes in response to sleep restriction or chronic (often mostly paradoxical) sleep deprivation (e.g., Gonzalez-Castaneda et al., 2016; Matos et al., 2013), they will not be reviewed here.
4.1. Rebound in NREMS amount
Typically, sleep deprivation generates an increase in time spent in NREMS during the following 12 h in comparison to baseline amount (Franken et al., 2006; Hor et al., 2019; Koehl et al., 2006; Kostin et al., 2020; Paul et al., 2006), which can be referred to as the NREMS rebound. On the one hand, a lower NREMS rebound has been reported in male mice compared to females (Paul et al., 2006). This sex difference, which was abolished by GDX (Paul et al., 2006), could originate from the longer time spent in NREMS in males under baseline (Fig. 1A) rendering more difficult for males to increase NREMS time under recovery from sleep loss. Sex chromosomes were shown to modulate time spent in NREMS following sleep deprivation (Ehlen et al., 2013; Nichols et al., 2020), with a delayed NREMS rebound being increased by the presence of the Y chromosomes in females (Ehlen et al., 2013). On the other hand, an absence of sex difference in NREMS rebound in WT mice was reported by two studies (Franken et al., 2006; Koehl et al., 2006), although the KO of Npas2 was shown to reveal a sex difference, with Npas2−/− males having a lower NREMS rebound than Npas2−/− females (Franken et al., 2006).
In rats, a lower NREMS rebound was reported in adult (3-4 mo) males in comparison to females in estrus when considering the first 3 h following sleep deprivation (Kostin et al., 2020). Interestingly, no sex difference was observed in an older (24-25 mo) group (Kostin et al., 2020). Considering that older females are acyclic, these results suggest that sex differences in young animals are due to hormonal fluctuations in females. However, the percent increase from baseline in the duration and number of individual NREMS episodes after sleep deprivation was shown to be statistically similar between males and OVX females submitted or not to hormone replacement (Deurveilher et al., 2011), but the NREMS rebound was not specifically investigated in this study. Altogether, the aforementioned mouse and rat findings could suggest that the NREMS rebound after sleep deprivation is sensitive to biological sex (i.e., five rodent studies pointing to a sex difference versus one showing none), and that age, genotype, sex chromosomes and gonadal hormones contribute to differences between males and females.
4.2. Rebound in REMS amount
There is also support for a significant REMS rebound after acute sleep deprivation (Franken et al., 2006; Hor et al., 2019; Koehl et al., 2006; Paul et al., 2006). In mice, we found two datasets showing no sex difference in REMS rebound (Ehlen et al., 2013; Paul et al., 2006), versus another in which males showed a larger REMS rebound than females for both WT and Npas2−/− mice (Franken et al., 2006). While these findings are inconsistent, it is important to note that mice in the first two studies were sleep deprived for 6 h (Ehlen et al., 2013; Paul et al., 2006), whereas those in the later experienced 8 h of sleep deprivation (Franken et al., 2006). With regard to rats, sex differences in REMS rebound are definitely understudied. Even if REMS rebound (total time spent in REMS in recovery relative to baseline) was not directly investigated, the percent increase from baseline in the duration and number of individual REMS episodes after sleep deprivation was shown to be similar between males and OVX females receiving E2 with or without P replacement (Deurveilher et al., 2011). Overall, there is a need for more investigations to understand the effect of biological sex on the response of REMS to sleep deprivation, which includes EEG activity during REMS.
4.3. Rebound in NREMS EEG activity
The increase in EEG delta power is probably the most striking effect observed during recovery NREMS after sleep deprivation (Franken et al., 2006; Hor et al., 2019; Koehl et al., 2006; Kostin et al., 2020; Paul et al., 2006). In fact, during the first 30 min of recovery NREMS, EEG power is increased compared to baseline across all frequencies between 0.5 and 25 Hz in male mice, whereas in females, the 9.5–17.5 Hz range is unaffected by sleep deprivation (Franken et al., 2006). Nevertheless, this study shows that delta power is similarly increased in males and females (Franken et al., 2006), which also seems to be the case in another dataset (Koehl et al., 2006). In addition to the relative increase from baseline, an absence of sex difference in the absolute delta power level during the first 3 h of recovery NREMS after sleep deprivation was reported (Grønli et al., 2016).
Contrary to these three independent articles pointing to an absence of sex difference in delta power after sleep deprivation conducted early during the light period, another study has shown sex differences in delta power after acute sleep loss when sleep deprivation was scheduled at the end of the light period (Paul et al., 2006). Specifically, male mice expressed higher NREMS delta power than females in the first 2-h interval of recovery sleep, but lower NREMS delta power 7 to 10 h after the end of sleep deprivation (Paul et al., 2006). These differences were reduced by GDX (Paul et al., 2006). In fact, OVX appears to alter the dynamics of NREM delta power in females while castrated males were comparable to intact males (Paul et al., 2006), suggesting that circulating ovarian hormones are mainly responsible for sex differences in homeostatic sleep regulation in this study. However, sex chromosomes and gonadal phenotype were also shown to influence the NREMS delta power rebound after sleep deprivation in FCG mice (Ehlen et al., 2013), with XX females showing lower power than XX males at the beginning of recovery sleep, and higher power than XY females 4 h later (Ehlen et al., 2013). Together, these last findings could suggest that the buildup during prolonged wakefulness and the dissipation during recovery sleep of NREMS delta power is influenced both by ovarian hormones and sex chromosomes. This assumption could also be supported by findings in rats showing that NREMS delta power during the first 2-3 h of recovery following sleep deprivation is lower in males than females in estrus (Kostin et al., 2020), and affected by ovarian hormones in OVX females (Deurveilher et al., 2011).
It has also been shown that, when compared to baseline, male mice have a larger increase in NREMS sigma/spindle activity (11-16 Hz) after sleep deprivation in comparison to females (Franken et al., 2006). Unfortunately, no other studies have addressed sex differences in spindle activity during recovery sleep in rodents. Altogether, there are indications of sex differences in the EEG activity response to sleep deprivation in rodents. For NREMS delta activity in particular, four of the rodent datasets described above suggest a sex difference/effect of ovarian hormones, while three are rather providing support for an absence of difference between sexes. Given that sleep architecture is altered by the estrous cycle, future studies are needed to precisely determine whether the dynamics of EEG activity in a wide range of frequencies during recovery NREMS and REMS are similarly sensitive to fluctuating ovarian hormones.
5. Circadian functions
In this section, we present some of the sex differences that have been reported in variables associated to the functioning of the circadian timing system in rodents. The focus will be on these specific circadian variables derived from locomotor activity and wheel-running activity patterns: the phase angle of activity onset, the length of the endogenous period, and the shift in activity onset following light pulses. As such, variables related to, for instance, the adaptation to non-24 h light-dark cycle will not be covered. It should also be noted that many studies in rodents other than mice and rats are available and discussed here.
5.1. Phase angle of activity onset
The phase angle of activity onset defines the time interval between the start of the main period of locomotor (or wheel-running) activity and the beginning of the dark period for nocturnal rodents. The angle is calculated relative to the start of the light period for diurnal rodents (e.g., Octodon Degus, a diurnal rodent with longer estrous cycle and lifespan than other rodents). It generally reflects circadian entrainment to normal light-dark conditions. We identified three studies reporting no sex difference in prepubertal juvenile and adult degus (Lee et al., 2004), young adult degus (Hummer et al., 2007), and in adolescent mice (Stowie and Glass, 2015). However, an earlier activity onset (i.e., longer phase angle) was found in male degus in comparison to females when tested at 9-11 months old, a difference that was eliminated by GDX (Hummer et al., 2007). In contrast, adult and middle age male mice and adult hamsters (both generally nocturnal) were shown to have a later activity onset (i.e., shorter phase angle or larger negative angle) in comparison to females (Davis et al., 1983; Ruby et al., 2018; Stowie and Glass, 2015), a difference also reduced by GDX in hamsters (Davis et al., 1983). The phase angle of activity onset was not shown to significantly differ, however, between sexes in PER2::LUC (Period 2::Luciferase) knock-in mice, even if males have a more precise and constant activity onset than females (Kuljis et al., 2013). These datasets indicate that the direction of the sex difference varies with developmental age and species, and that the presence of a sex difference is influenced by gonadal hormones.
Despite an effect of GDX on sex difference in hamsters, the phase angle was shown not to fluctuate across the estrous cycle in females (Davis et al., 1983). However, female degus have an earlier activity onset in estrus than in metestrus (Labyak and Lee, 1995). Similarly, in rats, the activity onset of females in estrus was shown to be prior the start of the dark period, whereas in the other phases, it generally occurs after the dark period onset (Albers et al., 1981; Wollnik and Turek, 1988). An effect of gonadal hormones is supported by additional observations made independently in females and males. First, E2 replacement in OVX female mice and hamsters was shown to advance the activity onset (Blattner and Mahoney, 2014; Morin et al., 1977). Second, circulating androgens were shown to alter the activity onset of males, with a direction that seems to depend on whether the species is nocturnal or diurnal, but generally resulting in a lower magnitude of the phase angle of entrainment (i.e., higher magnitude under GDX). For instance, in male degus (diurnal), GDX was reported to advance the activity onset (and thus lengthen the phase angle; Jechura et al., 2000), whereas GDX in male mice (nocturnal) delays the activity onset (Brockman et al., 2011). Altogether, the literature supports a modulatory role of gonadal hormones on the entrainment of the activity rhythm to the light-dark cycle. It should be noted, however, that different light-dark cycles have been used in the aforementioned studies (e.g., 14-h light:10-h dark versus 12-h light:12-h dark), which may impact sex differences.
5.2. Endogenous period
The endogenous period of the circadian system is driven by the internal clock in the suprachiasmatic nucleus (SCN) of the hypothalamus in mammals (Moore and Eichler, 1972; Ralph et al., 1990). In constant darkness, locomotor activity in rodents follows a circadian rhythm with a period length of about 24 h (Pittendrigh and Daan, 1976). In hamsters, rats and mice (three different articles), males were reported to have a longer endogenous period than females, although this sex difference did not always reach statistical significance (Davis et al., 1983; Schull et al., 1989; Sterniczuk et al., 2010). In parallel, we found four studies reporting no significant sex difference for this circadian variable in adult and older mice (Iwahana et al., 2008; Kuljis et al., 2013, 2016; Wisor et al., 2005). In the Octodon Degus, the length of the endogenous period has been shown to be similar in males and females within the first year of life, but shorter in males than females at 12 month old (Hummer et al., 2007; Lee et al., 2004). This ‘later-life’ sex difference (i.e., at 12 months) was eliminated by GDX conducted at 5-6 week old (Hummer et al., 2007), which appears to impact males in particular (GDX males resembling both intact and GDX females). Similarly, in rats and golden hamsters, GDX animals were shown to have an equivalent length of the endogenous circadian period (Albers, 1981; Zucker et al., 1980). However, other studies have shown that in GDX hamsters and mice, males have a longer free-running period than females (Davis et al., 1983; Iwahana et al., 2008), even if the difference was not significant in intact animals (Iwahana et al., 2008). Taken together, while most findings are not supporting a sex difference in the endogenous circadian period in mice, some datasets could nevertheless suggest that the endogenous period is longer in males than females in other nocturnal rodents, such as rats and hamsters. Interestingly, together with sex differences in the phase angle of entrainment reported above, male gonadal hormones are likely to contribute to this sex difference. A more in depth survey of the effect of gonadal hormones on the endogenous period is specifically offered in the next paragraph.
There is support for the endogenous circadian period of females to be sensitive to the estrous cycle phase. In rats, the period was shown to be shorter during estrus and longer during metestrus (Albers et al., 1981; Wollnik and Turek, 1988). In addition, E2 was found to shorten the period in females in different rodent species, but not in males nor in females with masculinized brain organization (Albers, 1981; Blattner and Mahoney, 2014; Morin et al., 1977; Takahashi and Menaker, 1980; Zucker et al., 1980). An association between a higher level of E2 and a reduced endogenous period length in females is also supported by the observation of a shorter period in WT mice than in aromatase KO females lacking the E2 production enzyme (Brockman et al., 2011). As emphasized earlier, there is also support for an effect of androgens on the endogenous period in males. Hummer et al. (2007) showed that GDX increased the period in 12 month old male degus (but see Jechura et al., 2000). GDX was also shown to significantly lengthen the period in male mice (Brockman et al., 2011; Daan et al., 1975). This effect is due, at least in part, to the loss of androgens as the administration of testosterone or the non-aromatizable androgen dihydrotestosterone eliminates the effect of GDX on the circadian period in male mice (Daan et al., 1975; Iwahana et al., 2008). Therefore, the literature suggests that the endogenous circadian period length is influenced by testosterone in males and E2 in females.
5.3. Response to a light pulse
The circadian rhythm of locomotor (or wheel-running) activity measured under constant darkness conditions can be advanced by a light pulse applied during the late active period or delayed by a light pulse applied during the early active period (e.g., Davies et al., 1983). The phase shift response of the circadian system in particular to a delaying light pulse was shown to be modulated by sex. First, a full phase response curve to light constructed in hamsters has reported a higher magnitude (~30 min) of delaying shifts in males than in females (Davies et al., 1983). However, in mice, phase delaying shifts were reported to be smaller in males in comparison to females at approximately 6 month old (Blattner and Mahoney, 2013; Kuljis et al., 2016), but not at 3 months (Kuljis et al., 2016). It was also found, using the mouse FCG model, that phenotypic males (XY and XX with testes) were having smaller delaying phase shifts than phenotypic females (XX and XY with ovaries; Kuljis et al., 2013), suggesting that the smaller phase delays in males are mediated by gonadal hormones and not chromosomal sex. This is corroborated by their findings that the sex difference is eliminated by GDX (Kuljis et al., 2013). In fact, GDX male mice demonstrate a larger delaying phase shift when compared to intact males, an effect that is reversed by the administration of dihydrotestosterone (Karastoreos et al., 2011). It should be noted, however, that other mouse studies not directly comparing males and females have observed relatively similar magnitude of phase delays in the two sexes (Ruby et al., 2018; Sterniczuk et al., 2010). In addition, there could be some level of species specificity, as castration did not seem to significantly change the response to different light pulses in degus (Jechura et al., 2000).
There could also be indication of an effect of ovarian hormones on phase advancing light stimuli. In OVX mice, high (but not low) E2 was shown to facilitate phase-advancing shifts (Blattner and Mahoney, 2014), an effect that was not significant in estrogen receptor KO mice (Blattner and Mahoney, 2014). Altogether, it is difficult to establish a direction of sex differences in the circadian response to light pulse considering between-species differences, and the fact that many reports have focused on the effects of gonadal hormones within one sex, with few studies directly comparing the sexes. As such, a relatively large knowledge gap remains in terms of whether males and females respond differently to phase shifting stimuli (Lee et al., 2021), even if the literature may suggest an impact of sex.
6. Conclusion
This review summarizes the rodent literature concerning sex differences in sleep and circadian phenotypes. In general, males appear to spend less time in wakefulness and more in NREMS than females in mice and rats (Fig. 1). A similar sex difference is found for REMS, although not as consistent in mice, and sex differences in wake/sleep consolidation and fragmentation may point to more fragmentation in male rodents. With regard to EEG activity during wakefulness and sleep, male mice appear to express less delta power during NREMS than females, whereas in rats, sex differences are importantly influenced by the estrous cycle. Following acute sleep deprivation, NREMS and REMS rebound, and changes in NREMS EEG activity are different in males and females, but it is difficult to extract a clear direction of sex differences, especially because differences seem time-dependent. Finally, variables associated to the functioning of the circadian system are also modulated both by sex and gonadal hormones. In the circadian field, several studies have focused on the effects of hormones within one sex, and few have directly compared the sexes. Considering that mutations can have different effects in both sexes, future studies need to include sex-based analyses to better understand how sleep and circadian variables are regulated in both sexes. In line with this, a comprehensive sex-based approach when considering widespread genetic variability in sleep/circadian phenotyping is to be favored, such as recently applied to 30 inbred mouse strains for the phenotyping of metabolic and energy expenditure traits (König et al., 2020). This type of research will be useful to identify strain-dependent differences between sexes, and to allow refining the choice of genetic strain for sleep/circadian studies investigating sex differences (given the general focus on the C57BL/6 strain for sleep phenotyping in mice).
As discussed, gonadal hormones are likely contributing to these sex differences in multiple ways. Striking examples are the negative effect of E2 on the activation (indexed by c-Fos) of sleep-active neurons in the hypothalamic ventrolateral preoptic area (Hadjimarkou et al., 2008), and the positive effect of E2 on neuronal activation (c-Fos indexed) of forebrain arousal regions after sleep deprivation in female rats (Deurveilher et al., 2008). These effects can well explain the generally increased time spent awake in female rodents. In addition to the impact of circulating gonadal hormones, sex differences in sleep and circadian variables could originate from differences in brain organization (McCarthy et al., 2012; Mong et al., 2011), or differences in the expression of gonadal hormone receptors in sleep and circadian-related brain areas (Bailey and Silver, 2014). For instance, compared to females, male rodents were reported to have a larger SCN (Gorski et al., 1978; Kuljis et al., 2016; Robinson et al., 1986), more androgen receptors and less oestrogen receptors in the SCN (Iwahana et al., 2008; Mong et al., 2011; Vida et al., 2008). Moreover, the firing rate of neurons in the dorsal SCN was reported to be higher in males than females in the light period (Kuljis et al., 2013). These morphological and functional sex differences could partly explain sex differences in sleep (Fig. 3). Of interest is also that sex differences persist when electrophysiological features resembling sleep are measured in vitro in the absence of circulating gonadal hormones (Sigalas et al., 2017), which could support sex differences in neuronal network organization (hormone-dependent or -independent) or an effect of circulating hormones at the time of sacrifice that would maintain itself in sliced tissue. Future research should evaluate molecular mechanisms underlying sex differences in sleep and circadian phenotypes using a genome-wide approach as recently done for the locus coeruleus, an important sleep-regulatory region showing >100 genes differentially expressed between male and female mice (Mulvey et al., 2018).
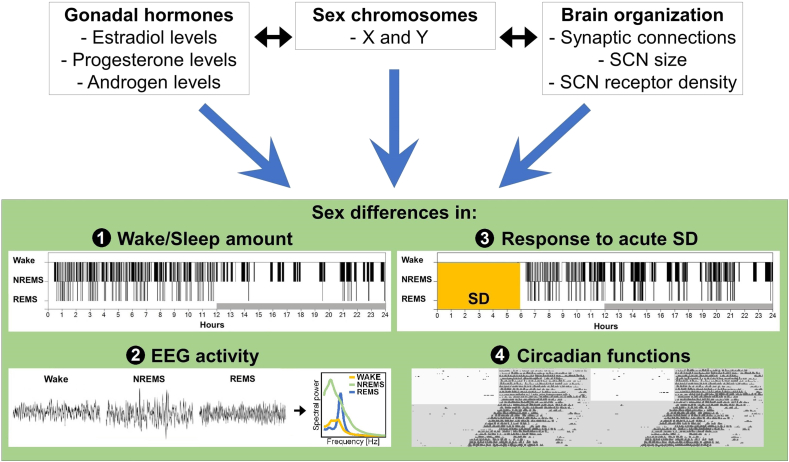
Fig. 3.
Schematic representation of the main factors implicated in sex differences in sleep and circadian phenotypes in rodents. Gonadal hormones, sex chromosomes and brain organization could all contribute to sex differences, which have been shown for sleep architecture (i.e., amount and consolidation of wakefulness, NREMS and REMS), and EEG activity during wakefulness and sleep. These factors could also explain sex differences observed in the EEG response to acute sleep deprivation (SD) and in circadian functions. SCN = suprachiasmatic nucleus of the hypothalamus.
While our review has focussed on sex differences in sleep and circadian phenotypes in (relatively young) adult rodents, sex differences have also been shown to be modulated by aging and pathology in humans and rodents (e.g., Carrier et al., 2017; Kostin et al., 2020; Kuljis et al., 2016; Sun et al., 2016; Wong et al., 2020), and to emerge in pathology-associated sleep disturbances (Gjerstad et al., 2007). As emphasized for the effect of targeted mutations, it is therefore tremendously important to consider sex difference in sleep/circadian functions in rodent models of pathology (or at least to investigate the two sexes). Albeit most rodent species are nocturnal and showing a highly fragmented alternation of wakefulness and sleep states in comparison to humans, and that rodent models of human pathologies usually recapitulate only few disease features, their use enables to identify molecular, cellular and circuit mechanisms contributing to sleep disturbances. Considering that adequate sleep is necessary for health and cognition (Durmer and Dinges, 2005; Klinzing et al., 2019), and that sleep problems can accelerate cognitive decline in the elderly and are linked to neurodegenerative disease (Lim et al., 2013; Osorio et al., 2011), the frequently reported insomnia in women, particularly important during menopause, could have psychological and physical consequences if untreated. Additionally, it has been suggested that ovarian hormones protect the brain from the detrimental cognitive effects of sleep deprivation (Gervais et al., 2017), which would place aging women in a particularly vulnerable position. Using rodent models to evaluate sex differences in insomnia and its consequences is primordial to identify sex-specific treatments against sleep disturbances.
Funding sources and acknowledgements
This work was supported by the Canada Research Chair in Sleep Molecular Physiology (VM), the Alzheimer's Association Research Fellowship co-sponsored by the Brain Canada Foundation (AARF-17-504715, NJG), and the Canadian Consortium on Neurodegeneration in Aging (CCNA-163902, NJG). Authors are thankful to Maria Neus Ballester Roig for help with Fig. 3 production.
Declaration of competing interest
The authors declare that they have no conflict of interest.
References
- Albers H.E. Gonadal hormones organize and modulate the circadian system of the rat. Am. J. Physiol. 1981;241(1):R62–R66. doi: 10.1152/ajpregu.1981.241.1.R62. [DOI] [PubMed] [Google Scholar]
- Albers H.E., Gerall A.A., Axelson J.F. Effect of reproductive state on circadian periodicity in the rat. Physiol. Behav. 1981;26(1):21–25. doi: 10.1016/0031-9384(81)90073-1. [DOI] [PubMed] [Google Scholar]
- Bailey M., Silver R. Sex differences in circadian timing systems: implications for disease. Front. Neuroendocrinol. 2014;35(1):111–139. doi: 10.1016/j.yfrne.2013.11.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baker A., Simpson S., Dawson D. Sleep disruption and mood changes associated with menopause. J. Psychosom. Res. 1997;43(4):359–369. doi: 10.1016/s0022-3999(97)00126-8. [DOI] [PubMed] [Google Scholar]
- Baker F.C., Driver H.S., Paiker J., Rogers G.G., Mitchell D. Acetaminophen does not affect 24-h body temperature or sleep in the luteal phase of the menstrual cycle. J. Appl. Physiol. 2002;92(4):1684–1691. doi: 10.1152/japplphysiol.00919.2001. [DOI] [PubMed] [Google Scholar]
- Baratta A.M., Buck S.A., Buchla A.D., Fabian C.B., Chen S., Mong J.A., Pocivavsek A. Sex differences in hippocampal memory and kynurenic acid formation following acute sleep deprivation in rats. Sci. Rep. 2018;8(1):6963. doi: 10.1038/s41598-018-25288-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bixler E.O., Papaliaga M.N., Vgontzas A.N., Lin H.M., Pejovic S., Karataraki M., Chrousos G.P. Women sleep objectively better than men and the sleep of young women is more resilient to external stressors: effects of age and menopause. J. Sleep Res. 2009;18(2):221–228. doi: 10.1111/j.1365-2869.2008.00713.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blattner M.S., Mahoney M.M. Photic phase-response curve in 2 strains of mice with impaired responsiveness to estrogens. J. Biol. Rhythm. 2013;28(4):291–300. doi: 10.1177/0748730413497190. [DOI] [PubMed] [Google Scholar]
- Blattner M.S., Mahoney M.M. Estrogen receptor 1 modulates circadian rhythms in adult female mice. Chronobiol. Int. 2014;31(5):637–644. doi: 10.3109/07420528.2014.885528. [DOI] [PubMed] [Google Scholar]
- Borbély A.A. A two process model of sleep regulation. Hum. Neurobiol. 1982;1(3):195–204. [PubMed] [Google Scholar]
- Borbély A.A., Daan S., Wirz-Justice A., Deboer T. The two-process model of sleep regulation: a reappraisal. J. Sleep Res. 2016;25(2):131–143. doi: 10.1111/jsr.12371. [DOI] [PubMed] [Google Scholar]
- Brankack J., Kukushka V.I., Vyssotski A.L., Draguhn A. EEG gamma frequency and sleep-wake scoring in mice: comparing two types of supervised classifiers. Brain Res. 2010;1322:59–71. doi: 10.1016/j.brainres.2010.01.069. [DOI] [PubMed] [Google Scholar]
- Brockman R., Bunick D., Mahoney M.M. Estradiol deficiency during development modulates the expression of circadian and daily rhythms in male and female aromatase knockout mice. Horm. Behav. 2011;60(4):439–447. doi: 10.1016/j.yhbeh.2011.07.011. [DOI] [PubMed] [Google Scholar]
- Brown A.M.C., Gervais N.J. Role of ovarian hormones in the modulation of sleep in females across the adult lifespan. Endocrinology. 2020;161(9):bqaa128. doi: 10.1210/endocr/bqaa128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brunner D.P., Münch M., Biedermann K., Huch R., Huch A., Borbély A.A. Changes in sleep and sleep electroencephalogram during pregnancy. Sleep. 1994;17:576–582. doi: 10.1093/sleep/17.7.576. [DOI] [PubMed] [Google Scholar]
- Cain S.W., Dennison C.F., Zeitzer J.M., Guzik A.M., Khalsa S.B., Santhi N., Duffy J.F. Sex differences in phase angle of entrainment and melatonin amplitude in humans. J. Biol. Rhythm. 2010;25(4):288–296. doi: 10.1177/0748730410374943. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Campbell S.S., Gillin J.C., Kripke D.F., Erikson P., Clopton P. Gender differences in the circadian temperature rhythms of healthy elderly subjects: relationships to sleep quality. Sleep. 1989;12:529–536. [PubMed] [Google Scholar]
- Cappuccio F.P., Stranges S., Kandala N.B., Miller M.A., Taggart F.M., Kumari M., Marmot M.G. Gender-specific associations of short sleep duration with prevalent and incident hypertension: the Whitehall II Study. Hypertension. 2007;50(4):693–700. doi: 10.1161/HYPERTENSIONAHA.107.095471. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carrier J., Land S., Buysse D.J., Kupfer D.J., Monk T.H. The effects of age and gender on sleep EEG power spectral density in the middle years of life (ages 20-60 years old) Psychophysiology. 2001;38:232–242. doi: 10.1111/1469-8986.3820232. [DOI] [PubMed] [Google Scholar]
- Carrier J., Semba K., Deurveilher S., Drogos L., Cyr-Cronier J., Lord C., Sekerovick Z. Sex differences in age-related changes in the sleep-wake cycle. Front. Neuroendocrinol. 2017;47:66–85. doi: 10.1016/j.yfrne.2017.07.004. [DOI] [PubMed] [Google Scholar]
- Cusmano D.M., Hadjimarkou M.M., Mong J.A. Gonadal steroid modulation of sleep and wakefulness in male and female rats is sexually differentiated and neonatally organized by steroid exposure. Endocrinology. 2014;155(1):204–214. doi: 10.1210/en.2013-1624. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Daan S., Beersma D.G., Borbély A.A. Timing of human sleep: recovery process gated by a circadian pacemaker. Am. J. Physiol. 1984;246(2 Pt 2):R161–R183. doi: 10.1152/ajpregu.1984.246.2.R161. [DOI] [PubMed] [Google Scholar]
- Daan S., Damassa D., Pittendrigh C.S., Smith E.R. An effect of castration and testosterone replacement on a circadian pacemaker in mice (Mus musculus) Proc. Natl. Acad. Sci. U.S.A. 1975;72(9):3744–3747. doi: 10.1073/pnas.72.9.3744. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davis F.C., Darrow J.M., Menaker M. Sex differences in the circadian control of hamster wheel-running activity. Am. J. Physiol. 1983;244(1):R93–R105. doi: 10.1152/ajpregu.1983.244.1.R93. [DOI] [PubMed] [Google Scholar]
- DeBruyne J.P., Weaver D.R., Reppert S.M. CLOCK and NPAS2 have overlapping roles in the suprachiasmatic circadian clock. Nat. Neurosci. 2007;10(5):543–545. doi: 10.1038/nn1884. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Deurveilher S., Cumyn E.M., Peers T., Rusak B., Semba K. Estradiol replacement enhances sleep deprivation-induced c-Fos immunoreactivity in forebrain arousal regions of ovariectomized rats. Am. J. Physiol. Regul. Integr. Comp. Physiol. 2008;295(4):R1328–R1340. doi: 10.1152/ajpregu.90576.2008. [DOI] [PubMed] [Google Scholar]
- Deurveilher S., Rusak B., Semba K. Female reproductive hormones alter sleep architecture in ovariectomized rats. Sleep. 2011;34(4):519–530. doi: 10.1093/sleep/34.4.519. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dijk D.J., Beersma D.G., Bloem G.M. Sex differences in the sleep EEG of young adults: visual scoring and spectral analysis. Sleep. 1989;12(6):500–507. doi: 10.1093/sleep/12.6.500. [DOI] [PubMed] [Google Scholar]
- Dijk D.J., Czeisler C.A. Contribution of the circadian pacemaker and the sleep homeostat to sleep propensity, sleep structure, electroencephalographic slow waves, and sleep spindle activity in humans. J. Neurosci. 1995;15(5):3526–3538. doi: 10.1523/JNEUROSCI.15-05-03526.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dijk D.J., Lockley S.W. Integration of human sleep-wake regulation and circadian rhythmicity. J. Appl. Physiol. 2002;92(2):852–862. doi: 10.1152/japplphysiol.00924.2001. [DOI] [PubMed] [Google Scholar]
- Dijk D.J., Shanahan T.L., Duffy J.F., Ronda J.M., Czeisler C.A. Variation of electroencephalographic activity during non-rapid eye movement and rapid eye movement sleep with phase of circadian melatonin rhythm in humans. J. Physiol. 1997;505(3):851–858. doi: 10.1111/j.1469-7793.1997.851ba.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Duffy J.F., Cain S.W., Chang A.M., Phillips A.J., Munch M.Y., Gronfier C., Czeisler C.A. Sex difference in the near-24-hour intrinsic period of the human circadian timing system. Proc. Natl. Acad. Sci. U.S.A. 2011;108(Suppl. 3):15602–15608. doi: 10.1073/pnas.1010666108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Durmer J.S., Dinges D.F. Neurocognitive consequences of sleep deprivation. Semin. Neurol. 2005;25(1):117–129. doi: 10.1055/s-2005-867080. [DOI] [PubMed] [Google Scholar]
- Ehlen J.C., Hesse S., Pinckney L., Paul K.N. Sex chromosomes regulate nighttime sleep propensity during recovery from sleep loss in mice. PloS One. 2013;8(5) doi: 10.1371/journal.pone.0062205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Erkkola R., Holma P., Jarvi T., Nummi S., Punnonen R., Raudaskoski T., Rehn K., Ryynänen M., Sipilä P., Tunkelo E., Virkkunen A. Transdermal oestrogen replacement therapy in a Finnish population. Maturitas. 1991;13(4):275–281. doi: 10.1016/0378-5122(91)90236-j. [DOI] [PubMed] [Google Scholar]
- Fang J., Fishbein W. Sex differences in paradoxical sleep: influences of estrus cycle and ovariectomy. Brain Res. 1996;734(1–2):275–285. [PubMed] [Google Scholar]
- Franken P., Dudley C.A., Estill S.J., Barakat M., Thomason R., O'Hara B.F., McKnight S.L. NPAS2 as a transcriptional regulator of non-rapid eye movement sleep: genotype and sex interactions. Proc. Natl. Acad. Sci. U.S.A. 2006;103(18):7118–7123. doi: 10.1073/pnas.0602006103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garner J.M., Chambers J., Barnes A.K., Datta S. Changes in Brain-derived neurotrophic factor expression influence sleep-wake activity and homeostatic regulation of rapid eye movement sleep. Sleep. 2018;41(2):zsx194. doi: 10.1093/sleep/zsx194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gervais N.J., Mong J.A., Lacreuse A. Ovarian hormones, sleep and cognition across the adult female lifespan: an integrated perspective. Front. Neuroendocrinol. 2017;47:134–153. doi: 10.1016/j.yfrne.2017.08.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gjerstad M.D., Wentzel-Larsen T., Aarsland D., Larsen J.P. Insomnia in Parkinson's disease: frequency and progression over time. J. Neurol. Neurosurg. Psychiatry. 2007;78(5):476–479. doi: 10.1136/jnnp.2006.100370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gonzalez-Castaneda R.E., Galvez-Contreras A.Y., Martinez-Quezada C.J., Jauregui-Huerta F., Grcia-Estrada J., Ramos-Zuniga R., Gonzalez-Perez O. Sex-related effects of sleep deprivation on depressive- and anxiety-like behaviors in mice. Exp. Anim. 2016;65(1):97–107. doi: 10.1538/expanim.15-0054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gorski R.A., Gordon J.H., Shryne J.E., Southam A.M. Evidence for a morphological sex difference within the medial preoptic area of the rat brain. Brain Res. 1978;148(2):333–346. doi: 10.1016/0006-8993(78)90723-0. [DOI] [PubMed] [Google Scholar]
- Grønli J., Clegern W.C., Schmidt M.A., Nemri R.S., Rempe M.J., Gallitano A.L., Wisor J.P. Sleep homeostatic and waking behavioral phenotypes in Egr3-deficient mice associated with serotonin receptor 5-HT(2) deficits. Sleep. 2016;39(12):2189–2199. doi: 10.5665/sleep.6324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hadjimarkou M.M., Benham R., Schwarz J.M., Holder M.K., Mong J.A. Estradiol suppresses rapid eye movement sleep and activation of sleep-active neurons in the ventrolateral preoptic area. Eur. J. Neurosci. 2008;27(7):1780–1792. doi: 10.1111/j.1460-9568.2008.06142.x. [DOI] [PubMed] [Google Scholar]
- Hellman K., Hernandez P., Park A., Abel T. Genetic evidence for a role for protein kinase A in the maintenance of sleep and thalamocortical oscillations. Sleep. 2010;33(1):19–28. doi: 10.1093/sleep/33.1.19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hor C.N., Yeung J., Jan M., Emmenegger Y., Hubbard J., Xenarios I., Naef F., Franken P. Sleep-wake-driven and circadian contributions to daily rhythms in gene expression and chromatin accessibility in the murine cortex. Proc. Natl. Acad. Sci. U.S.A. 2019;116(51):25773–25783. doi: 10.1073/pnas.1910590116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huitron-Resendiz S., Nadav T., Krause S., Cates-Gatto C., Polis I., Roberts A.J. Effects of withdrawal from chronic intermittent ethanol exposure on sleep characteristics of female and male mice. Alcohol Clin. Exp. Res. 2018;42(3):540–550. doi: 10.1111/acer.13584. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hummer D.L., Jechura T.J., Mahoney M.M., Lee T.M. Gonadal hormone effects on entrained and free-running circadian activity rhythms in the developing diurnal rodent Octodon degus. Am. J. Physiol. Regul. Integr. Comp. Physiol. 2007;292(1):R586–R597. doi: 10.1152/ajpregu.00043.2006. [DOI] [PubMed] [Google Scholar]
- Husby R., Lingjaerde O. Prevalence of reported sleeplessness in northern Norway in relation to sex, age and season. Acta Psychiatr. Scand. 1990;81(6):542–547. doi: 10.1111/j.1600-0447.1990.tb05009.x. [DOI] [PubMed] [Google Scholar]
- Hussain D., Shams W.M., Brake W.G. Estrogen and memory system bias in females across the lifespan. Transl. Neurosci. 2014;5(1):35–50. doi: 10.2478/s13380-014-0209-7. [DOI] [Google Scholar]
- Iwahana E., Karatsoreos I., Shibata S., Silver R. Gonadectomy reveals sex differences in circadian rhythms and suprachiasmatic nucleus androgen receptors in mice. Horm. Behav. 2008;53(3):422–430. doi: 10.1016/j.yhbeh.2007.11.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jechura T.J., Walsh J.M., Lee T.M. Testicular hormones modulate circadian rhythms of the diurnal rodent. Octodon degus. Horm. Behav. 2000;38(4):243–249. doi: 10.1006/hbeh.2000.1624. [DOI] [PubMed] [Google Scholar]
- Johnson E.O., Roth T., Schultz L., Breslau N. Epidemiology of DSM-IV insomnia in adolescence: lifetime prevalence, chronicity, and an emergent gender difference. Pediatrics. 2006;117(2):e247–256. doi: 10.1542/peds.2004-2629. [DOI] [PubMed] [Google Scholar]
- Juarez J., Corsi-Cabrera M., del Rio-Portilla I. Effects of prenatal testosterone treatment on sex differences in the EEG activity of the rat. Brain Res. 1995;694(1–2):21–28. doi: 10.1016/0006-8993(95)00725-6. [DOI] [PubMed] [Google Scholar]
- Karatsoreos I.N., Butler M.P., Lesauter J., Silver R. Androgens modulate structure and function of the suprachiasmatic nucleus brain clock. Endocrinology. 2011;152(5):1970–1978. doi: 10.1210/en.2010-1398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klinzing J.G., Niethard N., Born J. Mechanisms of systems memory consolidation during sleep. Nat. Neurosci. 2019;22(10):1598–1610. doi: 10.1038/s41593-019-0467-3. [DOI] [PubMed] [Google Scholar]
- Koehl M., Battle S.E., Turek F.W. Sleep in female mice: a strain comparison across the estrous cycle. Sleep. 2003;26(3):267–272. doi: 10.1093/sleep/26.3.267. [DOI] [PubMed] [Google Scholar]
- Koehl M., Battle S., Meerlo P. Sex differences in sleep: the response to sleep deprivation and restraint stress in mice. Sleep. 2006;29(9):1224–1231. doi: 10.1093/sleep/29.9.1224. [DOI] [PubMed] [Google Scholar]
- König C., Plank A.C., Kapp A., Timotius I.K., von Hörsten S., Zimmermann K. Thirty mouse strain survey of voluntary physical activity and energy expenditure: influence of strain, sex and day-night variation. Front. Neurosci. 2020;14:531. doi: 10.3389/fnins.2020.00531. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kostin A., Alam M.A., Siegel J.M., McGinty D., Alam M.N. Sex- and age-dependent differences in sleep-wake characteristics of Fisher-344 Rats. Neuroscience. 2020;427:29–42. doi: 10.1016/j.neuroscience.2019.11.046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kuljis D.A., Loh D.H., Truong D., Vosko A.M., Ong M.L., McClusky R., Colwell C.S. Gonadal- and sex-chromosome-dependent sex differences in the circadian system. Endocrinology. 2013;154(4):1501–1512. doi: 10.1210/en.2012-1921. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kuljis D.A., Gad L., Loh D.H., MacDowell Kaswan Z., Hitchcock O.N., Ghiani C.A., Colwell C.S. Sex differences in circadian dysfunction in the BACHD mouse model of Huntington's Disease. PloS One. 2016;11(2) doi: 10.1371/journal.pone.0147583. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Labyak S.E., Lee T.M. Estrus- and steroid-induced changes in circadian rhythms in a diurnal rodent, Octodon degus. Physiol. Behav. 1995;58(3):573–585. doi: 10.1016/0031-9384(95)00096-2. [DOI] [PubMed] [Google Scholar]
- Lee R., Tapia A., Kaladchibachi S., Grandner M.A., Fernandez F.X. Meta-analysis of light and circadian timekeeping in rodents. Neurosci. Biobehav. Rev. 2021;123:215–229. doi: 10.1016/j.neubiorev.2020.12.024. [DOI] [PubMed] [Google Scholar]
- Lee T., Hummer D.L., Jechura T.J., Mahoney M.M. Pubertal development of sex differences in circadian function: an animal model. Ann. NY Acad. Sci. 2004;1021:262–275. doi: 10.1196/annals.1308.031. [DOI] [PubMed] [Google Scholar]
- Li R.H., Wing Y.K., Ho S.C., Fong S.Y. Gender differences in insomnia--a study in the Hong Kong Chinese population. J. Psychosom. Res. 2002;53(1):601–609. doi: 10.1016/s0022-3999(02)00437-3. [DOI] [PubMed] [Google Scholar]
- Lim A.S., Kowgier M., Yu L., Buchman A.S., Bennett D.A. Sleep fragmentation and the risk of incident Alzheimer's disease and cognitive decline in older persons. Sleep. 2013;36(7):1027–1032. doi: 10.5665/sleep.2802. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lindberg E., Janson C., Gislason T., Bjornsson E., Hetta J., Boman G. Sleep disturbances in a young adult population: can gender differences be explained by differences in psychological status? Sleep. 1997;20(6):381–387. doi: 10.1093/sleep/20.6.381. [DOI] [PubMed] [Google Scholar]
- Matos G., Tenorio N.M., Bergamaschi C.T., Campos R.R., Cintra F., Tufik S., Andersen M.L. More than hormones: sex differences in cardiovascular parameters after sleep loss in rats. Prog. Neuro-Psychopharmacol. Biol. Psychiatry. 2013;44:34–38. doi: 10.1016/j.pnpbp.2013.01.009. [DOI] [PubMed] [Google Scholar]
- McCarthy M.M., Arnold A.P., Ball G.F., Blaustein J.D., De Vries G.J. Sex differences in the brain: the not so inconvenient truth. J. Neurosci. 2012;32(7):2241–2247. doi: 10.1523/JNEUROSCI.5372-11.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mong J.A., Baker F.C., Mahoney M.M., Paul K.N., Schwartz M.D., Semba K., Silver R. Sleep, rhythms, and the endocrine brain: influence of sex and gonadal hormones. J. Neurosci. 2011;31(45):16107–16116. doi: 10.1523/JNEUROSCI.4175-11.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mongrain V., Carrier J., Dumont M. Chronotype and sex effects on sleep architecture and quantitative sleep EEG in healthy young adults. Sleep. 2005;28(7):819–827. doi: 10.1093/sleep/28.7.819. [DOI] [PubMed] [Google Scholar]
- Mongrain V., Lavoie S., Selmaoui B., Paquet J., Dumont M. Phase relationships between sleep-wake cycle and underlying circadian rhythms in Morningness-Eveningness. J. Biol. Rhythm. 2004;19(3):248–257. doi: 10.1177/0748730404264365. [DOI] [PubMed] [Google Scholar]
- Moore R.Y., Eichler V.B. Loss of a circadian adrenal corticosterone rhythm following suprachiasmatic lesions in the rat. Brain Res. 1972;42(1):201–206. doi: 10.1016/0006-8993(72)90054-6. [DOI] [PubMed] [Google Scholar]
- Morin L.P., Fitzgerald K.M., Zucker I. Estradiol shortens the period of hamster circadian rhythms. Science. 1977;196(4287):305–307. doi: 10.1126/science.557840. [DOI] [PubMed] [Google Scholar]
- Mulvey B., Bhatti D.L., Gyawali S., Lake A.M., Kriaucionis S., Ford C.P., Dougherty J.D. Molecular and functional sex differences of noradrenergic neurons in the mouse locus coeruleus. Cell Rep. 2018;23(8):2225–2235. doi: 10.1016/j.celrep.2018.04.054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nichols I., Vincent S., Hesse S., Ehlen J.C., Brager A., Paul K. Sleep deprivation alters the influence of biological sex on active-phase sleep behavior. bioRxiv. 2020 doi: 10.1101/2020.02.21.958231. [DOI] [Google Scholar]
- Osorio R.S., Pirraglia E., Aguera-Ortiz L.F., During E.H., Sacks H., Ayappa I., de Leon M.J. Greater risk of Alzheimer's disease in older adults with insomnia. J. Am. Geriatr. Soc. 2011;59(3):559–562. doi: 10.1111/j.1532-5415.2010.03288.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paul K.N., Dugovic C., Turek F.W., Laposky A.D. Diurnal sex differences in the sleep-wake cycle of mice are dependent on gonadal function. Sleep. 2006;29(9):1211–1223. doi: 10.1093/sleep/29.9.1211. [DOI] [PubMed] [Google Scholar]
- Paul K.N., Laposky A.D., Turek F.W. Reproductive hormone replacement alters sleep in mice. Neurosci. Lett. 2009;463(3):239–243. doi: 10.1016/j.neulet.2009.07.081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paul K.N., Losee-Olson S., Pinckney L., Turek F.W. The ability of stress to alter sleep in mice is sensitive to reproductive hormones. Brain Res. 2009;1305:74–85. doi: 10.1016/j.brainres.2009.09.055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pittendrigh C.S., Daan S. A functional analysis of circadian pacemakers in nocturnal rodents. IV. Entrainment: pacemaker as Clock. J. Comp. Physiol. 1976;106:291–331. [Google Scholar]
- Port R.G., Gajewski C., Krizman E., Dow H.C., Hirano S., Brodkin E.S., Siegel S.J. Protocadherin 10 alters gamma oscillations, amino acid levels, and their coupling; baclofen partially restores these oscillatory deficits. Neurobiol. Dis. 2017;108:324–338. doi: 10.1016/j.nbd.2017.08.013. [DOI] [PubMed] [Google Scholar]
- Ralph M.R., Foster R.G., Davis F.C., Menaker M. Transplanted suprachiasmatic nucleus determines circadian period. Science. 1990;247(4945):975–978. doi: 10.1126/science.2305266. [DOI] [PubMed] [Google Scholar]
- Redline S., Kirchner H.L., Quan S.F., Gottlieb D.J., Kapur V., Newman A. The effects of age, sex, ethnicity, and sleep-disordered breathing on sleep architecture. Arch. Intern. Med. 2004;164(4):406–418. doi: 10.1001/archinte.164.4.406. [DOI] [PubMed] [Google Scholar]
- Robinson S.M., Fox T.O., Dikkes P., Pearlstein R.A. Sex differences in the shape of the sexually dimorphic nucleus of the preoptic area and suprachiasmatic nucleus of the rat: 3-D computer reconstructions and morphometrics. Brain Res. 1986;371(2):380–384. doi: 10.1016/0006-8993(86)90380-x. [DOI] [PubMed] [Google Scholar]
- Ruby C.L., Paye G., Fabi J.L., Zhang J., Risinger M.O., Palmer K.N., Swartzwelder H.S. Sex differences in photic entrainment and sensitivity to ethanol-induced chronodisruption in adult mice after adolescent intermittent Ethanol exposure. Alcohol Clin. Exp. Res. 2018;42(11):2144–2159. doi: 10.1111/acer.13867. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saré R.M., Lemons A., Song A., Smith C.B. Sleep duration in mouse models of neurodevelopmental disorders. Brain Sci. 2020;11(1):E31. doi: 10.3390/brainsci11010031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schull J., Walker J., Fitzgerald K., Hiilivirta L., Ruckdeschel J., Schumacher D., McEachron D.L. Effects of sex, thyro-parathyroidectomy, and light regime on levels and circadian rhythms of wheel-running in rats. Physiol. Behav. 1989;46(3):341–346. doi: 10.1016/0031-9384(89)90001-2. [DOI] [PubMed] [Google Scholar]
- Schwierin B., Borbely A.A., Tobler I. Sleep homeostasis in the female rat during the estrous cycle. Brain Res. 1998;811(1–2):96–104. doi: 10.1016/s0006-8993(98)00991-3. [DOI] [PubMed] [Google Scholar]
- Sigalas C., Konsolaki E., Skaliora I. Sex differences in endogenous cortical network activity: spontaneously recurring Up/Down states. Biol. Sex Differ. 2017;8:21. doi: 10.1186/s13293-017-0143-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spiegel K., Leproult R., Van Cauter E. Impact of sleep debt on metabolic and endocrine function. Lancet. 1999;354(9188):1435–1439. doi: 10.1016/S0140-6736(99)01376-8. [DOI] [PubMed] [Google Scholar]
- Sterniczuk R., Dyck R.H., Laferla F.M., Antle M.C. Characterization of the 3xTg-AD mouse model of Alzheimer's disease: part 1. Circadian changes. Brain Res. 2010;1348:139–148. doi: 10.1016/j.brainres.2010.05.013. [DOI] [PubMed] [Google Scholar]
- Stowie A.C., Glass J.D. Longitudinal study of changes in daily activity rhythms over the lifespan in individual male and female C57BL/6J mice. J. Biol. Rhythm. 2015;30(6):563–568. doi: 10.1177/0748730415598023. [DOI] [PubMed] [Google Scholar]
- Sun Q.Q., Zhou C., Yang W., Petrus D. Continuous spike-waves during slow-wave sleep in a mouse model of focal cortical dysplasia. Epilepsia. 2016;57(10):1581–1593. doi: 10.1111/epi.13501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Swift K.M., Keus K., Gonzalez Echeverria C., Carbrera Y., Jimenez J., Holloway J., Clawson B.C., Poe G.R. Sex differences within sleep in gonadally intact rats. Sleep. 2020;43(5):1–14. doi: 10.1093/sleep/zsz289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takahashi J.S., Menaker M. Interaction of estradiol and progesterone: effects on circadian locomotor rhythm of female golden hamsters. Am. J. Physiol. 1980;239(5):R497–R504. doi: 10.1152/ajpregu.1980.239.5.R497. [DOI] [PubMed] [Google Scholar]
- Ursin R., Bjorvatn B., Holsten F. Sleep duration, subjective sleep need, and sleep habits of 40- to 45-year-olds in the Hordaland Health Study. Sleep. 2005;28(10):1260–1269. doi: 10.1093/sleep/28.10.1260. [DOI] [PubMed] [Google Scholar]
- Vassalli A., Franken P. Hypocretin (orexin) is critical in sustaining theta/gamma-rich waking behaviors that drive sleep need. Proc. Natl. Acad. Sci. U.S.A. 2017;114(27):E5464–E5473. doi: 10.1073/pnas.1700983114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vida B., Hrabovszky E., Kalamatianos T., Coen C.W., Liposits Z., Kalló I. Oestrogen receptor alpha and beta immunoreactive cells in the suprachiasmatic nucleus of mice: distribution, sex differences and regulation by gonadal hormones. J. Neuroendocrinol. 2008;20(11):1270–1277. doi: 10.1111/j.1365-2826.2008.01787.x. [DOI] [PubMed] [Google Scholar]
- Wagner J., Makeig S., Hoopes D., Gola M. Can oscillatory alpha-gamma phase-amplitude coupling be used to understand and enhance TMS effects? Front. Hum. Neurosci. 2019;13:263. doi: 10.3389/fnhum.2019.00263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang C., Guerriero L.E., Huffman D.M., Ajwad A.A., Brooks T.C., Sunderam S., Seifert A.W., O'Hara B.F. A comparative study of sleep and diurnal patterns in house mouse (Mus musculus) and Spiny mouse (Acomys cahirinus) Sci. Rep. 2020;10(1):10944. doi: 10.1038/s41598-020-67859-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wisor J.P., Edgar D.M., Yesavage J., Ryan H.S., McCormick C.M., Lapustea N., Murphy G.M., Jr. Sleep and circadian abnormalities in a transgenic mouse model of Alzheimer's disease: a role for cholinergic transmission. Neuroscience. 2005;131(2):375–385. doi: 10.1016/j.neuroscience.2004.11.018. [DOI] [PubMed] [Google Scholar]
- Wollnik F., Turek F.W. Estrous correlated modulations of circadian and ultradian wheel-running activity rhythms in LEW/Ztm rats. Physiol. Behav. 1988;43(3):389–396. doi: 10.1016/0031-9384(88)90204-1. [DOI] [PubMed] [Google Scholar]
- Wong H., Hooper A.W.M., Niibori Y., Lee S.J., Hategan L.A., Zhang L., Karumuthil-Melethil S., Till S.M., Kind P.C., Danos O., Bruder J.T., Hampson D.R. Sexually dimorphic patterns in electroencephalography power spectrum and autism-related behaviors in a rat model of fragile X syndrome. Neurobiol. Dis. 2020;146:105118. doi: 10.1016/j.nbd.2020.105118. [DOI] [PubMed] [Google Scholar]
- Xu Y., Yang Z., Yuan H., Li Z., Li Y., Liu Q., Chen J. PCDH10 inhibits cell proliferation of multiple myeloma via the negative regulation of the Wnt/β-catenin/BCL-9 signaling pathway. Oncol. Rep. 2015;34(2):747–754. doi: 10.3892/or.2015.4056. [DOI] [PubMed] [Google Scholar]
- Yamaoka S. Modification of circadian sleep rhythms by gonadal steroids and the neural mechanisms involved. Brain Res. 1980;185(2):385–398. doi: 10.1016/0006-8993(80)91076-8. [DOI] [PubMed] [Google Scholar]
- Zucker I., Fitzgerald K.M., Morin L.P. Sex differentiation of the circadian system in the golden hamster. Am. J. Physiol. 1980;238(1):R97–R101. doi: 10.1152/ajpregu.1980.238.1.R97. [DOI] [PubMed] [Google Scholar]