Abstract
Background & Aims:
Endoscopic screening for gastric cancer is routine in some countries with high incidence and is associated with reduced gastric cancer-related mortality. Immigrants from countries of high incidence to low incidence of gastric cancer retain their high risk, but no screening recommendations have been made for these groups in the United States. We aimed to determine the cost effectiveness of different endoscopic screening strategies for noncardia gastric cancer, compared with no screening, among Chinese, Filipino, Southeast Asian, Vietnamese, Korean, and Japanese Americans.
Methods:
We generated a decision-analytic Markov model to simulate a cohort of asymptomatic 50-year-old Asian Americans. The cost effectiveness of 2 distinct strategies for endoscopic gastric cancer screening was compared with no screening for each group, stratified by sex. Outcome measures were reported in incremental cost-effectiveness ratios (ICERs), with a willingness to pay threshold of $100,000/quality-adjusted life-year (QALY). Extensive sensitivity analyses were performed.
Results:
Compared with performing no endoscopic gastric cancer screening, performing a 1-time upper endoscopy with biopsies, with continued endoscopic surveillance if gastric intestinal metaplasia was identified, was cost effective, whereas performing ongoing biennial endoscopies, even for patients with normal findings from endoscopy and histopathology, was not. The lowest ICERs were observed for Chinese, Japanese, and Korean Americans (all below $73,748/QALY).
Conclusions:
Endoscopic screening for gastric cancer with ongoing surveillance of gastric preneoplasia is cost-effective for Asian Americans ages 50 years or older in the United States. The lowest ICERs are for Chinese, Japanese, and Korean Americans (all below $73,748/QALY).
Keywords: Helicobacter pylori, early detection, public health, healthcare disparity, cost–benefit analysis
INTRODUCTION
Gastric cancer ranks as the 5th most common cancer and the 3rd leading cause of cancer-related mortality globally.1 The majority of the global burden is concentrated in Asian-Pacific, Latin American, and Eastern European countries, with over 50% of all new gastric cancer cases occurring in Asian-Pacific countries alone.2 Chronic Helicobacter pylori (H. pylori) gastritis is the strongest known risk factor for intestinal-type noncardia gastric adenocarcinoma (NCGA), the most common form of gastric cancer. NCGA develops as a result of ongoing chronic inflammation that, in a small percentage of people, progresses to neoplasia. Gastric intestinal metaplasia (GIM) is a preneoplastic mucosal stage that is associated with a baseline 0.16% annual increased risk of NCGA; as such, endoscopic surveillance among certain individuals with GIM may allow for earlier cancer detection and improved outcomes.3,4
In 2018, nearly 30% of the US population was comprised of foreign-born (first-generation) immigrants and their US-born (second-generation) offspring, most of whom originate from countries of high gastric cancer incidence and thus might plausibly benefit from targeted gastric cancer prevention and early detection interventions.5 In 2016, immigrants from the Asia-Pacific region formed the largest group and accounted for 27% of all foreign-born immigrants.5 Based on a recent meta-analysis, immigrants from high- to low-incidence countries retain their elevated risk of gastric cancer.6 While the US is overall considered a low-to-intermediate incidence country for gastric cancer, there are clear racial/ethnic differences in disease burden. Compared to the majority non-Hispanic white (NHW) US population, Hispanics and non-Hispanic blacks have approximately two-fold higher gastric cancer incidence rates7,8, while some Asian American (AA) ethnic groups have up to 6.6-fold higher rates.9-12 Indeed, rates of gastric cancer in some AA groups exceed even the rates of colorectal cancer (CRC), a cancer which is screened for on a population basis. Even more disconcerting, gastric cancer is among the top 5 causes of cancer incidence and mortality in several AA groups, including Japanese, Chinese, Korean, and Vietnamese, and accounts for up to 15% of their cancer deaths, compared to <2% of cancer deaths among NHWs.13 While population-based screening for gastric cancer does not occur in the US and is not cost-effective, our group recently demonstrated that among otherwise asymptomatic 50-year old AAs (as well as Hispanics and non-Hispanic blacks), performing a one-time esophagogastroduodenoscopy (EGD) for gastric cancer screening at the time of colonoscopy for CRC screening with subsequent EGDs only if indicated (e.g. surveillance of GIM), is cost-effective.14 Ongoing biennial EGD when the index screening EGD and histopathology were normal was not cost-effective for AAs as an aggregated group, although this is one of the accepted gastric cancer screening strategies in Japan and South Korea. As an aggregate, AAs represent over 30 different countries of origin with richly diverse cultural practices, dietary preferences, health behaviors, and lifestyles. Differences in these non-genetic factors, genetic factors, and their complex interaction might account, at least in part, for the observed differences in disease risk among AAs. Indeed, because gastric cancer incidence and mortality may vary several magnitudes between AA ethnicities9,13,15, and also between males and females, it is plausible that the cost effectiveness of certain screening strategies may also vary such that alternative screening approaches (or even no screening) might be more appropriate for certain groups.
We hypothesized that analyzing the cost effectiveness of gastric cancer screening strategies according to disaggregated AA ethnicities and separately for males and females might unmask important differences among the groups, which would be fundamental for informing gastric cancer prevention and early detection efforts. We constructed a Markov decision model to compare the cost effectiveness of two endoscopic strategies for gastric cancer screening with no screening (i.e. the current standard of care in the US), among a simulation cohort of 50-year old asymptomatic AA males and females overall and by disaggregated ethnic group. We focused on the six largest AA ethnicities in the US: Chinese, Filipino, Southeast Asian, Vietnamese, Korean, and Japanese Americans.
METHODS
Markov model
We constructed a transition state Markov decision model (TreeAge Pro software version 2017 (R 1.2)) that was similar to our previously published model analyzing the cost effectiveness of gastric cancer screening according to race/ethnicity, with the exception that we limited the current analysis to AAs only; our initial model was originally adapted from a model by Yeh et al, as we have previously described.14,16,17 We used the same method of validation to validate the current Markov model with one exception. Instead of using aggregated data inputs for AAs, we used disaggregated data inputs by ethnicity and sex wherever possible. Data inputs for the model were based on a systematic search of the published literature (see Supplemental Material for full strategy). First, we generated NCGA incidence rates for each of the six ethnicities based on our constructed Markov model and data inputs identified from the literature. We then compared these model outputs to recently published SEER data for disaggregated AAs (2001-2014)12, as well as a separate independent analysis of the California Cancer Registry (unpublished data, 2011-2015). Our model outputs were within the ranges of these population-based cancer registries suggesting that our model accurately represents the transition to NCGA for each of the AA ethnicities.
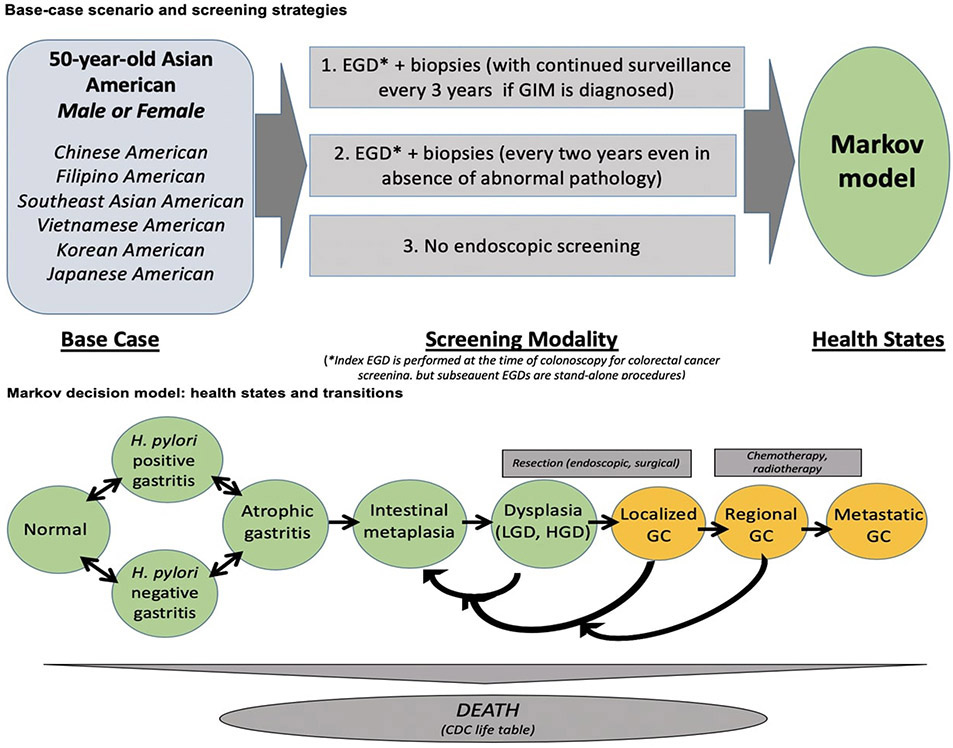
The model itself simulates a base case scenario of NCGA screening at age 50 years old for Chinese, Filipino, Southeast Asian, Vietnamese, Korean, and Japanese Americans. This model compared the incremental cost-effectiveness of NCGA screening for one of three “strategies”: 1) one-time upper endoscopy (EGD) with biopsies bundled at the time of colonoscopy for CRC screening with subsequent EGDs every three years for surveillance purposes only if GIM is identified, or appropriate management if higher-grade pathology is identified (hereafter referred to as: “one-time bundled EGD unless abnormal index exam”); 2) EGD with biopsies at the time of colonoscopy for CRC screening, with ongoing biennial screening EGDs even if the index examination is endoscopically and histologically normal (hereafter referred to as: “biennial EGD”); and 3) no endoscopic screening.
All individuals entered into the Markov model in one of the following initial “health states” based on the population probability for that health state for each of the disaggregated AA ethnic groups: normal gastric mucosa, gastritis (with or without H. pylori), atrophic gastritis, GIM, dysplasia, local (resectable) asymptomatic NCGA, regional asymptomatic NCGA, metastatic asymptomatic NCGA. As described below, a “screening” exam assumes that individuals are clinically asymptomatic. The population probabilities of the initial health state according to AA ethnicity for males and females were based on the available published literature. Individuals could then transition between health states, including the previously stated health states as well as local (resectable) symptomatic NCGA, regional symptomatic NCGA, metastatic NCGA, and death (Figure 1). Transition between health states depended on the assigned probabilities of an event, with probabilities disaggregated by ethnic group and sex as available based on the literature.
Figure 1. Markov model simulating gastric cancer screening for Asian American ethnic groups.
Adapted from Saumoy et al. Gastroenterology 2018; 155: 648–60.
In the first strategy, if GIM or early-stage neoplasia is identified during the index screening procedure at age 50 years, then individuals would enter into a structured surveillance program with subsequent surveillance EGDs and appropriate management as dictated by the diagnosed pathology (Figure 1). For example, if GIM is histologically confirmed, individuals would continue with surveillance every 3 years with biopsies of the antrum/incisura and body, so-called “mapping biopsies”.3,14,18 Individuals with histologically confirmed dysplasia or localized NCGA would undergo endoscopic submucosal dissection (ESD) or surgical resection (see below), followed by surveillance EGDs every 6 months for one year and then annually for three years. At this point, if there was no neoplastic recurrence, these individuals would have their surveillance interval extended to every 3 years, similar to individuals with GIM only.
In the second screening strategy, all individuals would still have a screening EGD with biopsies bundled with colonoscopy for CRC screening at age 50 years as in the first strategy, but these individuals would continue with biennial EGDs with biopsies even if the index screening examination and histopathology were normal. The third “screening strategy” was not performing an EGD for gastric cancer screening at age 50 years, which is the current standard of care in the US. (Figure 1)
Assumptions
Additional assumptions included that all individuals were otherwise healthy, asymptomatic, and appropriate candidates for endoscopy, since they were already deemed appropriate to undergo routine age-appropriate CRC screening with a colonoscopy. The model simulated that the initial screening EGD with biopsies would be bundled with, and performed at the same time as, an already scheduled screening colonoscopy without the need for additional facility utilization. All screening EGDs were assumed to also include biopsies from the antrum/incisura and body, along with the associated costs, in order to confirm the histologic presence or absence of GIM.18 Because of the sometimes-subtle endoscopic appearance of neoplastic lesions, we accounted for the possibility of missing neoplastic lesions on endoscopic examination, as well as conducted sensitivity analyses to determine the effect on the model when this probability is varied.
All individuals who were diagnosed with dysplasia were assumed to be eligible for ESD and would undergo ESD with subsequent surveillance intervals described above. Individuals who were diagnosed with local NCGA were assumed to be eligible for ESD or partial gastrectomy and would have the lesion resected by one of these methods. In the model, we accounted for the still relatively limited availability and expertise of ESD in the US by estimating that 20% of eligible lesions would be resected by ESD and the remaining 80% by surgery (gastrectomy). Because ESD has not yet undergone economic evaluation, there is no assigned CPT code and cost data are not publicly available. Therefore, based on expert opinion, we assigned a cost for ESD by using the CPT code for endoscopic mucosal resection (EMR) and adding an additional 50% to the cost of EMR in order to cover the presumed increase in procedural and anesthesia utilization time for ESD.14
No additional assumptions could be made based on immigrant-level details including generation of immigration, duration of US residence, or cultural practices, given that such granular data inputs for modeling purposes are not available in the literature.
Model Parameters
Transition probabilities, costs, and quality adjusted life years (QALY) were derived from published literature and available public data sources (Table 1; Supplemental Tables 1 and 2). The age-specific probability of all-cause mortality was estimated from the 2012 Center for Disease Control and Prevention US Life Tables.19 The Life Tables do not provide all-cause mortality separately for each AA ethnicity; for modeling purposes, we therefore assumed that there was no difference in background mortality between the ethnic groups. QALYs were used for utilities to describe the health-related quality of life for each state, ranging from 0 (death) to 1 (perfect health). Utility scores were taken from the literature for chronic medical health states (Supplemental Table 2). Utilities that were unavailable in the published literature were decided based on consensus. Direct procedural cost estimates were based on the Center for Medicare and Medicaid Services national average costs in US dollars, which are publicly available for 2015 (Supplemental Table 2).20 Costs and utilities were discounted at a rate of 3% per year.21 The base-case point estimates of cost were varied by ±50% for the sensitivity analysis.
Table 1.
Model input parameters for Asian American males: baseline probabilities
| Baseline probabilities according to ethnicity |
Base case | Sensitivity analysis range |
Monte Carlo distribution |
References |
|---|---|---|---|---|
| Japanese American | ||||
| ➢ HP gastritis | 0.27 | 0.1-0.6 | Beta | 30-34 |
| ➢ Non-HP gastritis | 0.03 | 0.03-0.12 | Beta | 35 |
| ➢ Atrophic gastritis | 0.24 | 0.3-0.87 | Beta | 34,36-38 |
| ➢ GIM | 0.36 | 0.18-0.58 | Beta | 41-43 |
| ➢ Dysplasia | 0.06 | 0.05-0.09 | Beta | 9,11,12,15,44 |
| ➢ Local NCGA | 0.00006 | 0.0001-0.00044 | Beta | 9,11,12,15,44 |
| ➢ Regional NCGA | 0.00012 | 0.00001-0.00011 | Beta | 9,11,12,15,44 |
| ➢ Distant NCGA | 0.000053 | 0.00001-0.00015 | Beta | 9,11,12,15,44 |
| Chinese American | ||||
| ➢ HP gastritis | 0.24 | 0.2-0.25 | Beta | 24,34 |
| ➢ Non-HP gastritis | 0.05 | 0.01-0.074 | Beta | 34,42,45 |
| ➢ Atrophic gastritis | 0.25 | 0.1-0.25 | Beta | 34,36,42,45 |
| ➢ GIM | 0.35 | 0.14-0.35 | Beta | 34,38,39,46 |
| ➢ Dysplasia | 0.06 | 0.05-0.065 | Beta | 41-43 |
| ➢ Local NCGA | 0.00004 | 0.00001-0.00044 | Beta | 9,11,12,15 |
| ➢ Regional NCGA | 0.000085 | 0.00001-0.00011 | Beta | 9,11,12,15 |
| ➢ Distant NCGA | 0.000036 | 0.00001-0.00015 | Beta | 9,11,12,15 |
| Korean American | ||||
| ➢ HP gastritis | 0.36 | 0.19-0.65 | Beta | 34,47-49 |
| ➢ Non-HP gastritis | 0.01 | 0.005-0.1 | Beta | 34,42,45 |
| ➢ Atrophic gastritis | 0.10 | 0.05-0.25 | Beta | 34,48 |
| ➢ GIM | 0.40 | 0.21-0.6 | Beta | 34,39,48,49 |
| ➢ Dysplasia | 0.08 | 0.05-0.09 | Beta | 41-43 |
| ➢ Local NCGA | 0.00013 | 0.0001-0.00044 | Beta | 9,11,12,15,44 |
| ➢ Regional NCGA | 0.00027 | 0.00001-0.00011 | Beta | 9,11,12,15,44 |
| ➢ Distant NCGA | 0.00011 | 0.00001-0.00015 | Beta | 9,11,12,15,44 |
| Vietnamese American | ||||
| ➢ HP gastritis | 0.34 | 0.26-0.7 | Beta | 24,34,50 |
| ➢ Non-HP gastritis | 0.03 | 0.01-0.25 | Beta | 34,42,45 |
| ➢ Atrophic gastritis | 0.24 | 0.08-0.85 | Beta | 34,50,51 |
| ➢ GIM | 0.28 | 0.2-0.4 | Beta | 34,39,50,51 |
| ➢ Dysplasia | 0.06 | 0.05-0.09 | Beta | 41-43 |
| ➢ Local NCGA | 0.000053 | 0.0001-0.000442 | Beta | 9,11,12,15 |
| ➢ Regional NCGA | 0.00011 | 0.00001-0.00011 | Beta | 9,11,12,15 |
| ➢ Distant NCGA | 0.000046 | 0.00001-0.00015 | Beta | 9,11,12,15 |
| Filipino American | ||||
| ➢ HP gastritis | 0.40 | 0.2-0.6 | Beta | 52 |
| ➢ Non-HP gastritis | 0.10 | 0.07-0.25 | Beta | 34,42,45 |
| ➢ Atrophic gastritis | 0.20 | 0.15-0.65 | Beta | 34,50,51 |
| ➢ GIM | 0.13 | 0.12-0.33 | Beta | 39 |
| ➢ Dysplasia | 0.06 | 0.05-0.09 | Beta | 41-43 |
| ➢ Local NCGA | 0.000018 | 0.00001-0.00005 | Beta | 9,11,12,15,44 |
| ➢ Regional NCGA | 0.000037 | 0.00001-0.00005 | Beta | 9,11,12,15,44 |
| ➢ Distant NCGA | 0.000015 | 0.00001-0.00005 | Beta | 9,11,12,15,44 |
| Southeast Asian American | ||||
| ➢ HP gastritis | 0.19 | 0.10-0.30 | Beta | 24,34,53-55 |
| ➢ Non-HP gastritis | 0.10 | 0.07-0.25 | Beta | 54-56 |
| ➢ Atrophic gastritis | 0.35 | 0.02-0.65 | Beta | 34 |
| ➢ GIM | 0.14 | 0.09-0.33 | Beta | 34,39 |
| ➢ Dysplasia | 0.06 | 0.05-0.09 | Beta | 41-43 |
| ➢ Local NCGA | 0.000019 | 0.0001-0.000442 | Beta | 9,11,12,15 |
| ➢ Regional NCGA | 0.000041 | 0.00001-0.00011 | Beta | 9,11,12,15 |
| ➢ Distant NCGA | 0.000017 | 0.00001-0.00015 | Beta | 9,11,12,15 |
| Overall Asian American, male | ||||
| ➢ HP gastritis | 0.22 | 0.10-0.26 | Beta | 14,34 |
| ➢ Non-HP gastritis | 0.12 | 0.07-0.25 | Beta | 14,34 |
| ➢ Atrophic gastritis | 0.29 | 0.07-0.65 | Beta | 14,34 |
| ➢ GIM | 0.25 | 0.10-0.40 | Beta | 14,34,39 |
| ➢ Dysplasia | 0.07 | 0.05-0.09 | Beta | 14,41-43 |
| ➢ Local NCGA | 0.00018 | 0.0001-0.000442 | Beta | 9,11,14,15 |
| ➢ Regional NCGA | 0.00005 | 0.00001-0.00011 | Beta | 9,11,14,15 |
| ➢ Distant NCGA | 0.00003 | 0.00001-0.00015 | Beta | 9,11,14,15 |
Abbreviations: HP = Helicobacter pylori; GIM = intestinal metaplasia; NCGA = noncardia gastric adenocarcinoma
Note: this is a consolidated list of the inputs used and represents the most pertinent transitions. Transition and baseline probabilities were altered according to Asian American ethnicity and sex whenever possible based on available data. While the literature differentiates the incidence of malignant states by sex, for some of the premalignant states (HP gastritis, non-HP gastritis, atrophic gastritis, GIM, dysplasia), there were no or limited data available differentiating incidence/prevalence by sex; in these instances, the same baseline probabilities were used for males and females along with appropriate sensitivity analyses (see Supplemental Material).
Note: for the purpose of the analysis, the base case probabilities were altered when the sum total of all probabilities exceeded “1” (100%), for example, in patients with HP gastritis and atrophic gastritis. Thus, each lesion was considered to be mutually exclusive with the more severe lesion assigned the higher probability in circumstances of overlap.
Analysis
A cohort of base-case 50-year-old AA males and females was simulated over a 30-year period, with each cycle lasting one year. The cost effectiveness of each of the three strategies was reported from a health care perspective, and reported separately for males and females according to AA ethnicity. We conducted two separate incremental analyses: 1) the main analysis analyzing all three strategies including the two endoscopic screening strategies and the no endoscopic screening strategy (reference arm), and 2) a secondary analysis comparing only the biennial endoscopy strategy to no endoscopic screening (reference arm). We planned a priori to conduct the latter analysis because of the possibility that biennial endoscopy could be “dominated” in the primary analysis, meaning that the strategy is less effective (caused more harm) and costlier. We were interested in analyzing biennial endoscopy specifically since this screening strategy is practiced in some Asian countries (e.g. Japan, South Korea), but it has not been specifically analyzed among AAs. Outcome measures were reported in incremental cost-effectiveness ratios (ICERs), with a willingness-to-pay (WTP) threshold of $100,000/QALY.22,23
One-way sensitivity analyses were performed using a Monte Carlo simulation to evaluate the effect of all defined variables according to each of ethnic groups. For the Monte Carlo probabilistic sensitivity analysis, 10,000 iterations were performed using gamma distributions for cost and beta distributions for transition probabilities and utilities.
RESULTS
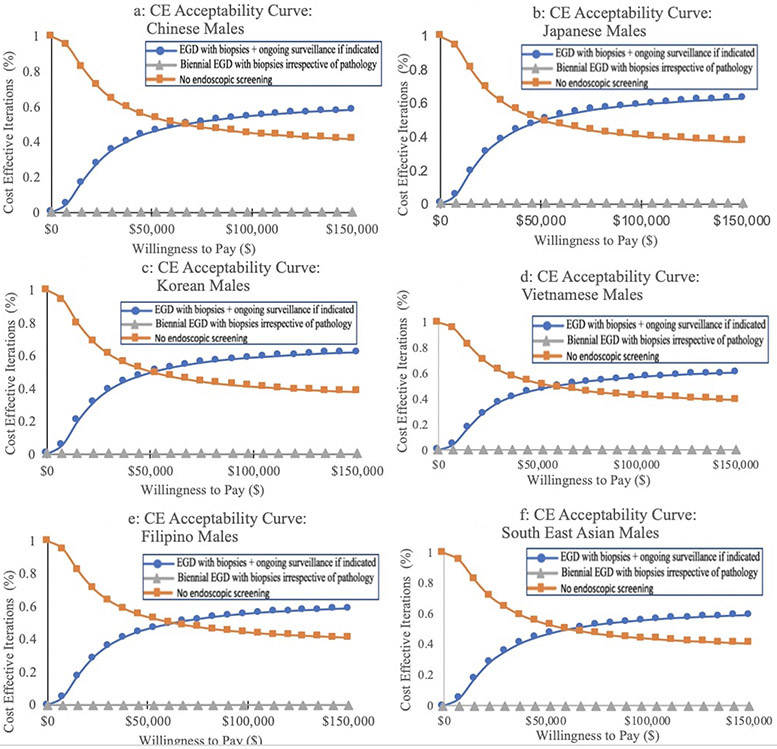
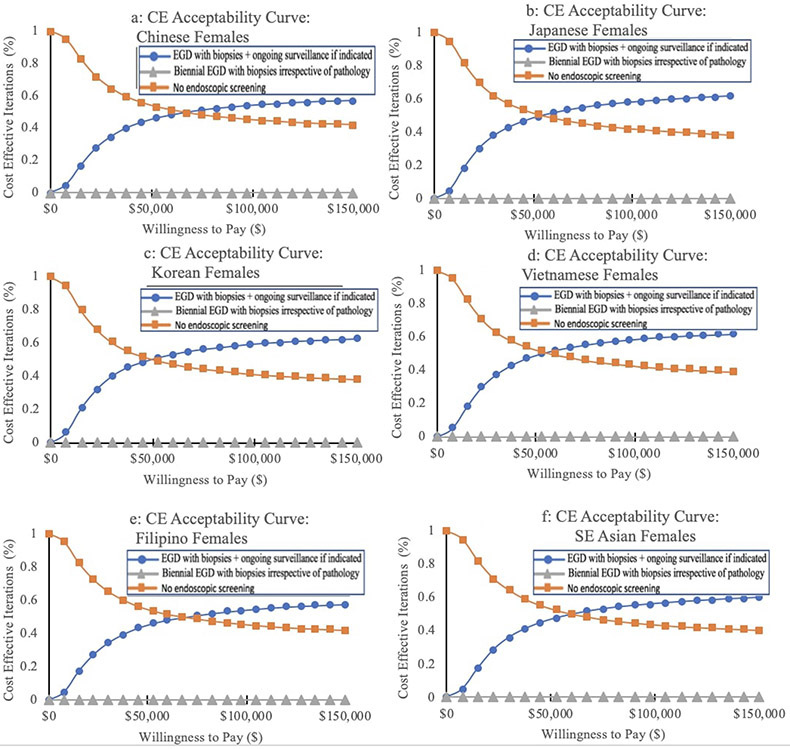
In the standard base case scenario of a healthy, asymptomatic 50-year old AA person, the “one-time bundled EGD unless abnormal index exam” strategy was the most cost-effective strategy for all AA ethnic groups, irrespective of sex, with an ICER of $75,959/QALY and $74,329/QALY for aggregated AA males and females, respectively. For both males and females, the lowest ICERs were among Chinese Americans (males and females: $68,256/QALY), Japanese Americans (males: $69,011/QALY, females: $73,748/QALY), and Korean Americans (males: $70,739/QALY, females: $70,236/QALY). Filipino American males and females had the highest ICERs, but these were still cost-effective at the predetermined WTP threshold (Table 2). The cost-effectiveness acceptability curves illustrate these same findings (Figures 2, 3).
Table 2.
Detailed analysis of the incremental cost-effectiveness ratio (ICER) of endoscopic screening strategies for gastric cancer compared to no endoscopic screening for Asian Americans (males, females)
| Ethnic Group (Males) |
Screening Strategy* | Cumulative Cost ($USD) |
Incremental cost ($USD) |
Effectiveness (QALYs) |
Incremental effectiveness (QALYs) |
ICER** ($USD/QALY) |
|---|---|---|---|---|---|---|
| Japanese American | • No screening | 4,050 | — | 27.48 | — | — |
| • One-time EGD +/− surveillance | 7,425 | 3,374 | 27.53 | 0.05 | 69,012 | |
| • Biennial EGD | 26,355 | 18,931 | 27.37 | −0.16 | Abs Dominated^ | |
| Chinese American | • No screening | 4,023 | — | 27.49 | — | — |
| • One-time EGD +/− surveillance | 7,362 | 3,339 | 27.53 | 0.05 | 68,738 | |
| • Biennial EGD | 26,336 | 18,974 | 27.37 | −0.16 | Abs Dominated^ | |
| Korean American | • No screening | 4,778 | — | 27.38 | — | — |
| • One-time EGD +/− Surveillance | 8,807 | 4,028 | 27.43 | 0.06 | 70,740 | |
| • Biennial EGD | 27,136 | 18,330 | 27.28 | −0.15 | Abs Dominated^ | |
| Vietnamese American | • No screening | 3,914 | — | 27.51 | — | — |
| • One-time EGD +/− surveillance | 7,082 | 3,168 | 27.55 | 0.04 | 74,146 | |
| • Biennial EGD | 26,075 | 18,994 | 27.39 | −0.16 | Abs Dominated^ | |
| Filipino American | • No screening | 3,642 | — | 27.60 | — | — |
| • One-time EGD +/− surveillance | 6,395 | 2,753 | 27.63 | 0.03 | 88,190 | |
| • Biennial EGD | 25,589 | 19,193 | 27.46 | −0.17 | Abs Dominated^ | |
| Southeast Asian American | • No screening | 3,671 | — | 27.62 | — | — |
| • One-time EGD +/− surveillance | 6,446 | 2,775 | 27.65 | 0.03 | 83,850 | |
| • Biennial EGD | 25,822 | 19,376 | 27.48 | −0.17 | Abs Dominated^ | |
| Asian American, overall | • No screening | 4,188 | — | 27.46 | — | — |
| • One-time EGD +/− surveillance | 7,529 | 3,341 | 27.51 | 0.04 | 75,959 | |
| • Biennial EGD | 26,461 | 18,931 | 27.35 | −0.16 | Abs Dominated^ | |
| Ethnic Group (Females) |
Screening Strategy* | Cumulative Cost ($USD) |
Incremental cost ($USD) |
Effectiveness (QALYs) |
Incremental effectiveness (QALYs) |
ICER** ($USD/QALY) |
| Japanese American | • No screening | 4,113 | — | 27.47 | — | — |
| • One-time EGD +/− surveillance | 7,547 | 3,434 | 27.52 | 0.05 | 73,748 | |
| • Biennial EGD | 26,470 | 18,923 | 27.36 | −0.16 | Abs Dominated^ | |
| Chinese American | • No screening | 4,016 | — | 27.49 | — | — |
| • One-time EGD +/− surveillance | 7,350 | 3,334 | 27.54 | 0.05 | 68,257 | |
| • Biennial EGD | 26,325 | 18,975 | 27.37 | −0.16 | Abs Dominated^ | |
| Korean American | • No screening | 4,812 | — | 27.37 | — | — |
| • One-time EGD +/− Surveillance | 8,827 | 4,015 | 27.43 | 0.06 | 70,236 | |
| • Biennial EGD | 27,145 | 18,318 | 27.28 | −0.15 | Abs Dominated^ | |
| Vietnamese American | • No screening | 3,916 | — | 27.51 | — | — |
| • One-time EGD +/− surveillance | 7,085 | 3,168 | 27.55 | 0.04 | 74,306 | |
| • Biennial EGD | 26,078 | 18,993 | 27.39 | −0.16 | Abs Dominated^ | |
| Filipino American | • No screening | 2,484 | — | 27.74 | — | — |
| • One-time EGD +/− surveillance | 4,348 | 1,864 | 27.76 | 0.02 | 83,732 | |
| • Biennial EGD | 24,318 | 19,970 | 27.58 | −0.18 | Abs Dominated^ | |
| Southeast Asian American | • No screening | 3,665 | — | 27.62 | — | — |
| • One-time EGD +/− surveillance | 6,438 | 2,772 | 27.65 | 0.03 | 83,267 | |
| • Biennial EGD | 25,815 | 19,377 | 27.48 | −0.17 | Abs Dominated^ | |
| Asian American, overall | • No screening | 4,170 | — | 27.47 | — | — |
| • One-time EGD +/− surveillance | 7,509 | 3,338 | 27.51 | 0.04 | 74,329 | |
| • Biennial EGD | 26,444 | 18,936 | 27.35 | −0.16 | Abs Dominated^ |
Abbreviations: $USD = US dollars; QALY = quality adjusted life
In the biennial endoscopy screening strategy, the index EGD was bundled with colonoscopy for colorectal cancer screening at 50 years of age. Subsequent EGDs were performed as stand-alone procedures. Please see text for full descriptions of each strategy.
ICERs might not calculate directly because of rounding.
Absolutely dominated (“Abs Dominated”) describes scenarios in which the strategy is less effective and costlier.
Figure 2. Cost-effectiveness (CE) acceptability curves for gastric cancer screening modalities in Asian American males.
One-time EGD at the time of colonoscopy for colorectal cancer screening with GIM surveillance if indicated (see text) is cost-effective for all six AA ethnicities (a: Chinese American; b: Japanese American; c: Korean American; d: Vietnamese American; e: Filipino American; f: Southeast Asian American). Biennial endoscopy and the no endoscopic screening strategies were not cost-effective for any group.
Figure 3. Cost-effectiveness (CE) acceptability curves for gastric cancer screening modalities in Asian American females.
One-time EGD at the time of colonoscopy for colorectal cancer screening with GIM surveillance if indicated (see text) is cost-effective for all six AA groups (a: Chinese American; b: Japanese American; c: Korean American; d: Vietnamese American; e: Filipino American; f: Southeast Asian American). Biennial endoscopy and the no endoscopic screening strategies were not cost-effective for any group.
Among all ethnic groups and irrespective of sex, the “biennial EGD” screening strategy was dominated—that is, this strategy was less effective (caused more harm) and was costlier compared to the other two strategies (Table 2)—including when compared to the no endoscopic screening arm alone (Supplemental Table 3).
Sensitivity analyses
One-way sensitivity analyses were also conducted separately for both males and females for each of the ethnic groups (Table 3 and Supplemental Material). Among males, all models were sensitive to: 1) yearly transition probabilities between each of the histopathologic stages from GIM stepwise through distal NCGA; 2) the probability that a dysplastic lesion would be eligible for ESD and ESD expertise was available; and 3) the probability of death related to EGD. All models were also sensitive to the cost of EGD, ESD, and surgery (gastrectomy). For each of these parameters, the threshold values below (probabilities) or above (costs) which the “one-time bundled EGD unless abnormal index exam” strategy is no longer cost-effective for NCGA screening varied for each AA ethnic group (Table 3 and Supplemental Material). For example, among Japanese American males, this screening strategy was no longer cost-effective if the yearly transition probability of GIM to dysplasia was below 0.18% per year or if the cost of EGD with biopsies exceeded $2190.
Table 3:
One-way sensitivity thresholds for input parameters for Asian Americans (males, females)
| Ethnicity Males |
Yearly transition probability: GIM to Dysplasia |
Yearly transition probability: Dysplasia to Local NCGA |
Yearly transition probability: Local to Regional NCGA |
Yearly transition probability: Regional to Distant NCGA |
Probability that a dysplastic lesion is eligible for ESD |
Probability of death due to EGD |
Cost EGD ($) |
Cost Surgery ($) |
Cost ESD ($) |
|---|---|---|---|---|---|---|---|---|---|
| Japanese American | 0.0018 | 0.024 | 0.144 | 0.030 | 0.57 | 0.00039 | 2,190 | 74,007 | 18,232 |
| Chinese American | 0.0017 | 0.024 | 0.144 | 0.03 | 0.56 | 0.00040 | 2,228 | 74,588 | 18,423 |
| Korean American | 0.0016 | 0.024 | 0.148 | 0.033 | 0.61 | 0.00039 | 2,046 | 68,250 | 16,650 |
| Vietnamese American | 0.0022 | 0.024 | 0.151 | 0.034 | 0.64 | 0.00032 | 1,929 | 63,374 | 15,063 |
| Filipino American | 0.0039 | 0.026 | 0.168 | 0.044 | 0.79 | 0.00017 | 1,351 | 39,357 | 7,634 |
| Southeast Asian American | 0.0031 | 0.025 | 0.164 | 0.041 | 0.75 | 0.00021 | 1,506 | 45,867 | 9,693 |
| Overall Asian American | 0.0020 | 0.025 | 0.155 | 0.037 | 0.67 | 0.00030 | 1,810 | 58,468 | 13,674 |
| Asian American ethnicity Females |
Yearly transition probability: GIM to Dysplasia |
Yearly transition probability: Dysplasia to Local NCGA |
Yearly transition probability: Local to Regional NCGA |
Yearly transition probability: Regional to Distant NCGA |
Probability that a dysplastic lesion is eligible for ESD |
Probability of death due to EGD |
Cost EGD ($) |
Cost Surgery ($) |
Cost ESD ($) |
| Japanese American | 0.0027 | 0.024 | 0.150 | 0.034 | 0.64 | 0.00033 | 1,956 | 64,300 | 15,194 |
| Chinese American | 0.0016 | 0.024 | 0.143 | 0.030 | 0.56 | 0.00040 | 2,228 | 75,689 | 18,761 |
| Korean American | 0.0015 | 0.024 | 0.148 | 0.032 | 0.60 | 0.00039 | 2,068 | 66,256 | 16,951 |
| Vietnamese-American | 0.0023 | 0.024 | 0.152 | 0.035 | 0.64 | 0.00031 | 1,922 | 63,054 | 14,963 |
| Filipino American | 0.0038 | 0.026 | 0.168 | 0.043 | 0.79 | 0.00018 | 1,363 | 39,901 | 7,810 |
| Southeast Asian American | 0.0029 | 0.025 | 0.163 | 0.041 | 0.74 | 0.00021 | 1,528 | 46,815 | 9,996 |
| Overall Asian American | 0.0016 | 0.024 | 0.153 | 0.035 | 0.65 | 0.00032 | 1,886 | 61,775 | 14,688 |
Note: On one-way sensitivity analysis, the tested parameter is varied while keeping all other inputs stable. These values represent the transition thresholds whereby the one-time EGD at the time of colonoscopy strategy (with ongoing surveillance if indicated) is no longer cost effective compared to the no endoscopic screening strategy.
DISCUSSION
AAs comprise the largest foreign-born population residing in the US, with this population expected to double by 2060.5 Collectively, AAs experience the highest rates of NCGA of all US racial/ethnic groups. It was recently demonstrated that performing a one-time EGD for gastric cancer screening (with subsequent EGDs only if indicated) bundled with colonoscopy for CRC screening at age 50 years old is cost-effective for AAs as an aggregated group.14 We extended this to now demonstrate that this strategy is cost-effective for each of the six most populous AA ethnicities in the US irrespective of sex. Moreover, performing biennial EGD in the absence of abnormal histopathology such as GIM—which is one of the accepted gastric cancer screening strategies in South Korea and Japan—not only incurred more cost than the other strategies but the potential harm exceeded the potential benefit, even when compared to the no screening arm only. Although our study focused on AA ethnicities, these findings can be reasonably extended to other US populations who experience higher rates of NCGA compared to the general NHW population. In fact, while five of the top ten origin countries of immigrants to the US are represented in our data (China, South Korea, Vietnam, Philippines, India), the remainder are Central and Latin American countries with similarly high gastric cancer incidence.1
In the US, initiation of targeted screening for at-risk groups according to predefined criteria with subsequent endoscopic intervals based on the findings at the index examination is a model used for other luminal GI tract cancers, specifically esophageal and colorectal adenocarcinoma. Here, we demonstrated that subsequent EGDs after the one-time bundled EGD are not cost-effective if the index screening EGD with gastric mucosal biopsies does not demonstrate at least GIM or higher-grade pathology. This is with the caveat that additional risk determinants such as family history, tobacco use, or persistent H. pylori infection, are not considered in the model since data regarding differential NCGA risk among AA ethnicities based on these factors are not available. Subsequent endoscopies for the purpose of early cancer detection was only cost-effective if GIM or higher-grade pathology was detected and if the rate of stepwise progression was above the thresholds we identified from the sensitivity analyses (Table 3); we previously demonstrated similar findings among Hispanic and black non-Hispanic Americans.14
Immigrants from high to low gastric cancer incidence countries retain an elevated risk of gastric cancer and related mortality.6 This elevated risk reflects the complex interaction between host genetic predisposition, environmental and cultural determinants, as well as H. pylori and non-H. pylori microbial determinants.6 Age at immigration, generation of immigration, duration of H. pylori infection (if applicable), level of acculturation, and other exposures or behaviors based on residence within ethnic enclaves might also importantly modulate gastric cancer risk and outcomes. The relative contribution of these constituents is not well-defined, however. For example, while the direct etiopathological role of chronic H. pylori infection in intestinal-type NCGA carcinogenesis is established, it is incompletely understood why populations in some Asian countries, such as India and Pakistan, have high H. pylori prevalence yet lower gastric cancer incidence and related mortality compared to populations from other Asian countries such as Japan and South Korea, which have lower H. pylori prevalence—an observation commonly referred to as the “Asian enigma”.24 This difference in risk among the native origin countries is reflected in AA ethnic groups, with differences in magnitude as high as 6.6-fold between some groups, such as between Korean American and Filipino American males, at least based on the most contemporary published disaggregated AA data (age-adjusted incidence rate, 2001-2014: 61.1 versus 9.2 per 100,000 males, respectively).9,11,12,15 Despite these differences, though, we demonstrated that the one-time bundled EGD unless abnormal index exam screening/surveillance strategy is cost-effective for each of the major AA ethnicities. The differences in cost-effectiveness according to AA ethnicity also relate to differences in cancer stage at diagnosis and, more specifically, the probability that curable stage disease is diagnosed. One recent population-based analysis of the SEER cancer registry demonstrated that among AAs, Korean Americans have the highest proportion of NCGA diagnosed in the localized stage.12 The higher probability of diagnosing resectable stage NCGA with the opportunity for potentially curative resection is a major driver of the cost effectiveness of endoscopic screening. The reasons for the observed differences in NCGA stage at diagnosis among AAs are not well-defined, but both biological (e.g. tumor behavior) and non-biological reasons (e.g. providers’ differential threshold for endoscopy based on origin country) are likely implicated.
The success of gastric cancer screening derives from the diagnosis of gastric neoplasia prior to submucosal invasion, which is typically asymptomatic, and when resection is usually curative. In marked contrast, diagnosis in the advanced stage when symptoms typically present is associated with <10% 5-year survival. In the US, less than 15% of all gastric cancers are diagnosed in the early-stage, which is reflected in the dismal 31% 5-year overall survival.25 With implementation of national screening in Japan and South Korea, nearly 60% of all gastric cancers are now diagnosed in a stage where endoscopic or surgical resection is curative. ESD for early gastric neoplasia is associated with a 96-100% and 97-100% 5-year overall- and disease-free survival respectively, based on large series from East Asia.26 While the duration of experience with ESD for early gastric cancer resection is acceptedly shorter among Western centers with less case volume, current data demonstrate that en bloc and curative resections, as well as complication rates are comparable to Eastern centers.27 Sensitivity analyses modeling the effect of varying availability of ESD and expertise of the endoscopist (i.e. complication rate, recurrence rate), did not significantly impact our overall conclusions. This further suggests that the major benefit of gastric cancer screening is a direct result of earlier identification of gastric neoplasia and reduced likelihood of metastatic disease, since this is the state that most impacts cost and utility.
This study has several strengths. As with any decision model, the robustness of the results depends on the quality of the data inputs. We conducted a systematic review to identify the best available data to inform the values for various health states and their transition probabilities. Most incidence data were derived from population-based cancer registry data from areas that are highly concentrated with AA groups in the US such as California, which represents perhaps the largest and most complete gastric cancer data according to disaggregated ethnic group. Furthermore, we confirmed the accuracy of our model with respect to data inputs and stepwise algorithms by demonstrating that the model output for each AA ethnicity corresponded to observed NCGA incidence rates for each group based on robust data from certified SEER cancer registries. In order to enhance the clinical relevance and interpretability of our data, as well as to account for possible variability and heterogeneity in the model inputs, we also conducted comprehensive sensitivity and probabilistic analyses separately for males and females of each ethnic group and reported each of these threshold values for the model’s cost effectiveness. Our detailed Markov model is practical for US clinicians and accounts for factors such as endoscopic miss rate and the availability of ESD. Our model additionally considers the overall therapeutic management of gastric neoplasia, including continued post-resection endoscopic surveillance over a 30-year time period. Notwithstanding, our study does have some limitations which mostly reflect inherent characteristics of any decision model, such as the need to make certain predefined assumptions. Because our model is specifically tailored for a US healthcare and payor system, generalizability might be limited. In Japan and South Korea, for example, endoscopy for gastric cancer screening, which also includes transnasal endoscopy, is often performed unsedated with remarkable efficiency and also oftentimes with a diagnosis of preneoplasia suspected based on image-enhancing endoscopic techniques but not confirmed with biopsies. These significant procedural cost differences might be one reason why biennial EGD is cost-effective in Japan and South Korea, but was not cost-effective in our model. Ongoing screening or surveillance exams with biopsies incurs significant costs, which are only meaningfully offset if early neoplasia is diagnosed and resected. Our model also assumes that individuals are at a certain baseline risk of gastric cancer based on their age, sex, and ethnic background, but other factors including family history of gastric cancer, smoking history, and immigration-related details such as immigrant generation, among other determinants that might modify the baseline risk are not considered by the model due to insufficient published data to inform model inputs. Our model also did not consider extent of GIM or histologic subtype given the insufficient high quality data from the US and for AAs specifically.3,4,18,28 That said, factors that increase gastric cancer risk would presumably enhance cost-effectiveness. We a priori designed our model with GIM diagnosed on index endoscopy as the decision node for whether or not to continue interval surveillance EGDs for early detection of gastric neoplasia. We fully acknowledge that even in the absence of concomitant GIM, atrophic gastritis and persistent H. pylori gastritis are both (reversible) histological findings associated with increased risk of intestinal-type NCGA; however, we are not able to comment on the cost-effectiveness of ongoing EGDs for early gastric cancer detection in the absence of GIM. We opted for GIM as the decision node because 1) GIM is generally considered to be the earliest irreversible histopathologic stage in the carcinogenic pathway for intestinal-type, although some controversy exists; 2) there is high interobserver agreement for the pathologic diagnosis of GIM; 3) there are more complete evidence profiles to inform data inputs for GIM epidemiology and progression.4,29 Furthermore, we felt that GIM as the decision node would be more clinically relevant and appropriate in light of the recent AGA guidelines focused specifically on GIM management.3 We developed our model based NCGA screening, but it warrants mentioning that other upper gastrointestinal malignancies including esophageal squamous cell cancer and adenocarcinoma, as well as gastroesophageal junction and cardia gastric adenocarcinoma, or precursor lesions such as Barrett’s esophagus, might also be diagnosed in parallel. The ‘off-target’ effect of diagnosing other upper GI (pre)malignant lesions on our model’s conclusions are uncertain. While not a limitation per se, but along the same line of clinical relevance, we did not model non-endoscopic screening strategies for NCGA since endoscopic screening has superior test characteristics and other modalities are either not available in the US (e.g. serum pepsinogen) or might not have appropriate test performance due to insufficient experience in the US (e.g. fluoroscopic imaging). Lastly, cost-effectiveness does not necessarily translate into clinical effectiveness, given that actualization of clinical benefit from a cancer screening program also depends on non-biological factors such as cultural barriers to uptake of screening (particularly if invasive, such as endoscopy), as well as access to the intervention and downstream management.
In summary, there are identifiable populations in the US who are at increased risk for NCGA and who therefore might benefit from endoscopic gastric cancer screening. Unfortunately, even though the US is a resource-replete country capable of supporting gastric cancer prevention and early detection programs, there remains an unmet and overdue need for selected gastric cancer screening among these high-risk populations. One-time endoscopic screening for gastric cancer performed at the time of colonoscopy for average-risk colorectal cancer screening with ongoing endoscopic surveillance only if indicated is a highly cost-effective intervention for Chinese Americans, Vietnamese Americans, Southeast AAs, Korean Americans, Japanese Americans, and possibly other racial/ethnic and immigrant groups who experience higher rates of gastric cancer. The potential health and economic detriment of continued inertia surrounding gastric cancer screening is amplified when considering that the pool of at-risk individuals is only expected to grow, with NHWs now considered the minority population in 35 of the 50 largest cities and projections that NHWs will no longer be the overall majority population by 2065. These findings could therefore have major public health implications.
Supplementary Material
Grant support:
Dr. Shah is funded by the Agency for Healthcare Research (AHRQ) and Quality and Patient-Centered Outcomes Research Institute (PCORI) under Award Number K12 HS026395, a 2019 American Gastroenterological Association Research Scholar Award, and a Veterans Affairs Career Development Award under award number ICX002027A. The content is solely the responsibility of the listed authors and does not necessarily represent the official views of the funding agencies listed.
Footnotes
Disclosures: There are no relevant disclosures
Writing assistance: None
Conflict of interest statement: Each author listed has no potential conflicts (financial, professional, nor personal) that are relevant to the content presented in this manuscript.
References
- 1.Bray F, Ferlay J, Soerjomataram I, Siegel RL, Torre LA, Jemal A Global cancer statistics 2018: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin 2018;68:394–424. [DOI] [PubMed] [Google Scholar]
- 2.Fock KM Review article: the epidemiology and prevention of gastric cancer. Aliment Pharmacol Ther 2014;40:250–60. [DOI] [PubMed] [Google Scholar]
- 3.Gupta S, Li D, El Serag HB, et al. AGA clinical practice guidelines on management of gastric intestinal metaplasia. Gastroenterology 2020;158:693–702. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Gawron AJ, Shah SC, Altayar O, et al. AGA Technical Review on Gastric Intestinal Metaplasia - Natural History and Clinical Outcomes. Gastroenterology 2019; published online December 3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.U.S. immigrants ∣ Pew Research Center. https://www.pewresearch.org/fact-tank/2019/06/17/key-findings-about-u-s-immigrants/ [Google Scholar]
- 6.Pabla BS, Shah SC, Corral JE, Morgan DR Increased Incidence and Mortality of Gastric Cancer in Immigrant Populations from High to Low Regions of Incidence: A Systematic Review and Meta-Analysis. Clin Gastroenterol Hepatol 2019; published online May 30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Miller KD, Goding Sauer A, Ortiz AP, et al. Cancer statistics for hispanics/latinos, 2018. CA Cancer J Clin 2018;68:425–45. [DOI] [PubMed] [Google Scholar]
- 8.Martinez Tyson D, Medina-Ramirez P, Flores AM, Siegel R, Aguado Loi C Unpacking Hispanic Ethnicity-Cancer Mortality Differentials Among Hispanic Subgroups in the United States, 2004-2014. Front Public Health 2018;6:219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.McCracken M, Olsen M, Chen MS, et al. Cancer incidence, mortality, and associated risk factors among Asian Americans of Chinese, Filipino, Vietnamese, Korean, and Japanese ethnicities. CA Cancer J Clin 2007;57:190–205. [DOI] [PubMed] [Google Scholar]
- 10.Kim Y, Park J, Nam B-H, Ki M Stomach cancer incidence rates among Americans, Asian Americans and Native Asians from 1988 to 2011. Epidemiol Health 2015;37 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Gomez SL, Noone AM, Lichtensztajn DY, et al. Cancer incidence trends among Asian American populations in the United States, 1990-2008. J Natl Cancer Inst 2013;105:1096–110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Huang RJ, Sharp N, Talamoa RO, et al. One Size Does Not Fit All: Marked Heterogeneity in Incidence of and Survival from Gastric Cancer among Asian American Subgroups. Cancer Epidemiol Biomarkers Prev 2020; published online March 9. [DOI] [PubMed] [Google Scholar]
- 13.Thompson CA, Gomez SL, Hastings KG, et al. The burden of cancer in asian americans: A report of national mortality trends by asian ethnicity. Cancer Epidemiol Biomarkers Prev 2016;25:1371–82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Saumoy M, Schneider Y, Shen N, Kahaleh M, Sharaiha RZ, Shah SC Cost effectiveness of gastric cancer screening according to race and ethnicity. Gastroenterology 2018;155:648–60. [DOI] [PubMed] [Google Scholar]
- 15.Lui FH, Tuan B, Swenson SL, Wong RJ Ethnic disparities in gastric cancer incidence and survival in the USA: an updated analysis of 1992-2009 SEER data. Dig Dis Sci 2014;59:3027–34. [DOI] [PubMed] [Google Scholar]
- 16.Yeh JM, Kuntz KM, Ezzati M, et al. Development of an empirically calibrated model of gastric cancer in two high-risk countries. Cancer Epidemiol Biomarkers Prev 2008; 17:1179–87. [DOI] [PubMed] [Google Scholar]
- 17.Yeh JM, Hur C, Ward Z, Schrag D, Goldie SJ Gastric adenocarcinoma screening and prevention in the era of new biomarker and endoscopic technologies: a cost-effectiveness analysis. Gut 2016; 65: 563–74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Shah SC, Gawron AJ, Li D Surveillance of gastric intestinal metaplasia. Am J Gastroenterol 2020; published online February 14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Arias E, Heron M, Xu J United states life tables, 2013. Natl Vital Stat Rep 2017;66:1–64. [PubMed] [Google Scholar]
- 20.Physician Fee Schedule Look-Up Tool ∣ CMS. https://www.cms.gov/Medicare/Medicare-Fee-for-Service-Payment/PFSlookup/index [Google Scholar]
- 21.Weinstein MC Recommendations of the Panel on Cost-Effectiveness in Health and Medicine.JAMA 1996;276:1253. [PubMed] [Google Scholar]
- 22.Braithwaite RS, Meltzer DO, King JT, et al. What does the value of modern medicine say about the $50,000 per quality-adjusted life-year decision rule? Med Care 2008;46:349–56. [DOI] [PubMed] [Google Scholar]
- 23.Marseille E, Larson B, Kazi DS, Kahn JG, Rosen S Thresholds for the cost-effectiveness of interventions: alternative approaches. Bull World Health Organ 2015;93:118–24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Hooi JKY, Lai WY, Ng WK, et al. Global Prevalence of Helicobacter pylori Infection: Systematic Review and Meta-Analysis. Gastroenterology 2017;153:420–9. [DOI] [PubMed] [Google Scholar]
- 25.National Cancer Institute. SEER Data. http://seer.cancer.gov [Google Scholar]
- 26.Akintoye E, Obaitan I, Muthusamy A, et al. Endoscopic submucosal dissection of gastric tumors: A systematic review and meta-analysis. World J Gastrointest Endosc 2016;8: 517–32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Tate DJ, Klein A, Sidhu M, et al. Endoscopic submucosal dissection for suspected early gastric cancer: absolute versus expanded criteria in a large Western cohort (with video). Gastrointest Endosc 2019;90:467–479 [DOI] [PubMed] [Google Scholar]
- 28.Shah SC, Gawron A, Mustafa R, Piazuelo MB Histologic subtyping of gastric intestinal metaplasia: overview and considerations for clinical practice. Gastroenterology. 2019; published online December 3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Altayar O, Davitkov P, Shah SC, et al. AGA Technical Review on Gastric Intestinal Metaplasia - Epidemiology and Risk Factors. Gastroenterology 2019; published online December 3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Hirayama Y, Kawai T, Otaki J, Kawakami K, Harada Y Prevalence of Helicobacter pylori infection with healthy subjects in Japan. J Gastroenterol Hepatol 2014;29 Suppl 4 [DOI] [PubMed] [Google Scholar]
- 31.Sugano K, Hiroi S, Yamaoka Y Prevalence of Helicobacter pylori Infection in Asia: Remembrance of Things Past? Gastroenterology 2018;154:257–8. [DOI] [PubMed] [Google Scholar]
- 32.Hiroi S, Sugano K, Tanaka S, Kawakami K Impact of health insurance coverage for Helicobacter pylori gastritis on the trends in eradication therapy in Japan. BMJ Open 2017;7:e015855. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Namekata T, Miki K, Kimmey M, et al. Chronic atrophic gastritis and Helicobacter pylori infection among Japanese Americans in Seattle. Am J Epidemiol 2000;151:820–30. [DOI] [PubMed] [Google Scholar]
- 34.Choi CE, Sonnenberg A, Turner K, Genta RM High prevalence of gastric preneoplastic lesions in east asians and hispanics in the USA. Dig Dis Sci 2015;60:2070–6. [DOI] [PubMed] [Google Scholar]
- 35.Naylor GM, Gotoda T, Dixon M, et al. Why does Japan have a high incidence of gastric cancer? Comparison of gastritis between UK and Japanese patients. Gut 2006;55:1545–52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Weck MN, Brenner H Prevalence of chronic atrophic gastritis in different parts of the world. Cancer Epidemiol Biomarkers Prev 2006; 15:1083–94. [DOI] [PubMed] [Google Scholar]
- 37.Asaka M, Sugiyama T, Nobuta A, Kato M, Takeda H, Graham DY Atrophic gastritis and intestinal metaplasia in Japan: results of a large multicenter study. Helicobacter 2001;6:294–9. [DOI] [PubMed] [Google Scholar]
- 38.Liu Y, Ponsioen CIJ, Xiao S-D, Tytgat GNJ, Ten Kate FJW Geographic pathology of Helicobacter pylori gastritis. Helicobacter 2005;10:107–13. [DOI] [PubMed] [Google Scholar]
- 39.Choi AY, Strate LL, Fix MC, et al. Association of gastric intestinal metaplasia and East Asian ethnicity with the risk of gastric adenocarcinoma in a U.S. population. Gastrointest Endosc 2018;87:1023. [DOI] [PubMed] [Google Scholar]
- 40.Uemura N, Okamoto S, Yamamoto S, et al. Helicobacter pylori infection and the development of gastric cancer. N Engl J Med 2001; 345:784. [DOI] [PubMed] [Google Scholar]
- 41.You WC, Zhang L, Gail MH, et al. Gastric dysplasia and gastric cancer: Helicobacter pylori, serum vitamin C, and other risk factors. J Natl Cancer Inst 2000;92:1607–12. [DOI] [PubMed] [Google Scholar]
- 42.You WC, Li JY, Blot WJ, et al. Evolution of precancerous lesions in a rural Chinese population at high risk of gastric cancer. Int J Cancer 1999;83:615–9. [DOI] [PubMed] [Google Scholar]
- 43.Jiang J-X, Liu Q, Zhao B, et al. Risk factors for intestinal metaplasia in a southeastern Chinese population: an analysis of 28,745 cases. J Cancer Res Clin Oncol 2016;143:409–18. [DOI] [PubMed] [Google Scholar]
- 44.Lee E, Liu L, Zhang J, et al. Stomach Cancer Disparity among Korean Americans by Tumor Characteristics: Comparison with Non-Hispanic Whites, Japanese Americans, South Koreans, and Japanese. Cancer Epidemiol Biomarkers Prev 2017;26:587–96. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.You WC, Blot WJ, Li JY, et al. Precancerous gastric lesions in a population at high risk of stomach cancer. Cancer Res 1993;53:1317–21. [PubMed] [Google Scholar]
- 46.Li WJ, Ang TL, Fock KM Prevalence and distribution of intestinal metaplasia and correlation with demographics in the singaporean chinese population. Clin Gastroenterol Hepatol 2015;13:e92. [Google Scholar]
- 47.Lim SH, Kwon J-W, Kim N, et al. Prevalence and risk factors of Helicobacter pylori infection in Korea: nationwide multicenter study over 13 years. BMC Gastroenterol 2013;13:104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Kim N, Park YS, Cho S-I, et al. Prevalence and risk factors of atrophic gastritis and intestinal metaplasia in a Korean population without significant gastroduodenal disease. Helicobacter 2008;13:245–55. [DOI] [PubMed] [Google Scholar]
- 49.Woo Y, Behrendt CE, Trapp G, et al. Screening endoscopy finds high prevalence of Helicobacter pylori and intestinal metaplasia in Korean American with limited access to health care. J Surg Oncol 2017;116:172–6. [DOI] [PubMed] [Google Scholar]
- 50.Nguyen TL, Uchida T, Tsukamoto Y, et al. Helicobacter pylori infection and gastroduodenal diseases in Vietnam: a cross-sectional, hospital-based study. BMC Gastroenterol 2010;10:114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Quach DT, Le HM, Nguyen TS, Hiyama T The Distribution of Incomplete Gastric Intestinal Metaplasia (GIM) Subtype among Biopsy Sites according to the Updated Sydney System and Its Association with GIM Extension. Gastroenterol Res Pract 2018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Destura RV, Labio ED, Barrett LJ, et al. Laboratory diagnosis and susceptibility profile of Helicobacter pylori infection in the Philippines. Ann Clin Microbiol Antimicrob 2004;3:25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Ang TL, Fock KM, Dhamodaran S, Teo EK, Tan J Racial differences in Helicobacter pylori, serum pepsinogen and gastric cancer incidence in an urban Asian population. J Gastroenterol Hepatol 2005;20:1603–9. [DOI] [PubMed] [Google Scholar]
- 54.Adlekha S, Chadha T, Krishnan P, Sumangala B Prevalence of helicobacter pylori infection among patients undergoing upper gastrointestinal endoscopy in a medical college hospital in kerala, India. Ann Med Health Sci Res 2013;3 559–63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Misra V, Misra SP, Dwivedi M, Singh PA Point prevalence of peptic ulcer and gastric histology in healthy Indians with Helicobacter pylori infection. Am J Gastroenterol 1997;92:1487–91. [PubMed] [Google Scholar]
- 56.Choudhuri G, Mohindra S Epidemiology of Helicobacter pylori in India. Indian J Gastroenterol 2000; 19 Suppl 1 [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.