SUMMARY
Chronic nonmalignant pain, sleep disturbances and sleep disorders are highly prevalent conditions among U.S. military veterans. Evidence summaries highlight the influence of sleep on pain outcomes in the general adult population but not for the military veteran population. This is a significant gap as U.S. military veterans are an exceedingly high-risk population for both chronic pain and sleep disturbances and/or disorders. We aimed to review the influence of sleep disturbances and sleep disorders on pain outcomes among veterans with chronic nonmalignant pain. A systematic scoping review was conducted using PubMed/Medline, EMBASE, Scopus, CINAHL, and PsycINFO. Twenty-six out of 1450 studies from initial search were included in this review resulting in a combined sample size of N = 923,434 participants. Sleep disturbances and sleep disorders were associated with worse pain outcomes among veterans with chronic pain. Treatment-induced sleep improvements ameliorated pain outcomes in veterans with sleep disorders and sleep disturbances. Research is indicated to address an overlooked pain treatment opportunity – that of sleep disturbance and sleep disorder management.
Keywords: Sleep disturbance, Sleep disorder, Sleep, Chronic pain, Veteran
Chronic nonmalignant pain, defined as pain unrelated to cancer that persists or recurs for 3-months or more [1], is a highly prevalent condition in the adult population [2]. Approximately 20% (50 million people) of adults in the United States (U.S.) experienced chronic pain in 2016 [2], with earlier prevalence estimates ranging from 11% to 40% [3]. Chronic pain is associated with restriction of daily life activities [4], poor quality of life [5–7], impaired social relationships [5], low productivity [7], lost wages [8], increased healthcare expenditures [8], opioid use disorder [9], depression and anxiety [7], and a wide range of health consequences [10].
Adults with chronic pain frequently report sleep disturbances such as impaired sleep duration and/or quality [11,12]. Sleep disorders are also common in adults with chronic pain [13–15]. When co-occurring, chronic pain and disturbed sleep and/or sleep disorders lead to substantially more impairment, further impacting overall health and quality of life. The current evidence supports a reciprocal relationship, where pain disrupts sleep, and sleep disturbances and disorders lead to increased pain [16,17], however support for the latter relationship is more robust [17,18]. The mechanisms underlying the sleep and pain association remain unclear. Given the complexity of the sleep and pain interaction, and based on findings from a growing, albeit relatively under-developed scientific literature it is reasonable to hypothesize that several biopsychosocial mediators explain the observed relationship between sleep and pain (Fig. 1).
Fig. 1.
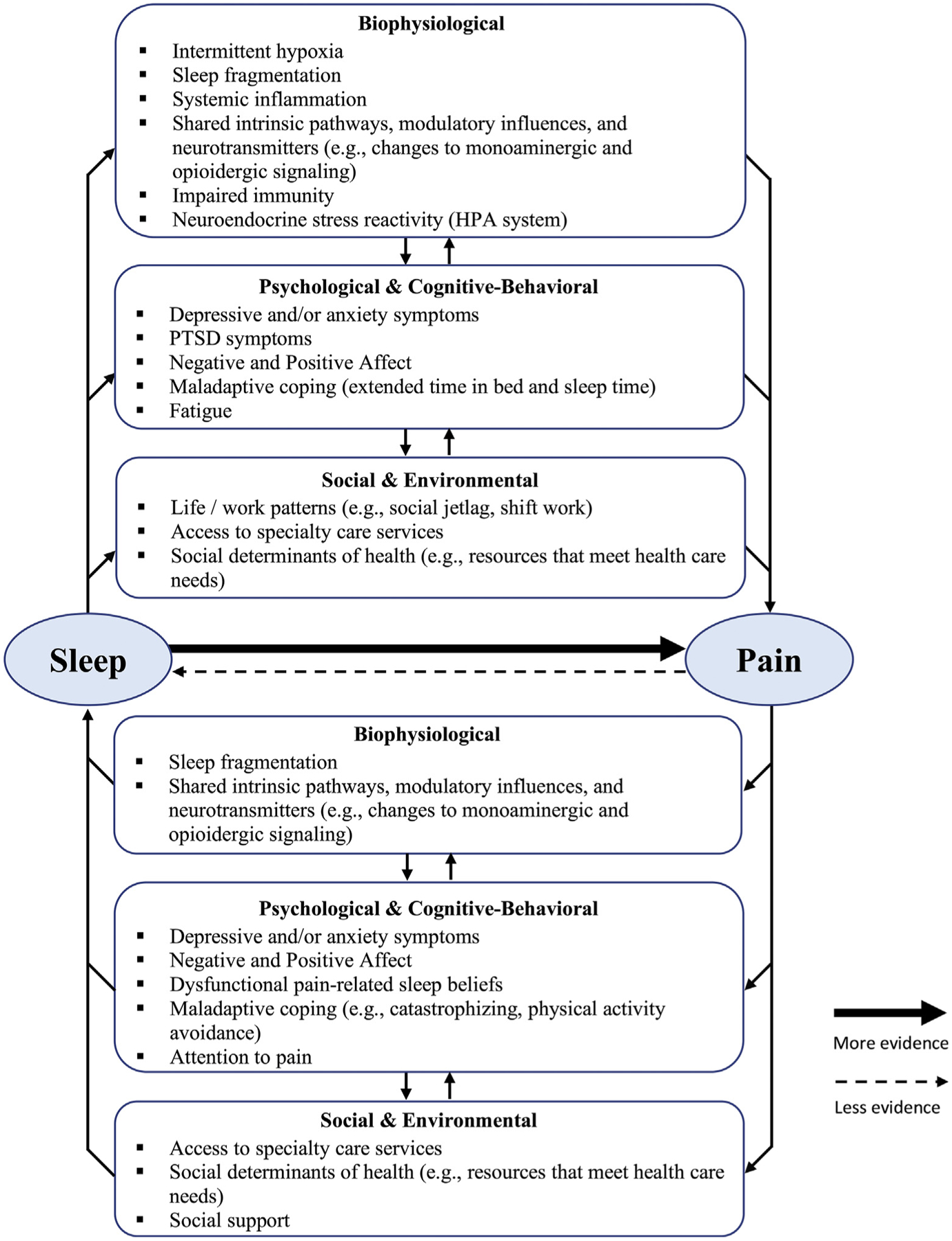
Hypothesized mediational mechanisms model underlying the association between sleep and pain. Multiple mediators may help explain the observed relationship between sleep and pain. Social determinants of health inclusive of many variables may be either/both mediator and/or moderator. HPA = hypothalamic pituitary adrenal. PTSD = post-traumatic stress disorder.
Veterans, defined as adults discharged from previous active service in the U.S. military forces [19], are uniquely vulnerable to both pain and sleep disorders. A comparative study found that veterans experience higher pain prevalence and greater pain severity than the general population; 65.5% of U.S. veterans reported the presence of pain over a prior three month period and 9.1% rated the pain as severe [20]. The etiology of painful conditions is unique among veterans, who are likely to suffer traumatic combat and training-related injuries, which are typified by chronic pain [21,22]. Musculoskeletal back and joint pain have been consistently reported as the most prevalent chronic pain diagnoses in veterans across different conflict cohorts [23–25]. Likewise, sleep disturbances and sleep disorders are common among veterans as deployment-related activities are associated with irregular sleep schedules, long and rotating shifts, unsafe sleep environments, psychological and physical trauma, and combat-related nightmares, all of which can chronically impact sleep [26]. The total prevalence of diagnosed sleep disorders among veterans seeking healthcare at Veteran Administration (VA) showed a six-fold relative increase from 2000 to 2010 (estimated prevalence of 6% in 2010), with higher rates of sleep disorders observed among veterans with comorbid chronic conditions [27].
In the veteran population, the sleep–pain relationship is further complicated by the high prevalence of mental health comorbidities, such as post-traumatic stress disorder (PTSD) [28], which predispose veterans to both sleep disorders [27] and increased pain [28,29]. Mental health comorbidities coupled with the high prevalence of chronic pain escalates veterans’ risk for opioid misuse and abuse [30] that further contribute to sleep disturbances and sleep disorders [31]. It is thereby important to understand the unique relationship between sleep and pain among the veteran population. The complex interaction between pain and associated chronic conditions, such as sleep disorders, is of high priority within the VA research agenda as is evidenced by VA’s dedicated Center of Innovation, PRIME (Pain research, Informatics, Multi-morbidities, and Education) [32].
Although extensive literature has been published on the association between sleep and pain [12,16,17,33], no study to date has summarized the evidence on the influence of disturbed and/or disordered sleep on pain outcomes in veterans. Thus, the primary aim of this systematic scoping review was to answer the question: “What is the influence of sleep disturbances and sleep disorders on chronic pain outcomes among military veterans?” For the purposes of this review, sleep disturbance was defined as a symptom characterized by decreased sleep duration and/or poor sleep quality. Sleep disturbances are not a synonym of sleep disorders, although sleep disturbances may occur as a consequence of a sleep disorder(s). Sleep disorders are pathological changes to one or more dimensions of the complex sleep-wake experience as defined by standard international disease classification systems. Secondary objectives were 1) to investigate how sleep disturbance is defined in the context of chronic pain, and 2) identify gaps in the evidence to guide future research.
This review is limited to studies investigating people with chronic nonmalignant, or noncancer pain (e.g., musculoskeletal and neuropathic disorders) as this cluster of pain conditions is believed to have shared neuropathophysiological mechanisms, which differs from the mechanisms of malignant, or cancer-related pain conditions [34]. Chronic nonmalignant pain is a broad category, and although there is no unanimously agreed upon pain classification system, pain disorders in this category are often further classified based on etiology (e.g., trauma, inflammation), system involved (e.g., musculoskeletal, cutaneous), region affected (e.g., cervical, back), and causal mechanisms (e.g., nociceptive, neuropathic) [35]. This approach for defining and categorizing chronic pain is consistent with clinical diagnostic criteria [36] and was used in this review to permit consideration of a wide range of studies consistent with the purpose of a systematic scoping review.
Methods
Design
A systematic scoping review methodology was employed as this method is ideal for a broad exploration of a topic area. Scoping methods are particularly useful for aggregating evidence that is complex and heterogeneous, and thus not amenable to traditional systematic reviews methods [37] that answer narrowly-circumscribed questions with a priori delimited intervention(s), comparator(s), and often a single, specific outcome(s) for which summary estimation is supported [38]. A scoping methodology may be used for surveying the state of the evidence to determine if a traditional systematic review is feasible. For lines of research that are relatively new, scoping review methods provide critical insight to the state of scientific development on a phenomenon of interest [37]. This study was conducted in accordance with the Joanna Briggs Institute guidelines for systematic scoping reviews [37] and conforms to the PRISMA Extension for Scoping Reviews (PRISMA-ScR) guideline [38].
Eligibility criteria
Studies included were 1) primary research, 2) on adults (≥18 y), 3) who were military veterans (worldwide), and 4) diagnosed with obstructive sleep apnea (OSA), insomnia or hypersomnia disorders – the three most prevalent sleep disorders among veterans [27], or with sleep disturbance symptoms such as poor sleep quality and/or decreased sleep duration. To be included, primary studies sampled participants with 1) documented or presumed chronic nonmalignant pain, or assessed chronic nonmalignant pain as the endpoint and 2) reported the association between sleep disorder diagnosis or sleep disturbance and a pain outcome. Inclusion criteria were not established for any specific pain domain(s) as such criteria would limit the usefulness of this scoping review which aimed to summarize the state of the science in a broad and integrated manner. Thus, we assess the influence of sleep disturbances and sleep disorders on a variety of pain outcomes including severity/intensity, prevalence and frequency, pain-related disability, and pain interference on relevant aspects of one’s life.
Exclusion criteria were: 1) non primary research papers (e.g., case series, opinion papers), 2) sleep disorders other than OSA, insomnia or hypersomnia, 3) active military or non-veteran individuals, and 4) pain of acute or malignant origin. Secondary data analyses that did not evaluate different sleep and/or pain variables than those reported in the primary study were excluded. If a conference abstract and equivalent journal manuscript were retrieved, the manuscript was used unless otherwise specified.
Search strategy
A systematic literature search was conducted with keywords such as “sleep”, “pain”, and “veteran” across the following databases from inception to March 18, 2020: PubMed/Medline, EMBASE, Scopus, CINAHL, and PsycINFO (see Appendix 1S). Ancestry search of relevant selected articles was performed to identify any potential references missed in the original database searches. Eligible gray literature sources such as conference abstracts and dissertations were included if indexed by one of the five databases and retrieved upon search. A biomedical librarian provided search process oversight.
Screening
Titles and abstracts were screened by the lead author (BS) using an investigator-developed checklist. Full texts of selected articles were further assessed for eligibility using a detailed checklist. A second reviewer was consulted (AMS) if uncertain regarding study eligibility. DistillerSR (Evidence Partners, Ottawa, Canada) was used to manage the screening procedures.
Data extraction and quality assessment
The lead investigator (BS) abstracted and double-entered data into an electronic database. In cases where data were missing or unclear, the corresponding author of the published source was contacted (up to three times). Clarification requests were sent to authors of 17 of the included studies; 13 provided additional information [39–51], and four did not respond or were unable to assist with the request [52–55].
The level of evidence and the methodological quality of included publications were independently assessed by two reviewers (BS and MM) using the Johns Hopkins Nursing Evidence-Based Practice (JHNEBP) Research Evidence Appraisal system [56]. The JHNEBP system classifies evidence by level as follows: I) experimental studies, randomized controlled trial (RCT), II) quasi-experimental studies, III) nonexperimental studies, IV) opinion and consensus statements, or V) experiential and non-research evidence. Methodological quality ratings range from high to low quality or major flaws based on appropriateness of sample size for proposed design, adequate control, generalizability of results, consistency of results and recommendations in the context of scientific evidence, and definitiveness of conclusions [56].
Data synthesis
Due to the diversity of pain outcomes measured across studies and the heterogeneity of study designs, association results were not quantitatively summarized. Thus, study results are narratively synthesized [57]. Association results were reported by type of sleep problem (sleep disorder or sleep disturbance) based on underlying study design: observational (i.e., without treating pain or sleep) or experimental, quasi-experimental, and observational studies of treated samples (pain or sleep treatment). The direction and magnitude of the effects on pain outcomes, when available, were further described in the context of good or improved sleep as differentiated from poor or worsened sleep. Pooled descriptive estimates were computed where appropriate for age and sample size.
Results
Study selection
A total of 1450 records were retrieved through initial electronic database search. Upon removal of duplicates, 1094 publications remained. After title/abstract level screening, 147 studies received full-text assessment. Two papers were manually added in lieu of equivalent abstracts retrieved from database searching. Most studies were excluded for assessing sleep and pain variables independently (45.3%) and assessing samples other than veterans (26.6%). No eligible review papers were identified. A final sample of 26 publications were selected for analysis (manuscripts [n = 20], abstracts [n = 5], and dissertations [n = 1]; Fig. 2).
Fig. 2.
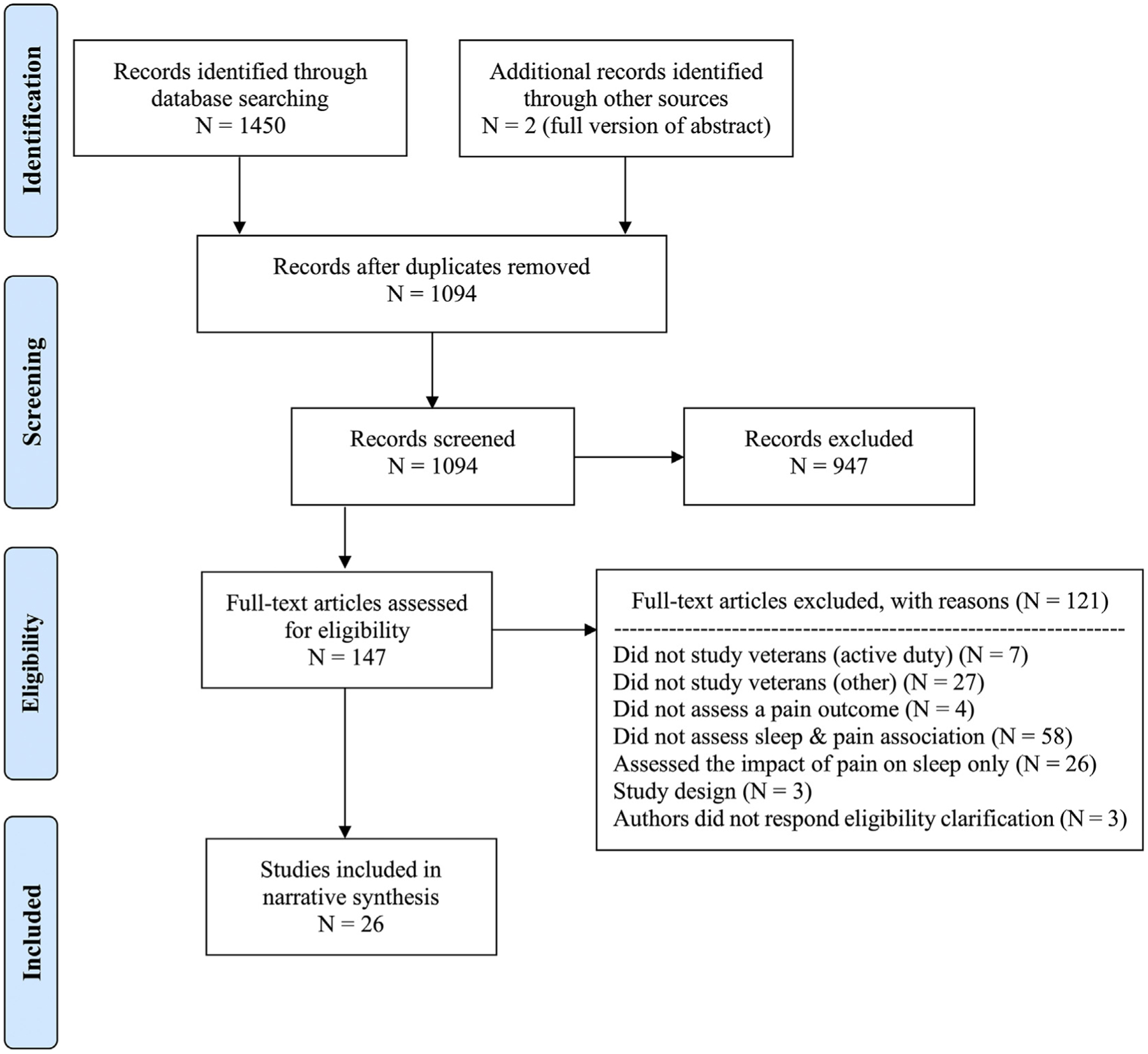
PRISMA flow diagram showing the study search and selection process.
Study characteristics
Across studies, a total of 923,434 participants were included with median sample size of 154 (range 7–858,226); 84.6% of studies reported samples below 700 participants (22/26) (Table 1). Only studies of U.S. veterans met all inclusion criteria; therefore, this set of evidence addresses U.S. veterans. Studies were predominantly prospective (69.2%, 18/26), cross-sectional (53.8%, 14/26), and observational in design (80.8%, 21/26). There were three observational studies of treated samples (14.3%, 3/21) – two of prazosin-treated sleep disturbance [58,59] and one of continuous positive airway pressure (CPAP)-treated OSA [60], and one qualitative study [61]. Pain was the primary outcome in 88.5% of studies (23/26); most studies assessed multiple outcomes. Studies were published between 2006 and 2020, with the majority published in the last 5 y (76.9%, 20/26).
Table 1.
Study characteristics.
| Citation (year) Publication Type | Study Design (Intervention, if applicable) | Veteran Cohort | Final sample (N) Gender Mean age ± SD | Ethnicity | Timing of Assessments |
|---|---|---|---|---|---|
| Amin et al., 2011 [40] Journal Article |
P, L, E Nasal CPAP vs. sham-CPAP |
Deployed to the Persian Gulf between 8/90 & 8/91 |
N = 18 100% male 42 ± 4 y |
89% white 11% black |
Baseline 3 wk posttreatment |
| Balba et al., 2018 [43] Journal Article |
P, C, O | NR | N = 639 91.7% male 55 ± 15 y§ |
85% white§ | Baseline |
| Burgess et al., 2019 [67] Journal Article |
P, L, Q Morning Bright Light |
NR | N = 37 73% male 48.4 ± 14.1 |
59% black 22% white 14% Hispanic 5% multiracial |
Baseline Mid-treatment (6 days on therapy) Final treatment assessment (13 days on therapy) |
| Burns et al., 2020 [68] Journal Article |
P, L, Q Morning Bright Light |
NR | N = 22 77% male 48.4 ± 13.9 y |
50% black 45.5% white 4.5% other |
Baseline Final treatment assessment (13 days on therapy) 30 days follow-up. |
| Card et al., 2018 [54] Abstract |
R, C, O | OEF, OIF, OND | N = 858,226 NR 30 ± NR years |
NR | Baseline |
| Chapman et al., 2006 [53] Journal Article |
P, L, O | NR | N = 201 88% male 56 ± 13 y |
87% white 5.5% black 5.5% Hispanic |
Baseline 2 mo |
| Cunningham & Oehlert, 2018 [48] Abstract |
R, C, O | NR | N = 9403 84% male† 59.4 ± NR years† |
72% white† 21% black† |
Baseline |
| Eakman et al., 2017 [39] Journal Article | P, L, Q Multicomponent CBT-I |
Post 9/11 veterans | N = 7 100% male 35.6 ± 7.4 y |
100% white | Baseline (twice; 1 wk apart) Posttreatment (within 48h of the end of an 8-wk intervention) |
| Hoot et al., 2018 [49] Journal Article |
P, C, O | OEF, OIF, OND | N = 454 86.78% male TBI group: 36 y (median) No TBI group: 38 y (median) |
67.6% white 22.7% black 9.7% other |
Baseline |
| Hughes et al., 2013 [50] Abstract |
P, C, O | NR | N = 39 95% male 78 ± NR years |
NR | Baseline |
| Jaoude et al., 2016 [60] Journal Article |
R, L, O Nasal CPAP on opiates vs. NASAL CPAP no opiates |
NR | N = 226 95.6% male 60 ± 10.8 y |
90.7% white 8.9% black |
Baseline 12 mo |
| Jaramillo et al., 2016 [44] Journal Article | R, L, O | OEF, OIF | N = 38.426 82.8% male§ 17–30 y (45.3%)§ 31–40y(27%)§ |
65.3% white§ 16.4% black§ 11.3% Hispanic§ |
Baseline (FY2008) Yearly until FY2011 |
| Koffel et al., 2016[45] Journal Article |
P, L, O Automated symptom monitoring to optimize analgesics | NR | N = 250 82.8% male 55 ± 8.45 y |
76.8% white | Baseline 3 mo 12 mo |
| Koffel et al., 2019[65] Journal Article |
P, L, O | NR | N = 238 87% male 57.05 ± 13.45 y§ |
86% white 7.5% black 6.5% other or multiple |
Baseline 3 mo 6 mo 9 mo 12 mo |
| Koffel, Amundson, & Wisdom, 2019 [61] Journal Article |
Qualitative semistructured interviews | NR | N = 17 82.35% male 35–45 y (18%) 45–54 y (18%) 55–65 y (29%) >65 y (35%) |
88% white | Baseline |
| Lang et al., 2014 [55] Journal Article |
R, C, O | OED, OIF | N = 137 95.6% male 30.9 ± 7.8 y |
52.4% white 21.1% black |
Baseline |
| Martin et al., 2017 [42] Journal Article |
P, C, O | NR | N = 660 0% male 50.9 ± 17.7 y |
49.4% white 30.1% black 17% Hispanic |
Baseline |
| Munds, 2017 [52] Doctoral Dissertation |
P, C, O | NR | N = 10 80% male 34.6 ± 8.25 y |
90% white 10% black |
Baseline |
| Powell et al., 2015 [63] Journal Article | R, C, O | OEF, OIF, OND | N = 171 86.5% male 33.26 ± 8.56 |
68.4% white 17% Hispanic 10.5% black |
Baseline |
| Ruff et al., 2009 [59] Journal Article |
P, L, O Oral prazosin at bedtime |
OEF, OIF | N = 74 93% male 29.4 ± 2.9 y |
NR | Baseline 9 wk 6 mo follow-up |
| Ruff et al., 2012 [58] Journal Article |
P, L, O Oral prazosin at bedtime |
OEF, OIF | N = 63 90.48% male 29.5 ± 0.92 y |
NR | Baseline 9 wk 6 mo follow-up |
| Sangani & Baker, 2016 [41] Abstract |
P, C, O | NR | N = 51 86% male 63 y (range 27–77) |
NR | Baseline |
| Tighe et al., 2020 [64] Journal Article |
P, C, O | NR | N = 517 72.9% male 63.72 ± 8.47 |
52% non-Hispanic black 48% non-Hispanic white |
Baseline |
| Taylor et al., 2019 [46] Abstract |
R, C, O | NR | N = 13,444 NR NR |
NR | Baseline |
| Travaglini et al., 2019 [51] Journal Article |
P, C, O | Vietnam (31.6%); between Vietnam & Gulf(45.6%); Gulf (14%), Gulf & OEF, OIF, OND (5.3%); OEF, OIF, OND (5.3%)y | N = 57 80.7% male 53.82 ± 7.83 y |
29.8% white 61.4% black 7% multiracial |
Baseline |
| Weiner et al., 2019 [47] Journal Article |
P, C, O | NR | N = 47 87.2% male 68 ± 6.5 y |
66% white 29.8% black |
Baseline |
Notes.
Data comes directly from the author or subsequent publication.
pooled estimate.
Cognitive behavioral therapy for insomnia (CBT-I), continuous positive airway pressure (CPAP), chronic low back pain (CLBP), cross-sectional (C), experimental (E), fiscal year (FY), Golf war illness (GWI), longitudinal (L), Millon behavioral medicine diagnostic (MBMD), not reported (NR), observational (O), obstructive sleep apnea (OSA), Operation Enduring Freedom (OEF), Operation Iraqi Freedom (OIF), Operation New Dawn (OND), prospective (P), qualitative (Qual), quasi-experimental (Q), post-traumatic stress disorder (PTSD), retrospective (R), sleep disordered breathing (SDB), standard deviation (SD), and traumatic brain injury (TBI).
Evidence quality appraisal
Studies were classified as level 1 (3.8% [1/26]), level 2 (11.5% [3/26]), and level 3 (84.6% [22/26]). The quality of evidence was high (19.2% [5/26]), good (38.5% [10/26]), and low/major flaws (42.3% [11/26]; Table 1S). Studies were downgraded for reasons such as insufficient control and/or small sample size for study design.
Subject characteristics
A pooled mean was computed [62] for studies reporting mean and standard deviations for age (69.2% [18/26]), which resulted in an average age of 53.3 ± 12.9 y (range of means 29.4–68 y). Eight studies were not included in this summary due to absence of reported standard deviations [41,48,50,54], reporting age categories only [44,61], reporting median ages for independent subgroups [49], or no report of participants’ ages [46]. Subjects were predominantly male (82.1%) and white/Caucasian; two studies did not report a sex ratio [46,54], and only one study exclusively included female veterans [42] (Table 1). Veteran cohorts were reported in 38.5% (10/26) of studies; studied cohorts were Operation Enduring Freedom (OEF), Operation Iraqi Freedom (OIF), and/or Operation New Dawn (OND) (26.9% [7/26]), mixed cohorts (7.7% [2/26]), and Gulf War veterans (3.8% [1/26]).
Sleep disturbances and sleep disorders
Fifteen studies assessed adults with sleep disturbances (57.7% [15/26]), and eleven studies assessed adults with sleep disorders (42.3% [11/26]; Table 2S). Sleep disturbances were most often measured using the Pittsburg Sleep Quality Index (PSQI) (40% [6/15]), the Insomnia Severity Index (ISI) (20% [3/15]), and the PROMIS Sleep Disturbance instrument (20% [3/15]). Sleep disorder diagnoses were established by medical chart review of codes from standard classification systems such as International Classification of Diseases (ICD)-9, ICD-10, and International Classification of Sleep Disorders-2 (45.4% [5/11]), polysomnography testing (PSG; 18.2% [2/11]), and subjective diagnostic criteria – e.g., pre-specified ISI cutoffs – (36.4% [4/11]). The most common sleep disorder diagnoses in this review were insomnia (54.5% [6/11]) and OSA (27.3% [3/11]). Of the five studies assessing samples with sleep disordered breathing (SDB), three established OSA diagnosis based on medical chart reviews of standard ICD diagnostic codes (Table 2S). One study defined OSA based on the presence of at least 5 obstructive respiratory events (apneas or hypopneas) per hour of sleep as summarized by the apnea hypopnea index (AHI; higher scores indicate greater OSA severity) from a full-night diagnostic PSG [60]. Lastly, SDB diagnosis was established based on PSG-derived AHI scores; however, no threshold criteria was defined (AHI mean ± SD: 19 ± 25) [40]. No other clinical criteria (e.g., daytime sleepiness, loud snoring) was accounted for with the diagnosis of OSA.
Pain outcomes
The most frequently assessed pain outcomes were self-reported pain severity/intensity (73.1% [19/26]), self-reported pain interference (38.5% [10/26]), self-reported pain-related disability (7.7% [2/26]), and likelihood/odds of a chronic pain diagnosis (7.7% [2/26]; Table 3S). Chronic pain duration at study onset was reported in ten studies (38.5% [10/26]) in which pain lasted for three months or more in 40% (4/10), and for six months or more in 60% (6/10). Multidimensional pain assessments were conducted in 42.3% of studies (11/26). When authors specified the type of chronic pain (50% [13/26]), musculoskeletal pain (38.5% [10/26]) or posttraumatic headaches (11.5% [3/26]) were the most common types (Table 3S). In instances where chronic pain was presumed but not diagnosed, the pain assessed was described as “current pain” (see Table 3S).
Influence of sleep disturbances and sleep disorders on pain outcomes
Observational studies (without sleep or pain treatment)
Veterans with sleep disorders.
Among studies assessing the influence of diagnosed sleep disorders on chronic pain outcomes, nine used an observational/non-experimental design (81.8% [9/11]; Tables 2 & 2S) [42,44,46–48,51,54,55,61]. Overall, the presence of a sleep disorder and/or worsening of sleep disorder-related symptoms resulted in worse pain outcomes across all nine studies (N = 920,417; Fig. 3).
Table 2.
Main findings on the influence of sleep disturbance and sleep disorders on pain outcomes among veterans.
| Citation (year) | Baseline adjusted variables | Independent (sleep) variable(s) | Dependent (self-reported pain) outcome(s) | Results: Poor or worsened sleep | Results: Good or improved sleep | |
|---|---|---|---|---|---|---|
| Group 1: Observational Studies (without treating pain or sleep) | ||||||
| Balba et al., 2018 [43] | n/a | Self-reported insomnia symptoms | Pain severity | ↑ pain severity across all studied groups | NR | |
| Card et al., 2018 [54] | Sociodemographic characteristics and mental health diagnoses | OSA diagnosis | Pain intensity | ↑ likelihood of reporting moderate/severe pain intensity | NR | |
| Chapman et al., 2006 [53] | Analysis #1: Opioid prescription, baseline pain, 2-month sleep med. prescription, and depressive symptoms | Self-reported sleep quality and sleep disturbance | Pain severity, Pain interference, and General activity level | No association | NR | |
| Analysis #2: Opioid prescription, baseline pain, sleep quality and disturbance, and depressive symptoms | Sleep medication prescription | Pain severity, Pain interference, and General activity level | NR | No association | ||
| Cunningham & Oehlert, 2018 [48] | n/a | Sleep disorder diagnoses (see list of codes on Table 2S) | Pain sensitivity | ↑ pain sensitivity*** | NR | |
| Hoot et al., 2018 [49] | Age, site, time since index injury, depression, PTSD, anxiety, combat exposure and duration, number of controlled and uncontrolled blast exposures | Self-reported sleep quality and sleep disturbance | Pain intensity & Pain interference | ↑ pain interference* ↑ pain intensity* |
NR | |
| Hughes et al., 2013 [50] | Analysis #1: n/a | Self-reported sleep quality and sleep disturbance | Pain intensity | ↑ pain intensity** | NR | |
| Analysis #2: n/a | Self-reported insomnia symptoms | Pain intensity | ↑ pain intensity*** | NR | ||
| Jaramillo et al., 2016 [44] | Demographic and military characteristics, TBI, PTSD, and/or postconcussive symptoms. | Insomnia disorder diagnosis | Posttraumatic headaches (PTHA) prevalence & PTHA persistence | Insomnia at baseline was associated with: ↑ PTHA prevalence*** ↑ headache persistence*** |
NR | |
| Koffel et al., 2016 [45] | Baseline covariates and depression & anxiety symptoms. | Changes in self-reported sleep disturbance (insomnia symptoms [difficulties falling and staying asleep] and lassitude [fatigue]) | Pain (intensity & interference, loaded as a single factor after EFA) | NR | Sleep disturbance improvement from baseline to 3 mo predicted ↓ pain (intensity & interference) at 12 mo*** | |
| Koffel et al., 2019 [65] | Baseline BPI values and treatment group assignment | Self-reported sleep disturbance | Pain severity & Pain interference | ↑ pain interference*** & ↑ pain severity*** ↓ improvement in pain interference*** & pain severity* |
NR | |
| Koffel, Amundson, & Wisdom, 2019 [61] | n/a | n/a | Pain perception | NR | Sleep improvement “enhanced functionality [and quality of life] in the context of chronic pain” | |
| Lang et al., 2014 [55] | Service connection (mediation models only) | Self-reported insomnia symptoms | Pain severity & Pain interference | ↑ pain severity** ↑ pain interference** Partially mediated relationship between PTSD symptoms and pain severity** & PTSD symptoms and pain interference** |
NR | |
| Martin et al., 2017 [42] | n/a | Insomnia disorder diagnosis | Pain interference with sleep | ↑ frequency of pain interference on sleep *** | NR | |
| Munds, 2017 [52] | None | Self-reported sleep disturbance | Pain-related disability | No change | NR | |
| Powel et al., 2015 [63] | PTSD severity, current mood disorder diagnosis, current anxiety disorder diagnosis, and alcohol use. | Self-reported sleep quality and sleep disturbance | Pain severity | ↑ pain severity** among the total sample (presumed chronic pain), but not among the subset (n = 65) with confirmed chronic pain | NR | |
| Sangani & Baker, 2016 [41] | n/a | Self-reported sleep disturbance | Pain intensity | ↑ pain intensity* | NR | |
| Taylor et al., 2019 [46] | Age, race, sex, combat exposure, Charlson comorbidity index, marital status, and combat exposure† | OSA diagnosis | Low back pain diagnosis | ↑ odds of LBP diagnosis OSA diagnosis mediated the effect of PTSD on risk of LBP diagnosis | NR | |
| Tighe et al., 2020 [64] | Sociodemographic and clinical characteristics | Self-reported sleep disturbance | Pain severity & Pain catastrophizing. | Frequency of sleep disturbance was associated with: ↑ pain severity*** ↑ pain catastrophizing*** |
NR | |
| Travaglini et al., 2019 [51] | n/a | Self-reported sleep quality and sleep disturbance | Pain intensity & Pain interference | ↑ pain interference* | NR | |
| Weiner et al., 2019 [47] | n/a | Insomnia disorder diagnosis | Pain disability & Pain severity | ↑ Back pain disability* | NR | |
| Group 2: Experimental, quasi-Experimental Studies, or observational studies with treatment of pain or sleep | ||||||
| Amin et al., 2011 [40] | n/a | Nasal CPAP (treatment) vs. sham CPAP (control) | Pain severity | NR | ↓ pain intensity*** | |
| Burgess et al., 2019 [67] | n/a | Morning bright light therapy | Pain intensity, pain behavior, pain interference, pain sensitivity (objectively measured), and physical function | NR | ↓ pain intensity* ↓ pain behavior* ↓ pain sensitivity* |
|
| Burns et al., 2019 [68] | The three study epochs: baseline, bright light treatment, and follow-up | Morning bright light therapy | Pain intensity, Pain intensity volatility, and Pain interference | NR | ↓ mean pain intensity*** ↓ pain intensity volatility*** |
|
| Eakmen et al., 2017 [39] | n/a | Multicomponent CBT-I | Pain interference | NR | ↓ pain interference | |
| Jaoude et al., 2016 [60] | n/a | Nasal CPAP | Pain intensity | Non-adherent participants reported ↑ pain intensity at baseline* and 12 mo (p = 0.05) | No change in pain intensity among adherers (≥4 h/night on 70% of nights) | |
| Ruff et al., 2009 [59] | n/a | Taking Prazosin (yes/no) | Headache severity & Headache frequency | None reported | ↓ pain severity and frequency (baseline to 9-wk posttreatment)*** ↓ pain severity and frequency among those who completed dosing of prazosin (9-wk posttreatment)*** ↓ pain severity and frequency among those taking prazosin (6-month follow-up)*** |
|
| Ruff et al., 2012 [58] | n/a | Daytime Sleepiness | Headache severity & Headache frequency | ↑ HA pain intensity at 9-wk*** and 6-mo*** ↑ HA pain frequency at 9-wk* and 6-mo*** |
Differences on daytime sleepiness from endpoint to baseline (Δ) were associated with: ↓ HA pain intensity at 9-wk***, and 6-mo*** ↓ HA pain frequency at 9-wk*, and 6-mo* |
|
Notes.
significant (p < 0.05);
significant (p < 0.01);
significant (p < 0.001);
Data from some of those columns come directly from the author or subsequent publication.
Adjusted odds ratio (AOR), brief pain inventory (BPI), continuous positive airway pressure (CPAP), exploratory factor analysis (EFA), fiscal year (FY), headache (HA), low back pain (LBP), non-applicable (n/a), none reported (NR), obstructive sleep apnea (OSA), osteoarthritis (OA), post-traumatic stress disorder (PTSD), sleep disordered breathing (SBD), standardized mean difference (SMD), traumatic brain injury (TBI).
Fig. 3.
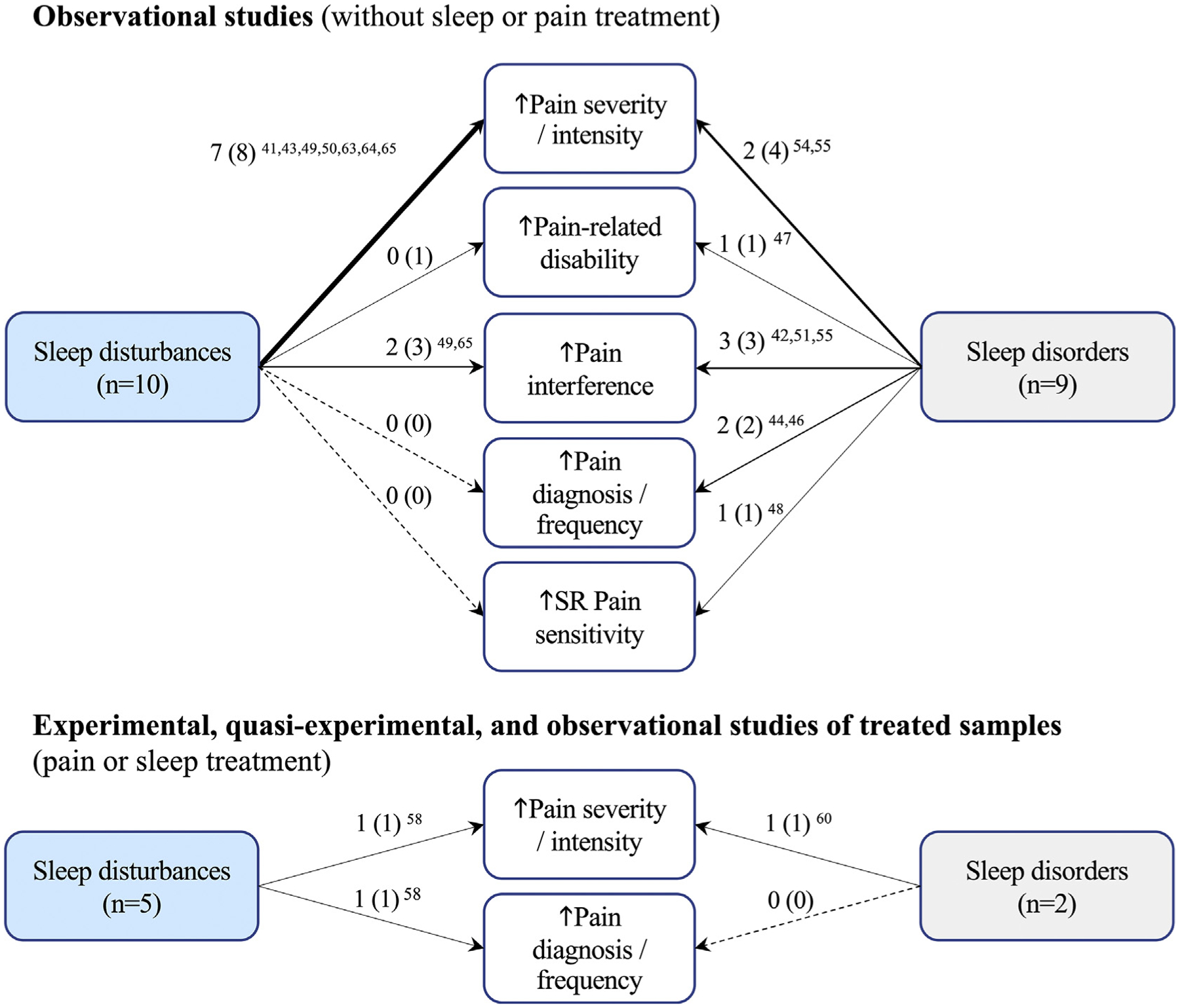
Summary of the total number of studies that supported the influence of sleep disturbances and sleep disorders on pain outcomes in the context of poor or worsened sleep. Notes: #A (#B) #A ref.: A = # of studies with findings that support the relationship (B = #total number of studies assessing the relationship) # references for A. Thicker arrows indicate higher frequency of evidence on the studied relationship. Dashed line indicates absence of evidence. Self-reported (SR).
Insomnia was associated with greater pain interference on sleep [42], increased odds of headache diagnosis [44], and greater pain-related disability [47]. Having insomnia was associated with almost a two-fold increase in the odds of being diagnosed with headache (aOR: 1.97 [1.81–2.15]; p < 0.001), and significantly increased odds of having persistent headache (aOR: 1.19 [1.02–1.39]; p < 0.001), even after controlling for characteristics such as traumatic brain injury (TBI) and PTSD [44]. Women veterans with insomnia reported greater pain interference on sleep than those without insomnia (p < 0.001) [42]. Older veterans with an insomnia diagnosis reported greater low-back pain disability [47] (p < 0.01). Compared to those without insomnia, veterans with insomnia were more likely to report a headache diagnosis, and experience greater chronic pain-related disability and interference.
Among veterans with insomnia and chronic pain, self-reported sleep quality and disturbances [51] and greater insomnia symptom severity [55] were associated with worse pain severity [55] and pain interference [51,55]. One study identified that insomnia partially mediated the relationship between PTSD and pain severity and pain interference [55]. Conversely, improved sleep through cognitive behavioral therapy for insomnia (CBT-I) in a qualitative study resulted in increased perceived functionality and quality of life in veterans with insomnia and chronic pain (Fig. 4) [61]. These findings suggest a positive association between insomnia severity and pain outcomes in veterans with insomnia, such that more severe insomnia symptoms result in greater pain severity and interference.
Fig. 4.
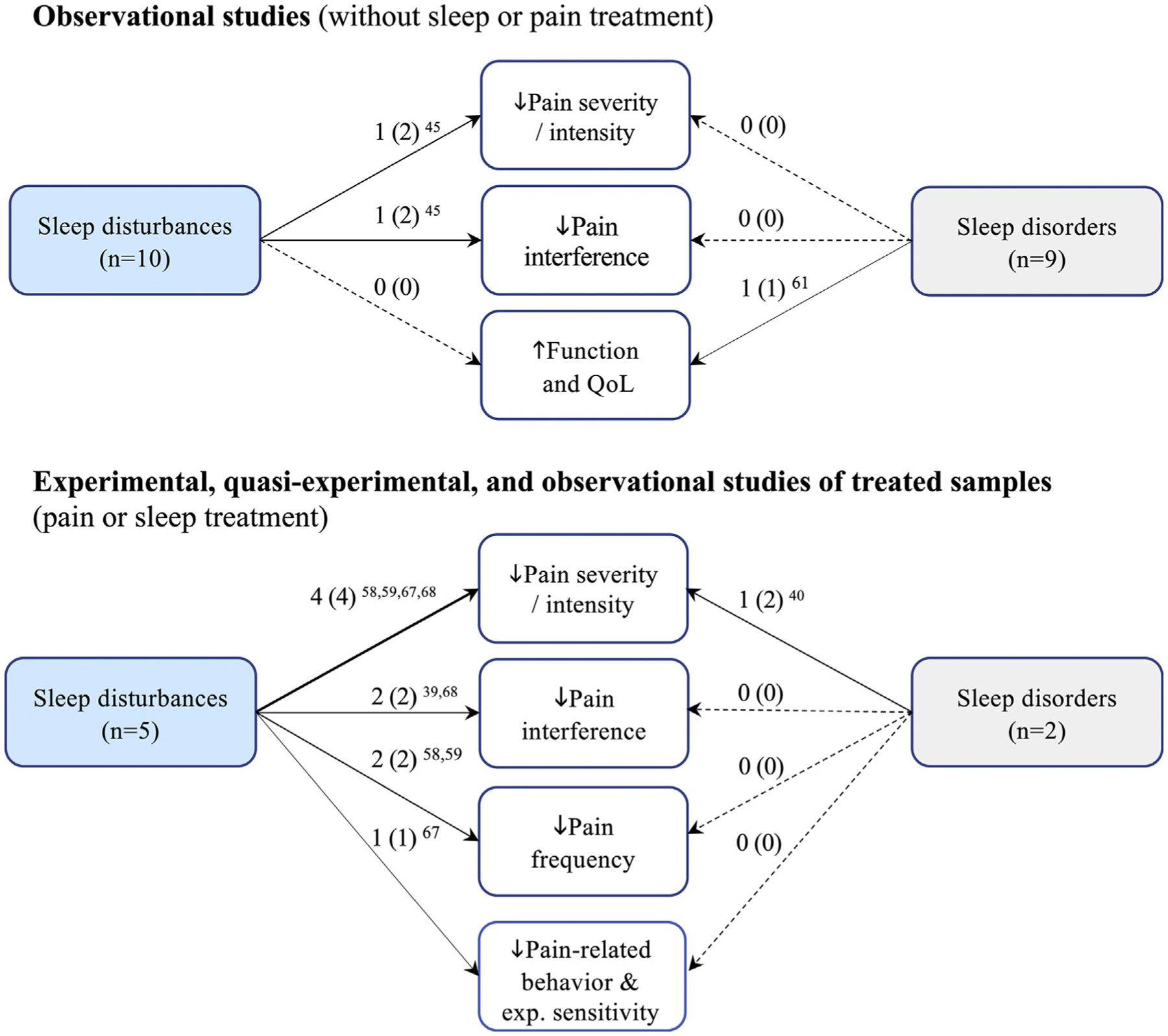
Summary of the total number of studies that supported the influence of sleep disturbances and sleep disorders on pain outcomes in the context of good or improved sleep. Notes: Quality of Life (QoL). #A (#B) #A ref.: A = # of studies with findings that support the relationship (B = #total number of studies assessing the relationship) # references for A. Thicker arrows indicate higher frequency of evidence on the studied relationship. Dashed line indicates absence of evidence. Experimental (Exp.).
Veterans with OSA were more likely to report moderate/severe pain intensity compared to those without the diagnosis (aOR = 1.28; 95% CI = 1.27–1.30) [54]. Taylor et al. [46] showed that OSA strongly predicted lower back pain (LBP) diagnosis (aOR 8.99; 95% CI = 7.07–11.35) and identified that OSA partially mediated the effect of PTSD on LBP [46]. Cunningham et al. [48] reported greater self-reported pain sensitivity among veterans with a diagnosed sleep disorder (t [9401] = 12.9; p < 0.001), wherein OSA and insomnia were the most common sleep diagnoses (31% and 22%, respectively; email communication, August 2019) [48]. Thus, having OSA significantly increased the odds of veterans reporting a chronic pain diagnosis and greater pain intensity and sensitivity.
Veterans with sleep disturbances.
Ten studies used an observational design to assess the influence of sleep disturbances on pain outcomes (66.6% [10/15]; Table 2 and 2S) [41,43,45,49,50,52,53,63–65]. Among veterans reporting disturbed sleep, worse pain responses were observed across seven studies (combined n = 2109; Fig. 3). Two studies (combined n = 211) did not find any influence of sleep disturbances on pain [52,53] (Table 2).
Poor sleep – defined by decreased quality and increased disturbance – as measured by the PSQI, was associated with higher chronic pain severity [41,49,63] and pain interference [49], and greater overall pain when assessed by a multidimensional scale [50]. Insomnia symptom severity, as measured by the ISI, was also associated with higher pain severity [43] and more overall pain when assessed by a multidimensional scale [50]. Though investigators did not qualify this sample as one with insomnia, by reported sample ISI (15.11 ± 6.32) and given the established threshold for clinically significant insomnia of ISI > 15 [66], this is in fact a sleep disordered sample. Disturbed sleep, when assessed by the PROMIS-sleep disturbance scale, was associated with greater chronic pain severity and interference [65], and with greater pain severity and catastrophizing as measured by a single item from the Patient Health Questionnaire-8 [64]. In a secondary analysis of data from a randomized trial, decreased self-reported sleep disturbance from baseline to three months predicted reduced pain intensity and interference at 12 months (β = 0.29, p < 0.001) [45].
Self-reported sleep disturbances were significantly associated with worse pain outcomes even after controlling for factors such as PTSD [43,49,63], anxiety [45,49,63,64], depression [42,62,65], and analgesic [43,64] and sleep medication [43] use. One study did not find disturbed sleep, measured by higher PSQI global scores, to be linked with pain severity and pain interference [53]. Within a small sample (n = 10), sleep disturbances failed to moderate the relationship between PTSD and chronic pain [52]. Overall, sleep disturbances were associated with intensified pain responses in the majority of studies reviewed.
Experimental, quasi-experimental, and observational studies of treated samples (pain or sleep treatment)
Veterans with sleep disorders.
Among studies assessing the influence of diagnosed sleep disorders on pain outcomes, two studies (18.2% [2/11]) evaluated samples with SDB treated with CPAP (Table 2 and 2S, Fig. 4) [40,60]. In a randomized, sham-controlled pilot trial (n = 18) of veterans with Gulf War Illness and SDB (mean apnea hypopnea index [AHI] 19 ± 25), Amin et al. [40] observed a 34% reduction in pain severity among those using CPAP (at least 5 h/night for 3 wk), compared to a sham-control group after 3-wk of treatment (p < 0.001). Although the authors suggest this is not a sleep apnea sample, 88.8% of participants would qualify as at least mild OSA (AHI ≥ 5). A retrospective case-control study of veterans with OSA on chronic opioid treatment found that CPAP treatment did not decrease pain severity, nor was opioid consumption reduced from baseline to 12-months follow-up [60]. However, the authors did report higher pain intensity among veterans who were non-adherent to CPAP (adherence criteria: ≥ 4 h/24 h for ≥ 70% days over 30 days) at baseline (p < 0.05) and at 12-month follow-up (p = 0.05) [60]. Findings from these studies suggest that CPAP treatment improves pain outcomes in veterans with SDB.
Veterans with sleep disturbances.
Of studies assessing the influence of sleep disturbances on pain outcomes, five evaluated treated samples (33.3% [5/15]; Table 2 and 2S) [39,58,59,67,68]. Across all five studies (total n = 203), treatment-induced improvements on sleep disturbance were associated with improved pain outcomes (see Fig. 4). In a single-arm feasibility trial of multimodal CBT-I among veterans with sleep disturbance (n = 7), Eakman et al. [39] observed non-significant trends towards reduced pain interference when comparing pretest to post-intervention scores (Cohen’s d = 0.45).
In an open, single arm pilot trial, morning bright light therapy (MBLT) resulted in significantly (p < 0.05) reduced pain intensity, pain-related behavior, and pain sensitivity following 13 days of daily (1 h/day) treatment [67]. In a secondary analysis, Burns et al. [68] assessed whether MBLT could affect pain volatility based on daily electronic diary data. In the study, MBLT was related to decreased mean pain severity (p < 0.001) and interference (p < 0.05), and reductions in the volatility of pain intensity (p < 0.001), but not in the volatility of pain interference.
In an observational study, Ruff et al. [59] investigated the effects of treating sleep with oral prazosin on headache frequency and severity in a sample of veterans with TBI and posttraumatic headache (n = 74). A significant reduction in pain intensity and interference at the end of the 9-wk intervention period (p < 0.001), and 6-month follow-up (p < 0.001) was identified. In a secondary analysis, Ruff et al. [58] assessed veterans (n = 63) with the triad of headaches, PTSD, and neurological deficits and found reductions in daytime sleepiness significantly correlated with decreased headache severity and frequency [58]. Together, these studies suggest that treating sleep disturbances with non-pharmacological [67,68] and pharmacological [58,59] interventions resulted in improved pain outcomes.
Sleep disturbance definitions
Among studies assessing samples with disturbed sleep, only one study (6.6%) provided a conceptual definition of sleep disturbances (Table 4S). However, the term sleep disturbance was used as a synonym for insomnia [39,43] and other sleep disorders such as OSA, hypersomnia, and circadian rhythm disorders [43].
Discussion
Sleep disturbances and sleep disorders were consistently associated with worse pain outcomes such as greater chronic pain frequency, severity, interference, and poorer functional outcomes among veterans with chronic nonmalignant pain. Sleep improvements with treatment ameliorated chronic pain outcomes; however, the evidence was limited and of moderate to low quality. These findings parallel the study results in the civilian population [18,19,21,66,67]. In a recent systematic review of longitudinal studies, adults with reduced sleep quality and duration experienced a two to three times greater risk of developing a pain condition, and a decline on self-reported pain-related physical health status [33]. Sleep improvements resulted in better physical functioning in the same analysis [33].
Similar to results of this scoping review, studies have identified that nonpharmacological treatments for sleep disorders have yielded improved pain outcomes [69,71,72] in non-veteran samples. A meta-analysis of randomized trials testing non-pharmacological interventions for insomnia in people with chronic pain found small reductions in pain post-treatment (standardized mean difference = 0.18; 95% CI = 0, 0.36, P < 0.05) [71]. Likewise, CPAP-treated OSA was associated with improvements in pain among samples with pre-existing and experimentally induced pain [69]. These results support the hypothesis that treatment of sleep disorders contributes to improved pain outcomes. Consistent with our results, existing evidence in non-veteran samples should also be considered with caution due to the observational nature of the evidence [69,73,74], modest sample sizes [69,73], and/or absence of control conditions [73].
The observed negative independent effects of OSA and insomnia on chronic pain outcomes are concerning, as these sleep disorders rank as the first and second most prevalent sleep disorders among veterans, respectively [27]. Insomnia is commonly co-morbid with OSA, with an estimated occurrence between 29 and 50% [75]. When compared to those with insomnia only, adults with comorbid OSA/insomnia experience increased rates of heart disease, more depressed mood, decreased quality of life [75], and higher chronic pain severity [76]. Considering the multi-morbidity associated with comorbid OSA/insomnia coupled with the extremely high prevalence of chronic non-malignant pain in veterans, prioritization of the management of these sleep disorders may be a real opportunity to improve veteran health outcomes.
None of the studies assessed the effect of circadian misalignment on chronic pain outcomes in veterans. Given the suggested modulatory effects of circadian processes on pain [90], future research will investigate the impact of circadian factors on chronic pain outcomes in this population. While natural light exposure history (intensity, frequency, and duration) in the context of military life may be a relevant factor to consider among veterans who recently departed from active duty and present circadian misalignments, no studies included in the review sampled recently separated from military duty samples. The samples included in this review were of middle-aged veterans, who were separated from active duty for several years and were established in civilian life and routine. As evidenced by the results of included studies (N = 2), the therapeutic use of light through MBLT holds great potential as a non-pharmacologic treatment for chronic pain and sleep disturbances even among veterans with no circadian misalignment.
Sleep disorder diagnoses are increasingly common among veterans, with the highest rates observed among those with PTSD and other mental health comorbidities [27]. A recent study by Dunietz et al. observed that anxiety symptoms, but not depressive symptoms, partially mediated the effect of insomnia symptoms on incident pain among a sample of older adults [77]. Given the high prevalence of mental illnesses and psychological symptoms in military veterans [28], there is a necessity to better understand how psychological factors may qualify the sleep and pain association. This knowledge may potentiate new intervention targets for chronic pain in veterans. Furthermore, future research will continue exploring the effects of hypothesized biopsychosocial mediators of the sleep and pain relationship, identifying moderators (e.g., the potential role of varied social determinants of health) and confounders of this association, as well as potential mechanistic pathways relating specific sleep and pain disorders.
Given the homogeneity of veteran samples included in this review (mostly white, middle-aged men), it is unclear whether sleep disturbances and/or disorders differentially influence pain outcomes based on sociodemographic factors such as age, sex, and race/ethnicity. Age and sex-based differences in the expression of sleep disorders [78,79] and chronic pain [80,81] are well-documented. Similarly, some racial/ethnic groups have higher prevalence of sleep disorders and/or disturbances [82,83], chronic pain [84], and comorbidities [85,86] that heighten likelihood for sleep disorders and/or chronic pain. Thus, as the veteran population is becoming increasingly more diverse [87,88] – with a greater proportion of female and less non-Hispanic white members– studies are needed to examine if demographic-based differences exist for the experience of chronic pain relative to sleep disturbances and/or disorders. This line of inquiry is consistent with VA Women’s Health Research statements that highlight the need for inclusion of women veterans in research [89]. Other opportunities for future studies include the influence of sleep disturbances and/or disorders on chronic pain outcomes by veteran cohorts (e.g., Vietnam, OIF, OEF, OND), and the time since separation from active duty. These veteran-centric factors were underreported in the included studies.
This systematic scoping review suggests that attention must be given to the operationalization and measurement of sleep disturbances and chronic pain. Most studies (93.4%) with a sleep disturbance(s) variable failed to provide a definition of this construct, resulting in a lack of conceptual clarity and thereby concerns for the validity of the results. This threat is exemplified in a study that classified the sample as having sleep disturbance, where in fact participants met the threshold for clinically significant insomnia [43]. It is essential that future work in the field prioritize conceptualizing a well-defined construct of sleep disturbance and then align an operational definition with appropriate measurement tools. This should be explicated in published study reports to permit more precise knowledge of the effect of sleep disturbance on pain will result.
Furthermore, sleep disturbances were most commonly assessed by using global scores (Table 2S). While self-reported instruments are important tools for assessing the sleep-wake experience, overreliance on global scores from such instruments as a summary measure of the complex sleep disturbance experience fails to accurately capture the distinct dimensions of the phenomena. Sleep disturbances are better characterized (and defined) by interference with multiple aspects of the complex sleep experience; if characterized in this manner, sleep disturbances variables such as total sleep time, sleep continuity, and perceived sleep quality are recommended. Respective measurement opportunities include actigraphy, sleep diary, and perceived sleep quality that are measured proximate to the sleep experience to minimize recall bias. If retrospective self-report measures are employed, standardized instructions that ask participants to reflect over a short recall period, ideally also proximate to the sleep experience, should be employed and reported to support reproducibility. Objective measurement of sleep disturbance dimensions should be prioritized for improved rigor but not to the exclusion of patient-reported outcomes.
Innovative measurement strategies such as ecological momentary assessment (EMA) have great potential for the assessment of night-to-night variability in sleep quality. In veterans, EMA and EMA-based techniques have been successfully employed to investigate the relationship between sleep and daily PTSD symptoms [91], daily alcohol use and PTSD symptoms [92], and the effect of sleep treatments on the volatility of pain, mood, and sleep [68]. Together, these studies demonstrate feasibility of employing EMA methods among veteran samples. Future studies will use EMA and electronic diaries to further investigate the influence of daily fluctuations of sleep parameters on chronic pain.
The systematic scoping review also revealed that chronic pain was inconsistently defined and operationalized; further, the duration of pain was often not reported. Only a few studies classified chronic nonmalignant pain beyond its duration. In instances where pain type was reported, chronic pain affecting the musculoskeletal system was predominant. Musculoskeletal pain is the most common type of chronic pain in veterans [23]. Future research will specify the type of chronic pain based on factors such as region affected, etiology, and causal mechanisms. This approach will support reproducibility but also pooled statistical estimation across the evidence to more robustly evaluate outcomes and effects. Historically, chronic pain diagnoses have not been systematically represented in widely used nosologic systems such as the ICD [36]. Chronic pain was not considered a primary diagnosis until the 11th ICD edition was published in 2018 [36]. The lack of an appropriate classification system may have contributed to the paucity of samples with clearly defined chronic pain diagnoses. With ICD-11, the landscape of future research will likely change as the new edition allows for better classification of a variety of chronic pain conditions. Of note, most studies featured unidimensional measures recording pain severity. Chronic pain is a complex phenomenon; thus, comprehensive biopsychosocial assessments – using a single comprehensive tool, or multiple unidimensional ones – must be prioritized [93,94].
Although preliminary results suggest that sleep management may improve pain, more rigorous and adequately powered studies are needed to establish the effect of non-pharmacologic sleep treatments such as PAP for OSA and MBLT on pain and sleep outcomes. Ideally, this will be accomplished with randomized controlled trials that include an adequate comparator. Only in this way will the field be able to determine causality and mechanisms. Data mining of clinical records stored within the VA’s Corporate Data Warehouse (CDW) should also be considered in future studies assessing the sleep-pain association among veterans. The VA has collected electronic health records (EHR) of more than 23.5 million veterans over 30 y of continual EHR use [95], and yet, this resource is underutilized by researchers [96]. Large observational studies on the phenomenon can be conducted using existing EHR data, and further optimized by using advanced data science methodologies such as propensity score matching for sample selection.
Our findings highlight sleep management as a potential opportunity for improving chronic pain outcomes among the veteran population. Pain assessment as the “fifth vital sign” has been a decades-long clinical practice in VA. Recognizing that sleep is a potential opportunity to improve chronic pain management, routine screening for sleep disorders and sleep disturbances in the care of veterans is recommended. This approach will necessitate that pain management and sleep experts develop collaborative practice approaches that align with the state of the science. At this point, the strongest recommendations for clinical care implications is provider awareness of the relationship between chronic pain and sleep disorders/disturbances and consistent employment of screening for sleep disorders and disturbances in the clinical setting. With the roll-out of the Mission Act, which, in part, supports veteran care by non-VA providers in community-based settings, chronic pain and sleep disorder “best practices” of the VA will necessarily need to be shared and communicated with civilian providers. By doing so, a consistent standard of care can be ensured for veterans, regardless of their care access point.
Limitations
This review could not pursue direct comparison across studies due to the heterogeneity of included studies and mixed level and quality of evidence. Most studies were classified as low quality/major flaws; readers should be mindful of the methodological limitations of the current evidence when interpreting the results of this systematic scoping review as more rigorous studies may not demonstrate similar findings. The findings should be interpreted in the context of the veteran samples included in this review – i.e., predominantly middle-aged, white, American male veterans. Furthermore, due to the high proportion of low quality, level 3 studies, uncontrolled variables could have introduced bias to study results. Although the presence of presumed or diagnosed chronic pain was carefully assessed, it is possible that some of the included studies had samples inclusive of veterans without chronic pain as defined by standardized diagnostic manuals. However, the existence of contextual cues such as the inclusion of a pain interference measure [39,42,49,51,55], discussion of results in the context of chronic pain [49,50], and personal communication with corresponding authors [39,51] supports the chronic nature of the pain symptoms assessed among participants included in those studies. Chronic pain definitions were absent or minimally developed or reported across studies. Thus, it is possible that the influence of sleep disturbances and/or sleep disorders on chronic pain outcomes may be different if chronic pain types were better specified.
Conclusion
This systematic scoping review has highlighted that sleep disturbances and sleep disorders are associated with worse pain outcomes among U.S. veterans with chronic nonmalignant pain. Insomnia and OSA were the most common diagnoses among studies assessing the effect of sleep disorders on pain outcomes. Having an OSA diagnosis significantly increased the odds of a reported chronic pain diagnosis and greater pain intensity and sensitivity, while having insomnia was associated with greater pain interference on sleep, increased odds of headache diagnosis, and greater pain-related disability. Preliminary evidence suggests that treatment-induced sleep improvements ameliorate pain. Thus, the relationship of sleep and pain should be considered for the management of chronic pain outcomes in veterans. Further research is needed to expand the current understanding of how sleep disturbances and sleep disorders influence pain, and subsequently inform the development of targeted interventions, particularly those that are non-pharmacological, for the management of chronic pain in veterans.
Supplementary Material
Practice points.
When sleep disturbances and/or sleep disorders co-occur with chronic pain in veterans:
Worse chronic pain outcomes are observed
When chronic pain is evaluated, screening for sleep disorders and sleep disturbances should be prioritized
Treatment-induced sleep improvements ameliorate pain
Sleep disturbance and/or disorder management are potential nonpharmacological opportunities for managing chronic pain
Research agenda.
Recruit & enroll large diverse veteran samples with respect to age, sex, race/ethnicity, and veteran cohort to support a priori planned sub-group comparisons
Carefully conceptualize and operationalize the accurate and precise measurement of distinct sleep disturbances and chronic pain dimensions
Prioritize higher level and quality study designs, including rigorous trials that are adequately powered to determine the magnitude of effect of non-pharmacologic interventions on pain outcomes
Use data mining approaches to analyze existing EHR within the VA’s Corporate Data Warehouse (CDW)
Acknowledgements
Support from American Lung Association (AMS), American Nurses’ Foundation (AMS), National Institutes of Health (RCP, PCC, STK, AMS), Pacira Pharmaceuticals (PCC), the University of Pennsylvania School of Nursing (PCC), VA HSR&D (STK, AMS), and the Van Ameringen Foundation (PCC). The views expressed in this article are those of the authors and do not necessarily reflect the position or policy of the Department of Veterans Affairs or the United States government. Acknowledgement of Richard James, M.L.S, B.A., Nursing Liaison Librarian, University of Pennsylvania Biomedical Library, for his contributions to the search strategy plan and refinements with search implementation.
Abbreviations
- AHI
apnea hypopnea index
- CPAP
continuous positive airway pressure
- CBT-I
cognitive behavioral therapy for insomnia
- EHR
electronic health record(s)
- ICD
International Classification of Diseases
- MBLT
morning bright light therapy
- OEF
Operation Enduring Freedom
- OIF
Operation Iraqi Freedom
- OND
Operation New Dawn
- OSA
obstructive sleep apnea
- PAP
positive airway pressure
- PTSD
post-traumatic stress disorder
- SDB
sleep disordered breathing
- TBI
traumatic brain injury
- US
United States
- VA
veteran administration
Footnotes
Conflicts of interest
The authors do not have any conflicts of interest to disclose.
Appendix A. Supplementary data
Supplementary data to this article can be found online at https://doi.org/10.1016/j.smrv.2020.101411.
References
* The most important references are denoted by an asterisk.
- [1].IASP Subcommittee on Taxonomy. Classification of chronic pain. Descriptions of chronic pain syndromes and definitions of pain terms. Prepared by the International Association for the Study of Pain, Subcommittee on Taxonomy. Pain Suppl 1986;3:S1–226 [Internet], [cited 2018 Dec 2]. Available from: http://www.ncbi.nlm.nih.gov/pubmed/3461421. [PubMed] [Google Scholar]
- [2].Dahlhamer JM, Lucas J, Zelaya C, Nahin R, Mackey S, Debar L, et al. Prevalence of chronic pain and high-impact chronic pain among adults — United States, vol. 67. Morbidity and Mortality Weekly Report. 2018; 2016. p. 1001–6 [Internet], [cited 2018 Dec 2]. Available from: http://www.cdc.gov/mmwr/volumes/67/wr/mm6736a2.htm?s_cid=mm6736a2_w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [3].NIH Interagency Pain Research Coordinating Committee. National pain strategy: a comprehensive population health-level strategy for pain. Available from: https://iprcc.nih.gov/National-Pain-Strategy/Overview; 2016.
- [4].Taylor AM, Phillips K, Patel KV, Turk DC, Dworkin RH, Beaton D, et al. Assessment of physical function and participation in chronic pain clinical trials: IMMPACT/OMERACT recommendations. Pain 2016;157(9):1836–50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [5].McCarberg BH, Nicholson BD, Todd KH, Palmer T, Penles L. The impact of pain on quality of life and the unmet needs of pain management: results from pain sufferers and physicians participating in an internet survey. Am J Ther 2008;15(4):312–20. [DOI] [PubMed] [Google Scholar]
- [6].Hadi MA, McHugh GA, Closs SJ. Impact of chronic pain on patients’ quality of life: a comparative mixed-methods study. J Patient Exp 2019;6(2):133–41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [7].Kawai K, Kawai AT, Wollan P, Yawn BP. Adverse impacts of chronic pain on health-related quality of life, work productivity, depression and anxiety in a community-based study. Fam Pract 2017;34(6):656–61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [8].Gaskin DJ, Richard P. The economic costs of pain in the United States. J Pain 2012;13(8):715–24. 10.1016/j.jpain.2012.03.009 [Internet], Available from:. [DOI] [PubMed] [Google Scholar]
- [9].Chou R, Turner JA, Devine EB, Hansen RN, Sullivan SD, Blazina I, et al. The effectiveness and risks of long-term opioid therapy for chronic pain: a systematic review for a national institutes of health pathways to prevention workshop. Ann Intern Med 2015;162(4):276–86. [DOI] [PubMed] [Google Scholar]
- [10].Fine PG. Long-term consequences of chronic pain: mounting evidence for pain as a neurological disease and parallels with other chronic disease states. Pain Med 2011;12(7):996–1004. [DOI] [PubMed] [Google Scholar]
- [11].Wu YL, Chang LY, Lee HC, Fang SC, Tsai PS. Sleep disturbances in fibro-myalgiaA meta-analysis of case-control studies. J Psychosom Res 2017;96: 89–97. 10.1016/j.jpsychores.2017.03.011 [Internet], (July 2016). Available from:. [DOI] [PubMed] [Google Scholar]
- [12].Stubbs B, Vancampfort D, Thompson T, Veronese N, Carvalho AF, Solmi M, et al. Pain and severe sleep disturbance in the general population: primary data and meta-analysis from 240,820 people across 45 low- and middle-income countries. Gen Hosp Psychiatr 2018;53(May):52–8. [DOI] [PubMed] [Google Scholar]
- [13].Calero K, Sanders S, Stachura Z, Anderson W. OSA common in veterans with chronic pain syndrome. Chest 2016. October;150(4):1288A [Internet], Available from: https://journal.chestnet.org/article/S0012-3692(16)57601-6/pdf. [Google Scholar]
- *[14].Mathias JL, Cant ML, Burke ALJ. Sleep disturbances and sleep disorders in adults living with chronic pain: a meta-analysis. Sleep Med 2018;52: 198–210. 10.1016/j.sleep.2018.05.023 [Internet], Available from:. [DOI] [PubMed] [Google Scholar]
- [15].Taylor DJ, Mallory LJ, Lichstein KL, Durrence HH, Riedel BW, Bush AJ. Comorbidity of chronic insomnia with medical problems. Sleep 2007. February 1;30(2):213–8. [DOI] [PubMed] [Google Scholar]
- *[16].Andersen ML, Araujo P, Frange C, Tufik S. Sleep disturbance and pain: a tale of two common problems. Chest 2018;154(5):1249–59. [DOI] [PubMed] [Google Scholar]
- [17].Finan PH, Goodin BR, Smith MT. The association of sleep and pain: an update and a path forward. J Pain 2013. December;14(12):1539–52 [Internet], Available from: http://www.ncbi.nlm.nih.gov/pmc/articles/PMC4046588/. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [18].Bonvanie IJ, Oldehinkel AJ, Rosmalen JGM, Janssens KAM. Sleep problems and pain: a longitudinal cohort study in emerging adults. Pain 2016;157(4): 957–63. [DOI] [PubMed] [Google Scholar]
- [19].38 U.C.S., §3.1(d) (Title 38 - pensions, bonuses, and veterans’ relief; definitions). 38 U.C.S., §3.1(d) United States of America 2020. [Google Scholar]
- [20].Nahin RL. Severe pain in veterans: the effect of age and sex, and comparisons with the general population. J Pain 2017;18(3):247–54. 10.1016/j.jpain.2016.10.021 [Internet], Available from:. [DOI] [PMC free article] [PubMed] [Google Scholar]
- *[21].Gauntlett-Gilbert J, Wilson S. Veterans and chronic pain. Br J Pain 2013;7(2): 79–84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [22].McGeary D, Moore M, Vriend CA, Peterson AL, Gatchel RJ. The evaluation and treatment of comorbid pain and PTSD in a military setting: an overview. J Clin Psychol Med Settings 2011;18(2):155–63. [DOI] [PubMed] [Google Scholar]
- [23].Veterans Health Administration. Analysis of VA health care utilization among OEF/OIF/OND veterans: cumulative from 1st Qtr FY 2002–3rd Qtr FY 2015 [Internet], Available from: http://www.publichealth.va.gov/epidemiology/reports/oefoifond/health-care-utilization/index.asp; 2017.
- [24].Frank JW, Carey E, Nolan C, Kerns RD, Sandbrink F, Gallagher R, et al. Increased nonopioid chronic pain treatment in the Veterans Health Administration, 2010–2016. Pain Med (United States) 2019;20(5):869–77. [DOI] [PubMed] [Google Scholar]
- [25].Kang HK, Mahan CM, Lee KY, Magee CA, Murphy FM. Illnesses among United States veterans of the Gulf war: a population-based survey of 30,000 veterans. J Occup Environ Med 2000;42(5) [Internet], Available from: https://journals.lww.com/joem/Fulltext/2000/05000/Illnesses_Among_United_States_Veterans_of_the_Gulf.6.aspx. [DOI] [PubMed] [Google Scholar]
- [26].Bramoweth AD, Germain A. Deployment-related insomnia in military personnel and veterans topical collection on sleep disorders. Curr Psychiatr Rep 2013;15(10). [DOI] [PMC free article] [PubMed] [Google Scholar]
- *[27].Alexander M, Ray MA, Hébert JR, Youngstedt SD, Zhang H, Steck SE, et al. The national veteran sleep disorder study: descriptive epidemiology and secular trends, 2000–2010. Sleep 2016;39(7):1399–410. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [28].Curtis I, Arnow B, Pomerantz A, Trivedi RB, Saxon AJ, Post EP, et al. Prevalence, comorbidity, and prognosis of mental health among US veterans. Am J Publ Health 2015;105(12):2564–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [29].Morasco BJ, Lovejoy TI, Lu M, Turk DC, Lewis L, Dobscha SK. The relationship between PTSD and chronic pain: mediating role of coping strategies and depression. Pain 2013. April;154(4):609–16 [Internet], Available from: http://content.wkhealth.com/linkback/openurl?sid=WKPTLP:landingpage&an=00006396-201304000-00013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [30].Edlund MJ, Steffick D, Hudson T, Harris KM, Sullivan M. Risk factors for clinically recognized opioid abuse and dependence among veterans using opioids for chronic non-cancer pain. Pain 2007;129(3):355–62. [DOI] [PubMed] [Google Scholar]
- [31].Morasco BJ, O’Hearn D, Turk DC, Dobscha SK. Associations between prescription opioid use and sleep impairment among veterans with chronic pain. Pain Med 2014. November 1;15(11):1902–10. [DOI] [PubMed] [Google Scholar]
- *[32].VA’s Health Services Research and Development Service (HSR&D). Center of inovation (COIN) for pain research, Informatics. West Haven, CT: Multi-morbidities, and Education (PRIME) Center; 2020. [Internet], [cited 2020 Apr 30]. Available from: https://www.hsrd.research.va.gov/centers/prime/default.cfm. [Google Scholar]
- *[33].Afolalu EF, Ramlee F, Tang NKY. Effects of sleep changes on pain-related health outcomes in the general population: a systematic review of longitudinal studies with exploratory meta-analysis. Sleep Med Rev 2018;39:82–97. 10.1016/j.smrv.2017.08.001 [Internet], Available from:. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [34].Gallagher R, Compton P, Popescu A. The pathophysiology of chronic pain and clinical interfaces with substance use disorder. In: Miller S, Fiellin D, Rosenthal R, Saitz R, editors. The ASAM principles of addiction medicine. 6th ed. Wolters Kluwer; 2019. [Google Scholar]
- [35].Sessle BJ. What is pain, and why and how do we experience pain? In: Lavigne G, Sessle BJ, Choinière M, Soja PJ, editors. Sleep and pain. Seattle: IASP Press; 2007. [Google Scholar]
- [36].Treede RD, Rief W, Barke A, Aziz Q, Bennett MI, Benoliel R, et al. Chronic pain as a symptom or a disease: the IASP classification of chronic pain for the international classification of diseases (ICD-11). Pain 2019;160(1):19–27. [DOI] [PubMed] [Google Scholar]
- [37].Peters MDJ, Godfrey CM, Khalil H, McInerney P, Parker D, Soares CB. Guidance for conducting systematic scoping reviews. Int J Evid Base Healthc 2015;13(3):141–6. [DOI] [PubMed] [Google Scholar]
- [38].Tricco AC, Lillie E, Zarin W, O’Brien KK, Colquhoun H, Levac D, et al. PRISMA extension for scoping reviews (PRISMA-ScR): checklist and explanation. Ann Intern Med 2018;169(7):467–73 [Internet], Available from: http://www.ncbi.nlm.nih.gov/pubmed/30178033. [DOI] [PubMed] [Google Scholar]
- [39].Eakman AM, Schmid AA, Henry KL, Rolle NR, Schelly C, Pott CE, et al. Restoring effective sleep tranquility (REST): a feasibility and pilot study. Br J Occup Ther 2017;80(6). [DOI] [PMC free article] [PubMed] [Google Scholar]
- [40].Amin MM, Gold MS, Broderick JE, Gold AR. The effect of nasal continuous positive airway pressure on the symptoms of Gulf War illness. Sleep Breath 2011;15(3). [DOI] [PubMed] [Google Scholar]
- [41].Sangani S, Baker JF. Sleep hygiene practices and associations with patient-reported outcomes in rheumatoid arthritis. Arthritis Rheum 2016;68(S10): 1906 [Internet], Available from: http://files/368/Sangani S. and Baker J.F. - Sleep hygiene practices and associations with pati.pdf. [Google Scholar]
- [42].Martin JL, Schweizer CA, Hughes JM, Fung CH, Dzierzewski JM, Washington DL, et al. Estimated prevalence of insomnia among women veterans: results of a postal survey. Wom Health Issues. 27(3). [DOI] [PMC free article] [PubMed] [Google Scholar]
- [43].Balba NM, Elliott JE, Weymann KB, Opel RA, Duke JW, Oken BS, et al. Increased sleep disturbances and pain in veterans with comorbid traumatic brain injury and posttraumatic stress disorder. J Clin Sleep Med 2018;14(11). [DOI] [PMC free article] [PubMed] [Google Scholar]
- [44].Jaramillo CA, Eapen BC, McGeary CA, McGeary DD, Robinson J, Amuan M, et al. A cohort study examining headaches among veterans of Iraq and Afghanistan wars: associations with traumatic brain injury, PTSD, and depression. Headache 2016;56(3). [DOI] [PubMed] [Google Scholar]
- *[45].Koffel E, Kroenke K, Bair MJ, Leverty D, Polusny MA, Krebs EE. The bidirectional relationship between sleep complaints and pain: analysis of data from a randomized trial. Health Psychol 2016;35(1). [DOI] [PMC free article] [PubMed] [Google Scholar]
- [46].Taylor KA, Schwartz SW, Sebastião YV, Anderson WM, Foulis PR. Does obstructive sleep apnea mediate the relationship between post-traumatic stress disorder and low back pain in veterans? Sleep. :A348. [Google Scholar]
- [47].Weiner DK, Gentili A, Coffey-Vega K, Morone N, Rossi M, Perera S. Biopsychosocial profiles and functional correlates in older adults with chronic low back pain: a preliminary study. Pain Med 2019. August 7;20(7):1300–10 [Internet], Available from: https://academic.oup.com/painmedicine/article/20/7/1300/4972841. [DOI] [PubMed] [Google Scholar]
- [48].Cunningham TJ, Oehlert ME. The impact of a sleep disorder diagnosis on patients’ medical prognosis in a veteran population. Sleep 2018:A341 [Internet], Available from: https://academic.oup.com/sleep/article/41/suppl_1/A341/4988968. [Google Scholar]
- [49].Hoot MR, Levin HS, Smith AN, Goldberg G, Wilde EA, Walker WC, et al. Pain and chronic mild traumatic brain injury in the US military population: a Chronic Effects of Neurotrauma Consortium study. Brain Inj 2018;32(10). [DOI] [PubMed] [Google Scholar]
- [50].Hughes JM, Jouldjian S, Dzierzewski JM, Vandenberg TZ, Fung CH, Alessi CA, et al. Sleep disturbance is related to poor quality of life, depression and pain in VA adult day health care participants. Sleep 2013:A391 [Internet], Available from: http://www.journalsleep.org/Resources/Documents/2013AbstractSupplement.pdf. [Google Scholar]
- [51].Travaglini LE, Cosgrave J, Klingaman EA. Pain and sleep problems predict quality of life for veterans with serious mental illness. Psychiatr Rehabil J 2019. September;42(3):229–37. 10.1037/prj0000358. Epub 2019 Mar 7. [DOI] [PubMed] [Google Scholar]
- [52].Munds M (2017). Sleep as a moderator of the association between PTSD and chronic pain (Order No. 10639221). Available from ProQuest Dissertations & Theses Global. (1994147102). Retrieved from https://proxy.library.upenn.edu/login?url=https://www-proquest-com.proxy.library.upenn.edu/dissertations-theses/sleep-as-moderator-association-between-ptsd/docview/1994147102/se-2?accountid=14707
- [53].Chapman JB, Lehman CL, Elliott J, Clark JD. Sleep quality and the role of sleep medications for veterans with chronic pain. Pain Med. 7(2). [DOI] [PubMed] [Google Scholar]
- [54].Card M, Charokopos A, Steffens C, Akgun KM, Gunderson C, Goulet J, et al. Obstructive sleep apnea and pain intensity among veterans of recent wars. Am J Respir Crit Care Med 2018:A2421. Meeting Abstracts. [Google Scholar]
- *[55].Lang KP, Veazey-Morris K, Andrasik F. Exploring the role of insomnia in the relation between ptsd and pain in veterans with polytrauma injuries. J Head Trauma Rehabil 2014;29(1):44–53. [DOI] [PubMed] [Google Scholar]
- [56].Dang D, Dearholt S. Johns Hopkins nursing evidence-based practice thrid edition: model and guidelines. In: Sigma theta tau international. 3rd. Indianapolis; 2018. [Google Scholar]
- [57].Popay J, Baldwin S, Arai L, Britten N, Petticrew M, Rodgers M, et al. Guidance on the conduct of narrative synthesis in systematic reviews. ESRC Methods Progr 2007; (January):13. [Google Scholar]
- [58].Ruff RL, Riechers RG, Wang XF, Piero T, Ruff SS. For veterans with mild traumatic brain injury, improved posttraumatic stress disorder severity and sleep correlated with symptomatic improvement. J Rehabil Res Dev 2012;49(9). [DOI] [PubMed] [Google Scholar]
- [59].Ruff RL, Ruff SS, Wang XF. Improving sleep: initial headache treatment in OIF/OEF veterans with blast-induced mild traumatic brain injury. J Rehabil Res Dev 2009;46(9). [DOI] [PubMed] [Google Scholar]
- [60].Jaoude P, Lal A, Vermont L, Porhomayon J, El-Solh AA. Pain intensity and opioid utilization in response to CPAP therapy in veterans with obstructive sleep apnea on chronic opioid treatment. J Clin Sleep Med 2016;12(8):1105–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [61].Koffel E, Amundson E, Wisdom JP. Exploring the meaning of cognitive behavioral therapy for insomnia for patients with chronic pain. Pain Med 2019:1–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [62].Chandler J, Higgins J, Deeks J, Davenport C, Clarke M. Cochrane handbook for systematic reviews of interventions. 2017. p. 1–12. [Google Scholar]
- [63].Powell MA, Corbo V, Fonda JR, Otis JD, Milberg WP, McGlinchey RE. Sleep quality and reexperiencing symptoms of PTSD are associated with current pain in U.S. OEF/OIF/OND veterans with and without mTBIs. J Trauma Stress 2015;28(4). [DOI] [PubMed] [Google Scholar]
- [64].Tighe CA, Youk A, Ibrahim SA, Weiner DK, Vina ER, Kwoh CK, et al. Pain catastrophizing and arthritis self-efficacy as mediators of sleep disturbance and osteoarthritis symptom severity. Pain Med 2019;21(August 2019): 501–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [65].Koffel E, Kats AM, Kroenke K, Bair MJ, Gravely A, DeRonne B, et al. Sleep disturbance predicts less improvement in pain outcomes: secondary analysis of the SPACE randomized clinical trial. Pain Med 2019:1–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [66].Morin CM, Belleville G, Bélanger L, Ivers H. The insomnia severity index: psychometric indicators to detect insomnia cases and evaluate treatment response. Sleep 2011;34(5):601–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- *[67].Burgess HJ, Rizvydeen M, Kimura M, Pollack MH, Hobfoll SE, Rajan KB, et al. An open trial of morning bright light treatment among US military veterans with chronic low back pain: a pilot study. Pain Med 2018:1–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [68].Burns JW, Gerhart J, Rizvydeen M, Kimura M, Burgess HJ. Morning bright light treatment for chronic low back pain: potential impact on the volatility of pain, mood, function, and sleep. Pain Med 2019:1–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [69].Charokopos A, Card ME, Gunderson C, Steffens C, Bastian LA. The association of obstructive sleep apnea and pain outcomes in adults: a systematic review. Pain Med 2018;19(suppl_1):S69–75 [Internet], Available from: https://academic.oup.com/painmedicine/article/19/suppl_1/S69/5089424. [DOI] [PubMed] [Google Scholar]
- [71].Cappuccio FP, Tang NKY, Boulton H, Lereya ST, Miller MA, Wolke D. Non-pharmacological treatments of insomnia for long-term painful conditions: a systematic review and meta-analysis of patient-reported outcomes in randomized controlled trials. Sleep 2015;38(11):1751–64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [72].Jungquist CR, O’Brien C, Matteson-Rusby S, Smith MT, Pigeon WR, Xia Y, et al. The efficacy of cognitive-behavioral therapy for insomnia in patients with chronic pain. Sleep Med 2010. March 1;11(3):302–9 [Internet], [cited 2017 Nov 20];Available from: http://www.sciencedirect.com/science/article/pii/S1389945710000146?via%3Dihub. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [73].Kallweit U, Hidalgo H, Uhl V, Sandor PS. Continuous positive airway pressure therapy is effective for migraines in sleep apnea syndrome. Neurology 2011. March 29;76(13):1189–91 [Internet], Available from: http://www.neurology.org/cgi/doi/10.1212/WNL.0b013e318212aad0. [DOI] [PubMed] [Google Scholar]
- [74].Goksan B, Gunduz A, Karadeniz D, Aǧan K, Tascilar FN, Tan F, et al. Morning headache in sleep apnoea: clinical and polysomnographic evaluation and response to nasal continuous positive airway pressure. Cephalalgia 2009;29(6):635–41. [DOI] [PubMed] [Google Scholar]
- [75].Cho YW, Kim KT, Moon HJ, Korostyshevskiy VR, Motamedi GK, Yang KI. Comorbid insomnia with obstructive sleep apnea: clinical characteristics and risk factors. J Clin Sleep Med 2018;14(3):409–17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [76].Mundt J, Eisenschenk S, Robinson M. (396) Severity and likelihood of chronic pain in individuals with obstructive sleep apnea and insomnia. J Pain 2017;18(4):S73. 10.1016/j.jpain.2017.02.246 [Internet], Available from:. [DOI] [Google Scholar]
- [77].Dunietz GL, Swanson LM, Jansen EC, Chervin RD, Brien LMO, Lisabeth LD, et al. Key insomnia symptoms and incident pain in older adults : direct and mediated pathways through depression and anxiety. Sleep 2018. September 1;41(July):1–8. 10.1093/sleep/zsy125[Internet], Available from:. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [78].Mallampalli MP, Carter CL. Exploring sex and gender differences in sleep health: a society for women’s health research report. J Wom Health 2014;23(7):553–62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [79].Harrington JJ, Avidan AY. Sleep and aging. Sleep deprivation causes. Eff Treat 2009:143–75. [Google Scholar]
- [80].Bartley EJ, Fillingim RB. Sex differences in pain: a brief review of clinical and experimental findings. Br J Anaesth 2013;111(1):52–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [81].Rustøen T, Wahl AK, Hanestad BR, Lerdal A, Paul S, Miaskowski C. Age and the experience of chronic pain. Clin J Pain 2005. November;21(6):513–23 [Internet], Available from: http://journals.lww.com/00002508-200511000-00008. [DOI] [PubMed] [Google Scholar]
- [82].Villaneuva ATC, Buchanan PR, Yee BJ, Grunstein RR. Ethnicity and obstructive sleep apnoea. Sleep Med Rev 2005;9(6):419–36. [DOI] [PubMed] [Google Scholar]
- [83].Tate DF, Cooper DB, Jaramillo CA, Wang C-P, York GE, Pugh MJ, et al. Subgroups of US Iraq and Afghanistan veterans: associations with traumatic brain injury and mental health conditions. Brain Imaging Behav 2015;9(3): 445–55. [DOI] [PubMed] [Google Scholar]
- [84].Rahavard BB, Candido KD, Knezevic NN. Different pain responses to chronic and acute pain in various ethnic/racial groups. Pain Manag 2017. September;7(5): 427–53. [DOI] [PubMed] [Google Scholar]
- [85].Petersen R, Pan L, Blanck HM. Racial and ethnic disparities in adult obesity in the United States: CDC’s tracking to inform state and local action. Prev Chronic Dis 2019. April 11;16:180579 [Internet], Available from: http://www.cdc.gov/pcd/issues/2019/18_0579.htm. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [86].González HM, Tarraf W, Whitfield KE, Vega WA. The epidemiology of major depression and ethnicity in the United States. J Psychiatr Res 2010;44(15): 1043–51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [87].Amaral EFL, Pollard MS, Mendelsohn J, Cefalu M. Current and future demographics of the veteran population, 2014–2024. Popul Rev 2018;57(1): 28–60. [Google Scholar]
- [88].Governance Data and Affairs Analytics. Department of veterans. Minority veterans report: military service history and VA benefit utilization statistics. 2017. March. p. 23. Available from: https://www.va.gov/vetdata/docs/SpecialReports/Minority_Veterans_Report.pdf.
- [89].VA’s Health Services Research and Development Service (HSR&D), VA Women’s Health Research Network (WHRN). VA women’s health research network (WHRN) - executive summary (october 2019), 21; 2019. [Internet], Available from: https://www.hsrd.research.va.gov/centers/whrn/WHRN-Exec-Summary.pdf.
- [90].Segal JP, Tresidder KA, Bhatt C, Gilron I, Ghasemlou N. Circadian control of pain and neuroinflammation. J Neurosci Res 2018;96(6):1002–20. [DOI] [PubMed] [Google Scholar]
- [91].DeViva JC, Rosen MI, Cooney NL, Black AC. Ecological momentary assessment of sleep and PTSD symptoms in a veteran sample. Psychol Trauma Theory, Res Pract Policy. 2019;12(2):186–92. [DOI] [PubMed] [Google Scholar]
- [92].Possemato K, Maisto SA, Wade M, Barrie K, McKenzie S, Lantinga LJ, et al. Ecological momentary assessment of PTSD symptoms and alcohol use in combat veterans. Psychol Addict Behav 2015;29(4):894–905. [DOI] [PubMed] [Google Scholar]
- [93].Dansie EJ, Turk DC. Assessment of patients with chronic pain. Br J Anaesth 2013;111(1):19–25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- *[94].Kroenke K, Krebs EE, Turk D, Von Korff M, Bair MJ, Allen KD, et al. Core outcome measures for chronic musculoskeletal pain research: recommendations from a veterans health administration work group. Pain Med 2018: 1–9. [DOI] [PubMed] [Google Scholar]
- [95].U.S. Department of Veteran Affairs. VA achieves critical milestone in its electronic health record modernization program [Internet], [cited 2020 Apr 17]. Available from: https://www.va.gov/opa/pressrel/pressrelease.cfm?=id5286; 2019.
- [96].Price LE, Shea K, Gephart S. The veterans affairs’s corporate data Warehouse: uses and implications for nursing research and practice. Nurs Adm Q 2015;39(4):311–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


