Abstract
Coronavirus disease 2019 (COVID-19) is caused by infection of the coronavirus severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) with typical respiratory symptoms. SARS-CoV-2 invades not only the respiratory system, but also other organs expressing the cell surface receptor angiotensin converting enzyme 2. In particular, the digestive system is a susceptible target of SARS-CoV-2. Gastrointestinal symptoms of COVID-19 include anorexia, nausea, vomiting, diarrhea, abdominal pain, and liver damage. Patients with digestive damage have a greater chance of progressing to severe or critical illness, a poorer prognosis, and a higher risk of death. This paper aims to summarize the digestive system symptoms of COVID-19 and discuss fecal-oral contagion of SARS-CoV-2. It also describes the characteristics of inflammatory bowel disease patients with SARS-CoV-2 infection and discusses precautions for preventing SARS-CoV-2 infection during gastrointestinal endoscopy procedures. Improved attention to digestive system abnormalities and gastrointestinal symptoms of COVID-19 patients may aid health care providers in the process of clinical diagnosis, treatment, and epidemic prevention and control.
Keywords: COVID-19, Digestive system, Liver function, Mechanisms, Inflammatory bowel disease, Endoscopy
Core Tip: Coronavirus infections can cause a series of digestive diseases and may also be accompanied by digestive manifestations. Furthermore, the potential mechanisms of coronavirus disease 2019 (COVID-19) on the digestive system, the fecal-oral contagion of severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2), the characteristics of inflammatory bowel disease patients with SARS-CoV-2 infection, and the management during gastrointestinal endoscopy procedures are also discussed. This review provides a new perspective to clinicians for the prevention and treatment of COVID-19.
INTRODUCTION
Coronavirus disease 2019 (COVID-19) is a neo-type respiratory infectious disease caused by severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2; previously known as 2019-nCoV). SARS-CoV-2 emerged in Wuhan, Hubei Province in late December 2019[1] and quickly spread throughout China and subsequently throughout over 213 countries, evolving into a pandemic and threatening global public health by human-to-human transmission. By December 29, 2020, over 79 million reported cases and over 1.7 million deaths have been confirmed globally since the start of the pandemic[2].
SARS-CoV-2 is a single-stranded positive-sense RNA virus belonging to the β-coronavirus family. SARS-CoV-2 shows over 88% homology with two bat-derived severe acute respiratory syndrome (SARS)-related coronaviruses[3] and is identified as the eighth coronavirus with human infection capacity[4]. Other similar coronaviruses with this capacity include SARS-CoV, which causes severe acute respiratory syndrome, and Middle East respiratory syndrome (MERS-CoV), which causes Middle East respiratory syndrome. In contrast to SARS-CoV and MERS-CoV, the new virus is highly transmissible between individuals even during the pre-clinical phase. It has higher transmission and infection potentiality but a reported lower mortality rate as compared to SARS-CoV and MERS-CoV[5]. Although respiratory compromise with dominant symptoms of fever and cough is the cardinal feature of the disease, involvement of the gastrointestinal (GI) tract and the hepatic system has been increasingly reported. In this review article, we discuss in detail GI symptoms and the role of liver involvement in COVID-19. We also discuss the possible effects of COVID-19 in inflammatory bowel disease (IBD) patients and precautions to be taken during GI endoscopy procedures.
DIGESTIVE TRANSMISSION OF COVID-19 OUTBREAKS
SARS-CoV-2 is spread and transmitted mainly through direct or indirect droplet exposure. The finding of SARS-CoV-2 nucleic acid in patients’ feces indicates that SARS-CoV-2 has the potential to be transmitted through the fecal-oral route. Several studies have reported the presence of viral RNA in feces or anal/rectal swabs of patients with COVID-19[6-9]. In a study about SARS-CoV-2 detection in the specimens of 205 COVID-19 patients, the live virus was detected in 29% of fecal specimens, implying that SARS-CoV-2 may be transmitted by the fecal route[10]. Guan et al[11] found that SARS-CoV-2 RNA was detected in four (6.5%) of 62 stool specimens, and four rectal swabs were positive for SARS-CoV-2 RNA. The percentage of positive stool samples has been reported up to 53.42% among hospitalized patients confirmed with COVID-19[12]. Chen et al[13] reported a special case of an infected COVID-19 patient with a positive virus nucleic acid result in a fecal specimen and negative findings on several pharyngeal and sputum samples. This case report contributes to the understanding of the infection route of SARS-CoV-2 by demonstrating that the virus can grow in the digestive tract and may be capable of spreading through fecal-oral transmission[13]. The duration time of positive stool results ranged from 1 to 12 d and 17 patients continued to have positive results in stool after showing negative results in respiratory samples[12]. Another systematic review and meta-analysis found that viral RNA was detected in stool samples from 48.1% of patients in the study sample, even in stool collected after respiratory samples had produced negative test results[14]. These findings indicate that viral gastrointestinal tract infections and potential fecal transmission may have persisted even after the virus was cleared in the respiratory tract. This positive detection of SARS-CoV-2 in stool specimens was a breakthrough because it demonstrated that the virus could replicate and exist in the digestive tract. The duration of viral nucleic acid in feces is longer than that in respiratory specimens, and the peak of viral load is later. Therefore, clinicians should consider the possibility of viral transmission through the fecal-oral route in the management of COVID-19. The importance of the high detection rate of viral RNA in fecal samples needs to be more carefully considered so that fecal-oral transmission of SARS-CoV-2 can be better controlled and prevented.
GASTROINTESTINAL SYMPTOMS OF COVID-19 PATIENTS
The most prominent clinical presentations of COVID-19 in the existing literature are respiratory symptoms such as fever, cough and sputum, and dyspnea. Digestive system symptoms in COVID-19 patients have been increasingly reported with the accumulation of case data as the pandemic continues to evolve[1,15-21] (Table 1). The most commonly reported gastrointestinal symptoms of the disease are diarrhea, anorexia, nausea, vomiting, abdominal discomfort, and gastrointestinal bleeding. Loss of appetite, diarrhea, and vomiting were the three most frequent digestive symptoms in patients with COVID-19. There have been some reports of a small number of patients presenting only with diarrhea and vomiting without fever or cough[22,23]. In a meta-analysis of 60 studies including 4243 patients, the pooled prevalence of all gastrointestinal symptoms was 17.6%[14]. Eleven studies in the meta-analysis compared the prevalence of gastrointestinal symptoms to COVID-19 disease severity; 11.8% of patients with non-severe COVID-19 and 17.1% of patients with severe COVID-19 had gastrointestinal symptoms, indicating that the prevalence of severe disease was more common in patients with gastrointestinal symptoms[14]. Redd et al[24] similarly found that gastrointestinal symptoms occurred in 61.3% of patients included in the study, and they were the predominant presenting complaint amongst 20.3% of patients. Importantly, gastrointestinal manifestations may be the only initial symptoms in some patients with COVID-19. In the study by An et al[25], 54 patients reported only gastrointestinal symptoms without fever or respiratory symptoms. Six (66.7%) patients had anorexia. None of the cases presented with fever at onset, and all cases presented with digestive symptoms occurring 1-3 d prior to admission[25]. Another study involving 1141 COVID-19 patients found that 183/1141 (16%) presented with GI symptoms only[18]. In addition, digestive symptoms appeared to be associated with worse prognoses. Multiple studies have reported a higher incidence of diarrhea, nausea, or vomiting in patients with severe disease as compared to those with non-severe disease. In addition, there is a connection between the presence of diarrhea and respiratory symptom severity; Li et al[26] reported that more patients with diarrhea required ventilator support and were admitted to the intensive care unit (ICU) than those without diarrhea. Pan et al[27] found that patients with digestive symptoms had a longer time from onset to hospital admission as compared to patients without digestive symptoms. Sixty percent of patients without digestive symptoms recovered and were discharged, while only 34.3% of patients with digestive symptoms recovered[27]. Digestive symptoms appeared to be tied to worse COVID-19 outcomes.
Table 1.
Summary of clinical features of coronavirus disease 2019 patients with digestive symptoms, n (%)
| Ref. | Total patients | Diarrhea | Anorexia | Nausea | Vomiting | Abdominal pain |
| Huang et al[1], 2020 | 38 | 1 (2.6) | ||||
| Chen et al[15], 2020 | 99 | 2 (2.0) | 1 (1.0) | 1 (1.0) | ||
| Zhou et al[105], 2020 | 141 | 9 (4.7) | 7 (3.7) | 7 (3.7) | ||
| Mao et al[19], 2020 | 6686 | 601 (9) | 1404 (21) | 401 (6) | 401 (6) | 200 (3) |
| Guan et al[11], 2020 | 1999 | 42 (3.8) | 55 (5.0) | 55 (5.0) | ||
| Pan et al[27], 2020 | 204 | 35 (17.2) | 4 (2.0) | 81 (39.7) | 2 (0.98) | |
| Holshue et al[20], 2020 | 1 (first case) | 1 (100) | 1 (100) | 1 (100) | 1 (100) | 1 (100) |
| Luo et al[18], 2020 | 1141 | 68 (6.0) | 180 (15.8) | 134 (11.7) | 119 (10.4) | 45 (3.9) |
| Han et al[9], 2020 | 206 | 67 (32.5) | 112 (49.5) | 24 (11.7) | 9 (4.4) | |
| Lin et al[106], 2020 | 95 | 23 (24.2) | 17 (17.9) | 17 (17.9) | 4 (4.2) | 2 (2.1) |
| Wang et al[32], 2020 | 138 | 55 (39.9) | 14 (10.1) | 14 (10.1) | 5 (3.6) | 3 (2.2) |
| Zhang et al[107], 2020 | 140 | 17 (12.2) | 18 (12.9) | 24 (17.3) | 7 (5) | 8 (5.8) |
| Liu et al[23], 2020 | 137 | 11 (8.0) |
This bank of published literature provides robust evidence for GI symptoms as common clinical manifestations of COVID-19. GI symptoms should attract the attention of both patients and doctors. It is crucial for clinicians to be on the alert for atypical symptoms to avoid missed COVID-19 diagnosis.
MECHANISMS OF GASTROINTESTINAL TRACT INVOLVEMENT
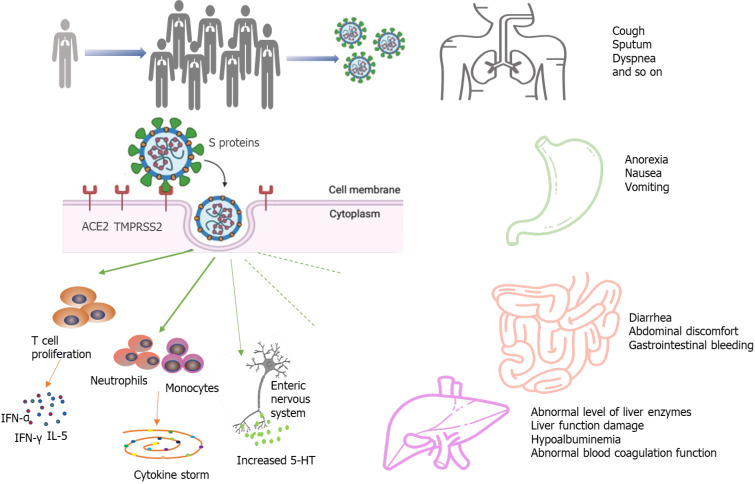
Intestinal damage caused by SARS-CoV-2 infection has been verified by autopsy and biopsy. A recent report described the intestinal autopsy from a COVID-19 patient who developed alternating segmental dilatation and stenosis of the small intestine[28]. However, the mechanism by which SARS-CoV-2 causes gastrointestinal symptoms remains unclear. The pathogenicity of COVID-19 is thought to be related to the angiotensin converting enzyme 2 (ACE2) receptor[29-32]. The SARS-CoV-2 virus consists of four main structural proteins: The spike (S) protein, membrane (M) proteins, nucleocapsid (N) proteins, envelope (E) proteins[33,34]. The S protein is the key component that mediates the entry of the virus into the host cell[35,36]. In the process of infecting cells, the S proteins of most coronaviruses are cleaved into S1 receptor-binding subunit and S2 fusion subunit by host cell Furin-like protease. S1 contains a receptor-binding domain (RBD) that plays an important role in recognizing and binding to the ACE2. Epigallocatechin gallate (EGCG), an active constituent of green tea, has been identified as a potential inhibitor of the RBD domain and other proteins of SARS-CoV-2[37]. The effect of green tea consumption may be explored for inhibition of S protein domains to prevent its binding with ACE2[38]. This may be particularly effective in the gastrointestinal tract, as the oral route implies maximum availability in the digestive tract. The S2 subunit is associated with the fusion of the viral membrane and the host cell membrane. The entry of coronavirus into susceptible cells is a complex process that requires ACE2 receptor binding and the enhancement of proteolytic distribution of protein S[36]. ACE2 is also closely related to the physiological processes of virus infection, virus killing, and natural immunity in the immune system (Figure 1). Transmembrane serine proteinase 2 (TMPRSS2) plays an indispensable role in the invasion and transmission of the virus for S protein priming. ACE2 is distributed in many tissues and organs of the human body. It is not only expressed in the heart, lung, kidney, and blood vessels but also in the digestive system, such as in the duodenum, jejunum, and liver[26]. Hoffmann et al[35] confirmed that the host cell entry of SARS-CoV-2 depends on the SARS-CoV receptor ACE2 and that the entry can be blocked by a clinically proven inhibitor of the cellular serine protease TMPRSS2. Xiao et al[12] observed the staining for viral nucleocapsid protein in the cytoplasm of gastric, duodenal, and rectum glandular epithelial cells and the positive staining for ACE2 and SARS-CoV-2 in the gastrointestinal epithelium from patients who tested positive for SARS-CoV-2 RNA in feces. Another study by Qian et al[39] also observed typical SARS-CoV-2 virus particles in the intestinal epithelial cells of a patient under electron microscopy and obtained direct evidence of active SARS-CoV-2 viral replication in the intestine. Virus particles were found in the cytoplasm of intestinal epithelial cells in a rectal adenocarcinoma patient with co-existing COVID-19 and the virions showed a typical morphology of coronavirus under electron microscopy[39]. These findings indicate that SARS-CoV-2 could directly target gastrointestinal cells, especially gastric and intestinal epithelial cells, leading to inflammatory reactions.
Figure 1.
A simplified diagram of the potential pathological mechanisms for gastrointestinal symptoms in severe acute respiratory syndrome coronavirus 2 infection. ACE2: Angiotensin converting enzyme-2; TMPRSS2: Transmembrane serine protease-2.
The “cytokine storm” has been considered as an essential mechanism of multiple organ dysfunction in COVID-19 patients. High expression of pro-inflammatory cytokines was detected in the serum of most COVID-19 patients, including interferon-γ, interferon-α, and interleukin (IL)-5[1]. The cytokine storm in COVID-19 infection is mainly caused by inducing varieties of pro-inflammatory signals through antigen-presenting cells and T cells against viral infection, and activated macrophages and dendritic cells[40-42]. These cells release chemokines and cytokines to attract more inflammatory cells, such as neutrophils, monocytes, and dendritic cells, which aggregate to the site of inflammation and activate the cascade of inflammatory response. The activated inflammatory cells release more cytokines, further worsening injury to the organs. The fierce cytokine-induced immunopathological attack contributes to the aggravation of patient's condition with a poorer prognosis.
LIVER INJURY IN COVID-19 PATIENTS
An increasing number of studies have reported liver damage in patients with COVID-19 and several have reported COVID-19 patients to have an increased risk of liver dysfunction[1,15,22,43,44]. COVID-19 patients may incur different degrees of liver function damage with elevated aspartate amino transaminase (AST), glutamate moderately amino transaminase (ALT), and total bilirubin (TBil)[45,46]. The risk of liver damage in severe and critically ill patients was higher than in mild patients in most studies. However, there was a subtle difference in the prevalence of lung injury and COVID-19 disease severity across studies, and the exact extent of liver involvement in the COVID-19 disease course remains uncertain[19,46]. In a meta-analysis of 12 studies comprised of 1267 patients, the pooled prevalence of liver injury was 19%, the prevalence of ALT elevation was 18%, the prevalence of AST elevation was 21%, and the prevalence of total bilirubin elevation was 6%[47]. Another cohort study of 1992 patients observed that 215 (11%) patients had an abnormal level of ALT or TBil[41]. Among patients with an abnormal ALT or TBil test result, 77% had a mild increase, 17% had a moderate increase, and 6% had a severe increase[48]. Additionally, the degree of liver function damage appears to correlate with the occurrence of gastrointestinal symptoms. Jin et al[47] reported that the incidence rate of elevated AST was significantly higher in patients with GI symptoms than in those without. Xu et al[49] observed moderate microvesicular steatosis and mild lobular and portal activity in the liver biopsy specimens of the patient with COVID-19, which provided evidence of liver injury. It is worth noting that the elevated prothrombin time among COVID-19 patients with digestive symptoms is common, and several studies have reported thromboembolism as a presenting clinical feature of COVID-19[50-53]. Therefore, liver function and the level of liver enzymes should be monitored early in COVID-19 patients with digestive symptoms (Figure 1).
COVID-19 may promote deterioration of liver function in patients who had been diagnosed with chronic liver disease previously and predict an increased risk for severe illness. Several studies have demonstrated that baseline liver disease severity is strongly associated with COVID-19-related morbidity and mortality; additionally, decompensated cirrhosis, hepatocellular carcinoma, and alcohol-related liver disease are risk factors for adverse outcomes from COVID-19[54-58]. A multi-center study involving 867 patients with chronic liver disease and COVID-19 reported that 14.0% of patients died, 60.4% were hospitalized, 23% were admitted to the ICU, and 7.7% developed hepatic decompensation[55]. Another study indicated that mortality was 32% in COVID-19 patients with a previous history of cirrhosis compared to 8% in those without (P < 0.001)[59]. Moon et al[60] found that 23.3% of patients with cirrhosis and COVID-19 were admitted to the ICU, 17.5% were treated with invasive ventilation, 18.6% were given non-invasive ventilatory support, 4.9% were given renal replacement therapy, and 39.8% died. Nowadays, accumulated data suggest that SARS-CoV-2 infection in patients with cirrhosis appears to be a particularly lethal combination. Compared to the patients without baseline liver disease, the patients with baseline liver disease are prone to unfavorable prognoses.
The mechanisms of liver injury in COVID-19 patients are complex. The higher overall mortality among patients with CLD and COVID-19 may be due to cirrhosis-associated immune dysfunction and metabolic syndrome[61,62], while it needs more research to confirm and explore.
IBD PATIENTS WITH SARS-COV-2 INFECTION
IBD, which mainly includes Crohn's disease (CD) and ulcerative colitis (UC), is a chronic, nonspecific inflammatory disease with unclear etiology and pathogenesis. At present, intestinal microbial growth disorder, intestinal mucosal barrier injury, abnormal immune response in intestinal mucosal tissue, genetic susceptibility, and environmental factors are considered to be involved in the occurrence of intestinal inflammation. Patients with IBD are prone to frequent and severe infections and are often treated with corticosteroids, which are immunosuppressive agents. Whether the clinical presentation in patients with IBD differs from that of non-IBD people and the risk of SARS-CoV-2 infection or development of COVID-19 in patients with active IBD are uncertain[63]. GI symptoms including diarrhea and abdominal pain appear to be more frequent in COVID-19 IBD patients than in the COVID-19 non-IBD group. Aziz et al[64] reported an incidence of 0.3% for COVID-19 in their cohort of 9177 patients with IBD, an ICU admission rate of 8.6% in IBD patients with COVID-19, and a mortality rate of 6.3% in IBD patients with COVID-19. In a multicenter study from eight major gastrointestinal centers in Lombardy, Italy, IBD patients were not reported to have an increased risk of COVID-19 specific symptoms or more severe disease as compared with a control group of gastroenterology patients[65]. Another observational study confirmed that patients with IBD had a higher risk of COVID-19 incidence as compared to the general population and that tumor necrosis factor (TNF) antagonists may reduce the severity of COVID-19[66]. Singh et al[67] indicated no significant differences in the composite outcome of hospitalization or mortality between COVID-19 patients with IBD and those without. In addition, IBD patients with COVID-19 on long-term biologics or non-steroid immunomodulatory therapies did not have a higher risk of worse COVID-19 outcomes[67]. As the pandemic continues, it is essential to continue the collection of descriptive data to determine if patients with IBD are more vulnerable to SARS-CoV-2 infection.
Due to the frequent use of biologics and immune suppressors in patients with IBD, questions arose on whether IBD treatment can be continued during SARS-CoV-2 infection and whether a potential threat from the application of biologics and immune suppressors would be present for patients. Several studies implied that thiopurines, steroids, and oral salicylates could be associated with an increased risk of developing severe COVID-19[68,69]. Feldmann et al[70] indicated that anti-TNF antibodies might reduce some of the processes that occur during COVID-19 lung inflammation. As well, Brenner et al[68] found that tumor necrosis factor antagonists did not appear to be associated with severe COVID-19. Future studies are required to investigate the impact of anti-TNF antibodies. The American Gastroenterological Association (AGA) recommended that patients with IBD who have been infected with SARS-CoV-2 but have not developed COVID-19 should hold thiopurines, methotrexate, and tofacitinib and should delay the biological therapies; on the other hand, patients with IBD who develop COVID-19 should hold thiopurines, methotrexate, tofacitinib, and biological therapies during the viral illness as well and restart previous treatment after complete symptom resolution or negative PCR tests for SARS-CoV-2[63]. Olendzki et al[71] propounded that the Anti-Inflammatory Diet (IBD-AID) was effective in some IBD patients as an adjunct dietary therapy. In the absence of biological therapies, IBD-AID and some identified anti-inflammatory aliments such as ginger, turmeric, and cinnamon may be provided to IBD patients due to their potential inhibitory effects on key pro-inflammatory cytokines such as TNF-α, IL-1β, and IL-6[72].
MALNUTRITIONAL RISK AND NUTRITIONAL INTERVENTION IN COVID-19 PATIENTS
Malnutrition has been associated with outcomes of COVID-19 patients and is correlated with a higher risk of mortality[73]. In a study of 348 severe patients with COVID-19, 161 patients had mild malnutrition and 139 suffered moderate-severe malnutrition. The patients with moderate-severe malnutrition had a lower survival rate and higher mortality compared to those with normal or mild malnutrition[74]. One of the reasons is that COVID-19 induces an acute inflammatory response, which accelerates the consumption of nutrients, such as protein, micronutrients, and glucose[75,76]. Gastrointestinal symptoms caused by SARS-CoV-2 further impacted the nutrition absorption and exacerbated malnutrition. In addition, patients’ anxiety and poor appetite were also potential contributors to malnutrition[11,77,78]. Some studies show that elderly patients with COVID-19 have a higher risk of developing moderate-severe malnutrition and multi-system organ dysfunction due to their weaker immune function and chronic comorbidities[79,80].
The albumin levels are also decreased in COVID-19 patients and are positively correlated with the infection severity[81]. Zhang et al[82] found that 54.78% of COVID-19 patients (63/115) present hypoalbuminemia and among the severe COVID-19 patients, the percentage is 90.32%. Decreased protein synthesis, inadequate nutrition intake, increased tissue catabolism, and nutrient overconsumption may be potential contributors to hypoalbuminemia. Albumin is synthesized in the liver and has several key physiologic functions, such as maintaining colloid oncotic pressure, binding and transporting substances, and sustaining acid-base equilibrium[83]. During the acute stress response, inflammatory mediators such as C-reactive protein, fibrinogen, and alpha 1-antitrypsin are increased, whereas albumin synthesis is decreased[84,85]. Due to the inflammation-induced higher endothelial permeability, albumin can escape to the extravascular space through capillaries, leading to lower serum albumin levels. Hypoalbuminemia may be related to a worse clinical outcomes for COVID-19 patients[73,86-89]. In a retrospective analysis of 181 patients with COVID-19, patients with higher albumin levels on admission were associated with a lower risk of developing ARDS, admission to the ICU, and for every 1 g/dL increase of albumin, there is a 72% decreased risk of developing venous thromboembolism[90]. In another retrospective analysis, the researchers showed significant differences in the rates of hypoalbuminemia (odds ratio = 5.68) between deceased and recovered patients[91].
Previous studies also show that micronutrients play important roles in boosting the immune system[92-94]. Several studies have demonstrated that low levels or decreased intake of micronutrients such as vitamins A, E, B6, B12, Zn, and Se were associated with adverse clinical outcomes of COVID-19[95-99]. Im et al[100] reported that 24.0% of the patients with COVID-19 had severe vitamin D deficiency, and among 12 patients with respiratory distress, 91.7% were deficient in at least one nutrient. They speculated that vitamin D deficiency might be an important risk factor for the poor prognosis of COVID-19. In order to maximize anti-infection nutritional defense, we suggest the daily provision of vitamins and trace elements to malnourished patients with COVID-19. The European Society for Clinical Nutrition and Metabolism (ESPEN) recommended reasonable provision of vitamins and trace elements to benefit anti-infection nutritional defense[101].
Plenty of scientists have emphasized the significance of various nutritional interventions to regulate immune function[102]. Nutrition support is of great importance for patients with severe COVID-19, which can elevate immune function, decrease the incidence of multiple organ failure, and improve the prognosis of the disease. Therefore, the nutritional status of COVID-19 patients should be taken into consideration after admission.
PRECAUTIONS FOR PREVENTING SARS-COV-2 INFECTION DURING GI ENDOSCOPY PROCEDURES
With the growing spread of COVID-19, concerns should be raised with respect to guaranteeing safety for endoscopy operators. Exposure of the respiratory tract and/or digestive tract during endoscopic examination inevitably provides the risk for patients’ secretions and excretions to become potential sources of infection, which will significantly increase the probability of nosocomial cross-infection during the pandemic. Therefore, it is important that endoscopy centers pay great attention to indications that most vitally warrant digestive endoscopy procedures. Iacucci et al[103] recommended four different urgent scenarios that could necessitate endoscopy: Confirmation of a new diagnosis, especially in a moderate-to-severe scenario; a severe acute flare-up in patients with ulcerative colitis; partial bowel obstruction in patients with IBD, which could be secondary to neoplasia or ileocolonic anastomotic stricture; and cholangitis and jaundice in patients with known primary sclerosing cholangitis with dominant bile duct stricture. Further, endoscopists and assistants must strictly execute hospital infection control requirements and take corresponding classified protective measures according to the actual situation of patients. The AGA recommended the use of N95 (or N99 or PAPR) masks instead of surgical masks as part of appropriate personal protective equipment[104]. It is essential to evaluate the risk of patients with suspected or confirmed COVID-19 before endoscopy, in order to defer unnecessary endoscopies to minimize concomitant exposure.
CONCLUSION
Patients with COVID-19 may also develop various gastrointestinal symptoms, which may be pre-existing or not accompanied by respiratory symptoms. The importance of recognizing and diagnosing the occurrence of gastrointestinal symptoms is immeasurable. Clinicians should be cautious in the management of this highly infectious disease and recognize that gastrointestinal symptoms may be characteristic of COVID-19. Careful consideration of gastrointestinal symptoms may enable early COVID-19 detection, diagnosis, isolation, and intervention. Evidence on fecal-oral contagion of SARS-CoV-2 continues to increase. It is therefore important to step up infection control measures to avoid fecal-oral transmission and standardize health care operational processes.
Footnotes
Conflict-of-interest statement: All authors who have affiliations with Zhongnan Hospital of Wuhan University declare that they do not have any competing interests. All authors who have affiliations listed with Dascena (Houston, TX, United States) are employees or contractors of Dascena.
Manuscript source: Invited manuscript
Peer-review started: February 28, 2021
First decision: April 5, 2021
Article in press: May 25, 2021
Specialty type: Gastroenterology and hepatology
Country/Territory of origin: China
Peer-review report’s scientific quality classification
Grade A (Excellent): 0
Grade B (Very good): B
Grade C (Good): 0
Grade D (Fair): 0
Grade E (Poor): 0
P-Reviewer: Khan ZA S-Editor: Gong ZM L-Editor: Wang TQ P-Editor: Wang LL
Contributor Information
Ting-Ting Cao, Department of Gastroenterology, Zhongnan Hospital of Wuhan University, Wuhan 430071, Hubei Province, China.
Gu-Qin Zhang, Department of Respiratory and Critical Care Medicine, Zhongnan Hospital of Wuhan University, Wuhan 430071, Hubei Province, China.
Emily Pellegrini, Dascena Inc., Houston, TX 77080, United States.
Qiu Zhao, Department of Gastroenterology, Zhongnan Hospital of Wuhan University, Wuhan 430071, Hubei Province, China.
Jin Li, Department of Gastroenterology, The Third Affiliated Hospital of Guangzhou Medical University, Guangzhou 510150, Guangdong Province, China.
Lin-jie Luo, Department of Experimental Radiation Oncology and Surgical Oncology, The University of Texas MD Anderson Cancer Center, Houston, TX 77030, United States.
Hua-Qin Pan, Department of Critical Care Medicine, Zhongnan Hospital of Wuhan University, Wuhan 430071, Hubei Province, China; Clinical Research Center of Hubei Critical Care Medicine, Wuhan 430071, Hubei Province, China. phq2012@whu.edu.cn.
References
- 1.Huang C, Wang Y, Li X, Ren L, Zhao J, Hu Y, Zhang L, Fan G, Xu J, Gu X, Cheng Z, Yu T, Xia J, Wei Y, Wu W, Xie X, Yin W, Li H, Liu M, Xiao Y, Gao H, Guo L, Xie J, Wang G, Jiang R, Gao Z, Jin Q, Wang J, Cao B. Clinical features of patients infected with 2019 novel coronavirus in Wuhan, China. Lancet . 2020;395:497–506. doi: 10.1016/S0140-6736(20)30183-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.National Comprehensive Cancer Network. NCCN Clinical Practice Guidelines in Oncology: Hepatobiliary Cancers. [cited 7 July 2020]. Available from: http://www.nccn.org/professionals/physician_gls . [DOI] [PMC free article] [PubMed]
- 3.Lu R, Zhao X, Li J, Niu P, Yang B, Wu H, Wang W, Song H, Huang B, Zhu N, Bi Y, Ma X, Zhan F, Wang L, Hu T, Zhou H, Hu Z, Zhou W, Zhao L, Chen J, Meng Y, Wang J, Lin Y, Yuan J, Xie Z, Ma J, Liu WJ, Wang D, Xu W, Holmes EC, Gao GF, Wu G, Chen W, Shi W, Tan W. Genomic characterisation and epidemiology of 2019 novel coronavirus: implications for virus origins and receptor binding. Lancet . 2020;395:565–574. doi: 10.1016/S0140-6736(20)30251-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Zheng J. SARS-CoV-2: an Emerging Coronavirus that Causes a Global Threat. Int J Biol Sci . 2020;16:1678–1685. doi: 10.7150/ijbs.45053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Liu Y, Gayle AA, Wilder-Smith A, Rocklöv J. The reproductive number of COVID-19 is higher compared to SARS coronavirus. J Travel Med . 2020;27 doi: 10.1093/jtm/taaa021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Wu Y, Guo C, Tang L, Hong Z, Zhou J, Dong X, Yin H, Xiao Q, Tang Y, Qu X, Kuang L, Fang X, Mishra N, Lu J, Shan H, Jiang G, Huang X. Prolonged presence of SARS-CoV-2 viral RNA in faecal samples. Lancet Gastroenterol Hepatol . 2020;5:434–435. doi: 10.1016/S2468-1253(20)30083-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Xu Y, Li X, Zhu B, Liang H, Fang C, Gong Y, Guo Q, Sun X, Zhao D, Shen J, Zhang H, Liu H, Xia H, Tang J, Zhang K, Gong S. Characteristics of pediatric SARS-CoV-2 infection and potential evidence for persistent fecal viral shedding. Nat Med . 2020;26:502–505. doi: 10.1038/s41591-020-0817-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Young BE, Ong SWX, Kalimuddin S, Low JG, Tan SY, Loh J, Ng OT, Marimuthu K, Ang LW, Mak TM, Lau SK, Anderson DE, Chan KS, Tan TY, Ng TY, Cui L, Said Z, Kurupatham L, Chen MI, Chan M, Vasoo S, Wang LF, Tan BH, Lin RTP, Lee VJM, Leo YS, Lye DC Singapore 2019 Novel Coronavirus Outbreak Research Team. Epidemiologic Features and Clinical Course of Patients Infected With SARS-CoV-2 in Singapore. JAMA . 2020;323:1488–1494. doi: 10.1001/jama.2020.3204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Han C, Duan C, Zhang S, Spiegel B, Shi H, Wang W, Zhang L, Lin R, Liu J, Ding Z, Hou X. Digestive Symptoms in COVID-19 Patients With Mild Disease Severity: Clinical Presentation, Stool Viral RNA Testing, and Outcomes. Am J Gastroenterol . 2020;115:916–923. doi: 10.14309/ajg.0000000000000664. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Wang W, Xu Y, Gao R, Lu R, Han K, Wu G, Tan W. Detection of SARS-CoV-2 in Different Types of Clinical Specimens. JAMA . 2020;323:1843–1844. doi: 10.1001/jama.2020.3786. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Guan WJ, Ni ZY, Hu Y, Liang WH, Ou CQ, He JX, Liu L, Shan H, Lei CL, Hui DSC, Du B, Li LJ, Zeng G, Yuen KY, Chen RC, Tang CL, Wang T, Chen PY, Xiang J, Li SY, Wang JL, Liang ZJ, Peng YX, Wei L, Liu Y, Hu YH, Peng P, Wang JM, Liu JY, Chen Z, Li G, Zheng ZJ, Qiu SQ, Luo J, Ye CJ, Zhu SY, Zhong NS China Medical Treatment Expert Group for Covid-19. Clinical Characteristics of Coronavirus Disease 2019 in China. N Engl J Med . 2020;382:1708–1720. doi: 10.1056/NEJMoa2002032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Xiao F, Tang M, Zheng X, Liu Y, Li X, Shan H. Evidence for Gastrointestinal Infection of SARS-CoV-2. Gastroenterology 2020; 158: 1831-1833. :e3. doi: 10.1053/j.gastro.2020.02.055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Chen L, Lou J, Bai Y, Wang M. COVID-19 Disease With Positive Fecal and Negative Pharyngeal and Sputum Viral Tests. Am J Gastroenterol . 2020;115:790. doi: 10.14309/ajg.0000000000000610. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Cheung KS, Hung IFN, Chan PPY, Lung KC, Tso E, Liu R, Ng YY, Chu MY, Chung TWH, Tam AR, Yip CCY, Leung KH, Fung AY, Zhang RR, Lin Y, Cheng HM, Zhang AJX, To KKW, Chan KH, Yuen KY, Leung WK. Gastrointestinal Manifestations of SARS-CoV-2 Infection and Virus Load in Fecal Samples From a Hong Kong Cohort: Systematic Review and Meta-analysis. Gastroenterology . 2020;159:81–95. doi: 10.1053/j.gastro.2020.03.065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Chen N, Zhou M, Dong X, Qu J, Gong F, Han Y, Qiu Y, Wang J, Liu Y, Wei Y, Xia J, Yu T, Zhang X, Zhang L. Epidemiological and clinical characteristics of 99 cases of 2019 novel coronavirus pneumonia in Wuhan, China: a descriptive study. Lancet . 2020;395:507–513. doi: 10.1016/S0140-6736(20)30211-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Zhou Z, Zhao N, Shu Y, Han S, Chen B, Shu X. Effect of Gastrointestinal Symptoms in Patients With COVID-19. Gastroenterology . 2020;158:2294–2297. doi: 10.1053/j.gastro.2020.03.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Wu Z, McGoogan JM. Characteristics of and Important Lessons From the Coronavirus Disease 2019 (COVID-19) Outbreak in China: Summary of a Report of 72 314 Cases From the Chinese Center for Disease Control and Prevention. JAMA . 2020;323:1239–1242. doi: 10.1001/jama.2020.2648. [DOI] [PubMed] [Google Scholar]
- 18.Luo S, Zhang X, Xu H. Don't Overlook Digestive Symptoms in Patients With 2019 Novel Coronavirus Disease (COVID-19) Clin Gastroenterol Hepatol . 2020;18:1636–1637. doi: 10.1016/j.cgh.2020.03.043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Mao R, Qiu Y, He JS, Tan JY, Li XH, Liang J, Shen J, Zhu LR, Chen Y, Iacucci M, Ng SC, Ghosh S, Chen MH. Manifestations and prognosis of gastrointestinal and liver involvement in patients with COVID-19: a systematic review and meta-analysis. Lancet Gastroenterol Hepatol . 2020;5:667–678. doi: 10.1016/S2468-1253(20)30126-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Holshue ML, DeBolt C, Lindquist S, Lofy KH, Wiesman J, Bruce H, Spitters C, Ericson K, Wilkerson S, Tural A, Diaz G, Cohn A, Fox L, Patel A, Gerber SI, Kim L, Tong S, Lu X, Lindstrom S, Pallansch MA, Weldon WC, Biggs HM, Uyeki TM, Pillai SK Washington State 2019-nCoV Case Investigation Team. First Case of 2019 Novel Coronavirus in the United States. N Engl J Med . 2020;382:929–936. doi: 10.1056/NEJMoa2001191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Zhang G, Hu C, Luo L, Fang F, Chen Y, Li J, Peng Z, Pan H. Clinical features and short-term outcomes of 221 patients with COVID-19 in Wuhan, China. J Clin Virol . 2020;127:104364. doi: 10.1016/j.jcv.2020.104364. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Wang D, Hu B, Hu C, Zhu F, Liu X, Zhang J, Wang B, Xiang H, Cheng Z, Xiong Y, Zhao Y, Li Y, Wang X, Peng Z. Clinical Characteristics of 138 Hospitalized Patients With 2019 Novel Coronavirus-Infected Pneumonia in Wuhan, China. JAMA . 2020;323:1061–1069. doi: 10.1001/jama.2020.1585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Liu K, Fang YY, Deng Y, Liu W, Wang MF, Ma JP, Xiao W, Wang YN, Zhong MH, Li CH, Li GC, Liu HG. Clinical characteristics of novel coronavirus cases in tertiary hospitals in Hubei Province. Chin Med J (Engl) . 2020;133:1025–1031. doi: 10.1097/CM9.0000000000000744. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Redd WD, Zhou JC, Hathorn KE, McCarty TR, Bazarbashi AN, Thompson CC, Shen L, Chan WW. Prevalence and Characteristics of Gastrointestinal Symptoms in Patients With Severe Acute Respiratory Syndrome Coronavirus 2 Infection in the United States: A Multicenter Cohort Study. Gastroenterology 2020; 159: 765-767. :e2. doi: 10.1053/j.gastro.2020.04.045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.An P, Chen H, Jiang X, Su J, Xiao Y, Ding Y, Ren H, Ji M, Feiyi Chen, Lv X, Chen M, Li J, Yin A, Kang J, Chen W, Liu S, Tan W, Wu L, Dong W, Cao J, Zhou Z, Shen L, Tan S, Chen G, Zhou J, Yang Y, Yu H. Clinical Features of 2019 Novel Coronavirus Pneumonia Presented Gastrointestinal Symptoms But Without Fever Onset (2/2/2020) Lancet . 2020 [Google Scholar]
- 26.Li MY, Li L, Zhang Y, Wang XS. Expression of the SARS-CoV-2 cell receptor gene ACE2 in a wide variety of human tissues. Infect Dis Poverty . 2020;9:45. doi: 10.1186/s40249-020-00662-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Pan L, Mu M, Yang P, Sun Y, Wang R, Yan J, Li P, Hu B, Wang J, Hu C, Jin Y, Niu X, Ping R, Du Y, Li T, Xu G, Hu Q, Tu L. Clinical Characteristics of COVID-19 Patients With Digestive Symptoms in Hubei, China: A Descriptive, Cross-Sectional, Multicenter Study. Am J Gastroenterol . 2020;115:766–773. doi: 10.14309/ajg.0000000000000620. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Liu Q, Wang RS, Qu GQ, Wang YY, Liu P, Zhu YZ, Fei G, Ren L, Zhou YW, Liu L. Gross examination report of a COVID-19 death autopsy. Fa Yi Xue Za Zhi . 2020;36:21–23. doi: 10.12116/j.issn.1004-5619.2020.01.005. [DOI] [PubMed] [Google Scholar]
- 29.Hikmet F, Méar L, Edvinsson Å, Micke P, Uhlén M, Lindskog C. The protein expression profile of ACE2 in human tissues. Mol Syst Biol . 2020;16:e9610. doi: 10.15252/msb.20209610. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Xu J, Chu M, Zhong F, Tan X, Tang G, Mai J, Lai N, Guan C, Liang Y, Liao G. Digestive symptoms of COVID-19 and expression of ACE2 in digestive tract organs. Cell Death Discov . 2020;6:76. doi: 10.1038/s41420-020-00307-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Chen L, Marishta A, Ellison CE, Verzi MP. Identification of Transcription Factors Regulating SARS-CoV-2 Entry Genes in the Intestine. Cell Mol Gastroenterol Hepatol . 2021;11:181–184. doi: 10.1016/j.jcmgh.2020.08.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Wang C, Wang S, Li D, Wei DQ, Zhao J, Wang J. Human Intestinal Defensin 5 Inhibits SARS-CoV-2 Invasion by Cloaking ACE2. Gastroenterology 2020; 159: 1145-1147. :e4. doi: 10.1053/j.gastro.2020.05.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Chan JF, Kok KH, Zhu Z, Chu H, To KK, Yuan S, Yuen KY. Genomic characterization of the 2019 novel human-pathogenic coronavirus isolated from a patient with atypical pneumonia after visiting Wuhan. Emerg Microbes Infect . 2020;9:221–236. doi: 10.1080/22221751.2020.1719902. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Tortorici MA, Veesler D. Structural insights into coronavirus entry. Adv Virus Res . 2019;105:93–116. doi: 10.1016/bs.aivir.2019.08.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Hoffmann M, Kleine-Weber H, Schroeder S, Krüger N, Herrler T, Erichsen S, Schiergens TS, Herrler G, Wu NH, Nitsche A, Müller MA, Drosten C, Pöhlmann S. SARS-CoV-2 Cell Entry Depends on ACE2 and TMPRSS2 and Is Blocked by a Clinically Proven Protease Inhibitor. Cell 2020; 181: 271-280. :e8. doi: 10.1016/j.cell.2020.02.052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Li W, Moore MJ, Vasilieva N, Sui J, Wong SK, Berne MA, Somasundaran M, Sullivan JL, Luzuriaga K, Greenough TC, Choe H, Farzan M. Angiotensin-converting enzyme 2 is a functional receptor for the SARS coronavirus. Nature . 2003;426:450–454. doi: 10.1038/nature02145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Chourasia M, Koppula PR, Battu A, Ouseph MM, Singh AK. EGCG, a Green Tea Catechin, as a Potential Therapeutic Agent for Symptomatic and Asymptomatic SARS-CoV-2 Infection. Molecules . 2021;26 doi: 10.3390/molecules26051200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Henss L, Auste A, Schürmann C, Schmidt C, von Rhein C, Mühlebach MD, Schnierle BS. The green tea catechin epigallocatechin gallate inhibits SARS-CoV-2 infection. J Gen Virol . 2021;102 doi: 10.1099/jgv.0.001574. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Qian Q, Fan L, Liu W, Li J, Yue J, Wang M, Ke X, Yin Y, Chen Q, Jiang C. Direct evidence of active SARS-CoV-2 replication in the intestine. Clin Infect Dis . 2020 doi: 10.1093/cid/ciaa925. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Ward SG, Westwick J. Chemokines: understanding their role in T-lymphocyte biology. Biochem J . 1998;333 (Pt 3):457–470. doi: 10.1042/bj3330457. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Moore JB, June CH. Cytokine release syndrome in severe COVID-19. Science . 2020;368:473–474. doi: 10.1126/science.abb8925. [DOI] [PubMed] [Google Scholar]
- 42.Tisoncik JR, Korth MJ, Simmons CP, Farrar J, Martin TR, Katze MG. Into the eye of the cytokine storm. Microbiol Mol Biol Rev . 2012;76:16–32. doi: 10.1128/MMBR.05015-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Gao Y, Li T, Han M, Li X, Wu D, Xu Y, Zhu Y, Liu Y, Wang X, Wang L. Diagnostic utility of clinical laboratory data determinations for patients with the severe COVID-19. J Med Virol . 2020;92:791–796. doi: 10.1002/jmv.25770. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Yang X, Yu Y, Xu J, Shu H, Xia J, Liu H, Wu Y, Zhang L, Yu Z, Fang M, Yu T, Wang Y, Pan S, Zou X, Yuan S, Shang Y. Clinical course and outcomes of critically ill patients with SARS-CoV-2 pneumonia in Wuhan, China: a single-centered, retrospective, observational study. Lancet Respir Med . 2020;8:475–481. doi: 10.1016/S2213-2600(20)30079-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Yao N, Wang SN, Lian JQ, Sun YT, Zhang GF, Kang WZ, Kang W. [Clinical characteristics and influencing factors of patients with novel coronavirus pneumonia combined with liver injury in Shaanxi region] Zhonghua Gan Zang Bing Za Zhi . 2020;28:234–239. doi: 10.3760/cma.j.cn501113-20200226-00070. [DOI] [PubMed] [Google Scholar]
- 46.Zhang C, Shi L, Wang FS. Liver injury in COVID-19: management and challenges. Lancet Gastroenterol Hepatol . 2020;5:428–430. doi: 10.1016/S2468-1253(20)30057-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Jin X, Lian JS, Hu JH, Gao J, Zheng L, Zhang YM, Hao SR, Jia HY, Cai H, Zhang XL, Yu GD, Xu KJ, Wang XY, Gu JQ, Zhang SY, Ye CY, Jin CL, Lu YF, Yu X, Yu XP, Huang JR, Xu KL, Ni Q, Yu CB, Zhu B, Li YT, Liu J, Zhao H, Zhang X, Yu L, Guo YZ, Su JW, Tao JJ, Lang GJ, Wu XX, Wu WR, Qv TT, Xiang DR, Yi P, Shi D, Chen Y, Ren Y, Qiu YQ, Li LJ, Sheng J, Yang Y. Epidemiological, clinical and virological characteristics of 74 cases of coronavirus-infected disease 2019 (COVID-19) with gastrointestinal symptoms. Gut . 2020;69:1002–1009. doi: 10.1136/gutjnl-2020-320926. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Chan KH, Poon LL, Cheng VC, Guan Y, Hung IF, Kong J, Yam LY, Seto WH, Yuen KY, Peiris JS. Detection of SARS coronavirus in patients with suspected SARS. Emerg Infect Dis . 2004;10:294–299. doi: 10.3201/eid1002.030610. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Xu Z, Shi L, Wang Y, Zhang J, Huang L, Zhang C, Liu S, Zhao P, Liu H, Zhu L, Tai Y, Bai C, Gao T, Song J, Xia P, Dong J, Zhao J, Wang FS. Pathological findings of COVID-19 associated with acute respiratory distress syndrome. Lancet Respir Med . 2020;8:420–422. doi: 10.1016/S2213-2600(20)30076-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Oxley TJ, Mocco J, Majidi S, Kellner CP, Shoirah H, Singh IP, De Leacy RA, Shigematsu T, Ladner TR, Yaeger KA, Skliut M, Weinberger J, Dangayach NS, Bederson JB, Tuhrim S, Fifi JT. Large-Vessel Stroke as a Presenting Feature of Covid-19 in the Young. N Engl J Med . 2020;382:e60. doi: 10.1056/NEJMc2009787. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Zhang L, Feng X, Zhang D, Jiang C, Mei H, Wang J, Zhang C, Li H, Xia X, Kong S, Liao J, Jia H, Pang X, Song Y, Tian Y, Wang B, Wu C, Yuan H, Zhang Y, Li Y, Sun W, Zhu S, Wang S, Xie Y, Ge S, Zhang L, Hu Y, Xie M. Deep Vein Thrombosis in Hospitalized Patients With COVID-19 in Wuhan, China: Prevalence, Risk Factors, and Outcome. Circulation . 2020;142:114–128. doi: 10.1161/CIRCULATIONAHA.120.046702. [DOI] [PubMed] [Google Scholar]
- 52.Cao T, Zhang G, Xie H, Pellegrini E, Li J, Chen X, Pan H. Case Report: The Coronavirus Disease 2019 (COVID-19) Pneumonia With Multiple Thromboembolism. Front Neurol . 2020;11:625272. doi: 10.3389/fneur.2020.625272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Chen S, Liu H, Li T, Huang R, Gui R, Zhang J. Correlation analysis of coagulation dysfunction and liver damage in patients with novel coronavirus pneumonia: a single-center, retrospective, observational study. Ups J Med Sci . 2020;125:293–296. doi: 10.1080/03009734.2020.1822960. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Qi X, Liu Y, Wang J, Fallowfield JA, Li X, Shi J, Pan H, Zou S, Zhang H, Chen Z, Li F, Luo Y, Mei M, Liu H, Wang Z, Li J, Yang H, Xiang H, Liu T, Zheng MH, Liu C, Huang Y, Xu D, Kang N, He Q, Gu Y, Zhang G, Shao C, Liu D, Zhang L, Kawada N, Jiang Z, Wang F, Xiong B, Takehara T, Rockey DC COVID-Cirrhosis-CHESS Group. Clinical course and risk factors for mortality of COVID-19 patients with pre-existing cirrhosis: a multicentre cohort study. Gut . 2021;70:433–436. doi: 10.1136/gutjnl-2020-321666. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Kim D, Adeniji N, Latt N, Kumar S, Bloom PP, Aby ES, Perumalswami P, Roytman M, Li M, Vogel AS, Catana AM, Wegermann K, Carr RM, Aloman C, Chen V, Rabiee A, Sadowski B, Nguyen V, Dunn W, Chavin K, Zhou K, Lizaola-Mayo B, Moghe A, Debes J, Lee TH, Branch A, Viveiros K, Chan W, Chascsa D, Kwo P, Dhanasekaran R. Predictors of Outcomes of COVID-19 in Patients with Chronic Liver Disease: US Multi-center Study. Clin Gastroenterol Hepatol . 2020 doi: 10.1016/j.cgh.2020.09.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Kuderer NM, Choueiri TK, Shah DP, Shyr Y, Rubinstein SM, Rivera DR, Shete S, Hsu CY, Desai A, de Lima Lopes G Jr, Grivas P, Painter CA, Peters S, Thompson MA, Bakouny Z, Batist G, Bekaii-Saab T, Bilen MA, Bouganim N, Larroya MB, Castellano D, Del Prete SA, Doroshow DB, Egan PC, Elkrief A, Farmakiotis D, Flora D, Galsky MD, Glover MJ, Griffiths EA, Gulati AP, Gupta S, Hafez N, Halfdanarson TR, Hawley JE, Hsu E, Kasi A, Khaki AR, Lemmon CA, Lewis C, Logan B, Masters T, McKay RR, Mesa RA, Morgans AK, Mulcahy MF, Panagiotou OA, Peddi P, Pennell NA, Reynolds K, Rosen LR, Rosovsky R, Salazar M, Schmidt A, Shah SA, Shaya JA, Steinharter J, Stockerl-Goldstein KE, Subbiah S, Vinh DC, Wehbe FH, Weissmann LB, Wu JT, Wulff-Burchfield E, Xie Z, Yeh A, Yu PP, Zhou AY, Zubiri L, Mishra S, Lyman GH, Rini BI, Warner JL COVID-19 and Cancer Consortium. Clinical impact of COVID-19 on patients with cancer (CCC19): a cohort study. Lancet . 2020;395:1907–1918. doi: 10.1016/S0140-6736(20)31187-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Singh S, Khan A. Clinical Characteristics and Outcomes of Coronavirus Disease 2019 Among Patients With Preexisting Liver Disease in the United States: A Multicenter Research Network Study. Gastroenterology 2020; 159: 768-771. :e3. doi: 10.1053/j.gastro.2020.04.064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Bajaj JS, Garcia-Tsao G, Biggins SW, Kamath PS, Wong F, McGeorge S, Shaw J, Pearson M, Chew M, Fagan A, de la Rosa Rodriguez R, Worthington J, Olofson A, Weir V, Trisolini C, Dwyer S, Reddy KR. Comparison of mortality risk in patients with cirrhosis and COVID-19 compared with patients with cirrhosis alone and COVID-19 alone: multicentre matched cohort. Gut . 2021;70:531–536. doi: 10.1136/gutjnl-2020-322118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Marjot T, Moon AM, Cook JA, Abd-Elsalam S, Aloman C, Armstrong MJ, Pose E, Brenner EJ, Cargill T, Catana MA, Dhanasekaran R, Eshraghian A, García-Juárez I, Gill US, Jones PD, Kennedy J, Marshall A, Matthews C, Mells G, Mercer C, Perumalswami PV, Avitabile E, Qi X, Su F, Ufere NN, Wong YJ, Zheng MH, Barnes E, Barritt AS 4th, Webb GJ. Outcomes following SARS-CoV-2 infection in patients with chronic liver disease: An international registry study. J Hepatol . 2021;74:567–577. doi: 10.1016/j.jhep.2020.09.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Moon AM, Webb GJ, Aloman C, Armstrong MJ, Cargill T, Dhanasekaran R, Genescà J, Gill US, James TW, Jones PD, Marshall A, Mells G, Perumalswami PV, Qi X, Su F, Ufere NN, Barnes E, Barritt AS, Marjot T. High mortality rates for SARS-CoV-2 infection in patients with pre-existing chronic liver disease and cirrhosis: Preliminary results from an international registry. J Hepatol . 2020;73:705–708. doi: 10.1016/j.jhep.2020.05.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Albillos A, Lario M, Álvarez-Mon M. Cirrhosis-associated immune dysfunction: distinctive features and clinical relevance. J Hepatol . 2014;61:1385–1396. doi: 10.1016/j.jhep.2014.08.010. [DOI] [PubMed] [Google Scholar]
- 62.Mehta P, McAuley DF, Brown M, Sanchez E, Tattersall RS, Manson JJ HLH Across Speciality Collaboration. UK COVID-19: consider cytokine storm syndromes and immunosuppression. Lancet . 2020;395:1033–1034. doi: 10.1016/S0140-6736(20)30628-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Rubin DT, Feuerstein JD, Wang AY, Cohen RD. AGA Clinical Practice Update on Management of Inflammatory Bowel Disease During the COVID-19 Pandemic: Expert Commentary. Gastroenterology . 2020;159:350–357. doi: 10.1053/j.gastro.2020.04.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Aziz M, Fatima R, Haghbin H, Lee-Smith W, Nawras A. The Incidence and Outcomes of COVID-19 in IBD Patients: A Rapid Review and Meta-analysis. Inflamm Bowel Dis . 2020;26:e132–e133. doi: 10.1093/ibd/izaa170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Maconi G, Bosetti C, De Monti A, Boyapati RK, Shelton E, Piazza N, Carvalhas Gabrielli AM, Lenti MV, Bezzio C, Ricci C, Greco S, Romeo S, Giangregorio F, Gridavilla D, Tagliani F, Massari A, Pastorelli L, Di Sabatino A, Saibeni S, Alicante S, Ferretti F, Rizzardini G, Galli M, Ardizzone S. Risk of COVID 19 in patients with inflammatory bowel diseases compared to a control population. Dig Liver Dis . 2021;53:263–270. doi: 10.1016/j.dld.2020.12.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Rizzello F, Calabrese C, Salice M, Calandrini L, Privitera H, Melotti L, Peruzzi G, Dussias N, Belluzzi A, Scaioli E, Decorato A, Siniscalchi A, Filippone E, Laureti S, Rottoli M, Poggioli G, Gionchetti P. COVID-19 in IBD: The experience of a single tertiary IBD center. Dig Liver Dis . 2021;53:271–276. doi: 10.1016/j.dld.2020.12.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Singh S, Khan A, Chowdhry M, Bilal M, Kochhar GS, Clarke K. Risk of Severe Coronavirus Disease 2019 in Patients With Inflammatory Bowel Disease in the United States: A Multicenter Research Network Study. Gastroenterology 2020; 159: 1575-1578. :e4. doi: 10.1053/j.gastro.2020.06.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Brenner EJ, Ungaro RC, Gearry RB, Kaplan GG, Kissous-Hunt M, Lewis JD, Ng SC, Rahier JF, Reinisch W, Ruemmele FM, Steinwurz F, Underwood FE, Zhang X, Colombel JF, Kappelman MD. Corticosteroids, But Not TNF Antagonists, Are Associated With Adverse COVID-19 Outcomes in Patients With Inflammatory Bowel Diseases: Results From an International Registry. Gastroenterology 2020; 159: 481-491. :e3. doi: 10.1053/j.gastro.2020.05.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Ungaro RC, Brenner EJ, Gearry RB, Kaplan GG, Kissous-Hunt M, Lewis JD, Ng SC, Rahier JF, Reinisch W, Steinwurz F, Underwood FE, Zhang X, Colombel JF, Kappelman MD. Effect of IBD medications on COVID-19 outcomes: results from an international registry. Gut . 2021;70:725–732. doi: 10.1136/gutjnl-2020-322539. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Feldmann M, Maini RN, Woody JN, Holgate ST, Winter G, Rowland M, Richards D, Hussell T. Trials of anti-tumour necrosis factor therapy for COVID-19 are urgently needed. Lancet . 2020;395:1407–1409. doi: 10.1016/S0140-6736(20)30858-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Olendzki BC, Silverstein TD, Persuitte GM, Ma Y, Baldwin KR, Cave D. An anti-inflammatory diet as treatment for inflammatory bowel disease: a case series report. Nutr J . 2014;13:5. doi: 10.1186/1475-2891-13-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Coelho MR, Romi MD, Ferreira DMTP, Zaltman C, Soares-Mota M. The Use of Curcumin as a Complementary Therapy in Ulcerative Colitis: A Systematic Review of Randomized Controlled Clinical Trials. Nutrients . 2020;12 doi: 10.3390/nu12082296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Huang J, Cheng A, Kumar R, Fang Y, Chen G, Zhu Y, Lin S. Hypoalbuminemia predicts the outcome of COVID-19 independent of age and co-morbidity. J Med Virol . 2020;92:2152–2158. doi: 10.1002/jmv.26003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Wei C, Liu Y, Li Y, Zhang Y, Zhong M, Meng X. Evaluation of the nutritional status in patients with COVID-19. J Clin Biochem Nutr . 2020;67:116–121. doi: 10.3164/jcbn.20-91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Stachowska E, Folwarski M, Jamioł-Milc D, Maciejewska D, Skonieczna-Żydecka K. Nutritional Support in Coronavirus 2019 Disease. Medicina (Kaunas) . 2020;56 doi: 10.3390/medicina56060289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Singer P, Blaser AR, Berger MM, Alhazzani W, Calder PC, Casaer MP, Hiesmayr M, Mayer K, Montejo JC, Pichard C, Preiser JC, van Zanten ARH, Oczkowski S, Szczeklik W, Bischoff SC. ESPEN guideline on clinical nutrition in the intensive care unit. Clin Nutr . 2019;38:48–79. doi: 10.1016/j.clnu.2018.08.037. [DOI] [PubMed] [Google Scholar]
- 77.Khan MA, Khan ZA, Charles M, Pratap P, Naeem A, Siddiqui Z, Naqvi N, Srivastava S. Cytokine Storm and Mucus Hypersecretion in COVID-19: Review of Mechanisms. J Inflamm Res . 2021;14:175–189. doi: 10.2147/JIR.S271292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Akhtar S, Das JK, Ismail T, Wahid M, Saeed W, Bhutta ZA. Nutritional perspectives for the prevention and mitigation of COVID-19. Nutr Rev . 2021;79:289–300. doi: 10.1093/nutrit/nuaa063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Liu K, Chen Y, Lin R, Han K. Clinical features of COVID-19 in elderly patients: A comparison with young and middle-aged patients. J Infect . 2020;80:e14–e18. doi: 10.1016/j.jinf.2020.03.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Recinella G, Marasco G, Serafini G, Maestri L, Bianchi G, Forti P, Zoli M. Prognostic role of nutritional status in elderly patients hospitalized for COVID-19: a monocentric study. Aging Clin Exp Res . 2020;32:2695–2701. doi: 10.1007/s40520-020-01727-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Viana-Llamas MC, Arroyo-Espliguero R, Silva-Obregón JA, Uribe-Heredia G, Núñez-Gil I, García-Magallón B, Torán-Martínez CG, Castillo-Sandoval A, Díaz-Caraballo E, Rodríguez-Guinea I, Domínguez-López J. Hypoalbuminemia on admission in COVID-19 infection: An early predictor of mortality and adverse events. A retrospective observational study. Med Clin (Barc) . 2021;156:428–436. doi: 10.1016/j.medcli.2020.12.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Zhang Y, Zheng L, Liu L, Zhao M, Xiao J, Zhao Q. Liver impairment in COVID-19 patients: A retrospective analysis of 115 cases from a single centre in Wuhan city, China. Liver Int . 2020;40:2095–2103. doi: 10.1111/liv.14455. [DOI] [PubMed] [Google Scholar]
- 83.Soni N. Wonderful albumin? BMJ . 1995;310:887–888. doi: 10.1136/bmj.310.6984.887. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Kim S, McClave SA, Martindale RG, Miller KR, Hurt RT. Hypoalbuminemia and Clinical Outcomes: What is the Mechanism behind the Relationship? Am Surg . 2017:83: 1220–1227. doi: 10.1177/000313481708301123. [DOI] [PubMed] [Google Scholar]
- 85.Margarson MP, Soni N. Serum albumin: touchstone or totem? Anaesthesia . 1998:53: 789–803. doi: 10.1046/j.1365-2044.1998.00438.x. [DOI] [PubMed] [Google Scholar]
- 86.Chen S, Zhang D, Zheng T, Yu Y, Jiang J. DVT incidence and risk factors in critically ill patients with COVID-19. J Thromb Thrombolysis . 2021;51:33–39. doi: 10.1007/s11239-020-02181-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Aziz M, Fatima R, Lee-Smith W, Assaly R. The association of low serum albumin level with severe COVID-19: a systematic review and meta-analysis. Crit Care . 2020;24:255. doi: 10.1186/s13054-020-02995-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Huang W, Li C, Wang Z, Wang H, Zhou N, Jiang J, Ni L, Zhang XA, Wang DW. Decreased serum albumin level indicates poor prognosis of COVID-19 patients: hepatic injury analysis from 2,623 hospitalized cases. Sci China Life Sci . 2020;63:1678–1687. doi: 10.1007/s11427-020-1733-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Chen B, Gu HQ, Liu Y, Zhang G, Yang H, Hu H, Lu C, Li Y, Wang L, Zhao Y, Pan H. A model to predict the risk of mortality in severely ill COVID-19 patients. Comput Struct Biotechnol J . 2021;19:1694–1700. doi: 10.1016/j.csbj.2021.03.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Kheir M, Saleem F, Wang C, Mann A, Chua J. Higher albumin levels on admission predict better prognosis in patients with confirmed COVID-19. PLoS One . 2021;16:e0248358. doi: 10.1371/journal.pone.0248358. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Zheng T, Liu X, Wei Y, Li X, Zheng B, Gong Q, Dong L, Zhong J. Laboratory Predictors of COVID-19 Mortality: A Retrospective Analysis from Tongji Hospital in Wuhan. Mediators Inflamm . 2021;2021:6687412. doi: 10.1155/2021/6687412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Gombart AF, Pierre A, Maggini S. A Review of Micronutrients and the Immune System-Working in Harmony to Reduce the Risk of Infection. Nutrients . 2020;12 doi: 10.3390/nu12010236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Elmadfa I, Meyer AL. The Role of the Status of Selected Micronutrients in Shaping the Immune Function. Endocr Metab Immune Disord Drug Targets . 2019;19:1100–1115. doi: 10.2174/1871530319666190529101816. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Wintergerst ES, Maggini S, Hornig DH. Immune-enhancing role of vitamin C and zinc and effect on clinical conditions. Ann Nutr Metab . 2006;50:85–94. doi: 10.1159/000090495. [DOI] [PubMed] [Google Scholar]
- 95.Pizzini A, Aichner M, Sahanic S, Böhm A, Egger A, Hoermann G, Kurz K, Widmann G, Bellmann-Weiler R, Weiss G, Tancevski I, Sonnweber T, Löffler-Ragg J. Impact of Vitamin D Deficiency on COVID-19-A Prospective Analysis from the CovILD Registry. Nutrients . 2020;12 doi: 10.3390/nu12092775. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Semba RD, Tang AM. Micronutrients and the pathogenesis of human immunodeficiency virus infection. Br J Nutr . 1999;81:181–189. doi: 10.1017/s0007114599000379. [DOI] [PubMed] [Google Scholar]
- 97.Papadimitriou-Olivgeris M, Gkikopoulos N, Wüst M, Ballif A, Simonin V, Maulini M, Nusbaumer C, Bertaiola Monnerat L, Tschopp J, Kampouri EE, Wilson P, Duplain H. Predictors of mortality of influenza virus infections in a Swiss Hospital during four influenza seasons: Role of quick sequential organ failure assessment. Eur J Intern Med . 2020;74:86–91. doi: 10.1016/j.ejim.2019.12.022. [DOI] [PubMed] [Google Scholar]
- 98.Goncalves-Mendes N, Talvas J, Dualé C, Guttmann A, Corbin V, Marceau G, Sapin V, Brachet P, Evrard B, Laurichesse H, Vasson MP. Impact of Vitamin D Supplementation on Influenza Vaccine Response and Immune Functions in Deficient Elderly Persons: A Randomized Placebo-Controlled Trial. Front Immunol . 2019;10:65. doi: 10.3389/fimmu.2019.00065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Zhang L, Liu Y. Potential interventions for novel coronavirus in China: A systematic review. J Med Virol . 2020;92:479–490. doi: 10.1002/jmv.25707. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Im JH, Je YS, Baek J, Chung MH, Kwon HY, Lee JS. Nutritional status of patients with COVID-19. Int J Infect Dis . 2020;100:390–393. doi: 10.1016/j.ijid.2020.08.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Barazzoni R, Bischoff SC, Breda J, Wickramasinghe K, Krznaric Z, Nitzan D, Pirlich M, Singer P endorsed by the ESPEN Council. ESPEN expert statements and practical guidance for nutritional management of individuals with SARS-CoV-2 infection. Clin Nutr . 2020;39:1631–1638. doi: 10.1016/j.clnu.2020.03.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Butler MJ, Barrientos RM. The impact of nutrition on COVID-19 susceptibility and long-term consequences. Brain Behav Immun . 2020;87:53–54. doi: 10.1016/j.bbi.2020.04.040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Iacucci M, Cannatelli R, Labarile N, Mao R, Panaccione R, Danese S, Kochhar GS, Ghosh S, Shen B. Endoscopy in inflammatory bowel diseases during the COVID-19 pandemic and post-pandemic period. Lancet Gastroenterol Hepatol . 2020;5:598–606. doi: 10.1016/S2468-1253(20)30119-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Sultan S, Lim JK, Altayar O, Davitkov P, Feuerstein JD, Siddique SM, Falck-Ytter Y, El-Serag HB AGA Institute. AGA Rapid Recommendations for Gastrointestinal Procedures During the COVID-19 Pandemic. Gastroenterology 2020; 159: 739-758. :e4. doi: 10.1053/j.gastro.2020.03.072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Zhou F, Yu T, Du R, Fan G, Liu Y, Liu Z, Xiang J, Wang Y, Song B, Gu X, Guan L, Wei Y, Li H, Wu X, Xu J, Tu S, Zhang Y, Chen H, Cao B. Clinical course and risk factors for mortality of adult inpatients with COVID-19 in Wuhan, China: a retrospective cohort study. Lancet . 2020;395:1054–1062. doi: 10.1016/S0140-6736(20)30566-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Lin L, Jiang X, Zhang Z, Huang S, Fang Z, Gu Z, Gao L, Shi H, Mai L, Liu Y, Lin X, Lai R, Yan Z, Li X, Shan H. Gastrointestinal symptoms of 95 cases with SARS-CoV-2 infection. Gut . 2020;69:997–1001. doi: 10.1136/gutjnl-2020-321013. [DOI] [PubMed] [Google Scholar]
- 107.Zhang JJ, Dong X, Cao YY, Yuan YD, Yang YB, Yan YQ, Akdis CA, Gao YD. Clinical characteristics of 140 patients infected with SARS-CoV-2 in Wuhan, China. Allergy . 2020;75:1730–1741. doi: 10.1111/all.14238. [DOI] [PubMed] [Google Scholar]