Abstract
Three new studies show that activity-dependent formation of myelin contributes to memory consolidation and recall, possibly by increasing functional coupling between neuronal ensembles encoding experience.
Synaptic plasticity is considered the cellular substrate of memory, but researchers have recently begun venturing beyond the synapse—indeed, beyond neurons—in search of how memories are made. Two studies published in this issue of Nature Neuroscience advance the growing evidence that experience-dependent formation of myelin in the circuits encoding memory is an important aspect of how memories are consolidated and recalled.
Traditionally myelin is regarded as static, inert insulation on axons, irrelevant to learning. Why would insulation change on axons that merely transmit the output of information processing? How would myelinating glia (oligodendrocytes in the CNS and Schwann cells in the peripheral nervous system) sense neural impulse activity flowing through axons? If action potentials are detected by myelinating glia, through what mechanism would this alter the formation of myelin?
The answers to these questions have recently begun to emerge. Studies have shown that neurotransmitters are released through vesicular and non-vesicular release mechanisms along axons that are firing action potentials and that these signals influence oligodendrocyte proliferation, differentiation and myelination. Activity-dependent myelination1 and remodeling of myelin2 are becoming recognized as new forms of nervous system plasticity, operating together with synaptic plasticity. MRI studies showing changes in the structure of myelinated tracts after learning in humans had implicated myelin plasticity in learning3, but definitive tests of this hypothesis were needed.
A systems-level perspective on learning suggests how myelin might contribute a non-synaptic cellular mechanism of memory. Complex tasks—such as learning to ride a bicycle and evoking rich memories of past experiences with proper context, place, sequence, sights, sounds, smells, emotions and appropriate links to other stored memories—must require coupling between neuron populations from many different brain regions. Precise spike-time arrival is critical for temporal summation of converging inputs, for synaptic plasticity and for coupling neuronal oscillations at appropriate frequencies4. Myelin, as the most effective mechanism of determining the rapid speed of impulse conduction, could help achieve optimal synchrony of spike-time arrival at synaptic relay points in complex neural circuits and thereby contribute to memory formation and recall.
Simon Pan and colleagues5 conducted studies using the well-established experimental model of contextual fear conditioning memory in mice, in which a fear memory is induced by a foot shock delivered in a specific visual, auditory and olfactory context. Crucially, in this study the authors experimentally arrested the formation of new myelin in adult animals. This was achieved by using transgenic mice in which expression of a gene encoding a transcription factor, Myrf, which is essential for the differentiation of mature oligodendrocytes from precursor cells, could be controlled by tamoxifen. This approach has also been used in studies on motor learning, where inhibiting oligodendrogenesis and the formation of new myelin impaired the ability of mice to learn to run on a modified running wheel with missing rungs6, as well as in a recent study on spatial learning in the Morris water maze by Steadman and colleagues7. The results of the new study5 show that remote memory of fear is impaired in adult mice when oligodendrogenesis and new myelin formation are inhibited after conditioning. Moreover, by using fiber photometry to monitor the activity of populations of neurons in prefrontal cortex—a hub of the neural circuitry that mediates fear conditioning—the researchers witnessed neuronal responses to conditioned context cues evolving over time, but not in animals that could not form new myelin. These effects could be reversed by treatment with the drug clemastine, which promotes myelination. The findings indicate that fear conditioning stimulates the proliferation of oligodendrocyte progenitor cells (OPCs), promotes their maturation into mature oligodendrocytes and increases myelin in appropriate brain regions (medial prefrontal cortex) over several weeks following training; they also show that remote recall is impaired when these processes are impeded (Fig. 1).
Fig. 1 |. Consolidation and/or recall of fear memory requires new myelin formation.
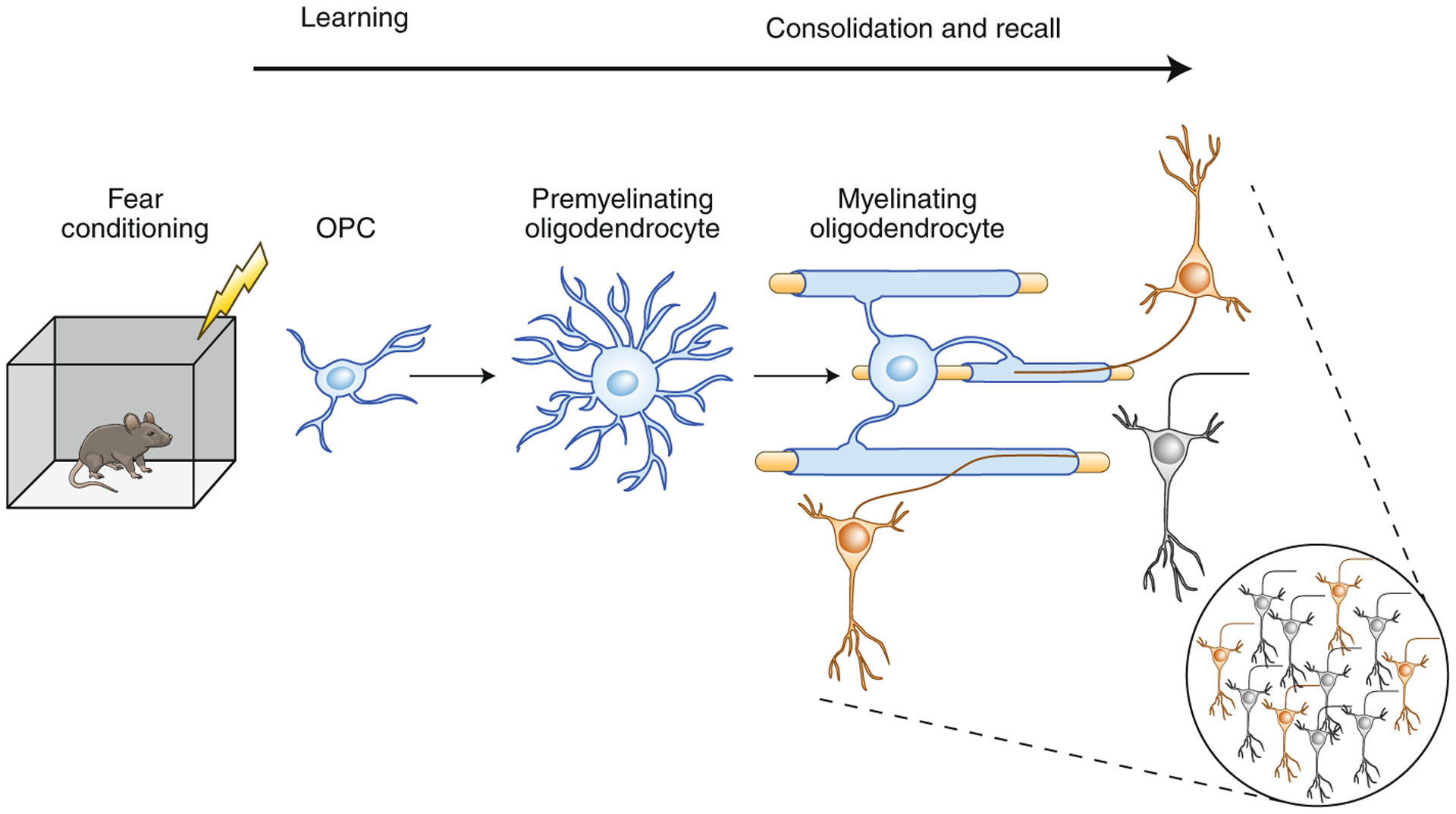
Fear learning induces the proliferation of OPCs, promotes their maturation into mature oligodendrocytes and thereby increases myelin. This process occurs over a timescale of weeks. The study by Pan and colleagues5 shows that memory consolidation and retrieval of remote memory requires new myelin formation, possibly to achieve appropriate conduction velocities for functional coupling among spatially distributed engrams.
An important outcome of these experiments is that interfering with the formation of new myelin impaired retrieval of remote fear memories, but not the ability to learn or to recall shortly after fear conditioning. This is consistent with the findings of Steadman et al., who found that recall in the Morris water maze could be impaired by inhibiting oligodendrogenesis immediately after training was over7. Thus, both studies implicate myelin formation in the process of memory consolidation, which involves coupling the activity of neuron populations across distant regions of the brain. The Steadman study7 further showed that oscillatory coupling between the prefrontal cortex and hippocampus following fear conditioning was impaired when differentiation of OPCs into oligodendrocytes was inhibited. The authors propose that formation of new myelin may promote the optimal conduction speed required to sustain coherent oscillations between the two brain regions.
Together these findings have implications for memory disorders such as post-traumatic stress disorder, in which frightening memories from a past traumatic event are evoked in an inappropriate context. If such memory abnormalities involve aberrations in the formation of new myelin, they may be amenable to treatment by drugs influencing myelination.
Another example of possible medical relevance of myelin formation contributing to memory is that loss of white matter correlates with cognitive decline in Alzheimer’s disease and with age-related cognitive impairment8. A second paper from the lab of Jonah Chan, by Fei Wang and colleagues, in this issue of Nature Neuroscience9 reports that deleting OPCs in young adult mice, by conditional knockout of the gene encoding the essential transcription factor Olig2, inhibits myelination and impairs spatial memory in a Morris water maze task and that clemastine treatment or deleting the muscarinic acetylcholine receptor type 1 in OPCs improved memory performance in aged mice.
Together, these three new studies build on previous research, which has shown that formation of new myelin is involved in implicit learning of a motor task, and now provide for a contribution of myelin formation in explicit learning and in reducing age-related memory decline. Moreover, they expand the role for activity-dependent myelination beyond learning to memory consolidation and recall.
Some differences in results between these studies suggest that oligodendroglial cells may contribute to learning through multiple mechanisms. Oligodendrocytes provide critical trophic support for axons10, which may be necessary to sustain axon function for memory consolidation and recall. This proposal is yet to be tested, but recent studies provide experimental evidence that activity-dependent myelination contributes to learning by promoting synchronous spike-time arrival. In studies in which mice were trained to pull a lever for a reward, myelination increased in subcortical white matter of motor cortex in proportion to increasing proficiency at late stages of learning, but not in transgenic mice with a mild myelin impairment11. Motor learning was restored by repetitive pairing of forelimb movements with optogenetic stimulation of thalamocortical axons to increase synchrony of spike-time arrival in motor cortex. These findings support the hypothesis that learning is enhanced by myelin plasticity promoting synchrony of spike-time arrival, and they demonstrate that brain stimulation combined with physical therapy could be therapeutic for learning and motor dysfunctions involving myelin.
However, synchrony of spike-time arrival also requires slowing conduction velocity from nearby inputs to avoid them arriving prematurely. Recent studies show that myelin can be thinned to reduce conduction velocity and that this is under control of perinodal astrocytes2; whether this process occurs during learning is not yet known. A major question in the field is how neural circuits determine optimal conduction velocities to promote synchrony of arriving inputs.
In addition to oligodendrocytes, the adult brain contains a large reservoir of OPCs, raising the question of whether these cells may have functions in memory beyond maturing into oligodendrocytes to make new myelin. A study by Xiao et al. showed that inhibiting oligodendrogenesis impaired motor learning rapidly, within 2–3 h (ref.12). Although action potentials can induce local translation of myelin basic protein and initiate myelination within tens of minutes13, differentiation into mature oligodendrocytes and the formation of compact myelin require days or weeks. Intriguingly, synapses can form on some OPCs, for reasons that are not well understood14, and these cells interact with neural networks to modulate NMDA receptor-dependent synaptic plasticity by cleavage of the transmembrane glycoprotein NG2 (ref.15).
A major factor that may contribute to some of the differences between these studies is that investigators studying the involvement of myelin plasticity in memory are confronting the fundamental complexity of learning and memory that all memory researchers face. There are many types and several phases of learning and recall, which involve distinct cellular mechanisms and brain regions. Memory consolidation, which involves the reorganization of memory networks shifting from hippocampus to distributed cortical ensembles, proceeds over a time scale compatible with the formation of new myelin, but oligodendrogenesis, myelin remodeling and other aspects of oligodendrocyte biology could also contribute on shorter time courses and/or in different types of memory.
The dogma that myelin is static and only clinically relevant to demyelinating disorders is no longer viable. The recent exploration of non-synaptic, non-neuronal contributions to neural network function and memory, including those of myelin, has not only expanded our understanding of memory but has also opened possibilities of developing novel therapeutic approaches to memory impairments and other cognitive disorders.
Footnotes
Competing interests
The authors declare no competing interests.
References
- 1.Fields RD Nat. Rev. Neurosci 16, 756–767 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Dutta DJ et al. Proc. Natl Acad. Sci. USA 115, 11832–11837 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Zatorre RJ, Fields RD & Johansen-Berg H Nat. Neurosci 15, 528–536 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Pajevic S, Basser PJ & Fields RD Neuroscience 276, 135–147 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Pan S, Mayoral SR, Choi HS, Chan JR & Kheirbek MA Nat. Neurosci 10.1038/s41593-019-0582-1 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.McKenzie IA et al. Science 346, 318–322 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Steadman PE et al. Neuron 105, 150–164.e6 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Bartzokis G et al. Neurobiol. Aging 25, 843–851 (2004). [DOI] [PubMed] [Google Scholar]
- 9.Wang F et al. Nat. Neurosci 10.1038/s41593-019-0588-8 (2020). [DOI] [Google Scholar]
- 10.Nave KA Nat. Rev. Neurosci 11, 275–283 (2010). [DOI] [PubMed] [Google Scholar]
- 11.Kato D et al. Glia 68, 193–210 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Xiao L et al. Nat. Neurosci 19, 1210–1217 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Wake H, Lee PR & Fields RD Science 333, 1647–1651 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Bergles DE, Roberts JD, Somogyi P & Jahr CE Nature 405, 187–191 (2000). [DOI] [PubMed] [Google Scholar]
- 15.Sakry D et al. PLoS Biol. 12, e1001993 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]


