Graphical abstract

Keywords: Cisplatin, Dabigatran, Thrombin, pERK1/2, PAR2
Abbreviations: BUN, Blood urea nitrogen; CDDP, Cisplatin; Dab, Dabigatran; Crcl, Creatinine clerance; GSH, Reduced Glutathion; H&E, Hematoxylin–Eosin; INR, International normalized ratio; KIM-1, kidney injury molecule-1; PAR, protease-activated receptor; Pt, Prothrombin time; Ptt, Partial thromboplastin time; ROS, Reactive oxygen species; Cr, creatinine; SOD, Superoxide dismutase; TF, Tissue factor; FXa, activated form of Factor X
Abstract
Introduction
Cisplatin (CDDP) nephrotoxicity is one of the most significant complications limiting its use in cancer therapy.
Objectives
This study investigated the pivotal role played by thrombin in CDDP-mediated nephrotoxicity. This work also aimed to clarify the possible preventive effect of Dabigatran (Dab), a direct thrombin inhibitor, on CDDP nephrotoxicity.
Methods
Animals were grouped as follow; normal control group, CDDP nephrotoxicity group, CDDP + Dab 15, and CDDP + Dab 25 groups. Four days following CDDP administration, blood and urine samples were collected to evaluate renal function. Moreover, tissue samples were collected from the kidney to determine apoptosis markers, oxidative stress and histopathological evaluation. An immunofluorescence analysis of tissue factor (TF), thrombin, protease-activated receptor-2 (PAR2), fibrin, pERK1/2 and P53 proteins expression was also performed.
Results
Thrombin, pERK, cleaved caspase-3, and oxidative stress markers were significantly elevated in CDDP-treated group. However, pretreatment of animals with either low or high doses of Dab significantly improved kidney function and decreased oxidative stress and apoptotic markers.
Conclusion
We conclude that thrombin is an important factor in the pathogenesis of CDDP kidney toxicity via activation of ERK1/2, P53 and caspase-3 pathway, which can be effectively blocked by Dab.
Introduction
Cisplatin (CDDP) is one of several drugs currently used as anticancer therapy for solid tumors of colon, lung, ovarian and tests [1]. Although CDDP has a high cure rate compared to other chemotherapeutic agents, nephrotoxicity is still one of the most significant limitations to its use in the treatment of cancer [2]. More than 30% of patients treated with CDDP suffer acute renal failure after one or two weeks of treatment [3].
Different hypotheses have been investigated and several studies performed to elucidate the etiology of CDDP nephrotoxicity [4]. It has been reported that CDDP can cause glutathione depletion, reactive oxygen species (ROS) production and an increase of inflammatory mediators and apoptotic markers resulting in cell injury and death [5], [6]. However, this serious problem continues, and many patients are unable to continue treatment with CDDP for a sufficient period of time. Therefore, deep searching for clarifying its mechanism and explore new approaches to diminish CDDP nephrotoxicity has become a necessity.
In our early work, we have shown the involvement of coagulation system activation in the progression of CDDP renal toxicity. Additionally, direct inhibition for activated Factor X (FXa) efficiently minimized CDDP nephrotoxicity [7].
The coagulation cascade controls hemostasis and balance thrombus formation [8]. However, there are reports implicating coagulation factors in inflammatory responses and tissue repair [9]. The coagulation cascade is a series of reactions that, once initiated, through tissue factor (TF) protein expression, continues to the final step of fibrin deposition and formation of fibrin clot [10]. Several proteins are involved in the coagulation cascade, and each one of them has an important role in the activation, amplification or stabilization of clotting [11].
Thrombin is considered to be the a key protein in the coagulation cascade as it promotes thrombus formation via platelets activation and transformation of fibrinogen to fibrin [12]. The role of thrombin is not only limited to coagulation cascade activation, but also stimulates the release of diverse mediators such as platelet-derived growth factor (PDGF), thromboxane A2 and transforming growth factor- β (TGF-β) from platelets [13]. Moreover, thrombin produces significant pro-inflammatory effects by increasing the level of numerous adhesion molecules, chemokines, and cytokines and stimulating ROS production [14].
Thrombin also acts as a chemo-attractant for neutrophils, monocytes and macrophages to the area of inflammation [15]. For these reasons, thrombin is described as a prospect driver of inflammatory reactions in diverse animal models of disease such as multiple sclerosis endotoxemia or sepsis [9]. The pathogenic role of thrombin has been previously reported in cerebral injury [16], liver injury [17], liver fibrosis [18] and neurotoxicity [19].
Dabigatran (Dab) is the first established oral direct thrombin inhibitor drug. Dab directly inhibits both free and clot-bound thrombin thus inhibiting the conversion of fibrinogen into fibrin, the positive feed-back amplification of coagulation activation, cross-linking of fibrin monomers, platelet activation and inhibition of fibrinolysis [12], [20]. Several studies have investigated the possible protective role of Dab in various diseases and toxicities. Dab has been showed to have a protective role against liver fibrosis, vascular endothelial disorder, and DNA damage through antioxidant, anti-inflammatory and anti-apoptotic mechanisms [21], [22].
To our knowledge, there have been no previous studies of the pathological role of thrombin in CDDP induced nephrotoxicity. Therefore, the aim of this study was to evaluate the potential role and mechanism by which thrombin may trigger CDDP induced renal injury and also evaluate the possible modulatory role of Dab as a direct thrombin inhibitor against CDDP nephrotoxicity.
Material and methods
Animals
Male adult albino rats approximately 14–16 weeks of age and weighing 230 ± 20 g at the start of experiment were obtained from animal facility of Nahda University, Beni-Suef, Egypt. Animals were maintained at a standard temperature (25 °C ± 0.5) and humidity (55% ± 1%) with 12 h light/dark cycles for a week prior to initiating the experiments. The rats were kept on standard diet pellets (El-Nasr, Abou-Zaabal, Egypt), and permitted to access water ad libitum. The experimental protocol was performed in accord with Nahda University guidelines and was approved by the Ethical Committee of Nahda University, Beni-Suef, Egypt, (Approval no. NUB-023-020).
Drugs, chemicals, kits and antibodies
Cisplatin was purchased from Mylan Institutional LLC- (Rockford, USA). The antigen retrieval solurion, Dako, was obtained from Dako (Carpinteria, USA). Dab was obtained from Boehringer Ingelheim Pharma and Co. (KG, Germany). Fluoromount and 4, 6-diamidino-2-phenylindole (DAPI) were obtained from Sigma-Aldrich Co. (USA).
Pyrogallol, 1,1–3,3-Tetramethoxypropane, 5,5′-dithiobis-2-nitrobenzoic acid (DTNB), glutathione (GSH) powder, thiobarbituric acid (TBA), N-(1-naphthyl)-ethylenediamine dihydrochloride (NNED), sulfanilamide were purchased from Sigma-Aldrich (U.S.A.). Vanadium chloride was obtained from Across (Belgium). All solvents and chemicals used in the current study were of high analytical grade.
Kits for determination of creatinine (Cr) and blood urea nitrogen (BUN) were obtained from Diamond Laboratory Reagents (Cairo, Egypt). The ELISA kit for rat cystatin-c was purchased from CUSABIO (MD, USA). The kits for partial thromboplastin (Ptt) and prothrombin (Pt) times were purchased from LABiTec GmbH (Ahrens Burg, Germany). Rabbit polyclonal antibody for cleaved caspase-3, Kidney injury molecule-1 (KIM-1) and lipocalin-2/NGAL as well as mouse monoclonal anti-β-actin antibodies were purchased from R&D Systems (MO, USA). Goat monoclonal TF and rabbit polyclonal fibrin antibodies were obtained from Thermo Fisher Scientific Pierce (IL, USA). Mouse monoclonal anti-P53, anti-thrombin, anti-pERK and anti-PAR2 antibodies were obtained from Santa Cruz Biotechnology (TX, USA). Rabbit anti-mouse Cy3, goat anti-rabbit Cy3, rabbit anti-mouse Alexa fluor 488, and donkey anti-goat Alexa fluor 488 conjugated secondary antibodies were obtained from Invitrogen Thermo Fisher Scientific (CA, USA).
Experimental design
A total of 32 male albino rats were classified randomly into 4 groups: Normal control received saline throughout the course of the experiment. The CDDP nephrotoxicity group received saline orally for seven constitutive days and then nephrotoxicity was induced as previously described [23]. Briefly, the rats were given a single dose of CDDP (6 mg/kg i.p.) and then animals were euthanized by cervical dislocation four days following CDDP injection. The CDDP + Dab 15 and CDDP + Dab 25 groups were treated orally with Dab (15 and 25 mg/kg b.w), respectively, seven days prior CDDP treatment and Dab was continued for additional four days following CDDP administration. All rats were kept in metabolic cages for urine collection 24 h before the end of the experiment. Blood samples were collected from the animal's eye orbital sinus under thiopental (50 mg/kg. i.p.) anesthesia and then euthanized by cervical dislocation.
Urine sample were analyzed for KIM-1, creatinine clearance (Crcl) and lipocalin-2 levels. WBCs and platelet count, Pt, Ptt, BUN, serum Cr and cystatin-c were measured using blood samples. Kidney tissue samples were stored in Davidson's fixing solution for the detection of TF and fibrin using immunofluorescence technique and histological analysis.
Biochemical analysis of kidney function
Serum Cr and BUN were measured following manufacturer’s instructions [24], [25] and Crcl was calculated using the following formulae “Crcl = (urine Cr X urine volume) / (serum Cr X 1440)” [26]. Serum cystatin-c was measured by ELISA kits following the manufacturer’s instructions.
Measuring WBCs and platelets count, Pt and Ptt
Total WBCs and platelet counts were measured with an automated hematology analyzer, ABX Micros 60 Analyzer (Montpellier, France). Pt and Ptt were determined using the CoaDATA (Ahrensburg, Germany) automated analyzer following the manufacturer’s instructions [27].
Determination of lipid peroxidation and renal malondialdehyde production (MDA)
Measuring of the of thiobarbituric acid reactive species (TBARS) level indicating the production of lipid peroxidation and determined spectrophotometrically as MDA in tissue homogenates at 520–535 nm as early mentioned [28].
Determination of renal GSH content
Measuring of GSH in the tissue homogenate was done in accordance with the method described earlier [29]. The reduction of DTNB by the sulfhydryl group of GSH is the principle of the resulted color that measure colorimetrically at 412 nm.
Determination of renal superoxide dismutase (SOD) activity
The activity of SOD in the tissue homogenate was measured following a previously described method [30], SOD inhibits the auto-oxidation of pyrogallol which is directly proportional to SOD activity.
Determination of renal nitric oxide (NOx) production
Nitric oxide is the metabolic end product of NO and its measurment depends on the activity of the activity of inducible nitric oxide synthase (iNOS). The resulted NOx was measured colorimetrically at 540 nm as previously mentioned [31].
Immunofluorescence
The immunofluorescence protocol in this study was in accordance with that previously reported [32]. In brief, paraffin was removed from renal tissue using xylol and dehydrated in a graded series of ethyl alcohol concentrations. Antigen were retrieved by boiling with DAKO solution (PH 6) for 20 min in microwave. After washing in 0.05% tween 80 in phosphate buffered saline (PBS), the slides were incubated in methanol for 15 min and blocked with PBS containing10% horse serum and 1% bovine serum albumin for 45 min. The sections were incubated for 2 h with the appropriate antibody and transferred to refrigerator for overnight incubation. After washing, the slides were incubated with the appropriate conjugated secondary antibody for 30 min. The nucleus was counterstained using DAPI, and the slides were mounted using fluoromount G. The slides were analyzed using Leica DM5000 B microscope and the mean fluorescence intensity for 4–6 zones was calculated using Image-J/ NIH software.
Western blot analysis
The expression of cleaved caspase-3 protein and urine lipocalin-2 or KIM-1 content were determined using the western blot method as previously reported [33]. In brief, equal amounts of protein from each samples were run on sodium dodecyl sulfate-polyacrylamide gel electrophoresis (SDS-PAGE) and blotted onto a nitrocellulose membrane using a semi-dry blotter (Bio-Rad). The blocking steps were performed using a blocking solution of tris buffered saline-tween (TBS-T) containing 5% skim milk and incubated for 1 h at room temprature. Incubation of the primary antibodies was performed overnight at 4 °C. The membranes were washed in TBS-T and then immersed in horseradish peroxidase--coupled alkaline phosphatase-coupled (HRP-coupled AP) coupled anti-mouse, anti-rabbit or anti-goat secondary antibodies for 1 h and then washed in TBS-T. The bands were detected using DAB detection kit (Chongqing Biospes Co., Cat. # BWR1069), or by BCIP/NBT detecting kit (Sigma Aldrich, USA), respectively. Quantification of the protein bands were achieved by Image J software and analyzed using Prism 5 software.
Histopathological analysis
Paraffin embedded tissues were sectioned into 5 μm thickness, prepared by slandered process and stained with hematoxylin and eosin (H&E). Slides were analyzed under blinded conditions using a light microscope. Abnormal histopathological features including distortion of the brush border, cell lysis, cast formation, and other features of nephrotoxicity injury were determined.
Statistical analysis of the results
Result are presented as mean ± SEM and analyzed using one-way ANOVA and Tukey's multiple for multiple comparisons. The statistics for the data was achieved using Graph Pad Prism 5 software (San Diego, USA) and the differences are considered significant at p < 0.05.
Results
Effect of CDDP in the presence or absence of Dab on renal function
Animals injected with CDDP showed a significant elevation in cystatin-c, serum Cr and BUN levels and a significant decrease in Crcl compared with normal control rats (Fig. 1A, 1B, 1C, 1D). Also, significant increases in urinary KIM-1 and lipocalin-2 were observed in CDDP -treated animals compared with normal control (Fig. 1E, 1F).
Fig. 1.
Effect of oral pre-treatment of animals with Dab 15 or 25 mg/kg on (A) serum Cr, (B) serum Cyst-C, (C) BUN, (D) Cr cl, (E) urinary KIM-1 and (F) Lipocaline-2 in the urine as compared to CP nephrotoxic group where (a) Differ significantly when compared to normal animals, (b) Differ significantly when compared to normal group nephrotoxic animals and (c) Differ significantly when compared to normal group CDDP + Dab 15.
Animals pretreated with both doses of Dab (15 and 25 mg/kg b.w) had significantly better kidney function. Both doses of Dab resulted in significantly lower BUN, serum Cr, and cystatin-c levels with significantly increased Crcl significantly as compared to CDDP nephrotoxicity group (Fig. 1A, 1B, 1C, 1D). There were no significant differences between Dab 15 and 25 groups except for Crcl, where the Dab 25 group significantly increased Crcl compared to Dab 15 group. Additionally, there was a marked reduction in urinary KIM-1 and lipocalin-2 protein levels in Dab pretreated animals compared with CDDP nephrotoxicity group (Fig. 1E, 1F).
Effect of CDDP alone or with Dab on WBCs, platelet count and coagulation profile
Cisplatin significantly altered blood coagulation profile, WBCs and platelet count of animals. In this study, we observed that injection of CDDP significantly elevated Pt, Ptt, WBCs count and international normalized ratio (INR) and significantly decreased the platelet count compared with control animals (Table 1).
Table 1.
Effect of CDDP alone or with Dab on hematological parameters (WBCs, platelets and coagulation profile).
| WBCs (103/mm3) | Platelet (103/mm3) | Pt (sec) | PC (mg/dl) | Ptt (sec) | INR | |
|---|---|---|---|---|---|---|
| N. control | 8.22 ± 0.39 | 745.1 ± 10.43 | 10.03 ± 0.15 | 124 ± 3.87 | 19.33 ± 0.24 | 0.90 ± 0.01 |
| CDDP nephrotoxic gp | 13.12 ± 0.54a | 410.38 ± 28a | 15.98 ± 0.40a | 55.01 ± 2.91a | 27 ± 1.05a | 1.42 ± 0.03a |
| CDDP + Dab 15 | 10.45 ± 1.300ab | 636.6 ± 23.97b | 10.58 ± 0.160b | 99.14 ± 5.470ab | 22.17 ± 1.190b | 1.010 ± 0.030b |
| CDDP + Dab 25 | 10.67 ± 0.710ab | 717.4 ± 14.31b | 10.63 ± 0.400b | 106.3 ± 8.680ab | 26.33 ± 1.330a | 1.010 ± 0.040b |
- Each value represents the mean of 6–8 experiments ± SEM.
- Statistical analysis was performed using one-way ANOVA followed by Tukey's multiple comparisons test.
aSignificantly different from N. control group value at p < 0.05, (
bSignificantly different from nephrotoxic group value at P < 0.05. where WBCs (White blood cells), PT (prothrombin time), PC (Prothrombin concentration), PTT (Partial thromboplastin time, INR (International normalization ratio).
Pretreatment of animals with a low or high dose of Dab significantly decreased the WBCs count compared with the CDDP nephrotoxicity group. Additionally, Dab pretreatment resulted in normal platelet count, Pt, Ptt and INR values compared with the CDDP nephrotoxicity group (Table 1).
Effect of CDDP alone or with Dab on oxidative stress parameters
Control rats had renal GSH, MDA, NOx production and SOD activity mean values of 95.73 ± 3.41 μmol/g tissue, 45.91 ± 3.21 nmol/g tissue, 124.31 ± 9.42 nmol/g tissue and 0.1 ± 0.001 U/g tissue, respectively. Rats treated with CDDP exhibited a significant reduction in renal GSH content to 23.58 ± 1.04 μmol/g tissue and SOD activity to 0.061 ± 0.002 U/g tissue. Additionally, CDDP significantly increased renal MDA content and NOx production to 72.44 ± 3.530 and 510.2 ± 10.21 nmol/g tissue, respectively compared with normal control rats (Table 2).
Table 2.
Effect of CDDP alone or with Dab on oxidative stress parameters:
| GSH (μmol/g tissue) | MDA (nmol/g tissue) | NOx (nmol/g tissue) | SOD (U/g tissue) | |
|---|---|---|---|---|
| N. Control | 95.73 ± 3.410 | 45.91 ± 4.360 | 124.1 ± 9.420 | 0.100 ± 0.001 |
| CDDP nephrotoxic | 23.58 ± 1.040a | 72.44 ± 3.530a | 510.2 ± 10.21a | 0.061 ± 0.002a |
| CDDP + Dab 15 | 79.04 ± 2.600b | 35.12 ± 2.760b | 295.9 ± 5.940ab | 0.085 ± 0.001ab |
| CDDP + Dab 25 | 60.30 ± 3.890ab | 39.68 ± 2.940b | 314.9 ± 6.360ab | 0.085 ± 0.001ab |
- Each value represents the mean of 6–8 experiments ± SEM.
- Statistical analysis was performed using one-way ANOVA followed by Tukey's multiple comparisons test.
Significantly different from N. control group value at p < 0.05.
Significantly different from CDDP nephrotoxic group value at P < 0.05.
Pretreatment of animals with Dab (15 and 25 mg/kg) markedly increased renal GSH content to 79.04 ± 2.600 and 60.30 ± 3.890 μmol/g tissue, respectively and increased SOD activity to 0.085 ± 0.001 U/g tissue with both doses as compared with CDDP nephrotoxicity group. Additionally, rats treated with Dab (15 and 25 mg/kg) showed significant decreases in renal MDA level to 35.12 ± 2.760 and 39.68 ± 2.940 nmol/g tissue, respectively, as well as renal NOx production to 295.9 ± 5.940 and 314.9 ± 6.360 nmol/g tissue, respectively, compared with the CDDP nephrotoxicity group.
Effect of CDDP alone or with Dab on the expression of TF, thrombin, PAR2 and fibrin
This study noted either negative or basal expression of PAR2, thrombin and fibrin in kidney tubules and glomeruli of the control animals with weak TF protein expression in the tubules (Fig. 2, Fig. 3A and 3B). CDDP injected rats exhibited a significant increase in PAR2 protein expression in renal tubular lumen tissue accompanied with baso-lateral and apical expression of TF protein in rat’s kidney tissue. CDDP also increased thrombin and fibrin expression in renal tubules (Fig. 3A, 3B)
Fig. 2.
Effect of oral pre-treatment of animals with Dab 15 or 25 mg/kg on the expression of coagulation proteins including protease activated receptor (PAR-2) and tissue factor (TF) in renal tissue as compared to CP nephrotoxic group where (a) Differ significantly when compared to normal group, (b) Differ significantly when compared to CP + Dab 15.
Fig. 3.
Effect of oral pre-treatment of animals with Dab 15 or 25 mg/kg on the expression of coagulation proteins including (A) fibrin and (B) Thrombin in renal tissue as compared to CP nephrotoxic group where (a) Differ significantly when compared to normal rats and (b) Differ significantly when compared to nephrotoxic rats.
Pretreatment of animals with Dab reduced PAR2 and TF protein expression in the lumen or baso-lateral location of the kidney (Fig. 2). Additionally, significant reduction in the expression of fibrin and thrombin proteins was observed in rats pretreated with either low or high doses of Dab compared with the CDDP nephrotoxicity group (Fig. 3A, 3B)
Effect of CDDP alone or with Dab on the expression of pERK, P53 and cleaved caspase-3
This current study found a significant increase in the expression of the pro-apoptotic proteins, pERK, P53 and cleaved caspase-3 caused by CDDP in the renal tubules. Pre-treatment with either low or high doses of Dab resulted in a significant decrease of pERK, P53 and cleaved caspase-3 proteins in renal tubules when compared with the CDDP nephrotoxicity group (Fig. 4A, 4B, 4C, 4D).
Fig. 4.
Changes of (A) pERK1/2, (B) P35 and (C) cleaved Caspase-3 proteins expression in renal tissue after oral pre-treatment of animals with Dab 15 or 25 mg/kg as compared to CP nephrotoxic group. (a) Differ significantly when compared to normal animals and (b) Differ significantly when compared to nephrotoxic group.
Effect of CDDP alone or with Dab on the histological characters of renal tissue
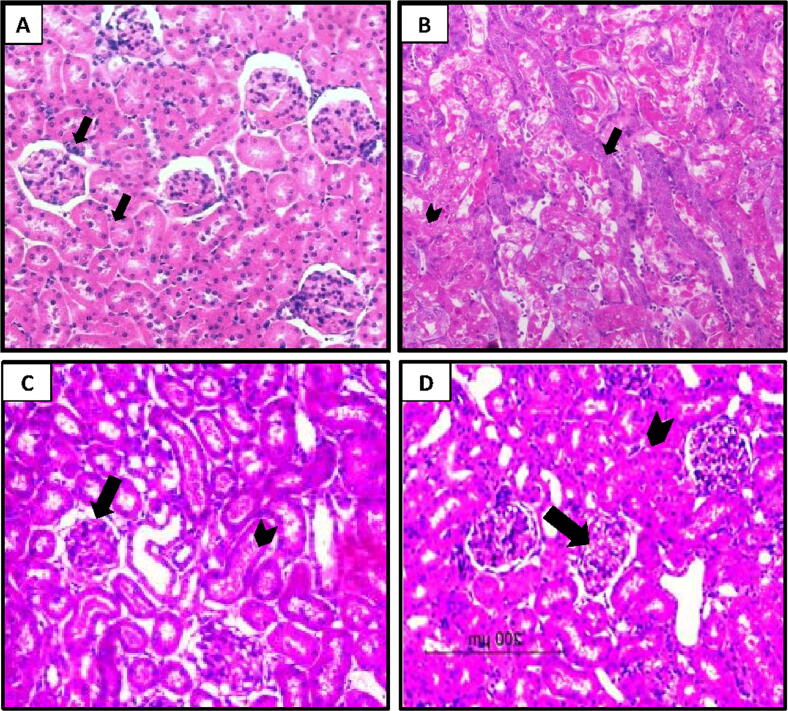
The renal tissues of control rats stained with H&E exhibited normal renal tubules and glomeruli (Fig. 5A). However, the histopathology of renal tissues from rats injected with CDDP showed structural distortion and desquamation of renal epithelial cells, and lumenal deposits of granules (Fig. 5B). In contrast, tissues from Dab (15 and 25 mg/kg) treated rats displayed moderate structural changes and epithelial desquamation and mild lumenal deposits of granules and glomerulonephrosis (Fig. 5C, 5D).
Fig. 5.
Rat kidney tissues of (A) control group; showed normal glomerular and tubular histological features (arrow in black color). (B) CDDP nephrotoxicity group displayed severe changes in the normal architectures and epithelium cells desquamation (arrow in black color) as well as deposits of granules in the apical parts (arrow head) and C,D) CP + Dab 15 and CP + Dab 25 displayed little changes in the structural architectures (arrow head) and mild deposits of the granules in the apical parts and glomerulonephrosis (arrow).
Discussion
This study is the first in-vivo study, to our knowledge, to examine the role of thrombin and its effect on molecular pathways involved in CDDP mediated nephrotoxicity. Moreover, this work is concerned with evaluating the possible protective effect of a direct thrombin inhibitor, Dab, against CDDP nephrotoxicity as a confirmatory evidence of our hypothesis.
In this study, CDDP caused severe nephrotoxicity in rats as evidenced biochemically by significant elevations of serum Cr, BUN, serum cystatin-c, urinary KIM-1 and lipocalin-2 levels as well as significant reduction in Crcl compared with control rats. Additionally, CDDP caused desquamation of the renal epithelium and necrosis and severe degenerative changes in glomerular tuft and tubules. These results are in agreement with a previous study [34] in which rats given a single i.p. dose of 7.5 mg/kg CDDP displayed an increase in nephrotoxicity biomarkers including serum Cr and BUN. Another report also noted that a single i.p. dose of 7.5 mg/kg CDDP significantly elevated renal toxicity biomarkers level and caused remarkable damage to glomerular and tubular structures [35]. Additionally, Dirican et al. in 2016 noted that serum cystatin-c and lipocalin-2 levels were markedly elevated in rats treated with a single i.p. dose of 7.5 mg/kg of CDDP when compared with normal control rat [36].
There are several possible mechanisms to explain CDDP nephrotoxicity, one of which is oxidative stress including over production of ROS and depletion of GSH [37]. This is caused by CDDP accumulation in proximal tubules where it is activated and react with molecules containing thiol thus causing GSH depletion [38]. This in turn causes an elevation of endogenous ROS production within the cells [1]. Additionally, CDDP suppresses renal antioxidant enzyme activity including catalase, SOD, and glutathione peroxidase and increases TBARS [39].
The results of this study confirm the role played by oxidative stress including the dramatic decrease in GSH content and SOD activity, as well as a significant increase in renal MDA content and the production of NOx in renal tissue. It was previously reported that a single injection of 7 mg/kg of CDDP in rats caused significant decreases in renal GSH content and SOD activity [40]. In addition, Tohamy et al. (2016) noted that rats receiving 5 mg/kg of CDDP had significant decreases in hepatic and renal GSH level and SOD activity with significant elevations in hepatic and renal production of nitric oxide and MDA after 5 days [41].
The current study found that apoptosis has an important role in CDDP induced renal injury. The expression of pERK1/2, P53 and cleaved caspase-3 in renal tubules was increased significantly in rats treated with CDDP. These results are in agreement with another study in which administration of 13 mg/kg of CDDP to male BALB/c mice caused activation of ERK1/2 and apoptosis in renal epithelial cells [42]. Another study reported that TNF-alpha expression and caspase-3 activation were reduced through the inhibition of ERK1/2 pathway in renal tissues resulting in a protective effect against CDDP nephrotoxicity [43]. Moreover, it has been noted that caspase-3 activation has an important effect on the initiation of CDDP induced renal injury in rats [33].
In a previous report from our lab, we noted that the activation of the coagulation system has an important role in the initiation of CDDP-mediated nephrotoxicity [7]. The results of this current study found that CDDP significantly elevated the expression of the coagulation proteins TF, fibrin, thrombin and PAR2 in renal tissues. This may be due to CDDP accumulation in renal tubular cells leading to increased expression of TF protein, which acts as the key triggering factor of the coagulation system. TF activates FXa that activates prothrombin (Factor II) to thrombin (Factor IIa) resulting in fibrin deposition [44]. Increased clot production resulted in reduction of the blood flow to the cells and subsequently cellular death [45].
In this study, increased PAR2 and thrombin proteins in the renal tissues of CDDP treated rats is one of the most interesting new findings. To our knowledge, increased thrombin activity in CDDP-treated animals has not been reported. In addition to being a key protein in the coagulation cascade, thrombin is also a potent inducer of platelet aggregation and regulator of inflammation [13].
On the other hand, CDDP caused significant changes in blood coagulation profile. It significantly increased WBCs count and decreased the platelet count resulting in thrombocytopenia. It also significantly prolonged the Pt, Ptt and INR. These results confirm those of Nasr in 2014 who observed a significant decrease in platelet count following i.p. administration of 7.5 mg/kg CDDP [46].
In the present study, CDDP increased ROS production, up regulated the expression of apoptotic proteins such as pERK1/2, P53 and cleaved caspase3, affected blood coagulation profile resulting in leukocytosis accompanied with thrombocytopenia and activated blood coagulation proteins TF, thrombin, PAR2, and fibrin in renal tubular cells. However, an important question is what is the connection between these pathways? Where does this toxicity start? To investigate these questions we blocked the coagulation system using Dab, a direct thrombin inhibitor to counteract thrombin activity.
Dabigatran is an anticoagulant classified as a direct thrombin inhibitor, and its action primarily depends on direct inhibition of thrombin by ionic binding at its active site [47]. In this study, animals pretreated with both low and high Dab doses had significantly reduced expression of thrombin protein in renal tubules. Moreover, Dab markedly down regulated the expression of other coagulation proteins TF, PAR-2, and fibrin that are induced by CDDP. This is similar to the results of Dittmeier et al. (2016) who noted that treatment of animals with 15 mg/kg of Dab significantly decreased thrombin production, inflammation and thrombus formation in ischemic stroke model [48]. It has also been reported that treatment of mice fed a high fatty diet with Dab resulted in significantly reduced fibrin deposition, inflammation and cellular injury in hepatocytes [49].
Dabigatran not only decreased the expression of coagulation proteins but also significantly decreased pro-apoptotic proteins expression. In this study, both doses of Dab significantly decreased pERK1/2 and P53 expression and cleaved caspase-3 protein in renal tubular cells. To our knowledge no earlier work has studied the antiapoptotic effect of Dab in-vivo. The potent anti-apoptotic effect of Dab is a result of the relationship between thrombin, PAR2 and pERK1/2 proteins. Thrombin has significant pro-apoptotic activity due to the activation of PAR which activates the inflammatory and pro-apoptotic effect of blood coagulation [50]. Therefore, PARs may represents the molecular connection between inflammation and coagulation [51]. Additionally, they contribute to tissue injury in multiple diseases including cancer progression, cardiovascular, and metabolic diseases [52], [53]. Four PARs subtypes have been characterized and were expressed on multiple different cell membranes including endothelial cells, leukocytes, and platelets [54].
It is known that thrombin receptors PAR-1, 3, and 4 are rapidly activated by thrombin [54]. Additionally, recent work by Mihara et al. (2016) described thrombin mediated direct activation of PAR2 as another target for thrombin signaling [55]. It has also been reported that PAR2 plays an important role in activating ERK1/2 protein [56]. Activation of ERK by PAR2 has important physiological roles in the nervous system [57] and in PAR2-stimulated cell migration [58] and this might explain the anti-apoptotic activity of Dab.
Another significant finding in this study is the potent antioxidant effect of Dab. As expected, we observed that Dab significantly increased renal content of GSH and SOD activity while producing a significant decrease in the production of renal NOx and MDA. This is similar to results of Yazici et al. (2015) who noted that 15 mg/kg of Dab significantly decreased MDA production in renal ischemia/reperfusion model [59].
It is well known that thrombin has a significant pro-inflammatory and pro-oxidant activity through generation of ROS by smooth muscle cells [14]. In addition, the pro-inflammatory role of thrombin through its promotion of atherosclerosis has been previously reported [60]. Tripathy et al. (2013) reported that thrombin is a key factor in progression of brain inflammation [61]. Moreover, thrombin increased superoxide production which is considered a key step in thrombin-induced atherogenesis [62].
These different mechanisms of Dab explain its reno-protective effect in this study through the reduction of BUN, Cr and cystatin-c in the serum and KIM-1 and lipocalin-2 in the urine as well as improvement noted of Crcl. In this study, Dab attenuated both necrosis and apoptosis through its reduction of the expression of apoptotic proteins; P 53 and cleaved caspase-3 as well as KIM-1 and Lipocaline-2 that considered specific markers for tubular necrosis [63]. Additionally, Dab administration resulted in a normal Pt, Ptt, INR and Platelet count with significant decrease in the WBCs count noted CDDP administration.
Conclusion
In conclusion, the current results found that thrombin has a pivotal role in the development of fibrin formation, the activation of ROS, the activation of apoptosis and consequently the pathogenesis of CDDP nephrotoxicity. Moreover, the direct thrombin inhibitor drug Dab has potent anti-coagulant, anti-apoptotic and anti-oxidant activities that can effectively protect against CDDP nephrotoxicity.
Compliance with ethic requirements
The experimental protocol was performed according to Nahda University guidelines and approved by the ethical Committee of Nahda University, Beni-Suef, Egypt, (Approval no. NUB-023-020).
Declaration of Competing Interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Acknowledgments
Acknowledgements
The authors appreciate the support of Dr. El-Shaymaa El-Nahass for performing the histopathological work and data analysis.
Funding
The authors received no financial support for the research, authorship, and/or publication of this article.
Footnotes
Peer review under responsibility of Cairo University.
References
- 1.Siddik Z.H. Cisplatin: mode of cytotoxic action and molecular basis of resistance. Oncogene. 2003;22:7265–7279. doi: 10.1038/sj.onc.1206933. [DOI] [PubMed] [Google Scholar]
- 2.Oun R., Moussa Y.E., Wheate N.J. The side effects of platinum-based chemotherapy drugs: A review for chemists. Dalt Trans. 2018;47:6645–6653. doi: 10.1039/c8dt00838h. [DOI] [PubMed] [Google Scholar]
- 3.Türk G., Ateşşahin A., Sönmez M., Çeribaşi A.O., Yüce A. Improvement of cisplatin-induced injuries to sperm quality, the oxidant-antioxidant system, and the histologic structure of the rat testis by ellagic acid. Fertil Steril. 2008;89:1474–1481. doi: 10.1016/j.fertnstert.2007.04.059. [DOI] [PubMed] [Google Scholar]
- 4.Pabla N., Murphy R.F., Liu K., Dong Z. The copper transporter Ctr1 contributes to cisplatin uptake by renal tubular cells during cisplatin nephrotoxicity. Am J Physiol Renal Physiol. 2009;296:F505–F511. doi: 10.1152/ajprenal.90545.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Abdel-Daim M.M., Mahmoud O.M., Al Badawi M.H., Alghamdi J., Alkahtani S., Salem N.A. Protective effects of Citrus limonia oil against cisplatin-induced nephrotoxicity. Environ Sci Pollut Res. 2020;27:41540–41550. doi: 10.1007/s11356-020-10066-x. [DOI] [PubMed] [Google Scholar]
- 6.Abdel-Daim M.M., Abdel-Rahman H.G., Dessouki A.A., El-Far A.H., Khodeer D.M., Bin-Jumah M., Alhader M.S., Alkahtani S., Aleya L. Impact of garlic (Allium sativum) oil on cisplatin-induced hepatorenal biochemical and histopathological alterations in rats. Sci Total Environ. 2020;710 doi: 10.1016/j.scitotenv.2019.136338. [DOI] [PubMed] [Google Scholar]
- 7.Ewees M.G., Messiha B.A.S., Abo-Saif A.A., Bayoumi A.M.A., Abdel-Bakky M.S. Interference with coagulation cascade as a novel approach to counteract cisplatin-induced acute tubular necrosis; an experimental study in rats. Front Pharmacol. 2018;9:1–10. doi: 10.3389/fphar.2018.01155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Schoenmakers S.H.H.F., Reitsma P.H., Spek C.A. Blood coagulation factors as inflammatory mediators. Blood Cells, Mol Dis. 2005;34:30–37. doi: 10.1016/j.bcmd.2004.09.001. [DOI] [PubMed] [Google Scholar]
- 9.Davalos D., Akassoglou K. Fibrinogen as a key regulator of inflammation in disease. Semin Immunopathol. 2012;34:43–62. doi: 10.1007/s00281-011-0290-8. [DOI] [PubMed] [Google Scholar]
- 10.Mackman N., Tilley R.E., Key N.S. Role of the extrinsic pathway of blood coagulation in hemostasis and thrombosis. Arterioscler Thromb Vasc Biol. 2007;27:1687–1693. doi: 10.1161/ATVBAHA.107.141911. [DOI] [PubMed] [Google Scholar]
- 11.DM, M., M, H., HR, R. Molecular biology and biochemistry of the coagulation factors and pathways of hemostasis. In Williams Hematology; McGraw-Hill Professional Publishing: New York NY, 2010; pp. 614–620.
- 12.Hankey G.J., Fracp F., Eikelboom J.W. MBBS; FRACP; FRCPA Dabigatran etexilate: A new thrombin inhibitor. Med. J. Aust. 2010;192:407–412. doi: 10.1161/CIRCULATIONAHA.110.004424. [DOI] [PubMed] [Google Scholar]
- 13.Li Z., Delaney M.K., O’Brien K.A., Du X. Signaling during platelet adhesion and activation. Arterioscler Thromb Vasc Biol. 2010;30:2341–2349. doi: 10.1161/ATVBAHA.110.207522. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Ma L., Dorling A. The roles of thrombin and protease-activated receptors in inflammation. Semin Immunopathol. 2012;34:63–72. doi: 10.1007/s00281-011-0281-9. [DOI] [PubMed] [Google Scholar]
- 15.Macfarlane S.R., Seatter M.J., Kanke T., Hunter G.D. Proteinase-activated receptors. Pharmacol Rev Pharmacol Rev. 2001;53:245–282. [PubMed] [Google Scholar]
- 16.Krenzlin H., Lorenz V., Danckwardt S., Kempski O., Alessandri B. The importance of thrombin in cerebral injury and disease. Int J Mol Sci. 2016;17 doi: 10.3390/ijms17010084. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Habib M., Roberts L.N., Patel R.K., Wendon J., Bernal W., Arya R. Evidence of rebalanced coagulation in acute liver injury and acute liver failure as measured by thrombin generation. Liver Int. 2014;34:672–678. doi: 10.1111/liv.12369. [DOI] [PubMed] [Google Scholar]
- 18.Dhar A., Sadiq F., Anstee Q.M., Levene A.P., Goldin R.D., Thursz M.R. Thrombin and factor Xa link the coagulation system with liver fibrosis. BMC Gastroenterol. 2018;18:1–9. doi: 10.1186/s12876-018-0789-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Mhatre M., Nguyen A., Kashani S., Pham T., Adesina A., Grammas P. Thrombin, a mediator of neurotoxicity and memory impairment. Neurobiol Aging. 2004;25:783–793. doi: 10.1016/j.neurobiolaging.2003.07.007. [DOI] [PubMed] [Google Scholar]
- 20.Weitz J.I. Factor Xa and thrombin as targets for new oral anticoagulants. Thromb Res. 2011;127:S5–S12. doi: 10.1016/S0049-3848(10)70147-X. [DOI] [PubMed] [Google Scholar]
- 21.Woźniak E., Broncel M., Bukowska B., Gorzelak-Pabiś P. The protective effect of dabigatran and rivaroxaban on DNA oxidative changes in a model of vascular endothelial damage with oxidized cholesterol. Int J Mol Sci. 2020;21 doi: 10.3390/ijms21061953. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Mahmoud N., Messiha B., Saleh I., Saif A., Abdel-Bakky M. Interruption of platelets and thrombin function as a new approach against liver fibrosis induced experimentally in rats. Life Sci. 2019;231 doi: 10.1016/j.lfs.2019.05.078. [DOI] [PubMed] [Google Scholar]
- 23.Hussein J., El-matty D.A., El-khayat Z., Abdel-latif Y. Therapeutic role of coenzyme Q10 in brain injury during experimental diabetes. J Appl Pharm Sci. 2013;3:213–217. doi: 10.7324/JAPS.2013.3636. [DOI] [Google Scholar]
- 24.Charles J.P., Crouch S.R. Spectrophotometric and kinetics investigation of the Berthelot reaction for the determination of ammonia. Anal Chem. 1977;49:464–469. doi: 10.1021/ac50011a034. [DOI] [Google Scholar]
- 25.Richard JH, Donald C, James WW. Clinical Chemistry: Principles and Techniques; 2nd ed.; Hagerstown, Md., Medical Dept., Harper & Row: New York, 1974.
- 26.Quiros Y., Ferreira L., Sancho-Martínez S.M., González-Buitrago J.M., López-Novoa J.M., López-Hernández F.J. Sub-nephrotoxic doses of gentamicin predispose animals to developing acute kidney injury and to excrete ganglioside M2 activator protein. Kidney Int. 2010;78:1006–1015. doi: 10.1038/ki.2010.267. [DOI] [PubMed] [Google Scholar]
- 27.Goyal V.K., Kakade S., Pandey S.K., Gothi A.K., Nirogi R. Determining the effect of storage conditions on prothrombin time, activated partial thromboplastin time and fibrinogen concentration in rat plasma samples. Lab Anim. 2015;49 doi: 10.1177/0023677215597137. [DOI] [PubMed] [Google Scholar]
- 28.Uchiyama M., Mihara M. Determination of malonaldehyde precursor in tissues by thiobarbituric acid test. Anal Biochem. 1978;86:271–278. doi: 10.1016/0003-2697(78)90342-1. [DOI] [PubMed] [Google Scholar]
- 29.Sedlak J., Lindsay R.H. Estimation of total, protein-bound, and nonprotein sulfhydryl groups in tissue with Ellman’s reagent. Anal Biochem. 1968;25:192–205. doi: 10.1016/0003-2697(68)90092-4. [DOI] [PubMed] [Google Scholar]
- 30.Marklund S.L. Product of extracellular-superoxide dismutase catalysis. FEBS Lett. 1985;184:237–239. doi: 10.1016/0014-5793(85)80613-X. [DOI] [PubMed] [Google Scholar]
- 31.Miranda K.M., Espey M.G., Wink D.A. A rapid, simple spectrophotometric method for simultaneous detection of nitrate and nitrite. Nitric Oxide. 2001;5:62–71. doi: 10.1006/niox.2000.0319. [DOI] [PubMed] [Google Scholar]
- 32.Abdel-Bakky M.S., Hammad M.A., Walker L.A., Ashfaq M.K. Tissue factor antisense deoxyoligonucleotide prevents monocrotaline/LPS hepatotoxicity in mice. Arch Toxicol. 2011;33:774–783. doi: 10.1002/jat.2728. [DOI] [PubMed] [Google Scholar]
- 33.Ewees M.G., Messiha B.A.S., Abdel-Bakky M.S., Bayoumi A.M.A., Abo-Saif A.A. Tempol, a superoxide dismutase mimetic agent, reduces cisplatin-induced nephrotoxicity in rats. Drug Chem Toxicol. 2018:1–8. doi: 10.1080/01480545.2018.1485688. [DOI] [PubMed] [Google Scholar]
- 34.Alhoshani A.R., Hafez M.M., Husain S., Al-sheikh A.M., Alotaibi M.R., Al Rejaie S.S., Alshammari M.A., Almutairi M.M., Al-Shabanah O.A. Protective effect of rutin supplementation against cisplatin-induced Nephrotoxicity in rats. BMC Nephrol. 2017;18:194. doi: 10.1186/s12882-017-0601-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Salihoglu Y.S., Elri T., Gulle K., Can M., Aras M., Ozacmak H.S., Cabuk M. Evaluation of the protective effect of agmatine against cisplatin nephrotoxicity with 99mTc-DMSA renal scintigraphy and cystatin-C. Ren Fail. 2016;38:1496–1502. doi: 10.1080/0886022X.2016.1227919. [DOI] [PubMed] [Google Scholar]
- 36.Dirican A., Sahin O., Tasli F., Sogut E., Kucukzeybek Y., Alacacioglu A., Gunes M., Saglam G., Coban E., Tarhan M.O. Thymoquinone enhances cisplatin-induced neprotoxicity in high dose. J Oncol Sci. 2016;1:17–24. [Google Scholar]
- 37.Vougiouka T., Boulikas M. Cisplatin and platinum drugs at the molecular level. Oncol Rep. 2003;10:1663–1682. doi: 10.3892/or.10.6.1663. [DOI] [PubMed] [Google Scholar]
- 38.Arany I., Safirstein R.L. Cisplatin nephrotoxicity. Semin Nephrol. 2003;23:460–464. doi: 10.1016/S0270-9295(03)00089-5. [DOI] [PubMed] [Google Scholar]
- 39.Yousef M.I., Hussien H.M. Cisplatin-induced renal toxicity via tumor necrosis factor-α, interleukin 6, tumor suppressor P53, DNA damage, xanthine oxidase, histological changes, oxidative stress and nitric oxide in rats: protective effect of ginseng. Food Chem Toxicol. 2015;78:17–25. doi: 10.1016/j.fct.2015.01.014. [DOI] [PubMed] [Google Scholar]
- 40.Ma X., Yan L., Zhu Q., Shao F. Puerarin attenuates cisplatin-induced rat nephrotoxicity: The involvement of TLR4/NF-κB signaling pathway. PLoS ONE. 2017;12 doi: 10.1371/journal.pone.0171612. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Tohamy A.A., Aref A.M., Moneim A.E.A., Sayed R.H., Esmat A., Moneim A. Cinnamic acid attenuates cisplatin-induced hepatotoxicity and nephrotoxicity. J Basic Environ Sci. 2016;3:1–9. [Google Scholar]
- 42.Potočnjak I., Škoda M., Pernjak-Pugel E., Peršić M.P., Domitrović R. Oral administration of oleuropein attenuates cisplatin-induced acute renal injury in mice through inhibition of ERK signaling. Mol Nutr Food Res. 2016;60:530–541. doi: 10.1002/mnfr.201500409. [DOI] [PubMed] [Google Scholar]
- 43.Jo S.K., Cho W.Y., Sung S.A., Kim H.K., Won N.H. MEK inhibitor, U0126, attenuates cisplatin-induced renal injury by decreasing inflammation and apoptosis. Kidney Int. 2005;67:458–466. doi: 10.1111/j.1523-1755.2005.67102.x. [DOI] [PubMed] [Google Scholar]
- 44.Greer JP. Wintrobe’s Clinical Hematology; 11 th.; Lippincott,Williams andWilkins: Philadelphia, PA, 2004.
- 45.Chambers R.C., Scotton C.J. Coagulation cascade proteinases in lung injury and fibrosis. Proc Am Thorac Soc. 2012;9:96–101. doi: 10.1513/pats.201201-006AW. [DOI] [PubMed] [Google Scholar]
- 46.Nasr A.Y. Protective effect of aged garlic extract against the oxidative stress induced by cisplatin on blood cells parameters and hepatic antioxidant enzymes in rats. Toxicol Reports. 2014;1:682–691. doi: 10.1016/j.toxrep.2014.09.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Lee C.J., Ansell J.E. Direct thrombin inhibitors. Br J Clin Pharmacol. 2011;72:581–592. doi: 10.1111/j.1365-2125.2011.03916.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Dittmeier M., Kraft P., Schuhmann M.K., Fluri F., Kleinschnitz C. Pretreatment with rivaroxaban attenuates stroke severity in rats by a dual antithrombotic and anti-inflammatory mechanism. Thromb Haemost. 2016;115:685–780. doi: 10.1160/TH15-08-0631. [DOI] [PubMed] [Google Scholar]
- 49.Kopec A.K., Joshi N., Towery K.L., Kassel K.M., Sullivan B.P., Flick M.J., Luyendyk J.P. Thrombin inhibition with dabigatran protects against high-fat diet-induced fatty liver disease in mice. J Pharmacol Exp Ther. 2014;351:288–297. doi: 10.1124/jpet.114.218545. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Rothmeier AS, Ruf W. Protease-activated receptor 2 signaling in inflammation. In Proceedings of the Seminars in immunopathology; Springer, 2012; Vol. 34, pp. 133–149. [DOI] [PubMed]
- 51.Schwartz R.S., Borissoff J.I., Spronk H.M.H., ten Cate H. The hemostatic system as a modulator of atherosclerosis. N Engl J Med. 2011;364:1746–1760. doi: 10.1056/NEJMra1011670. [DOI] [PubMed] [Google Scholar]
- 52.Owens P.I., Passam F.H., Antoniak S., Marshall S.M., Mcdaniel A.L., Rudel L., Williams J.C., Hubbard B.K., Dutton J., Wang J. Monocyte tissue factor-dependant activation of coagulation in hypercholesteolemic mice and monkeys is inhibited by simvastatin. J Clin Invest. 2012;122:558–568. doi: 10.1172/JCI58969DS1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Badeanlou L., Furlan-Freguia C., Yang G., Ruf W., Samad F. Tissue factor-PAR2 signaling promotes diet-induced obesity and adipose inflammation. Nat Med. 2012;17:1490–1497. doi: 10.1001/jamasurg.2014.1086.Feasibility. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Borensztajn K.S. Action and Function of coagulation FXa on cellular signaling. Trends Mol Med. 2009;2009:429–440. [Google Scholar]
- 55.Mihara K., Ramachandran R., Saifeddine M., Hansen K.K., Renaux B., Polley D., Gibson S., Vanderboor C., Hollenberg M.D. Thrombin-mediated direct activation of proteinase-activated receptor-2: another target for thrombin signaling. Mol Pharmacol. 2016;89:606–614. doi: 10.1124/mol.115.102723. [DOI] [PubMed] [Google Scholar]
- 56.Ungefroren H., Witte D., Fiedler C., Gädeken T., Kaufmann R., Lehnert H., Gieseler F., Rauch B.H. The role of PAR2 in TGF-β1-induced ERK activation and cell motility. Int J Mol Sci. 2017;18:1–13. doi: 10.3390/ijms18122776. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Nikolakopoulou A.M., Georgakopoulos A., Robakis N.K. Presenilin1 promotes trypsin-induced neuroprotection via the PAR2/ERK signaling pathway. Effects of presenilin1 FAD mutations. Neurobiol Aging. 2016;15:477–491. doi: 10.1586/14737175.2015.1028369.Focused. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Hu L., Xia L., Zhou H., Wu B., Mu Y., Wu Y., Yan J. TF/FVIIa/PAR2 promotes cell proliferation and migration via PKCα and ERK-dependent c-Jun/AP-1 pathway in colon cancer cell line SW620. Tumor Biol. 2013;34:2573–2581. doi: 10.1007/s13277-013-0803-2. [DOI] [PubMed] [Google Scholar]
- 59.Yazici S., Karahan O., Oral M.K., Bayramo lu Z., Unal M., Caynak B., Sagbas E. Comparison of renoprotective effect of dabigatran with low-molecular-weight heparin. Clin Appl Thromb. 2015;1–5 doi: 10.1177/1076029615571629. [DOI] [PubMed] [Google Scholar]
- 60.Borissoff J.I., Joosen I.A., Versteylen M.O., Spronk H.M., Ten Cate H., Hofstra L. Accelerated in vivo thrombin formation independently predicts the presence and severity of CT angiographic coronary atherosclerosis. JACC Cardiovasc Imaging. 2012;5:1201–1210. doi: 10.1016/j.jcmg.2012.01.023. [DOI] [PubMed] [Google Scholar]
- 61.Tripathy D., Sanchez A., Yin X., Luo J., Martinez J., Grammas P. Thrombin, a mediator of cerebrovascular inflammation in AD and hypoxia. Front Aging Neurosci. 2013;5:1–9. doi: 10.3389/fnagi.2013.00019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Wachowicz B., Olas B., Zbikowska H.M., Buczyński A. Generation of reactive oxygen species in blood platelets. Platelets. 2002;13:175–182. doi: 10.1080/09533710022149395. [DOI] [PubMed] [Google Scholar]
- 63.Liu X., Guan Y., Xu S., Li Q., Sun Y., Han R., Jiang C. Early predictors of acute kidney injury: a narrative review. Kidney Blood Press Res. 2016;41:680–700. doi: 10.1159/000447937. [DOI] [PubMed] [Google Scholar]