Graphical abstract
Keywords: Staphylococcus aureus, Virulence, Sub-MICs, Antibiotics
Highlights
-
•
Sub-MICs of antibiotics cause deformed S. aureus cells.
-
•
Antibiotics at sub-MICs affect the expression of S. aureus virulence factors.
-
•
Sub-MICs of antibiotics impact on the adhesion and invasion of S. aureus.
-
•
Sub-MICs of antibiotics moderate S. aureus biofilm formation.
-
•
Antibiotics at sub-MIC levels affect SCV production of S. aureus.
Abstract
Background
The treatment of patients with Staphylococcus aureus infections mainly relies on antistaphylococcal regimens that are established with effective antibiotics. In antibiotic therapy or while living in nature, pathogens often face the sub-inhibitory concentrations (sub-MICs) of antibiotics due to drug pharmacokinetics, diffusion barriers, waste emission, resistant organism formation, and farming application. Different categories of antibiotics at sub-MICs have diverse effects on the physiological and chemical properties of microorganisms. These effects can result in virulence alterations. However, the mechanisms underlying the actions of antibiotics at sub-MICs on S. aureus virulence are obscure.
Aim of review
In this review, we focus on the effects of sub-MICs of antibiotics on S. aureus virulence from the aspects of cell morphological change, virulence factor expression, bacterial adherence and invasion, staphylococcal biofilm formation, and small-colony variant (SCV) production. The possible mechanisms of antibiotic-induced S. aureus virulence alterations are also addressed.
Key scientific concepts of review
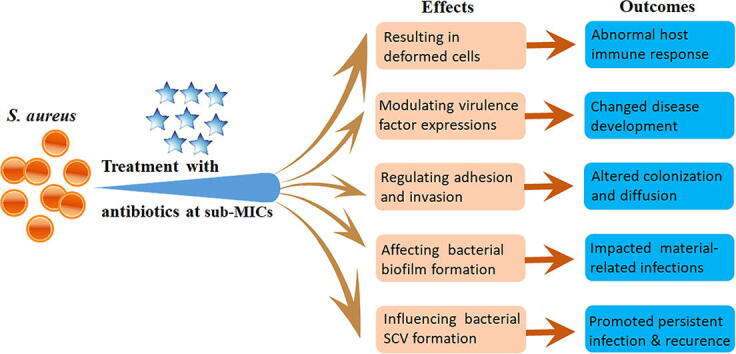
Five main aspects of bacterial virulence can be changed in S. aureus exposure to the sub-MIC levels of antibiotics, resulting in deformed bacterial cells to stimulate abnormal host immune responses, abnormally expressed virulence factors to alter disease development, changed bacterial adhesion and invasion abilities to affect colonization and diffusion, altered biofilm formation to potentate material-related infections, and increased SCV formation to achieve persistent infection and recurrence. These advanced findings expand our knowledge to rethink the molecular signaling roles of antibiotics beyond their actions as antimicrobial agents.
Introduction
The escalating crisis of antimicrobial resistance due to the extensive and indiscriminate use of antibiotics and their leakage into the environment has garnered appeal. The antibiotic resistance of a certain bacterium is phenotypically determined by minimum inhibitory concentrations (MICs), which are defined as the lowest antibiotic concentrations that can inhibit the growth of pathogens in a medium after 18–24 h of culture in vitro. During antibiotic therapy or while living in nature, however, pathogens may encounter the sub-inhibitory concentrations (sub-MICs) of antibiotics due to several situations [1], [2]. First, antibiotic concentrations decrease to sub-MICs over time after administration as a result of the pharmacokinetics of antibiotics [3]. Second, the occurrence of antibiotic sub-MICs in tissues or the internal milieu of bacterial biofilms is attributed to physical and chemical barriers to antibiotic penetration, as well as to drug–drug interactions in sites other than in the blood where pathogens are in direct contact with antibiotics [4], [5], [6], [7], [8]. Third, antibiotic sub-MICs may be generated after waste emissions from hospitals and other treatment facilities or pharmaceutical manufacturers. Fourth, antibiotic sub-MICs can occur owing to bacterial evolution and drug-resistant organism formation [1]. Lastly, in stock farming, antimicrobial drugs used at sub-MICs are still allowed in some countries to promote animal production or prevent bacterial infections [9]. The important effects of sub-MICs of antibiotics on the morphology, biofilm formation, and virulence expression of Gram-positive and Gram-negative bacteria are of interest and have been intensively investigated in the last decade [10].
Staphylococcus aureus is a representative of Gram-positive bacteria and the culprit pathogen of human beings; this bacterium causes a variety of infections ranging from local suppurative infections to fetal pneumonia, pseudomembranous enteritis, pyelonephritis, pericarditis, sepsis, and brain abscess [11]. The co-infections of S. aureus with other prevalent pathogens, such as Pseudomonas aeruginosa, human immunodeficiency virus, and SARS-CoV-2, have been reported [12], [13], [14]. The pathogenicity of S. aureus mainly depends on the expression of multiple virulence factors, such as hemolytic toxins, including α-, β-, γ-, and δ-toxins, that can damage platelets, destroy lysosomes, and cause local ischemia and necrosis; Panton-Valentine leucocidin (PVL), a pore-forming toxin that destroys white blood cells and macrophages; eight serotypes of enterotoxins, namely, A, B, C1, C2, C3, D, E, and F; toxic shock staphylococcal toxin 1 (TSST-1), coagulase, and staphylococcal protein A (SpA) [11]. In addition, the factors responsible for bacterial adherence, motility, and biofilm formation are associated with the virulence of S. aureus. Accumulated studies have demonstrated that S. aureus can develop genetic and phenotypic variations and alter its virulence expression to adapt to stress environments with the sub-MICs of antibiotics [1], [2].
The effect of antibiotics at sub-MIC concentrations on S. aureus was first investigated in 1940 by Gardner, who observed the morphological changes of S. aureus upon exposure to 1/4 MIC of penicillin in vitro [15]. Different categories of antibiotics may have diverse effects on bacterial morphology and virulence production. However, some research results are contradictory, and the mechanisms underlying antibiotic actions at sub-MICs remain obscure. The sub-MICs of β-lactams have been shown to enhance the expression of S. aureus exotoxins and adhesion factors positively [16], [17]. By contrast, the production of S. aureus toxins is significantly suppressed under exposure to the sub-MICs of antimicrobial agents, such as lincosamides and oxazolidinones, which target microbial ribosomes [18]. The most studies concerning the effects of antibiotics at sub-MIC concentrations on the virulence of S. aureus have been conducted in vitro, and the in vivo researches are rare and encouraging [1], [4], [5], [18]. In this review, we summarized the in vitro effects of the sub-MICs of antibiotics on the virulence of S. aureus from the aspects of morphology, virulence expression, bacterial adherence and invasion, biofilm formation, and small-colony variant (SCV) production. The in vivo effects of antibiotics at sub-MICs on the virulence of S. aureus were also addressed in a separate section.
Effects of the sub-MICs of antibiotics on the morphology of S. aureus
In general, intact bacteria are rod-shaped (bacilli), spherical (cocci), and spiral-shaped (spirilla). Bacilli are most common, followed by cocci, and spirilla are relatively rare. S. aureus is spherical. When cultured in agar plates, S. aureus colonies present round and swollen shapes, smooth surfaces, and neat edges with or without golden yellow pigments. Treatment with antibiotics at sub-MICs can cause three main types of morphological changes in S. aureus, specifically, cell morphology deformation, cell wall component changes, and cell wall breakdown [19], [20], [21]. For example, enlarged or damaged methicillin-resistant S. aureus (MRSA) subsp. aureus USA300_FPR3757 and methicillin-susceptible S. aureus (MSSA) subsp. aureus Rosenbach American Type Culture Collection (ATCC®) 25923™ cells with reduced adhesiveness, double cells attached to each other, or S. aureus cells with emerging holes are observed upon exposure to the 1/2 to 1/8 MIC of dicloxacillin, cefodizime, cefotaxime, or ceftriaxone [19], [20]. A 1/2 MIC concentration of cefodizime presents the greatest effect on the damage of S. aureus ATCC® 25923™, and the morphological changes are also observed at 1/4 and 1/8 MIC concentration of cefodizime but to a lesser extent [20]. The ciprofloxacin (CFX) at 1/2 MIC (64 μg/mL) and its membrane-targeting-modified derivatives CFX-ester-PPh3 (1/2 MIC = 5.56 μg/mL) and CFX-amide-PPh3 (1/2 MIC = 1.39 μg/mL) can induce dramatically morphological changes, such as thinned and irregular deformation, membrane disruption, loss of cell contents in the MRSA strain 5016 [22]. The cell membrane permeability increases in a dose-dependent manner in MRSA252 treated with berberine at sub-MICs (1/8 to 1/2 MIC), as a result, the doughnut shaped cells appear under the transmission electron microscope observation [23]. Juma et al. reported that a combination of 20% MIC of sophorolipid biosurfactant (0.4%, v/v) and 80% MIC of tetracycline (0.4 μg/mL) can result in significantly morphological changes in MRSA ATCC® 43300™ with larger cell diameters (from 758 ± 75 to 1276 ± 220 nm, P < 0.01) and increased bacterial core stiffness (from 205 ± 46 to 396 ± 66 mN/m, P < 0.01) compared with those in bacteria treated with tetracycline alone [24]. When exposure to 1/4 MIC of thioridazine (4 μg/mL), MRSA USA300_FPR3757 cells present thickened and irregular cell walls with a shortage of intracellular amino acids [19]. The 1/2 MIC of ceftaroline (0.25 μg/mL) can cause cell wall breakdown and cell-shape deformation in MRSA strains 06/1483 and 05/3291 [25]. Treatment with 1.5 μM of the membrane-active lipopeptide compound C10OOC12O, MRSA USA300 cells exhibit sustained mild membrane damages, and Hershkovits et al. speculated that such damaged bacterial membrane may not yet damaged enough at sub-MIC concentration of lipopeptide to the leakage of larger chemical molecules, such as ATP, which indeed happens at the MIC concentration of C10OOC12O (12.5 μM in Luria Bertani medium) [26].
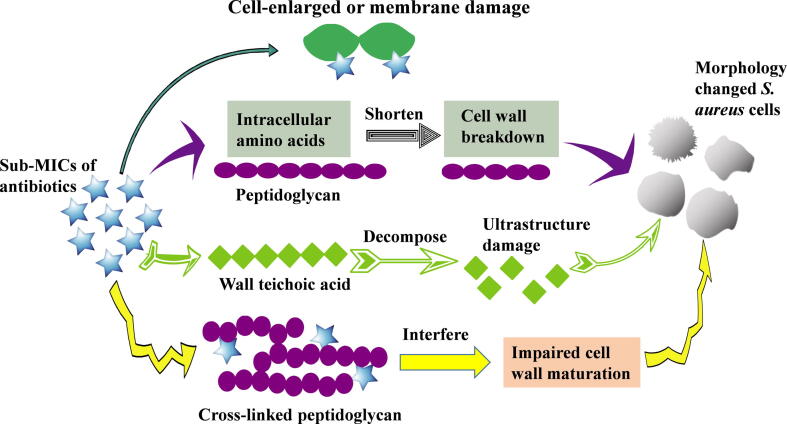
Different antibiotics at sub-MIC concentrations may have different effects on the morphology of S. aureus cells. The 1/2 MIC of desleucyl-oritavancin (10 μg/mL) can cause cell wall disorders with reduced cell wall cross-linking efficiencies and increased N-deacetylated muropeptides in S. aureus ATCC® 6538P™ [27], whereas the sub-MICs (1/2 to 1/32 MIC) of ciprofloxacin and amikacin have no effects on the morphology of a clinical S. aureus isolate [28]. In principle, the sub-MICs of certain antibiotics can weaken S. aureus cell walls through binding to penicillin-binding proteins (PBPs), such as methicillin and cefoxitin [29]. Moreover, some antimicrobial agents, such as vancomycin, may change peptidoglycan cross-linking via binding to the D-alanyl-D-alanine moieties of lipid II, a precursor of cell wall biosynthesis [27], [30]. Others, such as tunicamycin at sub-MICs of 2.5 and 5 μg/mL, can promote cell deformation by targeting and altering the cell wall teichoic acid in S. aureus strains ATCC® 29213™, ATCC® 43300™, ATCC® 25923™, and PRI 4656 [31]. Still others, like oritavancin, may cause cell wall disorders by binding to cross-linked peptidoglycans in cell walls to interfere with cell wall maturation [27]. All these possible mechanisms are summarized and presented in Fig. 1.
Fig. 1.
The potential mechanisms underlying the formation of S. aureus deformation cells upon treatment with antibiotics at sub-MICs.
Although the effects of the sub-MICs of antibiotics on S. aureus morphology may exhibit strain-to-strain differences, three main effects are exerted after the formation of deformed S. aureus upon treatment with the sub-MICs of antibiotics. First, in combination with antibiotics, S. aureus cell deformations may hinder antibiotic resistance or facilitate death, thus resulting in sub-MIC concentration of drug-induced antibiotic sensitivity. Thorsing et al. reported that 1/4 MIC of thioridazine can be able to sensitize MRSA USA300 to the antimicrobial effect of dicloxaillin by reduction of the cell viability by 3 log10 CFU/mL compared with that under dicloxaillin-treatment alone [19]. The combination of sub-MIC concentrations of sodium new houttuyfonate and berberine chloride offers a synergistic action against MRSA strains ATCC® 43300™ and ATCC® 33591™, as well as a vancomycin-intermediate S. aureus strain Mu50 [32]. Second, S. aureus with poorly cross-linked cell walls after treatment with antibiotics at sub-MICs may release a large number of toxins and other pathogenic factors to aggravate the inflammatory response in the host [33]. Lastly, changes in S. aureus cell wall components after sub-MICs of antibiotic treatment may affect the adhesion and aggregation capabilities of staphylococcal cells, resulting in decreased colonization [19], [21]. However, whether and how antimicrobial susceptibility, host immune response, and bacterial pathogenicity in deformed S. aureus bacteria are changed upon exposure to sub-MICs of antibiotics remain largely unknown, and further investigations are suggested to illustrate these points.
Effects of sub-MICs of antibiotics on the expression profiles of S. aureus virulence factors
Bacterial virulence refers to the degree of the pathogenicity of a certain pathogen to its host. Increasing data have shown that the sub-MICs of antimicrobial agents can alter the expression levels of bacterial toxins and those of factors responsible for colonization and invasion. Recently, Hodille et al. reviewed the effects of the diverse categories of antibiotics on S. aureus toxin production and host immune response [10]. They concluded that treatment with the sub-MICs of ribosome-targeting antibiotics and cell wall-active agents result in opposing manifestations of S. aureus virulence factor expression, with the former resulting in decreased production and the latter increasing expression. Such information is important for the establishment of valuable therapeutic regimens to improve patient outcomes in case of S. aureus infections. Table 1 lists the effects of the sub-MICs of different antibiotics on the expression of virulence factors in S. aureus.
Table 1.
The expression levels of virulence factors in S. aureus cultured in the presence of sub-MICs of antibiotics compared with those in S. aureus without antibiotic-treatment.
| Antibiotics | Hla | PVL | TSST-1 | PSM | SPA | Coagulase | Enterotoxins A and B | References |
|---|---|---|---|---|---|---|---|---|
| Penicillin | N | N | N | N | ↑ | N | N | [10] |
| Methicillin | ↑ | N | N | N | – | – | N | [34], [35] |
| Oxacillin | N | ↑ | N | ↓ | ↑ | N | N | [10] |
| Nafcillin | ↑ | ↑ | N | N | N | N | N | [2], [10] |
| Imipenem | N | ↑ | N | N | N | N | N | [10] |
| Gentamicin | N | N | ↓ | N | N | N | N | [10] |
| Erythromycin | ↓ | N | N | ↑ | N | N | N | [10] |
| Flucloxacillin | N | N | ↓ | N | N | N | N | [10] |
| Roxithromycin | N | N | N | N | N | ↓ | N | [35] |
| Enoxacin | ↓ | N | N | N | – | – | N | [35], [34] |
| Ciprofloxacin | ↓ | N | N | N | – | – | N | [35] |
| Azithromycin | ↓ | N | N | N | N | N | N | [36] |
| Lincomycin | N | N | N | N | N | ↓ | N | [34] |
| Clindamycin | ↓ | ↓ | ↓ | ↓ | ↓ | ↓ | N | [10], [34], [36], [37] |
| Fosfomycin (FOM) | ↓ | N | N | N | N | N | N | [38] |
| Tigecycline | ↓ | ↓ | ↓ | N | ↓ | N | N | [10], [39] |
| Vancomycin | N | – | – | N | – | N | N | [39] |
| Daptomycin | N | – | N | N | – | N | N | [39] |
| Linezolid | ↓ | ↓ | ↓ | ↑ | ↓ | N | ↓ | [10], [39] |
| Tedizolid | ↓ | ↓ | ↓ | ↓ | N | N | N | [2] |
| Mupirocin | ↓ | N | N | N | N | N | N | [37] |
| Costus oil | ↓ | N | ↓ | N | N | N | N | [21] |
| Fusidic acid | ↓ | N | N | N | N | N | N | [40] |
| C10OOc12O | ↓ | N | N | N | N | N | N | [26] |
| LP5 | ↓ | N | N | N | ↑ | N | ↓ | [41] |
| Thymol | ↓ | N | N | N | N | N | ↓ | [21] |
| Chlorogenic Acid | ↓ | N | N | N | N | ↓ | N | [21] |
| Sclareol | ↓ | N | N | N | N | N | N | [42] |
| L-NPDNJ | ↓ | N | N | N | N | N | N | [43] |
“↑”, the expression level increased; “↓”, the expression level decreased; “-”, comparable in the expression level; N, not determined.
The effects of antimicrobial agents on S. aureus virulence factor expression are commonly investigated by reporter fusion assays for specific gene promoters of interest, the mRNA quantitation of virulence factors, transcriptomic profiling, and the measurement of certain virulence proteins and proteomic profiles [10]. The effect of antibiotics on the production of α-toxin, a major virulence factor that contributes to staphylococcal disease, was first explored by Kernodle et al. [44]. They found that nafcillin-nonsusceptible and -susceptible S. aureus strains display increased α-toxin expression when treated with 0.006 to 0.1 μg/mL of nafcillin compared with the untreated bacteria. Further studies confirmed that α-toxin expression increases upon β-lactam exposure, and such β-lactams seemingly induce increased α-toxin production in MRSA by up to 30-fold relative to that in MSSA strains [45]. Other investigations have shown that α-toxin expression reduces after treatment with the sub-MICs of fusidic acid, erythromycin, or aminoglycosides (Table 1).
PVL is another S. aureus-produced pore-forming toxin that contributes to bacterial pathogenesis. The sub-MICs (ranging from 1/8 to 1/2 MIC) of β-lactams, including oxacillin and nafcillin, can induce an increase in the production of PVL against diverse S. aureus backgrounds (ST1, ST8, ST80, and ST59) [16], [17], [39], whereas other antibiotics, such as tedizolid and linezolid at 1/16 MIC, result in decreased PVL production in MRSA 1560, and the decreased mRNA levels of PVL are also observed in MRSA USA300 after treatment with sub-MICs (1/8 to 1/2 MIC) of clindamycin, linezolid, and tigecycline [2], [39]. Studies have demonstrated that the antibiotics active in protein synthesis inhibition exert varied effects on TSST-1 production with the most remarkable inhibitory effects (Table 1). Most recently, our group revealed that only the sub-MICs of β-lactam antibiotics, such as oxacillin, methicillin, cefoxitin, imipenem, and meropenem, but not vancomycin, chloramphenicol, erythromycin, and kanamycin, can promote the expression of a cluster of lipoprotein-like genes (sa2275-sa2273) encoding important virulence factors that contribute to MRSA pathogenicity [29]. A natural terpenoid (+)- nootkatone at a sub-MIC of 50 μg/mL suppresses the expression levels of sarA, agrA, RNAIII, and spa, which regulate the expression of toxins, in both MRSA strain SJTUF 20758 and MSSA strain ATCC® 25923™ [46].
The mechanisms underlying the actions of antibiotics at sub-MICs on S. aureus virulence expression are multifaceted. Antibiotics at sub-MICs can not only regulate the expression of toxins (e.g., α-toxin, PVL, SpA, PSM, and enterotoxins) through quorum sensing systems; two-component systems; or other regulators, such as SaeRS, SarA, Rot, and CcpA, but also directly bind to some amino acids of toxin proteins to affect their functions. For example, fosfomycin inhibits the activity of S. aureus α-toxin by binding to its Lys154 and Asp108 [38]. Furthermore, antibiotics targeting the 50S ribosome of bacteria can impair bacterial transcription and result in the accumulation of mRNA and transcription complex intermediates, thereby resulting in the accumulation of toxin mRNA [47].
Virulence factors implement their pathogenic roles either by host cell destruction or cell regulation pathway utilization. For example, S. aureus α-toxin may promote host inflammatory necrosis through the MAPK-associated signaling pathway or inflammasome-mediated inflammation [38]. An et al. reported that fosfomycin at a sub-MIC concentration (4 μg/mL) reduces the expression of α-toxin in S. aureus 8325–4, and the phosphorylation levels of p38, ERK, and JNK are remarkably decreased in THP-1 cells infected by fosfomycin-treated S. aureus relative to the untreated strain [38]. They also revealed that fosfomycin treatment can downregulate inflammasome-NLRP3-mediated inflammation in macrophages infected by α-toxin-positive S. aureus. These data are highly consistent with those of Morikawa et al., who observed that the sub-MICs of fosfomycin can greatly diminish IL-1β levels in LPS-treated human monocytes [48].
Effects of sub-MICs of antibiotics on the adhesion and invasion of S. aureus
The pathogenesis of S. aureus is closely associated with its capacity to adhere directly to host cells or to the extracellular matrix (ECM) [49]. Bacterial adhesion is the first step in the invasion of host cells and the formation of biofilms. A repertoire of adhesive molecules, including staphylococcal Fn-binding proteins A and B, clumping factors A and B, serine aspartate repeat-containing protein D, serine-rich adhesin for platelets, and staphylococcal autolysin, are responsible for S. aureus adhesion [50]. The effects of the sub-MIC levels of antibiotics on S. aureus adhesion and invasion may vary depending on the bacterial strain and host cell model used.
The overall roles of certain antibiotics at the sub-MICs in S. aureus adhesion and invasion are summarized in Table 2. Maioli et al. reported that the role of ceftibuten, a third-generation cephalosporin, at sub-MICs (1/4 MIC to 1/2 MIC) in the clinical treatment of bacterial infection is mainly attributed to its antiadherence characteristics [72]. Sasso et al. demonstrated that the fluoroquinolone agent gemifloxacin at the 1/32 MIC concentration significantly reduces the adhesiveness of S. aureus ATCC® 25923™ to primary human mucosal epithelial cells [3]. The strain-dependent modulatory effects of antibiotics at sub-MICs on the adhesion capabilities of S. aureus have been observed. Lázaro-Díez et al. revealed that the 1/2 to 1/4 MIC levels of ceftaroline can enhance bacterial adhesion of MRSA clinical isolates 06/1483 and 05/3291, whereas MRSA strain 05/2369 presents a reduced adhesive capability at the sub-MICs of ceftaroline tested [25]. The expression levels of adhesion-associated genes (fnbA, fnbB, clfA, clfB, and icaD) increase by more than 5-fold in S. aureus strain CCARM 3080 exposed to a half MIC of levofloxacin [51]. The relative expression levels of clfB and fnbB increase in S. aureus strain KACC 10778 treated with oxacillin (>3-fold), whereas those of icaA and icaD genes are downregulated by more than 5-fold under 1/2 MIC of levofloxacin or oxacillin treatment. When S. aureus ATCC® 15564™ is used, however, most adhesion-related genes are slightly upregulated in S. aureus cultured with levofloxacin or oxacillin at 1/2 MIC [51]. Sasso et al. showed that 1/32 and 1/64 MIC of gatifloxacin remarkably inhibits the adhesiveness of S. aureus ATCC® 25923™ to human epithelial cells [57]. Moreover, S. aureus may display an antibiotic-specific adhesion reaction under treatment with the sub-MICs of antimicrobial agents. Using fibronectin-coated microplates and human osteoblasts, Rasigade et al. observed that treatment with 1/2 MIC of oxacillin (0.125 μg/mL), linezolid (0.5 μg/mL), or moxifloxacin (0.06 μg/mL) results in a hyperadhesive phenotype of S. aureus reference strain 8325–4 with an increase in fnbA/B transcription. As a result, oxacillin-treated S. aureus cells present greatly increased adhesion capability (682% ± 374%) compared with the untreated strain (256% ± 128%) in the infection of osteoblastic MG-63 cells [52]. By contrast, exposure to 1/2 MIC of rifampin (0.003 μg/mL) decreases fibronectin adhesion in S. aureus 8325–4 and five clinical S. aureus isolates DU5883, SST2008 1028, ST2008 0563, HT2000 0594, and HT2001 0390. Gentamicin and vancomycin treatment exerts no effect on fibronectin binding or fnbA/B expression levels in S. aureus [52]. Braga et al. demonstrated that 1/2 MIC concentration of cefodizime or cefotaxime exhibits the greatest inhibitory effect on the adhesion of S. aureus ATCC® 25923™, with a gradual reduction from 1/2 to 1/16 MIC [20].
Table 2.
The biofilm formation, bacterial adherence, invasion, and SCV production of S. aureus cultured in the presence of sub-MICs of antibiotics compared with those in S. aureus without antibiotic-treatment.
| Antibiotics | Adhesion | Invasion | Biofilm formation | SCVs | Reference |
|---|---|---|---|---|---|
| Oxacillin | ↑ | N | ↑ | N | [51], [52], [53] |
| Methicillin | N | N | N | – | [54] |
| Ampicillin | – | ↑ | N | N | [55] |
| Amoxicillin | N | N | N | – | [54] |
| Ceftaroline | ↑ | N | ↑ | N | [25] |
| Cefalexin | N | N | ↑ | N | [35] |
| Cefodizime | ↓ | N | N | N | [20] |
| Ceftriaxone | ↓ | N | N | N | [20] |
| Gentamicin | N | N | N | ↑ | [54] |
| Kanamycin | N | N | ↓ | N | [56] |
| Amikacin | – | ↑ | N | N | [55] |
| Gemifloxacin | ↓ | N | N | N | [3] |
| Telithromycin | N | N | ↓ | N | [50] |
| Azithromycin | N | N | ↓ | N | [50] |
| Erythromycin | N | N | N | – | [54] |
| Ciprofloxacin | N | N | ↓ | – | [22], [54] |
| Enrofloxacin | N | N | ↓ | N | [56] |
| Moxifloxacin | ↑ | N | N | N | [52] |
| Gatifloxacin | ↓ | N | N | N | [57] |
| Rufloxacin | ↓ | N | N | N | [58] |
| Tetracyclin, | N | N | N | – | [54] |
| Clarithromycin | N | N | ↓ | – | [54], [56] |
| Clindamycin | ↑ | N | ↓ | ↑ | [36], [50], [54] |
| Minocycline | N | N | – | N | [59] |
| Vancomycin | – | N | ↓ | – | [52], [54], [53] |
| Daptomycin | N | N | ↓ | N | [50] |
| Linezolid | – | N | N | N | [52] |
| Mupirocin | N | N | ↑ | – | [54], [60] |
| Lincomycin | N | N | ↓ | N | [56] |
| Fusidic acid | ↓ | N | ↓ | N | [40] |
| Rifampicin | – | – | ↑ | ↑ | [52], [54], [59] |
| Tunicamycin | ↓ | ↓ | ↓ | N | [31] |
| Colistin sulfate | N | N | ↓ | N | [56] |
| Berberine | N | N | ↓ | N | [56] |
| RP557 | N | N | ↓ | N | [61] |
| Nisin | ↓ | N | ↓ | N | [62] |
| Glyceryl trinitrate | N | N | ↓ | N | [63] |
| Alpiniapurpuratalectin | N | N | ↓ | N | [64] |
| Carboxymethyl chitosan | N | N | ↓ | N | [65] |
| Eugenol | N | N | ↓ | N | [66] |
| Chlorogenic acid | ↓ | N | N | N | [21] |
| Acetylisovaleryltylosin tartrate | N | N | ↑ | N | [56] |
| Plantaricin GZ1-27 | N | N | ↓ | N | [67] |
| IMD0354 | N | N | ↓ | N | [68] |
| L-NPDNJ | N | N | ↓ | N | [43] |
| (+)-Nootkatone | N | N | ↓ | N | [47] |
| ZnO-Ag NPs | N | N | ↓ | N | [69] |
| Chitosan(CS) | N | N | ↓ | N | [70] |
| Syph-1 | N | N | ↓ | N | [71] |
“↑”, increased; “↓”, decreased; “-”, comparable (no effects); N, not determined.
S. aureus can invade and propagate in mammalian cells [36]. After adhesion, subsequent invasion may occur during the infection of suitable host cells by S. aureus. However, few studies have been performed to investigate the effects of the sub-MICs of antibiotics on S. aureus invasion partially because bacterial invasion is a dynamic process and difficult to observe. Hu et al. found that 1/2 MIC of clindamycin (0.1 μg/mL) but not azithromycin (1 μg/mL) results in the loss of the invasive capacity of S. aureus ATCC® 51650™ to enter primary human nasal epithelial cells; the infection rate of antibiotic-treated bacteria is 6.3% ± 0.76%, whereas that of bacteria without antibiotic treatment is 49% ± 9.8% [36]. In some cases, the sub-MICs of antibiotics that promote S. aureus adhesion capacity do not correlate well with bacterial invasion capability. Rasigade et al. observed that 1/2 MIC (0.125 μg/mL) of oxacillin-treated S. aureus strain 8325–4 presents increased bacterial adhesion to cultured osteoblasts, whereas oxacillin-treated bacteria exhibits comparable bacterial invasion capacity for osteoblast cells (9.2% ± 4.1%) relative to untreated S. aureus (6.0% ± 5.1%) [52]. These results imply that the relevance of S. aureus adhesion and invasion regulation during infections with or without antibiotic treatment remains unclear and requires further investigation.
Effects of sub-MICs of antibiotics on S. aureus biofilm formation
The biofilm is a microbial community that forms as microorganisms adhere to each other and grow on biotic or abiotic surfaces, such as artificial heart valves and catheters [73]. The developmental cycle of a biofilm usually includes four steps: (i) the attachment of microbial cells to a surface; (ii) the assembly of the attached cells into a microcolony; (iii) the growth of microbial cells into a mature biofilm; and (iv) the detachment of the fully developed biofilm [74], [75]. In addition to microbial cells, a typical biofilm contains microorganism-secreted adhesive ECM, which constitutes 90% of the biofilm volume [76]. The constituent of the biofilm ECM may vary depending on microbial species. The ECM of S. aureus comprises polysaccharides, extracellular DNA, and proteins and can protect pathogens from inactivation by antimicrobial agents, including antibiotics and chemical disinfectants [77], [78]. Killing pathogenic cells in biofilms with appropriate antibiotics is 1000-fold more difficult than killing planktonic cells [79]. Thus, pathogen-formed, biofilm-associated infections are commonly persistent and become a tremendous health burden in our world [80], [81], [82]. At least 65% of all chronic bacterial infections are related to biofilms, which can cause a wide variety of disease manifestations, including endocarditis, urinary tract infections, and catheter infections [83], [84], [85], [86]. The sub-MICs of antibiotics may affect biofilm formation by interfering with certain developmental steps, and many exploratory findings on the effects of sub-MICs of antibiotics on biofilm formation are crucial for setting up new options to treat biofilm-related S. aureus infections [87].
The effects of antibiotics at sub-MIC concentrations on S. aureus biofilm formation can vary depending on the tested strain, associated biofilm stage, and antibiotic used. The sub-MICs of most antibiotics inhibit the development of S. aureus biofilm, whereas those of several antibiotics, such as oxacillin, ceftaroline, mupirocin, and rifampicin (Table 2), can promote staphylococcal biofilm formation [25], [59], [60]. Sritharadol et al. compared the effects of sub-MICs of mupirocin with those of cefazolin, levofloxacin, gentamicin, and erythromycin on the biofilm formation of MRSA and MSSA [60]. Confocal laser microscopy revealed that the biofilm structure, cell viability, and cell density of the mupirocin-treated groups increase compared with those of untreated MRSA strains USA300 and ATCC® 43300™, and MSSA strain ATCC® 29213™. The expression level of the RNAIII gene in biofilms of MRSA USA300 displays the highest increase upon initial exposure (3 h) to mupirocin at 1/6 MIC level (0.17 mg/mL), and is then downregulated (6 h), whereas the expression levels of the other two biofilm formation-related regulatory genes, agrA and sarA, in strain USA300 shows no obvious difference in the presence or absence of mupirocin [60]. These findings indicate that sub-MICs of mupirocin can promote the strain-dependent biofilm formation of S. aureus and that the RNAIII gene may play important roles in the early attachment stage of staphylococcal biofilm formation. However, the exact function of RNAIII factor and the mechanism underlying S. aureus biofilm promotion remains unknown and is of great interest for later investigations.
The sub-MIC levels of the macrocyclic antibiotic rifampicin greatly promotes biofilm formation in seven out of 10 S. aureus isolates that are nonbiofilm producers in common tryptic soy broth (TSB) [59]. Multiplex PCR detection revealed that icaA and icaB genes are present in all 10 investigated S. aureus strains. The role of the ica operon, which encodes enzymes in rifampicin-induced S. aureus biofilm production, has yet to be elucidated given that biofilm formation by S. aureus can be mediated by either icaADBC-independent or -dependent pathways [88]. Haddadin et al. showed that a wide range of the sub-MICs (2.81%–45%) of cefalexin has great potential to induce the formation of S. aureus National Collection of Type Cultures (NCTC) 11962 biofilm [35]. The authors proposed that cephalexin-induced S. aureus biofilm formation is associated with bacterial hydrophobicity. Owing to its inhibitory role in cell wall synthesis, cefalexin at 1/16 MIC can increase the hydrophobicity of the cell surface of S. aureus strain NCTC 11962; this effect may lead to increased cell adhesion during biofilm formation [35].
Except for a few antibiotics with biofilm-promoting potential, most antistaphylococcal agents show inhibitory effects on S. aureus biofilm formation. Jo and Ahn evaluated the biofilm-forming capacity of S. aureus strains KACC 10778, ATCC® 15564™, and CCARM 3080 grown in the presence of oxacillin and levofloxacin at sub-MICs and found that the number of the biofilm-forming S. aureus strain KACC 10778 cells decrease by approximately 2 log10 CFU/mL under oxacillin treatment, whereas those of strains ATCC® 15564™ and CCARM 3080 decrease by 0.5–1 log10 CFU/mL under levofloxacin and oxacillin treatment [51]. The biofilm of MRSA strain ATCC® 43300™ treated with plantaricin GZ1-27 at 1/2 and 1/4 MIC decreases by 55.3% and 40.2%, respectively, accompanying with reduction of cell to cell connections [67]. Kang et al. showed that 1/2 MIC of CFX or CFX-PPh3 inhibits the biofilm formation of MSSA strain ATCC® 29213™ and MRSA strains 5016, 5013, and 3416 by 43.9% to 89.5% relative to the untreated strains [22]. Escober et al. demonstrated that the nuclear factor-kappa B inhibitor N-[3,5-Bis(trifluoromethyl) phenyl]-5-chloro-2-hydroxybenzamide at sub-MIC of 0.0313 μg/mL can inhibit the initial cell attachment and biofilm formation of vancomycin-resistant S. aureus strain VRS1 in a dose-dependent manner [68]. The sub-MICs of other antimicrobial compounds, such as N-nonyloxypentyl-L-DNJ, N4-benzyl-N-2-phenylquinazoline-2,4-diamine, terpenoid (+)-nootkatone, chitosan, marine steroid siphonocholin, zinc oxide, and silver nanoparticles, are found to be able to inhibit the biofilm formation of diverse S. aureus strains [43], [46], [69], [70], [71], [89].
S. aureus polysaccharide intercellular adhesin, the major component of biofilm ECM, is synthesized by ica operon enzymes, and their related regulators can be affected by certain antibiotics at sub-MICs. For example, Zheng et al. revealed that the 1/4 MIC of telithromycin is greatly superior to that of azithromycin and clindamycin, as well as that of vancomycin or daptomycin, in the inhibition of S. aureus biofilm formation; this antibiotic acts by downregulating the RNA expression levels of sigB, agrA, clfA, and icaA in MSSA and MRSA isolates [50]. The eDNA also plays important roles in the adhesion and maturation stages of biofilm-forming S. aureus. Andre et al. revealed that sub-MIC bacteriocin nisin effectively inhibits S. aureus biofilm by reducing eDNA amounts and polysaccharide compositions without significantly changing protein contents [62]. Exposure to the sub-MICs of tunicamycin (0.25–0.5 μg/mL) greatly reduces the biofilm-forming capability of S. aureus by decreasing eDNA levels [31].
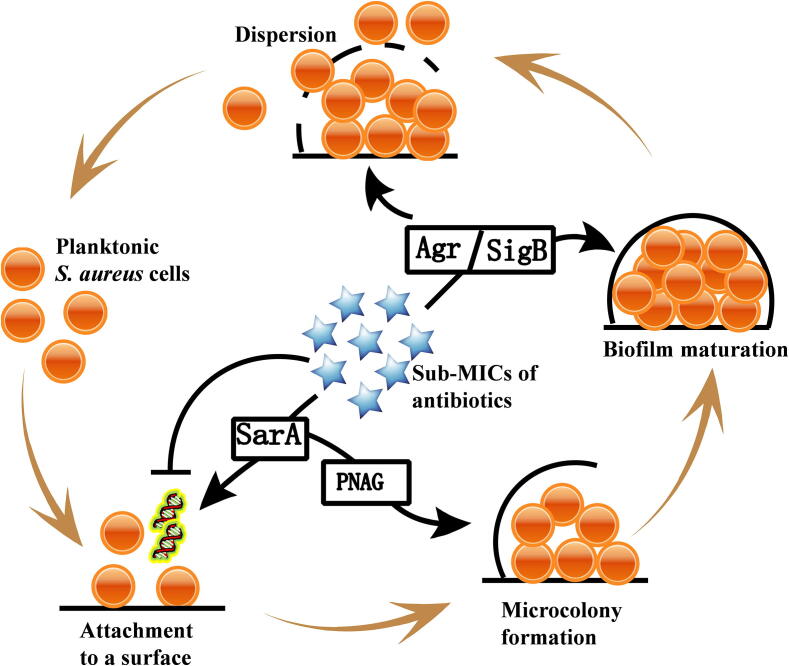
In addition to polysaccharides and eDNA, many exoproteins secreted by S. aureus contribute to biofilm formation; these exoproteins include accumulation-related, biofilm-associated, fibronectin-binding, extracellular-adhesive, and ECM proteins; iron-regulated surface determinant protein C; and clumping factors [50]. Antibiotics at sub-MICs may influence the formation of S. aureus biofilms through direct or indirect moderation of exoprotein expression. Schilcher et al. showed that clindamycin at the sub-MIC levels can trigger a transcriptional stress response in tested S. aureus strains via SigB and increases the expression levels of biofilm-associated genes, including fnbA, fnbB, atlA, irgA, psm, and agrA [90]. The effects of the sub-MIC levels of seven antimicrobial agents (kanamycin, enrofloxacin, lincomycin, clarithromycin, acetylisovaleryltylosin tartrate, colistin sulfate, and berberine) on biofilm formation by S. aureus were investigated in a recent study, and the results showed that all antibiotics tested, except for acetylisovaleryltylosin tartrate, can inhibit the biofilm formation of S. aureus clinical strain Hb0206 effectively [56]. RT-qPCR determination revealed that all antibiotics remarkably reduce the expression levels of six S. aureus biofilm-associated genes (fnbA, rbf, eno, lrgA, cidA, and sarA). However, acetylisovaleryltylosin tartrate promotes these genes. Taken together, antibiotics at the sub-MICs can affect S. aureus biofilm formation through directly regulating biofilm-associated exoprotein genes and global modulatory genes, such as sigB, sarA, and agrA, which then participate in biofilm formation regulation (Fig. 2). For example, SarA can induce the formation of S. aureus USA300 biofilms through promoting the synthesis of polymeric N-acetyl-glucosamine (PNAG), the major polysaccharide intercellular adhesions of staphylococcal ECM [60].
Fig. 2.
The mechanisms of the effects of the sub-MICs of antibiotics on the biofilm formation of S. aureus. Antibiotics at sub-MIC levels affect the formation of S. aureus biofilms through directly modulating biofilm-associated genes or global regulatory genes, including sigB, sarA, and agrA, which then take part in the biofilm formation regulation. PNAG, polymeric N-acetyl-glucosamine.
Although strain-to-strain differences can exist upon exposure to antibiotics at sub-MICs, several antibiotics do not exert effects on S. aureus biofilm formation. Examples of this phenomenon include the sub-MICs of minocycline on five clinical and five nonclinical S. aureus isolates [59], sub-MICs of rifampicin on nonclinical S. aureus H01 [59], and sub-MICs of ceftaroline on S. aureus clinical strain 05/3291 [25]. Providing a full explanation for the variable responses of biofilm formation by S. aureus strains to antibiotics at sub-MICs is difficult. S. aureus strains, including patient-derived clinical isolates and healthy volunteer-isolated strains, maintain heterogeneous biofilm producer phenotypes. Despite the existence of the ica operon, some S. aureus strains, such as clinical isolate C39, do not change their weak-biofilm producer phenotypes when exposed to sub-MICs of rifampicin but instead display increased biofilm production in medium supplemented with 1.0% glucose [59]. A clear understanding of the multiple biofilm production responses of S. aureus strains in the presence of antibiotics at sub-MIC levels may be established through the accumulation of additional exploratory data by using different strains, various antibiotics, and unified culture conditions.
Effects of sub-MICs of antibiotics on S. aureus SCV formation
Bacterial SCVs are characterized as subpopulations of bacteria that display slow growth rates and atypical colonies (nearly 1/10 of the wild-type strain) and possess unusual metabolic pathways (decreased oxidative phosphorylation, deficient electron transport, or insufficient thymidine biosynthesis) [91]. S. aureus SCVs were first investigated in 1951 by Dr. Hale, who described a mutant of S. aureus that requires carbon dioxide for normal growth [92]. Thereafter, S. aureus SCVs with deficiency of menaquinone-, heme-, thiamine-, and thymidine-biosynthetic pathways were identified, and such SCVs formed mainly due to the antibiotic-induced or spontaneous mutation of certain metabolic genes, including har, hemB, ctaA, and thyA [91], [93], [94], [95]. Currently, increasing evidence has demonstrated that S. aureus SCVs are of medical importance for facilitating clinically persistent and recurrent infections [54]. Approximately 70% of patients who underwent long-term antibiotic treatment exhibit S. aureus SCV infection [94].
Studies have shown that S. aureus SCVs can be induced from their parental strains by exposure to various antibiotics in vitro [54], [96]. Zhang et al. conducted an induction experiment wherein the capability of 11 antibiotics to stimulate SCVs in 66 clinical S. aureus isolates was investigated. They found that 26 out of 66 (33.3%) clinical strains grow small colonies (≤1 mm) after 24 h of culture in TSB agar plates with the sub-MICs of gentamicin and that seven out of 66 (10.6%) strains display mixed populations containing large and small colonies [54]. Zhang et al. revealed that the reference S. aureus strain ATCC® 51650™ can switch to the SCV phenotype after 1 h of culture under treatment with 1/2 MIC of gentamicin, whereas other antibiotics can not induce S. aureus SCVs at short incubation times [54]. Moreover, high clindamycin and rifampicin concentrations induce S. aureus SCVs after 24 h of culture, whereas clarithromycin, ciprofloxacim, amoxicillin, methicillin, tetracyclin, erythromycin, vancomycin, and mupirocin may not participate in S. aureus SCV induction. Further studies showed that aminoglycoside gentamicin-induced S. aureus SCVs exhibit hemin-auxotrophic phenotypes [94]. Weaver et al. showed that S. aureus can rapidly increase resistance to rishexylaminomelamine–trisphenylguanide via the selection of a menaquinone-auxotroph SCV, which became an exclusively SCV phenotype after continuous cell passage in media with increasing drug concentrations [97]. By using skin fibroblast and lung epithelial cell infection models, Häffner et al. demonstrated that S. aureus agr mutants present a higher percentage of SCV formation in comparison with their wild-type strain 6850 [98].
The in vivo effects of sub-MICs of antibiotics on S. aureus virulence
In contrast to in vitro studies, the in vivo investigations of roles of antibiotics at sub-MICs in the virulence alterations of S. aureus are rare [38], [61], [68]. The reasons may be ascribed to the limitation of detection methods for S. aureus in vivo, the dynamic variation of sub-MICs of antibiotics administered, and the complicated internal physiological and chemical environments. Using an otitis media-rat model, Song et al. showed the effect of a sub-MIC concentration of the phyto-compound eugenol on S. aureus colonization in vivo [66]. The authors found that 1/2 MIC of eugenol is able to decrease 88% of S. aureus ATCC® 29213™ colonization in the middle ear of rat. Scanning electron microscopy observed that the whole middle ears of the only S. aureus treated rats are coated with bacterial biofilms, which are not visible in the middle ears of rats treated with S. aureus and the eugenol at a half MIC, however, the alteration of virulence factor expression in S. aureus of the infected rat model was not determined [66].
In patients under long-term antibiotic treatment, S. aureus SCVs are commonly isolated [99], [100]. Loss et al. illustrated the evolution of a SCV from its wild-type strain in a patient fitted with a prosthetic joint and experiencing S. aureus infection relapse and rifampicin treatment [101]. Lånnergard et al. isolated menadione-auxotrophic S. aureus SCVs and their isogenic wild-type strains from three patients suffering from chronic osteomyelitis and receiving long-term antibiotic therapy [102]. Other studies reported thymidine-auxotrophic S. aureus SCVs from patients with cystic fibrosis and receiving trimethoprim-sulfamethoxazole therapy [103], [104]. Kussmann et al. demonstrated the emergence of dalbavancin- nonsusceptible and teicoplanin-resistant S. aureus SCVs in a patient with cardiac device-related endocarditis undergoing long-term dalbavancin treatment [105]. Their findings implied the existence of a new lipoglycopeptide resistance mechanism because the in vivo induced S. aureus SCVs are formed in the absence of lipopeptide- or glycopeptide-antibiotic treatment. Such a mechanism requires further investigation.
Concluding remarks
In addition to their antimicrobial functions, the signal induction roles of antibiotics at sub-MICs have gained increasing interest in recent years. The major in vitro effects of the sub-MIC levels of antibiotics on the virulence of the important pathogen S. aureus include five aspects: (i) inducing bacterial cell deformation to stimulate abnormal host immune responses; (ii) modulating the expression levels of S. aureus virulence factors to alter disease development; (iii) regulating strain-specified adhesion and invasion capabilities to affect bacterial colonization and diffusion; (iv) altering S. aureus biofilm formation to potentate implantation material-related infections; and (v) influencing bacterial SCV formation to achieve persistent infection and recurrence. However, whether these in vitro effects of antibiotic sub-MICs on S. aureus virulence can be expected to present in vivo is unclear, and would be a fruitful research area for additional investigation.
The manifestations of the overall effects of antibiotics at sub-MIC concentrations on S. aureus virulence are dependent on strains and vary by antibiotic types, and inconsistent effects have been observed when a certain antimicrobial agent is used against different S. aureus strains or the same strain is treated with various types of antibiotics. The antibiotic susceptibility, virulence profile, growth stage, and culture condition of S. aureus and the time of antibiotic application may work independently or synergistically to contribute to divergent results. Mechanically, antibiotics at sub-MICs affect S. aureus virulence through direct binding to certain molecules, such as cell wall synthetic factors PBP1 and PBP2 and virulence factor PVL, and/or via modulating global regulators, including sigB, sarA, and agrA, which form a regulatory network to control S. aureus virulence. Therefore, modern omics technologies, such as proteomics, transcriptomics, metabonomics, and interactomics, should be applied to explore the complex effects of sub-MICs of antibiotics on S. aureus virulence. Hopeful findings should help construct novel therapeutic strategies that facilitate the treatment of infections caused by S. aureus in the future.
Declaration of Competing Interest
The authors declared that there is no conflict of interest.
Acknowledgments
Acknowledgements
This work was supported by the National Natural Science Foundation of China (82071857).
Author contributions
Xiancai Rao and Rong Zhang proposed the idea for the article. Juan Chen and Huyue Zhou did the literature search and data analysis. Juan Chen drafted the work. Xiancai Rao, Jingbin Huang and Rong Zhang critically revised the manuscript.
Biographies

Ms. Juan Chen, works as experimenters in Department of Pharmacy, the Second Affiliated Hospital of Army Medical University. Her master degree was received from the Chinese Academy of Sciences with zoology. After graduation, she has been engaged in scientific research for 4 years. She has been engaged in the screening and mechanism research of small molecule compounds of anti-tumor cell activity. Now, she is mainly engaged in the research of vancomycin-intermediate Staphylococcus aureus and membrane vesicle of Staphylococcus aureus vaccine. She has published a total of eight papers as the first author or co-author, and involved in writing the chapters 16 and 18 of book “De Mandal – Recent Advancements in Microbial Diversity”

Ms. Huyue Zhou was born in 1994 in Chongqing, China. She is studying for a master's degree at the Army Medical University in China. Her area of interest mainly focuses on the mechanism of bacterial drug resistance.

Dr. Jingbin Huang is working as an assistant professor at Department of Pharmacy, The Second Affiliated Hospital of Army Medical University, China. He received his Ph.D from The Second Military Medical University in 2014. His research interests focus on designing biomimetic nanomedicine or targeted drug delivery systems for cancer therapy or infection diseases. His researches have been supported by several projects form the National Natural Science Foundation of China and Outstanding Talent Fund of Army Medical University. He also has published seven peer-reviewed articles and holds five issued patents.

Dr. Rong Zhang is working as a chief pharmacist and professor in Army Medical University. She currently serves as the director of Department of Pharmacy, The Second Affiliated Hospital of Army Medical University. She is the vice chairman of Clinical Pharmacy Branch of Military Pharmaceutical Committee and the associate editor of “China Pharmacy”. Prof. Zhang has long been specializing in the research of Hospital Pharmacy Management, Clinical Pharmacy and Pharmacology. Recent years, she has hosted 11 research projects, 14 issued patents and published 14 scientific articles as first or corresponding author. Meanwhile, she has been honored the Third Prize of Military Science and Technology Progress.

Dr. Xiancai Rao obtained his Ph.D from Third Military Medical University of China in 2002, and then worked as a Postdoc at Boston University Medical Center and Louisiana State University of United States. He is currently a Professor and Head of Department of Microbiology, Army Medical University (Third Military Medical University) of China. His broad research interests concern drug resistance of Staphylococcus aureus, pathogenicity of Chlamydia trachomatis, and vaccine development of Dengue virus. He has published more than 100 peer reviewed research articles and received three patents.
Contributor Information
Rong Zhang, Email: xqpharmacylab@126.com.
Xiancai Rao, Email: raoxiancai@126.com.
References
- 1.Yang H., Xu S., Huang K., Xu X., Hu F., He C. Anti-staphylococcus antibiotics interfere with the transcription of leucocidin ED gene in Staphylococcus aureus strain Newman. Front Microbiol. 2020;11:265. doi: 10.3389/fmicb.2020.00265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Katahira E.J., Davidson S.M., Stevens D.L., Bolz D.D. Subinhibitory concentrations of tedizolid potently inhibit extracellular toxin production by methicillin-sensitive and methicillin-resistant Staphylococcus aureus. J Med Microbiol. 2019;68(2):255–262. doi: 10.1099/jmm.0.000905. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Sasso M.D., Culici M., Bovio C., Braga P.C. Gemifloxacin: Effects of sub-inhibitory concentrations on various factors affecting bacterial virulence. Int J Antimicrob AG. 2003;21(4):325–333. doi: 10.1016/s0924-8579(02)00391-6. [DOI] [PubMed] [Google Scholar]
- 4.Hogan D., Kolter R. Why are bacteria refractory to antimicrobials? Curr Opin Microbiol. 2002;5:472–477. doi: 10.1016/s1369-5274(02)00357-0. [DOI] [PubMed] [Google Scholar]
- 5.Fux C.A., Costerton J.W., Stewart P.S., Stoodley P. Survival strategies of infectious biofilms. Trends Microbiol. 2005;13(1):34–40. doi: 10.1016/j.tim.2004.11.010. [DOI] [PubMed] [Google Scholar]
- 6.Thiele-Bruhn S. Pharmaceutical antibiotic compounds in soils - a review. J Plant Nutr Soil S. 2003;166:145–167. [Google Scholar]
- 7.Kümmerer K. Antibiotics in the aquatic environment - A review - Part I. Chemosphere. 2009;75(4):417–434. doi: 10.1016/j.chemosphere.2008.11.086. [DOI] [PubMed] [Google Scholar]
- 8.Chander Y., Kumar K., Goyal S.M., Gupta S.C. Antibacterial activity of soil-bound antibiotics. J Environ Qual. 2005;34(6):1952–1957. doi: 10.2134/jeq2005.0017. [DOI] [PubMed] [Google Scholar]
- 9.Hao H., Cheng G., Iqbal Z., Ai X., Hussain H., Huang L. Benefits and risks of antimicrobial use in food-producing animals. Front Microbiol. 2014;5:288. doi: 10.3389/fmicb.2014.00288. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Hodille E., Rose W., Diep B.A., Goutelle S., Lina G., Dumitrescu O. The role of antibiotics in modulating virulence in Staphylococcus aureus. Clin Microbiol Rev. 2017;30(4):887–917. doi: 10.1128/CMR.00120-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Zheng Y., Shang W., Peng H., Rao Y., Zhao X., Hu Z. Virulence determinants are required for brain abscess formation through Staphylococcus aureus infection and are potential targets of antivirulence factor therapy. Front Microbiol. 2019;10:68. doi: 10.3389/fmicb.2019.00682. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Limoli D.H., Hoffman L.R. Help, hinder, hide and harm: what can we learn from the interactions between Pseudomonas aeruginosa and Staphylococcus aureus during respiratory infections? Thorax. 2019;74(7):684–692. doi: 10.1136/thoraxjnl-2018-212616. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Hsu Y.Y., Wu D., Hung C.C., Huang S.S., Yuan F.H., Lee M.H. Methicillin-resistant Staphylococcus aureus nasal colonization among HIV-infected patients in Taiwan: prevalence, molecular characteristics and associated factors with nasal carriage. BMC Infect Dis. 2020;20(1):254. doi: 10.1186/s12879-020-04979-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Adeiza S.S., Shuaibu A.B., Shuaibu G.M. Random effects meta-analysis of COVID-19/S. aureus partnership in co-infection. GMS Hyg. Infect Control. 2020;15:Doc29. doi: 10.3205/dgkh000364. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Gardner A.D. Morphological effects of penicillin on bacteria. Nature. 1940;146(3713):837–838. [Google Scholar]
- 16.Stevens D.L., Ma Y.S., Salmi D.B., Mclndoo E., Wallace R.J., Bryant A.E. Impact of antibiotics on expression of virulence-associated exotoxin genes in methicillin-sensitive and methicillin-resistant Staphylococcus aureus. J Infect Dis. 2007;195:202–211. doi: 10.1086/510396. [DOI] [PubMed] [Google Scholar]
- 17.Dumitrescu O., Choudhury P., Boisset S., Badiou C., Bes M., Benito Y. Beta-lactams interfering with PBP1 induce Panton-Valentine leukocidin expression by triggering sarA and rot global regulators of Staphylococcus aureus. Antimicrob Agents Chemother. 2011;55(7):3261–3271. doi: 10.1128/AAC.01401-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Nau R., Eiffert H. Modulation of release of proinflammatory bacterial compounds by antibacterials: potential impact on course of inflammation and outcome in sepsis and meningitis. Clin Microbiol Rev. 2002;15(1):95–110. doi: 10.1128/CMR.15.1.95-110.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Thorsing M., Klitgaard J.K., Atilano M.L., Skov M.N., Kolmos H.J., SrR Filipe. Thioridazine induces major changes in global gene expression and cell wall composition in methicillin-resistant Staphylococcus aureus USA300. PLoS ONE. 2013;8(5) doi: 10.1371/journal.pone.0064518. e64518. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Braga P.C., Sasso M.D., Maci S. Cefodizime: effects of sub-inhibitory concentrations on adhesiveness and bacterial morphology of Staphylococcus aureus and Escherichia coli: comparison with cefotaxime and ceftriaxone. J Antimicrob Chemoth. 1997;39(1):79–84. doi: 10.1093/jac/39.1.79. [DOI] [PubMed] [Google Scholar]
- 21.Li G., Qiao M., Guo Y., Wang X., Xu Y., Xia X. Effect of subinhibitory concentrations of chlorogenic acid on reducing the virulence factor production by Staphylococcus aureus. Foodborne Pathog Dis. 2014;11(9):677–683. doi: 10.1089/fpd.2013.1731. [DOI] [PubMed] [Google Scholar]
- 22.Kang S., Sunwoo K., Jung Y., Hur J.K., Park K.H., Kim J.S. Membrane-targeting triphenylphosphonium functionalized ciprofloxacin for Methicliin-Resistant Staphylococcus aureus (MRSA) Antibiotics. 2020;9(11):758. doi: 10.3390/antibiotics9110758. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Zhang X., Sun X., Wu J., Wu Y., Wang Y., Hu X. Berberine damages the cell surface of Methicillin-Resistant Staphylococcus aureus. Front Microbiol. 2020;11:621. doi: 10.3389/fmicb.2020.00621. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Juma A., Lemoine P., Simpson A.B.J., Murray J., O’Hagan B.M.G., Naughton P.J. Microscopic investigation of the combined use of antibiotics and biosurfactants on Methicillin Resistant Staphylococcus aureus. Front Microbiol. 2020;11:1477. doi: 10.3389/fmicb.2020.01477. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Lázaro-Díez M., Remuzgo-Martínez S., Rodríguez-Mirones C., Acosta F., Icardo J.M., Martínez-Martínez L. Effects of subinhibitory concentrations of ceftaroline on methicillin-resistant Staphylococcus aureus (MRSA) biofilms. PLoS ONE. 2016;11(1) doi: 10.1371/journal.pone.0147569. e0147569. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Hershkovits A.S., Pozdnyakov I., Meir O., Mor A. Sub-inhibitory membrane damage undermines Staphylococcus aureus virulence. Biochim Biophys Acta, Biomembr. 2019;1861(6):1172–1179. doi: 10.1016/j.bbamem.2019.04.002. [DOI] [PubMed] [Google Scholar]
- 27.Chang J.D., Foster E.E., Thadani A.N., Ramirez A.J., Kim S.J. Inhibition of Staphylococcus aureus cell wall biosynthesis by desleucyl-oritavancin: a quantitative peptidoglycan composition analysis by mass spectrometry. J Bacteriol. 2017;199(15):e00278–e317. doi: 10.1128/JB.00278-17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Wojnicz D., Klak M., Adamski R., Jankowski S. Influence of subinhibitory concentrations of amikacin and ciprofloxacin on morphology and adherence ability of Uropathogenic strains. Folia Microbiol. 2007;52(4):429–436. doi: 10.1007/BF02932099. [DOI] [PubMed] [Google Scholar]
- 29.Shang W., Rao Y., Zheng Y., Yang Y., Hu Q., Hu Z. β-Lactam antibiotics enhance the pathogenicity of methicillin-resistant Staphylococcus aureus via SarA-controlled lipoprotein-like cluster expression. mBio. 2019;10(3):e00880–19. doi: 10.1128/mBio.00880-19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Kim S.J., Matsuoka S., Patti G.J., Schaefer J. Vancomycin derivative with damaged D-Ala-D-Ala binding cleft binds to cross-linked peptidoglycan in the cell wall of Staphylococcus aureus. Biochemistry. 2008;47(12):3822–3831. doi: 10.1021/bi702232a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Zhu X., Liu D., Singh A.K., Drolia R., Bai X., Tenguria S. Tunicamycin mediated inhibition of wall teichoic acid affects Staphylococcus aureus and Listeria monocytogenes cell morphology, biofilm formation and virulence. Front Microbiol. 2018;9:1352. doi: 10.3389/fmicb.2018.01352. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Li X., Wang P., Hu X., Zhang Y., Lu X., Li C. The combined antibacterial effects of sodium new houttuyfonate and berberin chloride against growing and persistent Methicillin-Resistant and Vancomycin-Intermediate Staphylococcus aureus. BMC Microbiol. 2020;20(1):317. doi: 10.1186/s12866-020-02003-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Muller S., Wolf A.J., Iliev I.D., Berg B.L., Underhill D.M., Liu G.Y. Poorly cross-linked peptidoglycan in MRSA due to mecA induction activates the inflammasome and exacerbates immunopathology. Cell Host Microbe. 2015;18(5):604–612. doi: 10.1016/j.chom.2015.10.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Doss S.A., Tillotson G.S., Amyes S.G. Effect of sub-inhibitory concentrations of antibiotics on the virulence of Staphylococcus aureus. Appl Bacteriol. 1993;75(2):123–128. doi: 10.1111/j.1365-2672.1993.tb02756.x. [DOI] [PubMed] [Google Scholar]
- 35.Haddadin R.N.S., Saleh S., Al-Adham I.D.S.I., Buultjens T.E.J., Collier P.J. The effect of subminimal inhibitory concentrations of antibiotics on virulence factors expressed by Staphylococcus aureus biofilms. J Appl Microbiol. 2010;108(4):1281–1291. doi: 10.1111/j.1365-2672.2009.04529.x. [DOI] [PubMed] [Google Scholar]
- 36.Hu H., Ramezanpour M., Hayes A.J., Liu S., Psaltis A.J., Wormald P.J. Sub-Inhibitory clindamycin and azithromycin reduce S. aureus exoprotein induced toxicity, inflammation, barrier disruption and invasion. J Clin Med. 2019;8(10):1617. doi: 10.3390/jcm8101617. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Jin Y., Li M., Shang Y., Liu L., Shen X., Lv Z. Sub-Inhibitory concentrations of mupirocin strongly inhibit alpha-toxin production in high-level mupirocin-resistant MRSA by down-regulating agr, saeRS, and sarA. Front Microbiol. 2018;9:993. doi: 10.3389/fmicb.2018.00993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.An Y., Wang Y., Zhan J., Tang X., Shen K., Shen F. Fosfomycin protects mice from Staphylococcus aureus pneumonia caused by alpha-hemolysin in extracellular vesicles by inhibiting MAPK-regulated NLRP3 inflammasomes. Front Cell Infect Microbiol. 2019;9:253. doi: 10.3389/fcimb.2019.00253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Otto M.P., Martin E., Badiou C., Lebrun S., Bes M., Vandenesch F. Effects of subinhibitory concentrations of antibiotics on virulence factor expression by community-acquired methicillin-resistant Staphylococcus aureus. J Antimicrob Chemother. 2013;68(7):1524–1532. doi: 10.1093/jac/dkt073. [DOI] [PubMed] [Google Scholar]
- 40.Liu L., Shen X., Yu J., Cao X., Zhan Q., Guo Y. Subinhibitory concentrations of fusidic acid may reduce the virulence of S. aureus by down-regulating sarA and saeRS to reduce biofilm formation and α-toxin expression. Front Microbiol. 2020;11:25. doi: 10.3389/fmicb.2020.00025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Gottschalk S., Ingmer H., Thomsen L.E. The lysine-peptoid hybrid LP5 maintain activity under physiological conditions and affects virulence gene expression in Staphylococcus aureus. Peptides. 2016;78:24–29. doi: 10.1016/j.peptides.2016.02.001. [DOI] [PubMed] [Google Scholar]
- 42.Ping O., Sun M., He X., Wang K., Yin Z., Fu H. Sclareol protects Staphylococcus aureus-induced lung cell injury via inhibiting alpha-hemolysin expression. J Microbiol Biotechn. 2017;27(1):19–25. doi: 10.4014/jmb.1606.06039. [DOI] [PubMed] [Google Scholar]
- 43.Gregorio E.D., Esposito A., Vollaro A., Fenza M.D., D’Alonzo D., Miglicaccio A. N-Nonyloxypentyl-l-Deoxynojirimycin inhibits growth, biofilm formation and virulence factors expression of Staphylococcus aureus. Antibiotics. 2020;9(6):362. doi: 10.3390/antibiotics9060362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Kernodle D.S., McGraw P.A., Baarg N.L., Menzies B.E., Voladri R.K.R., Harshman S. Growth of Staphylococcus aureus with nafcillin in vitro induces alpha-toxin production and increases the lethal activity of sterile broth filtrates in a murine model. J Infect Dis. 1995;172(2):410–419. doi: 10.1093/infdis/172.2.410. [DOI] [PubMed] [Google Scholar]
- 45.Ohlsen K., Ziebuhr W., Koller K.P., Hell W., Wichelhaus T.A., Hacker J. Effects of subinhibitory concentrations of antibiotics on alpha-toxin (hla) gene expression of methicillin-sensitive and methicillin-resistant Staphylococcus aureus isolates. Antimicrob Agents Chemother. 1998;42(11):2817–2823. doi: 10.1128/aac.42.11.2817. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Farha A.K., Yang Q., Kim G., Zhang D., Mavumengwana V., Habimana O. Inhibition of multidrug-resistant foodborne Staphylococcus aureus biofilms by a natural terpenoid (+)-nootkatone and related molecular mechanism. Food Control. 2020;112 107154. [Google Scholar]
- 47.Bryant A.E., Gomi S., Katahira E., Huang D.B., Stevens D.L. The effects of iclaprim on exotoxin production in methicillin-resistant and vancomycin-intermediate Staphylococcus aureus. J Med Microbiol. 2019;68(3):456–466. doi: 10.1099/jmm.0.000929. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Morikawa K., Watabe H., Araake M., Morikawa S. Modulatory effect of antibiotics on cytokine production by human monocytes in vitro. Antimicrob Agents Chemother. 1996;40(6):1366–1370. doi: 10.1128/aac.40.6.1366. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Josse J., Laurent F., Diot A. Staphylococcal adhesion and host cell invasion: fibronectin-binding and other mechanisms. Front Microbiol. 2017;8:2433. doi: 10.3389/fmicb.2017.02433. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Zheng J.X., Tu H.P., Sun X., Xu G.J., Chen J.W., Deng Q.W. In vitro activities of telithromycin against Staphylococcus aureus biofilms compared with azithromycin, clindamycin, vancomycin and daptomycin. J Med Microbiol. 2020;69(1):120–131. doi: 10.1099/jmm.0.001122. [DOI] [PubMed] [Google Scholar]
- 51.Jo A., Ahn J. Phenotypic and genotypic haracterization of multiple antibiotic-resistant Staphylococcus aureus exposed to subinhibitory levels of oxacillin and levofloxacin. BMC Microbiol. 2016;16(1):170. doi: 10.1186/s12866-016-0791-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Rasigade J.P., Moulay A., Lhoste Y., Tristan A., Bes M., Vandenesch F. Impact of sub-inhibitory antibiotics on fibronectin-mediated host cell adhension and invasion by Staphylococcus aureus. BMC Microbiol. 2011;11(1):263. doi: 10.1186/1471-2180-11-263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Mirani Z.A., Jamil N. Effect of sub-lethal doses of vancomycin and oxacillin on biofilm formation by vancomycin intermediate resistant Staphylococcus aureus. J Basic Microb. 2011;51(2):191–195. doi: 10.1002/jobm.201000221. [DOI] [PubMed] [Google Scholar]
- 54.Zhang G., Zhao Y., Hayes A.J., Psaltis A.J., Wormald P.J., Vreugde S. Staphylococcus aureus small colony variants: Prevalence in chronic rhinosinusitis and induction by antibiotics. Allergy. 2018;73(12):2403–2405. doi: 10.1111/all.13580. [DOI] [PubMed] [Google Scholar]
- 55.Selan L., Vrenna G., Ettorre E., Papa R., Artini M. Virulence of MRSA USA300 is enhanced by sub-inhibitory concentration of two different classes of antibiotics. J Chemother. 2018;30(6–8):384–388. doi: 10.1080/1120009X.2018.1533085. [DOI] [PubMed] [Google Scholar]
- 56.Yang B., Lei Z., Zhao Y., Ahmed S., Wang C., Zhang S. Combination susceptibility testing of common antimicrobials in vitro and the effects of Sub-MIC of antimicrobials on Staphylococcus aureus biofilm formation. Front Microbiol. 2017;8:2125. doi: 10.3389/fmicb.2017.02125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Sasso M.D., Bovio C., Culici M., Braga P.C. Interference of sub-inhibitory concentrations of gatifloxacin on various determinants of bacterial virulence. J Chemother. 2002;14(5):473–482. doi: 10.1179/joc.2002.14.5.473. [DOI] [PubMed] [Google Scholar]
- 58.Braga P.C., Piatti G. Favourable effects of sub-MIC rufloxacin concentrations in decreasing the pathogen-host cell adhesion. Pharmacol Res. 1993;28(1):11–20. doi: 10.1006/phrs.1993.1105. [DOI] [PubMed] [Google Scholar]
- 59.Lima-E-Silva A.A., Silva-Filho R.G., Fernandes H.M.Z., Saramago G.S.M., Viana A.S., Souza M.S. Sub-Inhibitory concentrations of rifampicin strongly stimulated biofilm production in S. aureus. Open Microbiol J. 2017;11:142–151. doi: 10.2174/1874285801711010142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Sritharadol R., Hamada M., Kimura S., Ishii Y., Srichana T., Tateda K. Mupirocin at subinhibitory concentrations induces biofilm formation in Staphylococcus aureus. Microb Drug Resist. 2018;24(9):1249–1258. doi: 10.1089/mdr.2017.0290. [DOI] [PubMed] [Google Scholar]
- 61.Woodburn K.W., Jaynes J.M., Clemens L.E. Evaluation of the antimicrobial peptide, RP557, for the broad-spectrum treatment of wound pathogens and biofilm. Front Microbiol. 2019;10:1688. doi: 10.3389/fmicb.2019.01688. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Andre C., Pimentel-Filho N.D.J., Costa P.M.D.A., Vanetti M.C.D. Changes in the composition and architecture of staphylococcal biofilm by nisin. Braz J Microbiol. 2019;50(4):1083–1090. doi: 10.1007/s42770-019-00135-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Abbas H.A., Elsheerbini A.M., Shaldam M.A. Glyceryl trinitrate blocks staphyloxanthin and biofilm formation in Staphylococcus aureus. Afr Health Sci. 2019;19(1):1376–1384. doi: 10.4314/ahs.v19i1.10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Ferreira G.R.S., Brito J.S., Procópio T.F., Santos N.D.L., Lima B.J.R.C., Coelho L.C.B.B. Antimicrobial potential of Alpinia purpurata lectin (ApuL): Growth inhibitory action, synergistic effects in combination with antibiotics, and antibiofilm activity. Microb Pathogenesis. 2018;124:152–162. doi: 10.1016/j.micpath.2018.08.027. [DOI] [PubMed] [Google Scholar]
- 65.Gonçalves R.C., Silva D.P., Signini R., Naves P.L.F. Inhibition of bacterial biofilms by carboxymethyl chitosan combined with silver, zinc and copper salts. Int J Biol Macromol. 2017;105:385–392. doi: 10.1016/j.ijbiomac.2017.07.048. [DOI] [PubMed] [Google Scholar]
- 66.Yadav M.K., Chae S.W., Im G.J., Chung J.W., Song J.J. Eugenol: A phyto-compound effective against methicillin-resistant and methicillin-sensitive Staphylococcus aureus clinical strain biofilms. PLoS ONE. 2015;10(3) doi: 10.1371/journal.pone.0119564. e0119564. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Du H., Zhou L., Lu Z., Bie X., Zhao H., Niu Y. Transcriptomic and proproteomic profiling response of methicillin-resistant Staphylococcus aureus (MRSA) to a novel bacteriocin, plantaricin GZ1-27 and its inhibition of biofilm formation. Appl Microbiol Biotechnol. 2020;104(18):7957–7970. doi: 10.1007/s00253-020-10589-w. [DOI] [PubMed] [Google Scholar]
- 68.Escobar I.E., White A., Kim W., Mylonakis E. New Antimicrobial Bioactivity against Multidrug-Resistant Gram-Positive Bacteria of Kinase Inhibitor IMD0354. Antibiotics. 2020;9(10):665. doi: 10.3390/antibiotics9100665. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Ali S., Delaramsadat R., Farzaneh R., Maria K., Sholoufeh M.O., Seyed-Alireza E. Evaluate the effect of zinc oxide and silver nanoparticles on biofilm and icaA gene expression in methicillin-resistant Staphylococcus aureus isolated from burn wound infection. Journal of Burn Care & Research. 2020;6(41):1253–1259. doi: 10.1093/jbcr/iraa085. [DOI] [PubMed] [Google Scholar]
- 70.Hemmati F., Salehi R., Ghotaslou R., Kafil H.S., Hasani A., Gholizadeh P. The assessment of antibiofilm activity of chitosan-zinc oxide-gentamicin nanocomposite on Pseudomonas aeruginosa and Staphylococcus aureus. J Biological Macromolecules. 2020;163:2248–2258. doi: 10.1016/j.ijbiomac.2020.09.037. [DOI] [PubMed] [Google Scholar]
- 71.Alam P., Alqahtani A.S., Husain F.M., Rehman M.T., Alajmi M.F., Noman O.M. Siphonocholin isolated from red sea sponge Siphonochalina siphonella attenuates quorum sensing controlled virulence and biofilm formation. Saudi Pharmaceutical J. 2020;28(1):1383–1391. doi: 10.1016/j.jsps.2020.09.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Maioli E., Marchese A., Roveta S., Cagnacci S., Cavallini F., Cassanelli C. In vitro activity of ceftibuten at sub-inhibitory concentrations in comparison with other antibiotics against respiratory and urinary tract pathogens. J Chemother. 2007;19(2):152–160. doi: 10.1179/joc.2007.19.2.152. [DOI] [PubMed] [Google Scholar]
- 73.Rao Y., Shang W., Yang Y., Zhou R., Rao X. Fighting mixed-species microbial biofilms with cold atmospheric plasma. Front Microbiol. 2020;11:1000. doi: 10.3389/fmicb.2020.01000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Boles B.R., Horswill A.R. Staphylococcal biofilm disassembly. Trends Microbiol. 2011;19(9):449–455. doi: 10.1016/j.tim.2011.06.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Foster T.J., Geoghegan J.A., Ganesh V.K., Höök M. Adhesion, invasion and evasion: the many functions of the surface proteins of Staphylococcus aureus. Nat Rev Microbiol. 2014;12(1):49–62. doi: 10.1038/nrmicro3161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Flemming H.C., Wingender J. The biofilm matrix. Nat Rev Microbiol. 2010;8(9):623–633. doi: 10.1038/nrmicro2415. [DOI] [PubMed] [Google Scholar]
- 77.Rice S.A., Wuertz S., Kjelleberg S. Next-generation studies of microbial biofilm communities. Microb Biotechnol. 2016;9(5):677–680. doi: 10.1111/1751-7915.12390. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Davey M.E., O’Toole G.A. Microbial biofilms: from ecology to molecular genetics. Microbiol Mol Biol R. 2000;64(4):847–867. doi: 10.1128/mmbr.64.4.847-867.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Gilbert P., Das J., Foley I. Biofilm susceptibility to antimicrobials. Adv Dent Res. 1997;11(1):160–167. doi: 10.1177/08959374970110010701. [DOI] [PubMed] [Google Scholar]
- 80.Andersson D.I., Hughes D. Evolution of antibiotic resistance at non-lethal drug concentrations. Drug Resist Update. 2012;15(3):162–172. doi: 10.1016/j.drup.2012.03.005. [DOI] [PubMed] [Google Scholar]
- 81.Wang S., Yang Y., Zhao Y., Zhao H., Bai J., Chen J. Sub-MIC tylosin inhibits Streptococcus suis biofilm formation and results in differential protein expression. Front Microbiol. 2016;7:384. doi: 10.3389/fmicb.2016.00384. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Jacobsen S.M., Shirtliff M.E. Proteus mirabilis biofilms and catheter-associated urinary tract infections. Virulence. 2011;2(5):460–465. doi: 10.4161/viru.2.5.17783. [DOI] [PubMed] [Google Scholar]
- 83.Van Laar T.A., Chen T., You T., Leung K.P. Sublethal concentrations of carbapenems alter cell morphology and genomic expression of Klebsiella pneumoniae biofilms. Antimicrob Agents Chemother. 2015;59(3):1707–1717. doi: 10.1128/AAC.04581-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Cairns J., Becks L., Jalasvuori M., Hultunen T. Sublethal streptomycin concentrations and lytic bacteriophage together promote resistance evolution. Philos Trans R Soc Lond B Biol. 2017;372:1712. doi: 10.1098/rstb.2016.0040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Westhoff S., Leeuwe T.M., Qachach O., Zhang Z., Wezel G.P., Rozen D.E. The evolution of no-cost resistance at sub-MIC concentrations of streptomycin in Streptomyces coelicolor. ISME J. 2017;11(5):1168–1178. doi: 10.1038/ismej.2016.194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Weiser J., Henke H.A., Hector N., Both A., Chritner M., Büttner H. Sub-inhibitory tigecycline concentrations induce extracellular matrix binding protein Embp dependent Staphylococcus epidermidis biofilm formation and immune evasion. Int J Med Microbiol. 2016;306(6):471–478. doi: 10.1016/j.ijmm.2016.05.015. [DOI] [PubMed] [Google Scholar]
- 87.Gullberg E., Cao S., Berg O.G., Ilback C., Sandegren L., Hughes D. Selection of resistant bacteria at very low antibiotic concentrations. PLoS Pathog. 2011;7(7) doi: 10.1371/journal.ppat.1002158. e1002158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.McCarthy H., Rudkin J.K., Black N.S., Gallagher L., O’Neill E., O’Gara J.P. Methicillin resistance and the biofilm phenotype in Staphylococcus aureus. Front Cell Infect Microbiol. 2015;5:1. doi: 10.3389/fcimb.2015.00001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Reis S.V., Ribeiro N.S., Rocha D.A., Fortes I.S., Trentin D.S., Andrade S.F. N4-benzyl-N2-phenylquinazoline-2,4-diamine compound presents antibacterial and antibiofilm effect against Staphylococcus aureus and Staphylococcus epidermidis. Chem Biol Drug Des. 2020;96(6):13745. doi: 10.1111/cbdd.13745. [DOI] [PubMed] [Google Scholar]
- 90.Schilcher K., Andreoni F., Dengler Haunreiter V., Seidl K., Hasse B., Zinkernagel A.S. Modulation of Staphylococcus aureus biofilm matrix by subinhibitory concentrations of clindamycin. Antimicrob Agents Chemother. 2016;60:5957–5967. doi: 10.1128/AAC.00463-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Proctor R.A., Eiff C., Kahl B.C., Becker K., McNamara P., Herrmann M. Small colony variants a pathogenic form of bacteria that facilitates persistent and recurrent infections. Nat Rev Microbiol. 2006;4:295–305. doi: 10.1038/nrmicro1384. [DOI] [PubMed] [Google Scholar]
- 92.Hale J.H. Studies on Staphylococcus mutation: a naturally occurring “G” gonidial variant and its carbon requirements. Br J Exp Pathol. 1951;32(4):307–313. [PMC free article] [PubMed] [Google Scholar]
- 93.Sherris J.C. Two small colony variants of Staphylococcus aureus isolated in pure culture from closed infected lesions and their carbon dioxide requirements. J Clin Pathol. 1952;5(4):354–355. doi: 10.1136/jcp.5.4.354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Melter O., RadojeviČ B. Small colony variants of Staphylococcus aureus-Review. Folia Microbiol. 2010;55(6):548–558. doi: 10.1007/s12223-010-0089-3. [DOI] [PubMed] [Google Scholar]
- 95.Tuchscherr L., Löffler B., Proctor R.A. Persistence of Staphylococcus aureus: Multiple metabolic pathways impact the expression of virulence factors in small-colony variants (SCVs) Front Microbiol. 2020;11:1028. doi: 10.3389/fmicb.2020.01028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Tuchscherr L., Kreis C., Hoerr V., Flint L., Hachmeister M., Geraci J. Staphylococcus aureus develops increased resistance to antibiotics by forming dynamic small colony variants during chronic osteomyelitis. J Antimicrob Chemother. 2015;71(2):438–448. doi: 10.1093/jac/dkv371. [DOI] [PubMed] [Google Scholar]
- 97.Weaver A.J., Peters T.R., Tripet B., Van Vuren A., Rakesh Lee RE. Exposure of methicillin-resistant Staphylococcus aureus to low levels of the antibacterial THAM-3ΦG generates a small colony drug-resistant phenotype. Sci Rep. 2018;8(1):9850. doi: 10.1038/s41598-018-28283-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Häffner N., Bär J., Dengler H.V., Mairpady S.S., Seidl K., Crosby H.A. Intracellular environment and agr system affect colony size heterogeneity of Staphylococcus aureus. Front Microbiol. 2020;11:1415. doi: 10.3389/fmicb.2020.01415. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Kahl B.C., Becker K., Löffler B. Clinical significance and pathogenesis of staphylococcal small colony variants in persistent infections. Clin Microbiol Rev. 2016;29(2):401–427. doi: 10.1128/CMR.00069-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Lin Y.T., Tsai J.C., Yamamoto T., Chen H.J., Hung W.C., Hsueh P.R. Emergence of a small colony variant of vancomycin-intermediate Staphylococcus aureus in a patient with septic arthritis during long-term treatment with daptomycin. J Antimicrob Chemother. 2016;71(7):1807–1814. doi: 10.1093/jac/dkw060. [DOI] [PubMed] [Google Scholar]
- 101.Loss G., Simões P.M., Valour F., Cortês M.F., Gonzaga L., Bergot M. Staphylococcus aureus small colony variants (SCVs): News from a chronic prosthetic joint infection. Front Cell Infect Microbiol. 2019;9:363. doi: 10.3389/fcimb.2019.00363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Lannergård J., von Eiff C., Sander G., Cordes T., Seggewiss J., Peters G. Identification of the genetic basis for clinical menadione-auxotrophic small-colony variant isolates of S. aureus. Antimicrob Agents Chemother. 2008;52(11):4017–4022. doi: 10.1128/AAC.00668-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Besier S., Smaczny C., von Mallinckrodt C., Krahl A., Ackermann H., Brade V. Prevalence and clinical significance of Staphylococcus aureus small-colony variants in cystic fibrosis lung disease. J Clin Microbiol. 2007;45(1):168–172. doi: 10.1128/JCM.01510-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Chatterjee I., Kriegeskorte A., Fischer A., Deiwick S., Theimann N., Proctor R.A. In vivo mutations of thymidylate synthase (encoded by thyA) are responsible for thymidine dependency in clinical small-colony variants of Staphylococcus aureus. J Bacteriol. 2008;190(3):834–842. doi: 10.1128/JB.00912-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Kussmann M., Karer M., Obermueller M., Schmidt K., Barousch W., Moser D. Emergence of a dabavancin induced glycopeptide/lipoglycopeptide non-susceptible Staphylococcus aureus during treatment of a cardiac device-related endocarditis. Emerg Microbes Infect. 2018;7(1):202. doi: 10.1038/s41426-018-0205-z. [DOI] [PMC free article] [PubMed] [Google Scholar]