Graphical abstract
Keywords: Nanoparticles, Nanotoxicology, Plant stress, Oxidative stress, Environmental pollution
Abstract
Background
Biostimulation and toxicity constitute the continuous response spectrum of a biological organism against physicochemical or biological factors. Among the environmental agents capable of inducing biostimulation or toxicity are nanomaterials. On the < 100 nm scale, nanomaterials impose both physical effects resulting from the core’s and corona’s surface properties, and chemical effects related to the core’s composition and the corona’s functional groups.
Aim of Review
The purpose of this review is to describe the impact of nanomaterials on microorganisms and plants, considering two of the most studied physical and chemical properties: size and concentration.
Key Scientific Concepts of Review
Using a graphical analysis, the presence of a continuous biostimulation-toxicity spectrum is shown considering different biological responses. In microorganisms, the results showed high susceptibility to nanomaterials. Simultaneously, in plants, a hormetic response was found related to nanomaterials concentration and, in a few cases, a positive response in the smaller nanomaterials when these were applied at a higher level. With the above, it is concluded that: (1) microorganisms are more susceptible to nanomaterials than plants, (2) practically all nanomaterials seem to induce responses from biostimulation to toxicity in plants, and (3) the kind of response observed will depend in a complex way on the nanomateriaĺs physical and chemical characteristics, of the biological species with which they interact, and of the form and route of application and on the nature of the medium -soil, soil pore water, and biological surfaces- where the interaction occurs.
Introduction
Biostimulation and toxicity constitute segments of the continuous response spectrum of a biological organism against a physicochemical or biological factor. Environmental agents that cause biostimulation or toxicity (or inhibition) can be physical, chemical, or biological. Typically, an excess of the factor or the presence of the factor at a level outside of a suitable range (e.g., irradiance, the concentration of an ion or a biomolecule) induces an inhibition response or even death of the organism; On the other hand, if the factor is at a certain level within the margins of an adequate range, then biostimulation occurs [1]. Toxicity is synonymous with inhibition, while biostimulation is synonymous with promotion.
Biostimulation as a biological activity must be distinguished from the concept of biostimulant that is applied in agricultural practice. There is no widely accepted definition of biostimulant [2]. Different authors and legislation point to varying definitions, agreeing that these are substances or microorganisms for agricultural use that facilitate the acquisition of nutrients or promote plant metabolism, but without belonging to the category of fertilizer, growth regulator or pesticide [2], [3]. The definition of biostimulant has a scientific basis, but its application has been directed more towards regulatory purposes.
Biostimulation, on the other hand, is a general biological response triggered by some environmental factor that causes the adaptive modification of metabolic processes, in such a way that the organism carries out adjustments that lead to more efficient use of environmental resources. Biostimulation occurs in response to virtually all environmental factors: physical, chemical, and biological [1]. When the level of the environmental triggering factor exceeds the appropriate threshold for the organism, it generates negative responses that are called toxicity (when it comes to chemical or biochemical compounds) or inhibition (in the case of physical factors such as temperature and irradiance). An example of the above is the toxicity of Cu when the concentration exceeds the levels of 25 mg kg−1 in plant tissues [4]. In this case, the toxicity is the result of the alteration of the functioning of the Ca2+ and K+ channels by interaction and blocking by Cu2+ [5]. This dysfunction changes the distribution and balance of Ca2+, K+ and Mg2+ in plant cells, leading to the production of free radicals and the resultant oxidative stress [6].
Among the environmental factors capable of inducing biostimulation or toxicity are nanomaterials (NMs) of metals, semi-metals, and non-metals, minerals (such as nanoclays and nanozeolites), organic (such as chitosan) and C (such as graphene). NMs are characterized by having at least one dimension<100 nm. On this scale, the materials have different properties from those of the bulk material of the same composition [7], showing physical effects associated with surface properties and chemical effects related to its composition [8].
Among the physical properties of NMs, it can be mentioned a high level of surface free energy, resulting from the exposure of electrons from the outer layers, which occurs when a material exhibits a high surface: volume ratio. The surface energy of an NM can be 3 to 6 times higher compared to the bulk material [9]. Chemical properties include the ability of NMs to enter cells and different cell compartments and release the ions or substances that make them up [10]. The above differential physical and chemical properties allow the NMs to show a broader range of impacts than those shown by the bulk material or the material in micron or ionic form [11], [12].
Different studies have compared the impact of different elements, especially metals and metalloids, in ionic and nanometric form. The effect in both cases is variable according to the concentration of the materials: hormesis, or stimulation with low level, and toxicity with high concentration [13]. The defense and adaptation mechanisms that the organisms exert in case of toxicity are different for bulk materials and NMs. What is known about the impact of bulk materials does not fully predict the effect of the NMs [8].
For ionic forms, the first line of defense in plants is the regulation of the concentration in the cytoplasm and other cellular compartments, using selective mechanisms based on transport proteins, channels, and endocytosis that mobilize the ions towards the vacuole or extrude them towards the apoplast [5], [14]. Additionally, the transformation of metals and metalloids into less toxic or non-toxic chemical species can occur in microorganisms. If the concentration regulation mechanisms are not enough, then the molecular and enzymatic antioxidant defenses and defenses that make use of chelating metabolites, peptides, and storage or detoxification proteins are activated in both plants and microorganisms [15], [16], [17].
In the case of nanometric species, the number of interactions with cell surfaces is greater compared to ionic forms and the response mechanisms seem to be more diverse. To the best of our knowledge, NMs can enter the different cell compartments, but not as a result of a sequestration or chelating process dependent on specialized transport mechanisms or complexing metabolites, as occurs with ionic forms. NMs can penetrate cell compartments, inducing damage to the membranes or pores through the reactivity of the NM surface or through endocytosis processes [18]. The formation of biofilms and cell aggregates of the same species or even of different species occurs in the microorganisms as a strategy to survive in stressful environments. The biopolymers and metabolites that are extruded by the cells and form the biofilm's extracellular matrix reduce the direct contact between the cells and the external environment, mitigating the cellular damage caused by NMs. On the other hand, cell aggregation, which may or may not be accompanied by a biofilm formation, reduces the net surface area of bacterial cells' exposure to the external media where the NMs are found. The strategy of cell aggregation reduces the damage caused by the NMs on the individual cells [19], [20].
Another kind of NM-cell interaction depends on the great surface free energy of the NM. The surface energy makes NMs highly reactive, rapidly inducing modifications in cell walls and membranes and causing membrane damage and formation of reactive oxygen species (ROS) and other reactive species, modification of the activity of different integral proteins, and changes in the redox balance, energy metabolism, and ion transport [5], [19]. The cell wall and membrane stimuli occur before NM is internalized to the cytoplasm and cell organelles. At low concentration, surface stimuli caused by NMs induce beneficial defense responses and modify positively the metabolism, analogously to a biostimulant [4]. However, when the concentration of NMs increases, it is supposed that excess surface interactions can induce a state of distress in cells, inhibiting metabolism, growth, or even causing cell death [8].
In addition to concentration and surface free energy, other characteristics as the composition, size, and charge, as well as more complex characters to quantify such as shape, crystallinity, roughness, porosity, hydrophobicity, and hydrophilicity, determine together the response of cellular components to the NMs [8], [21]. As with other environmental factors, such as nutrients, water deficit, cold, and UV radiation, the interaction of microorganisms and plants with the NMs determines changes in behavior at the subcellular, cellular, tissue, and organ levels, or the entire organism. The adaptive responses occur after a sequence of events of perception, transduction, and signaling, which modify gene expression or metabolism [22], [23].
An additional factor that increases the complexity of interactions between cells and NMs is the formation of the surface corona. In natural media: edaphic, aquatic, or internal volume of organisms, the surface of the NMs adsorbs organic molecules or biomolecules (proteins, peptides, polysaccharides, glycolipids, and small metabolites), forming a corona whose composition it is characteristic of each type of NM and of each medium where these materials are found. The formation of the corona radically modifies and extensively diversifies the interaction properties of the NMs with biological entities [21], [24], [25], [26], adding this character to the ability mentioned above to induce physical and chemical effects on cells [27].
The surface properties mentioned above are presented in all NMs; properties depend mainly on the nanodimensional magnitude of the material. Therefore, all NMs, regardless of their composition, operate in a spectrum of action from biostimulation to toxicity. This spectrum of responses of the cells to the NMs results from the interaction with the conglomerate of physical and chemical properties of the NMs. The initial responses mainly depend on the physical properties of the NM surface interacting with the cell surfaces, while the later responses are the result of the interaction of the internal cellular environment with the physical and chemical properties of the NM [8].
Different reviews have described the experimental information about the interaction between the NMs with biological organisms, pointing out the physical and chemical interactions [21], [24], [27], [28] and the different responses observed in different species of microorganisms [29], [30], fungi [31] and plants [32], [33], [34]; It has also been written about the transit of materials in ecosystems and food chains [12], [35]. We published a review in 2019, proposing that NMs be categorized as biostimulant compounds [1]. In our previous study, the emphasis was placed on the impact of surface charges and the electric double layer on the interaction of NMs with plant cells, pointing out the possibility that surface interactions were part of the explanation for the capacity of NMs to induce beneficial responses in plants.
As a complement to the previous reviews, the present manuscript tries to list, considering the cases of plants and microorganisms, published evidence about how the impact of the most studied physicochemical properties (e.g. concentration, size, surface charge, surface free energy, shape, roughness, hydrophobicity) of the NMs is framed in a continuum of responses that goes from biostimulation to toxicity, pointing out the potential use of NMs as biostimulants to improve biological processes.
The physical properties of NMs induce biostimulation or toxicity in microorganisms and plants
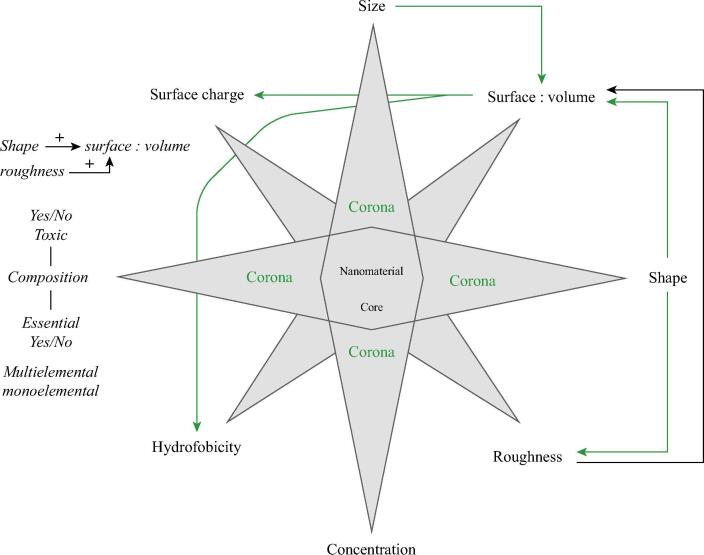
The first NM-microorganism or NM-plant interaction occurs through the surfaces or interfaces. The surface properties of the NMs and their corona define the primary impact that they have on cell walls and membranes (Fig. 1) and therefore, dictate the form and extent of the initial responses of the organisms [24], [36]. When NMs take contact with soils and water environments, atmosphere or biological fluids, immediately form a corona; therefore, the first NMs-cell interaction occurs between the NḾs corona and the receptors and surface components of cell walls and membranes [5], [26], [37]. For the above reason, the same type of NMs, with the same properties in shape, size, composition, etc., will have a different impact on an organism, depending on the corona composition. Likewise, NMs of the same composition but with variations in size, shape, or roughness, will form coronas with different components, and therefore with different biological impacts (Fig. 2) [25], [38]. Additionally, the adsorption of proteins on the surface of the NM can induce oxidation of proteins [36] or change their native form [37], causing cellular responses associated with the stresses that cause protein denaturation (e.g. unfolded protein response) [39]. The dynamic and complex nature of the corona makes the impact of an NM on an organism difficult to predict and has led to many studies on the functionalization of NMs with polymers, with antibodies, low weight organic and inorganic compounds, or with specific proteins to obtain preformed coronas. The objective has been to control the impact of the NMs on the cells or environment through a corona of known composition [37]. These studies, however, have been carried out mainly looking for applications in nanomedicine, and there is relatively little progress on this topic for the use of NMs in plants and microorganisms [40], [41], [42], [43].
Fig. 1.
Joint representation of eight the physicochemical characteristics of the NMs and the possible causal associations between these characters. The explanation of each characteristic is noted in the text.
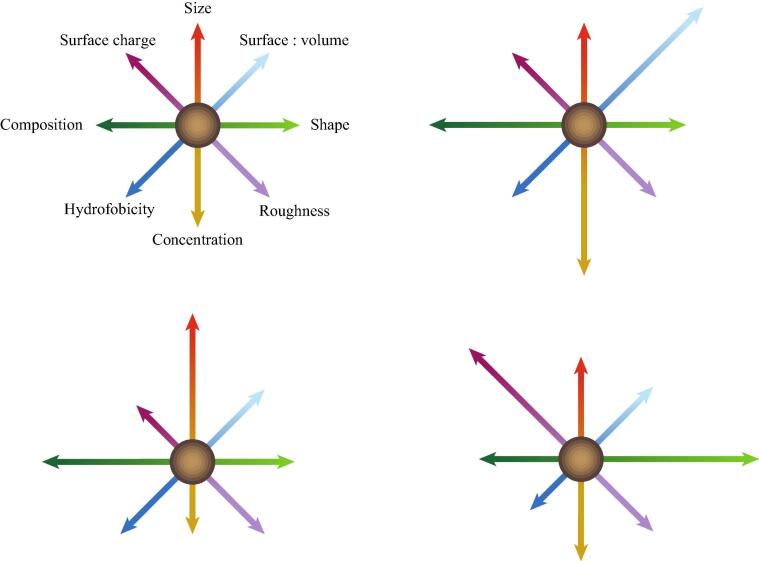
Fig. 2.
Representation of a possible way to classify NMs based on the magnitude (the length of each arrow) or category of each characteristic. The biological impact does not depend on a single feature. The effect depends on the set of interactions of the different properties of the NMs that define first the behavior in biological fluids and natural environments and, second, the biological impact exerted on cells when interacting with components of cell surfaces, cytoplasm, and organelles.
In the NMs, size, shape, aspect ratio, porosity and roughness are physical properties that determine the surface: volume ratio, which in turn specifies other properties such as the density of surface charges and surface free energy [44], [45]. A pristine NM has a high level of surface free energy, so in an environment without organic molecules or biomolecules, it tends to aggregate with itself and precipitate, thus reducing the exposed surface. On the other hand, when NM is found in soil or another natural environment, or a biological fluid, the NM through the surface charges will spontaneously associate with organic molecules or biomolecules to form a corona; this organic coating constitutes an automatic mechanism to decrease the free energy of the system in which the NM is immersed [38], [46]. Other types of NM's core interaction with the medium, such as the dissolution and redox reactions of the components and the reconstruction of the surface, also minimize free energy and, as the formation of the corona, completely change the response of cells to NM [47].
The above explains why functionalized NMs (coated with organic molecules such as citric acid) or those coated with a corona show less tendency to aggregation or aggregate reversibly [35], [48]. Each combination of (NM × physical properties × composition of the ecological or biological medium) results in a set of surface changes or a corona with a certain composition and the presence of functional groups from organic molecules or biomolecules in the medium. The aforesaid makes predicting the long-term behavior of NMs in the environment and when interacting with different species of organisms a significant challenge. Even in the case of plants, each organ has a specific proteome and metabolome, so depending on the route of entry of the NMs (seeds, roots, leaves, fruits), there will be variation in the impact on individuals [49], [50].
The functional groups of the corona’s biomolecules are those that will interact with the surface receptors of the cells in the first place [38], [51], [52]. The functional groups of both the corona and cell wall and membrane proteins protrude from the volume occupied by the electrical double layer (EDL). Therefore, their interaction occurs independently of the net surface charge of the EDL [1].
The identity of the corona is dynamic, changing through its passage through different environments and through time, until reaching the state of minimum free energy [24], [28], [46]. The corona can be formed by one or more layers of organic molecules which are agglutinated by means of Van der Waals forces, hydrogen bridges, electrostatic forces of functional groups, and hydrophobic interactions [47]. When the NM reaches a stable state of minimal energy through the formation of the corona, it is said to have formed a hard corona, characterized by biomolecules or organic molecules strongly bound to the NM that do not easily desorb of the surface. Before reaching a stable state, the NM is covered by a soft corona, which includes molecules adsorbed with less force [53]. The larger size of the NMs favors the formation of a soft corona, while the smaller NMs favor the fast formation of a hard corona since the greater surface: volume ratio results in more surface free energy and favors intermolecular interactions between biomolecules and the NMs [53], [54]. The hard corona is not presented in the functionalized NMs, where a soft corona with less stability is observed [25].
Because of the aforesaid, the initial impact of the NMs on the cells occurs through the corona-cell surface interaction [52], [55]. The initial interaction occurs between the surface charges of the NM-cell wall interface and subsequently at the NM-cell membrane interface and causes the non-specific activation of the cell wall and membrane receptors, energy transport or transduction proteins, and of mechanoreceptors. Furthermore, because of the high surface free energy, the union of the NM corona with the cell wall and membrane can cause alterations in these structures. The alterations are followed by the release of stress markers that in turn induce cascades of cellular defense responses [56], [57].
Through the mechanism described above, the size, shape, and aspect ratio of the NMs have been described as properties that have a decisive impact on cells [21], [52]. For example, in a range of 43 to 205 nm, Se nanoparticles (NPs) of 81 nm showed the highest antibacterial activity, and a variety of responses of the microorganisms depending on the different size of the NPs were observed, such as decreased internal ATP, production of ROS and disturbance of the membrane potential [58]. Regarding the shape, in a study with Arabidopsis thaliana with spherical (8 ± 2 nm), triangular (47 ± 7 nm) and decahedral (45 ± 5 nm) Ag NPs, it was found that all of them induced multiple biological responses such as, ROS accumulation, gene expression changes, and ethylene sensitivity, highlighting the spherical ones for being associated with higher accumulation of anthocyanins, the triangular ones for low antimicrobial activity and the decahedral ones for inducing more significant root growth [59].
The aspect ratio refers to the quotient between the length and the width of the NM. The aspect ratio is a manifestation of the shape of the NMs and its impact on organisms is variable. It is believed that the higher this ratio is, the more likely it is to induce toxicity on certain organisms [21], [60]. However, the need to consider other properties of the NMs, such as diameter, size, functionalization, and charge, has been highlighted, since they modify the biological impact of the aspect ratio [61], [62]. Most of the studies on the aspect ratio and its effect on cells have been carried out using animal models or human cells based on the asbestos paradigm [63], [64]; in plants they are relatively less available information.
In plants, it has been found, for example, that seed exposure from various plant species to carbon nanotubes (20 and 50 mg L-1), a class of NM with a high aspect ratio (10–20 nm × 10–30 μm), improved germination and seedling growth [65]. Carbon nanotubes have also been used successfully as vehicles for DNA delivery without transgene integration to leaves and protoplasts of several planta species [66]. In other studies, the application of carbon nanotubes by drench to tomato seedlings induced higher root biomass. It did not affect shoot biomass, while the foliar application did not affect the shoot and root biomass. Furthermore, in both application routes (foliar and drench), carbon nanotubes induced an increase in the content of chlorophylls, non-enzymatic antioxidants (ascorbic acid, glutathione, phenols, and flavonoids) and the activity of enzymes (APX, GPX, CAT, and PAL) [67].
Adverse effects were observed in shoot and root biomass in tomato seedlings grown from seeds soaked with carbon nanotubes; the impact being more negative as the concentration of these NMs increased (up to 1000 mg L-1). In contrast, the content of chlorophylls, vitamin C, phenols, flavonoids, H2O2, and the activity of the PAL, APX, GPX, SOD, and CAT enzymes was increased [68]. The above shows that in addition to the characteristics such as the aspect ratio, diameter, size, and charge of the NMs, the impact on the vegetables will also depend on the form and route of application. The above points on the aspect ratio of carbon NMs illustrate the necessity to carry out a higher amount of experimentation on the use of NMs as biostimulants in soils and crops since, as mentioned previously, the specific set of interactions between the characteristics of the NMs factors, the form and route of application, and the features of the microbial or plant species involved, determine the biological impact.
Roughness and porosity constitute two of the physical properties of pristine or functionalized NMs that increase the surface area of the NM [69], the distribution of charges on the surface of the NM [70], and, therefore, the ability to adsorb organic molecules in the corona [71]. The corona, apparently because of the complex and rough topography of the NM surface, has been found not to form a continuous coverage, allowing exposure of some regions of the NM surface [72]. These exposed regions are believed to, through hydrophobicity and hydrophilicity processes, modify the hydrodynamic properties of the NMs, the adsorption force of organic molecules to the surface of the NM [27] and the way of interaction with cell walls and membranes [70], [73].
Hydrophobicity is thought to be highly relevant to define the biological impact of the NMs [74] since the hydrophobicity: hydrophilicity ratio of the surfaces of the NMs determines the interaction and adhesion with cell walls and membranes. In general, hydrophobic surfaces tend to adhere to other hydrophobic surfaces, and the same occurs with hydrophilic-hydrophilic [73]. Hydrophobic interactions can occur on the surface of specific materials such as polystyrene or in the proteins or biomolecules of the NMs corona [27]. On the part of the cells of microorganisms and plants, the components that can cause hydrophobic interactions are compounds such as lipoteichoic acid, oligosaccharides, fibrils, and proteins with secondary and tertiary structures that contain hydrophobic residues (e.g., alanine, valine, and phenylalanine) [27], [73]. Even though roughness, porosity, and hydrophobicity are factors considered as determining factors during the NM-cell interfacial interaction [75], there are no studies in microorganisms and plants on the contribution of these characteristics to the biostimulation capacity or toxicity of NMs quantitatively.
Once the reciprocal actions between the NM-cell interfaces generate a set of responses, which can be called biophysical biostimulation or biophysical toxicity, the second phase of the interaction occurs, consisting of the internment of the NM or, where appropriate, definitive adhesion from NM to the cell surface [38]. It has been observed that when an NM shows a low level of surface coating, the surface interactions are strong, with less cellular internment. In contrast, high NM surface coverage reduces cellular responses dependent on surface interaction but increases cellular internment [76]. After admission or adhesion, the NM can be chemically modified by the biomolecules of the internal cellular medium, giving rise to the phenomena described in the following section where the composition of the NM comes to play an important role.
The chemical properties of NM’s corona and NM’s core induce biostimulation or toxicity in microorganisms and plants
The mechanisms by which NMs exert biostimulation and toxicity processes are not well understood. The most widely considered hypothesis is that they generate ROS [77], and that depending on the concentration of ROS they can activate cellular defense mechanisms (eustress) or can lead the cell to a state of distress and even cell death [78]. Other reactive species, such as nitrogen reactive species (RNS), also intervene in the signaling processes through NO, causing nitrosative stress [79], [80]. According to the intensity of stress, it can translate into cell responses from biostimulation to toxicity. RNS as a product of the interaction between nanomaterials and cells have been less studied than ROS, while reactive sulfur species (RSS) have not been studied in this regard.
When an NM comes into close contact with a cell, the NM-cell interfacial interaction and the subsequent cell signaling process induce a primary stimulus or first phase of cellular modulation responses: changes in membrane potential, modification of integral proteins for energy transduction, and generation of elicitors and antioxidants in response to the changes or damages in the membranes, followed by an alteration in the gene expression [52], [77]. The second phase of cellular responses occurs when the metallic NMs transform to ionic forms of the element, either in the growth medium of the microorganisms or in the plant apoplast; these extracellular ionic forms, depending on the concentration and the chemical speciation resulting from pH, ionic strength, oxidation–reduction potential, interaction with other inorganic ions and complexation with biomolecules, cause biostimulation or toxicity on cells by modifying the membrane potential and interacting with the membrane receptors [32], [81].
In the second phase of cellular response, the internalization into the cytoplasm of the NMs also occurs. This internalization seems to happen in general for all NMs (metallic, non-metallic, organic, and carbon). It gives rise to another series of events derived from biochemical responses that change cellular behavior [82]. The cellular responses triggered by NMs internalization occurs both by the presence of ions of the elements that were part of the core of the NM, by unfolded proteins adsorbed in the corona, as well as by cofactors of the corona that modifies cytoplasm metabolites or protein functional groups, or by induction of oxidative stress created by the interaction of the free energy on the surface of the NM with the internal membranes systems [83], [84], [85].
The NMs internalization occurs at the interface of the external environment with the cell wall or membrane. In cell walls, it happens through pre-existing pores up to 30 nm in diameter, modified pores with a larger diameter, or by induction of new pores resulting from interfacial interactions [86]. In the membranes, the NMs also induce openings that allow passage to the cellular medium; the passage through the membranes seems to result from membrane damage by the stimulation of the high density of surface charges, by hydrophobic or hydrophilic interactions, or through the action of endocytosis [18], [87]. Intracellular mobility has been described for metallic and non-metallic NMs, such as C nanotubes [32]. The literature indicates that the NMs come to maintain direct contact with the cytoplasm, with the internal membrane system and with the membranes of organelles and the nucleus. In this second phase of the biological response, which is now a mixture of biophysical and biochemical effects, the concentration of the NM, the elemental composition of the NM, whether it is an essential, beneficial or toxic element, as well as the identity of the corona components, are determining in cellular response [1].
From the above mentioned, it can be concluded that, under a certain range of concentrations, which depends on the specific type of NM and its above mentioned physical properties, an initial biophysical stimulus originates, resulting from the interaction of cell surfaces with cell charges, functional groups of the corona and hydrophobic or hydrophilic groups of the corona and of the exposed surfaces of the NM. In the right concentration, the initial stimulus can trigger a positive response in cellular metabolism and gene expression, that is, biostimulation. However, outside this concentration range, the NM causes toxicity [78], [88].
The result is a type of biological response to the concentration of a biphasic or hormetic nature [13], [89]. Agathokleous et al. [89] refers to this phenomenon as two faces of nanomaterials, where a low concentration would cause a stimulatory effect, and on the other hand, a high concentration would cause an inhibitory effect. The range of concentrations that define the boundary between biostimulation-toxicity will be variable, even for NM of the same composition, depending on the magnitude or category of the other NM variables such as shape, surface energy, the identity of the components of the corona, among others.
As with any other type of chemical compound, the definition of suitable concentration or size is specific for each kind of NM [32]. It is also possible that in the range from low to the high concentration of the NM, positive biological responses followed by negative and subsequently positive occur; this phenomenon derives on the one hand from the accumulated dose over time and, on the other hand, from the aggregation between NMs- NMs and NMs-cells that do not follow a linear trend [83]. The aggregation of the NMs that can occur when they reach a high concentration could partially explain the paradox that lower concentrations of NMs can induce more toxicity than higher concentrations [90]. Likewise, the non-linearity of the responses allows us to separate the biological impact of the NMs into two phases: one that depends on the concentration of the NMs in soil, water, or the atmosphere and that covers periods of time measured in days; and a second phase that can cover months to years, in which the impact is determined by the total accumulated dose, which is a function of the NM concentration, bioavailability and the time of exposure [35].
Phenotypic responses induced by NMs in microorganisms and plants
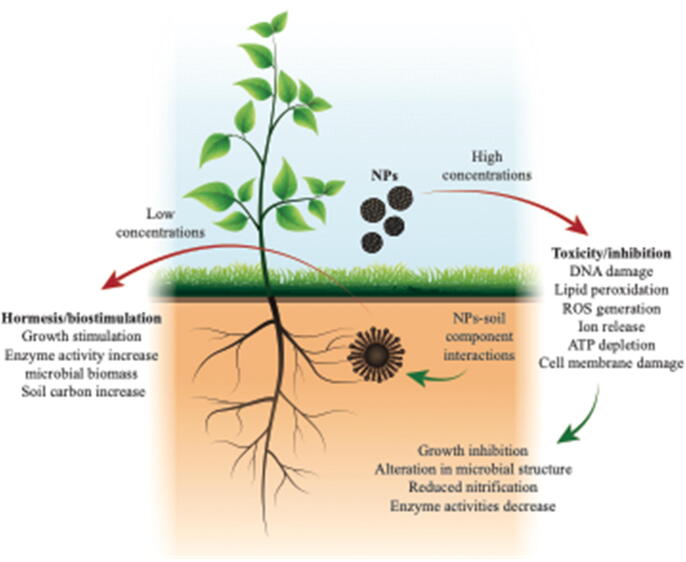
This section aims to describe employing a graphic model the magnitude of the responses of organisms to different NMs under different environmental conditions. Concentrations of NMs in the environment are still unknown. However, some studies estimate that the soil receives a significantly higher amount of polluting NMs compared to water and air [91]. In this regard, it has been widely documented that process associated to waste decomposition and biochemical cycles, organic mineralization, hydrolytic enzymes, nitrification and interaction with crops and wild plant in soil carried out by microorganisms, may be affected by NMs [30], [92]. On the other hand, positive responses also have been reported [13], however, the specific mechanisms have not been completely elucidated yet (Fig. 3).
Fig. 3.
Positive and negative responses on soil microbial communities caused by nanomaterials.
The term “magnitude” used in the title of this manuscript refers to the different classes of the impact of NMs on cells. For example, the higher effectivity in biostimulation occurs by increasing the concentration of the NMs in the medium from zero to a certain optimal level, from which the biostimulatory effect decreases until it becomes toxicity [89]. In the same sense, the higher effectivity in the impact of an NM on cells is inversely related to size, maybe because as it decreases, the more elevated surface: volume ratio determines more surface free energy and, on the other hand, because when the NM decreases its size below a certain threshold (typically at least one dimension < 15 nm) the NM begin to manifest electronic confinement and behave like quantum material. The latter implies the presence of new emerging properties, and consequently, additional biological responses to those shown with the largest non-quantum NMs [12], [93].
Biostimulation or toxicity additionally result from physical or chemical complex stimuli related to shape, roughness, hydrophobicity, and composition, variables for which little information is available or are qualitative (such as the fact that NM is a metal or an oxide). Therefore, concentration and size constitute a good preliminary approach to describe the impact of NMs on organisms. However, we consider that concentration and size do not fully explain the observed biological responses and that other variables for which little information is available (e.g., hydrophobicity) also contribute to the biological impact.
Impact on microorganisms
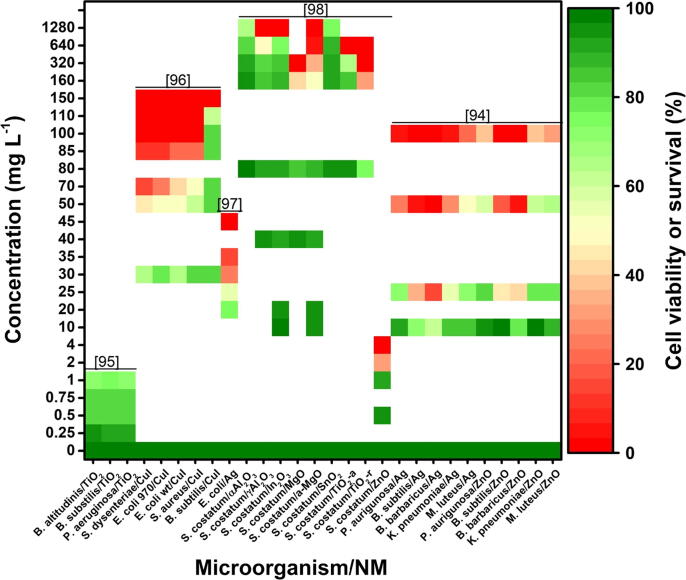
The results of the analysis carried out with the data obtained from 5 publications are presented below. Publications were considered by include results of at least four different concentrations plus a control. Additionally, the selected publications included information regarding the survival of the microorganisms evaluated (number of individuals or colonies) (Fig. 4). The information was used to construct a graph of biological responses refers to the concentration and type of NMs vs. the response in the growth or survival of microorganisms.
Fig. 4.
Survival of microorganisms exposed to different concentrations and types of NMs. Data from [94], [95], [96], [97], [98]. The species listed are: Bacillus altitudinis, Bacillus subtilis, Pseudomonas aeruginosa, Shigella dysenteriae, Escherichia coli, Staphylococcus aureus, Skeletonema costatum, Klebsiella pneumoniae, and Micrococcus luteus.
From the above, it is clear the capacity of NMs as antimicrobial agents. NMs have multiple modes of action on microorganisms, such as ROS production, direct damage to cell membranes, changes in metal/metal ion homeostasis, genotoxicity, among others [99]. Fig. 4 shows a direct negative impact of the NMs on the microorganisms, negatively affecting cell division or causing cell death, which is reflected in a decrease in individuals or colonies. Several authors suggest that the production of ROS is the main one of the different mechanisms that explain the impact of NMs on microorganisms [95], [96], [98], [99]. ROS overproduction results in damage to the cell membrane, proteins, and DNA, causing cytotoxicity in microorganisms [96]. On the other hand, several complex mechanisms are necessary to ensure a level of essential metals in microorganisms, which are mainly bounded to protein as a cofactor [100]. These same authors reported that input (influx) or output (efflux) of metal into cells is a consequence of activation of the metalloregulatory system, which can fail when the buffer capacity of a cell is exceeded. Microbial metal regulation can also fail when the cell membrane and its protein channels and transporters undergo conformational changes due to interaction with NM or suffer oxidative damage from reactive species that are generated when NMs interact with cell walls and membranes [5]. Changes in fatty acid methyl ester (FAME) profiles in the presence of metals for maintaining membrane fluidity have also been reported [101].
Cytotoxicity is directly dependent on the NM concentration to which the microorganisms are exposed [95]. Fig. 4 shows that, regardless of the type of NM, as the concentration increases, less survival of the microorganisms is observed. Cytotoxicity also depends on the type of NM applied, since in some cases even low concentrations of a specific NM e.g., copper iodide [96] and ZnO [98], can induce high toxicity, while in other cases higher concentrations of another NM such as SnO2 [98] may have less toxic impact.
Soil bacteria are considered a risk group due to the influence of the antimicrobial activity of some NPs, such as silver (AgNps) or copper (CuNps) nanoparticles [102], [103]. Many studies have been carried out about the direct effect of NPs on soil microorganisms, obtaining a great diversity of results, ranging from negative to positive effects [30]. According to cell structure, Gram-negative bacteria could be less sensitive to NMs than Gram-positive but is not always. [30] indicated that the toxicity of NPs on microorganisms depends on parent material, size, shape, and coating. Moreover, has been demonstrated that metal nanoparticles such as Zn oxides (ZnO-NPs), cerium oxide (CeO2-NPs), copper and copper oxides (CuNPs-CuO-NPs), iron oxide (Fe3O4-NPs), silver (Ag-NPs) or titanium dioxide (TiO2-NP) can modify the composition of soil microbial communities [92]. Carbon nanomaterials such as fullerenes and carbon nanotubes have also shown cause alteration in soil microbial communities, however, studies have demonstrated that fullerenes are less toxic to microorganisms than other carbon nanomaterials [104].
It also happens that the same species or strain of microorganism will react differently to different NMs, demonstrating that each type of NM has a differential capacity to generate cytotoxicity. For example, the growth of Skeletonema costatum presents different inhibition according to the type of NM to which it is exposed (Fig. 4). When exposed to NMs such as γ-Al2O3, In2O3, MgO, a-MgO, TiO2, and ZnO up to 100% growth inhibition can be observed, unlike when exposed to α-Al2O3 and SnO2 where it is not reached 100% inhibition. Also, the lowest concentration at which 100% growth inhibition occurred was 4 mg L-1 of ZnO, while concentrations as high as 1280 mg L-1 of α-Al2O3 and SnO2 only inhibited the growth of S. costatum in 35 and 24% respectively [98]. The growth of Escherichia coli is almost wholly inhibited when exposed to 45 mg L-1 of Ag NPs, while 20 mg L-1 of Ag slightly slows growth (approximately 20%) [97]. When E. coli is exposed to copper iodide nanoparticles, complete inhibition occurs at a dose of 100 mg L-1 [96]. In contrast, different microorganisms have different responses to the same type of NM (Fig. 4). For example, the observed sensitivity to copper iodide nanoparticles was Shigella dysenteriae > E. coli 970 > E. coli wt > Staphylococcus aureus > Bacillus subtilis, where B. subtilis required a dose of 150 mg L-1 of CuI NPs to achieve 100% inhibition, while for the rest of the strains this was achieved with 100 mg L-1 of CuI NPs [96]. The observed sensitivity to Ag NPs was Bacillus barbaricus > B. subtilis > Klebsiella pneumoniae ≈ Pseudomonas aeruginosa > Micrococcus luteus, where the growth of B. barbaricus was inhibited entirely with 50 mg L-1 of Ag NPs; when exposed to ZnO NPs sensitivity was B. barbaricus > B. subtilis > M. luteus > P. aeruginosa ≈ K. pneumoniae, where 100 mg L-1 of ZnO NPs were required to achieve 100% inhibition [94]. When TiO2 NPs were used, the sensitivity of the microorganisms was P. aeruginosa ≈ Bacillus altitudinis > B. subtilis; however, the doses used (0.25–1.0 ml L-1) failed to totally inhibit growth since it only decreased by approximately 20% [95]. As can be seen in Fig. 4, in the data set used, the impact of the NMs on the microorganisms was presented as neutral or deleterious and was found to be dependent on the concentration and the type of nanomaterial applied.
Notwithstanding the above, recently has been reported that quorum sensing could be used by soil bacteria as a strategy to produce hormesis in the presence of metals [105]. Then, if metals can cause a cascade of responses in bacterial cells, it would be logical to think that metal nanoparticles could generate the same adaptative responses. Not only the adverse effects of NMs on microorganisms have been reported, but also the increase in growth promotion, pesticide-degrading microorganisms, metabolic activity, and enzymes have been reported [104]. In this sense, [106] reported that TiO2-NPs and ZnO-NPs caused alteration in several microbial communities, but an increase in Sphingomonadaceae, Streptomycetaceae, and Streptomyces. More studies are necessary concerning molecular mechanisms that are triggered by microorganisms in the presence of metal nanoparticles, and less known is even the effects produced by nanomaterials such as carbon nanotubes or fullerenes.
Hormesis and biostimulation by nanoparticles or nanomaterials are not frequently and/or directly evaluated in soil microorganisms. Nevertheless, some studies have reported the diverse effects of NPs on microorganisms, either with modulating, activating, or suppressing activities [107]. In this way, [108] indicated that metal oxide NPs as CeO2, Fe3O4, and SnO2 did not alter the microbial biomass C and N significantly, while microbial C/N ratio increased after exposure to Fe3O4 and SnO2 due to the increase of ectomycorrhizae.
Very few studies were adjusted to the criteria described above for the construction of Fig. 4. This fact seems to indicate the need for more experimental information on the impact of NMs with different characteristics, composition, and concentration in different environmental situations and types of soil. On the other hand, the use of NMs as biostimulants could perhaps be directed towards crops in soilless or hydroponic systems. In this way, while more information is obtained on the use of NMs in agricultural soil, the techniques for their application as biostimulants in soilless systems can be tested and advanced.
Impact on plants
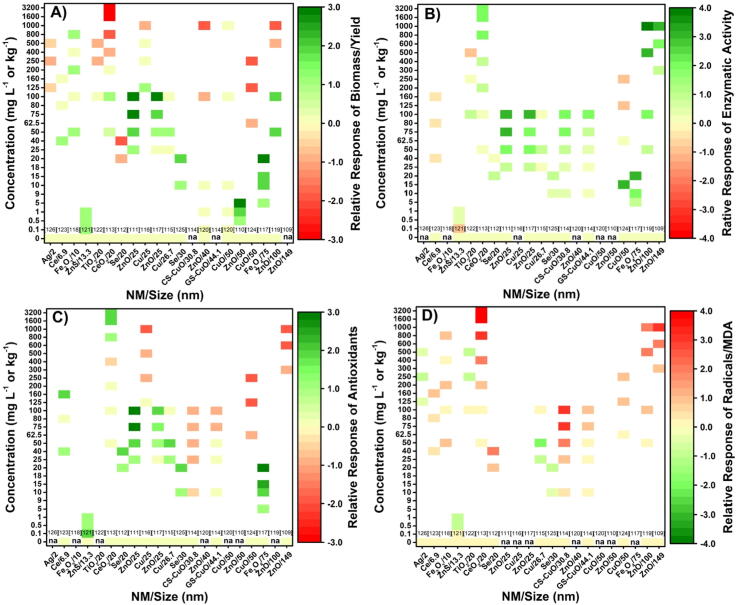
The results of the analysis carried out with the data obtained from 18 publications on the effects of NMs on different plant species are presented below. These publications were selected considering those that gave results of at least two concentrations of the evaluated NM plus a control, that the studied NM was applied without combining with another NM, and that the characterization of the NM (size and shape) was presented. The information used to construct the graph of biological responses refers to the concentration and type-size of the NMs vs. the response variables of plants: biomass production or yield, production of enzymatic or non-enzymatic antioxidants, free radicals, or Malondialdehyde, in whole plants, organs, or seeds, in different cultivation systems (Fig. 5).
Fig. 5.
Response graph A) of biomass production or crop yield, B) enzymatic activity, C) production of antioxidants, and D) production of free radicals or Malondialdehyde (MDA) of plants upon exposure to nanomaterials of different size and composition applied in different concentrations. na: data not available. Data and plant species from: [109]Solanum lycopersicum; [110]Oryza sativa; [111]Triticum aestivum; [112]Arachis hypogaea; [113]Calendula officinalis; [114]Nicotiana tabacum; [115]Triticum aestivum; [116]Vigna unguiculata; [117]Triticum aestivum; [118]Solanum lycopersicum; [119]Oryza sativa; [120]Raphanus sativus; [121]Vigna radiata; [122]Oryza sativa; [123]Phaseolus vulgaris; [124]Oryza sativa; [125]Fragaria × ananassa; [126]Raphanus sativus.
As previously described, the range of plant responses after exposure to various nanomaterials is dependent, among other things, on the concentration and size of the nanomaterial. The biostimulation or toxicity response can be described by the greater or lesser biomass production or plant yield (Fig. 5A). Regardless of the type of NM and its size, there is a threshold in the concentration that is applied in which, once it is exceeded, the observed effect is negative, obtaining a decrease in biomass production or yield. However, below this threshold, the observed response is positive. This range of biphasic responses dependent on the applied NM concentration presents the typical inverted U shape, in which as the concentration increases a positive response is observed until a certain threshold is reached [89]. From this point on, the response may be null or simply a negative response begins to be observed, such that as the NM concentration continues to increase, the negative effect increases in intensity (Fig. 5B).
The response of biomass production is dependent on multiple factors related to plant metabolism and physiology. In the first instance, biomass production depends on the efficiency of the photosynthesis process, since photosynthates which will be used in maintenance metabolism (respiration) and plant growth and development, are generated from this process. Therefore, any modification that is made in the photosynthesis or respiration process will lead to a redistribution of energy and biomass, modifying the phenotypic response of the plant.
Depending on their physicochemical characteristics, the NMs can modify the different metabolic processes of plants. Photosynthetic efficiency can be improved through some NMs [127], or overproduction of free radicals can be generated that will result in oxidative stress [113], [114], [120], [122]. In the first case, the observed effect will be a higher biomass production or plant yield, while in the second case, the opposite effect can be observed.
Free radical production is a necessary process in plants, functioning as signaling agents for environmental stimuli or as part of the signal network to trigger developmental events. Enzymatic or non-enzymatic processes produce free radicals in practically all cell compartments. The cellular redox state, dependent on the balance between the quantity of free radicals and antioxidant compounds, is a dynamic character that influences metabolism and gene expression. The redox balance can be modified by biotic or abiotic conditions that are outside the optimal limits for each plant species. In the case of NMs, low concentrations can induce a decrease in the amount of free radicals in plants [115], a response maybe derived from the NM-s ability to induce enzymatic and non-enzymatic antioxidant compounds (Fig. 5B–D). However, in most cases, the NMs induce overproduction of free radicals that can be beneficial if it does not exceed the plant́s metabolic limit [128], [129], [130]. If the metabolic threshold is surpassed, occurs the induction of oxidative stress due to the substantial accumulation of free radicals (Fig. 5D), causing damage to different cell structures [109], [113] and even causing damage to DNAs and RNAs [131]. The damage caused by an excessive concentration of free radicals is mitigated by the antioxidant defense system, increasing the production of enzymatic and non-enzymatic compounds (Fig. 5B and C) [113]. If the production of free radicals is extensive, a more significant amount of antioxidant compounds is required (Fig. 5B and D), which in some cases may be insufficient to neutralize the free radicals produced [113]. The above can affect the accumulation of non-enzymatic antioxidant compounds because some of these (ascorbate, GSH) are the necessary substrates for the functioning of antioxidant enzymes (Fig. 5C). The result is that the plant dedicates more energy to the maintenance of the antioxidant defense system, reducing the amount of energy for growth and yield (Fig. 5A).
The 18 publications included in the analysis shown in Fig. 5 provide data for 11 plant species under different cropping systems. As mentioned in the case of microorganisms, the amount of information is not high when considering the large volume of information available on the use of NMs in plants. However, unlike microorganisms, the results obtained in plants indicate the presence of a hormetic response [89], with biostimulation occurring up to a certain concentration threshold.
The response of plants to the size of the NMs seems less manifest, probably due to the diversity of the composition of the NMs. However, considering the relative response of biomass/yield, some NMs like Ce and Fe2O3 seems to show that smaller NMs have a positive impact with higher concentrations (Fig. 5A). This positive response to smaller NMs has been described for quantum NMs with dimensions smaller than 15 nm that positively impact photosynthesis [127]. Still, it requires more studies in the case of responses related to biomass and yield, since it is surely dependent on the NM class and the plant species in question.
Considering the results for microorganisms and plants together, we find that the smaller and more reactive NMs seem to have better results with plants. However, more reactive materials would also be expected to have a more significant negative impact on soil microorganisms. Therefore, except for the availability of more information, the use of NMs in soil cultivated crops would seem questionable. In this case, it would be advisable to use NMs mainly in seed priming or in soilless crops, where the impact on the soil microbiome would presumably be less. However, it would be necessary to analyze the potential effect of NMs on other microbiomes, e.g., on the endophytic microbiome of plants and, in the case of trophic transfer, on the microbiome of consumers.
Conclusions
Different mechanisms by which nanomaterials impact on microorganisms and plants were revised in the context of nanomaterial properties using a graphic model to represent the continuum of biostimulation and toxicity. The results indicate a high susceptibility of microorganisms to NMs. Simultaneously, in plants, a hormetic response was detected for the concentration of NMs, and a few cases where small-size NMs applied in high concentration induced favorable responses. Therefore, it is possible to use different NMs as a tool to increase growth and stress tolerance in plants. The minimum information necessary seems to be the concentration to be used, the composition, the size of the NMs, and the plant species to which it is directed. Regarding microorganisms, a more significant number of studies are recommended, primarily aimed at verifying the effect of NMs on edaphic microbiomes. Considering the previous points is advisable to increase the number of studies directed to using NMs as biostimulants for seed priming and in soilless cultivation systems. Further research is necessary to achieve a suitable assessment of the dose range, in combination with other nanomaterial properties, that will allow obtaining positive influences in biological processes related to microorganisms and plants.
Funding
REDES-ANID 180003, Project and Conicyt-Fapesp 2018/08194–2 Project; Universidad Autónoma Agraria Antonio Narro.
Declaration of Competing Interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Biographies

Antonio Juárez-Maldonado obtained his Doctor of Science degree in Production Systems Engineering at the Autonomous Agrarian University Antonio Narro in 2013. He is currently a full-time research professor in the Department of Botany of the same University. His research line focuses on the stimulatory effect of nanomaterials in agricultural crops, greenhouse crop production, disease control with the application of nanomaterials, induction of tolerance to biotic and abiotic stress.

Dr. Gonzalo Tortella F. Professor in the Universidad de La Frontera (Chile). He is the associated member of the Excellence Center in Biotechnological Research Applied to the Environment (CIBAMA). He received his PhD in Natural Resource Sciences in 2008 and the postdoctoral studies during 2008–2010. He has developed research in environmental biotechnology and microbiology, and nanotechnology with agricultural applications. In these research areas has developed several research projects and is author of book chapters and 50 scientific publications with a h- factor 15 and one patent. In the Academy, he has supervised doctoral and undergrad thesis. https://orcid.org/0000-0001-7069-7454.

Dr. Olga Rubilar A. is Environmental Engineering, Professor, and associated member of the Excellence Center in Biotechnological Research Applied to the Environment (CIBAMA) in La Frontera University-Chile. She received his PhD in Natural Resource Sciences at Universidad de la Frontera (2007), and postdoctoral studies in 2007–2009. She has developed research in environmental biotechnology, pollutant degradation in soil and wastewater. Currently, she is developing studies in nanobiotechnology with applications in plants. She has developed several research projects and is author of book chapters and 52 scientific publications. (h factor 18).

Dr. Paola Fincheira is a postdoctoral researcher at the Universidad de La Frontera. She is associated member of the Excellence Center in Biotechnological Research Applied to the Environment (CIBAMA). She received her PhD in Natural Resource Sciences in 2017, since then dedicating himself to her postdoctoral research to date. She has developed research in plant biotechnology, microbiology and nanotechnology with agricultural applications. In these research areas has developed research and application projects with scientific publications and a patent request. https://orcid.org/0000-0002-6234-4808.

Dr. Adalberto Benavides-Mendoza is a Biologist by Universidad Autónoma Agraria Antonio Narro and Doctor in Science by Universidad Autónoma de Nuevo León, Mexico. He is a professor and researcher in the Department of Horticulture at the Universidad Autónoma Agraria Antonio Narro. His research interest is led towards plant biostimulation, plant nutrition, and stress tolerance induction using nanometric or bulk species of essential and beneficial elements, biopolymers, and industrial or agricultural sludges. Research has a focus on the relationship between plant biostimulation, tolerance to stress, and the nutritional quality and biofortification of vegetables and fruits. http://orcid.org/0000-0002-2729-4315
Footnotes
Peer review under responsibility of Cairo University. *Corresponding author at: Departamento de Horticultura. Universidad Autónoma Agraria Antonio Narro, Saltillo, Mexico. Email address: adalberto.benavides@uaaan.edu.mx (A. Benavides-Mendoza)
References
- 1.Juárez-Maldonado A., Ortega-Ortíz H., Morales-Díaz A.B., González-Morales S., Morelos-Moreno Á., Cabrera-De la Fuente M. Nanoparticles and Nanomaterials as Plant Biostimulants. Int J Mol Sci. 2019 doi: 10.3390/ijms20010162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Drobek M., Frąc M., Cybulska J. Plant Biostimulants: Importance of the Quality and Yield of Horticultural Crops and the Improvement of Plant Tolerance to Abiotic Stress—A Review. Agronomy. 2019;9:335. doi: 10.3390/agronomy9060335. [DOI] [Google Scholar]
- 3.du Jardin P. Plant biostimulants: Definition, concept, main categories and regulation. Sci Hortic. 2015;196:3–14. doi: 10.1016/j.scienta.2015.09.021. [DOI] [Google Scholar]
- 4.Juárez-Maldonado A., González-Morales S., Cabrera-De la Fuente M., Medrano-Macías J., Benavides-Mendoza A. Nanometals as Promoters of Nutraceutical Quality in Crop Plants. In: Grumezescu A.M., Holban A.M., editors. Impact of Nanoscience in the Food Industry. Academic Press; 2018. pp. 277–310. 10.1016/B978-0-12-811441-4.00010-8. [Google Scholar]
- 5.Yin S., Liu J., Kang Y., Lin Y., Li D., Shao L. Interactions of nanomaterials with ion channels and related mechanisms. Br J Pharmacol. 2019;176:3754–3774. doi: 10.1111/bph.14792. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Liu J., Wang J., Lee S., Wen R. Copper-caused oxidative stress triggers the activation of antioxidant enzymes via ZmMPK3 in maize leaves. PLoS ONE. 2018;13 doi: 10.1371/journal.pone.0203612. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Judy J.D., Bertsch P.M. Bioavailability, Toxicity, and Fate of Manufactured Nanomaterials in Terrestrial Ecosystems. In: Sparks D.L., editor. Advances in Agronomy. Academic Press; 2014. pp. 1–64. 10.1016/B978-0-12-420225-2.00001-7. [Google Scholar]
- 8.Meng H., Leong W., Leong K.W., Chen C., Zhao Y. Walking the line: The fate of nanomaterials at biological barriers. Biomaterials. 2018;174:41–53. doi: 10.1016/j.biomaterials.2018.04.056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Nanda K.K., Maisels A., Kruis F.E., Fissan H., Stappert S. Higher Surface Energy of Free Nanoparticles. Phys Rev Lett. 2003;91 doi: 10.1103/PhysRevLett.91.106102. [DOI] [PubMed] [Google Scholar]
- 10.Boutchuen A., Zimmerman D., Aich N., Masud A.M., Arabshahi A., Palchoudhury S. Increased Plant Growth with Hematite Nanoparticle Fertilizer Drop and Determining Nanoparticle Uptake in Plants Using Multimodal Approach. J Nanomater. 2019;2019:1–11. doi: 10.1155/2019/6890572. [DOI] [Google Scholar]
- 11.Hochella M.F., Lower S.K., Maurice P.A., Penn R.L., Sahai N., Sparks D.L. Nanominerals, Mineral Nanoparticles, and Earth Systems. Science. 2008;319:1631–1635. doi: 10.1126/science.1141134. [DOI] [PubMed] [Google Scholar]
- 12.Lead J.R., Batley G.E., Alvarez P.J.J., Croteau M.-N., Handy R.D., McLaughlin M.J. Nanomaterials in the environment: Behavior, fate, bioavailability, and effects—An updated review. Environ Toxicol Chem. 2018;37:2029–2063. doi: 10.1002/etc.4147. [DOI] [PubMed] [Google Scholar]
- 13.Iavicoli I., Leso V., Fontana L., Calabrese E.J. Nanoparticle Exposure and Hormetic Dose–Responses: An Update. Int J Mol Sci. 2018;19:805. doi: 10.3390/ijms19030805. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Sharma S.S., Dietz K.-J., Mimura T. Vacuolar compartmentalization as indispensable component of heavy metal detoxification in plants. Plant, Cell Environ. 2016;39:1112–1126. doi: 10.1111/pce.12706. [DOI] [PubMed] [Google Scholar]
- 15.Shahid M., Dumat C., Khalid S., Schreck E., Xiong T., Niazi N.K. Foliar heavy metal uptake, toxicity and detoxification in plants: A comparison of foliar and root metal uptake. J Hazard Mater. 2017;325:36–58. doi: 10.1016/j.jhazmat.2016.11.063. [DOI] [PubMed] [Google Scholar]
- 16.Dubey S., Shri M., Gupta A., Rani V., Chakrabarty D. Toxicity and detoxification of heavy metals during plant growth and metabolism. Environ Chem Lett. 2018;16:1169–1192. doi: 10.1007/s10311-018-0741-8. [DOI] [Google Scholar]
- 17.Essa A.M.M., Abboud M.A.A., Khatib S.I. Metal transformation as a strategy for bacterial detoxification of heavy metals. J Basic Microbiol. 2018;58:17–29. doi: 10.1002/jobm.201700143. [DOI] [PubMed] [Google Scholar]
- 18.Palocci C., Valletta A., Chronopoulou L., Donati L., Bramosanti M., Brasili E. Endocytic pathways involved in PLGA nanoparticle uptake by grapevine cells and role of cell wall and membrane in size selection. Plant Cell Rep. 2017;36:1917–1928. doi: 10.1007/s00299-017-2206-0. [DOI] [PubMed] [Google Scholar]
- 19.Tang J., Wu Y., Esquivel-Elizondo S., Sørensen S.J., Rittmann B.E. How Microbial Aggregates Protect against Nanoparticle Toxicity. Trends Biotechnol. 2018;36:1171–1182. doi: 10.1016/j.tibtech.2018.06.009. [DOI] [PubMed] [Google Scholar]
- 20.Zhu N., Wang S., Tang C., Duan P., Yao L., Tang J. Protection Mechanisms of Periphytic Biofilm to Photocatalytic Nanoparticle Exposure. Environ Sci Technol. 2019;53:1585–1594. doi: 10.1021/acs.est.8b04923. [DOI] [PubMed] [Google Scholar]
- 21.Nel A.E., Mädler L., Velegol D., Xia T., Hoek E.M.V., Somasundaran P. Understanding biophysicochemical interactions at the nano–bio interface. Nature Mater. 2009;8:543–557. doi: 10.1038/nmat2442. [DOI] [PubMed] [Google Scholar]
- 22.Scandalios J.G. Oxidative stress: molecular perception and transduction of signals triggering antioxidant gene defenses. Braz J Med Biol Res. 2005;38:995–1014. doi: 10.1590/S0100-879X2005000700003. [DOI] [PubMed] [Google Scholar]
- 23.Ueda Y., Yanagisawa S. Perception, transduction, and integration of nitrogen and phosphorus nutritional signals in the transcriptional regulatory network in plants. J Exp Bot. 2019;70:3709–3717. doi: 10.1093/jxb/erz148. [DOI] [PubMed] [Google Scholar]
- 24.Docter D., Westmeier D., Markiewicz M., Stolte S.K., Knauer S.H., Stauber R. Vol. 44. 2015. The nanoparticle biomolecule corona: lessons learned – challenge accepted? pp. 6094–6121. (Chem Soc Rev). [DOI] [PubMed] [Google Scholar]
- 25.Foroozandeh P., Aziz A.A. Merging Worlds of Nanomaterials and Biological Environment: Factors Governing Protein Corona Formation on Nanoparticles and Its Biological Consequences. Nanoscale Res Lett. 2015;10:221. doi: 10.1186/s11671-015-0922-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.J. Chetwynd A, Lynch I. The rise of the nanomaterial metabolite corona, and emergence of the complete corona. Environmental Science: Nano 2020;7:1041–60. https://doi.org/10.1039/C9EN00938H.
- 27.Mahmoudi M., Bertrand N., Zope H., Farokhzad O.C. Emerging understanding of the protein corona at the nano-bio interfaces. Nano Today. 2016;11:817–832. doi: 10.1016/j.nantod.2016.10.005. [DOI] [Google Scholar]
- 28.Treuel L., Docter D., Maskos M., Stauber R.H. Protein corona – from molecular adsorption to physiological complexity. Beilstein J Nanotechnol. 2015;6:857–873. doi: 10.3762/bjnano.6.88. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Lewis R.W., Bertsch P.M., McNear D.H. Nanotoxicity of engineered nanomaterials (ENMs) to environmentally relevant beneficial soil bacteria – a critical review. Nanotoxicology. 2019;13:392–428. doi: 10.1080/17435390.2018.1530391. [DOI] [PubMed] [Google Scholar]
- 30.Parada J., Rubilar O., Fernández-Baldo M.A., Bertolino F.A., Durán N., Seabra A.B. The nanotechnology among US: are metal and metal oxides nanoparticles a nano or mega risk for soil microbial communities? Crit Rev Biotechnol. 2019;39:157–172. doi: 10.1080/07388551.2018.1523865. [DOI] [PubMed] [Google Scholar]
- 31.Tian H., Kah M., Kariman K. Are Nanoparticles a Threat to Mycorrhizal and Rhizobial Symbioses? A Critical Review. Front Microbiol. 2019;10 doi: 10.3389/fmicb.2019.01660. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Zuverza-Mena N., Martínez-Fernández D., Du W., Hernandez-Viezcas J.A., Bonilla-Bird N., López-Moreno M.L. Exposure of engineered nanomaterials to plants: Insights into the physiological and biochemical responses-A review. Plant Physiol Biochem. 2017;110:236–264. doi: 10.1016/j.plaphy.2016.05.037. [DOI] [PubMed] [Google Scholar]
- 33.Rastogi A., Zivcak M., Sytar O., Kalaji H.M., He X., Mbarki S. Impact of Metal and Metal Oxide Nanoparticles on Plant. A Critical Review. Front Chem. 2017;5 doi: 10.3389/fchem.2017.00078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Tripathi D.K., Shweta Singh S, Singh S., Pandey R., Singh V.P. An overview on manufactured nanoparticles in plants: Uptake, translocation, accumulation and phytotoxicity. Plant Physiol Biochem. 2017;110:2–12. doi: 10.1016/j.plaphy.2016.07.030. [DOI] [PubMed] [Google Scholar]
- 35.Morales-Díaz A.B., Ortega-Ortíz H., Juárez-Maldonado A., Cadenas-Pliego G., González-Morales S., Benavides-Mendoza A. Application of nanoelements in plant nutrition and its impact in ecosystems. Adv Nat Sci: Nanosci Nanotechnol. 2017;8 doi: 10.1088/2043-6254/8/1/013001. [DOI] [Google Scholar]
- 36.Shi T., Wei Q., Wang Z., Zhang G., Sun X., He Q.-Y. Photocatalytic Protein Damage by Silver Nanoparticles Circumvents Bacterial Stress Response and Multidrug Resistance. MSphere. 2019;4 doi: 10.1128/mSphere.00175-19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Cagliani R., Gatto F., Bardi G. Protein Adsorption: A Feasible Method for Nanoparticle Functionalization? Materials. 2019;12:1991. doi: 10.3390/ma12121991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Francia V., Yang K., Deville S., Reker-Smit C., Nelissen I., Salvati A. Corona Composition Can Affect the Mechanisms Cells Use to Internalize Nanoparticles. ACS Nano. 2019;13:11107–11121. doi: 10.1021/acsnano.9b03824. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Li D., Ji J., Yuan Y., Wang D. Toxicity comparison of nanopolystyrene with three metal oxide nanoparticles in nematode Caenorhabditis elegans. Chemosphere. 2020;245 doi: 10.1016/j.chemosphere.2019.125625. [DOI] [PubMed] [Google Scholar]
- 40.Avellan A., Yun J., Zhang Y., Spielman-Sun E., Unrine J.M., Thieme J. Nanoparticle Size and Coating Chemistry Control Foliar Uptake Pathways, Translocation, and Leaf-to-Rhizosphere Transport in Wheat. ACS Nano. 2019;13:5291–5305. doi: 10.1021/acsnano.8b09781. [DOI] [PubMed] [Google Scholar]
- 41.Santana I., Wu H., Hu P., Giraldo J.P. Targeted delivery of nanomaterials with chemical cargoes in plants enabled by a biorecognition motif. Nat Commun. 2020;11:2045. doi: 10.1038/s41467-020-15731-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Spielman-Sun E., Avellan A., Bland G.D., Clement E.T., Tappero R.V., Acerbo A.S. Protein coating composition targets nanoparticles to leaf stomata and trichomes. Nanoscale. 2020;12:3630–3636. doi: 10.1039/C9NR08100C. [DOI] [PubMed] [Google Scholar]
- 43.Yu X., Cao X., Yue L., Zhao J., Chen F., Wang Z. Phosphate induced surface transformation alleviated the cytotoxicity of Y2O3 nanoparticles to tobacco BY-2 cells. Sci Total Environ. 2020;732 doi: 10.1016/j.scitotenv.2020.139276. [DOI] [PubMed] [Google Scholar]
- 44.Gao X., Lowry G.V. Progress towards standardized and validated characterizations for measuring physicochemical properties of manufactured nanomaterials relevant to nano health and safety risks. NanoImpact. 2018;9:14–30. doi: 10.1016/j.impact.2017.09.002. [DOI] [Google Scholar]
- 45.Haryadi B.M., Hafner D., Amin I., Schubel R., Jordan R., Winter G. Nonspherical Nanoparticle Shape Stability Is Affected by Complex Manufacturing Aspects: Its Implications for Drug Delivery and Targeting. Adv Healthcare Mater. 2019;8:1900352. doi: 10.1002/adhm.201900352. [DOI] [PubMed] [Google Scholar]
- 46.Nasser F., Constantinou J., Lynch I. Nanomaterials in the Environment Acquire an “Eco-Corona” Impacting their Toxicity to Daphnia Magna—a Call for Updating Toxicity Testing Policies. Proteomics. 2020;20:1800412. doi: 10.1002/pmic.201800412. [DOI] [PubMed] [Google Scholar]
- 47.Wang Y., Cai R., Chen C. The Nano-Bio Interactions of Nanomedicines: Understanding the Biochemical Driving Forces and Redox Reactions. Acc Chem Res. 2019;52:1507–1518. doi: 10.1021/acs.accounts.9b00126. [DOI] [PubMed] [Google Scholar]
- 48.Hotze E.M., Phenrat T., Lowry G.V. Nanoparticle Aggregation: Challenges to Understanding Transport and Reactivity in the Environment. J Environ Qual. 2010;39:1909–1924. doi: 10.2134/jeq2009.0462. [DOI] [PubMed] [Google Scholar]
- 49.Prajitha N., Athira S.S., Mohanan P.V. Bio-interactions and risks of engineered nanoparticles. Environ Res. 2019;172:98–108. doi: 10.1016/j.envres.2019.02.003. [DOI] [PubMed] [Google Scholar]
- 50.Rajput V., Minkina T., Sushkova S., Behal A., Maksimov A., Blicharska E. ZnO and CuO nanoparticles: a threat to soil organisms, plants, and human health. Environ Geochem Health. 2020;42:147–158. doi: 10.1007/s10653-019-00317-3. [DOI] [PubMed] [Google Scholar]
- 51.D. Walkey C, W. Chan WC. Understanding and controlling the interaction of nanomaterials with proteins in a physiological environment. Chemical Society Reviews 2012;41:2780–99. https://doi.org/10.1039/C1CS15233E. [DOI] [PubMed]
- 52.Lipșa F.-D., Ursu E.-L., Ursu C., Ulea E., Cazacu A. Evaluation of the Antifungal Activity of Gold-Chitosan and Carbon Nanoparticles on Fusarium oxysporum. Agronomy. 2020;10:1143. doi: 10.3390/agronomy10081143. [DOI] [Google Scholar]
- 53.Kihara S., van der Heijden N.J., Seal C.K., Mata J.P., Whitten A.E., Köper I. Soft and Hard Interactions between Polystyrene Nanoplastics and Human Serum Albumin Protein Corona. Bioconjugate Chem. 2019;30:1067–1076. doi: 10.1021/acs.bioconjchem.9b00015. [DOI] [PubMed] [Google Scholar]
- 54.Shah J., Singh S. CHAPTER 1 Nanoparticle-Protein Corona Complex: Composition, Kinetics, Physico-Chemical Characterization, and Impact on Biomedical Applications. Nanoparticle-Protein Corona: Biophysics to Biology. Royal Soc Chem. 2019:1–30. [Google Scholar]
- 55.Liu N., Tang M., Ding J. The interaction between nanoparticles-protein corona complex and cells and its toxic effect on cells. Chemosphere. 2020;245 doi: 10.1016/j.chemosphere.2019.125624. [DOI] [PubMed] [Google Scholar]
- 56.Christen V., Fent K. Silica nanoparticles and silver-doped silica nanoparticles induce endoplasmatic reticulum stress response and alter cytochrome P4501A activity. Chemosphere. 2012;87:423–434. doi: 10.1016/j.chemosphere.2011.12.046. [DOI] [PubMed] [Google Scholar]
- 57.Vazquez-Muñoz R., Meza-Villezcas A., Fournier P.G.J., Soria-Castro E., Juarez-Moreno K., Gallego-Hernández A.L. Enhancement of antibiotics antimicrobial activity due to the silver nanoparticles impact on the cell membrane. PLoS ONE. 2019;14 doi: 10.1371/journal.pone.0224904. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Huang T., Holden J.A., Heath D.E., O’Brien-Simpson N.M., O’Connor A.J. Engineering highly effective antimicrobial selenium nanoparticles through control of particle size. Nanoscale. 2019;11:14937–14951. doi: 10.1039/C9NR04424H. [DOI] [PubMed] [Google Scholar]
- 59.Syu Y., Hung J.-H., Chen J.-C., Chuang H. Impacts of size and shape of silver nanoparticles on Arabidopsis plant growth and gene expression. Plant Physiol Biochem. 2014;83:57–64. doi: 10.1016/j.plaphy.2014.07.010. [DOI] [PubMed] [Google Scholar]
- 60.Fubini B., Fenoglio I., Tomatis M., Turci F. Effect of chemical composition and state of the surface on the toxic response to high aspect ratio nanomaterials. Nanomedicine. 2011;6:899–920. doi: 10.2217/nnm.11.80. [DOI] [PubMed] [Google Scholar]
- 61.Ji Z., Wang X., Zhang H., Lin S., Meng H., Sun B. Designed Synthesis of CeO2 Nanorods and Nanowires for Studying Toxicological Effects of High Aspect Ratio Nanomaterials. ACS Nano. 2012;6:5366–5380. doi: 10.1021/nn3012114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Wang J.W., Grandio E.G., Newkirk G.M., Demirer G.S., Butrus S., Giraldo J.P. Nanoparticle-Mediated Genetic Engineering of Plants. Molecular Plant. 2019;12:1037–1040. doi: 10.1016/j.molp.2019.06.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Kane A.B., Hurt R.H., Gao H. The asbestos-carbon nanotube analogy: An update. Toxicol Appl Pharmacol. 2018;361:68–80. doi: 10.1016/j.taap.2018.06.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Tran C.L., Tantra R., Donaldson K., Stone V., Hankin S.M., Ross B. A hypothetical model for predicting the toxicity of high aspect ratio nanoparticles (HARN) J Nanopart Res. 2011;13:6683–6698. doi: 10.1007/s11051-011-0575-9. [DOI] [Google Scholar]
- 65.Rao D.P., Srivastava A. Enhancement of seed germination and plant growth of wheat, maize, peanut and garlic using multiwalled carbon nanotubes. European Chem Bull. 2014;3:502–504. doi: 10.17628/ecb.2014.3.502-504. [DOI] [Google Scholar]
- 66.Demirer G.S., Zhang H., Matos J.L., Goh N.S., Cunningham F.J., Sung Y. High aspect ratio nanomaterials enable delivery of functional genetic material without DNA integration in mature plants. Nat Nanotechnol. 2019;14:456–464. doi: 10.1038/s41565-019-0382-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.González-García Y., López-Vargas E.R., Cadenas-Pliego G., Benavides-Mendoza A., González-Morales S., Robledo-Olivo A. Impact of Carbon Nanomaterials on the Antioxidant System of Tomato Seedlings. Int J Mol Sci. 2019;20:5858. doi: 10.3390/ijms20235858. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.López-Vargas E.R., González-García Y., Pérez-Álvarez M., Cadenas-Pliego G., González-Morales S., Benavides-Mendoza A. Seed Priming with Carbon Nanomaterials to Modify the Germination, Growth, and Antioxidant Status of Tomato Seedlings. Agronomy. 2020;10:639. doi: 10.3390/agronomy10050639. [DOI] [Google Scholar]
- 69.Yu W., Batchelor-McAuley C., Chang X., Young N.P., Compton R.G. Porosity controls the catalytic activity of platinum nanoparticles. Phys Chem Chem Phys. 2019;21:20415–20421. doi: 10.1039/C9CP03887F. [DOI] [PubMed] [Google Scholar]
- 70.Alan B.O., Barisik M., Ozcelik H.G. Roughness Effects on the Surface Charge Properties of Silica Nanoparticles. J Phys Chem C. 2020;124:7274–7286. doi: 10.1021/acs.jpcc.0c00120. [DOI] [Google Scholar]
- 71.Saikia J., Yazdimamaghani M., Hadipour Moghaddam S.P., Ghandehari H. Differential Protein Adsorption and Cellular Uptake of Silica Nanoparticles Based on Size and Porosity. ACS Appl Mater Interf. 2016;8:34820–34832. doi: 10.1021/acsami.6b09950. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Marichal L., Giraudon-Colas G., Cousin F., Thill A., Labarre J., Boulard Y. Protein-Nanoparticle Interactions: What Are the Protein-Corona Thickness and Organization? Langmuir. 2019;35:10831–10837. doi: 10.1021/acs.langmuir.9b01373. [DOI] [PubMed] [Google Scholar]
- 73.Krasowska A., Sigler K. How microorganisms use hydrophobicity and what does this mean for human needs? Front Cell Infect Microbiol. 2014;4 doi: 10.3389/fcimb.2014.00112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Valsesia A., Desmet C., Ojea-Jiménez I., Oddo A., Capomaccio R., Rossi F. Direct quantification of nanoparticle surface hydrophobicity. Commun Chem. 2018;1:1–11. doi: 10.1038/s42004-018-0054-7. [DOI] [Google Scholar]
- 75.Joo S.H., Aggarwal S. Factors impacting the interactions of engineered nanoparticles with bacterial cells and biofilms: Mechanistic insights and state of knowledge. J Environ Manage. 2018;225:62–74. doi: 10.1016/j.jenvman.2018.07.084. [DOI] [PubMed] [Google Scholar]
- 76.Gong L., Chen Y., He K., Liu J. Surface Coverage-Regulated Cellular Interaction of Ultrasmall Luminescent Gold Nanoparticles. ACS Nano. 2019;13:1893–1899. doi: 10.1021/acsnano.8b08103. [DOI] [PubMed] [Google Scholar]
- 77.Smerkova K., Dolezelikova K., Bozdechova L., Heger Z., Zurek L., Adam V. Nanomaterials with active targeting as advanced antimicrobials. WIREs Nanomed Nanobiotechnol. 2020 doi: 10.1002/wnan.1636. n/a:e1636. [DOI] [PubMed] [Google Scholar]
- 78.Zhao L., Lu L., Wang A., Zhang H., Huang M., Wu H. Nano-Biotechnology in Agriculture: Use of Nanomaterials to Promote Plant Growth and Stress Tolerance. J Agric Food Chem. 2020;68:1935–1947. doi: 10.1021/acs.jafc.9b06615. [DOI] [PubMed] [Google Scholar]
- 79.Balážová Ľ., Baláž M., Babula P. Zinc Oxide Nanoparticles Damage Tobacco BY-2 Cells by Oxidative Stress Followed by Processes of Autophagy and Programmed Cell Death. Nanomaterials. 2020;10:1066. doi: 10.3390/nano10061066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Wang Y., Yang Y., Shi Y., Song H., Yu C. Antibiotic-Free Antibacterial Strategies Enabled by Nanomaterials: Progress and Perspectives. Adv Mater. 2020;32:1904106. doi: 10.1002/adma.201904106. [DOI] [PubMed] [Google Scholar]
- 81.López-Moreno M.L., Cedeño-Mattei Y., Bailón-Ruiz S.J., Vazquez-Nuñez E., Hernandez-Viezcas J.A., Perales-Pérez O.J. Environmental behavior of coated NMs: Physicochemical aspects and plant interactions. J Hazard Mater. 2018;347:196–217. doi: 10.1016/j.jhazmat.2017.12.058. [DOI] [PubMed] [Google Scholar]
- 82.Lv J., Christie P., Zhang S. Uptake, translocation, and transformation of metal-based nanoparticles in plants: recent advances and methodological challenges. Environ Sci Nano. 2019;6:41–59. doi: 10.1039/C8EN00645H. [DOI] [Google Scholar]
- 83.Bell I.R., Ives J.A., Wayne B.J. Nonlinear Effects of Nanoparticles: Biological Variability from Hormetic Doses, Small Particle Sizes, and Dynamic Adaptive Interactions. Dose-Response. 2014;12 doi: 10.2203/dose-response.13-025.Bell. dose-response.13-025.Bell. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Sengupta B, E. Gregory W, Zhu J, Dasetty S, Karakaya M, M. Brown J, et al. Influence of carbon nanomaterial defects on the formation of protein corona. RSC Advances 2015;5:82395–402. https://doi.org/10.1039/C5RA15007H. [DOI] [PMC free article] [PubMed]
- 85.Wang D., Lin Z., Wang T., Yao Z., Qin M., Zheng S. Where does the toxicity of metal oxide nanoparticles come from: The nanoparticles, the ions, or a combination of both? J Hazard Mater. 2016;308:328–334. doi: 10.1016/j.jhazmat.2016.01.066. [DOI] [PubMed] [Google Scholar]
- 86.Nair R., Varghese S.H., Nair B.G., Maekawa T., Yoshida Y., Kumar D.S. Nanoparticulate material delivery to plants. Plant Sci. 2010;179:154–163. doi: 10.1016/j.plantsci.2010.04.012. [DOI] [Google Scholar]
- 87.Ahmad F., Ashraf N., Ashraf T., Zhou R.-B., Yin D.-C. Biological synthesis of metallic nanoparticles (MNPs) by plants and microbes: their cellular uptake, biocompatibility, and biomedical applications. Appl Microbiol Biotechnol. 2019;103:2913–2935. doi: 10.1007/s00253-019-09675-5. [DOI] [PubMed] [Google Scholar]
- 88.Park S., Choi K.S., Kim S., Gwon Y., Kim J. Graphene Oxide-Assisted Promotion of Plant Growth and Stability. Nanomaterials. 2020;10:758. doi: 10.3390/nano10040758. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Agathokleous E., Feng Z., Iavicoli I., Calabrese E.J. The two faces of nanomaterials: A quantification of hormesis in algae and plants. Environ Int. 2019;131 doi: 10.1016/j.envint.2019.105044. [DOI] [PubMed] [Google Scholar]
- 90.Shang H, Guo H, Ma C, Li C, Chefetz B, Polubesova T, et al. Maize (Zea mays L.) root exudates modify the surface chemistry of CuO nanoparticles: Altered aggregation, dissolution and toxicity. Science of The Total Environment 2019;690:502–10. https://doi.org/10.1016/j.scitotenv.2019.07.017. [DOI] [PubMed]
- 91.Bundschuh M., Filser J., Lüderwald S., McKee M.S., Metreveli G., Schaumann G.E. Nanoparticles in the environment: where do we come from, where do we go to? Environ Sci Eur. 2018;30:6. doi: 10.1186/s12302-018-0132-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.McKee M S., Filser J. Impacts of metal-based engineered nanomaterials on soil communities. Environmental Science. NANO. 2016;3:506–33. doi: 10.1039/C6EN00007J. [DOI] [Google Scholar]
- 93.Ball P. Quantum materials: Where many paths meet. MRS Bull. 2017;42:698–705. doi: 10.1557/mrs.2017.220. [DOI] [Google Scholar]
- 94.Dhas S.P., Shiny P.J., Khan S., Mukherjee A., Chandrasekaran N. Toxic behavior of silver and zinc oxide nanoparticles on environmental microorganisms. J Basic Microbiol. 2014;54:916–927. doi: 10.1002/jobm.201200316. [DOI] [PubMed] [Google Scholar]
- 95.Kumari J., Kumar D., Mathur A., Naseer A., Kumar R.R., Thanjavur Chandrasekaran P. Cytotoxicity of TiO2 nanoparticles towards freshwater sediment microorganisms at low exposure concentrations. Environ Res. 2014;135:333–345. doi: 10.1016/j.envres.2014.09.025. [DOI] [PubMed] [Google Scholar]
- 96.Pramanik A., Laha D., Bhattacharya D., Pramanik P., Karmakar P. A novel study of antibacterial activity of copper iodide nanoparticle mediated by DNA and membrane damage. Colloids Surf, B. 2012;96:50–55. doi: 10.1016/j.colsurfb.2012.03.021. [DOI] [PubMed] [Google Scholar]
- 97.Saxena A., Tripathi R.M., Zafar F., Singh P. Green synthesis of silver nanoparticles using aqueous solution of Ficus benghalensis leaf extract and characterization of their antibacterial activity. Mater Lett. 2012;67:91–94. doi: 10.1016/j.matlet.2011.09.038. [DOI] [Google Scholar]
- 98.Wong S.W.Y., Zhou G.-J., Kwok K.W.H., Djurišić A.B., Han J., Lee J.-S. In vivo toxicities of nine engineered nano metal oxides to the marine diatom Skeletonema costatum and rotifer Brachionus koreanus. Mar Pollut Bull. 2020;153 doi: 10.1016/j.marpolbul.2020.110973. [DOI] [PubMed] [Google Scholar]
- 99.Raghunath A., Perumal E. Metal oxide nanoparticles as antimicrobial agents: a promise for the future. Int J Antimicrob Agents. 2017;49:137–152. doi: 10.1016/j.ijantimicag.2016.11.011. [DOI] [PubMed] [Google Scholar]
- 100.Chandrangsu P., Rensing C., Helmann J.D. Metal Homeostasis and Resistance in Bacteria. Nat Rev Microbiol. 2017;15:338–350. doi: 10.1038/nrmicro.2017.15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Markowicz A., Plociniczak T., Piotrowska-Seget Z. Response of bacteria to heavy metals measured as changes in FAME profiles. Polish J Environ Studies. 2010;19:957–965. [Google Scholar]
- 102.Ameh T., Sayes C.M. The potential exposure and hazards of copper nanoparticles: A review. Environ Toxicol Pharmacol. 2019;71 doi: 10.1016/j.etap.2019.103220. [DOI] [PubMed] [Google Scholar]
- 103.Courtois P., Rorat A., Lemiere S., Guyoneaud R., Attard E., Levard C. Ecotoxicology of silver nanoparticles and their derivatives introduced in soil with or without sewage sludge: A review of effects on microorganisms, plants and animals. Environ Pollut. 2019;253:578–598. doi: 10.1016/j.envpol.2019.07.053. [DOI] [PubMed] [Google Scholar]
- 104.Chen M., Sun Y., Liang J., Zeng G., Li Z., Tang L. Understanding the influence of carbon nanomaterials on microbial communities. Environ Int. 2019;126:690–698. doi: 10.1016/j.envint.2019.02.005. [DOI] [PubMed] [Google Scholar]
- 105.Han J., Wang S., Fan D., Guo Y., Liu C., Zhu Y. Time-Dependent Hormetic Response of Soil Alkaline Phosphatase Induced by Cd and the Association with Bacterial Community Composition. Microb Ecol. 2019;78:961–973. doi: 10.1007/s00248-019-01371-1. [DOI] [PubMed] [Google Scholar]
- 106.Ge Y., Schimel J.P., Holden P.A. Identification of Soil Bacteria Susceptible to TiO2 and ZnO Nanoparticles. Appl Environ Microbiol. 2012;78:6749–6758. doi: 10.1128/AEM.00941-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Cecchin I., Reddy K.R., Thomé A., Tessaro E.F., Schnaid F. Nanobioremediation: Integration of nanoparticles and bioremediation for sustainable remediation of chlorinated organic contaminants in soils. Int Biodeterior Biodegrad. 2017;119:419–428. doi: 10.1016/j.ibiod.2016.09.027. [DOI] [Google Scholar]
- 108.Vittori Antisari L., Carbone S., Gatti A., Vianello G., Nannipieri P. Toxicity of metal oxide (CeO2, Fe3O4, SnO2) engineered nanoparticles on soil microbial biomass and their distribution in soil. Soil Biol Biochem. 2013;60:87–94. doi: 10.1016/j.soilbio.2013.01.016. [DOI] [Google Scholar]
- 109.Akanbi-Gada M.A., Ogunkunle C.O., Vishwakarma V., Viswanathan K., Fatoba P.O. Phytotoxicity of nano-zinc oxide to tomato plant (Solanum lycopersicum L.): Zn uptake, stress enzymes response and influence on non-enzymatic antioxidants in fruits. Environ Technol Innovation. 2019 doi: 10.1016/j.eti.2019.100325. [DOI] [Google Scholar]
- 110.Bala R., Kalia A., Dhaliwal S.S. Evaluation of Efficacy of ZnO Nanoparticles as Remedial Zinc Nanofertilizer for Rice. J Soil Sci Plant Nutr. 2019;19:379–389. doi: 10.1007/s42729-019-00040-z. [DOI] [Google Scholar]
- 111.Hussain A., Ali S., Rizwan M., Zia ur Rehman M., Javed M.R., Imran M. Zinc oxide nanoparticles alter the wheat physiological response and reduce the cadmium uptake by plants. Environ Pollut. 2018;242:1518–1526. doi: 10.1016/j.envpol.2018.08.036. [DOI] [PubMed] [Google Scholar]
- 112.Hussein H.-A.A., Darwesh O.M., Mekki B.B. Environmentally friendly nano-selenium to improve antioxidant system and growth of groundnut cultivars under sandy soil conditions. Biocatalysis and Agricultural. Biotechnology. 2019 doi: 10.1016/j.bcab.2019.101080. [DOI] [Google Scholar]
- 113.Jahani S., Saadatmand S., Mahmoodzadeh H., Khavari-Nejad R.A. Effect of foliar application of cerium oxide nanoparticles on growth, photosynthetic pigments, electrolyte leakage, compatible osmolytes and antioxidant enzymes activities of Calendula officinalis L. Biologia. 2019;74:1063–1075. doi: 10.2478/s11756-019-00239-6. [DOI] [Google Scholar]
- 114.Mahjouri S., Movafeghi A., Divband B., Kosari-Nasab M. Toxicity impacts of chemically and biologically synthesized CuO nanoparticles on cell suspension cultures of Nicotiana tabacum. Plant Cell Tiss Organ Cult. 2018;135:223–234. doi: 10.1007/s11240-018-1458-x. [DOI] [Google Scholar]
- 115.Noman M., Shahid M., Ahmed T., Tahir M., Naqqash T., Muhammad S. Green copper nanoparticles from a native Klebsiella pneumoniae strain alleviated oxidative stress impairment of wheat plants by reducing the chromium bioavailability and increasing the growth. Ecotoxicol Environ Saf. 2020;192 doi: 10.1016/j.ecoenv.2020.110303. [DOI] [PubMed] [Google Scholar]
- 116.Ogunkunle C.O., Bornmann B., Wagner R., Fatoba P.O., Frahm R., Lützenkirchen-Hecht D. Copper uptake, tissue partitioning and biotransformation evidence by XANES in cowpea (Vigna unguiculata L) grown in soil amended with nano-sized copper particles. Environ Nanotechnol Monit Manage. 2019;12 doi: 10.1016/j.enmm.2019.100231. [DOI] [Google Scholar]
- 117.Rizwan M., Ali S., Ali B., Adrees M., Arshad M., Hussain A. Zinc and iron oxide nanoparticles improved the plant growth and reduced the oxidative stress and cadmium concentration in wheat. Chemosphere. 2019;214:269–277. doi: 10.1016/j.chemosphere.2018.09.120. [DOI] [PubMed] [Google Scholar]
- 118.Shankramma K., Yallappa S., Shivanna M.B., Manjanna J. Fe2O3 magnetic nanoparticles to enhance S. lycopersicum (tomato) plant growth and their biomineralization. Appl Nanosci. 2016;6:983–990. doi: 10.1007/s13204-015-0510-y. [DOI] [Google Scholar]
- 119.Singh A., Prasad S.M., Singh S. Impact of nano ZnO on metabolic attributes and fluorescence kinetics of rice seedlings. Environ Nanotechnol Monit Manage. 2018;9:42–49. doi: 10.1016/j.enmm.2017.11.006. [DOI] [Google Scholar]
- 120.Singh D., Kumar A. Assessment of toxic interaction of nano zinc oxide and nano copper oxide on germination of Raphanus sativus seeds. Environ Monit Assess. 2019;191:703. doi: 10.1007/s10661-019-7902-5. [DOI] [PubMed] [Google Scholar]
- 121.Thapa M., Singh M., Ghosh C.K., Biswas P.K., Mukherjee A. Zinc sulphide nanoparticle (nZnS): A novel nano-modulator for plant growth. Plant Physiol Biochem. 2019;142:73–83. doi: 10.1016/j.plaphy.2019.06.031. [DOI] [PubMed] [Google Scholar]
- 122.Wu B., Zhu L., Le X.C. Metabolomics analysis of TiO2 nanoparticles induced toxicological effects on rice (Oryza sativa L.) Environ Pollut. 2017;230:302–310. doi: 10.1016/j.envpol.2017.06.062. [DOI] [PubMed] [Google Scholar]
- 123.Xie C., Ma Y., Yang J., Zhang B., Luo W., Feng S. Effects of foliar applications of ceria nanoparticles and CeCl3 on common bean (Phaseolus vulgaris) Environ Pollut. 2019;250:530–536. doi: 10.1016/j.envpol.2019.04.042. [DOI] [PubMed] [Google Scholar]
- 124.Yang Z., Xiao Y., Jiao T., Zhang Y., Chen J., Gao Y. Effects of Copper Oxide Nanoparticles on the Growth of Rice (Oryza Sativa L.) Seedlings and the Relevant Physiological Responses. Int J Environ Res Public Health. 2020;17:1260. doi: 10.3390/ijerph17041260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Zahedi S.M., Abdelrahman M., Hosseini M.S., Hoveizeh N.F., Tran L.-S.P. Alleviation of the effect of salinity on growth and yield of strawberry by foliar spray of selenium-nanoparticles. Environ Pollut. 2019;253:246–258. doi: 10.1016/j.envpol.2019.04.078. [DOI] [PubMed] [Google Scholar]
- 126.Zuverza-Mena N., Armendariz R., Peralta-Videa J.R., Gardea-Torresdey J.L. Effects of Silver Nanoparticles on Radish Sprouts: Root Growth Reduction and Modifications in the Nutritional Value. Front Plant Sci. 2016;7 doi: 10.3389/fpls.2016.00090. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Giraldo J.P., Landry M.P., Faltermeier S.M., McNicholas T.P., Iverson N.M., Boghossian A.A. Plant nanobionics approach to augment photosynthesis and biochemical sensing. Nat Mater. 2014;13:400–408. doi: 10.1038/nmat3890. [DOI] [PubMed] [Google Scholar]
- 128.López-Vargas E.R., Ortega-Ortíz H., Cadenas-Pliego G., De Alba R.K, Cabrera de la Fuente M., Benavides-Mendoza A. Foliar Application of Copper Nanoparticles Increases the Fruit Quality and the Content of Bioactive Compounds in Tomatoes. Appl Sci. 2018;8:1020. doi: 10.3390/app8071020. [DOI] [Google Scholar]
- 129.Morales-Espinoza M.C., Cadenas-Pliego G., Pérez-Alvarez M., Hernández-Fuentes A.D., Cabrera de la Fuente M., Benavides-Mendoza A. Se Nanoparticles Induce Changes in the Growth, Antioxidant Responses, and Fruit Quality of Tomato Developed under NaCl Stress. Molecules. 2019;24:3030. doi: 10.3390/molecules24173030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Pérez-Labrada F., López-Vargas E.R., Ortega-Ortiz H., Cadenas-Pliego G., Benavides-Mendoza A., Juárez-Maldonado A. Responses of Tomato Plants under Saline Stress to Foliar Application of Copper Nanoparticles. Plants. 2019;8:151. doi: 10.3390/plants8060151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Thabet A.F., Galal O.A., El-Samahy M.F.M., Tuda M. Higher toxicity of nano-scale TiO2 and dose-dependent genotoxicity of nano-scale SiO2 on the cytology and seedling development of broad bean Vicia faba. SN Appl Sci. 2019;1:956. doi: 10.1007/s42452-019-0960-z. [DOI] [Google Scholar]