Abstract
STUDY QUESTION
Has cumulative live birth rate (CLBR) improved over time and which factors are associated with such an improvement?
SUMMARY ANSWER
During an 11-year period, 2007–2017, CLBR per oocyte aspiration increased significantly, from 27.0% to 36.3%, in parallel with an increase in blastocyst transfer and cryopreservation by vitrification.
WHAT IS KNOWN ALREADY
While it has been shown that live birth rate (LBR) per embryo transfer (ET) is higher for fresh blastocyst than for fresh cleavage stage embryo transfer, CLBR per oocyte aspiration, including one fresh ET and all subsequent frozen embryo transfers (FET), does not seem to differ between the two culture strategies.
STUDY DESIGN, SIZE, DURATION
A national register study including all oocyte aspirations performed in Sweden from 2007 to 2017 (n = 124 700 complete IVF treatment cycles) was carried out. Oocyte donation cycles were excluded.
PARTICIPANTS/MATERIALS, SETTING, METHODS
Data were retrieved from the Swedish National Registry of Assisted Reproduction (Q-IVF) on all oocyte aspirations during the study period where autologous oocytes were used. CLBR was defined as the proportion of deliveries with at least one live birth per oocyte aspiration, including all fresh and/or frozen embryo transfers within 1 year, until one delivery with a live birth or until all embryos were used, whichever occurred first. The delivery of a singleton, twin, or other multiples was registered as one delivery. Cryopreservation of cleavage stage embryos was performed by slow freezing and of blastocyst by vitrification.
MAIN RESULTS AND THE ROLE OF CHANCE
In total, 124 700 oocyte aspirations were performed (in 61 313 women), with 65 304 aspirations in women <35 years and 59 396 in women ≥ 35 years, resulting in 38 403 deliveries with live born children. Overall, the CLBR per oocyte aspiration increased significantly during the study period, from 27.0% to 36.3% (odds ratio (OR) 1.039, 95% CI 1.035–1.043) and from 30.0% to 43.3% if at least one ET was performed (adjusted OR 1.055, 95% CI 1.050–1.059). The increase in CLBR was independent of maternal age, number of oocytes retrieved and number of previous IVF live births. The CLBR for women <35 and ≥35 years both increased significantly, following the same pattern. During the study period, a substantially increasing number of blastocyst transfers was performed, both in fresh and in FET cycles. Other important predicting factors for live birth, such as number of embryos transferred, could not explain the improvement. An increased single embryo transfer rate was observed with time.
LIMITATIONS, REASONS FOR CAUTION
The retrospective design implicates that other confounders of importance for CLBR cannot be ruled out. In addition, some FET cycles might be performed later than 1 year post oocyte aspiration for the last year (2017) and are, thus, not included in this study. In addition, no data on ‘dropouts’, i.e. patients that do not continue their treatment despite having cryopreserved embryos, are available, or if this drop-out rate has changed over time.
WIDER IMPLICATIONS OF THE FINDINGS
The results suggest that blastocyst transfer, particularly when used in FET cycles and in combination with vitrification, is an important contributor to the improved live birth rates over time. This gives a possibility for a lower number of oocyte aspirations needed to achieve a live birth and a shortened time to live birth.
STUDY FUNDING/COMPETING INTERESTS
The study was financed by grants from the Swedish state under the agreement between the Swedish government and the county councils, the ALF-agreement (ALFGBG-70940) and by Hjalmar Svensson’s research foundation. None of the authors declares any conflict of interest.
Keywords: IVF outcome, cumulative live birth rate, blastocyst, vitrification, IVF / ICSI
WHAT DOES THIS MEAN FOR PATIENTS?
This study aims to investigate the trend over time for success rates after IVF treatment, and which factors that might explain this trend.
Data from all 124 700 complete IVF treatment cycles performed in Sweden during 2007–2017 were analyzed. A complete IVF treatment cycle was defined as the total result from all embryo transfers generated from one oocyte aspiration (egg retrieval) and performed within 1 year.
During this time period, the chance of having a live born baby increased from 27% to 36.3% per complete IVF cycle. The most distinct difference was observed if a blastocyst (an embryo 5–6 days old) was transferred compared to an embryo 2–3 days old.
In recent years, the techniques for embryo culture to the blastocyst stage and for embryo cryopreservation (i.e. freezing) have greatly improved, allowing more blastocysts available for transfer. This implies that a smaller number of oocyte aspirations might be needed and that the time to achieve a pregnancy and birth of a live born baby may be shortened.
Introduction
Results after IVF have historically been calculated in many different ways; most commonly, pregnancy rate or live birth rate (LBR) per started cycle, per oocyte aspiration or per embryo transfer (Wilkinson et al., 2016). To minimize multiple births and thereby improve neonatal and maternal outcome, the concept of ‘birth of a single (healthy) child’ has been suggested (ESHRE Capri Workshop 2000). The most efficient way to achieve this goal is to transfer only one embryo at a time (Thurin et al., 2004).
Cryopreservation was for many years considered a secondary treatment during IVF, with rather poor embryo survival rates and low LBRs compared to fresh embryo transfer. In the last few years, the situation has changed. The use of extended embryo culture to the blastocyst stage has increased, and cryopreservation of blastocysts by vitrification has considerably improved cryosurvival rates (Li et al., 2014; Rienzi et al., 2017).
Cumulative live birth rates (CLBR), defined as the proportion of deliveries with at least one live birth per started cycle or per oocyte aspiration, including all fresh and/or frozen embryo transfers until one delivery with a live birth or until all embryos were used, whichever occurs first, may, therefore, be considered a more relevant variable, even though it may take some time from initiation of a cycle until these data can be collected.
Indeed, some studies propose that the ‘freeze-all’ strategy should be preferred in all IVF cycles. However, while use of vitrified/warmed blastocysts has led to significantly higher pregnancy and LBRs after frozen embryo transfer (FET) compared to slow-frozen/thawed cleavage stage embryos, conflicting results have been reported after the use of the freeze-all strategy. Five large randomized controlled trials (RCTs), using cleavage stage embryos as well as blastocysts, have investigated the differences in LBR following fresh embryo transfer (ET) and FET in freeze-all cycles (Chen et al., 2016; Shi et al., 2018; Vuong et al., 2018; Wei et al., 2019; Stormlund et al., 2020). Four of these studies were performed in Asia. Significantly higher live birth rates were found in anovulatory patients (Chen et al., 2016), while for ovulatory patients, most studies showed no difference in LBR between freeze-all and fresh transfer (Shi et al., 2018; Vuong et al., 2018). In a recent study, performed in European countries and applying single embryo transfer (SET), no difference in ongoing pregnancy rate or LBR was observed when comparing the freeze-all blastocyst group to the fresh blastocyst group in ovulatory women (Stormlund et al., 2020). Furthermore, in another recent RCT from the Netherlands, a strategy with freeze-all of blastocysts was inferior to fresh blastocyst transfer followed by FET, concerning ongoing cumulative pregnancy rate, in unselected infertile women (Wong et al., 2021).
The aim of this study was to investigate if CLBR has improved over time, using an 11-year period from the Swedish National Quality Registry for ART (Q-IVF) and, if an improvement is observed, to examine which factors are associated with such an improvement.
Materials and methods
A population-based register study was performed with data derived from the Swedish Q-IVF registry during the period 2007–2017 (www.qivf.se).
The Q-IVF registry was launched in 2007 and includes treatment data and pregnancy outcomes from all IVF cycles carried out in Sweden, both in public and private clinics, and with full identification of the patients, making it possible to collect cumulative data from all fresh and FET cycles from individual patients. Results are public and posted as aggregated data on the registry website. All patients are informed about the registry and, although voluntary, it is very rare that patients choose not to have their data included. Hence, the registry has an almost complete coverage.
Data on all oocyte aspirations performed in Sweden during the time period 2007–2017 (n = 124 700) were included in the study, however, oocyte donation cycles were excluded. All ET (fresh and/or frozen) from one oocyte aspiration and performed within 1 year after the oocyte aspiration were included. The time limit of 1 year was chosen since very few patients, less than 3% of the cycles in the Q-IVF database, still have cryopreserved embryos left, mainly because the patients already had a baby from this oocyte retrieval, meaning that a further FET and a further baby would not contribute to the CLBR for that particular oocyte aspiration cycle. Furthermore, cryopreserved embryos mainly derive from the last year(s) meaning that if anything, transferring, and including also these embryos would further, to a small extent, increase the CLBR.
Transfers of cleavage stage embryos (Says 2-3) as well as blastocysts (Days 4–6) were included. Natural cycles, modified (aromatase inhibitor stimulated) cycles or programmed cycles were used for FET. Cryopreservation of cleavage stage embryos was performed by slow freezing on Days 2–3 and cryopreservation of blastocysts by vitrification on Days 5–6 after oocyte aspiration.
In the analysis, CLBR per oocyte aspiration was defined as the proportion of deliveries with at least one live birth per oocyte aspiration, including all fresh and/or frozen ETs, until one delivery with a live birth or until all embryos were used within 1 year, whichever occurred first. The delivery of a singleton, twin, or other multiples was registered as one delivery (Zegers-Hochschild et al., 2017). Calculations were performed both for the entire cohort of patients undergoing oocyte aspiration, as well as separately for the cohort of patients who achieved at least one ET (fresh or frozen-thawed cycle). Pregnancies conceived naturally were excluded.
The primary outcome was CLBR and secondary outcomes were LBR in fresh and FET cycles.
Ethical approval
The study was approved by the Regional Ethics Committee at Gothenburg University, D nr T 892-18.
Statistical analysis
Descriptive statistics are given by number and percentage with 95% CI obtained using normal approximation. Crude odds ratios (OR) and adjusted odds ratios (AOR) with 95% CI were obtained using General Estimation Equation (GEE) models, adjusting for dependence within each woman (statistical package SPSS version 25, IBM Corp., Armonk, NY, USA) The ORs and AORs for live birth after a fresh cycle, and cumulatively after fresh and subsequent FET cycles, respectively, were obtained by including year of oocyte retrieval in the models as a continuous variable. Adjustments were performed for maternal age (continuous), blastocyst transfer (yes/no), number of oocytes retrieved (continuous) and number of previous live births after IVF treatment (continuous), where specified. The ORs obtained from the multivariable analyses were used to produce estimates of the live birth probabilities shown in the figures: Adjusted live birth probability= eg(x)/(1+eg(x)), where g(x)=β0+β1*x1+β2*x2+β3*x3+…+βn*xn, and β0 is the intercept, and the coefficients β1–βn, are the logarithms for the obtained ORs, and the x2 to xn are the population means for the possible confounders 2 to n (supposing that x1 is the year of oocyte retrieval, which is the variable of interest in the current study). The models were checked regarding linearity and no signs of any interactions were found. Different models for ‘number of oocytes retrieved’ were tested—but none of these had any impact on the variable of interest (= the year of oocyte retrieval) in relation to treatment outcome. Missing information on maternal age and number of oocytes retrieved (0.4% and 0.04% of all observations, respectively) were replaced by the overall means. For the variable number of previous IVF live births, missing data was 0%. For the variable live birth, the missing number was 0.2%. Missing data for live birth were replaced by no live birth. A p-value less than 0.05 was considered significant.
Results
During the period 2007–2017, 124 700 oocyte aspirations were performed (in 61 313 women), with 65 304 aspirations in women <35 years and 59 396 in women ≥ 35 years, resulting in 38 403 deliveries with live born children. The number of oocyte aspirations per year showed a slow increase during the time period (Supplementary Table SI). Treatment characteristics are presented in Table I. The rate of blastocyst transfers increased rapidly in Sweden during the time period, for fresh transfers from 5% in 2007 to 31% in 2017 and for FET from 6% to 88.0% (Table I). Mean maternal age at oocyte aspiration was 33.5 (SD 6.3) years, and did not change substantially during the study period, neither did the number of oocytes retrieved per oocyte aspiration (Supplementary Table SII), while the rate of SET increased.
Table I.
Maternal and treatment characteristics in a study of trend in cumulative live birth rate after IVF over time.
| Year of treatment | 2007 | 2011 | 2017 |
|---|---|---|---|
| Oocyte aspirations, n | 9782 | 11 467 | 11 931 |
| Maternal age≥ 35 years, n (%) | 3766 (38.5) | 4965 (43.3) | 4784 (40.1) |
| ICSI, n (%) | 4508/9533 (48) | 5199/10 542 (50) | 5335/10 943 (48) |
| SET, fresh, n (%) | 5973/8536 (70) | 7071/9317 (74) | 7203/8576 (84) |
| SET, FET, n (%) | 3024/3909 (74) | 4508/5085 (89) | 6584/6718 (98) |
| Blastocyst transfer rate, fresh, n (%) | 427/8536 (5) | 1025/9317 (11) | 2659/8576 (31) |
| Blastocyst transfer rate, FET, n (%) | 235/3909 (6) | 1525/5085 (30) | 5912/6718 (88) |
| Number of oocytes retrieved, median (IQR) | 9 (7) | 8 (7) | 9(7) |
Data from www.qivf.se.
SET, single embryo transfer; FET, frozen embryo transfer; IQR, interquartile range.
Cumulative live birth rate
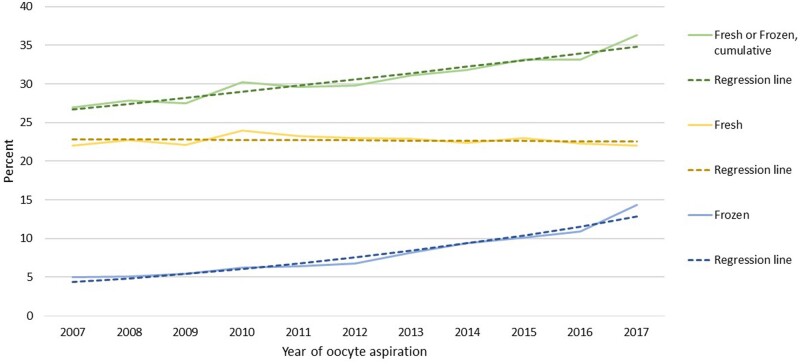
The CLBR per oocyte aspiration increased significantly during the study period, from 27.0% to 36.3%, OR for increase per year 1.039 (95% CI 1.035–1.043) (Fig. 1, Supplementary Table SIII). Adjustment for maternal age, maternal age and number of retrieved oocytes, or maternal age and number of retrieved oocytes and number of previous IVF live births, did not change the estimate of the increase per year (AOR 1.038, 95% CI 1.034–1.042, AOR 1.038, 95% CI 1.034–1.042, and AOR 1.034, 95% CI 1.030–1.039, respectively). The contribution from fresh transfers to the CLBR stayed fairly constant during the time period, at around 23%, while the contribution from FET increased steadily (Fig. 1). The observed and the predicted regression curves in the statistical analyses for LBR after one oocyte aspiration fitted well, both for fresh ET, FET and for cumulative results (Fig. 1).
Figure 1.
Cumulative live brith rate (CLBR) per oocyte aspiration by year of treatment, including all embryo transfers performed within 1 year. Green line: cumulative live birth rate (CLBR) per oocyte aspiration. Data separated for live birth rate after fresh embryo transfer (yellow line) and the additive live birth rate after transfer of frozen embryos (blue line) from the same oocyte retrieval. Actual percentages and results from general estimation equations (GEE) analysis.
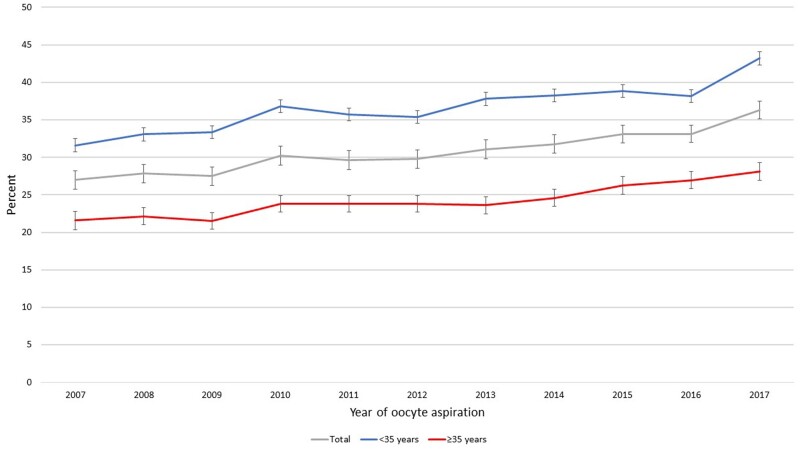
Delivery rates were negatively affected by age, AOR for 1-year-age-increase: 0.918 (95% CI 0.915–0.921), however the CLBR for women <35 and ≥35 years, respectively, increased for both age groups, following the same pattern (Fig. 2). Similarly, the number of oocytes retrieved was significantly associated with LBR and CLBR (P < 0.001) but did not affect the AOR per year.
Figure 2.
CLBR by year of treatment, categorized in age groups <35 and ≥35 years, and total group. CLBR, cumulative live birth rate.
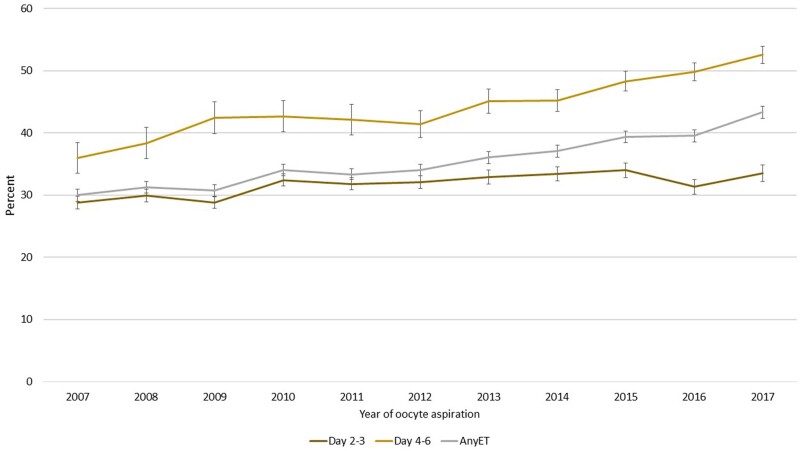
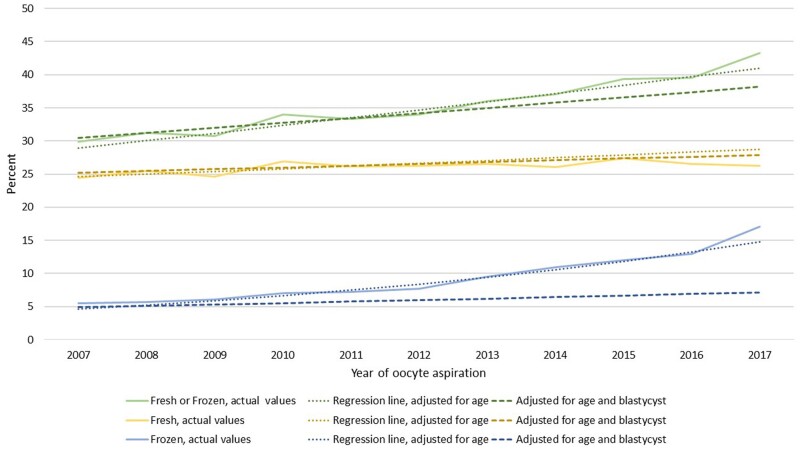
CLBR among oocyte aspirations leading to at least one ET, either fresh or FET, and separated into cleavage and blastocyst stage ET is presented in Fig. 3. Thus, in this graph, oocyte aspiration resulting in no ET (fresh or FET) is excluded. The number of culture days shown in the graph represent the latest performed ET, either leading to live birth or being the latest ET within 1 year after one oocyte aspiration. CLBR when at least one ET was performed increased during the study period independent of embryo stage at the last ET; after cleavage stage ET from 28.8% to 33.5% and after blastocyst transfer from 36.0% to 52.5%. Including both types of transfers, CLBR after at least one ET increased from 30.0% to 43.3%. The increases over time in live birth after any kind of ET, and live birth when the last ET was a blastocyst transfer, were highly significant (AOR 1.055, 95% CI 1.050–1.059) and (AOR 1.658, 95% CI 1.609–1.709) (Fig. 3). The observed and the age-adjusted predicted regression curves in the statistical analyses for delivery rates fitted well, both for fresh ET, FET and for cumulative results. When including adjustment also for blastocyst transfer, the increasing trend in CLBR by year was substantially weakened for FETs (Fig. 4, Supplementary Table SIII), indicating that the increase in LBR, particularly for FET, is highly associated with blastocyst transfer.
Figure 3.
CLBR per oocyte aspiration, where oocyte aspirations not amounting to any embryo transfer are excluded. Categorized by day of transfer, Days 2 and 3 (cleavage stage), Days 4–6 (blastocyst stage) and both groups altogether (any embryo transfer: ET). All three graphs show CLBR. The number of culture days in the graph represents the latest performed ET, either leading to live birth or being the latest ET within 1 year after one oocyte aspiration. CLBR, cumulative live birth rate. ET, embryo transfer.
Figure 4.
CLBR per oocyte aspiration by year of treatment, with oocyte aspirations not amounting to any ET excluded. Data separated for live birth rate after fresh ET (yellow) and the additive live birth rate after transfer of frozen embryos (blue) from the same oocyte retrieval, and live birth rate after fresh or frozen cycles from the same oocyte retrieval (green). Actual rates and regression lines with adjustment for age and blastocyst transfer. CLBR, cumulative live birth rate. ET, embryo transfer.
Discussion
In this large national observational study, we demonstrate a substantial increase over time in CLBR. This increase took place despite an increased SET rate and independent of maternal age, number of oocytes retrieved or earlier IVF live births. Although there may be several possible contributing factors for this positive development, the most striking changes during this more than 10-year period are the partial shifts from transfer of cleavage stage embryos to blastocysts for fresh embryos, and from slow-freezing of early cleavage stage embryos to vitrification of blastocysts as the cryopreservation method. The observation that the increase over time in CLBR was substantially reduced, and for FET almost disappeared, when adjusting for blastocyst transfer suggests that blastocyst transfer has a major impact on the increase in CLBR seen during the study period. An important contributor, however, and included in the blastocyst strategy, may be the extended culture of the total cohort of embryos from one oocyte aspiration to reach the blastocyst stage. It is known that a certain number of poor quality embryos on Days 2 and 3 will develop into good quality blastocyst (20–35%) with good implantation rates (Poulain et al., 2014; Kaartinen et al., 2015; Sallem et al., 2018). Considering that these embryos previously often were discarded at early cleavage stages, they now contribute to the total number of available blastocysts and may increase the chance of a live birth within that oocyte aspiration cycle. In addition, the use of vitrification as cryopreservation method has shown improved cryosurvival rates, adding even further to the numbers of blastocysts available for transfer from the cohort (Li et al., 2014; Fernandez-Shaw et al., 2015). Other possible confounding variables, such as culture media, have changed during these years and may have contributed to improved LBRs, although not shown in any controlled trial.
Since the cumulative data include a mix of blastocyst and cleavage stage transfers from the same oocyte aspiration with several combinations, we chose to analyze trend over time, instead of comparing blastocyst versus cleavage stage embryos. Even though a large randomized trial comparing blastocyst versus cleavage stage embryos for CLBR would give the best evidence, large observational studies have their value in reflecting real life data.
A major change in culture duration and cryopreservation technique have taken place in recent years, where a combination of blastocyst culture and vitrification of surplus embryos has been shown to be successful and resulted in high pregnancy rate and LBR. Although a Cochrane review found higher LBR after blastocyst compared to cleavage stage transfer in fresh cycles, the situation was less clear for CLBR (Glujovsky et al., 2016). No difference in CLBR was demonstrated, however, all but one small study in this review used slow freezing for blastocysts. In a study from Spain, including 120 patients, a significantly higher ongoing pregnancy rate was found for blastocysts, also on a cumulative basis, when using vitrification as cryopreservation method (Fernandez-Shaw et al., 2015).
In a retrospective study from Belgium, no difference in CLBR for Day 3 versus Day 5 transfer was found when including both fresh ET and FET from the same oocyte aspiration (n = 1000 patients) (De Vos et al., 2016). However, the early transfer policy required a higher number of transfers and thus a longer time to reach a live birth. Another more recent and larger retrospective study, also from Belgium, showed a similar CLBR for Days 3 and 5 transfers, however, when adjusting for age and number of retrieved oocytes, a significantly higher CLBR after Day 5 transfer was found, given that patients received a fresh blastocyst (De Croo et al., 2019). It is important to note that the study by de Vos et al. (2016) included only young women in their first cycle having >4 oocytes aspirated, while the de Croo et al. (2019) study compared unselected patients from two time periods with only cleavage stage transfers performed in one period and only blastocyst transfers in a later period. The improved CLBR for those receiving transfer in the de Croo study suggests that a selective use of blastocyst culture in cycles with a higher number of oocytes/embryos may be superior to performing blastocyst culture for all women. Our present study where a substantial increase in CLBR over time is noticed when using blastocyst culture selectively in fresh cycles and frequently in FET cycles, also support such a selective regime. In a recent large population-based retrospective study from UK, CLBR was compared between cleavage stage embryos and blastocysts for first cycles. Although a higher CLBR was achieved in the blastocyst group, this difference disappeared in the adjusted analysis (Cameron et al., 2020). However, although including around 100 000 patients, there were several limitations to this study, as acknowledged by the authors. The transfer stage was only based on the fresh cycle and no information was available on embryo stage for cryopreserved embryos or cryopreservation method. Furthermore, the dataset includes data only up to 2010.
Whether all patients would benefit from blastocyst culture for the fresh transfer, also when very few oocytes are retrieved, cannot be answered in the present study. To answer that question a randomized trial is needed. However, the idea behind blastocyst culture/blastocyst transfer is to select potentially more viable embryos, and if only one embryo is available on Day 2/3 then no selection problem exists. Even if an RCT was initiated comparing live birth after blastocyst versus cleavage stage embryos, it is doubtful that poor prognosis patients, where only a few oocytes are retrieved, would be included.
For many years, there has been a debate on how to best express efficacy in ART (Min et al., 2004). A systematic review, including RCTs performed in IVF treatments, found more than 800 different combinations of numerator and denominator, and no specific outcome measure appeared in the majority of studies. Furthermore, in only a minority of the studies, the entire randomized cohort was included when calculating the primary outcomes, such as LBR, while most trials used subgroups for their analyses (Wilkinson et al., 2016). The issues with the lack of common reporting standards have been addressed by the CoRe Outcomes in WomeŃs health CROWN initiative regarding all diagnoses in women’s health, claiming that this is a hindrance in the synthesis of evidence (Khan 2014). The ‘Improving the Reporting of Clinical Trials of Infertility Treatments (IMPRINT) recommendations’ is to always present LBR and CLBR in infertility trials (Legro et al., 2014; Maheshwari et al., 2015).
However, in ART, safety issues also have to be addressed. A shift to more blastocyst culture results in a higher proportion of children being born after FET. Several large studies and meta-analyses have shown better perinatal outcome in terms of lower rates of preterm birth (PTB), low birthweight and small for gestational age (SGA) following FET in comparison to fresh transfer (Wennerholm et al., 2013; Pinborg et al., 2013; Maheshwari et al., 2018). However, higher rates of high birthweight and large for gestational age (LGA) (Pinborg et al., 2013; Wennerholm et al., 2013; Berntsen and Pinborg, 2018; Maheshwari et al., 2018) have been reported for children born after cryopreservation. While the late consequences of PTB or SGA are quite well known, with increased risk of cardiovascular and metabolic diseases (Barker et al., 1993), the risks in the longer term of being LGA or born with high birthweight are less well documented. For maternal outcomes, the most prominent risk in pregnancies after FET compared to fresh ET seems to be higher risk of hypertensive disorders of pregnancy, as demonstrated in several large studies and meta-analyses (Ishihara et al., 2014; Opdahl et al., 2015; Maheshwari et al., 2018). Large Nordic population-based studies on children born after blastocyst transfer compared to children born after cleavage stage transfer have found similar risks for most perinatal outcomes between the two techniques while some placenta-related complications seemed increased (Ginström Ernstad et al., 2016; Spangmose et al., 2020). Also, change of cryopreservation method to vitrification of blastocysts seemed reassuring, indicating that the freezing technique per se had no major influence on the perinatal and maternal outcomes (Ginström Ernstad et al., 2019).
The strengths of our study are the size and completeness of the data, with all IVF cycles performed in Sweden during an 11-year period being included. The main weakness is the retrospective design where unmeasured confounders might play a role. A further weakness is the follow up time for CLBR being limited to 1 year, which might amount to some FET cycles being performed later and thus not being included in the study. In addition, no data on ‘dropouts’, i.e. patients that do not continue their treatment despite having cryopreserved embryos, are available, or if this drop-out rate has changed over time.
In conclusion, a substantial increase in CLBR has taken place over time, in parallel with an increase in blastocyst transfer, particularly when used in FET cycles. This development is observed in parallel with a high and increasing use of SET, resulting in a low multiple birth rate.
Supplementary data
Supplementary data are available at Human Reproduction Open online.
Data availability
Data cannot be shared publicly directly because use of the data from Sweden was only approved by the ethical committee for this particular use and for this research group. Approval of use for other purposes would be needed from the ethical committee as well as from the National Quality Register of Assisted Reproduction. Contact information is given in the Abstract.
Authors’ roles
All authors were responsible for the conception and design of the study. K.K. performed the statistical analyses. All authors contributed to the interpretation of data. Z.S. wrote the first draft of the manuscript and all authors revised and approved the final version.
Funding
The study was financed by grants from the Swedish state under the agreement between the Swedish government and the county councils, the ALF-agreement (ALFGBG-70940) and by Hjalmar Svensson’s research foundation.
Conflict of interest
None.
Supplementary Material
Contributor Information
Zoha Saket, Department of Reproductive Medicine, Sahlgrenska University Hospital, Göteborg, Sweden.
Karin Källén, Unit of Reproduction Epidemiology, Department of Obstetrics and Gynecology, Tornblad Institute, Institution of Clinical Sciences, Lund University, Lund, Sweden.
Kersti Lundin, Department of Reproductive Medicine, Sahlgrenska University Hospital, Göteborg, Sweden; Reproductive Medicine, Department of Obstetrics and Gynecology, Institute of Clinical Sciences, Sahlgrenska Academy, Sahlgrenska University Hospital, Göteborg University, Göteborg, Sweden.
Åsa Magnusson, Department of Reproductive Medicine, Sahlgrenska University Hospital, Göteborg, Sweden; Reproductive Medicine, Department of Obstetrics and Gynecology, Institute of Clinical Sciences, Sahlgrenska Academy, Sahlgrenska University Hospital, Göteborg University, Göteborg, Sweden.
Christina Bergh, Reproductive Medicine, Department of Obstetrics and Gynecology, Institute of Clinical Sciences, Sahlgrenska Academy, Sahlgrenska University Hospital, Göteborg University, Göteborg, Sweden.
References
- Barker DJ, Hales CN, Fall CH, Osmond C, Phipps K, Clark PM.. Type 2 (non-insulin-dependent) diabetes mellitus, hypertension and hyperlipidaemia (syndrome X): relation to reduced fetal growth. Diabetologia 1993;36:62–67. [DOI] [PubMed] [Google Scholar]
- Berntsen S, Pinborg A.. Large for gestational age and macrosomia in singletons born after frozen/thawed embryo transfer (FET) in assisted reproductive technology (ART). Birth Defects Res 2018;110:630–642. [DOI] [PubMed] [Google Scholar]
- Cameron NJ, Bhattacharya S, McLernon DJ.. Cumulative live birth rates following blastocyst- versus cleavage-stage embryo transfer in the first complete cycle of IVF: a population-based retrospective cohort study. Hum Reprod 2020;35:2365–2374. [DOI] [PubMed] [Google Scholar]
- Chen Z-J, Shi Y, Sun Y, Zhang B, Liang X, Cao Y, Yang J, Liu J, Wei D, Weng N.. et al. Fresh versus frozen embryos for infertility in the polycystic ovary syndrome. N Engl J Med 2016;375:523–533. [DOI] [PubMed] [Google Scholar]
- De Croo I, Colman R, De SP, Tilleman K.. Blastocyst transfer for all? Higher cumulative live birth chance in a blastocyst-stage transfer policy compared to a cleavage-stage transfer policy. Facts Views Vis Obgyn 2019;11:169–176. [PMC free article] [PubMed] [Google Scholar]
- De Vos A, Van Landuyt L, Santos-Ribeiro S, Camus M, Van de Veide H, Tournaye H, Verheyen G.. Cumulative live birth rates after fresh and vitrified cleavage-stage versus blastocyst-stage embryo transfer in the first treatment cycle. Hum Reprod 2016;31:2442–2244. [DOI] [PubMed] [Google Scholar]
- Fernández-Shaw S, Cercas R, Braña C, Villas C, Pons I. Ongoing and cumulative pregnancy rate after cleavage-stage versus blastocyst-stage embryo transfer using vitrification for cryopreservation: impact of age on the results. J Assist Reprod Genet 2015;32:177–184. 10.1007/s10815-014-0387-9 25403438 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ginström Ernstad E, Bergh C, Khatibi A, Källén K, Nilsson S, Westlander G, Wennerholm UB.. Neonatal and maternal outcome after blastocyst transfer – a population-based registry study. Am J Obstet Gynecol 2016;214:378.e1–378.e10. [DOI] [PubMed] [Google Scholar]
- Ginström EE, Spangmose AL, Opdahl S, Henningsen AA, Romundstad LB, Tiitinen A, Gissler M, Wennerholm UB, Pinborg A, Bergh C.. et al. Perinatal and maternal outcome after vitrification of blastocysts: a Nordic study in singletons from the CoNARTaS group. Hum Reprod 2019;34:2282–2289. [DOI] [PubMed] [Google Scholar]
- Glujovsky D, Farquhar C, Quinteiro RA, Alvarez SC, Blake D.. Cleavage stage versus blastocyst stage embryo transfer in assisted reproductive technology. Cochrane Database Syst Rev 2016;CD002118. [DOI] [PubMed] [Google Scholar]
- Ishihara O, Araki R, Kuwahara A, Itakura A, Saito H, Adamson GD.. Impact of frozen-thawed single-blastocyst transfer on maternal and neonatal outcome: an analysis of 277,042 single-embryo transfer cycles from 2008 to 2010 in Japan. Fertil Steril 2014;101:128–133. [DOI] [PubMed] [Google Scholar]
- Kaartinen N, Das P, Kananen K, Huhtala H, Tinkanen H.. Can repeated IVF-ICSI-cycles be avoided by using blastocysts developing from poor-quality cleavage stage embryos? Reprod Biomed Online 2015;30:241–247. [DOI] [PubMed] [Google Scholar]
- Khan K. The CROWN Initiative: journal editors invite researchers to develop core outcomes in women’s health. BJOG 2014;121:1181–1182. [DOI] [PubMed] [Google Scholar]
- Legro RS, Wu X, Barnhart KT, Farquhar C, Fauser BCJM, Mol B, Legro RS, Wu X, Barnhart K, Niederberger C, The Harbin Consensus Conference Workshop Group. et al. Improving the reporting of clinical trials of infertility treatments (IMPRINT): modifying the CONSORT statement. Hum Reprod 2014;29:2075–2082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li Z, Wang YA, Ledger W, Edgar DH, Sullivan EA.. Clinical outcomes following cryopreservation of blastocysts by vitrification or slow freezing: a population-based cohort study. Hum Reprod 2014;29:794–2801. [DOI] [PubMed] [Google Scholar]
- Maheshwari A, McLernon D, Bhattacharya S.. Cumulative live birth rate: time for a consensus? Hum Reprod 2015;30:2703–2707. [DOI] [PubMed] [Google Scholar]
- Maheshwari A, Pandey S, Amalraj Raja E, Shetty A, Hamilton M, Bhattacharya S.. Is frozen embryo transfer better for mothers and babies? Can cumulative meta-analysis provide a definitive answer? Hum Reprod Update 2018;24:35–58. [DOI] [PubMed] [Google Scholar]
- *Min JK, Breheny SA, MacLachlan V, Healy DL.. What is the most relevant standard of success in assisted reproduction? The singleton, term gestation, live birth rate per cycle initiated: the BESST endpoint for assisted reproduction. Hum Reprod 2004;19:3–7. [DOI] [PubMed] [Google Scholar]
- Opdahl S, Henningsen AA, Tiitinen A, Bergh C, Pinborg A, Romundstad PR, Wennerholm UB, Gissler M, Skjærven R, Romundstad LB.. Risk of hypertensive disorders in pregnancies following assisted reproductive technology: a cohort study from the CoNARTaS group. Hum Reprod 2015;30:1724–1731. [DOI] [PubMed] [Google Scholar]
- Pinborg A, Wennerholm UB, Romundstad LB, Loft A, Aittomaki K, Soderstrom-Anttila V, Nygren KG, Hazekamp J, Bergh C.. Why do singletons conceived after assisted reproduction technology have adverse perinatal outcome? Systematic review and meta-analysis. Hum Reprod Update 2013;19:87–104. [DOI] [PubMed] [Google Scholar]
- Poulain M, Hesters L, Sanglier T, de Bantel A, Fanchin R, Frydman N, Grynberg M.. Is it acceptable to destroy or include human embryos before day 5 in research programmes? Reprod Biomed Online 2014;28:522–529. [DOI] [PubMed] [Google Scholar]
- Rienzi L, Gracia C, Maggiulli R, LaBarbera AR, Kaser DJ, Ubaldi FM, Vanderpoel S, Racowsky C.. Oocyte, embryo and blastocyst cryopreservation in ART: systematic review and meta-analysis comparing slow-freezing versus vitrification to produce evidence for the development of global guidance. Hum Reprod Update 2017;23:139–155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sallem A, Santulli P, Barraud-Lange V, Le Foll N, Ferreux L, Maignien C, Bourdon M, Chapron C, de Ziegler D, Wolf JP.. et al. Extended culture of poor-quality supernumerary embryos improves ART outcomes. J Assist Reprod Genet 2018;35:311–319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shi Y, Sun Y, Hao C, Zhang H, Wei D, Zhang Y, Zhu Y, Deng X, Qi X, Li H.. et al. Transfer of fresh versus frozen embryos in ovulatory women. N Engl J Med 2018;378:126–136. [DOI] [PubMed] [Google Scholar]
- Spangmose AL, Ginström Ernstad E, Malchau S, Forman J, Tiitinen A, Gissler M, Opdahl S, Romundstad LB, Bergh C, Wennerholm UB.. et al. Obstetric and perinatal risks in 4601 singletons and 884 twins conceived after fresh blastocyst transfers: a Nordic study from the CoNARTaS group. Hum Reprod 2020;35:805–815. [DOI] [PubMed] [Google Scholar]
- Stormlund S, Sopa N, Zedeler A, Bogstad J, Prætorius L, Nielsen HS, Kitlinski M, Skouby SO, Mikkelsen A, Spangmose AL.. et al. Freeze-all versus fresh embryo transfer in IVF: a multicentre randomised trial in women with a regular menstrual cycle. BMJ 2020;370:m2519.doi:10.1136/bmj.m2519. [DOI] [PMC free article] [PubMed] [Google Scholar]
- The ESHRE Capri Workshop Group. Multiple gestation pregnancy. The ESHRE Capri Workshop Group. Hum Reprod 2000;15:1856–1864. [PubMed] [Google Scholar]
- Thurin A, Hausken J, Hillensjö T, Jablonowska B, Pinborg A, Strandell A, Bergh C.. Elective single-embryo transfer versus double-embryo transfer in in vitro fertilization. N Engl J Med 2004;351:2392–2402. [DOI] [PubMed] [Google Scholar]
- Vuong LN, Dang VQ, Ho TM, Huynh BG, Ha DT, Pham TD, Nguyen LK, Norman RJ, Mol BW.. IVF transfer of fresh or frozen embryos in women without polycystic ovaries. N Engl J Med 2018;378:137–147. [DOI] [PubMed] [Google Scholar]
- Wei D, Liu JY, Sun Y, Shi Y, Zhang B, Liu JQ, Tan J, Liang X, Cao Y, Wang Z.. et al. Frozen versus fresh single blastocyst transfer in ovulatory women: a multicentre, randomised controlled trial. Lancet 2019;393:1310–1318. [DOI] [PubMed] [Google Scholar]
- Wennerholm UB, Henningsen AK, Romundstad LB, Bergh C, Pinborg A, Skjaerven R, Forman J, Gissler M, Nygren KG, Tiitinen A.. Perinatal outcomes of children born after frozen-thawed embryo transfer: a Nordic cohort study from the CoNARTaS group. Hum Reprod 2013;28:2545–2553. [DOI] [PubMed] [Google Scholar]
- Wilkinson J, Roberts SA, Showell M, Brison DR, Vail A.. No common denominator: a review of outcome measures in IVF RCTs. Hum Reprod 2016;31:2714–2722. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wong KM, van Wely M, Verhoeve HR, Kaaijk EM, Mol F, van der Veen F, Repping S, Mastenbroek S.. Transfer of fresh or frozen embryos: a randomised controlled trial. Hum Reprod 2021; 36:998–1006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zegers-Hochschild F, Adamson GD, Dyer S, Racowsky C, de Mouzon J, Sokol R, Rienzi L, Sunde A, Schmidt L, Cooke I.. et al. The international glossary on infertility and fertility care, 2017. Hum Reprod 2017;32:1786–1801. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
Data cannot be shared publicly directly because use of the data from Sweden was only approved by the ethical committee for this particular use and for this research group. Approval of use for other purposes would be needed from the ethical committee as well as from the National Quality Register of Assisted Reproduction. Contact information is given in the Abstract.