Abstract
Background
Concurrent general psychopathology (GP) and eating disorder psychopathology (EDP) are commonly reported among youth with overweight/obesity and may impact weight change.
Purpose
We identified patterns of GP and EDP in children with overweight/obesity and examined the impact on weight change following family-based behavioral obesity treatment (FBT) and maintenance interventions.
Methods
Children (N = 172) participated in 4 month FBT and subsequent 8 month weight maintenance interventions. GP and EDP were assessed prior to FBT (baseline). Child percentage overweight was assessed at baseline, post-FBT (4 months), and post-maintenance (12 months). Latent profile analysis identified patterns of baseline GP and EDP. Linear mixed-effects models examined if profiles predicted 4- and 12-month change in percentage overweight and if there were two-way and three-way interactions among these variables, adjusting for relevant covariates.
Results
Results indicated a three-profile structure: lower GP and EDP (LOWER); subclinically elevated GP and EDP without loss of control (LOC; HIGHER); and subclinically elevated GP and EDP with LOC (HIGHER + LOC). Across profiles, children on average achieved clinically meaningful weight loss (i.e., ≥9 unit change in percentage overweight) from baseline to 4 month FBT and sustained these improvements at 12 month maintenance. There was no evidence that latent profiles were related to percentage overweight change from baseline to FBT (p > .05) or baseline to maintenance (p > .05). There was no evidence for two-way or three-way interactions (p > .05).
Conclusion
Concurrent GP and EDP do not portend differential short- or long-term weight change following FBT and maintenance. Future research is warranted on the durability of weight change among youth with GP and EDP.
Trial registration
Keywords: Childhood obesity, Treatment, Eating disorder psychopathology, General psychopathology
Regardless of concurrent general psychopathology and eating disorder psychopathology at baseline, treatment-seeking children with obesity demonstrate clinically significant short- and long-term weight change.
Higher general psychopathology (GP; i.e., symptoms of depression and anxiety) [1] and eating disorder psychopathology (EDP; i.e., eating disorder attitudes and behaviors) [2] are commonly reported among youth with overweight/obesity. Although GP and EDP are theoretically distinct constructs, research suggests that they tend to co-occur in youth with overweight/obesity [3–5]. Our prior work in a larger sample from which the current sample was drawn indicated that approximately 40% of children met the cutoff for clinical depressive symptoms and/or anxiety symptoms, and children’s reports of depressive and/or anxiety symptoms were positively associated with their reports of EDP [6].
Studies in youth have shown mixed findings regarding whether either form of psychopathology affects weight loss; while some studies suggest a link between baseline GP or EDP and suboptimal weight loss [7–9], other studies do not [10, 11]. Results from our prior work suggest that regardless of patterns of EDP, children achieved clinically significant short-term weight loss following 4 month family-based behavioral obesity treatment (FBT); however, children with high EDP achieved less weight loss compared to children with low EDP, controlling for baseline weight [7]. No studies have examined the impact of concurrent GP and EDP in relation to short- and long-term weight change. Examination of concurrent psychopathology in relation to weight change is important in light of cross-sectional data suggesting that a constellation of psychological symptoms may compound risk for poor weight-related outcomes, such as greater serum leptin production and components of the metabolic syndrome [12, 13].
To extend prior research that has examined separate, but not concurrent, forms of psychopathology in relation to weight change, the present study sought to identify distinct profiles of children with varying patterns of GP and EDP using latent profile analysis. Latent profiles were then examined in relation to weight change following FBT and weight maintenance interventions. Based on our prior work [6, 7], we hypothesized that children with high levels of concurrent psychopathology would demonstrate less short- and long-term weight change compared to children with low levels of concurrent psychopathology. An exploratory aim was to examine the interactions between profile, maintenance condition, and time on weight change following the maintenance interventions.
Methods
Participants
Two-hundred forty-one children aged 7–11 years with overweight/obesity (body mass index [BMI; kg/m2] ≥85th percentile for age and sex [14]) and at least one parent with overweight/obesity (BMI ≥25 kg/m2) were assessed at baseline. Of the 241 parent–child dyads, 172 participated in a multisite, randomized-controlled trial of weight loss maintenance interventions following 4 months of FBT (Clinical Trials Registration: NCT00759746) [15]. Parents and children were excluded for the following reasons: current participation in another weight management program; use of weight/appetite- altering medications; and presence of medical or psychiatric conditions. The details of the trial, including study participant flow and attrition, are reported elsewhere [15].
Interventions
Phase 1: FBT
All families completed 4 months of FBT, which is regarded as the first-line treatment for childhood obesity due to sustainable improvements in child and parent weight [16]. FBT is a multicomponent intervention that promotes parent and child weight change through addressing dietary intake, physical activity, parenting behaviors, behavior modification, and changes to the home environment [17].
Phase 2: weight maintenance interventions
After completing FBT, parent–child dyads were randomized to a high or low dose of enhanced social facilitation maintenance or to an educational control condition. Social facilitation maintenance is a multiphase intervention designed to help families apply the skills learned in FBT to various socioenvironmental contexts (e.g., school, home, and social settings) [18]. Both maintenance doses had similar content delivered over 30 min family sessions (parent and child dyads) and 45 min parent-only and child-only group sessions. The difference between the two doses was the intensity of the intervention. Low dose of social facilitation maintenance was 16 every-other-week sessions, whereas high dose of social facilitation maintenance was 32 weekly sessions. The group-based education control condition was similar to low-dose social facilitation maintenance in length (75 min/session) and dose (16 every-other-week sessions). Families in the control condition received information on nutrition and exercise that was not previously discussed in FBT.
Assessments
Child weights and heights were assessed at baseline, following FBT (4 months) and following the weight maintenance interventions (12 months). Demographic variables and all other measures were collected at baseline.
Demographics
Parents completed demographic questionnaires at baseline that assessed parent and child race, ethnicity, age, and sex. Parental occupational status and education were assessed using the Barratt Simplified Measure of Social Status [19], a proxy for socioeconomic status (SES). The total score ranges from 8 to 66, with higher numbers indicating more years of education and higher-status occupations.
Body composition
Trained research staff measured weight using a calibrated electronic scale and height using a stadiometer at baseline, 4 months, and 12 months. Child percentage overweight (i.e., the percentage that the child’s BMI was above the median for age and sex) was calculated using CDC 2000 growth charts [14].
Depressive symptoms
Child-reported depressive symptoms were assessed at baseline with the Short Mood and Feelings Questionnaire [20], a 13-item questionnaire that assesses child depressive symptoms in the past 2 weeks. Items are summed to form a total score, with higher scores corresponding to greater depressive symptoms. A total score ≥8 indicates clinically elevated symptoms of depression. The Short Mood and Feelings Questionnaire has demonstrated good internal consistency [20].
Anxiety symptoms
Child-reported anxiety symptoms were assessed at baseline with the Screen for Child Anxiety Related Disorders [21], a 41-item questionnaire that assesses for the presence of anxiety symptoms that map onto diagnostic criteria. Items are summed to form a total score, with higher scores indicative of greater anxiety symptoms. A total score ≥25 indicates clinically elevated symptoms of anxiety. The Screen for Child Anxiety Related Disorders has demonstrated good internal consistency in clinical samples of youth [21].
Loss of control eating episodes
The number of child-reported loss of control (LOC) eating episodes, defined as the subjective inability to stop eating regardless of the amount of food consumed, was assessed at baseline using the child version of the Eating Disorder Examination—Overeating Section [22]. Adapted from the adult Eating Disorder Examination, the child version is a semistructured interview that assesses diagnostic features of eating disorders. A total number of LOC episodes in the past 3 months was calculated among children who reported experiencing at least one episode of overeating (subjective or objective) with the presence of LOC. Children with no LOC eating episodes in the past 3 months were coded as 0. Report of experiencing at least one LOC eating episode is considered clinically meaningful based on prior data that show a prospective association between baseline report of LOC eating and partial- or full-syndrome binge eating disorder [23].
Shape concern and weight concern
Child shape concern and weight concern were assessed at baseline using the child-report Youth Eating Disorder Examination Questionnaire [24]. The Youth Eating Disorder Examination Questionnaire is a 39-item youth version of the adult Eating Disorder Examination Questionnaire; it assesses the frequency of engaging in ED attitudes and behaviors in the past month. Response options were 0 to 6, with higher numbers indicating greater ED attitudes and behaviors. The five-item Shape Concern subscale and the eight-item Weight Concern subscale were included in the current analysis given the high prevalence of ED attitudes in treatment-seeking samples of youth, which suggests that these constructs may be the most important to assess [25]. Prior data in adults indicate that a mean score ≥2.3 on either subscale is considered clinically elevated [26]. The Shape Concern and Weight Concern subscales have excellent internal consistency in samples of youth with overweight/obesity [27].
Statistical Analyses
All analyses were conducted using R (version 3.6.2) [28]. Latent profile analysis was performed using the Mclust library [29]. GP variables (i.e., depressive symptoms and anxiety symptoms) and EDP variables (i.e., LOC eating episodes, shape concern, and weight concern) were screened for normality and were subsequently cube root transformed to reduce positive skewness and rescaled using min–max normalization (i.e., minimum value = 0 and maximum value = 1). Missing data were imputed using maximum likelihood estimation. Under the assumption that scores on the GP and EDP continuous measures are generated from unobserved latent profiles, participants were assigned probabilistically as belonging to the distinct profiles. The number of potential profiles was evaluated iteratively from one to nine profiles using Bayesian Information Criterion (BIC) [30] and Integrated Completed Likelihood (ICL) [31] values to assess model fit. BIC and ICL values in the Mclust library package are inverted; thus, higher BIC and ICL values in the current analyses are indicative of better model fit [29].
After determining the optimal latent profile model, analyses of variance (ANOVAs), for continuous variables, or chi-square analyses, for categorical variables, were conducted to examine profile differences in demographics and baseline GP and EDP, with follow-up Tukey-corrected HSD (for ANOVAs) or Bonferroni-corrected (for chi-square analyses) post hoc tests. A linear mixed-effects model was fit with random intercept and fixed effects of time, profile, maintenance condition, and all two- and three-way interactions involving time, profile, and maintenance condition. Child sex, age, race, ethnicity, household SES, and baseline percentage overweight were also included as fixed effects to adjust for these covariates. Contrasts were conducted to examine within- and between-profile comparisons in change in percentage overweight across time and by maintenance condition. All tests were two tailed. Significance was defined at p < .05 (linear mixed-effects model, Tukey-adjusted ANOVAs) or p < .008 (Bonferroni-adjusted chi-square).
Results
Participant Characteristics
One hundred seventy-two children were randomized to maintenance treatment following FBT and, therefore, were included in the latent profile analysis. Participant demographics are included in Table 1. Of the total sample, approximately 28% of children demonstrated clinically elevated symptoms of depression, 28% of children demonstrated clinically elevated symptoms of anxiety, 30% of children reported at least one LOC eating episode in the past 3 months, and 32% and 39% of children reported clinically elevated shape concerns and weight concerns, respectively. There were (by design) significant profile differences in GP and EDP (Table 1). There were no significant differences in demographics among the profiles after adjusting for multiple comparisons (Table 1).
Table 1.
Baseline differences in demographics, general psychopathology (GP), and eating disorder psychopathology (EDP) by profile
| Variable | Total sample (n = 172) | Lower pathology (n = 35, LOWER) | Higher pathology (n = 84, HIGHER) | Higher pathology + LOC (n = 53, HIGHER+ LOC) | Test statistic (df), p-valuec, effect sized |
|---|---|---|---|---|---|
| Age (years) | 9.40 (1.30) | 9.30 (1.30)a | 9.60 (1.30)a | 9.30 (1.20)a | F(2,169) = 1.63, p = .20, η 2 = .02 |
| Female | 106 (62.4%) | 22 (62.9%)a | 44 (52.4%)a | 40 (75.5%)a | χ 2(2) = 7.35, p = .03, V = .21 |
| Caucasian | 134 (77.9%) | 28 (80.0%)a | 59 (70.2%%)a | 47 (88.7%)a | χ 2(2) = 6.53; p = .04, V = .20 |
| Non-Hispanic | 153 (89.0%) | 31 (88.6%)a | 75 (89.3%)a | 47 (88.7%)a | χ 2(2) = 0.02, p = .99, V = .01 |
| SES | 44 (10.20) | 45.0 (8.10)a | 44.2 (11.10)a | 42.8 (10.20)a | F(2,169) = 0.54 p = .59, η 2 = .01 |
| Baseline percentage overweight | 64.15 (25.21) | 63.0 (22.1)a | 63.1 (24.0)a | 66.5 (29.1)a | F(2,169) = 0.34, p = .71, η 2 = .00 |
| Depressive symptoms | 5.73 (5.34) | 1.26 (1.98)a | 6.65 (4.48)b | 7.23 (6.49)b | F(2,169) = 18.87, p < .001, η 2 = .18 |
| Anxiety symptoms | 19.36 (14.43) | 7.18 (6.67)a | 21.73 (12.98)b | 23.66 (16.10)b | F(2,169) = 19.37, p < .001, η 2 = .19 |
| LOC eating episodes | 4.24 (11.92) | 0.00 (0.00)a | 0.00 (0.00)a | 13.77 (18.26)b | F(2,169) = 33.91, p < .001, η 2 = .29, |
| Shape concern | 1.84 (1.55) | 0.68 (1.10)a | 2.08 (1.41)b | 2.24 (1.67)b | F(2,169) = 14.54, p < .001, η 2 = .15 |
| Weight concern | 2.02 (1.44) | 0.84 (1.15)a | 2.29 (1.32)b | 2.39 (1.40)b | F(2,169) = 17.87, p < .001, η 2 = .18 |
N = 172. Values are mean (standard deviation) or n (%). GP and EDP values are nontransformed and nonnormalized to aid interpretation.
LOC loss of control; SES household socioeconomic status.
a,bFor variables with a significant difference across profiles, superscripts that are not shared among groups indicate significantly different group means.
cSignificance defined as p < .05 (Tukey-adjusted analyses of variance [ANOVAs]) or p < .008 (Bonferroni-adjusted chi-square).
dEffect sizes are η 2 (ANOVAs) or Cramer’s V (chi-square).
LPA Model Identification
BIC and ICL values across one to nine profiles were compared for model fit. Both BIC and ICL criteria selected a VEV Gaussian model (i.e., a model with covariances that were equal in shape, variable in volume, and ellipsoidal in distribution) with three profiles (BIC = 785.57 and ICL = 775.77). As a sensitivity analysis, we fit the three-group profile structure with VEV variance to the complete baseline sample (N = 241). The resulting group profiles largely resembled those presented herein and, furthermore, group membership did not predict FBT completion, X2(2, N = 241) = 0.56, p = .76. Profile 1 (LOWER) comprised 20.3% of the sample (n = 35) and were characterized by lower levels on all forms of GP and EDP relative to the other profiles (i.e., lower depressive and anxiety symptoms, lower shape and weight concern, and no LOC eating episodes). Profile 2 (HIGHER) comprised 48.8% of the sample (n = 84) and demonstrated subclinically elevated GP (i.e., higher depressive and anxiety symptoms) and subclinically elevated ED attitudes (i.e., higher shape concern and weight concern) without LOC eating episodes. Profile 3 (HIGHER + LOC) comprised 30.8% of the sample (n = 53) and were subclinically elevated on all forms of GP and EDP plus LOC eating episodes. Posterior probabilities of belonging to Profile 1 (LOWER; .97), Profile 2 (HIGHER; .97), and Profile 3 (HIGHER + LOC; .99) were excellent. Overall discrimination among profiles was excellent (i.e., relative entropy = .93, where values approaching 1 indicate clear profile discrimination).
Relation of Latent Profiles With Short- and Long-Term Weight Change
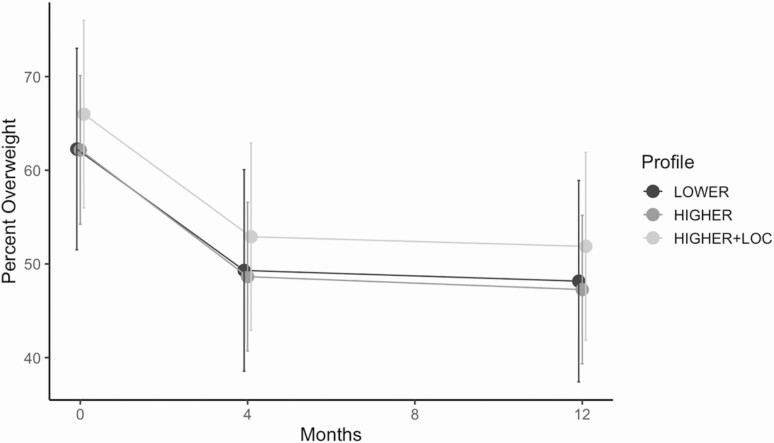
After adjusting for relevant covariates, there was no evidence that latent profiles were related to percentage overweight change from baseline to FBT or baseline to maintenance; furthermore, there was no evidence for an interaction between profile and maintenance condition or three-way interaction between profile, maintenance condition, and time (Table 2). Across profiles, children on average achieved clinically meaningful weight loss (i.e., ≥9 unit change in percentage overweight [15]) from baseline to 4 month FBT and sustained these improvements at 12 month maintenance. Figure 1 depicts the change in percentage overweight by profile over the course of the study period, collapsed across maintenance condition and at average levels of the covariates.
Table 2.
Summary of adjusted mixed-effects model examining baseline latent profiles as predictors of change in percentage overweight
| Fixed-effects term | Test statistic (df), p-valuea, effect sizeb |
|---|---|
| Time | F(2,326) = 162.24, p < .001, η 2 = .47 |
| Profile | F(2,158) = 0.41, p = .66, η 2 = .001 |
| Maintenance condition | F(2,158) = 0.07, p = .93, η 2 = .00 |
| Age | F(1,158) = 1.04, p = .31, η 2 = .001 |
| Sex | F(1,158) = 1.19, p = .28, η 2 = .002 |
| Race | F(1,158) = 2.03, p = .16, η 2 = .003 |
| Ethnicity | F(1,158) = 1.22, p = .27, η 2 = .002 |
| SES | F(1,158) = 9.10, p = .002, η 2 = .01 |
| Two-way interactions | |
| Time by profile | F(4,326) = 0.06, p = .99, η 2 = .00 |
| Time by maintenance condition | F(4,326) = 4.29, p = .002, η 2 = .03 |
| Profile by maintenance condition | F(4,158) = 0.75, p = .56, η 2 = .004 |
| Three-way interactions | |
| Time by profile by maintenance condition | F(8,326) = 0.55, p = .82, η 2 = .01 |
df degrees of freedom; SES socioeconomic status.
aSignificance defined as p < .05 (Tukey-adjusted analyses of variance).
bEffect sizes are η 2.
Figure 1.
Change in percentage overweight by profile over the course of the intervention period. Change in percentage overweight over the intervention period is collapsed across maintenance condition and at average levels of covariates. Error bars are 95% confidence intervals.
Discussion
This study identified a three-profile structure of concurrent GP and EDP in treatment-seeking children with overweight/obesity: lower GP and EDP (LOWER); subclinically elevated GP and EDP without LOC (HIGHER); and subclinically elevated GP and EDP with LOC (HIGHER + LOC). We did not observe differences in initial weight loss or subsequent weight maintenance across the three profiles, nor did profile membership alter the effects of the treatment conditions on weight maintenance. Our findings extend prior work by examining how patterns of concurrent GP and EDP affect short- and long-term weight change. In line with some studies [10, 11], but in contrast to others [7, 9], children achieved clinically meaningful weight loss regardless of baseline subclinical psychopathology.
Mixed findings on the association between baseline psychopathology and weight change may be due to the varying ways in which pediatric weight management programs’ curriculum does or does not address GP and EDP. Although FBT and maintenance are not standalone mental health treatments, both interventions address risk factors for internalizing symptoms (e.g., lack of parental support) and ED symptoms (e.g., unhealthy restraint) at the parent and child level, thereby increasing the opportunity to mitigate risk for the development and maintenance of child internalizing disorders and eating disorders. Thus, the nonsignificant association between baseline psychopathology and weight change in the current study could be a result of improvements in GP and EDP over the course of treatment and maintenance. Indeed, meta-analytic findings indicate that participation in professionally delivered obesity interventions with a healthy dietary component is associated with reductions in symptoms of anxiety and depression and ED prevalence, risk, and symptoms [32, 33]. Prospective data are needed to examine whether GP and EDP change over the course of FBT and maintenance and examine the durability of these improvements. Furthermore, future research should elucidate underlying mechanisms that explain the association between baseline GP and EDP and weight change.
Mixed findings may also arise due to differences in sample characteristics. Less than half of children endorsed either clinically elevated symptoms of GP or EDP at baseline. Future research should examine whether short- and long-term weight change varies among children with threshold versus subthreshold symptoms of GP and EDP. Additionally, replication of these profiles in other treatment-seeking samples of youth with greater severity of psychopathology could inform whether augmenting FBT or maintenance interventions to specifically focus on decreasing GP or EDP risk factors has the potential to improve weight status.
The current study had several strengths. To our knowledge, this study is the first to use latent profile analysis to examine the co-occurrence of multiple forms of psychopathology in relation to short- and long-term weight change among treatment-seeking children with overweight/obesity. Given the exploratory nature of latent profile analysis, future research should replicate these profiles in other treatment-seeking samples of youth, particularly among youth with more severe psychopathology. The present sample was not recruited for the presence of a psychiatric diagnosis, nor were the measures selected for their ability to diagnose psychiatric illnesses. Thus, our findings may not generalize to other treatment-seeking children with full-syndromal symptoms. Nevertheless, our results demonstrate that concurrent GP and EDP symptoms do not portend differential short- or long-term weight change. Prospective data are needed to examine the durability of weight change among youth with GP and EDP.
Acknowledgments
Funding: This research was funded by the Eunice Kennedy Shriver National Institute of Child Health and Human Development (R01HD036904) and the National Heart, Lung, and Blood Institute (T32HL130357).
Compliance with Ethical Standards
Authors’ Statement of Conflict of Interest and Adherence to Ethical Standards The authors have no conflicts of interest to report.
Authors’ Contributions: Conceptualization: A.C.G., J.R.B., and D.E.W.; methodology: R.I.S., B.E.S., R.R.W., M.G.P., L.H.E., and D.E.W.; data collection: J.R.B., R.I.S., R.P.K.C., R.R.W., and D.E.W.; formal data analysis: A.C.G. and J.R.B.; writing—original draft preparation: A.C.G.; writing—review and editing: A.C.G., J.R.B., L.A.F., K.N.B., R.I.S., R.P.K.C., B.E.S., and D.E.W.; Funding: D.E.W., R.I.S., and B.E.S.
Ethical Approval: All procedures performed in studies involving human participants were in accordance with the ethical standards of Washington University School of Medicine, Seattle Children’s Research Institute, and with the 1964 Helsinki declaration and its later amendments or comparable ethical standards.
Informed Consent: Informed consent was obtained from all individual participants included in the study.
References
- 1.Quek YH, Tam WWS, Zhang MWB, Ho RCM. Exploring the association between childhood and adolescent obesity and depression: A meta-analysis. Obes Rev. 2017;18:742–754. [DOI] [PubMed] [Google Scholar]
- 2.Hayes JF, Fitzsimmons-Craft EE, Karam AM, Jakubiak J, Brown ML, Wilfley DE. Disordered eating attitudes and behaviors in youth with overweight and obesity: Implications for treatment. Curr Obes Rep. 2018;7:235–246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Tanofsky-Kraff M, Yanovski SZ, Wilfley DE, Marmarosh C, Morgan CM, Yanovski JA. Eating-disordered behaviors, body fat, and psychopathology in overweight and normal-weight children. J Consult Clin Psychol. 2004;72:53–61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Eddy KT, Tanofsky-Kraff M, Thompson-Brenner H, Herzog DB, Brown TA, Ludwig DS. Eating disorder pathology among overweight treatment-seeking youth: Clinical correlates and cross-sectional risk modeling. Behav Res Ther. 2007;45:2360–2371. [DOI] [PubMed] [Google Scholar]
- 5.Glasofer DR, Tanofsky-Kraff M, Eddy KT, et al. . Binge eating in overweight treatment-seeking adolescents. J Pediatr Psychol. 2007;32:95–105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Sheinbein DH, Stein RI, Hayes JF, et al. . Factors associated with depression and anxiety symptoms among children seeking treatment for obesity: A social-ecological approach. Pediatr Obes. 2019;14:e12518. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Balantekin KN, Hayes JF, Sheinbein DH, et al. . Patterns of eating disorder pathology are associated with weight change in family-based behavioral obesity treatment. Obesity (Silver Spring). 2017;25:2115–2122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Conlon RPK, Hurst KT, Hayes JF, et al. . Child and parent reports of children’s depressive symptoms in relation to children’s weight loss response in family-based obesity treatment. Pediatr Obes. 2019;14:e12511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Wildes JE, Marcus MD, Kalarchian MA, Levine MD, Houck PR, Cheng Y. Self-reported binge eating in severe pediatric obesity: Impact on weight change in a randomized controlled trial of family-based treatment. Int J Obes (Lond). 2010;34:1143–1148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Levine MD, Ringham RM, Kalarchian MA, Wisniewski L, Marcus MD. Overeating among seriously overweight children seeking treatment: Results of the children’s eating disorder examination. Int J Eat Disord. 2006;39:135–140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Goossens L, Braet C, Van Vlierberghe L, Mels S. Weight parameters and pathological eating as predictors of obesity treatment outcome in children and adolescents. Eat Behav. 2009;10:71–73. [DOI] [PubMed] [Google Scholar]
- 12.Byrne ME, Tanofsky-Kraff M, Jaramillo M, et al. . Relationships of trait anxiety and loss of control eating with serum leptin concentrations among youth. Nutrients. 2019;11(9): 2198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Byrne ME, Tanofsky-Kraff M, Kelly NM, et al. . Pediatric loss-of-control eating and anxiety in relation to components of metabolic syndrome. J Pediatr Psychol. 2019;44:220–228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Kuczmarski RJ, Ogden CL, Guo SS, et al. . 2000 CDC growth charts for the United States: Methods and development. Vital Health Stat. 2002;11(246):1–190. [PubMed] [Google Scholar]
- 15.Wilfley DE, Saelens BE, Stein RI, et al. . Dose, content, and mediators of family-based treatment for childhood obesity: A multisite randomized clinical trial. JAMA Pediatr. 2017;171:1151–1159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Epstein LH, Paluch RA, Roemmich JN, Beecher MD. Family-based obesity treatment, then and now: Twenty-five years of pediatric obesity treatment. Health Psychol. 2007;26:381–391. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Coppock JH, Ridolfi DR, Hayes JF, St Paul M, Wilfley DE. Current approaches to the management of pediatric overweight and obesity. Curr Treat Options Cardiovasc Med. 2014;16:343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Wilfley DE, Stein RI, Saelens BE, et al. . Efficacy of maintenance treatment approaches for childhood overweight: A randomized controlled trial. JAMA. 2007;298:1661–1673. [DOI] [PubMed] [Google Scholar]
- 19.Barratt W. The Barratt simplified measure of social status (BSMSS). Available at http://socialclassoncampus.blogspot.com/2012/06/barratt-simplified-measure-of-social.html. Accessibility verified January 24, 2020.
- 20.Angold A, Costello EJ, Messer SC, Pickles A. Development of a short questionnaire for use in epidemiological studies of depression in children and adolescents. Int J Methods Psychiatr Res. 1995;5(4):237–249. [Google Scholar]
- 21.Birmaher B, Brent DA, Chiappetta L, Bridge J, Monga S, Baugher M. Psychometric properties of the Screen for Child Anxiety Related Emotional Disorders (SCARED): A replication study. J Am Acad Child Adolesc Psychiatry. 1999;38:1230–1236. [DOI] [PubMed] [Google Scholar]
- 22.Bryant-Waugh RJ, Cooper PJ, Taylor CL, Lask BD. The use of the Eating Disorder Examination with children: A pilot study. Int J Eat Disord. 1996;19:391–397. [DOI] [PubMed] [Google Scholar]
- 23.Tanofsky-Kraff M, Shomaker LB, Olsen C, et al. . A prospective study of pediatric loss of control eating and psychological outcomes. J Abnorm Psychol. 2011;120:108–118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Goldschmidt AB, Doyle AC, Wilfley DE. Assessment of binge eating in overweight youth using a questionnaire version of the Child Eating Disorder Examination with Instructions. Int J Eat Disord. 2007;40:460–467. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Doyle AC, le Grange D, Goldschmidt A, Wilfley DE. Psychosocial and physical impairment in overweight adolescents at high risk for eating disorders. Obesity (Silver Spring). 2007;15:145–154. [DOI] [PubMed] [Google Scholar]
- 26.Mond JM, Hay PJ, Rodgers B, Owen C, Beumont PJ. Validity of the Eating Disorder Examination Questionnaire (EDE-Q) in screening for eating disorders in community samples. Behav Res Ther. 2004;42:551–567. [DOI] [PubMed] [Google Scholar]
- 27.Kass AE, Theim Hurst K, Kolko RP, et al. . Psychometric evaluation of the Youth Eating Disorder Examination Questionnaire in children with overweight or obesity. Int J Eat Disord. 2017;50:776–780. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.R Core Team. R: A language and environment for statistical computing. Available at https://www.R-project.org. Accessibility verified May 2, 2020.
- 29.Scrucca L, Fop M, Murphy TB, Raftery AE. Mclust 5: Clustering, classification, and density estimation using gaussian finite mixture models. RJ. 2016;81:289–317. [PMC free article] [PubMed] [Google Scholar]
- 30.Schwarz G. Estimating the dimension of a model. Ann Stat. 1978;6(2):461–464. [Google Scholar]
- 31.Biernacki C, Celeux G, Govaert G. Assessing a mixture model for clustering with the integrated completed likelihood. INRIA. 2000;22(7):719–725. [Google Scholar]
- 32.Jebeile H, Gow ML, Baur LA, Garnett SP, Paxton SJ, Lister NB. Association of pediatric obesity treatment, including a dietary component, with change in depression and anxiety: A systematic review and meta-analysis. JAMA Pediatr. 2019:e192841. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Jebeile H, Gow ML, Baur LA, Garnett SP, Paxton SJ, Lister NB. Treatment of obesity, with a dietary component, and eating disorder risk in children and adolescents: A systematic review with meta-analysis. Obes Rev. 2019;20:1287–1298. [DOI] [PMC free article] [PubMed] [Google Scholar]