Abstract
Background
Over the life course, African American (AA) women have faster telomere attrition, a biological indicator of accelerated aging, than White women. Race, sex, age, and composite socioeconomic status (SES) modify associations of institutional racial discrimination and telomere length. However, interactions with everyday racial discrimination have not been detected in AA women, nor have interactions with individual socioeconomic predictors.
Purpose
We estimated statistical interaction of institutional and everyday racial discrimination with age, education, employment, poverty, and composite SES on telomere length among midlife AA women.
Methods
Data are from a cross-section of 140 AA women aged 30–50 years residing in the San Francisco Bay Area. Participants completed questionnaires, computer-assisted self-interviews, physical examinations, and blood draws. Adjusted linear regression estimated bootstrapped racial discrimination–relative telomere length associations with interaction terms.
Results
Racial discrimination did not interact with age, poverty, or composite SES measures to modify associations with telomere length. Interactions between independent SES variables were nonsignificant for everyday discrimination whereas institutional discrimination interacted with educational attainment and employment status to modify telomere length. After adjusting for covariates, we found that higher institutional discrimination was associated with shorter telomeres among employed women with lower education (β = −0.020; 95% confidence interval = −0.036, −0.003). Among unemployed women with higher education, higher institutional discrimination was associated with longer telomeres (β = 0.017; 95% confidence interval = 0.003, 0.032). Factors related to having a post-high school education may be protective against the negative effects of institutional racism on cellular aging for AA women.
Keywords: African Americans, Aging, Educational status, Employment, Telomeres, Women’s Health
For African American women with a post high school education who report experiences of institutional racism, telomeres were shorter for those in the workforce and longer for those who were not.
Introduction
African Americans (AAs) have a higher risk of aging-related illnesses in early life, worse disease sequelae in late life, and higher mortality than their White counterparts [1]. Compared with Whites, AAs experience higher mortality rates across all stages of the life course from numerous leading causes of death such as heart disease, cancer, vascular disease, and diabetes [1]. Furthermore, risk factors for development of chronic disease before age 50 are more prevalent in AAs including physical inactivity, obesity, and hypertension [1]. Consequently, life expectancy at birth for Whites is 3.5 years longer than AAs (78.8 and 75.3, respectively) [2].
Such adverse health outcomes have been partially linked to accelerated aging—when physiologic deterioration (i.e., biological aging) at a given chronological age is faster than the expected pace of aging [3]. Biological aging is commonly measured by telomere length. Telomeres are the protective end caps on chromosomes made up of a repeating sequence of six nucleotides that shorten naturally as humans age. As cells divide, telomeres lose nucleotides (30–120 base pairs per cycle) [4]. When telomeres run critically short of these protective nucleotides, cells enter senescence and no longer divide, resulting in a loss of functionality that can ultimately lead to cell death and other pathogenic states [5]. This is usually when physical signs of aging begin. Indeed, a variety of biomarkers indicative of cellular senescence have been shown to accumulate in the tissues of aging individuals [5].
Studies show that AAs have longer telomeres than Whites at birth, but experience a faster rate of telomere attrition over the life course [6, 7]. Telomere attrition is associated with cancer, diabetes, inflammatory diseases, cardiovascular disease, and mortality [8]. Shorter telomeres and faster attrition are linked to numerous socioeconomic and behavioral risk factors (e.g., low educational attainment, lack of physical exercise) and psychological stress [9, 10]. In addition, AA women, in particular, are disproportionately burdened with over twice the levels of allostatic load—a predictor of cumulative physiologic dysregulation resulting from chronic adaptation to stress—compared with AA men at ages 55–64 and White women ages 35–64 [11]. By midlife (ages 49–55), AA women have shown shorter telomeres than White women (i.e., 7.5 biological years older) [12] and have faster telomere attrition than White and Hispanic women [7]. Hence, racial disparities in telomere shortening, or biological weathering, could result in part from exposure to lifetime race- and gender-related stressors [11, 12].
AA women report racial discrimination as a prominent psychosocial stressor [13, 14], which may explain racial disparities in accelerated telomere shortening among this group. Chronic stressors can provoke inflammation and oxidative stress—an imbalance of a cell’s normal redox state—which contributes to telomere erosion and accelerated aging [15, 16]. Racial discrimination—when members of a racial/ethnic group are treated unfairly based on their race/ethnicity—has been linked to oxidative stress, greater epigenetic aging, and shorter telomeres among AAs, but the association between racial discrimination and telomere length are scarce among AA women [17–23]. Further, interactions of various forms of discrimination with age and sex have predicted shorter telomeres. No association with day-to-day racial discrimination was detected [20, 21]. However, previous data show disparate effects of different types of racial discrimination on biological dysregulation [3]. Since chronic everyday racial discrimination has been linked to increased cardiovascular risk and depression in AA women [24], it is plausible that everyday discrimination may contribute to shortening of telomeres in this group.
Research also suggests that educational attainment, poverty, and employment status may be important independent socioeconomic predictors of telomere length among AA women. Various studies show that higher educational attainment, particularly post-high school (HS), predicts longer telomeres [25, 26], and significant interactions with race show additional protective effects for AAs [26]. Poverty is a chronic stressor that is consistently associated with shorter telomeres [9]. However, the role of employment status on telomere length is less clear, and the variation is likely due to multiple factors. For example, mixed findings may reflect different stressors related to needing and not needing to work. It could also reflect differences in how employment status is measured. Moreover, studies examining interactions with racial discrimination routinely use composite socioeconomic status (SES) measures (e.g., education and income) [20, 21]. If independent SES measures influence telomere attrition in different magnitudes and directions, then composite SES measures could conflate and mask individual predictors that may help inform more targeted interventions. No studies have yet examined the interaction of racial discrimination with educational attainment, poverty status, or employment status as an independent or composite socioeconomic predictors of telomere length.
To address this gap, we estimated statistical interaction of institutional and everyday racial discrimination with age, educational attainment, employment status, and poverty on telomere length, and then compared a composite SES measure among midlife AA women. We hypothesized that (a) being older predicts inverse associations given that telomeres shorten with age, (b) post-HS education predicts longer telomeres since higher education is generally protective, (c) being employed predicts shorter telomeres because AA women report workplace racial discrimination as a considerable source of stress [27], (d) poverty predicts shorter telomeres since economic disadvantage is a chronic stressor, and (e) composite SES measures estimate weaker associations than independent measures by conflating different SES associations.
Materials and Methods
Study Sample
Data are from the African American Women’s Heart & Health Study (AAWHHS), a cross-sectional study designed to investigate associations between social–environmental stress and mental and physical health among a community sample of 208 AA women aged 30–50 years. AAWHHS sample recruitment and study participation has been described in detail elsewhere [27]. In short, to maximize variability across key exposures of interest (e.g., racial discrimination, socioeconomic indicators), purposive sampling using multiple recruitment strategies was employed to recruit women from five San Francisco Bay Area counties in 2013. Participants completed computer-assisted self-interviews, interviewer-administered surveys, physical examinations, and blood draws. Incentives included health-promoting materials and a cash gift card. The Committee for the Protection of Human Subjects at the University of California Berkeley provided study approval.
Exposure Variables
Everyday racial discrimination
We used a modified version of the Everyday Discrimination Scale asking respondents how often they experienced day-to-day unfair treatment based on their race, ethnicity, or skin color in 10 everyday life situations (e.g., followed around in stores, treated with less respect than others; α = .95) [28]. Responses were scored using a six-point Likert scale ranging from “never” to “almost every day.” Scores were summed across the 10 items to create a summary score (10–60) with higher scores representing greater experiences of everyday racial discrimination. Everyday racial discrimination was assessed as a continuous variable.
Institutional racial discrimination
The Experiences of Discrimination scale asked respondents if they have ever been unfairly treated, judged, limited, hassled, or made to feel inferior based on their race, ethnicity, or skin color in eight domains (e.g., by police/courts, getting hired; α = .92) [29]. Responses were measured on a five-point Likert scale ranging from “never” to “6 or more times.” A summary score was generated by summing the eight survey items (8–40) with higher scores reflecting more institutional experiences. Institutional racial discrimination was evaluated continuously.
Outcome Variable
DNA extraction and measurement
Genomic DNA (gDNA) was extracted from buffy coats using QiaAmp DNA blood mini kit (Qiagen) according to manufacturer recommendations. The concentration of extracted gDNA was measured by absorbance at 260 nm, and the A260/280 was used to determine its purity using NanoDrop 2000 Spectrophotometer (Thermo Scientific). Only gDNA samples having 260/280 ratios close to 1.8 were used for quantitative polymerase real-time chain reaction (qPCR). All gDNA samples were stored at −20°C for long-term storage.
Relative telomere length measurement
qPCR was used to assess relative telomere length (RTL) for each participant using previously implemented protocols adapted for this study [30–32]. For qPCR assays 1x Power and Fast SYBR Green Master Mixes, primers (sequence available upon request) [31], and 20 ng of DNA per reaction well were used. A standard curve was created using threefold serial dilutions of standardized reference DNA ranging from 81 to 1 ng [31, 32]. Genomic DNAserved as template, and the amount of telomere repeats (T) and the hemoglobin gene (S) that exists as a single copy across all chromosomes was measured. Analysis of melting curves was used to verify specificity of products. The RTL was determined as the ratio of telomere repeat to single-gene products (T/S) [30].
Covariates
Theoretical and empirically based covariates expected to confound the association between racial discrimination and telomere length were identified using a directed acyclic graph (DAG; Supplementary Fig. S1) [6, 9, 17, 22, 33]. Age, SES, marital status, and perceived stress were identified as confounders. Identified mediators (e.g., behaviors) were excluded from the analysis to avoid bias (i.e., over-controlling). All covariates were coded such that higher values represent higher risk. The Perceived Stress Scale asked respondents how often they felt various stress-related feelings or thoughts over the past month across 10 items (e.g., felt nervous or stressed, unable to control important things in your life) using a five-point Likert scale ranging from “never” to “very often,” and a summary score was generated (10–50; α = 0.88) [34]. Age and perceived stress were assessed continuously, while data restrictions required dichotomous variables (0/1) for the remaining covariates; marital/partnership status (1 = unmarried/not partnered), educational attainment (1 = ≤HS diploma), employment status (1 = unemployed), and poverty status (1 = ≤100% federal poverty threshold). A composite SES variable comprised of all three SES predictors was generated (range 0–3) and assessed continuously.
Statistical Analysis
All statistical analyses were conducted using STATA IC 16 (College Station, TX). Among the 208 respondents, blood was collected from 140 women that consented to DNA analysis. Bivariate analyses (e.g., χ 2) assessed statistical differences between all variables and differences in sample characteristics between DNA consenting groups. Confounders were selected a priori using our DAG. Adjusted linear regression was used to estimate associations of racial discrimination and RTL with interaction terms using bootstrapped standard errors (1,000 repetitions). Nonlinearity of the main associations was assessed using cubic splines and quadratic discrimination measures. Wald tests evaluated the presence of interactions. Postestimation analyses assessed the presence of outliers and point estimates for interaction coefficients. Sensitivity analyses evaluated the influence of adding identified mediators to our final models. Only significant interactions are interpreted if present (p < .05).
Results
Sample Characteristics
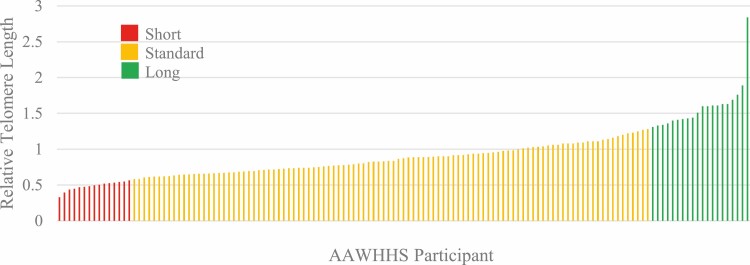
Sample distributions of participant characteristics and RTL are presented in Table 1 and Fig. 1, respectively (N = 140). Precisely 75% of participant RTL fell within one standard deviation of the mean and were considered to be in the “standard” range, 11% were below the standard range (i.e., “short”), and 14% were above the standard range (i.e., “long”).
Table 1.
AAWHHS study sample characteristics (N = 140)
| n | % | |
|---|---|---|
| Everyday discrimination (EDS)a | ||
| (range 10–60), mean (SD) | 28.69 (13.14) | |
| Experiences of discrimination (EOD)a | ||
| (range 8–40), mean (SD) | 18.39 (8.55) | |
| Age | ||
| (range 31–51), mean (SD) | 41.58 (5.88) | |
| Educational attainment | ||
| >High school diploma | 93 | 66.43 |
| ≤High school diploma | 47 | 33.57 |
| Employment status | ||
| Employed | 74 | 52.86 |
| Not employed | 66 | 47.14 |
| Poverty status | ||
| >100% federal household threshold | 112 | 80.00 |
| ≤100% federal household threshold | 28 | 20.00 |
| Socioeconomic status | ||
| (range 0–3), mean (SD) | 1.00 (0.93) | |
| Marital/partnership status | ||
| Married/partnered | 41 | 29.29 |
| Unmarried/not partnered | 99 | 70.71 |
| Perceived stress | ||
| (range 10–50), mean (SD) | 30.10 (7.27) | |
| Relative telomere length | ||
| (range 0.3–2.8), mean (SD) | 0.93 (0.36) |
AAWHHS African American Women’s Heart & Health Study; EDS Everyday Discrimination Scale; EOD Experiences of Discrimination; SD standard deviation.
aN = 138.
Fig. 1.
Relative telomere length (RTL) distribution among African American Women’s Heart & Health Study (AAWHHS) participants (N = 140). Participant RTL was considered “standard” if within one standard deviation of the mean, “short” if below one standard deviation of the mean, “long” if above one standard deviation of the mean.
Bivariate Analyses
Significant bivariate associations are reported at p < .05. There were no differences in RTL by everyday and institutional racial discrimination, or covariates (Supplementary Table S1). Everyday and institutional experiences were highly but not perfectly correlated (r = .76), institutional racial discrimination was weakly correlated with age (r = .17) and perceived stress (r = .28), everyday racial discrimination was weakly correlated with marital/partnership status (r = .20) and perceived stress (r = .28), and educational attainment and employment status were moderately correlated (r = .33; Supplementary Table S2), and were the only categorical covariates that were associated using cross tabulation. Apart from racial discrimination, there were no differences in the distribution of sample characteristics between DNA consenting groups (Supplementary Table S3). We later discuss the potential implications of consenting group variation in self-reported discrimination.
Multivariable Linear Regression
Linear trends between the main associations were detected using cubic splines and quadratic discrimination terms, hence discrimination measures were evaluated continuously. Significant interactions were detected in institutional but not everyday racial discrimination models (p < .05). Postestimation analyses identified four observations as extreme outliers (beyond 3 standard deviations of the mean), which were consequently dropped from all models. Final analyses were conducted on observations for which there were complete data, resulting in an analytic sample of 134 women.
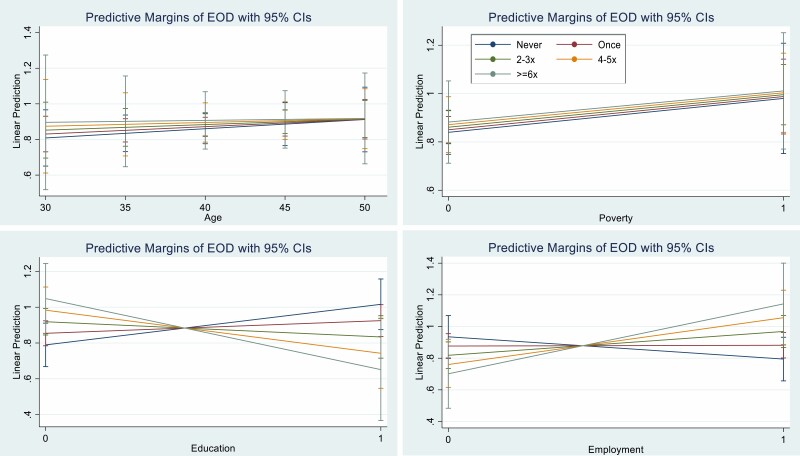
Tables 2 and 3 show everyday and institutional racial discrimination–interaction estimates, respectively. No racial discrimination interactions were found in models using the composite SES measures. Everyday racial discrimination did not interact with any independent SES measures. Significant interactions were found for institutional racial discrimination with educational attainment and employment status whereas age and poverty interactions were nonsignificant. Interaction plots illustrate statistical interaction of institutional racial discrimination with education and employment (Fig. 2). Our sensitivity analyses showed that adding mediators to our models did not meaningfully change study estimates or statistical precision, lending support for our decision to exclude them from the analysis (Supplementary Tables S4 and S5).
Table 2.
Linear regression of everyday racial discrimination on relative telomere length in AAWHHS, including interactions (N = 134)
| aModel A | bModel B (SES) | |||||
|---|---|---|---|---|---|---|
| β | LB | UB | β | LB | UB | |
| 95% CI | 95% CI | 95% CI | 95% CI | |||
| EDS × age | 0.000 | −0.001 | 0.001 | 0.000 | 0.000 | 0.001 |
| EDS × ≤HS diploma | −0.008 | −0.018 | 0.002 | |||
| EDS × not employed | 0.006 | −0.005 | 0.016 | |||
| EDS × poverty | 0.000 | −0.009 | 0.008 | |||
| EDS × SES | −0.001 | −0.005 | 0.003 | |||
| EDS | −0.001 | −0.025 | 0.024 | −0.005 | −0.029 | 0.018 |
| Age | 0.004 | −0.017 | 0.024 | 0.000 | −0.019 | 0.019 |
| ≤HS diploma | 0.262 | −0.050 | 0.573 | |||
| Not employed | −0.109 | −0.416 | 0.197 | |||
| Poverty | 0.165 | −0.149 | 0.479 | |||
| SES | 0.096 | −0.039 | 0.231 | |||
| Marital status | 0.043 | −0.067 | 0.152 | 0.055 | −0.054 | 0.164 |
| Perceived stress | −0.003 | −0.011 | 0.004 | −0.003 | −0.010 | 0.004 |
| Intercept | 0.720 | 0.841 | ||||
Note: Estimates correspond to high-risk groups. AAWHHS African American Women’s Heart & Health Study; CI confidence interval; EDS Everyday Discrimination Scale; HS high school; LB lower bound; SES socioeconomic status; UB upper bound.
aIntercept: 95% CI = −0.135, 1.58.
b Intercept: 95% CI = 0.017, 1.67.
Table 3.
Linear regression of institutional racial discrimination on relative telomere length in AAWHHS, including interactions (N = 134)
| aModel A | bModel B (SES) | |||||
|---|---|---|---|---|---|---|
| β | LB | UB | β | LB | UB | |
| 95% CI | 95% CI | 95% CI | 95% CI | |||
| EOD × age | 0.000 | −0.001 | 0.001 | 0.000 | −0.001 | 0.001 |
| EOD × ≤HS diploma | *−0.019 | −0.036 | −0.003 | |||
| EOD × not employed | *0.018 | 0.002 | 0.035 | |||
| EOD × poverty | 0.000 | −0.014 | 0.014 | |||
| EOD × SES | 0.000 | −0.007 | 0.007 | |||
| EOD | 0.005 | −0.042 | 0.052 | 0.002 | −0.043 | 0.048 |
| Age | 0.006 | −0.017 | 0.029 | 0.004 | −0.018 | 0.027 |
| ≤HS diploma | *0.383 | 0.077 | 0.688 | |||
| Not employed | −0.286 | −0.608 | 0.036 | |||
| Poverty | 0.143 | −0.186 | 0.473 | |||
| SES | 0.061 | −0.090 | 0.212 | |||
| Marital status | 0.047 | −0.061 | 0.154 | 0.050 | −0.059 | 0.160 |
| Perceived stress | −0.004 | −0.011 | 0.003 | −0.003 | −0.010 | 0.003 |
| Intercept | 0.656 | 0.687 | ||||
Note: Estimates correspond to high-risk groups. AAWHHS African American Women’s Heart & Health Study; CI confidence interval; EOD Experiences of Discrimination; HS high school; LB lower bound; SES socioeconomic status; UB upper bound.
aIntercept: 95% CI = −0.315, 1.63.
bIntercept: 95% CI = −0.241, 1.62.
*p < .05.
Fig. 2.
Interaction plots of the adjusted estimates for relative telomere length for institutional racial discrimination (EOD) and age, educational attainment, employment status, and poverty among African American Women’s Heart & Health study participants (N = 134). The 5-level ordinal EOD categories represent increasing lifetime exposure to institutional racial discrimination; never (<9), once (9–16), 2–3 times (17–24), 4–5 times (25–32), and 6 or more times (33–40). Nonparallel lines provide evidence of statistical interaction.
Given that age and poverty were nonsignificant in our final institutional racial discrimination model, we applied the change in mean-squared error (MSE) approach to assess whether a model without age and poverty interactions would improve model fit [35]. The change in MSE was negative, therefore excluding both nonsignificant interactions decreased the MSE. Put another way, removing age and poverty interactions increased model precision without introducing bias. Hence, our final estimates are reported with education and employment interactions only (Supplementary Table S6).
Table 4 shows the point estimates for associations of institutional racial discrimination and RTL at the remaining four levels, adjusted for covariates. We found no association between institutional experiences and RTL among employed women with higher education or among unemployed women with lower education. Among employed women with lower education, a one-unit increase in institutional racial discrimination was associated with shorter RTL (β = −0.020; 95% confidence interval = −0.036, −0.003). Among unemployed women with higher education, a one-unit increase in institutional racial discrimination was associated with longer RTL (β = 0.017; 95% confidence interval = 0.003, 0.032).
Table 4.
Associations of institutional racial discrimination with telomere length within strata of education and employment in AAWHHS (N = 134)
| β | LB | UB | |
|---|---|---|---|
| 95% CI | 95% CI | ||
| High education and employed | 0.000 | −0.008 | 0.008 |
| Low education and employed | *−0.020 | −0.036 | −0.004 |
| High education and unemployed | *0.017 | 0.003 | 0.032 |
| Low education and unemployed | −0.002 | −0.012 | 0.008 |
Note: Estimates are adjusted for covariates. AAWHHS African American Women’s Heart & Health Study; CI confidence interval; LB lower bound; UB upper bound.
*p < .05.
Discussion
Summary of Findings
We found statistical interaction of institutional racial discrimination with educational attainment and employment status associated with cellular aging in a community sample of middle-aged AA women. Racial discrimination did not interact with age, poverty, or composite SES measures to modify associations with telomere length. Interactions between independent SES variables were nonsignificant for everyday discrimination whereas institutional discrimination interacted with educational attainment and employment status to modify telomere length. Among employed women with lower education, increased racial discrimination was associated with shorter telomere length. Among unemployed women with higher education, increased discrimination was associated with longer telomere length. Overall, our findings suggest that factors related to having a post-HS education are protective against the negative effects of racism on cellular aging for AA women whether they are in or out of the workforce.
Interaction With Educational Attainment
Building on prior studies [17–21] and consistent with our hypothesis, we found inverse institutional racial discrimination–telomere length associations among employed women with less education, a previously unreported SES predictor. Using data from the Healthy Aging in Neighborhoods of Diversity across the Life Span study, Pantesco et al. and Beatty Moody et al. found inverse associations between institutional racial discrimination and telomere length in higher SES women using a composite measure comprised of education and income (i.e., poverty) in AAs [21] and in AA and White women [20], respectively. Our result more closely aligns with prior work among AAWHHS participants where educational attainment modified the association of institutional racial discrimination and allostatic load leaving lower educated women at higher risk of disease [27]. Together, these findings provide additional evidence that having a post-HS education in itself may play a unique role in buffering the association between institutional racism and worse health for AA women, including accelerated aging among working women.
Interaction With Employment Status
Our finding that nonworking women with higher education reporting more experiences of institutional racial discrimination had longer telomeres was novel while aligning with our hypothesis for testing this association. Racial discrimination is a prominent psychosocial stressor for AA women [13, 14]. Further, workplace discrimination is among the most frequently reported form of racial discrimination among AA women [14, 36], and racial discrimination is linked to shorter telomeres [19, 20]. Pervasive racial discrimination in the workplace may be especially deleterious for educated Black women compared with other institutional domains that it leads to faster telomere attrition for those in the workforce. This may explain why we found longer telomeres among educated yet unemployed women reporting higher institutional racial discrimination. It is also conceivable that highly educated women who are unemployed may have less exposure to social and economic stressors in general and have better access to resources to cope with the stressors to which they are exposed. Indeed, unemployed women in our sample were more likely to be married/partnered than employed women (76% and 63%, respectively), and therefore may have greater financial resources and social support. Our result suggests that educated AA women in the workforce may have an additional burden of telomere shortening related to institutional racism, though the mechanism is unclear.
Interaction With Poverty
To our knowledge, we are the first to evaluate whether poverty modifies associations of racial discrimination and telomere length. Our nonsignificant findings were surprising, given that poverty, a strong predictor of shorter telomeres [9], has been shown to interact with race/ethnicity to influence telomere length among Black, White, and Mexican adults in Detroit, after controlling for socioeconomic, psychosocial, coping, and behavioral factors [33]. We identified psychosocial, coping, and behavioral factors as mediators. However, this does not explain our differing finding: including mediators did not change our estimates. It is more conceivable that our nonsignificant result is due to our sample being restricted to midlife AA women in the Bay Area, suggesting that poverty may interact with racism differentially across racial/ethnic and gender groups, and by region to impact telomere length.
Interaction With SES
Consistent with our hypothesis, the composite SES measure did not modify any associations between racial discrimination and telomere length despite strong interactions of racial discrimination with independent SES measures. As previously described [20, 21], prior studies have shown that SES modifies the association of institutional racial discrimination and telomere length however these SES measures were comprised of two indicators: education and poverty. Our conflicting results may be explicated by our use of SES comprised of three indicators; education, poverty, and employment, since employment and education had different associations. In the current study, educational attainment was protective for working and nonworking women, which was masked when combined in our composite measure. Future studies should minimize the use of SES composite measures when examining the effects of racism on cellular aging and focus on specific socioeconomic factors associated with extending the life expectancy of AA women.
Interaction With Age
Contrary to our hypothesis, we found no age interaction with racial discrimination. This finding is consistent with other work reporting no significant age interaction between everyday racial discrimination and other forms of discrimination (e.g., major lifetime experiences) associated with telomere length [37, 38]. However, one investigation reported that higher institutional racial discrimination predicted shorter telomeres in younger White males and longer telomeres in older White men [21]. Given our nonsignificant findings among AA women, telomere attrition associated with everyday and institutional racism-related stress may be considerable for AA women by midlife, conceivably resulting in middle-aged groups having more similar telomere lengths. To assess this assumption in our sample, we estimated unadjusted and adjusted models including quadratic and cubic age-transformed variables to assess potential variation in associations, and the findings remained nonsignificant, offering some empirical support for this notion (Supplementary Fig. S2).
Institutional Versus Everyday Racial Discrimination
Unlike institutional racial discrimination, no socioeconomic indicators modified the association between everyday racial discrimination and telomere length, which was also unexpected yet similar to prior findings among AA women [20, 21]. Since AA women report racial discrimination as a particularly salient chronic stressor across SES groups [13, 14], everyday experiences, as connoted by the name, may be more commonplace than institutional experiences over the life course. Stressors that are commonplace can become more predictable and hence less stressful [39]. Similarly, cumulative exposure to daily racism for AA women over time can plausibly become more predictable, less stressful, and less impactful on telomeres independent of socioeconomic position. In other words, everyday racism that occurs in public (e.g., poorer service in restaurants, being followed around in stores) may be less differential between high and low SES AA women by midlife. This may explain, in part, why the link between experiences of everyday racial discrimination and telomere length was not modified by educational attainment, employment, or poverty status among women in our sample.
Potential Pathways and Recommendations
Our work adds to a growing body of evidence demonstrating important biopsychosocial modifiers and mediators along the physiologic stress regulation pathway that may buffer the negative impacts some forms of racism appear to have on telomeres. For instance, Chae et al. found that institutional racial discrimination interacts with having an anti-Black bias [18] and with depression [17] to predict shorter telomeres among AA men, implying potential cognitive and affective pathways. Furthermore, Lu et al. reported that AA women who did not discuss their experiences of everyday racial discrimination had shorter telomeres, suggesting coping strategies may be important [19]. We too reported that actively coping with chronic racism modified the association between everyday racial discrimination and hypertension risk among AAWHHS participants [40]. Finally, Alexeeff et al. found that individual and neighborhood SES were associated with shorter telomeres, albeit among Whites, proposing socio-environmental mechanisms [41]. Our findings suggest that factors related to higher education could reflect better access to health-promoting resources to cope with institutional racism-related stress for working and nonworking AA women, though the mechanism is not well understood. Thus, multiple biopsychosocial mechanisms may minimize racism-related telomere shortening across various racial/gender groups, meriting further study in nationally representative samples of AA women.
Given the totality of the current evidence, we offer two recommendations for future investigations to better understand how higher education and being unemployed “get under the skin” to protect AA women against racism-related biological deterioration: Researchers should (a) examine SES variables as independent (vs. composite) predictors of telomere length, especially post-HS education, to avoid masking the unique effect that each may have in relation to experiences of racial discrimination, and (b) evaluate the effect of psychosocial mediators on the biological stress regulation pathway (e.g., coping strategies) related to experiencing racist events to elucidate whether and to what extent various forms of racism contribute to cellular aging for AAs, particularly women.
Limitation and Strengths
Due to the cross-sectional study design, causal inference is precluded. We used purposive sampling hence study findings are not generalizable. However, according to the 2013 American Community Survey [42] the distribution of the educational attainment and employment status of middle-aged AA women residing in the same San Francisco Bay Area counties were comparable to our sample. Importantly, average racial discrimination scores were slightly yet significantly higher among women who did not (vs. did) give consent for DNA analysis: everyday (32.7 vs. 28.7) and institutional racial discrimination (21.5 vs. 18.4), which could bias study estimates. For selection bias to occur, both racial discrimination and telomere length must be a cause of consent, and telomere length as a cause is implausible. Nevertheless, if an unmeasured confounder contributed to both telomere length and consent, specifically health status or worry of irresponsible handling of one’s DNA, selection bias could occur. Since all women consented to physical exams and blood draws, it is unlikely that health status impacted consent. If worry impacted consent, we likely accounted for it, at least in part, by adjusting for perceived stress. Additionally, while poor recall is a limitation for any observational design using self-report, our utilization of two commonly used validated discrimination scales strengthened internal validity and allowed for comparability across studies. Internal validity was further strengthened by our theory-based approach to address confounding, though unmeasured confounding certainly exists. Last, potential misclassification of the outcome was considerably reduced by using the ratio of telomere repeat to hemoglobin gene products because the hemoglobin gene is conserved as a single copy in all persons [30].
Conclusion
Racial discrimination contributes to accelerated aging in AA women. The current study provides novel evidence that post-HS education may offer protection against the adverse effects that institutional racism has on cellular aging for AA women whether they are in or out of the workforce, though the mechanism is unclear. Mediation analyses using longitudinal data from a nationally representative sample would help elucidate important psychosocial and socioeconomic factors that connect racism to telomere length among healthy AA women.
Supplementary Material
Acknowledgments
We wish to thank the HEARTs (Health Effects Associated with Racism Threat) Research Group at the University of California Berkeley’s Center for Social Medicine for comments regarding the refinement of this manuscript, particularly Eli Michaels, Dr. Rachel Berkowitz, Elleni Hailu, Kevin Lee, and Dr. Thu Thi Nguyen. We also thank Dr. Eric Vittinghoff for comments on statistical analysis as well as the four anonymous reviewers for comments on prior drafts of this manuscript.
Funding
M.D. Thomas was partially supported by a Ford Foundation Predoctoral Fellowship administered by the National Academies of Sciences, Engineering, and Medicine, USA, and by an award made from NIGMS grant UL1GM118985, USA. This grant also funds the Health Equity Research lab at SF State, thereby supporting the work of S. Sohail, R.M. Mendez, and L. Márquez-Magaña on this study. A.M. Allen contributions were partially supported by NIMHD grant P60MD006902, USA. Study sponsors did not participate in study design, data collection, data analysis, interpretation of study results, or drafting of the manuscript.
Compliance with Ethical Standards
Authors’ Statement of Conflict of Interest and Adherence to Ethical Standards Authors Marilyn D. Thomas, Saba Sohail, Rebecca M. Mendez, Leticia Márquez-Magaña, and Amani M. Allen declare that they have no conflict of interest.
Authors’ Contributions M.D.T. primary investigator, conceptualized the study, coordinated all logistics related to data collection, cleaning, and coding, designed and performed all statistical analyses (and takes responsibility for the integrity of the data analyzed), and took final responsibility for drafting the manuscript; S.S. performed biological data extraction, purification, and measurement, and assisted in data cleaning, coding, and analysis, and editing and approval of the manuscript; R.M.M. assisted in developing and leading the study design for biological data extraction, purification, and measurement protocols, and editing and approval of the manuscript; L.M.-M. assisted in developing and managing the study design for biological data extraction, purification, and measurement protocols, and editing and approval of the manuscript; A.M.A. assisted with the design of the study, data analysis and interpretation, and editing and approval of the manuscript.
Ethical Approval All procedures performed in studies involving human participants were in accordance with the ethical standards of the Committee for the Protection of Human Subjects at the University of California, Berkeley and with the 1964 Helsinki declaration and its later amendments or comparable ethical standards.
Informed Consent All research subjects gave their informed consent for inclusion prior to their participation in the study.
References
- 1.Cunningham TJ, Croft JB, Liu Y, Lu H, Eke PI, Giles WH. Vital signs: racial disparities in age-specific mortality among blacks or African Americans—United States, 1999–2015. MMWR. 2017;66(17):444. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Arias E, Heron M, Xu J.. United States Life Tables, 2014: National Vital Statistics Reports (Report No. 4). Vol. 66. Hyattsville, MD: Natl Center Health Stat; 2017. [PubMed] [Google Scholar]
- 3.Levine ME, Crimmins EM. Evidence of accelerated aging among African Americans and its implications for mortality. Soc Sci Med. 2014;118:27–32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Vaziri H, Schächter F, Uchida I, et al. Loss of telomeric DNA during aging of normal and trisomy 21 human lymphocytes. Am J Hum Genet. 1993;52(4):661–667. [PMC free article] [PubMed] [Google Scholar]
- 5.Bernardes de Jesus B, Blasco MA. Assessing cell and organ senescence biomarkers. Circ Res. 2012;111(1):97–109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Rewak M, Buka S, Prescott J, et al. Race-related health disparities and biological aging: does rate of telomere shortening differ across blacks and whites? Biol Psychol. 2014;99:92–99. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Brown L, Needham B, Ailshire J. Telomere length among older U.S. adults: differences by race/ethnicity, gender, and age. J Aging Health. 2017;29(8):1350–1366. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Fok WC, Batista LFZ. Stressed ends: telomere attrition in chronic diseases. Rev Bras Hematol Hemoter. 2017;39(2):98–99. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Oliveira BS, Zunzunegui MV, Quinlan J, Fahmi H, Tu MT, Guerra RO. Systematic review of the association between chronic social stress and telomere length: a life course perspective. Ageing Res Rev. 2016;26:37–52. [DOI] [PubMed] [Google Scholar]
- 10.Epel ES, Blackburn EH, Lin J, et al. Accelerated telomere shortening in response to life stress. Proc Natl Acad Sci U S A. 2004;101(49):17312–17315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Geronimus AT, Hicken M, Keene D, Bound J. “Weathering” and age patterns of allostatic load scores among blacks and whites in the United States. Am J Public Health. 2006;96(5):826–833. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Geronimus AT, Hicken MT, Pearson JA, Seashols SJ, Brown KL, Cruz TD. Do US black women experience stress-related accelerated biological aging? A novel theory and first population-based test of black-white differences in telomere length. Hum Nat. 2010;21(1):19–38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Cunningham TJ, Seeman TE, Kawachi I, et al. Racial/ethnic and gender differences in the association between self-reported experiences of racial/ethnic discrimination and inflammation in the CARDIA cohort of 4 US communities. Soc Sci Med. 2012;75(5):922–931. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Nuru-Jeter A, Dominguez TP, Hammond WP, et al. “It’s the skin you’re in”: African-American women talk about their experiences of racism. an exploratory study to develop measures of racism for birth outcome studies. Matern Child Health J. 2009;13(1):29–39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Khansari N, Shakiba Y, Mahmoudi M. Chronic inflammation and oxidative stress as a major cause of age-related diseases and cancer. Recent Pat Inflamm Allergy Drug Discov. 2009;3(1):73–80. [DOI] [PubMed] [Google Scholar]
- 16.Kurz DJ, Decary S, Hong Y, Trivier E, Akhmedov A, Erusalimsky JD. Chronic oxidative stress compromises telomere integrity and accelerates the onset of senescence in human endothelial cells. J Cell Sci. 2004;117(11):2417–2426. [DOI] [PubMed] [Google Scholar]
- 17.Chae DH, Epel ES, Nuru-Jeter AM, et al. Discrimination, mental health, and leukocyte telomere length among African American men. Psychoneuroendocrinology. 2016;63:10–16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Chae DH, Nuru-Jeter AM, Adler NE, et al. Discrimination, racial bias, and telomere length in African-American men. Am J Prev Med. 2014;46(2):103–111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Lu D, Palmer JR, Rosenberg L, et al. Perceived racism in relation to telomere length among African American women in the Black Women’s Health Study. Ann Epidemiol. 2019;36:33–39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Beatty Moody DL, Leibel DK, Darden TM, et al. Interpersonal-level discrimination indices, sociodemographic factors, and telomere length in African-Americans and Whites. Biol Psychol. 2019;141:1–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Pantesco EJ, Leibel DK, Ashe JJ, et al. Multiple forms of discrimination, social status, and telomere length: interactions within race. Psychoneuroendocrinology. 2018;98:119–126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Brody GH, Miller GE, Yu T, Beach SR, Chen E. Supportive family environments ameliorate the link between racial discrimination and epigenetic aging: a replication across two longitudinal cohorts. Psychol Sci. 2016;27(4):530–541. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Szanton SL, Rifkind JM, Mohanty JG, et al. Racial discrimination is associated with a measure of red blood cell oxidative stress: a potential pathway for racial health disparities. Int J Behav Med. 2012;19(4):489–495. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Michaels E, Thomas M, Reeves A, et al. Coding the Everyday Discrimination Scale: implications for exposure assessment and associations with hypertension and depression among a cross section of mid-life African American women. J Epidemiol Community Health. 2019;73(6):577–584. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Robertson T, Batty GD, Der G, Fenton C, Shiels PG, Benzeval M. Is socioeconomic status associated with biological aging as measured by telomere length? Epidemiol Rev. 2013;35(1):98–111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Adler N, Pantell MS, O’Donovan A, et al. Educational attainment and late life telomere length in the Health, Aging and Body Composition Study. Brain Behav Immun. 2013;27:15–21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Allen AM, Thomas MD, Michaels EK, et al. Racial discrimination, educational attainment, and biological dysregulation among midlife African American women. Psychoneuroendocrinology. 2019;99:225–235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Williams DR, Yu Y, Jackson JS, Anderson NB. Racial differences in physical and mental health: socio-economic status, stress and discrimination. J Health Psychol. 1997;2(3):335–351. [DOI] [PubMed] [Google Scholar]
- 29.Krieger N, Smith K, Naishadham D, Hartman C, Barbeau EM. Experiences of discrimination: validity and reliability of a self-report measure for population health research on racism and health. Soc Sci Med. 2005;61(7):1576–1596. [DOI] [PubMed] [Google Scholar]
- 30.Cawthon RM. Telomere measurement by quantitative PCR. Nucleic Acids Res. 2002;30(10):e47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Lin J, Epel E, Cheon J, et al. Analyses and comparisons of telomerase activity and telomere length in human T and B cells: insights for epidemiology of telomere maintenance. J Immunol Methods. 2010;352(1–2):71–80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Ramirez J, Elmofty M, Castillo E, et al. Evaluation of cortisol and telomere length measurements in ethnically diverse women with breast cancer using culturally sensitive methods. J Community Genet. 2017;8(2):75–86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Geronimus AT, Pearson JA, Linnenbringer E, et al. Race-ethnicity, poverty, urban stressors, and telomere length in a Detroit community-based sample. J Health Soc Behav. 2015;56(2):199–224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Cohen S, Kamarck T, Mermelstein R. Perceived stress scale. In: Measuring Stress: A Guide for Health and Social Scientists. Vol. 10. 1994:1–2. [Google Scholar]
- 35.Greenland S, Daniel R, Pearce N. Outcome modelling strategies in epidemiology: traditional methods and basic alternatives. Int J Epidemiol. 2016;45(2):565–575. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Thomas MD, Michaels EK, Reeves AN, et al. Differential associations between everyday versus institution-specific racial discrimination, self-reported health, and allostatic load among black women: implications for clinical assessment and epidemiologic studies. Ann Epidemiol. 2019;35:20–28.e3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Lee DB, Kim ES, Neblett EW. The link between discrimination and telomere length in African American adults. Health Psychol. 2017;36(5):458–467. [DOI] [PubMed] [Google Scholar]
- 38.Liu SY, Kawachi I. Discrimination and telomere length among older adults in the United States. Public Health Rep. 2017;132(2):220–230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Sapolsky RM. Why is psychological stress stressful? In: Sapolsky RM, ed. Why Zebra’s Don’t Get Ulcers. 3rd ed. New York, NY: WH Freeman; 1994:252–270. [Google Scholar]
- 40.Michaels EK, Reeves AN, Thomas MD, et al. Everyday racial discrimination and hypertension among midlife African American women: disentangling the role of active coping dispositions versus active coping behaviors. Int J Environ Res Public Health. 2019;16(23):4759. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Alexeeff SE, Schaefer CA, Kvale MN, et al. Telomere length and socioeconomic status at neighborhood and individual levels among 80,000 adults in the Genetic Epidemiology Research on Adult Health and Aging cohort. Environ Epidemiol. 2019;3(3):e049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.United States Census Bureau. General demographic characteristics.2018. Available at https://factfinder.census.gov/faces/nav/jsf/pages/index.xhtml. Accessibility verified August 8, 2018.
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.