Abstract
Pancreatic islet beta cells (β-cells) synthesize and secrete insulin in response to rising glucose levels and thus are a prime target in both major forms of diabetes. Type 1 diabetes ensues due to autoimmune destruction of β-cells. On the other hand, the prevailing insulin resistance and hyperglycemia in type 2 diabetes (T2D) elicits a compensatory response from β-cells that involves increases in β-cell mass and function. However, the sustained metabolic stress results in β-cell failure, characterized by severe β-cell dysfunction and loss of β-cell mass. Dynamic changes to β-cell mass also occur during pancreatic development that involves extensive growth and morphogenesis. These orchestrated events are triggered by multiple signaling pathways, including those representing the transforming growth factor β (TGF-β) superfamily. TGF-β pathway ligands play important roles during endocrine pancreas development, β-cell proliferation, differentiation, and apoptosis. Furthermore, new findings are suggestive of TGF-β’s role in regulation of adult β-cell mass and function. Collectively, these findings support the therapeutic utility of targeting TGF-β in diabetes. Summarizing the role of the various TGF-β pathway ligands in β-cell development, growth and function in normal physiology, and during diabetes pathogenesis is the topic of this mini-review.
Keywords: TGF-β, pancreas, β cells, islets, development, function, diabetes
Pancreas Development
Overview of the Pancreas
The pancreas synthesizes and secretes digestive enzymes and hormones principally involved in glucose homeostasis. These functions are performed by multiple unique cell types within the pancreas. Moreover, these cells work in coordination with other organs as part of an integrated glucose homeostasis regulatory network. Specifically, cells within the exocrine and endocrine pancreas are the primary conductors, along with supporting cells such as vascular, immune, mesenchymal, and neuronal cells. The exocrine pancreas is composed of acinar cells, which act as a factory that produces digestive enzymes. On the other hand, the endocrine pancreas is represented by the islets of Langerhans (islets) that comprise distinct cell types which produce hormones that regulate glucose homeostasis. Islets are primarily composed of alpha cells, beta cells, delta cells, and pancreatic polypeptide (PP) cells, which produce glucagon, insulin, somatostatin, and pancreatic polypeptide, respectively.
Pancreatic cells are derived from a common endodermal progenitor cells that also give rise to the liver (1). In general, rodent pancreatic development can be classified into 3 stages (2). Pancreas specification occurs at embryonic day (E) 7.5 in the mouse. During primary transition (E8.5 to E12.5), the pancreas originates from the foregut endoderm as dorsal and ventral pancreatic buds, wherein the pancreatic progenitor cells form a stratified epithelium with a centrally located lumen. These progenitor cells maintain multipotency with the ability to give rise to endocrine, acinar, and duct cells. At E12.5, the pancreatic ductal epithelium gets compartmentalized into tip and trunk domains, with multipotent progenitor cells located in the tip domain and the endocrine precursors residing in the trunk domain (3). The tip domain will eventually give rise to acinar cells, while the trunk domain cells retain both duct and endocrine differentiation potential. It appears that the number of progenitor cells assigned to the pancreatic anlage dictate the final size of the mature pancreas (4). The secondary transition (E12.5 to E16.5) is a period of significant expansion and branching differentiation of the pancreatic bud. Specifically, the pancreatic ductal network arises via the fusion of microlumens into a ramified epithelial network, and this morphological remodeling coincides with the formation of the 3 main pancreatic cell types (endocrine, exocrine/acinar, and ductal cells). Progenitor cells are destined to become either acinar, endocrine, or duct cells. Between E13.0 and E15.0, fully differentiated insulin-expressing β-cells and glucagon-expressing α-cells arise from the epithelial trunk (5). The tertiary transition (E16.5 to birth) leads to further maturation and expansion of the pancreas, which continues postnatally. The individual differentiated endocrine cells delaminate from the epithelium to form islets (6). In rodents, 90% of islet cells are β cells that are clustered in the islet core and are surrounded by α-, δ- and PP-cells.
Relative to our understanding of pancreas development in rodents, knowledge about human pancreas development is less complete, because of limited access and ethical constraints (4, 7). Human embryonic development is classified into 23 Carnegie stages (8). Similar to rodent pancreas, the human pancreas originates from the foregut endoderm, at Carnegie stage (CS) 9, to generate ventral and dorsal buds. In contrast to the rodent, the human pancreas does not appear to undergo endocrine differentiation, also referred to as the “primary transition” (4, 7, 9). Thus, distinct endocrine precursor cells are not found in humans during a stage equivalent to the primary transition in mouse pancreatic development. Tip-like and trunk-like cell populations appear at CS19 (10), pancreatic-duodenal homeobox 1 (PDX1)+ pancreatic progenitors are found in the epithelium from CS20 and β-cells first appear at CS23 (4, 7). These observations represent hallmarks of a “secondary transition” in human pancreas development. Further maturation and expansion continue after CS23 as part of the “tertiary transition.” At that stage, glucagon-expressing cells emerge (10, 11), endocrine clustering begins, and all endocrine cell types are found in the developing islets (12). We point readers to a recently compiled review that describes human pancreas development in greater detail (13). All data in the mini-review refer to rodents unless specified as human and we point readers to a recently compiled review that describes human pancreas development in greater detail (13).
TGF-β Superfamily Signaling
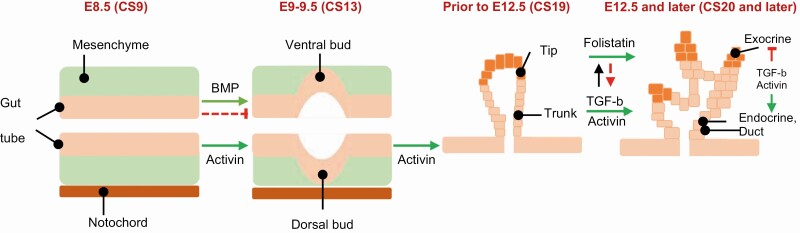
Signaling pathways initiated by transforming growth factor-β (TGF-β), fibroblast growth factor, retinoic acid, vascular endothelial growth factor, and Notch, Wnt, sonic hedgehog ligands are involved during various stages of pancreas development. Before pancreas formation is initiated (primary transition), the notochord secretes a variety of potent inducing molecules—including the TGF-β family ligands (Fig. 1)—that are required for initiation of pancreas organogenesis (14). The mammalian TGF-β isoforms (TGF-β1, -β2, and -β3) are expressed in the pancreatic epithelium during early pancreas development (15). TGF-β receptor type I (TβR-I) appears in early pancreatic epithelium and later is restricted to the pancreatic ductal area. TGF-β receptor type II (TβR-II) exhibits an expression pattern similar to that of TβR-I. Related TGF-β superfamily members, such as activins and bone morphogenetic proteins (BMPs), share receptors and inhibitors in many cases (16, 17). Activins are expressed in the notochord. Tissue culture experiments with chick embryos have revealed that isolated notochord or purified activin induce expression of key pancreas genes Pdx1 and insulin through inhibition of endodermal sonic hedgehog (SHH) (18, 19).
Figure 1.
Presumptive role of the TGF-β superfamily in pancreas organogenesis and lineage specification. At E8.5 (rodent)/CS9 (human) signals from the notochord, including activin, initiate development of pancreatic buds. BMP signals reportedly exert both negative (dashed, red line) and positive (solid green arrow) effects on pancreatic development. Initial pancreatic bud outgrowth occurs between E9-E9.5/CS13. Around E12.5/CS19, multipotent “tip” and “trunk” domains get established. During the secondary transition (E12.5/CS20 and later), the “tip” domain gives rise to exocrine acinar cells, whereas the “trunk” domain gives rise to duct and endocrine cells. TGF-β1 and activin promote endocrine differentiation and repress exocrine differentiation. Follistatin, an antagonist of activin, promotes exocrine cell differentiation and reduces differentiation of endocrine cells. Established interactions between molecules are indicated by solid lines with green arrows indicating positive regulation and red dash lines indicating negative regulation.
Compared with TGF-β ligand signaling, research into the role of BMP-specific signaling pathways in pancreatic development has been limited (20). BMPs were reported to be involved in A-P patterning of endoderm and BMP induces posterior transformation of the endoderm (21, 22). The lateral plate mesoderm adjacent to the presomitic mesoderm strongly expresses BMP4, which can induce Pdx1 expression in the ventral prepancreatic endoderm (22). However, BMP4 promotes reprogramming of ventral endoderm to a liver fate and inhibits pancreas development (23). Instead, lack of BMP4 allows the endodermal cells to activate pancreatic differentiation pathways. BMP4 inhibits pancreatic endocrine cells differentiation while promoting cellular proliferation via inhibitor of differentiation 2 (Id2) transcription factor, which in turn inactivates Neuro-D, which is required for endocrine cell maturation (24).
TGF-β superfamily also plays additional roles in the differentiation of distinct cell types that form the mature pancreas (Fig. 1). In vitro exposure of pancreatic dorsal bud tissue to TGF-β1 promoted endocrine differentiation, particularly β-cells, and disrupted epithelial branching and acinar formation (18). Blocking TGF-β signaling in the embryonic pancreas, by overexpressing a dominant-negative TGF-βRII (DNTβRII), increased endocrine precursors and proliferation of endocrine cells (15). Activin was shown to promote endocrine differentiation while blocking branching and proliferation of the pancreatic bud and repressing exocrine differentiation (25). On the other hand, follistatin, an antagonist of activin, promoted exocrine cell differentiation and reduced differentiation of endocrine cells. Transgenic mice overexpressing either a dominant-negative or null mutation in type II activin receptors ActRIIA and ActRIIB exhibited abnormal pancreatic tissue with severely altered architecture and pancreatic islet hypoplasia. Thus, these studies provide evidence consistent with regulation of crucial aspects of pancreas development by TGF-β1 superfamily signaling.
β-Cell Development
Pancreas induction and specification
Endocrine islets scattered throughout the exocrine pancreas produce hormones which primarily regulate glucose levels and cellular function (26, 27). As mentioned above, during the secondary transition, the pancreatic epithelium undergoes significant expansion and differentiation. At this point, most of the cells get specified to either acinar, duct, or endocrine cells. This pancreatic cell fate is determined by intrinsic transcription factors. The common pancreatic progenitor expresses Pancreatic Transcription Factor 1A (Ptf1A), Pancreatic-Duodenal Homeobox 1 (Pdx1), Sry-related HMG box (Sox9), Neurogenin 3 (Ngn3) and NK Homeobox 6.1 (Nkx6.1) (9, 28, 29). Ptf1A and Ngn3 are required for exocrine and endocrine progenitor cell differentiation during the secondary transition. Pdx1 is expressed in the endoderm and is required for pancreatic development beyond initial bud formation. Upon embryonic deletion of Pdx1 in mouse, pancreatic bud formation occurs, but expansion and branching of the tissue does not proceed (30, 31). In the mature pancreas, Pdx1 expression is limited to differentiated β- and δ-cells. Without Pdx1, acini do not form, although reduction of Pdx1 is required for acinar cell differentiation and maturation (32).
Islet β-cell development
β-Cells arise from Ngn3-positive pancreatic endocrine progenitors. Orderly expression of a cascade of transcription factors are required for the functional maturity of β-cells. The earliest endocrine cells observed during primary transition are either glucagon-positive, insulin-positive, double hormone-positive, or multihormonal-positive (14). While insulin+:glucagon+ cells appear at E10.5, immature insulin single-positive β-cells appear around E13.5, although they exhibit a reduced capacity to synthesize and secrete insulin in response to secretagogues (2, 33, 34). In mature β-cells, Pdx-1 transactivates key β-cell genes, including the insulin gene and other genes involved in glucose sensing and metabolism such as glucose transporter 2 (Glut2) and glucokinase (Gck). Deletion of Pdx1 in mice resulted in reduced β-cell function and early-onset diabetes, and in humans PDX1 haploinsufficiency is associated with maturity-onset diabetes of the young (MODY4), indicating the importance of PDX1 activity in β-cell identity and function (35). MafA is a β-cell-specific transcription factor that binds to the insulin promoter, regulating the expression of genes involved in insulin biosynthesis and secretion (36, 37). In addition, MafA interacts with Pdx-1 and NeuroD1 to activate insulin transcription. MafA deficiency in β-cells leads to impaired insulin secretion in response to glucose or arginine (38). NeuroD1 is required for β cell maturation and maintenance of glucose-responsive β-cells. Mice lacking NeuroD1 exhibit reduced insulin secretion, display immature β-cells, and are diabetic due to a reduction in all endocrine subtypes (39).
Mechanisms involved in proliferation and differentiation of postnatal β-cells are slightly different when compared with those involved during the embryonic phase. Particularly, the roles of cell cycle proteins in proliferation, differentiation, and regeneration of β-cells are instructive. For example, mice lacking the cyclin-dependent kinase Cdk4 exhibit defects prior to and after the secondary transition due to paucity of Pdx1+ pancreatic progenitors and Ngn3+ endocrine progenitors. In contrast, constitutive activation of Cdk4, via a Cdk4R24C mutant, leads to increased Nkx2.2+ and Nkx6.1+ cells along with increased proliferation of Ngn3+ endocrine precursors, resulting in expansion of β-cell mass (40). Cdk4-dependent phosphorylation of its chief substrate retinoblastoma protein (RB) regulates the stability of Pdx1 via competition between Pdx1 and E2F1 for binding to RB. Binding to RB stabilizes Pdx1 by precluding its degradation via the ubiquitin proteasome complex. Accordingly, RB deficiency in vivo results in reduced size of the pancreas due to decreased proliferation of Pdx-1 pancreatic progenitors (41). These interactions of Cdk4 in controlling 2 key transcription factors—Pdx1 and Ngn3—thus regulate the embryonic endocrine β-cell mass via modulating the numbers of pancreatic progenitors and endocrine progenitors, respectively. Consistent with this, Cdk4-deficient mice develop age-dependent β cell hypoplasia and diabetes (42). Mice lacking cyclin D1 and/or cyclin D2 also display similar phenotypes (43, 44). In contrast, Cdk4R24C mice develop β-cell hyperplasia (42). The β-cell mass can regenerate following streptozotocin (STZ)-mediated injury or partial pancreatectomy, at least part of which reflects neogenesis from preexisting duct cells or acini (45, 46). Lineage-tracing offers evidence for contribution of both β-cell neogenesis and β-cell replication during Cdk4-dependent increase in postnatal β-cell regeneration (47). Thus, dynamic changes to the pancreatic and β-cell specific cell cycle machinery appears to modify the embryonic and postnatal β-cell mass and its regeneration potential. Further, we recently showed that Cdk2 promotes β-cell function and its loss promotes β-cell dysfunction along with an age- and/or diet-induced deterioration of β-cell mass (48).
TGF-β signaling in β-cell development
TGF-β superfamily members play important roles in regulating β cell development, postnatal mass and function (Table 1). In ex vivo cultures of embryonic mouse pancreas, TGF-β1 promoted islet β cell differentiation, while inhibiting exocrine cell differentiation, thereby mimicking TGF-β’s action during early pancreatic development (18). In addition, TGF-β2 induced differentiation of mouse embryonic stem cells (mESCs) into Pdx1-expressing endodermal cells but failed to induce insulin expression (49). In contrast, transgenic mice that overexpress TGF-β1 under the rat insulin II promoter develop massive fibrosis of the pancreas but maintain normoglycemia with normal β cell mass (50), or with smaller islets (51). In addition, TGF-β1 transcriptionally represses the expression of β-cell specific transcription factors in human pancreatic duct cells (52). Interestingly, inhibition of TGF-β signaling by BMP-7 stimulates exocrine cell differentiation toward endocrine β cell fate in human organoid cultures, evidence of a crosstalk between 2 superfamily ligands (53). Dominant-negative TGF-β receptor 2 (DNTβRII) transgene increased the number of endocrine precursors (54). Similar to TGF-β1, activin regulates endocrine islet differentiation, enabling insulin secretion in vivo and in vitro (55-58). Deletion of the Fstl3 gene (an antagonist of activin) in vivo results in larger islets with β cell hyperplasia accompanied by improved glucose tolerance and insulin sensitivity in the absence of altered β cell proliferation (59, 60). Further, treatment with activin repress the α-cell transcription factors and induces expression of β-cell transcription factors wherein islet β cells can be generated by using a TGF-β receptor kinase inhibitor (61). In contrast, mice with β cell specific overexpression of either a dominant-negative activin type II receptor (ActRII) or an activated activin type I receptor (ActRI) mutant display hypoplastic islets and a decrease in insulin secretion (62). TGF β1, acting through Smad3, represses insulin transcription (63). While overexpression of Smad3 in human islets reduced insulin content, dominant-negative Smad3 enhanced insulin levels. In agreement, Smad3-deficiency in mice induced moderate hyperinsulinemia and mild hypoglycemia in vivo. Overexpression of Smad7 in β cells under a Pdx-1 promoter results in reversible diabetes in mice (64). TGF β signaling also modified glucose induced insulin secretion in β cells (63, 65). Smad2 deficient mice show increased insulin release from islet cells and Smad2 deficiency impaired insulin secretion in response to glucose (66, 67).
Table 1.
TGF-β Signaling in Pancreas, Specifically β Cells
| Model | Phenotype | Sp | Refs |
|---|---|---|---|
| TGF-β | |||
| TGF-β1 | Promotes the development of endocrine cells Increased insulin secretion with/without glucose |
m, r | (18, 66) |
| TGF-β inhibitor | Increase β cell replication by repression of Ink4a, improved C-peptide secretion |
h, m | (90) |
| Transgenic overexpression of TGF-β under the insulin promoter | Decreased development of exocrine pancreas and islets, maintenance of glucose control | m | (51, 91) |
| Transgenic overexpression of TGF-β under glucagon promoter | Impaired glucose tolerance, low insulin secretion, decreased size of endocrine and whole pancreas | m | (92) |
| Transgenic overexpression of dominant-negative TβRII | Increase in endocrine precursors, proliferating endocrine cells and insulin-positive cells | m | (93) |
| Double TβRI/TβRII KO | Reduced β-cell proliferation | (77) | |
| TβRI inhibition, expanded human β cells in culture | Inhibition of proliferation and dedifferentiation and stimulation of β cell redifferentiation | h | (76) |
| Activin | |||
| Activin A | Induced pancreatic β cell differentiation from hES cells | h | (57, 58) |
| Increased insulin secretion with/without glucose | r | (55) | |
| Activin B | Induced pancreatic β cell differentiation from murine embryonic stem cells | m | (94, 95) |
| Follistatin (activin inhibitor) | Reduced insulin-positive cell differentiation | m | (25) |
| Increased exocrine cell differentiation from embryonic mesenchyme | |||
| Activin B KO | Hyperinsulinemia | m | (96) |
| Double heterozygous ActRIIA/ActRIIB KO | Hypoplastic pancreatic islets, hypoinsulinemia, and impaired glucose tolerance | m | (56) |
| ActRIIB KO | Hypoplastic islets | m | (56, 97) |
| Double heterozygous ActRIIB/Smad2 KO | Hypoplastic islets, decreased islet area, decreased insulin content, impaired glucose tolerance | m | (97) |
| Transgenic overexpression of dominant-negative ActRII | Impaired differentiation of endocrine and exocrine cells of the pancreas, Hypoplastic islets | m | (62, 98) |
| BMP | |||
| Transgenic overexpression of BMP4 | Improved glucose tolerance, increased insulin secretion | m | (99) |
| Smad | |||
| Hetero Smad2 KO Smad2 KO in β cells |
Increased numbers of Ngn3+ cells, reduced β-cell numbers Impaired insulin secretion in response to glucose |
m | (67, 100) |
| Smad3 KO, transgenic overexpression of DN-Smad3 under insulin promoter Transgenic overexpression of CA-Smad3 under insulin promoter |
Improved glucose tolerance, increased insulin secretion Increased β cell apoptosis, impaired glucose tolerance |
m | (63, 74) |
| Transgenic overexpression of Smad7 under Pdx-1 promoter | Reduced pancreatic insulin content and hypoinsulinemia | m | (64) |
| Transgenic overexpression of Smad7 or KO under insulin promoter | Loss of SMAD7 in β cells inhibited proliferation, and SMAD7 overexpression enhanced β cell proliferation, no defect in β cell function in either model | m | (78) |
Abbreviations: h, human; m, mouse; r, rat; Sp, species.
Taken together, these results suggest a central role for TGF-β signaling in regulating β-cell function. However, considering the shared receptors and downstream effectors or inhibitors, it is not entirely clear whether TGF-β superfamily signals augments β-cell differentiation from endocrine progenitors. It appears that the local concentration and length of exposure of the ligands as well as the combination of receptors, effectors and antagonists locally and/or temporally might determine the final fate of the β-cell.
Diabetes and Connections to TGF-β Signaling
Pancreatic islet β-cells are a target in both major forms of diabetes. Type 1 diabetes is a result of autoimmune destruction of insulin-producing β-cells. Genetic, environmental and autoimmune factors promote infiltration of self-reactive lymphocytes in the pancreas to target and eliminate pancreatic β cells (68, 69). In contrast, T2D has a multifactorial origin and disease propagation with insulin resistance in peripheral organs such as skeletal muscle and liver. Initially, insulin resistance stimulates compensatory β-cell proliferation and/or increased insulin production from β-cells (70, 71). Over time and in the face of continued insulin resistance, β-cell dysfunction and deterioration of β-cell mass results in progressive β-cell failure. Chronic exposure to hyperglycemia leads to the accumulation of reactive oxygen species (ROS) activating damaging pathways which trigger nitro-oxidative stress (NOS) and endoplasmic reticulum stress (72). Autophagy is a catabolic process for recycling cellular components and damaged organelles, but hyperactivation of β-cell autophagy leads to β-cell apoptosis (73), ultimately leading to reduction in functional β-cell mass.
TGF-β signals and β-cell proliferation, differentiation, and apoptosis
β-cell proliferation and differentiation are tightly controlled by intrinsic and extrinsic factors that control how the β cells adjust to the metabolic demand. Diabetes ensues when genetic and/or environmental triggers disrupt the β cell compensation mechanism leading to β cell failure. While loss of β cell mass plays a central role in diabetes progression, the mechanisms underlying the loss of β cell mass are not entirely clear. β-cell apoptosis has been observed in rodent and human pancreas (1, 15-19). However, in comparison to β-cell proliferation (10-12), the process of β-cell apoptosis is poorly understood. A study of human cadaveric pancreas underscored the role of apoptosis in the observed deterioration of β-cell mass in T2D, regardless whether the T2D subjects were lean or obese (15). In that same study, the levels of β-cell replication and neogenesis within the cadaveric human T2D pancreas were very low and indistinguishable when compared with nondiabetic (ND) human pancreas specimens. We recently showed that mice with β-cell specific constitutively active TGF-β/Smad3 signals display increased susceptibility to β-cell apoptosis (74). In contrast, reductions in TGF-β/Smad3 signaling protected from β-cell apoptosis. These findings support a role for controlled inhibition of TGF-β/Smad3 signals in prevention of β-cell apoptosis and restoration of β-cell mass. Further studies on mechanisms of β-cell apoptosis will likely identify additional drug targets for prevention of β cell failure in type 2 diabetes.
Overexpressing TGF-β under an insulin promoter in the type 1 diabetes susceptible nonobese diabetic (NOD) mice prevents development of diabetes by inhibiting islet antigen-specific Th1 cells (51). However, TGF-β overexpression does not prevent the ultimate islet destruction in the NOD mice. Transgenic mice overexpressing TGF-β1 exhibit massive fibrosis of the pancreas—similar to chronic pancreatitis—leading to hyperglycemia and β-cell failure. Enhanced TNF-a signaling is observed associated with the fibrosis, suggesting that intact TGF-β signaling plays an important role in modulating the β-cell’s response to inflammatory stimuli (50).
Failure of compensatory increase of β-cell mass in the face of metabolic stress promotes glucose intolerance. Furthermore, chronic metabolic stress likely stimulates dedifferentiation of β-cells. Growing evidence suggests that that β-cell dedifferentiation may be a contributing factor for β-cell failure (75). Dedifferentiating β-cells show abnormal levels of key β-cell transcription factors and reduction in insulin production. As described, TGF-β signaling regulates adult β-cell dedifferentiation. Deletion of TGF-β Receptor 1 (TβRI) using shRNA inhibits β-cell proliferation and dedifferentiation in human islet cell in vitro, and mice with combined deletions of TβRI and TβRII in the pancreas show a reduction of β-cell proliferation (76, 77). Transgenic mice with pancreas-specific overexpression of Smad7, an inhibitor of TGF-β signaling, show reduced insulin and MafA transcripts and hyperglycemia (64). However, a recent report using transgenic mice with β-cell-specific overexpression or depletion of Smad7 shows that loss of SMAD7 inhibited proliferation whereas SMAD7 overexpression enhanced cell proliferation, without altering β-cell function in either model (78).
TGF-β in Human Induced Pluripotent Stem Cell-Derived β-Cells
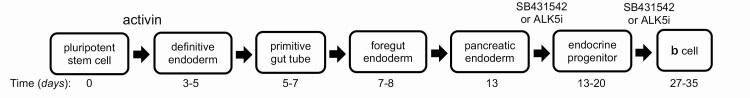
Induced pluripotent stem cells (iPSC) provide a potentially tractable model to investigate developmental processes and produce therapeutically deliverable cells. Insulin-secreting human β-cells generated via iPSC techniques represent potential therapeutic options for diabetes patients. Protocols have been continuously developed and improved over the past decade using variations of the method initially described by D’Amour et al (79-83). Although these protocols generated pancreatic progenitors, only a minimal amount of C-peptide release was detected in response to glucose. Modified approaches using activin A have been adopted to differentiate human embryonic stem cells toward insulin-producing cells (57, 58). These investigations show that human embryonic stem cell–derived cells express islet specific genes PDX1, INS, SLC2A2, GCG, and AMY1A. However, the differentiated endocrine cells not only tended to produce both glucagon and insulin but also displayed transcriptional similarities between human fetal β-cells and iPSC-derived β-cells (84). To address this issue, the TGF-β receptor antagonist SB431542 was included in the pancreatic differentiation protocol (85). Combination of appropriate anterior-posterior patterning and inhibition of TGF-β signaling using SB431542 enabled efficient differentiation of hPSCs to C-peptide+ cells. Similarly, TGF-βRI kinase inhibitor IV induced generation of pancreatic progenitors from CyT49 hPSCs (86). Also, the ALK5 inhibitor ALK5iII can induce expression of NGN3, NEUROD1, INS, and GCG and promote mature endocrine cell differentiation (87). A follow-up study by the same group showed that ALK5iII (and no other ALK5 inhibitors) downregulates expression of NGN3 while increasing INS, GCG, and SST at a later phase of differentiation of human iPSCs (hiPSCs) (88). Subsequent protocols have undergone significant improvement to generate mature endocrine cells (single hormonal cells) (88, 89). For example, a robust protocol using ALK5iII also generated mature and functional human pancreatic β-cells from hiPSCs (89). Overall, TGF-β signal modulation showed variable maturity levels of β-cells from hiPSCs depending on the protocol (Fig. 2). Further research is needed to investigate the exact effects of TGF-β signals during pancreatic development and β-cell maturation ex vivo.
Figure 2.
Schematic of in vitro differentiation from hPSC into INS+ cells. Timeline indicates days in culture to progress through the different stages of differentiation when cultured in the presence of TGF-β signaling ligands (activin) and inhibitors (SB431542 or ALK5i).
Unanswered Questions
The past decade has seen remarkable progress in our understanding of TGF-β signaling mediated mechanisms in pancreas development and β-cell proliferation, differentiation, and regeneration. Earlier studies emphasized the importance of this signaling pathway during development, and more recent studies implicate the TGF-β signaling pathway in adult β-cell function and maturity. Together, these observations underscore that members of TGF-β signaling play important roles in maintaining β-cell homeostasis. Nevertheless, there are a number of unanswered questions. It is not clear whether the effects of TGF-β are conserved in human and rodents and whether TGF-β superfamily ligand production is dependent on physiological and pathological conditions. It is also unclear whether TGF-β’s actions on β-cells in vivo are autocrine in nature or generated by non-β-cells within the pancreatic islet, pancreatic exocrine cells, or from nonpancreatic cells. Whether alterations in TGF-β signaling can increase progenitor cell formation and development to mature β-cells also remains to be established. Answers to these open questions will help better our understanding of β cell development and function.
Perspectives
The regulatory roles of TGF-β superfamily signals in normal pancreatic development, β-cell differentiation and function, apoptosis, and regeneration are complex. Furthermore, the amplitude and specificity of the signals appear to be context dependent, and not limited to the cell type or the timing and dosage of the signal. Similarly, during diabetes pathogenesis, more nuanced roles of TGF-β signaling might regulate compensatory responses of β-cells to sustained metabolic demand. Taken together, targeting TGF-β signaling is a promising therapeutic opportunity, albeit one that needs to be carefully investigated. Despite numerous studies, further confirmation seems necessary, including defining the exact functional relationship between TGF-β superfamily signals and external signals along with defining the underlying molecular mechanisms that promote maturity and functionality of β cells.
Acknowledgments
We apologize for any omission in citing relevant publications due to space limitations. This work was supported by intramural funds (ZIA DK075073-09 and ZIA DK055106-14) from the National Institutes of Diabetes and Digestive and Kidney Diseases to Sushil G. Rane.
Glossary
Abbreviations
- β-cells
pancreatic islet beta cells
- BMP
bone morphogenetic protein
- Cdk
cyclin-dependent kinase
- CS
Carnegie stage
- E
embryonic day
- iPSC
induced pluripotent stem cell
- Ngn3
Neurogenin 3
- PDX1
pancreatic-duodenal homeobox 1
- RB
retinoblastoma protein
- T2D
type 2 diabetes
- TβR
TGF-β receptor
- TGF-β
transforming growth factor-β
Additional Information
Disclosures: The authors have nothing to disclose and declare no actual or potential competing financial interests.
Data Availability
Data sharing is not applicable to this article as no datasets were generated or analyzed during the current study. All requests for data and information will be addressed by the corresponding author.
References
- 1. Deutsch G, Jung J, Zheng M, Lóra J, Zaret KS. A bipotential precursor population for pancreas and liver within the embryonic endoderm. Development. 2001;128(6):871-881. [DOI] [PubMed] [Google Scholar]
- 2. Pictet RL, Clark WR, Williams RH, Rutter WJ. An ultrastructural analysis of the developing embryonic pancreas. Dev Biol. 1972;29(4):436-467. [DOI] [PubMed] [Google Scholar]
- 3. Zhou Q, Law AC, Rajagopal J, Anderson WJ, Gray PA, Melton DA. A multipotent progenitor domain guides pancreatic organogenesis. Dev Cell. 2007;13(1):103-114. [DOI] [PubMed] [Google Scholar]
- 4. Pan FC, Wright C. Pancreas organogenesis: from bud to plexus to gland. Dev Dyn. 2011;240(3):530-565. [DOI] [PubMed] [Google Scholar]
- 5. Ben-Othman N, Courtney M, Vieira A, et al. From pancreatic islet formation to beta-cell regeneration. Diabetes Res Clin Pract. 2013;101(1):1-9. [DOI] [PubMed] [Google Scholar]
- 6. Pan FC, Bankaitis ED, Boyer D, et al. Spatiotemporal patterns of multipotentiality in Ptf1a-expressing cells during pancreas organogenesis and injury-induced facultative restoration. Development. 2013;140(4):751-764. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Pan FC, Brissova M. Pancreas development in humans. Curr Opin Endocrinol Diabetes Obes. 2014;21(2):77-82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. O’Rahilly R, Müller F. Developmental stages in human embryos: revised and new measurements. Cells Tissues Organs. 2010;192(2):73-84. [DOI] [PubMed] [Google Scholar]
- 9. Bastidas-Ponce A, Scheibner K, Lickert H, Bakhti M. Cellular and molecular mechanisms coordinating pancreas development. Development. 2017;144(16):2873-2888. [DOI] [PubMed] [Google Scholar]
- 10. Jennings RE, Berry AA, Kirkwood-Wilson R, et al. Development of the human pancreas from foregut to endocrine commitment. Diabetes. 2013;62(10):3514-3522. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Jeon J, Correa-Medina M, Ricordi C, Edlund H, Diez JA. Endocrine cell clustering during human pancreas development. J Histochem Cytochem. 2009;57(9):811-824. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Meier JJ, Köhler CU, Alkhatib B, et al. Beta-cell development and turnover during prenatal life in humans. Eur J Endocrinol. 2010;162(3):559-568. [DOI] [PubMed] [Google Scholar]
- 13. Jennings RE, Berry AA, Strutt JP, Gerrard DT, Hanley NA. Human pancreas development. Development. 2015;142(18):3126-3137. [DOI] [PubMed] [Google Scholar]
- 14. Lin CL, Vuguin PM. Determinants of pancreatic islet development in mice and men: a focus on the role of transcription factors. Horm Res Paediatr. 2012;77(4):205-213. [DOI] [PubMed] [Google Scholar]
- 15. Tulachan SS, Tei E, Hembree M, et al. TGF-beta isoform signaling regulates secondary transition and mesenchymal-induced endocrine development in the embryonic mouse pancreas. Dev Biol. 2007;305(2):508-521. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Manova K, De Leon V, Angeles M, et al. mRNAs for activin receptors II and IIB are expressed in mouse oocytes and in the epiblast of pregastrula and gastrula stage mouse embryos. Mech Dev. 1995;49(1-2):3-11. [DOI] [PubMed] [Google Scholar]
- 17. Verschueren K, Dewulf N, Goumans MJ, et al. Expression of type I and type IB receptors for activin in midgestation mouse embryos suggests distinct functions in organogenesis. Mech Dev. 1995;52(1):109-123. [DOI] [PubMed] [Google Scholar]
- 18. Sanvito F, Herrera PL, Huarte J, et al. TGF-beta 1 influences the relative development of the exocrine and endocrine pancreas in vitro. Development. 1994;120(12):3451-3462. [DOI] [PubMed] [Google Scholar]
- 19. Hebrok M, Kim SK, Melton DA. Notochord repression of endodermal Sonic hedgehog permits pancreas development. Genes Dev. 1998;12(11):1705-1713. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Gittes GK. Developmental biology of the pancreas: a comprehensive review. Dev Biol. 2009;326(1):4-35. [DOI] [PubMed] [Google Scholar]
- 21. McCracken KW, Wells JM. Molecular pathways controlling pancreas induction. Semin Cell Dev Biol. 2012;23(6): 656-662. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Kumar M, Jordan N, Melton D, Grapin-Botton A. Signals from lateral plate mesoderm instruct endoderm toward a pancreatic fate. Dev Biol. 2003;259(1):109-122. [DOI] [PubMed] [Google Scholar]
- 23. Rossi JM, Dunn NR, Hogan BL, Zaret KS. Distinct mesodermal signals, including BMPs from the septum transversum mesenchyme, are required in combination for hepatogenesis from the endoderm. Genes Dev. 2001;15(15):1998-2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Hua H, Zhang YQ, Dabernat S, et al. BMP4 regulates pancreatic progenitor cell expansion through Id2. J Biol Chem. 2006;281(19):13574-13580. [DOI] [PubMed] [Google Scholar]
- 25. Miralles F, Czernichow P, Scharfmann R. Follistatin regulates the relative proportions of endocrine versus exocrine tissue during pancreatic development. Development. 1998;125(6):1017-1024. [DOI] [PubMed] [Google Scholar]
- 26. Kim SK, Hebrok M. Intercellular signals regulating pancreas development and function. Genes Dev. 2001;15(2):111-127. [DOI] [PubMed] [Google Scholar]
- 27. Murtaugh LC, Melton DA. Genes, signals, and lineages in pancreas development. Annu Rev Cell Dev Biol. 2003;19:71-89. [DOI] [PubMed] [Google Scholar]
- 28. Gradwohl G, Dierich A, LeMeur M, Guillemot F. neurogenin3 is required for the development of the four endocrine cell lineages of the pancreas. Proc Natl Acad Sci U S A. 2000;97(4):1607-1611. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Zaret KS. Genetic programming of liver and pancreas progenitors: lessons for stem-cell differentiation. Nat Rev Genet. 2008;9(5):329-340. [DOI] [PubMed] [Google Scholar]
- 30. Offield MF, Jetton TL, Labosky PA, et al. PDX-1 is required for pancreatic outgrowth and differentiation of the rostral duodenum. Development. 1996;122(3):983-995. [DOI] [PubMed] [Google Scholar]
- 31. Gannon M, Ables ET, Crawford L, et al. pdx-1 function is specifically required in embryonic beta cells to generate appropriate numbers of endocrine cell types and maintain glucose homeostasis. Dev Biol. 2008;314(2):406-417. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Hale MA, Kagami H, Shi L, et al. The homeodomain protein PDX1 is required at mid-pancreatic development for the formation of the exocrine pancreas. Dev Biol. 2005;286(1):225-237. [DOI] [PubMed] [Google Scholar]
- 33. Herrera PL. Adult insulin- and glucagon-producing cells differentiate from two independent cell lineages. Development. 2000;127(11):2317-2322. [DOI] [PubMed] [Google Scholar]
- 34. Prasadan K, Daume E, Preuett B, et al. Glucagon is required for early insulin-positive differentiation in the developing mouse pancreas. Diabetes. 2002;51(11):3229-3236. [DOI] [PubMed] [Google Scholar]
- 35. Stoffers DA, Ferrer J, Clarke WL, Habener JF. Early-onset type-II diabetes mellitus (MODY4) linked to IPF1. Nat Genet. 1997;17(2):138-139. [DOI] [PubMed] [Google Scholar]
- 36. Kataoka K, Han SI, Shioda S, Hirai M, Nishizawa M, Handa H. MafA is a glucose-regulated and pancreatic beta-cell-specific transcriptional activator for the insulin gene. J Biol Chem. 2002;277(51):49903-49910. [DOI] [PubMed] [Google Scholar]
- 37. Aramata S, Han SI, Kataoka K. Roles and regulation of transcription factor MafA in islet beta-cells. Endocr J. 2007;54(5):659-666. [DOI] [PubMed] [Google Scholar]
- 38. Zhang C, Moriguchi T, Kajihara M, et al. MafA is a key regulator of glucose-stimulated insulin secretion. Mol Cell Biol. 2005;25(12):4969-4976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Gu C, Stein GH, Pan N, et al. Pancreatic beta cells require NeuroD to achieve and maintain functional maturity. Cell Metab. 2010;11(4):298-310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Kim SY, Rane SG. The Cdk4-E2f1 pathway regulates early pancreas development by targeting Pdx1+ progenitors and Ngn3+ endocrine precursors. Development. 2011;138(10):1903-1912. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Kim YC, Kim SY, Mellado-Gil JM, et al. RB regulates pancreas development by stabilizing Pdx1. EMBO J. 2011;30(8):1563-1576. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Rane SG, Dubus P, Premkumar Reddy E, Barbacid M. Loss of Cdk4 expression causes insulin-deficient diabetes and Cdk4 activation results in β-islet cell hyperplasia. Nat Genet. 1999;22:44-52. [DOI] [PubMed] [Google Scholar]
- 43. Georgia S, Bhushan A. Beta cell replication is the primary mechanism for maintaining postnatal beta cell mass. J Clin Invest. 2004;114(7):963-968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Kushner JA, Ciemerych MA, Sicinska E, et al. Cyclins D2 and D1 are essential for postnatal pancreatic beta-cell growth. Mol Cell Biol. 2005;25(9):3752-3762. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Bonner-Weir S, Weir GC. New sources of pancreatic beta-cells. Nat Biotechnol. 2005;23(7):857-861. [DOI] [PubMed] [Google Scholar]
- 46. Lardon J, Bouwens L. Metaplasia in the pancreas. Differentiation. 2005;73(6):278-286. [DOI] [PubMed] [Google Scholar]
- 47. Lee JH, Jo J, Hardikar AA, Periwal V, Rane SG. Cdk4 regulates recruitment of quiescent beta-cells and ductal epithelial progenitors to reconstitute beta-cell mass. PLoS One. 2010;5(1):e8653. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Kim SY, Lee JH, Merrins MJ, et al. Loss of cyclin-dependent kinase 2 in the pancreas links primary β-cell dysfunction to progressive depletion of β-cell mass and diabetes. J Biol Chem. 2017;292(9):3841-3853. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Shiraki N, Lai CJ, Hishikari Y, Kume S. TGF-beta signaling potentiates differentiation of embryonic stem cells to Pdx-1 expressing endodermal cells. Genes Cells. 2005;10(6):503-516. [DOI] [PubMed] [Google Scholar]
- 50. Sanvito F, Nichols A, Herrera PL, et al. TGF-beta 1 overexpression in murine pancreas induces chronic pancreatitis and, together with TNF-alpha, triggers insulin-dependent diabetes. Biochem Biophys Res Commun. 1995;217(3):1279-1286. [DOI] [PubMed] [Google Scholar]
- 51. Grewal IS, Grewal KD, Wong FS, et al. Expression of transgene encoded TGF-beta in islets prevents autoimmune diabetes in NOD mice by a local mechanism. J Autoimmun. 2002;19(1-2):9-22. [DOI] [PubMed] [Google Scholar]
- 52. Shin JA, Hong OK, Lee HJ, et al. Transforming growth factor-β induces epithelial to mesenchymal transition and suppresses the proliferation and transdifferentiation of cultured human pancreatic duct cells. J Cell Biochem. 2011;112(1):179-188. [DOI] [PubMed] [Google Scholar]
- 53. Klein D, Álvarez-Cubela S, Lanzoni G, et al. BMP-7 induces adult human pancreatic exocrine-to-endocrine conversion. Diabetes. 2015;64(12):4123-4134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Böttinger EP, Jakubczak JL, Roberts IS, et al. Expression of a dominant-negative mutant TGF-beta type II receptor in transgenic mice reveals essential roles for TGF-beta in regulation of growth and differentiation in the exocrine pancreas. Embo J. 1997;16(10):2621-2633. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. Verspohl EJ, Ammon HP, Wahl MA. Activin A: its effects on rat pancreatic islets and the mechanism of action involved. Life Sci. 1993;53(13):1069-1078. [DOI] [PubMed] [Google Scholar]
- 56. Kim SK, Hebrok M, Li E, et al. Activin receptor patterning of foregut organogenesis. Genes Dev. 2000;14(15):1866-1871. [PMC free article] [PubMed] [Google Scholar]
- 57. Jiang W, Shi Y, Zhao D, et al. In vitro derivation of functional insulin-producing cells from human embryonic stem cells. Cell Res. 2007;17(4):333-344. [DOI] [PubMed] [Google Scholar]
- 58. Cho YM, Lim JM, Yoo DH, et al. Betacellulin and nicotinamide sustain PDX1 expression and induce pancreatic beta-cell differentiation in human embryonic stem cells. Biochem Biophys Res Commun. 2008;366(1):129-134. [DOI] [PubMed] [Google Scholar]
- 59. Mukherjee A, Sidis Y, Mahan A, et al. FSTL3 deletion reveals roles for TGF-beta family ligands in glucose and fat homeostasis in adults. Proc Natl Acad Sci U S A. 2007;104(4):1348-1353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60. Brown ML, Bonomi L, Ungerleider N, et al. Follistatin and follistatin like-3 differentially regulate adiposity and glucose homeostasis. Obesity (Silver Spring). 2011;19(10):1940-1949. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61. Lima MJ, Muir KR, Docherty HM, et al. Suppression of epithelial-to-mesenchymal transitioning enhances ex vivo reprogramming of human exocrine pancreatic tissue toward functional insulin-producing β-like cells. Diabetes. 2013;62(8): 2821-2833. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62. Yamaoka T, Idehara C, Yano M, et al. Hypoplasia of pancreatic islets in transgenic mice expressing activin receptor mutants. J Clin Invest. 1998;102(2):294-301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63. Lin HM, Lee JH, Yadav H, et al. Transforming growth factor-beta/Smad3 signaling regulates insulin gene transcription and pancreatic islet beta-cell function. J Biol Chem. 2009;284(18):12246-12257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64. Smart NG, Apelqvist AA, Gu X, et al. Conditional expression of Smad7 in pancreatic beta cells disrupts TGF-beta signaling and induces reversible diabetes mellitus. PLoS Biol. 2006;4(2):e39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65. Sjöholm A, Hellerström C. TGF-beta stimulates insulin secretion and blocks mitogenic response of pancreatic beta-cells to glucose. Am J Physiol. 1991;260(5 Pt 1):C1046-C1051. [DOI] [PubMed] [Google Scholar]
- 66. Totsuka Y, Tabuchi M, Kojima I, Eto Y, Shibai H, Ogata E. Stimulation of insulin secretion by transforming growth factor-beta. Biochem Biophys Res Commun. 1989;158(3):1060-1065. [DOI] [PubMed] [Google Scholar]
- 67. Nomura M, Zhu HL, Wang L, Morinaga H, Takayanagi R, Teramoto N. SMAD2 disruption in mouse pancreatic beta cells leads to islet hyperplasia and impaired insulin secretion due to the attenuation of ATP-sensitive K+ channel activity. Diabetologia. 2014;57(1):157-166. [DOI] [PubMed] [Google Scholar]
- 68. Brusko T, Atkinson M. Treg in type 1 diabetes. Cell Biochem Biophys. 2007;48(2-3):165-175. [DOI] [PubMed] [Google Scholar]
- 69. Jin Y, Chen X, Podolsky R, et al. APC dysfunction is correlated with defective suppression of T cell proliferation in human type 1 diabetes. Clin Immunol. 2009;130(3):272-279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70. Ahrén B. Type 2 diabetes, insulin secretion and beta-cell mass. Curr Mol Med. 2005;5(3):275-286. [DOI] [PubMed] [Google Scholar]
- 71. Heit JJ, Apelqvist AA, Gu X, et al. Calcineurin/NFAT signalling regulates pancreatic beta-cell growth and function. Nature. 2006;443(7109):345-349. [DOI] [PubMed] [Google Scholar]
- 72. Robertson RP. Chronic oxidative stress as a central mechanism for glucose toxicity in pancreatic islet beta cells in diabetes. J Biol Chem. 2004;279(41):42351-42354. [DOI] [PubMed] [Google Scholar]
- 73. Hur KY, Jung HS, Lee MS. Role of autophagy in β-cell function and mass. Diabetes Obes Metab. 2010;12(Suppl 2):20-26. [DOI] [PubMed] [Google Scholar]
- 74. Lee JH, Mellado-Gil JM, Bahn YJ, Pathy SM, Zhang YE, Rane SG. Protection from β-cell apoptosis by inhibition of TGF-β/Smad3 signaling. Cell Death Dis. 2020;11(3):184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75. Talchai C, Xuan S, Lin HV, Sussel L, Accili D. Pancreatic β cell dedifferentiation as a mechanism of diabetic β cell failure. Cell. 2012;150(6):1223-1234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76. Toren-Haritan G, Efrat S. TGFβ pathway inhibition redifferentiates human pancreatic islet β cells expanded in vitro. PLoS One. 2015;10(9):e0139168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77. Xiao X, Wiersch J, El-Gohary Y, et al. TGFβ receptor signaling is essential for inflammation-induced but not β-cell workload-induced β-cell proliferation. Diabetes. 2013;62(4): 1217-1226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78. Sehrawat A, Shiota C, Mohamed N, et al. SMAD7 enhances adult β-cell proliferation without significantly affecting β-cell function in mice. J Biol Chem. 2020;295(15):4858-4869. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79. D’Amour KA, Agulnick AD, Eliazer S, Kelly OG, Kroon E, Baetge EE. Efficient differentiation of human embryonic stem cells to definitive endoderm. Nat Biotechnol. 2005;23(12):1534-1541. [DOI] [PubMed] [Google Scholar]
- 80. D’Amour KA, Bang AG, Eliazer S, et al. Production of pancreatic hormone-expressing endocrine cells from human embryonic stem cells. Nat Biotechnol. 2006;24(11):1392-1401. [DOI] [PubMed] [Google Scholar]
- 81. Kroon E, Martinson LA, Kadoya K, et al. Pancreatic endoderm derived from human embryonic stem cells generates glucose-responsive insulin-secreting cells in vivo. Nat Biotechnol. 2008;26(4):443-452. [DOI] [PubMed] [Google Scholar]
- 82. Chen S, Borowiak M, Fox JL, et al. A small molecule that directs differentiation of human ESCs into the pancreatic lineage. Nat Chem Biol. 2009;5(4):258-265. [DOI] [PubMed] [Google Scholar]
- 83. Johannesson M, Ståhlberg A, Ameri J, Sand FW, Norrman K, Semb H. FGF4 and retinoic acid direct differentiation of hESCs into PDX1-expressing foregut endoderm in a time- and concentration-dependent manner. PLoS One. 2009;4(3):e4794. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84. Pagliuca FW, Melton DA. How to make a functional β-cell. Development. 2013;140(12):2472-2483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85. Nostro MC, Sarangi F, Ogawa S, et al. Stage-specific signaling through TGFβ family members and WNT regulates patterning and pancreatic specification of human pluripotent stem cells. Development. 2011;138(5):861-871. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86. Schulz TC, Young HY, Agulnick AD, et al. A scalable system for production of functional pancreatic progenitors from human embryonic stem cells. PLoS One. 2012;7(5):e37004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87. Rezania A, Riedel MJ, Wideman RD, et al. Production of functional glucagon-secreting α-cells from human embryonic stem cells. Diabetes. 2011;60(1):239-247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88. Rezania A, Bruin JE, Arora P, et al. Reversal of diabetes with insulin-producing cells derived in vitro from human pluripotent stem cells. Nat Biotechnol. 2014;32(11):1121-1133. [DOI] [PubMed] [Google Scholar]
- 89. Pagliuca FW, Millman JR, Gürtler M, et al. Generation of functional human pancreatic β cells in vitro. Cell. 2014;159(2):428-439. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90. Xiao X, Fischbach S, Song Z, et al. Transient suppression of TGFβ receptor signaling facilitates human islet transplantation. Endocrinology. 2016;157(4):1348-1356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91. Lee MS, Gu D, Feng L, et al. Accumulation of extracellular matrix and developmental dysregulation in the pancreas by transgenic production of transforming growth factor-beta 1. Am J Pathol. 1995;147(1):42-52. [PMC free article] [PubMed] [Google Scholar]
- 92. Moritani M, Yamasaki S, Kagami M, et al. Hypoplasia of endocrine and exocrine pancreas in homozygous transgenic TGF-beta1. Mol Cell Endocrinol. 2005;229(1-2):175-184. [DOI] [PubMed] [Google Scholar]
- 93. Hanley S, Rosenberg L. Transforming growth factor beta is a critical regulator of adult human islet plasticity. Mol Endocrinol. 2007;21(6):1467-1477. [DOI] [PubMed] [Google Scholar]
- 94. Ku HT, Zhang N, Kubo A, et al. Committing embryonic stem cells to early endocrine pancreas in vitro. Stem Cells. 2004;22(7):1205-1217. [DOI] [PubMed] [Google Scholar]
- 95. Frandsen U, Porneki AD, Floridon C, Abdallah BM, Kassem M. Activin B mediated induction of Pdx1 in human embryonic stem cell derived embryoid bodies. Biochem Biophys Res Commun. 2007;362(3):568-574. [DOI] [PubMed] [Google Scholar]
- 96. Bertolino P, Holmberg R, Reissmann E, Andersson O, Berggren PO, Ibáñez CF. Activin B receptor ALK7 is a negative regulator of pancreatic beta-cell function. Proc Natl Acad Sci U S A. 2008;105(20):7246-7251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97. Goto Y, Nomura M, Tanaka K, et al. Genetic interactions between activin type IIB receptor and Smad2 genes in asymmetrical patterning of the thoracic organs and the development of pancreas islets. Dev Dyn. 2007;236(10):2865-2874. [DOI] [PubMed] [Google Scholar]
- 98. Shiozaki S, Tajima T, Zhang YQ, Furukawa M, Nakazato Y, Kojima I. Impaired differentiation of endocrine and exocrine cells of the pancreas in transgenic mouse expressing the truncated type II activin receptor. Biochim Biophys Acta. 1999;1450(1):1-11. [DOI] [PubMed] [Google Scholar]
- 99. Goulley J, Dahl U, Baeza N, Mishina Y, Edlund H. BMP4-BMPR1A signaling in beta cells is required for and augments glucose-stimulated insulin secretion. Cell Metab. 2007;5(3):207-219. [DOI] [PubMed] [Google Scholar]
- 100. Harmon EB, Apelqvist AA, Smart NG, Gu X, Osborne DH, Kim SK. GDF11 modulates NGN3+ islet progenitor cell number and promotes beta-cell differentiation in pancreas development. Development. 2004;131(24):6163-6174. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Data sharing is not applicable to this article as no datasets were generated or analyzed during the current study. All requests for data and information will be addressed by the corresponding author.