Abstract
OBJECTIVE.
Vascular Ehlers-Danlos syndrome (vEDS) is a rare connective tissue disorder due to pathogenic variants in COL3A1 that lead to impaired type III collagen production. We aim to describe the contemporary multi-institutional experience of aortic and arterial pathology in individuals with vEDS, to evaluate disease patterns, and to refine management recommendations.
METHODS.
This is a cross-sectional retrospective study of individuals with genetically confirmed vEDS between 2000 and 2015 at multiple institutions participating in the Vascular Low Frequency Disease Consortium. Aortic and arterial events including aneurysms, pseudoaneurysms, dissections, fistulae, or ruptures were studied. Demographics, COL3A1 variants, management, and outcomes data were collected and analyzed. Individuals with and without arterial events were compared.
RESULTS.
Eleven institutions identified 86 individuals with pathogenic variants in COL3A1 (47.7% male, 86% Caucasian, median age 41 [IQR 31– 49.5] years, 65.1% missense COL3A1 variants). The median follow up from the time of vEDS diagnosis was 7.5 [IQR 3.5–12] years. A total of 139 aortic/arterial pathologies were diagnosed in 53 (61.6%) individuals (50.9% Male, 88.5% Caucasian, median age 33 [IQR 25–42.3] years. The aortic/arterial events presented as an “emergency” in 52 (37.4%) cases. The most commonly affected arteries were the mesenteric arteries (31.7%), followed by cerebrovascular (16.5%), iliac (16.5%), and renal arteries (12.2%). The most common management was medical management. When undertaken, the predominant endovascular interventions were arterial embolization of medium sized arteries (13.4%), followed by stenting (2.5%). Aortic pathology was noted in 17 (32%) individuals (58.8% male, 94.1% Caucasian, median age 38.5 [IQR 30.8–44.7] years). Most notably, 4 individuals underwent successful abdominal aortic aneurysm (AAA) repair with excellent results on follow up. Individuals with missense mutations, in which glycine was substituted with a large amino acid, had an earlier onset of aortic/arterial pathology (Median age 30 IQR 23.5–37] years) compared to the other pathogenic COL3A1 variants (Median age 36 [IQR 29.5–44.8] years, P=.065). There were 12 deaths (22.6%) at a median age of 36 [IQR 28–51] years.
CONCLUSIONS.
Most of vEDS arterial manifestations were managed medically in this cohort. When intervention is required for an enlarging aneurysm or rupture, embolization and less frequently stenting, appear to be well tolerated. Open repair of AAA appears to be as well tolerated as those without vEDS; vEDS should not be a deterrent to offering an operation. Future work to elucidate the role of surgical interventions and refine management recommendations in the context of patient centered outcomes is warranted.
Keywords: Vascular Ehlers-Danlos Syndrome, COL3A1 mutation, Arterial dissection, Arterial aneurysm, Arterial rupture
INTRODUCTION
Vascular Ehlers-Danlos syndrome (vEDS) is a rare syndrome in which type III collagen production is reduced or the collagen produced is defective because of autosomal dominant mutations in COL3A1.1–3 The syndrome, which was previously called Ehlers-Danlos syndrome type IV is one of 13 subtypes of Ehlers-Danlos syndrome.4 In addition to spontaneous intestinal perforation or rupture of a gravid uterus, the hallmark of the disease is spontaneous arterial dissections, aneurysms, and rupture at a young age.1,2,5
Up to 40% of individuals with vEDS experience their first major arterial complication by age 40.2,6 The classically reported arteries include mesenteric, renal, iliac, femoral and/or the abdominal aorta, followed by the carotid, subclavian, ulnar, popliteal, and tibial arteries.7 A spontaneous carotid-cavernous fistula (CCF) is pathognomonic of vEDS and estimated to occur in 9.8% of individuals with vEDS.8
The predominant challenges to studying vEDS are driven by the rarity of the disease, the heterogeneous presentation of aortic and arterial pathology, the large number of pathogenic variants in COL3A1 leading to vEDS, lack of robust longitudinal data, and under or misdiagnosis. While we have a basic understanding of the natural history of the disease based on seminal work2 and a limited understanding of the genotype-phenotype correlation6,9–11, we do not have a detailed natural history of aortic and arterial aneurysms and dissections in this population. Nor do we have a clear understanding of the risk of complications, once diagnosed and treated.12 The aim of this study is to describe the contemporary multi-institutional experience of aortic and arterial pathology in individuals with vEDS, to evaluate disease patterns, and refine management recommendations to improve our understanding of genotype-phenotype correlations.
METHODS
The Vascular Low Frequency Disease Consortium
This is a multi-institutional retrospective cross-sectional cohort study of individuals diagnosed with vEDS between January 1, 2000 and December 31, 2015. The 11 institutions were recruited through the Vascular Low Frequency Disease Consortium (University of California-Los Angeles Division of Vascular Surgery).13 Each participating center obtained its own Institutional Review Board (IRB) approval. The IRBs waived the patient consent process due to minimal patient risk. Data were collected by each institute’s respective investigator(s), deidentified, and then submitted and stored using a password-encrypted database maintained by the University of Washington.
Identification of individuals with vEDS, Inclusion/Exclusion Criteria
Individuals were initially identified with International Classification of Diseases (ICD)-9 -CM code 756.83 or ICD-10-CM code Q79.6 for Ehlers-Danlos syndrome. Confirmatory molecular testing results were then reviewed by a geneticist (PHB) to confirm that the COL3A1 variant is a pathogenic variant in keeping with the ACMG guidelines.2,9,10 Individuals were included for analysis only if they had a pathogenic variant in COL3A1. The pathogenic COL3A1 variants were grouped into missense mutations (glycine substations), exon skip and splice site variants, and haploinsufficiency (null mutations). 6,9–11,14–16 The type of amino acid substitution was noted as a large or small amino acid.6,15
Demographics, current age, age at diagnosis, family history, clinical diagnostic criteria17, aortic and arterial pathology, management of aortic and arterial pathology, and outcomes were collected. Family history was defined as a family history of vEDS, aortic or arterial aneurysms and dissections, and/or sudden death. Arterial pathology included aortic and arterial aneurysms, dissections, pseudoaneurysms, fistulae, thrombosis, or ruptures. Arterial pathology was noted as emergent if it was life threating on presentation. This included aortic or arterial rupture, symptomatic aortic/arterial dissections, and CCF.
Given that subject data was collected locally at each participating institution and then submitted as de-identified data to the consortium, a comparison of all the presentations, COL3A1 variants, and demographics was performed to assure that there were no duplicated cases. Data were analyzed using Microsoft Excel 2007 software (Microsoft, Redmond, WA) and SPSS version 19 for Windows (SPSS, Inc., Chicago, IL). Continuous data are presented as medians and interquartile ranges (IQR).Continuous data were compared using Wilcoxon rank-sum (Mann-Whitney) test. Categorical data were compered by Chi square or Fisher’s exact test where appropriate. The comparisons of the onset of the arterial pathology and survival were performed using Kaplan–Meier survival curves with Log-rank test. All statistical tests were two-sided and a P-value < .05 was considered statistically significant.
RESULTS
Eight-six individuals had molecular confirmation of vEDS (47.7% male, 86% Caucasian, median age 41 years, IQR 31– 49.5 years, range 1–88 years). The cohort included 19 (22.1%) individuals who were diagnosed as children (age < 18 years old). The median follow up from the time of vEDS diagnosis was 7.5 (IQR 3.5–12) years.
A total of 139 aortic/arterial pathologies were diagnosed in 53 (61.6%) individuals (50.9% Male, 88.5% Caucasian, median age 33 years, IQR 25–42.3 years). The aortic/arterial events presented as an “emergency” in 52 of (37.4%) cases.
The diagnosis of vEDS was already established in 20 (37.3%) individuals prior to the diagnosis of aortic/arterial pathology. The diagnosis of vEDS was less likely be known in the emergent setting compared to the elective stetting (18.2% vs. 55.2%, P=.007).
There were no differences between men and women in the age of the initial aortic/arterial pathology diagnosis (Median 32 [IQR 23–42] years among men, median 36 [IQR 26.8–43.3 years among women, P=393), or the time of first aortic/ arterial rupture (Median 33 [IQR 15.5–49] years among men, median 39 [IQR 30–46.7] years among women, P=.540). The individuals with aortic/arterial pathology were significantly older than those without (Table 1). In addition, the group without aortic/arterial pathology included 14 children compared to only one child who presented with type B aortic dissection (TBAD) at age 12 years old (had missense mutation, p.Gly244Arg). Those patients with aortic/arterial pathology had more hypertension and deep vein thrombosis. There were no differences in the type of pathogenic COL3A1 variants or minor clinical diagnostic criteria between those with and without aortic/arterial pathology, with the exception of a lower frequency of hypermobile small joints (32.1% vs. 54.5%, P=.039).
Table 1.
A comparison of individuals with vascular Ehlers-Danlos syndrome with and without a diagnosis of aortic/arterial pathology
| N (%), Median [IQR] | Aortic/Arterial pathology (n=53) | No aortic/arterial pathology (n=33) | P |
|---|---|---|---|
| Current Age | 41 [31–49.5] | 25 [15–41.5] | <.001 |
| Age range | 19–79 | 1–88 | - |
| Age at diagnosis | 32 [23–43.3] | 18 [10–31] | <.001 |
| Male | 27 (50.9) | 14 (42.4) | 0.442 |
| Caucasian | 47 (88.7) | 27 (81.8) | 0.491 |
| BMI | 24.6 [21.6–27.4] | 22.9 [17.1–25.3] | 0.068 |
| Hypertension | 17 (32.1) | 2 (6.1) | 0.005 |
| Deep vein thrombosis | 12 (22.6) | 1 (3) | 0.014 |
| Intestinal perforation | 9 (17) | 5 (15.2) | 0.823 |
| Spontaneous PTX/HTX | 7 (13.2) | 5 (15.2) | 0.800 |
| Current or past smoker | 12 (22.6) | 5 (15.2) | 0.396 |
| Family history of VEDS | 24 (45.3) | 18 (54.5) | 0.403 |
| Follow up post vEDS diagnosis | 7.5 [3.5–12] | 5 [1–11] | 0.152 |
| Died | 13 (24.5) | 0 | 0.002 |
| Age at death | 39 [27.8–51.3] | - | - |
Arterial pathology
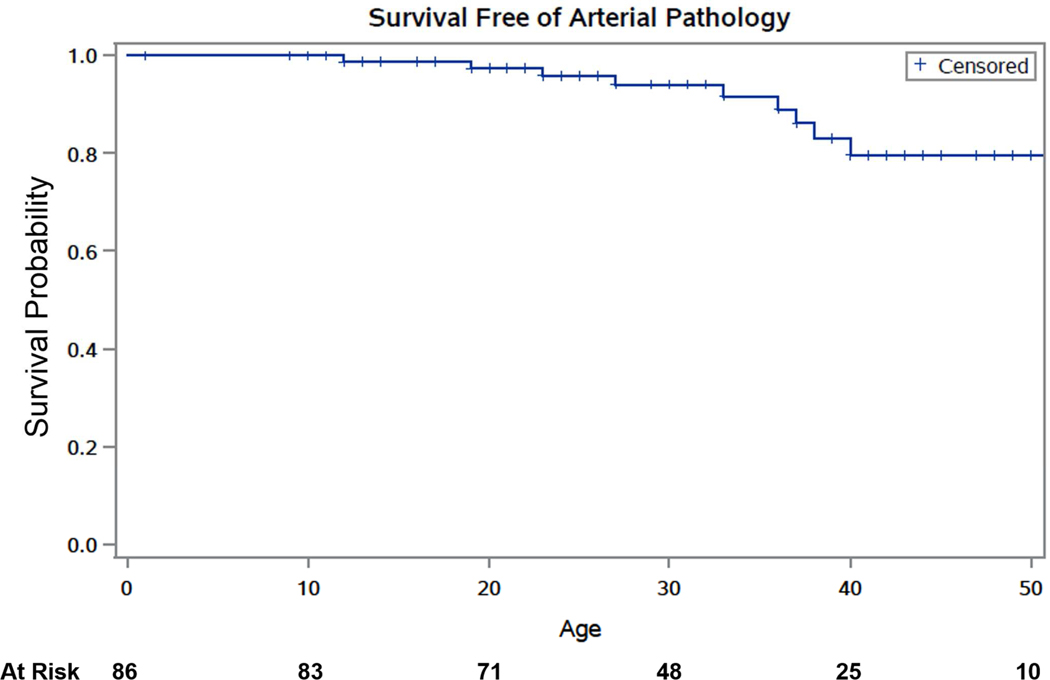
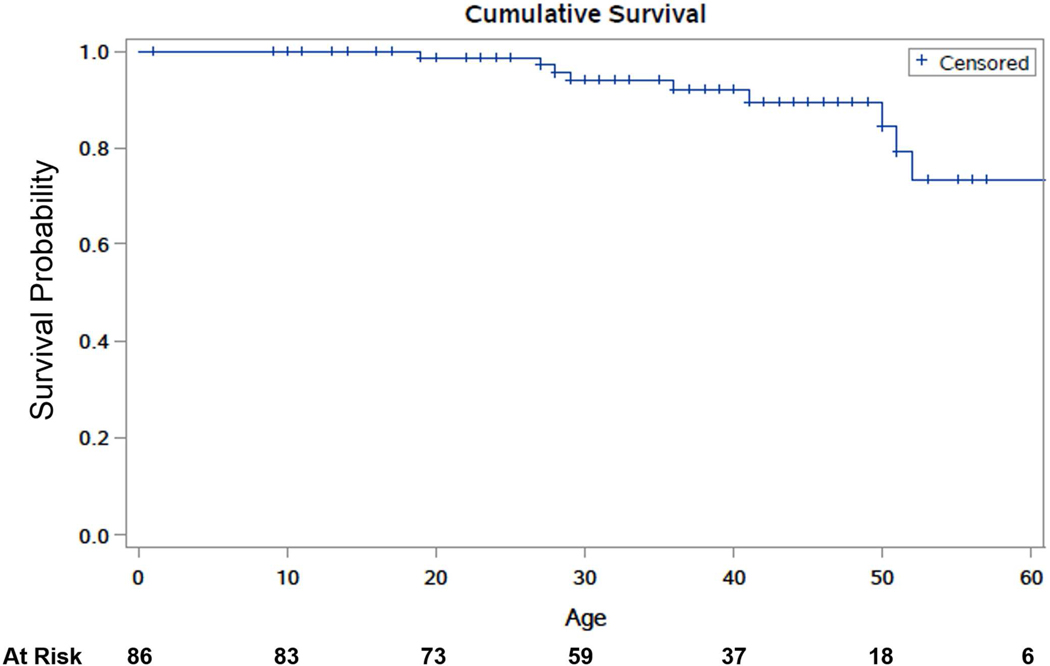
The most commonly affected arteries were the mesenteric arteries (31.7%), followed by cerebrovascular and iliac arteries (16.5% each), and renal arteries (12.2%). Arterial pathology included aneurysms (53.8%), dissections (35.3%), rupture (10.1%), pseudoaneurysms (3.4%), thrombosis (2.5%), and CCF (4.2%). Figure 1 shows Kaplan-Meier estimates of cumulative survival free of any arterial pathology. Table 2 summarizes the presentation and management of the arterial pathologies.
Figure 1.
Kaplan-Meier estimates of cumulative survival free of any arterial pathology in a cohort of individuals with vascular Ehlers-Danlos syndrome.
Table 2.
Presentation and management of arterial pathology in patients with vascular Ehlers-Danlos syndrome by artery involved. The % given is the percentage of all arterial pathology
| Arteries N (%) | % Male | Median Age (IQR) | Known vEDS diagnosis | Missense mutation | Dissection | Aneurysm, Pseudoaneur ysm | Rupture | Medical Management | Endovascular Embolization | Endovascular Stenting | Open Repair |
|---|---|---|---|---|---|---|---|---|---|---|---|
| Carotid N=17 (12.2) | 5 (41.7) | 27 (24–36) | 4 (33.3) | 8 (66.7) | 6 (50) | 5 (41.7), 1 (8.3) | 0 | 100% | 0 | 0 | 0 |
| Vertebral N=6 (4.3) | 2 (33.3) | 27.5 (22–32.5) | 3 (50) | 5 (83.3) | 4 (66.6) | 1 (16.7) | 0 | 100% | 0 | 0 | 0 |
| Celiac N=14 (10.5) | 6 (40) | 44 (33–50) | 12 (80) | 11 (73.3) | 7 (46.7) | 7 (46.7), 2 (13.3) | 2 (13.3) | 13 (86.7) | 1 (6.7)* | 0 | 1 (6.7) |
| Gastric N=3 (2.2) | 0 | 37 (36–37) | 2 (66.7) | 2 (66.7) | 1 (33.3) | 0 | 0 | 3 (100) | 0 | 0 | 0 |
| Phrenic N=1 (0.7) | 0 | 58 | 1 (100) | 1 (100) | 0 | 1 (100) | 0 | 1 (100) | 0 | 0 | 0 |
| Splenic N=11 (7.9) | 3 (27.3) | 44 (31–51) | 6 (54.5) | 6 (54.5) | 2 (18.2) | 8 (72.7) | 4 (36.4) | 4 (36.4) | 6 (54.5) | 0 | 1 (9.4) |
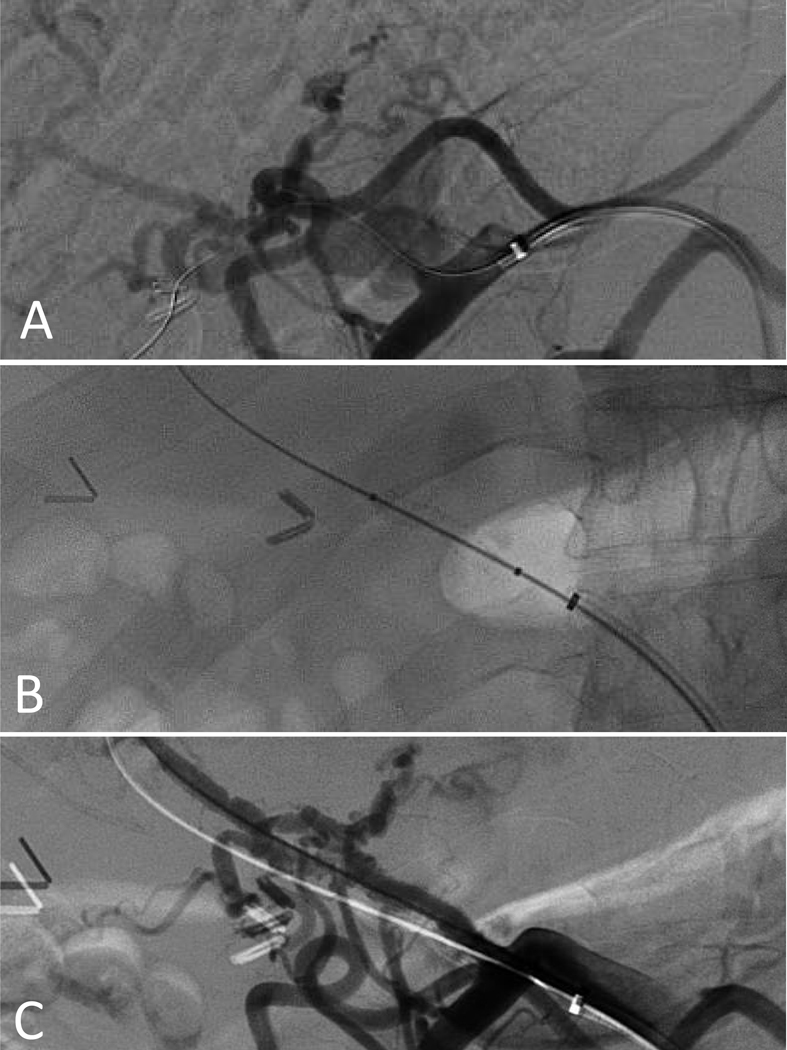
| Hepatic N=8 (5.8) | 5 (62.5) | 47 (38.5–50.5) | 5 (62.5) | 4 (50) | 0 | 7 (87.5), 1 (12.5) | 1 12.5) | 5 (62.5) | 2 (25) | 1 (12.5) (Figure 3) | 0 |
| Superior mesenteric N=6 (4.3) | 1 (16.7) | 45.5 (39–54.4) | 5 (83.3) | 3 (50) | 2 (33.3) | 2 (33.3) | 1 (16.7) | 5 (83.3) | 1 (16.7)* | 0 | 0 |
| Renal N=17 (12.2) | 12 (70.6) | 37 (30.5–41) | 9 (52.9) | 13 (76.5) | 7 (41.2) | 8 (47.1), 1 (5.9) | 0 | 14 (82.4) | 1 (5.9) | 0 | 2 (11.8) |
| Iliac N=23 (16.5) | 18 (78.3) | 41 (30–48) | 12 (52.2) | 19 (82.6) | 7 (30.4) | 16 (69.6) | 1 (4.3) | 17 (73.9) | 1 (4.2) | 2 (8.7) | 3 (13) |
| Femoral N=2 (1.4) | 1 (50) | 27,33 | 0 | 2 (100) | 1 | 1 | 0 | 1 | 0 | 0 | 1 |
| Popliteal N=1 (0.7) | 0 | 26 | 0 | 1 (100) | 0 | 1 | 0 | 1 (100) | 0 | 0 | 0 |
| Posterior Tibial N=3 (2.2)** | 2 (100) | 19,32 | 1 (50) | 1 (50) | 0 | 3 | 0 | 2 | 0 | 0 | 1 |
Same individual, death due to multiple mesenteric arterial ruptures
One individual had bilateral posterior tibial arteries aneurysms
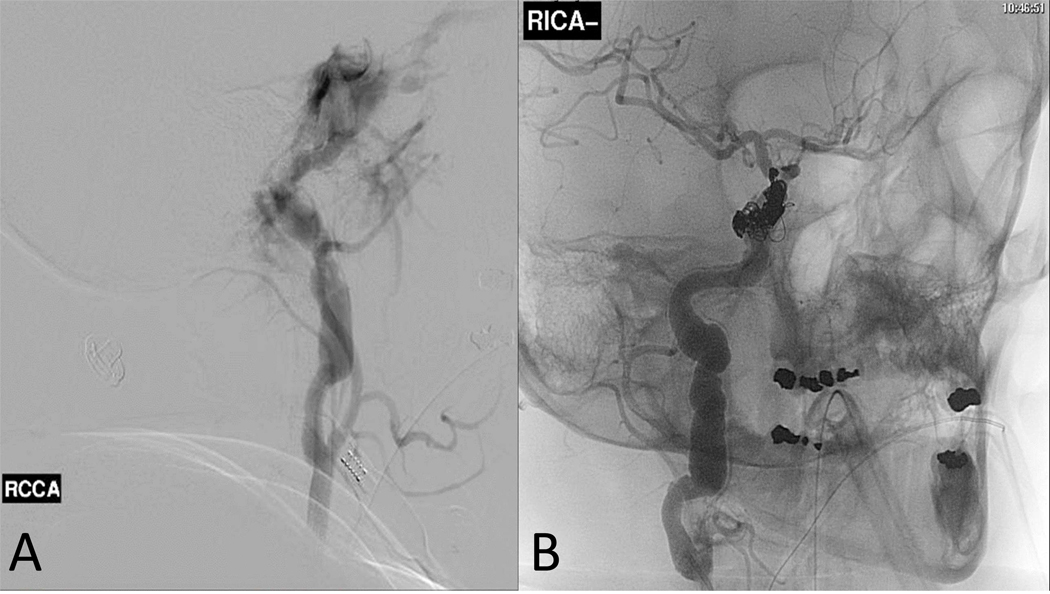
Carotid cavernous fistulae. CCF occurred in 4 individuals ((Table 3). Management was predominantly via embolization with satisfactory outcomes (Figure 2). None of the CCFs were associated with mortality.
Carotid and vertebral pathology other than CCF. These patients presented with small aneurysms or dissections. All were managed medically, as detailed in table 2. Most had no complications, with the exception of one patient with a vertebral artery dissection leading to a lateral medullary infarct and death.
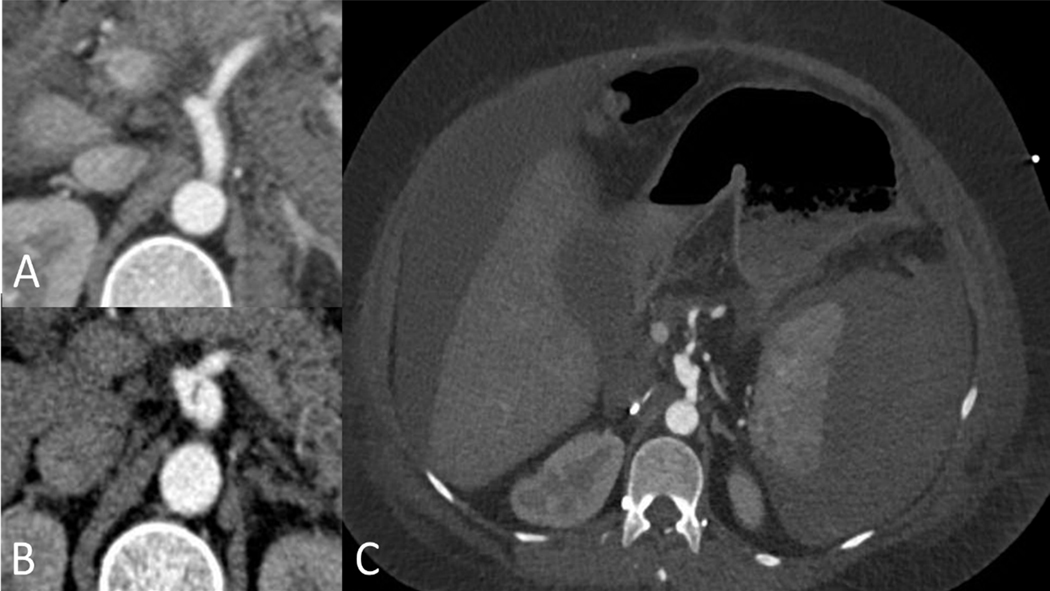
Mesenteric arteries. The celiac artery was the most commonly affected mesenteric artery (Figure 3), however, the splenic artery was most frequently affected by rupture (36.4%). Medical management was the most common approach (Table 2) with the exception of cases in which rupture or pseudoaneurysms occurred. These cases were treated most commonly with endovascular embolization, and in one case with stenting (Figure 4), with satisfactory results. There was one mortality in this group (a patient who presented with multiple mesenteric arterial ruptures).
Renal arteries. Renal arteries presented with nearly equal frequency of dissections and aneurysms. Aneurysm sizes were recorded in 4 cases (0.8 cm, 0.8 cm, 1.2 cm, and 3 cm). The predominant approach to treatment was medical management (Table 2), with the exception of two patients requiring intervention: a 40-year-old male who underwent embolization (no additional detail), and a 29-year-old male (c.1124G>A, p.Gly375Glu) who presented with left renal artery thrombosis that was treated with thrombolysis. This approach was complicated by splenic hemorrhage requiring a splenectomy. He presented 5 months later with aneurysmal degeneration of the renal artery and underwent a successful nephrectomy (6 year follow-up).
- Iliac arteries. Twenty-three common, external, and internal iliac arteries had pathology, which was diagnosed as isolated iliac disease in 9 (16.9%), and in association with abdominal aortic aneurysm in 8 (15.1%) individuals (Table 2). Most were managed medically. Stenting was performed in 2 cases (c.3847C>T, p.Gln1283Ter and c.3320G>A, Gly107Glu) for common iliac artery aneurysms, using femoral artery exposure and stent repair, with satisfactory results. Open iliac repair was performed in 3 cases:
- ○ For an iliac artery rupture in a 50-year-old male (c.1295G>A, p.Gly432Asp), which was complicated with post-operative wound dehiscence with development of enterocutaneous fistula
- ○ For an iliac artery dissection with thrombosis treated with an uncomplicated iliofemoral bypass in a 30-year-old male (c.674G>C, p.Gly225Ala). The patient has had 6 years of follow-up post procedure without complication.
- ○ For an iliac artery aneurysm treated with an uncomplicated open bypass in a 41-year-old male (c.2356G>A, p.Gly786Arg). Of note, this individual had a prior open repair for a ruptured abdominal aortic aneurysm repair at age 33. The patient has had 10 years of follow up post procedure without complication.
Posterior tibial arteries: Two patients had posterior tibial artery aneurysms (3.8%); one was managed medically. The other individual was a 19-year-old male with bilateral posterior tibial artery aneurysms (1.5 and 2 cm respectively). Open repair with saphenous vein bypass graft was performed for the larger aneurysm. This procedure was complicated by hematoma due to vein graft disruption, requiring re-operation. Other rare presentations included involvement of the cerebral and coronary arteries as follows:
Cerebral arteries. Only one individual was affected (1.9%). This was a 39-year-old female (Haploinsufficiency/Null mutation) who presented with a left middle cerebral artery dissection. The dissection was managed medically and she is alive at age 43.
Coronary arteries: There were two cases (3.8%) of coronary artery dissection. One occurred in a 45-year-old woman (c.4360C>T, p.Gln1454Ter) who was initially managed medically but subsequently underwent coronary artery bypass. She has had one-year follow-up without complications. The second case was in a 41-year-old woman (c.2337+2T>C, p.Gly762_Lys779del) who was hospitalized for a spontaneous perforation of the colon. She developed a left anterior descending coronary artery dissection and subsequent cardiac arrest.
Table 3.
Presentation and management of carotid cavernous fistulae in patients with vascular Ehlers-Danlos syndrome
| Age/Sex | COL3A1 variant | Presentation | Management, access site | Length of stay (days), disposition |
|---|---|---|---|---|
| 49F | c.2131G>A, p. Gly544Ser (missense) | Rupture | Embolization, Percutaneous, 5 Fr, manual pressure closure | 7, Home, Alive at 63 14 years follow up |
| 51M | IVS8+5 G>A (Exon skip) | N/A | Embolization, Percutaneous, 5 Fr, manual pressure closure | 3, Home, alive at 70 |
| 41F | c.2337+2T>C, p.Gly762_Lys779del (splice site) | Ipsilateral retro-orbital pain, blurry vision, and tinnitus | Embolization, Percutaneous, 6 Fr, closure with angioseal (Figure 3) | 4, Home. Less than 1 year follow up. Died from unrelated complications |
| 42F | Pathogenic | Bilateral | Non-operative | N/A |
Figure 2.
The angiographic findings of a carotid cavernous fistula in a 41-year-old woman with vascular Ehlers-Danlos syndrome due to a splice site mutation (c.2337+2T>C/p.Gly762_Lys779del) presenting with sudden onset ipsilateral retro-orbital pain, blurry vision, and tinnitus. (A) Pre-embolization. (B) Post coil embolization.
Figure 3.
Celiac artery dissection in 36 year old woman with vascular Ehlers-Danlos syndrome due to a splice site mutation (c.2337+2T>C/p.Gly762_Lys779del) managed medically. A. axial CT imaging obtained one month prior to the dissection when she presented with a spontaneous hemoperitoneum managed medically. B. Axial CT imaging demonstrating focal dissection and mild enlargement of the celiac artery. This remained unchanged on follow up imaging over the next 5 years. C. Axial imaging demonstrating a large spontaneous subcapsular splenic hematoma and hemoperitoneum. This occurred following a complicated hospitalization for perforated sigmoid diverticulitis requiring sigmoid resection, transverse colostomy and Hartmann’s pouch.
Figure 4.
Angiogram showing a proper hepatic artery pseudoaneurysm in a 49 year old man with vascular Ehlers-Danlos syndrome due to a haploinsufficiency mutation (A). The pseudoaneurysm was excluded with 5-mm x 2.5-cm Viabahn stent (B and C)
Endovascular Procedures for medium sized arteries
Embolization of medium sized arteries was the predominant endovascular procedure performed (n=16, 13.4%), followed by stenting (n=3, 2.5%) as described previously. Percutaneous access was used in 14 cases for sheath sizes of 4–7 French. Manual pressure post procedure was used in 7 cases (4 in which the vEDS diagnosis was not known at the time); the closure was satisfactory in all 7 cases, with one (14.3%) hematoma reported. A closure device was used in 5 cases: Angio-Seal™ (n=2), StarClose (n=1), Perclose ProGlide (n=1). All were reported to be successful. Open femoral artery exposure and repair was performed in an additional 3 cases, with one complicated by hematoma.
Aortic Pathology
Aortic pathology was identified in 17 (32%) individuals (58.8% male, 94.1% Caucasian, median age 38.5 [IQR 30.8–44.7] years). Hypertension was noted in 52.9% (n=9) and smoking in 29.4% (n=5) of the cases. The most common COL3A1 variant was a missense mutation with glycine substitution with a large amino acid residue (n=11, 64.7%).
Thoracic aortic pathology was reported in 10 individuals and abdominal aortic pathology was reported in 7 individuals (Table 4). Notably, there were two thoracic endovascular aneurysm repairs (TEVAR) performed for descending thoracic aortic aneurysm ruptures in which the diagnosis of vEDS was unknown at the time. One individual died while the other had a successful repair (Table 4). In one case, an open thoracoabdominal aortic aneurysm (TAAA extent II) was performed successfully in a 19-year-old male with a missense mutation (Table 4).
Table 4.
Presentation and management of aortic pathology in 17 individuals with vascular Ehlers-Danlos syndrome.
| Age/Sex | COL3A1 variant* | Location /type | Management Known Outcomes vEDS diagnosis | ||
|---|---|---|---|---|---|
| Thoracic Aorta (N=10) | |||||
| 33M | c.601G>C, p.Gly201Arg | Thoracic aneurysm | Medical | Yes | Died at age 51 due to stroke |
| 44F | c.970G>A, p.GlyS24Ser | ATA aneurysm | Medical | Yes | Alive at age 48 |
| 37F | c. 764G>A,p.Gly255 Glu | Ruptured type A aortic dissection | Medical | Yes | Cause of death |
| 79M | c.SS20G>A, p.Gly1107Glu | Arch PAU | Medical | Yes | - |
| 40F | c.2222G>A, p.Gly574Asp | DTAA | Medical | Yes | Diameter 4.2 cm. Alive at age 41 |
| 53M | c.S966delG, p.Lys1S2SArgfs*64 (null) | TBAD/rupture | Medical | Yes | Cause of death, Ruptured due to aortitis |
| 40M | c.926G>A, p.Gly142Glu | TBAD, mild ATA dilation | Medical | No | Alive at age 49 |
| 12M | c.547G>C, p.Gly18SArg | DTAA rupture | TEVAR | No | Discharged home after 2 days. Died at age 27 from multi-organ failure |
| 27F | c.1024G>A, p.GlyS42Arg | DTAA rupture | TEVAR | No | Cause of death |
| 19M | c.12S1G>C, p.Gly244Arg | TBAD | Open Repair | Yes | Extent II TAAA repair at age 21. Discharged home after 10 days. Alive at age 29 |
| Abdominal Aorta (N=7) | |||||
| 33F | c.996+2G>A, p.Gly318_Pro332del (splice site) | Infrarenal abdominal aortic dissection | Medical | No | Aortic diameter 1.9 cm. Alive at age 34 |
| 30F | c.1330G>A, p.Gly444Arg | Abdominal aortic dissection | Medical | Yes | - |
| 45M | Pathogenic | AAA/ Dissection | EVAR | No | Alive at age 46 |
| 27M | c.2113G>A, p.Gly705Arg | AAA/Dissection | Open Repair | No | Discharged home after 8 days. alive at age 31 |
| 33M | c.2356G>A, p.Gly786Arg | Ruptured AAA | Open Repair | No | Takeback for a ruptured gallbladder. Alive at age 51 |
| 43F | Pathogenic | Ruptured AAA | Open Repair | No | Discharged to rehab after 24 days, wound complicated by infection. Alive at age 48 |
| 48M | c.3847C>T, p.Gln1283Ter | Ruptured AAA | Open Repair | Yes | Take back right limb ischemia. Discharged home after 7 days. Alive at age 56 |
AAA: Abdominal Aortic Aneurysm. ATA: Ascending Thoracic Aorta. DTAA: Descending Thoracic Aortic Aneurysm. EVAR: Endovascular Aneurysm Repair. PAU: Penetrating aortic ulcer. TBAD: type B aortic dissection. TEVAR: Thoracic Endovascular Aneurysm Repair.
All are missense mutations unless specifically noted.
Among the abdominal aortic aneurysm cases, four individuals underwent successful open abdominal aortic aneurysm (AAA) repair with excellent long-term follow up (3 had ruptured AAA, table 4). In one case, an endovascular aneurysm repair (EVAR) was performed for aortic dissection prior to diagnosing vEDS. This patient had no short-term complications, with one-year follow-up.
Aortic rupture occurred in 7 individuals (41.4%) and was the cause of death in 2 (11.7%) individuals: a ruptured type A aortic dissection and a ruptured TBAD complicated by infectious aortitis.
Mortality
The median follow up after the first arterial event was 5 [IQR 2.5–12] years. There were 12 deaths (22.6%) at a median age of 36 [IQR 28–51] years (58.3% male, 83.3% Caucasian). Table 5 summarizes the characteristics and causes of mortality and figure 5 shows Kaplan-Meier estimates of cumulative survival.
Table 5.
Age and causes of death among 12 individuals with vascular Ehlers-Danlos syndrome along with the age of diagnosis, pneumothorax, colon perforation, first arterial pathology and rupture
| Age | |||||||
|---|---|---|---|---|---|---|---|
| Age/Sex | Col3A1 Variant | Diagnosis | Pneumo thorax | Colon Perforation | Arterial pathology | Arterial Rupture | Cause of Death |
| 19M | Pathogenic | 19 | 19 | 19 | Myocardial infarction with associated ventricular rupture | ||
| 27F | c.1024G>A, p.Gly342Arg | 17 | 23 | 27 | 27 | Hemothorax | |
| 28F | c.2069G>T, p.Gly690Val | 23 | 23 | 26 | Lateral medullary infarct due to vertebral artery dissection | ||
| 29M | c.547G>C, p.Gly183Arg | 12 | 12 | 12 | Multiple organ failure | ||
| 36F | c.764G>A, p.Gly255Glu | 36 | 36 | 36 | Ruptured type A aortic dissection | ||
| 41F | c.2337+2T>C, | 24 | 36 | 36 | 41 | Cardiac arrest due to left anterior | |
| 50F | IVS34+2T>C, p.Gly762_Lys779del Pathogenic | 32 | 33 | 46 | 38 | 46 | descending coronary artery dissection multiple visceral arteries ruptures |
| 51M | c.601G>C, p.Gly201Arg | 29 | 25 | 33 | Stroke | ||
| 53M | c.3966delG, p.Lys1323Argfs*64 | 43 | 36 | 53 | ruptured type B aortic dissection due to Infectious aortitis | ||
| 28M | c.2445+2dupT, IVS37+2dupT, p.Gly798 Pro815del | 24 | 22 | 22 | unknown | ||
| 52M | 1295G>A, p.Gly432Asp | 51 | 40 | 50 | unknown | ||
| 70M | IVS8+5 G>A, p.Gly195_Ser212del | 58 | 50 | 51 | unknown | ||
Figure 5.
Kaplan-Meier estimates of cumulative survival in individuals with vascular Ehlers-Danlos syndrome
Genotype-phenotype correlation
The molecular confirmation in this cohort was performed by genetic testing, showing a pathogenic variant in COL3A1 (n=81) or by skin biopsy (N=5). Missense mutations with a substitution of glycine with a large amino acid were the most common type of pathogenic variant (n=44, 51.2%) followed by missense mutations with a substitution of glycine residue with a small amino acid (n=12, 14%). Null mutations occurred in only 7 individuals (8.1%).
While not statistically significant, individuals with missense mutations in which glycine was substituted with a large amino acid had an earlier onset of aortic/arterial pathology (median 30 [IQR 23.5–37] years) compared to the other pathogenic COL3A1 variants (median 36 [IQR 29.5–44.8] years, P=.065). There were no differences between the variant groups in terms of arterial/aortic pathology or rupture.
DISCUSSION
A substantial challenge to evaluating treatment of arterial/aortic pathology in individuals with vEDS is the rare frequency of the disease (1:50,000), such that few centers have any significant experience.5,11,12,18 This multi-institutional study is a step in the direction of better understanding the natural history in vEDS and management outcomes.
Several generalizations can be made based on this cohort. Most of the carotid (other than CCF), vertebral, mesenteric, and renal manifestations of the syndrome can be managed medically. When management of arterial manifestations is required due to spontaneous rupture, enlarging aneurysms, or pseudoaneurysms, an endovascular approach, mostly with coil embolization, and less frequently with stenting, appears to be well tolerated and these results are consistent with previous reports.18,19 Access site management in this series had few complications, including those managed with manual compression and closure devices. This finding is to be interpreted with caution, since we usually recommend open femoral artery exposure and primary repair as previously described18,20 for access puncture sites in patients with known vEDS diagnosis, as this allows the most control of the artery. While thrombolysis for arterial thrombosis was performed in one case in this series, it was associated with a spontaneous hemorrhagic complication. Given the risk of spontaneous hemorrhage, we do not recommend thrombolysis in individuals with vEDS.
CCF embolization appears to offer satisfactory results. This finding confirms previous reports; a referral to a neuro-interventionalist is recommended in these cases.21,22 While we did not find a consistent approach for embolization in these cases, the transvenous approach appears to offer a decreased risk of vascular injury.21,22
Iliac arteries dissections without thrombosis can be managed medically, while aneurysm repairs are based on association with an AAA. Endovascular stenting of an aneurysmal iliac artery has been reported to be successful.23
Several observations can be made in relation to aortic pathology in this cohort. First, aortic disease seems more frequent in thoracic segment than in the abdominal differently that the observed frequency in the general population. Given the small numbers, it is difficult to draw any additional conclusions from this observation but it is worth noting with plans for further evaluation. Second, an open repair of ruptured AAA appears to be as well tolerated as those without vEDS. Therefore, vEDS should not be a deterrent to offering an operation for a ruptured AAA. We recommend Teflon or felt reinforcements for the anastomoses in all aortic repairs. Third, two TEVARs were reported in this cohort (one associated with death) and one EVAR with a short post-operative follow up duration. These repairs were performed prior to knowing the vEDS diagnosis. There has been a single case report of successful of TEVAR24 in an individual with vEDS. However, we recommend against the use of TEVAR or EVAR in individuals with vEDS, based on extrapolating from other connective tissues disorders such as Marfan’s syndrome. TEVAR and EVAR in this population carry a significant risk of use perforation and erosion at the fixation zones, due to the fragility of the aortic wall, and, with TEVAR, the risk for retrograde aortic dissection.18,25
An interesting finding was the observation of a higher frequency of hypertension and deep vein thrombosis in the vEDS patients with arterial pathology. We were not able to ascertain if these diagnoses were pre or post diagnosis of the aortic/arterial pathology. We also did not ascertain if the affected individuals were taking an antihypertensive. Another interesting finding was a significantly higher frequency of small joint hypermobility in the individuals who did not have arterial pathology, but the significance of this finding is unclear.
We did not find sex differences at initial arterial presentation, similar to what has been previously reported.6 Other studies have shown an increased risk of sudden death related to arterial ruptures in males younger than 20 years.10 A plausible explanation that unites these disparate findings is the bias toward those who survived to have a diagnosis.
Similar to prior studies, the missense mutations (glycine substitutions) in COL3A1 were over-represented in this cohort. Missense mutations are the most common type of variant affecting COL3A1. The variant results in a substitution of a glycine residue in the Gly-X-Y repeats of the triple helical domain thus disrupting the type III collagen folding. As a consequence a minimal amount of normal collagen (10–15%) is excreted from the cell into the extracellular matrix.14 Similarly, exon skip and splice site variants create a frameshift that results in exon (s) deletion and production of defective collagen similar to the effect of pathogenic missense variants.6,16 The biologic explanation is related to the disruptive effect this type of variant has on the type III collagen folding process.6,10While, haploinsufficiency/Null mutations lead to the creation of a premature termination codon thus the affected gene is essentially silent resulting in the production of half the amount of normal type III collagen. Thus missense variants cause a more severe phenotype of vEDS, 10 while haploinsufficiency mutations lead to milder phenotype.6,9–11 In our cohort, individuals with missense mutations in which the substitution of glycine is with a large amino acid appeared to present with aortic/arterial pathology an at earlier age, compared to the other types of variants, including missense mutations with substitution of glycine with a small amino acid. This is plausible as prior work demonstrated increased disruption of the collagen triple helix when Gly is replaced by a large amino acid rather than by a smaller amino acid.6,15 However, our study was underpowered to detect a statistically significant difference and therefore replication in a larger cohort is warranted.
We recommend a raised index of suspicion at the time of initial presentation of aneurysms and dissections in young individuals especially with a family of aneurysms/dissections and presence of clinical diagnostic features of vEDS. This then should lead to confirmatory genetic testing rather than relying on clinical criteria alone, for vEDS diagnosis given the overlap in clinical features with other forms of Ehlers-Danlos syndrome and other genetically triggered aortopathies.4,6 Additionally, understanding the type of COL3A1 variant allows for counseling on the effect of the variant type on an individual.6,9–11,26 Moreover, individuals with an established diagnosis preoperatively have improved outcomes when undergoing elective surgical repairs or interventions compared to those who require emergency operative repair.11 Our work and others substantiate that the genetic testing is no longer experimental or investigational and has real consequences to the affected individual, in terms of surveillance, the operative approaches, perioperative care, and pregnancy planning. These consequences are relevant to family members as well.
Once the diagnosis is confirmed, we recommend establishing a multidisciplinary care team locally and at a tertiary care center, to coordinate care including diagnostic testing, surveillance, and surgical repairs.12 The care team should include a vascular surgeon, a geneticist, and a primary care provider who organizes the variable levels of care needed by the patient. Baseline arterial imaging should be performed during the initial consultation visits. There are no trials delineating the frequency of surveillance imaging, nor did we ascertain the frequency of imaging in this study. In general, we recommend that surveillance imaging be tailored to the symptoms and presentation of each individual.
This study has several limitations. We did not evaluate the role of medical management in mitigating the risk of aortic/arterial pathology, using beta-blockers, losartan, vitamin C, or adjunctive measures during open repairs such as the use of desmopressin (DDAVP).5,27–29 We were also unable to ascertain if the diagnosis of aneurysms and dissections was incidental, made because of surveillance, or because of symptoms. Additionally, the retrospective nature of this study limits an understanding of the decision-making used in management or the indication for the elective operations beyond what has been presented in the results. Lastly, the imaging for all the individuals in this cohort was not available to review in a standardized manner. Review of imaging is highly relevant to understanding the natural history of vEDS and should be included in future study designs.
Future directions include work within the vEDS Research Collaborative to enroll individuals with genetically confirmed vEDS into a large natural history study.30 This is necessary to obtain detailed longitudinal data to better characterize the natural history and outcomes to guide future management recommendations.
CONCLUSIONS
Most of the arterial manifestations of vEDS were managed medically in this cohort. When intervention is required due to spontaneous rupture or enlarging aneurysms, embolization (and less frequently stenting) appears to be well tolerated. Open repair AAA was tolerated and as such, the diagnosis of vEDS should not be a deterrent to offering an operation. Future directions include enrolling patients prospectively into the vEDS Research Collaborative to further ascertain the natural history, elucidate the role of surgical interventions, and to refine management recommendations in the context of patient centered outcomes.
Highlights.
Type of Research:
Cross-sectional retrospective study of the Vascular Low Frequency Disease Consortium
Key Findings: :
In this group of 86 individuals with genetically confirmed vascular Ehlers-Danlos Syndrome (vEDS), with pathogenic COL3A1 variants, most patients were managed medically. For treatment of an enlarging aneurysm or rupture, embolization and stenting were well tolerated. Four patients underwent successful open abdominal aortic aneurysm repair.
Take home message:
Genetic confirmation of pathogenic COL3A1 variants of vEDS is essential for counseling affected individuals on the effect of their variant type and directing care. When intervention is required, embolization and stenting are acceptable options. Open repair of abdominal aortic aneurysms is also well tolerated.
TOC Summary:
In this multi institutional retrospective study of 86 individuals with genetically confirmed vascular Ehlers-Danlos Syndrome, with pathogenic COL3A1 variants, most patients were managed medically. When intervention was required for an enlarging aneurysm or rupture, embolization and stenting were well tolerated. Four patients underwent successful open abdominal aortic aneurysm repair.
Acknowledgments:
The authors acknowledge Binod Shrestha (Department of Cardiovascular and Vascular Surgery, at the University of Texas Health Science Center at Houston) for assisting with data collection at the University of Texas Health Science Center at Houston.
Funding: research was supported in part the National Center for Advancing Translational Sciences of the National Institutes of Health under Award Number UL1TR000423 (SS), in part by funds from the Freudmann Fund for Translational Research in Ehlers-Danlos syndrome at the University of Washington (PHB), and in part by the National Institute of Health (NIDDK 1K08DK107934) (KW). The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health.
Presentation: This work was partially presented at the Society for Clinical Vascular Surgery 45th Annual symposium, March 2017
Footnotes
Conflicts of Interest: None
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Pope FM, Martin GR, Lichtenstein JR, Penttinen R, Gerson B, Rowe DW, et al. Patients with Ehlers-Danlos syndrome type IV lack type III collagen. Proc Natl Acad Sci U S A 1975; 72: 1314–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Pepin M, Schwarze U, Superti-Furga A, Byers PH. Clinical and genetic features of Ehlers-Danlos syndrome type IV, the vascular type. N Engl J Med 2000; 342: 673–80. [DOI] [PubMed] [Google Scholar]
- 3.Pyeritz RE. Ehlers-Danlos syndrome. N Engl J Med 2000; 342: 730–2. [DOI] [PubMed] [Google Scholar]
- 4.Malfait F, Francomano C, Byers P, Belmont J, Berglund B, Black J, et al. The 2017 international classification of the Ehlers-Danlos syndromes. Am J Med Genet C Semin Med Genet 2017; 175: 8–26. [DOI] [PubMed] [Google Scholar]
- 5.Eagleton MJ. Arterial complications of vascular Ehlers-Danlos syndrome. J Vasc Surg 2016; 64: 1869–80. [DOI] [PubMed] [Google Scholar]
- 6.Frank M, Albuisson J, Ranque B, Golmard L, Mazzella JM, Bal-Theoleyre L, et al. The type of variants at the COL3A1 gene associates with the phenotype and severity of vascular Ehlers-Danlos syndrome. Eur J Hum Genet 2015; 23: 1657–64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Oderich GS, Panneton JM, Bower TC, Lindor NM, Cherry KJ, Noel AA, et al. The spectrum, management and clinical outcome of Ehlers-Danlos syndrome type IV: a 30-year experience. J Vasc Surg 2005; 42: 98–106. [DOI] [PubMed] [Google Scholar]
- 8.Adham S, Trystram D, Albuisson J, Domigo V, Legrand A, Jeunemaitre X, et al. Pathophysiology of carotid-cavernous fistulas in vascular Ehlers-Danlos syndrome: a retrospective cohort and comprehensive review. Orphanet J Rare Dis 2018; 13: 100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Leistritz DF, Pepin MG, Schwarze U, Byers PH. COL3A1 haploinsufficiency results in a variety of Ehlers-Danlos syndrome type IV with delayed onset of complications and longer life expectancy. Genet Med 2011; 13: 717–22. [DOI] [PubMed] [Google Scholar]
- 10.Pepin MG, Schwarze U, Rice KM, Liu M, Leistritz D, Byers PH. Survival is affected by mutation type and molecular mechanism in vascular Ehlers-Danlos syndrome (EDS type IV). Genet Med 2014; 16: 881–8. [DOI] [PubMed] [Google Scholar]
- 11.Shalhub S, Black JH 3rd, Cecchi AC, Xu Z, Griswold BF, Safi HJ, et al. Molecular diagnosis in vascular Ehlers-Danlos syndrome predicts pattern of arterial involvement and outcomes. J Vasc Surg 2014; 60: 160–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Byers PH, Belmont J, Black J, De Backer J, Frank M, Jeunemaitre X, et al. Diagnosis, natural history, and management in vascular Ehlers-Danlos syndrome. Am J Med Genet C Semin Med Genet 2017; 175: 40–7. [DOI] [PubMed] [Google Scholar]
- 13.Harlander-Locke MP, Lawrence PF. The Current State of the Vascular Low-Frequency Disease Consortium. Ann Vasc Surg 2017; 38: 8–9. [Google Scholar]
- 14.Smith LT, Schwarze U, Goldstein J, Byers PH. Mutations in the COL3A1 gene result in the Ehlers-Danlos syndrome type IV and alterations in the size and distribution of the major collagen fibrils of the dermis. J Invest Dermatol 1997; 108: 241–7. [DOI] [PubMed] [Google Scholar]
- 15.Mizuno K, Boudko S, Engel J, Bachinger HP. Vascular Ehlers-Danlos syndrome mutations in type III collagen differently stall the triple helical folding. J Biol Chem 2013; 288: 19166–76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Schwarze U, Goldstein JA, Byers PH. Splicing defects in the COL3A1 gene: marked preference for 5’ (donor) spice-site mutations in patients with exon-skipping mutations and Ehlers-Danlos syndrome type IV. Am J Hum Genet 1997; 61: 1276–86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Beighton P, De PA, Steinmann B, Tsipouras P, Wenstrup RJ. Ehlers-Danlos syndromes: revised nosology, Villefranche, 1997. Ehlers-Danlos National Foundation (USA) and Ehlers-Danlos Support Group (UK). Am J Med Genet 1998; 77: 31–7. [DOI] [PubMed] [Google Scholar]
- 18.Brooke BS, Arnaoutakis G, McDonnell NB, Black JH 3rd. Contemporary management of vascular complications associated with Ehlers-Danlos syndrome. J Vasc Surg 2010; 51: 131–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Okada T, Frank M, Pellerin O, Primio MD, Angelopoulos G, Boughenou MF, et al. Embolization of life-threatening arterial rupture in patients with vascular Ehlers-Danlos syndrome. Cardiovasc Intervent Radiol 2014; 37: 77–84. [DOI] [PubMed] [Google Scholar]
- 20.Lum YW, Brooke BS, Arnaoutakis GJ, Williams TK, Black JH 3rd. Endovascular procedures in patients with Ehlers-Danlos syndrome: a review of clinical outcomes and iatrogenic complications. Ann Vasc Surg 2012; 26: 25–33. [DOI] [PubMed] [Google Scholar]
- 21.Kanner AA, Maimon S, Rappaport ZH. Treatment of spontaneous carotid-cavernous fistula in Ehlers-Danlos syndrome by transvenous occlusion with Guglielmi detachable coils. Case report and review of the literature. J Neurosurg 2000; 93: 689–92. [DOI] [PubMed] [Google Scholar]
- 22.Huynh TJ, Morton RP, Levitt MR, Ghodke BV, Wink O, Hallam DK. Successful treatment of direct carotid-cavernous fistula in a patient with Ehlers-Danlos syndrome type IV without arterial puncture: the transvenous triple-overlay embolization (TAILOREd) technique. BMJ Case Rep 2017; 2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Tonnessen BH, Sternbergh WC 3rd, Mannava K, Money SR. Endovascular repair of an iliac artery aneurysm in a patient with Ehlers-Danlos syndrome type IV. J Vasc Surg 2007; 45: 177–9. [DOI] [PubMed] [Google Scholar]
- 24.Khalique Z, Lyons OT, Clough RE, Bell RE, Reidy JF, Schwarze U, et al. Successful endovascular repair of acute type B aortic dissection in undiagnosed Ehlers-Danlos syndrome type IV. Eur J Vasc Endovasc Surg 2009; 38: 608–9. [DOI] [PubMed] [Google Scholar]
- 25.Shalhub S, Eagle KA, Asch FM, LeMaire SA, Milewicz DM, Gen TAC Investigators. Endovascular thoracic aortic repair in confirmed or suspected genetically triggered thoracic aortic dissection. J Vasc Surg 2018; 68: 364–71. [DOI] [PubMed] [Google Scholar]
- 26.Schwarze U, Schievink WI, Petty E, Jaff MR, Babovic-Vuksanovic D, Cherry KJ, et al. Haploinsufficiency for one COL3A1 allele of type III procollagen results in a phenotype similar to the vascular form of Ehlers-Danlos syndrome, Ehlers-Danlos syndrome type IV. Am J Hum Genet 2001; 69: 989–1001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Ong KT, Perdu J, De Backer J, Bozec E, Collignon P, Emmerich J, et al. Effect of celiprolol on prevention of cardiovascular events in vascular Ehlers-Danlos syndrome: a prospective randomised, open, blinded-endpoints trial. Lancet 2010; 376: 1476–84. [DOI] [PubMed] [Google Scholar]
- 28.Mast KJ, Nunes ME, Ruymann FB, Kerlin BA. Desmopressin responsiveness in children with Ehlers-Danlos syndrome associated bleeding symptoms. Br J Haematol 2009; 144: 230–3. [DOI] [PubMed] [Google Scholar]
- 29.Malfait F, De Paepe A. The Ehlers-Danlos syndrome. Adv Exp Med Biol 2014; 802: 129–43. [DOI] [PubMed] [Google Scholar]
- 30.Vascular Ehlers-Danlos Syndrome (vEDS) Collaborative. http://www.becertain.org/projects/patient-engagement/vascular-ehlers-danlos-syndrome-veds-collaborative (accessed 05/01/2018 2018).