Abstract
Introduction
Previous studies have indicated that low-intensity extracorporeal shockwave therapy (Li-ESWT) may improve male erectile dysfunction (ED) of vascular aetiology.
Aim
To investigate penile rehabilitation of Li-ESWT in a randomized, placebo-controlled trial in men with ED following robotic nerve-sparing radical prostatectomy (RARP).
Methods
Included were men with ED following nerve-sparing RP with a score <22 in the 5-item International Index of Erectile Function (IIEF-5) questionnaire. Participants were divided into an active A (n = 20) and a placebo/sham B group (n = 18). They were randomized consecutively upon study entry. Each study arm had one treatment a week for 5 weeks.
Main outcome measures
Sexual outcomes were assessed by international validated questionnaires, Erection Hardness Score (EHS) and IIEF-5 at baseline and at 4 and 12 weeks after treatment.
Results
A total of 38 (n = 38) participants were enrolled; there were no dropouts. A significant increase was observed in IIEF-5 and EHS in group A at both 4 and 12 weeks. At 12 weeks, the mean IIEF-5 score had increased by 3.45 points (P = .026), while the mean EHS score had increased by 0.5 points (P= .019).
Conclusion
This randomized study indicates that Li-ESWT for ED in men undergone RP might be effective and safe. However, further and more robust research is needed before Li-ESWT can be characterized as a reliable treatment modality. Ladegaard PBJ, Mortensen J, Skov-Jeppesen SM, et al. Erectile Dysfunction A Prospective Randomized Placebo-Controlled Study Evaluating the Effect of Low-Intensity Extracorporeal Shockwave Therapy (LI-ESWT) in Men With Erectile Dysfunction Following Radical Prostatectomy. Sex Med 2021;9:100338.
Key Words: Erectile dysfunction, Extracorporeal shockwave, Penis, Radical prostatectomy
Introduction
Erectile dysfunction (ED) is a well-known complication to radical prostatectomy (RP) due to lesion to the erectile nerves.1 Post-RP histological examination of the cavernous bodies shows increasing fibrosis in the penile organ leading to ED.2 During the past decade, increasing use of nerve-sparing techniques, including robotic assisted radical surgery (RARP), has improved postoperative morbidity and lowered complication rates.1,3, 4, 5 Despite efforts at preserving nerve function, studies indicate that 40%–70% of patients having undergone RP suffer from erectile impairment 12 months after surgery.1,6,7
The mental burden of men with ED has been quantified by Lee et al. in the CaPSURE (Cancer of the Prostate Strategic Urologic Research Endeavour database) cohort. The outcomes assessed were sexual function and sexual bother. Lee et al. found that sexual desire was preserved in men after RP and that the greatest decline in sexual function was the ability to achieve erection and erection frequency. These parameters also accounted for most sexual bother.8
The current treatment for ED following RP remains oral 5-phosphodiesterase inhibitors (5-PDEi) or intracavernosal injection (ICI) therapy with vasodilating agents.9 However, 5-PDEi and ICI have shown not to be efficient in all patient having undergone RP. In addition, patients lack spontaneous erection and must endure several adverse effects.10,11 In addition, they are contraindicated in patients suffering from various heart diseases and prescribed concomitant nitrates and other antihypertensive drugs because of their blood-pressure-lowering effects.12 Other erectogenic aids (EA) include the penile ring, penile vacuum pump or penile prosthesis, which can be applied before sexual intercourse to achieve sufficient erection.13,14 Stem-cell treatment is a novel treatment for ED still being investigated for its relevance to ED following RARP. Preliminary results in one study have shown sufficient penile restoration in 8 of 17 men following RARP.15,16
Recent studies have shown promising results of low-intensity extracorporeal shockwave therapy (Li-ESWT) on patients suffering from mild to severe ED, basically in ED of vascular aetiology.17, 18, 19 Several meta-analyses conclude that Li-ESWT might induce a statistically significant increase in erectile function (EF), though claim a need for robust studies investigating what is a clinically meaningful effect of Li-ESWT.20,21 Frey et al. found a slight effect of Li-ESWT in men with ED of neurogenic aetiology. One year following treatment, four patients in a cohort of 17 had achieved improved EF.22 Li-ESWT has previously been investigated in animal models with neurogenic lesions and been found to increase levels of essential neurogenic and angiogenic markers of tissue repair in tissue exposed to Li-ESWT.23 In this study, we tested the following null hypothesis: there is no difference in mean IIEF-5 or EHS scores in men with ED following RP after Li-ESWT.
Materials and Methods
Study population
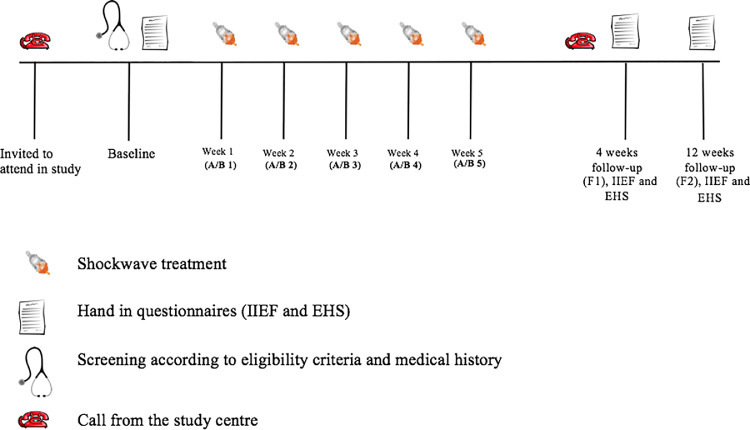
We prospectively included 38 participants from the Region of Southern Denmark covering 1.2 mill. People; the study was conducted from the autumn of 2017 until the spring of 2019. Participants were mainly included based on interviews conducted at our outpatient clinic of sexology, where many of the patients having undergone RARP are offered a postoperative follow-up visit 6 months after surgery. During the first visit, participants underwent consultations focused on their medical history and current health status, and they were then screened for inclusion according to the eligibility criteria (Figure 1).
Figure 1.
Overview of participant visits and due dates for handing in questionnaires. A = group A; B = group B (sham).
Inclusion criteria included men with ED for more than 6 months post-operatively (IIEF-5 < 22), at least unilateral nerve-sparing operation, no impaired EF prior to RP (no use of 5-PDEi, ICI or other erectogenic aid), no active cancer or radiation therapy to the pelvic area. Exclusion criteria were any de novo use of EA that could conflict with shockwave therapy. For ethical reasons, we decided that it was not prohibited to continue existing use of EA during the study period. All participants gave written and oral informed consent before inclusion. The regional ethics committee (ID: S-20170074) and the national data protection agency approved the study (ID:19/16374). The trial is registered at www.clinicaltrials.gov (ID: NCT03192917).
Study design
The study was carried out as a blinded, 1:1 randomized, placebo-controlled trial. Before inclusion, all participants completed EHS and IIEF-5 questionnaires (Figure 1). The IIEF-5 is a five-item questionnaire providing a score between 5 and 25, which translates into the categories severe, moderate, mild to moderate, mild or no ED. The EHS is a one-entity statement soliciting information on erection quality. The EHS provides a score between 0 and 4. At the consultations, questionnaires were examined to ensure that there were no misunderstandings. Four and 12 weeks following treatment, the participants completed identical questionnaires using Survey-Xact software. Assuming they were not taking regular EA, all patients were instructed in answering the questions. The primary outcome measure was changes in mean IIEF-5 and EHS score at 12 weeks. The secondary outcome measure included pain during shockwave application and other adverse effects.
Randomization and blinding
To ensure valid randomization, we used in-house software (OPEN randomizes) customized to create block randomization with a fixed block size unknown to the investigator. Block randomization was utilized due to the small sample size. Participants were randomized consecutively to either group A or B (sham) upon entering the intervention. They were blinded throughout the trial.
The manufacturer (Storz Medical, Switzerland) provided gel pads used for shockwave transmission; sham pads to prevent shockwaves from being transmitted, and active pads for active transmission. Each set of pads was initially labeled A or B, applied to group A or B, respectively. The shockwave machine was set at identical options and made the same noise during both treatments. The patients could feel no discomfort during active or placebo treatment, as shown by other studies.24
Treatment protocol
Treatments were performed once a week in 5 weeks using a handheld Duolith SD1 machine (Storz Medical, Switzerland). The machine was set at 0.15 mJ/mm2, 5Hz, with a total of 4,000 shock impulses, resulting in a total energy of 12.8 J per treatment. An electromagnetic system in the machine generated shockwaves, which were then focused by a parabolic reflector for maximum energy inside the tissue. A water-based gel was used for optimal conductivity. Five hundred impulses were given to the distal, middle and proximal penile shaft, respectively, changing side for every other treatment to a total of 3,000 impulses. A cone-shaped pad was used for optimal energy depth in the penile tissue. Additionally, 500 impulses were given to each of the penile crurae, to a total of 1,000 impulses, using a round plane stand-off. Please note that the machine generates shockwaves different from common known acoustic waves used for instance in ultrasonic diagnostic tools.
During each session, participants were asked about any discomfort during application. Between treatment sessions, participants were encouraged to masturbate and have sexual intercourse with their partner.
Statistics
For statistics, the SPSS version 26.0 tool was used. Baseline demographics and sexual outcomes between the groups were compared using a χ2 (chi-square) and Mann-Whitney U test. The data was analyzed for distribution of normality, which was not found. The scores of IIEF-5 and EHS within groups were compared using a Wilcoxon signed rank. A 2-sided P-value < .05 was considered statistically significant.
Previous studies of Li-ESWT estimate that a true increase in IIEF score of 66% or more can be detected with a sample of 32 participants in each study arm with a power of 80% and a significance level of 5%.18
Results
Baseline variables
A total of 38 patients were recruited to the study and randomized to group A (n = 20) or group B (n = 18) (Table 1). Patients were mean 3.5-year older age in group B than in group A, but the difference was not statistically significant (P = .11). The mean time since patients had RP performed was also relatively longer in group B. However, one patient in group B had undergone RP surgery 74 months before study inclusion. In comparison, in group A, the longest time since RP was 20 months (Table 1). All other patients in group B had undergone RP surgery between 6 and 22 months before study inclusion. Seven out of 20 (35%) patients in group A were operated by a bilateral nerve-sparing procedure, which is comparable to the proportion of patients operated by the same procedure in group B (28%). In both groups, the most common and highest histological grade found in the prostate after RP was Gleason score seven in both groups (Table 1). Most of the patients had received previous oral medication and there was no difference between the groups in this respect. However, a few more patients in group A were classified as responders to previous oral medication, meaning that they had experienced an adequate, satisfactory effect of oral medications. In group A, 67% of the patients responded to previous oral medication compared with 56% in group B. Eleven patients (55%) were diagnosed with cardiovascular disease in group A as opposed to only 6 patients (33%) in group B (ie, coronary heart disease, previous acute myocardial infarction, cardiac failure, peripheral arterial disease). On the other hand, twice as many patients were diagnosed with diabetes mellitus in group B (22%) (n = 4) as in group A (10%; n = 2). Both groups counted three current smokers, and none of the patients had high alcohol intake (Table 1).
Table 1.
Baseline parameters
| Group A (n = 20) |
Group B (n = 18) |
Comparison between groups | |
|---|---|---|---|
| Age (years) | 60.8 (6.8) | 64.3 (4.8) | P = .11 |
| Time since RP (months) | 11.2 (4.5) | 14.3 (16.4) | P = .77 |
| RP procedure | |||
| Bilateral nerve-sparing | 7 (35%) | 5 (28%) | P = .63 |
| Unilateral nerve-sparing | 13 (65%) | 13 (72%) | |
| Gleason score* | |||
| 6 | 6 (30%) | 6 (33%) | P = .40 |
| 7 | 14 (70%) | 12 (67%) | |
| Previous oral medication (5-PDEi)† | 18 (90%) | 16 (89%) | P = .91 |
| Responders to oral medication (5-PDEi)‡ | 12 (67%) | 9 (56%) | P = .53 |
| Side effects to oral medication (5-PDEi)§ | 6 (33%) | 7 (44%) | P = .56 |
| Comorbidities5 | 7 (35%) | 7 (39%) | P = .80 |
| Cardiovascular disease | 11 (55%) | 6 (33%) | P = .18 |
| Diabetes mellitus | 2 (10%) | 4 (22%) | P = .30 |
| Current smokers | 3 (15%) | 3 (17%) | P = .89 |
| Alcohol consumption (weekly) | |||
| 1–7 | 12 (60%) | 12 (67%) | P = .67 |
| 8–14 | 8 (40%) | 6 (33%) | |
| >14 | 0 (0%) | 0 (0%) |
Data are displayed as mean (SD) or n (%). The statistical comparison between groups was performed with Mann Whitney U test, Pearson chi-square test or Fisher's exact test as appropriate.
Bold text represent P < 0.05.
Final histology Gleason score as determined in prostate specimen after surgery.
Number of patients who had previously received oral 5PDEi following operation.
The patients were asked if they had experienced an adequate response to previous oral medication (5-PDEi). Proportions were calculated from the number of patients who had received previous oral medication (5-PDEi) (Group A: 12/18 and Group B: 9/15).
The patients were asked if they had experienced side effects of any kind to previous oral medication (5-PDEi). Proportions were calculated from the number of patients who had received previous oral medication (Group A: 6/18 and Group B: 7/15).
EHS and IIEF-5
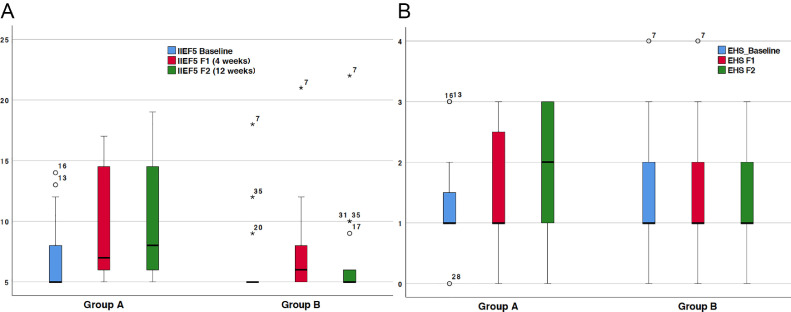
At baseline, the mean EHS was 1.30 (standard deviation [SD] 0.73) in group A and 1.44 (SD 0.98) in group B (Table 2). In group A, mean EHS was improved by 0.35 (SD 0.59) at 4 weeks of follow-up and by 0.50 (0.76) at 12 weeks of follow-up. EHS was found to be slightly decreased at both 4 weeks and 12 weeks of follow-up in group B. A comparison between group A and group B revealed statistically increased EHS at both follow-up visits after Li-ESWT (Table 2). Only in group A did the EHS change significantly compared with baseline in a within-group analysis. In group A, the mean IIEF-5 score improved gradually by 2.40 (SD 3.30) points at four weeks of follow-up and by 3.45 (SD 4.01) points at 12 weeks of follow-up, up from a mean baseline score of 6.80 (SD 3.04; Table 2). On the other hand, in group B, the mean IIEF-5 score was only improved by 1.28 (SD 1.90) points at 4 weeks of follow-up and by 0.65 (SD 2.03) points at 12 weeks of follow-up, up from a mean baseline score of 6.83 (SD 3.87) (Table 2). Group A had a significantly higher IIEF-5 score than group B at 12 weeks of follow-up. However, the between-group difference in IIEF-5 score was not statistically significant at four weeks of follow-up. Compared with baseline in group A, the within-group effect was significant in regard to the IIEF-5 score at both four and 12 weeks of follow-up. In group B, the patients’ EF seemed to improve spontaneously since there was a slight, significant increase in IIEF-5 score at four weeks of follow-up in comparison with the baseline score. However, at 12 weeks of follow-up, the mean increase in IIEF-5 score was not significantly different from baseline in group B (Table 2). The median changes in IIEF-5 score and EHS are illustrated in Figure 2A and B, respectively.
Table 2.
IIEF-5 and EHS
| Group A (n = 20) |
Group B (n = 18) |
Comparison between groups | |
|---|---|---|---|
| EHS | |||
| Baseline | 1.30 (0.73) | 1.44 (0.98) | P = .828 |
| Increase at 4 weeks | 0.35 (0.59)* | -0.17 (0.51) | P = .033 |
| Increase at 12 weeks | 0.50 (0.76)* | -0.17 (0.71) | P =.019 |
| IIEF-5 score | |||
| Baseline | 6.80 (3.04) | 6.83 (3.87) | P = .633 |
| Increase at 4 weeks | 2.40 (3.30)† | 1.28 (1.90)* | P = .228 |
| Increase at 12 weeks | 3.45 (4.01)† | 0.65 (2.03) | P = .026 |
Data are displayed as mean (SD). The statistical comparison between groups was performed with Mann-Whitney U test.
Indication of a significant increase compared with baseline within group (Wilcoxon signed rank test P < .05).
Indication of a highly significant increase compared with baseline within group (Wilcoxon signed rank test P < .005).
Figure 2.
Boxplots of A) 5-item International Index of Erectile Function (IIEF-5) score and B) Erection Hardness Score (EHS) at baseline and at the 4-week follow-up (F1) for the active (Group A) and the placebo (Group B) group. Asterisk and unfilled-circles symbolise single observations.
Discussion
In this prospective placebo-controlled study, we found a slight, positive effect of Li-ESWT in male ED after RP. We found a significant increase in both EHS and IIEF-5 score at 12 weeks of follow-up. Rosen et al. demonstrated that a change of 2, 5 and 7 points in the IIEF Erectile Function (IIEF-ef) score (mild, moderate, severe ED) corresponded to a clinically significant improvement in EF.25 The IIEF-ef is a questionnaire containing 6 items, while the IIEF-5 has only 5 items. Consequently, the IIEF-5 and the IIEF-ef are not exactly comparable, and we do not know which score change in either the IIEF-5 or the EHS is needed to confirm a clinically significant improvement. To our knowledge no consensus when to use IIEF-5 or IIEF-ef questionnaires exists. We used the IIEF-5 because it is validated for short follow ups and because Frey et al. also used this metric to obtain data.25 Although we saw an increase in the IIEF-5 and EHS score, no participants were cured of ED (no one had an IIEF-5 score >22 at F2). In addition, we are aware that the increase in mean EHS (0.50) and IIEF-5 (3.45) at 12 weeks of follow-up does not necessarily imply the ability to have sexual intercourse. The findings in our study are almost similar to those obtained by Frey et al. in a pilot cohort study where 69% responded to treatment at the 1-month follow-up, 44% of whom had improved their ED category. At the 1-year follow-up, 25% had improved their ED category, and 12.5% were categorized as having no ED.22
The primary knowledge on Li-ESWT in treatment of ED rests on clinical trials of ED of vascular aetiology. Results from these trials have been conflicting. For instance, Vardy et al. found a 6.7 (P= .032) point increase in mean IIEF-ef score at 1 month of follow-up following Li-ESWT, while Olsen et al. found no increase in IIEF-ef at 1, 3, or 6 months of follow-up.18,19 Conversely, they found a significant increase in EHS at 1 month (P= .0001), but the effect faded 3 and 6 months after Li-ESWT. The most recent study of LI-ESWT was published by Kim et al who measured outcome by IIEF-ef and EHS in a heterogeneous group of 81 participants with ED.26 A significant increase in IIEF-ef score of 5.1 (medians) was seen in the active group at 7 weeks of follow-up. In addition, the proportion of participant in the active group with EHS >3 improved from 52.6% at baseline to 89.5% at 7 weeks of follow-up. In the present study, six participants (30%) in the active group had an EHS>2 at 12 weeks follow-up versus 2 participants (10%) at baseline. A novel meta-analysis (including ten RCT studies of Li-ESWT in ED of vascular origin) found an overall slightly significant effect (the mean difference in IIEF was 3.97).20 However, the RCTs evaluated only the short-term effect of Li-ESWT, and the study protocols showed large heterogeneity and described randomization and blinding inadequately. Beside Frey et al., the only study assessing the effect of Li-ESWT following RP was conducted by Zerwin et al. The set-up in this trial was a little different as they included and treated patient instantly after radical cystoprostatectomy. The participants were followed for 9 months postoperatively. At 9 months of follow-up, they saw a significant improvement in penile restoration to 76.2% (Li-ESWT) and 79% (Li-ESWT + erectogenic aids) compared with 60.5% in the control group.27
The strengths of our study include that all participants completed the 5-week treatment sessions, and none were lost to follow-up. Thus, all of the participants returned filled-out questionnaires at follow ups. In previous studies, dropout rates have been low as well (5% in study by Olsen et al.18 and 15% in the study by Vardi et al.19). The blinding and randomization processes of the present study were robust. No adverse effects were reported in this study. Few participants described a mild burning sensation in relation to Li-ESWT, particularly in the perineal region. No conditions needed medical support or led to sustained discomfort for participants.
The set-up of this study has several limitations. We did not prohibit the use of currently used EA. Other trials have managed to run a drug free set-up with good results and still have low dropout rates, which ensure that parameters are comparable across cohorts.18,19 We are fully aware that there could be a difference of the use of 5-PDE5 among patients, but the same bias exists in both groups. Another limitation was the low number of participants. In the protocol, we described that we wished to recruit 70 participants. Assuming a dropout rate of 7%, similar to rates encountered in previous studies, we would have wished to have 32 participants in each treatment arm.18 We performed an interim analysis with the present results and decided to stop recruitment ending up with a total of 38 participants. Surprisingly, 76% of our participants suffered from severe ED at baseline (according to IIEF-5 score), while the proportion of participants with severe ED at baseline was 31% in Frey et al..22 This difference likely arises because our participants were recruited from a sexology clinic where the referred patients probably have worse ED than the average RP patient. The preoperatively ED was assessed by patient reports. The statement from patient were held up against drug prescription history, where previous use of 5-PDEi or other EA were not accepted. Potentially, participant might be confused about their preoperatively ED function. However, it is obvious the RP made a great impact on their erectile function, since they seek help in our clinic.
Participants might still have a potential for physiological penile regeneration and hence natural erection. According to Seddon's nerve injury classification, axonometric injury may recover with great variation ranging from 8 to 24 months.4 Patients could potentially achieve restored EF because our cohort was mixed with a different length of time after surgery although we used a 6-month post-operative inclusion criterion because early initiation of therapy may prevent pelvic and penile fibrosis, which could make the ED Li-ESWT resistant.2 We did not start therapy immediately after RARP because we did not intent to treat and expose patients to the inconvenient treatment protocol if they had a potential for natural regeneration. The study by Frey et al. included subjects 12 months postoperatively, but they lacked a control group.22 We found a significant, equal distribution of total time since surgery between groups. Since we found no improvement of EF in the placebo group, we consider the spontaneous regeneration of ED 6 months after surgery to be minimal.
Perspectives
Future research should focus on which patient subgroups can benefit from Li-ESWT. A study performed by Yee et al. found a positive effect in a subgroup with severe ED of vascular origin,28 while subgroup analysis performed in a meta-analysis revealed that only the group with mild ED had significant effect of Li-ESWT.21 To further explore this issue, international multicentre studies must be conducted in a suitable study population with narrowly defined inclusion criteria.
Still, the most optimal treatment protocol for Li-ESWT remains to be defined. In the present trial, we examined the efficacy of Li-ESWT applied at 6 different sites of the penile shaft in combination with the penile crurae over a 5-week period, which is comparable to the approach applied by Olsen et al.18 Other trials have sought to extend the treatment period or reduce the intervals between treatment sessions but achieved no additional beneficial outcome.19,21,28,29 Furthermore, the effect of linear Li-ESWT on ED of vascular aetiology was tested by Fojecki et al.29 and Sramkova et al.17 Their protocols gave lower density (0.06 mj/mm2, 600 shockwaves per treatment) but over a longer period. The 2 studies show conflicting results. Fojecki et al found no effect of linear Li-ESWT, while an effect on IIEF outcome was observed in the study by Sramkova et al.
No studies have directly assessed the dose dependency of Li-ESWT in ED. Hence, the energy level of Li-ESWT seems to be based on the empirical experience of different research groups and the manufacturer's recommendations. More treatment sessions or higher energy densities may result in better outcomes, but this needs to be investigated in a head-to-head study design. We chose the use of 0.15 mj/mm2 because most other trials were successful using this protocol.18 Still, it remains unclear if RP patients can benefit from subsequent treatments. Frey et al. found a declining EF in their patients 1 year after Li-ESWT, and the authors suggested that patients could possibly improve or at least maintain EF achieved by Li-ESWT by additional treatment after a treatment-free period.22
Conclusion
This randomized study showed a slight increase in mean IIEF-5 and EHS in men with ED following RARP treated with Li-ESWT. The mean IIEF-5 and EHS scores increased significantly, but only by few points, doubtful to give a meaningful sexual effect. We therefore question whether Li-ESWT has a major role to play in the treatment of ED in men following RARP. We found Li-ESWT to be safe. However, future research in Li-ESWT following RP should focus on initial timing of treatment, stratification of ED subgroups most susceptible to treatment, the efficiency of Li-ESWT in combination with other treatment modalities (eg, 5-PDEi or stem cell therapy) and defining the most optimal time for shockwave application. However, we aim to get a more accurate answer after our current initiated prospective randomized study where 70 men receive treatment 3 weeks after RARP.
STATEMENT OF AUTHORSHIP
Peter Bill Juul Ladegaard: conceived and performed experiments, wrote the manuscript; Jonas Mortensen: conceived and performed experiments; Sune Møller Skov-Jeppesen: provided reagents and statistical analysis, provided expertise and feedback; Lars Lund: provided reagents and statistical provided expertise and feedback, had the overall responsibility for the handling of participants and ethics.
Footnotes
Conflicts of interest: The authors report no conflicts of interest.
Funding: None.
References
- 1.Campbell JD, Burnett AL. Neuroprotective and nerve regenerative approaches for treatment of erectile dysfunction after cavernous nerve injury. Int J Mol Sci. 2017;18:1794. doi: 10.3390/ijms18081794. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Iacono F, Giannella R, Somma P. Histological alterations in cavernous tissue after radical prostatectomy. J Urol. 2005;173:1673–1676. doi: 10.1097/01.ju.0000154356.76027.4f. [DOI] [PubMed] [Google Scholar]
- 3.Skarecky DW. Robotic-assisted radical prostatectomy after the first decade: surgical evolution or new paradigm. ISRN Urol. 2013;2013 doi: 10.1155/2013/157379. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Kumar A, Tandon S, Samavedi S. Current status of various neurovascular bundle-sparing techniques in robot-assisted radical prostatectomy. J Robot Surg. 2016;10:187–200. doi: 10.1007/s11701-016-0607-7. [DOI] [PubMed] [Google Scholar]
- 5.Haglind E, Carlsson S, Stranne J. Urinary incontinence and erectile dysfunction after robotic versus open radical prostatectomy: a prospective, controlled, nonrandomised trial. Eur Urol. 2015;68:216–225. doi: 10.1016/j.eururo.2015.02.029. [DOI] [PubMed] [Google Scholar]
- 6.Haahr MK, Azawi NH, Andersen LG. A retrospective study of erectile function and use of erectile aids in prostate cancer patients after radical prostatectomy in denmark. Sex Med. 2017;5:e156–e162. doi: 10.1016/j.esxm.2017.06.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Tal R, Alphs HH, Krebs P. Erectile function recovery rate after radical prostatectomy: a meta-analysis. J Sex Med. 2009;6:2538–2546. doi: 10.1111/j.1743-6109.2009.01351.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Le JD, Cooperberg MR, Sadetsky N. Changes in specific domains of sexual function and sexual bother after radical prostatectomy. BJU Int. 2010;106:1022–1029. doi: 10.1111/j.1464-410X.2010.09231.x. [DOI] [PubMed] [Google Scholar]
- 9.Goldstein I, Lue TF, Padma-Nathan H. Oral sildenafil in the treatment of erectile dysfunction. Sildenafil Study Group. N Engl J Med. 1998;338:1397–1404. doi: 10.1056/NEJM199805143382001. [DOI] [PubMed] [Google Scholar]
- 10.Montorsi F, Brock G, Stolzenburg JU. Effects of tadalafil treatment on erectile function recovery following bilateral nerve-sparing radical prostatectomy: a randomised placebo-controlled study (REACTT) Eur Urol. 2014;65:587–596. doi: 10.1016/j.eururo.2013.09.051. [DOI] [PubMed] [Google Scholar]
- 11.Wang X, Wang X, Liu T. Systematic review and meta-analysis of the use of phosphodiesterase type 5 inhibitors for treatment of erectile dysfunction following bilateral nerve-sparing radical prostatectomy. PLoS One. 2014;9:e91327. doi: 10.1371/journal.pone.0091327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Webb DJ, Freestone S, MJ Allen. Sildenafil citrate and blood-pressure-lowering drugs: results of drug interaction studies with an organic nitrate and a calcium antagonist. Am J Cardiol. 1999;83:21C–28C. doi: 10.1016/s0002-9149(99)00044-2. [DOI] [PubMed] [Google Scholar]
- 13.Bratu O, Oprea I, Marcu D. Erectile dysfunction post-radical prostatectomy - a challenge for both patient and physician. J Med Life. 2017;10:13–18. [PMC free article] [PubMed] [Google Scholar]
- 14.Menard J, Tremeaux JC, Faix A. Erectile function and sexual satisfaction before and after penile prosthesis implantation in radical prostatectomy patients: a comparison with patients with vasculogenic erectile dysfunction. J Sex Med. 2011;8:3479–3486. doi: 10.1111/j.1743-6109.2011.02466.x. [DOI] [PubMed] [Google Scholar]
- 15.Reed-Maldonado AB, Lue TF. The current status of stem-cell therapy in erectile dysfunction: a review. World J Mens Health. 2016;34:155–164. doi: 10.5534/wjmh.2016.34.3.155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Haahr MK JC, Toyserkani NM, Andersen DC. Safety and potential effect of a single intracavernous injection of autologous adipose-derived regenerative cells in patients with erectile dysfunction following radical prostatectomy: an open-label phase I clinical trial. EBioMedicine. 2016:204–210. doi: 10.1016/j.ebiom.2016.01.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Sramkova T, Motil I, Jarkovsky J. Erectile dysfunction treatment using focused linear low-intensity extracorporeal shockwaves: single-blind, sham-controlled, randomized clinical trial. Urol Int. 2019:1–8. doi: 10.1159/000504788. [DOI] [PubMed] [Google Scholar]
- 18.Olsen AB, Persiani M, Boie S. Can low-intensity extracorporeal shockwave therapy improve erectile dysfunction? A prospective, randomized, double-blind, placebo-controlled study. Scand J Urol. 2015;49:329–333. doi: 10.3109/21681805.2014.984326. [DOI] [PubMed] [Google Scholar]
- 19.Vardi Y, Appel B, Kilchevsky A. Does low intensity extracorporeal shock wave therapy have a physiological effect on erectile function? Short-term results of a randomized, double-blind, sham controlled study. J Urol. 2012;187:1769–1775. doi: 10.1016/j.juro.2011.12.117. [DOI] [PubMed] [Google Scholar]
- 20.Sokolakis I, Hatzichristodoulou G. Clinical studies on low intensity extracorporeal shockwave therapy for erectile dysfunction: a systematic review and meta-analysis of randomised controlled trials. Int J Impot Res. 2019;31:177–194. doi: 10.1038/s41443-019-0117-z. [DOI] [PubMed] [Google Scholar]
- 21.Lu Z, Lin G, Reed-Maldonado A. Low-intensity extracorporeal shock wave treatment improves erectile function: a systematic review and meta-analysis. Eur Urol. 2017;71:223–233. doi: 10.1016/j.eururo.2016.05.050. [DOI] [PubMed] [Google Scholar]
- 22.Frey A, Sonksen J, Fode M. Low-intensity extracorporeal shockwave therapy in the treatment of postprostatectomy erectile dysfunction: a pilot study. Scand J Urol. 2016;50:123–127. doi: 10.3109/21681805.2015.1100675. [DOI] [PubMed] [Google Scholar]
- 23.Qiu X, Lin G, Xin Z. Effects of low-energy shockwave therapy on the erectile function and tissue of a diabetic rat model. J Sex Med. 2013;10:738–746. doi: 10.1111/jsm.12024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Kitrey ND, Gruenwald I, Appel B. Penile low intensity shock wave treatment is able to shift pde5i nonresponders to responders: a double-blind, sham controlled study. J Urol. 2016;195:1550–1555. doi: 10.1016/j.juro.2015.12.049. [DOI] [PubMed] [Google Scholar]
- 25.Rosen RC, Allen KR, Ni X. Minimal clinically important differences in the erectile function domain of the International Index of Erectile Function scale. Eur Urol. 2011;60:1010–1016. doi: 10.1016/j.eururo.2011.07.053. [DOI] [PubMed] [Google Scholar]
- 26.Kim KS, Jeong HC, Choi SW. Electromagnetic low-intensity extracorporeal shock wave therapy in patients with erectile dysfunction: a sham-controlled, double-blind, randomized prospective study. World J Mens Health. 2020;38:236–242. doi: 10.5534/wjmh.190130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Zewin TS, El-Assmy A, Harraz AM. Efficacy and safety of low-intensity shock wave therapy in penile rehabilitation post nerve-sparing radical cystoprostatectomy: a randomized controlled trial. Int Urol Nephrol. 2018;50:2007–2014. doi: 10.1007/s11255-018-1987-6. [DOI] [PubMed] [Google Scholar]
- 28.Yee CH, Chan ES, Hou SS. Extracorporeal shockwave therapy in the treatment of erectile dysfunction: a prospective, randomized, double-blinded, placebo controlled study. Int J Urol. 2014;21:1041–1045. doi: 10.1111/iju.12506. [DOI] [PubMed] [Google Scholar]
- 29.Fojecki GL, Tiessen S, Osther PJ. Effect of low-energy linear shockwave therapy on erectile dysfunction-a double-blinded, sham-controlled, randomized clinical trial. J Sex Med. 2017;14:106–112. doi: 10.1016/j.jsxm.2016.11.307. [DOI] [PubMed] [Google Scholar]