Abstract
Objective
To investigate the relationship between α1-antitrypsin deficiency (AATD), a disorder resulting in protease activity imbalances, and the risk of ascending aortic aneurysm.
Methods
In this single-center study, from August 1, 2018, to February 25, 2019, demographic data were retrospectively collated for patients with AATD-associated emphysema (AATD group) or non–AATD-associated emphysema (control group) with available high-resolution computed tomography scans. Mean ascending aortic diameter was compared between the groups, and the correlation of diameter with age was analyzed.
Results
Patients with AATD (n=51; mean AAT level, 20.3 mg/dL [to convert to μmol/L, multiply by 0.184]) were approximately 10 years younger than those in the control group (n=93; mean AAT level, 172.0 mg/dL), with a mean age of 55 vs 65 years. Overall and grouped by sex, the mean ascending aortic diameter in patients with AATD was not different from that in the control group (overall, 3.34 vs 3.37 cm; P=.68); however, ascending aortic diameter was significantly associated with age for patients in the AATD group (r=0.43; P=.0016), whereas no correlation was observed between age and aortic diameter in the control group (r=0.16; P=.11).
Conclusion
Results of this study suggest that there is a pathologic association between AATD and aortic distention and that AATD may increase the risk of ascending aortic aneurysm. These data provide a basis for the regular assessment of aortic diameter in patients with AATD as well as for the testing of patients with aortic distention or aneurysm for AATD.
Abbreviations and Acronyms: AAT, α1-antitrypsin; AATD, α1-antitrypsin deficiency; COPD, chronic obstructive pulmonary disease; CT, computed tomography
α1-antitrypsin deficiency (AATD), a genetic disorder characterized by low serum levels of the antiprotease α1-antitrypsin (AAT), is caused by mutations in the SERPINA1 gene that encodes AAT.1 The result is an imbalance between AAT antiprotease activity and neutrophil elastase/protease activity in the lung, leading to the irreversible degradation of lung tissue.2 It predisposes individuals, particularly smokers, to early-onset chronic obstructive pulmonary disease (COPD)/emphysema.1 Recognized extrapulmonary manifestations include liver disease, panniculitis, and vasculitis.1 A less common but fatal extrapulmonary manifestation associated with AATD is aneurysmal disease, particularly in the aorta and cerebral arteries.3,4
Although the association between AATD and abdominal aortic aneurysm has been historically studied, it is not fully understood.4,5 Furthermore, the increased risk of aortic aneurysm in smokers may confound the data linking abdominal aortic aneurysm with AATD.6 Data regarding the potential association of AATD with ascending aortic aneurysm are limited.
An arterial aneurysm is a permanent focal distention of an arterial blood vessel to 1.5 times the usual diameter.7 The diameter of the ascending aorta in healthy individuals ranges from 3.5 to 3.8 cm in adults (<60 years of age) and naturally increases with age.8 An ascending aortic diameter of 4 cm or more is considered dilated, and a diameter of 5 cm or more is classed as an aneurysm.7 Known risk factors associated with ascending aortic aneurysm include age, male sex, general health and familial history, and smoking.9 Of these factors, age is the most strongly correlated with aortic dilation.9
Aortic aneurysms are fundamentally caused by a loss of elasticity in the vessel walls, leading to vessel stiffness and an increased propensity for dilation.10 With age, the natural degradation of elastin and smooth muscle fibers increases the risk of aortic aneurysms.10 Similarly, the risk of aneurysmal disease in patients with AATD may be increased because of uninhibited enzymatic breakdown of structural elastin, leading to loss of elasticity and weakening of the aortic wall.11
It has been observed that ascending aorta diameter was greater, with the aortic wall exhibiting abnormal elastic tissue properties, in patients with AATD compared with matched healthy controls.11 In addition, in the case of a patient with a thoracic aortic aneurysm, AATD was postulated to have been a contributing factor.12
This study assessed the relationship between ascending aortic diameter and AATD to investigate the risk that AATD confers for aortic distention and potentially aortic aneurysm.
Methods
A retrospective cohort study was performed at a single tertiary academic center. During 7 months, from August 1, 2018, to February 25, 2019, patients’ demographic data were collated. Male and female patients of any age with panlobular emphysema with available high-resolution computed tomography (CT) scans eligible for assessment were included in the study. The study was Institutional Review Board approved (Temple: 25217; Western: 20181599), and the decision to include patients was made by F.K.
The patient cohort included those with AATD-associated emphysema (AATD group) or non–AATD-associated emphysema (control group). The diagnosis of COPD/emphysema was established by clinical criteria, high-resolution CT scan of the thorax, and pulmonary function tests. The diagnosis of AATD was made in all participants by isoelectric focusing, specific genotyping by polymerase chain reaction, or next-generation sequencing or a combination of the 3 methods. The quantitative determination of AAT serum level was made by radial immunodiffusion or immunoturbidimetry (Roche AAT2). Patients’ demographic characteristics for age, sex, serum AAT levels, and SERPINA1 genotype were collated. The patient’s history of cigarette smoking was also recorded.
All high-resolution CT scans were performed with the same equipment as part of the clinical work-up for all patients and were not performed specifically for this study. The diameter of the mid-ascending aorta from outer wall to outer wall was measured by nongated axial CT scans, with or without contrast enhancement (Figure 1), by F.D., A.M., and S.S. without knowledge of whether the patient was part of the AATD or control group. The mean diameter was calculated for the AATD group and control group, with subgroup analysis by sex and age. Two-sample t-tests were used to compare between the AATD and control groups. Pearson correlation coefficient was used to analyze the association of ascending aortic diameter and age.
Figure 1.

Example of a nongated axial computed tomography scan of the mid-ascending aorta from a patient in the control group of this study.
Results
A total of 144 patients were included in the analysis; 51 patients (34%) had a confirmed AATD diagnosis, with all but 1 patient (PI∗SZ) having the phenotype PI∗ZZ (Table). The percentage of male and female patients was the same in the AATD group and the control group. There was a significant difference (P<.0001) in the number of cigarettes smoked between the groups as the mean total pack-years for patients in the AATD group was less than half that in the control group (Table). In the AATD group, there were 15 never-smokers.
Table.
| AATD group (n=51) | Control group (n=93) | |
|---|---|---|
| Serum AAT level (mg/dL) | 20.3±7.1d | 172.0±33.0 |
| Age (y) | 55.0±9.5 | 65.0±7.2 |
| Sex | ||
| Male | 29.0 (56.9) | 53 (57.0) |
| Female | 22.0 (43.1) | 40 (43.0) |
| SERPINA1 genotype/phenotype | ||
| PI∗ZZ/Z null | 50 | – |
| PI∗SZ | 1 | – |
| PI∗MM | – | 93 |
| Smoking history | ||
| Total pack-years | 30.6±19.0 | 63.0±32.2 |
AAT, α1-antitrypsin; AATD, α1-antitrypsin deficiency.
To convert α1-antitrypsin values to μmol/L, multiply by 0.184.
Categorical variables are presented as number (percentage). Continuous variables are presented as mean ± standard deviation.
Mean AAT levels calculated on the basis of 36/51 patients.
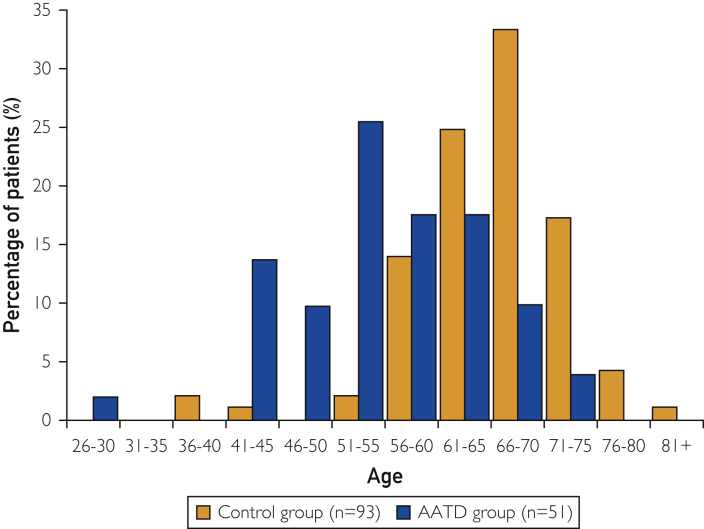
Mean serum AAT level was approximately 8.5 times lower for patients with AATD than for those in the control group (Table). At the time of assessment, patients with AATD were approximately 10 years younger than those in the control group, with a mean age of 55 vs 65 years (Table; Figure 2). In the AATD group, the age range of most patients was 51 to 55 years; in the control group, most patients were aged 66 to 70 years (Figure 2). These results are as expected, corroborating the established data that AATD leads to early-onset COPD/emphysema in individuals.
Figure 2.
Age of patients in the α1-antitrypsin deficiency (AATD) and control groups at the time of assessment.
The overall mean aortic diameter observed in patients with AATD was 3.34 cm, which aligns with the reference value of 3.38 cm reported in the American College of Radiology Imaging Network arm of the National Lung Screening Trial.13 No differences were observed in aortic diameter between patients with AATD (mean ± SD, 3.34±0.43 cm) and those in the control group (3.37±0.39 cm; P=.68). Similarly, no differences were observed in aortic diameter between the AATD group and the control group when analysis was performed for the study cohort grouped by sex (male: 3.49±0.45 cm vs 3.50±0.39 cm, respectively [P=.90]; female: 3.14±0.32 cm vs 3.18±0.31 cm, respectively [P=.68]).
For patients with AATD, a significant positive correlation was observed between aortic diameter and age (r=0.43; P=.0016; Figure 3). This relationship was not observed in the control group (r=0.16; P=.11). These data indicate that reduced serum AAT levels can lead to arterial distention in patients with AATD and that the risk of arterial distention is increased with increasing age of patients with AATD.
Figure 3.
Correlations between age and aortic diameter in the α1-antitrypsin deficiency (AATD) and control groups.
Discussion
We believe that this is the first controlled study to assess the relationship between AATD and ascending aortic diameter. Results of this study provide evidence for the use of aortic diameter measurement as a predictor for development of aortic aneurysms in patients with AATD.
Age is the most significant risk factor for the development of aortic aneurysms in healthy individuals, and it is established that AATD causes the onset of emphysema and related comorbidities in patients at an earlier age than in non–AATD-associated emphysema.1,9 Data that ascending aortic distention is significantly correlated with age of patients with AATD suggest that there is a pathologic association between AATD and aortic distention.
Although the incidence of sequelae of aortic aneurysms in PI∗ZZ and PI∗MZ AATD patients is unclear because data are limited, case reports have identified an association between AATD and ascending aortic dissection as a clinical consequence of aortic wall degeneration and distention.14, 15, 16, 17 A previous study reported that abdominal aortic distention in male patients with the PI∗ZZ genotype was due to significantly reduced elasticity of arterial walls.18 In addition, a study that examined proteomic properties of ascending aortic wall samples of aneurysms, acute dissections, and control samples found that the most significant differentially expressed protein between the pathologic and control samples was AAT (P<.05).19 Hence, in patients with AATD, the lack of AAT antiprotease activity can result in the overactivity of proteases that degrade elastin and connective tissues, resulting in a weakening of the wall of the ascending aorta and leading to aortic distention. Therefore, patients with AATD may have an increased risk for development of ascending aortic aneurysms and potentially fatal conditions such as aortic dissection.
These data echo the findings that AATD may be associated with abdominal aortic aneurysms and cerebral aneurysms, conferred from studies concluding that correlations have been observed but causality has not been confirmed.5 A study of patients with abdominal aortic aneurysms found that the proportion of patients with AATD was 14%, whereas in a study of patients with cerebral aneurysms, the proportion of patients with AATD was 11%.20,21 Furthermore, in an observational study of patients with intracranial aneurysms, the incidence of individuals with AATD with heterozygous genotype (PI∗MS and PI∗MZ) was 16% compared with 7% in the general population, and 1% (1 patient) were AATD homozygous genotype (PI∗ZZ) compared with an expected 1.5%.22 These observations indicate that the incidence of AATD is higher than expected in patients with aneurysms than in the general population, suggesting that AATD is a genetically associated risk factor for the development of aneurysmal disease. Thus, it is recommended that patients with aortic aneurysms be tested for AATD.
In this study, almost all patients in the AATD group were homozygous PI∗ZZ genotype (1 patient was PI∗SZ), a genotype that has previously been associated with ascending aortic aneurysms.11,23,24 In contrast, a number of studies have not found an association between AATD and the PI∗ZZ genotype for abdominal aortic aneurysm but have observed an association with heterozygous genotypes, including PI∗MS and PI∗MZ.21,25,26 In addition, it has been reported that both heterozygous and homozygous AATD genotypes may be risk factors for the development of cerebral aneurysms.3,20,22 Therefore, further investigation of AATD genotypes and the relationship with aortic distention is warranted.
Building on evidence from previous reports, the findings of this study suggest that routine assessment and follow-up of patients with AATD should be amended to include reporting of the aortic diameter and the evaluation of aortic diameter changes followed longitudinally in individuals. Because patients with AATD often have regular CT scans for assessment of respiratory disease progression, it would not be a great burden to the patient or the physician for these data to be collected and analyzed. Implementing these suggested changes in clinical practice may provide significant benefit by identifying patients with AATD at risk of aneurysmal disease.
Conclusion
α1-antitrypsin deficiency is commonly associated with lung and liver diseases; however, some patients can present with vascular disorders. In a cohort of patients with COPD/emphysema, significant age-related acceleration of ascending aortic distention was observed in only those with AATD-associated disease, suggesting that AATD has a pathologically relevant role in aortic wall degradation. More frequent assessment of aortic diameter may be warranted in patients with AATD to analyze the risk for development of aortic aneurysm. Moreover, individuals with known aortic distention or aortic aneurysm who present with a history of or current respiratory complications should be tested for AATD to confirm or to rule out AATD diagnosis.
Acknowledgments
Medical writing support was provided by Amrita Shergill of Meridian HealthComms (Plumley, United Kingdom) in accordance with good publication practice (GPP3), funded by CSL Behring.
Footnotes
Grant Support: Medical writing support for this manuscript was funded by CSL Behring. The funder had no role in the study design; in the collection, analysis, and interpretation of data; and in the decision to submit the article for publication.
Potential Competing Interests: The authors report no competing interests.
References
- 1.Miravitlles M., Dirksen A., Ferrarotti I. European Respiratory Society statement: diagnosis and treatment of pulmonary disease in α1-antitrypsin deficiency. Eur Respir J. 2017;50(5):1700610. doi: 10.1183/13993003.00610-2017. [DOI] [PubMed] [Google Scholar]
- 2.Dżeljilji A., Rokicki W., Karuś K. Selected metabolic aspects of elastin and collagen fiber proteolysis in diseases of the respiratory system—the significance of α1 antitrypsin deficiency. Kardiochir Torakochirurgia Pol. 2016;13(3):242–247. doi: 10.5114/kitp.2016.62614. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Schievink W.I., Prakash U.B., Piepgras D.G., Mokri B. Alpha 1-antitrypsin deficiency in intracranial aneurysms and cervical artery dissection. Lancet. 1994;343(8895):452–453. doi: 10.1016/s0140-6736(94)92693-x. [DOI] [PubMed] [Google Scholar]
- 4.Cohen J.R., Mandell C., Margolis I., Chang J., Wise L. Altered aortic protease and antiprotease activity in patients with ruptured abdominal aortic aneurysms. Surg Gynecol Obstet. 1987;164(4):355–358. [PubMed] [Google Scholar]
- 5.Dżeljilji A., Chorostowska-Wynimko J., Szewczyk D., Popiołek W., Kierach A., Andziak P. The role of alpha-1-antitrypsin protein in the pathogenesis of abdominal aortic aneurysm. Acta Angiologica. 2018;24(1):30–33. [Google Scholar]
- 6.Wilmink T.B., Quick C.R., Day N.E. The association between cigarette smoking and abdominal aortic aneurysms. J Vasc Surg. 1999;30(6):1099–1105. doi: 10.1016/s0741-5214(99)70049-2. [DOI] [PubMed] [Google Scholar]
- 7.Hiratzka L.F., Bakris G.L., Beckman J.A. 2010 ACCF/AHA/AATS/ACR/ASA/SCA/SCAI/SIR/STS/SVM guidelines for the diagnosis and management of patients with thoracic aortic disease. Circulation. 2010;121(13):e266–e369. doi: 10.1161/CIR.0b013e3181d4739e. [DOI] [PubMed] [Google Scholar]
- 8.Mao S.S., Ahmadi N., Shah B. Normal thoracic aorta diameter on cardiac computed tomography in healthy asymptomatic adults: impact of age and gender. Acad Radiol. 2008;15(7):827–834. doi: 10.1016/j.acra.2008.02.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Sawabe M., Hamamatsu A., Chida K., Mieno M.N., Ozawa T. Age is a major pathobiological determinant of aortic dilatation: a large autopsy study of community deaths. J Atheroscler Thromb. 2011;18(2):157–165. doi: 10.5551/jat.6528. [DOI] [PubMed] [Google Scholar]
- 10.Saliba E., Sia Y. The ascending aortic aneurysm: when to intervene? Int J Cardiol Heart Vasc. 2015;6:91–100. doi: 10.1016/j.ijcha.2015.01.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Vizzardi E., Corda L., Pezzali N. Elastic properties of the ascending aorta in patients with α1-antitrypsin deficiency (Z homozygotes) Heart. 2012;98(18):1354–1358. doi: 10.1136/heartjnl-2012-302144. [DOI] [PubMed] [Google Scholar]
- 12.Pizarro C., Skowasch D., Nickenig G., Thomas D., Kaminski M. Spontaneous rupture of aneurysmatic pulmonary artery in homozygotic alpha-1-antitrypsin deficiency [in German] Dtsch Med Wochenschr. 2015;140(1):39–41. doi: 10.1055/s-0040-100417. [DOI] [PubMed] [Google Scholar]
- 13.McComb B.L., Munden R.F., Duan F., Jain A.A., Tuite C., Chiles C. Normative reference values of thoracic aortic diameter in American College of Radiology Imaging Network (ACRIN 6654) arm of National Lung Screening Trial. Clin Imaging. 2016;40(5):936–943. doi: 10.1016/j.clinimag.2016.04.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Blair H.C., Zorn G., Young K.R., McGiffin D.C. Aortic degeneration in alpha1-antitrypsin deficiency. Histopathology. 2000;37(4):377–378. doi: 10.1046/j.1365-2559.2000.00997-2.x. [DOI] [PubMed] [Google Scholar]
- 15.Ibrahem D.A., Eljaili D.A., Butt D.U.A., Eley D.W., Vangara D.J.I., Hanna D.A. Aortic dissection in patient with alpha 1 antitrypsin deficiency. https://www.eurorad.org/case/15525
- 16.Leppard W.M., Ikonomidis J.S. Type A aortic dissection in association with alpha-1-antitrypsin deficiency. Am Surg. 2011;77(7):E136–E137. [PubMed] [Google Scholar]
- 17.Tatsumi K., Hamai Y., Mizota T., Fukuda K. Intraoperative aortic dissection during lung transplantation in a patient with alpha-1 antitrypsin deficiency. Masui. 2017;66(5):530–534. [PubMed] [Google Scholar]
- 18.Ahlgren A.R., Piitulainen E., Sonesson B., Lanne T. Changes in aortic wall stiffness in men with alpha 1-antitrypsin deficiency. Eur J Vasc Endovasc Surg. 1997;14(4):252–257. doi: 10.1016/s1078-5884(97)80236-5. [DOI] [PubMed] [Google Scholar]
- 19.Schachner T., Golderer G., Sarg B. The amounts of alpha 1 antitrypsin protein are reduced in the vascular wall of the acutely dissected human ascending aorta. Eur J Cardiothorac Surg. 2010;37(3):684–690. doi: 10.1016/j.ejcts.2009.07.025. [DOI] [PubMed] [Google Scholar]
- 20.Kueppers F., Sultan F., Weaver M., Sanders C. Alpha-1 antitrypsin levels in patients with cerebral aneurysms. Chest. 2015;148:498A. [Google Scholar]
- 21.Pini L., Bonardelli S., Ferrarotti I. Alpha-1 antitrypsin deficiency and abdominal aortic aneurysms: does this association really esisti? Am J Respir Crit Care Med. 2015;191:A2745. [Google Scholar]
- 22.Schievink W.I., Katzmann J.A., Piepgras D.G., Schaid D.J. Alpha-1-antitrypsin phenotypes among patients with intracranial aneurysms. J Neurosurg. 1996;84(5):781–784. doi: 10.3171/jns.1996.84.5.0781. [DOI] [PubMed] [Google Scholar]
- 23.Cohen J.R., Sarfati I., Ratner L., Tilson D. Alpha 1-antitrypsin phenotypes in patients with abdominal aortic aneurysms. J Surg Res. 1990;49(4):319–321. doi: 10.1016/0022-4804(90)90029-2. [DOI] [PubMed] [Google Scholar]
- 24.Ramsbottom D., Fitzgerald P., Grace P.A. Biochemical and molecular genetic studies of abdominal aortic aneurysm in an Irish population. Eur J Vasc Surg. 1994;8(6):716–722. doi: 10.1016/s0950-821x(05)80652-8. [DOI] [PubMed] [Google Scholar]
- 25.Schardey H.M., Hernandez-Richter T., Klueppelberg U., Tutsch-Bauer E., Lauterjung L. Alleles of the alpha-1-antitrypsin phenotype in patients with aortic aneurysms. J Cardiovasc Surg (Torino) 1998;39(5):535–539. [PubMed] [Google Scholar]
- 26.Elzouki A.N., Eriksson S. Abdominal aortic aneurysms and alpha 1-antitrypsin deficiency. J Intern Med. 1994;236(5):587–591. doi: 10.1111/j.1365-2796.1994.tb00850.x. [DOI] [PubMed] [Google Scholar]